Aquaculture
journal homepage: www.elsevier.com/locate/aquaculture
Effects of dietary different canthaxanthin levels on growth performance, antioxidant capacity, biochemical and immune-physiological parameters of white shrimp (Litopenaeus Vannamei)
Samia Fawzy a, f, 1 , Weilong Wang a, b, c, 1 , Meiqin Wu d , Ganfeng Yi a, e , Xuxiong Huang a, b, c, *
a Centre for Research on Environmental Ecology and Fish Nutrition of the Ministry of Agriculture and Rural Affairs, Shanghai Ocean University, China
b Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-culture of Aquaculture Animals, Shanghai Ocean University, China
c National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, China
d College of Marine Ecology and Environment, Shanghai Ocean University, Shanghai 201306, China
e Beijing Dabeinong Technology Group Co., Ltd, Beijing 100080, China
f Department of Animal Wealth Development, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
ARTICLE INFO
Keywords:
Canthaxanthin
Growth performance
Pigmentation
Antioxidant
Litopenaeus vannamei
ABSTRACT
An 8-week feeding trial was conducted to evaluate the effects of different dietary canthaxanthin (CX) levels on growth performance, pigmentation, antioxidant capacity, hemolymph biochemical parameters, immune response, and resistance to hypoxia stress of white shrimp (Litopenaeus vannamei). Juveniles (initial weight 1.15 ± 0.12 g) were fed with five iso nitrogenous and isolipidic diets supplemented with/without CX: 0 (control), 50, 100, 200, and 400 mg kg 1 diet. Results showed that the growth performance, survival, and feed conversion ratio improved significantly in CX supplemented treatments compared to the control. The redness of cooked shrimp tended to increase with increasing CX level; however, no significant difference was observed among the treatments that received CX of more than 100 mg kg 1 Further, the activities of digestive enzymes, total antioxidant capacity, and peroxidase increased significantly (P < 0.05), while the activities of glutathione peroxidase, catalase, and malondialdehyde decreased significantly (P < 0.05) in the hepatopancreas with increasing dietary CX levels. The immune-related enzymes (alkaline phosphatase, lysozyme, alanine aminotransferase, and aspartate aminotransferase) and hemolymph biochemical parameters (cholesterol, high-density, and low-density lipoprotein) are significantly affected by different dietary CX levels. Total carotenoids and astaxanthin contents in shrimp muscle, shell, head, and whole-body showed an upward trend with the increment of dietary CX. After exposure to hypoxia stress, juveniles in 200 mg kg 1 supplementation treatment exhibited the highest LT50 value among all the treatments. Moreover, broken-line regression analysis indicated that a dose of 173.73 to 202.13 mg kg 1 of CX was suitable in the diet of L. vannamei as a potential carotenoid source for substituting dietary astaxanthin in the shrimp feed industry.
1. Introduction
Shrimp has tremendous importance among seafood products due to the significant economic benefits it yields (Mialhe et al., 2013). White shrimp (L. vannamei) is a major cultivated species which exhibits rapid growth rate and tolerance to a wide range of environmental conditions such as temperature and salinity (Landsman et al., 2019; Li et al., 2017). Nowadays, intensive shrimp farming is widely practiced to meet the
market demand and generate more profit (Shinji et al., 2019). As a result of this intensification, shrimp are losing their natural red color and suffering from “blue disease” (Chien and Jeng, 1992). Unfortunately, crustaceans do not have the pathway for de novo synthesis of the pigments (Goodwin, 1984). Under such conditions, only the dietary intervention through carotenoids supplementation can maintain the desired color, improve the product quality and further raise their market price (Kalinowski et al., 2005; Parisenti et al., 2011). The typical red color of
* Corresponding author at: Centre for Research on Environmental Ecology and Fish Nutrition of the Ministry of Agriculture and Rural Affairs, Shanghai Ocean University, China.
E-mail address: xxhuang@shou.edu.cn (X. Huang).
1 These authors have contributed equally to this work and share first authorship.
https://doi.org/10.1016/j.aquaculture.2022.738276
Received 3 January 2022; Received in revised form 30 March 2022; Accepted 18 April 2022
Availableonline21April2022
0044-8486/©2022PublishedbyElsevierB.V.
crustaceans could be obtained by incorporating astaxanthin into their diets (Niu et al., 2009; Wade et al., 2017a). Nevertheless, astaxanthin is an expensive carotenoid supplement that leads to a significant increase in feed costs despite constituting a small portion from their diet (Hansen et al., 2016). In this regard, several trials have been done to find other carotenoid sources that can come up with results similar to those of astaxanthin but at lower prices. Ample evidence had proven that canthaxanthin (CX) could be as effective as, if not better than, astaxanthin in pigmentation of Atlantic salmon (Buttle et al., 2001) and red porgy (Kalinowski et al., 2015). Additionally, it has been reported that shrimp could efficiently utilize dietary synthetic CX and deposit it as astaxanthin in their tissues (Yamada et al., 1990). Consequently, CX would be a substrate for astaxanthin, the most abundant carotenoid in crustaceans.
CX (β,β-carotene-4,4′ -dione), also known as red ketocarotenoid, is a member of xanthophylls and is widely used as a feed additive in aquaculture (Rebelo et al., 2020). Its market value was estimated at 75 million dollars in 2017 and is expected to hit 85 million dollars by 2024, with aquaculture and livestock sectors utilizing about 40% of the total volume (Mussagy et al., 2021). Moreover, synthetic CX is considered the primary dietary source available in the market that could provide sufficient supply as the natural sources are limited (Pi et al., 2020; Sanchez et al., 2013). Due to its attractive color and pigmentation property, CX was added predominantly to the diets of Salmon, Rainbow trout (Baker et al., 2002; Buttle et al., 2001), and Penaeus monodon shrimp (Niu et al., 2012) for their flesh color enhancement. In addition to color improvement, previous studies have reported multiple benefits for CX, such as growth promotion, antioxidant activities, and immune modulation properties (Elia et al., 2019; Shahidi and Brown, 1998; Venugopalan et al., 2013). The antioxidative role of carotenoids is closely associated with improving the resistance of aquatic animals to the stressors during the culture period (Niu et al., 2009). Oxygen depletion (hypoxia) generally results in oxidative stress and increased ROS release, which negatively affects the survival and growth performance of shrimp (Zhang et al., 2013). It had been reported that the increase in dietary and body carotenoids could alleviate the destructive effects of hypoxia, so CX as a carotenoid may be effective in such cases (Chien and Shiau, 2005).
Therefore, CX, with its numerous properties and relatively low market price compared with astaxanthin, may be a potential carotenoid source in the shrimp diet. As long as the utilization efficiency differs from one species to another, it was essential to study the effect of dietary CX on L. vannamei Based on this background, the current study was carried out to investigate the effect of different dietary CX levels on growth performance, pigmentation, antioxidant activity, immune response, and resistance to hypoxia stress of L. vannamei The findings of the current study would contribute to achieving a cost-effective feed formulation to control the blue disease of farmed shrimp.
2. Materials and methods
2.1. Diet formulation and preparation
A chemically synthesized pigment (CarophyllⓇ yellow) containing 10% CX produced by DSM Nutrition, Switzerland, was used as a source for CX in the current experiment. Five semi-purified pelleted diets were formulated to be iso nitrogenous and isolipidic as described in Table 1 and supplemented with different levels of CX (0, 50, 100, 200, 400 mg kg 1 of diet). Casein was used as the major protein source. Crystalline amino acids were added to meet the amino acid requirement of this species according to Zhou et al. (2012) Fish oil, soybean lecithin, cholesterol, and PUFA were used as the lipid source. The mineral and vitamin mixtures were added according to Xie et al. (2012) with slight modifications. The diet-making process started with weighing all dry ingredients and mixing thoroughly with a mixer (B10, Rudong, China) for 10–15 min. Lipid ingredients and fat-soluble components were also
Table 1
Formulation and composition of the experimental diets (g
, dry matter basis).
Ingredients Treatments
a Yuehai Feed Group Co., Ltd., Zhejiang, China.
b Shanghai Macklin Biochemical Co., Ltd., Shanghai, China.
c PUFA: (eicosapentaenoic acid) EPA 4 g and (docosahexaenoic acid) DHA 4 g, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China.
d Vitamin mixture (12 g kg 1 diet) (vitamin A free): p-aminobenzoic acid 0.092 g; biotin 0.004 g; inositol 3.668 g; nicotinic acid 0.368 g, Ca-pantothenate 0.552 g; pyridoxine-HCl 0.112 g; riboflavin 0.072 g; thiamine-HCl 0.036 g; menadione 0.036 g, α-tocopherol 0.184 g; cyanocobalamin 0.0008 g; cholecalciferol 0.012 g; stay-C 1.36 g, folic acid 0.008 g; choline chloride 5.5 g.
e Mineral mixture (34.38 g kg 1 diet): C6H10CaO6 5H2O, 1.75; K2HPO4, 11.98; MgSO4.7H2O, 12.28; NaH2PO4, 7.04; C6H5O7Fe 5H2O, 0.23; CuSO4.5H2O, 0.34; ZnSO4 7H2O, 0.48; CoCl2, 0.07; MnSO4 H2O, 0.21.
f Canthaxanthin: CarophyllⓇ Yellow containing 10% canthaxanthin made by DSM Nutrition, Switzerland.
premixed and then added to the dry ingredients with continuous mixing. Subsequently, water (30% of dry ingredients mixture) was added and carefully mixed for another 10 min. Finally, the blend was transferred into a single-screwed mincer to produce pellets (1.5 mm in diameter). The pellets were then dried in a dryer mechanical convection oven at 35 ◦ C until moisture content was reduced to about 10%. Once the experimental diets were dried, they were packed and stored at 20 ◦ C until use. The approximate analysis of the diets is shown in Table 2
2.2. Shrimp and culture conditions
Juvenile white shrimp (L. vannamei) were
Table 2
Proximate composition (g kg 1 , dry matter basis) of the experimental diets.a Parametersa Treatments
a Data were expressed as mean ± S.E.M from triplicate groups. CX, canthaxanthin.
b ND: not detected.
Haixingnong Aquaculture Cooperatives (Shanghai, China). Juveniles were maintained in a cement pool at 27 ± 0.5 ◦ C and fed the control diet for two weeks to acclimate to water conditions. After the acclimation, six hundred juveniles with mean initial weight (1.15 ± 0.12 g) were selected and randomly distributed into 15 PVC net cages (Length = 1 m, Width = 1 m, and Height = 1.2 m) fitted inside a cement pond (Length = 10 m, Width = 5 m, and Height = 1.5 m) corresponding to 40 shrimp/ cage. The cement tanks used in the study were provided with a drainage system and water flow system. Each tank’s water was changed every two days to two-thirds of its capacity. Each experimental diet was tested in triplicate in a randomized design. The experiment was carried out indoor and lasted for 56 days. During this period, juveniles were manually fed four times per day (7:00, 12:00, 17:00, and 22:00). A fixed feeding method was applied in which the shrimp were fed at 5–8% of their body weight. In order to determine the amount of feed required, a weekly random sampling was done to check the average body weight of shrimp. The diet was introduced through disk-shaped feeders placed inside the rearing nets. Therefore, the uneaten feed was kept inside the feeders and could be easily collected. Three hours after feeding, the uneaten feed was collected while the fecal matter was siphoned out of the cages. All uneaten food was freeze-dried to calculate feed intake and feed efficiency ratio. During the experiment, water conditions were measured everyday: temperature, 29–32 ◦ C; dissolved oxygen concentration, > 5 mg L 1; ammonia nitrogen, < 0.05 mg L 1; pH, 8.0–8.5; salinity, 1–2‰.
2.3. Determination of growth performance parameters
At the end of 8 weeks feed trial, all the shrimp were fasted for 24 h, and the survivors from each cage counted and weighed to evaluate the growth performance based on the following formulae:
Body weight gain (BWG, %) = [(final weight-initial weight)/initial weight] × 100.
Specific growth rate (SGR, % day 1) = [(Ln final weight-Ln initial weight)/duration] × 100.
Feed conversion ratio (FCR) = dry weight of feed consumed (g)/live weight gain (g).
Survival (%) = (final number of shrimp/initial number of shrimp) × 100.
2.4. Sampling techniques
After counting and weighing, 15 shrimp from each treatment (5 shrimp per cage × 3 replicates) were randomly sampled and used to analyze whole-body composition and carotenoid content. Using a 1-mL sterile syringe, hemolymph was collected from the ventral sinus of 10 shrimp per cage. Also, the hepatopancreas of 6 shrimp from each cage were dissected out and collected separately in 5 mL tubes. Hemolymph and hepatopancreas samples were frozen immediately in liquid nitrogen, while whole-body shrimp samples were chilled in ice. Finally, all samples were transported to the laboratory and stored at 80 ◦ C until analysis.
