The Reason I Married Him Meghan Quinn
https://ebookmass.com/product/the-reason-i-married-him-meghan-quinn/
ebookmass.com
Watertreatmentandenvironmental remediationapplicationsofcarbon-based nanomaterials
XiaoliTan,XinWang
MOEKeyLaboratoryofResourcesandEnvironmentalSystemsOptimization,CollegeofEnvironmental ScienceandEngineering,NorthChinaElectricPowerUniversity,Beijing,P.R.China
1.Introduction
Pollutionistermedasthepresenceofundesirablechemicalmatterspreventingthenatural processorcausingadverseeffectstolivingorganismsandtheenvironment[1 4].Identi ficationandtreatmentofenvironmentalpollutants andtheirpreventionmeasurementsarekey stepsintheprotectionoftheenvironment. Materialscienceplaysavitalroleincleaning environmentalpollutants,andmaterialsscience technologyhasprogressedexponentiallyinthe lastdecadeespeciallynanomaterials[5,6].The pureandcleanwaterisgettingscarcedueto industrialization,andtheworldisfacinga shortageofcleanwaterespeciallyinthedevelopingworld.Watercontaminantscanbeorganics,bacteria,viruses,dyes,heavymetalions andradionuclides[7,8],theheavymetalsuch aslead(Pb),cadmium(Cd),zinc(Zn),nickel (Ni),arsenic(As),chromium(Cr),andmercury (Hg)withnonbiodegradablenatureposinga greatrisktohumanhealth[9,10].Asa
distinguishednewenergysource,nuclearpower,frequentlyreferredtoin “solvingtheenergy crisis,” hasrecentlyreceivedattentionforitsabilitytosatisfybasicenergyrequirementsand relieveenergypressures.Nevertheless,the extensiveoperationandutilizationofnuclearenergywillundisputedlyproduceradioactive pollution,therebyresultingindifferentlevels ofenvironmentalpollutionandpotentialtoxicologicaleffects[8].Therefore,itisofgreaturgency todevelophighlyefficientandenvironmentally friendlymethodsfortheremovalortheeliminationofradionuclidesfromaqueoussolutions. Radioactiveelementssuchasuranium(U),europium(Eu),strontium(Sr),technetium(Tc), andamericium(Am)haveraisedconcernsabout potentialhazardstohumanhealthandenvironmentalstabilityduetotheirsevereperniciousnessanddurability[11,12].
Becauseofthesesevereadverseeffects, removalofheavymetalionsandradionuclides fromwaterisofprimeimportanceforsaving thehumanlivesfromsuchproblematichealth
6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials
issues.Toxicmetalionscouldberemovedby numerousmethods,likeionexchange,reverse osmosis,precipitation filtration,biosorption, coagulation,andextraction[13 15].Adsorption isconsideredthebestmethodasitiscosteffective,highlyefficient,andeasytooperate forremovingtracelevelsofheavymetalions orradionuclides.Avarietyofmaterialshave beentestedasadsorbentsforwatercontaminationremediation.Commonlyusednanomaterialsforadsorptionofpollutantsaremetal oxides(Fe3O4,CuO,TiO2,etc.)[16,17],transition metalchalcogenides(NiS/Ni3S4,MoS2,ZnS, ZnSe,etc.)[18 21],transitionmetalcarbides andcarbonitrides(MXenes)[22],layereddouble hydroxides(LDHs)[23],magneticnanomaterials [24],polymernanocomposites[25]andcarbon nanomaterials,etc.[26].However,inthescientificcommunity,carbon-basednanomaterialsare gainingpopularityasnanosorbentsforwater treatmentduetotheirsizeandshapedependent properties,environmentallybenignnature,abundance,andeaseofhandling[17,27].Inthischapter,wewilltrytoreviewthelatestadvancement intheapplicationofcarbonnanomaterials, namelyactivatedcarbons(ACs),carbonnanotubes(CNTs),graphene,anditsderivatives includinggrapheneoxide(GO)andcarbonnanofibers(CNFs)inthepurificationofheavymetal andradionuclidesinion-contaminatedwater.
