Contributors
TarunKumarBarik DepartmentofPhysics,AchhruramMemorialCollege,Jhalda,WestBengal, India
AbidBhat DepartmentofPharmacology,JSS CollegeofPharmacy,JSSAcademyofHigher Education & Research,Mysuru,Karnataka,India
RameshChandra DrugDiscovery & Development Laboratory,DepartmentofChemistry,University ofDelhi,Delhi,India;Dr.B.R.AmbedkarCentre forBiomedicalResearch,UniversityofDelhi,Delhi, India
VivekanandChatap DepartmentofPharmaceutics, H.R.PatelInstituteofPharmaceuticalEducation andResearch,Shirpur,Maharashtra,India
SaravanaBabuChidambaram DepartmentofPharmacology,JSSCollegeofPharmacy,JSSAcademy ofHigherEducation & Research,Mysuru,Karnataka,India
JørnB.Christensen DepartmentofChemistry,UniversityofCopenhagen,FrederiksbergC,Denmark RolfDaniels DepartmentofPharmacy,Eberhard KarlsUniversit€ atT € ubingen,T € ubingen,Germany
XavierDelgadillo CentreMédicoChirurgicalVolta, LaChauxdeFonds,Switzerland
KoyelDey DepartmentofEngineeringDesign, IndianInstituteofTechnologyMadras,Chennai, TamilNadu,India
NamdevDhas InstituteofPharmacy,Nirma University,Ahmedabad,Gujarat,India
SunilKumarDubey DepartmentofPharmacy,Birla InstituteofTechnologyandScience,Pilani, Rajasthan,India
SachinDubey Formulation,AnalyticalandDrug ProductDevelopment,GlenmarkPharmaceuticals, LaChauxdeFonds,Switzerland
MysorePrakashGowrav DepartmentofPharmaceutics,JSSCollegeofPharmacy,JSSAcademyof HigherEducation & Research,Mysuru,Karnataka, India
SiddhanthHejmady DepartmentofPharmacy, BirlaInstituteofTechnologyandScience,Pilani, Rajasthan,India
PadamatiJagadeeswari DepartmentofPharmacology,JSSCollegeofPharmacy,JSSAcademyof HigherEducation & Research,Mysuru,Karnataka, India
PaulJoyce DepartmentofPhysics,ChalmersUniversityofTechnology,Gothenburg,Sweden
SrabaniKar DepartmentofElectricalEngineering, UniversityofCambridge,Cambridge,United Kingdom
Kavitha DepartmentofEngineeringDesign,Indian InstituteofTechnologyMadras,Chennai,Tamil Nadu,India
AljoschaKoenneke DepartmentofPharmacy,BiopharmaceuticsandPharmaceuticalTechnology, SaarlandUniversity,Saarbr € ucken,Germany
AmoghKumar DepartmentofEngineeringDesign, IndianInstituteofTechnologyMadras,Chennai, TamilNadu,India
ArehallyMarappaMahalakshmi Departmentof Pharmacology,JSSCollegeofPharmacy,JSS AcademyofHigherEducation & Research, Mysuru,Karnataka,India
TahliaR.Meola SchoolofPharmacyandMedical Sciences,UniversityofSouthAustralia,Adelaide, SA,Australia;ARCCentreofExcellencein ConvergentBio-NanoScienceandTechnology, UniversityofSouthAustralia,Adelaide,SA, Australia
L.Mohan DepartmentofEngineeringDesign, IndianInstituteofTechnologyMadras,Chennai, TamilNadu,India
SrinivasMutalik DepartmentofPharmaceutics, ManipalCollegeofPharmaceuticalSciences, ManipalAcademyofHigherEducation,Manipal, Karnataka,India
FrancisKamauMwiiri DepartmentofPharmacy, EberhardKarlsUniversit€ atT € ubingen,T € ubingen, Germany
MoetoNagai DepartmentofMechanicalEngineering,ToyohashiUniversityofTechnology,Toyohashi,Aichi,Japan
AbhijeetPandey DepartmentofPharmaceutics, ManipalCollegeofPharmaceuticalSciences, ManipalAcademyofHigherEducation,Manipal, Karnataka,India
KamlaPathak UttarPradeshUniversityofMedical Sciences,Saifai,UttarPradesh,India
MarcelPourasghar DepartmentofPharmacy, BiopharmaceuticsandPharmaceuticalTechnology, SaarlandUniversity,Saarbr € ucken,Germany
CliveA.Prestidge SchoolofPharmacyandMedical Sciences,UniversityofSouthAustralia,Adelaide, SA,Australia;ARCCentreofExcellenceinConvergentBio-NanoScienceandTechnology,University ofSouthAustralia,Adelaide,SA,Australia
VamshiKrishnaRapalli DepartmentofPharmacy, BirlaInstituteofTechnologyandScience,Pilani, Rajasthan,India
KamalSinghRathore DepartmentofPharmaceutics,BNCollegeofPharmacy,BNUniversity, Udaipur,Rajasthan,India
BipulRay DepartmentofPharmacology,JSS CollegeofPharmacy,JSSAcademyofHigher Education & Research,Mysuru,Karnataka,India
MeenaKishoreSakharkar CollegeofPharmacy andNutrition,UniversityofSaskatchewan, Saskatoon,SK,Canada
TuhinSubhraSantra DepartmentofEngineering Design,IndianInstituteofTechnologyMadras, Chennai,TamilNadu,India
MarcSchneider DepartmentofPharmacy,BiopharmaceuticsandPharmaceuticalTechnology, SaarlandUniversity,Saarbr € ucken,Germany
HayleyB.Schultz SchoolofPharmacyandMedical Sciences,UniversityofSouthAustralia,Adelaide, SA,Australia;ARCCentreofExcellencein ConvergentBio-NanoScienceandTechnology, UniversityofSouthAustralia,Adelaide,SA, Australia
JavadSharifi-Rad FoodSafetyResearchCenter (salt),SemnanUniversityofMedicalSciences, Semnan,Iran
PallaviShinde DepartmentofEngineeringDesign, IndianInstituteofTechnologyMadras,Chennai, TamilNadu,India
DebjaniSingh PharmaceuticalTechnologyCentre, CadilaHealthcareLtd.,Ahmedabad,Gujarat,India; DepartmentofPharmaceutics,BNCollegeofPharmacy,BNUniversity,Udaipur,Rajasthan,India
GautamSinghvi DepartmentofPharmacy,Birla InstituteofTechnologyandScience,Pilani, Rajasthan,India
TuladharSunanda DepartmentofPharmacology, JSSCollegeofPharmacy,JSSAcademyofHigher Education & Research,Mysuru,Karnataka,India
AkhileshKumarTewari MylanLaboratoriesLtd., Bangalore,Karnataka,India
PhilippeWuthrich CliniquedeGenolier,Arzier-Les Muids,Switzerland
Preface
Thebookseriestitled Expectationsand RealitiesofMultifunctionalDrugDelivery Systems coversseveralimportanttopicson drug-deliverysystems,regulatoryrequirements, clinicalstudies,intellectualpropertiestrends, newadvances,manufacturingchallenges,etc. writtenbyleadingindustryandacademic experts.Overall,thechapterspublishedinthis seriesreflectthebroadnessofnanopharmaceuticals,microparticles,otherdrugcarriersandthe importanceoftherespectivequality,regulatory, clinical,GMPscaleup,andregulatoryregistrationaspects.
Thisseriesisdestinedto filltheknowledge gapthroughinformationsharingandwithorganizedresearchcompilationbetweendiverse areasofpharma,medicine,clinical,regulatory practices,andacademics.
ExpectationsandRealitiesofMultifunctionalDrug DeliverySystems isdividedintofourvolumes:
Volume1:Nanopharmaceuticals
Volume2:DeliveryofDrugs
Volume3:DrugDeliveryTrends
Volume4:DrugDeliveryAspects
Thespecificobjectivesofthisbookseriesareto:
(1)provideaplatformtodiscussopportunities andchallengesindevelopmentof nanomedicineandotherdrug-delivery systems; (2)discusscurrentandfuturemarkettrends; (3)facilitateinsightsharingwithinvarious areasofexpertise;and (4)establishcollaborationsbetweenacademic scientists,andindustrialandclinical researchers.
Innovativecutting-edgedevelopmentsin micro-nanotechnologyoffernewwaysof preventingandtreatingdiseaseslikecancer, malaria,HIV/AIDS,tuberculosis,andmany more.Theapplicationsofmicro-nanoparticles indrugdelivery,diagnostics,andimagingare vast.Hence, Volume2:DeliveryofDrugs, in thebookseriesmainlyreviewsadvancesin siteandorganspecifi ctargetingapproaches, technologiesusedinprep arationofmicro-nanoparticles,challengesofcomplextypeofdrug deliveryformsandroleofphysicalmethodsin achievingtargeteddrugeffect.
OneofthecontributionsbyJoyceetal. (Chapter1) discussestheopportunitiesand challengesofdevelopingpolymerlipidhybrid (PLH)formulationsfororaldeliveryofdrugs. Thistypeofsynergisticdrug-deliveryapproach canovercomegastricstabilityandsolubility challengesassociatedwithconventionalnanoparticlesandexhibitimprovedperformance. Theauthorsalsobrieflydiscussnewadvances indesigningandengineeringofthesurfaceand matrixofPLHsystems.
InthenextchapterbyChristensen (Chapter2),thecurrentstatusofdendrimer developmentandpotentialchallengesthe industryisfacingarehighlighted.Although dendrimershavelotsofpotentialasdrugdeliverysystems,simplechallengessuchas reproducibility,scalability,andavailabilityof CROs/CMOs(contractresearchorganizations andcontractmanufacturingorganizations)are hinderingitsmarketentry.
ThecontributionbyMwiiriandDaniels (Chapter3) describestheapplicationsofelectrospunnano fi bersforbiomedicalapplications Furthermore,widerapplicationsofthis techniqueintissueengineering,drugdelivery, andwoundhealingarediscussedindetail. Theauthorsalsohighlighttechnological, regulatorychallengesofthistechniquealong withupcomingtrendsusingelectrospinning technology.
Psoriasisisachronicauto-immuneskin disorderaffectingmillionsworldwide,andthe potentialofnanomedicinesfortreatmentof thisconditioniscoveredbySinghvietal. (Chapter4).Theauthorsdiscussimpressive researchoutcomesinusingdrugdelivery, includingliposomes,micelles,andsolidlipid nanoparticles(SLNs).
TheworkbyChidambarametal. (Chapter5) isaimedatdiscussingnewcellulartargets,i.e., mitochondria.Theteamhashighlightedthe importanceoftargetingmitochondriainvarious neurogenerativediseases.Theapplicationsofa varietyofdrug-deliverysystemsincludingliposomes,niosomes,polymericnanoparticles,metal nanoparticles,anddendrimersarereviewedin thischapter.
ThechapterbyKoennekeetal. (Chapter6) highlightsopportunitiesandchallengesfor nanoparticulatecarriers,especiallyaspherical microparticlesinpulmonarytherapy.The teamhasdevelopedcylinder-shapednanostructuredmicrorods,whichcouldsuccessfully enableselectiveuptakeofparticlesbyalveolar macrophages,resultingintargeteddeliveryof plasmidDNAandlysosomalbufferingagent (bPEI).Thisresearchshouldovercomesomeof manylimitationsassociatedwithpulmonary drugtherapy.
ThetopicpresentedbyShindeetal. (Chapter7) describesthepotentialofphysicalapproaches likephotoporation,geneguns,electroporation, andmechanoporationinenhancingthelimitationsofcurrentnanomedicines.Thischapter discussesindetailtheworkingmechanisms,advantages,limitationsoftheappliedtechniques.
