ChemicalApproachesforGreenand SustainableDrugs1stEditionTörökM.(Ed.)
https://ebookmass.com/product/contemporary-chemicalapproaches-for-green-and-sustainable-drugs-1st-editiontorok-m-ed/
Green Approaches for Chemical Analysis Emanuela Gionfriddo
https://ebookmass.com/product/green-approaches-for-chemical-analysisemanuela-gionfriddo/
ebookmass.com
Green Sustainable Process for Chemical and Environmental Engineering and Science: Green Solvents for Biocatalysis 1st Edition Inamuddin (Editor)
https://ebookmass.com/product/green-sustainable-process-for-chemicaland-environmental-engineering-and-science-green-solvents-forbiocatalysis-1st-edition-inamuddin-editor/
ebookmass.com
Green Approaches in Medicinal Chemistry for Sustainable Drug Design 1st Edition Bimal K. Banik (Editor)
https://ebookmass.com/product/green-approaches-in-medicinal-chemistryfor-sustainable-drug-design-1st-edition-bimal-k-banik-editor/
ebookmass.com
Chronic Renal Disease 2nd Edition Edition Paul Kimmel
https://ebookmass.com/product/chronic-renal-disease-2nd-editionedition-paul-kimmel/
ebookmass.com
https://ebookmass.com/product/deadly-passion-holly-bloom/
ebookmass.com
5 Steps to a 5: AP Calculus AB 2023 (5 Steps to a 5)
William Ma
https://ebookmass.com/product/5-steps-to-a-5-ap-calculusab-2023-5-steps-to-a-5-william-ma/
ebookmass.com
Economics for Sustainable Prosperity 1st ed. Edition
Steven Hail
https://ebookmass.com/product/economics-for-sustainableprosperity-1st-ed-edition-steven-hail/
ebookmass.com
Entrepreneurship: The Art, Science, and Process for Success 4th Edition Charles Bamford
https://ebookmass.com/product/entrepreneurship-the-art-science-andprocess-for-success-4th-edition-charles-bamford/
ebookmass.com
Discovering John: Essays by John Ashton 1st Edition
John Ashton
https://ebookmass.com/product/discovering-john-essays-by-johnashton-1st-edition-john-ashton/
ebookmass.com
https://ebookmass.com/product/twisted-emotions-the-camorra-chroniclesbook-2-cora-reilly/
ebookmass.com
ContemporaryChemicalApproaches forGreenandSustainableDrugs AdvancesinGreenand SustainableChemistry Contemporary ChemicalApproaches forGreenand SustainableDrugs SeriesEditor
Be´laTo¨ro¨k
TimothyDransfield
Editedby
MariannaTo¨ro¨k
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright 2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandtheCopyright LicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions .
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightby thePublisher(otherthanasmaybenotedherein).
Notices Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professional practices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatter ofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-12-822248-5
ForinformationonallElsevierpublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: GabrielaCapille
EditorialProjectManager: ClarkEspinosa
ProductionProjectManager: SujathaThirugnanaSambandam
CoverDesigner: MarkRogers
TypesetbyTNQTechnologies
Listofcontributors xiii
1.Usingthezebrafishmodelsystemtoidentifythe healtheffectsofpharmaceuticalpollutants
ChristinaKaucic,AnushaLakshmiDharmavathi andJenniferL.Freeman
1.Introduction 1
2.Thezebrafishmodelsystem 2
3.Useofthezebrafishmodelsystemindrugdiscovery 3
4.Significanceofpharmaceuticalpollution 6
5.Useofthezebrafishmodelsystemtoassesspharmaceutical pollutanttoxicity 7
6.Methodsandapproachestoassesspharmaceuticalpollutant toxicityusingthezebrafishmodelsystem 13
6.1Acutedevelopmentaltoxicityassessmentswiththe developingzebrafish13
6.2High-throughputscreenings(HTS)fordevelopmentaltoxicity assessments14
6.3Zebrafishdevelopmentalandadultbehavioralassaystoassess pharmaceuticalpollutanttoxicity16
6.4Cellularandmolecularassaystoidentifymechanismsof pharmaceuticalpollutanttoxicity18
7.Futuredirectionsfortheuseofzebrafishindefining pharmaceuticalpollutanttoxicity 20
8.Conclusions 21 AcknowledgmentsandFundingsources 21 References 21
2.Analysisofpharmaceuticalsintheenvironment
AdityaKulkarniandScottE.Miller
1.Introduction 27
2.Sourcesofpharmaceuticalpollutants 28
2.1Effectsoftracelevelpharmaceuticalpollutantson humans29
2.2Effectsoftracelevelpharmaceuticalpollutantson aquaticenvironments30
3.Analyticalmethodsfortracelevelanalysisofwatersamples
3.Leakingofantibioticsintheaquaticenvironment
Indu,ManishaSharmaandKashyapKumarDubey
4.Advancesindrugdevelopmentwiththe applicationofartificialintelligence
ManuelaSouzaLeite,AndersonAllesdeJesus, PauloJardelLeiteAraujoandBrunnoFerreiradosSantos
1.Machinelearningandartificialintelligenceinthe
4.Supportvectormachines(SVM)indrugdiscoveryand
5.Virtualscreeningtechniquesinpharmaceutical research
JustineC.Williams,StanleyOpare,SenthilKumarSugadoss, AravindhanGanesanandSubhaKalyaanamoorthy
1.Introduction 89
2.Structure-baseddrugdesign(SBDD) 91 2.1Proteinstructureprediction92
3.Moleculardocking 96
3.1Searchalgorithms96
3.2Scoringfunctions97
3.3Targetflexibility101
3.4Denovodrugdesign101
3.5Bindingenergyestimation103
3.6Machine/deeplearningmethodsinSBVS104
4.Ligand-baseddrugdiscovery(LBDD) 107
4.1Similaritysearching108
4.2Ligand-basedpharmacophoremapping113
4.3Quantitativestructure-activityrelationship(QSAR) modeling115