2.Propertiesofcarbon-based nanomaterials
2.1Activatedcarbon
ACisrecognizedasoneofthemostpopular andwidelyusedadsorbentinwaterandwastewatertreatmentthroughouttheworld[1,28]. ACisacommontermusedtodescribecarbonbasedmaterialswhichcontainswell-developed internalporestructure.ACisproducedfroma varietyofcarbonaceousrichmaterialssuchas wood,coal,ligniteandcoconutshell[29].The credittodevelopcommercialACgoestoaSwedishchemistvonOstreijkowhoobtainedtwo
patents,in1900and1901,oncoveringthebasic conceptsofchemicalandthermal(orphysical) activationofcarbon,withmetalchloridesand withcarbondioxideandsteam,respectively [30].Theprocessofchemicalactivationof sawdustwithzincchloridewascarriedoutfor the firsttimeinanAustrianplantatAussingin 1914onanindustrialscale,andinthedyeplant ofBayerin1915[31].Inthistypeofactivation, pyrolyticheatingofthecarbonaceousmaterial wasperformedinthepresenceofdehydrating chemicalssuchas,zincchlorideorphosphoric acid[32,33].Despitetheefficiencyofcommercial ACishigh,therehavebeensomedrawbackson itsusageowingtoitsexpensivenature[34]. Currentresearchconcentratesonproducing ACsusingreadilyavailableandcheapmaterials withhighcarboncontentandeasypreparation, likeplantwastes,agriculturalwastesandbyproductsfromindustries[35 37].Production ofadsorbentsusingsuchwasteproductsforwater/wastewatertreatmentwillhelpinreducing theexpensesthatmaybeincurredondisposing ofsuchwastes,andthegeneratingofACs.
Asacarbonaceousmaterial,ACisoftenproducedbypyrolysisofcellulose-basedsubstances orbituminouscoalathightemperatureinthe absenceofair.Highsurfacearea,porousstructure,andsurfacereactivityaresomeofthe importantcharacteristicsofACs[29].The adsorptioncapacityofACisdependentgreatly ontheactivationprocess.Thepurposeofthe activationstepistoimproveporosityandthe combustionofthetarspresentduringcarbonization[38].Theprocessesofactivationarebased onthereactionofvariouscomponentsthat makeupthecarbonstructureandcanbecategorizedintophysical,chemical,andphysical/ chemicalactivation.The firststepoftheprocess involvesremovalofthetarswhichcausepore blockage.Thishelpstoeasethecontactbetween thesurfaceofthecarbonandtheactivation agent.Thisisfollowedbythesecondstepin whichsmallcrystalsofcarbonarecombusted. Inthethirdphase,theoxidationofthecarbon particlestakesplace[39].
