https://ebookmass.com/product/coronavirus-disease-from-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak Hussin A. Rothan
https://ebookmass.com/product/the-epidemiology-and-pathogenesis-ofcoronavirus-disease-covid-19-outbreak-hussin-a-rothan/
ebookmass.com
2019 novel coronavirus (2019-nCoV) outbreak: A new challenge Tommaso Lupia
https://ebookmass.com/product/2019-novel-coronavirus-2019-ncovoutbreak-a-new-challenge-tommaso-lupia/
ebookmass.com
COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses Muhammad Adnan Shereen
https://ebookmass.com/product/covid-19-infection-origin-transmissionand-characteristics-of-human-coronaviruses-muhammad-adnan-shereen/
ebookmass.com
Greek Gods Box Set: Books 1 - 4 Holly Rayner
https://ebookmass.com/product/greek-gods-box-set-books-1-4-hollyrayner/
ebookmass.com
Set Lighting Technicianu2019s Handbook: Film Lighting Equipment, Practice, and Electrical Distribution 4th Edition, (Ebook PDF)
https://ebookmass.com/product/set-lighting-technicians-handbook-filmlighting-equipment-practice-and-electrical-distribution-4th-editionebook-pdf/
ebookmass.com
John Locke and the Grounds for Toleration 1st Edition Flavio Fontenelle Loque
https://ebookmass.com/product/john-locke-and-the-grounds-fortoleration-1st-edition-flavio-fontenelle-loque/
ebookmass.com
Butler, Vermont Series: Boxed Set, Books 1-8 Force
https://ebookmass.com/product/butler-vermont-series-boxed-setbooks-1-8-force/
ebookmass.com
Economics of Social Issues, 21e 21st Edition Charles A. Register
https://ebookmass.com/product/economics-of-social-issues-21e-21stedition-charles-a-register/
ebookmass.com
Sustainable Horticulture: Microbial Inoculants and Stress Interaction Seymen
https://ebookmass.com/product/sustainable-horticulture-microbialinoculants-and-stress-interaction-seymen/
ebookmass.com
Biology. Concepts & Applications 10th Edition Cecie Starr
https://ebookmass.com/product/biology-concepts-applications-10thedition-cecie-starr/
ebookmass.com
CoronavirusDisease
Thispageintentionallyleftblank
CoronavirusDisease FromOrigintoOutbreak
Editedby AdnanI.Qureshi
ZeenatQureshiInstitutesandDepartmentofNeurology, UniversityofMissouri,Columbia,MO,UnitedStates
OmarSaeed
DepartmentofNeurology,UniversityofTennesseeHealthScienceCenter, Memphis,TN,UnitedStates
UzmaSyed
SouthShoreInfectiousDiseases,Bayshore;TravelMedicineConsultants andAntibioticInfusionCenter,Syosset,NY,UnitedStates
AcademicPressisanimprintofElsevier
125LondonWall,LondonEC2Y5AS,UnitedKingdom 525BStreet,Suite1650,SanDiego,CA92101,UnitedStates 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
Copyright 2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyright LicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions .
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby thePublisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatter ofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-824409-8
ForinformationonallAcademicPresspublicationsvisitour websiteat https://www.elsevier.com/books-and-journals
Publisher: StacyMasucci
AcquisitionsEditor: KattieWashington
EditorialProjectManager: PatGonzalez
ProductionProjectManager: MariaBernard
CoverDesigner: MatthewLimbert
TypesetbyTNQTechnologies
Timescaleoftransmission75
Susceptiblegroups76
Clinicalmanifestations 77
Initialphase78
Pulmonaryphase78
Inflammatoryphase80
Extrapulmonarymanifestations80
Clinicalclassificationofsymptomaticpatients85
Riskfactorsforseveredisease85
Diseasecourseinspecialgroups87
Diagnosis 89
Specificdiagnostictests89 Laboratoryfindings92
Radiologicalfindings94
Casedefinitions97
PrecautionaryguidelinessetupbytheCentersfor DiseaseControlandPreventionregardingthetesting processofCOVID-19andlaboratorybiosafety 101
Useofpersonalprotectiveequipment101
Collecting,handling,andtestingclinicalspecimensfor COVID-19103 COVID-19laboratorybiosafety105 References 107
8.Treatmentandtherapeuticagents
IqraNaveedAkhtar
EmergencyuseauthorizationsduringtheSARS-CoV-2 pandemic 123
Whatisanemergencyuseauthorization?123
EUAforvaccinedevelopment124
PartI:Antiviraldrugtherapy 126
