https://ebookmass.com/product/comprehensive-organometallic-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Comprehensive Pharmacology 7 Volume Set Terry P. Kenakin (Editor)
https://ebookmass.com/product/comprehensive-pharmacology-7-volume-setterry-p-kenakin-editor/ ebookmass.com
Comprehensive Renewable Energy (Nine Volume Set) 2nd Edition Trevor M. Letcher
https://ebookmass.com/product/comprehensive-renewable-energy-ninevolume-set-2nd-edition-trevor-m-letcher/
ebookmass.com
Organometallic Chemistry of Five-Membered Heterocycles 1st Edition Dr. Alexander Sadimenko
https://ebookmass.com/product/organometallic-chemistry-of-fivemembered-heterocycles-1st-edition-dr-alexander-sadimenko/ ebookmass.com
American and British Soft Power in Iran, 1953-1960: A 'Special Relationship'? Darius Wainwright
https://ebookmass.com/product/american-and-british-soft-power-iniran-1953-1960-a-special-relationship-darius-wainwright/ ebookmass.com
A Republican Theory Of Free Speech: Critical Civility 1st
Edition Suzanne Whitten
https://ebookmass.com/product/a-republican-theory-of-free-speechcritical-civility-1st-edition-suzanne-whitten/
ebookmass.com
Managing Fuzzy Projects in 3d: A Proven, Multi-Faceted Blueprint for Overseeing Complex Projects Lavagnon Ika
https://ebookmass.com/product/managing-fuzzy-projects-in-3d-a-provenmulti-faceted-blueprint-for-overseeing-complex-projects-lavagnon-ika/
ebookmass.com
The Psychology of Humor: An Integrative Approach Rod A. Martin & Thomas E. Ford
https://ebookmass.com/product/the-psychology-of-humor-an-integrativeapproach-rod-a-martin-thomas-e-ford/
ebookmass.com
Understanding Unix-Linux Programming Bruce Molay
https://ebookmass.com/product/understanding-unix-linux-programmingbruce-molay/
ebookmass.com
The Ninth Life of a Diamond Miner: Grace Tame Memoir Grace Tame
https://ebookmass.com/product/the-ninth-life-of-a-diamond-miner-gracetame-memoir-grace-tame/
ebookmass.com
Tailgreat John Currence [Currence https://ebookmass.com/product/tailgreat-john-currence-currence/
ebookmass.com
COMPREHENSIVE ORGANOMETALLIC CHEMISTRYIV COMPREHENSIVE ORGANOMETALLIC CHEMISTRYIV EDITORS-IN-CHIEF
GERARDPARKIN
DepartmentofChemistry,ColumbiaUniversity,NewYork,NY,UnitedStates
KARSTENMEYER
DepartmentofChemistryandPharmacy,Friedrich-Alexander-Universität,Erlangen,Germany
DERMOTO’HARE
DepartmentofChemistry,UniversityofOxford,Oxford,UnitedKingdom
VOLUME1 FUNDAMENTALS VOLUMEEDITOR
PATRICKL.HOLLAND
YaleUniversity,NewHaven,UnitedStates
Elsevier Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright © 2022ElsevierLtd.Allrightsreserved
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,including photocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhow toseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuchasthe CopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybenoted herein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding,changesin researchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmayalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation,methods, compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthe safetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjuryand/or damagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN978-0-12-820206-7
Forinformationonallpublicationsvisitour websiteat http://store.elsevier.com
Publisher: OliverWalter
AcquisitionEditor: BlerinaOsmanaj
ContentProjectManager: ClaireByrne
AssociateContentProjectManager: FahmidaSultana Designer: ChristianBilbow
CONTENTSOFVOLUME1 EditorBiographies
ContributorstoVolume1
1.01Introduction:VolumeI 1 PatrickLHolland
1.02ModelsforUnderstandingMainGroupandTransitionMetalBonding 2 AaronLOdom
1.03ReversibleHomolysisofMetal-CarbonBonds 31 MaximeMichelas,ChristopheFliedel,andRinaldoPoli
1.04VeryLowOxidationStatesinOrganometallicChemistry 86 CGunnarWerncke
1.05VeryHighOxidationStatesinOrganometallicChemistry
MoritzMalischewski
1.06CharacterizationMethodsforParamagneticOrganometallicComplexes 135 AleksaRadovic,ShilpaBhatia,andMichaelLNeidig
1.07ComputationalMethodsinOrganometallicChemistry 176 SChantalEStieber
1.08f-ElementOrganometallicSingle-MoleculeMagnets 211 RichardALayfield,ChristopherGTPrice,andSiobhanRTemple
1.09ElectrochemistryinOrganometallicChemistry
JulieAHopkinsLeseberg,WadeCHenke,andJamesDBlakemore
1.10OrganometallicPhotosensitizers
ThomasSTeetsandYanyuWu
1.11OrganometallicChemistryofNHCsandAnalogues
LiangDengandZhenboMo
1.12LigandsFeaturingCovalentlyTetheredModeratetoWeaklyCoordinatingAnions 373 AntonWTomich,VarunTej,SergioLovera,IsaacBanda,StevenFisher,MatthewAsay,andVincentLavallo
1.13Redox-ActiveLigandsinOrganometallicChemistry 421 ErrikosKounalisandDaniëlLJBroere
1.14ProtonResponsiveandHydrogenBondingLigandsinOrganometallicChemistry
ElizabethTPapish,SanjitDas,WeerachaiSilprakob,ChanceMBoudreaux,andSonyaManafe
1.15IntroductiontotheOrganometallicChemistryofCarbonDioxide
CharlesWMachan
1.16Alkane s-Complexes
RowanDYoung
1.17DinitrogenBindingandFunctionalization
JeremyEWeber,SamuelMBhutto,AlexandreT-YGenoux,andPatrickLHolland
1.18LewisAcidParticipationinOrganometallicChemistry
521
555 JuliaBCurley,NilayHazari,andTanyaMTownsend
1.19OrganometallicChemistryonOxideSurfaces
583 MatthewPConley,JiaxinGao,WinnHuynh,JessicaRodriguez,andKavyasripriyaKSamudrala
1.20SeparationStrategiesinOrganometallicCatalysis
609 FernandaGMendonçaandRTomBaker
1.21ImpuritiesinOrganometallicCatalysis
635 NicholasELeadbeater
EDITORBIOGRAPHIES EditorsinChief KarstenMeyer studiedchemistryattheRuhrUniversityBochumandperformed hisPh.D.thesisworkonthemolecularandelectronicstructureoffirst-row transitionmetalcomplexesunderthedirectionofProfessorKarlWieghardtat theMaxPlanckInstituteinMülheim/Ruhr(Germany).Hethenproceededto gainresearchexperienceinthelaboratoryofProfessorChristopherCumminsat theMassachusettsInstituteofTechnology(USA),whereheappreciatedtheartof synthesisanddevelopedhispassionforthecoordinationchemistryandreactivity ofuraniumcomplexes.In2001,hewasappointedtotheUniversityofCalifornia, SanDiego,asanassistantprofessorandwasnamedanAlfredP.SloanFellowin 2004.In2006,heacceptedanoffer(C4/W3)tobethechairoftheInstituteof Inorganic&GeneralChemistryattheFriedrich-Alexander-UniversityErlangenNürnberg(FAU),Germany.Amonghisawardsandhonors,hewaselecteda lifetimehonorarymemberoftheIsraelChemicalSocietyandafellowofthe RoyalSocietyofChemistry(UK).KarstenreceivedtheElhuyar-GoldschmidtAwardfromtheRoyalSocietyof ChemistryofSpain,theLudwigMondAwardfromtheRSC(UK),andtheChugaevCommemorativeMedal fromtheRussianAcademyofSciences.Hehasalsoenjoyedvisitingprofessorshippositionsattheuniversitiesof Manchester(UK)andToulouse(F)aswellastheNagoyaInstituteofTechnology(JP)andETHZürich(CH). TheMeyerlabresearchfocusesonthesynthesisofcustom-tailoredligandenvironmentsandtheirtransition andactinidemetalcoordinationcomplexes.Thesecomplexesoftenexhibitunprecedentedcoordinationmodes, unusualelectronicstructures,and,consequently,enhancedreactivitiestowardsmallmoleculesofbiological andindustrialimportance.Interestingly,Karsten’sfavoritemoleculeisonethatexhibitslittlereactivity:the Th symmetricU(dbabh)6
DermotO’Hare wasborninNewry,CoDown.HestudiedatBalliolCollege, OxfordUniversity,whereheobtainedhisB.A.,M.A.,andD.Phil.degreesunder thedirectionofProfessorM.L.H.Green.In1985,hewasawardedaRoyal Commissionof1851ResearchFellowship,duringthisFellowshiphewasa visitingresearchfellowattheDuPontCentralResearchDepartment,Wilmington,Delawarein1986–87inthegroupledbyProf.J.S.Millerworkingon molecular-basedmagneticmaterials.In1987hereturnedtoOxfordtoa short-termuniversitylectureshipandin1990hewasappointedtoapermanent universitypositionandaSeptcentenaryTutorialFellowshipatBalliolCollege. HehaspreviouslybeenhonoredbytheInstitütdeFrance,AcadémiedesSciences asaleadingscientistinEuropeunder40years.Heiscurrentlyprofessorof organometallicandmaterialschemistryintheDepartmentofChemistryatthe UniversityofOxford.Inaddition,heiscurrentlythedirectoroftheSCG-Oxford CentreofExcellenceforchemistryandassociateheadforbusiness&innovationintheMathematics,Physical andLifeSciencesDivision.Heleadsamultidisciplinaryresearchteamthatworksacrossbroadareasofcatalysis andnanomaterials.Hisresearchisspecificallytargetedatfindingsolutionstoglobalissuesrelatingtoenergy, zerocarbon,andthecirculareconomy.Hehasbeenawardednumerousawardsandprizesforhiscreativeand
ground-breakingworkininorganicchemistry,includingtheRoyalSocietyChemistry’sSirEdwardFrankland Fellowship,LudwigMondPrize,TildenMedal,andAcademia–IndustryPrizeandtheExxonEuropeanChemicalandEngineeringPrize.