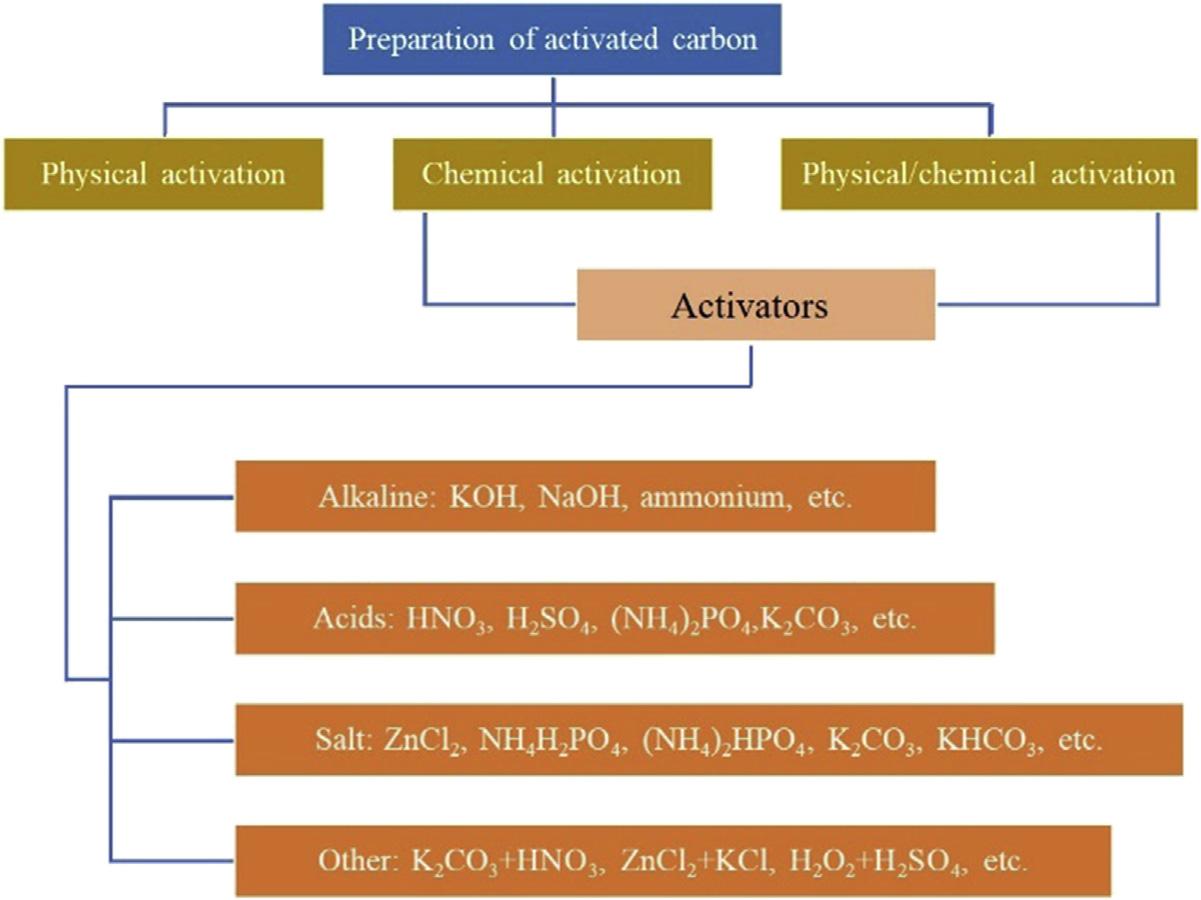
Physicaltypeofactivationisalsocalledthermalactivationandisdonebyoxidizingprecursorwiththehelpofactivatingagentusually withinthetemperaturerangesof800 1100 C toobtainparticularcompositionofAC[40]. Thephysicalactivationprocessconsistsoftwo phases[40];the firstphaseentailstheburning oftheprecursors,whileatthesecondphase,activationiscarriedoutusingactivatingagentssuch assteam,orcarbondioxide[41,42].Thevolatile mattersinthecharareexcludedwhichbrings abouttheobservationoftheporoustextureof theACs.Theparamountaimofgasificationis toincreasethepores.Nevertheless,thetemperaturemustbesetwithcaution.Atadecreased temperature,reactionstakeplaceinsidethecarbonsurfaceduringtheinitialstate.Whileat increasedtemperature,thereactionsbecome controlledbydiffusionoutsidethecarbonparticles[39].TheACsyieldproducedduringphysicalactivationisabitlow,whichlimitsitsusage. Chemicaltypeofactivation,thematerialsare subjectedtoactivationaswellascarbonization. Theprecursorsareinjectedwithacertainquantityofactivatingagents.Theactivatingagents usuallyusedarepotassiumcarbonate,zinc
chloride,potassiumhydroxide,calciumcarbonate,sodiumhydroxide,andphosphoricacid, etc.[32,33,43],asshownin Fig.6.1.Thechemical activationprocessisnormallycarriedoutata lowertemperature,unlikethephysicalactivationprocess.Thecarboncontentobtainedfrom chemicalactivationisrelativelyhigherthanthe quantityobtainedduringthephysicalactivation process.Butitrequiresfurtherwashingto