DevelopmentsinClayScience ClayMineralsand SyntheticAnalogous asEmulsifiersof PickeringEmulsions
Volume10
Editedby FernandoWypych
DepartmentofChemistry,FederalUniversityofParana ´ , CentroPolitecnico,Curitiba,PR,Brazil
RiltonAlvesdeFreitas
DepartmentofChemistry,FederalUniversityofParana ´ , CentroPolitecnico,Curitiba,PR,Brazil
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,Oxford,OX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierLtd.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangements withorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency, canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribedherein.In usingsuchinformationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyof others,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproducts liability,negligenceorotherwise,orfromanyuseoroperationofanymethods,products, instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-91858-9
ISSN:1572-4352
ForinformationonallElsevierPublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: CandiceJanco
AcquisitionsEditor: JennetteMcClain
EditorialProjectManager: MariaElaineDesamero
ProductionProjectManager: SruthiSatheesh
CoverDesigner: MarkRogers
TypesetbySTRAIVE,India
Part1 Introduction
1.Clayminerals:Classification,structure,and properties3
FernandoWypychandRiltonAlvesdeFreitas
1.1Basicconcepts,classification,andnomenclature3
1.1.1Aluminosilicate3
1.1.2Basalsurface3
1.1.3Basalreflection4
1.1.4Bentonite4
1.1.5Brunauer–Emmett–Teller(BET)4
1.1.6Cationexchange5
1.1.7Clay5
1.1.8Clayminerals5
1.1.9Interlayerdistance5
1.1.10Layer5
1.1.11Phyllosilicate5 1.1.12Kaolin6
1.1.13Serpentine-kaolin6
1.1.14Smectite6
1.1.15Talc-pyrophyllite7
1.2Claymineralswithneutralstructures8
1.2.1Claymineralsofthekaolin/serpentinegroup8
1.2.2Claymineralsofthetalc/pyrophyllitegroup12
1.3Claymineralswithnegativelychargedlayers13
1.3.1Claymineralsofthesmectitegroup14
1.3.2Claymineralsofthemicagroup15
1.3.3Claymineralsofthevermiculitegroup16
1.3.4Claymineralsofthechloritegroup17
1.3.5Claymineralsofthesepiolite/palygorskitegroup17
1.4Physicalandchemicalmodificationsofclayminerals18
1.5Concludingremarks30 Acknowledgments31 References31
2.Fundamentalsofemulsionformationandstability37
CarolineE.P.SilvaandWatsonLoh
2.1Definitions37
2.2Thermodynamicsofemulsification40
2.3Kineticstability(metastability)ofemulsions41
2.4Instabilityphenomenainemulsions42
2.4.1Sedimentationandcreaming:Gravitation separation43
2.4.2Flocculation44
2.4.3Ostwaldripening44
2.4.4Coalescence45
2.5Preparationandstabilizationofemulsions46
2.5.1Disruptingdropletsbymechanicalenergy (comminutionmethods)46
2.5.2Low-energyemulsificationmethods50
2.5.3Protectingtheoil–waterinterface51
2.5.4Electrostaticstabilization52
2.5.5Stericstabilization52
2.5.6Choosingtheemulsifieraccordingtothetypeof emulsion53
2.5.7Rheologymodifiers55 References56
3.Pickeringemulsions:Historyandfundamentals61
BaptisteRobin,FlorenceAgnely,NicolasTsapis,and NicolasHuang
3.1AbriefhistoryofPickeringemulsions61
3.1.11990s:Theoriginsofsolid-stabilizedemulsions61
3.1.2Fromthe1910stothe1980s:80yearsofmodest advances62
3.1.3Fromthe1990s:TheboomofPickering emulsions63
3.2FormationandmaincharacteristicsofPickering emulsions68
3.2.1Stabilityofemulsions68
3.2.2Dropletsize75
3.2.3Emulsiontype78
3.3Conclusion79 References79
4.Experimentalmultiscaleapproachandinstrumental techniquesforthecharacterizationofPickering emulsions87
VeroniqueSchmittandValerieRavaine
4.1Introduction87
4.2Emulsioncharacterizationatthemacroscopicand mesoscopicandmicroscopiclevels88
4.2.1Abilityofparticlestostabilizeanemulsion88
4.2.2Typeofobtainedemulsions89
4.2.3Creamingorsedimentationanddispersionstateofthe emulsion90
4.2.4Dropsizedistribution95
4.2.5Limitedcoalescence95
4.3Particlesattheinterface98
4.3.1Characterizationofindividualparticlesattheliquid interface99
4.3.2Organizationofparticlesattheliquidinterface101
4.3.3Organizationofparticlesatamodelliquid interface104
4.3.4Mechanicalpropertiesofparticle-ladenmodelliquid interface105
4.3.5Mechanicalpropertiesofparticle-ladendropsurfaces inemulsions111
4.4Conclusion115 References115
Part2
Pickeringemulsionbasedonclayminerals
5.Physicalandchemicalpropertiesoflayeredclay mineralparticlesurfaces125
