https://ebookmass.com/product/corrosion-of-aluminium-2nd-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
High Temperature Oxidation and Corrosion of Metals 2nd Edition David John Young
https://ebookmass.com/product/high-temperature-oxidation-andcorrosion-of-metals-2nd-edition-david-john-young/
ebookmass.com
International Practice Theory 2nd ed. Edition Christian Bueger
https://ebookmass.com/product/international-practice-theory-2nd-ededition-christian-bueger/
ebookmass.com
Learning Scientific Programming with Python 2nd Edition Christian Hill
https://ebookmass.com/product/learning-scientific-programming-withpython-2nd-edition-christian-hill-2/
ebookmass.com
From Charity to Justice: How NGOs Can Revolutionise Our Response to Extreme Poverty Fang
https://ebookmass.com/product/from-charity-to-justice-how-ngos-canrevolutionise-our-response-to-extreme-poverty-fang/
ebookmass.com
The University as a Site of Resistance: Identity and Student Politics 1. ed. Edition Gaurav J Pathania
https://ebookmass.com/product/the-university-as-a-site-of-resistanceidentity-and-student-politics-1-ed-edition-gaurav-j-pathania/
ebookmass.com
Applications of Nanofluids in Chemical and Bio-medical Process Industry Shriram S. Sonawane
https://ebookmass.com/product/applications-of-nanofluids-in-chemicaland-bio-medical-process-industry-shriram-s-sonawane/
ebookmass.com
Fundamentals of Body MRI 2nd Edition Christopher G. Roth
https://ebookmass.com/product/fundamentals-of-body-mri-2nd-editionchristopher-g-roth/
ebookmass.com
Clinical
Ethics, 8th Edition: A Practical
Approach
to Ethical
Decisions
in Clinical Medicine, 8E 8th Edition, (Ebook PDF)
https://ebookmass.com/product/clinical-ethics-8th-edition-a-practicalapproach-to-ethical-decisions-in-clinical-medicine-8e-8th-editionebook-pdf/
ebookmass.com
Clinical Obstetrics and Gynaecology - E-Book Elizabeth A. Layden
https://ebookmass.com/product/clinical-obstetrics-and-gynaecology-ebook-elizabeth-a-layden/
ebookmass.com
Handbook of Modern Coating Technologies: Applications and Development Mahmood Aliofkhazraei
https://ebookmass.com/product/handbook-of-modern-coating-technologiesapplications-and-development-mahmood-aliofkhazraei/
ebookmass.com
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2020ElsevierLtd.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical, includingphotocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfrom thepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbe foundatourwebsite: www.elsevier.com/permissions .
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanas maybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshould bemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessional responsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforany injuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseor operationofanymethods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-08-099925-8
ForinformationonallElsevierpublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: AnitaAKoch
EditorialProjectManager: MonaZahir
ProductionProjectManager: StalinViswanathan
CoverDesigner: VictoriaPearson
TypesetbyTNQTechnologies
Formygrandchildren,AlexandreVargel,RomainVargel,AlexandraBourgoin, andLaureBourgoin
Foreword
Ifthereisametalofthefuture,itisaluminium.
Aluminium’ssuccesshasbeenconsistentforover100years.Mostrecently,atthestartofthe21st century,annualconsumptionofaluminiumreached29millionmetrictons;by2017,thatfigurehad risento80millionmetrictons.Andsomearepredictingconsumptioncouldreachabout120million metrictonsin2030.
Thisishardlyasurprise.Aluminium’sintrinsicpropertiesmakeitauniqueandextremelyvirtuous materialthatrespondstonumerouschallengesinoursocietylinkedtoclimatechangeandresponsible managementandprotectionoftheEarth’sresources.Aluminium’sabilitytobeendlesslyrecyclable withoutlosingitsinitialpropertiesisanexcellentexampleofthis.
Furthermore,thankstoitslightweightcapability,aluminiumallowstransportationvehiclesonland, intheairandatseatosignificantlydecreasefuelconsumptionandthusdrasticallyreduceemissionsof greenhousegases.Italsocombinesductilityandahighlevelofresistancetobothimpactsand corrosion.
Beyonditsspecificproperties,theinexorablefascinationwithaluminiumisfirstandforemostthe resultofdecadesofresearchandinnovation.Generationsofresearchershaveworkedonthedevelopmentofincreasinglyadvancedalloysandever-moreinnovativemanufacturingprocesses.
Thisisparticularlythecasewiththetechniqueoffrictionstirwelding,whichmakesitpossibleto assembledifferentmaterialswithouttheneedforrivetsoradditivemanufacturingprocesses,also knownas3Dprinting.
AtConstellium asuccessorofthePechiney,AlcanandAlusuissegroupsandagloballeaderin innovative,highvalue-addedaluminiumsolutions wemakesureengineersandtechniciansinour R&Dcentrehaveaccesstothemostsophisticatedresourcesandmethodsavailable.Thanksto manyclosecollaborativeprojectswithourcustomersandnumerouslaboratoriesanduniversities worldwide,ConstelliumhasdesigneduniqueandrevolutionarysolutionssuchasAirwarefortheaerospacesector,SecuralexandHSA6fortheautomotiveindustryandAeralforaerosolpackaging.
Althoughtheyareusedindifferentapplicationsandcomefromawiderangeoffamiliesofalloys, thesenewproductshighlightourindustry’squestforexcellenceandourabilitytoimaginetheworldof thefuturethroughcontinuousoptimizationofthepropertiesofaluminium.
Oneofthefundamentalareasofresearchiscorrosion,thesubjectofthisbook.Althoughitis naturallyresistant,aluminiumneedstoofferever-higherperformance.ThisneweditionofChristian Vargel’sbook, CorrosionofAluminium,reflectstheknowledgehehasaccumulatedoverhis40yearsat Pechineyasanengineerinourresearchcentre.Asaspeakerandauthorofseveralbooksonthemetal, ChristianVargelisarecognizedexpertinthefieldofaluminiumcorrosion,orashewouldputit,a corrosionpractitioner.
