DeliveringLow-Carbon Biofuelswith BioproductRecovery
Editedby LakhveerSingh
DepartmentofEnvironmentalScience,SRMUniversity-AP, Amaravati,India
DurgaMadhabMahapatra
TERIDeakinNanobiotechnologyCenter(TDNBC),TERI,India
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2021ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress ISBN:978-0-12-821841-9
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher:BrianRomer
AcquisitionsEditor:GrahamNisbet
EditorialProjectManager:RubyGammell
ProductionProjectManager:PoulouseJoseph
CoverDesigner:MatthewLimbert
TypesetbyMPSLimited,Chennai,India
ListofContributors..................................................................................................xi
CHAPTER1Electricalenergyproducedbymicrobialfuelcells usingwastewatertopoweranetworkofsmart sensors .......................................................................... 1
P.M.D.SerraandA.Esp´ırito-Santo
1.1 Introduction....................................................................................1
1.2 Microbialfuelcells........................................................................4
1.2.1Microbialfuelcellstheoreticalanalysis.............................4
1.2.2Energyextractionfrommicrobialfuelcells......................6
1.3 Energyproduction,regulationandstorage..................................21
1.3.1Energyregulationandstorage..........................................21
1.4 Smartsensorstructureandoperation...........................................25
1.5 Conclusions..................................................................................27 Acknowledgments.......................................................................27 References....................................................................................28
CHAPTER2Applicationofbioelectrochemicalsystemsin wastewatertreatmentandhydrogenproduction ....... 31 SanthanaKrishnan,AbudukeremuKadier,MohdFadhilBin MDDin,MohdNasrullah,NurulNazleatulNajiha, ShazwinMatTaib,ZularisamAbWahid,YuYouLi,YuQin, KamalKishorePant,ShreesivadasanChelliapan, HesamKamyab,ImranAhmadandLakhveerSingh
2.1 Introduction..................................................................................31
2.2 MECfundamentalsandworkingprinciples................................32
2.3 Electrontransfermechanism........................................................34
2.4 MECtechnologyinhydrogenproductionusingwastewater......35
2.5 Agrowastewater...........................................................................36
2.6 Domesticwastewater..................................................................36
2.7 Industrialwastewater....................................................................38
2.8 Fermentationeffluent...................................................................38
2.9 NutrientandheavymetalsremovalsinMEC.............................39
2.10 IntegratedMECapproach............................................................40
2.11 Conclusions..................................................................................42 Acknowledgments.......................................................................42 References....................................................................................42 v
CHAPTER3Nutrientremovalandrecoveryin bioelectrochemicalsystems ....................................... 45 AryamaRaychaudhuriandManaswiniBehera
3.1 Introduction..................................................................................45
3.2 Nitrogenremovalandrecovery...................................................47
3.2.1Issuesrelatedtoconventionaltechnologies.....................48
3.2.2Nitrogenremovalinbioelectrochemicalsystem..............49
3.2.3Ammoniarecovery............................................................59
3.2.4Challengesinnitrogenremovalandrecovery..................62
3.3 Phosphorusremovalandrecovery...............................................66
3.3.1Issuesrelatedtobiologicalphosphorusremoval.............66
3.3.2Struviteprecipitation.........................................................68
3.3.3Phosphorusremovalandrecoveryin bioelectrochemicalsystem................................................69
3.3.4Challengesinphosphorusremovalandrecovery.............71
3.4 Conclusionandfutureperspectives.............................................74
CHAPTER4Roleofbioelectrochemicalsystemsfor bioremediationofwastewatersandbioenergy production ................................................................... 85 MuhammadFaisalSiddiqui,ZahidUllah,LakhveerSingh, FarhanaMaqbool,SadiaQayyum,IhsanUllah, ZiaurRahmanandFazalAdnan
4.1 Introduction..................................................................................85 4.2 Principleofbioelectrochemicalsystems.....................................86 4.3 Kindsofbioelectrochemicalsystems..........................................87
4.3.1Microbialfuelcells...........................................................87
4.3.2Microbialelectrolysiscellsforenergy.............................88
4.3.3Microbialelectrosynthesisforenergyproduction............88
4.3.4Enzymaticfuelcellsforenergyproduction.....................88
4.3.5Microbialsolarcellsforenergyproduction.....................89
4.3.6Plantmicrobialfuelcellsforenergyproduction.............89
4.3.7Microbialdesalinationcellsforenergyproduction.........90
4.4 Roleofbioelectrochemicalsystemsinremediationof pollutants......................................................................................90
4.4.1Remediationoforganicxenobiotics.................................90
4.4.2Treatmentofinorganicpollutants....................................92
4.5 Sustainabilityofthetechnology..................................................93
4.6 Scalingupofthetechnology.......................................................94
4.7 Conclusion....................................................................................94
Acknowledgments.......................................................................95 References....................................................................................95
CHAPTER5Energygenerationfromfish-processingwaste usingmicrobialfuelcells ........................................ 101
A.R.AbdulSyukor,SuryatiSulaiman,JadhavPramod Chandrakant,PuranjanMishra,MohdNasrullah, LakhveerSinghandA.W.Zularism
5.1 Introduction................................................................................101
5.2 NationalGreenTechnologyPolicy............................................103
5.2.1Wastefromfreshmarkets...............................................103
5.2.2Fish-processingwastewatercharacteristics....................105
5.3 Microbialfuelcellsystem..........................................................107
5.3.1Substratesusedinmicrobialfuelcell.............................109
5.3.2Fish-processingwasteassubstrate.................................109
5.4 Treatmentmethodologyoffish-wasteusingmicrobialfuel cell(aMalaysiancasestudy).....................................................110
5.4.1Preparingthesubstrate....................................................110
5.4.2Testingforphysical,chemical,andbiological parameters.......................................................................110
5.4.3Electrode.........................................................................111
5.5 Resultsobservation....................................................................113
5.5.1Voltageproduction..........................................................113
5.5.2Biochemicaloxygendemandremoval...........................115