CliffT.Johnston,MarikaSantagata,and MohammadhasanSasar
5.1Introduction125
5.2Surface-activeclayminerals127
5.2.1Structuralconsiderations128
5.2.2Isomorphoussubstitution129
5.2.3Surfacestructures131
5.2.4Particlemorphologies134
5.3Surfacewettabilityofclayminerals138
5.3.1Watersorptionisotherms138
5.3.2Molecularmodelingofclay-waterinteractions139
5.3.3Surfaceswithvariablehydrophobic/hydrophilic characteristics141
5.4Surfacemodification142
5.4.1Siloxanesurfaceofkaoliniteandhalloysite142
5.4.2Aluminahydroxylsurfaceofkaoliniteandhalloysite (lumen)144
5.4.3Modificationofedgesite147
5.5Particle-particleinteractions148
5.5.1SignificancetoPickeringemulsions148
5.5.2Perspectivesonthefactorscontrollingthestructure andfabricofclaydispersions149
5.6Conclusions156 References159
6.Pickeringemulsionsandfoamsstabilizationbasedon clayminerals169 YongfengZhuandAiqinWang
6.1Introduction169
6.2Pickeringemulsionorfoamstabilizedwithclaymineral171
6.2.1Layeredchainstructure171
6.2.2Tubularstructure174
6.2.3Layerstructure177
6.3Stabilizationmannerofclaymineralinpickeringemulsions orfoams184
6.3.1Synergisticallystabilizedwithclaymineralandsmall molecular185
6.3.2Synergisticallystabilizedwithclaymineraland polymers187
6.3.3Synergisticallystabilizedwiththeclaymineraland otherparticles188
6.4EffectfactorsofPickeringemulsionorfoamsstabilizedwith claymineral190
6.4.1Ionstrength190
6.4.2Claymineralparticlesconcentration192
6.4.3Claymineralparticlesize193
6.4.4Claymineralshape194
6.4.5DispersionpH196
6.5Applicationoftheclaymineralstabilizedpickeringemulsion orfoam196
6.5.1Applicationinenhancedoilrecovery(EOR)196
6.5.2Applicationinpreparationofclay-basedpolymeric nanoparticles198
6.5.3Applicationinpreparationoftheporousmaterial205
6.5.4Applicationincatalysisreaction208
6.5.5Applicationinhistoricpreservation210
6.6Conclusionandoutlook212
6.6.1Theunclearstabilizationmechanism212
6.6.2Theuniquenessofclaymineralsisunrealized213 Acknowledgments213 Declarationofcompetinginterest213 References213
7.Pickeringemulsionsbasedonlayeredclayminerals withneutralstructures,scrolls,andnanotubes morphologies229 PriscilaGrittenSieben,FernandoWypych,and RiltonAlvesdeFreitas
7.1Claymineralswithneutralstructures229
7.1.1Kaolinite231
7.1.2Halloysite232
7.1.3Talc234
7.2Claymineralswithneutralstructuresappliedin emulsions236
7.3Emulsionscontainingnonionicclaymineralsappliedin biomedicalandpharmaceuticalareas243
7.4Dermatologyandcosmeticsapplications245
7.5Environmentalapplications247
7.6Conclusion247 References248
8.Pickeringemulsionsbasedoncation-exchanged layeredclayminerals253 RiltonAlvesdeFreitasandFernandoWypych
8.1Introduction253
8.2Smectitegroupofminerals257
8.2.1Laponite262
8.2.2Montmorillonite266
8.3Conclusions271 References272
9.Roleofsurfactantsandpolymersforclaymineralsas stabilizerofPickeringemulsion277
AnneAimable,Gise ` leLecomte-Nana,andCecilePagnoux
9.1Introduction277
9.2Interactionsbetweenclaymineralsandsurfactantor polymersforPickeringemulsification278
9.2.1Shortintroductiononmainlayeredclaymineralsand descriptionoftheirsurfaceproperties278
9.2.2Characterizationtechniquestoevaluateinteractions betweensurfactantsandpolymersandclaymineral surfaces285
9.3AnonexhaustiveoverviewofPickeringemulsionsstabilized byclaymineralswithsurfactantsandpolymers292
9.3.1SomeexamplesusingkaoliniteforPickering emulsions292
9.3.2HalloysiteasaPickeringstabilizerforenhancedoil recovery293
9.3.3Montmorillonite,akeymaterialforclaystabilized Pickeringemulsions295
9.3.4Laponite,amodelclayforaversatileuseinPickering emulsions297
9.3.5Pickeringclayemulsionswithbiopolymers299
9.4NewmaterialsderivedfromclaymineralsPickering emulsions300
9.4.1Emulsionpolymerizationforthesynthesisofclay polymernanocomposites(CPN)300
9.4.2Colloidosomesandliquidmarbles303
9.4.3PorousmaterialsderivedfromPickeringclay emulsions305
9.5Conclusion307 References307
Pickeringemulsionbasedonsyntheticlayered hydroxides
10.Layereddoublehydroxidesandhydroxidesalts: Structureandproperties317
FernandoWypychandRiltonAlvesdeFreitas
10.1Layeredcompounds:Basicconceptsand nomenclature317
10.2Layereddoublehydroxides324
10.2.1Layereddoublehydroxideswiththecomposition
[M2+ 1 xM3+ x (OH)2](An )x/n yH2O324
