CO2 Hydrogenation Catalysis
Edited by Yuichiro Himeda
Editor
Dr. Yuichiro Himeda
National Institute of Advanced Industrial Science and Technology
AIST Tsukuba West, 16‐1 Onogawa
305‐8569 Tsukuba, Ibaraki
Japan
Cover
Cover Design: Wiley
Cover Image: Courtesy of Yuichiro Himeda
All books published by Wiley-VCH are carefully produced. Nevertheless, authors, editors, and publisher do not warrant the information contained in these books, including this book, to be free of errors. Readers are advised to keep in mind that statements, data, illustrations, procedural details or other items may inadvertently be inaccurate.
Library of Congress Card No.: applied for
British Library Cataloguing-in-Publication Data
A catalogue record for this book is available from the British Library.
Bibliographic information published by the Deutsche Nationalbibliothek
The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at <http://dnb.d‐nb.de>.
© 2021 WILEY‐VCH GmbH, Boschstr. 12, 69469 Weinheim, Germany
All rights reserved (including those of translation into other languages). No part of this book may be reproduced in any form – by photoprinting, microfilm, or any other means – nor transmitted or translated into a machine language without written permission from the publishers. Registered names, trademarks, etc. used in this book, even when not specifically marked as such, are not to be considered unprotected by law.
Print ISBN: 978‐3‐527‐34663‐9
ePDF ISBN: 978‐3‐527‐82409‐0
ePub ISBN: 978‐3‐527‐82410‐6
oBook ISBN: 978‐3‐527‐82411‐3
Typesetting SPi Global, Chennai, India
Printing and Binding
Printed on acid‐free paper
10 9 8 7 6 5 4 3 2 1
Contents
Preface xi
1 Introduction 1
Yuichiro Himeda and Matthias Beller
1.1 Direct Use of CO2 1
1.2 Chemicals from CO2 as a Feedstock 2
1.3 Application and Market Studies of CO2 Hydrogenation Products 4
1.3.1 Formic Acid/Formate 4
1.3.2 Methanol 4
1.3.3 Methanation 5
1.3.4 Energy Storage 6
1.4 Supply of Materials 6
1.4.1 CO2 Supply 6
1.4.2 Energy and H2 Supply 8
1.5 Political Aspect: Tax 9
1.6 Conclusion and Perspectives 9
References 10
2 Homogeneously Catalyzed CO2 Hydrogenation to Formic Acid/Formate by Using Precious Metal Catalysts 13
Wan-Hui Wang, Xiujuan Feng and Ming Bao
2.1 Introduction 13
2.2 Ir Complexes 14
2.2.1 Ir Complexes with N,N-ligands 14
2.2.1.1 Tautomerizable N,N-ligands with OH Groups 14
2.2.1.2 N,N-ligands with NH Group 30
2.2.1.3 Tautomerizable N,N-ligands with OH and NH Groups 32
2.2.1.4 Tautomerizable N,N-ligands with Amide Group 33
2.2.2 Ir Complexes with C,N- and C,C-ligands 34
2.2.3 Ir Complexes with Pincer Ligands 35
2.3 Ru Complexes 37
2.3.1 Ru Complexes with Phosphorous Ligands 38
2.3.2 Ru Complexes with N,N- and N,O-ligands 40
2.3.3 Ru Complexes with Pincer Ligands 41
2.4 Rh Complexes 46
2.5 Summary and Conclusions 49 References 49
3 Homogeneously Catalyzed CO2 Hydrogenation to Formic Acid/Formate with Non-precious Metal Catalysts 53 Luca Gonsalvi, Antonella Guerriero and Sylwia Kostera
3.1 Introduction 53
3.2 Iron-Catalyzed CO2 Hydrogenation 55
3.2.1 Non-pincer-Type Iron Complexes 56
3.2.2 Pincer-Type Iron Complexes 63
3.3 Cobalt-Catalyzed CO2 Hydrogenation 69
3.4 Nickel-Catalyzed CO2 Hydrogenation 73
3.5 Copper-Catalyzed CO2 Hydrogenation 77
3.6 Manganese-Catalyzed CO2 Hydrogenation 78
3.7 Other Non-precious Metals for CO2 Functionalization 81
3.8 Conclusions and Perspectives 85 References 86
4 Catalytic Homogeneous Hydrogenation of CO2 to Methanol 89 Sayan Kar, Alain Goeppert and G. K. Surya Prakash
4.1 Carbon Recycling and Methanol in the Early Twenty-First Century 89
4.2 Heterogeneous Catalysis for CO2 to Methanol 91
4.3 Homogeneous Catalysis – An Alternative for CO2 to Methanol 92
4.3.1 Benefits of Homogeneous Catalysis 92
4.3.2 CO2 Hydrogenation to Methanol Through Different Routes 92
4.3.3 The First Homogeneous System for CO2 Reduction to Methanol 93
4.3.4 Indirect CO2 Hydrogenation 94
4.3.5 Direct CO2 Hydrogenation 97
4.3.5.1 Through Formate Esters 97
4.3.5.2 Through Oxazolidinone or Formamides 100
4.3.6 CO2 to Methanol via Formic Acid Disproportionation 108
4.4 Conclusion 109
References 110
5 Theoretical Studies of Homogeneously Catalytic Hydrogenation of Carbon Dioxide and Bioinspired Computational Design of Base-Metal Catalysts 113 Xiuli Yan and Xinzheng Yang
5.1 Introduction 113
5.2 H2 Activation and CO2 Insertion Mechanisms 114
5.2.1 Hydrogen Activation 114
5.2.2 Insertion of CO2 115