GerardParkin receivedhisB.A.,M.A.,andD.Phil.degreesfromtheQueen’ s College,OxfordUniversity,wherehecarriedoutresearchundertheguidanceof ProfessorMalcolmL.H.Green.In1985,hemovedtotheCaliforniaInstituteof TechnologyasaNATOpostdoctoralfellowtoworkwithProfessorJohn E.Bercaw.HejoinedtheFacultyofColumbiaUniversityasassistantprofessor in1988andwaspromotedtoassociateprofessorin1991andtoprofessorin 1994.HeservedaschairmanoftheDepartmentfrom1999to2002.Hehasalso servedaschairoftheNewYorkSectionoftheAmericanChemicalSociety,chair oftheInorganicChemistryandCatalyticScienceSectionoftheNewYorkAcademyofSciences,chairoftheOrganometallicSubdivisionoftheAmericanChemicalSocietyDivisionofInorganicChemistry,andchairoftheGordonResearch ConferenceinOrganometallicChemistry.
HeisanelectedfellowoftheAmericanChemicalSociety,theRoyalSocietyof Chemistry,andtheAmericanAssociationfortheAdvancementofScience,andistherecipientofavarietyof internationalawards,includingtheACSAwardinpurechemistry,theACSAwardinorganometallicchemistry, theRSCCordayMorganMedal,theRSCAwardinorganometallicchemistry,theRSCLudwigMondAward,and theRSCChemSocRevLectureAward.HeisalsotherecipientoftheUnitedStatesPresidentialAwardfor ExcellenceinScience,MathematicsandEngineeringMentoring,theUnitedStatesPresidentialFacultyFellowshipAward,theJamesFlackNorrisAwardforOutstandingAchievementintheTeachingofChemistry,the ColumbiaUniversityPresidentialAwardforOutstandingTeaching,andtheLenfestDistinguishedColumbia FacultyAward.
Hisprincipalresearchinterestsareintheareasofsynthetic,structural,andmechanisticinorganicchemistry.
VolumeEditors SimonAldridge isprofessorofchemistryattheUniversityofOxfordand directoroftheUKRICentreforDoctoralTrainingininorganicchemistryfor FutureManufacturing.OriginallyfromShrewsbury,England,hereceivedboth hisB.A.andD.Phil.degreesfromtheUniversityofOxford,thelatterin1996for workonhydridechemistryunderthesupervisionofTonyDowns.After post-doctoralworkasaFulbrightScholaratNotreDamewithTomFehlner, andatImperialCollegeLondon(withMikeMingos),hetookuphisfirstacademicpositionatCardiffUniversityin1998.HereturnedtoOxfordin2007, beingpromotedtofullprofessorin2010.Prof.Aldridgehaspublishedmorethan 230paperstodateandisapastwinneroftheDaltonTransactionsEuropean Lectureship(2009),theRoyalSocietyofChemistry’sMainGroupChemistry (2010)andFranklandAwards(2018),andtheForschungspreisoftheAlexander vonHumboldtFoundation(2021).Prof.Aldridge ’sresearchinterestsareprimarilyfocusedonmaingrouporganometallicchemistry,andinparticularthedevelopmentofcompoundswith unusualelectronicstructure,andtheirapplicationsinsmallmoleculeactivationandcatalysis(website: http:// aldridge.web.ox.ac.uk).
(Picturecredit:JohnCairns)
EszterBoros isassociateprofessorofchemistryatStonyBrookUniversitywith courtesyappointmentsinradiologyandpharmacologyatStonyBrookMedicine. EszterobtainedherM.Sc.(2007)attheUniversityofZurich,Switzerlandandher Ph.D.(2011)inchemistryfromtheUniversityofBritishColumbia,Canada.She wasapostdoc(2011–15)andlaterinstructor(2015–17)inradiologyatMassachusettsGeneralHospitalandHarvardMedicalSchool.In2017,Eszterwas appointedasassistantprofessorofchemistryatStonyBrookUniversity,where herresearchgroupdevelopsnewapproachestometal-baseddiagnosticsand therapeuticsattheinterfacesofradiochemistry,inorganicchemistryandmedicine.Herlab’sworkhasbeenextensivelyrecognized;Eszterholdsvariousmajor federalgrants(NSFCAREERAward,NIHNIBIBR21Trailblazer,NIHNIGMS R35MIRA)andhasbeennameda2020MooreInventorFellow,the2020 JonathanL.SesslerFellow(AmericanChemicalSociety,InorganicDivision), recipientofa2021ACSInfectiousDiseases/ACSDivisionofBiologicalChemistryYoungInvestigatorAward (AmericanChemicalSociety),andwasalsonameda2022AlfredP.SloanResearchFellowinchemistry.
ScottR.Daly isassociateprofessorofchemistryattheUniversityofIowain theUnitedStates.Afterspending3yearsintheU.S.Army,heobtainedhisB.S. degreeinchemistryin2006fromNorthCentralCollege,asmallliberalarts collegeinNaperville,Illinois.HethenwentontoreceivehisPh.D.atthe UniversityofIllinoisatUrbana-Champaignin2010undertheguidanceof ProfessorGregoryS.Girolami.Histhesisresearchfocusedonthesynthesisand characterizationofchelatingborohydrideligandsandtheiruseinthepreparation ofvolatilemetalcomplexesforchemicalvapordepositionapplications.In2010, hebeganworkingasaSeaborgpostdoctoralfellowwithDrs.StoshA.Kozimor andDavidL.ClarkatLosAlamosNationalLaboratoryinLosAlamos,New Mexico.HisresearchthereconcentratedonthedevelopmentofligandK-edge X-rayabsorptionspectroscopy(XAS)toinvestigatecovalentmetal–ligandbondingandelectronicstructurevariationsinactinide,lanthanide,andtransition metalcomplexeswithmetalextractants.Hestartedhisindependentcareerin2012atGeorgeWashington UniversityinWashington,DC,andmovedtotheUniversityofIowashortlythereafterin2014.
Hiscurrentresearchinterestsfocusonsyntheticcoordinationchemistryandliganddesignwithemphasison thedevelopmentofchemicalandredoxnoninnocentligands,mechanochemicalsynthesisandseparation methods,andligandK-edgeXAS.HisresearchandoutreacheffortshavebeenrecognizedwithanOutstanding Faculty/StaffAdvocateAwardfromtheUniversityofIowaVeteransAssociation(2016),aNationalScience FoundationCAREERAward(2017),andaHawkeyeDistinguishedVeteransAward(2018).Hewaspromotedto associateprofessorwithdistinctionasaCollegeofLiberalArtsandSciencesDeansScholarin2020.
LenaJ.Daumann iscurrentlyprofessorofbioinorganicandcoordination chemistryattheLudwigMaximilianUniversitätinMunich.Shestudiedchemistry attheUniversityofHeidelbergworkingwithProf.PeterCombaandsubsequently conductedherPh.D.attheUniversityofQueensland(Australia)from2010to 2013holdingIPRSandUQCentennialfellowships.In2013shewaspartofthe AustralianDelegationforthe63rdLindauNobelLaureatemeetinginchemistry. FollowingpostdoctoralstaysatUCBerkeleywithProf.KenRaymond(2013–15) andinHeidelberg,fundedbytheAlexandervonHumboldtFoundation,she startedherindependentcareerattheLMUMunichin2016.Herbioinorganic researchgroupworksonelucidatingtheroleoflanthanidesforbacteriaaswellas onironenzymesandsmallbiomimeticcomplexesthatplayaroleinepigenetics andDNArepair.Daumann’steachingandresearchhavebeenrecognizedwith numerousawardsandgrants.Amongthemarethenational ArsLegendiPrize for chemistryandthe TheresevonBayernPrize in2019andthe Dozentenpreis ofthe “FondsderChemischen Industrie“ in2021.In2018shewasselectedasfellowforthe KlausTschiraBoostFund bythe GermanScholars Organisation andin2020shereceivedaStartinggrantoftheEuropeanResearchCounciltostudytheuptakeof lanthanidesbybacteria.
DerekP.Gates hailsfromHalifax,NovaScotia(Canada)wherehecompleted hisB.Sc.(HonoursChemistry)degreeatDalhousieUniversityin1993. HecompletedhisPh.D.degreeunderthesupervisionofProfessorIanManners attheUniversityofTorontoin1997.HethenjoinedthegroupofProfessor MauriceBrookhartasanNSERCpostdoctoralfellowattheUniversityofNorth CarolinaatChapelHill(USA).Hebeganhisindependentresearchcareerin1999 asanassistantprofessorattheUniversityofBritishColumbiainVancouver (Canada).Hehasbeenpromotedthroughtheranksandhasheldtheposition ofprofessorofchemistrysince2011.AtUBC,hehasreceivedtheScienceUndergraduateSociety TeachingExcellenceAward,theCanadianNationalCommitteeforIUPACAward,andtheChemicalSocietyofCanada StremChemicals Awardforpureorappliedinorganicchemistry.Hisresearchinterestsbridgethe traditionalfieldsofinorganicandpolymerchemistrywithparticularfocuson phosphoruschemistry.Keytopicsincludethediscoveryofnovelstructures,unusualbonding,newreactivity, alongwithapplicationsincatalysisandmaterialsscience.