Thankstoitsscientificapproachtothetopic,thisbookisbothanexplorationofthephenomenonof aluminiumcorrosionandapracticalguidetotheuseofaluminiumalloysbasedontheircorrosion properties.Aimedatbothacademicsandprofessionals,itisunrivalledinitsfieldandIwouldlike tooffertheauthormywarmestthanksforentrustingitsforewordtome.
Jean-MarcGermain ChiefExecutiveOfficer,Constellium
Forewordtotheoriginaledition
Withanannualconsumptionof25millionmetrictons,aluminiumisthesecondmostcommonlyused metalintheworldaftersteel.Itslightnessisveryoftenthemostimportantadvantageforthecommercialdevelopmentofaluminium,whichexplainswhyitisextensivelyusedforgroundtransport, aerospaceandshipbuilding.Thisisalsothereasonwhytheautomotiveindustryiscurrentlyveryinterestedinaluminium:lightnessisbecomingapriority.
Thesecondadvantageofaluminiumisitscorrosionresistance.Thisexplainsitsimportantposition inconstruction,civilengineering,transport,heatexchangersandsoon.
In1890,navalarchitectshadconsideredaluminiumforreducingweightinvessels.Butinorderfor aluminiumtobeuseableforshipbuilding,metallurgistsandcorrosionspecialistsinthe1930sfirsthad todevelopaluminiummagnesiumalloys.Thesealloyshaveanexcellentcorrosionresistanceinthe marineenvironment,andtheyareweldable.Since1960,allhigh-speedferrieshavebeenbuiltinthese alloys.
Asimilartrendwasobservedwithheatexchangers:aluminiumwasrecognizedasanobvioussolution,especiallyforautomotiveheatexchangerssince1970.Infact,severalalloyshaveverygoodthermal conductivityandexcellentresistancetoenginecoolants,makingitpossibletomanufactureheat exchangersthatarecheaperandofcourselighterthantraditionalheatexchangersincopperalloys.
Projectsfordevelopingrenewableenergysources(solar,etc.)haveoftenbeenbasedontheuseof aluminiumheatexchangersforseveralreasons:amuchlowercostthantitanium,goodthermal conductivityandexcellentcorrosionresistance.
ChristianVargel,throughouthislongcareerwithinPechiney,hasbeenapractitionerofaluminium corrosionandarecognizedexpertinthisfield.Hisfirstbook, LeComportementdel’Aluminiumetde SesAlliages (TheBehaviorofAluminiumandItsAlloys),waspublishedbyDunodin1979.
Sincethen,hisexperiencehasgrownsteadily.Hehasfollowedmarineapplicationsandautomotive heatexchangersandhasparticipatedinmanydamageassessmentsinvolvingcorrosioninservice.He hasalsogivenmanytalksonthecorrosionresistanceofaluminium,andhascontributedtomanyof PechineyRhenalu’stechnicaldocumentsandbrochures,suchas‘AluminiumandtheSea’and ‘AluminiuminIndustrialVehicles’.Wethereforeencouragedhiminhisprojecttowriteasecond book:hisrecognizedexperienceinthefieldofaluminiumcorrosiondeservedtobemorewidelyknown anddisseminated.Thisbookwillcertainlycontributetomeetingthisgoal.
Corrosionisadifficulttopic.Iamdeeplyconvincedthatthepractitioner’sapproach,basedon expertiseandexperience,isbestforassessingthecorrosionresistanceofaluminium,anassessment thatisobviouslyoneofthemainconditionsforthedevelopmentofmanyusesofaluminiumin transportandconstructionpowertransmission.
ChristianVargel’sbookpresentsthereaderwithaglobalapproachtocorrosion,comprisingthe selectionofalloys,designprinciplesandserviceconditions.Iamconvincedthatitwillcontribute tothedevelopmentofaluminiuminthosefieldswhereresistancetocorrosionisanessentialproperty.
BernardLegrand FormerDeputyChiefExecutiveOfficer
Pechiney,September1998 xxxvii
Preface
MylongcareerinthePechineyGroupfrom1957to1997andsubsequentlyasanindependentconsultantprincipallyfocusedonaluminiumcorrosionhasenabledmetoacquireextensiveexperienceinthis field.
Myexperienceisbasedonthetreatmentofthemanycasesofin-servicecorrosionthatIhavehadto dealwithoverthepast50years,andontheelectrochemicalandmetallurgicalfundamentalsof aluminiumcorrosion.
Thisdualapproachhasmademeacorrosionpractitioner,inthesensethatIhadtofindanexplanationforcasesofcorrosioninserviceorthatIhadtoplanhowtoavoiditthroughthechoiceofalloys, operatingconditionsandsoon.
Indeed,corrosionremainsacomplexsubjectbecauseitdependsonmanyparameters,whichmakes itnecessarytohaveapracticalapproachtobeusefulandexplainableonthebasisoffundamentaldata.
Thisengineeringactivityhasallowedmetoacquirewideexperienceinaluminiumapplications, particularlywherethequestionofcorrosionresistanceofbuildings,heatexchangers,renewable energysystems(solar,OTEC,etc.),transport,shipbuildingandsoonisconcerned.
Ihavefrequentlysharedmyexperienceofaluminiumcorrosionatnumerousconferencesandin severalbrochurespublishedbyPechiney:“AluminiumandtheSea,”“AluminiumintheAutomotive Industry,”“AluminiuminIndustrialVehicles,”and“AluminiumSemi-finishedProducts.”
Followingarequestofthepublisher,Elsevier,Iresumedthereeditingofthefirstversionof Corrosion ofAluminium publishedin2004,whichwasthetranslationof Corrosiondel’Aluminium publishedin FrenchbyDunodin1999.
Inthisnewedition,Ihavereviewedandincorporatedtheknowledgeacquiredrecentlybymany laboratoriesthathavepublishedonthesubjectofaluminiumcorrosionanditsmetallurgicalaspects. In20years,newinvestigativemethodshavealsomadeitpossibletoexplainmanyofthephenomena involvedinstructuralcorrosion.