Asystemicreviewofthefutureprospectsand real-timeapplicationsofthesetechniquesisgiven atappropriatesectionsinthechapter.
ThechapterbyPandeyetal. (Chapter8) reviewstheopportunitiesandchallengesof complexinjectabledrugsystems.TheFDA (FoodandDrugAdministration)haslaiddown variousguidelinesforcomplexinjectable productsfortheindustrytofollow.Thischapter highlightsanindustrialviewofthecombination product(drug device)andchallengesassociatedwithit,alongwithmanufacturingcontrols implementedasperregulatoryrequirements ofspecificproducts.Anoverviewofstability challengesforbiopharmaceuticalsisalso provided.
The finalchapter,byDelgadilloandWuthrich (Chapter9),reviewsanewapproachforeffectivedrugdeliveryusingPIPAC(pressurized intraperitonealaerosolizedchemotherapy) technologyincolorectalcancertreatment.This routecandeliverdrugseffectivelyatthetarget sitewithimproveddrugtherapeuticperformanceincolorectalcarcinomaswithimproved survivalratesandqualityoflife.Theauthors con firmpositiveoutcomesofseveralclinical trialsusingPIPACtherapy.
Insummary,Iamsurethisbookvolumeand thecompletebookserieswillprovideyougreat insightsinareasofmicro-nanomedicines,drug deliverysciences,newtrends,andregulatory aspects.
M.Aragon,C.Ashley,J.Brinker,andthe NationalCancerInstitutearegratefully acknowledgedforthebookcoverimage,which representsthepotentialofinnovativenanoparticleplatforms,thatis,poroussilicananoparticlesloadedwithmulticomponentcargos, coveredwithalipidbilayerthatactviaatargeteddeliverymechanismtoreleasetheircontentsdirectlyintocancercells.
Alltheeffortsofexperts,scientists,andauthorsarehighlyacknowledgedforsharingtheir knowledge,ideas,andinsightsaboutthetopic.
RanjitaShegokar,PhD Editor
Polymerlipidhybrid(PLH)formulations:
PaulJoyce1,HayleyB.Schultz2,3,TahliaR.Meola2,3,
CliveA.Prestidge2,3
1DepartmentofPhysics,ChalmersUniversityofTechnology,Gothenburg,Sweden; 2SchoolofPharmacy andMedicalSciences,UniversityofSouthAustralia,Adelaide,SA,Australia; 3ARCCentreofExcellence inConvergentBio-NanoScienceandTechnology,UniversityofSouthAustralia,Adelaide, SA,Australia
1.Introduction
Oraldeliveryiswidelyconsideredthe preferredandmostconvenientrouteofdrug administration,owingtohighpatientcompliance, flexibility,limitedregulatoryhurdlesand cost-effectivedosageforms,limitedpackaging considerations,andsimplicityofmanufacturing [1].However,oraladministrationcanpresenta numberoflimitations,speci ficallywithregard tobiologicalbarriersthatpreventabsorptionof complextherapeutics,suchasproteins,peptides, andlipophilicmolecules,intothesystemic bloodstream [2].Oralbioavailabilityis controlledbythreevitalfactors,namely,dissolution,solubility,andpermeability [3];allofwhich aredependentonanumberofintrinsicfactors associatedwithgastrointestinal(GI)processing. Thisincludesacidhydrolysis,pHgradients,
enzymaticdegradation,mucosalbarriers,and microbialinteractions [1,4,5];alongwithinnate physicochemicalpropertiesassociatedwiththe therapeuticofinterest,suchasaqueoussolubility,intestinalpermeability,andchemicaland enzymaticresistance [6].
Whileconventionalsolubilityenhancement (e.g.,micronizationandextrusion)canbe employedinsomeinstancestoimproveoral bioavailability,however,theemergenceofnanotechnologyinformulationdesignhasofferedunlimitedpotentialforovercomingbiological barriersassociatedwithoraladministration [7] Lipid-baseddrug-deliverysystems(LBDDSs) (e.g.,lipidnanoemulsions,liposomes,andsolid lipidnanoparticles)andpolymervehicles(e.g., polymericnanoparticles,polymericmicelles, dendrimers)aretwoofthemostcommonly employednanocarriersystemsforconquering
biodegradation,dissolution,andsolubilityissuesassociatedwithcomplexpharmaceuticals. LBDDSshavebeenextensivelyusedasoraldeliveryvehiclesduetotheirhighbiocompatibility,lowcost,easeoffabrication,andability tofacilitateimproveddissolutionwithinthe gastrointestinaltract(GIT)bymimickingthe pharmaceuticalfoodeffect [8,9].Despitethe wealthofpreclinicalresearchthathighlights thepotentialofLBDDSstoenhancebiopharmaceuticalperformance,commercialsuccesshas beenlimited.SeveralintrinsicpropertiesassociatedwithLBDDSshavepreventedtheirtranslationintoclinicallyrelevantformulations, includinglimitedphysicochemicalstabilityand portabilityofliquid-lipidformulations,propensityfordrugcrystallizationandprecipitation invivo,lowdrugloading,susceptibilitytoburst release,andpoorinvitro invivocorrelations [10 13]
Polymericsystemsofferarangeofalternate opportunitiesandchallengestolipid-basedcarriers.Suchpolymericvehiclesexhibithighstructuralstabilityandphysicochemicalstability, especiallyduringlong-termstorage,andoffer thepotentialforcontrolled-releasemechanisms tobeimpartedwithintheirdesign [14].Unlike LBDDS,whicharesusceptibletoburstrelease ofencapsulatedcargo,polymericsystemsoften pertainmatrix-dependentreleasemechanisms thatcanprolongdrugreleaseforanextended periodoftime [15].Furthermore,thechemical integrityofpolymersandfabricationtechniques thatcanbeemployedvaryconsiderably,enabling precisecontrolovernanostructureandsurface chemistrytobeinduced,whichultimatelycontrolsinvivobiophysicalinteractions [16].However,aswithlipid-basedapproaches,the therapeuticpotentialofpolymericformulations hasbeencharacteristicallylimitedbyinsufficient encapsulationefficienciesandlowdrug-loading levels [17].Furthermore,drugmoleculesretained onthesurfaceofpolymericnanocarriersareoften
susceptibletoburst-releasemechanisms [18], whilereleaseofcargowithinthepolymermatrix maybeincompleteduetoretentionwithin aqueousenvironments [19].Inthischapter,the focusistowardpolymervehicles,wherebydrug isencapsulatedwithinapolymericphase,rather thandrug polymerconjugatesinwhichthe drugischemicallyconjugatedtothepolymersurface,sincethisisdeemedasanewchemicalentity fromaregulatorystandpoint [4].
Inanefforttounlockthecommercialpotential ofbothsystems,recentfocushasbeenattributed toengineeringnanostructuredconjugateformulationsthatmergethebene fitsoflipidsandpolymers.Byintelligentlydesigningpolymer lipid hybrid(PLH)formulations,thefundamental drawbacksthatlimitthetherapeuticpotential oflipidandpolymersystemscanbeeradicated orreduced,whilesuccessfullyovercomingthe multifacetedchallengesassociatedwithoraldelivery [20].Duetothehighphysicochemical andstructuralversatilityoflipidandpolymer systems,anextensiverangeofmultifunctional PLHformulationshavebeenfabricated,which varyconsiderablyinstructure,composition, andpharmacokineticperformance.Subsequently,theaimofthischapteristosystematicallyreviewtheplethoraofinnovativePLH systemsthatexistasoralformulations,while highlightingtheirpotentialtoimprovepharmacokineticpro filesofchallengingtherapeuticsin contrasttotheirlipid-orpolymer-basedcounterparts.Specificemphasisisplacedontheengineeringanddesignconsiderationsthat in fluencephysicochemicalandbiopharmaceuticalperformanceofPLHformulations,inan attempttoguideauthorstowardselectingthe correctapproachwhenformulatingtheirtherapeuticofinterest.Ultimately,theinsights revealedfromthedesignanddevelopmentof intelligentPLHsystemscanbeharnessedfor thefabricationofnext-generation,hybridized oraldeliveryvehicles.
2.Structureandcompositionof polymer lipidhybrid(PLH)systems
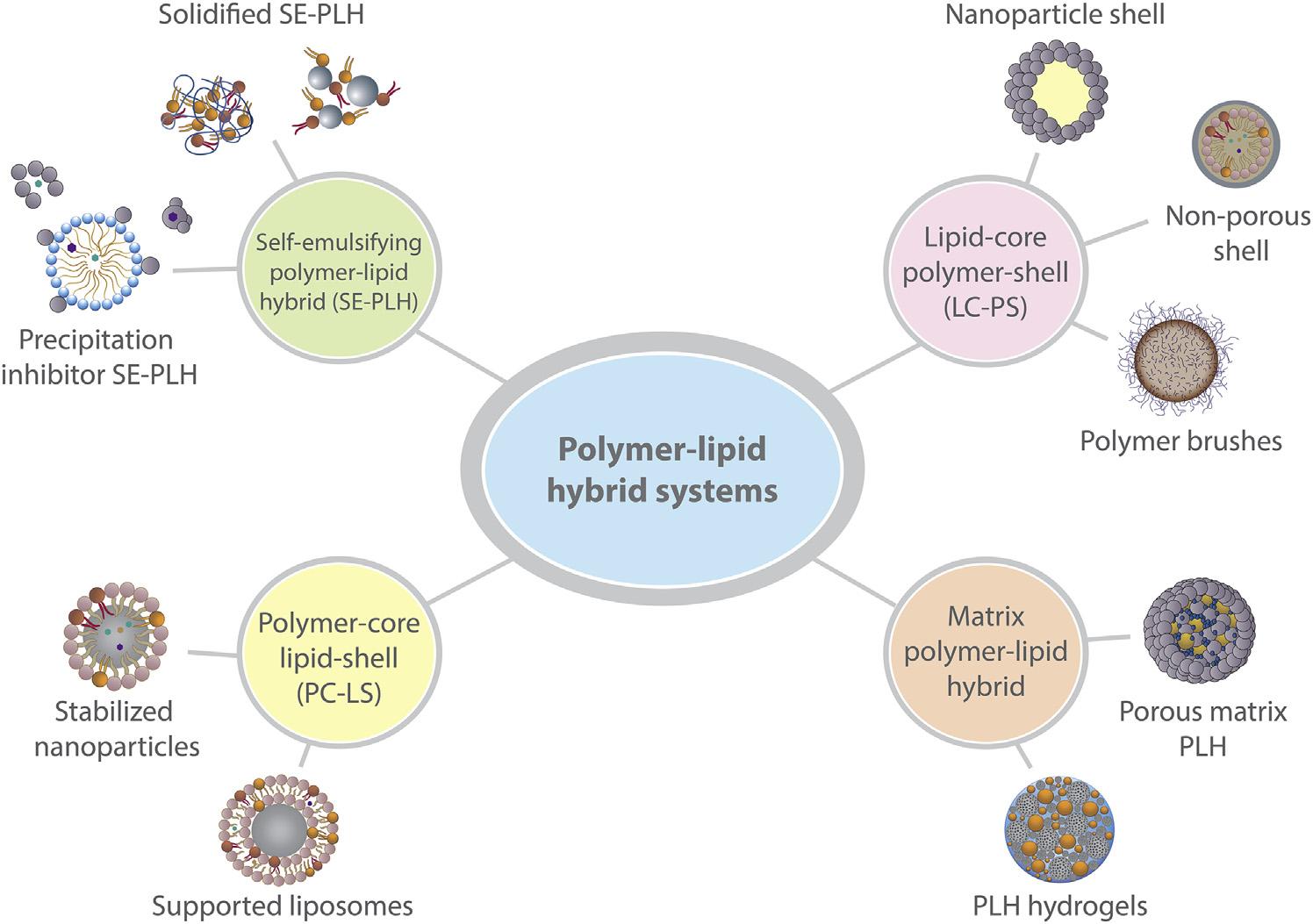
Whiletheopportunitiesandchallengesassociatedwithlipidandpolymersystemsvary, bothformulationapproachesbene fitfromhigh physicochemicalandstructuralversatility.Subsequently,thisaffordsunlimitedvariationsin thestructureandcompositionofPLHsystems, whichultimatelypermitsthetailoringof invivopropertiesthroughvarioussimplemodificationtechniques,wherecharacteristicssuchas gastricretentionandincreasedresidencetimes [21],controlledandstimuli-responsiverelease [22],anddrugtargeting [23] canbeinduced. TherangeofPLHsystemsthatcurrentlyexist toimprovethebiopharmaceuticalperformance oforallyadministereddrugcompoundscanbe categorizedinto:self-emulsifyingPLH(SEPLH);polymer-corelipid-shell(PC-LS);lipidcorepolymer-shell(LC-PS);andmatrixstructuredPLH(matrix-PLH)systems. Fig.1.1 comparesandcontraststhestructuresofthe variousPLHsystems,whileexpandingtopresentsubcategoriesfortheaforementionedsystems.Furthermore, Table1.1 summarizesthe multitudeofdrugcompoundsthathavebeen successfullyformulatedwithvariousPLHtypes forimprovedoraldeliveryperformance;thus, providingabriefoverviewonthemechanisms involvedinrespectivePLHstructuresfor enhancingdrugabsorption.Thefollowingsectionelaboratesonthese findingsandprovides anin-depthandcriticaloverviewofstructural characteristics,biopharmaceuticaladvantages, andlimitationsthatareaffordedbyeachPLH category.