PatrickHolland performedhisPh.D.researchinorganometallicchemistryatUC BerkeleywithRichardAndersenandRobertBergman.Hethenlearnedabout bioinorganicchemistrythroughpostdoctoralresearchoncopper-O2 and copper-thiolatechemistrywithWilliamTolmanattheUniversityofMinnesota. HisindependentresearchattheUniversityofRochesterinitiallyfocusedon systematicdevelopmentofthepropertiesandreactionsofthree-coordinate complexesofironandcobalt,whichcanengageinarangeofbondactivation reactionsandorganometallictransformations.Sincethen,hisresearchgrouphas broadeneditsstudiestoiron-N2 chemistry,reactivemetal–ligandmultiple bonds,iron–sulfurclusters,engineeredmetalloproteins,redox-activeligands, andsolarfuelproduction.In2013,Prof.HollandmovedtoYaleUniversity, whereheisnowConkeyP.WhiteheadProfessorofChemistry.Hisresearchhas beenrecognizedwithanNSFCAREERAward,aSloanResearchAward,Fulbright andHumboldtFellowships,aBlavatnikAwardforYoungScientists,andwaselectedasfellowoftheAmerican AssociationfortheAdvancementofScience.IntheareaofN2 reduction,hisgrouphasestablishedmolecular principlestoweakenandbreakthestrongN–Nbond,inordertousethisabundantresourceforenergyand synthesis.Hisgrouphasmadeaparticularefforttogainaninsightintoironchemistryrelevanttonitrogenase, theenzymethatreducesN2 innature.Hisgroupalsomaintainsanactiveprogramintheuseofinexpensive metalsfortransformationsofalkenes.MechanisticdetailsareacentralmotivationtoProf.Hollandandthe wonderfulgroupofover80studentswithwhomhehasworked.
SteveLiddle wasborninSunderlandintheNorthEastofEnglandandgained hisB.Sc.(Hons)andPh.D.fromNewcastleUniversity.AfterpostdoctoralfellowshipsatEdinburgh,Newcastle,andNottinghamUniversitieshebeganhisindependentcareeratNottinghamUniversityin2007withaRoyalSocietyUniversity ResearchFellowship.ThiswasheldinconjunctionwithaprolepticLectureship andhewaspromotedthroughtherankstoassociateprofessorandreaderin2010 andprofessorofinorganicchemistryin2013.HeremainedatNottinghamuntil 2015whenhewasappointedprofessorandheadofinorganicchemistryand co-directoroftheCentreforRadiochemistryResearchatTheUniversityofManchester.HehasbeenarecipientofanEPSRCEstablishedCareerFellowshipand ERCStarterandConsolidatorgrants.HeisanelectedfellowofTheRoyalSociety ofEdinburghandfellowoftheRoyalSocietyofChemistryandheisvice presidenttotheExecutiveCommitteeoftheEuropeanRareEarthandActinide Society.Hisprincipalresearchinterestsarefocusedonf-elementchemistry,involvingexploratorysynthetic chemistrycoupledtodetailedelectronicstructureandreactivitystudiestoelucidatestructure-bonding-property relationships.Heistherecipientofavarietyofprizes,includingtheIChemEPetronasTeamAwardfor ExcellenceinEducationandTraining,theRSCSirEdwardFranklandFellowship,theRSCRadiochemistry
GroupBillNewtonAward,a41stICCCRisingStarAward,theRSCCorday-MorganPrize,anAlexandervon HumboldtFoundationFriedrichWilhelmBesselResearchAward,theRSCTildenPrize,andanRSCDalton DivisionHorizonTeamPrize.Hehaspublishedover220researcharticles,reviews,andbookchapterstodate.
DavidLiptrot receivedhisMChem(Hons)inchemistrywithIndustrialTrainingfromtheUniversityofBathin2011andremainedtheretoundertakeaPh.D. ongroup2catalysisinthelaboratoryofProfessorMikeHill.Aftercompletingthis in2015hetookupaLindemannPostdoctoralFellowshipwithProfessorPhilip PowerFRS(UniversityofCalifornia,Davis,USA).In2017hebeganhisindependentcareerreturningtotheUniversityofBathandin2019wasawardedaRoyal SocietyUniversityResearchFellowship.Hisinterestsconcernnewsynthetic methodologiestointroducemaingroupelementsintofunctionalmolecules andmaterials.
DavidP.Mills hailsfromLlanbradachandCaerphillyintheSouthWales Valleys.HecompletedhisMChem(2004)andPh.D.(2008)degreesatCardiff University,withhisdoctorateinlowoxidationstategalliumchemistrysupervisedbyProfessorCameronJones.HemovedtotheUniversityofNottingham in2008toworkwithProfessorStephenLiddleforpostdoctoralstudiesin lanthanideandactinidemethanediidechemistry.In2012hemovedtothe UniversityofManchestertostarthisindependentcareerasalecturer,wherehe hassincebeenpromotedtofullprofessorofinorganicchemistryin2021. Althoughheisinterestedinallaspectsofnonaqueoussyntheticchemistryhis researchinterestsarecurrentlyfocusedonthesynthesisandcharacterizationof f-blockcomplexeswithunusualgeometriesandbondingregimes,withtheaim ofenhancingphysicochemicalproperties.Hehasbeenrecognizedforhiscontributionstobothresearchandteachingwithprizesandawards,includinga Harrison-MeldolaMemorialPrize(2018),theRadiochemistryGroupBillNewtonAward(2019),andaTeam MemberoftheMolecularMagnetismGroupfortheDaltonDivisionHorizonPrize(2021)fromtheRoyal SocietyofChemistry.HewasaBlavatnikAwardsforYoungScientistsintheUnitedKingdomFinalistin Chemistryin2021andhecurrentlyholdsaEuropeanResearchCouncilConsolidatorGrant.
IanTonks istheLloydH.ReyersonprofessorattheUniversityofMinnesotaTwinCities,andassociateeditorfortheACSjournal Organometallics HereceivedhisB.A.inchemistryfromColumbiaUniversityin2006andperformedundergraduateresearchwithProf.GedParkin.HeearnedhisPh.D.in 2012fromtheCaliforniaInstituteofTechnology,whereheworkedwithProf. JohnBercawonolefinpolymerizationcatalysisandearlytransitionmetal-ligand multiplybondedcomplexes.AfterpostdoctoralresearchwithProf.ClarkLandis attheUniversityofWisconsin,Madison,hebeganhisindependentcareeratthe UniversityofMinnesotain2013andearnedtenurein2019.Hiscurrentresearch interestsarefocusedonthedevelopmentofearthabundant,sustainablecatalytic methodsusingearlytransitionmetals,andalsooncatalyticstrategiesforincorporationofCO2 intopolymers.Prof.Tonks’ workhasrecentlybeenrecognized withanOutstandingNewInvestigatorAwardfromtheNationalInstitutesof Health,anAlfredP.SloanFellowship,aDepartmentofEnergyCAREERaward,andtheACS Organometallics DistinguishedAuthorAward,amongothers.Additionally,Prof.Tonks’ servicetowardimprovingacademic safetyculturewasrecentlyrecognizedwiththe2021ACSDivisionofChemicalHealthandSafetyGraduate FacultySafetyAward.
TimothyH.Warren istheRosenbergprofessorandchairpersonintheDepartmentofChemistryatMichiganStateUniversity.HeobtainedhisB.S.fromthe UniversityofIllinoisatUrbana-Champaignin1992andPh.D.fromtheMassachusettsInstituteofTechnologyin1997.After2yearsofpostdoctoralresearchat theOrganicChemistryInstituteoftheUniversityofMünster,GermanywithProf. Dr.GerhartErker,Dr.WarrenstartedhisindependentcareeratGeorgetown Universityin1999wherehewasnamedtheRichardD.Vorisekprofessorof chemistryin2014.HemovedtoMichiganStateUniversityin2021.
Prof.Warren’sresearchinterestsspansyntheticandmechanisticinorganic, organometallic,andbioinorganicchemistrywithafocusoncatalysis.His researchgroupdevelopsenvironmentallyfriendlymethodsfororganicsynthesis viaC–Hfunctionalization,explorestheinterconversionofnitrogenandammoniaascarbon-freefuels,anddecodeswaysthatbiologycommunicatesusingnitric oxideasamolecularmessenger.Mechanisticstudiesonthesechemicalreactionscatalyzedbymetalionssuchas iron,nickel,copper,andzincenablenewinsightsforthedevelopmentofusefulcatalystsforsynthesisand energyapplicationsaswellaslaythemechanisticgroundworktounderstandbiochemicalnitricoxidemisregulation.Dr.WarrenreceivedtheNSFCAREERAward,chairedthe2019InorganicReactionMechanisms GordonResearchConference,andhasservedontheACSDivisionofInorganicChemistryexecutiveboardand ontheeditorialboardsof InorganicSynthesis, InorganicChemistry,and ChemicalSocietyReviews.