Asaresult,thisneweditionof CorrosionofAluminium,whilemaintainingitspracticalorientation tomeettheneedsofaluminiumusers,givesalargeplacetotheresultsofthehigh-qualityscientific publicationsofthemanyresearchersdedicatedtothestudyofaluminiumcorrosion.1
Likethepreviousversion,thisneweditionaimsatawidereadershiprangingfromaluminium userstoacademia.Bothwillfindusefulinformationonaluminiumcorrosionbasedonthestateof knowledgeacquireduptothetimeofwritingthisnewedition.
Iwouldliketothankallthosewhohavegivenmetheirprecioussupportinthewritingofthisbook:
➢ DrLionelPeguet,corrosionandsurfaceR&Dengineer,CTECConstelliumTechnologyCentre, Voreppe,France
➢ Jean-SylvestreSafrany,researchengineer,SurfaceTreatments,CTECConstelliumTechnology Centre,Voreppe,France
➢ Franc¸oiseSaillard,informationspecialist,CTECConstelliumTechnologyCentre,Voreppe,France
➢ DamienFe ´ ron,CEASaclay,France,presidentoftheEuropeanCorrosionFederation
➢ BrunoSavelli,CEASaclay,France,DirectiondelaRechercheFondamentale,Servicede Valorisationdel’Information
1Over2000publicationsonaluminiumcorrosionpublishedfromthebeginningofthe20thcenturyuptothepresentdayhave beencitedinthisbook.
➢ PhilippeMarcus,directorofresearchattheCentreNationaldeRecherchesScientifique(CNRS), ChimieParisTech
➢ MichelJannier,expertinaluminiumsurfacetreatmentprocesses
➢ MichelPinc¸on,expertinaluminiumsurfacetreatmentprocesses
➢ MichelGarat,aluminiumfoundryconsultant,formerPechineyR&Dmanager
➢ HassinaFounas,executiveassistantoftheFrenchAnti-CorrosionCentre(CEFRACOR),Paris
IwouldliketothankHughDunlopforagreeingtotranslatemytextintoEnglish.IchoseHugh becauseofhisexperienceandknowledgeofaluminiumsurfacephenomenaandsurfacetreatments, havingbeenanengineerandgroupleaderintheConstelliumCTECVoreppeResearchCentre (formerlyPechiney & Alcan)for27yearsandhimselftheauthorofmanypublications.
IwouldalsoliketoexpressmythankstoGeoffScamans,chiefscientificofficeratInnoval Technology(Banbury,UK)forhisexpertreviewofseveralchapters,particularlythoserelatingto thedifferenttypesofaluminiumcorrosion.Iamverygratefulforhisknowledgeableinput.
IwouldliketoexpressmygratitudeonceagaintoAndre ´ Guilhaudis(1918 2008),whowas Pechiney’scorrosionexpertfrom1945to1980.HewelcomedmetothePechineyResearchCentre inChambe ´ ryin1957andsharedwithmehispassionforaluminiumandhisexperienceincorrosion.
C.VARGEL
Inge ´ nieurENSEEG 15November2019
Introductoryremarks
Itiscustomary,andforconvenience,torefertoaluminium,butwhatismeantinmostcasesare aluminiumalloys.Itshouldberecalledthatunalloyedaluminiumaccountsforjustover10%ofthe world’sannualconsumptionofallaluminiumproducts.
However,forthesakeofsimplicity,Iuse‘aluminium’insteadofthetraditionalexpressions ‘aluminiumanditsalloys’or‘aluminiumandal uminiumalloys’.Itshouldnotbeconcludedthat thecorrosionresistanceofallaluminiumalloys isthesameinanyenvironment!Therearecertainly somesimilarities,buttherearealsoimportantdifferencesbetween2XXXand7XXXseriesalloys andthoseoftheotherfamilies.ThatiswhyIhavemadethisdistinctionwheneverappropriate.
Whenoneormorealloyshavebeenusedforcorrosiontestsinagivenenvironment,itseemed desirabletometomentionthembecausetheywerepartofthetestprotocolchosenbythescientists andcontributedtoitsvalidation.Similarly,itseemsessentialtoindicatethealloyscommonlyused inanapplication.Thesearereferencesthathelptoestablishtheuseandstrengthenthechoiceof prescribersandusers.
ThedesignationofwroughtandcastalloysemployedisthatoftheAluminumAssociation1 [1].To facilitatethereadingofPartsEandFdealingwiththecorrosionresistanceofaluminiuminchemicals, Ihavequoted(between{})theirADRnumber,2 whichistheUnitedNationsfour-digitcodeforthe substanceinquestion.ThedesignationoforganicchemicalslistedinPartsMandNshallpreferablybe thataccordingtotherulesofIUAPCnomenclature.3
Excludedfromthescopeofthisworkarealuminiumpowderproducts,powderandgranuleswhose propertiesandapplicationsarenotrelatedtotheapplicationsofcastproducts,wroughtsemifinished products,rolledproducts,extrudedproductsandsoon.Alsoexcludedaresinteredaluminiumpowder compositesaswellasaluminizedsteel.
Reference
[1]KaufmanJB.Understandingwroughtandcastaluminiumalloysdesignations.[Chapter3],ASM International,p.23 37. https://doi.org/10.1361/iaat2000p023.
1TheAluminumAssociation,1400CrystalDriveSuite,430ArlingtonVA22202. 2ADR,accordfordangerousgoodsbyroad.
3IUAPC,InternationalUnionofPureandAppliedChemistry.
Historicalreviews A.1
Chapteroutline
1.1Chemicallyproducedaluminium........................................................................................................4
1.2Electrochemicallyproducedaluminium..............................................................................................4 References..............................................................................................................................................6
ThechemistLouisGuytondeMorveau(1736 1816),aco-workerofAntoineLaurentLavoisier (1743 1794),coinedtheword‘alumine’foroneofthesulphatescontainedinalum. Alumine is derivedfromtheLatinword alumen,whichissaidtohavebeenusedforpotassiumalum KAl(SO4)2 12H2OduringtheRomanperiod.Aluminiumcompoundswereusedinlargequantitiesin antiquepottery,asdyestuffandasanastringentinmedicine [1].Itisnottheword‘alumine’thathas cometodesignatealuminiumorebuttheword‘bauxite’.Thisisbecausein1821PierreBerthier (1782 1867),aminingengineer,discoveredthattheredsoilofthevillageLes-Baux-de-Provencein Francecontained40 50wt.%ofwhathetermed‘hydratedalumina’ [2].