10.2.2Layereddoublehydroxideswiththecomposition [Li(Al(OH)3)2](An )1/n yH2O326
10.2.3Layereddoublehydroxideswiththecomposition M2+(Al(OH)3)nA2 yH2O327
10.2.4Layereddoublehydroxideswiththecomposition [M2+ 6 Al3+ 3 (OH)18][B+(H2O)6(X2 )2] 6H2O327
10.3Layeredhydroxidesalts330
10.3.1Layeredhydroxidessalts:Simonkolleite: Zn5(OH)8Cl2 H2O331
10.3.2Layeredhydroxidessalts:zinchydroxidenitrate dihydrate:Zn5(OH)8(NO3)2 2H2O331
10.3.3Otherlayeredhydroxidesalts332
10.3.4Layeredhydroxidessalts:sodium-gordaite: Zn4(OH)6(SO4)Cl Na(H2O)6 332
10.3.5Layeredhydroxidessalts:Osakaite/Namuwite family:Zn4(OH)6(SO4) nH2O333
10.4Methodsofsynthesis334
10.4.1CoprecipitationwithconstantandvariablepH334
10.4.2Reconstructionorstructuralmemoryeffect337
10.4.3Mechanochemicalapproach337
10.4.4Hydrolysisofsaltsandoxides337
10.4.5Exchangereactions338
10.4.6Diadochyreactions339
10.4.7Sol/gelmethod339
10.5Someapplicationsandfutureperspectives340 Acknowledgments341 References341
11.Pickeringemulsionsbasedonlayereddouble hydroxidesandmetalhydroxides351 VanessaPrevot,CedricGastaldi,andClaudeForano
11.1Introduction351
11.1.1LDH-andLHS-stabilizingparticlesusedfor Pickeringemulsions352
11.2Oil-in-wateremulsions355
11.2.1Water-in-oilemulsions360
11.2.2Water-in-wateremulsions363
11.3ApplicationsofPickeringemulsionstabilizedbyLDH/LSH particles368
11.3.1LDHpreparation368
11.3.2Catalysis369
11.3.3PorousLDHmaterials369
11.3.4Towardnanocompositecolloids369
11.4Conclusions371 References372 Index377
Contributors
FlorenceAgnely(61),Universite Paris-Saclay,CNRS,InstitutGalienParis-Saclay, Orsay,France
AnneAimable(227),UniversityofLimoges,CNRS,IRCER,UMR7315,Limoges, France
RiltonAlvesdeFreitas(3,229,253,317),DepartmentofChemistry,Federal UniversityofParana ´ ,CentroPolitecnico,Curitiba,PR,Brazil
ClaudeForano(351),Universite ClermontAuvergne,CNRS,ICCF,ClermontFerrand,France
CedricGastaldi(351),Universite ClermontAuvergne,CNRS,ICCF,ClermontFerrand,France
NicolasHuang(61),Universite Paris-Saclay,CNRS,InstitutGalienParis-Saclay, Orsay,France
CliffT.Johnston(125),DepartmentsofEarth,AtmosphericandPlanetarySciences andAgronomy,PurdueUniversity,WestLafayette,IN,UnitedStates
Gise ` leLecomte-Nana(277),UniversityofLimoges,CNRS,IRCER,UMR7315, Limoges,France
WatsonLoh(37),DepartmentofPhysicalChemistry,InstituteofChemistry, UniversityofCampinas,Campinas,Brazil
CecilePagnoux(277),UniversityofLimoges,CNRS,IRCER,UMR7315,Limoges, France
VanessaPrevot(351),Universite ClermontAuvergne,CNRS,ICCF,ClermontFerrand,France
ValerieRavaine(87),Univ.Bordeaux,CNRS,BordeauxINP,ISM,Talence,France
BaptisteRobin(61),Universite Paris-Saclay,CNRS,InstitutGalienParis-Saclay, Orsay,France
MarikaSantagata(125),LylesSchoolofCivilEngineering,PurdueUniversity,West Lafayette,IN,UnitedStates
MohammadhasanSasar(125),LylesSchoolofCivilEngineering,PurdueUniversity, WestLafayette,IN,UnitedStates
VeroniqueSchmitt(87),CentredeRecherchePaulPascal(CRPP),Univ.Bordeaux, CNRS,Pessac,France
PriscilaGrittenSieben(229),DepartmentofChemistry,FederalUniversityofParana ´ , CentroPolitecnico,Curitiba,PR,Brazil
CarolineE.P.Silva(37),DepartmentofPhysicalChemistry,InstituteofChemistry, UniversityofCampinas,Campinas,Brazil
NicolasTsapis(61),Universite Paris-Saclay,CNRS,InstitutGalienParis-Saclay, Orsay,France
AiqinWang(169),KeyLaboratoryofClayMineralAppliedResearchofGansu Province,CenterofEco-materialandGreenChemistry,LanzhouInstituteof ChemicalPhysics,ChineseAcademyofSciences,Lanzhou,P.R.China
FernandoWypych(3,229,253,317),DepartmentofChemistry,FederalUniversityof Parana ´ ,CentroPolitecnico,Curitiba,PR,Brazil
YongfengZhu(169),KeyLaboratoryofClayMineralAppliedResearchofGansu Province,CenterofEco-materialandGreenChemistry,LanzhouInstituteof ChemicalPhysics,ChineseAcademyofSciences,Lanzhou,P.R.China
Clayminerals:Classification, structure,andproperties
FernandoWypych andRiltonAlvesdeFreitas DepartmentofChemistry,FederalUniversityofParana´,CentroPolitecnico,Curitiba,PR,Brazil
1.1Basicconcepts,classification,andnomenclature
Sincethedefinitionsinclaysciencearestillnotunanimous,dueespeciallyto theinterdisciplinarynatureofthisfieldofresearch,sometermsusedthroughoutthetextwillbefirstdefinedfollowingtheterminologyasindicatedby BergayaandLagaly(2013).