2.1Self-emulsifyingpolymer lipid hybrid(SE-PLH)systems
2.1.1Rationale
Conventionalself-emulsifyingdrug-delivery systems(SEDDSs)arecomposedofanisotropic
mixtureoflipids,surfactants,andoptionallycosurfactantsandcosolvents,thatform finelipid emulsionsupondispersionandagitationwithin theGIT [47].Onceanemulsionisformed,lipophilicdrugmoleculesaresolubilizedwithinthe lipidphase,whichistypicallypronetolipasemediateddigestion.Theonsetoflipidhydrolysis triggersthereleaseoftheencapsulatedcargo alongwithdigestionproducts,specificallyfree fattyacidsandmonoglycerides,whichpartition towardtheaqueousphaseintheformofmixed micellesandvesicles [9].Theformationofthese colloidalphasespromotestheresolubilization ofthelipophilicdrugspecies,whicharethen absorbedacrosstheintestinalepitheliumin conjunctionwiththedigestionproducts [48].
DuetothepropensityforSEDDSstoimprove theintestinalsolubilizationofawiderangeof poorlysolublebioactives,theyexistasoneof themostexploredandutilizedlipid-based formulationapproaches [47].However,likethe majorityofliquid-lipiddeliveryvehicles, SEDDSssufferfromsigni ficantlimitationsthat preventtheirtranslationintowidespreadcommercialuse,including:poordrugloading,drug crystallizationandprecipitationchallenges,and poorstoragestability [10 13].Suchlimitations canbeovercomebyformulatingselfemulsifyinglipidswithpolymers,toformSEPLHsystems.Theprimaryfocusofthisstrategy istoeither(1)formasolid-SEDDSor(2)increase drugsolubilitywithintheGIT.Arecentreview highlightedtheopportunitiesandchallenges associatedwithsolid-SE-PLHsystems [49],and therefore,thischapterwillfocusmorespecificallyonSE-PLHsystemsthatovercomedrug crystallizationandprecipitationchallengesof conventionalSEDDSs.
2.1.2Synthesisapproach
Facilesynthesisapproachesareutilizedto fabricateSE-PLHsystems,sincetheirmechanismofactionreliesonself-emulsi fication invivo.SE-PLHsystemsareproducedby firstly solubilizingdrugmoleculeswithinSEDDS
1.Polymerlipidhybrid(PLH)formulations:asynergisticapproach
FIGURE1.1 SchematicrepresentationofthevariousPLHstructuresthatcanbefabricatedforimprovingtheoralbiopharmaceuticalperformanceofchallengingtherapeutics.
TABLE1.1 CasestudiesdemonstratingtheabilityofPLHsystemsofvariouscompositionsandstructurestoimprove thebiopharmaceuticalperformanceofawiderangeoftherapeuticcompounds [4,24].
DrugcompoundPolymerLipidImprovedoraldrugdeliverypropertiesReferences
Self-emulsifyingPLH(SE-PLH)systems
InsulinChitosanLecithinA12-foldenhancementinpharmacological bioavailabilitywasachievedbyencapsulation withinSE-PLH
GriseofulvinHydroxypropyl methylcellulose (HPMC)
Oleicacid,LabrifilTween20,Labrafac PG
Invitrostudiesrevealeda >2-foldincreasein supersaturationfactorcanbeachievedby combiningconventionalself-emulsifying drug-deliverysystems(SEDDS)withHPMC Thedegreeofdrugsupersaturationwas dependentonHPMCviscositylevels,with lowerviscositiesprovidinggreater supersaturation
ProgesteroneMicrocrystalline cellulose
Lecithinand polysorbate80
Increaseddrugpermeabilityasdemonstrated bymonitoringdrugabsorptionacrossa Caco-2cellmonolayerandratintestine
TABLE1.1 CasestudiesdemonstratingtheabilityofPLHsystemsofvariouscompositionsandstructurestoimprove thebiopharmaceuticalperformanceofawiderangeoftherapeuticcompounds [4,24]. cont'd
DrugcompoundPolymerLipidImprovedoraldrugdeliverypropertiesReferences
TetrahydrocurcuminSodiumstarch glycolate
LovastatinSodiumalginate andHPMC
Polymer-corelipid-shell(PC-LS)systems
Bovineserum albumin(BSA) PLGA nanoparticles
Labrasol, CremophorEL, Capryol90, LabrafacPG
Saquinavir mesylate PLGA nanoparticles
CapmulMCM andCapryol90
Controlled-releasemechanisminducedby inclusionofsodiumstarchglycolate,leading toanextendedreleaseprofileover8hin simulatedgastric fluid.Slowreleasebehavior wasattributedto floatingmechanismofSE-PLH system,whereby93%oftheformulation remained floatingingastricmediaafter6h
Thearea-under-the-curveoflovastatinplasma concentrationprofileswasmorethandoubled ininvivoanimalstudies,comparedtothepure drug
PhosphatidylcholineTheburstreleaseofencapsulatedcargowas reducedbycoatingPLGAnanoparticleswith alipidshell,leadingtoanincreasein transcytoticefficiencywithinMcells comparedtobarePLGAspheres
Glyceryltributyrate andPEGsuccinate mixture;or,E200 phospholipid mixture
ImprovedpermeationacrossaCaco-2cell monolayerforlipid-coatedPLGA nanoparticles,comparedtoareference nanoemulsionsofthesamelipid composition
Cromolyn sodium PLGA nanoparticles
CabazitaxelPoly(ε-caprolactone) andpoloxamer188
Lecithin
VardenafilHClGantrezAN-119 (poly-methylvinyl ether-co-maleic anhydride)
Lipid-corepolymer-shell(LC-PS)systems
Medium-chain triglycerides, octadecylamine andlecithin
Polyglycerl-6distearateor glyceryltristearate
w12-foldimprovementinoralbioavailability comparedtothepuredrug,attributedtoan extendedreleasemechanismthatprolonged cromolynsodiumreleaseovera48hperiodin simulatedGI fluid
Thearea-under-the-curveofcabazitaxel plasmaconcentrationprofileswasmorethan doubledininvivoanimalstudies,compared tothebarepolymersystem [33]
Almosta2-foldincreaseinoralbioavailability andprolongedresidencetimeswithin circulationcomparedtothecommercially availableformulation [34]
IndometacinChitosanLecithinRetentiontimewithinGITofratswas increasedcomparedtouncoatedliposomes [35]
PaclitaxelPoly(acrylicacid) andpoly(allylamine) HCl
Lecithinandstearyl amine
Drugreleasewassustainedovera24h period,whichcontributedtoa w4-fold improvementinoralbioavailability comparedtopurepaclitaxel [36] (Continued)
TABLE1.1 CasestudiesdemonstratingtheabilityofPLHsystemsofvariouscompositionsandstructurestoimprove thebiopharmaceuticalperformanceofawiderangeoftherapeuticcompounds [4,24]. cont'd
DrugcompoundPolymerLipidImprovedoraldrugdeliverypropertiesReferences
DanazolPoly(ethylene glycol)
DoxorubicinPoly(ethylene glycol)
OvalbuminPoly(ethylene glycol)
Medium-chain triglycerides
Drugsolubilizationwasenhancedduring invitrolipolysiscomparedtotheuncoated triglycerideemulsion,leadingtoa1.2 1.8 enhancedoralbioavailability
MonostearinEnhancedmucoadhesivepropertieswere inducedbycoatingsolidlipidnanoparticles withPEG,leadingtoa >7-foldincreaseinoral bioavailabilityand >6-foldincreaseinmean residencetime,comparedtothepuredrug
Distearoylphosphatidycholine
Increasedresidencetimeintheintestinal lumenwasenhancedbycoatingliposomes withPEG,leadingtoagreaterintestinal immuneresponsecomparedtouncoated liposomes
CalcitoninChitosan-aprotininDistearoylphosphatidycholine
Increasedmucoadhesionwasinducedby coatingliposomeswithchitosan-aprotinin, leadingtoa15-foldincreaseintheareaabove thebloodcalciumconcentration timecurve [40]
Matrix-structuredPLH(matrix-PLH)systems
CinnarizinePLGAMiglyol812and lecithin
VerapamilDextranCompritol,ATO 888,Tween80and poloxamer188
AsynergisticeffectininhibitingpH-provoked drugprecipitationwasobservedwhenthe polymerandlipidphasewerecombinedwithin theoneformulation,leadingtoa >2-fold increaseindrugdissolutionduringinvitro digestion InvivostudiesrevealedthattheAUCofplasma drugconcentrationswasmorethandoubled comparedtoalternateorallipid-based formulations [15,41]
IncreaseddrugpermeabilityacrossaCaco-2 cellmonolayerandprolongeddrugreleaseof upto72hinsimulatedGI fluid
SilymarinChitosanandPLGA nanoparticles Lecithin w1.2-foldgreaterrelativebioavailability comparedtobarePLGAnanoparticles
DoxorubicinGantrezAN-119Plurol (Polyglyceryl-6distearate) w4-foldincreaseinoralbioavailability comparedtothepuredrug
IbuprofenPoly(methacrylic acid)
GuargumoleateControlleddrug-releasemechanismwas provokedbyincorporationofpH-sensitive polymerwithinthePLHsystem,allowingfor site-specificreleasewithinthesmallintestine
TABLE1.1 CasestudiesdemonstratingtheabilityofPLHsystemsofvariouscompositionsandstructurestoimprove thebiopharmaceuticalperformanceofawiderangeoftherapeuticcompounds [4,24]. cont'd
DrugcompoundPolymerLipidImprovedoraldrugdeliverypropertiesReferences b-CaroteneAlginateCornoil stabilizedbywhey proteinisolate
Therateandextentofinvitrolipolysiswas dependentonpolymerconcentration,with increasingalginateconcentrationsleadingtoa decreaseinlipolysiskinetics.Indoingso, chemicalandlipolyticdegradationofthe encapsulatecargoreducedwithincreasing alginatelevels [46]
excipients,priortoadditionofthepolymerphase throughgentlephysicalmixingandheatingto produceauniformsuspension [50].Theconcentrationofpolymeraddedtotheconventional SEDDSisexcipient-andsystem-dependent,but frequentlyrangesfrom0.5 5wt% [51].Generally,well-formulatedSE-PLHsystemsrapidly andhomogeneouslydispersewithinminutes, andcanbesubsequentlyspray-orfreeze-dried tocreateasolid-dosageform [11].Furthermore, SE-PLHsystemscanbeencapsulatedwithinor adsorbedonanalternatepolymermatrixthat doesnotprovideadditionalsupersaturationcapacity,suchashigh-molecular-weightpoly(ethyleneglycol)(PEG) [52,53].