5.Summaryandperspectives
6.Insilicomodelingofenvironmentaltoxicity ofdrugs
KabiruddinKhanandKunalRoy
1.Introduction 129
2.Pharmaceuticalecotoxicityanalysis:generalconsiderations 129 2.1Overviewofconcerns130
3.Releaseofpharmaceuticalstotheenvironment 131
3.1Thesourcesofpharmaceuticalspollutiontoenvironment131
4.AssessmentofecotoxicityofAPIs,limitations,andsolutions: adatascientist’sperspective 131
5.Currentadvancementinecotoxicitymodelingof pharmaceuticals 132
5.1Insilicotoolsreportedindifferentresearcharticles133
6.Onlineexpertsystemsforecotoxicityprediction 141 7.Conclusion
7.Sustainableseparationsinpharmaceutical manufacturing
GergoIgnacz,RobertOrkenyi,ArpadKonczolandGyorgySzekely
1.Introduction 155
1.1Separationconceptsinthepharmaceuticalindustry156
1.2Continuousandautomatedseparationprocesses158
2.Chromatography-basedseparations 159
2.1Sustainableaspectsofchromatography159
2.2Towardgreenchromatographictechniques159
2.3Strategiestowardgreenliquidchromatography162
2.4Relevantindustrialapplications:casestudies166
2.5Conclusions167
3.Membranebasedseparations 167
3.1Membraneseparationinthepharmaceuticalindustry: aliquiddominantsector167
3.2Organicsolventnanofiltration(OSN)168
4.Continuouspurificationprocesses 174
4.1Classificationofthecontinuousflowpurification processes174
4.2Continuouscrystallization(CC)175
4.3Centrifugalpartitionchromatography177
4.4Simulatedmovingbedchromatography179
4.5Comparisonofthepreviouslydiscussedcontinuous purificationmethods180
5.ForecastingthefutureofAPIseparations 183 Listofabbreviations 184 References 185
8.Greensyntheticmethodsindrugdiscoveryand development
GuoshuXie,RitaBernadettVlocsko ´ andBe ´ laTo ¨ ro ¨ k
1.Introduction 201
2.Catalysis 203
2.1Homogeneouscatalysis204
2.2Heterogeneouscatalysis217
3.Nontraditionalactivationmethodsandenergyefficiencyof chemicalprocesses 238
3.1Microwave-assistedorganicsynthesis238
3.2Ultrasonicactivation242
3.3Photochemicalactivation248
3.4Electrochemicalactivation256
4.Conclusions 260 References 261
9.Characterizingtheenvironmentallybenignnatureof chemicalprocesses:greenchemistrymetrics
DavidDaggett,YizhouShiandBe ´ laTo ¨ ro ¨ k
3.Sustainableproductionofpharmaceuticalsandtheirbuilding blocks:quantitativegreenmetricstoevaluatechemical processes
3.1Mass-relatedmetrics283
3.2Energy-relatedmetrics294
10.Greenchemistryapproachestodrugsthattreat epidemicandpandemicdiseases BerkeleyW.Cue 1.Introduction
3.Drugstotreatmalaria
5.AntiviralstotreatCOVID-19
11.Dynamiceffectsoforganicmoleculesfordrug deliveryinmicelles
DebanjanaGhosh,RiaRamoutarandShainazLandge
3.Moleculardrugdeliverybyorganizedself-assemblies 337
3.1Smallmolecule-surfactantbaseddrugdelivery337
3.2Liposomalsystemasdrugdeliveryvehicle347
3.3Reversemicellesnanocarriersfordrugdelivery360 4.Conclusion
12.Antibody-drugconjugatesfortargeteddelivery
GarimaPandey,SunilK.TripathiandVivekBulbule
1.Introduction 377
1.1Whatisanantibody-drugconjugate?377 1.2ImportanceofADCsintargeteddrugdelivery379 1.3CurrentADCsapprovedorinclinicaltrials380
2.Composition 385 2.1Targetandantibody385 2.2Linkers386 2.3Payloads395
3.Antibody-drugconjugation 396
3.1Throughsidechainlysineresidue397
3.2Throughsidechaincysteineresidue398
3.3Drugantibodyratio(DAR)400
3.4Site-specificconjugation400
4.Physicalstabilityofantibody-drugconjugates 403
5.Chemicalstabilityofantibody-drugconjugate
6.Formulationdevelopmentofantibody-drugconjugate
7.FutureaspectsofADCs
13.Towardthegreensynthesisofpeptidesand peptidicdrugs
Do ´ raBogda´n,LeventeKa ´ rpa´tiandIstva´nM.Ma´ndity
1.Peptidesandpeptidesynthesis:theformationofpeptide bond 421 1.1Historicalperspectiveofsolutionphasepeptidesynthesis421 1.2Solid-phasepeptidesynthesis(SPPS)422 1.3Solid-phasefragment/segmentcondensationinthe1980s424 1.4AutomatizationoftheSPPS424
2.Improvementsinthesolid-phasemethod 426 2.1Optimizationofthelinkersandpolymericsupportsin SPPS426
2.2DevelopmentoftheN-terminalaminoprotecting groups427
2.3Onresinmonitoringofthecouplingefficiency428
2.4Developmentofcouplingagentsandcoupling methodologies428
3.NovelmethodsfordissolvinglimitationsofSPPS 430
3.1Nativechemicalligation(NCL)430
3.2Continuous-flowsolid-phasepeptidesynthesis(CF-SPPS)431
3.3Solution-phasecontinuous-flowpeptidesynthesis435
3.4Microwave-assistedpeptidesynthesis436
4.Large-scalepeptidesynthesismethods 439
4.1Continuous-flowlargescalepeptidesynthesis439
4.2Solution-phaselarge-scalepeptidesynthesis440
4.3Solid-phaselarge-scalepeptidesynthesis440
4.4Hybridlarge-scalepeptidesynthesis441
5.Greenchemistryaspectsofpeptidesynthesis 441
6.Summary 445
14.Themultitargetapproachasagreentoolin medicinalchemistry
RitaBernadettVlocsko
´ ,SinemApaydın,Be ´ laTorok andMariannaTorok
1.Introduction 457
2.Thegreenimpactofmultitargetdrugdiscovery 460
2.1Greensynthesesinmultitargetdrugdevelopment(MTDD)462
2.2Combininginsilicoandexperimentalmethods: inherentlygreenimprovementtodesignand testingefficiency464
3.Multitargetleadgeneration screening-versus knowledge-basedapproaches 465
3.1Knowledge-basedrationaldesign466
3.2Screening-basedmultitargetleadgeneration468
3.3Naturalproduct-inspiredscaffoldsfordesigningMTDs472