1.1.1Aluminosilicate
Ingeneral,silicateswhichcontaintetrahedrallycoordinatedaluminumare calledaluminosilicatesincontrasttosilicatescontainingoctahedrallycoordinatedaluminumforwhichthetermaluminumsilicateisused.Phyllosilicates aregenerallyconsideredaluminosilicates,becausemostphyllosilicatesdo haveAlsubstitutionforSi,butnotalldo(andthosemineralswithouttetrahedralAlpresent,butwithAlinotherpolyhedralcoordinationsaremoreproperlyreferredtoas“aluminumsilicates”).
1.1.2Basalsurface
Basalsurfacetheterminatingsurface(orbasalplane)paralleltotheatom planesintherepeatinglayersinclaysandlayeredminerals.Commonusage hasbroadenedthemeaningtoinclude(internal)surfacesthatparallelthe terminatingsurface.Iftherepeatinglayershaveastackingdirectionalong [001],theccrystallographicaxis,thentheatomplanesintherepeatinglayers arethe(00l)planes(paralleltotheplanecontainingthetwolateralaxes, aandb).
1.1.3Basalreflection
BasalreflectionadiffractionX-raypeakfromalayermaterialoriginatingfrom thoseatomicplaneswhichcomprisethelayers(i.e.,paralleltocleavage).For mostlayersilicates,basalreflectionsareoftheMillerindextype:00l,where lisaninteger.
1.1.4Bentonite
1.1.4.1Mineralogical/petrologicalterm
Asoft,plastic,light-coloredrockcomposedprimarilyofclaymineralsofthe smectitegroup,particularlytheclaymineralmontmorillonite,whichtypically formsfromchemicalalterationofglassyvolcanicashortuffundermarineor hydrothermalconditions.Bentonitemaycontainaccessorycrystalgrainsthat wereoriginallyphenocrystsintheparentrockaswellassecondaryantigenic mineralphasessuchasK-richfeldspar.Diageneticorlow-grademetamorphic alterationcanmodifythesmectitetoavarietyofinterstratifiedillite-smectite minerals,resultinginmaterialsknownasK-bentonites.
1.1.4.2Industrialterm
Ahighlycolloidalandplasticclaymaterial,primarilycomposedoftheclay mineralmontmorillonite,thatiscommonlyusedindrillingmud,asafoundry sandbinder,incatlitter,animalfeed,cements,ceramicsandvariousother industrialactivitiesandproducts.Sodiumbentoniteswellssignificantlywhen exposedtowater(to 12 )whereascalciumbentonitehasminimalswelling capability(to 3 ).
1.1.5Brunauer–Emmett–Teller(BET)
Forspecificsurfaceareaanalysissurfaceareadeterminationbysorptionanalysisofnon-polargases,typicallyN2,onasolid,ascalculatedfromthelinear formoftheBETequationformulti-layergasadsorptiononthesurfaceofa sampleofknownweight.Thetechniquerequiresremovalofsorbedgasesfrom thesamplepriortoBETanalysis.ObjectionsinvolvingtheuseofBETanalysis forclayscontainingH2Oinclude(1)platysurfacesofthephyllosilicateparticlesprotectunderlyingadjacentsurfacesfromgasadsorption,and(2)interlayer regionsmaybecomeinaccessibletoN2 owingtopretreatmentsthatremove interlayerH2O,whichcollapsesanyswellingclayspresent,andthusresults maybeaffectedbypreparationtechniques.Itiscommonlyconsideredtomeasureexternalsurfaceareaand,assuch,shouldnotbeusedfortotalspecificsurfaceareaorasanindicatoroftheamountofchemicallyaccessibleinternal surfacearea.
1.1.6Cationexchange
Aprocesswherebyacationboundtoasiteonasurfaceisreplacedbyacation fromasolution.Inbothphyllosilicatesandzeolites,thecationmaybelocated oneitherexternalsurfacesorinternalsurfaces;thus,thefullprocessmay involvecationsfromtheinteriorthatdiffusetowardthesurface,andarein turnreplacedbycationsfromthesolutionwhichdiffuseinward.
1.1.7Clay
Anaturallyoccurringmaterialcomposedprimarilyoffine-grainedminerals, whichisgenerallyplasticatappropriatewatercontentsandwillhardenwhen driedorfired.Althoughclayusuallycontainsphyllosilicates,itmaycontain othermaterialsthatimpartplasticityandhardenwhendriedorfired.