5.5.3Chemicaloxygendemandremoval................................115
5.5.4Nitrogen...........................................................................116
5.5.5Phosphorous....................................................................118
5.6 Conclusion..................................................................................119 References..................................................................................120
CHAPTER6Microbialelectrosynthesis:Recoveryof high-valuevolatilefattyacidsfromCO2 .................. 123 NarnepatiKrishnaChaitanya,AkashTripathiand PrithaChatterjee
6.1 Introduction................................................................................123
6.2 Basicprincipleofmicrobialelectrosynthesiscell.....................124
6.3 Factorsaffectingproducttiter....................................................124
6.3.1TheeffectofpH..............................................................125
6.3.2Fluctuationsinelectricitysupply....................................126
6.3.3Impactofinoculum.........................................................127
6.3.4Electrodematerials..........................................................128
6.3.5Effectofelectrodepotential...........................................129
6.3.6Effectofreactordesign...................................................130
6.4 Strategiestoimproveproducttiter............................................131
6.5 Economicevaluation..................................................................134
6.6 Futurescopeofwork.................................................................136
6.7 Conclusion..................................................................................137
CHAPTER7Lowcarbonfuelsandelectro-biocommodities ....... 143
BahaaHemdan,S.BhuvaneshandSurajbhanSevda
7.1 Introduction................................................................................143
7.2 Workingmechanismofbioelectrochemicalsystems................144
7.3 Applicationofmicrobialelectrochemicaltechnologiesin wastewatertreatment..................................................................146
7.4 Electro-biocommoditiesandvalue-addedbiochemical’s production...................................................................................148
7.4.1Biohydrogenproduction.................................................149
7.4.2Biomethaneproduction...................................................149
7.4.3Bioethanolproduction.....................................................151
7.4.4Acetateproduction..........................................................152
7.4.5Hydrogenperoxideproduction.......................................153
7.4.6Othervalue-addedbiochemicalproduction....................153
7.5 Recentprogressforelectro-biocommoditiesgenerationina bioelectrochemicalsystem.........................................................154
7.6 Conclusion..................................................................................156 Acknowledgment.......................................................................156 References..................................................................................157
CHAPTER8Potentialofhighenergycompounds:Biohythane production ................................................................. 165
SurajbhanSevda,VijayKumarGarlapati,SwatiSharma andT.R.Sreekrishnan
8.1 Introduction................................................................................165
8.2 Mainaspectsofthebiohythanegenerationin bioelectrochemicalsystem.........................................................166
8.3 Substrateforbiohythanegeneration..........................................167
8.4 Recentprogressforbiohythanegenerationin bioelectrochemicalsystem.........................................................169
8.5 Useofbiohythane......................................................................172
8.6 Futureprospectsandconcludingremarks.................................173
Acknowledgment.......................................................................173 References..................................................................................173
CHAPTER9Biologicalandchemicalremediationoftreated woodresidues ........................................................... 177
LaisGonc¸alvesdaCosta,YonnyMartinezLopez, VictorFassinaBroccoandJuarezBenignoPaes
9.1 Introduction................................................................................177
9.2 Environmentalrisksoftreatedwood.........................................178
9.3 Remediationandrecoveryoftreatedwood...............................180
9.3.1Bioremediation................................................................180
9.3.2Mechanismsusedbyfungiintheremediation process.............................................................................183
9.3.3Chemicalremediation.....................................................186
9.4 Concludingremarks...................................................................189 References..................................................................................189
CHAPTER10Anoverviewondegradationkineticsoforganic dyesbyphotocatalysisusingnanostructured electrocatalyst .......................................................... 195 RishuKatwal,RichaKothariandDeepakPathania
10.1 Introduction................................................................................195
10.2 Organicdyes...............................................................................196
10.3 Classificationoforganicdyes....................................................196
10.4 Methodsfortheremovalofpollutants......................................196
10.5 Advancedoxidationprocesses...................................................197
10.6 Photocatalysis.............................................................................198
10.7 Photocatalysts.............................................................................200
10.8 Photocatalystsurfacemodifications..........................................201
10.9 Kineticsofphotocatalyticdegradation......................................202
10.10 Photocatalyticreactionparameters............................................203
10.11 Photocatalyticactivityofnonmetalsandmetalloids supportednanophotocatalyst......................................................204
10.12 Photocatalyticactivityofpolymersupported nanophotocatalyst.......................................................................206
10.13 Conclusions................................................................................207 References..................................................................................207 Index......................................................................................................................215
ListofContributors
A.R.AbdulSyukor
FacultyofCivilEngineeringTechnology,UniversitiMalaysiaPahang(UMP), Kuantan,Malaysia
FazalAdnan
AttaurRahmanSchoolofAppliedBiosciences,NationalUniversityofSciences &Technology,Pakistan
ImranAhmad
DepartmentofEngineering,RazakFacultyofTechnologyandInformatics, UniversitiTeknologiMalaysia,JalanSultanYahyaPetra,KualaLumpur, Malaysia
ManaswiniBehera
SchoolofInfrastructure,IndianInstituteofTechnologyBhubaneswar, Bhubaneswar,India
S.Bhuvanesh
Director’sResearchCell,CSIR-NationalEnvironmentalEngineeringResearch Institute,Nagpur,India
VictorFassinaBrocco
CenterforHigherStudiesofItacoatiara,AmazonasStateUniversity(CESIT/ UEA),Itacoatiara,Brazil
NarnepatiKrishnaChaitanya
DepartmentofCivilEngineering,IndianInstituteofTechnologyHyderabad, Hyderabad,India