4.Selectedcasestudieswithmultitargetfocus 476
4.1Neurodegenerativediseases Alzheimer’sdisease476
4.2Infectiousdiseases477
4.3Cancer478
4.4Epigeneticpolypharmacology479
5.Challengesandlimitationsinmultitargetdrugdesignand development 480
6.Conclusions 481 Listofabbreviations 482
15.Directedevolution:anewpowerfultoolindrug development
AndreaBaierandRyszardSzyszka
1.Introduction 493
2.Methodsindirectedevolution 494
2.1Methodsforgenemanipulation495
2.2Methodsforscreeningandselectionofenzymelibraries501
3.Applicationofdirectedevolutionindrugdevelopment 502
3.1SimvastatinsynthaseLovD502
3.2TransaminaseATA-17inthesynthesisofsitagliptin504
3.3 Candidaantarctica lipaseA(CALA)inthesynthesisof ibuprofen505 4.Perspectives
16.Conventionalandadvancedtreatmentmethods fortheremovalofpharmaceuticalsandrelated compoundsinwastewater
JamesA.Noblet
1.Introduction 511
2.Overviewofconventionalwastewatertreatment 513
3.Effectivenessofconventionalwastewatertreatmentin removingpharmaceuticals 514
4.Advancedtreatmentprocessesfortheremovalof pharmaceuticalsfrommunicipalwastewater 516
4.1Overview516
4.2Ozonation516
4.3Activatedcarbonmethods517
4.4Membrane-basedtechnologies518
4.5Newermethods:advancedoxidativeprocesses521
5.CaseStudy#1:SanBernardinomunicipalwater department 524
5.1Rapidinfiltrationandextraction(RIX)facility524
6.CaseStudy#2:theOrangeCountySanitationDistrict, FountainValley,CA 529
6.1Groundwaterreplenishmentsystem(GWRS)529
7.Conclusion 529
Listofcontributors AndersonAllesdeJesus,CoordinationoftheInterdisciplinaryBachelorofScience andTechnology,UniversidadeFederaldoMaranha ˜ o,Balsas-MA,Brazil
SinemApaydın,DepartmentofChemistry,UniversityofMassachusettsBoston, Boston,MA,UnitedStates
PauloJardelLeiteAraujo,ICONEducacional,Aracaju-SE,Brazil
AndreaBaier,TheJohnPaulIICatholicUniversityofLublin,Lublin,Poland
Do ´ raBogda ´ n,DepartmentofOrganicChemistry,FacultyofPharmacy,Semmelweis University,Budapest,Hungary;TTKLendu ¨ letArtificialTransportersResearch Group,InstituteofMaterialsandEnvironmentalChemistry,ResearchCentrefor NaturalSciences,Budapest,Hungary
VivekBulbule,AdesisInc.,NewCastle,DE,UnitedStates
BerkeleyW.Cue,BWCPharmaConsulting,LLC,Nottingham,NH,UnitedStates; DepartmentofChemistry,UniversityofMassachusettsBoston,Boston,MA, UnitedStates
DavidDaggett,DepartmentofChemistry,UniversityofMassachusettsBoston,Boston, MA,UnitedStates
BrunnoFerreiradosSantos,DepartmentofChemicalandMaterialsEngineering (DEQM),PontificalCatholicUniversityofRiodeJaneiro(PUC-Rio),Riode Janeiro,Brazil
JenniferL.Freeman,SchoolofHealthSciences,PurdueUniversity,WestLafayette, IN,UnitedStates
AravindhanGanesan,ArGan’sLab,SchoolofPharmacy,UniversityofWaterloo, Waterloo,ON,Canada
DebanjanaGhosh,DepartmentofChemistryandBiochemistry,GeorgiaSouthern University,Statesboro,GA,UnitedStates
GergoIgnacz,AdvancedMembranesandPorousMaterialsCenter,PhysicalScience andEngineeringDivision(PSE),KingAbdullahUniversityofScienceand Technology(KAUST),Thuwal,SaudiArabia
Indu,BioprocessEngineeringLaboratory,CentralUniversityofHaryana, Mahendergarh,Haryana,India
SubhaKalyaanamoorthy,DepartmentofChemistry,UniversityofWaterloo, Waterloo,ON,Canada
Listofcontributors
LeventeKa ´ rpa ´ ti,DepartmentofOrganicChemistry,FacultyofPharmacy,SemmelweisUniversity,Budapest,Hungary
ChristinaKaucic,SchoolofHealthSciences,PurdueUniversity,WestLafayette,IN, UnitedStates
KabiruddinKhan,DepartmentofPharmaceuticalTechnology,JadavpurUniversity, Kolkata,WestBengal,India
ArpadKonczol,RotaChromTechnologiesLLC,Kecskemet,Hungary
AdityaKulkarni,908Devices,Boston,MA,UnitedStates
KashyapKumarDubey,BioprocessEngineeringLaboratorySchoolofBiotechnology,JawaharlalNehruUniversity,NewDelhi,India
AnushaLakshmiDharmavathi,SchoolofHealthSciences,PurdueUniversity,West Lafayette,IN,UnitedStates
ShainazLandge,DepartmentofChemistryandBiochemistry,GeorgiaSouthern University,Statesboro,GA,UnitedStates
Istva ´ nM.Ma ´ ndity,DepartmentofOrganicChemistry,FacultyofPharmacy,SemmelweisUniversity,Budapest,Hungary;TTKLenduletArtificialTransporters ResearchGroup,InstituteofMaterialsandEnvironmentalChemistry,Research CentreforNaturalSciences,Budapest,Hungary
ScottE.Miller,908Devices,Boston,MA,UnitedStates
JamesA.Noblet,DepartmentofChemistryandBiochemistry,CaliforniaState UniversitySanBernardino,SanBernardino,CA,UnitedStates
StanleyOpare,DepartmentofChemistry,UniversityofWaterloo,Waterloo,ON, Canada
RobertOrkenyi,RotaChromTechnologiesLLC,Kecskemet,Hungary;EvonetixLtd, Cambridge,UnitedKingdom
GarimaPandey,OrganixInc.,Woburn,MA,UnitedStates
RiaRamoutar,DepartmentofChemistryandBiochemistry,GeorgiaSouthern University,Statesboro,GA,UnitedStates
KunalRoy,DepartmentofPharmaceuticalTechnology,JadavpurUniversity,Kolkata, WestBengal,India
ManishaSharma,BioprocessEngineeringLaboratory,CentralUniversityofHaryana, Mahendergarh,Haryana,India
YizhouShi,DepartmentofChemistry,UniversityofMassachusettsBoston,Boston, MA,UnitedStates
ManuelaSouzaLeite,GraduatePrograminProcessEngineering,Tiradentes University,Aracaju-SE,Brazil;InstituteofTechnologyandResearch,Aracaju-SE, Brazil
SenthilKumarSugadoss,DepartmentofChemistry,UniversityofWaterloo,Waterloo,ON,Canada
Listofcontributors xv