DrugsthatinhibitSARS-CoV-2cellentry,endocytosis,and membranefusion126
DrugsthatinhibitproteolysisofSARS-CoV-2134
DrugsthatinhibittheRNA-dependentRNA-polymerase (RdRp)ofSARS-CoV-2136
Drugswithunspecifiedantiviralactivity142
PartII:Immunomodulatoryagents 144
Corticosteroids144
PartIII:Convalescentplasma,Intravenousimmunoglobulin, andCell-basedtherapies 155
ClinicalResearch 156
Wuhan,China156
TheNetherlands(CONCOVID)156
Contributors
IqraNaveedAkhtar,ZeenatQureshiStrokeInstitute,Columbia,MO,UnitedStates
YaseminAkinci,ZeenatQureshiStrokeInstitute,UniversityofMissouri,Columbia, MO,UnitedStates;IstanbulUniversity-Cerrahpasa,CerrahpasaSchoolof Medicine,DepartmentofNeurology,Istanbul,Turkey
ImaanBashir,AlbirrMedicalResearchConsultants,Gainesville,FL,UnitedStates
MohammadRaufA.Chaudhry,DepartmentofNeurology,TexasTechUniversity HealthScienceCenter,ElPaso,TX,UnitedStates;ZeenatQureshiStrokeInstitute, St.Cloud,MN,UnitedStates
IrynaLobanova,ZeenatQureshiStrokeInstituteandDepartmentofNeurology, UniversityofMissouri,Columbia,MO,UnitedStates
AhmedA.Malik,DepartmentofInternalMedicine,UCF-COM/HCAGME Consortium,NorthFloridaRegionalMedicalCenter,Gainesville,FL,United States;ZeenatQureshiStrokeInstitutes,Columbia,MO,UnitedStates
AbhiPandhi,UniversityofTennesseeHealthScienceCenter,Memphis,TN,United States
AdnanI.Qureshi,ZeenatQureshiStrokeInstituteandDepartmentofNeurology, UniversityofMissouri,Columbia,MO,UnitedStates
IhteshamQureshi,FellowshipPhysician,Epilepsy,DepartmentofNeurology, UniversityofTexasHealthScienceCenteratHouston,Houston,TX,UnitedStates
UsmanSaeed,IndependentAdvisoronInternationalsPoliticalEconomy
IshitaVasudev,SirGangaRamHospital,NewDelhi,Delhi,India
GhaidaZaid,DepartmentofNeurology,UniversityofTennesseHealthScience Center,Memphis,TN,UnitedStates
Thispageintentionallyleftblank
Chapter1 Introduction
AdnanI.Qureshi
ZeenatQureshiStrokeInstituteandDepartmentofNeurology,UniversityofMissouri,Columbia, MO,UnitedStates
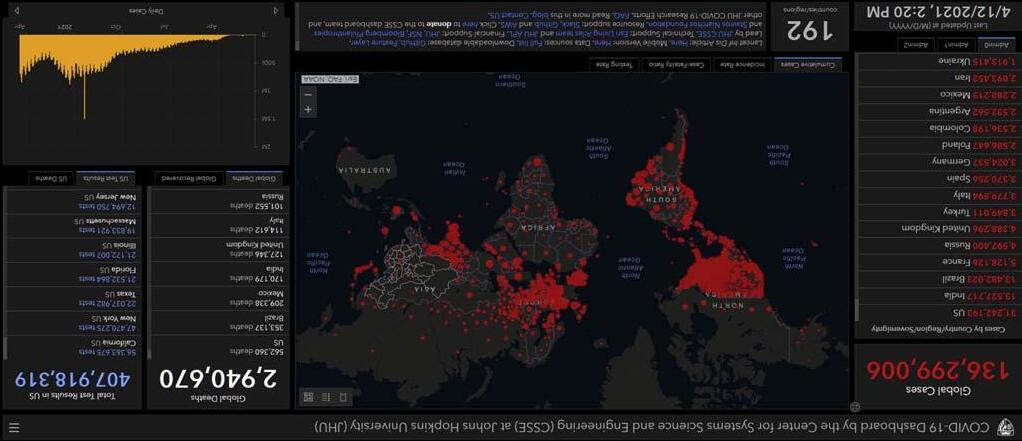
Thegoalofthisbookistoprovideadetaileddescriptionwitheasy-to-understandaccountsofoneofthefastestgrowinginfectionsintheworld.An outbreakofrespiratorydiseasewascausedbyanovelcoronavirusthatwas firstdetectedinChinaandwhichhasnowbeendetectedinalmostevery locationinternationally.Therespiratorydiseasecausedbyvirushasbeen named“coronavirusdisease2019”(COVID-19).AnoutbreakofCOVID-19 beganinWuhan,HubeiProvince,China,inDecember2019.OnJanuary30, 2020,theWorldHealthOrganizationdeclaredtheChineseoutbreakof COVID-19tobeaPublicHealthEmergencyofInternationalConcernposinga highrisktocountrieswithvulnerablehealthsystems.ByFebruary23,2020, therewere76,936reportedcasesinmainlandChinaand1875casesinlocationsoutsidemainlandChina.ByMarch5th, 2020,360casesofCOVID-19 werereportedintheUnitedStates.AsofApril2021,136millionpersonshad beeninfectedbythenovelcoronaviruswith2.94millionpersonsdyingfrom theinfectionworldwide.Severalweb-basedresourceshavebeencreatedto providereal-timeupdatesontheoccurrenceofCOVID-19.Oneofthemost widelyusedisdevelopedatJohnsHopkinsUniversityavailableat COVID-19 Map-JohnsHopkinsCoronavirusResourceCenter(jhu.edu).Theinterfaceis shownin Fig.1.1.
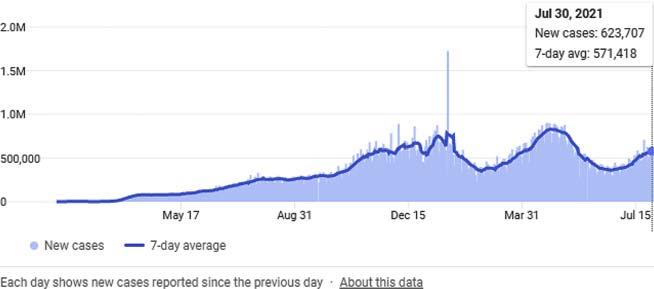
TheprogressionofCOVID-19overtimeisshownin Fig.1.2 adaptedfrom Wikipedia.
ThetopfivecountrieswiththehighestratesofCOVID-19areshownin Table1.1 (adaptedfromWikipedia):
Paradoxically,thereisadisproportionatelyhighburdenfacedbysomeof themostdevelopedcountriesintermsofbothhealthcareandeconomic infrastructureintheCOVID-19pandemic.Thisisverydifferentfromprevious pandemicssuchasthosecausedbyEbolavirusorDenguevirus.Also,there appearstobedifferencesinCOVID-19-relatedmortalitybetweencountries. ThedifferencesinratesofCOVID-19-relateddeathsbetweencountriesarea functionofthetotalnumberofcases,theproportionofthepopulationwhoare athighriskforsevereCOVID-19,theimplementationofprecautionary
CoronavirusDisease. https://doi.org/10.1016/B978-0-12-824409-8.00010-2 Copyright © 2022ElsevierInc.Allrightsreserved. 1
FIGURE1.1 InterfaceofJohnsHopkinsCoronavirusResourceCenter.
