DRUGDELIVERYASPECTS
EXPECTATIONSANDREALITIESOF MULTIFUNCTIONALDRUG DELIVERYSYSTEMS
VOLUME4
Editedby
RANJITA SHEGOKAR,PHD
CapnomedGmbH,Zimmern,Germany
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,including photocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher. Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswith organizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybe notedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding, changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation, methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheir ownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjury and/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationof anymethods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-821222-6
ForinformationonallElsevierpublicationsvisitourwebsite at https://www.elsevier.com/books-and-journals
Publisher: AndreGerhardWolff
AcquisitionsEditor: ErinHill-Parks
EditorialProjectManager: PatGonzalez
ProductionProjectManager: KiruthikaGovindaraju
CoverDesigner: MarkRogers
TypesetbySPiGlobal,India
Contributors
HendAbd-Allah DepartmentofPharmaceutics andIndustrialPharmacy,FacultyofPharmacy, AinShamsUniversity,Cairo,Egypt
SaraM.AbdelSamie DepartmentofPharmaceutics andIndustrialPharmacy,FacultyofPharmacy,Ain ShamsUniversity,Cairo,Egypt
MonaM.A.Abdel-Mottaleb DepartmentofPharmaceuticsandIndustrialPharmacy,Facultyof Pharmacy,AinShamsUniversity,Cairo,Egypt; PEPITEEA4267,Univ.BourgogneFranche-Comte, Besanc ¸on,France
AbidRiazAhmed MerckHealthcareKGaA, Darmstadt,Germany
AkashChavrasiya DepartmentofPharmacy,Birla InstituteofTechnologyandScience,Pilani, Hyderabad,India
YanpingChen CenterofEmphasisinInfectious Diseases,DepartmentofMolecularandTranslationalMedicine,PaulL.FosterSchoolofMedicine, TexasTechUniversityHealthSciencesCenterEl Paso,ElPaso,TX,UnitedStates
JoaoDias-Ferreira DepartmentofPharmaceutical Technology,FacultyofPharmacy,Universityof Coimbra,Coimbra,Portugal
DianaDiaz-Arevalo ImmunologyFunctional Group,FoundationInstituteofImmunologyof Colombia-FIDIC,SchoolofMedicineandHealth Sciences,UniversidaddelRosario,Bogota,D.C., Colombia
SachinDubey Formulation,AnalyticalandDrug ProductDevelopment,GlenmarkPharmaceuticals, LaChauxdeFonds,Switzerland
AlessandraDurazzo CREA-ResearchCentrefor FoodandNutrition,Rome,Italy
ThomasD € urig R&DandInnovation,Ashland PharmaandHealth&Wellness,AshlandSpecialty IngredientsG.P.,Wilmington,DE,UnitedStates
RihamI.El-Gogary DepartmentofPharmaceutics andIndustrialPharmacy,FacultyofPharmacy, AinShamsUniversity,Cairo,Egypt
MuhammadIrfan DepartmentofPharmaceutics, FacultyofPharmaceuticalSciences,GCUniversity Faisalabad,Faisalabad,Pakistan
NirmalJayabalan DepartmentofPharmacy,Birla InstituteofTechnologyandScience,Pilani, Hyderabad,India
AnđelkaB.Kovacevic DepartmentofPharmaceuticalTechnology,FacultyofBiologicalSciences, InstituteofPharmacy,Friedrich-SchillerUniversity Jena,Jena,Germany
DharmeshMehta BusinessDevelopment,Gangwal Chemicals,Mumbai,India
JoanaPortugalMota Lecifarma—Laborato ´ rio Farmac^ eutico,Lda,Va ´ rzeadoAndrade—Cabec ¸o deMontachique,Lousa;CBIOS-ResearchCenter forBiosciencesandHealthTechnologies,Luso ´ fona University,Lisbon,Portugal
MostafaNakach SanofiR&D,VitrysurSeine, France
MahaNasr DepartmentofPharmaceuticsand IndustrialPharmacy,FacultyofPharmacy,Ain ShamsUniversity,Cairo,Egypt
RidahunlangNongkhlaw Department ofPharmacy,BirlaInstituteofTechnologyandScience, Pilani,Hyderabad,India
ParameswarPatra DepartmentofPharmacy,Birla InstituteofTechnologyandScience,Pilani, Hyderabad,India
AntonelloSantini DepartmentofPharmacy, UniversityofNapoli“FedericoII”,Napoli,Italy
AhmadAbdul-WahhabShahba KayyaliChairfor PharmaceuticalIndustries,DepartmentofPharmaceutics,CollegeofPharmacy,KingSaudUniversity,Riyadh,SaudiArabia
RanjitaShegokar CapnomedGmbH,Zimmern, Germany
VaibhavSihorkar Formulations,NCEandInnovation,SaiLifeSciencesLimited,ICICIKnowledge Park,GenomeValley,Hyderabad,Telangana, India
SarabjitSingh FormulationResearch,CIPLA, Mumbai,India
ElianaB.Souto DepartmentofPharmaceutical Technology,FacultyofPharmacy,Universityof Coimbra,Coimbra;CEB—CentreofBiological Engineering,UniversityofMinho,GualtarCampus,Braga,Portugal
HaiyanWang KeyLaboratoryofOralMedicine, GuangzhouInstituteofOralDisease,Stomatology HospitalofGuangzhouMedicalUniversity, Guangzhou,Guangdong,People’sRepublicof China;CenterofEmphasisinInfectiousDiseases,
DepartmentofMolecularandTranslationalMedicine,PaulL.FosterSchoolofMedicine,TexasTech UniversityHealthSciencesCenterElPaso,ElPaso, TX,UnitedStates
YongyongYan KeyLaboratoryofOralMedicine, GuangzhouInstituteofOralDisease,Stomatology HospitalofGuangzhouMedicalUniversity, Guangzhou,Guangdong,People’sRepublicof China;CenterofEmphasisinInfectiousDiseases, DepartmentofMolecularandTranslationalMedicine,PaulL.FosterSchoolofMedicine,TexasTech UniversityHealthSciencesCenterElPaso,ElPaso, TX,UnitedStates
MingtaoZeng CenterofEmphasisinInfectious Diseases,DepartmentofMolecularandTranslationalMedicine,PaulL.FosterSchoolofMedicine, TexasTechUniversityHealthSciencesCenterEl Paso,ElPaso,TX,UnitedStates
Versatilehyaluronicacidnanoparticles forimproveddrugdelivery
MonaM.A.Abdel-Mottaleb a,b,HendAbd-Allah a , RihamI.El-Gogarya,MahaNasra
aDepartmentofPharmaceuticsandIndustrialPharmacy,FacultyofPharmacy,AinShamsUniversity, Cairo,Egypt
bPEPITEEA4267,Univ.BourgogneFranche-Comte,Besanc ¸ on,France
1Introduction
Althoughmorethan80yearshavepassedsince thediscoveryofhyaluronicacid(HA),itstillsurprisesresearcherswithitsuniquephysicochemical propertiesandphysiologicalrolesinthehuman body.HAwasfirstdiscoveredbyKarlMeyer andJohnPalmerin1954 [1].Theyisolatedan unknownmaterialfromthevitreousbodyofa bovineeye,containingtwosugarmolecules including“uronicacid.”So,byconnectingthesubstitutenameforthevitreous—“hyaloid”—with thenameofacomponentofthatpolysaccharide—“uronicacid”—thenameofHAwas adoptedforthismaterial.HAwasfirstusedcommerciallyasasubstituteforeggwhiteinbakery products.Lateron,itsfirstmedicalapplication forhumanswasinitiatedasavitreousreplacement duringeyesurgeryinthelate1950s [2]
HAbelongstoagroupofsubstancescalled mucopolysaccharidesbelongingtotheglycosaminoglycans(GAGs)family [1,2].HAincludes
severalthousandrepeatingdisaccharidesmoleculesinthebackbone.Themolecularweightof HAmoleculesdiffersowingtothevariable numberoftheserepeatingdisaccharideunits ineachmolecule,itsmolecularweightranges from1to10,000kDa [3].HAhasanunusual mechanismofbiosynthesisandexceptional physicalproperties.Sodiumhyaluronateisthe predominantformofHAatphysiologicalpH. SodiumhyaluronateandHAarecollectively referredtoashyaluronan.Duetothefactthat HAexistsasapolyanion,itcanself-associate andcanalsobindwatermoleculesgivingita stiff,viscousqualitywithjelly-likeconsistency thatcausesittobehavelikealubricant [2].
HAwasfoundtobeabundantlydistributed incellularsurfaces,inthebasicextracellular substancesoftheconnectivetissuesofvertebrates,inthesynovialfluidofjoints,inthevitreoushumoroftheeye,andinthetissueofthe umbilicalcord;allthisattractedsignificant attentionregardingitsmedicalapplications
[4].AlthoughHAhasaverysimplestructure, almosteverythingelseconcerningthemolecule isunusual.Sometimesitsroleismechanicaland structural,suchasinsynovialfluid,thevitreous humor,ortheumbilicalcord.Inothercases,it caninteractinlowconcentrationswithcellsto triggerimportantcellularresponses.HA’scharacteristics,includingitsconsistency,biocompatibility,andhydrophilicity,havemadeitan excellentmoisturizerincosmeticdermatology andskin-careproducts.Moreover,itsunique viscoelasticityandlimitedimmunogenicity haveledittobeusedforviscosupplementation inosteoarthritistreatment,asasurgicalaidin ophthalmology,andforsurgicalwoundregenerationindermatology.Inaddition,HAhascurrentlybeenexploredasadrugdeliveryagentfor differentroutessuchasnasal,pulmonary,ophthalmic,topical,andparenteral [2].Hencethe useofnanotechnologywouldcombinetheoutstandingpropertiesofHAbeingbiocompatible, biodegradable,nontoxic,andabletobindspecificreceptorswiththedifferentadvantagesof nanoparticlessuchasenhancedtherapeutic effectsandtargetability.Inthischapter,the useofHAnanoparticlesasaversatiledrug deliverysystemwillbediscussedbyhighlightingthedifferentproductiontechniquesbased onthechemicalandbiologicalpropertiesofHA.