GyorgySzekely,AdvancedMembranesandPorousMaterialsCenter,PhysicalScience andEngineeringDivision(PSE),KingAbdullahUniversityofScienceandTechnology(KAUST),Thuwal,SaudiArabia
RyszardSzyszka,TheJohnPaulIICatholicUniversityofLublin,Lublin,Poland
Be ´ laTo ¨ ro ¨ k,DepartmentofChemistry,UniversityofMassachusettsBoston,Boston, MA,UnitedStates
MariannaTo ¨ ro ¨ k,DepartmentofChemistry,UniversityofMassachusettsBoston, Boston,MA,UnitedStates
SunilK.Tripathi,UniversityofMichigan,AnnArbor,MI,UnitedStates
RitaBernadettVlocsko ´ ,DepartmentofChemistry,UniversityofMassachusetts Boston,Boston,MA,UnitedStates
JustineC.Williams,DepartmentofChemistry,UniversityofWaterloo,Waterloo,ON, Canada
GuoshuXie,DepartmentofChemistry,UniversityofMassachusettsBoston,Boston, MA,UnitedStates
Chapter1 Usingthezebrafishmodel systemtoidentifythehealth effectsofpharmaceutical pollutants ChristinaKaucic,AnushaLakshmiDharmavathiandJenniferL.Freeman SchoolofHealthSciences,PurdueUniversity,WestLafayette,IN,UnitedStates
1.Introduction
Withalleyesshiftingtowardtheimpactsofglobalclimatechangeand pollutiononhumanandecologicalhealth,sustainabilityandenvironmental safetyareeminenttopicsthatneedtobeaddressed.Specifically,thereismuch focustowardhowpharmaceuticalwasteandbuildupintheenvironmentmay affectthegeneralhealthandwellnessofexposedorganisms.1 Pharmaceutical pollution,whichreferstothepresenceofsyntheticdrugsandtheirmetabolites intheenvironment,islargelyunregulatedandhasthepotentialtobedetrimentaltohumanandecologicalhealth.Inthepast,environmentaltoxicology studies,includingthoseassessingpharmaceuticalpollution,havereliedprimarilyonrodentorothermammalianmodelstoperformtoxicityassessments. Limitationsandhigheconomiccostsoflarge-scalemammalianmodelsfor assessingenvironmentaltoxicityhaveledtoanexpansionofothervertebrate modelsystemsbeingappliedinthisresearcharea.Asselectingtheoptimal animalmodelisvitaltounderstandingenvironmentaltoxicology,researchers haveenthusiasticallyturnedtothezebrafishmodeltostudytheeffectsof environmentalpollutantsincludingpharmaceuticalpollutionasananimal modelcomplementarytothetraditionalmammalianassays.Inthischapter,we discusstheadvantages,strengths,andlimitationsforutilizingthezebrafish modelsystemintoxicologicalstudiesandinparticular,thosestudiesassessing theadverseeffectsofpharmaceuticalpollution.Inaddition,wedetailcommon zebrafishassaysemployedtoassessdevelopmentaltoxicity,morphology, behavior,andmolecularperturbationsinducedbypharmaceuticalpollution exposure.
2.Thezebrafishmodelsystem Zebrafish(Daniorerio)areanotableandpopularvertebratemodelindevelopmentalandmolecularbiologyduetotheirsuitabilityforgenomicstudies andtransparentembryos,whichallowforeasydevelopmentalassessment.2 Basedonanumberofstrengths,theirvalueasacomplementarymodelsystem fortoxicitystudieshassteadilyincreasedoverthepast20yearsincluding thoseaddressingenvironmentalpollutionandtheiruseindrugdevelopment.3,4 Inadditiontobeingasmall,economicalmodelincomparisonwith mammaliancounterparts,thezebrafishhashighfecundity,easyhusbandry,is near-transparentduringtheearlystagesofdevelopment,and,perhapsmost importantly,hasahighdegreeofmolecularsyntenywithhumans.Infact,71% ofallhumanproteinsand82%ofdisease-causingproteinshaveanorthologin thezebrafish.5 Coupledtothewell-conservedphysiologywithhumans,the zebrafishisanidealmodelforhumandrugdiscoveryandtoxicological studies.
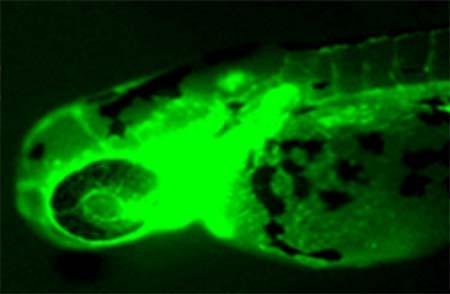
Afewofthemostsimplisticbenefitsofusingthezebrafishasatoxicologicalmodelaretheirsmallsizeandhusbandry.Unlikeothermodelspecies, adultzebrafisharefullygrownat1 1.5incheslong.Thisaloneallowsforan increasedsamplesizeinlaboratorieswithlimitedspaceforhousingand breeding.Thesmallsizeofthefishalsoallowsforsmallerquantitiesofdosing solutionsintoxicologicalstudieswhich,inturn,minimizeslaboratorycosts andchemicalwaste.Additionally,zebrafishmatingpairshaveahighfecundity andproducemanysmallembryosthatcanincreasesamplesizesandnumber ofreplicatesindose-responseassaysandhigh-throughputtoxicitytesting. Majorreasonsforthepopularityofthezebrafishmodelindevelopmental biologyarebasedontheirhighfecundityandembryosthathavetransparent chorions.Transparencyallowsfordevelopmentalstagingstudiesandidentificationofphenotypicabnormalitiesattheearliestdevelopmentalstages.The transparentchorionsofzebrafishembryosproveevenmorevaluablewhen usedwithinsituhybridizationandimmunohistochemistrytechniques.Insitu hybridizationandimmunohistochemistryaretypicallyusedindevelopmental biologytoinvestigategeneandproteinexpressioninembryonictissues;the clarityofthezebrafishembryoallowslabeledprobestobeeasilyidentified.In addition,developmentoftransgeniczebrafishmodelswithfluorescenttags (e.g.,greenfluorescentprotein,GFP)(Fig.1.1)alsopermitsassessmentof geneexpressionpatterns,tissue/organdevelopment,cellularlocalizationof proteins,andotherapplicationsattheearliestdevelopmentalstages.6,7 Theuse ofthesetechniqueshasspurredquestionsabouttherelevanceofgeneand proteinexpressionstudiesinthezebrafishandtherelationshiptothehuman genome.Understandingthissyntenicrelationshipbetweenthetwospecies allowsforhuman-diseasemodelstudiestotakeplaceusingthezebrafish.