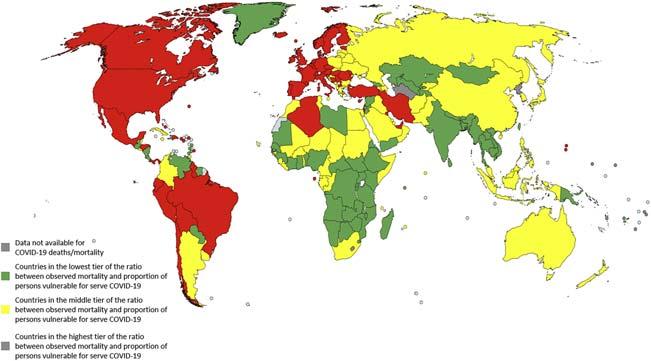
measuresbyrespectivegovernmentsandpopulations,andeffectivenessof medicaltreatment.Countriescanbedividedbasedonratioofbetween observedmortalityandvulnerabilityindextoquantifyhoweffectivethepreventivemeasuresandmedicaltreatmentwereinreducingmortality(measure ofperformance)[1].Thethreegroupsofcountriesarepresentedontheworld map(see Fig.1.3)withcountriesdepictedingreenasthosewithhighperformanceinreducingmortality,inyellowasmoderateperformance,andredas lowperformance.Countriesforwhichnostatisticsordatawasavailableon COVID-19-relateddeathsormortalityper1,000,000personshavebeen markedingrayonthemap.Countriesinthehigh-performancegroupincluded severalAfricanandsouth-eastAsiannationsthataretypicallyresourcedeprivedandarethoughttofacetheworstbruntofanyinfectiousdisease.
FIGURE1.2 GlobalprogressionofCOVID-19overtime.
FIGURE1.3 PerformanceofvariouscountriesinreducingCOVID-19-relatedmortality.
TABLE1.1 FivecountrieswiththehighestratesofcasesofCOVID-19and associateddeaths.
AnotherinterestingfindingwasthatTaiwanwasinthehigh-performance groupdespitetheislandcomprising23millioninhabitantsislocatedjust81 milesfrommainlandChina.FrequenttravelbackandforthbetweenChinaand TaiwanoccursonadailybasisandthousandsofTaiwanesenationalsliveand workinChina.Despitethechallenges,theCOVID-19-relatedmortalitywas lowinTaiwanafteradjustingforvulnerabilitytosevereCOVID-19infection. Amongcountriesinthelow-performancetierwerethewealthyandresourceful countriesofwesternEuropeandNorthAmerica,supportingtheargumentthat merehealthcareresourcesandfinancesarenotenoughwhenitcomesto effectivelydealingwiththecurrentpandemic.Thesecountrieshavehigh proportionofpersonsatriskforsevereCOVID-19andpoorperformancewas stillidentifieddespiteadjustmentforvulnerabilityindex.
CoronavirusesarealargefamilyofvirusesthatareresponsibleforMiddle EastRespiratorySyndromeCoronavirus(MERS-CoV)andSevereAcute RespiratorySyndromeCoronavirus(SARS-CoV).Thecausativeagentwas identifiedfromthroatswabsamplesconductedbytheChineseCenterfor
DiseaseControlandPreventiononJanuary7,2020andwassubsequently namedSevereAcuteRespiratorySyndromeCoronavirus2(SARS-CoV-2). TheSARS-CoV-2virusisabetacoronavirus,likeMERS-CoVandSARS-CoV. Allthreeoftheseviruseshavetheiroriginsinbats.TheSARS-CoVwas transmittedfromcivetcatstohumansandMERS-CoVfromdromedary camelstohumans.
Commonsignsofinfectionincluderespiratorysymptoms,fever,cough, andshortnessofbreath.Inmoreseverecases,infectioncancausepneumonia, severeacuterespiratorysyndrome,andevenrespiratoryfailureleadingto death.COVID-19isnotlimitedtopulmonarysystembutresultsinmultiorgan dysfunctioninvolvinggastrointestinal,cardiac,hepatic,neurologic,andrenal systems.Anotherfeaturethatgainedprominencewasinflammatorythrombosis,whichresultedinischemicstroke,pulmonaryembolisms,cardiac ischemia,andperipheralvenousthromboembolism.Therewasasecondary component2 4weeksafterprimaryinfectionattributedtoexcessiveimmunologicalresponse(cytokinestorm)resultinginmultisysteminflammatory syndromeconsistingofshock,cardiacinvolvement,andgastrointestinal symptoms.Anecdotaldatasuggeststhataproportionofpersonsaftercontact withCOVID-19-infectedindividualsdevelopsymptomsofCOVID-19butdo nothavethedisease,anentitywetermasCOVID-19mimic.Thepooled prevalenceofCOVID-19mimicwas16per100personsundersurveillance (95%confidenceinterval11 23per100persons)[2].Intheanalysisofa priorisubgroups,byregionofthestudies,prevalenceofCOVID-19mimicwas 16(95%CI11 23)inNorthAmerica,15(95%CI4 40)inEurope,and15 (95%CI7 32)per100personsinAsia.