Subsequently,in1861FrenchchemistHenriSainte-ClaireDevillenamedtheore‘bauxite’.In fact,bauxitecompositionsvarydependingonthesourceoforebuttheyusuallycontainaluminium hydroxidessuchasgibbsiteAl(OH)3,boehmite g-AlO(OH)anddiaspore a-AlO(OH),therestbeing composedessentiallyofironoxidesandhydroxides,mainlyhaematite, a-Fe2O3 andgoethite FeO(OH)(thesourceofitsredcolour),andthealuminosilicateclaymineralkaolinite,Al2Si2O5(OH)4. EventhoughitisthemostabundantmetalintheEarth’scrust(83,000ppm)andthethirdmost abundantelementafteroxygenandsilicon,aluminiumdidnotbecomeanindustrialmetalbeforethe endofthe19thcentury.Aluminaisoneofthemoststableofalloxides,withanenthalpyofformation, DG,of 1,582kJ$mol 1 (theenthalpyofironoxideis 1,015kJ$mol 1).Itishenceverydifficultto reducealumina.
ThediscoveryofmetallicaluminiumisattributedtoSirHumphreyDavy(1778 1829).He referredtoitusingtheterm‘aluminium’in1809.Byelectrolysisofmoltenaluminiumsalts,he obtainedanalloyofaluminiumwithiron,becausehehadusedanironcathode [3].ThechemistHans ChristianOersted(1777 1851)andlaterFriedrichWo ¨ hler(1800 1882)chosetoreducealuminium chloridewithpotassium.Thechloridehadbeenpreparedbychlorinationofbauxiteinthepresenceof carbon.ItwasWo ¨ hlerwho,in1827,succeededinproducingasufficientlypuremetaltodetermine someofitsproperties,mostnotablyitslowdensity.
CorrosionofAluminium. https://doi.org/10.1016/B978-0-08-099925-8.00001-6
Therewereinitiallytworoutesfortheindustrialproductionofaluminium:
•Byachemicalmethod(1856 1889);
•Followedbytheelectrochemicalprocessinventedin1886andstillinusetoday.
1.1Chemicallyproducedaluminium
In1854,HenriSainte-ClaireDeville(1817 1881)improvedWo ¨ hler’sprocess.Hereplacedpotassium withsodiumfortworeasons:thereductionof1molofAluses3molofsodium,totalling60g,instead of3molofpotassiumamountingto117g.Atthattime,sodiumwaslessexpensivethanpotassium.He alsoreplacedaluminiumchloride,whichisrathervolatile,withasodiumaluminiumchloride.
ThefirstplantwascreatedinParis,in1856,inthe‘LaGlacie ` re’area,butsoonitwasshutdown:
‘ThesmallplantofLaGlacie`re,locatedinaninnersuburbofParis,amidsthousesandmarket gardens,releasingintotheatmospheresmokeladenwithsodaandchlorine,wasforcedtoceaseits aluminiumproductionafternumerouscomplaints’ [4].
Inthespringof1857,Sainte-ClaireDevillemovedtheplanttoNanterre(nearParis),farawayfrom residentialareas.In1859,productionreached500kg.Thatsameyear,whenLouisLeChatelierhad patentedareductionprocessofaluminawithsodiumcarbonate,aplantwasbuiltinSalindres,closeto Ale ` sintheGardFrenchdepartment,notfarfromthebauxitesupplyandthesaltfieldsofLaCamargue. TheproductioninSalindreshadvariedfrom505kg,whentheplantwasstartedin1860,to2,959kgin 1880,whenthisprocesswasdiscontinued.
Thefirstkilogramsofaluminium,producedin1856,weresoldslightlymoreexpensivethansilver, around300francs,equivalenttoUS$3,200in2016.Duringthedecade1880 1889,themetal producedinSalindreswassoldforbetween60and70francsperkilo,theequivalentofUS$750 800 in2016.Aluminium,whichSainte-ClaireDevillelikedtocomparetosilver,wasmainlyusedfor silverwareandjewellery.CharlesCristofle(1805 1863),thecelebratedParisiansilversmith,produced castartworkmadeofaluminiumalloyedwith2%wt.copper.In1858,thesonofNapoleonIIIwas offeredarattlemadeofaluminium.
1.2Electrochemicallyproducedaluminium
In1871,Ze ´ nobeGramme(1826 1901)inventedthefirstrevolvingmachinecalleda‘dynamo’.The useofpowerfulsourcesofdirectcurrentmadeitpossibletoenvisionproductionmethodsbasedon electrolysis.Sainte-ClaireDevillehadtriedunsuccessfullytoelectrolysemoltenaluminiumchloride. ThemanufacturingprocessofaluminiumbyelectrolysisofmoltenaluminawasdevelopedinFrance byPaulLouisToussaintHe ´ roult(1863 1914),whofiledapatenton23April1886,andintheUnited StatesbyCharlesMartinHall(1863 1914),whofiledhispatenton9July1886.Bothhadsucceeded indissolvingalumina(meltingpoint2030 C)incryolite3NaF AlF3,whichmeltsat977 C,withthe industrialmeltcontainingabout2 3wt.%alumina.