2Hyaluronicacid
2.1Chemistry
TheexactchemicalstructureofHAwas determinedbyWeissmanandMeyerin1954. Asalreadynoted,HAbelongstoagroupofsubstancescalledmucopolysaccharidesbelonging totheGAGsfamily.ItisanunbranchednonsulfatedGAGcomposedofrepeatingdisaccharides [β-1,4-D-glucuronicacid(knownasuronicacid) and β-1,3-N-acetyl-D-glucosamide],asshown in Fig.1.1.Bothsugarsarespatiallyrelatedto glucoseinthebetaconfiguration,thusallowing
allitsbulkygroups(thehydroxyls,thecarboxylatemoietyandtheanomericcarbononthe adjacentsugar)tobeinstericallyfavorableequatorialpositions,whileallthesmallhydrogen atomsoccupythelessstericallyfavorableaxial positions.Thus,thestructureofthedisaccharide isenergeticallyverystable [3].
TheHAbackboneisstiffenedinphysiological solutionviaacombinationofinternalhydrogen bonds,interactionswithsolvents,andthechemicalstructureofthedisaccharide.HAmolecular investigationssuggestedthattheaxialhydrogen atomsformanonpolarface(relativelyhydrophobic)andtheequatorialsidechainsformamore polarface(hydrophilic)whichleadstoatwisted ribbonstructureforHAcalledacoiled structure [4]
Owingtothisconformationalbehavioras wellasitshighmolecularweight,thesolutions ofHAareveryviscousandelastic.Atvery lowconcentrations,chainsentanglewitheach other,leadingtoamildviscosity(molecular weightdependent).However,HAsolutionsat higherconcentrationshaveahigherthanexpectedviscosityduetogreaterHAchainentanglementthatisshear-dependent.Forinstance,a1% solutionofhighmolecularweightHAcan behavelikejelly,butwhenshearstressis applied,itwilleasilyshearthinlyandcanbe administeredviaafineneedle [2].HAisthereforea“pseudo-plastic”material.Thisrheologicalproperty(concentrationandmolecular weightdependent)ofHAsolutionshasmade
FIG.1.1 Chemicalstructureofhyaluronicacidunit.
itidealasalubricantinbiomedicalapplications. Thereisevidencethathyaluronanseparates mosttissuesurfacesthatslidealongeachother. Theextremelylubriciouspropertiesofhyaluronan,meanwhile,havebeenshowntoreduce postoperativeadhesionformationfollowing abdominalandorthopedicsurgery.
HAhasseveralinterestingmedical,pharmaceutical,food,andcosmeticusesinitsnaturally occurringlinearform.However,chemicalmodificationsoftheHAstructurerepresentastrategytoextendthepossibleapplicationsofthe polymer,obtainingbetterperformingproducts thatcansatisfyspecificdemandsandcanbe characterizedbyalongerhalf-life.Duringthe designofnovelsyntheticderivatives,particular attentionispaidtoavoidthelossofnativeHA propertiessuchasbiocompatibility,biodegradability,andmucoadhesivity [5].HAcanbechemicallymodifiedbycrosslinkingorconjugation reactions.Thesechemicalmodificationsmainly involvetwofunctionalsitesofthebiopolymer: thehydroxyl(probablytheprimaryalcoholic functionofthe N-acetyl-D-glucosamine)andthe carboxylgroups [6].Furthermore,synthetic modificationscanbeperformedafterthedeacetylationofHAN-acetylgroups [7].
ConjugationreactionsusuallyconsistofaddingamonofunctionalmoleculeontooneHA chainbyasinglecovalentbond,whilecrosslinkingemployspolyfunctionalcompoundstolink togetherdifferentchainsofnativeorconjugated HAbytwoormorecovalentbonds.Crosslinked hyaluronancanbepreparedfromnativeHA (directcrosslinking)orfromitsconjugates. Crosslinkingisnormallyintendedtoimprove themechanical,rheological,andswellingpropertiesofHAandtoreduceitsdegradationrate, todevelopderivativeswithalongerresidence timeinthesiteofapplicationandcontrolled releaseproperties [5]
2.2Sources
HAisanaturalpolymerbiologicallysynthesizedbycellsinthebodybyanenzymaticprocess.HAproductionisaunique,highly controlled,andcontinuousprocess.Approximatelyhalfofourbody’sHAisdistributedin thecutaneousregion.Itisproducedand secretedbycellsincludingfibroblasts,keratinocytes,orchondrocyteswithvaryingmolecular weightsbetween50and3000kDa.TheGolgi networkistheproductionsiteformostGAGs. IntissuessuchasskinandcartilagewhereHA comprisesalargeportionofthetissuemass, HAissynthesizedinlargeamounts.Itisnaturallysynthesizedbyhyaluronansynthases (HAS1,HAS2,andHAS3),aclassofintegral membraneproteins [10].Thethreeenzymes arelocatedondifferentchromosomes,producingHAwithdifferentmolecularweights. HAS1andHAS2proteinsareresponsiblefor thesynthesisofhighmolecularweightHA ( 2 106 Da)withthelattermoreactivecatalyticallythantheformer,whereastheenzyme HAS3isthemostactivebutcanonlysynthesize shortHAchainsfrom200,000to300,000Da.The differentmolecularweightsofHAchainscan leadtodifferenteffectsoncellbehavior.HAperformsitsbiologicalactionsaccordingtotwo basicmechanisms:itcanactasapassivestructuralmoleculeandasasignalingmolecule.Both mechanismsofactionhavebeenshowntobe size-dependent [11].
Asmentionedabove,HAhasanessential functionalcomponentofalmostalltissuesin thevertebrateorganism.Thus,variousanimal
ConjugationofdrugstoHAwasreportedas earlyas1991.ThisapproachaimedtoformaprodrugbycovalentlybindingadrugtotheHA backbonethroughabondthatideallyshould bestableduringthebloodcirculationand promptlycleavedataspecifictargetsite [8]. OwingtoHAsolubility,itispossibletoperform thereactioninwater.However,intheaqueous phase,somereactionsarepH-dependentand needtobeperformedinacidicoralkalineconditions,whichhavebeenshowntoinducesignificantHAchainhydrolysis [8,9].
tissues,forexample,inroostercombs,sharkskin, andbovineeyeshavebeenusedassourcesofisolationandproductionofhighmolecularweight HA.SinceHAinbiologicalmaterialsisusually presentinacomplexlinkedtootherbiopolymers, severalseparationproceduresmustbeappliedto obtainapurecompound,suchasproteasedigestion [10].HAwasinitiallyisolatedfrombovine vitreoushumorandlaterfromroostercombs andhumanumbilicalcords [11].Themean molecularweightofthecommerciallyavailable “extractive”HApreparationsobtainedfromanimaltissuesismostlyintherangefromseveral hundredthousandDauptoapproximately2.5 MDa [12].However,ithasbeenobservedthat theHAproductsobtainedfromroostercombs causedsomeallergicresponses.Further,thetechnologyhasbeendevelopedrecentlytowardbacterialfermentationtoreducetheproductioncost andcomplexpurificationprocesses.Suchalternativesourcesincludeattenuatedstrainsof Streptococcuszooepidemicus and Streptococcusequi for theproductionofHA.Thebacteriumsecretes theHAintofermentationbrothandthisbehavior isanadditionaladvantageforisolationoftheHA directlyfrombrothwithouttheneedforhomogenizingbacterialcells [13,14].However,therisk ofmutationofthebacterialstrains,andpossible co-productionofvarioustoxins,pyrogens,and immunogens,decreasestheapplicationoffermentativeHAinclinicalpractice.Thisisalso whyHAsamplesoriginatingfromroostercombs arestillcurrentlypreferredforhumantreatment incaseswhentheHAmaterialisdesignatedfor injection,intheeyesorsynovialjoints.However, thesearealsonotidealsourcesofHA,asallHA productsobtainedfromroostercombsareobligatedtocarrywarningsforthosewhoareallergic toavianproducts.Thus,alternativesourcesfor productionofHAarepresentlyasubjectof research [12]
Oneofthepromisingpotentialcandidatesis ageneticallymodifiedbacterialstrain, Bacillus subtilis ,carryingtheAgenefrom Streptococcus equisimilis encodingtheenzymeHAsynthase.
SuchanengineeredstraincouldproduceHA withthemolecularweightinthe1MDarange. Theadvantageofusing B.subtilis isthatitis easilycultivatedonalargescaleanddoes notproduceexotoxinsorendotoxins,and manyproductsmanufacturedbythismicroorganismhavereceivedaGRAS(generallyrecognizedassafe)designationintheearly 1960s.Atpresent,microbiologicallyproduced HAhasbeenapprovedfortreatmentofsuperficialwoundsaswellasforuseinthecosmetic industry [12] .
2.3Physiologicalrole
HAdiffersfromothersyntheticpolymersin thatitisbiologicallyactive.Togetherwith HA’soutstandingviscoelasticnature,itsbiocompatibilityandnonimmunogenicityhave ledtoitsuseinseveralclinicalapplications. HAwasdescribedasanubiquitouscarbohydratepolymerthatispartoftheextracellular matrix [15].Ahumanbodyweighing70kgcontains15gofHA.ThegreatestamountofHAis presentintheskin,followedbythesynovial fluid,thevitreousbody,andtheumbilicalcord. Itcanalsobefoundinplaceswherefriction occurs:thejoints,tendons,sheaths,pleura,and pericardium [16].