Thezebrafishgenomeiscomplexwithahighdegreeofsimilaritytothe humangenome.8 Inastudyinvestigatingthedifferencesbetweenthehuman
FIGURE1.1 Zebrafishfli1[(Tg(fli1:eGFP)y1]transgeniclarva.Thistransgeniclineinvolvesa transgenicinsertionofenhancedgreenfluorescentprotein(eGFP)inthevasculatureundercontrol ofthefli1promoter.Thistransgeniclineisusedtoeasilyvisualizevasculardefectsinzebrafish followingchemicalexposures.
genomeandthezebrafishgenome,itwasdiscoveredthataround80%ofgenes andexpressedsequencetagsareconserved.9 Inalattermoreextensivestudy, resultsshowed82%ofhumandiseasegeneshaveatleastonezebrafish ortholog.5 Moreover,genemutationsinzebrafishhavebeenshowntoproduce lessembryoniclethalitythanthesamegenemutationsinmammalianmodels, largelyduetoagenomeduplicationevent.10 Assuch,researchersarebetter abletostudytheaffectedgenefunctionandtheassociatedsignalingpathways inpathogenesis.Intoxicologicalstudies,thisfactisvitalasweareableto studydevelopmentalchangesandthemolecularmechanismsofthexenobiotic exposures.
3.Useofthezebrafishmodelsystemindrugdiscovery Thescientificdiscoveryofnewdrugsbeingmanufacturedfrompharmaceuticalcompanieshasdeclinedovertheyears,whiletheproductioncostshave steadilyincreased.Amajorcauseisthetedioustestingthatisneededinorder toputanewdrugonthemarket.Usually,numerousinvitrobiochemicaland cellassaysfollowedbyinvivotestingwithmammalianmodelsarerequired beforehumantrialscancommence.Thisprocessoftenleadstoonlyafew drugsonthemarket.Theseprospectivedrugscangetterminatedatanyphase ofdrugdevelopmentduetoadversehealthoutcomes,lackofefficacy,or excessivetoxicityproblems.Mostofthefailuresofdrugtoxicitycomeatthe levelofusinganimalmodels.Thecausesofdrugtoxicityobservedare organizedintomechanism-based(on-target),immunehypersensitivity,offtargettoxicity,andcovalentmodification.Therefore,drugdevelopmentisan importantprocessthatservesasanessentialwaytoevaluatethesafetyand toxicityofpotentialharmfuldrugsthatcanbefoundinourenvironment.
4 ContemporaryChemicalApproachesforGreenandSustainableDrugs
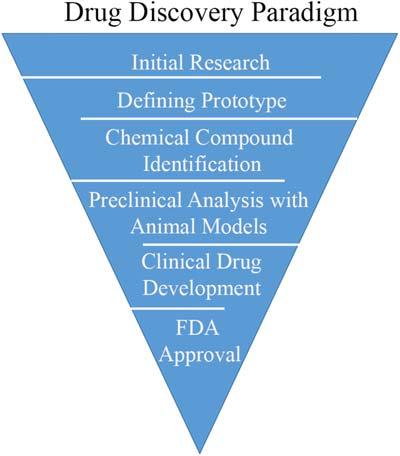
Althoughcurrentmethodsworkinpreventingthetoxiccompoundsfrom theclinicalsetting,therearestillfalsenegativesobservedduringpreclinical toxicity.Thisisseenbecausedrugsafetyisinitiallyevaluatedpreclinicallyin animalmodels.Thisincreaseinpreclinicaltoxicityaswellastheadverse eventsthatcomefromhumantoxicityaccountforone-thirdofthecasesof attritionforprospectivedrugs.Assuch,itisimportanttoidentifytoxic compoundsaccuratelytoreducepotentialtoxicityeffects.Stepsinthedrugdevelopmentparadigmsupportthatearlyinterventionwithremovingtoxic compoundscanhelpsaveresourcesandreducecosts(Fig.1.2).Additionally, intheearlyphaseofdrugdevelopmentbetween40%and80%ofthecompoundsarehaltedindevelopmentduetosafetyconcerns,4 supportingthe investigationofthistime-point.Ingeneral,theincidenceofadversedrugreactionsvarieswiththetypeofdruganddosage,supportingthatdrugtoxicity canbeamajorlimitingfactortotheefficacyofwidelyusedagents.
Drugtoxicityhasprovidedscientificandpracticalapplications,but threemajordiscrepanciesstillexistthatcanhelpreducethetoxiceffectsof thechemicalsthroughinvestigation.Thesethreemajorareasoffocusare findingusefulbiomarkersoftoxicity,establishingusefulinvitro/invivorelationships,andlinkinganimalmodelswithhumantoxicitymoreaccurately.11 Throughdrugdiscovery,wehavebeenabletofindmethodstoapproachthese threemainissuesinordertoassessdrugsafetyandtoxicity.Animportant invivorelationshipthatallowsustoimproveoveralltoxiceffectsindrug
FIGURE1.2 Thegeneralized drugdiscoveryanddevelopment pathwaybeforeanoveldrugpasses throughtheUSFoodandDrug Administration(FDA)approvalfor safetytesting.