2.1.3Biopharmaceuticaladvantagesof SLH-PEsystems
2.1.3.1Improvedsolubilizationandprecipitation inhibition
Thevariableandunpredictableinvivopharmacokineticsassociatedwithconventional SEDDSsisprimarilyattributedtodrugcrystallizationandprecipitationuponemulsificationin theGIT [54].Polymericprecipitationinhibitors (PPIs)canbecombinedwithself-emulsifying lipidsforasynergisticeffect,wherebythepolymerphasestabilizesthemetastablesaturated andsupersaturatedstatesofawiderangeof lipophilicdrugs.ExamplesofPPIsincludePluronics [55],cellulosederivatives [56],poloxamers [57],andpolyvinylpyrrolidone(PVP) [58].Gao etal. [56] were firsttoreportonemploying
hydroxypropylmethylcellulose(HPMC),a water-solublenonioniccelluloseether,asaprecipitationinhibitorinaconventionalpaclitaxel self-emulsifyingformulation [56].Invitrodissolutionstudiesrevealedthattheincorporationof HPMCprolongedthesupersaturatedstatefor 2hcomparedtoSEDDSswithoutHPMC.Concentrations31-foldhigherthantheequilibrium solubilityofpaclitaxelinthesimulatedGImediumwerereached,suggestingHPMCwas effectiveatgeneratingandsustainingasupersaturateddrugsolution.Itishypothesizedthat hydrogenbondingand/orhydrophobicinteractionsbetweendrugmoleculesandpolymers, suchasHPMC,increasethenucleationactivationenergy,leadingtodelayedcrystalnucleationandgrowthwithinthesmallintestine [59].Indoingso,thedegreeofdrugmolecules dissolvedandbioavailableforabsorptionacross theintestinalepitheliumisenhanced considerably.
TheabilityofSE-PLHsystemstoovercome thecrystallizationandprecipitationchallenges ofpoorlysolubledugspresentstwoadditional, interrelatedadvantagesoverconventional SEDDSs,being(1)improveddrugloadingand (2)reducedsurfactantconcentrations [49]. SEDDSsaretypicallyassociatedwithhighsurfactantconcentrations(>30wt%)toproducemicro/nano-sizedemulsiondropletswithhigh surfaceareasandhighsolubilizationcapacities [60,61].Whilethismayimprovetheoral bioavailabilityofpoorlysolublecompounds,
studieshaveindicatedthathighsurfactantdoses canbepoorlytoleratedinchronicuse [54].By employingbiocompatibleandbiodegradable polymersasstabilizingandsupersaturating agents,itispossibletomaintainorincrease oralabsorptionlevelswithlowersurfactantconcentrations.Forexample,instudiesbyGaoetal. [56,62],thesurfactantconcentrationwastoolow toachievecompletesolubilizationoftheencapsulatedcargofortheconventionalSEDDSs. However,drugmoleculesweresuf ficientlysolubilizedwithintheSE-PLHsystem,usingHPMC, whichallowedforcompletedissolution.By replacingasignificantportionofthesurfactants requiredtoachievecompletedissolutionand improvedabsorption,thepotentialsafetyand toxicityconcernsofhighsurfactantconcentrationswerereduced/eliminated [49].
2.1.3.2Soliddosageform
Thebene fitsofenhancedGIsolubilitycanbe combinedwiththeadvantagesofsolid-statesystemsbyfabricatingSE-PLHsystemsthatutilize thePPIasasolidcarrier.Chenetal. [63] investigatedtheeffectsofdifferentPPIsincluding HPMC,PVP,andAvicel(colloidalmicrocrystallinecellulose)onthebiopharmaceuticalperformanceofdocetaxelwhenencapsulatedwithin solid-SE-PLH.HPMCsignificantlypromotedsupersaturationcomparedtoalternativePPIsand thuswasusedincombinationwithlactoseasa carrierforsolid-SEDDSs.Invivostudiesindicateda1.45-foldincreaseinthearea-under-thecurve(AUC)forthesolid-SEDDSswithHPMC comparedtowithoutHPMC,suggestingthe presenceofHPMCasaPPIsignificantly improveddrugsolubilityallowingforenhanced dissolutionandbioavailability.
Morerecently,Quanetal. [64] includedthe innovativeamphiphilicpolymer,Soluplus(polyvinylcaprolactam polyvinylacetate polyethyleneglycolgraftcopolymer)asasolid carrierincombinationwithmesoporoussilica, tocombinethebene fitsofenhancedGIsolubility (facilitatedbytheinclusionofaPPI),withthe
advantagesofsolid-statesystems(facilitatedby theinclusionofmesoporoussilica).SE-PLHcontainingSolupluswasmosteffectiveatretarding drugprecipitationinvitro,comparedtoabroad rangeofalternateSE-PLHsystems,showing consistentlyhigherapparentdrugconcentrationsovera2hperiod.Invivoresultswerein agreementwitha1.4-foldimprovementin bioavailability,implyingthatthesupersaturable formulationwasabletogeneratehigherdrug concentrationsintheGIT.Itissuggestedthat thesuperiorprecipitationinhibitionprovided bySoluplusisachievedboththermodynamically andkinetically.Acombinatorialmechanismallowsforanincreasedapparentsaturationsolubilityduetoitssurfactantaction,whilealso delayingnucleationandcrystalgrowth [64].
2.1.3.3Controlleddrugrelease
ByfabricatingSE-PLHsystems,thepotential forcontrolleddrugreleaseisintroduced.Zhang etal. [65] developedasustained-releaseSE-PLH formulationofpuerarin,anisoflavoneusinga combinationofHPMCandmicrocrystallinecelluloseinordertoenhancebioavailability. Controlleddrugreleasewasobtainedduring invitrodissolutionstudieswherebythepellet formulationsustainedreleaseover10h.Oral administrationtobeagledogsrevealeda2.6foldenhancementinAUCandimportantly,a threefolddelayintimetoreachmaximumconcentration(Tmax)whichindicatedsuccessfulGI retention [66].Theunderlyingmechanismofactionwashypothesizedtobeafavorableinteractionbetweenthepolymericcarrierandgastric epithelialcells,resultinginalongerresidence timeinthestomachallowingforprolonged dissolution.Furthermore,Taoetal. [21] demonstratedthatalteringthemolecularweightofthe polymercanfurthercontroltherateofdrug releasefromSE-PLHsystems.Utilizingahighmolecular-weightHPMCcontributedtoaslow releaseofthedrug,sirolimus,asalongertime wasrequiredtocorrodeHPMCandallowdrug diffusion.Comparatively,alow-molecular-
weightHPMCshowedthemostrapidandsustainedreleaseovera12hperiod,highlighting thatmodificationstothepolymeritselfcanrefine thereleaserate.
2.1.4Keytherapeuticsofinterest
Perhapsthemostsignificantapplicationof SE-PLHsystemsliesintheirabilitytoprevent pH-provokedprecipitationofpoorlysoluble weakbases(PSWBs).PSWBs,suchascinnarizine andalbendazole,exhibitpH-dependentsolubilityduetotheirprotonationwithinacidicenvironments.Theionizationofsuchcompounds leadstodrugsupersaturationwithinthegastric environment,withdrugsolubilityrapidly decreasingasthedrugtransitstowardneutral conditionsofthesmallintestine [15,49].Subsequently,oralbioavailabilityofPSWBsissignificantlyretardedduetotheirpropensityfor precipitationwithintheintestinalphase.ConventionalLBDDSs,suchasSEDDSs,have limitedefficaciesindeliveringPSWBsdueto theirinabilitytoovercomeequilibriumsolubility discrepancieswithintheGIT [67].However, encapsulationwithintheSE-PLHsystemhas beenshowntobeaneffectiveapproachinovercomingthepH-mediatedprecipitationchallenges ofPSWBs,sincehydrophobicinteractionsbetweenthedrugandPPIsrestrictstheconversion ofthesupersaturateddrugmoleculetothemore thermodynamicallystablecrystallinestateupon gastricemptying [15,41,68 74].Indoingso,the rateandextentofdrugdissolutionissignificantly enhancedattheprimarysiteofdrugabsorption (i.e.,thesmallintestine).
2.1.5LimitationsofSE-PLHsystems
ThemajorlimitationaffectingSE-PLHsystems isthelackofmechanisticunderstandingwithregardtoPPImodeofaction.Variousanalytical methodshavebeenusedtoelucidatethepotential forPPIstostabilizethesupersaturatedstateand/ orinhibitprecipitation;however,theexactmechanismofactionisstillnotclear.Theoverarching hypothesisisthatdrug polymerinteractions
(i.e.,hydrogenbonding,hydrophobicinteractions etc.):(1)increasenucleationactivationenergy, effectivelyinhibitingthe firststepforcrystallization,and/or(2)delayandimpedecrystalgrowth [75].Thatis,polymersthatarehydrogen-bond donorsarelikelytoinhibitcrystallizationofdrugs whicharehydrogen-bondacceptors,andvice versa [59,76].Additionally,Gaoetal. [77] demonstratedthattheeffectivenessofnucleationinhibitionbyvariousgradesofHPMC(withequivalent viscosities)inanSE-PLHsystemwasdependent onthedegreeofmethylsubstitution(orhydrophobicity).Thatis,HPMC-Eserieswith29% methylsubstitutionwassuperiortoHPMC-K serieswith22%methylsubstitutioninsustaining supersaturationofthedrug,AMG517.Thisis supportedbyadditionalstudiesthathavehighlightedtheroleofhydrophobicityindelaying crystalgrowth [53,78,79]
Whilethecommonhypothesisofpolymer druginteractionspreventingprecipitationis supportedbynumerousstudies, findingsfrom Ilevbareetal. [80,81] contradictedthis,ascrystal growthofritonavirwasnotdirectlycorrelated forover15PPIswithvaryinghydrophobicities. Assuch,alternatedeterminingfactorsofcrystallizationinhibitionthatarehypothesizedtoexist includesolutionviscosity,polymermolecular weight,andsterichindrance [75].Theneedfor improvedmechanisticunderstandingofPPIsis requiredtoallowforadequateidenti fication, selection,anddesignofSE-PLHsystemsthatprovideoptimalsupersaturationandprecipitation inhibitionofthetherapeuticofinterest.ThedemandforsystematicstudiesthatanalyzethemolecularinteractionsisamplifiedforSE-PLH systems,sinceitishypothesizedthatfurther drug lipidinteractionsexist,whichmaysubsequentlyaltertheirsolubilizationcapacity [49] ThepreclinicaldevelopmentofSE-PLHsystems iscurrentlyheavilydependentontime-and energy-exhaustiveinvivostudiestovalidate oraldrugperformance.Improvingtheunderstandingofstructure activityrelationshipsfor SE-PLHsystemswillaccommodatethe
1.Polymerlipidhybrid(PLH)formulations:asynergisticapproach
developmentofaplethoraofnovelSE-PLHsystemswithimprovedactivitiestowardoralsolubilizationandabsorption,whilereducingtheneed forextensiveinvivostudies.