5.3 Hydrogenation of CO2 to Formic Acid/Formate 118
5.3.1 Catalysts with Precious Metals 118
5.3.2 Catalysts with Non-noble Metals 128
5.4 Hydrogenation of CO2 to Methanol 133
5.5 Summary and Conclusions 142 References 145
6 Heterogenized Catalyst for the Hydrogenation of CO2 to Formic Acid or Its Derivatives 149 Kwangho Park, Gunniya Hariyanandam Gunasekar and Sungho Yoon
6.1 Introduction 149
6.2 Molecular Catalysts Heterogenized on the Surface of Grafted Supports 150
6.3 Molecular Catalysts Heterogenized on Coordination Polymers 157
6.4 Molecular Catalysts Heterogenized on Porous Organic Polymers 161
6.5 Concluding Remarks and Future Directions 172 References 173
7 Design and Architecture of Nanostructured Heterogeneous Catalysts for CO2 Hydrogenation to Formic Acid/Formate 179 Kohsuke Mori and Hiromi Yamashita
7.1 Introduction 179
7.2 Unsupported Bulk Metal Catalysts 180
7.3 Unsupported Metal Nanoparticle Catalysts 181
7.3.1 Metal Nanoparticles Without Stabilizers 181
7.3.2 Metal Nanoparticles Stabilized by Ionic Liquids 182
7.3.3 Metal Nanoparticles Stabilized by Reverse Micelles 183
7.4 Supported Metal Nanoparticle Catalysts 184
7.4.1 Metal Nanoparticles Supported on Carbon-Based Materials 184
7.4.2 Metal Nanoparticles Supported on Nitrogen-Doped Carbon 185
7.4.3 Metal Nanoparticles Supported on Al2O3 189
7.4.4 Metal Nanoparticles Supported on TiO2 191
7.4.5 Metal Nanoparticles Supported on Surface-Functionalized Materials 194
7.5 Embedded Single-Atom Catalysts 198
7.6 Summary and Conclusions 202 References 203
8 Heterogeneously Catalyzed CO2 Hydrogenation to Alcohols 207 Nat Phongprueksathat and Atsushi Urakawa
8.1 Introduction 207
8.2 CO2 Hydrogenation to Methanol – Past to Present 207
8.2.1 Syngas to Methanol 207
8.2.2 CO2 to Methanol 208
8.2.3 Thermodynamic Consideration – Chemical and Phase Equilibria 212
8.2.4 Catalyst Developments 215
8.2.5 Active Sites and Reaction Mechanisms: The Case of Cu/ZnO Catalysts 217
8.2.6 Beyond Industrial Cu/ZnO/Al2O3 Catalysts 223
8.3 CO2 Hydrogenation to Ethanol and Higher Alcohols – Past to Present 226
8.3.1 Background 226
8.3.2 Catalysts, Active Sites, and Reaction Mechanisms 227
8.3.2.1 Modified-Methanol Synthesis Catalyst 227
8.3.2.2 Modified Fischer–Tropsch Catalysts 230
8.3.2.3 Rhodium-Based Catalysts 231
8.3.2.4 Modified Molybdenum-Based Catalysts 232
8.4 Summary 232
References 233
9 Homogeneous Electrocatalytic CO2 Hydrogenation 237
Cody R. Carr and Louise A. Berben
9.1 CO2 Reduction to C H Bond-Containing Compounds: Formate or Formic Acid 237
9.1.1 Survey of Catalysts 238
9.1.1.1 Group 9 Metal Complexes 238
9.1.1.2 Group 8 Metal Complexes 241
9.1.1.3 Nickel Complexes 244
9.1.1.4 Iron and Iron/Molybdenum Clusters 246
9.1.2 Hydride Transfer Mechanisms in CO2 Reduction to Formate 247
9.1.2.1 Terminal Hydrides 247
9.1.2.2 Bridging Hydrides 248
9.1.3 Kinetic Factors in Catalyst Design 249
9.1.3.1 Roles of Metal–Ligand Cooperation 249
9.1.3.2 Roles of Multiple Metal–Metal Bonds 250
9.1.4 Thermochemical Considerations in Catalyst Design 253
9.1.4.1 Selectivity for Formate over H2 as a Function of Hydricity 254
9.1.4.2 Solvent Dependence of Hydricity 255
9.2 Prospects in Electrocatalysis: CO2 Reduction Beyond Formation of One C H Bond 255
References 257
10 Recent Advances in Homogeneous Catalysts for Hydrogen Production from Formic Acid and Methanol 259
Naoya Onishi and Yuichiro Himeda
10.1 Introduction 259
10.2 Formic Acid Dehydrogenation 260
10.2.1 Organic Solvent Systems 260
10.2.1.1 Ru 260
10.2.1.2 Ir 266
10.2.1.3 Fe 268
10.2.2 Aqueous Solution Systems 270
10.2.2.1 Ru 270
10.2.2.2 Ir 272
10.3 Aqueous-phase Methanol Dehydrogenation 275
10.3.1.1 Ir 279
10.3.1.2 Non-precious Metals 279
10.4 Conclusion 281 References 282
Index 285
Preface
Carbon dioxide is widely considered to be primarily responsible for global climatic changes. Presently, scientists are facing enormous challenges in mitigating the global CO2 emissions. Significant progress has recently been achieved in the research topic of the catalysis of CO2 hydrogenation, as one of the most important subjects in chemistry. In addition, the paradigm shift from fossil fuels to low‐carbon renewable energy (solar photovoltaics and wind) in recent years will allow for the competition between the CO2 emission by energy consumption and its fixation by CO2 conversion. In future, advancement in the fields of carbon capture and utilization is expected.