2.5.
Determination of biochemical parameters
According to the Association of Official Analytical Chemists, the proximate composition (crude protein, crude lipid, and ash) of whole shrimp and experimental diets were determined after freeze-drying and grinding (AOAC, 2006). Briefly, crude protein was analyzed using the Kjeldahl method (2300-Autoanalyzer, Foss Tecator, Sweden). Crude lipid was determined by the ether extraction method using Soxtec System HT (Soxtec System HT6, Sweden). Ash content was determined by pre-incineration on a hot plate followed by 6 h in a muffle furnace at 550 ◦ C.
For assaying enzymatic activities, serum was obtained from the supernatant by centrifuging the hemolymph at 10000 rpm, 4 ◦ C for 10 min. Parameters including urea, cholesterol, triglycerides, high-density
lipoprotein (HDL), and low-density lipoprotein (LDL) were measured by the colorimetric method performed using a chemistry analyzer (iChem340, China). Serum was also used for the determination of nonspecific immune parameters, including lysozymes (LZM), aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatase (AKP). All enzymes were assayed using Nanjing Kits (Nanjing Jiancheng Bioengineering Institute, China) according to the instructions of the manufacturer.
Furthermore, hepatopancreas was prepared by homogenization, dilution with shrimp saline solution and centrifugation at 8000 rpm, 4 ◦ C for 15 min (Wang et al., 2019). Hepatopancreas samples were used for assaying the activities of antioxidant enzymes, including total antioxidant capacity (T-AOC), peroxidase (POD), glutathione peroxidase (GSH-Px), Malondialdehyde (MDA), catalase (CAT), and digestive enzymes (protease, lipase, and amylase). Nanjing Kits (Nanjing Jiancheng Bioengineering Institute, China) were used according to the instructions of the manufacturer.
2.6. Color reading
The color was assessed using a colorimeter (Chroma Meter CR400, Konica Minolta Sensing Inc., Osaka, Japan) after putting shrimp samples into plastic bags and placed into a boiling water bath for 3 min and cooling in tap water (about 5 ◦ C) (Ju et al., 2011). The color values were expressed according to CIE L * a*b* color space (Nickell and Bromage, 1998), with L * , a * , and b* representing lightness, redness, and yellowness of the cooked shrimp, respectively.
2.7. Determination of carotenoid content
Total carotenoid content in the whole-body shrimp, shell (including carapace, telson, and uropod), muscle, and head tissues (without hepatopancreas) was quantified spectrophotometrically. Dried and crushed samples of the whole body (1.5 g), muscle (2 g), shell (1 g), and head (1 g) were weighed and placed separately into 50 mL polypropylene tubes in triplicates. Chloroform was added to the samples, thoroughly mixed for 10 min, centrifuged at 4 ◦ C at 10000 rpm for 5 min, and stored for 2 h in darkness. This step was repeated three times until the extract became completely clear. The extracts were collected and pooled into new tubes, then dried in a vacuum evaporator (Eyela SB 1100, Japan). After that, 8 mL acetone was added to each dried sample. 3 mL of the solution were used for measuring the carotenoid concentration using a spectrophotometer (Puxi T6, China) at 475 nm and E1% value of 2100 against a blank of acetone (Wang et al., 2021).
The remaining 5 mL were dried by N2 and delivered for Ultra Performance Liquid Chromatography (UPLC, Waters ACQUITY, USA) analysis after re-dissolving in 2 mL of mobile phase solution (Acetonitrile: Methanol, 70:30 v/v). UPLC was equipped with Waters ACQUITY H-Class BEH C18 column (1.7 μm, 2.1 mm × 150 mm). The mobile gradient phase consisted of A (100% dH20), B (Acetonitrile: Methanol, 70:30 v/v), and C (100% methyl tert-butyl ether). Initial ratio of 5% A: 95% B for 16 min, 5% A: 10% B: 85% B for 39 min, then returned to initial ratio over 4 min at a flow rate of 0.2 mL/min. An ultravioletvisible detector set quantified relative amounts of carotenoids to 475 nm by calculating the area under the curve of each peak and it to a known standard. The standards of astaxanthin, CX, echinenone, zeaxanthin, and β-carotene were purchased from Sigma-Aldrich (USA), and the β-cryptoxanthin standard was purchased from CaroteNature (Swit). Since relatively significant differences were detected in the amount of free and esterified carotenoids in shrimp tissues, only the free-type carotenoids were considered and presented in the current study.
2.8. Hypoxia stress resistance test
Fifteen shrimp from each treatment were randomly selected after the feeding trial and distributed into 5 L white plastic buckets in triplicates
with five shrimp per bucket. Each bucket was filled with the same water (temperature 28 ◦ C, salinity 1‰, pH 8.2, and DO 1 mg L 1). DO in the test buckets was measured using an oxygen meter (HACH HQ30d, USA), while low DO conditions were maintained by nitrogen gas bubbling. Shrimp survival was recorded and expressed as LT50 for each hour during the resistant test. LT50 (the time required for 50% mortality of tested shrimp by a stressor) was calculated by a regression equation according to Yokoyama et al. (2005). The equation is as the following:
Y = a X + b
X = time to individual death of shrimp.
LT50 (X) was obtained when Y = log10 (50) =1.7.
The equation resulted from the plotting of values of log survival against the time of mortality to determine LT50 for each treatment. The values obtained from the equation were statistically compared, and the higher value indicated a greater tolerance to hypoxia stress.
2.9. Statistical analysis
The statistical analyses were performed using SPSS 20.0. All data are presented as means ± standard error of the mean (S.E.M., n = 3). Oneway analysis of variance was used to test the differences among the treatments at p-value (P < 0.05), followed by Duncan’s multiple range test to compare the treatments’ means. The broken-line analysis was performed using OriginPro 2021. The relationships among parameters were estimated by Pearson coefficient of correlation, thus visualized by heatmap of correlation, using ggcorrplot package (ver. 0.1.2.999) and heatmaply package (ver. 1.3.0) with R version 4.1.2.
2.10. Ethical statement
The present experimental procedures were carried out in strict accordance with the recommendations in the ethical guidelines of EU Directive 2010/63/EU for animal experiments.
3. Results
3.1. Growth performance parameters and feed utilization
Growth performance (FBW, BWG, and SGR), FCR, and the survival rate of shrimp fed with experimental diets are given in Table 3 The results indicated that all the parameters as mentioned above improved significantly in shrimp fed with CX supplemented diets compared to those fed the control diet (P < 0.05). CX400 treatment had the lowest FBW, BWG, and SGR among CX supplemented treatments, while there was no significant difference (P > 0.05) in survival among them.
Table 3
Growth parameters and nutrient utilization of Litopenaeus vannamei fed the experimental diets for 56 days.
Parametersa Treatments
3.2.
Shrimp whole-body composition
The findings of the shrimp whole-body composition analysis are presented in Table 4 CX50 and CX100 treatments differed significantly (P < 0.05) from the control in crude protein content. In contrast, dietary CX feeding did not significantly affect (P > 0.05) the crude lipid and ash content of the shrimp body.
3.3. Body color reading
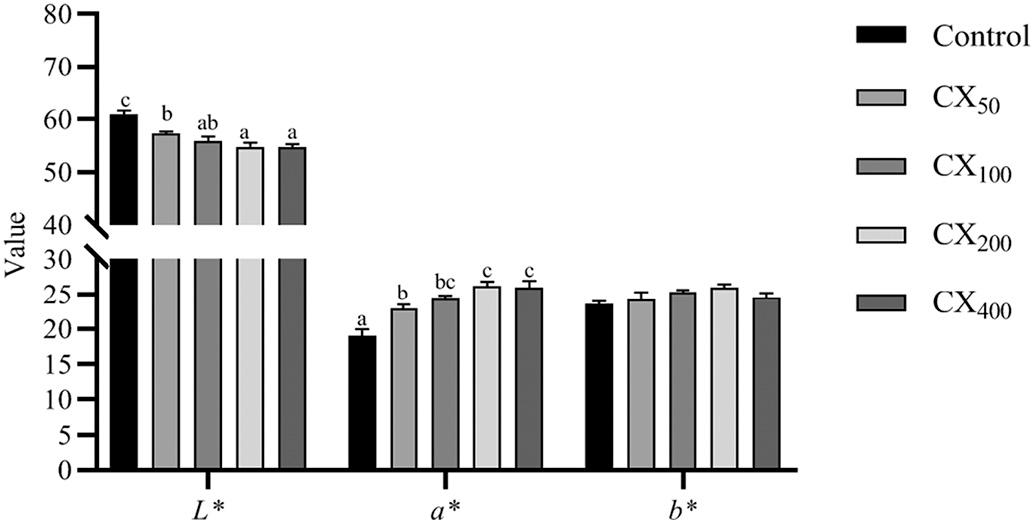
The color reading values of cooked shrimp fed different dietary CX levels are shown in Fig. 1 Shrimp fed with CX-containing diets exhibited higher (P < 0.05) redness (a *) values than the control treatment, however, lightness (L * ) showed an opposite trend. Further, CX100, CX200, and CX400 treatments were similar in a* and L* values. Although yellowness (b*) values were higher in CX fed treatments than in the control, no significant difference (P > 0.05) was observed among treatments.
3.4. Antioxidant capacity
Dietary manipulation significantly affected the hepatopancreatic antioxidant parameters of L. vannamei (Table 5). T-AOC showed a significant increase (P < 0.05) in shrimp-fed CX-containing diets compared to those fed the control diet. POD activity showed a similar trend, but CX400 treatment did not exhibit a significant difference with the control treatment. On the contrary, GSH-Px, MDA, and CAT levels decreased significantly in CX supplemented treatments (P < 0.05) compared to the control treatment.
3.5. Hemolymph biochemical and hemato-immunological parameters
The immunological parameters in the hemolymph of L. vannamei fed with different CX levels were represented in Table 6 The findings cleared that the AKP and LZM activities tended to increase markedly (P < 0.05) when dietary CX levels increased, while AST and ALT showed an opposite trend. However, a higher dose of CX (400 mg kg 1) caused neither a further significant increase in AKP and LZM nor a significant decrease in AST and ALT.
The results of hemolymph biochemical parameters of the studied shrimp are shown in Table 7. The findings showed that with increasing dietary CX levels, cholesterol showed a significant decreasing trend compared to the control treatment (P < 0.05). The lowest levels of HDL, LDL, and TP were observed in the control treatment, which significantly differs (P < 0.05) from the higher CX supplemented treatments (CX200 and CX400). In contrast, the parameters of triglycerides and urea in experimental treatments did not show a significant difference (P > 0.05) among all the treatments.
3.6. Digestive enzymes activities
The effect of dietary CX on digestive enzymes activities is shown in
Table 4
Whole-body proximate analysis (g kg 1 , dry matter basis) of Litopenaeus vannamei fed with experimental diets for 56 days.
a FBW, final body weight; FCR, feed conversion ratio; BWG, body weight gain; SGR, specific growth rate. Data were expressed as mean ± S.E.M from triplicate groups. Mean values in the same row with different letters are significantly different (P < 0.05).
a Data were expressed as mean ± S.E.M from triplicate groups. Mean values in the same row with different letters are significantly different (P < 0.05).
Fig. 1. Color parameters of cooked whole-body shrimp fed with different levels of canthaxanthin for 56 days. Data were expressed as mean ± S.E.M from triplicate groups. Bars with different letters represented significant differences between various treatments (P < 0.05).
Table 5
Effect of dietary different canthaxanthin levels on hepatopancreatic antioxidant statuses of Litopenaeus vannamei.
Parametersa
Treatments
a Data were expressed as mean ± S.E.M from triplicate groups. Mean values in the same row with different letters are significantly different (P < 0.05). T-AOC, total antioxidant capacity; POD, peroxidase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde. CAT: catalase.
Table 6
Effect of dietary different canthaxanthin levels on hemato-immunological parameters of Litopenaeus vannamei.
Parametersa
a Data were expressed as mean ± S.E.M from triplicate groups. Mean values in the same row with different letters are significantly different (P < 0.05). AKP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, Alanine aminotransferase; LZM, lysozyme.