CONTRIBUTORSTOVOLUME1 MatthewAsay
UniversalDisplayCorporation,Ewing,NJ,UnitedStates
RTomBaker
DepartmentofChemistryandBiomolecularSciencesand CentreforCatalysisResearchandInnovation,University ofOttawa,Ottawa,ON,Canada
IsaacBanda UniversityofCaliforniaRiverside,Riverside,CA,United States
ShilpaBhatia
DepartmentofChemistry,UniversityofRochester, Rochester,NY,UnitedStates
SamuelMBhutto
DepartmentofChemistry,YaleUniversity,NewHaven, CT,UnitedStates
JamesDBlakemore
DepartmentofChemistry,UniversityofKansas,Lawrence, KS,UnitedStates
ChanceMBoudreaux DepartmentofChemistry,TheUniversityofAlabama, Tuscaloosa,AL,UnitedStates
DaniëlLJBroere OrganicChemistryandCatalysis,DebyeInstitute forNanomaterialsScienceFacultyofScience, UtrechtUniversity,Universiteitsweg99,Utrecht, TheNetherlands
MatthewPConley
DepartmentofChemistry,UniversityofCalifornia, Riverside,CA,UnitedStates
JuliaBCurley DepartmentofChemistry,YaleUniversity,NewHaven, CT,UnitedStates
SanjitDas DepartmentofChemistry,TheUniversityofAlabama, Tuscaloosa,AL,UnitedStates
LiangDeng
StateKeyLaboratoryofOrganometallicChemistry, ShanghaiInstituteofOrganicChemistry,Chinese AcademyofSciences,Shanghai,PRChina
StevenFisher
LawrenceLivermoreNationalLaboratory,Livermore,CA, UnitedStates
ChristopheFliedel LaboratoiredeChimiedeCoordination,UPRCNRS8241, Toulouse,France
JiaxinGao DepartmentofChemistry,UniversityofCalifornia, Riverside,CA,UnitedStates
AlexandreT-YGenoux DepartmentofChemistry,YaleUniversity,NewHaven, CT,UnitedStates
NilayHazari DepartmentofChemistry,YaleUniversity,NewHaven, CT,UnitedStates
WadeCHenke DepartmentofChemistry,UniversityofKansas,Lawrence, KS,UnitedStates
PatrickLHolland DepartmentofChemistry,YaleUniversity,NewHaven, CT,UnitedStates
JulieAHopkinsLeseberg DepartmentofChemistry,UniversityofKansas,Lawrence, KS,UnitedStates
WinnHuynh DepartmentofChemistry,UniversityofCalifornia, Riverside,CA,UnitedStates
ErrikosKounalis
OrganicChemistryandCatalysis,DebyeInstitutefor NanomaterialsScienceFacultyofScience,Utrecht University,Universiteitsweg99,Utrecht,TheNetherlands
VincentLavallo
UniversityofCaliforniaRiverside,Riverside,CA,United States
RichardALayfield
DepartmentofChemistry,SchoolofLifeSciences, UniversityofSussex,Brighton,UnitedKingdom
NicholasELeadbeater
DepartmentofChemistry,UniversityofConnecticut, Storrs,CT,UnitedStates
SergioLovera UniversityofCaliforniaRiverside,Riverside,CA, UnitedStates
CharlesWMachan DepartmentofChemistry,UniversityofVirginia, Charlottesville,VA,UnitedStates
MoritzMalischewski
FreieUniversitätBerlin,InstitutfürChemie undBiochemie AnorganischeChemie,Berlin, Germany
SonyaManafe DepartmentofChemistry,TheUniversityofAlabama, Tuscaloosa,AL,UnitedStates
FernandaGMendonça DepartmentofChemistryandBiomolecularSciences andCentreforCatalysisResearchandInnovation, UniversityofOttawa,Ottawa,ON,Canada
MaximeMichelas
LaboratoiredeChimiedeCoordination,UPRCNRS8241, Toulouse,France
ZhenboMo
StateKeyLaboratoryandInstituteofElemento-Organic Chemistry,CollegeofChemistry,NankaiUniversity, Tianjin,PRChina
MichaelLNeidig DepartmentofChemistry,UniversityofRochester, Rochester,NY,UnitedStates
AaronLOdom DepartmentofChemistry,MichiganStateUniversity, EastLansing,MI,UnitedStates
ElizabethTPapish DepartmentofChemistry,TheUniversityofAlabama, Tuscaloosa,AL,UnitedStates
RinaldoPoli
LaboratoiredeChimiedeCoordination,UPRCNRS8241, Toulouse,France
ChristopherGTPrice DepartmentofChemistry,SchoolofLifeSciences, UniversityofSussex,Brighton,UnitedKingdom
AleksaRadovic DepartmentofChemistry,UniversityofRochester, Rochester,NY,UnitedStates
JessicaRodriguez DepartmentofChemistry,UniversityofCalifornia, Riverside,CA,UnitedStates
KavyasripriyaKSamudrala DepartmentofChemistry,UniversityofCalifornia, Riverside,CA,UnitedStates
WeerachaiSilprakob DepartmentofChemistry,TheUniversityofAlabama, Tuscaloosa,AL,UnitedStates
SChantalEStieber DepartmentofChemistry&Biochemistry,CaliforniaState PolytechnicUniversity,Pomona,CA,UnitedStates
ThomasSTeets
DepartmentofChemistry,UniversityofHouston,Houston, TX,UnitedStates
VarunTej UniversityofCaliforniaRiverside,Riverside,CA,United States
SiobhanRTemple DepartmentofChemistry,SchoolofLifeSciences, UniversityofSussex,Brighton,UnitedKingdom
AntonWTomich
UniversityofCaliforniaRiverside,Riverside,CA, UnitedStates
TanyaMTownsend DepartmentofChemistry,YaleUniversity,NewHaven, CT,UnitedStates
JeremyEWeber DepartmentofChemistry,YaleUniversity,NewHaven, CT,UnitedStates
CGunnarWerncke ChemistryDepartment,Philipps-UniversityMarburg, Marburg,Germany
YanyuWu DepartmentofChemistry,UniversityofHouston,Houston, TX,UnitedStates
RowanDYoung NationalUniversityofSingapore,Singapore,Singapore
PREFACE Published40yearsagoin1982,thefirsteditionof ComprehensiveOrganometallicChemistry (COMC)providedan invaluableresourcethatenabledchemiststobecomeefficientlyinformedofthepropertiesandreactionsof organometalliccompoundsofboththemaingroupandtransitionmetals.Thisareaofchemistrycontinuedto developatarapidpacesuchthatitnecessitatedthepublicationofsubsequenteditions,namely Comprehensive OrganometallicChemistry II(COMC2)in1995and ComprehensiveOrganometallicChemistry III(COMC3)in2007. Organometallicchemistryhascontinuedtobevibrantinthe15yearsfollowingthepublicationofCOMC3,not onlybyaffordingcompoundswithnovelstructuresandreactivitybutalsobyhavingimportantapplications inorganicsynthesesandindustrialprocesses,asillustratedbytheawardingofthe2010NobelprizetoHeck, Negishi,andSuzukiforthedevelopmentofpalladium-catalyzedcrosscouplingsinorganicsyntheses. ComprehensiveOrganometallicChemistry IV(COMC4)thusservesthesameimportantroleasitspredecessorsby providinganindispensablemeansforresearchersandeducatorstoobtainefficientlyanup-to-dateanalysisof aparticularaspectoforganometallicchemistry.
COMC4comprises15volumes,ofwhichthefirstprovidesareviewoftopicsconcernedwithtechniques andconceptsthatfeatureprominentlyincurrentorganometallicchemistry,while5volumesaredevotedto applicationsthatincludeorganicsynthesis,materialsscience,bio-organometallics,metallo-therapy,metallodiagnostics,medicine,andenvironmentalchemistry.Inthisregard,weareverygratefultothevolumeeditors fortheirdiligentefforts,andtheauthorsforproducinghigh-qualitychapters,allofwhichwerewrittenduring theCOVID-19pandemic.Finally,wewishtothankthemanystaffatElsevierfortheireffortstoensurethatthe project,initiatedinthewinterof2018,remainedonschedule.
KarstenMeyer,Erlangen,March2022
DermotO’Hare,Oxford,March2022
GerardParkin,NewYork,March2022
1.01 Introduction:VolumeI PatrickLHolland,DepartmentofChemistry,YaleUniversity,NewHaven,CT,UnitedStates
©2022ElsevierLtd.Allrightsreserved.
ThefieldoforganometallicchemistryhasundergonesubstantialevolutionsincethepublicationoftheoriginalComprehensive OrganometallicChemistryin1982,andevensincetheappearanceofComprehensiveOrganometallicChemistryIIIin2007.This hasencouragedustocompileafreshversionofVolume1thatintroducesthereadertocross-cuttingconceptsthatareinvolvedin thesubsequentvolumes.ThetopicshavebeenchosentominimizeoverlapwiththechaptersinVolumeIofComprehensive OrganometallicChemistryIII,andtohighlightemergingareas.Forexample,valencebondtheorymodelsforunderstanding bondinghavebeenusedincreasinglyduetotheirintuitivecontent(Odom),andthepopularizationofredox-activeligands (Broere)hasencouragedorganometallicchemiststolookbeyondtheformaloxidationstate.Chemistshavecontinuedtopush theboundariesofoxidationstateswithhighlyoxidized(Malischewski)andhighlyreduced(Werncke)complexes,whichdisplay amazingreactivityaswell.Inorganometallicchemistry,chemistsalsostrivetowardthebindingandactivationofweaksubstrates likealkanes(Young),carbondioxide(Machan),anddinitrogen(Holland).Growingattentiontotheuseofabundantfirst-row metalshasfedarenaissanceofparamagneticorganometalliccomplexesandadvancedspectroscopictechniques,whicharecovered inchaptersoncomputations(Stieber)andonspectroscopy(Neidig).Organometallicchemistryhasremainedlargelydrivenby liganddesign,andanumberofchaptersexplorepopularligandtypessuchas N-heterocycliccarbenes(Deng)andligands withchargeseparatedfromthemetal(Lavallo).Inaddition,influencesfromoutsidethecoordinationsphereareincreasingly utilized,andaccordinglyweincludechaptersonorganometallicsystemsthatincorporateLewisacidparticipation(Hazari), proton-responsiveligands(Papish),andattachmenttooxidesurfaces(Conley).Theuseoforganometalliccomplexeshasalso expandedinnon-traditionalapplicationslikeelectrochemistry(Blakemore),single-moleculemagnets(Layfield),photosensitizers (Teets),andradicalreactions(Poli).Finally,practicalconsiderationsincatalysishavefueledresearchintostrategiesforheterogenizationandseparation(Baker),andonprinciplesthathelpthechemisttorecognizespuriousresultsfromcatalystimpurities (Leadbeater).
