https://ebookmass.com/product/current-trends-and-futuredevelopments-on-bio-membranes-reverse-and-forward-osmosisprinciples-applications-advances-angelo-basile/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Current Trends and Future Developments on (Bio-) Membranes Angelo Basile
https://ebookmass.com/product/current-trends-and-future-developmentson-bio-membranes-angelo-basile/
ebookmass.com
Current Trends and Future Developments on (Bio-)
Membranes: Recent Advances on Membrane Reactors Angelo Basile
https://ebookmass.com/product/current-trends-and-future-developmentson-bio-membranes-recent-advances-on-membrane-reactors-angelo-basile/
ebookmass.com
Current Trends and Future Developments on (Bio-) Membranes: Silica Membranes: Preparation, Modelling, Application, and Commercialization Angelo Basile (Editor)
https://ebookmass.com/product/current-trends-and-future-developmentson-bio-membranes-silica-membranes-preparation-modelling-applicationand-commercialization-angelo-basile-editor/
ebookmass.com
Football Fandom in Europe and Latin America: Culture, Politics, and Violence in the 21st Century Bernardo Buarque
https://ebookmass.com/product/football-fandom-in-europe-and-latinamerica-culture-politics-and-violence-in-the-21st-century-bernardobuarque/
ebookmass.com
A
Daughter Forged in Fire (Chronicles of the Tuatha, Book One) Jessica Leigh
https://ebookmass.com/product/a-daughter-forged-in-fire-chronicles-ofthe-tuatha-book-one-jessica-leigh/
ebookmass.com
Conversion to Islam in the Premodern Age: A Sourcebook
Nimrod Hurvitz
https://ebookmass.com/product/conversion-to-islam-in-the-premodernage-a-sourcebook-nimrod-hurvitz/
ebookmass.com
Career Development and Counseling: Theory and Practice in a Multicultural World (Counseling and Professional Identity) 1st Edition, (Ebook PDF)
https://ebookmass.com/product/career-development-and-counselingtheory-and-practice-in-a-multicultural-world-counseling-andprofessional-identity-1st-edition-ebook-pdf/ ebookmass.com
Investment Analysis & Portfolio Management 11th Edition
Frank Reilly
https://ebookmass.com/product/investment-analysis-portfoliomanagement-11th-edition-frank-reilly/
ebookmass.com
Rights As Security: The Theoretical Basis Of Security Of Person 1st Edition Rhonda Powell
https://ebookmass.com/product/rights-as-security-the-theoreticalbasis-of-security-of-person-1st-edition-rhonda-powell/
ebookmass.com
My First Knock-Knock Jokes Jimmy Niro
https://ebookmass.com/product/my-first-knock-knock-jokes-jimmy-niro/
ebookmass.com
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
©2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(other thanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,or fromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN: 978-0-12-816777-9
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionEditor: KostasKIMarinakis
EditorialProjectManager: VincentGabrielle
ProductionProjectManager: AnithaSivaraj
CoverDesigner: MathewLimbert
TypesetbySPiGlobal,India
Contributors
FynnJ.Aschmoneit TechnicalUniversityofDenmark,Kgs.Lyngby,Denmark
AngeloBasile InstituteonMembraneTechnology,ITM-CNR,Rende,Italy
MuhammadRoilBilad ChemicalEngineeringDepartment,UniversitiTeknologi PETRONAS,BandarSeriIskandar,Malaysia
CristianoP.Borges ChemicalEngineeringProgram,AlbertoLuisCoimbraInstitutefor GraduateStudiesandResearchinEngineering(COPPE),TechnologyCenter,Federal UniversityofRiodeJaneiro(UFRJ),RiodeJaneiro,Brazil
AlfredoCassano InstituteonMembraneTechnology,ITM-CNR,Rende,Italy
M.Cunnington CSIRO,Pullenvale,QLD,Australia
SirshenduDe DepartmentofChemicalEngineering,IndianInstituteofTechnology Kharagpur,Kharagpur,India
RaminFarnood DepartmentofChemicalEngineeringandAppliedChemistry,University ofToronto,Toronto,ON,Canada
GimunGwak SchoolofCivil,EnvironmentalandArchitecturalEngineering,Korea University;CenterforWaterResourceCycleResearch,KoreaInstituteofScienceand Technology,Seoul,RepublicofKorea
ClausHelix-Nielsen TechnicalUniversityofDenmark,Kgs.Lyngby,Denmark
SeungkwanHong SchoolofCivil,EnvironmentalandArchitecturalEngineering,Korea University,Seoul,RepublicofKorea
G.P.SyedIbrahim MembraneTechnologyLaboratory,DepartmentofChemistry, NationalInstituteofTechnologyKarnataka,Surathkal,India
ArunM.Isloor MembraneTechnologyLaboratory,DepartmentofChemistry,National InstituteofTechnologyKarnataka,Surathkal,India
DavidInhyukKim SchoolofCivil,EnvironmentalandArchitecturalEngineering,Korea University,Seoul,RepublicofKorea
SihangLi CollegeofFoodScienceandEngineering,NorthwestA&FUniversity,Yangling, China
ZhenyuLi CollegeofFoodScienceandEngineering,NorthwestA&FUniversity,Yangling, China
ShengLi GuangzhouInstituteofAdvancedTechnology,ChineseAcademyofSciences, Guangzhou,China
SouravMondal DepartmentofChemicalEngineering,IndianInstituteofTechnology Kharagpur,Kharagpur,India
SaraR.Osipi ChemicalEngineeringProgram,AlbertoLuisCoimbraInstitutefor GraduateStudiesandResearchinEngineering(COPPE),TechnologyCenter,Federal UniversityofRiodeJaneiro(UFRJ),RiodeJaneiro,Brazil
NavinK.Rastogi DepartmentofFoodEngineering,CentralFoodTechnologicalResearch Institute,CouncilofScientificandIndustrialResearch,Mysore,India
ArgimiroR.Secchi ChemicalEngineeringProgram,AlbertoLuisCoimbraInstitutefor GraduateStudiesandResearchinEngineering(COPPE),TechnologyCenter,Federal UniversityofRiodeJaneiro(UFRJ),RiodeJaneiro,Brazil
S.Su CSIRO,Pullenvale,QLD,Australia
R.Thiruvenkatachari CSIRO,Pullenvale,QLD,Australia
DasTrishitman DepartmentofFoodEngineering,CentralFoodTechnologicalResearch Institute,CouncilofScientificandIndustrialResearch,Mysore,India
YusufWibisono BioprocessEngineeringDepartment,UniversitasBrawijaya,Malang, Indonesia
Preface
Thepracticalapplicationofreverseosmosis(RO)wasrealizedinCaliforniaintheearly 1960s.Sincethen,thedevelopmentofnew-generationmembranes,suchasthethin-film compositemembraneswithhighlyimprovedwaterfluxandsoluteseparationcharacteristics,hasresultedinmanyROapplications.Inadditiontothetraditionalseawaterand brackishwaterdesalinationprocesses,ROmembraneshavefoundusesinwastewater treatment,productionofultrapurewater,watersoftening,foodprocessing,andmany others.Asignificantdevelopmenthasbeenwitnessedinthistechnologywithrespect tomaterials,synthesistechniques,modifications,andmodulesoverthelastfewdecades.
Forwardosmosis(FO)isanemergingmembranetechnology,whichhasbeenimplementedwithfull-scaledemonstrationonlyinthepast3–5years.Ithasshownpromising outcomesinavarietyofapplicationsincludingseawaterandbrackishwaterdesalination, powergeneration,foodprocessing,andwastewaterreclamation.
ThecombinationofFOandRO,includingdirectandindirectapproaches,canplaya promisingroleforthecomprehensivemanagementofwaterresourceandpromotethe sustainabilityinwater-energynexus.
Thisbookprovidesacomprehensiveandthoroughcoverageofrecentdevelopmentsof FO,RO,andtheircombinationinintegratedsystemsforspecificapplicationsincluding waterdesalination,wastewatertreatment,powergeneration,andfoodprocessing.These applicationsareagoodexampleofstrategiesforengineeringdevelopmentandprocess intensificationofinterestforbothindustrialanddevelopingcountries.
Generalprinciples,membranemoduledevelopments,membranefoulingandmodeling,simulation,andoptimizationofbothtechnologiesarealsocovered.
Goingintothedetails,thebookisdividedinthreeparts:
I—Forwardosmosis,withfivechapters
II—Reverseosmosis,withfivechapters
III—IntegratedRO/FO,withfourchapters
Thefirstone, Chapter1 (Rastogi),considersthatthedemandofconsumersforhighestqualityconcentratedproductswithnaturalflavorandtaste,freefromchemicaladditives andpreservatives,triggeredthedevelopmentofanovelmembrane-basedprocessforthe concentrationofliquidfoods.Inthiscontext,FOisavalidalternative:itsvariousapplicationsinfoodprocessing,desalination,andpharmaceuticalsaredemonstratedbythe increaseinscientificreportsinthespecializedliterature.ThemostvitalfeaturesofFO areconcentrationofliquidfoodsatambienttemperatureandpressurewithoutconsiderablefoulingofmembrane.Lowenergyconsumption,lowfoulingpropensity,reducedor
easycleaning,lowcost,highsaltrejection,andhighwaterfluxarethefewadvantagesof FOovertheothermembraneprocesses.ThecurrentlimitationsofFOareconcentration polarization,foulingofmembrane,backdiffusionofdrawsolute,andthedesignandfabricationofmostsuitablemembrane.So,thischapterdiscussesthecurrentadvancesinFO andfocusesontheopportunitiesandchallengesassociatedwiththistechnology.