Inthehumanbody,HAoccursinmany diverseforms,circulatingfreely,decoratedwith avarietyofHA-bindingproteins(hyaladherins), tissue-associated,intercolatedintotheextracellularmatrixbyelectrostaticorcovalentbindingtoothermatrixmolecules.Itcomprisesa majorportionoftheintimateglycocalyxthat surroundsallcells.HAcanbetetheredtocell surfacesbyanyofthemembrane-associated receptors.RecentevidenceindicatesthatHA alsoexistswithincells,thoughlittleisknown oftheformorfunctionofsuchHA [17]
HAisamajorcomponentofthesynovial fluid,andwasfoundtoincreasetheviscosity ofthefluid.Alongwithlubricin,itisoneof thefluid’smainlubricatingcomponents.Itis
consideredanimportantcomponentofarticular cartilage,presentingacoataroundindividual chondrocytesandprovidingitsresistanceto compression.Themolecularweight(size)of HAincartilagedecreaseswithage,butthe amountofitincreases [18].HApossessesanumberofprotectivephysiochemicalfunctionsthat mayprovidesomeadditionalchondroprotectiveeffectsinvivo,explainingitslonger-term effectsonarticularcartilage.HAdecreasesthe nerveimpulsesandnervesensitivityassociated withpain.Inexperimentalosteoarthritis,HA hasprotectiveeffectsoncartilage [19].ExogenousHAenhancesitssynthesistogetherwith proteoglycaninchondrocyte,reducestheproductionandactivityofproinflammatorymediatorsandmatrixmetalloproteinases,andalters thebehaviorofimmunecells.Thesefunctions aremanifestedbythescavengingofreactive oxygen-derivedfreeradicals,theinhibitionof immunecomplexadherencetopolymorphonuclearcells,andtheinhibitionofleukocyteand macrophagemigrationandaggregation [20]
Alubricatingroleofhyaluronaninmuscular connectivetissuestoenhanceslidingbetween adjacenttissuelayershasalsobeensuggested. Aparticulartypeoffibroblasts,embeddedin densefascialtissues,hasbeenproposedasbeing cellsspecializedforthebiosynthesisofthe hyaluronan-richmatrix.Theirrelatedactivity couldbeinvolvedinregulatingthesliding abilitybetweenadjacentmuscularconnective tissues [18].
HAisalsoamajorcomponentofskin.More thanhalfofthetotalbody’sHAispresentin theskin [8],whereitplaysastructuralrolethat dependsonitsuniquehydrodynamicproperties anditsinteractionswithotherextracellular matricesmolecules(ECM)components.HA excellentconsistencyandtissue-friendliness andbeingoneofthemosthydrophilicmolecules innaturehascausedittobedescribedas nature’smoisturizer [4].Itgivestheskinits propertiesofresistanceandmaintenanceof theshape,andsupportsthepreservationof
thenaturaldegreeofhydrationoftheskincells. Itsconcentrationinthebodytendstodecrease withaging,andalackofitleadstoaskinweaknesspromotingtheformationofwrinkles [7]
Whileitisabundantinextracellularmatrices, HAalsocontributestotissuehydrodynamics, movementandproliferationofcells,andparticipatesinanumberofcellsurfacereceptorinteractions,notablythoseincludingitsprimary receptors,CD44andRHAMM.Upregulation ofCD44itselfiswidelyacceptedasamarker ofcellactivationinlymphocytes.HA’scontributiontotumorgrowthmaybeduetoitsinteractionwithCD44.ThereceptorCD44participates incelladhesioninteractionsrequiredbytumor cells.
Onthecellularlevel,HAishighlyhygroscopicandthispropertyisbelievedtobeimportantformodulatingtissuehydrationand osmoticbalance.Becauseofitshygroscopic properties,hyaluronansignificantlyinfluences hydrationandthephysicalpropertiesofthe extracellularmatrix.Hyaluronanisalsocapable ofinteractingwithseveralreceptors,resultingin theactivationofsignalingcascadesthatinfluencecellmigration,proliferation,andgene expression [21].
2.4Turnoverandeliminationpathways
TheconcentrationofHAinthehumanbody variesfromahighconcentrationof4g/kgin umbilicalcord,2–4g/Linsynovialfluid,0.2 g/kgindermis,about10mg/Linthoracic lymph,andthelowestof0.1–0.01mg/Linnormalserum [10].Dependingonthelocationof HAinthebody,mostofitiscatabolizedwithin days.Studiessuggestedthatthenormalhalf-life ofHAvariesfrom1–3weeksininerttissues suchascartilages,to1–2daysintheepidermis ofskin,to2–5mininbloodcirculation.ThepathwaysinvolvedinHAcatabolismincludeturnover(internalizationanddegradationwithin tissue)andreleasefromthetissuematrix,
1.Versatilehyaluronicacidnanoparticlesforimproveddrugdelivery
drainageintothevasculature,andclearancevia lymphnodes,liver,andkidneys.
Instructuraltissueslikeboneorcartilage withnoorlittlelymphaticdrainage,HAdegradationoccursinsituwithotherECMsuchascollagensandproteoglycans.Ontheotherhand,in skinandjoints,aminimalfraction(approximately20%–30%)ofHAdegradesinsitu.Since HAisrestrictedtothesmallintracellularspace ofskintissue,itshalf-lifeisslightlylongerfor daysandweeks [22].
3Preparationofhyaluronicacid nanoparticles
3.1Conjugateformation
Oneofthemostcommonlyusedtechniques forthepreparationofHAnanoparticlesisthe preparationofHA-drugnano-conjugatesby establishingacovalentbondbetweenthedrug ofinterestandHAwhichcouldimprovesolubility,pharmacokineticprofile,andinvivoplasma half-lifeoftheconjugateddrugs.Inmostcases, theconjugationisdesignedtobecleavedafter reachingthetargetsite,andincasesofcancer, theseconjugatesusuallyhavehigheraccumulationinthetumorsitesduetotheenhancedpermeationandretentioneffect(EPR) [23].Dueto thepresenceofmultiplefunctionalgroupson thebackboneofHAlikehydroxylandcarboxylic acidgroups,itwaspossibletogetconjugatedto variouscompoundsandmacromoleculessuchas paclitaxel [24,25],sodiumbutyrate [26,27],and ovalbumin [28].
TheuseofHAdrugconjugatesiskindofconvertingthedrugintoaprodrugderivativein whichthelinkbetweentheHAandthedrug moleculeshouldachievecertaincriteria.The mostimportantisthatthebondshouldbestable extracellularlytogivetherequiredinvivohalflife,andshouldbecleavedeasilyintracellularly toachievethedesiredeffect.Itisevidentthatthe releaseofintactdrugmoleculewithoutaffecting
thechemicalstructureisanotherrequiredproperty [29].Thetwomostcommonsitesforthe chemicalconjugationofdrugstoHAarethe hydroxylgroupsandthecarboxylicacidfunctionalitieswhiletheterminalaldehydegroup canonlybeusedtoprepareterminallymodified HA-ligandconjugatesaswellastograftHA oligomerstoanotherpolymercarryingamino groups.
3.2Self-assembliesformation
AnothertypeofHAnanoparticlesarethe onespreparedbyHAassociationwithhydrophobicpolymerswiththeresultingconjugates abletoself-aggregateintheformofhydrophobic micelles.Examplesincludethehydrophobic associationofHAtopoly(lactic-co-glycolicacid) (PLGA) [30],poly(ethyleneglycol)-poly (ε-caprolactone)copolymers(PEG-PCL) [31], ortetradecylamine [32].Theseamphiphilic self-assemblingHAderivativeshavebeenpreparedbycouplingthecarboxylicgroupsofthe hydrophilicHAthroughcarbodiimidechemistrytodifferenthydrophobicmoietiessuchas PLGA [33],tetradecylamine [32],andPCL [31]. Thiskindofnanoparticlewouldcombinethe hydrophobiccoreneededfortheencapsulation ofmanytherapeuticagents,andthehydrophilic shellprovideslongercirculationtimesbyreducingunwantedproteinadsorption [23].
HydrophobicallymodifiedHA(HMH)was preparedbythecovalentconjugationtothe hydrophobictetradecylamine(TDA)using 1-ethyl-3(3-dimethylaminopropyl)carbodiimide (EDC)andhydroxysulfosuccinimide(sulfoNHS).Thisreactionwasabletoproducenano self-aggregatesuponitsdissolutionandsonicationintoaqueousphosphatebuffersolution [32] Theself-associatedHMHnanoparticlesranged insizebetween197and285nmdependingon thedegreeofsubstitutionwithminorinfluence fromthepolymerconcentrationused.Thehigher thedegreeofsubstitution,thesmallertheparticles produced.AmphiphilicHA-5b-cholanicacid
conjugates(HA-CAconjugates)weresynthesized bychemicalconjugationofhydrophobicbileacid (5b-cholanicacid)tothehydrophilicHAbackbonethroughamideformationinthepresence ofEDCandNHS.VaryingthemolarratioofCA tothecarboxylicacidofHAvariedthedegreeof substitutionfrom2to10.Fortheproductionof HAnanoparticles,theproducedamphiphilic HA-CAconjugatesweredissolvedinaphosphate bufferedsalineandthesolutionwassonicated usingaprobe-typesonificationsystemfollowed byfiltrationstep.Theproducedparticlesranged insizefrom237to424nm [34].