JadhavPramodChandrakant
FacultyofCivilEngineeringTechnology,UniversitiMalaysiaPahang(UMP), Kuantan,Malaysia
PrithaChatterjee
DepartmentofCivilEngineering,IndianInstituteofTechnologyHyderabad, Hyderabad,India
ShreesivadasanChelliapan
DepartmentofEngineering,RazakFacultyofTechnologyandInformatics, UniversitiTeknologiMalaysia,JalanSultanYahyaPetra,KualaLumpur, Malaysia
LaisGonc¸alvesdaCosta
DepartmentofForestandWoodScience,FederalUniversityofEsp´ıritoSanto, Jero ˆ nimoMonteiro,Brazil
A.Esp´ırito-Santo
DepartmentofElectromechanicalEngineering,UniversityofBeiraInterior, Covilha ˜ ,Portugal;IT—InstituteofTelecommunications,Covilha ˜ ,Portugal
MohdFadhilBinMDDin
CentreforEnvironmentalSustainabilityandWaterSecurity(IPASA),Research InstituteofSustainableEnvironment(RISE),SchoolofCivilEngineering, FacultyofEngineering,UniversitiTeknologiMalaysia,Skudai,Malaysia
VijayKumarGarlapati
DepartmentofBiotechnologyandBioinformatics,JaypeeUniversityof InformationTechnology,Waknaghat,India
BahaaHemdan
DepartmentofBiosciencesandBioengineering,IndianInstituteofTechnology Guwahati,Guwahati,India;WaterPollutionResearchDepartment, EnvironmentalResearchDivision,NationalResearchCentre,Giza,Egypt
AbudukeremuKadier
LaboratoryofEnvironmentalScienceandTechnology,TheXinjiangTechnical InstituteofPhysicsandChemistry,Key LaboratoryofFunctionalMaterialsand DevicesforSpecialEnvironments,ChineseAcademyofSciences,Urumqi,China
HesamKamyab
DepartmentofEngineering,RazakFacultyofTechnologyandInformatics, UniversitiTeknologiMalaysia,JalanSultanYahyaPetra,KualaLumpur, Malaysia
RishuKatwal
Departmentofchemistry,CSKHPKV,Palampur,India
RichaKothari
DepartmentofEnvironmentalSciences,CentralUniversityofJammu,Bagla (Rahya-Suchani),Samba,Jammu&Kashmir,India
SanthanaKrishnan
CentreforEnvironmentalSustainabilityandWaterSecurity(IPASA),Research InstituteofSustainableEnvironment(RISE),SchoolofCivilEngineering, FacultyofEngineering,UniversitiTeknologiMalaysia,Skudai,Malaysia
YuYouLi
DepartmentofCivilandEnvironmentalEngineering,GraduateSchoolof Engineering,TohokuUniversity,Sendai,Japan
YonnyMartinezLopez
DepartmentofForestandWoodScience,FederalUniversityofEsp´ıritoSanto, Jero ˆ nimoMonteiro,Brazil
FarhanaMaqbool
DepartmentofMicrobiology,HazaraUniversity,Mansehra,Pakistan
PuranjanMishra
FacultyofCivilEngineeringTechnology,UniversitiMalaysiaPahang(UMP), Kuantan,Malaysia
NurulNazleatulNajiha
CentreforEnvironmentalSustainabilityandWaterSecurity(IPASA),Research InstituteofSustainableEnvironment(RISE),SchoolofCivilEngineering, FacultyofEngineering,UniversitiTeknologiMalaysia,Skudai,Malaysia
MohdNasrullah
FacultyofCivilEngineeringTechnology,UniversitiMalaysiaPahang(UMP), Kuantan,Malaysia
JuarezBenignoPaes
DepartmentofForestandWoodScience,FederalUniversityofEsp´ıritoSanto, Jero ˆ nimoMonteiro,Brazil
KamalKishorePant
DepartmentofChemicalEngineering,IITDelhi,NewDelhi,India
DeepakPathania
DepartmentofEnvironmentalSciences,CentralUniversityofJammu,Bagla (Rahya-Suchani),Samba,Jammu&Kashmir,India;DepartmentofChemistry, SardarVallabhbhaiPatelClusterUniversity,Mandi,HimachalPradesh,India
SadiaQayyum
DepartmentofMicrobiology,HazaraUniversity,Mansehra,Pakistan
YuQin
DepartmentofCivilandEnvironmentalEngineering,GraduateSchoolof Engineering,TohokuUniversity,Sendai,Japan
ZiaurRahman
DepartmentofMicrobiology,AbdulWaliKhanUniversityMardan,Khyber Pakhtunkhwa,Pakistan
AryamaRaychaudhuri
SchoolofInfrastructure,IndianInstituteofTechnologyBhubaneswar, Bhubaneswar,India
P.M.D.Serra
DepartmentofElectromechanicalEngineering,UniversityofBeiraInterior, Covilha ˜ ,Portugal;IT—InstituteofTelecommunications,Covilha ˜ ,Portugal
SurajbhanSevda
DepartmentofBiotechnology,NationalInstituteofTechnologyWarangal, Warangal,India
SwatiSharma
DepartmentofBiotechnologyandBioinformatics,JaypeeUniversityof InformationTechnology,Waknaghat,India
MuhammadFaisalSiddiqui
DepartmentofMicrobiology,HazaraUniversity,Mansehra,Pakistan
LakhveerSingh
DepartmentofEnvironmentalScience,SRMUniversity-AP,Amaravati,India
T.R.Sreekrishnan
DepartmentofBiochemicalEngineeringandBiotechnology,IndianInstituteof TechnologyDelhi,NewDelhi,India
SuryatiSulaiman
FacultyofCivilEngineeringTechnology,UniversitiMalaysiaPahang(UMP), Kuantan,Malaysia
ShazwinMatTaib
CentreforEnvironmentalSustainabilityandWaterSecurity(IPASA),Research InstituteofSustainableEnvironment(RISE),SchoolofCivilEngineering, FacultyofEngineering,UniversitiTeknologiMalaysia,Skudai,Malaysia
AkashTripathi
DepartmentofCivilEngineering,IndianInstituteofTechnologyHyderabad, Hyderabad,India
IhsanUllah
DepartmentofBiologicalSciences,FacultyofScience,KingAbdulaziz University,Jeddah,SaudiArabia
ZahidUllah
DepartmentofMicrobiology,HazaraUniversity,Mansehra,Pakistan
ZularisamAbWahid
FacultyofCivilEngineeringTechnology,UniversitiMalaysiaPahang(UMP), Kuantan,Malaysia
A.W.Zularism
FacultyofCivilEngineeringTechnology,UniversitiMalaysiaPahang(UMP), Kuantan,Malaysia
Electricalenergyproduced bymicrobialfuelcellsusing wastewatertopowera networkofsmartsensors
P.M.D.Serra1,2 andA.Esp´ırito-Santo1,2
1DepartmentofElectromechanicalEngineering,UniversityofBeiraInterior,Covilha,Portugal 2IT—InstituteofTelecommunications,Covilha,Portugal
1.1 Introduction
Humanpopulationisthriving,anditsnumberscontinuetogrow,evenindevelopingcountries,whereitcouldreach9billionpeopleby2050(WWAP,2014).Our planet’snaturalresourcescontinuebeingexploredand,morefrequentlythannot, irresponsiblyspent.Insomedevelopedcountries,technologicaladvancementsare beingthoughtofwithenvironmentallyfriendlyconcernsgaininggroundtodisposablesolutionsandcounterbalancingnegligentbehavior.Food,water,and energysuppliesarealsobeingstudiedforadequatemanagement,beingthemost basicnecessitiesforthesustainabilityanddevelopingoflife.Foodcannotbeproducedwithoutwater;watercannotbemadeavailablewithoutenergy;andenergy andelectricityproductionareextremelylimitedwithoutwater.Thiscloseinterconnectioncreatedanewresearchtopic,referredtoastheenergy-waternexus. Consideringcurrenttrendsofresourcedepletionandclimatechanges,waterand energywillleveragefoodproduction,resourcesustainability,andtechnology development,balancingsupplyanddemand(USDepartmentofEnergy,2014).
Thebusinessofenergyimpliesmoremoneythanthewatercounterpart:direct costswithenergyarerelatedtoexploration,treatmentorgeneration,distribution andenvironmentaltaxeswhilewater-relatedcostsaremostlyconnectedwith treatmentanddistribution,sincewaterisafreeendogenousrawmaterial.Ontop ofthis,energyexpendituredataisricherthanwaterexpenditureanddistribution (Walshetal.,2015).By2012,around700millionpeopledidnothaveaccessto animprovedwatersource,while1.3billionpeoplehadnoaccesstoelectricity (Walshetal.,2015;Halsteadetal.,2014).Allthesefactorshaveasignificant influenceonpolicies,ensuingstricterenergymanagementproceduresthanwater regulations.However,acloserlookatenergyorwaterexpendituredatashows theirrelationship,andrecentdatapointstotheneedofapplyingsimilarcontrol strategiestohinderresourceandwaterscarcity.Waterisusedinalmostevery
sectorofhumanactivity,butfromthemall,theenergysectorcomesinsecond. Onlywateruseforirrigatedculturescomesfirst.InEurope,in2015,of247,000 millioncubicmetersofwater,44%wasspentonenergyproduction.Forenergyrelatedactivities,waterisusedforfuelextraction,refining,andprocessing.It alsoplaysasignificantpartonthecoolingofthermalpowerplants,accounting for50%ofthetotaloffreshwaterwithdrawalsintheUnitedStatesandover10% inChina.Itisalsovitalforbioenergyandbiofuelfeedstockcrops,anenergy solutioneasilybelievedtobeeco-friendlyandthatisestimatedtoachieve7.5% ofglobalelectricityproductionby2050(Walshetal.,2015;Halsteadetal.,2014; Wakeeletal.,2016;HightowerandPierce,2008).