Chapter2 (Gwak,Kim,andHong)underlinesthatwhileFOprocesseshaveundergone greattechnologicaladvancements,thecontrolofreversesolutediffusionandtheeffective recoveryofdrawagentsarethemajorchallengesforthesystematicallysustainableand economicallyfeasibleuseofFOremain.Thischapterreviewspolymerhydrogel-and nanoparticle-baseddrawagentsthatcan(1)induceahighosmoticpressure,(2)result ininsignificantreversediffusionofsolutes,and(3)beeffectivelyregeneratedbysimple separationmethods. Chapter3 (WibisonoandBilad)introducesthedesignofFOsystems. Inparticular,FO,consideredanemergingmembraneprocess,haspotentialtobea“lowenergy”footprintforwaterandwastewatertreatment.However,theterm“lowenergy” mayonlybesuitablewhenFOisappliedasastand-aloneprocesswithoutrequiring thedrawsoluterecovery.Forthisreason,thischapterdiscussesthedesignsystemsfor FOimplementation,aseitherastand-alonesystemviatheosmoticconcentrationoras ahybridsystemforrecoveryofthedrawsolute,includingthethermalprocessviaevaporation,membranedistillationorcrystallization,stimuli-responseusing“smart”draw solute,pressure-drivenmembraneprocess,andotherrecoveryprocesses.Finally,the summaryofthecurrentreportedsystemdesignandfutureresearchtrendonFOsystem isprovided.Chapter4(AschmoneitandHelix-Nielsen)presentsthecomputationalfluid dynamics(CFD)processsequenceforthedevelopmentofaCFDmodel,designedforthe analysisofidealpackingdensitiesinFOhollow-fibermodules.EachCFDprocessisindividuallyoutlined,followedbyitsapplicationinbuildingtheCFDmodel,givingagood overviewofCFDmodelinginFOprocesses.Themodelshowsgoodagreementwithexperimentaldata,fordrawconcentrationofupto1M.Thesubsequentpackingdensityanalysisshowssevereexternalconcentrationpolarization(ECP)effectsforincreasingpacking densities,yieldingamaximumwaterfluxforpackingdensitiesintherange45%–80%. Nevertheless,thetotalpermeationratestillgrowswithincreasingpackingdensity:the effectofincreasingmembranesurfaceexceedstheincreasingECP,asthepackingdensity isincreased.However,theenormouspressuredroprisealongthemembranemodule limitsefficientapplicationswithhighpackingdensities.In Chapter5 (Rastogi),theapplicationsofFOprocessinfoodprocessingandfutureimplicationsarepresented.Infact,FO isconsideredasapromisingmembranetechnologythatfindsitsapplicationinfood industriesforliquidfoodconcentrationwhilepreservingtheheat-sensitivecompounds. Lowhydraulicpressure,lowtreatmenttemperature,lowfoulingtendency,andhighsolid contentarethemainadvantagesofFOcomparedwithboththermalandconventional membraneprocessing.Thechaptershowsacomprehensiveaccountofrecentadvances inFOtechnologyasrelatedtothemajorissuesofconcerninitsrapidlygrowingapplicationsinfoodprocessingsuchasconcentrationoffruitsandvegetablesjuices(grape,pineapple,redraspberry,orange,tomato,andredradishjuices)andnaturalfoodcolorants
(anthocyaninandbetalainextracts).Italsoprovidesaclearoutlineforresearchersonthe recentdevelopmentsinFO.
With Chapter6 (Ibrahim,Isloor,andFarnood)startsPartII.Thischapterendowsan inclusiveintroductiontothefundamentalsandbasicsoftheROprocessstartingfrom theshorthistory,plants,andtheoreticalcontextualofRO.Aconcisesummaryofthe recentadvancesinROmembranesandmaterialsthenfollows.Lastly,theusageofdifferenttypesofmodulessuchasspiralwoundpreparedfromflatsheetmembranesand hollow-fibermembranesusedinROdesalinationisdiscussedindetail.Inrecenttimes, manycommercial-scaleseawaterdesalinationplantshavebeenconstructedinwaterstressedcountries,whichisexpectedtoincreaseinthenearfuturetoincreasetheavailabilityofpotablewater.Inspiteofmanydevelopmentsinthedesalinationtechnologies, seawaterdesalinationusingROmembraneisbeingconsideredasthestate-of-the-art technology.Inthiscontext, Chapter7 (SyedIbrahim,Isloor,andFarnood)introduces thepretreatmenttechniques,foulingandcontrolstrategies.Theauthorssuggesttotake intoconsiderationthattherearealsosomelimitationstotheROmembranedesalination bytheunavoidablemembranefouling,whichincreasetheoperatingcostandtransmembranepressure.Here,thechapterreviewsthepotentialwaytotheROmembranefouling controlstrategies,whichincludestheroleofadvancedmaterials,surfacemodification, andfeedwaterpretreatment.In Chapter8,theauthors(MondalandDe)attributetheprimaryreasonbehindthedevelopmentofsciencefortheROmembranetoitspotentialin desalinationofbrackishwater.Thoughthetechnologyhasbeenwellestablishedformore thanadecadenow,thereareseveralscopesofimprovementinitsscaling-upandsmooth operation,whicharelinkedtotheunderstandingandmodelingoftheprocessdynamics. RO-basedprocessesarealsosuitableforseparation(andremoval)ofhighvaluedlow–molecularweightcompounds,whichisotherwisedifficultthroughchemicalroutes.In recentyears,therehasbeengrowthintheresearchofnewmembranematerialstargeted towardhighflux/highselectivity,showingpromiseingreatereconomicbenefitand reducedenergycosts.Inthiscontext,itisquiteimperativetounderstandthephysicalphenomenainvolved,withinthepurviewofmathematicaldesignequations,simulatingthe performanceforthegivenoperatingconditions. Chapter9 (Trishitman,Cassano,Basile, andRastogi)isdedicatedtotheROforindustrialwastewatertreatments.Consideringthat thefundamentalresourceofeconomic,social,andculturaldevelopmentoftheworldis water,itisevidentthataverysmallamountoftheglobalwaterisavailablefordirectaccessibility.Unfortunately,thislimitedamountofavailableglobalwaterhasbeenpollutedby industrialplants.Theever-evolvingandever-increasingstrictregulatorystandardsfordischargingeffluentsfromindustriesposehugeenvironmentalandeconomicimplications becauseofbulkdepositionintheenvironment.Whiletheworldfacesanincreasedscarcityinfreshwatersupply,itisgreatimportancethatthewastewaterfromtheindustries canbetreated,recycled,andreused.ROmembranescanbeappliedtoprocessorconvert thewastewaterintocleanwaterorintoeffluentsthatcanreturntothewatercycledirectly totheenvironmentorforreuse.Thischapterincludesthemajorsourcesofindustrial wastewater,theircharacterizationandevaluation,thesuppliersofROwastewater
treatmenttechnologies,andapplicationsofROsystemfordifferentindustrialwastewaters.Thenextwork, Chapter10 (Cassano,Rastogi,andBasile),isdedicatedtoROinfood processing.RO,recognizedasaleadingtechnologyindesalinationprocesses,isalsoa well-establishedapplicationinfoodprocessingduetoitsseveraladvantagesoverconventionalmethodologies(suchaspreservationofthermosensitivecompounds,lowenergy consumption,andlowinvestmentcosts).Thechapterfocusesonthemainapplications ofROinfoodprocessing,includingconcentrationoffruitandvegetablejuices,preconcentrationofmilkandwhey,improvementofmustqualityandwinedealcoholization. ThecontributionofROtotherecoveryofbiologicallyactivecompoundsfromagrofood wastewaters,accordingtothenewindustrialecologyandbiorefineryconcepts,isalsodiscussed.Inthesefields,thecombinationofROandothermembranefiltrationsystemswell contributestoredesignthetraditionalprocessingoffoodandbeverageswithinthelogicof theprocessintensificationstrategy,withremarkablebenefitsintermsofproductquality, plantcompactness,environmentalimpact,andenergeticaspects.Theseaspectsare addressedindetailaccordingtothelatestliteratureinputs.
With Chapter11 (S.LiandZ.Li)startsPartIII.ItconsidersbothFOandROinintegrated systems.SincetheosmoticpressureasdrivingforceinFOiscreatedbytheosmoticgradientbetweenfeedsolutionanddrawsolution,aposttreatmentofdrawsolutionisgenerallyrequiredandconductedbyROtorecoverproductwaterandreusedrawsolutes. Therefore,FOandROareoftencombinedinanintegratedmembranesystemtostudy salinewaterdesalination,wastewatertreatment,andrenewableenergygeneration.The harshfoulingofconventionalROprocesscanbeconvertedtoreversiblefoulingofFO intheintegratedsystem.Itispossibletoperformthedesalination,watertreatment, andenergygenerationsimultaneouslybyemployingdifferentwater(suchasquality impairedwater,salinewater,andconcentratedbrine)intheintegratedsystem.TheintegratedFO-ROmembranesystemcanplayapromisingroleforthecomprehensivemanagementofwaterresourceandpromotethesustainabilityinwater-energynexus.
Chapter12 (S.LiandZ.Li)describesFOandROindesalinationmembranesystems.After morethan40years’development,ROmembranedesalinationisawell-establishedtechnology,withsimilarmarketshareasconventionalthermaltechnologies.However,membranefouling,especiallybiofouling,stillremainsachallengefortheROdesalination. Transparentexopolymerparticlesrecentlyarereportedasakeymembranefoulingelement.FOcanbeusedfordesalinationifcombinedwithotherseparationtechnologies. TheFO-ROhybridsystemconsumesslightlymoreenergythanconventionalROunit, butitslifespancanbeextendedduetothereductionofROmembranefouling.In Chapter13 (Osipi,Secchi,andBorges),acostanalysisofFOandROisperformedbyconsideringacasestudy.Costsensitivityanalysisisanimportanttooltostudywatertreatmentprocessesandunderstandhowvariablesandparametersaffectwatercost.Itcan alsobeusedtosetthedriversforfutureresearch,sincethemembraneparameters,for example,canalsobeinvestigated.Inthischapter,FOandROasstand-aloneprocesses and,sequentially,thecombinedFO-ROprocessareinvestigatedusingsensitivityanalysis. Itisobservedthatthestructuralparameterisoneofthekeyinfluencersonproducedwater
costforthecombinedprocess.Additionally,severalparametersindicatethatFO-ROfeasibilityishighlysensitivetoROconditionsandsaltrepositioncost.Thisresultsuggests thatdifferentrecoverymethodsmaybemoresuitableforconcentratingthedrawsolution, especiallywhenthefeedisahigh-salinitystream.In Chapter14 (Thiruvenkatachari,Su, andCunnington),acombinedFO-ROprocessforminingwastewatertreatmentispresentedanddiscussed.Waterinteractionsinminingprocess,wastewatercharacteristics, treatmentmethodsadopted,andtheresearchtechnologygapsarebrieflydiscussed.A lab-scaleintegratedFO-ROwasusedtoestablishtheproofofconceptinapplyingthis technologyforminewatertreatment.Theintegratedsystemhasbeentestedunder extendedoperationwithvariousminewaters.TheFOcombinedwithROcouldminimize numberofpretreatmentstepsrequiredfortheRO.Thistechnologycanreducethebrine volumebyatleast80%andrecoverconsistentqualityreusablewater.Apilot-scaleFO-RO model(capacityof1m3/day)wasdesignedbyincorporatingtwodifferenttypesofFO membranes.Thestudyshowsthatthisprocesscanpotentiallyofferasignificantreduction inenergyuse,chemicaluse,pipinginfrastructure,andoperatingcostsindesalinating minewater,andthesuccessofthistechnologyisintheefficientprocessdesign,FOmembranemoduleconfiguration,membranechemistry,andthestructure.