I would like to thank all the authors, who are all acknowledged as world expert in their area of CO2 hydrogenation, for their enthusiastic efforts to present recent advances in CO2 hydrogenation. Their state‐of‐the‐art research gives exceptionally beneficial information to the researchers, teachers, and students who are interested in the research field of CO2 hydrogenation. I anticipate that their contributions will stimulate further study in CO2 utilization as well as CO2 hydrogenation. I would also like to thank the Wiley‐VCH team for their continuous support. Finally, I deeply appreciate the members of my research group for their valuable assistance, especially Dr. Ryoichi Kanega for the cover design, and Dr. Hide Kambayashi for data survey.
In the spring and summer of 2020, the world has been hit by the COVID‐19 pandemic. Despite these difficult times, I am delighted that this book could be completed.
July 2020 Yuichiro Himeda
National Institute of Advanced Industrial Science and Technology, Global Zero Emission Research Center, Tsukuba, Japan
Introduction
Yuichiro Himeda1 and Matthias Beller2
1National Institute of Advanced Industrial Science and Technology, Global Zero Emission Research Center, AIST Tsukuba West, 16-1 Onogawa, Tsukuba, Ibaraki, 305-8569, Japan
2Leibniz-Institut für Katalyse, Applied Homogeneous Catalysis, Albert-Einstein Straße 29a, 18059, Rostock, Germany
Of the final products of the combustion of carbon-based fossil fuels, carbon dioxide (CO2) has the highest oxidation state and is known as the major cause of global warming. Annual CO2 emissions from anthropogenic activity in 2018 were approximately 33.1 Gton, an increase of 1.7% compared with 2017 [1]. Since the Industrial Revolution, two trillion tons of CO2 have accumulated in the atmosphere, and the current atmospheric concentration of CO2 has reached an unprecedented level of over 400 ppm (Figure 1.1) [2]. The anthropogenic emission of CO2 is associated with energy consumption, i.e. the combustion of carbon-based fossil fuels, which currently account for around 85% of the world’s energy.
According to the Paris Agreement of the United Nations, an overall limit on total cumulative CO2 emissions is crucial for our future development [3, 4]. According to the 2 °C scenario, further cumulative emissions should be limited to below one trillion ton of CO2 The spread of renewable energy (35%), advances in energy conservation (40%), and carbon capture and sequestration (CCS) technologies (14%) are sure to contribute to addressing the problem (Figure 1.2) [3]. However, it is clear that these methods will not completely solve the issues arising from the vast quantities of emitted CO2. In 2017, the International Energy Agency (IEA) presented the Energy Technology Perspectives (Beyond 2 °C Scenario: B2DS), which placed a much greater emphasis on the role of CO2 utilization for reducing emissions [3]. Indeed, in the next decade, we will still rely on carbon-based products for fuels, polymers, commodity chemicals, cosmetics, detergents, and fabrics in modern life. If these chemicals were to be derived from CO2 instead of fossil oils, a sustainable carbon cycle will be possible.
1.1 Direct Use of CO2
Apart from chemical applications, already today, CO2 is used directly in enhanced oil recovery (EOR), beverage carbonation, food processing (e.g. coffee decaffeination and
Figure 1.1 Atmospheric CO2 concentration at Mauna Loa Observatory. Source: Data from National Oceanic and Atmospheric Administration, Global Monitoring Laboratory [2].
Figure 1.2 IEA 2 °C Scenario (2DS) in Energy Technology Perspectives 2017. Source: Data from Market-driven future potential of Bio-CC(U)S [3].
drinking water abstraction), welding, as a cleaning agent for textiles, and as a solvent in the electronics industry [5]. These approaches are commercially viable. In particular, 70–80 Mton of CO2 is consumed for EOR in the oil sector. Although such direct utilization of CO2 addresses a significant amount of CO2 emissions, these topics are beyond the scope of this book.
1.2 Chemicals from CO2 as a Feedstock
CO2 has been recognized as an inexpensive and abundant industrial C1 carbon source. The various chemicals that can be produced by CO2 conversion are shown in Table 1.1 [6]. The largest chemical use of CO2 is in the production of urea from ammonia. However, since a
Table 1.1 Chemicals produced commercially from CO2
Chemical
Anthropogenic CO2 emissions (2018)
Scale of production/ton
33 100 000 000
Urea [7] 181 000 000
Diphenyl carbonate (Asahi Kasei Process) [8]
1 070 000
Salicylic acid 90 000
Cyclic carbonate
Polypropylene carbonate
80 000
76 000
Acetylsalicylic acid 16 000
Methanol (CRI process) [9] 4000
Source: From Omae [6]. © 2012 Elsevier.
huge amount of CO2 is emitted during methane steam reforming to supply H2, urea production does not contribute to carbon sequestration at present.