3.3Ionicnanocomplexesformation
SinceHAisapolyanionicpolysaccharide,it canbeeasilyallowedtoreactwithcationiccompoundsandformsuccessfulioniccomplexes. ThisreactioncanbeinducedbydirectinteractionofHAwithpositivelychargedcargomoleculeslikeinthecaseofDNAandplasmids,orit canhappeninthepresenceofanotherpositively chargedpolymerlikechitosan [35].Examples includetheionicnanocomplexbetweenHA andTRAIL(TumornecrosisfactorRelatedApoptosisInducingLigand),whichwashighlystableandlongcirculatingcomparedtothe nativeTRAIL [36].Similarly,biopolymeric amphiphileswerepreparedfromtheEDC mediatedcouplingreactionbetweenHAand deoxcycholicacidproducingself-assembled nanocarriersinthesizerangeof100–600nm. ThestronginteractionbetweenHAandchitosan (CS)evenledtothefasterreleaseofthe entrappednegativelychargedcargomolecules, whosereleasecouldhavebeenhinderedincase ofinteractionwithchitosanalone.CS-HAplasmidnanoparticleswerepreparedbysimple mixingofbothsolutionsundermagneticstirring andDNAwasthenaddedtoformcomplexes. Themixturewasvortexedfor3–5sandthenleft atroomtemperatureforthecomplexestoform completely [37].Itwasfoundthatthesizeof thenanoparticlesincreasedandthezeta
potentialdecreaseduponincreasingtheratio ofHAtoCS.Similarly,HAcouldionicallyinteractwithpositivelychargedmetalliccompounds suchastheanticancerdrugcisplatin.CisplatinHAnanoparticleswerepreparedbysimple mixingofHAandthedrug,formingnanoparticlesinthesizerangeof80–160nm.Being largeinamount,thereleaseofthedrugfrom theparticlematrixwasaccompaniedbydisintegrationoftheparticles [38].
3.4Nanogelsformation
HAnanogelscanbepreparedbyeitherphysicalorchemicalcrosslinkingofHAtoprovide colloidalstableparticlesinthemicroornano range.Thephysicalcrosslinkingwoulddepend onnoncovalentattractiveforcesbetweenthe polymerchainssuchashydrophobicinteractions,hydrogenbondingandionicinteractions. However,chemicalcrosslinkingwouldprovide particlesofhigherstabilityandsubsequently longerhalf-lives.Topreparechemicallycrosslinkednanogelparticles,itispreferableto spatiallylocalizetheHAmoleculesandcrosslinkersinverysmallvolumestoachievethe requiredreactioninthenanorange.Thiscould besuccessfullyachievedusingtheinverse w/omicroemulsiontechnique,whichis describedin Fig.1.2[29].Hyaluronanmicrosphereswerepreparedbythesurfactant-aided homogenizationofHAaqueoussolutionsand crosslinkerinmineraloil.Thisstepisfollowed bytheinitiationofcrosslinkingbyadding EDC [39].Otherexamplesofnanogelparticles formationbyamidationcrosslinkingwere describedintheliterature [40,41].However,this microemulsionchemicalcrosslinkingtechniquesdemandshighenergymechanicalstirringorultrasonicationandtheuseoforganic solvents,whicharenotconsideredfavorable conditionsforthelabilemoleculessuchasproteinsandnucleicacids.Physicalcrosslinkingis consideredtobemuchmilderintermsofaffectingthelabilestructuresofdrugmolecules [29].
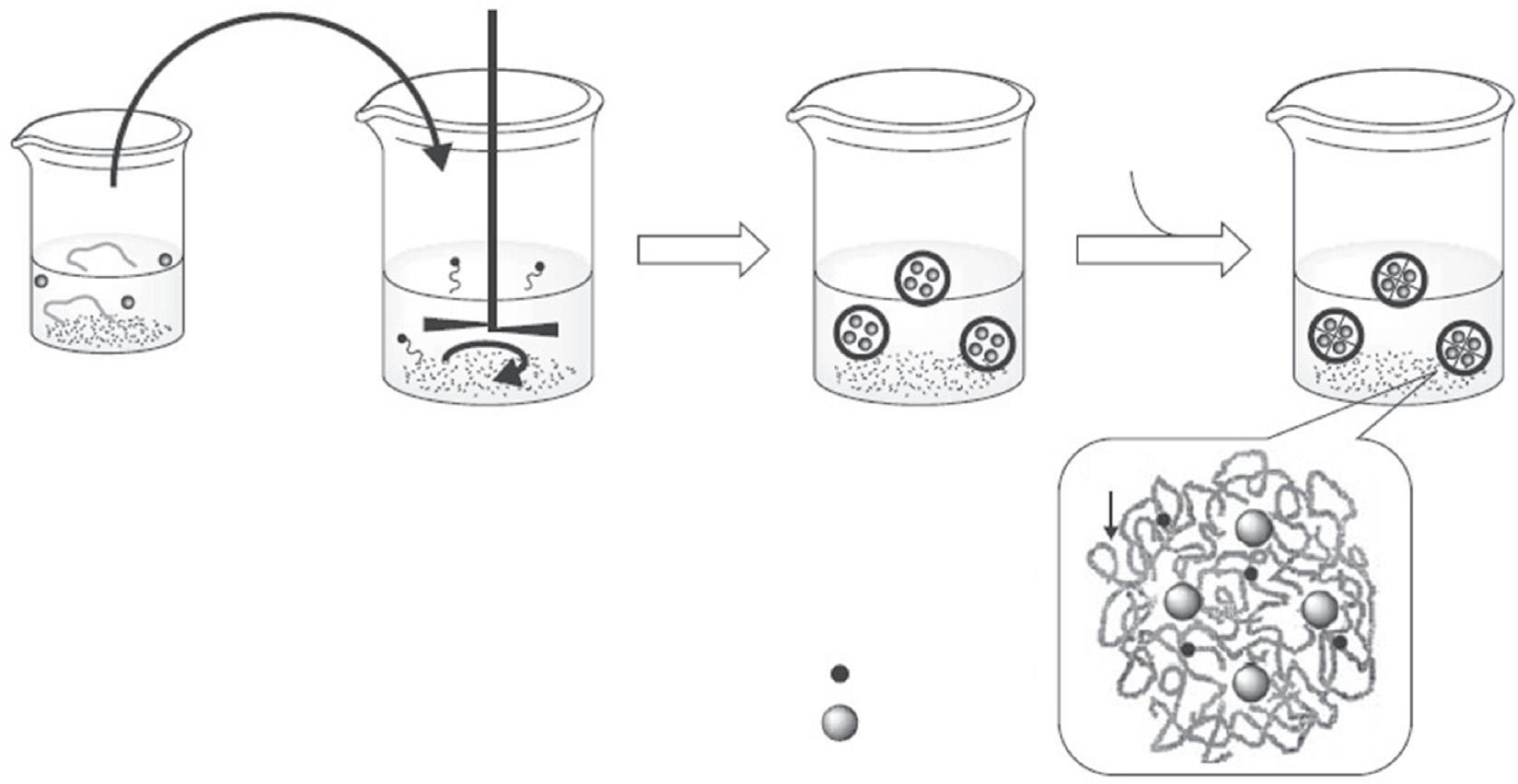
FIG.1.2 InversephasewaterinoilmicroemulsiontechniqueforthepreparationofchemicallycrosslinkedHAnanogels. ReprintedwithpermissionfromOssipovDA.Nanostructuredhyaluronicacid-basedmaterialsforactivedeliverytocancer.ExpertOpin DrugDeliv2010;7:681–703. https://doi.org/10.1517/17425241003730399 andTaylorandFrancisLtd.
4.1Skinapplications
AlthoughHAispresentinmostofthebiologicalfluidsandtissuesandextracellular matrixofsoftconnectivetissues,skinisconsideredtobemostHA-abundanttissueinthe humanbody.Uponaging,especiallyafterthe ageof20,skincontentofHAcontinuously decreases [42]
TheuniquepropertiesofHA,includingits biocompatibility,biodegradability,viscoelasticity,andnonimmunogenicity,havemadeitan idealmaterialforcosmeticandbiomedical applications.Itexertsahydratingeffectonthe skin,whichmayhelptoenhancethepenetration ofdifferentdrugsthroughtheskin.However,its ownpenetrationisverylimitedduetothehigh molecularweightaswellastheenzymatic degradationrisk.ItwasreportedthatcrosslinkedHAprovedtopermeatethroughtheskin
deeperlayersmakingitsuitablecarrierfortransdermalapplications [42].HAitselfasmacromoleculeisnotabletopenetratetheskin beyondthesurfacelayersduetothestrongbarrierproperties [43].HAnanoparticlesprepared bytheanionicinteractionwiththecationicpolymerprotaminewereabletopenetratetheskin anddelivertheHAtothedermiswhilefree HApenetratednofurtherthestratumcorneum. Therefore,thesenanoparticleswereconsidered promisingfortheeffectiveskindeliveryofHA tocontributetobarrierrecoveryfollowingUV irradiation [44].Similarly,nanoparticlesofquaternizedcyclodextrin-graftedchitosanassociatedwithHAhavebeenalsoproven promisingforcosmeticsandskinhydration applications.Theirskinhydratingabilityaswell astheirsafetyonhumanskinfibroblastswere demonstratedinvitro [45].
Besidestheuseofnanoparticlesforthedrug deliverypurposes,somecosmeticapplications
HA and drug Aqueous phase
Surfactant Organic phase
Nano gel
Crosslinks
Drug
Crosslinker or coupling agent
HA
havebeenproposed.Preparationsofslightly crosslinkedHAareusedasfillersforaugmentation,tofillfacialwrinklesanddepressedscars. SuchHAgelsaremoreeffectiveinmaintaining cosmeticcorrectionsthancollagen-basedproducts.Unlikecollagen-basedfillers,HAis extremelyelastic,providingtheelasticity requiredbyspacesinwhichitisinjectedand thehyaluronatepreparationsaremoresustained.ExamplesoftheuseofHANPsforcosmeticapplicationsaredescribedin Table1.1.
4.2Osteoarthritis
Theuseofintra-articularHAinjectionsasa viscosupplementtorestorethenormalviscosity ofsynovialfluidinosteoarthritispatientsisa well-establishedtherapeuticstrategy [11]. Cationicpolymericnanoparticleslinkedtohyaluronateprovedtobeeffectiveintheproduction ofionicallycrosslinkedhydrogelsinsituto increasetheretentiontimeofamodeldrugin thesynovialcavity [50].