Wewishtogreatlythankalltheauthorsofthechaptersfortheirexcellentworkandalso fortheirpatiencetocarefullyfollowoursuggestionsandcomments.WealsoaddourspecialthankstoallthestaffofElsevierfortheirconstantandprofessionallyhighqualified help.Wetrustthatthisbookwillbehelpfultothescientificcommunityinproviding in-depthviewandpromotingthepromisingdevelopmentofmembranetechnologyfor sustainablefuture.Besides,thisbookcanserveasanessentialreferencesourcetostudentsandresearchersattheuniversitiesandresearchinstitutions.
AlfredoCassano
Forwardosmosis:Principles, applications,andrecent developments
NavinK.Rastogi
DEPARTMENTOFFOODENGINEERING,CE NTRALFOODTECHNOLOGICALRESEARCH
INSTITUTE,COUNCILOFSCIENTIFICAND INDUSTRIALRESEARCH,MYSORE,INDIA
Listofacronyms
DS drawsolution
ECP externalconcentrationpolarization
FO forwardosmosis
ICP internalconcentrationpolarization
NF nanofiltration
RO reverseosmosis
UF ultrafiltration
Listofsymbols
AandB activelayerpermeabilitycoefficientsofthemembraneforwaterandsolute,respectively
Jw flux
k masstransfercoefficient
Κ soluteresistivityfordiffusionwithintheporoussupportlayer
Greeksymbols
ΔP differenceinpressure
π bulkosmoticpressure
π* osmoticpressureonmembranesurface
π0 osmoticpressureinsidetheactivelayer
Subscripts
d drawsolution
f feedsolution
w water
1Introduction
Osmosishasbeenasubjectofstudyinvariousscientificandengineeringdisciplines.Itis well-definedasamethodfortransportofwaterbasedonthedifferenceinosmotic CurrentTrendsandFutureDevelopmentson(Bio-)Membranes. https://doi.org/10.1016/B978-0-12-816777-9.00001-0
© 2020ElsevierInc.Allrightsreserved.
pressureacrossasemipermeablemembrane.Forwardosmosis(FO)isdefinedasamembraneprocessinwhichthedifferenceinosmoticpressureproducesmovementofwater fromthefeed(dilute)solution(lowosmoticpressure)sidetothedraw(concentrated) solution(highosmoticpressure)side,throughaselectivelypermeablemembrane.FO allowshigherconcentrationsofjuiceswithoutsignificantfoulingofthemembrane (Beaudry&Lampi,1990a; Nayak&Rastogi,2010a; Rastogi,2016).
Alsoknownbyothernamessuchasdirectosmosis,engineeredosmosis,andmanipulated osmosis,FOconcentratesliquidfoodsatambienttemperature.Theprocessutilizesan osmoticsolutiontoisolatewaterfromafeedthroughasemipermeablemembrane.Incontrast,thereverseosmosisprocessrequireshydraulicpressureasthedrivingforcefor separation.
Duetoitslowhydraulicpressurerequirement,FOhasseveralpotentialadvantagesin comparisontoprocessessuchasreverseosmosis(RO),nanofiltration(NF),andultrafiltration(UF).Itsmainadvantagesovertheconventionalprocessingmethodscanbesummarizedas(Babu,Rastogi,&Raghavarao,2006; Cath,Adams,&Childress,2005; Cath, Gormly,etal.,2005):
• Retentionoffreshfruitflavorandnutritional/bioactivecomponents
• Minimumcolordegradationandimprovedproductquality
• Concentrationatambientpressureandtemperature
• Absenceofthermaleffects,socookedtasteisnotimpartedtothefood
• Reductioninmembranefoulingpropensityoverpressure-drivenmembraneprocesses
• Useofsimpleequipmentandenergyefficiency
Manyfruitjuicescontainbioactivecompoundssuchasvitamins,phenoliccompounds, anthocyanins,andcarotenoids.Oncethecolorantsareextractedfromplantsources,the juiceisverydiluted( Thomas,1984).Thesejuicesaregenerallyconcentratedbyemploying thermalevaporationundervacuumtoavoidgrowthofmicroorganisms,toextendtheshelf life,andtoreducethecostofstoringandshipping.However,thisalsoresultsinthelossof thefreshjuicearomaandcolordeterioration(lossofhueandchroma),besidesthedevelopmentofacookedtaste,subsequentlyleadingtodegradationinthequalityoftheproduct (Bhaskaran&Mehta,2006; Patil&Raghavarao,2007).FOhasbeensuccessfullyusedforthe concentrationoffruitjuicesaswellasnaturalcolors(Babuetal.,2006; Bolin&Salunke, 1971; Loeb&Bloch,1973; Nayak&Rastogi,2010a,2010b; Nayak,Valluri,&Rastogi,2011; Popper,Camirand,Nury,&Stanley,1966; Rodriguez-Saona,Giusti,Durst,&Wrolstad, 2001; Zhao,Zou,Tang,&Mulcahy,2012),wastewatertreatment(Holloway,Childress,Dennett,&Cath,2007),andfreshwaterrecoveryfromseawater(Kravath&Davis,1975).
Thethermalconcentrationofliquidfoodsisveryenergyintensiveandresultsin lossofsensoryandnutritionalproductquality(Lazarides,Iakovidis,&Schwartzberg, 1990; Petrotos&Lazarides,2001).Increasingconsumerdemandforhigh-quality concentratedfruitjuicesandnaturalcolora ntshasmotivatedthedevelopmentofinnovativemethodssuchasfreezeconcentrationorreverseosmosisforconcentration,for improvedqualityandmoreefficientenergy utilization.Theseprocessesarecapital intensiveandalsosufferfrommanylimitati ons,e.g.,high-pressurerequirements,
aceilingonthemaximumattainableconcentration,andconcentrationpolarization (Babuetal.,2006; Rastogi&Nayak,2011).
Cost-effectivemethodsbasedonmembranesforwaterpurificationanddesalinating seawaterareneededtoproducepurifiedwaterfromsalinewater.Thedevelopmentof aneffectiveandefficientdrawsolutionwithalow-costenergyrecoverysystemisessential forincreasedperformanceofthisprocess,aswellasachievingalargerscaleforFO.The noveltyoftheprocessliesinutilizinganaturalosmoticprocessfordesalinationrather thanhydraulicpressure,asinRO.FOhasthepotentialtoachievehighwaterfluxalong withhighwaterrecovery,whichreducesthevolumeofdesalinationbrine,amajorenvironmentalissue(McCutcheon,McGinnis,&Elimelech,2005).
EasternEuropeanfarmershaveshownthatextractingjuiceintoabagandsubjectingit toaNaClsolutionresultsinahigherconcentrationoffruitjuice.However,thistechnique requiredalongertimetoachieveconcentrationofthejuices(Cussler,1984). Scott(1975) developedaprocessfortheconcentrationofliquidfoods,e.g.,fruitjuicesordairyproducts,byimmersingthepackedbagsofmembranematerial(celluloseorpolysulfones)into adrawsolutionatarelativelyhightemperaturewithcontinuousstirring.
SincethefeedduringFOisnotsubjectedtopressureortemperature,itmaintainsthe color,taste,aroma,andnutritionoftheoriginalproduct(Cath,Childress,&Elimelech, 2006;Yang,Wang,&Chung,2009a). Popperetal.(1966) indicatedthatfruitjuiceconcentrationusingtheFOprocesswaspossiblebyutilizingcelluloseacetatereverseosmosis membranes.However,thisresultedinloweraveragetransmembraneflux.Modifiedthin filmcompositeROmembranes(thickness25–85 μm)withincreasedturbulenceyield higherosmoticfluxandreducedfouling(Beaudry&Lampi,1990a,1990b; Herron, Beaudry,Jochums,&Medina,1994).Subsequently, Petrotos,Quantick,andPetropakis (1998,1999), PetrotosandLazarides(2001) showedthatuseofathinnermembranewith lessviscousosmoticmedium,e.g.,saltsolution,demonstratedimprovedperformanceas comparedtoamoreviscousosmoticmedium.Microfiltrationorultrafiltrationclarificationofthejuicebeforefiltrationledtohigherfluxes.FOconcentratedjuicewasfoundto beofimprovedqualityincomparisontojuiceconcentratedbyevaporation(Herron etal.,1994).
Inordertoimplementthistechnologyonalargescale,itisnecessarytoworkout strategieseitherforrecoveryoradisposaldrawsolution,whichisconsideredtobeanecessaryevilthatrequireshighenergyand/orcapitalcosts(Garcia-Castello,McCutcheon,& Elimelech,2009; McCutcheonetal.,2005).FOpromisestoemergeasalow-energy solutionifmethodsfortheregenerationofdrawsolutescanbefoundthatareeconomicallyandtechnicallysound(McGinnis&Elimelech,2007).Anumberofpublicationsare availableintheliteratureontheconcentrationofbeveragesandliquidfoodsusingFO; however,thesearegenerallyoflaboratoryscale(Raghavarao,Nagaraj,Patil,Babu,&Niranjan,2005).Reversedrawsolutefluxisoneoftheimportantparametersthatmustbe assessedwhenevaluatingtheperformanceoftheFOprocess.Thereversesaltdiffusion phenomenoncandecreasethenetosmoticpressureacrossthemembrane,whichresults influxdeclineandcanjeopardizetheprocess.Moreover,reversesalttransportisnotonly aneconomicalloss,butcanalsocomplicateconcentratemanagement.
