Reviewers
Lisa M Anderson, MHSA, MT (ASCP) SBB Armstrong Atlantic State University Savannah, Georgia
Lisa Baker B.S. (ASCP) Tyler Junior College Tyler, Texas
Dorothy A. Bergeron, M.S., MLS (ASCP) CM University of Massachusetts–Dartmouth North Dartmouth, Massachusetts
Melanie Chapman, M.Ed., MLS (ASCP) The University of Louisiana at Monroe Monroe, Louisiana
Janice Costaras, M.S., MT (ASCP) SC Cuyahoga Community College Cleveland, Ohio
Daniel P deRegnier, M.S., MT (ASCP) Ferris State University Big Rapids, Michigan
Lynne Fantry, MLA, MT (ASCP) York Technical College Rock Hill, South Carolina
Dorothy J. Fike, M.S., MLS (ASCP) SBB Marshall University Huntington, West Virginia
Amy Lundvall, B.S., MT (ASCP) Harrisburg Area Community College Harrisburg, Pennsylvania
Linda E. Miller, Ph.D. SUNY Upstate Medical University Syracuse, New York
Valerie Polansky, M. Ed, MT (ASCP) St. Petersburg College St. Petersburg, Florida
Diane Schmaus, M.A., MT (ASCP) McLennan Community College Waco, Texas
Sherri Sterling B.S., M.B.A., CLS (NCA), MT (ASCP) University of Massachusetts–Dartmouth North Dartmouth, Massachusetts
Wendy Sweatt, M.S., CLS Jefferson State Community College Birmingham, Alabama
Dick Y. Teshima, MPH, MT (ASCP) University of Hawai‘i at Manoa Honolulu, Hawaii
Sandra L. Tijerina, M.S., MT (ASCP) SBB, CLSpH (NCA) University of Texas–Pan American Edinburg, Texas
M. Lorraine Torres, MT (ASCP), Ed.D. University of Texas El Paso El Paso, Texas
Jeffrey Wolz, M.Ed., MT (ASCP) Arizona State University Phoenix, Arizona
■ Objectives—LeveL i (continued )
26. Compare and contrast the T-cell receptor on a T cell and the surface immunoglobulin on a B cell.
27. Define human leukocyte antigen (HLA) and major histocompatibility complex (MHC)
28. Differentiate T cell subsets on the basis of antigenic structure and function.
29. Explain how natural killer cells differ from cytotoxic T cells.
30. Discuss the principles involved in the analysis of cells by flow cytometry.
31. Analyze data obtained using a flow cytometer.
32. Interpret the use of gating in a particular flow cytometer analysis.
33. Apply the information in this chapter concerning CD markers on particular cell types, and evaluate the significance of flow scattergram data.
34. Define apoptosis.
■ Objectives—LeveL ii
After this chapter, the student should be able to:
1. Name seven types of pathogen-associated molecular patterns that trigger the innate immune response.
2. Describe pathogen recognition receptors.
3. Describe Toll-like receptors.
4. Describe antimicrobial peptides, and name two families.
Key terms
acquired immune system
acute phase proteins
acute phase reactants
adaptive immune system
alpha-1 acid glycoprotein
antigen antigen presentation antimicrobial peptides
autocrine response
basophil
bone marrow
C-reactive protein (CrP)
cathelicidins
chemotactic factors
complement proteins
complement system
cytokine defensins
dendritic cells
diapedesis
endocrine response
eosinophil
fibrinogen
flow cytometry
haptoglobin
humoral
immune system
immunity immunology
inflammation
innate immune system lectins
macrophages
mast cells
natural immune system
natural killer (nK) cells
neutrophils
opsonin
paracrine response
pathogen-associated molecular patterns (PamPs)
pattern recognition receptors (Prrs) phagocytosis
plasma
polymorphonuclear leukocyte
serum
serum amyloid a t cells
toll-like receptor (tLr)
Tonsil
Lymph nodes
Thymus
Spleen
Peyer’s patches in intestine
Lymph nodes
Lymphatics
Bone marrow
Earwax
Lysozyme in tears
Mucous
Sweat
Stomach acid
Normal flora
Vaginal secretions
Urine
Skin
an antibiotic. Because antibiotics work systemically, the bacteria in the throat are not the only ones killed, and the removal of the normal gut flora can result in overgrowth of resistant bacteria and the resultant nausea and diarrhea. We will not talk much more about innate defenses, but their importance should not be forgotten.