4.3Tissueengineering
HAasoneofthemaincomponentsofbody tissueshasfrequentlybeeninvestigatedfor tissueengineeringapplications.HA-based sheetsserveasamatrixforsofttissue,cartilage, bone,andskingrowth,andasasubstratefor tissueregenerationandremodeling.ThreedimensionalscaffoldsofHA-basedmaterials canfacilitaterestructuringoftissuesandassist inregainingfunction.Thesematerialsareideal fortissuereconstruction,asthereisnohost immuneresponse,andareparticularlyuseful
Genetherapyhasbeenproposedasatreatmentmodalityfortargetingspecificpathological mechanismsandhencehelpingtotreatthe underlyingdiseaseorigin.HA-CS-plasmid nanoparticleswerepreparedasnovelnonviral genedeliveryvectorforthetreatmentofosteoarthritis.TheyutilizetheabilityofHAtobindtothe CD44tobeinternalizedbythetargetedcellsby theendocytosispathway.ThetransfectionefficiencyoftheseNPswasfoundtobesuperiorto theCS-plasmidNPS,suggestingthemasasafe andeffectivenonviralgenedeliveryvectorto chondrocytes [37].
TABLE1.1 CosmeticapplicationsofHAandHANPs.
HAPolyioncomplexformationwiththe cationicpolymerProtamine
HAPolyioncomplexformationwith quaternizedcyclodextrin-grafted chitosan
barrierpropertiesindamagedskin [44]
moisturizingproperties [45] HAHA/lysineNPsbyionicinteraction betweenHAandlysine
injectiontechnique
nanoparticlesbyionicgelation
[46]
forcellularskinlayersregeneration [47]
[48]
percutaneousabsorption [49]
forburnandtraumapatients.Stemcellsrequire anHA-richenvironmentformaintainingthe undifferentiatedstate.Vascularendothelialcells canbeselected,aswellasaorticsmoothmuscle cellsfortheconstructionofheartvalves,by seedingontoHAsheetsandmembranes.However,duetoHA’shighsolubilityandfastelimination,itsuseforscaffoldfabricationand structuralstabilityhasbeenchallenging.To overcometheselimitations,modificationand crosslinkingofHAhavebeenproposed.Various examplesontheuseofcrosslinkedHAnanoparticlesfordrugdeliveryarepresentedin Table1.2.Water-solublecarbodiimidecrosslinking,polyvalenthyadrazidecrosslinking,and othertechniqueshavebeenintroducedfortissue engineeringapplicationsofHA.ChemicalcrosslinkingisexpectedtoextendtheHAdegradationprocessinvivoandprovidelong-term stabilityforthevariousapplicationsinorthopedics,cardiovascularmedicine,anddermatology. Elsewhere,photocrosslinkedHAhydrogels havebeenalsointroducedfordifferentapplicationssuchascartilagetissueengineering,cardiac repair,moleculedelivery,valvularengineering, controlofstemcellbehavior,andmicrodevices [10].HAasanaturalpolymerhasbeenmixed withpoly(lactic-co-glycolicacid)nanoparticles todevelopaninsitucrosslinkablesystemwith drugdeliverypotential.Althoughsuchasystem hasshownfavorablemechanicalpropertiesfor tissueengineering,itsbiocompatibilityandtoxicityinvivoisstillamajorconcern [64].
4.4Cancertargeting
Self-assembledpolymericnanoparticleshave beeninvestigatedforcancertherapyduetotheir abilitytoencapsulatethechemotherapeutic agentsandreleasethemonasustainedmanner. Thisisevenenhancedbyrenderingtheirsurface hydrophilicwhichwouldenhancetheircirculationtimeleadingtohigheraccumulationinto thetumortissuewiththeknownEPReffectof nanoparticles [34,65].Inadditiontothispassive
targetingstrategy,activetargetingcanbealso achievedbybindingtheseNPstotargeting moietiestorecognizeandbindtothetumorcells andbeinginternalizedbyreceptor-mediated endocytosis.SinceHAisdistinguishedbyits abilitytobindtovariouscancercellsthat overexpressCD44,ithasbeenconjugatedto variousdrugloadednanoparticlesasatargeting moiety [66].CD44isoverexpressedinmanycancersofepithelialoriginandthereforetheuseof HAnanocarrierscouldincreasethetargetability andtheretentionintothecancertissue [66]. However,theuseofHAnanoparticlesasatargetingmoietyanddrugcarrierisanewtrend, andextensivestudiesareneededtounderstand thefactorsaffectingtheaffinitytoCD44andthe internalizationmechanismsofHAnanoparticles.ItwasfoundthattheslowCD44representation(24–48h)ledtolimitedavailabilityofHA internalizationreceptors.Therefore,ahigher affinitynanoparticleandahigherdegreeofclusteringwouldleadtoalowernumberofinternalizedparticles.Ontheotherhand,loweraffinity systemsmightleadtolessclusteringwithmore efficientHA-mediateddeliveryofdrugpayloads [67].AmphiphilicHA-CAconjugates nanoparticles,whichself-assembleintohydrophobiccorenanosizedparticles,surrounded byahydrophilicHAshellefficientlyaccumulate intothetumorsitecomparedtothepurewatersolubleHAaftersystemicadministration.Thein vivobiodistributionoftheseNPsintumorbearingmicewasinvestigatedusinganoninvasivenear-infraredopticalimagingtechnique, whichrevealedasignificantaccumulationof theHANPsinthetumorsite.Theaccumulation wasmuchstrongerthaninnormaltissuesand wassizedependent,whichembracestherole oftheEPRpassivetargetingpathway.However, whenanimalswerepreinjectedwithhighdoses ofHApolymerbeforetheinjectionofHANPs, theNPsaccumulationinthetumorsitewas remarkablyattenuated,suggestingthatthe interactionbetweentheHAfromtheNPsto theCD44receptorsonthecancercellsurfaceis
TABLE1.2 VariousapplicationsofHANPsindrugdelivery.
Drug HAnanoparticles Particle size (nm)Application Reference
PlasmidDNAPolyioncomplexofHAandchitosan andplasmidDNA 100–300Nonviralvectorforgene deliveryforchondrocytes [37]
siRNA Inversew/oemulsion(nanogel)200–500SelectivetargetingofHCT-116cells [51]
PlasmidDNADihydrazidemediatedcrosslinking5–20 μmControlledrateDNAdelivery [39]
DoxorubicinHA-PEG-PLGApolymeric nanoparticlesbynanoprecipitation 93–186Selectivetumortargeting [33]
HA
Self-assembliesofhydrophobic 5β-cholanicacid-HA
HA Self-assembliesofhydrophobic 5β-cholanicacid-HA
DoxorubicinBioreduciblecore-crosslinked polymericmicellebasedon hyaluronicacid
Cy5.5and doxorubicin
Self-assembliesofamphiphilic iodinatedhyaluronicacid
–400Tumortargeting [52]
–424Passiveandactivetumortargeting [34]
[53]
200Theranosticsystemforcancer [54]
Paclitaxel Hyaluronate-cholanicacidmicelles258TargetingCD44overexpressionin cancercells [55]
Cy5.5 (PEG)-conjugatedself-assembledHA nanoparticles 217–269Cancertherapyanddiagnosis [56]
Cy5.5 Self-assembliesofhydrophobic 5β-cholanicacid-HA
Cy7or 89ZrEDCandsulfo-NHScrosslinkedHA andcholanicacid
237–424Targetingstabilin-2andCD44 receptorsoverexpressedin atherosclerosis [57]
90Theranosticapplicationin atherosclerosis [58]
pEGFPorpβ-gal asmodelplasmid Hyaluronicacid-chitosanionotropic gelation 100–235Genetransferandtargetingtoocular cells [59]
DexamethasoneHA-chitosannanoparticles – Enhancedocularbioavailability [60]
InsulinReverse-emulsion-freeze-drying182Oralinsulindelivery [61]
PerfluoropentaneOil-in-water(O/W)emulsification350Ultra-long-acting,liver-specific, Ultrasoundcontrastagent [62]
CuSandCy5.5EDCandsulfo-NHScrosslinkedHA andcholanicacid
227Image-guidedphotothermal therapyofcancer [63]
alsoresponsibleforthehighaccumulationon thetumorsite [34,52].TheuseofcorecrosslinkedHAmicellespreparedwithasimple methodofdisulfidebondformationloadedwith doxorubicinhasshownanenhancedtherapeutic efficiencyandtumoraccumulationaswellas improvedstabilityinvivocomparedtothe uncrosslinkedmicellesandthefreedrug.The superioractivitywascorrelatedtotheability ofthesecarrierstounloadthedruginsidethe tumorcellonlywiththemicellarstructuredissociatedinresponsetotheglutathioneattheintracellularlevel.2,3,5-Triiodobenzoicacid(TIBA) wasconjugatedtoanHAoligomerasa computedtomography(CT)imagingmodality andahydrophobicresidueandself-woven nano-assemblieswereproducedforthetumortargeteddeliveryofdoxorubicinmwhich presentedapromisingtheranosticsystemfor cancerdiagnosisandtherapyoftumorsthat expressCD44receptors [54].PolymericnanoparticulatemicellesofHA-CApaclitaxelwere foundtobespecificandefficientchemotherapeutictreatmentforCD44overexpressing tumorsandcancercells [55].However,amajor drawbackofHA-basedconjugatesornanoparticlesforcancertargetingistheirpreferential accumulationintheliveraftersystemicadministration.PEGylatedHA-NPsformedselfassemblednanoparticlesinthesizerangeof 217–269nmofimprovedcanceraccumulation andtargetabilitycomparedtoHA-NPswhen testedintumor-bearingmice [56].