The catalytic copolymerization of CO2 with epoxides, which provides a thermodynamic driving force due to the strained three-membered ring, is the most prominent example of the synthesis of CO2-based polymers without formal reduction of the carbon oxidation state. Another example, the manufacture of diphenyl carbonate from ethylene oxide, bisphenol A, and CO2 instead of phosgene was developed beginning in 1977 by Asahi Kasei Chemical Corporation to address environmental and safety issues. The first commercial facility started operation in 2002 [8]. This process produces high-quality polycarbonate and high-purity monoethylene glycol in high yields without waste or wastewater. In addition, the phosgene-free process emits approximately 2.32 ton/tonPC less CO2 than the phosgene process according to life-cycle assessment (LCA). Diphenyl carbonate has a large market (3.6 Mton in 2016) for use in automotive parts and accessories, glazing, and medical devices. The phosgene-free technology has already been licensed to Taiwan, South Korea, Saudi Arabia, China, and Russia.
Since 2011, in Iceland, carbon recycling international (CRI) operated the first commercial plant for methanol production from CO2 via syngas by the reverse water-gas shift (rWGS) reaction (George Olah Renewable Methanol Plant) [9]. At present, more than five million liters of methanol per year is produced using low-cost electricity and high-concentration CO2 in the flue gas from an adjacent geothermal power plant. It should be noted that this technology is at present only viable in Iceland; however, if there is a surplus of green electricity in the future from an excess of renewable energy, then this process will be attractive at other places, too.
Notably, the amount of CO2 utilized by all these approaches, including urea and carbonate production, is very small compared with the magnitude of anthropogenic emissions. Therefore, CO2 conversion into chemicals is unlikely to significantly reduce emissions. Comparatively, it should be noted that fuels are produced and consumed on a much larger scale than these chemicals.
1.3
Application and Market Studies of CO2 Hydrogenation Products
Hydrogenation of CO2 could be an efficient option for developing more environmentfriendly products as alternatives to fossil-based ones. In terms of practicality, the distribution infrastructure of carbon-based chemicals is well established. However, their manufacturing is currently several times more expensive than their conventionally produced counterparts, mainly due to the costs associated with H2 production. Some of the key features of CO2 hydrogenation products and conventional fuels are given in Table 1.2.
1.3.1 Formic Acid/Formate
Formic acid is the first carboxylic acid and is naturally occurring produced by ants, bees, and some plants. In 2016, the global production of formic acid was 1.02 Mton [10]. The general production process of formic acid involves the formal carbonylation of water in a two-step synthesis via methyl formate. Formic acid and its salts (formate) are valuable chemical products used for silage and animal feed (27%), leather and tanning (22%), pharmaceuticals and food chemicals (14%), textile (9%), natural rubber (7%), and drilling fluids (4%) [11]. Recently, formic acid has been recognized as a promising liquid organic H2 carrier (LOHC) because of its low toxicity, low combustibility, stability, environmental friendliness, and 4.4 wt% (53 g l−1) H2 content [12–14]. In addition, compressed hydrogen gas can be supplied only by heating of formic acid using catalysts as a chemical compressor [15]. Therefore, advances in the efficient production of formic acid/formates may eventually lead to their large-scale use as LOHCs (see Chapter 10).
1.3.2 Methanol
Methanol, the industrial production of which is mainly from syngas, is in high global demand as a fuel and bulk chemical (Figure 1.3) [17]. One ton of methanol produced by the
Table 1.2 Characteristics of various energy vectors.
Compound
(GJ m−3)
price per energy (US$/GJ)
point/ melting point (°C)
point/ flash point (°C) Vapor pressure at 25 °C (kPa)
Methanol15.815 64.55/–97.68470/15 (open)16.9
Formic acid6.3100 100.56/8.27520/59 (open)43.1
Natural gas (CH4) 8.1 (20 MPa)2 –161/–183537/–188147 (15 °C)
Gasoline34.530 17–220/≤–40300/≤–4350–93 (37.8 °C) Diesel oil36.323 140–400/–29 to –18 250/40–70 ≤0.35 (37.8 °C)
Hydrogen5.1 (70 MPa)120 –252.87/–259.14500–571/—1.65 × 105
Figure 1.3 Global methanol demand in 2018. Source: Data from Global methanol demand (Methanol Institute) [16].
established process consumes 37.5 GJ of natural gas and emits 1.49 ton of CO2 [18]. In 2018, the global production of methanol was approximately 91.7 Mton, and since 2015, its production has grown by approximately 16% [16]. Approximately 26% and 8% of the methanol produced worldwide is consumed to produce formaldehyde and acetic acid, respectively, as the conventional demands. Methanol can be used as a fuel for internal combustion engines and fuel cells because it has a comparably high-octane number of 113 and a density approximately half that of gasoline. In addition, methanol can be transformed into gasoline through the methanol-to-gasoline (MTG) process developed by Mobil in the 1970s [19]. Another growing market for methanol is the production of light olefins (i.e. ethylene (152 Mton yr−1) and propylene (103 Mton yr−1) in 2017), [20] which are monomer feedstocks for polyethylene and polypropylene as basic products of the plastics industry [21].
The concept of a so-called methanol economy was independently proposed by Olah and Asinger due to the chemical’s promising characteristics for use as an energy vector and chemical feedstock [22–24]. Therefore, the production of methanol by CO2 conversion is regarded as an attractive and potentially profitable route for CO2 utilization.