2.2Lipid-corepolymer-shell(LC-PS) systems
2.2.1Rationale
LiquidLBDDSs(e.g.,lipid-in-wateremulsions, liposomes)sufferfrompoorthermodynamicand kineticstabilities,whichleadtodistinctphase separationduringshort-termstorage [82].
Additionalchallengesariseforlipidcarriers withregardtocontrollingdrugreleasewhen administeredorally.Drugreleasefromlipidsystemsisdiffusion-anddigestion-dependent, whichcanleadtorapid/burstreleaseofdrug moleculesintotheaqueousphaseoftheGIT [83,84].Incontrast,drugreleasefrommostpolymericsystemsistypicallymatrix-dependent, whichleadstoslowreleasekineticsasafunction ofpolymererosion [17].Subsequently,polymeric coatingscanbeadsorbedontothelipid-in-water interfacetoimprovethestabilityoflipidcolloids duringstorageandGIprocessing [85],whilealso impartingthepotentialtofacilitatecontrolledand targeteddrugrelease [86].LC-PSsystemsare typicallyengineeredtoencapsulatethebioactive compoundwithinthelipidcomponent,while thepolymerphaseactsasaphysicalbarrierprotectingthedrugcargo.Sincethelipidcomponent mayexistaslipidparticles/dropletsorliposomes,itispossibletofabricateLC-PSsystems fordrugswitharangeofpolarities.
2.2.2Synthesisapproach
ThefabricationapproachofLC-PScanbe broadlycategorizedaseither:
(1) One-stepsynthesis
LC-PScanbepreparedusingaone-step methodwherebyanamphiphilicpolymeric emulsifierisusedtostabilizelipiddroplets/ particlesbyreducingtheinterfacialtension
betweentheoilandwaterphases [87].For example,PEGylatedsolidlipidnanoparticles (PEG-SLN)canbefabricatedbydissolvinga mixtureofsolidlipid,suchastripalmitin, andPEGwithinanorganicsolution [88]. Emulsificationoftheorganicsolutionwithin anaqueousphase,andsubsequent evaporationoftheorganicphase,leadsto theprecipitationofPEG-SLNparticles, wherebyPEGpartitionstothelipid-in-water interfaceduetoitsamphiphilicnature.The particlesizeandsurfacechargeofthePEGSLNparticlescanbealteredbyvaryingthe relativeconcentrationofPEG.Yuanetal. [38] observedthatareductionofPEG-SLN particlesizewasobservedwhenthemass fractionofPEGtosolidlipidincreasedfrom 0%to20% [4].Furthermore,theinternal structureofsolidlipidnanoparticles(SLN) canbeconvertedtonanostructuredlipid carriers(NLC)bysubstitutingaportionof solidlipidcontentwithaliquidlipid,such asmedium-chainlengthtriglycerides [88]
(2) Two-stepsynthesis
Presynthesizedandloadedlipidmicro/ nanoparticlescanbecoatedbyapolymeric shellthroughself-assemblyprocesses mediatedbyelectrostaticattractive interactionsbetweenthelipidandpolymer phases.ChitosanLC-PSarecommonly preparedusingthistwo-stepmethod, wherebycoincubationofachitosansolution withlipidparticlestriggerstheself-assembly ofchitosanatthelipid-in-waterinterface [89,90].Layersomesorpolyelectrolytestabilizedliposomes,extendthistwo-step approachbyemployingalayer-by-layer technique [36,91],wherebyaminesare initiallyutilizedtoinferapositivechargeon aphospholipid-basedliposomecore [4].The liposomecoreisthencoatedwithananionic polymer,suchaspoly(acrylicacid)(PAA), throughelectrostaticinteractions,followed bycoatingwithacationicpolymer,suchas poly(allylamine)hydrochloride(PAH) [4].
2.2.3BiopharmaceuticaladvantagesofLC-PS systems
2.2.3.1EnhancedGIstability
Aplethoraofstudieshavedemonstratedthe abilityforlipidnanocarriers,includingliposomes,nano-/microemulsions,andSLN,to enhancedrugsolubilizationofpoorlywatersolublebioactiveswithintheGIT,bycreatinga lipophilicenvironmentthatmaintainsthedrug inadissolvedstate,asreviewedpreviously [9,48,92].However,thepropensityforLBDDS todegradeanddestabilizewhenexposedto theharshacidicandenzymaticconditionsof theGIenvironmentcantriggera(1)changein solubilizationcapacity,(2)burstreleaseof encapsulatedcargo,and(3)presystemicdrug metabolism [85].Ultimately,thishasprevented theirwidespreadtranslationintoacommercial andclinicallyrelevantformulation.
ToovercometheGIstabilitydrawbacks, LBDDSscanbecoatedwithanexteriorshell composedofpolymerexcipientsthatarestable duringGIprocessingandprotecttheconfined lipidanddrugmoleculesfromdegradationand prematurerelease [4].ThischaracteristicofLCPSsystemsisespeciallyimportantforpoorly water-solubledrugs(PWSDs)pronetoprecipitationintheGIT,aswellaspH-andenzymesensitivetherapeutics,suchasproteinsandpeptides.Agrawaletal. [93] recentlydemonstrated thisbycoatingliposomeswithamultilayer coatingofpoly(acrylicacid)(PAA)andpoly(allylamine)hydrochloride(PAH)fortheoral administrationoffolate-functionalizedinsulin. Theinsulinentrappedwithinthemultilayer LC-PSsystemwasfoundtobechemicallyand thermodynamicallystableduringpreparation andlyophilization,aswellasduringbiological processinginsimulatedGI fluids.ImprovedGI stabilityledtoasustaineddrug-releasemechanism,whereby72%ofinsulinwasreleased overa24hperiod.Incontrast,completedrug releasewasobservedforuncoatedliposomes overthisperiodand,interestingly,thenature
ofthereleasepro fileswasshowntobeindependentofpHandtypeofsimulatedGI fluid.Ultimately,invivopharmacokineticstudiesin Sprague Dawleyratsrevealeda4.8-foldincreaseinAUCfortheLC-PSsystem,compared touncoatedliposomes [93].Pharmacodynamic studiesdemonstrateda1.92-foldimprovement incumulativehypoglycemiaforthemultilayeredliposomes,comparedtoasubcutaneousinjectionofunformulatedinsulin.Thishighlights thepotentialforLC-PSsystemstosuccessfully protectsensitivetherapeuticsfromtheGIT, allowingforefficientoraladministration.
2.2.3.2Improvedmucosalinteractions
Coatinglipidnanocarrierswithapolymer layerintroducestheabilitytomanipulateand promoteinteractionswithmucuslayerswithin theGIT [94].Improvingmucosalinteractions hasbeenshowntoenhancethetherapeuticwindowforabsorptionbyprolonginggastricresidencetimesand,therefore,increasingexposure toabsorptivesites,aswellasincreasingpenetrationacrossmucosalbarriersthatpreventabsorptionthroughtheintestinalepithelium [95]. Carbohydratepolymers,suchaschitosanand alginate,arethemostef ficientcategoryofpolymersinexertingmucoadhesiveandmucopenetratingpropertiesofLC-PSsystemsdueto favorableelectrostaticandhydrogenbondinginteractionsthatformbetweenthepolymershell andGImucus [96 98].Speci fically,positively chargedchitosanhasdemonstratedstrong mucoadhesionpropertiesduetotheelectrostatic attractiveforcesthatexistbetweenthecationic polymerandanionicmucusmembrane [99].A studybyLuoetal. [100] revealedthatpositively chargedchitosan-coatedSLNexerteda5.7-fold greateraffinityformucin,comparedtouncoated SLN.Itwassuggestedthatthehydrophilicnatureandpositivechargeofthechitosansurface wereequallyimportantforstrengtheningtheaffinitybetweenSLNandthemucinlayer [100].
Carefuldesignconsiderationsmustbeimplementedtoensureanoptimalbalanceexists
1.Polymerlipidhybrid(PLH)formulations:asynergisticapproach
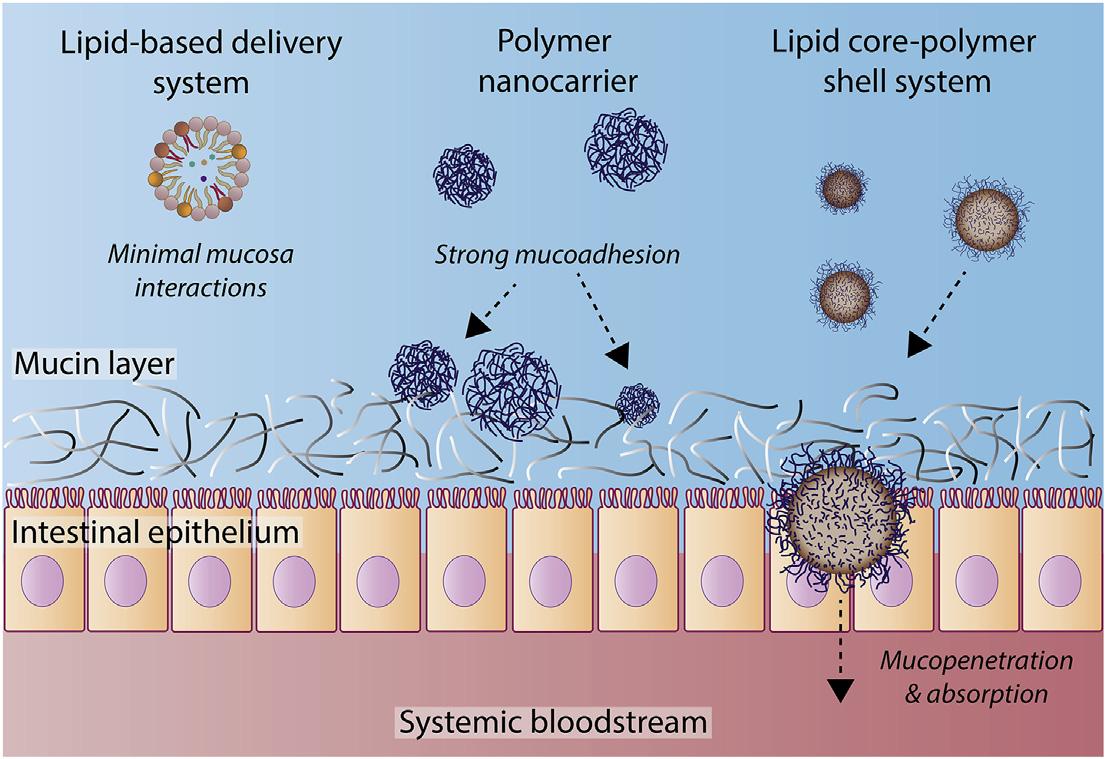
betweenmucoadhesionforprolongedresidence timesandmucopenetrationforimproveddrug absorptionacrosstheintestinalepitheliumand intothesystemicbloodstream [39].Prolonged GIresidencecanbebeneficialfordrugswith shorthalf-lives;however,strongmucoadhesion maylimitdrugdiffusionandpermeationacross mucuslayers,restrictingdrugaccesstotheprimaryabsorptionsite [4].Bachhavetal. [101] attemptedtobalancemucoadhesionwithmucopenetratingpropertiesofSLNbycoatingvarious concentrations/thicknessesofthesynthetic polymer,GantrezAN119.Creatingthehybrid formulationincreasedthehydrophobicityof thesystemand,therefore,weakermucoadhesion interactionsformedbetweenthehybridsystem andmucuslayers,comparedtopurepolymer nanoparticlesofequivalentsize.However,the LC-PSsystemwasshowntobemoreproneto penetrationacrossmucosalbarriers,which contributedtogreaterPeyer’spatchuptakeand plasmadrugconcentrationscomparedtothe precursorpolymer [101].Thus,byoptimizing thebalancebetweenmucoadhesionand
mucopenetrationofLC-PSsystems,itispossible toenhanceabsorptionacrosstheintestinal epitheliumseveral-fold,incomparisontothe precursorLBDDSsandpolymernanocarriers (Fig.1.2).