removethechemicalwhichlimitsitsapplication [44,45].Severalkindsofresearchcarriedoutby previousauthorsfocusedonthephysicalor chemicaltypeofactivationprocess.ACsobtainedfromeitherofthesetwomethodspossess microporesorareofalowsurfacearea[46,47]. Hence,acombinationofbothphysicalaswell aschemicaltypesofactivationisnecessary.Khalilietal.opinedthatwithphysicalandchemical activationprocesses,thereisalikelihoodofgettingACwithdistinctsurfacecharacteristics.ACs withhighsurfaceareaaredeemedimportantin solvingenvironmentalissues.Forphysicalor chemicalactivationprocesstotakeplace, burningofthebiomassisdoneandfollowed byactivationwithphysicalandchemicalactivatingagents[48].Inaddition,therearemany
6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials
researcherstoloadmetaloxideorfunctional compoundsonthesurfaceoftheACs[49,50].
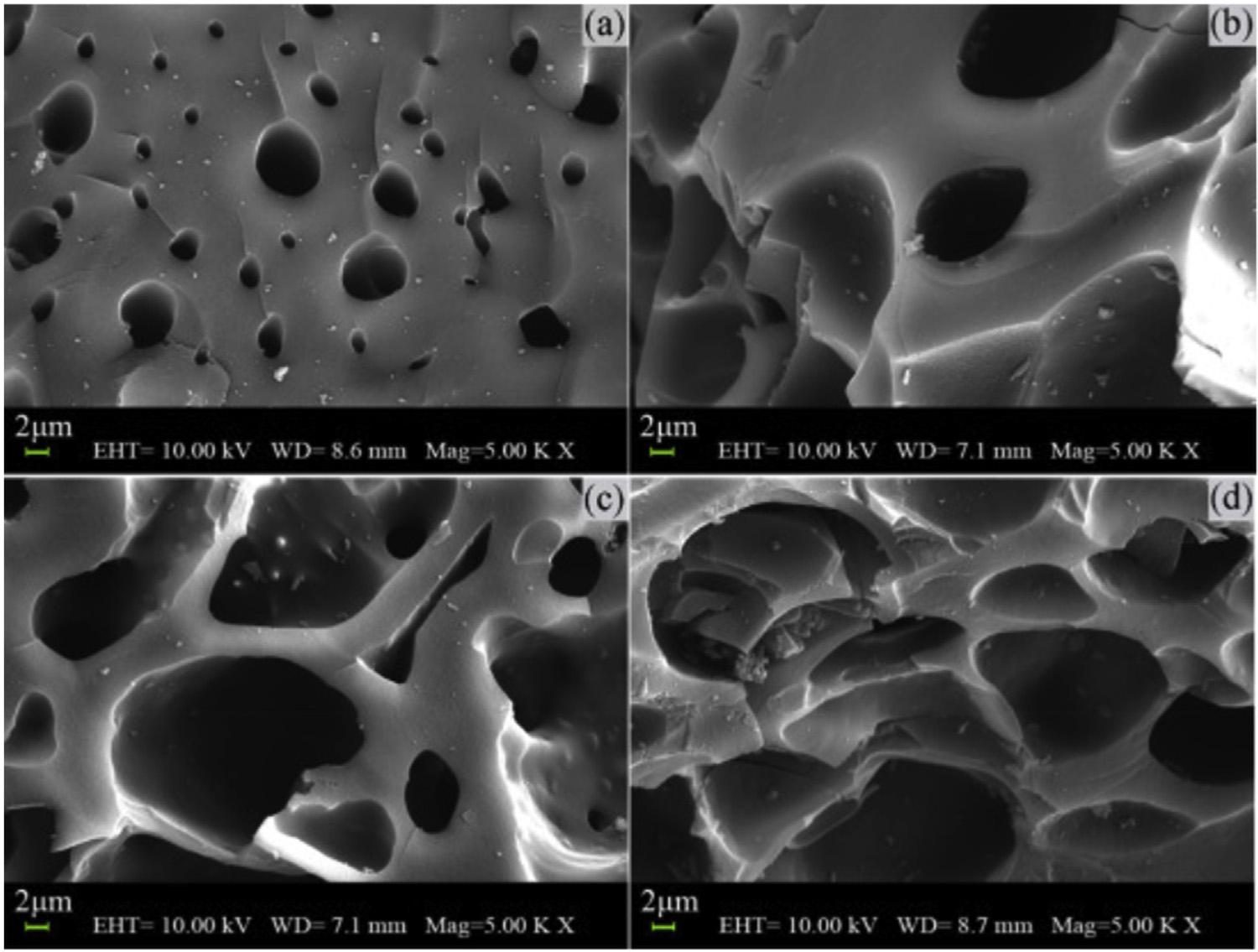
ThestructureofACsishighlycomplexand dependsontherawmaterialusedtoproduce it,themethodofproduction,andpretreatment procedure.ACissometimesdescribedashavinga “ crumpled ” layeredsurface,inwhich fl atsheetsarebrokenandcurvedbackupon themselves.Itgenerallyconsistsofsmall graphitecrystalliteswithhighlydisordered, irregular,rough,andheterogeneoussurfaces [51 ].ThesurfaceareaofACscanrangefrom 500to1400m 2 g 1 ,withvaluesashighas 2636m 2 g 1 [ 29 ,52 ].TheporosityofACcanbe increasedbypretreatmentwithacidsorbases thatcausesreorganizationoftheirsurfaceand pores[ 29].Theporousstructuralcharacteristics ofACareclearlyshownbySEMimagesofcoconutshellACsobtainedatdifferentH 3 PO4 impregnationratios( Fig.6.2 ).TheACprepared