4.5Atherosclerosis
TheuseofHA-NPshasbeenproposedasa potentialtoolforbothdiagnosticandtherapeuticapplicationsinatherosclerosis.Amajor observationinthepathogenicprocessofatherosclerosisistheoverexpressionofreceptorsof HAsuchasstabilin-2andCD44.SelectivestrongeraccumulationofHA-NPsinatherosclerotic lesionswasobserved,whichwasprobably explainedbyanactivetargetingmechanism
aftersystemicadministration [57].Aminefunctionalizedoligometrichyaluronanconjugated withcholanicesterandlabeledwithfluorescent orradioactivenanoparticleswastestedfor targetingatherosclerosisassociatedinflammation.The90nmparticlesaccumulationwas 30%higherinatheroscleroticaortasthanwild typecontrols.TheplaquestreatedwiththeHA nanoparticlescontained30%fewermacrophagescomparedtocontrolandfreeHAtreated groups.Therefore,thesenanoparticleswere proposedforPETimagingofatherosclerosisassociatedinflammationduetotheirfavorable targetingpotential [58].
4.6Oculardrugdelivery
Duetothestrongdefensivemechanismsofthe eye,thetransportofdrugsviatopicalinstillation intheeyesislimited,causingrestrictedbioavailability.Theuseofhyaluronan-coatedchitosannanoparticleswasinvestigatedforthe enhancedoculardeliveryofdexamethasone. TheHA-coatednanoparticleshaveshown 2.14-foldhigherAUC0-24h comparedtodexamethasonesolution,whichwasexplainedby theprolongedprecornealretentioncausedby thehighlymucoadhesiveHA [60].HA-chitosan nanoparticleswithsizesbetween100and235 nmwerealsotestedforoculargenedelivery andwereabletoachievehightransfection efficiencywithoutaffectingcellviability [59]. HAisalsoparticularlyusefulasaspace-filling matrixintheeyetomaintaintheshapeof theanteriorchamberduringsurgeries.HAsolutionsalsoserveasaviscosity-enhancingcomponentofeyedropsandasanadjuvanttoeyetissue repair.
4.7Insulinsensitivityanddiabetes
Recently,ithasbeendemonstratedthatCD44 receptorsinpro-inflammatorycellsinobese adiposetissuesareinvolvedinthedevelopment ofadiposetissueinflammationandinsulin
resistanceintype2diabetespatients.Therefore, emptyHAnanoparticleswereusedasatherapeutictoolforadiposetissueinflammationand insulinresistancebyselectivelyaccumulating andclusteringtheCD44receptorsininhibiting theinteractionofthelowmolecularweightHA withthesereceptors,leadingtoimprovedinsulin sensitivityandglycemiccontrol.TheHAnanoparticlesarehenceproposedasatherapeutic agentinthetreatmentoftype2diabetespatients [68].HAnanoparticleshavealsobeenproposed fortheoraldeliveryofinsulinasanalternative forinsulininjectionsforthetreatmentofdiabetes.Insulin-loadedHAnanoparticleswerepreparedbyareverseemulsionfreeze-drying methodinsizesofapproximately180nm.The particlesproducedhadveryhighentrapment efficiencyupto95%.ThesepH-sensitivenanoparticlesprovidedtherequiredprotectionfor insulinfromtheacidicenvironmentofthestomachandatthesametimehadnoeffectonthejunctionintegrityofepithelialcells,whichisan importantparametertoconsiderincaseof chronicuseofmedicaments,whichistheusual caseofinsulin.ThepresenceofHAenhanced thetranscellulareffluxofinsulintransported throughCaco-2cellmonolayersviathetranscellularpathways.Theresultsofpermeability througharatsmallintestineshowedthatinsulin transportthroughtheduodenumandileumwas enhanced,andthetherapeuticefficiencyofthe producednanoparticleswasalsoprovedinadiabeticratsmodel [61].
4.8Theranosticandimaging
Forhigherdiagnosticprocedures’efficiency fordifferentdiseases,thereisagreatneedfor instantreal-timeimagingtechniquesthatcould favorablytargetcertainorgansortissuetypes. Oneofthemostcommonlyusedimagingtechniquesinmedicalfieldsisultrasoundimaging. NewechogenicHAnanoparticleshavebeen developedandpresentedasanultra-longacting,liver-specificultrasoundcontrastagent.
Theparticleswerepreparedwiththeoilina wateremulsificationmethodandcontainedperfluoropentaneasanultrasoundgasprecursor. Theparticlesweremorestablecomparedtothe conventionalmicrobubblesusedforultrasound (US)imaging.Theirlongcirculatingproperties allowedforseveralsystemiccirculationsfollowedbyintenseaccumulationattheliver, whichembracedtheirpotentialasatargeting imagingsystemfordiagnosisofliverdiseases. Thetargetabilitytotheliverwasrelatedtoboth thesmallhydrodynamicsizeoftheparticlesas wellasthehighaffinityofHAtotheliver.More interestingly,thepreparedparticleswereableto discriminatebetweenthenormallivertissues andlivercancerinalivertumor-bearingmice model.Thecanceroustissuewasfoundtobe morecompactthanthenormaltissue,andhence lowerUSsignalswereobserved [62]
Ironoxide-basedmagneticnanoparticles bearingHAonthesurfacehavebeendeveloped totargetactivatedmacrophagesforimagingand therapeuticapplicationsininflammatorydiseases.Theparticleswerepreparedby co-precipitationproceduresfollowedbypostsyntheticfunctionalizationwithbothHAand fluorescein.Particlesexpressedasignificantbiocompatibilityandstabilityinserum.Significant uptakebyactivatedmacrophageswasobserved, whichwasHAdependent.Inaddition,themagneticcoreoftheparticleswasfoundtobeonly transientlypresentinthecells,whichindicates lowerrisksoftoxicity.Fluoresceinwasfound tobesuccessfullydeliveredtothecellularnuclei, whichshowedthepotentialofusingthesenanocarriersalsoasatargeteddrugdeliveryand molecularimaging [69].HAnanoparticleswere synthesizedandlabeledwiththenearinfrared dyeCys5.5forimagingpurposes.Forusingthe sameparticlesbutforphotothermalpurposes, anadditionalstepofloadingtheparticleswith CuSwasperformed.Theobtainedparticleshad asizerangeofaround200nm.Thefluorescent signalofCys5.5wasquenchedbythepresence ofCuS.Whentheenzymehyaluronidasewas
addedtotheparticles,thefluorescencesignal wasrestoredinatime-dependentmanner,which suggestedthattheparticlescouldbeusedasa nanoprobetobeactivatedbythehighlyoverexpressedhyaluronidaseinthecanceroustumor areas.Therefore,theCuS-loadedHAnanoparticleswereusedforphotoacousticimaging, utilizingthestrongabsorbanceoftheCuS.After intravenousadministration,theparticlesaccumulatedinthetumorareaovertime,andwhen irradiatedwithalaser,agoodtumorinhibition rateof89.74%wasobservedonday5,indicating thegreatpotentialofthesenanoparticlesfor theranosticapplications [63].
5Clinicalstatus
Asalreadydiscussed,HAseemstobeavery promisingmoleculeforutilizationasavehicleor asanactiveingredientformanydrugdelivery purposes.TheuseofHAasadermalfillerand forintra-articulardeliveryisalreadywellestablished,andseveralproductshavebeenproved safeandapprovedbytheFDAforclinicaluse [70].However,mostofthescientificresearch conductedontheuseofHAandHANPsfor clinicalapplicationsinotherareasisstillinthe phaseoflaboratoryresearchandpreclinical evaluation.Thismaybeduetotheconsideration ofHAasanewchemicalentityeverytimeitis linkedorconjugatedtoadrugoranymolecules forchangingtheirproperties.Duetothecomplexprocessesinvolvedinsuchreactionsand thevariousfactorstobestudied,HAandits derivativesarealsodifficulttoindustrialize [1] However,severalproductshavereachedphase IIandphaseIIIclinicaltrials.AlchemicaoncologyinAustraliahaveproducedseveral HA-basedsystemsforthemanagementof cancersuchasHA-irinotecan,HA-DOX,and HA-5FU.AphaseIclinicaltrialwasconducted on12patients,andHA-Irinotecanhasproved tobesafeandwelltoleratedwhilepreserving
theanticanceractivityofirinotecan [23].Another phaseIItrialwasdoneon41patients,and showedtheadvantagesoftheHAnanoformulationsintermsofprogression-freesurvivaland safety [71].Unfortunately,phaseIIIclinicaltrials didnotachievetheexpectedresultsandthe reasonforthisisnotyetclear [1].Therefore, theindustrializationandwidespreadclinical utilizationofHAandHANPsasdrugcarriers arestillrichareasthatrequiremoreextensive researchandhavealongwaytogo.
6Conclusion
HAisoneofthemostimportantnaturalcomponentsofhumantissues,makingitabiocompatible,biodegradable,andpromisingcarrier forvariousdrugdeliveryapplications.Theuse ofnanotechnologyhasenhancedtoagreat extentthebenefitsachievedfromthisbiomaterial.Thiscouldbebyusingsystemsthatcan activelytargetcertaintissuesorachieve enhancedpenetrationorpermeationthrough certainbodybarriers.Futurestudiesareneeded toenhancethefabricationtechniquesand ensuretherequiredefficacyandsafetyofthe producednanosystems.
References
[1]HuangG,HuangH.Applicationofhyaluronicacidas carriersindrugdelivery.DrugDeliv2018;25:766–72. https://doi.org/10.1080/10717544.2018.1450910.
[2] NecasJ,BartosikovaL,BraunerP,KolarJ.Hyaluronic acid(hyaluronan):areview.VetMed(Praha) 2008;53:397–411.