developmentwasaccomplishedbyexpandingtheuseofanimalmodels beyondmammalsincludingutilizationofthezebrafishmodel.Thezebrafishis showinggreatpotentialtoeliminateunsafecompoundsduringtheearlystages ofdrugdevelopmentandevaluatethepreclinicaltoxicityofnewdrugsata loweroveralleconomiccost.4
Toxicityisamajorattritionindrugdevelopmentleadingtothewithdrawal ofcertaindrugs.Assuch,researchhasbeendonetoaccuratelyfindthepotentialadversehealthoutcomesthatmayoccur.Cardiotoxicityisoneofthe majorconcernsforpharmaceuticalcompaniesalongwithhepatic,oncological, andneurologicaldrugsduetotheirunfavorablecomplications.Cardiotoxicity hasindicateditsrelevanceparticularlyinchildrenandadolescentsastheyare generallymoresusceptibletotoxiceffects.Cardiotoxicityhasremainedan issueasunforeseenheartattacks,strokes,orarrhythmiasmayoccuraswas seenforRofecoxib,anantiarthriticdrug,andPropulsid,agastrointestinal prokineticagent.12 Inordertounderstandthistoxicity,thezebrafishmodel systemwasappliedasareliablecardiotoxicologicaltool.Despitesomeofthe physiologicaldifferencesbetweenthezebrafishandmammalianheart,useof zebrafishpresentsanoptimalassessmentoforgandevelopment.Theheartis thefirstorganthatdevelopsinzebrafishduringtheearlystagesofembryogenesissupportinghighconservationandexhibitingsimilarfunctionalcharacteristics,whichallowstomoreaccuratelylinkanimalmodelswithhuman toxicity.Thisiswhyzebrafishareparticularlysuitablefordetermining developmentaltoxicity,generaltoxicity,andtoperforminitialhigh-throughput drugscreeningsinordertoaccomplishandvalidatedrugdevelopmentstudies. Additionalpaststudieshaveevaluatedcardiotoxicityandcomparedthe zebrafishwithotherinvivoandinvitromodels.Thedatahaveconcluded supportforthezebrafishmodelsystemasanefficientwaytoevaluatedrug toxicityandgivecomprehensiveknowledgefornewgenerationsofdrugs.13
Asecondexampleoftheapplicationofthezebrafishmodelsystemin addressingconcernsduringdrugdevelopmentrevolvesarounddrug-induced liverinjury(DILI).Inclinicalmedicine,amajorchallengeoccursfromthe deliberateoverdoseofacetaminophen.Intermsofdrugdevelopment,DILI remainsaproblembecauseofsafetyconcerns.Theoverdosefromacetaminophenispredictablebecauseitisdose-dependent,whereasidiosyncratic livertoxicityoccursinthelaterphasesofdrugdevelopmentmakingithardto predictduringtheearlystagesofdevelopment.Thischallengewasaddressed byutilizingthezebrafishmodelsysteminhigh-throughputscreenings(HTS) toadvancethefurtherstudyofDILIandshowedmechanisticsimilarityfor DILIinhumans.14 Thesemethodscanbeusefultoidentifynewmediatorsfor predictinghumanDILIwithpotentialtherapeuticcompoundsandearly identificationofhepatotoxiccompoundstohelpreducecostsintheprocessof drugdevelopment.15,16
Inrecentyears,manydrugshavebeenwithdrawnfromthemarketdueto safetyconcernsandthecostsofnewdrugdevelopmenthaverisen.Theuseof
biomarkerscanaddressseveralmajorproblemsassociatedwithdrugdevelopment.Biomarkersenhancedeterminationofwhetherdrugcandidatesare promisingearlyindevelopmentaswellasreducethecostsofdrugdevelopment.Useofbiomarkerscanalsoidentifyapplicabledrugswithenhanced safetyandfindtreatmentsforthosepatientswhoneedthebestbalanceofrisk andbenefit.Moreover,translationalbiomarkersbetweenfishandmammalian modelscanbeestablishedtofurtherimprovetheaccuracyandourunderstandingofpreclinicaltoxicityduringdrugdevelopment.Asdonewiththe DILIstudies,biomarkersshowtobeausefultoolthatmayhavegreatvaluein drugdiscovery.14,16 Biomarkerscanprovideabridgebetweenfish,rodents, andhumans.Thus,itisimportanttoexpandupontheuseofbiomarkersinthe processofdrugdiscoverywhenworkingwithanimalmodels.Thisexpansion canhelpdetectfuturedrugtoxicityissuesinhumans,whichcanmakethe processmoreefficientandlowerthecoststowardamoresuccessfuloutcome inthefuture.Overall,theseapplicationssupporttheuseofthezebrafishin futurestudiesprogressingtowardpromisingdrugsduringtheearlydevelopmentalphaseofdrugdiscovery.Furthermore,thesestudiesprovideconfidence forapplicationofthezebrafishtoaddresstheenvironmentaltoxicityquestions surroundingpharmaceuticalpollution,providingaconnectionbetweenthe overalltoxicityofthesepharmaceuticalswhetherintheearlystagesofdrug discoveryoratthelatterstagesevaluatingthetoxicityofpharmaceutical pollution.
4.Significanceofpharmaceuticalpollution Pharmaceuticalsareahugepartofmodernhealthcare.Infact,statistical analysisoftheglobalpharmaceuticalindustryshowsthat,in2017,the worldwidemarketwasworth$934.8billionUSDandwasprojectedtoreach $1170billionUSDby2021,17 butinfactwasgreaterthan$1250billionUSD by2019.17 Thereareafewreasonsforthissignificantgrowth.Firstand foremost,technologicaladvancementsinindustrializedcountrieshavemade drugdesignanddevelopmenteasierandmuchlesstime-consuming.Aging societiesandadvancesinresearchhavealsoledtoasignificantincreasein globalpharmaceuticalconsumption.Thismayseemencouragingtosomeasit isdifficulttopicturemodernmedicineandanyadvancementswithinmodern medicinewithouttheuseofpharmaceuticals.18 However,drugdesign, development,anddistributionarenotwithoutconsequences.Theoccurrence ofbothhumanandveterinarypharmaceuticalsintheenvironmenthasbeena concernsincetheearly1990s.Sincethen,amyriadofstudieshavedemonstratedthepotentialdangersthatthepresenceofpharmaceuticalsinthe environmentmayhaveonthehealthofwildlifeandhumans.19
Atthispoint,thereisnoquestionaboutthepresenceofpharmaceuticalsin theenvironment.Studieshavedemonstratedtheoccurrenceofsignificant concentrationsofmanypharmaceuticalsinsurfacewater,subsurfacewater,
groundwater,andwastewater.1,20 Itisknownthatmostoftenhumanpharmaceuticalsentertheenvironmentviaeffluentfromwastewatertreatment plants.Ina2007study,samplesofeffluentfromawastewatertreatmentplant inIndiawerecollected.21 DrugssuchasEnrofloxacin,Aspirin,Citalopram, andRanitidinewerejustafewexamplesofthosepresentinthesamples.Not onlywerethesepharmaceuticalspresent,buttheywerepresentin“thehighest levelsofpharmaceuticalsreportedinanyeffluent.”Concentrationsranged from90to31,000partsperbillion(ppb; mg/L).Anotherstudymonitored samplesofinfluentandeffluentcollectedfromwastewatertreatmentplantsin Greece.UsingLC-MSanalysis,researchersidentifiedFurosemide,thebetablockersAtenololandMetoprolol,analgesicsincludingparacetamol(acetaminophen)andNimesulide,salicylicacid,Trimethoprim,Ciprofloxacin, Diclofenac,andcaffeine.22 Otherstudiesofwastewatertreatmentplantsfrom aroundtheworldhaveidentifiedthepresenceofibuprofen,methylparaben, Naproxen,tetrahydrocannabinol(THC),Diazepam,tetracycline,erythromycin,andCefoxitintonameafew.23 Asthedrinkingwatersupplyformost communitiesisobtainedfromsurfacewaterorgroundwater,manyhave questionedwhatsubstancesarepresentintheirownwatersuppliesandwhat effectslowdoseexposureshaveonhealth.