the innate immune system internaL cOmpOnents
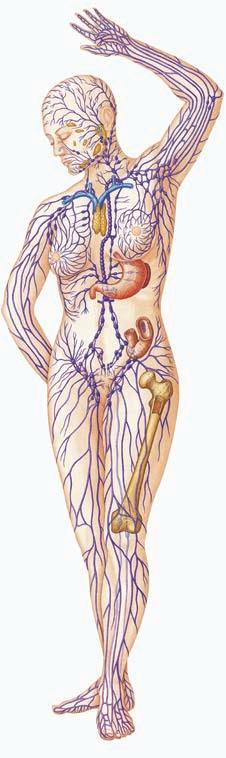
■ Figure 1.3 (a) Blue arrows (right side): External components of the innate immune system: human body with skin, mucous and the associated cilia, earwax, lysozyme in tears; the acid pH of sweat, stomach acids, urine, and vagina fluids; and the normal bacteria of the skin and gastrointestinal tract labeled. (b) Red arrows (left side): organs of the acquired immune system: tonsil, lymph node, thymus, spleen, Peyer’s patches, lymphatics, bone marrow. Source: “Human Body image” by Joanna Cameron. DK Human Body Books, reprinted by permission of Dorling Kindersley.
vinegar,” or some other expression that indicates that they will not change the protective low pH of the vagina. However, even with this lower pH, vaginal douches still disrupt the mucous layer and the normal flora of the vagina. Perhaps because of this disruption of the innate immune system, vaginal douching has been linked to bacterial vaginosis, pelvic inflammatory disease, cervical cancer, and increased transmission rates of HIV (8).
The normal bacteria that colonize an individual are called their normal flora. Alteration of the normal flora through the use of antibiotics can upset the balance in an area and lead to an infection with a pathogen that normally would not be able to grow. An example of this is the gastrointestinal distress that can result after treatment of strep throat or any infection with
The internal components of the innate immune system include both a cellular and a molecular component that is in the fluid phase of the blood. This fluid phase is called the humoral component of the blood, and when blood has been allowed to clot, the fluid phase is called serum. If anticoagulants have been added, the fluid phase is different and is called plasma. Clotting factors are no longer in the serum because they have joined the clot, but these factors remain in plasma because the blood was not allowed to clot. These internal components of the innate immune system categorized into the cellular components and the humoral components will be discussed separately.
the cells of the innate immune system
The cells of the innate immune system are all white blood cells and include granulocytes (neutrophils, eosinophils, and basophils), monocytes/macrophages, mast cells, dendritic cells, natural killer (NK) cells, and lymphokine-activated killer (LAK) cells. Surface proteins of white blood cells are used along with the cells’ microscopic appearance to characterize and differentiate the cells. These surface protein markers were identified using monoclonal antibodies and are numbered beginning with the designation CD for cluster of differentiation. An example of the use of CD markers is the commonly used term, the CD4/CD8 ratio, to discuss an AIDs diagnosis (9, 10, 11).
All white blood cells express CD45. Granulocytes, which express CD45 and CD15, have granules in their cytoplasm
■ Figure 1.4 This image shows the spread of droplets from an uncovered sneeze. Source: © CDC/Brian Judd.
■ Figure 1.5 The blood cells are shown in (a)–(f) and the tissue white blood cells are shown in (g), (h), and (i). The 3 types of granulocytes: (a) neutrophils, (b) eosinophils, and (c) basophils. As well as a (d) monocyte, (e) lymphocyte, and (f) red blood cell. Next are white blood cells of tissue: (g) macrophage, (h) mast cells, and (i) dendritic cells. Source: GOODENOUGH, JUDITH; MCGUIRE, BETTY A., BIOLOGY OF HUMANS: CONCEPTS, APPLICATIONS, AND ISSUES WITH MASTERINGBIOLOGY®, 4th, ©N/A. Printed and Electronically reproduced by permission of Pearson Education, Inc., Upper Saddle River, New Jersey.