1.3.3 Methanation
CO2 methanation, also known as the Sabatier process, affords methane by the exothermic reaction of CO2 with H2. The commercial methanation of CO2 is performed at 300–550 °C and above 5 bar. Most CO2 methanation processes are considered to be a linear combination of rWGS and CO methanation. The process is expected to be a power-to-gas concept for converting renewable electrical energy into methane as chemical energy. In other words, the main goal of methanation is the intermediate storage of renewable electricity in methane as an energy carrier. Since fossil-based natural gas is a common fuel, there would be easy access to existing infrastructure. Due to the significant interest in CO2 methanation, the first pilot plant capable of producing 0.5 Nm3 h−1 of synthetic natural gas was built in Japan [25]. In terms of commercial
installations, Audi has an operational CO2 methanation facility (max. 325 Nm3 h−1) using renewable H2 (max. 1300 Nm3 h−1) from electrolysis (max. 6.0 MW) in Germany [26, 27].
1.3.4 Energy Storage
The two most growing renewable energy sources, solar and wind, are intermittent and thus provide highly fluctuating electrical energy. In addition, the region’s best suited areas for the production of renewable energy are often far from consumption areas, i.e. cities. These cause the two key problems of storage and transport. Certainly, electrical energy is an effective way to transfer energy within 1000 km and can be stored in batteries. However, lowcost solutions for the large-scale storage and long-range transport of electrical energy must be developed to improve energy security and balance energy prices.
The transformation of excess renewable energy into chemical energy by converting CO2 is one promising option. CO2-based compounds, such as methane, methanol and formic acid, can store energy as gas or liquids with comparably high-energy densities. Especially liquids can be easily transported and release energy as H2 or electricity through oxidation and fuel cells when there is a greater demand. In other words, CO2 can act as an energy vector between electrical and chemical energy. Recently, the electroreduction of CO2 to chemical fuels has been receiving increasing attention because it allows for the direct use of renewable electricity without conversion to high-cost H2 by water electrolysis (see Chapter 9). Much more CO2 is in demand as a feedstock for fuels than for chemicals and mineralization. In addition, related photo-catalytic processes gain more and more interest.
1.4 Supply of Materials
The CO2 hydrogenation approach requires H2, CO2, and an energy supply. In particular, how much the energy-intensive hydrogenation process contributes to mitigating CO2 emissions will be dominated by the H2 source. Obviously, H2 must be produced with the help of a renewable electricity source such as water electrolysis and not from fossil fuels.
1.4.1 CO2 Supply
Capture, purification, and transport of CO2 are essential for its utilization. Table 1.3 lists several large CO2 sources with their typical amounts and concentrations of CO2 as well as impurities. In present CO2 merchant market (approximately 230 Mton, US$7.7 billion), the fermentation process (i.e. bioethanol production) and ammonia production, which provide close to 100% CO2, are predominantly CO2 sources [5, 30]. The CO2 generated from ethanol fermentation commercially supplies roughly 270 000 ton of CO2 annually for EOR through pipeline from Kansas to Texas [28]. On the other hand, the production of electricity and heat accounts for 41% of global CO2 emissions (Figure 1.4), and the transport and industrial sectors account for an additional 25% and 19%, respectively [32]. However, suitable sources of CO2 for use in chemical transformation are limited. The gases contain various impurities, the separation of which is both energy and cost intensive. To supply CO2 of an
Table 1.3 Concentration of CO2 and contaminants from various sources.
Source
Ethanol fermentation [28, 30] 50 99EtOH, MeOH, H2O, H2S
Anhydrous ammonia 30 >95NH3, CO, H2, H2O
Natural deposits 13 90–100N2, O2, He
Power plants 4287 10–15N2, H2O, SOx, NOx, CO
Steelmaking 266 18–20N2, SOx, NOx, O2
Cement production [31] 220 14–33SOx, NOx, O2
Atmosphere 3 200 000 0.04N2, O2, SOx, NOx
Source: Carbon Recycling International; Capturing and Utilizing CO2 from Ethanol: Adding Economic Value and Jobs to Rural Economies and Communities While Reducing Emissions (2017); and Greenhouse Gas Inventory Data [9, 28, 29].
1.4 CO2 emissions from fuel combustion. Source: Data from IEA, CO2 emissions from fuel combustion, 2020 [32].
appropriate quality for use in chemical conversion processes, capture and separation are required (Table 1.4) [33]. The most effective CO2 capture method as the current industrial standard is chemical absorption in an aqueous solution of an amine-based organic compound. However, the cost (35 US$/ton) and energy consumption (2.5 GJ ton−1) of amine capture must still be reduced to provide economically viable routes from carbon dioxide to fuels [34, 35].
Recently, the direct capture of CO2 from ambient air, called direct air capture (DAC), has received increasing attention [36]. One of the advantages of DAC is that it can be located anywhere, because it is unnecessary for CO2 transport. However, from both engineering and chemistry views, there remains much room for improvements to the sorbents and processes. Additionally, thorough techno-economic analyses of DAC processes are necessary [37].
Figure
Table 1.4
capture technologies.
Capture technologyTechnical principle
Chemical absorptionChemical reaction between CO2 and absorbent by a temperature swing.
Physical absorptionDissolution of CO2 into a liquid, the efficiency of which depends on the solubility of CO2 in the liquid.
Solid absorptionAbsorption into solid absorbents, which include porous materials impregnated with amines for low-temperature separation or other solid absorbents for high-temperature separation.
Physical adsorptionAdsorption onto porous solids such as zeolites by a pressure or temperature swing.
Membrane separation
Permeation through a membrane with selective permeability for different gas species.
Source: Based on Styring [33].