2.2.3.3Site-specificrelease
Thetherapeuticef ficiencyoforaldrugdeliverysystemscanbeimprovedbyimparting controloverthespeci ficlocationofdrugrelease [102].ThiscanbeachievedinLC-PSsystemsby coatinglipidnanocarrierswithstimuliresponsivepolymers.Mostapplicabletooral administrationistheuseofpolymersthattrigger drugreleaseinresponsetochangesinpH, wherebytheencapsulatedcargoisprotected fromtheacidicgastricenvironmentandrelease isinducedbythechangeinpHupongastric emptying [103].Thisisfundamentallyimportant fordrugsthatexertpH-dependentsolubilities andthosethatdegrade/denatureunderacidic conditions.
Polymerswithahighconcentrationofcarboxylicgroups,suchascelluloseandchitosan,are
FIGURE1.2 SchematicrepresentationoftherationaleassociatedwithcoatedLBDDSswithpolymericcoatingsforoptimal mucosainteractions.Polymernanocarriersexertsignificantlystrongermucoadhesionproperties,incontrasttoLBDDSs,dueto favorableelectrostaticandhydrogenbondinginteractions.BycoatingLBDDSs,itispossibletobalancemucoadhesionpropertieswithmucus-penetratingcharacteristicsforenhanceduptakeacrossintestinalepithelia.
idealcoatingexcipientsforinducingpHresponsivenesstoLC-PSsystemsduetotheir abilitytoswellanddeswelluponionization deionizationofcarboxylgroups [104].Zhang etal. [86] investigatedtheabilityforacarboxymethylchitosancoatingtoinduceapHmediatedreleasemechanismofdocetaxelloaded withinstabilizedliposomes.Itwasestablished thatdrugreleasefromuncoatedliposomeswas morethanfourfoldgreaterthanthecarboxymethylchitosan-coatedliposomes,duetothe chitosancoatingdeswellinginacidicconditions andformingadenselayeronthestabilizedliposomes.Whenexposedtosimulatedintestinal conditions,theneutralaqueousmediaprovoked swellingofthepolymercoating,allowingdrug diffusionoutoftheliposomes.Thiswasdemonstratedbysustaineddrugreleaseof w80%over a25hperiod [86].Thus,notonlycandrug releaseduringgastricprocessingbeprevented, additionalcontrolcanbeimplementedtosustain intestinaldrugreleasebycoatinglipidnanocarrierswithpolymershellsthatswellupon changesinpH.
2.2.4Keytherapeuticsofinterest
ItcanbearguedthatLC-PSsystemsarethe mostversatilePLHcarriersandhavebeen exploredforawiderangeoftherapeutics.Since thecoreoftheseparticlescanexistasalipid phase,anaqueousphase,orashollowparticles, itintroducestheabilitytodeliverpoorlysoluble andsolubledrugmolecules [4].However,of greatestimportanceandimpactisthesuperior abilityforLC-PSsystemstoorallydeliversensitivemacromolecules,suchasproteinsandpeptides,duetotheprotectionprovidedtothe confinedlipidnanocarriersanddrugmolecules bythepolymericcoating [25].Forexample, Tooriskaetal. [105] demonstratedtheabilityto orallyadministerinsulinthroughconfinement withinaninnovativeLC-PSsystem.Speci fically, NLCwerecoatedwiththepH-responsivechitosanderivative,hydroxypropylmethylcellulose phthalate(HPMCP).pH-mediatedreleasewas observedduringinvitroassessments,whereby
insulinremainedencapsulatedwithintheNLC matrixduringsimulatedgastricprocessingand wasrapidlyreleasedduringtheintestinalphase asaresultofHPMCPsheddingfromthelipid surface [105].Inacomparablestudy,Fonte etal. [90] designedanddevelopedchitosancoatedSLNtoimprovetheoralabsorptionofinsulin.An w1.4-foldand w3.3-foldincreaseininsulintransportacrossaCaco-2cellmonolayer wasobservedforchitosan-coatedSLN, comparedtouncoatedSLNandfreeinsulin, respectively.Subsequently,atwofoldenhancementinrelativepharmacologicalbioavailability wasobservedforchitosan-coatedSLN, comparedtouncoatedSLN.Indoingso,the studydemonstratedtheabilityforthechitosan coatingtoserveasaprotectiveboundaryforinsulindegradationduringgastricprocessing,as wellasimprovedmuco-adhesionand-penetrationcomparedtouncoatedSLN.
2.2.5LimitationsofLC-PSsystems
Whilesigni ficantresearchhasfocusedon employingandoptimizingLC-PSsystemsfor oraldrugdelivery,translationintoclinicallyrelevantmaterialshasbeenlimitedduetothe complexityofengineeringprotocols,complex structure/compositions,anddrug-releasemechanisms.Fromamanufacturingperspective, facileone-stepfabricationtechniquesaredesirableforalldrugformulationsdueto:simplified manufacturing,cost-effectiveness,andreduced batch-to-batchvariations [4].However,onestepmethodologiesarelimitedtoLC-PSsystems withamphiphilicpolymercoatings [106].Twosteptechniquesaremorecommonlyemployed forLC-PSfabrication,whichareassociated withdifficultiesmeetingqualitycontrolstandardsandscaling-upissuesforcommercialprocessing [107].Additionallimitationsexistfor coatinglipidnanocarrierswithsyntheticpolymers,suchaspoly(lactic-co-glycolicacid) (PLGA),sinceorganicsolventsaretypically employedtosynthesizeand/ordissolvethe polymerphasepriortoadsorptionontothelipid nanoparticlesurface [108].
2.3Polymer-corelipid-shell(PC-LS) systems
2.3.1Rationale
PWSDreleasefrompolymernanocarriersis matrix-dependentandreliesonerosionofthe polymernetworktofacilitatepartitioningof thedrugtowardtheaqueousphase [109].This typicallytriggersaslowandsustaineddrugreleasingmechanismthatisidealforpreventing saturationofthedrugwithintheGIT [110]. However,hydrophilicdrugsaresusceptibleto burstreleasemechanismswhencon finedwithin polymernanocarriers,sinceaqueousmediacan diffuseintothepolymermatrixandpromptthe outwarddiffusionofencapsulateddrugmolecules [111].Indoingso,thisservesasakeylimitationfortheuseofpolymersystemsin deliveringsolublebioactives.Asuccessful approachthatcanbeusedtosafeguardrapid andmassdrugleakagethroughdiffusionisto coatthepolymericnanocarrierswithalipid layer,whichservesasaphysicalbarriertothe aqueousenvironment [112].Furthermore,by preventingwaterpenetration,thelipidshell canretardpolymerdegradation,whilethepolymercorecanimpartstabilityandstructural integritytothelipidlayer [4].Thisintroduces theabilitytoinducesustainedandcontrolled drugreleaseforsolubledrugswhenencapsulatedwithinpolymernanocarriers,whichistypicallydependentonthedigestiblenatureandGI stabilityofthelipidlayeradsorbedonthepolymersurface.
2.3.2Synthesisapproach
SimilartoLC-PSsystems,fabricationofPCLSsystemscaninvolveeitheraone-stepor two-stepapproach.Themostcommonly employedmethodologyisanemulsionevaporationapproachwherebythepolymer andlipid,typicallyaphospholipidemulsifier, aredissolvedwithinawater-immisciblesolvent. Anemulsionisformedbyadditionofthe organicphasetoanaqueoussolution,triggering
theamphiphiliclipidstoself-assembleatthe polymer-in-waterinterfacetoimpartthermodynamicstabilitytotheemulsion [31].Thatis,the hydrophobiclipidtailattachestothepolymer core,whilethepolarheadgroupextendstoward theaqueousphase.Uponremovaloftheorganic solventthroughevaporation,precipitatedPC-LS nanoparticlesremain,whichcanbedried throughlyophilization [113].Thisone-step approachisconsideredfavorableoveralternate two-stepsynthesis,sinceittakesadvantageof conventionalemulsion-evaporationpolymer nanoparticlefabricationthatutilizesemulsifiers tostabilizetheorganicphase [32].Incontrast, two-stepfabricationrequiresseparatesynthesis ofthepolymernanocarrierusinganonlipid, ionicemulsifier,whichisthencoincubated withalipidphasecarryinganalternatecharge totheionicemulsifier,allowingfor electrostatic-mediatedself-assemblyofalipid layeratthepolymersurface.Regardlessofthe approachemployed,thesurfacechemistryof PC-LScolloidscanbeeasilycontrolledbythe chemistryandcompositionofthelipidemulsifier(s) [34,113].
2.3.3BiopharmaceuticaladvantagesofPC-LS systems
2.3.3.1Improveddrugencapsulation
Adsorbingalipidshellontopolymernanocarriersisaprovenwaytoincreaseencapsulation ef ficiencyanddrugloading,specificallyfor water-solubledrugs [114].However,thedegree thatPC-LSsystemsincreasedrugencapsulation isprimarilydependentonthefollowingtwofactors [4,115]:
(1) Synthesisapproach
Two-stepPC-LSfabricationapproachesmaybe consideredcounterproductiveforenhancing solubledrugencapsulationefficiency,since coincubationandself-assemblyofthe lipidshellontothedrug-loadedpolymer nanocarriermaytriggerwatertopenetratethe
polymercoreand,thus,outwarddiffusionof drugmoleculesintothebulkorganicphase [115].Incontrast,one-stepsynthesisof PC-LSsystemsrequiresthepolymer,lipid, anddrugtobedissolvedwithinawaterimmisciblesolvent.Uponemulsification,the drugisretainedwithinthepolymer/organic phase,whichisimmediatelycoatedbythe lipidshellandprotectedfromdiffusionprovokeddrugleakage [114,116].
(2) Drugphysicochemicalproperties
Theionicityandhydro/lipophilicityofthe therapeuticofinteresthasbeenshowntoplay afundamentalroleinitsencapsulation efficiencywithinPC-LSsystems.Cheowetal. [115] successfullydemonstratedthisby investigatingthepercentdrugencapsulation forthreedifferent fluoroquinoloneantibiotics withvaryingphysicochemicalproperties(i.e., levofloxacin,ciprofloxacin,andofloxacin)in phospholipid-coatedPLGAnanoparticles.It wasrevealedthatonlythetwozwitterionic compounds,levofloxacinandofloxacin,were successfullyencapsulatedwithinthePC-LS system,whereasthecationiccompound, ciprofloxacin,triggeredlargeaggregation betweenthepolymerandlipidphase.The percentagelevofloxacinandofloxacin encapsulationwasenhanced wtwofoldin thePC-LSsystem,comparedtothe uncoated,precursorPLGAparticles, demonstratingtheabilityofthelipidlayer toserveasaphysicalbarrierandprevent potentialleakageinthepreparationprocess [115].