TheCOVID-19pandemicresultedinwidespreadandunprecedented institutionofmandatedsocietallockdown.Mandatedsocialdistancing comprisingacombinationoftravelrestrictions,closureofnonessentialgroup meetingvenues(restaurants,schools,shops),andstepstoavoidclosecontact atessentialmeetingvenues(hospitals,foodsupply,pharmacies).Using publiclyavailabledata,wehadexaminedtheeffectoftimingofmandated socialdistancingontherateofCOVID-19in119geographicregionsderived from41stateswithintheUnitedStatesand78countries[3].Theprimary outcomewasthehighestnumberofnewCOVID-19casesperdayrecorded withineachgeographicunit.WefoundthathighestnumberofnewCOVID-19 casesperdaypermillionpersonswassignificantlyassociatedwithtotal numberofCOVID-19casespermillionpersonsonthedaybeforemandated socialdistancing(b ¼ 0.66, P < .0001).Ourfindingssuggestedthatthe initiationofmandatedsocialdistancingafterdoublinginnumberofexisting COVID-19caseswouldresultineventualpeakwith58%highernumberof COVID-19casesperday.Initiatingmandatedsocialdistancingwithsmaller numberofCOVID-19caseswithinaregionsignificantlyreducesthenumber ofdailynewCOVID-19casesandperhapsalsoreducesthetotalnumberof casesintheregion.
Wearingfacemasktocovermouthsandnoseswithfilteringmaterialshas beenwidelyusedtopreventinhalationofparticulatescontainingSARS-CoV-2 virus.ByFebruary2020,CentersforDiseaseControlandPreventionhad recommendedthatpersonswithsuspectedSARS-CoV-2infectionshouldwear facemasks[4].ByJuly2020,CentersforDiseaseControlandPreventionhad recommendedfacemaskuseduringallpublicencountersforallpersons.A studyfromalargehealthcaresysteminMassachusettswithmorethan75,000 employeesevaluatedtheeffectofmandatorypolicyofuniversalmaskingforall healthcareworkersandforallpatients[5].Aftertheuniversalmaskingpolicy wasinplace,theproportionofsymptomatichealthcareworkerswithpositive testresultssteadilydeclined,from14.7%to11.5%(ameandecreaseof0.49% perday).Anotherstudythatlookedattransmissionamong139clientsexposed totwohairstylistwithCOVID-19foundnocaseofSARS-CoV-2transmission whenbothhairstylistsandclientswerewearingfacemasks[6].
Oneoftheuniqueaspectsofdevelopingdiagnostictests,vaccines,and medicationsforpreventionandtreatmentofSARS-CoV-2infectionwasthe useofEmergencyUseAuthorization(EUA)byFoodandDrugAdministration (FDA).OnFebruary4,2020,pursuanttosection564(b)(1)(C)oftheFD&C Act(21U.S.C.360bbb3(b)(1)(C)),theSecretaryofHealthandHumanServicesdeterminedthatthereisapublichealthemergencythathasasignificant potentialtoaffectnationalsecurityorthehealthandsecurityofUScitizens livingabroad,andthatinvolvesthevirusthatcausesCOVID-19.Onthebasis ofsuchdetermination,onMarch27,2020,theSecretarythendeclaredthat circumstancesexistjustifyingtheauthorizationofemergencyuseofdrugsand biologicalproductsduringtheCOVID-19pandemic,pursuanttosection 564(b)(1)oftheFD&CAct(21U.S.C.360bbb-3(b)(1)).Acopyofthenotice isprovidedin Fig.1.4.
Severalinvitrodiagnostic(IVD)deviceswereapprovedunderEUAfor performingtestsonsamplessuchasswabsofmucusfrominsidethenoseor backofthethroatorbloodtakenfromaveinorfingerstick.TheFDAclassifies theseIVDsasfollows:
DiagnosticTests: Moleculartestsandantigenteststhatdetectcomponents oftheSARS-CoV-2todiagnoseinfectionwiththeSARS-CoV-2.
Serology/AntibodyandOtherAdaptiveImmuneResponseTests: Tests thatdetectIgMandIgGantibodiestotheSARS-CoV-2virusorthatmeasurea differentadaptiveimmuneresponse(suchasTcellimmuneresponse)tothe SARS-CoV-2virus.Thesetypesoftestsarebestsuitedforidentifying previousinfection.
TestsforManagementofCOVID-19Patients: Teststhatareauthorized foruseinthemanagementofpatientswithCOVID-19,suchastodetect biomarkersrelatedtoinflammationandguidepatientmanagementdecisions.
SeveralmedicationswereapprovedforuseinpatientswithCOVID-19 underEUA.Alistisprovidedin Table1.2 asadaptedfrom https://www.fda. gov/medical-devices/coronavirus-di sease-2019-covid-19-emergency-useauthorizations-medical-devices/ .
FIGURE1.4 Emergencyuseauthorizationdeclaration[7].
TABLE1.2 MedicationswereapprovedforuseinpatientswithCOVID-19 underEUA.