(see Figure 1.5 ■ for the three types of granulocytes (neutrophils, eosinophils, and basophils) as well as monocytes, mast cells, and macrophages, which are shown with a lymphocyte and a red blood cell for relative size comparisons). The three types of granulocytes are named for their characteristic staining pattern when using Wright’s stain. All granulocytes can be recruited from the blood by chemotaxic factors to enter the tissues that stimulate them to move to the site of an infection. Neutrophils are the most abundant type of granulocyte, and they contain neutrally staining granules, that is, their granules do not stain when Wright’s stain is utilized. Fifty to 70% of white blood cells in the blood are neutrophils. The nucleus of a neutrophil is irregular in shape with multiple lobes, which gives the neutrophil the alternative name polymorphonuclear leukocyte with the shortened names of either polys or PMNs. Neutrophils are the first cells that enter the site of an acute infection. They reside only about 12 hours in the circulation or one to two days after migration to the tissue. These cells are in the pus of an infected area, and eventually macrophages come into the infected area as well. Neutrophils are very active in phagocytosis (engulfment and digestion) of foreign cells and particles. They have recently been found to be involved in the presentation of the antigen to T cells, which is one type of cell of the acquired immune system. Antigen
presentation, is a process in which a cell of the innate immune system shows or presents the antigen to the cells (lymphocytes) of the acquired immune system. The numbers of neutrophils can increase in an acute infection or inflammation. The eosinophil contains red-stained granules after Wright’s staining, which indicates that the granules are acidic. These cells are involved in antiparasitic responses and allergic reactions. The numbers of eosinophils can increase during an allergic reaction, parasitic infection, and skin inflammation. In blood, 1 to 3% of the white blood cells are eosinophils. The basophil, the rarest of the granulocytes, composes only 0.4 to 1% of white blood cells. The numbers of basophils can be elevated in leukemia, in some allergic responses, in patients with chronic inflammation, and in patients following radiation therapy (9, 10, 11). After Wright’s staining, basophils have blue-black-stained granules, indicating that the granules are basic. The function of basophils is not completely defined, but we do know that they play a role in inflammation and allergy. Mast cells are very similar in appearance to basophils but come from a different lineage. Mast cells have a surface receptor that binds IgE (the antibody molecule involved in allergy) with a high affinity, and this relates to their primary role in allergic and antiparasitic reactions. Mast cells contain granules of histamine and heparin and, when bound to IgE,
Tissue
erythrocyte sedimentation rate (ESR) that is seen with inflammation. The cytokine IL-6 stimulates the production of acute phase proteins, which include C-reactive protein, alpha-1 acid glycoprotein, haptoglobin, fibrinogen, serum amyloid A, and complement proteins (which are discussed separately). An acute phase reactant that is frequently used to assess inflammation is C-reactive protein, the PRR mentioned earlier.
C-reactive protein (CRP) was so named because it reacts with the C-polysaccharide of Streptococcus pneumonia. Sensitive assays for increases in this marker have recently been approved to measure risk of cardiovascular disease. CRP is a sensitive indicator of inflammation, rising up to 1000 fold quickly after inflammation and rapidly falling when the inflammation resolves. This marker is involved in the immune system at many levels: It activates complement, is an opsonin, and this enhances cell-mediated cytotoxic effects on the pathogen. Alpha-1 acid glycoprotein is found to be elevated in some autoimmune disorders. Like many of the acute phase proteins, the liver produces alpha-1 acid glycoprotein. Its primary function may be the inhibition of progesterone and other drugs. It has also been called orosomucoid. Haptoglobin is an acute phase protein that removes free hemoglobin that has been released through injury and red blood cell lysis. The haptoglobin molecule acts as an antioxidant. Fibrinogen is an acute phase protein molecule that is involved in the coagulation pathway. It is converted to fibrin and then fibrin is cross-linked to form a clot. Serum amyloid A is an apolipoprotein, which is associated with highdensity lipoprotein (HDL) in the blood stream. Serum amyloid A is involved with the transport of cholesterol to the liver and in the induction of extracellular matrix degrading enzymes that are involved in repair after infection-induced tissue damage. Additionally, it is a chemoattractant, bringing cells of the
✪ Table 1.3
The Molecules of the innate immune response
Molecules of the innate immune System
Recognition molecules
Pattern recognition receptors
Effector molecules
Cytokines
Effector molecules
Antimicrobial peptides
Effector molecules
Acute phase proteins
Effector molecules
Complement
Targets or Molecules recognized
Pathogen-associated molecular patterns
Immune cells
Bacterial cell walls
Produced in response to infection
3 pathways, recognize (1) surfaces of bacteria, fungi, viruses, tumor cells or (2) mannose (3) antibody bound to antigen
innate and acquired immune systems to the site of the infection (1, 3, 6, 7, 13, 14).