Waterextraction,distribution,treatment,anddisposalareverydifferentin countrieswithandwithoutlimitedenergyaccess.By2025,800millionpeople willliveinwater-scarceregionsandaround65%oftheglobalpopulationwill liveinseverewaterstressconditions.By2050,waterusedforirrigationwillbe surpassedbythewaterwithdrawnforenergy,industrialprocesses,andmunicipal applications.Energyisneededtoextractandconveywater,fromsourcetodestination,anddependsmostlyontopography,distanceandtherelationshipbetween thesource’svolumeofwaterandtheamountneededatthedestination(small aquifersimplymoreenergyforwaterpumping).Watertreatmentalsodealswith largeamountsofenergytoconvertwastage,rain,orseawatertoausefulandsafe version,bothforhumansandfortheenvironment:thedirtierthewater,themore energyisneededtocleanit(Walshetal.,2015;Halsteadetal.,2014;Wakeel etal.,2016;doAmbiente,2012;Copeland,2014).
Theconceptofenergy-waternexuswasfirstexploredby Gleick(1994).For sometime,sincethen,severaleventshavehighlightedtheneedforstricterregulationsinwatermanagement.In2001,therewasasignificantperiodofwatershortageinCalifornia,inaneventknownas“TheCalifornianEnergyCrisis.”Political decisionstakenatthetimefavoredshort-termhumancomfortindetrimentforthe environmentandamoresustainablewatermanagement.Thisincident,andthe likesofthese,showthatifnopoliciesareappliedonwatermanagement,the onsetofwaterscarcitywillhappensooner.China,theMiddleEast,NorthAfrica, andSpainaretheregionsatmostperil.Simplemeasureslikeefficientwater appliancesandreducedleaksinwaterdistributionareagoodwaytostart. Sourcingwaterbodyfarfromitsdestination,moreenergywillbespentonwater transport.ThisishappeningallacrossEurope,makingwatersupplymoreenergy intensiveintheseterritoriesthaninAsiancountries.Trailingthis,governments shouldinvestonaugmentingthewatersupplywithadditionalsources,treating andreusingstormwater,andinvestigatingtechnologicalsolutionsforfreshwater production,withreverseosmosiswaterrecyclingsystemsanddesalinationplants. Onpowerplants,alternativewatersourcescanbeusedforcoolingandwastewatercanalsobeusedforenergyrecovery(Walshetal.,2015;Wakeeletal.,2016; Hamicheetal.,2016).
InEurope,legislationisinplaceforenergymanagement,withtheEuropean directive2012/27/EU(the“EnergyEfficiencyDirective”)andtheadoptionofthe
ISO50001.Thisdirectiveestablishesenergyutilizationtargets,enforcesperiodical energyauditsandrequiressustainedupgradesonenergymanagementanddeliverytools.Forwater,similardirectivesarebeingstudiedandimplemented.The waterfootprintassessmentisaconcept,verysimilartothecarbonfootprint,used toquantifythewaterusedbasedonlifecycleassessments(LCA)andimplementedwithaninternationalstandard,theISO14046.TheISO14046evaluatesthe waterexpenditureanditsimpactontheenvironmentindifferentlifecycles stages,informingusersandindustryplayersofthewaterimpactsoftheiractivitiesandchoices(Walshetal.,2015;InternationalOrganizationfor Standardization,2017).
ByadoptingtheISO50001,severalorganizationsshowedsignificantdecreases onenergyandwaterusagelevelsaswell.InIreland,forinstance,theUniversity ofCork,whichadoptedthestandardin2011,sawan18%reductionofthespent water,thoughanincreasedstudentactivitywasregisteredduringthatyear.This decreasecouldhavebeenhigherifthestandardhadsimilarmeasuresforwater relatedissues,likeleakages,oldpipes,faultymetersandoperationofsanitary facilitiesinlowoccupationperiods.Theimplementationofthissamestandardby CocaColaresultedina10%decreaseinwaterand16.5%inenergyconsumption (Walshetal.,2015;Johnsonetal.,2012).
Followingthepreviousbackgrounddiscussion,thischapterdiscussestheviabilityofproducingelectricityasabyproductofwastewaterprocessing.Thisdiscussion allowstodemonstratethattheextractedenergycanbemadeusefultopowersensors thatintegrateanetworkofsmarttransducerscompatiblewiththeIEEE1451standard.Thisisaclearexampleoftheinterconnectionthatispossibletoachieve betweentwoessentialgoods,suchasenergyandwater:orthewater-energynexus.
Aftertheintroductorysection,thechapterdescribestheoperationofamicrobialfuelcell(MFC)andtheenergyconversionprocessassociatedwithit,which useswastewatertoproduceenergy,while,atthesametime,removestheorganic contentofthewater. Section1.3 demonstratestheabilitytosimultaneouslyobtain twoproductsofhighfinancialandenvironmentalvalue:electricityandwater withaloworganicmattercontent.Awastewatertreatmentplantcanthusbeseen notonlyasanenergyconsumer,butalsoashavinganenergyproductionpotential associatedwithitsoperation.AnMFC’soperationefficiencydependsontheits operationmode.Asfarassubstrateflowisconcerned,therearetwopossibilities: batchprocessingorcontinuousprocessing.
FortheMFC’sbatchoperatingmode,amaximumoperatingpointisidentified in Section1.3.Theefficiencyoftheconversionprocessdependsofseveralfactors:theelectricalloadobservedbytheMFC,itstemperatureofoperation,or organicmatteravailabilitytothebacteriaareafewexamples.Theseparameters willchangecontinuouslywithtimeandcontributetochangetheoperatingpoint continuously.ToincreasetheextractionofenergyfromtheMFC,atracking mechanism,thatadjuststheelectricalloadtotheMFC’sinternalimpedance, needstobeadopted.Properregulationandstorageoftheharvestedenergywill supporttheoperationofasmartsensornetwork.