Constructionoftheseextensivereviewsrequiresanimmenseamountofeffort,andallorganometallicchemistsoweadebtof gratitudetothesegenerousauthorsforthetimetheydevotedtoteachingusaboutmodernaspectsoforganometallicchemistry. Theyhaveallthoughtfullyrevisedtoincorporatemysuggestionsforclarification,andIamconfidentthattheresultingchaptersgive insightsandtrendsthatwillguidethenextgenerationoforganometallicchemiststoevengreaterheights.
1.02 ModelsforUnderstandingMainGroupandTransitionMetalBonding AaronLOdom,DepartmentofChemistry,MichiganStateUniversity,EastLansing,MI,UnitedStates
©2022ElsevierLtd.Allrightsreserved.
1.02.1Introduction 2
1.02.2Primogenicrepulsion:Orbitalsizeeffectsinbondingintheperiodictable2 1.02.3The2-center2-electronheterocovalentbondandpolarcovalencetheory5 1.02.4The2-center2-electrondativebond12 1.02.5Complementarityofqualitativehybridizationtheoryandmolecularorbitaltheory14 1.02.6Lonepairbondweakeninganditsinfluenceonorganometallicchemistry18 1.02.7Beyondthe2-center2-electronbond20 1.02.8ExamplesofusingBent’srule,hybridization,andstructureinthemaingroup23 1.02.9Hybridizationtheoryandthetransitionmetals25 1.02.10Practicalevaluationofmetal-ligandbondinteractionsforapplicationsincatalysis27 1.02.11Concludingremarks 29 Acknowledgments 29 References 29
1.02.1Introduction
Structureisacentralconceptthatdefinesandempowersfunctioninbiology,chemistry,andphysics.Paulingsaid, “Thewhole problemofunderstandingscienceis,Ibelieve,theproblemofrelatingfactstotheconceptofstructure,firstintermsofatomsand thenintermsofsomethingstillsmaller,suchasnucleons ... Itisstructurethatwelookforwheneverwetrytounderstandanything. Allscienceisbuiltuponthissearch:weinvestigatehowthecellisbuiltofreticularmaterial,cytoplasm,chromosomes;howcrystals aggregate;howatomsarefastenedtogether;howelectronsconstituteachemicalbondbetweenatoms.Weliketounderstand,andto explain,observedfactsintermsofstructure.”1
Structureforbeginnersinchemistryusuallyconsistsofviewingmoleculeswitha “hubandstrut” model,wheretheatomsactasa hubinthestructureconnectedbybondingstrutsrepresentedbylines.Uponthisbasis,weaddlayersofcomplexitydependingon thetypesofatomsandbonds,butmolecularstructureremainsthebasisofourmodelsforunderstandingelectronicstructure, reactivity,andproperties.
Theatomic “hubs,” fromachemicalperspective,areoftenquiteadjustable,andthesameelementmayvarydramaticallyinthe numberandtypesofbondsfromonecompoundtothenext.Whatchemistsmeanbyalinebetweenatomsisalsowonderfully diverse,rich,andcomplexaswell.Becauseafullquantum-mechanicaltreatmentiscomplexanddifficulttoconceptualize, organometallicchemistsseeksimplermodelsthataremoreintuitive,yetgroundedinquantummechanics.Thesesimpler expressionsofnaturemayhavelimitations,butasRoaldHoffmannsaid, “Chemistryisnotmathematics,andevenifitbothers somepeoplelikehell,therearenotheoremsofchemistry.Chemicalargumentsarenotfalsifiedbyanexception.”2 Ifone approximationforunderstandingthebondingfails,weattempttouseabetter(sometimesjustassimple,sometimesmore complex)approximationtounderstandthesystem.
Thischapterisaperspectiveon “whatthelinesmean” insomechemicalstructures.Itfocusesonrelativelysimplemodelsfor understandingbondingthatarereadilyapplied,asthesemoreoftenhelponedesignthenextexperiment.Thisseemsfitting,assaid inarecentreviewbySchwerdtfeger,Frenking,andcoworkers(emphasistheirs), “Chemicalbondingmodelsarenotrightorwrong,they aremoreorlessuseful ”3 Inaddition,somefundamentalprincipleswillbecoveredthatarebeneficialforunderstandingbonding throughouttheperiodictable.Therewillbelittlediscussionofhistoricalcontext,basicsofmolecularorbitaltheory,andothertopics thatarecommontoundergraduatetexts.Whatiscoveredwillbenolessfundamental,buttheattemptismadetoboildownthe discussiontoafew(largelypenandpaper)modelstheauthorfindsparticularlyhelpful;thesearesometimessupplementedwith quantumchemicalresults,e.g.,densityfunctionaltheory(DFT)andnaturalbondorbital(NBO)calculations.
1.02.2Primogenicrepulsion:Orbitalsizeeffectsinbondingintheperiodictable Westartwiththetaskofexplainingthe “primogeniceffect,” whichwillclarify:whythe1strowissodifferentfromtherestofthe p-block;whythe1strowtransitionmetalsshowdifferentbehavior(e.g.,weakerbondsandweakerligandfields)thanthe2ndand 3rdrow;andwhythelanthanidesshowlittle f-orbitalparticipationinbondingwhiletheactinidesshowsignificantlymore.
Beforedescribingthiseffect,weneedtoreviewthreesimpleideas:
(1)Alltheatomicorbitalsofanatomareorthogonaltooneanother.Thisorthogonalityismaintained,notthroughspatial separation,butthroughcancelationofbondingandantibondinginteractions.Thenetoverlapofanyatomicorbitalwith anotheriszeroduetothiscancelation.
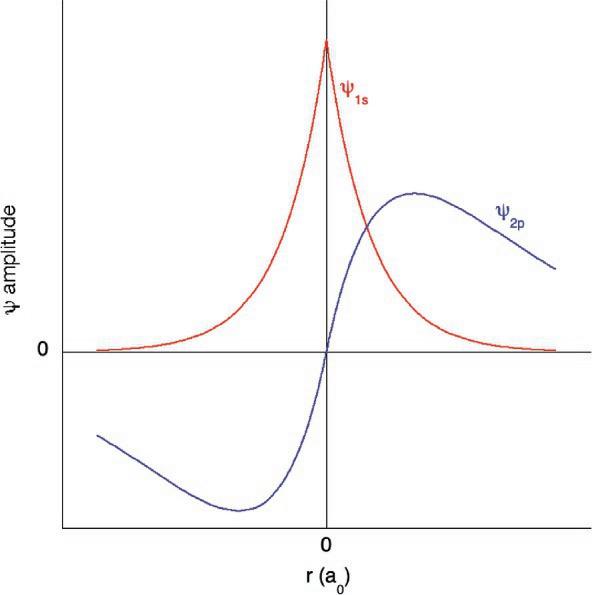
(2)Thisorthogonalityismaintainedthroughacombinationofangularandradialnodes.The1s orbital,forexample,maintains orthogonalitywiththe2s orbitalbecausethe2s orbitalhasanode,achangeofsigninthewave,sothatthetwoorbitalshaveno netoverlap.(Moreonthistofollow.)Forangulardifferences,thecancelationisreadilyseeninsaytheoverlapofa p orbitalwith an s onthesameatom(Fig.1).Theconstructiveoverlap,unshadedwithunshaded,isexactlycanceledbythedestructive overlap,unshadedwithshaded,whichistrueofanycombinationoforbitalswithdifferentangularmomentumonthesame atom.
(3)Finally,itisusefultohaveanexpectationforthesizeoforbitalsofdifferentangularmomentumwiththesameprincipal quantumnumber(n).Forexample,when n ¼ 4thereare4s,4p,4d,and4f orbitals.Whichorbitaldoweexpecttobethe smallestandwhichthelargestinradius?Theangularmomentumquantumnumber l correspondstotheshapeoftheorbital, withshapeshavinggreatercurvaturebeingassociatedwithhigherangularmomentum.Thinkingfromaclassicalperspectivefor amoment,wecanmodeltheelectron’smotionaroundthenucleusasaball(electron)rotatingonanelasticstring(Coulombic attractiontothenucleus).Ifweincreasetheangularmomentum,theelasticstringshouldstretchfurtherfromthecentralpoint. Inotherwords,weexpectthatthe4s orbital,whichhasalowestangularmomentum,wouldbemostcompact(closestto nucleus)whilethe4f orbital,whichhasthehighestangularmomentum,wouldbethemostdiffuse(farthestfromnucleus). Whencomparingfilledorbitalswiththesameprincipalquantumnumber,lowerangularmomentumorbitalsareexpectedto havesmallerradii.