The complement system contains about 25 proteins; with the origin of some of these proteins found back as far as insects in evolution. Within this system, the roles of the innate and the acquired immune system are truly blended. Three pathways of activation of complement proteins exist: the classical pathway, the alternative pathway and the lectin pathway. These pathways differ in the way that the C3 convertase is formed, and after this step, the pathways are the same with a cascade of proteins each in turn activating other proteins until pathogen lysis occurs. The classical pathway functions only with antibody bound to antigen at the onset, so this pathway is linked to the acquired immune system. The alternative pathway begins with the spontaneous hydrolysis of some of the C3 that is in the serum, which may bind to surfaces of bacteria, fungi, viruses, or tumor cells and subsequently bind the next molecules of the pathway. The lectin pathway begins with the binding of a mannose binding lectin (a PRR) to the mannose on the surface of the pathogen. The mannose-binding lectin is associated with enzymes that bind and cleave the complement component C4. The cleaved C4 subsequently binds to C3 and, thus, complement is activated. Complement will be discussed further in Chapter 4 (1, 3, 6, 7, 13, 14). See Table 1.3 ✪ for a summary of the information about the molecules of the innate immune response (1, 3, 6, 7, 13, 14) and Table 1.4 ✪ for a summary of the information about acute phase proteins (1, 3, 6, 7, 13, 14).
✓
Checkpoint! 1.3
These molecules of the innate immune system can bind to PAMPs.
Primary Function example
Recognition
Enhance or decrease immune response
Protect at epithelial cell surface
Different proteins, different functions
Include activating complement to removing free hemoglobin
Target cell lysis, improve phagocytosis, increase vascular permeability
Toll-like receptors (TLR)
Interleukins
Interferons
Defensins
Cathelicidins
C-reactive protein
Haptoglobin
Alternative Lectin or classical pathways
phagolysosome. In this the pathogen is usually killed and broken down by cellular enzymes and the reaction with peroxide anions, hydroxyl radicals, and singlet oxygen produced by a respiratory burst by the phagocytic cell. Some microbes can survive phagocytosis and can actually spread throughout the body while riding in these phagocytic cells (1, 3, 6, 7, 13, 14).
This concludes the discussion of the innate immune system as a separate entity. All further discussions of these cells and molecules will be as they interact with the acquired immune system.
the acquired immune system
To begin this section, please remember that the hallmarks of the acquired immune system are that it is specific, has a large scope, can discriminate, and has a memory. See Table 1.1 for a summary of the differences between the innate and the acquired immune response.
the ceLLs Of the acquired immune system
The key cells involved in the acquired immune response are two types of lymphocytes, the T lymphocyte and the B lymphocyte, which are also called the T and the B cells. Lymphocytes are about 20% of the circulating white blood cells and are composed of very little cytoplasm with the resting cell almost full of the nucleus alone. The nucleus can have a slight dent, making it look kidney shaped. These cells get their names from their location of maturation; although they are both derived from the same hematopoietic progenitor, T cells have matured to become T cells in the thymus, while in mammals the B cells have matured to become B cells in the bone marrow and in birds B cells mature in the bursa of Fabricius. This
distinction is important because this easily removable organ in chickens led to the understanding that B cells produce antibody (see Box 1.1 ✪). The acquired immune system is said to have two arms, the humoral and the cellular. The humoral arm is antibody-mediated immunity, and the cellular arm is T-cell-mediated immunity. Both B and T cells recognize antigens through a specific molecule on their surfaces; for the B cell, this is surface immunoglobulin; for T cells, it is called the T-cell receptor (2, 4, 5, 6, 7, 8, 10, 15, 16).