Dateoffirst EUA issuance Mostrecentletterof authorization(PDF)Authorizeduse 04/30/2020FreseniusMedical, multiFiltratePROSystem andmultiBic/multiPlus Solutions(171KB) [also listedunderMedical DeviceEUAs]
January05, 2020 RemdesivirforCertain HospitalizedCOVID-19 Patients(423KB) (ReissuedAugust28, 2020,October1,2020, andOctober22,2020)
Toprovidecontinuousrenalreplacement therapy(CRRT)totreatpatientsinan acutecareenvironmentduringthe COVID-19pandemic
Foremergencyusebylicensedhealthcare providersforthetreatmentofsuspected orlaboratory-confirmedCOVID-19in hospitalizedpediatricpatientsweighing 3.5kgtolessthan40kgorhospitalized pediatricpatientslessthan12yearsof ageweighingatleast3.5kg. OnOctober22,2020,FDAapproved
TABLE1.2 MedicationswereapprovedforuseinpatientswithCOVID-19 underEUA. cont’d
Dateoffirst
EUA issuance
Mostrecentletterof authorization(PDF)Authorizeduse
Veklury(remdesivir)foruseinadultsand pediatricpatients(12yearsofageand olderandweighingatleast40kg)forthe treatmentofCOVID-19requiring hospitalization.Vekluryshouldonlybe administeredinahospitalorina healthcaresettingcapableofproviding acutecarecomparabletoinpatient hospitalcare.Thisapprovaldoesnot includetheentirepopulationthathad beenauthorizedtouseVekluryunderan emergencyuseauthorization(EUA) originallyissuedonMay1,2020.Inorder toensurecontinuedaccesstothe pediatricpopulationpreviouslycovered undertheEUA,theEUAforVeklury continuestoauthorizeVekluryfor emergencyusebylicensedhealthcare providersforthetreatmentofsuspected orlaboratory-confirmedCOVID-19in hospitalizedpediatricpatientsweighing 3.5kgtolessthan40kgorhospitalized pediatricpatientslessthan12yearsof ageweighingatleast3.5kg.
August05, 2020
FreseniusKabiPropoven 2%(209KB)
08/13/2020REGIOCITreplacement solutionthatcontains citrateforregional citrateanticoagulation (RCA)ofthe extracorporealcircuit (92KB)
08/23/2020COVID-19convalescent plasma(284KB) (ReissuedFebruary23, 2021andMarch9, 2021)
Tomaintainsedationviacontinuous infusioninpatientsolderthanage16 withsuspectedorconfirmedCOVID-19 whorequiremechanicalventilationinan intensivecareunit(ICU)setting
Tobeusedasareplacementsolutiononly inadultpatientstreatedwithcontinuous renalreplacementtherapy(CRRT),and forwhomregionalcitrateanticoagulation isappropriate,inacriticalcaresetting
Forthetreatmentofhospitalizedpatients withcoronavirusdisease2019 (COVID-19) Continued
TABLE1.2 MedicationswereapprovedforuseinpatientswithCOVID-19 underEUA. cont’d
Dateoffirst
EUA issuance
September 11,2020
Mostrecentletterof authorization(PDF)Authorizeduse
Bamlanivimab(339KB) (reissuedFebruary9, 2021andMarch2, 2021)
11/19/2020Baricitinib(Olumiant)in combinationwith remdesivir(Veklury) (322KB)
Forthetreatmentofmild-to-moderate COVID-19inadultandpediatricpatients withpositiveresultsofdirectSARS-CoV-2 viraltestingwhoare12yearsofageand olderweighingatleast40kg(about88 pounds),andwhoareathighriskfor progressingtosevereCOVID-19and/or hospitalization.
Foremergencyusebyhealthcare providersforthetreatmentofsuspected orlaboratory-confirmedCOVID-19in hospitalizedadultsandpediatricpatients 2yearsofageorolderrequiring supplementaloxygen,invasive mechanicalventilation,orextracorporeal membraneoxygenation(ECMO).
11/21/2020REGEN-COV (Casirivimaband Imdevimab)(232KB) (ReissuedFebruary3, 2021andFebruary25, 2021)
September 02,2021
Bamlanivimaband Etesevimab(344KB) (ReissuedFebruary25, 2021)
Casirivimabandimdevimabtobe administeredtogetherforthetreatmentof mildtomoderatecoronavirusdisease 2019(COVID-19)inadultsandpediatric patients(12yearsofageandolder weighingatleast40kg)withpositive resultsofdirectSARS-CoV-2viraltesting, andwhoareathighriskforprogressingto severeCOVID-19and/orhospitalization.
Forthetreatmentofmild-to-moderate COVID-19inadultandpediatricpatients withpositiveresultsofdirectSARS-CoV-2 viraltestingwhoare12yearsofageand olderweighingatleast40kg(about88 pounds),andwhoareathighriskfor progressingtosevereCOVID-19and/or hospitalization.
December 03,2021
Propofol-Lipuro 1%(344KB)
Tomaintainsedationviacontinuous infusioninpatientsgreaterthanage16 withsuspectedorconfirmedCOVID-19 whorequiremechanicalventilationinan ICUsetting.
DevelopmentofvaccineforpreventionofSARS-CoV-2infectionwasa healthcareprioritywiththefirstclinicaltrialofavaccinecandidateforSARSCoV-2beginninginMarch2020[8].TheFDAprespecifiedsomeoftherequirementsforapprovalunder“DevelopmentandLicensureofVaccinesto PreventCOVID-19”guidance,whichincludedapointestimateforaplacebocontrolledefficacytrialofatleast50%,withalowerboundoftheappropriatelyalpha-adjustedconfidenceintervalaroundtheprimaryefficacyendpoint pointestimateof >30%withadditionalsafetyandeffectivenessdata. Messengerribonucleicacid(mRNA)-basedvaccinesassumedamajorrolein vaccinecandidatesforSARS-CoV-2.Thegeneticinformationfortheantigen isdeliveredbymRNA(withmodifications)oraself-replicatingRNA.The antigenisthenexpressedinthecellsofthevaccinatedindividualinvokingan immuneresponse.
SeveralvaccineswereapprovedforuseunderEUA.Alistisprovidedin Table1.3 asadaptedfrom https://www.fda.gov/medical-devices/coronavirusdisease-2019-covid-19-emergency-use-authorizations-medical-devices/.
TABLE1.3 VaccinesapprovedforuseforpreventionofSARS-CoV-2 infectionunderEUA.