ThecapabilitiesandlimitationsofFOhavebeenextensivelyreviewed(Cathetal.,2006; Chung,Zhang,Wang,Su,&Ling,2012; Jiao,Cassano,&Drioli,2004; McCutcheon&Elimelech,2007; Rastogi,2016; Rastogi&Nayak,2011; Wilf,2010; Wong&Winger,1999; Zhao etal.,2012).ThisreviewmainlyconcentratesonthevariousaspectsandbenefitsofFOin foodprocessing.ItfocusesondevelopmentsinFOmembranes,mechanismsofwater transport,andcharacteristicsofosmoticsolutions;theFOmembranesthemselvesaswell astheirusesarediscussedindetail.
2Transportacrosstheforwardosmosismembrane
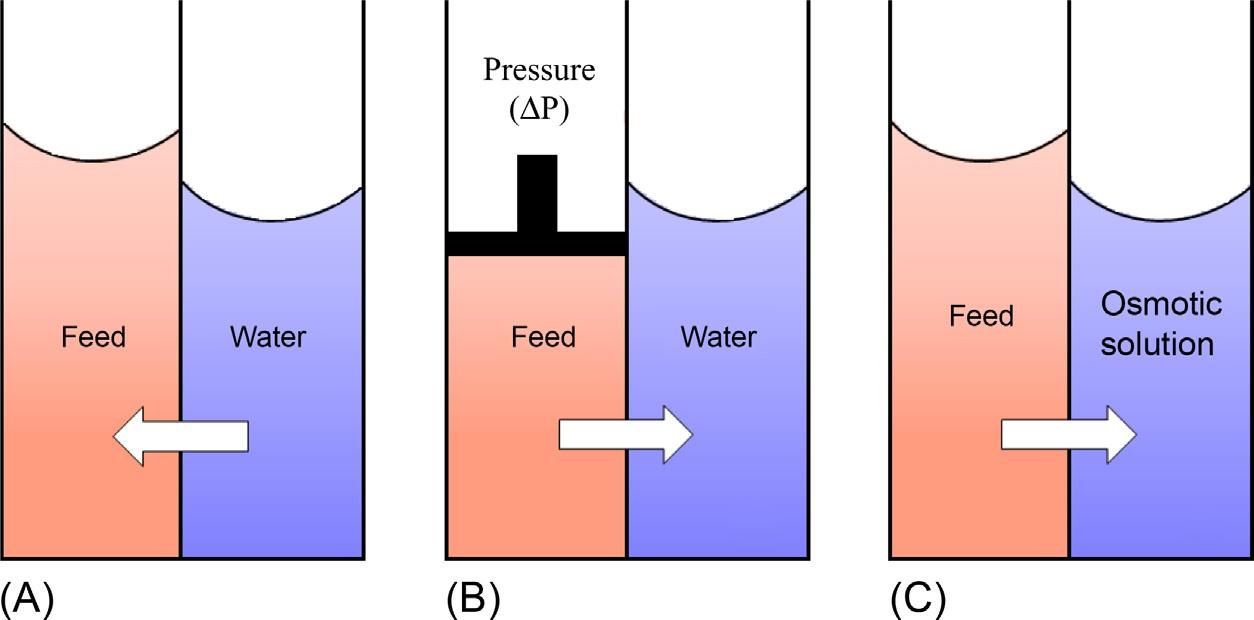
Osmosisisreferredtoasthediffusion-drivenmigrationofwatermoleculesfromalow concentrated(hypotonic)toahighlyconcentrated(hypertonic)solutionthroughaselectivelypermeablemembranewiththeaimofequalizingthechemicalpotential(soluteconcentrations)onbothsides.Thewateristransportedacrossthemembranesothatthe chemicalpotentialoneithersidebecomesidentical(Fig.1A).Thedifferenceinthepressureacrossthemembraneleadstotheconcentrationofliquidfoodswithoutaphase change.Hydraulicpressureisusedasadrivingforcetooffsettheosmoticpressuredifferential(Δπ)(Fig.1B).FOisanosmoticprocessinvolvingasemipermeablemembraneto separatethefeedfromthedrawsolution.Theosmoticpressuregradientbetweenthefeed andthedrawsolutionactsasthedrivingforcefortheseparationofwaterfromthefeed towardthedrawsolution,resultingintheconcentrationofthefeedsolution(Fig.1C) (Cathetal.,2006; Rastogi&Nayak,2011).
Therelationshipamongwaterflux,osmoticpressure,andhydraulicpressurescanbe describedas:
FIG.1 Migrationofwaterduring(A)osmosis,(B)reverseosmosis,and(C)forwardosmosis.The arrows indicatethe directionofmasstransfer. 4 Pisthehydraulicpressureand πf, πd, πw aretheosmoticpressuresoffeedsolution,draw solution,andwater. FromRastogi,N.K.(2016).Opportunitiesandchallengesinapplicationofforwardosmosisin foodprocessing. CriticalReviewsinFoodScienceandNutrition, 56,266–291.
where,Jw,A,and ΔParetheflux,membranepermeability,anddifferenceinpressure, respectively(Rastogi,2016; Xu,Peng,Tang,ShiangFu,&Nie,2010).
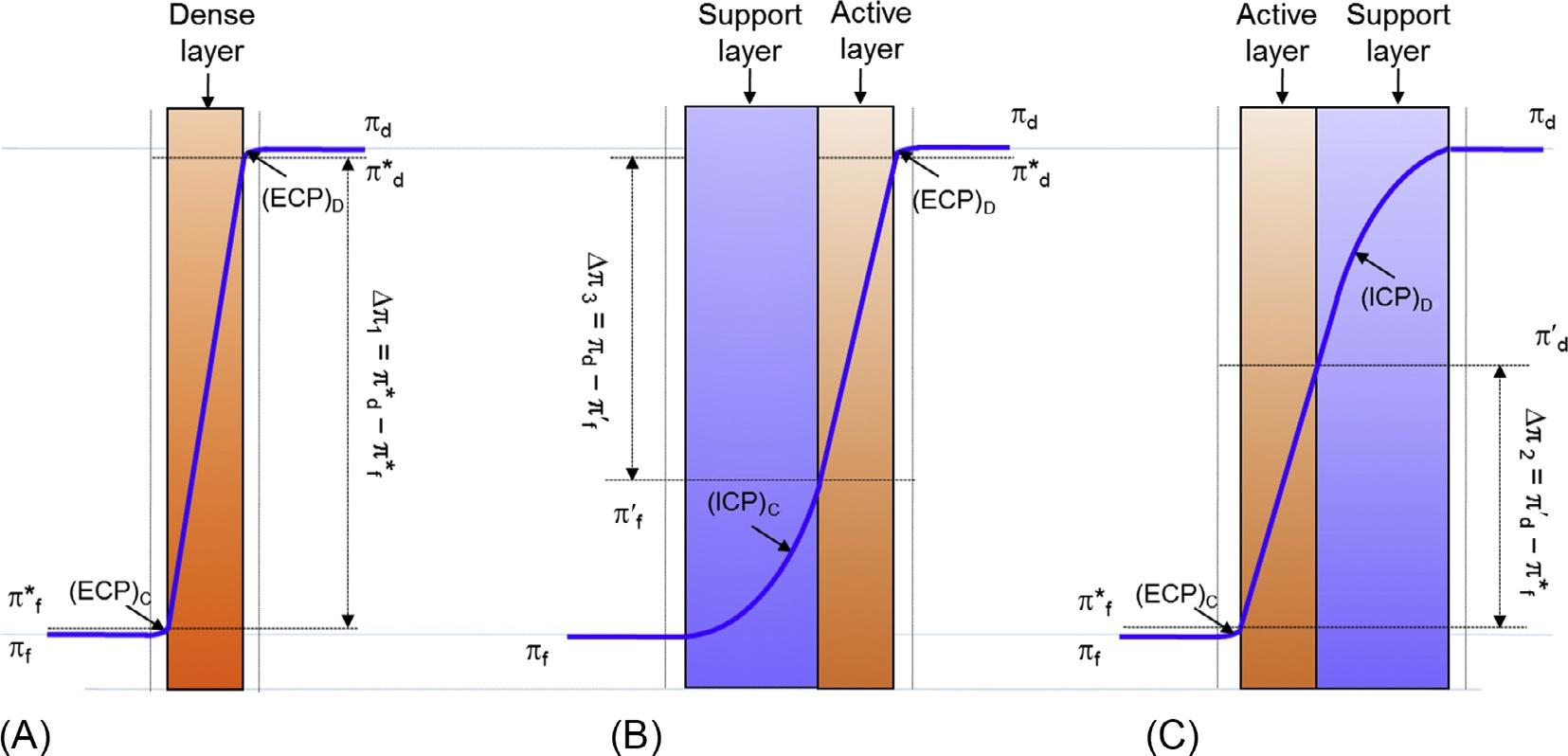
Concentrationpolarizationisabuildupofretainedmoleculesatthesurfaceofthe membrane,resultinginadecreaseinosmoticpressuredifferenceacrossthemembrane (reductionintransmembraneflux),whichisaprobleminpressure-drivenmembrane processes,e.g.,RO.InthecaseofadenseFOsymmetricmembrane,thisphenomenon canariseonbothsidesofthemembrane(towardboththefeedanddrawsolutions). Thesoluteisconcentratedonthefeedsideanddilutedonthedrawsolutionside,resulting inconcentrativeanddilutiveexternalconcentrationpolarization(ECP),respectively (Fig.2A).ThefluxequationforFOcanbegivenas:
Eq. (2) canbemodifiedforhigherfluxestoquantifythedilutiveaswellasconcentrative (feed/drawside)ECP(McCutcheon&Elimelech,2007):
FIG.2 Mechanismofforwardosmosisindicatingwatertransport(A)withadensesymmetricmembrane;with asymmetricmembrane(B)feedtowardthesupportlayer(modeI)and(C)feedtowardactivelayer(modeII); πd and πf arethebulkosmoticpressuresofdrawandfeedsolutions,respectively; π* d and π* f theosmoticpressureson membranesurfaceofdrawandfeedsolutions,respectively; π0 f and π0 d aretheosmoticpressuresofthefeedanddraw solutionsontheinsideoftheactivelayerwithintheporoussupportforconcentrativeinternalconcentration polarizationonfeedsideanddilutiveinternalconcentrationpolarizationondrawsideformodeIandmodeII, respectively.Notethat Δπ1, Δπ2,and Δπ3 arethecorrespondingeffectivedrivingforcein(A),(B),and(C)situations, respectively. FromRastogi,N.K.(2016).Opportunitiesandchallengesinapplicationofforwardosmosisinfood processing. CriticalReviewsinFoodScienceandNutrition, 56,266–291.
whereJw,kf,andkd arethewaterfluxandmasstransfercoefficientsonfeedaswellasdraw solutionsides,respectively; πf and πd arethebulkosmoticpressuresoffeedanddrawsolutions,respectively; π* f ¼ πf exp Jw kf and π* d ¼ πd exp Jw kd aretheosmoticpressuresof feedanddrawsolutions,respectively.