antibOdy intrOductiOn—GeneraL structure
Antibody molecules are also called immunoglobulin molecules and gamma globulins. The derivation of the first name is obvious because they have an immune function and are globular proteins, but the second name—gamma globulin—takes a little explanation. In serum protein electrophoresis, serum is placed in an electric field in agarose at pH 8.6, and the proteins in the serum, mostly with a negative charge at this pH, are separated into five groups. Albumin is the most anionic protein and thus is the fastest-moving protein toward the anode. Next are the alpha 1 globulins, alpha 2 globulins, then the beta globulins, and finally the gamma globulins (see Figure 1.9 ■). The antibody activity is found in the gamma region, hence, the name gamma globulins. The serum protein electrophoresis assay is performed to yield information about certain clinical diseases, such as decreased antibody production (hypogammaglobulinemia) and increased antibody production due to the cancer of antibody-producing cells (myeloma), so this assay will be discussed again later. The 5 types of antibody molecules are IgG, IgM, IgA, IgE, and IgD. There are some similarities between these molecules and some physical differences with the physical differences resulting in biological differences as
The Accidental Discovery of the role of the Bursa in B-Cell Development
The discovery of the role of the bursa for B cell development in birds, was one of the many serendipitous moments in which pivotally important information was discovered accidentally, and not immediately respected. Bruce Glick in 1952 was a scientist in the field of poultry science and was trying to discover the role of the bursa in birds. He had bursectomized several chickens so that he could study the physiologic function of the bursa. A graduate student asked if he could have a few of these chickens to show an undergraduate class that vaccination results in the production of antibodies that can agglutinate Salmonella. A week after this vaccination, much to the hilarity of the undergraduates, when the graduate student mixed the drop of blood with the bacteria nothing happened in the first few samples tried. However, as the graduate student continued, samples from other chickens did agglutinate the bacteria.
The graduate student, Tim Chang, went to talk to Bruce Glick about this and they realized that the animals whose sera did not agglutinate had been bursectomized when they were very young. Further studies showed a link of the bursa to antibody production. Science magazine did not accept this manuscript because they wanted the mechanism explained. This data was subsequently published in the Journal of Poultry Science, and has become a Citation Classic (13).
✪ box 1.1
■ Figure 1.9 Serum protein electrophoresis is a technique in which serum components separate into 5 different major groups based on their mobility in an electric field. The sample was applied on the right side of the figure. The peak farthest from the origin is albumin, the next contains the alpha 1 globulins, and then the alpha 2, followed by the beta globulins, and finally the gamma globulin peak, which contains the immunoglobulin.
well (Figure 1.10 ■). These will be discussed in Chapter 2. B cells produce antibody in response to the antigen specifically binding to their surface immunoglobulin and can respond to soluble antigen alone. B cells are characterized by this surface immunoglobulin and by the presence of the surface molecules CD19, CD20, and CD21 (2, 4, 5, 6, 7, 8, 10, 15, 16).
Checkpoint! 1.4
Serum has been taken from a patient and analyzed by serum protein electrophoresis. The patient is immunized with a number of vaccines to prepare for travel and to meet the requirements for college entrance. Blood is drawn 2 weeks later, giving the patient enough time to make antibody. Which peak in the serum protein electrophoresis will be increased?
the ceLLuLar arm
The cellular arm of the immune response is due to the functions of T cells. T cells respond to antigens that specifically bind to their T-cell receptor (TCR) and that are presented by an antigen-presenting cell in either a major histocompatibility
■ Figure 1.10 The basic structure of an antibody molecule. Two heavy chains are joined by two light chains. The antibody contains two binding sites, each of which is formed by a heavy and a light chain. The constant portion of each chain is shown in blue and the variable region in green.
complex (MHC) class I molecule or MHC class II molecule. Additional signals of costimulation through binding of other molecules on the antigen-presenting cells (APCs) and cytokines are also required. The major histocompatibility complex was so named because these antigens were first discovered as a result of their role in the rejection or acceptance (compatibility) of tissue (histo) grafts. These genetically inherited molecules have been found to be important in antigen presentation and in the immune response.