Inthischapter,theenergyproductionprocessisassociatedwithanartificial wetland,aswhatoccursinsidetheMFCissimilartosomestagesinwastewater treatmentprocesses.Ontheotherhand,thistypeofinfrastructureislocatedoutsidelargeurbancenters.Intheseplaces,energyavailabilityisreduced,whichis whytheproductionofenergyinsituisanasset.Atthesametime,intermsof monitoring,thegeographicalareatobecoveredisvast.Consideringtheprevious arguments,thechapterproposes,in Section1.4,theuseoftheenergyproduced bytheMFCtopowerwirelesssensornodesthatintegrateasensornetwork,and thatcollecttheinformationnecessaryforthemanagementofthetreatmentprocess.Thesensornetworkthusbecomesenergyindependentasitobtainsitspower fromtheprocessitself.Thisavoidstheneedtouseconventionalbatteries,and theirreplacementwork,whichcanbedifficulttoperform,duetothehighnumber ofnetworknodes,andbecausetheycanbeplacedindifficultordangerouslocationsinsidethetreatmentplant.Theapplicationscenariowillillustratewhatdata needstobeacquiredatthewastewatertreatmentplant(waterlevels,pH,ORP, O2),andthenintroducetheIEEE1451standardanditsusefulnessinthedevelopmentofthesolution.
1.2 Microbialfuelcells
1.2.1
Microbialfuelcellstheoreticalanalysis
Balancebetweenenergyproductionandexpenditureisfragile.Althoughanoverallenergysurplusisdesirable,energydeficitconditionsarefarmoreproneto occur.Thedevelopmentofwastewaterusealternatives,namelyforenergyproduction,cantipthescales.MFCsbeganbeingexploredwiththeknowledgegatheredfromtraditionalfuelcelltechnologyandthepreviouslymentionedgoal. Thesedevicesoperateonoxidation-reductionreactions,manipulatedinawaythat theproducedelectronscanberedirectedforenergyproduction.
FuelcellresearchbeganwiththeworksbyWilliamGrovein1839.Onlyafter 1950didthetechnologyreachadevelopmentleveladequateforindustryuse. Specifically,forthefirstAmericanspaceprograms,followedbythesameprograms inJapanandinEurope.Environmentalconcernssteeredstudiesonefficiencyoptimizationandreducedemissionsinmorerecentyears.Astheseareasentaildifferent knowledgeareas,aplethoraoffuelcelltypologiesrose.Theelectrolytenatureultimatelydeterminesthefuelcelltype,sinceallfuelcellssharethesamefunctional elements:ananode,acathode,andanelectrolyte(thatcanbesolidorliquid) (LarminieandDicks,2003;Scottetal.,2016).
MFCsareoneofthosespecificfuelcelltypeswheretheelectrolytecanbe anywastewatertype:domestic,piggerywastewater,brewerywastewater,and dyeingwastewaterareafewexamples(SerraandEspirito-Santo,2016).For MFCs,theanodealsohasamajordifferencefromotherfuelcelltypes:itmust becolonizedbyexoelectrogenicanaerobicbacteria.Infact,nousefulenergy
productionispossiblefromMFCsbeforetheanodicbiofilmisadequatelydevelopedandmatured.Thebacterialcolonywillbesolelyresponsibleforconverting thewastewaterorganicsource(biomoleculesasglucoseandacetate)toenergy. Thereactorappliesconditions,anaerobiosis,suchthatbacterianeedboththeelectrodestofinishthemetabolicpathwayfromwhichtheyproduceenergy.The overallreactioncanbedescribedasfollows(Serraetal.,2020a):
1. Theelectrolyteorganicsourceissequesteredbybacteria.
2. Throughanaerobicrespiration,theorganicsourceisconvertedtocarbon dioxideandprotons,withconcomitantelectronproduction.
3. Thefinalacceptoroftheseelectrons(O2)ismadeavailableatthecathode;the anodeandcathodeareconnectedoutsideoftheelectrolyte,andthecathode materialischosensothatithasahigherpotentialthantheanode;thisultimately forcestheexoelectrogenicbacteriatodelivertheelectrontotheanode.
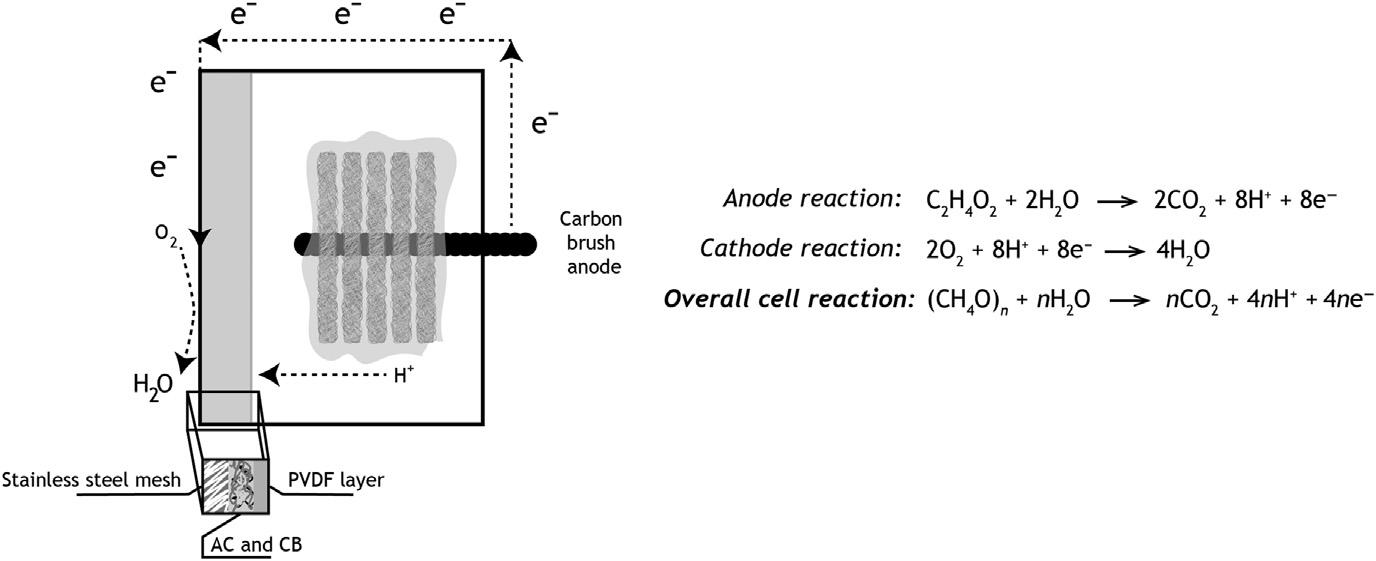
Theimageon Fig.1.1 presentsagraphicaloverviewofthisprocess,highlightingtheoverallchemicalreaction.