2.3.3.2Sustaineddrugrelease
Preventingburstreleaseofdrugsencapsulatedwithinpolymericnanocarriersiscritical toimprovingoralbioavailability,especiallyfor pH-andenzyme-sensitivedrugs(e.g.,proteins andpeptides)thatundergodegradationwhen exposedtotheGIenvironment.Lietal. [94] exploredthepotentialforlipid-coatedchitosan
nanoparticlestosustainthereleaseandrestrict protease-mediateddegradationoforallyadministeredinsulin.After1and2hexposureto trypsinandchymotrypsin,respectively,unformulatedinsulinandinsulinencapsulatedwithin uncoatedchitosanparticlescompletely degraded.Incontrast,insulincon finedwithin lipid-coatedchitosannanoparticleswasprotectedfrom w40%to w60%trypsin-and chymotrypsin-induceddegradationafterthecorrespondingexposureperiods;highlightingthe importanceofthelipidcoronainpreventingoutwardandinwarddiffusionofinsulinandhydrolyticenzymes,respectively.Ultimately,this contributedtoa10-foldincreaseininsulinpermeationacrosstheintestinalepitheliacomparedto uncoatedchitosannanoparticles [94].
Lipidbilayershavealsosuccessfullyshown theabilitytoshieldsensitivecargowithin PLGAnano-andmicroparticles [30,114].Yu etal. [112] investigatedthepotentialforlipidcoatedPLGAmicroparticles(w210 mmindiameter)toimprovetheoraldeliveryefficacyofinsulinbyprotectingagainstchemicaland enzymaticbarriers.ThePLHparticlesdemonstratedexcellententrapmentefficiencyofover 90%,whichremainedstableovera3-monthstorageperiod.Furthermore,asustained-release mechanismwasinducedbythelipidbilayeron theparticlesurface,whichallowedforcontrolled releaseovera24hperiodinsimulatedintestinal conditions.Theprotectionofinsulinanda sustained-releasemechanismallowedforan w fourfoldimprovementinCaco-2cellularuptake, aswellasaprolongeddecreaseinbloodglycemiclevels,comparedtopureinsulin [112].
2.3.4Keytherapeuticsofinterest
Coatingpolymericnanoparticleswithalipid layeraffordstheabilitytoef ficientlyencapsulate anddeliverwater-solubledrugswithouttriggeringdiffusion-mediatedburstreleasemechanisms.Indoingso,acontrolled-release mechanismcanbeinduced,allowingforsustainedsystemicabsorptionandtherapeutic
response.Thiswasrecentlydemonstratedby Pateletal. [32] whopreparedlecithin-coated PLGAnanoparticlesfororaldeliveryofthehighlywater-solubledrug,cromolynsodium.Cromolynsodiumisusedforthetreatmentof multipleallergysymptomsbutisassociated withdose-dependentpharmacologyandtransientirritationwhenadministeredlocally(i.e., nasalandpulmonaryroutes).Subsequently,an improvedandidealdeliveryapproachforcromolynsodiumisviaacontrolledoraladministrationmechanismthatregulatesdrug concentrationsinsystemiccirculation.By loadingcromolynsodiuminlecithin-coated PLGAnanoparticles,drugreleasewasextended overa48hperiodinsimulatedGIconditions.In doingso,theoptimizedPLHformulationtriggeredan w seven-foldincreaseinexvivodrug permeationacrossratintestinaltissueandan 11.9-foldenhancementinoralbioavailability comparedtothepuredrug [32]
2.3.5LimitationsofPC-LSsystems
PoorstorageandGIstabilityarethefundamentallimitationsassociatedwithcoatingpolymericnanocarrierswithalipidlayer,sincePCLSsystemsarepronetoagglomerationand phaseseparation [115].Adsorbingioniclipids ontochargedpolymersurfacesinducesshorttermthermodynamicstabilitytothesystem, butfewstudieshavedemonstratedthelongtermstoragestabilityofPC-LSsystems.Subsequently,adryingmethod,suchasfreezedrying, istypicallyrequiredtoimpartstoragestabilityto PC-LSsystems,butacryoprotectantistypically requiredtoprotectthelipidphaseduringharsh dryingconditions [117,118].Additionally,lipids areliabletohydrolysisandoxidationwhen exposedtotheacidicandenzymaticconditions oftheGIT [119],whichcanleadtostructural rearrangementspriortoreachingthedesired siteofdrugrelease.Subsequently,limited studieshaveexploredthepotentialforPC-LS systemsfororaldrugdeliveryandhaveinstead
focusedmoresigni ficantlyonintravenous administration.
2.4Matrix-structuredpolymer lipid hybrid(matrix-PLH)systems
2.4.1Rationale
Encapsulationoflipidnanocarrierswithin particle-basedmatricesandscaffoldsaffords numerousphysicochemicalandbiopharmaceuticaladvantagesoverconventionalLBDDSs, includinggreaterthermodynamicandstorage stability,dosageform,controloverlipiddigestibility,andsubsequentdrugreleaseandsolubilization [120,121].Thekeyspecificinterestfororal deliveryistheabilitytocreateahybridformulation,wherebythedrugisencapsulatedwithin twoormorephasesforamulticomponentdeliverymechanism.Byformulatingmatrix-PLHsystems,itispossibletoencapsulateone,or multipledrugswithinboththelipidandpolymerphasesofthehybridsystem,whichhas beenshowntoimpartfavorablepharmacokineticpro files [15].Furthermore,inherentstorage stabilitychallengesassociatedwithnanoparticle deliverysystemshavelimitedtheirwidespread translationintoclinicalapplication [122].Nanoin-microhybridsystemsovercomestabilityissuesbystabilizingandcon finingthelipidnanocarriersystemwithinapolymermatrix,thereby transformingtheformulationintoasoliddosage formthatishighlystableunderstorageconditions [4,123].
2.4.2Synthesisapproach
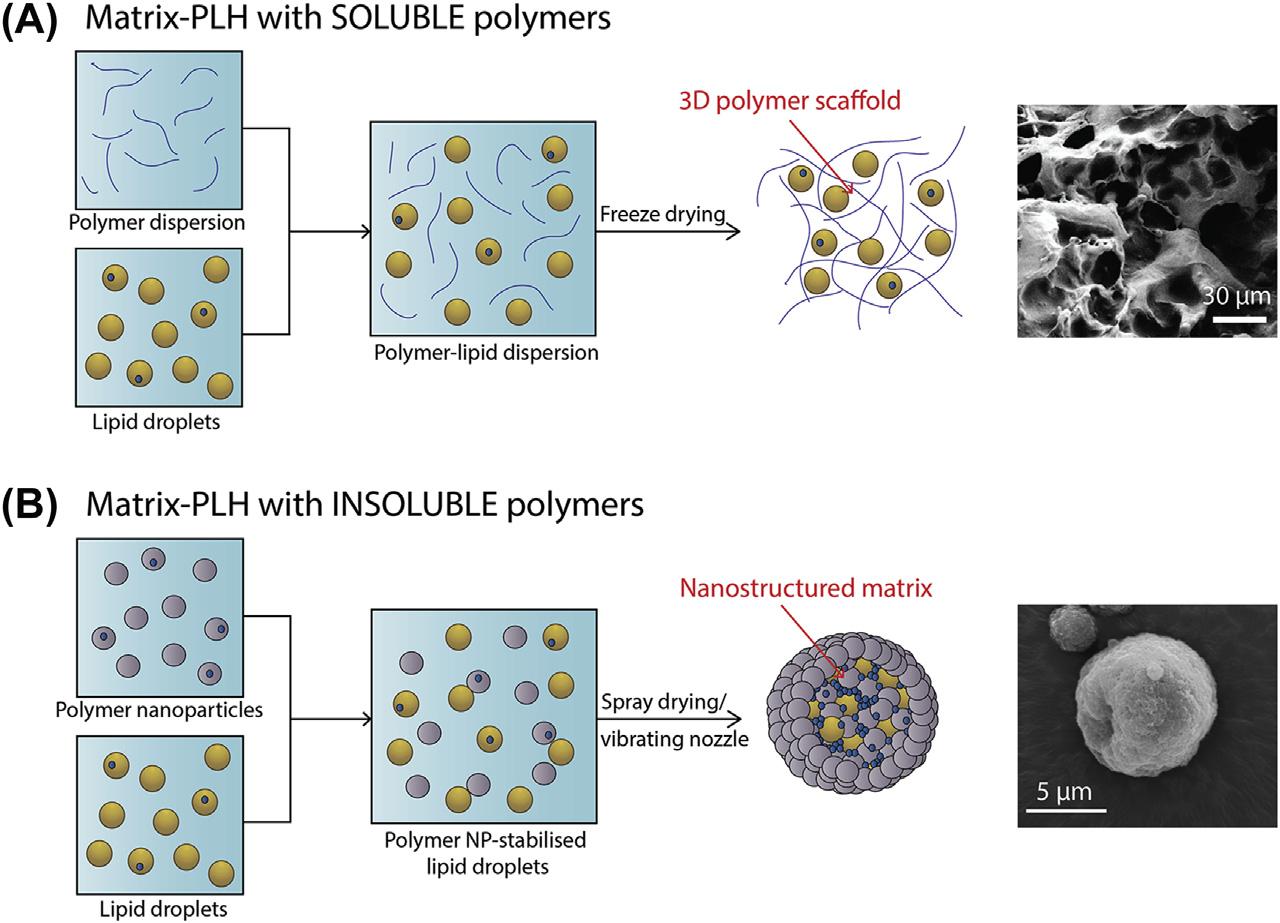
Thesynthesisapproachandtypeofpolymer utilizedinmatrix-PLHsystemsultimatelycontrolsthenanostructureoftheformulationand, therefore,carefulconsiderationshouldbeattributedtoformulationdesignpriortofabrication. Typically,matrix-PLHsystemsarecomposed oflipidnanocarriersencapsulatedwithinasemiporouspolymermatrix [20].Matrix-PLHfabricationcanbecategorizedbasedonthesolubilityof
FIGURE1.3 Schematicrepresentationofthesynthesisapproachandsubsequentnanostructureofmatrix-PLHsystems formedwith(A)solubleand(B)insolublepolymers. AdaptedwithpermissionfromJoyceP,PrestidgeCA.Synergisticeffectof PLGAnanoparticlesandsubmicrontriglyceridedropletsinenhancingtheintestinalsolubilisationofalipophilicweakbase.EurJPharm Sci2018;118:40 8;MaYH,YangJ,LiB,JiangYW,LuX,ChenZ.Biodegradableandinjectablepolymer liposomehydrogel:apromisingcellcarrier.PolymChem2016;7:2037 44.Copyright2018Elsevierand2016RoyalSocietyofChemistry.
thepolymerphase.Thatis,thepredominantsynthesisapproachesemployedinvolvecodispersionoflipiddropletsandeithersolubleor insolublepolymers,followedbyasuitable microencapsulationtechnique(Fig.1.3) [4].For solublepolymers,coincubationwithlipiddropletsistypicallyfollowedbylyophilization,formingathree-dimensionalpolymermatrixthat swellsanddeswellsinresponsetodispersion inaqueousmedia [103].Incontrast,lipiddropletsand(insoluble)polymernanoparticlesare preparedseparatelyandthendispersedtogether inaqueousmediatoformastabilizedlipidemulsion [68].Microencapsulationisachievedby eitherspraydryingthepolymer lipiddispersion [15,20,41] orutilizingavibratingnozzle technique[124 126].Indoingso,microparticles areformedwithnanostructurednetworks wherebylipiddropletsareencapsulatedwithin apolymernanoparticlematrix.Upon
redispersioninaqueousmedia,thisgroupof matrix-PLHbreaksdownintoindividualand smallagglomeratesofprecursorlipiddroplets andpolymernanoparticles [20].