1.4.2 Energy and H2 Supply
Another consideration is the energy required to capture and convert CO2, which must certainly be derived from renewable sources (Figure 1.5) [38]. If this energy comes from fossil oils, much more CO2 will be emitted than separated. Fortunately, the renewables now account for over 25% of global power output (hydro: 16%, wind: 5%, PV: 2%), [1] and the costs of PV and wind power become even lower than that of fossil fuels (natural gas and coal) (Figure 1.6) [39]. Thus, electricity from renewable sources can be converted into H2 by water electrolysis, which can be performed on an industrial scale. Nevertheless, H2 produced by electrolysis systems (2.5–6 US$/kgH2) is at present more expensive than that from
CO2
Figure 1.5 Low-carbon electricity generation by source in 2017. Source: Data from explore energy data by category, indicator, country or region (IEA) [38].
Figure 1.6 Levelized cost of energy comparison: Renewable energy versus conventional generation. Source: Data from Lazard.com, Lazard’s levelized cost of energy analysis [39].
current industrial production based on conventional fossil sources, like natural gas reforming and coal gasification (<1 US$/kg H2) [40, 41].
1.5 Political Aspect: Tax
The future prospects for CO2 utilization on large scale will mainly depend on policy support. The carbon tax, a fee imposed on the burning of carbon-based fuels (coal, oil, gasoline, and natural gas), is one policy for reducing the use of fossil fuels. To reduce CO2 emissions, as many as 29 countries have implemented carbon taxes as of 2019. Tax rates, including energy taxes, differed according to use and fuel type in 2017. For example, high tax rates are imposed on gasoline in every country, from yi SOLL in the United States to yy i SOLL i IST - in the Netherlands [42]. On the other hand, there are also significant differences in tax rates for the industrial sector depending on the country (Figure 1.7). An increasing of the tax rates of carbon-based fuels seems to be necessary to motivate our societies to switch to clean energy.
1.6 Conclusion and Perspectives
CO2 utilization will play a crucial role in achieving the internationally agreed climate and energy goals. In particular, the conversion of CO2 to fuels and chemicals will be of significant importance. However, these technologies are still in their infancy, and the following issues require consideration and technological improvements:
(i) Supply of H2 and power from renewables.
(ii) Cost reduction, mainly for the supply of low-carbon H2
(iii) Political support to shift from a fossil-based to CO2-based economy.
(iv) Highly efficient catalysts to minimize energy usage for the valorization of CO2
oil (industrial)
(industrial)
gas (industrial)
Figure 1.7 Carbon tax rate per ton of emitted CO2. Source: Data from the Ministry of the Environment of Japan [42].
In addition, critical evaluation from an LCA perspective will be necessary. In any case, the spread and expansion of renewable energy are essential, which, in turn, require energy storage and transport. CO2-based fuels produced by CO2 hydrogenation will contribute to these needs. Therefore, further research into CO2 hydrogenation is necessary from a standpoint of both fundamental science and application. In this respect, we believe the focus of this book on the hydrogenation/electroreduction of CO2 to formic acid and methanol as chemicals and fuels using homogeneous and heterogeneous catalysts will be of interest to many scientists. It will serve as motivation for studying the development of catalysts for the hydrogenation of CO2 as a fuel and bulk chemical. In addition, the challenge of activating unreactive CO2 will stimulate the curiosity and creativity of chemists.
References
1 IEA. (2019). Global energy & CO2 status report 2019. The latest trends in energy and emissions in 2018. https://www.iea.org/reports/global-energy-co2-status-report-2019/ emissions#abstract (accessed 8 September 2020).
2 National Oceanic and Atmospheric Administration. Global Monitoring Laboratory. https:// www.esrl.noaa.gov/gmd/ccgg/trends/data.html (accessed 5 June 2020).
3 Kristin Onarheim, Antti Arasto (2017). Market-driven future potential of Bio-CC(U)S, http://task41project5.ieabioenergy.com/publications/market-driven-future-potential-bioccus/ (accessed 5 June 2020).
4 Rogelj, J., Huppmann, D., Krey, V. et al. (2019). Nature 573 (7774): 357–363.
5 IEA. (2019) Putting CO2 to use – Creating value from emissions. https://www.iea.org/ reports/putting-co2-to-use (accessed 8 September 2020).
6 Omae, I. (2012). Coord. Chem. Rev. 256 (13): 1384–1405.
7 Short-Term Fertilizer Outlook 2019–2020. (2019). https://www.ifastat.org/market-outlooks (accessed 16 June 2020).
8 Fukuoka, S., Fukawa, I., Adachi, T. et al. (2019). Org. Process Res. Dev. 23 (2): 145–169.
9 Carbon Recycling International. https://www.carbonrecycling.is/ (accessed 17 June 2020).
10 2018–2023 Global Formic Acid Consumption Market Report. (2018). https://www. marketinsightsreports.com/reports/1120988312/2018-2023-global-formic-acidconsumption-market-report (accessed 12 June 2020).
11 Hietala, J., Vuori, A., Johnsson, P. et al. (2016). Formic Acid. In: Ullmann’s Encyclopedia of Industrial Chemistry, 1–22. Wiley-VCH.