athighimpregnationratiodisplayedporeswith largeporesize.Thiswasunderstandable.As theimpregnationratioincreased,moreH3 PO 4 couldbeembeddedintothecarbonmatrix andparticipateinthecarbongasi fi cationreactions[ 53].Togetaninsightintotheeffectof temperatureontheporosity,AChoneycomb monolith(ACH)waspreparedfrombituminouscoal.Ahighercarbonizationtemperature resultingincharsmoreresistanttosteamactivation,andyieldingACHwithlesstotal-pore volume,higherpercentageofmicroporevolume,andhighermechanicalstrength.Alonger steamactivationtimeresultedintheconversion ofahigherproportionofmicroporestomesopores[ 54 ].
ThechemicalcharacteristicsofACsare largelydeterminedbyacertaindegreeofsurface chemicalheterogeneity,whichisrelatedtothe presenceofheteroatoms,i.e.,atomspresentin
FIGURE6.2 SEMimagesof(A)AC-S-1-CO2,(B)AC-S-2-CO2,(C)AC-S-3-CO2,and(D)AC-S-4-CO2 (theas-obtainedAC wasnamedAC-S-X-CO2,whereX(¼1,2,3,or4)referredtotheH3PO4/precursorimpregnationratiousedtopreparethe sample)[53].
thecarbonstructurethatarenotonlycarbon, suchasoxygen,nitrogen,hydrogen,sulfur,and phosphorus,whicharederivedeitherfromthe natureofstartingmaterialorintroducedduring theactivationprocess[55].Surfacefunctional groups(whichareformedfromtheseheteroatoms)andthedelocalizedelectronsofthecarbonstructuredeterminetheacidicorbasic characteroftheACsurface[56].