ThereareseveraltypesofMFCs,sortedbyreactortypeorelectrodedisposition[Serraetal.(2020b) tobepublishedin InstrumentsandExperimental Techniques,Springer].Theuseofsingle-chamberreactorsprovideamoreattractivesolutionforincreasedpowergenerationsincedecreasedinternalresistances havebeenregisteredinsuchconfigurations(Fanetal.,2008).Thebestperformancepairfortheelectrodesistohaveabrushanodeandaplanarcathode.The brushshapegreatlyincreasesthereactionarea,whiletheplanarcathodeissuccessfulinguaranteeingthereactorwatertightness.Thissetisalsoveryusefulfor fixingtheelectrodedistance,whichhasbeenfoundtobeoptimized,forenergy production,for1cmbetweenthelastbrushbristlesandthecathodesurface
FIGURE1.1
Agraphicalrepresentationofamicrobialfuelcellwithadetailofthecathodecomposition. AC,Activatedcarbon; CB,Carbonblack.Thegoverningequationoftheoperationprocess isalsopresented,atright.Detailsonthiscanbefoundin Serraetal.(2020a).
(WatsonandEstadt,2007).Whenrelatingthecathodeproductioncost,durability andpowerproductioncapabilities[asitisthebottleneckelementforMFCpower production(Fanetal.,2008)],theactivated-carbonaircathodesproducedin (Yangetal.,2014)presentasatisfactorycompromise.Theresearchteamofthis workiscurrentlyworkingwithsingle-chamberair-cathodereactors,asdescribed in Serraetal.(2020a),thatfollowtheaforementionedrecommendations.
1.2.2 Energyextractionfrommicrobialfuelcells
1.2.2.1
Generalprinciple
Themaximumvoltageproducedbyafuelcellcorrespondstoitselectromotive force,or Eemf ,derivedfromtheNernstequation:
In Eq.(1.1), Ecat representsthecathodepotentialand Ean theanodepotential.To determinethesepotentials,thereactionequation,theconcentrationofeachoxidizingandreducingagent,andthespecifictemperatureareneeded.Whenknown, theelectrodepotentialscanbededucedfrom Eqs.(1.2)and(1.3):
where, E 0 cat and E 0 an representtheelectrodepotentialinstandardconditions (298.15K,1barpressure,1Mforallspecies), R correspondstotheuniversalgas constant, T isthetemperatureinKelvin,and L isthereactionquotient.
ForMFCs,consideringapHof7,andthegeneralgoverningequationin Fig.1.1,thetheoretical Eemf is1.1V.Thistheoretical Eemf value,however,cannotbeachievedbecauseMFCs,asotherfuelcells,inherentlyloseenergydueto, forinstance,unbalancedproportionsbetweenreactantsandproducts,thereversibilityoftheoxidation-reductionreaction,thecell’sinternalresistance—aresult fromthematerialsandgeometrieschosenforthereactor,thebacterialprofile, andthesubstratecomposition.AllthesevariablesplayaroleontheMFC’slosses andintroduceamplevariabilityandcontroldegrees.
TheMFCenergylossesneedtobeminimizedtoincreasethedevice’senergy production.Todoso,theyfirstneedtobeidentifiedandmeasured.Thisstepis crucialifefficientadjustmentsaretobeapplied.Measuresofthecell’svoltage andcurrentwithrespecttoexternalresistanceandelectrode’spotentialasafunctionoftimearekeystoneforsuchstudies.PolarizationreportsarethemostfundamentalstudiesthatcanbeconductedonanMFC.Thecellcanbeanalyzedall altogetherorstudiescanbemadeindependentlytoeachelectrode.Forprobing thecell,usingapotentiometerandavoltmeter,thoughsimpleandextremely
accessible,produceslowdetaileddataandcanbeacumbersomeprocess,whether duetothelackofprecisioninthemagnitudeofchangesintheexternalload,or duetothelackoftimeprecisionofthosechanges.Adigitalpotentiometerand adigital-to-analogconverter(DAC)canhelpimprovethereliabilityofsucha methodevenmorewhencombinedwithamicroprocessor.Dataretrievedthrough thismethodwillallowforanadequatecellelectriccharacterization,althoughnot usefuliftheanalysisofbacterialchangesareofinterest.Whenneedingtoconductthesestudies,theuseofapotentiostatismoreadequate.Theresultsofusing thismeasuringinstrumentgowellbeyondtheelectricalcharacterizationofan MFCandcanalsocontributetotheelectrochemicalstudyofthecell’selectrodes andmicrobialcommunities.Allandall,apotentiostatcanretrievetheelectrochemicalactivityofmicrobialstrains,determinethestandardredoxpotentialsof redoxactivecomponents,testtheperformanceofcathodematerials,acquire polarizationcurves,quantifythe(overpotentialsandohmic)lossesofanMFC, conductcurrentinterrupttechniques,electrochemicalimpedancespectroscopy, linear,differentialpulse,cyclicvoltammetry,andchronostudies,namelychronoamperometryandchronopotentiometry(Loganetal.,2006;Logan,2012;Zhao etal.,2009).Nonetheless,thepotentiostatusefulnessandthefollowingdiscussion willconsiderpolarizationstudiesconductedwithapotentiometerandaDACona mbedLPC1768duetotheirprice,accessibility,andeaseofuse.
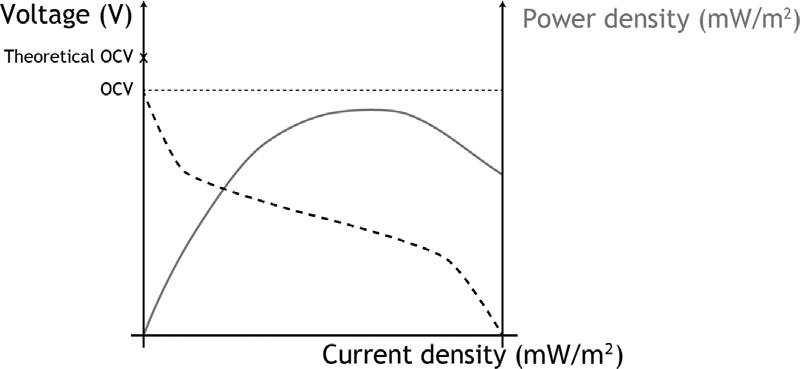
Atypicalpolarizationcurveisshownin Fig.1.2.
Thiscurvecanbebuiltbyapplyingasingle-cycleormultiple-cyclemethod. Asingle-cyclemethodisusedwhenseveralresistancechangesaremadeduring onbatchofwastewater,orifthecellisoperatedincontinuousflow.Multiplecyclemethodsareappliedinfedbatchreactorsandimplyaresistancechangeper batch,wherethenumberofbatcheswillbedependentontheresolutionneeded forthepolarizationcurve.Acommonoccurrencefoundinpolarizationcurvesis powerovershoot.Apowerovershootbehaviorcanbeidentifiedafterthemaximumpowerpointinpowergraphs,whentwodifferentpowerdensityvaluescan betracedbacktothesamecurrentdensity.Itisusuallyassociatedwiththeanode andimmaturebiofilms;theanodiccommunityisunabletomaintaintheincreasingelectrondemand;orinadequatemeasurementprocedureswithresistance
FIGURE1.2 Anexampleofapolarizationcurve.
changestooquickthatdonotallowthebiofilmtoadjust.Thiscanhappenfor anyreactortype,geometry,orevenforreactorsoperatedasastack.Tobypass suchevent,multiple-cyclemethodsarethebestchoice,since,providedtheadequatereadinessofthebiofilm,fullacclimationtotheexternalloadonthereactor isguaranteed(Logan,2012;Prakashetal.,2010;Vicarietal.,2017;Watsonand Logan,2011;Boghanietal.,2013;Winfieldetal.,2011;Hongetal.,2011).The weightanddiscussionofeachoftheMFClossesidentifiableinapolarization curvecanbefoundin(Serraetal.,2020a).Polarizationcurvesarefundamentalto adequatelycomparetheperformanceofdifferentMFCsetups.