In1887,Bayerfiledapatentforamethodtoextractaluminafrombauxitebasedontheattackof bauxitebyhotcausticsoda.He ´ roultwenttoNeuhausen,Switzerla nd,inordertosetuphisprocess. Theyearafter,hereturnedtoFranceandin1888createdtheFrogesplantnearGrenobleinFrance wheretheproductionwas1,100kgin1889.SeveralplantsweresetupinFrance,Switzerland, andtheUnitedStates.Sixthousandmetrictonswereproducedin1900,andthesalespriceof
TableA.1.1Worldproductionofprimaryaluminium
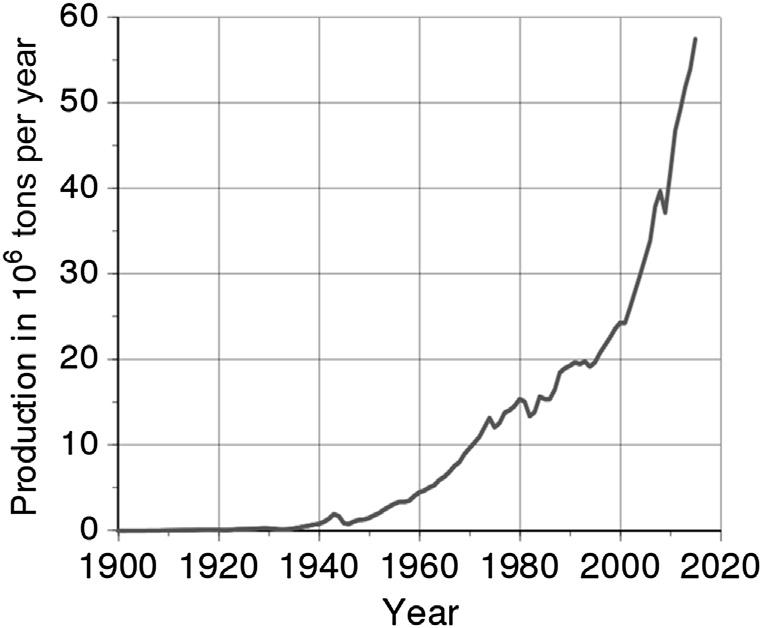
19006 191044 1920125 1930270 1940780 19501,500230 19604,450790 197010,5002,230 198015,4004,670 199019,5008,470 200024,65012,700 201042,30020,200 201557,70025,000 201659,900 201763,400
FrompublishedrecordsoftheWorldAluminium InternationalAluminiumInstitute.
aluminiumstabilizedataroundUS$3.30perkilogr am.Thiswasthestartoftheindustrialadventure ofaluminium.Theworldproductionofso-calle dprimaryaluminiumamountedto6,000tonnes in1900(see TableA.1.1 )andhassteadilyincreased(see Fig.A.1.1),especiallysince1950.More recentlytherehasbeenafurthersharpincreaseassociatedwiththedevelopmentofChinese production(<5,000kTin2000to >30,000kTby2015).
FIGUREA.1.1 Worldproductionofprimaryaluminium.
Itisworthnotingthat,duetoitsinherentresistancetocorrosionandmorerecentlythroughthe developmentofaluminiumrecycling,75%oftotalworldaluminiumproduction(w750MT)isstillin productiveuse(eversince1888).
References
[1]Dictionnairehistoriquedelalanguefranc¸aisesousladirectiond’AlainRey.Paris:EditionsRobert;1994. p.55.
[2]BerthierP.Analysedel’aluminehydrate ´ edesBauxdeProvence.AnnalesdesMines1829;5(2):531 4.
[3]PascalP.Nouveautraite ´ deChimiemine ´ rale.e ´ diteurs,Paris:MassonetCie;1960.
[4]Saint-ClairDevilleH.Del’aluminium.Sesproprie ´ te ´ s,safabrication,etsesapplications.Paris:MalletBachelier,Imprimeur-Libraire;1859.p.8.
CHAPTER
Physicalpropertiesof aluminium A.2
Theprincipalphysicalpropertiesofunalloyedaluminiumarelistedin TableA.2.1
TableA.2.1Propertiesofunalloyedaluminium
Longitudinalelasticmodulus,EMPa69,000
Poisson’sratio, n 0.33
aThisisthegenerallyacceptedvalueforthedensityofmetalbetween99.65%and99.99%pure.At700 C,thedensityof moltenmetalof99.996%purityis2,357kg.m 3 CorrosionofAluminium. https://doi.org/10.1016/B978-0-08-099925-8.00002-8
Thereflectivityofbareandanodizedaluminiumdependsbothonthesurfaceaspectandonthe wavelength(Fig.A.2.1).Reflectivityincreaseswithpurity:withbrightmetalanodizedtoanoxide thicknessof5 mm,reflectivityincreasesfrom75%onmetalwithapurityof99.6%to85%reflectivity fora99.99%puremetal(Fig.A.2.2).
FIGUREA.2.1
Reflectivityofaluminium,silver,goldandcopper.
FIGUREA.2.2
Effectofpurityonthereflectivityofanodizedaluminium(5 mmthickanodizationlayer).
Theadvantagesofaluminium A.3
Chapteroutline
3.1Thehymnofthecannonball
PresidentBarbicanhad,withoutlossoftime,nominatedaworkingcommitteeoftheGunClub.The dutyofthiscommitteewastoresolvethethreegrandquestionsofthecannon,theprojectile,andthe powder.
Gentlemen,saidhe,wehavetoresolveoneofthemostimportantproblemsinthewholeofthenoble scienceofgunnery.Itmightappear,perhaps,themostlogicalcoursetodevoteourfirstmeetingtothe discussionoftheenginetobeemployed.
Theproblembeforeus,continuedthepresident,ishowtocommunicatetoaprojectileavelocityof 12,000yardspersecond.
Thesideswillrequireathicknessoflessthantwoinches. Willthatbeenough?askedthemajordoubtfully. Clearlynot!repliedthepresident.
Whatistobedone,then?saidElphinstone,withapuzzledair. Employanothermetalinsteadofiron. Copper?saidMorgan.
No!thatwouldbetooheavy.Ihavebetterthanthattooffer. Whatthen?askedthemajor.
Aluminium!repliedBarbican. Aluminium?criedhisthreecolleaguesinchorus.
Unquestionably,myfriends.Thisvaluablemetalpossessesthewhitenessofsilver,theindestructibilityofgold,thetenacityofiron,thefusibilityofcopper,thelightnessofglass.Itiseasilywrought, isverywidelydistributed,formingthebaseofmostoftherocks,isthreetimeslighterthaniron,and seemstohavebeencreatedfortheexpresspurposeoffurnishinguswiththematerialforour projectile.
Whatwilltheprojectileweighthen?askedMorgan.
Hereistheresultofmycalculations,repliedBarbican.Ashotof108inchesindiameter,andtwelve inchesinthickness,wouldweigh,incast-iron,67,440pounds;castinaluminium,itsweightwillbe reducedto19,250pounds.