TheFOasymmetricmembrane(looselyboundedsupportlayerandthecompacted activemembranelayer)canbeemployedintwoalignments,withfeedtowardthesupport layer(normalmode,modeI)andfeedtowardtheactivelayer(reversemode,modeII), (Fig.2BandC)(Gray,McCutcheon,&Elimelech,2006; Nayak&Rastogi,2010a).Formode I,waterfromthefeedarrivesattheporoussupportlayeranddiffusesthroughtheactive layerintothedrawsolution.However,thesaltalsogoesinwithwaterintotheopenstructureandistransportedandretainedbytheactivelayer,resultinginariseinconcentration onthesideofthesupportlayer,whichiscalledconcentrativeinternalconcentration polarization(ICP).Thesupportlayercreatesnoresistancetowatermigrationandallows freepassageofthesolutethroughit.Hence,itisassumedthatnoconcentrativeECP occursonthesideofthesupportlayer(Cathetal.,2006).Onthepermeateside,dilutive ECPoccursduetotransferofdissolvedosmoticagentfromthemembranesurfaceleading tothereductionofeffectivedrivingforce(Fig.2B).
InthecaseofmodeII,whenfeedisonthesideoftheactivelayer,thedilutiveICPtakes placetowardthepermeateside.Themovementofwatertowardthedrawsolutionsideled toahigherconcentrationofsoluteontheactivelayerofthemembrane(increasesthe osmoticpressure),resultinginadecreaseinthedrivingforce.Thiseffectcannotevenbe reducedbyhydrodynamicssuchasturbulence,andhenceradicallylessenstheosmotic drivingforce(McCutcheon&Elimelech,2007).NoECPtranspiresatthedrawside(Fig.2C).
ThefluxesduringFOthroughanasymmetricmembrane,inthecasesofmodeI(feed towardthesupportlayer)andmodeII(feedtowardtheactivelayer),arerepresentedby Eqs. (4),(5),respectively.
Symbols π0 f and π0 d aretheosmoticpressuresofthefeedanddrawsolutionsonthe insideoftheactivelayerwithintheporoussupportforconcentrativeICPonthefeedside anddilutiveICPonthedrawsideformodeIandmodeII,respectively: π0 d ¼ πd exp( JwKd) and π0 f ¼ πf exp(JwKf );Kf andKd arethesoluteresistivitieswithintheporoussupportlayer formodeIandmodeII,respectively.FormodeI,concentrativeICPanddilutiveECPoccur simultaneously.Likewise,formodeII,dilutiveICPandconcentrativeECPexistatthe sametime.
NayakandRastogi(2010a) illustratedtheprocessofwatermigrationinFOfromafeed solutioncontainingamixtureoflowaswellashighmolecularweightcompounds.For modeI,highmolecularweightcompoundsareretainedonthesurfaceofthesupport layer,leadingtodevelopmentofmarkedECPonthefeedside.Atthesametime,low
molecularweightcompoundsdiffusewithinthesupportlayer,resultinginconcentrative ICP.However,theECPtowardtheosmoticagentsidewasalmostinsignificant.Formode II,thewaterfromthefeedisdiffusedintotheactivelayerandthenistransportedtothe bulkviathesupportlayer.ECPonthefeedsideisinsignificantincomparisontoICP (Nayaketal.,2011). MiandElimelech(2008,2010) alsoindicatedthatdepositionofhigh molecularweightcompoundsintheporousstructureresultsintheformationofacake layer.ThefollowingequationscouldbeemployedforthecalculationofmembraneresistivityduringtheFOprocessformodeIandII(Grayetal.,2006; Loeb,Titelman,Korngold,& Freiman,1997; McCutcheon&Elimelech,2007; Tang&Ng,2008):
whereJw isthetransmembranefluxduringFO,Kc andKd arethemembraneresistivity (s/m)formodesIandII,respectively; πd and πf aretheosmoticpressureofdrawandfeed solutions,respectively.TheconstantsAandBaretheactivelayerpermeabilitycoefficients ofthemembraneforwaterandsolute,respectively(Grayetal.,2006; Loebetal.,1997). Further,theeffectofinternalconcentrationpolarizationonthefluxbehaviorduring FOwasstudiedbymanyresearchers(Gruberetal.,2011; Lietal.,2011; Sagiv&Semiat, 2011; Tang,She,Lay,Wang,&Fane,2010; Zhao&Zou,2011a,2011b).
3Drawsolutionsforforwardosmosis
Recently,FOhasgainedrenewedinterestasanemergingtechnologyleadingtoalowenergydesalinationprocess.VitaltotheFOprocessarethedrawsolution(DS)and themembrane,becausebothplayanimportantroleinitsperformance.Hence,the selectionofanappropriateDSisessentialinobtainingprocessefficiency.FOemploys thenaturalosmoticpressuregradientbetweenadrawsolutionandafeedsolutionfor theproductionofpotablewater,offeringalow-energy,low-costalternativetomoreconventionaltreatmentmethods.Asapotentialsolutiontothecrisesofenergyand resources,FOhasbeenlimitedbythedevelopmentofdrawagents.Anidealdrawagent shouldgeneratehighosmoticpressureandbeeasilyrecoverable.Transferofsalttothe feedside(reversedrawsolutediffusion)reducesthewaterfluxandcontaminatesthe feedsolution.Thedrawsolutionshouldbewatersolubleanditshouldprovidegreater osmoticpressurethanthefeedsolutiontoobtainincreasedwaterfluxandminimal reversesaltdiffusion,andtoyieldasuitableprocessforeconomicalreconcentration. Reversesaltdiffusioncontaminatesthefeedsolution,diminishesthedrivingforce, andmayadverselyaffecttheproductquality.Inthecaseofpotablewater,drawsolutes shouldbenonexistentinthefinalproduct(water)oratleastbelowthemaximumallowablecontaminantlevel.
Achilli,Cath,andChildress(2010) establishedaprocedurefortheselectionofideal osmoticsolutionsforFOapplicationsbyevaluatingtheamountofwatertransport,salt diffusiontothefeedside,ROpermeateconcentration,andcostofreplenishment.Similarly, Kimetal.(2012) alsoplannedalogicalapproachforaviableandoptimaldrawsolute foranFOdesalinationprocess.AconcentratedNaClsolutioniscommonlyadoptedasan osmoticagentsolutionbecauseofitshighsolubility,nontoxicnature,andthepossibility ofconcentratingbyinvolvingconventionaldesalinationtechniques.Otherdrawsolutes, suchasCaCl2,MgCl2,KHCO3,andNaHCO3,canalsobeused.
Grayetal.(2006) demonstratedthattheconcentrationandmolecularweightofosmotic agentssuchasNaCl,sucrose,ordextroseusedasadrawsolutioninthecaseofmodeIdid nothaveasubstantialeffectonthetransmembranefluxbecauseofnosubstantialICPor ECP.However,inthecaseofmodeII,itledtodecreasedfluxduetosignificantICP. Petrotos etal.(1998,1999) examinedtheconcentrationoftomatojuicewithFOusingCaCl2, Ca(NO3)2,andNaCl. McCutcheonetal.(2005) and McCutcheon,McGinnis,andElimelech (2006) reportedamethodforseawaterdesalinationusingathermolyticdrawsolution basedonammoniaandcarbondioxide.
Mondal,Mahto,etal.(2015) and Mondal,Nataraj,etal.,2015 showedthefeasibilityof employingseveralapproachesfordeepeutecticsolventsasadrawagenttoenrichlow abundanceDNAandproteinsusinganFOprocess. DuttaandNath(2018a,2018b) discussedtheprospectofionicliquids(ILs)anddeepeutecticsolventsasnew-generation drawsolutions.ILswithsmallmolecularweightandlowviscosityshowedacomparable waterpermeabilitytotheNaClaqueoussolution,aswellasthepossibilityofrepeateduse. Itresultsinhighwaterrecoveryefficiencyandminimalreversediffusion.FOdesalination withapolyelectrolyte-baseddrawsolutiongainedmoreattentionduetolowreversesolutefluxandeasyrecoveryofthedrawsolution.
Hydrogelsarepromisingandinnovativeosmoticagents,whichmayrendertheFOprocesssimplerandmoreenergyefficient.Theycanbeadoptedasapossiblematerialfor hydrationbagsforfastandrepeatableproductionoffreshwaterfromsalinewateror wastewater( Yu,Zhang,&Yang,2017a,2017b). Li,Gao,andTang(2011) establishedthat polymerhydrogelparticleswithlight-absorbingcarbonparticlescouldbeusedasosmotic agentssuitabletoextractwaterthroughFOmembranes,whichcouldthenberecuperated byheatingorbyapplyingpressure,oracombination.Chemicalstructuremodificationof ahydrogelnetworkbyintegratingionicgroupsandlight-absorbingparticlescansignificantlyincreasetheeffectivenessofhydrogels.Largehydrogelparticlesresultedingreater liquidwaterrecoveryamounts,whereassmallerparticlesledtomorewaterrecovery underathermalstimulus(Razmjou,Simon,&Wang,2015).Electric-sensitivehydrogels asdrawagentscanreducereversesaltleakageandmaketheFOprocesssimpler.Similarly, stimuli-responsivehydrogelscancompletelyevadereversesolutediffusionandwatercan bereleasedsimplyunderexternalstimuli(Cui,Zhang,Jiang,&Yang,2018; Cui,Zhang,& Yang,2017; Cui,Zhang,Yu,&Yang,2018).Thepropensityofthesurfactantstoaggregate intomicellesandtoadsorbatinterfacesprovidesintriguingosmoticpressuresandoffers usablepropertiesbywhichosmoticsolutionscanberegenerated(Roach,Al-Abdulmalek, Al-Naama,&Haji,2014). 10CurrentTrendsandFutureDevelopmentson(Bio-)Membranes
Ling,Wang,andChung(2010) demonstratedthattheuseofhighlywater-solublemagneticnanoparticles(MNPs)cappedwithpolyacrylicacidasosmoticsolutesresultedin higherwaterflux.Amagneticfieldwasusedtocapturethenanoparticlesattheendfor recycling. LingandChung(2011a,2011b) developedanintegratedFO-ultrafiltrationsystemfordesalinationinvolvingsuperhydrophilicnanoparticlesasosmoticsolute,which wererecoveredfromtheosmoticsolutionbyultrafiltration. Kim,Han,andHong(2011) and Liu,Bai,Lee,andSun(2011) alsousednaturallynontoxicmagnetoferritinasadraw soluteforrecoveringdrinkingwaterinanFOprocesswithoutreversesaltdiffusion.These featuresoftheosmoticsolutionrenderFOasanecosustainableprocedure. Alejo,Arruebo, Carcelen,Monsalvo,andSebastian(2017) indicatedthatMNPsarethemostsuitable osmoticsolutionfordesalinationbecauseoftheirquickrecoverybyapplyingamagnetic fieldorbymembraneprocesses.Thetypesofdrawsolutesusedbymanyresearchersare presentedin Table1.