Theterm “primogenicrepulsion” wasfirstpublishedbyPyykköin1979,4 andheattributedthephrasetoacolleagueinthe DepartmentofEnglish. Theprimogeniceffectreferstotheabilityoforbitalsofaspecificangularmomentumappearingforthefirsttimeinthe Aufbausequence,i.e.,1s,2p,3d,and4f,toshrinktowardsthenucleusmorethanmightbeexpected,simplybecausetherearenoorbitalsof thesameangularmomentuminthecorebelowthem.Allorbitalsinaparticularshellareorthogonalbytheirshapeduetothe l and ml quantumnumberdifferences(videsupra).Bythisprinciple,mostorbitalsthenhavetheirsizeset,tosomeextent,bythenecessityof beingorthogonaltotheorbitalsofthesameangularmomentumbelowthem.Inotherwords,thesizeofthe1s-orbitalaffectsthe sizeofthe2s orbital;the2s musthaveitsnodefitpreciselywiththe1s sothatthereiszerooverlap.Ifthe1s orbitalcontracted,the 2s orbitalwouldalsohavetocontract.Incontrast,the2p orbitals,becausethereisno “1p orbital,” havenosuchrestrictionandcan lowertheirenergybycontractingtowardthenucleus.5,6
Fig.1 Atomicorbitalsofdifferentangularmomentumarealwaysorthogonalwithequalamountsofconstructiveanddestructiveinterference.Asanexample,(top) a1s orbitaland2p orbitalonthesameatomhaveequalamountsofconstructive(unshadedwithunshaded)anddestructive(unshadedwithshaded)interference. (Bottom)The1sand2pwavefunctionsareplotted.Centeredaroundtheorigin(nucleus),theconstructiveinterference(rightoftheorigin)isequivalenttothe destructiveinterference(leftoftheorigin).
Theresultofthe2p-orbitalcontraction,andprimogenicexpansionof2s,isthatthe2s and2p orbitalsforthefirst-row p-block elementsareessentiallythesamesize.(Fortherestofthe p-block,thevalence s-orbitalisabout80%thesizeof p becauseboth orbitalsareprimogenicallyexpandedand p hasahigherangularmomentum.)Sincethe2s and2p orbitalsaresimilarinsize,they moreeffectivelyhybridizethan,forexample,3s and3p.Thus,thedirectionalbondingandmultiplebondingthatdominateorganic chemistryaredueinlargeparttotheprimogeniceffect.
Toillustrate,considertheveryfamiliargeometryofacetyleneversusAr–GeGe–Ar(Fig.2).7 Whileacetylenehasthefamiliar lineargeometrywith sp hybridizationoncarbon,alltheheaviercongenersarebent.Theexperimentalgeometryofthegermanium complexhasGe–Ge–Aranglesof128.7(1) ,closertothatexpectedfor sp2 than sp.NaturalBondOrbitalcalculationsonthe H–GeGe–Hmodelcompound(Fig.2)identifythehybridizationoftheorbitalusedbygermaniumtoformtheGe–Ge s-bondas essentially sp;however,theGe–Hbondisformedbyan sp2 hybrid,whichgivesabentgeometry.Theprimogeniceffectlimitsthe sp hybridizationsomewhatfortheheaviercongener,givingapreferenceforbondingwithgreater p character.(Thisisonlypartofthe answerasthelowerelectronegativityoftheelementsbelowcarbonalsoaffectsthehybridization,Bent’sRule,videinfra.)
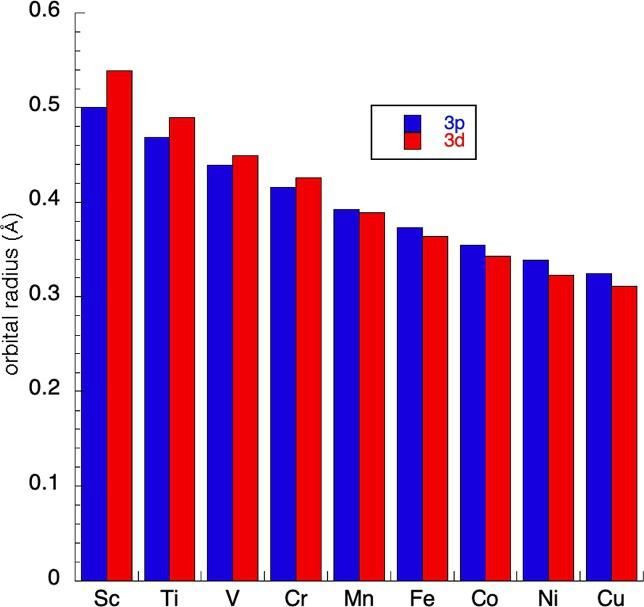
Therearemanyothercommonchemicalobservationsthatmaybeattributedtoprimogenicrepulsion,orlackthereof. Ofparticularimportanceforourpurposeshereisthecontractionofthe3d orbitalsofthefirstrowofthetransitionseries.The 3d orbitalsaresmallerthanmightbeexpectedfromtheirheaviercongeners.Asaresult,the3d orbitalsareonlyabout1/3thesizeof thevalence4s-orbitalsforthesemetals.8 Inaddition,the3d-orbitalsareveryclosetothesamesizeasthecore3p orbitals;for example,the3p-orbitalsofironhaveradiia of0.373A ˚ ,whilethe3d orbitalshaveradiiof0.364A ˚ ,slightlysmallerthanthesecore orbitals.8 So,asligandsapproachthemetalforbondingwiththe3d orbitals,thereisrepulsionbycoreorbitalsofessentiallythe samesize.Therelationshipbetweenthe3d and3p orbitalsforthefirst-row d-blockelementsisshownin Fig.3.Theearlytransition metalshave d-orbitalsslightlyextendedfromthecore,whilethelatermetalshavevalence d-orbitalsslightlysmallerthanthesecore orbitals.
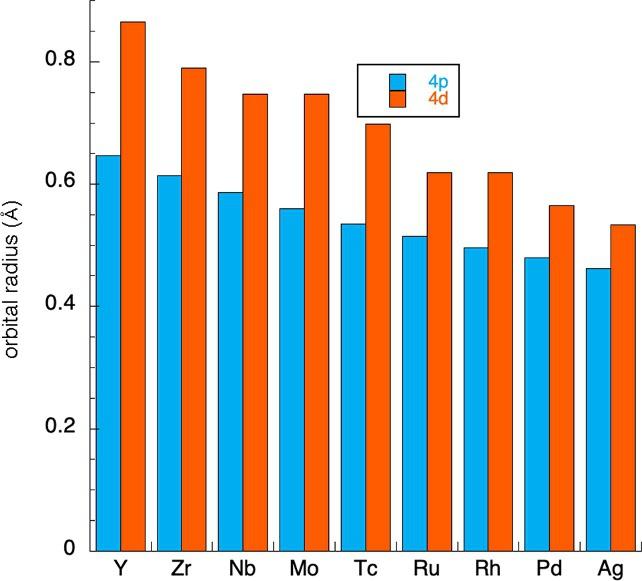
Incontrasttothefirst-rowtransitionmetals,thesecondandthirdrowelementshave d orbitalsthatextendwellbeyondthecore. Forexample,rutheniumhas4d orbitalradiiof0.616A ˚ ,whilethe4p orbitalsaresmallerat0.515A ˚ .Thesizesoftheseorbitals areshownin Fig.4,andthevalence4d-orbitalsarealwayslargerthanthecoreorbitalsfortheelements.(Thethird-rowmetalsare slightlylargerbutprovidesimilarratios:e.g.,osmiumhas5d-orbialswithradiiof0.682A ˚ ,with5p radiiof0.550A ˚ .)
Animportantconsequenceofprimogenicrepulsiononthevalenceorbitalsforthe2ndand3rdrowtransitionelements,andthe lackthereofforthe1strow,isthatthebondsformedbytheheaviercongenersarestrongerthanthe1strowforsimilarcompounds. Thesmallsizeandcorerepulsioneffectsinthe1strowresultinrelativelyweakbondsandweakerligandfieldsthanfoundforthe 2ndand3rdrows.
Carbon has sp-hybridization
Experimental geometry of Ge2Ar2
Ge Ge Ar Ar
Calculated
Fig.2 (Topleft)Thefamiliargeometryofacetylene,whichhas sp-hybridizedcarbonsduetogoodhybridizationbetween2s and2p,aresultoftheprimogenic effect.(Topcenterandbottom)LinedrawingandORTEPdiagramfromtheX-raydiffractiondataonAr–GeGe–Ar,whereAr ¼ 2,6-(2,6-diisopropylphenyl)phenyl. (Topright)ThecalculatedgeometryofH–GeGe–HusingM06L/aug-cc-PVTZonG19.Ar ¼ 2,6-(2,6-Pri2C6H3)2C6H3
aWaberandCromercalculatedthe “maximuminthechargedensityanditscorrespondingradius” fortheatomicvalenceorbitals,whichI’llsimplyrefertoasthe “orbitalradius.”8
Theprimogeniceffectdramaticallyaffectsthechemistryofthe f-blockaswell.The f-orbitalsofthelanthanidescontracttoward thenucleussothattheyarelessthan20%thesizeofthevalence s-orbital,farsmallerthansomeofthecoreorbitals.Forexample,for cerium,the f-orbitalsare0.366A ˚ ,whilethecore5p-orbitalisovertwiceaslargeat0.825A ˚ .Asaresult,the f-orbitalsofthe lanthanidesaresosmallthattheyoftencannotoverlapwithligandorbitalsandarerarelyinvolvedinbonding.Incontrast,the actinideshaveprimogenicallyexpanded5f-orbitalsthatmayparticipateinbonding.8
1.02.3The2-center2-electronheterocovalentbondandpolarcovalencetheory
Aseriesofconceptswearetaughtfromourinitialindoctrinationaschemistsregardcovalent2-center2-electron(2c2e)bonds,such asthosefoundinH2 andHCl.AccordingtoMolecularOrbital(MO)theory,championedbyMulliken,overlapleadstobonding andantibondingmolecularorbitalswherethelowerenergybondingorbitalisoccupied.InHybridizationtheory,championedby Pauling,thebondisviewedasaseriesofresonanceforms,withsomeioniccontributions.Intheend,thetwoviewpointsare mathematicallyequivalentattheirlimits,9 andthechoiceofonemodelovertheotherisbasedonexpediencyinaddressingthe problemathand.