Capital!criedthemajor;butdoyouknowthat,atninedollarsapound,thisprojectilewillcost
Adopted!repliedthethreemembersofthecommittee.Soendedthefirstmeeting.Thequestionofthe projectilewasdefinitelysettled.
From TheEarthtotheMoon,Chapter7 JulesVerne,1865.
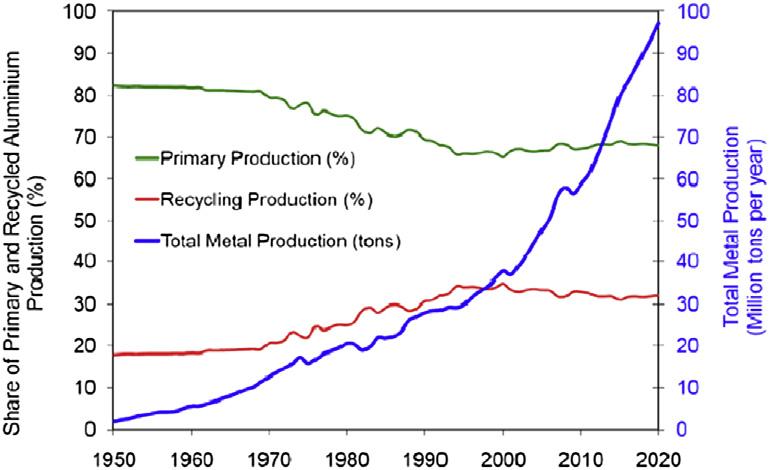
Withanannualworldconsumptionof59milliontonsin2016,aluminiumistheleaderinthe metallurgyofnon-ferrousmetals.Theproductionofaluminiumhasbeenincreasingsteadilysince 1950(see Fig.A.3.1).
Shareofprimaryandrecycledaluminiumintotalaluminiumproduction.
FromWorldAluminium InternationalAluminiumInstitute.
FIGUREA.3.1
Thedevelopmentofapplicationsforaluminiumanditsalloys,aswellasthesustainedrisein consumption,canbeattributedtoseveralofitspropertieswhicharedecisivecriteriainusers’choiceof metals,especiallyinthefieldsoftransport,building,electricalengineeringandpackaging. Theseadvantageouspropertiesare:
➢ Lightness;
➢ Thermalconductivity;
➢ Electricalconductivity;
➢ Suitabilityforsurfacetreatments;
➢ Corrosionresistance;
➢ Diversityofaluminiumalloys;
➢ Diversityofsemi-products;
➢ Functionaladvantagesofextrudedandcastsemi-products;
➢ Easewithwhichaluminiumcanbeformed;and
➢ Easeofrecycling.
3.2Lightness
Thediscoverersofaluminiumwereparticularlyimpressedbythelowdensityofthismetal.
‘Aluminiumismuchlighterthananyothercommonmetal,andthekindofsensationwhichitgivesyouto carryaningotofthismetalisalwaysamazing,evenifyoualreadyknowaboutthispeculiaraspect’ [1]
Lightnessisthepropertyofaluminiumthatfirstspringstomind,somuchsothatforalongtimethe term‘lightalloy’wasusedforwhatisnowcalled‘aluminiumalloys’.Aluminiumisthelightestofall commonmetals(TableA.3.1).Itsdensityis2,700kg.m 3,whichisalmostthreetimeslessthanthatof steel.Thedensityofaluminiumalloysrangesfrom2,600to2,800kg.m 3
Experiencehasshownthatanaluminiumalloystructurecanbeupto50%lighterthanits equivalentmadefrommildsteelorstainlesssteel.Thistakesintoaccountthemodulusofelasticity (one-thirdofthemodulusforsteel)andthefatiguelimitsofweldedorboltedstructuresmadeof aluminiumalloys.Itisnotappropriatetosimplytransposetherulesforsteeldesigntoaluminium. Rather,thespecificpropertiesofaluminiumneedtobetakenintoaccount.
Severalareasoftechnologytakeadvantageofaluminium’slightness:
➢ Transportbyland,seaorair.Theneedtolimitemissionsofcarbondioxideandotherpollutinggaseous emissionsmeansthatthefuelconsumptionofcarsmustbereduced.Theyneedtobelightened. Aluminiumisthematerialchosenmoreandmorefrequentlytomeetthischallengefacingthe automotiveworld.Inordertoachieveweightsavingsinhigh-speedferries,shipyardshaveturned toaluminiumalloysofthe5XXXand6XXXseries.Experiencehasshownthatweightsavingsin hullstructurescanalsobeashighas40 50%comparedtoanequivalenthullstructuremadeofsteel;
➢ Ontheotherhand,aluminiumhasalwaysbeenthemainmaterialusedforthefabricationofaircraft load-bearingstructures(wings,fuselageandempennage)andforspaceapplications.Althoughnow undercompetitionfromcarbonfibrereinforcedplasticcomposites(CFRP)forcertainaircraft applicationssuchaswingsincivilairliners,itisstillmaintainingitsleadershipduetothe developmentofnewhigh-performancealloyssuchasAlCuLialloyslikeAA2198,AA2050, AA2090andothers.ItalsoholdsseveralundeniableadvantagesoverCFRP,includingthoseof damagetoleranceandisotropicmechanicalproperties,recyclability,electricalandthermal conductivityandcost;
TableA.3.1Comparisonoftypicalproperties
Meltingpointor meltingrange( C)
Volumicmass, r (kg.m 3)2,7002,7902,6602,8107,8207,9008,940
Coefficientoflinearexpansion, a1, at20 100 C(10 6 K 1)
Thermalconductivity, l at 20 C(W.m 1.K 1)
Electricconductivityat 20 C(mS.m 1)
Proofstress, Rp0.2 (MPa)80275215505240 >23069
Tensilestress, Rm (MPa)115425305570410 700235
Elongation, A (%)621101024 5045
Elasticmodulus(MPa)69,00072,50071,00072,000210,000200,000115,000
Brinellhardness(HB)351109016011020045
Meanvalues,givenforguidance.