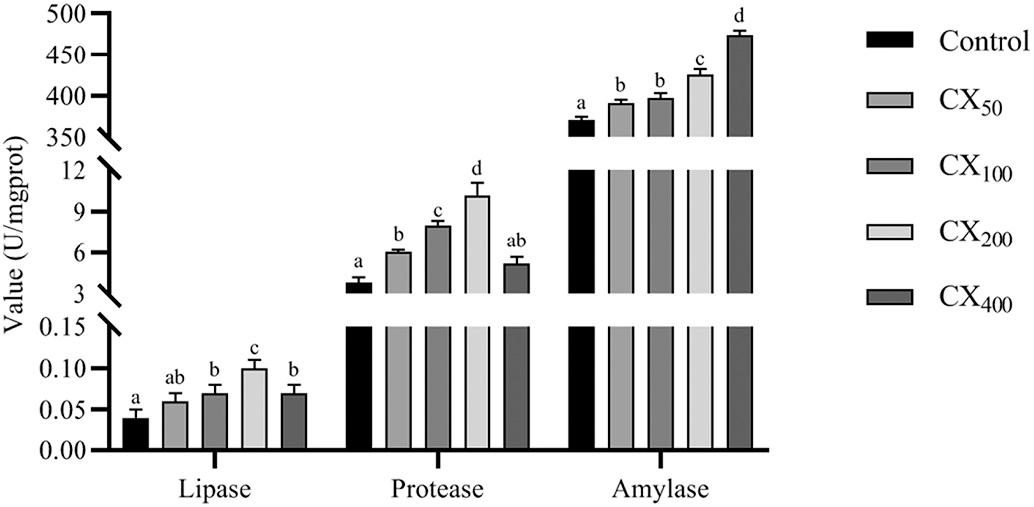
Fig. 2 It was observed that the dietary CX inclusion significantly (P < 0.05) affected the activities of digestive enzymes in shrimp hepatopancreas. The highest (P < 0.05) protease and lipase activities were detected in CX200 treatment, while amylase was detected in the CX400 treatment. The control treatment had the lowest levels (P < 0.05) of the three enzymes.
3.7. Carotenoid contents
The total carotenoid contents of different tissues of L. vannamei fed
Table 7
Effect of dietary different canthaxanthin levels on hemolymph biochemical parameters of Litopenaeus vannamei.
a Data were expressed as mean ± S.E.M from triplicate groups. Mean values in the same row with different letters are significantly different (P < 0.05). HDL, high-density lipoprotein; LDL, low-density lipoprotein; CHO, cholesterol; TG, triglycerides; TP, total protein.
Fig. 2. Effect of dietary different canthaxanthin levels on digestive enzymes activities in hepatopancreas of Litopenaeus vannamei Data were expressed as mean ± S.E.M from triplicate groups. Bars with different letters represented significant differences between various treatments (P < 0.05).
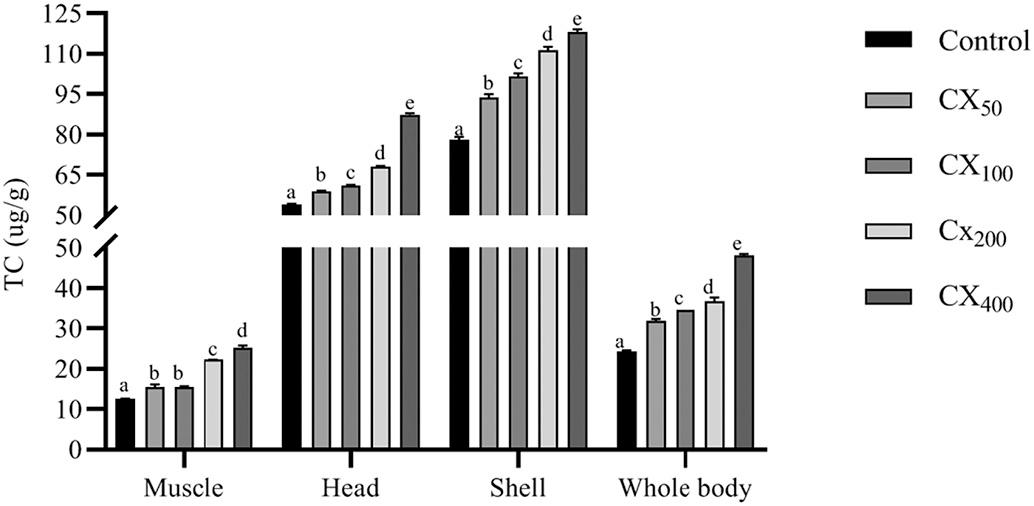
with experimental diets for 56 days are shown in Fig. 3 With increasing the dietary CX levels, the total carotenoid content in different tissues significantly increased (P < 0.05). The highest carotenoid content in the muscle, head, shell, and whole-body was observed in shrimp fed with 400 mg kg 1 CX. Meanwhile, the lowest content was found in the control treatment.
The profile of carotenoids in various tissues of L. vannamei (muscle, shell, head, and whole-body) analyzed by UPLC was given in Table 8.
Fig. 3. Effect of different dietary canthaxanthin levels on total carotenoid content in different tissues of Litopenaeus vannamei. Data were expressed as mean ± S.E.M from triplicate groups. Bars with different letters represented significant differences between various treatments (P < 0.05). TC, total carotenoid.
S. Fawzy
Table 8
Effect of dietary canthaxanthin levels on carotenoids composition in tissues (μg g 1) of Litopenaeus vannamei.
Tissue CDa Treatments
Muscle Astaxanthin
β-Carotene 0.09 ± 0.01a 0.15 ± 0.01b 0.18 ± 0.01b 0.23 ± 0.02c 0.31 ± 0.02d
Head
β
Shell
±
β-Cryptoxanthin 1.26 ± 0.10a 1.63 ± 0.18ab 1.77 ± 0.06b 1.80 ± 0.12b 2.74 ± 0.11c Echinenone 1.65 ± 0.21a 3.02 ± 0.18b 3.06 ± 0.41b 3.76 ± 0.23b 5.03 ± 0.01c
β-Carotene 2.77 ± 0.15a 3.88 ± 0.36ab 4.07 ± 0.34b 4.31 ± 0.25b 5.07 ± 0.54b
Whole body
Astaxanthin 0.54 ± 0.05a 0.99 ± 0.04b 1.39 ± 0.10c 1.64 ± 0.02d 2.13 ± 0.02e
Canthaxanthin 0.05 ± 0.01a 0.17 ± 0.01b 0.21 ± 0.01b 0.30 ± 0.01c 0.34 ± 0.03c
Zeaxanthin 0.04 ± 0.01a 0.08 ± 0.01b 0.10 ± 0.01bc 0.12 ± 0.01c 0.15 ± 0.01d
β-Cryptoxanthin 0.42 ± 0.06a 0.47 ± 0.03a 0.57 ± 0.04a 0.81 ± 0.07b 0.84 ± 0.06b
Echinenone 0.66 ± 0.10a 0.76 ± 0.08a 0.94 ± 0.16ab 1.18 ± 0.10bc 1.34 ± 0.12c
β-Carotene 0.58 ± 0.02a 0.65 ± 0.03ab 0.84 ± 0.01b 0.98 ± 0.01b 1.22 ± 0.02c
a Data were expressed as mean ± S.E.M from triplicate groups. Mean values in the same row with different letters are significantly different (P < 0.05). CD, carotenoids.
Astaxanthin, CX, zeaxanthin, β-cryptoxanthin, echinenone, and β-carotene were detected as the main carotenoids in the tissues of L. vannamei. The six carotenoids mentioned above were significantly (P < 0.05) affected by the dietary manipulation and showed increasing trends in different tissues by increasing dietary CX supplementation. The most abundant carotenoid accumulated in various tissues was astaxanthin. The highest astaxanthin content in all tissues was given by CX400 treatment, which was significantly different (P < 0.05) from other treatments in muscle, shell, and whole-body but did not differ markedly
(P > 0.05) from CX100 and CX200 treatments in the head. Relative to astaxanthin concentration, CX content in L. vannamei tissues was lower.
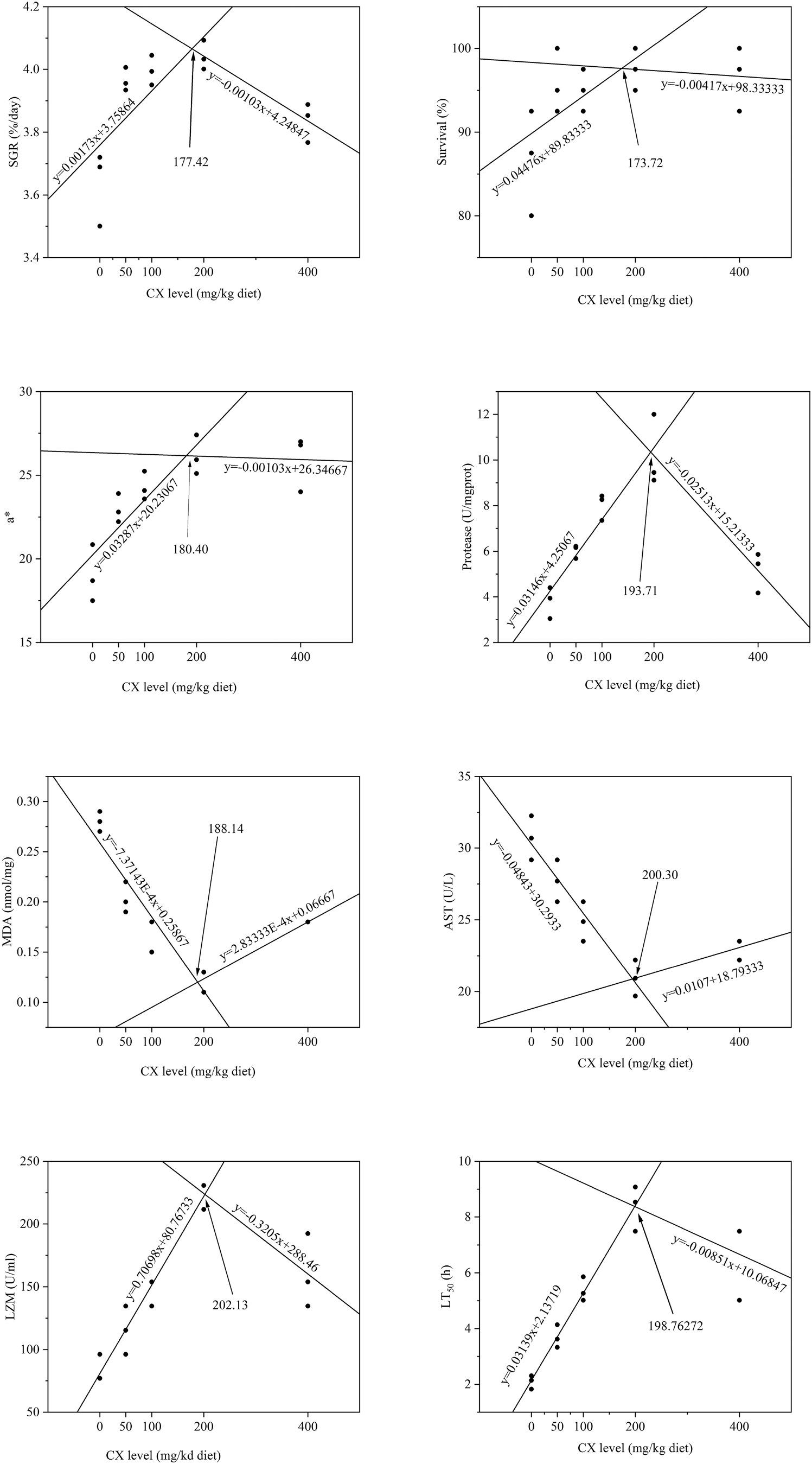
3.8. Quantification of optimum dietary CX level for L. vannamei
Fig. 4 presented the findings obtained after performing the brokenline regression analysis to determine the optimum dietary CX levels. The analysis was based on SGR, survival, a* (redness), protease, MDA, AST, lysozyme, and LT50 These parameters figured out that the optimal dietary CX levels were 177.42, 173.72, 180.40, 193.71, 188.14, 200.30, 202.13, and 198.76 mg kg 1 diet, respectively.
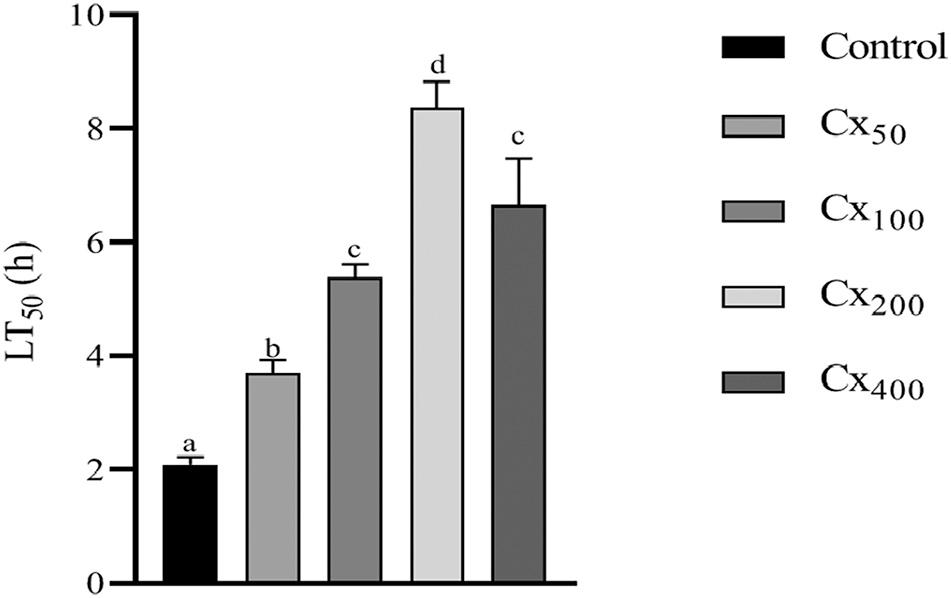
3.9. Hypoxia stress resistance test
After exposure to low DO stress, the time required for the death of 50% of the stressed shrimp (LT50) was calculated and presented in Fig. 5. LT50 values were significantly (P < 0.05) influenced by the dietary inclusion of CX. The maximum LT50 value was observed in the CX200 treatment with significant differences from the other treatments, while the minimum value was given by the control treatment (P < 0.05). Further, no significant difference was observed in LT50 between CX100 and CX400 treatments.