1.1.8Clayminerals
Referstophyllosilicatemineralsandtomineralswhichimpartplasticityto clayandwhichhardenupondryingorfiring.Claymineralsmaybeofanycrystallitesizesothattheterm“claymineral”isconsistentwiththedefinitionof “mineral,”whichisunrelatedtocrystallitesize.However,theuniqueproperties ofclaysarepartlyrelatedtotheirsmallparticlesizeandhighsurfacearea.
1.1.9Interlayerdistance
Ismoreprecisetodescribethedistancebetweentheadjacentlayers(tetrahedralsheettotetrahedralsheet,andismeasuredbytakingtheaverageofthez coordinateofthebasaloxygenplane.The“interlayerdisplacement”describes thedisplacementportionorlateralshiftfromtetrahedralsheettotetrahedral sheetacrosstheinterlayerspace.Comparelayer,layerdisplacement.
1.1.10Layer
Forphyllosilicates,alayercontainsoneormoretetrahedralsheetsandan octahedralsheet.Therearetwotypesoflayers,dependingontheratiosof thecomponentsheets:a“1:1layer”hasonetetrahedralsheetandoneoctahedralsheet,whereasa“2:1layer”hasanoctahedralsheetbetweentwoopposingtetrahedralsheets.
1.1.11Phyllosilicate
Familyofmineralscontainscontinuoustwo-dimensionaltetrahedralsheetsof compositionT2O5 (T ¼ Si,Al,Be, …)withtetrahedralinkedbysharingthree
cornersofeach,andwithafourthcornerpointinginanydirection.Thetetrahedralsheetsarelinkedintheunitstructuretooctahedralsheets,ortogroups ofcoordinatedcations,orindividualcations.Althoughcontinuoustetrahedral sheetsoftenformsixfoldrings,otherringconfigurationsareconsideredpart ofthephyllosilicatefamily.
1.1.12Kaolin
1.1.12.1Petrologicterm
Rockcomposedprimarilyofkaolinite,nacrite,dickite,orhalloysite(i.e.,mineralsofthekaolingroup).Inmostcase,theidentificationofthespecificspeciesis unknown.Therockiscommonlywhite,earthy,andsoft.
1.1.12.2Mineralogicterm
Asub-groupname(withinthegroup“serpentine-kaolin”)forthosephyllosilicatesthataredioctahedral,with1:1layers,andwithanetlayerchargeof approximately0.Speciesofthissub-groupincludekaolinite,nacrite,dickite andhalloysite.Previously,thegroupnamewas“serpentine-kaolinite,”andthe subgroupnamewas“kaolinite,”butthisschemecreatedconfusionbecauseit wasunclearif“kaolinite”wasreferringtothemoregeneralsub-grouporthe species“kaolinite.”
1.1.13Serpentine-kaolin
Agroupnameforplatyphyllosilicatesof1:1layerandalayerchargeof 0per formulaunit.Generally,the d(001)spacingisapproximately7.1–7.3A ˚ .The groupisfurtherdividedintosubgroupsthatareeithertrioctahedral(serpentine) ordioctahedral(kaolin),andthesesubgroupsarefurtherdividedintomineral speciesbasedonchemicalcomposition.The1:1layersarebondedbylong hydrogenbonds( 2.9A ˚ )andpossibleCoulombicinteractionsbetweenthe octahedralsheetsofonelayerandthetetrahedralsheetoftheadjacentlayer.
1.1.14Smectite
Smectiteagroupnameforplatyphyllosilicatesof2:1layerandalayercharge ofapproximately 0.2to 0.6performulaunit.Generallyfornaturalsamples,the d(001)spacingisapproximately14.4–15.6A ˚ ,althoughotherspacing mayoccurdependingonH2Oretentionandinterlayeroccupancy.Thegroup isfurtherdividedintosubgroupsthatareeithertrioctahedralordioctahedral withdifferentmineralspeciesbasedonchemicalcomposition.Smectite mineralshavelargespecificsurfaceareas(10–700m2/g)andexhibitahigh
expansion(swelling)capabilityinthepresenceofH2O.Smectiteandvermiculitemineralsareoftenreferredtoas“swelling”or“expandable”clayminerals.Cation-exchangecapacityorsolvationofpolarmoleculesislarge. Smectiteiscommonlyaprimaryconstituentofbentonite(seebentonitefor respectivegenesisinformation)andpeliticsediments(e.g.,shales)andoccurs insoils.Veryearly,smectitewasusedasatermforfuller’searth(initially), montmorillonite,andcertainbentoniticclaydeposits.
1.1.15Talc-pyrophyllite
Agroupnameforplatyphyllosilicatesof2:1layerandalayerchargeof 0performulaunit.Generally,the d(001)spacingisapproximately 9.1–9.4A ˚ .Thegroupisfurtherdividedintosubgroupsthatareeithertrioctahedral(talc)ordioctahedral(pyrophyllite),andthesesubgroupsarefurther dividedintomineralspeciesbasedonchemicalcomposition.Thelayersare bondedbyweakvanderWaalsinteractions.
Moredefinitionsrelatedwithlayeredmaterialsandintercalationcompoundscanbefoundin Chapter10
ThestructureoftheclaymineralswasreproducedusingtheVisualization forElectronicandStructuralAnalysis(VESTA)program,version3.4.8 (MommaandIzumi,2011),andusingtheCrystallographicInformationFile (CIF)availableintheCrystallographyOpenDatabase(http://www. crystallography.net/cod/).