➢ Mechanicalengineering.Thereplacementofasteelcomponentbyoneofthesamesizemadefrom a2XXX,7XXX,5XXXor6XXXseriesalloyleadstoaweightsavingontheorderofthedensity ratio,thatis,60%.Aluminiumiswidelyusedformovingparts,forexampleinrobots,inorderto minimizeinertia.
Lightnessisnotonlyanadvantagefortheapplicationitself,butalsoaffectsfactoryoperationsand workingconditions.Handlingsemi-productsandcomponentsmadeofaluminiumalloysiseasier, potentiallycuttingthecapitalcostofhandlingequipment.
3.3Thermalconductivity
Unalloyedaluminiumisanexcellentheatconductor,withroughly60%ofthethermalconductivityof copper,theoptimumperformeramongcommonmetals.Thethermalconductivityofaluminiumalloys dependsontheircompositionandmetallurgicaltemper.Asearlyastheendofthe19thcentury,this propertyledtoreplacingtin-platedcopperwithaluminiumalloysinthemanufactureofkitchen utensils,bothfordomesticandprofessionaluse.
Wheneverthereisaproblemrelatedtoheatexchange,theuseofaluminiumisalwaystakeninto consideration,underthecondition,ofcourse,thatthemediumisappropriatewhenliquid liquidor liquid gaseousexchangeisenvisaged.Therearemanyapplicationsofaluminiumheat-exchangers: cars,commercialvehicles,refrigerators,airconditioning,desalinationofseawater,solarenergy, coolersinelectronicdevices,andsoon.
3.4Electricalconductivity
Theelectricalconductivityofaluminiumisaroundtwo-thirdsthatofcopper,whichitisreplacingfor manyelectricalapplications.OverheadpowertransmissionlinesmadeofaluminiumorAlmelec-type aluminium,onthemarketinFrancesince1927 [2],areusedthroughouttheworld.Aluminiumbars andtubesarealsowidelyusedinconnectingstationsforhigh-andmedium-voltageoutdoornetworks.
3.5Resistancetocorrosion
Sainte-ClaireDevilleobservedthataluminiumhadgoodresistancetoatmosphericcorrosion,which includedtheparticularatmosphereofgaslamps(usedforstreetlightingduringthe19thcentury), whichwasladenwithhydrogensulphide(H2S).Healsorecognizedtheverygoodresistanceof aluminiumincontactwithwater.
Manydecadesofexperiencewithitsuseinbuildings,publicworks,shipbuilding,andsoonhave confirmedtheobservationsofthe19thcenturychemists.Aluminiumandthealloysofthe1XXX, 3XXX,5XXX,6XXXand8XXXserieshaveexcellentresistancetoatmosphericcorrosioninthe marine,urbanandindustrialenvironments(seeChapterH.5).
Thisverygoodresistancetocorrosion,asmuchaslightness,explainsthedevelopmentof numerousaluminiumapplicationsandoffersusersanumberofmajoradvantages:
➢ Equipmentandcomponentscanhaveaverylongservicelife,extendingtoseveraldecades; 1
➢ Maintenanceisminimal,evenwhennoextraprotection(painting,anodizing)isprovided;
➢ Appearanceispreservedlonger,becauseofaverygoodresistancetocorrosion.2
3.6Suitabilityforsurfacetreatments
Aluminiumsurfacetreatmentscanserveseveralpurposes,including:
➢ Protectingcertainalloysiftheirnaturalcorrosionresistanceisdeemedinsufficient,oftenthecase withcopper-containingalloysofthe2XXXand7XXXseries;
➢ Preservingthesurfaceaspect,inordertoavoidpittingcorrosionorblackening;
➢ Modifyingcertainsurfacepropertiessuchassuperficialhardness;
➢ Decoratingthemetal.
3.7Thediversityofaluminiumalloys
Witheightseriesorfamilies,aluminiumalloysareverynumerousandofferawiderangeofcompositions,propertiesanduses.
Thecontinuingprogressinthemetallurgyofaluminiumhasproducedhigh-performancealloys thatarewellsuitedtoalltypesofapplications,usingconventionalorspecialfabricationtechniques amongothers.Whilealloysinthesameseriessharecommonproperties,oneseriescandiffergreatly fromanother,andcertainpropertiescanvarywidely.Thusalloysinthe5XXXseriesareweldableand generallyhavegoodcorrosionresistance,whilealloysinthe2XXXserieshavebettermechanical properties,butcannotbeweldedusingconventionaltechniques,andtheirresistancetoatmospheric corrosionispoor.
Howevertemptingtheprospect,thismeansthatitisnotalwayspossibletoswitchfromoneseries toanotherinthesearchforbettermechanicalproperties.Forexample,onewouldnotreplaceAA6061T6byAA2017A-T4withoutathoroughanalysisoftheprevailingserviceconditions.Otherwise,a choicebasedonasinglecriterion,heremechanicalproperties,maywellpenalizetheuseronother propertiesincludingcorrosionresistance.
3.8Thediversityofsemi-products
Thetransformationtechniquesofaluminiumrolling,extrusionandcastingpresentdesignersand manufacturerswithaverywiderangeofsemi-products:
➢ Castings:sandcastingsforsmallseries,mouldcastingsforlargeseries;
➢ Flatrolledproducts:plateandsheet,treadplate,coil-coatedsheet,andothers;
1Itisnotuncommontofindinserviceasroofing[includingthatofachurchinRome(seeChapterH.5, x1)],claddings, marinaequipmentandboatsthathavehadseveraldecadesofservice.
2Aluminiumcorrosionproductsarewhite.Theydonotstainbareorpaintedmetalsurfaceslikerustdoesonsteel.
➢ Extrusions:holloworfullprofiles,instandardorcustomizedshapes;
➢ Die-forgedorhand-forgedproducts.
Severalalloysarecompatibleforweldingpurposes.Rolledorextrudedsemi-productsofthe 3XXX,5XXXand6XXXXseriescanbejoinedwithcastingsinalloys356.0,357.0and360.0by meansofTIGandMIGwelding.