12 Perez-Fortes, M., Schoneberger, J.C., Boulamanti, A. et al. (2016). Int. J. Hydrogen Energy 41 (37): 16444–16462.
13 Sordakis, K., Tang, C.H., Vogt, L.K. et al. (2018). Chem. Rev. 118 (2): 372–433.
14 Wang, W.-H., Himeda, Y., Muckerman, J.T. et al. (2015). Chem. Rev. 115 (23): 12936–12973.
15 Müller, K., Brooks, K., and Autrey, T. (2018). Energy Fuels 32 (9): 10008–10015.
16 Global methanol demand (Methanol Institute). https://www.methanol.org/methanolprice-supply-demand/ (accessed 15 June 2020).
17 Dalena, F., Senatore, A., Marino, A. et al. (2018). Chapter 1. Methanol production and applications: an overview. In: Methanol (eds. A. Basile and F. Dalena), 3–28. Elsevier.
18 DECHEMA. (2017). Low carbon energy and feedstock for the European chemical industry. https://dechema.de/Low_carbon_chemical_industry.html (accessed 8 September 2020).
19 Chang, C.D. and Silvestri, A.J. (1977). J. Catal. 47 (2): 249–259.
20 Production capacity, production and demand on petrochemical products. (2019). Ministry of Economy, Trade and Industry of Japan. https://www.meti.go.jp/policy/mono_info_ service/mono/chemistry/downloadfiles/04_2019syouhinbetudeta.pdf (accessed 16 June 2020).
21 Market Analytics: Methanol and Derivatives (2019). https://www.nexantsubscriptions. com/reports/market-analytics-methanol-and-derivatives-2019 (accessed 13 June 2020).
22 Olah, G.A. (2005). Angew. Chem. Int. Ed. 44 (18): 2636–2639.
23 Asinger, F. (1986). Methanol—Chemie- und Eneigierohstoff. Berlin Heidelberg: Springer-Verlag.
24 Olah, G.A., Goeppert, A., and Prakash, G.K.S. (2018). Beyond Oil and Gas: The Methanol Economy, 3e. Wiley-VCH.
25 Bailera, M., Lisbona, P., Romeo, L.M. et al. (2017). Renewable Sustainable Energy Rev. 69: 292–312.
26 The first industrial PtG plant–Audi e-gas as driver for the energy turnaround. (2014). http://www.cedec.com/files/default/8-2014-05-27-cedec-gas-day-reinhard-otten-audi-ag. pdf (accessed 10 June 2020).
27 Jarvis, S.M. and Samsatli, S. (2018). Renewable Sustainable Energy Rev. 85: 46–68.
28 Capturing and Utilizing CO2 from Ethanol: Adding Economic Value and Jobs to Rural Economies and Communities While Reducing Emissions (2017). http://www.kgs.ku.edu/ PRS/ICKan/2018/March/WhitePaper_EthanolCO2Capture_Dec2017_Final2.pdf (accessed 10 June 2020).
29 Greenhouse Gas Inventory Data. https://di.unfccc.int/detailed_data_by_party (accessed 12 June 2020).
30 Xu, Y., Isom, L., and Hanna, M.A. (2010). Bioresour. Technol. 101 (10): 3311–3319.
31 Bosoaga, A., Masek, O., and Oakey, J.E. (2009). Energy Proc. 1 (1): 133–140.
32 CO2 Emissions from Fuel Combustion (2019). https://www.iea.org/subscribe-to-dataservices/co2-emissions-statistics (accessed 12 June 2020).
33 Styring, P. (2015). Carbon dioxide capture agents and processes (Chapter 2). In: Carbon Dioxide Utilisation: Closing the Carbon Cycle (eds. P. Styring, E.A. Quadrelli and K. Armstrong), 19–32. Elsevier.
34 Shell Cansolv Deploying CCS Worldwide. (2013). https://ieaghg.org/docs/General_Docs/ PCCC2/Secured%20pdfs/3_PCCC2-Just-September2013.pdf (accessed 16 June 2020).
35 Survey on the Carbon Capture and Storage process (2017). Center for Low Carbon Society Strategy, Japan Science and Technology Agency. https://www.jst.go.jp/lcs/pdf/ fy2016-pp-06.pdf (accessed 16 June 2020).
36 Sanz-Perez, E.S., Murdock, C.R., Didas, S.A. et al. (2016). Chem. Rev. 116 (19): 11840–11876.
37 Direct Air Capture (2020). https://www.iea.org/reports/direct-air-capture (accessed 18 September 2020).
38 Explore energy data by category, indicator, country or region (IEA). https://www.iea.org/ data-and-statistics?country=WORLD&fuel=Energy%20supply&indicator=Low-carbon%20 electricity%20generation%20by%20source (accessed 13 June 2020).
39 Lazard’s Levelized Cost of Energy Analysis (2018). https://www.lazard.com/ media/450784/lazards-levelized-cost-of-energy-version-120-vfinal.pdf (accessed 12 June 2020).
40 Alvarez, A., Bansode, A., Urakawa, A. et al. (2017). Chem. Rev. 117 (14): 9804–9838.
41 IEA. (2019). The Future of Hydrogen – Seizing today’s opportunities. https://www.iea.org/ reports/the-future-of-hydrogen (accessed 7 September 2020).
42 Status of carbon taxes in various countries (2017). https://www.env.go.jp/policy/tax/ misc_jokyo/attach/intro_situation.pdf (accessed 12 June 2020).