[3]JinY-J,UbonvanT,KimD-D.Hyaluronicacidindrug deliverysystems.JPharmInvestig2010;40:33–43. https://doi.org/10.4333/KPS.2010.40.S.033
[4]SzeJH,BrownlieJC,LoveCA.Biotechnologicalproductionofhyaluronicacid:aminireview.3Biotech2016;6: https://doi.org/10.1007/s13205-016-0379-9
[5]FallacaraA,BaldiniE,ManfrediniS,VertuaniS.Hyaluronicacidinthethirdmillennium.Polymers 2018;10:701. https://doi.org/10.3390/polym10070701
[6]TripodoG,TrapaniA,TorreML,GiammonaG, TrapaniG,MandracchiaD.Hyaluronicacidandits
derivativesindrugdeliveryandimaging:recent advancesandchallenges.EurJPharmBiopharm 2015;97:400–16. https://doi.org/10.1016/j.ejpb.2015. 03.032
[7]WolfKJ,KumarS.Hyaluronicacid:incorporatingthe biointothematerial.ACSBiomaterSciEng2019. https://doi.org/10.1021/acsbiomaterials.8b01268.
[8]MeroA,CampisiM.Hyaluronicacidbioconjugatesfor thedeliveryofbioactivemolecules.Polymers 2014;6:346–69. https://doi.org/10.3390/polym6020346.
[9]SchanteCE,ZuberG,HerlinC,VandammeTF.Chemicalmodificationsofhyaluronicacidforthesynthesisof derivativesforabroadrangeofbiomedicalapplications.CarbohydrPolym2011;85:469–89. https://doi. org/10.1016/j.carbpol.2011.03.019.
[10]FakhariA,BerklandC.Applicationsandemerging trendsofhyaluronicacidintissueengineering,asadermalfillerandinosteoarthritistreatment.ActaBiomater 2013;9:7081–92. https://doi.org/10.1016/j.actbio. 2013.03.005
[11] FakhariA.Biomedicalapplicationofhyaluronicacid nanoparticles.UniversityofKansas;2012.
[12]KoganG,S ˇ oltesL,SternR,GemeinerP.Hyaluronic acid:anaturalbiopolymerwithabroadrangeof biomedicalandindustrialapplications.BiotechnolLett 2006;29:17–25. https://doi.org/10.1007/s10529-0069219-z
[13]MendichiR,GiacomettiSchieroniA.Fractionationand characterizationofultra-highmolarmasshyaluronan. 2.On-linesizeexclusionchromatographymethods. Polymer2002;43:6115–21. https://doi.org/10.1016/ S0032-3861(02)00586-4
[14]ShiedlinA,BigelowR,ChristopherW,ArbabiS, YangL,MaierRV,WainwrightN,ChildsA,MillerRJ. Evaluationofhyaluronanfromdifferentsources: Streptococcuszooepidemicus,roostercomb,bovinevitreous,and humanumbilicalcord.Biomacromolecules2004; 5:2122–7. https://doi.org/10.1021/bm0498427.
[15]SalwowskaNM,BebenekKA,Za˛dłoDA,WcisłoDziadeckaDL.Physiochemicalpropertiesandapplicationofhyaluronicacid:asystematicreview.JCosmet Dermatol2016;15:520–6. https://doi.org/10.1111/ jocd.12237
[16]TooleBP.Hyaluronanisnotjustagoo!.JClinInvest 2000;106:335–6. https://doi.org/10.1172/JCI10706
[17]KoganG,S ˇ oltesL,SternR,SchillerJ,MendichiR.Hyaluronicacid:itsfunctionanddegradationininvivosystems.In:Studiesinnaturalproductschemistry. Elsevier;2008.p.789–882. https://doi.org/10.1016/ S1572-5995(08)80035-X
[18]HolmesMWA,BaylissMT,MuirH.Hyaluronicacidin human articularcartilage.Age-relatedchangesincontentandsize.BiochemJ1988;250:435–41. https://doi. org/10.1042/bj2500435
[19]AkmalM,SinghA,AnandA,KesaniA,AslamN, GoodshipA,BentleyG.Theeffectsofhyaluronicacid onarticularchondrocytes.JBoneJointSurg(Br) 2005;87-B:1143–9. https://doi.org/10.1302/0301-620X. 87B8.15083
[20]IturriagaV,Va ´ squezB,ManterolaC,delSolM.Roleof hyaluronicacidinthehomeostasisandtherapeuticsof temporomandibularjointosteoarthritis.IntJMorphol 2017;35:870–6. https://doi.org/10.4067/S0717-95022 017000300012.
[21]DechertTA,DucaleAE,WardSI,YagerDR.Hyaluronaninhumanacuteandchronicdermalwounds:hyaluronaninhumandermalwounds.WoundRepair Regen2006;14:252–8. https://doi.org/10.1111/j.17436109.2006.00119.x.
[22]DruryJL,MooneyDJ.Hydrogelsfortissueengineering: scaffolddesignvariablesandapplications.Biomaterials 2003;24:4337–51. https://doi.org/10.1016/S0142-9612 (03)00340-5
[23]ChoiKY,SaravanakumarG,ParkJH,ParkK.Hyaluronicacid-basednanocarriersforintracellulartargeting: Interfacialinteractionswithproteinsincancer.Colloids SurfBBiointerfaces2012;99:82–94. https://doi.org/ 10.1016/j.colsurfb.2011.10.029
[24]LuoY,PrestwichGD.Synthesisandselectivecytotoxicityofahyaluronicacid-antitumorbioconjugate.BioconjugChem1999;10:755–63. https://doi.org/ 10.1021/bc9900338
[25]RosatoA,BanzatoA,DeLucaG,RenierD,BettellaF, PaganoC,EspositoG,ZanovelloP,BassiP.HYTAD1p20:anewpaclitaxel-hyaluronicacidhydrosoluble bioconjugatefortreatmentofsuperficialbladdercancer. UrolOncol2006;24:207–15. https://doi.org/10.1016/j. urolonc.2005.08.020 [ProcAnnuMeetSocUrolOncol May2005PartIIMolApproachesEarlyDetectProgn DetermProsateCancerSystTherProstateCancer AngiogenicTargetsClin.TrialData].
[26] CoradiniD,PellizzaroC,MiglieriniG,DaidoneMG, PerbelliniA.Hyaluronicacidasdrugdeliveryforsodium butyrate:improvementoftheanti-proliferativeactivity onabreast-cancercellline.IntJCancer1999;81:411–6.
[27] CoradiniD,ZorzetS,RossinR,ScarlataI,PellizzaroC, TurrinC,BelloM,CantoniS,SperanzaA,SavaG. Inhibitionofhepatocellularcarcinomasinvitroand hepaticmetastasesinvivoinmicebythehistone deacetylaseinhibitorHA-But.ClinCancerRes 2004;10:4822–30.
[28]KimKS,KimH,ParkY,KongWH,LeeSW,KwokSJJ, HahnSK,YunSH.Noninvasivetransdermalvaccinationusinghyaluronannanocarriersandlaseradjuvant. AdvFunctMater2016;26:2512–22. https://doi.org/ 10.1002/adfm.201504879
[29]OssipovDA.Nanostructuredhyaluronicacid-based materialsforactivedeliverytocancer.ExpertOpin
1.Versatilehyaluronicacidnanoparticlesforimproveddrugdelivery
DrugDeliv2010;7:681–703. https://doi.org/ 10.1517/17425241003730399
[30]LeeH,AhnC-H,ParkTG.Poly[lactic-co-(glycolic acid)]-graftedhyaluronicacidcopolymermicellenanoparticlesfortarget-specificdeliveryofdoxorubicin. MacromolBiosci2009;9:336–42. https://doi.org/ 10.1002/mabi.200800229.
[31]YadavAK,MishraP,JainS,MishraP,MishraAK, AgrawalGP.Preparationandcharacterizationof HA-PEG-PCLintelligentcore-coronananoparticlesfor deliveryofdoxorubicin.JDrugTarget2008;16:464–78. https://doi.org/10.1080/10611860802095494.
[32]ChoiKY,LeeS,ParkK,KimK,ParkJH,KwonIC, JeongSY.Preparationandcharacterizationofhyaluronicacid-basedhydrogelnanoparticles.JPhysChem Solids2008;69:1591–5. https://doi.org/10.1016/j. jpcs.2007.10.052.
[33]YadavAK,MishraP,MishraAK,MishraP,JainS, AgrawalGP.Developmentandcharacterizationofhyaluronicacid-anchoredPLGAnanoparticulatecarriersof doxorubicin.NanomedNanotechnolBiolMed 2007;3:246–57. https://doi.org/10.1016/j.nano.2007. 09.004
[34]ChoiKY,ChungH,MinKH,YoonHY,KimK,ParkJH, KwonIC,JeongSY.Self-assembledhyaluronicacidnanoparticlesforactivetumortargeting.Biomaterials 2010;31:106–14. https://doi.org/10.1016/j.biomaterials. 2009.09.030
[35]DuceppeN,TabrizianM.Factorsinfluencingthetransfectionefficiencyofultralowmolecularweightchitosan/ hyaluronicacidnanoparticles.Biomaterials 2009;30:2625–31. https://doi.org/10.1016/j.biomaterials. 2009.01.017.
[36]NaSJ,ChaeSY,LeeS,ParkK,KimK,ParkJH,KwonIC, JeongSY,LeeKC.StabilityandbioactivityofnanocomplexofTNF-relatedapoptosis-inducingligand.Int JPharm2008;363:149–54. https://doi.org/10.1016/j. ijpharm.2008.07.013.
[37]LuH-D,ZhaoH-Q,WangK,LvL-L.Novelhyaluronic acid-chitosannanoparticlesasnon-viralgenedeliveryvectorstargetingosteoarthritis.IntJPharm2011;420:358–65. https://doi.org/10.1016/j.ijpharm.2011.08.046.