Dateoffirst EUA issuance
Mostrecentletterof authorization(PDF)Authorizeduse November 12,2020 Pfizer-BioNTech COVID-19Vaccine (455KB)(Reissued February25,2021)
LetterGrantingEUA Amendment(January6, 2021)(164KB)
LetterGrantingEUA Amendment(January22, 2021)(190KB)
LetterGrantingEUA Amendment(April6, 2021)(166KB)
12/18/2020ModernaCOVID-19
Vaccine(392KB) (ReissuedFebruary25, 2021)
LetterGrantingEUA Amendment(April1, 2021)(193KB)
02/27/2021JanssenCOVID-19
Vaccine(183KB)
LetterGrantingEUA Amendment(March29, 2021)(152KB)
Forthepreventionof2019coronavirus disease(COVID-19)forindividuals16 yearsofageandolder
Forthepreventionofcoronavirusdisease 2019(COVID-19)forindividuals18years ofageandolder
Forthepreventionofcoronavirusdisease 2019(COVID-19)forindividuals18years ofageandolder
Anotherissuethatisgainingimportanceisreinfection.AbetterunderstandingofreinfectionbecameoneoftheprioritiesforCentersforDisease ControlandPreventiontoinformpublichealthaction[9].andtheEuropean CentreforDiseasePreventionandControl[10]duetoimplicationsfor durationofacquiredimmunity.TheEuropeanCentreforDiseasePrevention andControl[10]andCentersforDiseaseControlandPrevention[11] emphasizethatindividualsthathavebeeninfectedoncewithSARS-CoV-2are notalwaysimmuneandinfectionprevention/controlandcontactprinciples shouldbefollowedevenaftertheinfection.ByOctober2020,fivecasesof reinfectionwithSARS-CoV-2hadbeenreportedfromHongKong,Belgium, theNetherlands,Ecuador,andtheUnitedStates[12 16]whenover37million SARS-CoV-2-infectedpersonshadbeenreportedworldwide[17].Reinfection wasidentifiedin0.7%(n ¼ 63,95%confidenceinterval0.5% 0.9%)during follow-upof9119patientswithSARS-CoV-2infectionamong62healthcare facilitiesintheUnitedStatesbetweenDecember1,2019andNovember13, 2020[18].Themeanperiod( standarddeviation[SD])betweentwopositive testswas116 21days.SimilarresultswerereportedbySARS-CoV-2 ImmunityandReinfectionEvaluation(SIREN)[19]Thestudyidentified44 reinfections(twoprobable,42possible)inthebaselinepositivecohortof6614 healthcareworkers.Theseobservationsstronglysuggestthatsurvivorsfrom SARS-CoV-2infectionmustnotrelaxcompliancewithproveninterventionsin preventionofSARS-CoV-2transmissionsuchassocialdistancing[3]and universalfacemaskuse[20].Duetoconcernsforreinfection,theCentersfor DiseaseControlandPrevention[11]currentlyrecommendsvaccinationfor patientswhohadSARS-CoV-2infectionafter90daysbutacknowledgesthe limiteddataisavailabletosupporttherecommendation.
References
[1]QureshiAI,JilaniT,HuangW,etal.PerformanceofvariouscountriesinreducingCOVID19mortalityafteradjustmentforvulnerability.HealthCareResJ2020;1(1):9 10.
[2]QureshiAI,JaniV,AkhtarI,etal.OccurrenceofCOVID-19mimicinpersonsunder surveillanceafterCOVID-19exposure:asystematicreview.HealthCareResJ 2020;1(1):2 8.
[3]QureshiAI,SuriMFK,ChuH,SuriHK,SuriAK.Earlymandatedsocialdistancingisa strongpredictorofreductioninpeakdailynewCOVID-19cases.PublicHealth 2021;190:160 7.
[4]PatelA,JerniganDB,nCoVCDCResponseTeam.Initialpublichealthresponseand interimclinicalguidanceforthe2019novelcoronavirusoutbreak-UnitedStates, December31,2019 February4,2020.MMWRMorbMortalWklyRep 2020;69(5):140 6.
[5]WangX,FerroEG,ZhouG,HashimotoD,BhattDL.Associationbetweenuniversal maskinginahealthcaresystemandSARS-CoV-2positivityamonghealthcareworkers. JAmMedAssoc2020;324(7):703 4.
[6]HendrixMJ,WaldeC,FindleyK,RTrotman.Absenceofapparenttransmissionof SARS-CoV-2fromtwostylistsafterexpos ureatahairsalonwithauniversalface coveringpolicy Springfield,Missouri,May2020.MMWR(MorbMortalWklyRep). 2020;69(28):930-932.
[7]DepartmentofHealthandHumanServices.Emergencyuseauthorizationdeclaration.2020. UpdatedApril1,2020.[Accessed13April2021], https://www.federalregister.gov/ documents/2020/04/01/2020-06905/emergency-use-authorization-declaration
[8]KrammerF.SARS-CoV-2vaccinesindevelopment.Nature2020;586(7830):516 27.
[9]ReinfectionwithCOVID-19.2020.UpdatedOctober27,2020.[Accessed7Febaruary 2021], https://www.cdc.gov/coronavirus/2019-ncov/your-health/reinfection.html
[10]EuropeanCenterforDiseasePreventionandControl.ReinfectionwithSARS-CoV-2: considerationsforpublichealthresponse.2020.AccessedApril4,2021, https://www.ecdc. europa.eu/sites/default/files/documents/Re-infection-and-viral-shedding-threat-assessmentbrief.pdf.
[11]FrequentlyaskedquestionsaboutCOVID-19vaccination.2021.AccessedFebaruary10, 2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html#:w:text¼Yes.,already %20had%20COVID%2D19%20infection.
[12]Firstcaseofcovid-19reinfectiondetectedintheus;n.d. https://www.ajmc.com/view/firstcase-of-covid-19-reinfection-detected-in-the-us.[Accessed7February2021].