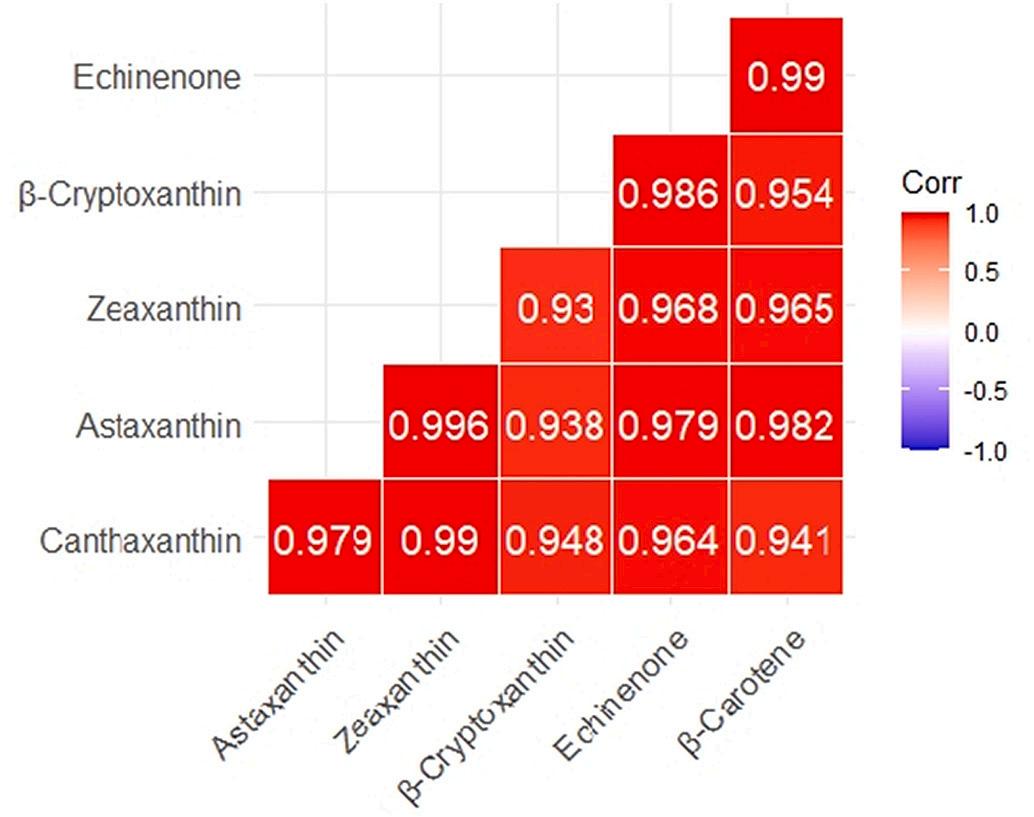
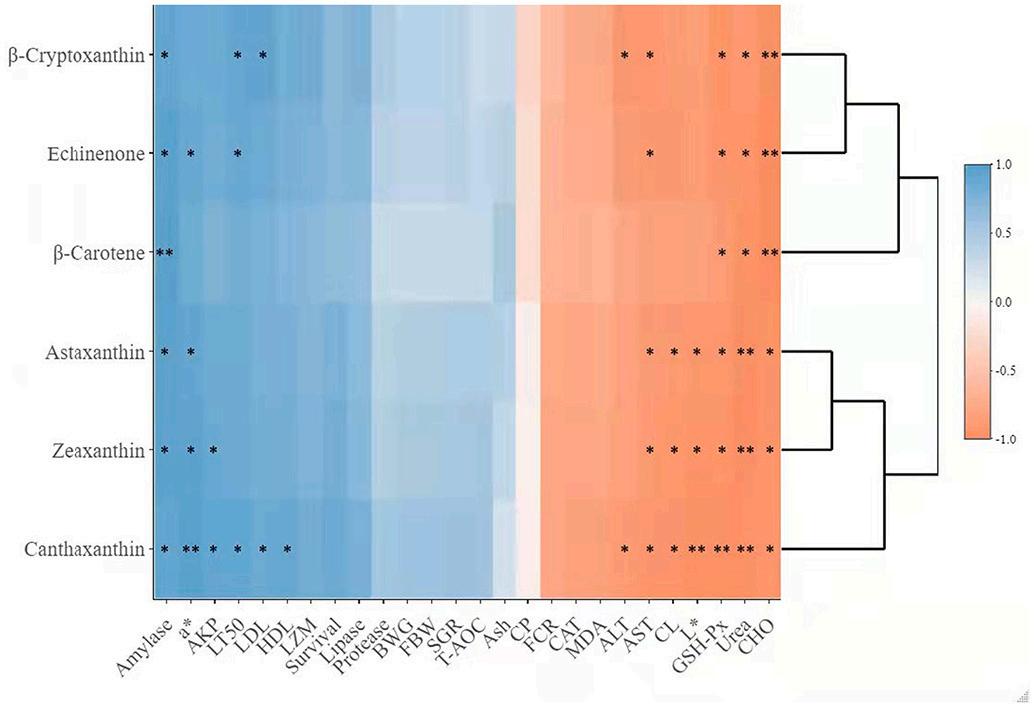
3.10. Person correlation analysis
A heat map visualization was used to present the correlation association between the carotenoid composition in shrimp whole body (Fig. 6) and whole-body carotenoids with biochemical and immunephysiological parameters (Fig. 7). The results indicated significant and positive associations between carotenoids in shrimp whole body and amylase, a* (redness), AKP, LT50, LDL, and HDL. While, ALT, AST, L * , CL, GSH-Px, Urea, and CHO showed an opposite pattern.
4. Discussion
Carotenoid pigments are multi-functional feed additives. They are added to the diets of aquatic animals, essentially for improving their organoleptic properties (De Carvalho and Caramujo, 2017; Pereira da Costa and Campos Miranda-Filho, 2020) and consequently providing better market prices (Parisenti et al., 2011). Moreover, carotenoids were recognized to perform many physiological and metabolic functions with evidence for their beneficial effects on physical performance (Manikandan et al., 2020; Wade et al., 2017b). Our results indicated that dietary inclusion of CX dramatically improved growth performance, enhanced survival, and decreased the FCR of Litopenaeus vannamei. The results were consistent with the previous studies on giant tiger shrimp (P. monodon) fed with CX supplemented diet (Niu et al., 2012), oriental river prawn (Macrobrachium nipponense) fed with lutein supplemented diet (Ettefaghdoost and Haghighi, 2021); and swamp crayfish, Procambarus clarkii (Cheng and Wu, 2019), L. vannamei (Flores et al., 2007; Liu et al., 2018; Wang et al., 2020), and kuruma shrimp, Marsupenaeus japonicas (Wang et al., 2018a) fed with astaxanthin supplemented diets. Furthermore, the studies carried out on a variety of fish species showed that dietary CX supplementation could boost the growth and survival of Lake Kurumoi rainbowfish, Melanotaenia parva (Allen) (Meilisza et al., 2017), Atlantic salmon (Torrissen, 1984), and red porgy, Pagrus pagrus (Kalinowski et al., 2015).
The growth-promoting role of carotenoids such as CX could be related to their capability on activating digestion and metabolism (Amar et al., 2001), shortening the molting cycles intervals (Petit et al., 1997), inhibition of nicotinamide adenine dinucleotide phosphoric acid (NADPH) reductase, decreasing energy consumption, and further promoting the biosynthesis and growth (Ohno et al., 2011) in aquatic animals. Additionally, the higher survival rate of shrimp fed with CX supplemented diets could be associated with the superior antioxidant activity of carotenoids which ameliorates the effect of stressors and
Fig. 4. Broken-line analysis of optimal dietary CX levels based on SGR, survival, a* (redness), protease, MDA, AST, lysozyme, and LT50
Fig. 5. Time to 50% mortality (h) after exposure to hypoxia stress tolerance of Litopenaeus vannamei fed with different levels of canthaxanthin for 56 days. Data were expressed as mean ± S.E.M. from triplicate groups. Bars with different letters represented significant differences between various treatments (P < 0.05).
Fig. 6. The correlation heatmap characterizes the Pearson correlation coefficients (r) between the carotenoid composition in shrimp whole body. The red bar means the positive correlation, while the blue bar means the negative correlation. Moderate correlation was defined following (0.5 < |r| < 0.8) and high correlation (0.8 < |r| < 1), respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
prolongs the shrimp life (Cheng and Wu, 2019; Niu et al., 2012). Wade et al. (2017a) demonstrated that carotenoid supplementation could improve only the weight gain but not the survival of P. monodon Moreover, other counterpart studies on kuruma shrimp Penaeus japonicus (Yamada et al., 1990), P. monodon (Boonyaratpalin et al., 2001), tropical spiny lobster Panulirus ornatus (Barclay et al., 2006), L. vannamei (Ju et al., 2011) and Chinese mitten crab Eriocheir sinensis (Jiang et al., 2020) indicated that the growth and survival were similar regardless of addition or omission of dietary carotenoids. The dissimilarity of these studies with ours could be attributed to the variations in diet composition, culture conditions, state of animal health, species-specific carotenoid requirements, source of carotenoid supplement, and the initial carotenoid level in shrimp (Chien and Jeng, 1992; Wade et al., 2017b).
In the aquaculture industry, dietary carotenoid additives constitute about 15–20% of the salmon feed cost (Forsberg and Guttormsen, 2006), which dramatically increase the total production costs of some aquatic animals (Hansen et al., 2016). Therefore, it was necessary to determine
Fig. 7. The heatmap characterizes the Pearson correlation coefficients (r) between the whole-body carotenoids with other measured parameters in the current study. The blue bar suggests that the whole-body carotenoids positively correlate with the other parameters, while the orange bar means the negative correlation. The asterisk (* and **) indicates P < 0.05 and P < 0.01, respectively. Moderate correlation was defined following (0.5 < |r| < 0.8) and high correlation (0.8 < |r| < 1), respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
the optimum CX level for better physical performance. In such a way, only the requirements of the species will be provided, wastage will be avoided, and the feed cost will be maintained.
One of the most critical indicators for the nutritive value of the cultured species is the proximate composition (Shao et al., 2014). According to Kawamura et al. (2020), carotenoids intake could stimulate protein synthesis leading to an increase in protein content and muscle mass. This agrees with our study where the protein content in the body of shrimp fed CX-containing diets was significantly higher than that fed the control diet, which was reflected by the growth performance. A similar finding was observed for P. monodon (Niu et al., 2014) and E. sinensis (Long et al., 2017). The authors suggested that the efficiency of carotenoids in reducing oxidative stress and hence, energy expenditure is possibly the cause for the elevation of protein concentration.