4Membranesforforwardosmosis
FOisdevelopingrapidlyandhasrevealeditsadvantagesinmanyapplications.However, challengessuchasreversesolutediffusion,concentrationpolarization,andmembrane foulingarethecurrentnecessaryevilsinFOprocesses.Tolessentheseproblems,substantialeffortshavebeenmadeinrecentyearstodesignsuitableFOmembranes.Chemical modificationofexistingmembraneshasbecomeavitalmethodinnovelFOmembrane studies(Xu,Chen,&Ge,2017).
ThemostcriticalaspectsindesigningFOprocessesaretheselectionofthemembrane. Membranesusedforreverseosmosis(dense,nonporous,andselectivelypermeable)can beusedforFO.However,thethicksupportlayerofROresultsinenhancedconcentration polarization,requiringaveryhighosmoticpressuredifferencetowithstandsatisfactory waterflux(Dova,Petrotos,&Lazarides,2007a,2007b;Grayetal.,2006; McCutcheon etal.,2005;McGinnis,McCutcheon,&Elimelech,2007).Thefeasibilityofadoptingbladdersofpigs,cattle,andfish,collodion(nitrocellulose),rubber,porcelain,andgoldbeater’s skinhasalsobeendemonstrated(Cathetal.,2006; Tang&Ng,2008).
IntheFOprocess,theconcentrationpolarizationormembranefoulingtakesplaceon thefeedaswellaspermeatesidesofthemembrane(Grayetal.,2006; McCutcheonetal., 2006).Thesupportfabriclayeradheredtothesupportlayerofasymmetricmembranes wasremovedandthesemembranesusedintheosmosisprocessby Loebetal.(1997), whoestablishedthatthemembranesupportlayersubstantiallyinfluencedthewater transport(McCutcheon&Elimelech,2008).
McGinnisandElimelech(2007) showedthat,thoughthewaterpermeabilityofthecellulosetriacetatemembranewasslightlylessthanthatofthereverseosmosismembranes, therewasanenormousdifferenceintheosmoticflux.Membranessuitableforreverse osmosiswereconsistingofaveryslimactivelayer(<10 μm)andabulkyporoussupport layer.ThefabricandsupportlayerspresentinROmembranesresultinsevereICPand, therefore,theysignificantlylessentheresultingwaterflux.
Table1 Severalnoveldrawsolutesusedbyvariousresearchersforforwardosmosis
ProductDrawsolutes
Ionicliquids
References
Protonatedbetainebis(trifluoromethylsulfonyl)imide Zhonget al. (2016)
Monocationicimidazolium,phosphonium,andammonium salts Zeweldietal.(2018)
1-Acetate-2,3-dimethylimidazoleionicliquidsodiumsalt
Polyelectrolyte
Poly(N-isopropylacrylamide-co-acrylic acid)
Hydrolyzedpoly(isobutylene-alt-maleicanhydride)
Cationicstarchbygrafting2,3-epoxyproplytrimethyl ammoniumchlorideontobackboneofcornstarch
Hydrogels
Chen,Ge,Xu,andPan(2019)
Wang etal.(2016)
Kumar,Al-Haddad,Al-Rughaib,and Salman(2016)
Laohaprapanonetal.(2017)
Polydiallyldimethylammoniumchlorideanditsmonomer HamadandChirwa(2019)
Acrylicacidandsodium p-styrenesulfonatemonomerswith hydrophilicgroup Cui,Zhang,Jiang,andYang(2018), Cui,Zhang,Yu,andYang(2018)
Ethyleneoxide-propyleneoxidecopolymer-thermoresponsive polyelectrolyte Ahmed,Kumar,Garudachari,and Thomas(2019)
Compositehydrogels:Reducedgrapheneoxideand hydrogels, e.g.,poly(sodiumacrylate)orpoly(sodium acrylate)-poly(N-isopropylacrylamide)
Polymerizationofmonomersodiumacrylate(SA)and cross-linker N,N0 -methylenebisacrylamide
Compositehydrogelmonolithscontainingthermoplastic polyurethane(TPU)microfibers
Hydrogel-polyurethaneinterpenetratingnetworkwith monolithformwaspreparedbycontrollingtheradical polymerizationofthemonomers(N-isopropylacrylamideand sodiumacrylate)
Grapheneoxidenanosheetscovalentlycross-linkedtosodium alginateformeda3-Dandhighlyporousaerogel
Electric-sensitivepoly(vinylalcohol)/poly(acrylicacid) (PVA/PAAc)hydrogels
Electric-responsivehydrogelsbyaqueoussolution polymerizationof2-acrylamido-2-methyl-1-propanesulfonic acidand2-(dimethylamino)ethylmethacrylate
Thermo-responsive N-isopropylacrylamidepolymer copolymerizedwithsuperabsorbentacrylicacidmonomer
Polymerhydrogel—Carboxymethylcelluloseandacrylicacid andquaternarygrapheneoxidenanomodifier
Copolymermicrogelsof N-isopropylacrylamideand acrylamide
Magneticnanoparticles
Citrate-coatedmagneticnanoparticles
Zengetal.(2013)
Li,Zhang,Simon,andWang(2013)
Ou,Zhang,Simon,andWang(2016)
Wei,Low,Ou,Simon,andWang (2016)
Yuetal.(2017a,2017b)
Cuietal.(2017)
Cui,Zhang,Jiang,andYang(2018), Cui,Zhang,Yu,andYang(2018)
GawandeandMungray(2015)
Shakerietal.(2019)
Hartanto,Zargar,Cui,Jin,andDai (2019)
Na, Yang, and Lee(2014), Kadhim, Al-Abodi,andAl-Alawy(2018)
Table1 Severalnoveldrawsolutesusedbyvariousresearchersfor forwardosmosis—cont’d
ProductDrawsolutes
Surfactants
Salts
References
Chitosan-anddehydroascorbicacid-coatedFe3O4 nanoparticles ShabaniandRahimpour(2016)
Hyperbranchedpolyglycerolcarboxylate-coatedmagnetic nanoparticles Yangetal.(2016)