Fig.3 Thesizesofthe valence3d (red)and core3p-orbitals (blue)forthefirst-rowtransitionelements.8
Fig.4 Thesizesofthe valence4d (orange)and core4p-orbitals (lightblue)forthesecond-rowtransitionelements.8
PaulingwassuchaneffectiveadvocatethatValenceBond(VB)theorywasregardedasthe “correct” methodforunderstanding chemicalbondinguntiltherewasaseriesofinstanceswhereVBtheorywasthoughttofailtogivethecorrectanswerwhereMO theorywassuccessful.CommonexampleswhereVBtheorywasthoughttofailareindescribingtheelectronicstructureofO2 and photoelectronspectrumofCH4.Inactuality,these “failures” ofVBtheoryareattributablemoretopooruseofthetheorythanthe theoryitself.10,11
AmajoradvantageofVBtheoryisthatthelocalizedbondsaremoreeasilycorrelatedwiththelinesinourLewisstructuresthan delocalizedmolecularorbitalsofMOtheory.Despitethisadvantage,VBtheoryfelloutoffavorbecauseMOtheorywaseasierto adapttocomputationalmethods.ComputerswerepowerfulalliestoMOtheory,leavingVBmethodsbehindformanyyears.
InsomewaysrelatedtotheorbitalsusedinVBtheory,NaturalOrbitalswerefirstdiscussedbyLöwdinin1955,butWeinhold andLandisturnedthemintoapowerfultoolformodernchemists.Effectively,NaturalBondOrbital(NBO)theoryallowstheuseof moderncomputationmethods(DFT,coupledclustertheory,etc.)toadoptmanyadvantagesofVBtheory.Inthischapter,Iwillrefer to “Hybridizationtheory” asageneraltermthatincorporatestheunderlyingconceptsofVBandNBOtheory.Theunderlying conceptualunderpinningsofVBtheory(andNBOtheory)regainrelevanceasquantitativemethodscanbeusedinconjunctionwith theback-of-the-envelopecalculations.
Inthissection,itisadvantageoustooutlineaHybridizationtheorysystemforunderstandingheterocovalentbonds,Polar Covalencetheory.Thetheoryisnotputforwardasareplacementforaccuratequantummechanicalcalculationsorexperimental bondenergies.Instead,itisamethodforcalculatingbondenergiesbyhandthatillustratesseveralimportantconceptsabout bonding.Noderivationsoftheequationsaregiventoprovideroomforadiscussionofconceptsthatunderliethesedescriptionsof covalentbonds.
PolarCovalencetheorywasdevelopedbyR.T.Sandersonasamethodforthecalculationofbondenergies.12,b Themethoduses onlyafewdescriptorsfortheelementsandthebonddistance,R0,inthecompoundtocalculatethebondenergy,typicallywithin 5%ofexperimentalvalues.ThetabulatedvaluesareSandersonelectronegativity(wS),thechangeinelectronegativityofan elementwithunitcharge(DwS),covalentradii(rc),andthehomonuclearbondenergiesoftheelements.Thehomonuclearbond energiesaredescribedasbeing “fullyunweakened” (E000 ), “partiallyweakened” (E00 ),and “fullyweakened” (E0 ).Aselectionofthese valuesisshownin Table1
Thecauseoftheweakening(differencebetween E000 , E00 ,and E0 )isaveryinterestingonethatexplainsmanychemical phenomena.Unfortunately,itwillalsorequiresomebuildingoffoundationsbeforetheexplanationcanbegivenandthecause described.Forthemoment,wewillcallitLonePairBondWeakening(LPBW),whichwasSanderson’snamefortheeffect,aneffect thatwasn’tfullyelucidateduntil1996.15
Onthesurface,Sandersonelectronegativity(wS)hasnothingtodowithbondenergiestotheelement,unlikePauling electronegativity,whichisderivedfrombondenergies.Incontrast,Sandersonelectronegativityisderivedfromelectrondensity oftheatoms(z)andtheirrelationshiptoacalculatedidealelectrondensity(zi).Theelectrondensityaroundtheatomissimplyas showninEq. (1).TheelectrondensityofanatomXistheatomicnumberdividedbythevolumeofthespheredefinedbythe covalentradiusoftheatominunitsofelectrons/A ˚ 3 .
Theidealelectrondensitywasfoundbymakinganinterestingassumption.Itwasassumedthatthenoblegaseswerenotonly unreactivebecauseofaclosedshell,butalsobecausetheyhadanidealelectrondensityaroundtheatom.Theidealelectrondensity (zi)fornon-noblegasatomswasfoundbylinearinterpolationbetweenthenearestnoblegases;inotherwords,thetwonoblegases thatarethebookendsforanatomicshellwereusedtodefinealineofelectrondensityrelativetoatomicnumberthatwasidealfor theatomsinbetween.
Sandersonelectronegativityissimplytheratiobetweentheelectrondensityofanatom(z)andtheidealelectrondensity(zi),i.e., w S ¼ (z)/(zi).Originally,thesevalueswerecalled “stabilityratios” astheygaveameasureofhowtheatomcouldbestabilizedby additionorsubtractionofelectrondensity,andfluorinehadavalueof5.75onthisscale.Later,Sandersonscaledallhisvaluestothe morefamiliarPaulingvalueof wF ¼ 4.00,andthenumbersbecameknownasSandersonelectronegativity.16
Atthisstage,theequationsfordeterminationofbondenergiesusingPolarCovalencetheorywillbegiven(Table2)andabrief explanationofeachwillbeprovidedwiththeunderlyingconcept.
Asmentioned,PolarCovalencetheoryisbasedonHybridizationtheoryandsuggeststhatthebondinaheterocovalent compoundcanbedescribedusingonlytworesonanceforms(Fig.5).Intheleftform,thereisapurelycovalentbondbetween atomsAandX,signifiedbyaline.Intherightform,thereisapurelyionicformwherethelesselectronegativeatom(A)hasa positivecharge,andthemoreelectronegativeatom(X)hasanegativecharge.Analternative,minor,resonanceformwheretheless electronegativeatomhasanegativechargeisdisregarded.
Theoverallbondenergy(theaverageenthalpyofthebondsinthecompound)accordingtoPolarCovalencetheoryiscomprised ofthecovalentbondenergy, Ec,andtheionicbondenergy, Ei,withtwoweightingcoefficients tc and ti.Thesumofthecontributions fromallresonanceformsshouldbeunity,andthesearetheonlyformsbeingconsidered,so tc +ti ¼ 1(Eq.3in Table2).Theoverall
bHere, “bondenergy” referstotheaveragebondenthalpyforhomolyticcleavageofthebondsinacompound,e.g.,1/3oftheenthalpyrequiredtoatomize BF3 toB+3F.Amoredetailedexplanationandexamplesaregivenbelow.