CX has been reported to have potent antioxidant and immune modulation properties (Venugopalan et al., 2013). T-AOC is an essential index for both enzymatic and non-enzymatic antioxidant activities, and its higher value indicates an improved antioxidant capacity. The current study revealed that CX feeding significantly increased T-AOC compared to the control diet. This is in the line of findings on P. monodon (Niu et al., 2012) and O. mykiss (Cui et al., 2009; Elia et al., 2019). The authors concluded that the antioxidant activity was greatly enhanced after feeding a diet supplemented with CX compared to CX-free diet. POD, a pivotal antioxidant enzyme, also increased in the current study with increasing dietary CX supplementation which is similar to the finding of Long et al. (2017) on E. sinensis Another major enzyme in the antioxidant defense system, CAT, acts as a scavenger for hydrogen peroxide (H2O2) and catalyzes its decomposition into molecular oxygen and water to protect the cell from oxidative damage (Chien et al., 2003; Ighodaro and Akinloye, 2018). In the current study, CAT activity decreased significantly by increasing the dietary CX levels. Similar findings were observed on astaxanthin fed L. vannamei (Zhang et al., 2013), P. monodon (Niu et al., 2014), Oncorhynchus mykiss (Rahman et al., 2016), and lutein fed M. nipponense (Ettefaghdoost and Haghighi, 2021). The GSH-Px enzyme also participates in the removal of H2O2. Its decreasing activity in CX supplemented treatments compared to the control treatment indicates that higher cell protection was provided by CX as reported on yellow perch, Perca flavescens (El-Gawad et al., 2019), and characins, Hyphessobrycon callistus (Wang et al., 2006). Additionally, Pearson correlation analysis indicated that GSH-Px activity was
S. Fawzy
significantly and positively reflected by carotenoid level in shrimp body (Fig. 7). These authors suggested that carotenoid pigments, including CX, have a vigorous O2 quenching activity. Therefore, they could effectively neutralize and remove the free radicals and hence, lower the need to produce endogenous antioxidant enzymes like CAT and the GSH-Px, which is consistent with the findings of the current study. Further, increased CX supplementation level resulted in a significant decrease in MDA level in L. vannamei. According to Munoz et al. (2000), MDA level reflects the degree of cellular damage and lipid peroxidation. So, the decreased MDA level in our study indicates CX’s effective role in the inhibition of lipid peroxidation and protection of the cell against free radicals (Sahin et al., 2014). Combining these data, the efficient antioxidant properties of CX can be concluded.
It is well-known that shrimp, as a crustacean, rely entirely on the innate immune system to defend themselves against the invading pathogens (Zhao et al., 2009). Innate immunity includes two main components, the cellular and humoral immune responses. Hemocytes are the critical element that mediates cellular immunity and are involved in the secretion of various humoral components in the hemolymph (Li and Xiang, 2013; Liu et al., 2020). In this respect, non-specific immune biomarkers (AKP, AST, ALT, and lysozyme) in the serum were used to evaluate the immune response of L. vannamei after feeding with CX. AKP is a pivotal regulatory enzyme that participates in phosphorylation and dephosphorylation processes in organisms and its role in improving the absorption and utilization of nutrients in shrimp. Lysozyme is another important index of non-specific immunity in crustaceans due to its function as a potent killer for Gram-positive bacteria (Wang et al., 2018c). The activity of lysozyme points out an enhancement of the immune response of aquatic animals. The results of the current study showed that the activities of AKP and lysozyme significantly increased in shrimp fed with CX supplemented diets compared to those fed the control diet. Our findings were in agreement with the studies carried out on P. clarkii (Cheng and Wu, 2019), E. sinensis (Jiang et al., 2020), and crucian carp, Carassius auratus (Wu and Xu, 2021). The similarity between the present and the previous studies clarifies that CX, like other carotenoids, exerts a significant role in improving the nonspecific immunity of shrimp. Given the vital role of AST and ALT enzymes in protein metabolism, they are widely used to evaluate the health status of aquatic animals (Haque et al., 2021). The activities of these enzymes in serum reflect the physiological status of hepatopancreatic cells because their higher levels in hemolymph indicate severe damage and increased leakage of hepatopancreatic cells (Ettefaghdoost and Haghighi, 2021; Mohankumar and Ramasamy, 2006). The current study demonstrated that shrimp fed with CX exhibited reduced levels of AST and ALT in comparison to those fed the control diet. Similarly, Ettefaghdoost and Haghighi (2021), Lim et al. (2017), and Niu et al. (2012) demonstrated that carotenoids supplementation significantly decreased the activities of AST and ALT in the serum of shrimp. Combining the results, dietary CX could be a beneficial immunostimulant for enhancing the health status of the studied shrimp.
Hepatopancreas is the primary digestive gland in shrimp. It combines the functions of the intestine, liver, and pancreas, taking part in the synthesis of digestive enzymes, absorption of nutrients, and their metabolism (Vogt, 2019). Therefore, the hepatopancreatic digestive enzymes were used as indicators for nutritional physiology in the studied shrimp. Evaluation of digestive enzymes activities gives an indication about the digestion processes and nutrient utilization efficiency, which is reflected by the growth of aquatic animals (Abolfathi et al., 2012; Lovett and Felder, 1990). Lutein, a carotenoid pigment, had been reported to significantly increase the activities of the digestive enzymes of M. nipponense (Ettefaghdoost and Haghighi, 2021). Likewise, Wang et al. (2018b) noticed an improvement in the activities of digestive enzymes in kuruma shrimp, M. japonicus after feeding astaxanthin added diet. Similarly, our findings revealed that the dietary incorporation of CX markedly increased protease, amylase, and lipase activities more than CX-free diet. Together with the previous reports, these
findings support the positive role of carotenoids in enhancing the digestion process and nutrient utilization, which probably explains the better growth performance of shrimp in the treatments received CX compared to those in the control treatment.
Due to the disability to synthesize carotenoids de novo, L. vannamei and other crustaceans mainly depend on the dietary carotenoids to acquire the attractive reddish coloration (Goodwin, 1984; Wade et al., 2017b). Moreover, the color of crustaceans’ bodies is determined by factors such as the concentration and composition of carotenoids, species, and environmental conditions (Long et al., 2017; Niu et al., 2012). Our results clearly indicated that the total carotenoids and astaxanthin contents in the shell, muscle, head, and whole body of L. vannamei increased significantly and positively with increasing dietary CX levels (Fig. 6). Deposition of CX primarily in the form of astaxanthin supports the findings on P. japonicus (Yamada et al., 1990) and P. monodon (Boonyaratpalin et al., 2001), which implied that crustaceans could transform or modify the pigments precursors like CX into astaxanthin and accumulate them in different tissues. Jiang et al. (2020) also stated that the red color is related to astaxanthin content in the exoskeleton of E. sinensis. Consistent with this report, Pearson correlation analysis indicated that the redness (a *) value of cooked shrimp in treatment received CX was significantly and positively affected by astaxanthin levels in shrimp body in the current study (Fig. 7). Additionally, CX had been proved to be as effective as astaxanthin in the pigmentation of Atlantic salmon (Buttle et al., 2001). On the contrary, the L* value of cooked shrimp showed a decreasing trend with increasing dietary CX, and it was also found that carotenoids included in diets decreased the lightness of red king crab, Paralithodes camtschaticus (Daly et al., 2013), and red porgy, P. pagrus (Kalinowski et al., 2005). The results confer that L. vannamei could utilize the dietary CX effectively to improve their red appeal and other biological functions. One more observation is that the lowest total carotenoids and astaxanthin contents were observed in muscle tissue which is consistent with the findings reported earlier (Niu et al., 2012). Nevertheless, the carotenoid deposition in muscle is beneficial for the consumer’s health since carotenoids are effective antioxidants besides having other properties that make them an essential human food supplement (De Carvalho and Caramujo, 2017).
Shrimp culture under an intensive system is constantly subjected to different environmental stressors such as hypoxia (Chien and Shiau, 2005) that can arise from temperature fluctuations or the formation of algal blooms due to the presence of organic pollutants (Belao et al., 2011). Hypoxia causes significant economic losses and is considered a threat to productivity due to its negative impact on shrimp survival (Diaz and Rosenberg, 2011). Previous studies showed that the enhancement of resistance in penaeid shrimp to hypoxia stress is closely related to dietary carotenoids uptake (Niu et al., 2012; Niu et al., 2009). Moreover, a positive correlation was detected between the improvement of shrimp resistance to stressors and pigment concentration in the diet and tissue (Chien et al., 2003). Similarly, in the current study, LT50 values increased with increasing dietary CX levels, with the maximum values observed for the shrimp-fed diets containing the higher CX levels (200 and 400 mg kg 1). Therefore, it can be concluded that dietary CX pigment could enhance the resistance of the studied shrimp against hypoxia stress and prolong its life, as reported by Chien et al. (1999) and Niu et al. (2012) on P. monodon, and Zhang et al. (2013) on L. vannamei This is probably associated with the antioxidant property of carotenoids (Table 5), which is closely linked to stress resistance (Niu et al., 2014). They play a vital role in protecting sensitive structures of the cell from oxidative damage, which is reflected by an increase in shrimp survival.
5. Conclusion
Overall, the findings of the current study revealed that supplementation of the diet with CX in the range of 173.73 to 202.13 mg kg 1 is of primary importance to shrimp growth performance and health status. Therefore, with its numerous properties and relatively low market price,
S. Fawzy
antioxidant and metabolic conversion characters, CX could be a potential astaxanthin precursor in the diet of L. vannamei.
Credit author statement
Weilong Wang finished the data analysis and worked on paper revision; Fawzy Samia finished the biochemical analysis and drafted the manuscript; Meiqin Wu put forward relevant experimental guidance; Ganfeng Yi and Xuxiong Huang designed the research. All authors read and gave final approval of the manuscript.
Declaration
of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgments
The work was supported by the Shanghai Agriculture Applied Technology Development Program, China (No. 202102080012F00761); National Science Foundation of China (31902385); Chinese Postdoctoral Science Foundation (219724); Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-culture of Aquaculture Animals, Key Laboratory of Aquatic Functional Feed and Environmental Regulation of Fujian Province Open Project (FACE20200001); and China Scholarship Council (CSC) supported this research through a Ph.D scholarship (No.2018 GBJ008472).
References
Abolfathi, M., Hajimoradloo, A., Ghorbani, R., Zamani, A., 2012. Effect of starvation and refeeding on digestive enzyme activities in juvenile roach, Rutilus rutilus caspicus Comp. Biochem. Physiol. A Mol. Integr. Physiol. 161, 166–173
Amar, E.C., Kiron, V., Satoh, S., Watanabe, T., 2001. Influence of various dietary synthetic carotenoids on bio-defence mechanisms in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 32, 162–173
Association of Official Analytical Chemists (AOAC), 2006. Official Methods of Analysis, Eighteenth ed. AOAC, Arlington, VA
Baker, R., Pfeiffer, A.M., Schoner, F.J., Smith-Lemmon, L., 2002. Pigmenting efficacy of astaxanthin and canthaxanthin in fresh-water reared Atlantic salmon, Salmo salar Anim. Feed Sci. Technol. 99, 97–106
Barclay, M., Irvin, S., Williams, K., Smith, D., 2006. Comparison of diets for the tropical spiny lobster Panulirus ornatus: astaxanthin-supplemented feeds and mussel flesh. Aquac. Nutr. 12, 117–125
Belao, T., Leite, C., Florindo, L., Kalinin, A., Rantin, F., 2011. Cardiorespiratory responses to hypoxia in the African catfish, Clarias gariepinus (Burchell 1822), an air-breathing fish. J. Comp. Physiol. B. 181, 905–916. Boonyaratpalin, M., Thongrod, S., Supamattaya, K., Britton, G., Schlipalius, L., 2001. Effects of β-carotene source, Dunaliella salina, and astaxanthin on pigmentation, growth, survival and health of Penaeus monodon Aquac. Res. 32, 182–190
Buttle, L., Crampton, V., Williams, P., 2001. The effect of feed pigment type on flesh pigment deposition and colour in farmed Atlantic salmon, Salmo salar L Aquac. Res. 32, 103–111
Cheng, Y., Wu, S., 2019. Effect of dietary astaxanthin on the growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkii Aquaculture. 512, 734341
Chien, Y.H., Jeng, S.C., 1992. Pigmentation of kuruma prawn, Penaeus japonicus bate, by various pigment sources and levels and feeding regimes. Aquaculture. 102, 333–346
Chien, Y.H., Shiau, W.C., 2005. The effects of dietary supplementation of algae and synthetic astaxanthin on body astaxanthin, survival, growth, and low dissolved oxygen stress resistance of kuruma prawn, Marsupenaeus japonicus bate J. Exp. Mar. Biol. Ecol. 318, 201–211
Chien, Y.H., Chen, I.M., Pan, C.H., Kurmaly, K., 1999. Oxygen depletion stress on mortality and lethal course of juvenile tiger prawn Penaeus monodon fed high level of dietary astaxanthin. J. Fish. Soc. Taiwan 26, 85–93
Chien, Y.H., Pan, C.H., Hunter, B., 2003. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture. 216, 177–191
Cui, W., Leng, X., Li, X., Li, X., Xu, J., 2009. Effects of astaxanthin and canthaxanthin on pigmentation of muscle and total antioxidant capacity of liver in rainbow trout (Oncorhynchus mykiss). J. Fish. China 33, 987–995.