Thisdiversityofsemi-productsmakesitpossibleto:
➢ Selecttherightlocationforstressesoncomponentsofboltedorweldedstructures;
➢ Simplifyfinishingprocessesbyusingcoil-coatedorpre-anodizedsheets;
➢ Saveonthetimeneededforassembly,whichcancompensatefortheaddedrawmaterialcostof structuresmadefromaluminiumalloyscomparedwithequivalentsteelstructures.
3.9Thefunctionalityofcastingsandextrusionsfunctionality
Castingmakesitpossibletomanufacturepieceswithcomplexshapesandseveralfunctions,reducing complexmachiningtosimplemachiningorsurfacemilling.Extrusionallowsmanufacturingprofiles withaverywiderangeofdimensionsandshapes,profilesthatarewellsuitedtotheneedsofdesigners whoneedtoselecttherightlocationforstressesonstructures.Extrusiondiesarenormallyeasyto manufactureatamoderatecost.3
3.10Easeofuse
Providedthatcertainrulesspecifictoaluminiumalloysareobserved,aluminiumalloyscanbeprocessedusingthesameconventionaltechniquesofshaping,bending,fabrication,deepdrawingand machiningasusedforothercommonmetalssuchasmildorstainlesssteel.
Aluminiumalloyscanusuallybeprocessedwithouttheneedforspecificequipmentormachine tools.Itisadvisable,however,tosetupaworkshopdedicatedtoaluminiumalloyprocessing;this workshopshouldbeseparatedfromtheworkshopprocessingsteelandespeciallycopperalloys.Like allothercommonmetals,aluminiumalloyslendthemselvestojoiningtechniquessuchaswelding, brazing,riveting,bondingandsoon.
3.11Recycling
Aluminiumrecyclingisundercontinualdevelopment,bothinthecontextofenergysavingsandfor economicreasons.Aluminiumre-meltingrequiresonly5%oftheenergythatisneededtoextractthe primarymetalfromitsore.Decadesofexperiencewithscrapcollectinghaveshownthataluminium scrapalwayshasahighermarketvaluethansteelscrap.Alsosignificantisthesteadyriseinthe consumptionofrecycledmetaloverthelast20years;itnowstandsatabout30%oftheproductionof primaryaluminium(Fig.A.3.1,p.10).
3Itispossibletomaketools(dies)forareasonablecosttomakecustomizedprofilesadaptedtoaspecificuse.
References
[1]Saint-ClairDevilleH.Del’aluminium.Sesproprie ´ te ´ s,safabrication,etsesapplications.Paris:MalletBachelier,Imprimeur-Libraire;1859.p.15.
[2]SuhrJ.Unalliaged’aluminium-magne ´ sium-silicium,l’Almelec.Revuedel’AluminiumAvril1927;(18): 412 22.
Aluminiumalloyseries A.4
Chapteroutline
4.1Alloyseries....................................................................................................................................18
4.2Alloyingelements...........................................................................................................................19
4.3Additives.......................................................................................................................................19
4.4Impurities......................................................................................................................................19
4.5Designationofaluminiumalloys................................................................................................
Unalloyedmetalsgenerallyhaveonlyfewapplications:whiletheyexhibitver yparticularproperties, thesepropertiesareoftenlimitedtoaverynarrowfieldofapplication.Theartofthemetallurgististo createalloysfromagivenbasemetal,beitcopper,ironoraluminium,byaddingcontrolledamounts ofothermetals(ormetalloids)inordertoimprove ormodifycertainpropertiessuchasmechanical properties,formabilityorweldability.Thisishow,morethan5000yearsago,themostancient metallurgistsdiscoveredthatbyaddin gtintocopper,theyproducedanalloy bronze thatwas easytomouldandthatofferedanoutstandingresistancetomarinecorrosion.
Sainte-ClaireDevillepreparedanaluminiumalloycontaining10wt.%silicon.Thesilversmith CharlesCristofle 1 usedanalloycontaining2wt.%copper,thusharderandeasiertochisel,for objectshemanufacturedinaluminiumdu ringtheSecondEmpireinFrance(1852 1870). Immediatelyafterthedevelopmentof electrolysisofmoltensaltsbyPaulHe ´ roultinFranceand CharlesMartinHallintheUnitedS tatesin1866,metallurgiststrie dtoimprovethepropertiesof aluminiumsuchasmechanicalresis tance,resistanceathightemper atures,machinability,corrosion resistanceandsoon.
Researchanddevelopmentworkcarriedoutforover100 yearsonthecompositionofalloys, theirtransformationprocesses,theheattreatmen tprocessesandonoptimiz ingallthesefactorshas resultedinawiderangeofalloys,fromwhichuser scanselectthosebestsuitedtotheirspecific requirements.
1CharlesChristofle(1805 1863)wasaParisiansilversmithandfounderofthecompanyofthesamename.Hemadesome famousaluminiumcutlery.
CorrosionofAluminium. https://doi.org/10.1016/B978-0-08-099925-8.00004-1 Copyright © 2020ElsevierLtd.Allrightsreserved.
Amplitudeofthemechanicalpropertiesofaluminiumalloys(minimumvaluesonsheets1.5 3mmthick, accordingtostandardEN485-2).
Thediversityofalloysandthewiderangeofcertainpropertiessuchastheirtensilestrengthranging from100to700MPaexplainsthegrowthinapplications,bothnumerousandvaried,fromaeronautics topackaging(Fig.A.4.1).
4.1Alloyseries
Allaluminiumproductsbelongtooneofeightalloyseries.Theyareavailableascastingsandwrought semi-productsthatareflatrolled,extrudedorforged.
Alloysbelongingtothesameseriesexhibitasetofcommonpropertiessuchascastability, mechanicalproperties,extrudability,corrosionresistanceandothers.Thesepropertiescanvary considerablyfromoneseriestoanother.Foragivenuse,itisthereforenotalwayspossibleor desirabletoswitchtoanotherseries.
Thesameappliestometallurgicaltempers.Astr ain-hardenedtemper(temperH1X)willnothave thesamedeformationcapacityasasofttemper(te mperO)instrain-hardenablealloys,andasan agedtemper(temperT4)inage-hardenablealloys.Thechemicalcompositionofindustrialalloysis influencedbyalloyingelements,additivesandimpurities.
FIGUREA.4.1