1.2.2.2 Improvingenergyproductionfrommicrobialfuelcells
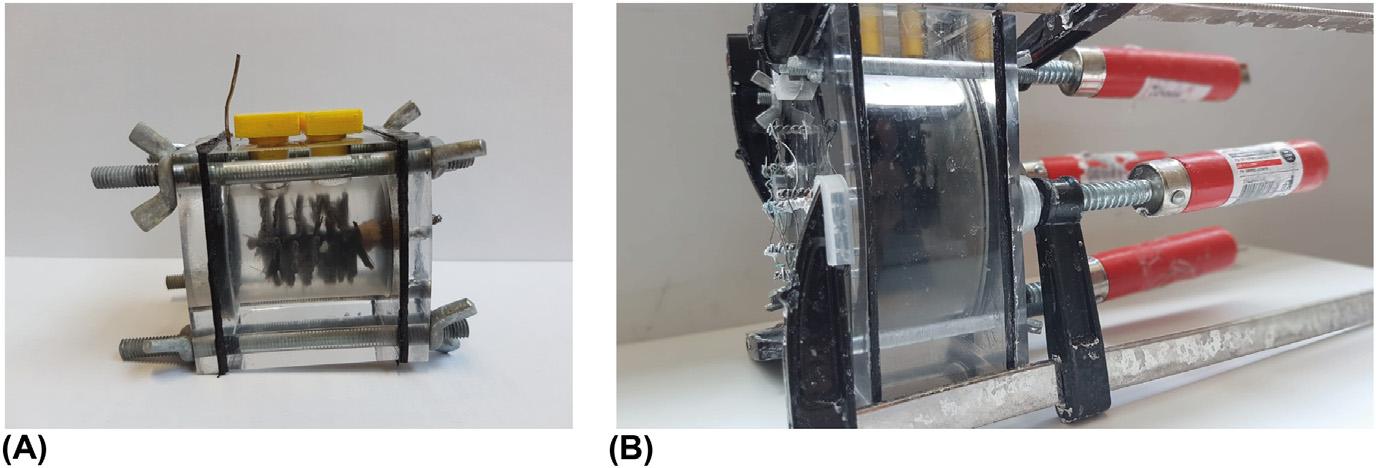
ThepowerproductioncapabilitiesofMFCsaredependentonamyriadoffactors, namelyonreactorandelectrodecharacteristics,andsubstratecomposition.To makeanunblemishedcomparison,thenextthreesubsectionsandrespective assumptionsaresupportedonpolarizationdataconductedontwotypesofreactors,picturedin Fig.1.3:smallvolumereactors, Rx,andbigvolumereactors, RBigx,where x denotesthereactornumber.
Theteamhasaccesstosixsmalltypereactorsandtwobigvolumereactors. Bothreactorssharethesameelectrodes:acarbonbrushanodeandanactivatedcarbonair-cathode,asdescribedinSection2.1.Theirinnerchamberalsohasthe samerectangularshapealthoughwithdifferentsizes.Theshapewaskeptinorder tomaintainthebestperformingelectrodedistance. Rx typereactorshaveanempty bedvolumeof28mL,whilethe RBigx topologyaccomplishesalmostninetimes thatvolume,reaching250mL.Anotherdifferencebetweenthetworeactortypesis ontheelectrodenumberandarea.ThesmallMFCshaveoneelectrodeofeach type,withacathodeareaof7cm2.Thebiggerversionhassixinterconnected anodes—disposedinapentagonshapewithacentralpoint—andalsoasinglecathode.Theinterconnectedanodesworkasasingleelectrodeandthelargecathodehas atotalareaofapproximately64cm2.Thisdataissummarizedin Table1.1.
FIGURE1.3
Ontheleft(A),apictureofoneofthesmallvolume(Rx)MFCs.Ontheright(B),the pictureofabigvolumereactor(RBigx). MFC,Microbialfuelcell.
Table1.1 Summaryofthemicrobialfuelcelltypesusedforpower improvementstudies.
Reactor type Anode (number) Cathode (number) Cathode area(cm2)
Emptybed volume (mL) Electrode distance
7281cm,measured fromtheanode’s lastbristles
Big: RBigx Carbon brush(6) 64250
Small: Rx Carbon brush(1) Activatedcarbonaircathode (1)
Intotal,thenumberofreactorsavailabletotheteamissix Rx (R1toR6)and two RBigx (R7andR8).
Thedataforbuildingthepolarizationcurveswasretrievedbythemulti-cycle method,throughvoltagemonitoringonasetofsevendifferentresistancesconnectedbetweeneachelectrodeofanindividualMFC(orbetweenelectrodesof differentreactorswhentestingforseriesandparallelassociations).Theloadsetis comprisedofresistancesof1000,500,200,100,68,50,and20 Ω.TheDACon anmbedLPC1768wasprogrammedfordataacquisitionevery2minutes.The retrieveddatapointswerefurtherprocessedinMATLAB2018b.Thevaluesused foreverypolarizationcurvecorrespondtothemaximumvalueretrievedfromthe hourlyaverageofthe2minutesacquiredvoltagevalues.Thisprocessingstep guaranteesanadequateshieldingfrommeasurementartifactsthatmayoriginate onpowerfluctuationsofmicroprocessorand/orloadconnectiondefects.
Thetrialsconductedforcontinuousversusintermittentmodeofoperationare notpolarizationcurvesandusethe2minutesacquiredvalues.Thediscussionon thistrialtypewillfollowintheappropriatesection.
Whicheverthetrial,thereactorswerealwaysfedwithanartificialwastewater (AW)preparation,a50mMsodiumphosphatebufferatpH7.Thefeedingsolutionfollowsthecomposition:
• CH3COONa—1g/L;
• NaH2PO4.2(H2O)—3.12g/L;
• Na2HPO4—4.26g/L;
• NH4Cl—0.31g/L;and
• KCl—0.13g/L.
Asolutionwithsuchcompositionhasaround9.8JofenergypermL (Eq.1.5),whichcanbeprovedbyusingtheorganicsourceconcentration,the equationdescribingtheenergyproductionprocess(Eq.1.4)andtheindividual standardGibbsfreeenergyofformation,displayedin Table1.2:
Thisvaluewillbeusedtoaccuratelypinpointtheefficiencyofeachtrial.