Homogeneously Catalyzed CO2 Hydrogenation to Formic Acid/Formate by Using Precious Metal Catalysts
Wan-Hui Wang1,2, Xiujuan Feng1 and Ming Bao1,2
1Dalian University of Technology, State Key Laboratory of Fine Chemicals, 2 Linggong Road, Ganjingzi District, Dalian, 116023, China
2Dalian University of Technology, School of Chemical Engineering, 2 Dagong Road, Liaodongwan New District, Panjin, 124221, China
2.1 Introduction
CO2 hydrogenation to formic acid (FA)/formate has a long history, and heterogeneous catalytic transformation even dates back to 1914 [1]. Inoue et al. in 1976 reported CO2 hydrogenation by using Ru-, Rh-, and Ir-based complexes and has paved the way for the homogeneous catalytic hydrogenation of CO2 [2]. Considering that CO2 emission has increased significantly and causes severe environmental problems, CO2 fixation and utilization have attracted attention in the last three decades [3], and CO2 hydrogenation to FA or formate has been revived. Considerable efforts have been devoted to this transformation, and a remarkable progress has been achieved.
Developing highly efficient complexes requires modulation of the electronic and steric environments of the metal center. Adjusting the ligands and/or metals generates effective complexes for the catalysis of certain reactions. Phosphorous ligands had been widely used to develop catalysts during the initial CO2 hydrogenation research. C,N- and N,N-chelated ligands, N-heterocyclic carbene (NHC) ligands, and pincer ligands have also been recently developed. Among these ligands, functionalized N,N-ligands and non-innocent pincer ligands effectively promote the catalytic activity of the complexes and usually contain tautomerizable moieties, including pyridinol, imidazole or imidazoline, amide, and alkyl pyridine as shown in Scheme 2.1. Tautomerizable ligands can transfer electrons coupled with proton migration. The acidity of the active protons increases when these ligands coordinate with the metal center by donating electrons. At a certain pH, the active proton can be abstracted by a base. Therefore, the complexes, especially those bearing OH or NH groups, show proton-responsive property. Deprotonation also strengthens electron-donating ability of the ligands and facilitates electron transfer from ligand to metal. Additional functions, such as acting as a pendant base, are gained in some cases. These unique properties
Scheme 2.1 Tautomerizable ligands for functional complexes.
of tautomerizable ligands endow complexes with versatility for catalytic hydrogenation. In this chapter, the important role of ligands, especially tautomerizable ligands, is presented. CO2 hydrogenation is commonly promoted using inorganic or organic base, and further acidification is required to obtain pure FA. The direct hydrogenation of CO2 to FA under base-free conditions has recently been achieved albeit with a relatively low activity. Process design and development enable the production of pure FA and the recycling of homogeneous catalysts. The important progresses in catalyst activity, reaction mechanism, and process development are discussed herein.
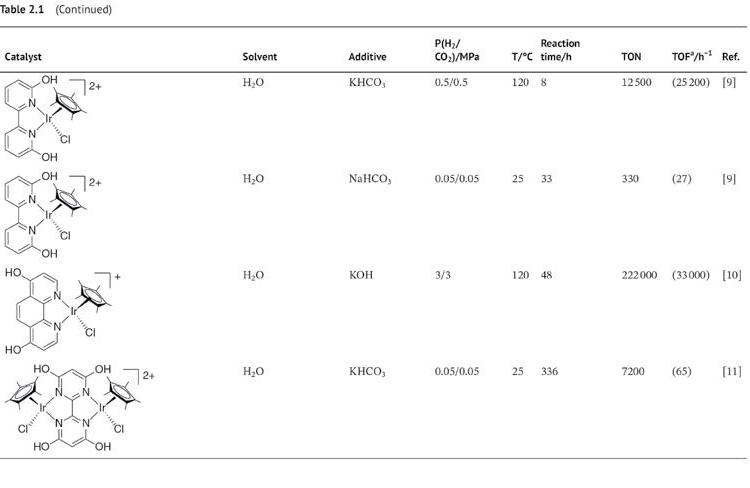
CO2 hydrogenation is a popular topic well reviewed by Leitner, Jessop, Himeda, Beller, and Ding et al. [4]. Although remarkable progress in CO2 reduction to FA/formate using hydrogen sources, such as alcohols, boranes, and silanes, has been reported [5], we focus on the recent advances in the homogeneous hydrogenation of CO2 to FA/formate catalyzed by noble metal–based complexes using molecular hydrogen. In this chapter, we briefly introduce previous studies and mainly focus on the progress in last decades. Table 2.1 lists the selected catalytic systems for CO2 hydrogenation with precious metals. Notably, CO2 and bicarbonate can serve as hydrogenation substrates alone or in combination with others. State-of-the-art homogeneous CO2 hydrogenation to FA/formate catalyzed by precious metals is introduced in this chapter.
2.2 Ir Complexes
Among the commonly used precious metal–based catalysts, Ir catalysts exhibit high activity for CO2 hydrogenation. Ir complexes with functionalized ligands show remarkable catalytic activity. This section covers the development of different types of ligands, especially tautomerizable ligands (i.e. pyridinol, diazole, amide, and pincer ligands) for highly efficient Ir complexes.
2.2.1 Ir Complexes with N,N-ligands
2.2.1.1 Tautomerizable N,N-ligands with OH Groups
Himeda et al. reported half-sandwich Ir complexes with N,N-ligand-bearing OH functional groups, including 4DHBP (4,4′-dihydroxy-2,2′-bipyridine) [47] and DHPT (4,7-dihydroxy1,10-phenanthroline) [48] for CO2 hydrogenation. These complexes exhibit high activity