2.4.3Biopharmaceuticaladvantagesof matrix-PLHsystems
2.4.3.1Bioactivitiestowarddigestiveenzymes
LipiddigestionwithintheGITiscontrolled bygastricandpancreaticlipases;twointerfaciallyactiveenzymesthathydrolyzetriglyceridesintomorepolarandabsorbablelipid species(i.e.,freefattyacids,mono-anddiglycerides) [128].Duetotheinterfacialnatureofthese enzymes,theirrelativeactivitiescanbemanipulatedthroughchangesininterfacialstructure andcomposition[129 131].Thishasimportant implicationsfordrugdeliverysincesolubilizationkineticsofdrugsencapsulatedwithin
LBDDSs,especiallythosethatarepoorlywatersoluble,canbecontrolledbytherateandextent ofGIlipolysis [9].Thatis,theonsetoflipasemediatedhydrolysistriggerstheconcurrent releaseofdigestionproductsanddrugmoleculesintotheaqueousenvironment.Freefatty acidsandglyceridesformmixedmicellesand variouscolloidalvesiclesthatcanfurthersolubilizethedrugandtherebypromoteabsorptionof thedissolveddrugacrosstheintestinalepitheliumandintothesystemicbloodstream [132]. Thus,itispossibletocontrolandenhancedrug absorptionandbioavailabilitybyengineering LBDDSsthatcontroltheactivitiesoflipase enzymes.
Amultitudeofinnovativematrix-PLHsystemshavedemonstratedtheabilitytomanipulate lipase-mediateddigestionthroughtheconfinementoflipiddropletswithinathreedimensionalpolymernetwork [20,68,105,124] Severalstudieshaveestablishedthatthepolymer chemistryandnanostructurearetheintegral physicochemicalpropertiesofhybridsystems thatcontrollipiddigestion [20,133 136].This wasemphasizedinarecentstudywhereby PLGA lipidhybridmicroparticleswerefabricatedbyspraydryingalipidemulsionstabilized witheitherpositivelychargedornegatively chargedPLGAnanoparticles [20].Forbothsystems,spraydryinginducedathree-dimensional matrixstructurewherebythesubmicronlipid dropletswereconfinedwithinaPLGAnanoparticlenetwork.Invitrolipolysisstudies,performed undersimulatedintestinalconditions,revealed thattheformationofhybridparticlesreduced theinitialrateofdigestion,comparedtotheprecursoremulsions,irrespectiveofsurfacecharge. Thiswasattributedtotherestrictedabilityfor lipasetoaccessandsubsequentlyhydrolyzetriglyceridesatthelipid-in-waterinterface.However,theextentoflipiddigestionwasgreaterthan theprecursoremulsions,duetothereducedinterferenceeffectofsurface-activedigestionproducts withinthehybridformulations.Thiseffectwas maximizedforthenegativelychargedPLGA
lipidhybridsystemduetotheelectrostaticrepulsionbetweenthenegativelychargedfreefatty acidsandthenegativelychargedPLGAnanoparticlesurface.Incontrast,positivelycharged PLGA lipidmicroparticlesretainedahigherdegreeofdigestionproductswithinthematrix structure,whichenabledgreaterimpedimentof lipiddigestion [20].
Extensiveworkhasalsobeenperformedto controllipiddigestionbyencapsulatinglipid nanoparticleswithinnonporousbiopolymer matrices [135,137,138],includingalginate [46,133,139],chitosan [140 143],andstarch [134,144,145].Lietal. [139] preparedlipid-filled calciumalginatebeadsbycoincubatingalipid nanoemulsionwithinanalginatesolution,prior tostepwisedroppingthedispersionintoan aqueouscalciumchloridesolution.Encapsulatingthelipiddropletswithinthealginatematrixservedasahighlyeffectivemethodfor inhibitinglipiddigestion,asdemonstratedby an w14-foldreductioninlipolysiscomparedto freedropletsandalginatebeadsinsolution. Lipiddigestionkineticswerecomparablebetweenfreelipiddropletsintheabsenceandpresenceofalginatebeadsintheaqueousphase. Subsequently,itwashypothesizedthatthesurfacechemistryofalginatedidnotimpedeon lipaseactivity.Rather,thegelalginatematrix inthePLHsystemincreasedthediffusionpath lengthoflipasetotheencapsulatedlipiddroplets,aswellasthesubsequentreleaseofdigestionproducts,whichsubsequentlydelayedand retardedlipolysis [139].Furthermore,lipasemediateddigestionkineticswereshowntobe dependentonthelipiddropletsizewithinthe alginatehydrogels.Thatis,therateandextent offreefattyacidreleasewasgreatestforsmaller lipiddropletsduetotheincreaseininterfacial surfaceareaandthus,bioaccessibilityofthelipid droplets.Thisstudysucessfullydemonstrated theroleandinfluenceofmatrix-PLHnanoarchitectureonlipiddigestionkinetics,whereby smallchangesinstructureandcompositioncan triggersignificantchangesinthereleaseoffree
2.Structureandcompositionofpolymer
fattyacidsandencapsulatedbioactive compounds.
Readersaredirectedtowardthefollowing excellentreviewsthatcriticallydetailand discussthevariousengineeringapproaches thatcanbeemployedtofabricatebiomaterials withcontrolledactivitiestowarddigestiveenzymes [84,120,121,146 148].
2.4.3.2Multifunctionaldrug-releasemechanisms
Formono-therapy,deliveringtherapeutics throughtwodifferentmechanismsmayserve asafavorableapproachto(1)increasedrugabsorptionacrosstheintestinalepithelium,(2)alter solubilization,dissolution,andabsorptiondynamics,and(3)improvepharmacokineticprofiles [149].Joyceetal. [15] recentlyexploredthe
multifunctionaldrug-releasemechanismofa matrix-PLHformulation,composedofsubmicronlipiddropletscon finedwithinaPLGA nanoparticlematrix,intheoraldeliveryofcinnarizine.Cinnarizinewasencapsulatedwithin boththelipidandpolymerphases,whichtriggereddual-componentdrugdissolutionkinetics invitro.Thatis,undersimulatedintestinal digestion,drugreleasefromsubmicronlipid dropletswasrapid,whichwasfollowedbya sharpdecreaseinsolubilizationduetopHinduceddrugprecipitation.Incontrast,drugsolubilizationfromPLGAnanoparticleswasslow andsustained,withonly w22%ofthedrugbeingreleasedoverthecourseof2h.Drugrelease andsolubilizationfromthePLGA lipidsystem mimickedacombinationofthesetwosystemsas
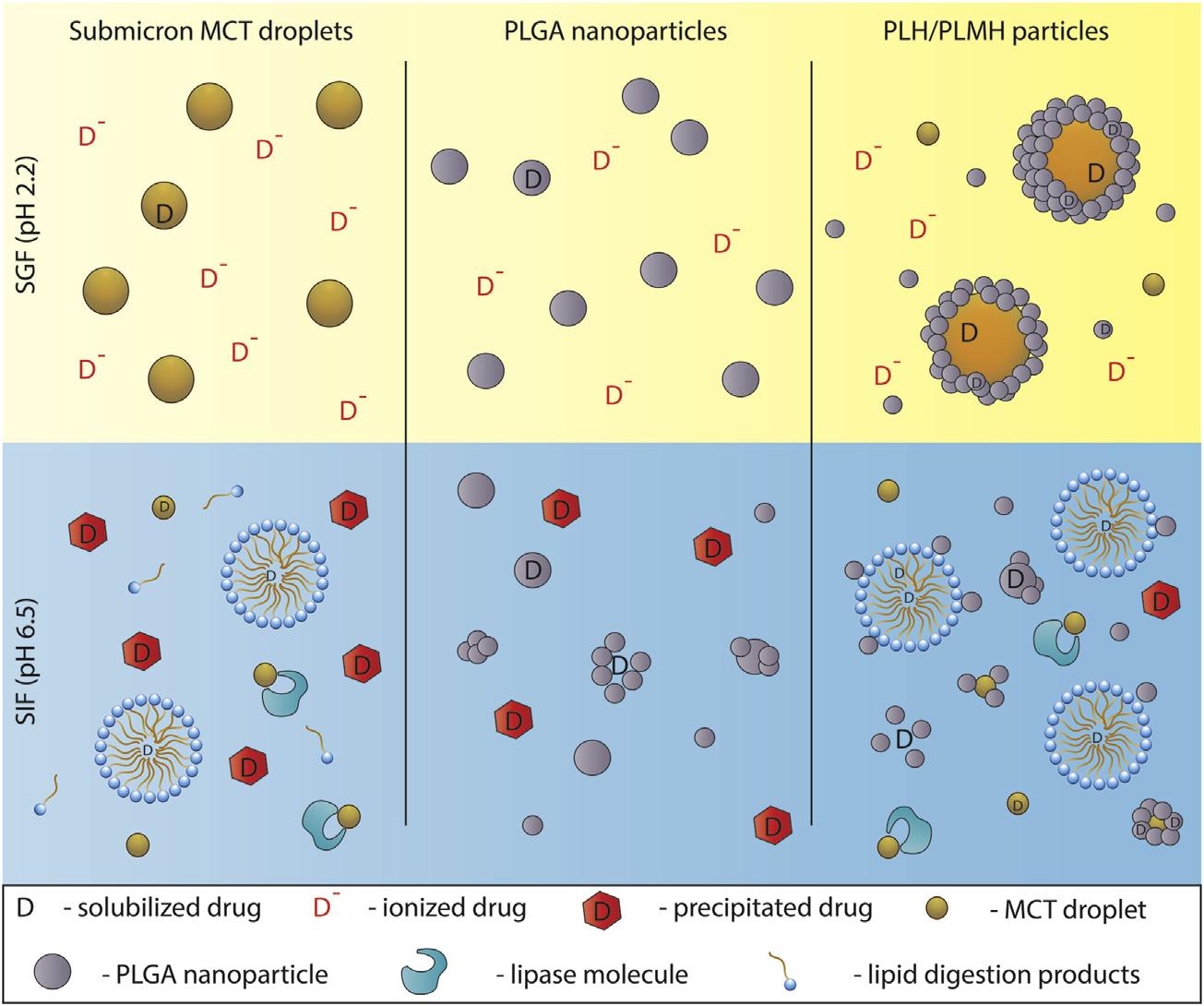
FIGURE1.4 Schematicrepresentationofmulticomponentreleasemechanismofamatrix-PLHsystem,incomparisontothe precursorlipidnanoemulsionsandPLGAnanoparticles,whencinnarizinewasencapsulatedwithinboththelipidandpolymer phase. UsedwithpermissionfromJoyceP,PrestidgeCA.SynergisticeffectofPLGAnanoparticlesandsubmicrontriglyceridedropletsin enhancingtheintestinalsolubilisationofalipophilicweakbase.EurJPharmSci2018;118:40 8.Copyright2018Elsevier.