Table1.2 SummaryofthestandardGibbsfreeenergyofformationper compoundof Eq.(1.4)
1.2.2.2.1 Thebenchmark:polarizationcurveonasmallvolume microbialfuelcell
Thepolarizationtrialon Fig.1.4 hasbeenretrievedonasmallvolumereactor, R6.Theanodicbiofilmhas674daysandthecathodeappliedwasfreshlyproduced,withoutanyaerobicbiofilmdeposition.Theinoculationprocedurefollowedthemethodologyavailableon Serraetal.(2020a).Thisdatawillbeused tobenchmarkalltheothertrials.
Theanalysisofthedataon Fig.1.4 showsthatamaximumpowerdensityof 822mW/m2 (0.58mW)wasproducedat0.34mA/cm2 or100 Ω.Byapplyingthe maximumpowertransfertheory,thisloadvaluecanbeusedtotracethereactor’s internalresistance(Serraetal.,2020a).Alowerpowerdensitythanthecurve wouldanticipateisnoticeableatabout0.4mA/cm2.This,however,isnotcorroboratedbythetotalenergyextracted,asshownin Fig.1.5.
Theanalysisofthetotalextractedenergyperload,consideringtrialsrununtil substratedepletion,alsoshowsthatthemaximumenergyharvesteddoesnot occurattheloadformaximumextractedpower.Infact,betweenthetwoloads (200and100 Ω),moreenergyisextractedatthelowestcurrentvalue.Thedifferencecorrespondstoapproximately0.05J/h,wherethetrialfor200 Ω runfor 21hoursandthetrialfor100 Ω for17hours,bothuntilcurrentvaluesremained over0.1mA.Themaximumefficiencyofthereactorwasdeterminedtobe8.9%, with24.5Jextractedfrom28mLoftheAW(274.4J).
1.2.2.2.2 Increasingthesizeofthereactor
Todetermineifanincreaseinthereactorvolumecanberelatedwithanincrease inproducedpower,apolarizationtrialwasrunonabigvolumereactor,RBig8, andispresentedwith Fig.1.6.AswiththetrialonR6,theloadwasuninterruptedlyconnectedbetweentheelectrodesuntilsubstrateexhaustion.
Incomparisonwiththesmallvolumereactor,allthevoltagevaluesarehigher, althoughcurrentandpowerdensityfiguresarelower.As Table1.3 shows,the samecannotbesaidforabsolutepowerandcurrentvalues,whichpresenthigher valuesthanthesmallvolumereactorforloadsunder100 Ω,inclusive.Thecurve shapehintsthatthepolarizationrunloadvalueswerenotadequatetofindthe reactor’sinternalresistanceand,consequently,itsmaximumpowerproduction
Polarization curve for benchmarking: small reactor, R6.
FIGURE1.4
Polarizationcurveforasmallvolumereactor,R6,withafreshcathodeandananodic biofilmof674days(overone-and-a-halfyears).
FIGURE1.5
Stackedviewofthepowercurveandextractedenergyvaluesperloadforthesmall volumereactor,R6.
capabilities.Thelowestloadvalueontheset,20 Ω,seemstobeanoverestimationofthereactor’sinternalresistance.Nevertheless,andfor20 Ω,themaximum powerfoundforthetrialwasof487mW/m2 (3.1mW).As Fig.1.7 clearlyrepresents,thetotalextractedenergywashigherfor68 Ω,207J,whichalsocorroboratestheconclusionforthetrialwiththesmallreactor.
Polarizationcurveforabigvolumereactor,RBig8,withafreshcathodeandananodic biofilmof415days(over1year).
Thevolumerelationshipbetweenthetworeactortypesisninefold.However, neitherpowernorextractedenergyincreasedinthatproportion.Infact,power densitydecreasedandthereactorperformanceatthemaximumenergyextraction was5.8%lower.Atthemaximumpowerproductionload,thedifferencewas even3.3%higher,at9.1%.Noninefoldproportionwasfoundbetweenthereactor volumeincreaseandanyotherenergyproductionparameter,althoughtheanode numberandcathodesurfacewereincreased.
1.2.2.2.3 Seriesandparallelassociation
Anotherstrategythatcanbeusedtoimprovetheenergyproductionlevelsfrom MFCsistheseriesorparallelassociationoftworeactors.Theoretically,considering tworeactorsastwoequalvoltagesources,theirseriesassociationwillimprovethe totalvoltageoftheset,whiletheparallelgroupingwillcontributetohighercurrent levels.Thechoiceofthebestassociationwillultimatelyrelyontheapplication. Nevertheless,andconsideringthe100 Ω internalresistanceofthesmallreactors foundwiththebenchmarkingtrial,theseriesassociationisexpectedtoproducethe highestpowerlevelsfor200 Ω andtheparallelcombinationat50 Ω.Accordingto thisexpectation,seriesandparalleltrialswereconductedonlyforthreeloadvalues: 200,100,and50 Ω.Thebiofilmforthesetrialshasthesametime,674days.
TheseriestrialdatapointsaresuperimposedonthesmallMFCpolarization curvein Fig.1.8.
Thepolarizationcurveispresentedwithabsolutevaluesofpowerandcurrent sincenoassumptioncanbemadeonthereferenceareaforreaction:thereareno guaranteesthatthereactorsperformedthesamewayand,therefore,thecathode areacannotbedoubledonthatassumption.
Polarization curve for big volume reator: RBig8.
FIGURE1.6
Table1.3 Summaryonthedatausedtobuildtheplotssupportingtheconclusionsrelatedwithpowerimprovementintrialswith biggervolumereactors,seriesandparallelassociationandcommutedloadconnection.
VariableUnitTrial
CathodeDays0
Thistableshouldnotbeusedasreferencewithoutthetextreferringtoit. aCommutedloadoperation. bAftercommutedloadoperation. benchmark. 1 continuousoperation.
Stackedviewofthepowercurveandextractedenergyvaluesperloadforthesmall volumereactor,RBig8.
Polarizationcurveofthesmallreactor(R6)anddatapointsforseriesassociationoftwo smallvolumereactors(R5andR6).
Overall,theseriesassociationoftworeactorsproducedhighervoltagevalues, exceptfor50 Ω.Asfarascurrentisconcerned,thevalueswereapproximatelythe sameforasinglereactorortwoseriesconnectedMFCs.Thesedatapointsseemto pointthat,forlowerloads,theseriesbehaviorapproachestheperformanceofasingleMFC.Asfaraspowerisconcerned,duetothehighervoltagesresultingfrom theseriesassociation,valuesarealsohigherforhigherloads.Infact,forthe200 Ω load—correspondingtotheseriesassociationoftworeactorswithaninternalload
FIGURE1.7
Polarization data comparison between a single small reactor and a series association of 2 small volume reactors.
FIGURE1.8