[13]TillettRL,SevinskyJR,HartleyPD,etal.GenomicevidenceforreinfectionwithSARSCoV-2:acasestudy.LancetInfectDis2021;21(1):52 8.
[14]Prado-VivarB,Becerra-WongM,GuadalupeJJ,etal.COVID-19re-infectionbya phylogeneticallydistinctSARS-CoV-2variant,firstconfirmedeventinSouthAmerica. September3,2020.
[15]ToKK,HungIF,IpJD,etal.COVID-19re-infectionbyaphylogeneticallydistinctSARScoronavirus-2strainconfirmedbywholegenomesequencing.ClinInfectDis 2020:ciaa1275.
[16]VanElslandeJ,VermeerschP,VandervoortK,etal.SymptomaticSARS-CoV-2reinfection byaphylogeneticallydistinctstrain.ClinInfectDis2020;73(2):354 6.
[17]Coronavirusdisease(COVID-19).2020.UpdatedOctober11,2020.AccessedFebaruary7, 2021, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201012weekly-epi-update-9.pdf
[18]QureshiAI,BaskettWI,HaungW,etal.Re-infectionwithSARS-CoV-2inpatientsundergoingseriallaboratorytesting.ClinInfectDis2021:ciab345.
[19]HallV,FoulkesS,CharlettA,etal.Doantibodypositivehealthcareworkershavelower SARS-CoV-2infectionratesthanantibodynegativehealthcareworkers?Largemulti-centre prospectivecohortstudy(theSIRENstudy),England:JunetoNovember2020.medRxiv 2021;01.13.21249642. https://doi.org/10.1101/2021.01.13.21249642.
[20]BrooksJT,ButlerJC,RedfieldRR.UniversalmaskingtopreventSARS-CoV-2 transmission-thetimeisnow.JAmMedAssoc2020;324(7):635 7.
HistoryofSARS-CoV-2
IrynaLobanova
ZeenatQureshiStrokeInstituteandDepartmentofNeurology,UniversityofMissouri,Columbia, MO,UnitedStates
OnDecember31,2019,theWorldHealthOrganization(WHO)wasformally notifiedaboutaclusterofcasesofpneumoniainWuhanCity,hometo11 millionpeopleandtheculturaland economichubofcentralChina[1 ].By January5th,59caseswereidentifiedandnonehadbeenfatal[ 1 ,2 ].Tendays later,WHOwasawareof282confirmedcases,ofwhichfourwereinJapan, SouthKorea,andThailand[ 1, 3].TherehadbeensixdeathsinWuhan,51 peoplewereseverelyill,and12wereinacriticalcondition.Thevirus responsiblewasisolatedonJanuary7thanditsgenomesharedonJanuary 12th[1 ,4 ].Thecauseofthesevereacuterespiratorysyndromethatbecame knownascoronavirusdisease2019(COVID-19)wasanovelcoronavirus, severeacuterespiratorysyndromeco ronavirus2(SARS-CoV-2).PhylogeneticanalysissuggeststhatSARS-CoV-2originatedinanimals,probably bats,andwastransmittedtootheranimalsbeforecrossingintohumansatthe HuananwetmarketinWuhanCity[ 1, 5 7 ].AsofFebruary22,2021,more than111millioncaseshavebeenconfirmed,withmorethan2.46million deathsattributedtoCOVID-19[8 ].
Thehistoryofhumancoronavirusesbeganin1965afterTyrrellandBynoe [9,10]identifiedavirusnamedB814.Theviruswasfoundinhumanembryonictrachealorganculturesobtainedfromtherespiratorytractofanadultwith acommoncold.Ataboutthesametime,HamreandProcknow[9,11]were abletogrowaviruswithunusualpropertiesintissueculturefromsamples obtainedfrommedicalstudentswithcolds.BothB814andHamre’svirus, whichshecalled229E,wereether-sensitiveandthereforepresumablyrequired alipid-containingcoatforinfectivity.Whileworkinginthelaboratoryof RobertChanockattheNationalInstitutesofHealth,McIntoshetal.[9,12] reportedtherecoveryofmultiplestrainsofether-sensitiveagentsfromthe humanrespiratorytractbyusingatechniquesimilartothatofTyrrelland Bynoe[9,12].Thesevirusesweretermed“OC”todesignatethattheywere growninorgancultures.Withinthesametimeframe,AlmeidaandTyrrell [9,13]performedelectronmicroscopyonfluidsfromorganculturesinfected
CoronavirusDisease. https://doi.org/10.1016/B978-0-12-824409-8.00007-2
withB814andfoundparticlesthatresembledtheinfectiousbronchitisvirusof chickens.Inthelate1960s,Tyrrellwasleadingagroupofvirologistsworking withthehumanstrainsandanumberofanimalviruses.Thisnewgroup ofviruseswasnamedcoronavirus(corona denotingthecrown-likeappearance ofthesurfaceprojections)andwaslaterofficiallyacceptedasanewgenusof viruses[9,14].
Epidemiologicandvolunteerinoculationstudiesfoundthatrespiratory coronaviruseswereassociatedwithavarietyofrespiratoryillnesses;however, theirpathogenicitywasconsideredtobelow[9,11,15 17].Thepredominant illnessassociatedwithinfectionswasanupperrespiratoryinfectionwithoccasionalcasesofpneumoniaininfantsandyoungadults[9,18,19].These viruseswerealsoshowntobeabletoproduceasthmaexacerbationsinchildrenaswellaschronicbronchitisinadultsandtheelderly[20 22].