Polyglycerol-graftedsilica-encapsulatedsuperparamagnetic ironoxidenanoparticles Nazarietal.(2017)
Magneticnanoparticle-crosslinkedferrohydrogel Shakeri,Salehi,Khankeshipour, Nakhjiri,andGhorbani(2018)
Micellarsolutionsofcetylpyridiniumchloride,sodiumdodecyl sulfate, and Triton X-100
Anionic(sodiumdodecylsulfate,1-octanesulfonicacid sodiumsalt)andcationicsurfactants(meristyltrimethyl ammoniumbromide,trimethyloctylammoniumbromide,or tetraethylammoniumbromide)
Ethylenediaminetetraaceticacid(EDTA)-2Nacoupledwith TritonX-100
TritonX100andNa3PO4
Poly(propyleneglycol)andTritonX100
Hexavalentphosphazenesalts
Oxalicacidcomplexes
Poly(asparticacidsodiumsalt)
EDTAcomplexes(EDTA-MgNa2,EDTA-CaNa2,EDTA-MnNa2, andEDTA-ZnNa2)
Chlorhexidinegluconate–basedmouthwashhaving antifungalandantimicrobialactivity
Gluconatesaltsforjuicereconcentration
Triethylenetetraminehexapropionicacidsodium (TTHP-Na)—Carboxyethylaminesodiumsalts
Ferric-lactatecomplex
Antiscalant-poly(asparticacidsodiumsalt)blendedsolution
Roachetal.(2014)
Gadelhaetal.(2014)
Nguyenetal.(2018)
Nguyenetal.(2015)
Rayetal.(2018)
Stone,Rae, Stewart, andWilson (2013)
GeandChung(2015)
Gwak,Jung,Han,andHong(2015)
Zhaoetal.(2016)
Rayetal.(2016)
Long,Qi,andWang(2016)
LongandWang(2016)
Yuetal.(2017a)
GwakandHong(2017)
Poly(4-styrenesulfonicacid-co-maleicacid)sodiumsalt Huang,Long,Xiong,Shen,and Wang(2017)
Glaubersalt—Sodiumsulfatedecahydrate
Oligomericcarboxylates—Polymaleicacidsodiumand poly(itaconate-co-acrylate)sodiumsalt
DuttaandNath(2018a,2018b)
Long,Huang,Xiong,Shen,and Wang(2018)
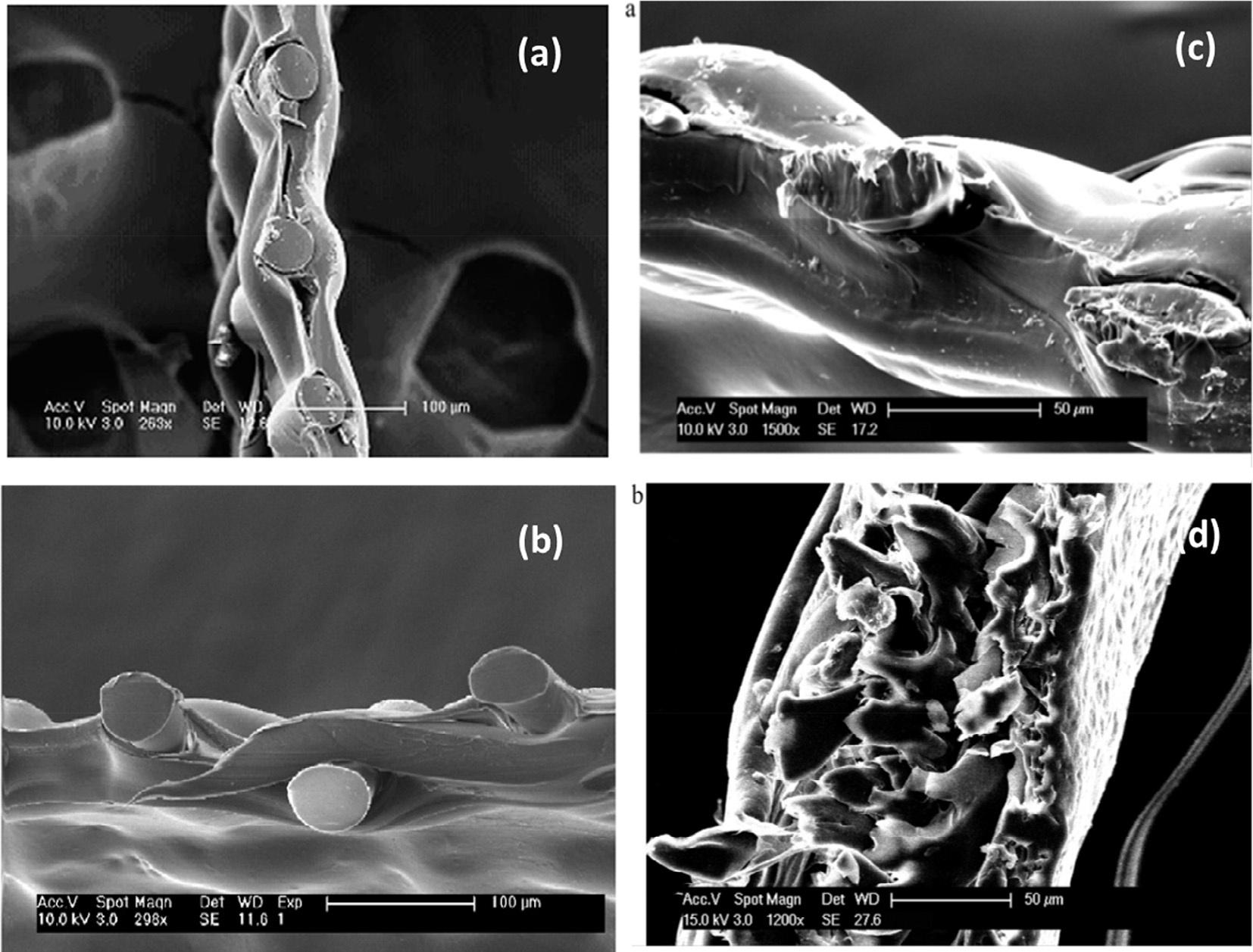
Subsequently,HydrationTechnologiesInc.intheUnitedStatesmanufacturedasuperior membraneforFOusingcellulosetriacetate,whichwasfixedinapolyestermeshtogivesupport,andasupportlayerwasprovided(<50 μm, Fig.3AandB)(McCutcheonetal.,2005;Ng, Tang,&Wong,2006).Thecross-sectionalSEMimagesofverythin( 50m)andthick
FIG.3 (A–D)SEMimagesofcross-sectionsofcellulosicforwardosmosismembrane(CA).Apolyestermeshis embeddedwithinthepolymermaterialformechanicalsupport.Themembranethicknessislessthan50 μm. From McCutcheon,J.R.,McGinnis,R.L.,&Elimelech,M.(2005).Anovelammonia–carbondioxideforward(direct)osmosis desalinationprocess. Desalination, 174,1–11;Garcia-Castello,E.M.,McCutcheonJ.R.,&Elimelech,M.(2009). Performanceevaluationofsucroseconcentrationusingforwardosmosis. JournalofMembraneScience, 338,61–66; Zhao,S.,Zou,L.,Tang,C.Y.,&Mulcahy,D.(2012).Recentdevelopmentsinforwardosmosis:Opportunitiesand challenges. JournalofMembraneScience, 396,1–21.
(>100m)asymmetricFOmembranesmadeofcellulosetriacetate(CTA)areshownin Fig.3C andD(Cathetal.,2006;Herronetal.,1994; Zhao&Zou,2011a,2011b).
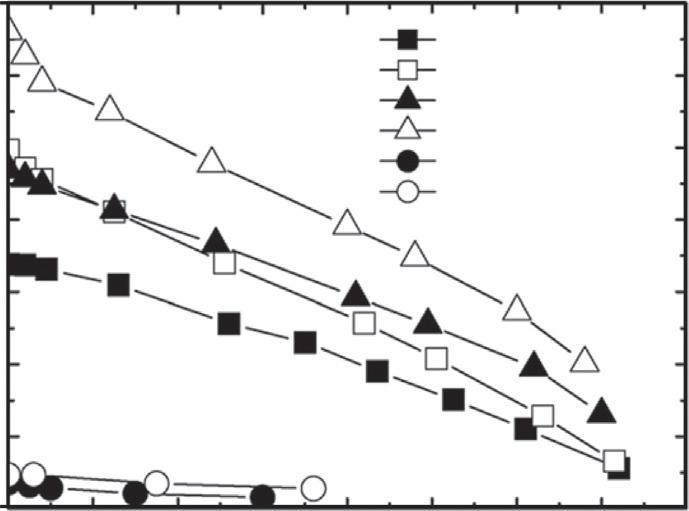
TheperformanceofthecellulosicmembraneproducedbyHydrationTechnologiesInc. (designatedasCA)andthereverseosmosismembranefromGeneralElectricInc. (Osmonics)(designatedasAG)wascompared,anditwaspointedoutthatthewaterfluxes fortheAGmembranewereonly7.7%oftheCAmembrane(Fig.4, Garcia-Castelloetal.,2009).
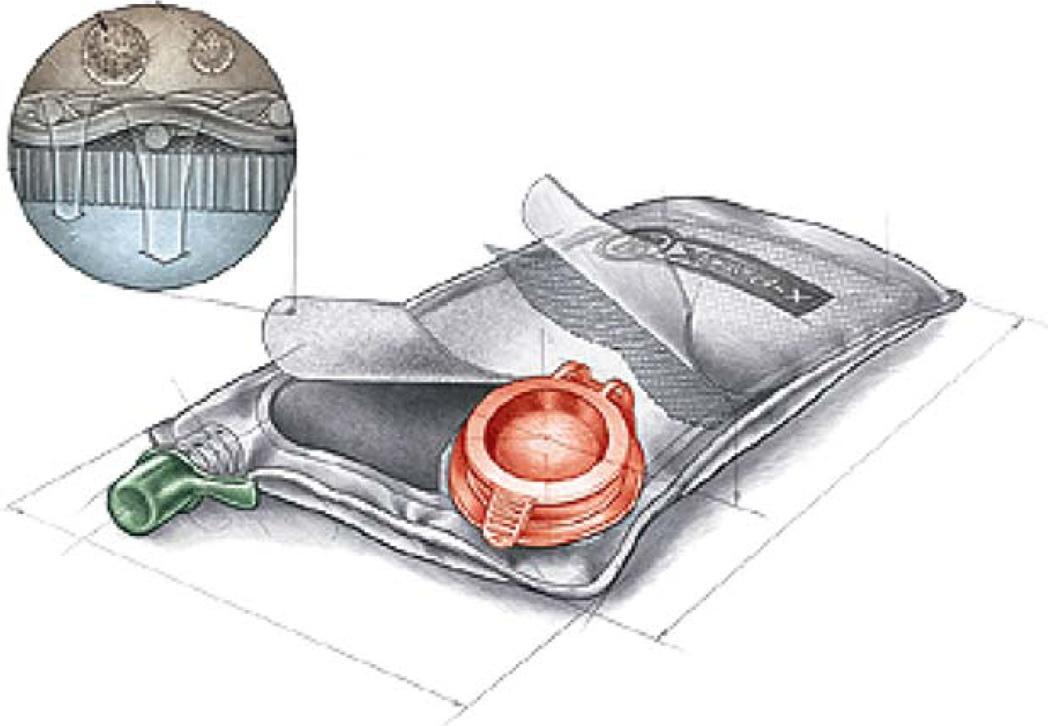
Cathetal.(2006) indicatedthatFOmembranecanbeusedformakingahydrationbag filledwithflavoredsucrose.Subjectingthebagtoanaqueoussolutiondiffuseswaterinto thebag(Fig.5)duetothedifferenceinosmoticpressureandslowlydilutesthedrawsolution.Thistechniquecanbeusedfortherecoveryofwater.Thehydrationbagsweredevelopedformilitaryandemergencyreliefsituationswherereliabledrinkingwaterwasnot available.
CA, 2 M DS, 20°C
CA, 2 M DS, 30°C
CA, 4 M DS, 20°C
CA, 4 M DS, 30°C
AG, 4 M DS, 20°C
AG, 4 M DS, 30°C
00.20.40.60.81.01.21.4 1.6
Sucroseconcentration (M)
FIG.4 Effectoftemperature,drawsolutionconcentration,andsucrosefeedsolutionconcentrationonpermeate waterfluxforboththeCAandAGmembranes.Experimentalconditions:feedsolution(sucrose)concentrationof 0–1.65M(0–48 °Brix),drawsolutionconcentrationof2or4MNaCl,andcross-flowratefordrawandfeedsolutionsof 1Lmin 1 (1.67 10 5 m3 s 1). FromGarcia-Castello,E.M.,McCutcheonJ.R.,&Elimelech,M.(2009).Performance evaluationofsucroseconcentrationusingforwardosmosis. JournalofMembraneScience, 338,61–66.
FIG.5 Illustrationofwaterpurificationhydrationbag. FromCath,T.Y.,Childress,A.E.,&Elimelech,M.(2006). Forwardosmosis:Principles,applications,andrecentdevelopments. JournalofMembraneScience, 281,70–87.
AhollowfiberFOmembranehavingaduallayerforproteinenrichmentconsistingofa fineselectiveskin( 10 μm),open-cellwaterchannels,andaporousspongesupportstructurewasdeveloped;itachievedincreasedpurityforlysozyme( Yangetal.,2009a;Yang, Wang,&Chung,2009b).