Daly, B., Swingle, J., Eckert, G., 2013. Dietary astaxanthin supplementation for hatcherycultured red king crab, Paralithodes camtschaticus, juveniles. Aquac. Nutr. 19, 312–320
De Carvalho, C.C., Caramujo, M.J., 2017. Carotenoids in aquatic ecosystems and aquaculture: a colorful business with implications for human health. Front. Mar. Sci. 4, 93
Diaz, R.J., Rosenberg, R., 2011. Introduction to environmental and economic consequences of hypoxia. Int. J. Water Resour. Dev. 27, 71–82
El-Gawad, A., Eman, A., Wang, H.P., Yao, H., 2019. Diet supplemented with synthetic carotenoids: effects on growth performance and biochemical and immunological parameters of yellow perch (Perca flavescens). Front. Physiol. 10, 1056
Elia, A.C., Prearo, M., Dorr, A.J.M., Pacini, N., Magara, G., Brizio, P., Abete, M.C., 2019. Effects of astaxanthin and canthaxanthin on oxidative stress biomarkers in rainbow trout. J. Toxicol. Environ. Health Part A. 82, 760–768
Ettefaghdoost, M., Haghighi, H., 2021. Impact of different dietary lutein levels on growth performance, biochemical and immuno-physiological parameters of oriental river prawn (Macrobrachium nipponense). Fish Shellfish Immunol. 115, 86–94
Flores, M., Díaz, F., Medina, R., Re, A.D., Licea, A., 2007. Physiological, metabolic and haematological responses in white shrimp Litopenaeus vannamei (Boone) juveniles fed diets supplemented with astaxanthin acclimated to low-salinity water. Aquac. Res. 38, 740–747.
Forsberg, O.I., Guttormsen, A.G., 2006. A pigmentation model for farmed Atlantic salmon: nonlinear regression analysis of published experimental data. Aquaculture. 253, 415–420
Goodwin, T., 1984. The Biochemistry of the Carotenoids Volume II: Animals, 2. vyd. Chapham and Hall, New York
Hansen, Ø.J., Puvanendran, V., Bangera, R., 2016. Broodstock diet with water and astaxanthin improve condition and egg output of brood fish and larval survival in Atlantic cod, Gadus morhua L Aquac. Res. 47, 819–829
Haque, R., Sawant, P.B., Sardar, P., Xavier, K.M., Varghese, T., Chadha, N., Naik, V.A., 2021. Synergistic utilization of shrimp shell waste-derived natural astaxanthin with its commercial variant boosts physio metabolic responses and enhances colouration in discus (Symphysodon aequifasciatus). Environ. Nanotechnol. Monit. Manag. 15, 100405
Ighodaro, O., Akinloye, O., 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54, 287–293
Jiang, X., Zu, L., Wang, Z., Cheng, Y., Yang, Y., Wu, X., 2020. Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab, Eriocheir sinensis Fish Shellfish Immunol. 102, 499–510
Ju, Z.Y., Deng, D.F., Dominy, W.G., Forster, I.P., 2011. Pigmentation of Pacific white shrimp, Litopenaeus vannamei, by dietary astaxanthin extracted from Haematococcus pluvialis. J. World Aquacult. Soc. 42, 633–644.
Kalinowski, C., Robaina, L., Fernandez-Palacios, H., Schuchardt, D., Izquierdo, M., 2005. Effect of different carotenoid sources and their dietary levels on red porgy (Pagrus pagrus) growth and skin colour. Aquaculture. 244, 223–231
Kalinowski, C.T., Socorro, J., Robaina, L.E., 2015. Effect of dietary canthaxanthin on the growth and lipid composition of red porgy (Pagrus pagrus). Aquac. Res. 46, 893–900 Kawamura, A., Aoi, W., Abe, R., Kobayashi, Y., Wada, S., Kuwahata, M., Higashi, A., 2020. Combined intake of astaxanthin, β-carotene, and resveratrol elevates protein synthesis during muscle hypertrophy in mice. Nutrition. 69, 110561
Landsman, A., St-Pierre, B., Rosales-Leija, M., Brown, M., Gibbons, W., 2019. Impact of aquaculture practices on intestinal bacterial profiles of Pacific Whiteleg shrimp Litopenaeus vannamei Microorganisms. 7, 93
Li, E., Wang, X., Chen, K., Xu, C., Qin, J.G., Chen, L., 2017. Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev. Aquac. 9, 57–75
Li, F., Xiang, J., 2013. Recent advances in researches on the innate immunity of shrimp in China. Dev. Comp. Immunol. 39, 11–26
Lim, K.C., Yusoff, F.M., Shariff, M., Kamarudin, M.S., 2017. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 10, 738–773
Liu, M., Jiang, X., Chen, A., Chen, T., Cheng, Y., Wu, X., 2020. Transcriptome analysis reveals the potential mechanism of dietary carotenoids improving antioxidative capability and immunity of juvenile Chinese mitten crabs Eriocheir sinensis Fish Shellfish Immunol. 104, 359–373
Liu, X., Wang, B., Li, Y., Wang, L., Liu, J., 2018. Effects of dietary botanical and synthetic astaxanthin on E/Z and R/S isomer composition, growth performance, and antioxidant capacity of white shrimp, Litopenaeus vannamei, in the nursery phase [for this article an erratum has been published]. Invertebr. Surviv. J. 15, 131–140
Long, X., Wu, X., Zhao, L., Liu, J., Cheng, Y., 2017. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis Aquaculture. 473, 545–553
Lovett, D.L., Felder, D.L., 1990. Ontogenetic change in digestive enzyme activity of larval and postlarval white shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol. Bull. 178, 144–159
Manikandan, K., Felix, N., Prabu, E., 2020. A review on the application and effect of carotenoids with respect to canthaxanthin in the culture of fishes and crustaceans. Int. J. Fish. Aquat. Stud. 8, 128–133
Meilisza, N., Jusadi, D., Zairin Jr., M., Artika, I.M., Priyo Utomo, N.B., Kadarini, T., Suprayudi, M.A., 2017. Digestibility, growth and pigmentation of astaxanthin, canthaxanthin or lutein diets in Lake Kurumoi rainbowfish, Melanotaenia parva (Allen) cultured species. Aquac. Res. 48, 5517–5525
Mialhe, F., Gunnell, Y., Mering, C., 2013. The impacts of shrimp farming on land use, employment and migration in Tumbes, northern Peru. Ocean Coast. Manag. 73, 1–12
Mohankumar, K., Ramasamy, P., 2006. Activities of membrane bound phosphatases, transaminases and mitochondrial enzymes in white spot syndrome virus infected tissues of Fenneropenaeus indicus Virus Res. 118, 130–135
S. Fawzy
Munoz, M., Cedeno, R., Rodriguez, J., van der Knaap, W.P., Mialhe, E., Bachere, E., 2000. Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, Penaeus vannamei Aquaculture. 191, 89–107
Mussagy, C.U., Khan, S., Kot, A.M., 2021. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 1–15
Nickell, D., Bromage, N., 1998. The effect of timing and duration of feeding astaxanthin on the development and variation of fillet colour and efficiency of pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture. 169, 233–246
Niu, J., Tian, L.X., Liu, Y.J., Yang, H.J., Ye, C.X., Gao, W., Mai, K.S., 2009. Effect of dietary astaxanthin on growth, survival, and stress tolerance of postlarval shrimp, Litopenaeus vannamei J. World Aquacult. Soc. 40, 795–802
Niu, J., Li, C.H., Liu, Y.-J., Tian, L.X., Chen, X., Huang, Z., Lin, H.Z., 2012. Dietary values of astaxanthin and canthaxanthin in Penaeus monodon in the presence and absence of cholesterol supplementation: effect on growth, nutrient digestibility and tissue carotenoid composition. Br. J. Nutr. 108, 80–91
Niu, J., Wen, H., Li, C.H., Liu, Y.J., Tian, L.X., Chen, X., Lin, H.Z., 2014. Comparison effect of dietary astaxanthin and β-carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture. 422, 8–17
Ohno, M., Darwish, W.S., Ikenaka, Y., Miki, W., Ishizuka, M., 2011. Astaxanthin can alter CYP1A-dependent activities via two different mechanisms: induction of protein expression and inhibition of NADPH P450 reductase dependent electron transfer. Food Chem. Toxicol. 49, 1285–1291
Parisenti, J., Beirao, L.H., Tramonte, V.L., Ourique, F., da Silveira Brito, C.C., Moreira, C. C., 2011. Preference ranking of colour in raw and cooked shrimps. Int. J. Food Sci. Technol. 46, 2558–2561
Pereira da Costa, D., Campos Miranda-Filho, K., 2020. The use of carotenoid pigments as food additives for aquatic organisms and their functional roles. Rev. Aquac. 12, 1567–1578
Petit, H., N` egre-Sadargues, G., Castillo, R., Trilles, J.P., 1997. The effects of dietary astaxanthin on growth and moulting cycle of postlarval stages of the prawn, Penaeus japonicus (Crustacea, Decapoda). Comp. Biochem. Physiol. A 117, 539–544
Pi, S., Xi, M., Deng, L., Xu, H., Feng, C., Shen, R., Wu, C., 2020. Practical synthesis of canthaxanthin. J. Iran. Chem. Soc. 17, 493–497
Rahman, M.M., Khosravi, S., Chang, K.H., Lee, S.-M., 2016. Effects of dietary inclusion of astaxanthin on growth, muscle pigmentation and antioxidant capacity of juvenile rainbow trout (Oncorhynchus mykiss). Prev. Nutr. Food Sci. 21, 281.
Rebelo, B.A., Farrona, S., Ventura, M.R., Abranches, R., 2020. Canthaxanthin, a red-hot carotenoid: applications, synthesis, and biosynthetic evolution. Plants. 9, 1039
Sahin, K., Yazlak, H., Orhan, C., Tuzcu, M., Akdemir, F., Sahin, N., 2014. The effect of lycopene on antioxidant status in rainbow trout (Oncorhynchus mykiss) reared under high stocking density. Aquaculture. 418, 132–138
Sanchez, S., Ruiz, B., Rodríguez-Sanoja, R., Flores-Cotera, L., 2013. Microbial Production Of Carotenoids, Microbial Production of Food Ingredients, Enzymes and Nutraceuticals. Elsevier, pp. 194–233
Shahidi, F., Brown, J.A., 1998. Carotenoid pigments in seafoods and aquaculture. Crit. Rev. Food Sci. Nutr. 38, 1–67 Shao, L., Wang, C., He, J., Wu, X., Cheng, Y., 2014. Meat quality of Chinese mitten crabs fattened with natural and formulated diets. J. Aquat. Food Prod. Technol. 23, 59–72 Shinji, J., Nohara, S., Yagi, N., Wilder, M., 2019. Bio-economic analysis of superintensive closed shrimp farming and improvement of management plans: a case study in Japan. Fish. Sci. 85, 1055–1065 Torrissen, O.J., 1984. Pigmentation of salmonids effect of carotenoids in eggs and startfeeding diet on survival and growth rate. Aquaculture. 43, 185–193 Venugopalan, V., Tripathi, S.K., Nahar, P., Saradhi, P.P., Das, R.H., Gautam, H.K., 2013. Characterization of canthaxanthin isomers isolated from a new soil Dietzia sp. and their antioxidant activities. J. Microbiol. Biotechnol. 23, 237–245
Vogt, G., 2019. Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 280, 1405–1444
Wade, N.M., Cheers, S., Bourne, N., Irvin, S., Blyth, D., Glencross, B.D., 2017a. Dietary astaxanthin levels affect colour, growth, carotenoid digestibility and the accumulation of specific carotenoid esters in the Giant Tiger shrimp, Penaeus monodon Aquac. Res. 48, 395–406
Wade, N.M., Gabaudan, J., Glencross, B.D., 2017b. A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquac. 9, 141–156
Wang, W., Ishikawa, M., Koshio, S., Yokoyama, S., Dawood, M.A., Zhang, Y., 2018a. Effects of dietary astaxanthin supplementation on survival, growth and stress resistance in larval and post-larval kuruma shrimp, Marsupenaeus japonicus Aquac. Res. 49, 2225–2232
Wang, W., Ishikawa, M., Koshio, S., Yokoyama, S., Hossain, M.S., Moss, A.S., 2018b. Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture. 491, 197–204.