Table1 Sandersonelectronegativities(wS),changeinelectronegativitywithunitcharge(DwS),covalentradii(rc)inA ˚ ( 10 10 m),homonuclearfully unweakened(E000 ),partiallyweakened(E00 ),andfullyweakened(E0 )bondenergiesinkcal/mol.a
H2.5922.5280.320104.2
Li0.6701.2851.33624.6
Be1.8102.1120.88767.6
B2.2752.3680.82276.7
C2.7462.6020.77285.4
N3.1942.8060.73494.966.938.8
O3.6543.0010.702104.068.833.6
F4.0003.1400.681113.176.838.2b
Na0.5601.2751.53916.4
Mg1.3181.8021.37342.3
Al1.7142.0551.25848.2
Si2.1382.2961.16954.1
P2.5152.4901.10760.056.953.7
S2.9572.7001.04965.960.454.9
Cl3.4752.9270.99471.864.958.0
K0.4451.0471.96213.1
Ca0.9461.5271.7430.8
Sc(II)0.641.2561.69531.3
Sc(III)1.021.5861.69531.3
Ti(II)0.731.3411.65031.8
Ti(III)1.091.6391.65031.8
Ti(IV)1.501.9231.65031.8
V(II)0.691.3041.60532.3 V(III)1.391.8511.60532.3 V(IV)1.892.1581.60532.3
V(V)2.512.4871.60532.3
Cr(II)1.241.7481.56032.8
Cr(III)1.662.0231.56032.8
Cr(IV)2.292.3761.56032.8
Cr(V)2.832.6411.56032.8
Cr(VI)3.372.8821.56032.8
Mn(II)1.662.0231.51633.3
Mn(III)2.202.3291.51633.3
Mn(IV)2.742.5991.51633.3
Mn(V)3.282.8431.51633.3
Mn(VI)3.823.0691.51633.3
Fe(II)1.642.0111.47133.8 Fe(III)2.202.3291.47133.8
Co(II)1.962.1981.42634.3 Co(III)2.562.5121.42634.3 Co(IV)3.102.7641.42634.3 Ni(II)1.942.1871.38134.8
Ni(III)2.732.5941.38134.8
Ni(IV)3.272.8391.38134.8
Ni(V)3.813.0651.38134.8
Cu(II)1.982.2091.33635.3 Zn2.2232.3411.29235.8
Ga2.4192.4421.25640.3
Ge2.6182.5401.22344.8
As2.8162.6351.29449.345.140.9
Se3.0142.7261.16753.846.038.2
Br3.2192.8171.14258.352.246.1
Rb0.3120.8772.1612.4
Sr0.7211.3331.9124.6
Y(II)0.400.9931.86825.1
Y(III)0.651.2661.86825.1
Zr(II)0.521.1321.82625.7
Zr(III)0.791.3951.82625.7
Zr(IV)0.901.4891.82625.7
Nb(II)0.771.3781.78426.3
Nb(III)1.021.5861.78426.3
Nb(IV)1.251.7551.78426.3
Continued )
Table1 (Continued)
Nb(V)1.421.8711.78426.3
Mo(II)0.901.4891.74226.9
Mo(III)1.151.6841.74226.9
Mo(IV)1.401.8581.74226.9
Mo(V)1.732.0651.74226.9
Mo(VI)2.202.3291.74226.9
Cd1.9782.2081.49330.4
In2.1382.2961.45533.1
Sn(IV)2.2982.3801.42035.8
Sn(II)1.4771.9081.42035.8
Sb2.4582.4611.38938.535.833.0
Te2.6182.5401.36041.239.237.2
I2.7782.6171.33343.940.036.1
Cs0.2200.7362.3510.8
Ba0.6511.2671.9822.2
Hf(II)0.310.8741.8015.6
Hf(III)0.561.1751.8015.6
Hf(IV)0.811.4131.8015.6
Ta(II)0.441.0411.7614.7
Ta(III)0.691.3041.7614.7
Ta(IV)0.941.5221.7614.7
Ta(V)1.171.6981.7614.7
W(II)0.731.3411.7313.8
W(III)0.981.5541.7313.8
W(IV)1.231.7411.7313.8
W(V)1.481.9101.7313.8
W(VI)1.672.0291.7313.8
Tl(III)2.2462.3531.49016.6
Tl(I)0.981.5601.4916.6
Pb(IV)2.2912.3761.4824.2
Pb(II)1.9002.1641.4824.2
Bi2.3422.4031.4732.2
aValuesarefromSanderson’sreferencesonPolarCovalence. 12,13
bValuefromHuberandHerzberg.14
Table2 PolarCovalenceTheoryequationsfordetermininghomolyticbondenergies.
DeterminationoftheA–Xbondenergy
X ¼ tcEc + ttEt (2)a
Sumoftheionicandcovalentcoefficientsisunity1 ¼ tc + tt (3)
Covalentbondenergy
(4)b
Bondorder b Single1.000 Double1.488 Triple1.787
Ionicbondenergy
Estimationoftheioniccoefficient
Chargeonanatominamolecule
Molecularelectronegativity
atc ¼ covalentcoefficient, ti ¼ ioniccoefficient, Ec ¼ covalentbondenergy, E ¼ ionicbondenergy. bRc ¼ covalentbonddistancefromsumofcovalentradii(RA + RX), R0 ¼ bonddistance, EAA ¼ covalentbondenergyof A, EXX ¼ covalentbondenergyofX.
cd ¼ partialcharge.
d wM ¼ molecularelectronegativity, wA ¼ electronegativityof A
eN ¼ numberoftotalatoms, NA ¼ numberofatoms A
Fig.5 ResonanceformsconsideredinPolarCovalencetheory.
bondenergyinA–Xisthen EAX ¼tcEc +tiEi (Eq.2in Table2).Since tc and ti arerelatedbyEq.(3)in Table2,byfindingonlythree values Ec,Ei,and ti wecancalculatethebondenergyforthesystem.Allthreevaluesarerelativelysimpletocalculate,aswill beshown.
First,Sandersonshowedthattheapparentbondorder(thenumberofbondsachemistwouldnormallydrawforthe compound,e.g.,asinglebondforH2 andadoublebondforO2)hastheeffectofaddingascalar(b)to Ec and Ei.Asanychemist willtellyou,adoublebondisnottwiceasstrongasasinglebond,andatriplebondisnotthreetimesstrongerthanasinglebond. Sandersonfoundforadoublebondthat b ¼ 1.488andforatriplebond b ¼ 1.787.Thereis,however,anadditionalcorrectionfor thebondshorteningthatoccursinthesehigherbondordersthatleadstosomestrengtheningoverthesefactors(videinfra).
Sandersonproposedthatthecovalentbondenergy(Ec)wassimplythegeometricmeanofthehomolyticbondenergiesforthe elementsinvolved.Forexample,thepurelycovalentbondenergyofNaClwouldbethegeometricmeanoftheNa2 andCl2 bond energies.Inessence,thissuggeststhatthereisaprimalenergyassociatedwithaspecificatominvolvedinpurelycovalentbonding thatisfullymanifestedwhentheatombondstoitself.Whentheatombondstoanatomofadifferenttype,e.g.,A–X,thenthe covalentbondenergyisthegeometricmeanoftheseprimalcovalentenergiesofAandX.(Thissameassumptionwasusedby Paulinginthegenerationofhiselectronegativityvalues,withoutthecorrectivevalueforthebonddistance.Therearesomecaveats tothishavingtodowithLPBWeffect,whichwillbediscussedinduecourse.)Tothistherewasappliedacorrectioninvolvingthe ratiooftheactualbonddistanceinthecompoundandthesumofthecovalentradii.Shorterbonddistancesincreaseoverlapand generallyincreasebondenergy.This,inadditiontothefactorforthebondorder(b),iswhatleadstotheequationfor Ec (Eq.4in Table2).
Theionicbondenergy Ei isverysimpleandcomesdirectlyfromCoulomb’sLaw.Theionicbondenergy(Eq.5in Table2)is simplyaconstant(332kcalA ˚ /mol)dividedbythebonddistance,R0.Thisequationgives Ei inkcal/molifthebonddistanceisinA ˚
Sofar,twoofthethreevalues(Ec,Ei, and ti)neededtocalculatethebondenergyhavebeenfound;allthatremainsistheionic weightingcoefficient, ti.Thecontributionoftheionicformtotheoverallelectronicstructureisrelatedtothepartialchargesinthe system.IftherearelargerpartialchargesonAandX,then ti shouldbelarger.IfthereisafullpositivechargeonA(dA ¼ +1)andafull negativechargeonX(dX ¼ 1),then ti ¼ 1andonlytheionicformcontributes.Asaresult, ti canbeestimatedastheaverageofthe absolutevaluesofthepartialchargesonthetwoatoms,i.e.,(| dA |+| dX |)/2 ¼ ti (Eq.6in Table2).
Tofind ti,weneedthepartialchargesontheatoms,whicharerelatedtotheelectronegativitiesoftheatomsinvolved.Thereare manychargeschemesthathavebeendevelopedovertheyearsusingcomputationalmethods.Here,Sandersondevelopedasimple methodforcalculatingchargesinamoleculebyhand.Themethodreliesonabasicprincipleofcovalentsystems,electronegativity equalization.12
Considerasimplediatomicinthegasphase,e.g.,HCl.Chlorineismoreelectronegativethanhydrogen,meaningitwillattract moreelectrondensityinthebond.Asaresult,thechlorineatomaccumulatessomenegativecharge,andthehydrogenaccumulates somepositivecharge.Apartialpositivechargemakeshydrogenmoreelectronegativethanneutralhydrogen,andapartialnegative chargemakeschlorinelesselectronegativethanneutralchlorine.Inotherwords,chargetransfersbetweenthetwoatomsuntilthe electronegativitiesofHandClinHClequalize.Electronegativityequalizationsimplysuggeststhatinasymmetriccovalentsystem, theelectronegativityofalltheatomsisthesame.
Sanderson’ssimplechargeschemereliesonelectronegativityequalizationtofindthemolecularelectronegativity,andthe chargesonindividualatomsarethenrelatedtothedifferencebetweenthemolecularelectronegativityandtheelectronegativity oftheindividualatoms.Theelectronegativityofthemolecule(wM)isfoundbysimplytakingthegeometricmeanofthe electronegativitiesofalltheatomsinthemolecule(Eq.8in Table2).
Thepartialchargeonanindividualatom(dA)isthenthedifferencebetweenthemolecularelectronegativity(wM)andtheatomic electronegativity(wA)dividedbythechangeinelectronegativityperunitcharge(DwA)forthatelement.Sandersonfound empiricallythatthechangeinelectronegativitywithunitchargewasequalto1.57timesthesquarerootoftheneutralatom’ s electronegativity,i.e., DwA ¼ 1.57 wA p .Theelectronegativitywouldgoupwithapositivechargebythatamountanddownwitha negativecharge.Usingfluorineasanexample, wF ¼ 4.000,and DwF ¼ 1.57(2) ¼ 3.140.Inotherwords,theelectronegativityofF+ wouldbe7.140,butF hasanestimatedelectronegativityof0.86.
Itisimportanttonotethelimitationsofthechargescheme.First,thissimpleschemedoesnotworkformoleculesthathavethe sameatomintwoverydifferentenvironments,e.g.,H3Si–SiF3.Theschemeistooprimitivetodistinguishbetweenthedifferent chargesonsiliconinH3Si– and –SiF3.Second,thischargeschemedoesnotworkformoleculeswithdativebonds.(Muchmoreon thislater.)Asaruleofthumb,thisschemeworksbestforbinary,covalentcompoundswithanAXn formula.Despiteitslimitations, therearethousandsofcompoundswherethissimplemethodcanbeappliedverysuccessfully,anditillustratesmanybonding principles.