T cells can be divided into helper T cells, cytotoxic T cells, and regulatory T cells. The products of helper T cells are cytokines that can upregulate the immune response; the product of cytotoxic T cells is their direct cytotoxicity (cell killing) of cells bearing the antigen; and the products of regulatory T cells are cytokines that downregulate the immune response when the pathogen is cleared and help prevent autoimmunity. Helper T cells respond to a specific antigen that binds to their TCR in association with the MHC class II molecule, cytotoxic T cells respond to a specific antigen that binds to their TCR in association with the MHC class I molecule, and regulatory T cells bind to their specific antigen through their TCR usually in association with MHC class II molecules but sometimes in association with MHC class I molecules. T cells can be identified by the CD3 marker on their surface, which is part of the T-cell receptor. In addition, helper T cells are CD4 + , cytotoxic T cells are CD8 + , and regulatory T cells are usually CD4 + . CD8 + regulatory cells can also be found with Foxp3 + serving as the marker that characterizes these cells (Figure 1.11 ■) (2, 4, 5, 6, 7, 8, 10, 15, 16). More about these cells and their functions and controls will be described in Chapter 4.
HUMORAL
(ANTIBODY-MEDIATED) IMMUNE SYSTEM
Control of freely circulating pathogens
Extracellular antigens
A B cell binds to the antigen for which it is specific. A T-dependent B cell requires cooperation with a T helper (TH) cell.
The B cell, often with stimulation by cytokines from a TH cell, differentiates into a plasma cell. Some become memory cells.
Cytokines activate T helper (TH) cell
CELLULAR (CELL-MEDIATED) IMMUNE SYSTEM
Control of intracellular pathogens
Exposure to a processed intracellular antigen: intracellular antigens expressed on the surface of a cell infected by a virus, bacterium, or parasite (also may be expressed on the surface of an APC).
1 A T cell binds to MHC–antigen complexes on the surface of the infected cell, activating the T cell (with its cytokine receptors).
Cytokines
Cytokines from the TH cell transform B cells into antibody-producing plasma cells.
Plasma cell
Plasma cells proliferate and produce antibodies against the antigen.
Memory cell
Some T and B cells differentiate into memory cells that respond rapidly to any secondary encounter with an antigen.
Cytotoxic T lymphocyte
Cytokines
Lysed target cell
2 Activation of macrophage (enhanced phagocytic activity).
3 The CD8+ T cell becomes a cytotoxic T lymphocyte (CTL) able to induce apoptosis of the target cell.
Regulatory T cell
■ Figure 1.11 The humoral and cellular immune response. At the left is the humoral (or B-cell mediated); at the right is the cellular (or T-cell mediated) immune system. B cells become antibody secreting plasma cells or memory cells with antigen stimulation. T cells are of three general types: (1) helper T cells that secrete cytokines, which upregulate the immune response, (2) cytotoxic T cells that kill target cells after making direct contact with their target, and (3) regulatory T cells, which serve to downregulate the immune response.
Source: TORTORA, GERARD J.; FUNKE, BERDELL R.; CASE, CHRISTINE L., MICROBIOLOGY: AN INTRODUCTION, 10th, ©2010. Printed and Electronically reproduced by permission of Pearson Education, Inc., Upper Saddle River, New Jersey.
Checkpoint! 1.5
Which T cells seem to be on opposite sides of a battle?
the LymphOid OrGans
The primary lymphoid organs, the bone marrow and the thymus, are where the lymphocytes mature into either T or B cells. Organs in which these white blood cells meet antigens, respond, proliferate, and interact with other lymphocytes are
called secondary lymphatic organs. The secondary lymphatic organs include lymph nodes, the spleen, tonsils, mucosalassociated lymphoid tissue (MALT), and skin-associated lymphoid tissue (SALT). MALT includes Peyer’s patches in the intestine, tonsils, and the appendix (2, 4, 5, 6, 7, 8, 10, 15, 16).
the primary Lymphatic OrGans
The primary lymphoid organs are those in which lymphocytes are generated and the initial differentiation of the lymphoid cells occurs to form mature T cells, B cells, and NK cells. Antigen contact in primary lymphatic organs results in cell
B cell