Wang, W., Ishikawa, M., Koshio, S., Yokoyama, S., Dawood, M.A., Hossain, M.S., Zaineldin, A.I., 2019. Interactive effects of dietary astaxanthin and cholesterol on the growth, pigmentation, fatty acid analysis, immune response and stress resistance of kuruma shrimp (Marsupenaeus japonicus). Aquac. Nutr. 25, 946–958
Wang, W., Liu, M., Fawzy, S., Xue, Y., Wu, M., Huang, X., Lin, Q., 2021. Effects of dietary phaffia rhodozyma astaxanthin on growth performance, carotenoid analysis, biochemical and immune-physiological parameters, intestinal microbiota, and disease resistance in Penaeus monodon Front. Microbiol. 12
Wang, Y., Wang, B., Liu, M., Jiang, K., Wang, M., Wang, L., 2020. Comparative transcriptome analysis reveals the potential influencing mechanism of dietary astaxanthin on growth and metabolism in Litopenaeus vannamei Aquac. Rep. 16, 100259
Wang, Y.J., Chien, Y.H., Pan, C.H., 2006. Effects of dietary supplementation of carotenoids on survival, growth, pigmentation, and antioxidant capacity of characins, Hyphessobrycon callistus Aquaculture. 261, 641–648
Wang, Z., Cai, C.F., Cao, X.M., Zhu, J.M., He, J., Wu, P., Ye, Y.T., 2018c. Supplementation of dietary astaxanthin alleviated oxidative damage induced by chronic high pH stress, and enhanced carapace astaxanthin concentration of Chinese mitten crab Eriocheir sinensis Aquaculture. 483, 230–237
Wu, S., Xu, B., 2021. Effect of dietary astaxanthin administration on the growth performance and innate immunity of juvenile crucian carp (Carassius auratus). 3. Biotech. 11, 1–6
Xie, F., Zeng, W., Zhou, Q., Wang, H., Wang, T., Zheng, C., Wang, Y., 2012. Dietary lysine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture. 358, 116–121
Yamada, S., Tanaka, Y., Sameshima, M., Ito, Y., 1990. Pigmentation of prawn (Penaeus japonicus) with carotenoids: I. effect of dietary astaxanthin, β-carotene and canthaxanthin on pigmentation. Aquaculture. 87, 323–330
Yokoyama, S., Koshio, S., Takakura, N., Oshida, K., Ishikawa, M., Gallardo-Cigarroa, F.J., Teshima, S.I., 2005. Dietary bovine lactoferrin enhances tolerance to high temperature stress in Japanese flounder Paralichthys olivaceus Aquaculture. 249, 367–373
Zhang, J., Liu, Y.J., Tian, L.X., Yang, H.J., Liang, G.Y., Yue, Y.R., Xu, D.H., 2013. Effects of dietary astaxanthin on growth, antioxidant capacity and gene expression in Pacific white shrimp Litopenaeus vannamei Aquac. Nutr. 19, 917–927
Zhao, D., Song, S., Wang, Q., Zhang, X., Hu, S., Chen, L., 2009. Discovery of immunerelated genes in Chinese mitten crab (Eriocheir sinensis) by expressed sequence tag analysis of haemocytes. Aquaculture. 287, 297–303
Zhou, Q.C., Zeng, W.P., Wang, H.L., Wang, T., Wang, Y.L., Xie, F.J., 2012. Dietary arginine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei Aqauculture 364-365, 252–258
Other documents randomly have different content
The year 1870 came and went; for Sonia it had been a year of study, and nothing more. Her sleep had become shorter and more broken, and she neither knew nor cared what she ate, when suddenly, in the spring of the following year, she was sent for by her sister in Paris. Anjuta had fallen passionately in love with a young Parisian, who was a member of the Commune; he had just been arrested, and was in danger of losing his life. Sonia and Valdemar succeeded in penetrating through the line of troops, found Anjuta, and wrote to their father. General Krukovsky came at once, and it was only then that he discovered what his daughters were doing abroad, and learned for the first time that his eldest daughter had been living alone in Paris, for Anjuta had always been careful to send her letters through Sonia, with the Berlin postmark. Anjuta showed great spirit, and after an interview with Thiers they succeeded in helping this very undesirable son-in-law to escape. Throughout the whole affair their father’s behavior is a rare proof of the nobility of the race from which Sonia sprang. This stern man not only forgave—he also admired his daughters for what they had done. The cold manner and grandfatherly authority with which he had hitherto treated them was superseded by a cordial sympathy such as would have been impossible before. He was much impressed by Anjuta’s passion, but Sonia’s platonic marriage distressed him greatly.
In the year 1874 Sonia took the degree of doctor at Göttingen, as the result of three mathematical treatises, of which one especially, her thesis “On the Theory of Partial Differential Equations,” is reckoned one of her most prominent works. Immediately after this, the whole family assembled on the old estate of Palibino. Sonia was completely worn out, and it was a long time before she was able to resume any severe brain work. Her holiday was cut short by her father’s death a few months later, and the following winter was spent with her family at St. Petersburg. Until now Sonia’s brain was the only part of her which was thoroughly awakened. She had been entirely absorbed in her studies, and had worked with the obstinate tenacity of auto-suggestion, more commonly found in women, especially girls, than in men. Marie Bashkirtseff had done the same,
year in, year out; she had worked breathlessly, feverishly, with an incomprehensible, unwearied power of production,—while failing health was announcing the approach of death in her frail young body. Suddenly the end came.
Thousands of girls in middle-class families work themselves to death in the same way. Badly paid to begin with, they lower the prices still more by competing with one another. Others, placed in better circumstances, work with the same insistency at useless handicrafts, while a large number of women of the poorer classes work because they are driven to it by dire necessity. The result is the same in all cases; they lose the power of enjoyment, and forget what happiness means.
Sonia’s stay in St. Petersburg was the occasion of the first great change which took place in her, to be followed later on by many like changes. Mathematics were thrust aside; she did not want to hear any more about them, she wanted to forget them.
Mind and body were undergoing a healing process, struggling to attain an even balance in her fresh young nature. She felt the need of change, she required companionship, and she threw herself into the midst of all social and intellectual pursuits. It was then that the woman awoke in her
During the period of nervous excitement and sorrow which followed after the death of her beloved father, she had become the wife of her husband, after having been nominally married for nearly seven years. Since then they had drawn closer to one another; and now that her fortune, as long as her mother lived, was not sufficient for her support, she and Valdemar invested their money in various speculations. With true Russian enthusiasm they set to work building houses, establishing watering-places, and starting newspapers, besides lending their aid to every imaginable kind of new invention. The first year all went well, and in 1878 a daughter was born. After that came the crash. Kovalevsky was bitten with the rage for speculation, and although he was nominated Professor of Paleontology at Moscow in 1880, and in spite of all that his wife could do to dissuade him, he took shares in a company connected
with petroleum springs in the south of Russia. The company was a swindle, the undertaking proved a failure, and he shot himself.
Sonia had left him some time before. She knew what was coming, having been warned by bad dreams and presentiments, and as she had lost her influence over him, and was anxious to provide for her own and her child’s future, she left him and went to Paris. Just as she was recovering from the nervous fever to which she succumbed on hearing the news of her husband’s sudden death, she received the summons to go to Stockholm.
The invitation had been sent by the representatives of a Woman’s Rights movement which was then in full swing. It was an exceedingly narrow society of the genuine bourgeois kind, and as it was to them that she owed her appointment, they were anxious to bind her firmly to their cause. Sonia soon won their hearts by the sociability of her Russian nature, but as one term after the other passed by, she grew more and more weary of it, and whenever her course of lectures was over she hurried away as quickly as possible to Russia, Italy, France, England,—no matter where, if only she could escape out of Sweden into a freer atmosphere. She never looked upon her stay there as anything more than an episode in her life, and she longed to be back in Paris; but the years passed by, and she received no other appointment.
Her lectures at the university began to pall upon her; it gave her no pleasure to be forever teaching the students the same thing in a dreary routine. She needed an incentive in the shape of some highly gifted individual whom she could respect, and whose presence would call forth her highest faculties; but even the esteem in which she held some few people was not of long duration.
Her friendship with Fru Edgren-Leffler dates from this period. It was this lady’s renown as an authoress which roused Sonia’s talent for writing, for her life had been rich in experiences, and never wanting in variety until now, when, in a period of comparative leisure, she allowed her thoughts to dwell upon the past. She began by persuading Fru Edgren-Leffler to dramatize the sketches which she gave her, and “The Struggle for Happiness” was the first result of this
collaboration. But Sonia soon realized that the honest, simpleminded Swede was not in sympathy with this department of literature; so she wrote a story on her own account, entitled “The Sisters Rajevsky,” which was a sketch of her own youth, followed by an excellent novel called “Vera Barantzova;” after which she began another novel called “Vae Victis,” which was never finished.
III
U� till now we have followed this remarkable woman’s life along a clear, though somewhat agitated course; but from henceforward there is something uncomfortable, something strange and distorted about it. It is very difficult for us to ascertain the cause of her increasing distraction of mind, and early death, and the difficulty is intensified by the fact that the material contributed by Fru Leffler is poor and contradictory, and also because her work is disfigured by the peculiar inferences which she draws.
I have seen four portraits of Sonia Kovalevsky, and they are all so entirely different that no one would imagine that they were intended to represent the same person. She had none of the fascinating, though irregular beauty of Marie Bashkirtseff, who carried on an artistic cult with her own person. Sonia’s powerful head, with the short hair, massive forehead, and short-sighted eyes of the color of “green gooseberries in syrup,” was placed on a delicate child-like body. Her chief charm lay in her extraordinary liveliness and habit of giving herself up entirely to the interest of the moment; but she was completely unversed in the art of dress, and did not know how to appear at her best; she never gave any thought to the subject at all until she was thirty; and although she paid more attention to it then, she never learned the secret. She aged early, and a celebrated poet has described her to me as being a withered little old woman at the age of thirty. These external circumstances stood more in her way in Sweden, among a tall, fair people, than would have been possible either in Russia or in Paris. Between herself and the Swedish type there was a wide gulf fixed, which allowed no encouragement to the finer erotic emotions to which she was very strongly disposed; she
felt crushed, and her impressionable, unattractive nature suffered acutely from being so unlike the ordinary victorious type of beauty. The picture of her when she was eighteen bears a strong resemblance to the late King Louis II. of Bavaria; not only are her features like his, but also the expression in the eyes and the curve of the lips. The second picture dates from the year 1887. It has something wearied and disillusioned about it, and she seems to be making an effort to appear amiable. It was taken at the time when she was struggling to accustom herself to the stiff, prudish, and somewhat pretentious ways of Stockholm society The third portrait was taken at the time when she won the Prix Bordin in Paris, and it is a regular Russian face, with a much more cheerful expression than the former ones. But in the last picture, taken in the year 1890, which was, to a certain extent, official and very much touched up, how ill she looks; how disappointed and how weary! These four portraits are, to my mind, four different women; they show us what Sonia was once, and what she became after living for several years in an uncongenial atmosphere.
Sonia Kovalevsky was a true Russian genius, with an elastic nature. She was lavish and careless in her ways, and she thrived best upon a torn sofa in an atmosphere of tea, cigarettes, and profusion of all kinds,—intellectual, spiritual, and pecuniary; she needed to be surrounded by people like herself, who were in sympathy with her, and the inhabitants of Stockholm were never that. She had been torn away from the Russian surroundings in which she had lived in Berlin. She, who never could endure solitude, found herself alone among strangers, who forced themselves upon her,—hard, angular, women’s rights women, who expected her to be their leader, and to fulfil a mission. She seldom rebelled against the duties which were constantly held before her eyes, partly because her vanity was flattered by the public position which she occupied, and also because her livelihood depended upon it, now that her private means were not sufficient for her support, and for the numerous journeys which she undertook.
A great deal of her time was spent in travelling to and fro between Stockholm and St. Petersburg, where she went to visit Anjuta, whose
marriage had turned out most unhappily, and who was suffering from a severe illness, of which she afterwards died. After her sister’s death Sonia took a great interest in the study of Northern literature, which was then just beginning to attract attention. She also wrote books, and solved some mathematical problems. Every time that she returned to Stockholm, after spending her holidays in Russia or the South, she had almost entirely forgotten her Swedish, and every year that passed by called forth fresh lamentations over her exile. The tone of society in Stockholm was unendurable to her; but she was of too disciplined a character, and too gentle, too submissive in her loneliness, to rebel against it. Her life became monotonous, which it had never been before, and her courage began to give way. She yearned for sympathy, for excitement, for her native land,—for everything, in fact, which was denied her.
She also longed for something else, which was the very thing that she could not have. She was seized with an eager, nervous longing to be loved. She wanted to be a woman, to possess a woman’s charm. She had lived like a widow for years during her husband’s lifetime, and for years after his death as well. As long as her mathematical studies produced a tension in her mind, she asked for nothing better, but buried herself in her work, and was perfectly contented. When she started being an authoress, a change came over her character. The development of the imagination created a need for love, and because this devouring need could not be satisfied, she became exacting, discontented, and mistrustful of the amount of affection which was accorded her In her younger days she had asked for nothing more than that curious kind of mystic love, known only to Russians, which had run its course in mutual enthusiasm of a purely intellectual and spiritual character. It was otherwise now. She lamented her lost youth, and the time wasted in study; she regretted the unfortunate talent which had deprived her womanhood of its attractiveness. She wanted to be a woman, and to enjoy life as a woman.