5.Useofthezebrafishmodelsystemtoassess pharmaceuticalpollutanttoxicity
Aimingtoprovideanswerstotoxicityquestionsrelatedtopharmaceutical pollution,toxicologistshavefocusedresearchinthepastdecadeonstudying howbothterrestrialandaquaticorganismsareinfluencedbypharmaceutical pollution.Thisworkhasexpandedtoincludeanimalmodelsofhumanhealth includingrodentandzebrafishstudies.Asdiscussedabove,itisnow commonlyunderstoodandacceptedthatthezebrafishexhibitssimilarphysiologicalresponsestomammalsintoxicitytestingandthatzebrafisharebeing usedinthedrugdiscoverypipeline.Thus,itislogicaltoapplythezebrafishto definetheadversehumanhealtheffectsoflow-doseexposuretopharmaceuticalsandenvironmentalpollutantsduetothehighgenomeandphysiological functionconservation.2,3 Sincehumanandveterinarypharmaceuticalsare designedtoelicitspecificphysiologicalresponsesatlowdoses,thezebrafishis anidealmodelsystemforpharmaceuticalpollutionstudies.Whilethereisan abundanceofstudiesusingzebrafishtoaddressvariousaspectsofdrug toxicity,inthissectionwespecificallyfocusonexamplestudieswhere zebrafishwereusedtoaddressquestionssurroundingpharmaceuticalpollution demonstratingfeasibility(Table1.1).Thesestudieshaveincludedvarious experimentalendpointsinbothdevelopingandadultzebrafish,whichare furtherdetailedinthenextsectionscoveringmethodsandapproaches. Inmultiplestudies,Galusetal.24 26 investigatedorgan-specificand/or developmentaleffectsofexposuretopharmaceuticalandpersonalcare
TABLE1.1 Summaryofexamplestudiesdefiningpharmaceuticalpollutanttoxicityusingthezebrafishmodel.
PharmaceuticalsExposureparametersResultsImplicationsReferences
n Acetaminophen
n Carbamazepine
n Gemfibrozil
n Venlafaxine
n Acetaminophen
n Carbamazepine
n Gemfibrozil
n Venlafaxine
Adultzebrafish(6weeks duration)orembryos (1 72hpf)
0.5or10ppbsingle exposureofeach pharmaceutical
n adultfemaleoocyteatresiaandaltered ovarianhistologywithallcompounds
n alteredkidneytubulemorphologyinboth sexeswithallcompounds
n alteredliverhistologyinbothsexeswith exposuretoacetaminophenorGemfibrozil
n decreasedplasma11-ketotestosteronein bothsexeswithexposuretoCarbamazepine
n embryonicacetaminophenexposure increaseddevelopmentalabnormalities
n increasedmortalityinembryonicexposure withallcompoundsatlowconcentration (0.5ppb)
Lowconcentrationchronic exposuretopharmaceuticals resultsinnegativeimpactsto multipleorgansystems includingreproductive system,kidney,andliver, alongwithembryo mortality.
24
n Carbamazepine
n Gemfibrozil
Adultzebrafish(6weeks duration)orembryos(1 72hpf)
Mixtureoffour pharmaceuticalsat0.5or 10ppbor5%or25% dilutedwastewatereffluent
Adultzebrafish(6weeks duration)thencrossedto produceF1generation, whichwasrearedincontrol waterandthenassessedas adults 10ppbsingleexposureof eachpharmaceutical
n adultfemaleoocyteatresiaandaltered ovarianhistologyinallexposures
n alteredkidneytubulemorphologyinboth sexeswithallexposures
n developmentalabnormalitiesinhigh exposureconcentration
n increasedembryomortalityinhigh concentrationofmixture
n decreasedreproductiveoutputinadult offspringfromparentswithpharmaceutical treatment
n reciprocalcrossesindicatedmoreimpactsto maleswithdrug-specificeffectsonsperm morphology(Carbamazepine:longer midpieces;Gemfibrozil:shorterhead lengthsandmidpieces)
n noeffectsonorganpathologyofliver, kidney,orgonads
Environmentallyrelevant concentrationsofthese pharmaceuticalsinmixture resultsinmultipleorgan systemimpactsincluding ovaryinfemalesandkidney inbothsexes,alongwith mortalityinembryos.
Parentalchroniclow-dose pharmaceuticalexposure wassufficienttocause significantreproductive effectsinmaleoffspring.
25
26
n Carbamazepine
n Dilitiazem
n Fluoxetine
n Gemfibrozil
n Metformin
Developmentalexposure(6 144hpf)
Exposuresincludedsingle pharmaceuticalatsub-acute concentrations(0.001,0.01, 0.1,or1ppb)oramixtureof 5pharmaceuticals,along withnegative(embryo media),solvent(0.001% ethanol),andpositive(17bestradiol)controls
n nosignificanteffectsonmortality,hatching rate,ormorphology
n Carbamazepineresultedinasignificant increaseinallthreegenes(cyp19a2, er-a, and gnrh3),butonlyatconcentrations greaterthanthosefoundinthewater samples(>0.1ppb)
n DiltiazemandFluoxetineincreased expressionof cyp19a2 inalltreatment concentrations,while er-a and gnrh3 expressionalterationswereonlyathigher concentrations(>0.1ppb)
n Gemfibrozilonlyimpacted cyp19a2 expressionat0.01and1ppb
n Metformincausedsignificantexpression changesof cyp19a2 and gnrh3 at1ppb andgreaterandof er-a at10ppbandgreater
n mixturestudiesrepresentingdifferent locationsinLakeMichiganshoweda significantincreaseintheexpressionofall threegenesforfiveofthesevendifferent chemicalmixturestested
Singleandmixture treatmentsrepresenting pharmaceuticalpollution canusegeneexpression biomarkerstoprovide locationandmixturespecifictoxicityprofiling.