Claymineralsareveryabundant,andmanydifferentaluminosilicate resourcesexistaroundtheworld.Claymineralsareverycommoninsoils andinfine-grainedsedimentaryrocks,andbelongtoaveryimportantclass ofcompoundshavingvariablechemicalcompositions.Ingeneral,theyare definedbytheircrystallographyandlayercharges.Claymineralsnormally donotoccurasasinglepurephase,insteadbeingassociatedwithdifferent impuritiessuchasquartz,amorphoussilica,cristobalite,alunite,ironoxides, anatase,magnesite,andorganicmatter.Theimpuritiesdependontheclay minerals’genesis,andtheiridentificationandremovalareveryimportantto aggregatevalueandenabletheiruseinmanyindustrialprocesses.Inspite oftheseimpurities,tofacilitatethecomprehension,claymineralsareconsideredas“pure”materialsinthepresentchapter.
Duetotheirabundance,lowprice,diversityofstructuresandproperties, lowenvironmentalimpact,andgoodmechanical/heatstability,thesematerials havebeenusedsinceantiquity.Nowadays,demandisstillstrongduetothe growingnumberofindustrialapplications,attractingincreasinginterestfrom researchersintheinterdisciplinaryareaofclaysciencedueespeciallytothe sui-generisparticleshabitsandproperties.
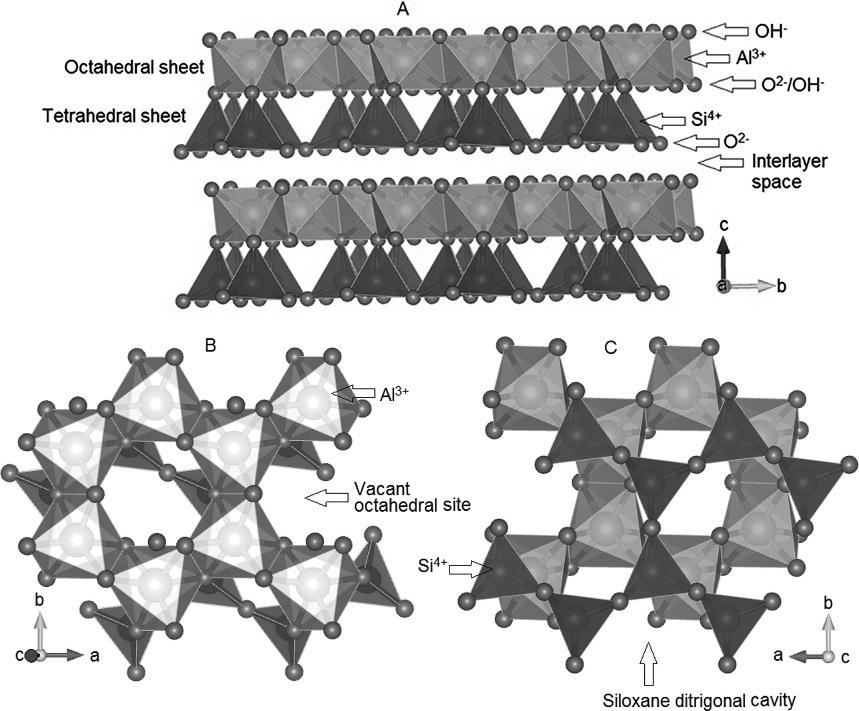
Twoimportantgeometricunitsarepresentinclayminerals’structures: SiO4 tetrahedrawhenSi4+ ispositionedinthecenterandO2 anionsareat
thevertices;andM(OH)6 octahedra(M ¼ M2+ orM3+,frequentlyAl3+)when metalcationsarepositionedinthecenterwithOH anionsatthecorners.In general,smallercationsoccurintetrahedralsitesandlargercationsinoctahedralsites,andwhenavailablespacesarepresentbetweentheintercalatedspecies,normallytheyarehydratedandtheoccupiedspaceismeasuredbythe hydratedcations’sizes.
WhenAl(OH)6 orMg(OH)6 octahedrasharetheedges,atwo-dimensional octahedralsheetisformed.Initially,thefirst2/3oftheoctahedraareoccupied andthestructureiscalleddioctahedral.WhenMg(OH)2 ispresent,allthe octahedralsitesareoccupiedandthestructureistrioctahedral.Moredetails aboutthestructureofgibbsite(Al(OH)3)andbrucite(Mg(OH)2)canbefound in Chapter10
TetrahedralsheetsareobtainedwhenSiO4 tetrahedrasharethethreecorners offouroxygens,composinghexagonalrings,withemptysitescalledsiloxane ditrigonalcavities.WhentheapicalO2 anionsofthetetrahedraareconnected tothecornersoftheM(OH)6 octahedra,two-dimensionallayersareobtained. Thesmallmismatchbetweenthetwounitsiscompensatedbysmallrotations oftheSiO4 tetrahedra.Whenonetetrahedral(T)sheetisconnectedtooneoctahedral(O)sheet,mineralsofthe1:1subgroupareformed(TO),andwhenone octahedralsheetisconnectedatthetopandbottomofthetetrahedralsheet, mineralsofthe2:1subgroupsareobtained(TOT).