She had also another wish, just as passionate in its way and as difficult of fulfilment as the former one, and this was her wish to receive an appointment in Paris. It was to a certain extent fulfilled
when she was awarded the Prix Bordin on Christmas Eve, 1888, on the occasion of a solemn session of the French Academy of Science, in an assembly which was largely composed of learned men. It was the highest scientific distinction which had ever been accorded to a woman, and from henceforth she was an European celebrity, with a place in history. But it gave her no pleasure. She was as completely knocked up as she had been after receiving her doctor’s degree. She had worked day and night for days beforehand, and during the weeks that followed she took part in the social functions which were given in her honor She left no pleasure untasted, and yet she was not satisfied, for by this time her yearning for love had reached its highest pitch.
A short time before, Sonia had made the acquaintance of a cousin of her late husband’s, “fat M.,” as she called him. The companionship of a sympathetic fellow-countryman put her in the height of good humor, and she soon found it so indispensable that she wanted to have him always at her side, and was never happy except when he was there. M. K. did not return this strong affection; he was, however, quite willing to marry her, and the result was that a most unfortunate relationship sprang up between them. Sonia could not exist without him, so they travelled from Stockholm to Russia, and from Russia to Paris or Italy, in order to spend a few weeks together, and then separated, because by that time they were mutually tired of each other. It was on one of these journeys, when Sonia had come out of the sunshine of Italy into the winter of Sweden, that she caught cold, and no sooner had she arrived at Stockholm than she did everything to make her condition worse. In a desperate mood of indifference she immediately commenced her lectures, and went to all the social entertainments that were given. Dark presentiments and dreams, in which she always believed, had foretold that this year would be fatal to her. Longing for death, yet fearing it, she died suddenly in the beginning of the year 1891.
T���� who know something about Russian women, without having any very detailed knowledge, divide them into two types, and a superficial observer would class Sonia Kovalevsky as belonging to one or the other of these. The first type consists of luxurious, languishing, idle, fascinating women, with passionate black eyes, or playful gray ones, a soft skin, and a delicate mouth, which is admirably adapted for laughing and eating. These women have a most seductive charm; their movements suggest that they are wont to recline on soft pillows, dressed en négligé, and their power of chattering is unlimited, and varies in tone from the most enchanting flattery to the worst temper imaginable. They are, in fact, the most womanly of women, as little to be depended upon in their amiability as in their anger; they are quick to fall in love, and men are as quickly enthralled by them. But Sonia Kovalevsky was not one of these.
The women of the second type present the greatest contrast that it is possible to imagine. They are honest and straightforward, and essentially what is called “a good fellow,” plain, sensible, brave, energetic, as strong in soul as in body,—thinking heads, flat figures; they have none of that grace of form which is peculiar to a large number of Russian women. Their faces are generally sallow, and their skin is clammy, but thoroughly Russian in spite of it. There is something lacking in them, which for want of a better expression I shall call a want of sweetness. There is a curious neutrality about them; it takes one some time to realize that they are women. And they themselves are but dimly conscious of it, and then only on rare occasions. They are generally people with a mission,—working people, people with ideas.
It is these women who have furnished the largest contingent to the ranks of the Nihilists. It is they who chose to lead the lives of hunted wild beasts, and who found ample compensation in mental excitement for all that they had renounced as women and as persons of refinement. But although this last is a genuine Russian type, it is by no means confined to Russia. It is a type peculiar to the age. The class of women who become Nihilists in Russia are the champions for women’s rights in Sweden, and it is they who agitate
for women’s franchise in England, who start women’s clubs in America, and become governesses in Germany.
The type is universal, but it is left to circumstances to decide which special form of mania it is to take,—a form of mania which calls itself “a vocation in life.” In Russia the woman, in whom sex lay dormant, felt it her calling to become a murderess, and that merely from a general desire to promote the popular welfare; in Germany this philanthropic spirit took the form of wishing to prune little human plants in the Kindergarten. But this is a long chapter, which I cannot pursue any further at present, and which, like many others on the characteristics of the woman of to-day, I shall keep for a separate book. We must include Sonia Kovalevsky in this latter type: she considered herself as belonging to it, and the whole course of her life is in itself sufficient to prove that she was one of them. The nature of her friendships with men furnishes us with yet another proof. She had a large circle of acquaintances, amongst whom were some of the best known and most talented men of Russia, Scandinavia, England, Germany, France, and Italy,—all of whom enjoyed her society, although not one of them fell in love with her, and not one among those thousands said to her, “I cannot exist without you.”
She belonged to the class of women with brains, and she was numbered amongst them. She was their triumphant banner, the emblem of their greatest victory, and their appointed Professor. “She did not need the lower pleasures; her science was her chief delight.” She stood on the platform and taught men, and believed it to be her vocation. Was it not for this that she had toiled during long years of overwork and study, whilst concealing her real purpose under the threadbare cloak of a feigned marriage?
She was a woman of genius with a man’s brain, who had come into the world as an example and a leader of all sister brains. She was, and she was not! Sometimes she felt that she was, and then again she did not. In her latter years she disclaimed the whole of her former life, and silence reigned among the aggrieved sisterhood whenever her name was mentioned; if these latter years had never been, they would have sent the hat round in order to erect
a monument in her memory But that became impossible; silence was best.
She was a woman. She was a woman in spite of all—in spite of a feigned marriage which lasted nearly ten years, in spite of a widowhood which lasted just as long, in spite of her Doctor’s degree and the Professorship of Mathematics and the Prix Bordin—she was a woman still; not merely a lady, but an unhappy, injured little woman, running through the woods with a wailing cry for her husband.
She was far more of a woman than those luxurious, prattling, sweatmeat-eating young ladies whose languid movements lead us to suppose that they have only just got out of bed; she was more of a woman than the great majority of wives, whose sole occupation it is to increase the world, and to obliterate themselves in so doing. She, who never charmed any man, was more of a woman than the charmers who turn love into a vocation. She was a new kind of woman, understood by no one, because she was new; she did not even understand herself, and made mistakes for which she was less to blame than the spirit of the age, by whose lash she was driven. And when she became free at last, it was too late to map out a future of her own.
Who knows whether it would have been better for her had she been free from the first? A woman has no destiny of her own; she cannot have one, because she cannot exist alone. Neither can she become a destiny, except indirectly, and through the man. The more womanly she is, and the more richly endowed, all the more surely will her destiny be shaped by the man who takes her to be his wife. If then, even in the case of the average woman, everything depends upon the man whom she marries, how much more true must this be in the case of the woman of genius, in whom not only her womanhood, but also her genius, needs calling to life by the embrace of a man. And if even the average woman cannot attain to the full consciousness of her womanhood without man, how much less can the woman of genius, in whom sex is the actual root of her being, and the source from whence she derives her talent and her ego. If her womanhood
remains unawakened, then however promising the beginning may be, her life will be nothing more than a gradual decay, and the stronger her vitality, the more terrible will the death-struggle be.
That was Sonia’s life. No man took her in his arms and awoke the whole harmony of her being. She became a mother and also a wife, but she never learned what it is to love and be loved again.
VA� I write, the air is filled with a sweet penetrating fragrance, which comes from a tuberose, placed near me on the window-sill. The narrow stalk seems scarcely strong enough to support its thick, knob-like head with the withered buds and sickly, onion-shaped leaves. A tuberose is a poor unshapely thing at the best of times, but this plant is unhealthy because it has lived too long as an ornament in a dark corner of the room under the chandeliers, among albums and photographs. It was dying visibly, decaying at the roots, and there was no help for it. Of course it was a rare flower, but it grew uglier from day to day.
They put it on the window-sill, where there was just room for one plant more, and a pot of mignonette was fetched out of the kitchen garden, attired in an artistic ruffle of green silk paper, and placed under the chandelier in its stead. It fulfilled its duty well, and seemed to thrive admirably among the albums, visiting-cards, and photographs. Nobody looked after the tuberose on the window-sill until it suddenly reminded them of its existence by a strong smell, and even then they only cast a hasty glance and noticed how sickly it looked. When I examined it more closely, I discovered three blossoms in full flower, and quite healthy; the stem was bent forward, and the blossoms were pressing against the window-pane, doing their best to catch the rays of the sun as long as the short autumn day lasted. It thrust forth its dying blossoms and renewed itself now that the great warmer of life was shining on, and embracing it. To me this flower is an emblem of Sonia Kovalevsky.
She was a rare, strange being in this world of mignonette pots and trivialities. Everything about her was out of proportion, from her thin little body, with its large head, to the sweet fragrance of her genius. She, too, stood in the place of honor under the chandelier, among fashionable poets and thinkers who wrote and thought in accordance with the spirit of the age; and she, too, sickened, as though she desired something better, and the nervous blossoms which her mind thrust forth grew more and more withered, and the thin stem which carried her stretched more and more towards the greater warmer of life, which shines upon and embraces the just and the unjust,—only not her, only not her!
What was the reason? Why did she get none of that love which is rained down upon the most insignificant women in so lavish a manner by impetuous mankind?
“She was not in the least pretty, that is it,” reply her several women admirers.
But we women know well that it is not the prettiest women who are the most loved, and that, on the contrary, the most ardent love always falls to the share of those in whom men have something to excuse. Barbey d’Aurevilly, the greatest women’s poet, has told us so in his immortal lines.
“She was too old,—that is to say, she aged too early,”—say her women admirers, still anxious to find an explanation.
But that is ridiculous. Sonia Kovalevsky died at the age of forty, and that is the age when a Parisian grande mondaine is at the height of her popularity; and as for aging early—! A woman of genius does not grow old as quickly as a teacher in a girl’s school, and the fading tuberose which thrusts forth fresh blossoms has a far sweeter and more penetrating fragrance than her white knob-headed sisters.
“She asked too much,” asserts Fru Anne Charlotte Edgren-Leffler, Duchess of Cajanello, who was of the same age as Sonia, and married at the time when she died; and her entire book on Sonia is founded on the one argument, that she asked too much of love.
But how is it possible? Does not experience teach us that it is just the women who ask most who receive most? Always make fresh claims,—that is the motto of the majority of ladies in society, and with this solid principle to start from they have none of them failed.
“She had everything that a human being can desire,” said that worthy writer, Jonas Lie, in an after-dinner speech. “She had genius, fame, position, liberty, and she took the lead in the education of humanity. But when she had all this it seemed to her as nothing; she stretched out her hand like a little girl, and said, ‘Oh, do but give me also this orange.’”
It was kindly said, and also very true. Father Lie was the only person who understood Sonia, and saw that she remained a little girl all her life,—a woman who never reached her maturity. But, tell me, dear Father Lie, do you consider love to be worth no more than an orange?
No, these explanations will never satisfy us; they are far too shallow and simple. The true reason lies deeper; it is more a symptom of the time in which she lived than those who knew her will allow. Even so friendly and intelligent an exponent as Ellen Key, her second biographer, does not seem to be aware of the fact that, although Sonia is a typical woman of her time,—typical of the more earnest upholders of women’s rights, and the representative of the highest intellectual accomplishments to which women have attained,—she is also typical of that which the woman of this century loses in the struggle, and of that in which the woman of the future will be the gainer.
If Sonia failed to please,—she whose personal charm was so great, whose vivacity was so prepossessing, as all who knew her declared that it was; if she failed where so many lesser women have succeeded, her failure was entirely due to her ignorance of the art of flirtation,—an art which is as old as sex, and to which men have been accustomed since the world began. Even the most refined, the most highly-developed men, are not geniuses in this matter, where everything has always been most carefully arranged for them. And if they did not fall in love with Sonia, it was due to a kind of purity with
which she unconsciously regarded the preliminaries of love,—a kind of nobility which existed in her more modern nature, and a lack of the ancient instinct which had been a lost heritage to her.
Sonia belonged to a class of women who have only been produced in the latter half of our century, but in such large numbers that it is they who have determined the modern type. We cannot help hoping that they are but transitory, so greatly do their assumptions seem opposed to their sex, and yet they are formed of the best material that the age supplies. They are the women who object to begin life by fulfilling their destinies as women, and who consider that they have duties of greater importance than that of becoming wives and mothers; they are the “clever” daughters of the middle-class families, who, as governesses and teachers, swarm in every country in Europe. The popular opinion about them is that they do not want to marry; and as that, by the majority of men, is interpreted to mean that they are no good as wives, they turn to the herd of geese who are driven yearly to the market, and who go cackling to meet their fate. And although the descendants of such fathers and such mothers present a very small amount of intelligence capable of development, yet it is they who form the majority, and the majority is always right. Formerly, it was people’s sole object to get their daughters married, clever and stupid alike; it was an understood thing. But nowadays, the ones with “good heads” are set apart to lead celibate lives, while those who are “hard of understanding” are brought into the marriage market. This method of distribution has already become one of the first principles of middle-class economy The daughters who are considered capable of providing for themselves are given a good education, accompanied by numerous hints as to the large sums which their parents have spent on them; while, together with the inevitable marriage portion, every effort is made to find husbands for the others with as little delay as possible. The first named are “the clever women,” but the latter make “the best wives;” and man’s sense of justice in the distribution of the good things of this life has fixed a stern practical barrier between these two classes.