In2004,vanderHoeketal.[9 ,23]reportedthediscoveryofanewhuman coronavirus,NL63,isolatedfroma7-month-oldgirlwithcoryza,conjunctivitis,fever,andbronchiolitis.Usinganovelgenomicamplificationtechnique,theseinvestigatorswereablet osequencetheentireviralgenome. PhylogeneticanalysisdemonstratedthatthisviruswasagroupIcoronavirus relatedto229Eandtransmissiblegastroenteritisvirus,avirusofpigs. Screeningof614respiratoryspecimenscollectedbetweenDecember2002 andApril2003identifie dsevenadditionalindividualswithupperorlower respiratorytractdiseaseorboth.Shortlyafter,Fouchieretal.[ 9 ,24 ]reported theidentificationofacoronavirus,namedNL,isolatedfroman8-month-old boywithpneumoniaandgrownfromaclinicalspecimenthatwasobtainedin April1988.FullgenomicsequenceanalysisofNLshowedthatthisviruswas alsoagroupIcoronavirusandclosely relatedtoNL63.Thediscoveryofboth NL63andNLdependedonthepropagationofthevirusesincellculture. Withtheuseofmolecularprobesthattargetedconservedregionsofthe coronavirusgenome,monthslater,Esperetal.[ 9].foundevidenceofahumanrespiratorycoronavirusinrespira toryspecimensobtainedfromchildren youngerthan5yearsofage,whichwasdesignatedtheNewHavencoronavirus(HCoV NH).Graf[25 ]detectedthepresenceofapeptidecorrespondingtothespikeglycoproteinofNL63,thecloselyrelatedvirus identifiedintheNetherlands,intissu efromindividualswithKawasakidisease.ThesummationofthesefindingssuggeststhatHCoV-NHmayplaya roleinthepathogenesisofKawasakidisease.
Twononendemiccoronaviruseshave causedseriousdisease.Averynew coronavirus,severeacuterespiratorysyndrome,calledsevereacuterespiratorysyndrome(SARS),emergedin2002 03asacoronavirusfromsouthern Chinaandspreadthroughouttheworldwithquantifiablespeed[9 ,26, 27]. Duringthe2002 03outbreak,SARS-CoVinfectionwasreportedin29 countriesinNorthAmerica,SouthAme rica,Europe,andAsia.Thisvirus wasresponsibleforSARS,aflu-likeilln ess,thoughdiarrheawascommon.It couldprogresstopneumoniaandrespiratoryfailurein2weeks,and25%of peopleinfectedrequiredintensivecare[ 1].SARS-CoVwastransmittedvia
HistoryofSARS-CoV-2 Chapter|2 15
dropletsinrespiratoryaerosol,con tactwithsurfaces,andpossiblyvia fecal oralcontact[ 28 ].Within1monthof55indexcasesbeingrecognized inHongKong,Hanoi,andSingapore,atotalof3000caseshadbeen confirmedgloballywithapeakreportingrateof200newcasesperday[29].
Overall,8098infectedindividualswereidentified,with774SARS-related fatalities[30 ].ItisstillunclearhowthevirusenteredthehumanpopulationandwhethertheHimalayanpalmcivetswerethenaturalreservoirforthe virus.SequenceanalysisofthevirusisolatedfromtheHimalayanpalmcivets revealedthatthisviruscontaineda29-nucleotidesequencenotfoundinmost humanisolates,inparticularthoseinvolvedintheworldwidespreadofthe epidemic[31 ].TheSARSepidemicgavetheworldofcoronavirusesresearch anenormousinfusionofenergyandactivitythatcontributedtothelarge amountalreadyknownaboutthevirologyandpathogenesisofcoronavirus infectionsfromtheexpandingareaofveterinaryvirology[ 32 ].
ThesecondseriousinfectionduetoacoronaviruswasMiddleEastern respiratorysyndrome(MERS).TheMERS-CoVviruswasfirstidentifiedas thecauseofafatalinfectioninSaudiArabiain2012[9 ,33 ].Itspreadto27 countries.UnlikeSARS,MERSisstillprevalent,andasofNovember2019, 2494infectionshadbeennotified,ofwhich858provedfatal[ 9 ,34].Like SARS,MERScausesaflu-likeillnes swithsymptomsrangingfrommild (withaboutone-quarterofpeoplealsohavingdiarrhea)toseverepneumonia, acuterespiratorydistresssyndrome ,septicshock,andmultiorganfailure. MERS-CoVisbelievedtohavereachedhumansviadromedarycamels, whichappeartobeareservoirinseveralMiddleEaststates.Theoriginal sourcespeciesisnotknown,butbatsarethemostlikely.SARS-CoV-2more closelyresemblesthebatwildvirusthanitdoeseitherSARS-CoVorMERSCoV,stronglysuggestingthatitisanovelcoronavirusinhumans[6 , 9]. OutbreaksofMERS-CoVinfectionnow occurmostlyduetoanimal-to-humantransmission(probablyduringthecamelcalvingseason)[9 , 35 ].Personto-personspreadseemstodependonclosecontact,suchasprovidingcareto aninfectedpersonorwithinahospitalsetting.Inall,40%ofconfirmedcases havebeenacquirednosocomially on1dayinMay2015,anindividualwith MERSvisitedseveralhospitalsinKoreaandinfected186people[ 9, 33].No vaccinesareyetavailablethatcanprotectagainstMERS-CoVinfection[33].
Astudyofthefirst41casesofconfirmedCOVID-19,publishedinJanuary 2020in TheLancet,reportedtheearliestdateofonsetofsymptomsas December1,2019[36].OfficialpublicationsfromtheWHOreportedthe earliestonsetofsymptomsasDecember8,2019[37].Human-to-human transmissionwasconfirmedbytheWHOandChineseauthoritiesbyJanuary 20,2020[38].
Duringtheearlystagesoftheoutbreak,thenumberofcasesdoubled approximatelyeverysevenandahalfdays[39].Inearlyandmid-January 2020,thevirusspreadtootherChineseprovinces,helpedbytheChinese NewYearmigrationandWuhanbeingatransporthubandmajorrail