Zhangetal.(2010),Wang,Ong,andChung(2010),and Wangetal.(2010) prepareda celluloseacetateFOmembraneconsistingofaporoussublayerthatwassqueezedin betweentwoselectiveskinlayersforpreventingdrawsolutefrompenetratingintothe membranesupport,whichcausedincreasedwaterfluxandlesssaltmigration.Theactive layersshowedexcellentseparationpropertiesandgoodmechanicalstrength.
Wang,Yang,Chung,andRajagopalan(2009) fabricatedanFOmembranehavinghigh fluxandrejectionfordesalinationusingpolybenzimidazolenanofiltrationhollowfiber membranes,whichwerecross-linkedwith p-xylylenedichloride.Later, Su,Yang,Teo, andChung(2010) developedcelluloseacetatenanofiltrationmembranesbyexposing themtothermaltreatment,whicheffectivelyshrunkthemembraneporesleadingtoa higherrejectionofNaClandMgCl2 andlowpurewaterpermeability. Yip,Tiraferri,Phillip, Schiffman,andElimelech(2010) madeahigh-performance,thin-filmcompositemembranehavingaselectivepolyamideactivelayerformedbyinterfacialpolymerization ontopofapolysulfonesupportlayerontoathin(40-μm)polyesternonwovenfabric.
Augmentationofzwitterionwasfoundtoenhancethehydrophilicityandsurface roughnessofanFOmembrane,whichresultedinanimprovementinthewaterpermeability.Theanionicmoietyofthezwitterionwasexposedoutside,whilethecationicmoietiesofthezwitterionweremostlyretainedtowardthecore,assuggestedbynegativezeta potentialandproteinabsorption.Incorporationofzwitterionwasfoundtoenhancethe antifoulingpropertyofthemembrane(Chiaoetal.,2019). Jia,Li,Wang,Wu,andHu(2010) demonstratedthatcarbonnanotubemembranescanbeusedforseawaterdesalination usingFO,leadingtooptimumsaltrejectionandhigherwaterflux,besideshavingantifoulingabilityandgoodmechanicalstrength.Highwaterfluxesareattributedtothecapillarity ofZigzaggraphyne-3nanotubesandthehugeosmoticpressuredifferencebetweenthe feedsolutionanddrawsolution( Wu,Zhang,Chen,Chen,&Gai,2016).Thereasonfor thehighosmoticwaterfluxofthenanotubesisthelowtortuosity,highporosity,and lowthickness,resultinginareductionintheinternalconcentrationpolarizationphenomenon(An,Lee,&Park,2017).ToimprovetheperformanceofFOmembranes,researchers haveuseddifferentmaterialssuchaszwitterionicpolymer,graphene,nanomaterials (fiberandsheets),nanoparticles,andnanotubes.Thelistofdifferentmaterialsusedfor FObyvariousresearchersispresentedin Table2.
5Applicationsofforwardosmosis
ThediversifiedapplicationsofFOprocesshavebeenstudied.FOresultsinincreased rejectionandlessmembranefoulingincomparisontopressure-drivenmembraneprocesseslikereverseosmosis.FOwasusedfortheconcentrationandrecoveryofmanyproducts,suchasgrapejuice,pineapplejuice,raspberryjuice,orangejuice,tomatojuice, naturalfoodcolorant(Garciniaindica extract,beetrootjuice,redradish,etc.),modelfood systems,wheyproteins,ethanolproduction,microalgae,molassesdistillerywaste,sugars concentration,tunacookingjuice,organicacids,fattyacids,andinorganicsalts.The resultsobtainedbyseveralFOresearchersaresummarizedin Table3.
Exploitationofseawaterusingconventionaldesalinationtechnologiesislimiteddueto thesignificantenergyconsumption,highunitcosts,andenvironmentalimpacts.FOmay havepotentialforproducingpotablewaterinanenergy-efficientstyle.Itisamembranebaseddesalinationprocessandaviablealternativetoreverseosmosis,asalower-costand moreenvironmentallyfriendlydesalinationtechnology.Drivenbyosmoticpressure,FO hasattractedgrowingattentionforwaterforrecovery.FOisaneconomicalprocessthat
Table2 Differentmaterialusedbyvariousresearchersforforwardosmosismembrane
TypeMembranematerial
Zwitterionicpolymer
Poly(sulfobetainemethacrylate) (PSBMA)brushes
Hydrophilicsilicananoparticlesand zwitterionicpolymerbrushes
Poly[3-(N-2methacryloylxyethyl-N,Ndimethyl)ammonatopropanesulfonate]
N-Aminoethylpiperazinepropane sulfonate
Features
Super hydrophilic andultralowoil-adhesion properties
Higherfoulingresistance,reducedsurface roughness,enhancedhydrophilicity,and lowersurfacecharge
Lowermembranefoulingrate,lowerenergy requirement,andhigherwaterrecoveryrate
Superiorseparationperformanceand antifoulingproperty
Zwitterion-silvernanocompositeImprovedhydrophilicityandtransport properties;biofoulingresistancedueto antiadhesiveandantimicrobialproperty
Zigzaggraphyne-3nanotubesHigherwaterfluxes
Grapheneoxide-silver nanocomposites
Polyvinylpyrrolidonemodified grapheneoxide(PVP-GO)
NanofillersofTiO2 andgraphene oxide
Reducedgrapheneoxide laminatescoatedwithhydrophilic polydopamine(pDA-coatedrGO)
Polyamide-crosslinkedgraphene oxide(PA-GO)membrane
Magneticallyresponsivegraphene oxide(GO)/Fe3O4 nanohybrid
Nanosizedbactericidalgraphene quantumdots
Sulfonatedgrapheneoxide polyamidethin-filmcomposite membranes
Nanomaterials—Fiberandsheets
Nanocompositeofmesoporous silica nanoparticles andnanofibers
Antibiofoulingpropertywithoutsacrificing themembraneintrinsictransportproperties
Enhanceddesalinationperformance— Higherwaterfluxandlowerreversesolute flux
References
Zhang,Huang,Meng,Li, andCai(2017), Zhang, Tian,etal.(2017)
Liu,Lee,Ma,and Elimelech(2017), Liu, Wu,Liu,andWang (2017)
Lee,Goh,Lau,Ong,and Ismail(2018)
Wangetal.(2018)
QiuandHe(2018)
Wu et al. (2016)
Fariaetal.(2017)
Wu,Field,Wu,and Zhang(2017), Wu, Yoshioka,etal.(2017)
Improvedhydrophilicity,greaterporosity, higherwaterflux,andenhancedantifouling ability Lietal.(2017)
Outstandingwaterfluxandhighsalt rejectionrate
Highwaterfluxandlowsoluteflux
Higherfoulingresistance
Improvedsurfacehydrophilicity, antimicrobialactivity,andFOperformance
Highwaterflux,lowreversesaltflux,and decreasedfoulingpropensity
Yangetal.(2017)
Jin,Wang,Zheng,and Mi(2018)
Rastgar,Shakeri,Bozorg, Salehi,andSaadattalab (2018)
Seyedpour,Rahimpour, Shamsabadi,and Soroush(2018)
Galagedaraetal.(2018)
Osmoticwaterpermeabilityandsodium chlorideselectivityincreasedby7-foldand 3.5-fold,respectively
BuiandMcCutcheon (2016)
Graphene
18CurrentTrendsandFutureDevelopmentson(Bio-)Membranes
Table2 Differentmaterialusedbyvariousresearchersforforwardosmosis membrane—cont’d
TypeMembranematerial
Electrospunpolysulfone(PSf)/ titaniumdioxide(TiO2) nanocompositefibers
Positivechitosanandnegative grapheneoxide(GO)nanosheets
LaminarMoS2 nanosheet
Porousmetalorganiccopper1,4benzenedicarboxylatenanosheets
Nanoparticles
Al2O3 nanoparticlesintoboth substrateandpolyamideactive layer
Features
TiO2 additionresultedinhigher hydrophilicity,porosityandporesizeofthe substratesleadingtohigherwaterfluxthan commercialmembranes
Improvedwaterfluxandselectivityand foulingresistance
References
Zhang,Huang,etal. (2017), Zhang,Tian, etal.(2017)
Salehi,Rastgar,and Shakeri(2017)
Synergisticeffectsofsurfacehydrophilicity, porosityandfouling-releaseproperties Lietal.(2018)
Higherwaterpermeabilityandlowerreverse solutefluxforwaterdesalinationand wastewatertreatment
EnhancementofFOperformanceand stability
Fe3O4 magneticnanoparticlesImprovementofhydrophilicityand roughnessofthemembranes
ZnOandstableZnO-SiO2 coreshellnanoparticles
Longfinger-likeporeswithinthemembrane supportlayersresultedinpermeateflux enhancements
ZeolitenanoparticlesReducedinternalconcentration polarization,increasedsurfaceporosity,and enhancedwaterflux
Highlycompatiblepolyrhodanine nanoparticles
Nanotubes
Membranesurfacewassmootherandmore hydrophilic,providingimprovementin antimicrobial,antifouling,andtransport properties
Dai,Zhang,Liu,Wu,and Wang(2019)
Dingetal.(2017)
Chi,Zhang,Guo,andXu (2017)
Rastgar,Shakeri,Bozorg, Salehi,andSaadattalab (2017)
Salehi,Peyravi, Jahanshahi,Lau,andRad (2018)
Rahimpouretal.(2018)
Titanatenanotubes(TNT) Twotimesimprovementinwaterflux without sacrificing saltrejection Emadzadehetal.(2015)
Imologitenanotubes(INT) Enhancedthehydrophilicity,purewater flux,overallporosity,surfaceporosity,and roughness
Cyclicpeptidenanotubes(CPNT)Highselectivelybetweenwatermolecules andions
Aminefunctionalizedmultiwalled carbonnanotubes(CNTs)
Improvedsurfacehydrophilicitywithhigh waterpermeabilityandacceptablesalt rejection
Pan,Zhao,Gu,andWu (2017)
Wu,Field,etal.(2017), Wu,Yoshioka,etal. (2017)
Amini,Jahanshahi,and Rahimpour(2013)
candesalinatesalinewatersourcesatanotablylessercost.Itisanincreasinglyimportant technologythatishighlypromisingtoaddresswaterscarcityaroundtheglobe.Rapidprogressoverthepastdecadehasbeenmarkedbysignificantinnovationsinmembrane developmentandprocessdesign.Thekeyideaistodevelophybridsystemsinwhich theFOprocesscanreallyaddvalue.