Inanidealworld,tetrahedraandoctahedrausedasbuildingblocksinclay minerals’structureswouldberegular,butinnature,substantialstrainsand distortionsoccur.Thereasonsforthesedistortionsarethe“misfits”between theoctahedralandtetrahedralsheetsandbonddistortionsattributedtotheisomorphicsubstitutionintheoctahedraland/ortetrahedralsheets.Thesedistortionsareexpectedinthebulkandalsointheexfoliatedsinglelayers,where thesurfacesarepuckeredratherthantotallyflat.Allthesedistortionsare small,andfordidacticpurposes,theseunitscanbeconsideredasregular geometricunits.
Whenthelayersarepackedalongthebasalaxis,layeredcrystalsare formedwithhighaspectratio,whichistheratiobetweentheheightandthe diameteroftheplatelet-likeparticles.
1.2Claymineralswithneutralstructures
1.2.1Claymineralsofthekaolin/serpentinegroup
Kaolinite,withtheidealformulaAl2Si2O5(OH)4,isdioctahedralclaymineral belongingtothephyllosilicatefamilyandthekaolin/serpentinegroup.Other polymorphsalsohavethesameformulation,namelydehydratedhalloysite, dickite,andnacrite.WhenAl(OH)6 octahedrasharetheedges,two-dimensional octahedralsheetsareformedandtheelectro-neutralityofthesheetisonlypossiblewhenone-thirdoftheoctahedraareleftvacant,asingibbsite(Al(OH)3). Inkaolinite’sstructure,onetetrahedralsheetisconnectedtooneoctahedral
sheetthroughO2 /OH bonds(Fig.1.1)usingtheapicaloxygensofthe SiO4 tetrahedraandonecorneroftheAl(OH)6 octahedra(mineralsofthe1:1 typeorTO).
Kaolinitelayersareanisotropic,andthetopsideofeachlayer(asdepicted in Fig.1.1A)ispopulatedwithhydroxideanions,alongwithvacantoctahedralsites,resultinginamorehydrophilicsurface(Fig.1.1B).Theunderside ofthelayersispopulatedwithoxideanions,aditrigonalsiloxanecavityis presentandthesideofthelayersismorehydrophobic(Fig.1.1C)(Bishand VonDreele,1989).Inthetridimensionalstructure,thelayersareheldtogether byhydrogenbonds(Fig.1.1A),andtheoverallstructureiselectrostatically neutral.Inkaolinite,thepartialreplacementofAl3+ byFe3+ iscommon,givingkaoliniteatypicalyellowishcolorduetothenormallylowcontentofFe3+ (Lombardietal.,2002).
Otherimpuritieslikeferruginousandtitanoferrousminerals,ironoxides, hydroxides,oxyhydroxides,sulfides,carbonates,quartz,andanatasearealso commonlyfoundinkaolin(ChandrasekharandRamaswamy,2002).Dueto theweakhydrogenbondsbetweenthelayers,thedistortionsdescribed abovecausesomerandomdisplacementsinthestackingofthelayers,giving risetokaolinite(orotherpolymorphs)withdifferentdegreesofdisorderor crystallinities.
FIG.1.1 Schematicpolyhedralrepresentationofthekaolinitestructure,orientedalongtheindicatedaxis.Sideview(A),topview(B),andbottomview(C)(BishandVonDreele,1989).
Independentoftheparticlesizes,degreeofcrystallinity,andnumberof packedlayers(evensinglelayerswithsolventdispersion),theoctahedralside ofthekaolinitesurfacecanbegraftedwithorganicmoietiestoobtainorganic functionalizedsurfaceswithdifferentchemicalfunctionswhendispersedin organicsolvents.Anotherpossibilityistointercalatethebulkstructurewith somekeyorganicmoleculestoformkaoliniteintercalationcompounds,where theorganicmoleculesarelocatedondefinedcrystallographicpositions betweenthekaolinitelayersandstabilizedbyhydrogenbonds(Gardolinski etal.,1999,2000; Fukamachietal.,2007; Lerf,2014).
Halloysiteisascrolledpapyrus-likepolymorphicvarietyhavinghydrated (Al2Si2O5(OH)4 2H2O)anddehydratedformulas(Al2Si2O5(OH)4).Halloysite isfrequentlydescribedintheliteratureashavingtubularstructures,butthese arenotrealtubeslikeincarbonnanotubes,butratherscrolledtwo-dimensional layers.
DuetothemismatchoftheAl OandSi Obonds,thepackingofevena singlelayertendstoscrollalongthe“a” axisin preferencetothe“b” axis, formingnanoscrolls. Since theoutersurfacehasSi Obondsandtheinner surfacehasAl OHbonds,theinnersurfacecanbegraftedwithorganicmoietieswhiletheoutercannot. Innature,thenumberofscrolledlayersisaround 10–15units,andtheresultisnanoscrollswithouterdiameterof40–70nm, innerdiameterof10–40nm,andlengthsof0.2–40 μm.Thisnumbercanvary dependingonthehalloysitegenesis.