n Caffeine
n Ibuprofen
n Carbamazepine
n Tamoxifen Larvalexposurefor96h(72 168hpf)
Exposuresincludedsingle pharmaceuticals(Caffeine, ibuprofen,or Carbamazepineat0.05or
5 mM;Tamoxifenat0.003or
0.3 mM)oraloworhigh mixturetreatmentwith negative(embryomedia), solvent(0.001%ethanol), andpositive(17aethinylestradiolor phenanthrene)controls
n decreasedexpressionof cyp1a inallsingle pharmaceuticaltreatments,butincreased expressioninhighmixturetreatment
n expressionof vtg decreasedinTamoxifen treatments
n ERODactivitydecreasedinloweribuprofen treatmentandincreasedinthehighmixture treatment
Noadditivetoxicitywas observed,butdifferences seeninbetweenthe individualandmixture treatmentssignifyingneedto includemixturetoxicity assessments. 28
TABLE1.1 Summaryofexamplestudiesdefiningpharmaceuticalpollutanttoxicityusingthezebrafishmodel. cont’d
PharmaceuticalsExposureparametersResultsImplicationsReferences
n Bezafibrate
n Caffeine
n Carbamazepine
n Clarithromycin
n Diclofenac
n Ibuprofen
n Sulfamethoxazole
n Triclosan
n paracetamol (acetaminophen)
n Ciprofloxacin
Embryonicexposure(2 50 hpf)
Singlepharmaceutical exposureat0.01,0.1,1,10, or100ppb
Low(1X),medium(10X),or high(100X)mixtureofall8 pharmaceuticals
Developmentalexposure (<3 96hpf)
Paracetamolat5,25,125, 625,or3125ppb
Ciprofloxacinat0.005, 0.013,0.031,0.078,0.195, or0.488ppb
Treatmentwaterrefreshedat 48hpf
n novisibleacutetoxicityforanysingleor mixturetreatments
n delayedhatchat72hpfforTriclosan(1ppb) andibuprofen(100ppb)
n decreasedswimmingspeedat100ppbof Triclosan,Clarithromycin,andthehigh mixturetreatmentat118hpf
n increasedswimmingspeedat100ppbof Carbamazepineat118hpf
n nondoseresponsemorphologicalalterations at48,72,and96hpfforparacetamol
n hyper-and/orhypoactivityobservedat144 hpfforparacetamolandCiprofloxacin dependentonphaseandconcentration
n paracetamolcausedincreaseinAChE,total GPx,andGSTactivity;alterationsinGSH metabolism;andincreasedlipid peroxidation
n CiprofloxacinresultedinincreasedAChE activityandadecreaseincatalaseactivity andlipidperoxidation
n increasedDNAmethylationfollowing paracetamolexposure
Delayedbehavioral alterationswereobserved forseveralpharmaceuticals andthehighmixture treatment. 29
Environmentallyrelevant concentrationsofthese pharmaceuticalsresultedin behavioralandbiomarker alterations,while morphologicalandDNA methylationchangeswere observedonlyfor paracetamol.
30
AChE:acetylcholinesterase; cyp1a:cytochromeP4501a; cyp19a2:cytochromeP45019a2; er-a:estrogenreceptor-alpha;EROD:ethoxyresorufin-O-deethylase; gnrh3: gonadotropinreleasinghormone;GPx:glutathioneperoxidase;GST:glutathionetransferase;hpf:hourspostfertilization;ppb:partsperbillion(mg/L); vtg:vitellogenin.
productsinsingularormixtureexposuresofacetaminophen,Carbamazepine, Gemfibrozil,orVenlafaxineinadultandembryoniczebrafishtomimic environmentallyrelevantconcentrations(Table1.1).First,singularexposures ofthefourpharmaceuticalswerecompletedinadultzebrafishandresultedin oocyteatresiaandalteredovarianhistologyinfemalesandalteredkidney histologyinbothsexes.24 LiverhistologywasalteredbyexposuretoacetaminophenorGemfibrozil,whileexposuretoCarbamazepinedecreased plasma11-ketotestosteroneinbothsexes.Noalterationswereobservedin developmentalabnormalitiesinoffspringfromparentalexposure,butembryonicexposuretoacetaminophencauseddevelopmentalabnormalitiesand increasedmortalitywasobservedforallfourpharmaceuticalsat0.5ppb.Ina secondstudybythesamegroup,25 amixtureofthefourpharmaceuticalsor dilutedeffluentfromwastewaterwherethesefourpharmaceuticalswerethe mostprevalentwasinvestigatedagaininzebrafishadultsandembryos (Table1.1).Exposuretothesesolutionsresultedinmanyofthesameperturbationsobservedbythesinglepharmaceuticalexposuresintheprevious study24 includingimpactstoovarianhistologyinfemalesandalteredkidney proximaltubulemorphologyinbothsexes.Directexposureinembryoscaused developmentalabnormalities,specificallyspinalmalformationsandpericardialedemawithincreasedembryomortalityinthehighestconcentrationofthe pharmaceuticalmixture.25 Furthermore,inathirdstudy,chronicparental exposuretoCarbamazepineorGemfibrozilat10ppbresultedinareductionin thenumberofclutchesinadultoffspring(F1)withadrug-specificeffecton spermmorphologyofadultmaleoffspring26 (Table1.1).Thisstudyshowed thataparentalexposurewassufficienttoresultinreproductiveeffectsintheir offspring.Anadditionalstudywiththesameexposureparameters(i.e., parentalexposurefor6weeksto10ppbCarbamazepineorGemfibrozil) furthersupportedthemultigenerationalfindingindicatingpaternalinfluence onsexualdifferentiationandmalereproductiveeffectsintheF1offspring.31 ThemalereproductiveeffectswerereportedtobelimitedtotheF1generation withnotransgenerationaleffectsobservedinsubsequentF2,F3,orF4generationswiththe10ppbGemfibrozilexposure(i.e.,6weeksintheF0generationonly).32
Developmentaltoxicityassessmentsincludingmorphometricsarebeing coupledtomolecularendpointstolinkgeneexpressionalterationsand phenotypicperturbations.Forexample,estrogen-relatedtranscriptanalysis wasappliedtoassessthedevelopmentaltoxicityoffivepharmaceuticals (Carbamazepine,Diltiazem,Fluoxetine,Gemfibrozil,andMetformin)reported tobethemostcommonandhighlyconcentratedinwatersamplescollected fromvariouslocationsinLakeMichigan.27,33 Assessmentswerecompleted forsingularpharmaceuticalexposuresandinmixtures.Nosignificantmortalityormorphologicalalterationswereobservedforanypharmaceuticalor pharmaceuticalmixtures,butsignificanttranscriptexpressionalterationsof estrogenreceptor-alpha(er-a),brainaromatase(cyp19a2),andgonadotropin Usingthezebrafishmodelsystemtoidentify Chapter|1 11