[38]JeongY-I,KimS-T,JinS-G,RyuH-H,JinY-H,JungT-Y, KimI-Y,JungS.Cisplatin-incorporatedhyaluronicacid nanoparticlesbasedonion-complexformation.JPharm Sci2008;97:1268–76. https://doi.org/10.1002/jps.21103
[39]YunYH,GoetzDJ,YellenP,ChenW.Hyaluronan microspheresforsustainedgenedeliveryandsitespecifictargeting.Biomaterials2004;25:147–57. https://doi.org/10.1016/S0142-9612(03)00467-8
[40]KumarA,SahooB,MontpetitA,BeheraS,LockeyRF, MohapatraSS.Developmentofhyaluronicacid-Fe2O3 hybridmagneticnanoparticlesfortargeteddeliveryof peptides.NanomedNanotechnolBiolMed 2007;3:132–7. https://doi.org/10.1016/j.nano.2007. 03.001
[41]PitarresiG,CraparoEF,PalumboFS,CarlisiB, GiammonaG.Compositenanoparticlesbasedonhyaluronicacidchemicallycross-linkedwith α,β-polyaspartylhydrazide.Biomacromolecules2007;8:1890–8. https://doi.org/10.1021/bm070224a.
[42]Berko ´ S,MarodaM,Bodna ´ rM,Ero ˝ sG,HartmannP, SzentnerK,Szabo ´ -ReveszP,KemenyL,BorbelyJ, Csa ´ nyiE.Advantagesofcross-linkedversuslinearhyaluronicacidforsemisolidskindeliverysystems.Eur PolymJ2013;49:2511–7. https://doi.org/10.1016/j. eurpolymj.2013.04.001.
[43]Abdel-MottalebMMA,LamprechtA.Invivoskinpenetrationofmacromoleculesinirritantcontactdermatitis.IntJPharm2016;515:384–9. https://doi.org/ 10.1016/j.ijpharm.2016.10.042
[44]TokudomeY,KomiT,OmataA,SekitaM.Anewstrategyforthepassiveskindeliveryofnanoparticulate, highmolecularweighthyaluronicacidpreparedbya polyioncomplexmethod.SciRep2018;8:2336. https://doi.org/10.1038/s41598-018-20805-3
[45]SakulwechS,LourithN,RuktanonchaiU, KanlayavattanakulM.Preparationandcharacterization ofnanoparticlesfromquaternizedcyclodextrin-grafted chitosanassociatedwithhyaluronicacidforcosmetics. SpecIssuePharmInnov2018;13:498–504. https://doi. org/10.1016/j.ajps.2018.05.006
[46]CarneiroJ,D € oll-BoscardinPM,FiorinBC,NadalJM, FaragoPV,dePaulaJP.Developmentand characterizationofhyaluronicacid-lysinenanoparticles withpotentialasinnovativedermalfilling.Braz JPharmSci2016;52:645–51. https://doi.org/10.1590/ s1984-82502016000400008.
[47]PereiraGG,DetoniCB,BalducciAG,RondelliV, ColomboP,GuterresSS,SonvicoF.Hyaluronatenanoparticlesincludedinpolymerfilmsfortheprolonged releaseofvitaminEforthemanagementofskin wounds.EurJPharmSci2016;83:203–11. https://doi. org/10.1016/j.ejps.2016.01.002
[48]MorgantiP,PalomboM,TishchenkoG,YudinV, GuarneriF,CardilloM,DelCiottoP,CarezziF, MorgantiG,FabriziG.Chitin-hyaluronannanoparticles: amultifunctionalcarriertodeliveranti-agingactiveingredientsthroughtheskin.Cosmetics2014;1:140–58. https://doi.org/10.3390/cosmetics1030140
[49]KongM,ChenXG,KweonDK,ParkHJ.Investigationson skinpermeationofhyaluronic acidbasednanoemulsionas transdermalcarrier.CarbohydrPolym2011;86:837–43. https://doi.org/10.1016/j.carbpol.2011.05.027
[50]MorgenM,TungD,BorasB,MillerW,MalfaitA-M, TortorellaM.Nanoparticlesforimprovedlocalretentionafterintra-articularinjectionintothekneejoint. PharmRes2013;30:257–68. https://doi.org/10.1007/ s11095-012-0870-x.
[51]LeeH,MokH,LeeS,OhY-K,ParkTG.Target-specific intracellulardeliveryofsiRNAusingdegradablehyaluronicacidnanogels.JControlRelease2007;119:245–52. https://doi.org/10.1016/j.jconrel.2007.02.011.
[52]ChoiKY,MinKH,NaJH,ChoiK,KimK,ParkJH, KwonIC,JeongSY.Self-assembledhyaluronicacid nanoparticlesasapotentialdrugcarrierforcancertherapy:synthesis,characterization,andinvivobiodistribution.JMaterChem2009;19:4102. https://doi.org/ 10.1039/b900456d
[53]HanHS,ChoiKY,KoH,JeonJ,SaravanakumarG, SuhYD,LeeDS,ParkJH.Bioreduciblecore-crosslinked hyaluronicacidmicellefortargetedcancertherapy. JControlRelease2015;200:158–66. https://doi.org/ 10.1016/j.jconrel.2014.12.032
[54]LeeJ-Y,ChungS-J,ChoH-J,KimD-D.Iodinatedhyaluronicacidoligomer-basednanoassembliesfor tumor-targeteddrugdeliveryandcancerimaging. Biomaterials2016;85:218–31. https://doi.org/10.1016/ j.biomaterials.2016.01.060
[55]ThomasRG,MoonM,LeeS,JeongYY.Paclitaxelloaded hyaluronicacidnanoparticlesfortargetedcancer therapy:Invitroandinvivoanalysis.IntJBiol Macromol2015;72:510–8. https://doi.org/10.1016/j. ijbiomac.2014.08.054.
[56]ChoiKY,MinKH,YoonHY,KimK,ParkJH,KwonIC, ChoiK,JeongSY.PEGylationofhyaluronicacidnanoparticlesimprovestumortargetabilityinvivo.Biomaterials 2011;32:1880–9. https://doi.org/10.1016/j.biomaterials. 2010.11.010.
[57]LeeGY,KimJ-H,ChoiKY,YoonHY,KimK,KwonIC, ChoiK,LeeB-H,ParkJH,KimI-S.Hyaluronicacid nanoparticlesforactivetargetingatherosclerosis. Biomaterials2015;53:341–8. https://doi.org/10.1016/j. biomaterials.2015.02.089
[58]BeldmanTJ,SendersML,AlaargA,Perez-MedinaC, TangJ,ZhaoY,FayF,DeichmollerJ,BornB, DesclosE,vanderWelNN,HoebeRA,KohenF, KartvelishvilyE,NeemanM,ReinerT,CalcagnoC, FayadZA,deWintherMPJ,LutgensE,MulderWJM, KluzaE.Hyaluronannanoparticlesselectivelytarget
plaque-associatedmacrophagesandimproveplaque stabilityinatherosclerosis.ACSNano2017;11:5785–99. https://doi.org/10.1021/acsnano.7b01385
[59]delaFuenteM,SeijoB,AlonsoMJ.Novelhyaluronic acid-chitosannanoparticlesforoculargenetherapy. InvestOphthalmolVisSci2008;49:2016–24. https:// doi.org/10.1167/iovs.07-1077
[60]KalamMA.Thepotentialapplicationofhyaluronicacid coatedchitosannanoparticlesinoculardeliveryof dexamethasone.IntJBiolMacromol2016;89:559–68. https://doi.org/10.1016/j.ijbiomac.2016.05.016.
[61] HanL,ZhaoY,YinL,LiR,LiangY,HuangH,PanS, WuC,FengM.Insulin-loadedpH-sensitivehyaluronic acidnanoparticlesenhancetranscellulardelivery. AAPSPharmSciTech2012;13:836–45. https://doi.org/ 10.1208/s12249-012-9807-2.
[62]MinHS,SonS,LeeTW,KooH,YoonHY,NaJH,ChoiY, ParkJH,LeeJ,HanMH,ParkR-W,KimI-S,JeongSY, RheeK,KimSH,KwonIC,KimK.Liver-specificand echogenichyaluronicacidnanoparticlesfacilitatingliver cancerdiscrimination.AdvFunctMater2013;23:5518–29. https://doi.org/10.1002/adfm.201301131
[63]ZhangL,GaoS,ZhangF,YangK,MaQ,ZhuL.Activatablehyaluronicacidnanoparticleasatheranosticagent foroptical/photoacousticimage-guidedphotothermal therapy.ACSNano2014;8:12250–8. https://doi.org/ 10.1021/nn506130t
[64]YeoY,ItoT,BellasE,HighleyCB,MariniR,KohaneDS. Insitucross-linkablehyaluronanhydrogelscontaining polymericnanoparticlesforpreventingpostsurgical adhesions.AnnSurg2007;245:819–24. https://doi. org/10.1097/01.sla.0000251519.49405.55
[65]RamzyL,NasrM,MetwallyAA,AwadGAS.Cancer nanotheranostics:areviewoftheroleofconjugated ligandsforoverexpressedreceptors.EurJPharmSci 2017;104:273–92. https://doi.org/10.1016/j.ejps.2017. 04.005.
[66]PlattVM,SzokaFC.Anticancertherapeutics:targeting macromoleculesandnanocarrierstohyaluronanor CD44,ahyaluronanreceptor.MolPharm2008;5:474–86. https://doi.org/10.1021/mp800024g.
[67]AlmalikA,KarimiS,OuastiS,DonnoR,WandreyC, DayPJ,TirelliN.Hyaluronicacid(HA)presentationasa tooltomodulateandcontrolthereceptor-mediateduptake ofHA-coatednanoparticles.Biomaterials2013;34:5369–80. https://doi.org/10.1016/j.biomaterials.2013.03.065
[68]RhoJG,HanHS,HanJH,LeeH,NguyenVQ,LeeWH, KwonS,HeoS,YoonJ,ShinHH,LeeE,KangH, YangS,LeeEK,ParkJH,KimW.Self-assembled hyaluronicacidnanoparticles:implicationsasa