Whenkaoliniteparticlesareintercalated/graftedwithprimary n-alkylamines anddeintercalationoccursintolueneundersonication,theparticlesdelaminate/ exfoliateandnanoscrollswithminimumouterdiameterofaround25nmare obtained(GardolinskiandLagaly,2005; Kurodaetal.,2011; Yuanetal., 2013).Thissyntheticprocedurecanbeachievedwithdifferentintercalated moleculestosimulatethescrollingprocessthatoccursinnaturetogenerate halloysite.
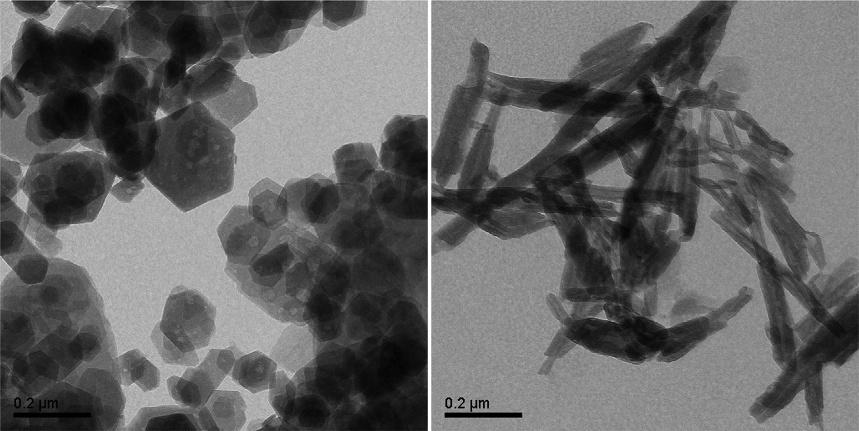
Micrographsofkaolinite(left)andhalloysitesamples(right)fromPara ´ state,Amazonregion,Brazil,obtainedbytransmissionelectronmicroscopy (TEM),areshownin Fig.1.2.
Thedifferencesbetweenthemorphologiescanbeclearlyseen,wherekaoliniteappearsassubmicrometricplatelet-likeparticleswithsharpborderswith anglescloseto120degrees,resemblingapseudo-hexagonalstructure,while halloysiteappearsasrolledscrollswithsomedelaminatedlayersresembling crumpledsheetsofpaperorrags.Theaspectratioofkaoliniteisintherange of10–40(Weberetal.,2014).
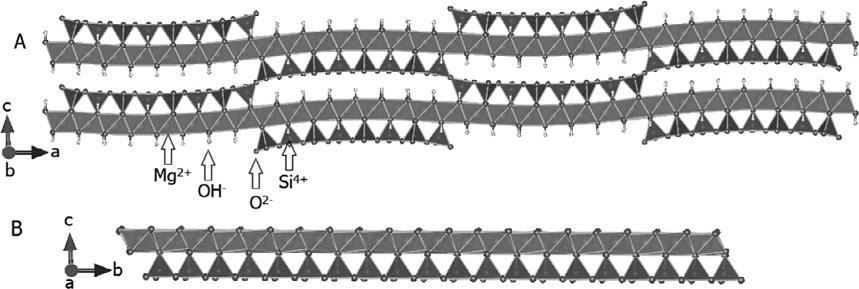
Othermorphologiesarealsoknownforhalloysite,occurringduetoincompletescrollingprocess,orevenintheformofpseudo-spheres.Inthecaseof serpentines,twomaintrioctahedralmagnesium-richspeciescanbedescribed withthesameformula,chrysotilepolymorphsorwhiteasbestos(clinochrysotile,orthochrysotile,andparachrysotile)(Whittaker,1956a,b,c),having scrolledpapyrus-likestructures(Falinietal.,2004),andlizardite,having alternatingwave-likestructures(Fig.1.3).
FIG.1.2 Micrographsofkaolinite(left)andhalloysitesamples(B)fromtheAmazonregion, Brazil,obtainedbyTEM.
FIG.1.3 Schematicpolyhedralrepresentationofantigorite(A)andlizardite(B)structures, orientedalongtheindicatedaxis(Mellini,1982; CapitaniandMellini,2006).
TheidealcompositionofchrysotileisMg3Si2O5(OH)4,whichissimilarto kaolinite(Al2Si2O5(OH)4),whenAl3+ isreplacedwithMg2+.Duetothe highermisfitbetweenthetetrahedraofSiO4 andoctahedraofMg(OH)6,the structureisrolledintheformoffibrils,whichaggregatetoformbundles.In general,thesefiberbundlescontaintensorhundredsoffibrils,witheachfibril havingdiameterintherangeofhundredsofnanometersandhollowlumens withadiameterofabout5–8nm.Theycanbeemptyorfilledwithamorphous silicaoriron-richamorphousphases.
Thescrollingofchrysotilelayersexposestheoctahedralsheetpopulated withhydroxideanions,whichcanbemodifiedbygraftingorganicmoieties likeintheoctahedralsheetofkaolinite.Manyothervarietiesbelongtothis groupofcompounds,whensubstitutionoccursnotonlyinthetetrahedral sheetsbutalsoinoctahedralsheetsandsometimesinbothsimultaneously. OthermembersofthesamegroupareobtainedwhenpartoftheMg2+ in