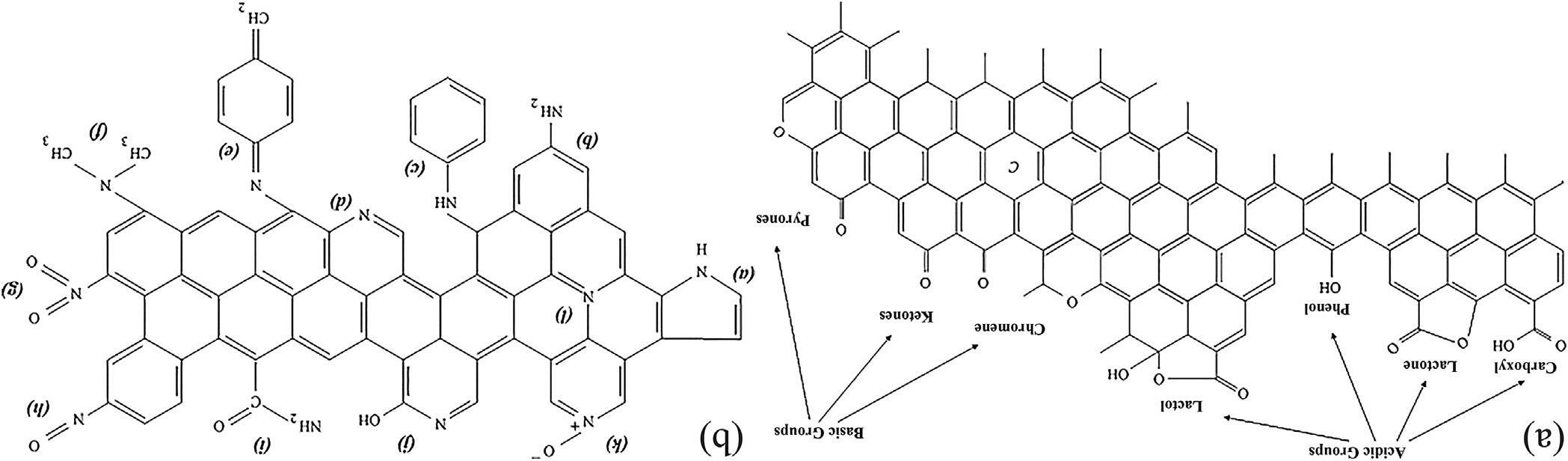
Theacidic/basiccharacterofACsurfacesis closelyrelatedtotheoxygen-containingsurface groups[57,58].Thesegroupswhicharemainly presentontheoutersurfaceoredgeofthebasal planecontributetowardthechemicalnatureof thecarbon.Astheseoutersitesconstitutethemajoritiesoftheadsorptionsurface,theconcentrationofoxygenonthesurfacehasagreatimpact ontheadsorptioncapabilitiesofAC[59].Some examplesofoxygen-containingfunctionalities detectedonthecarbonsurfaceincludethe following:carboxylic,lactone,phenol,carbonyl, pyrone,chromene,quinone,andethergroups (Fig.6.3A).Functionalgroupssuchascarboxylic acid,lactone,andphenolichydroxylhavebeen postulatedasthesourcesofsurfaceacidity[62]. Oxygen-containingfunctionalgroupsarecreated whenthecarbonsurfaceisoxidized[43,63,64]. Theactivationmethodscommonlyusedtointroduceoxygen-containingacidicgroupsareoxidationbygasesandwetoxidants.Carbondioxide andsteamcanbeusedinthegasphasetreatment. ACsshownahighdegreeofaromaticitywiththe presenceofoxygenfunctionalgroups(carboxylates,lactonesandphenols)onitssurface[64]. Wetoxidationscanintroduceahigheramount ofoxygenintothecarbonsurfaceatmuchlower temperaturescomparedwiththegasphase treatment.HNO3 modificationgeneratesasignificantlylargenumberofsurfacefunctionalgroups suchascarbonyl,carboxyl,andnitrategroups. NaOHcausesanincreaseinthecontentof hydroxylgroups.TheHCltreatmentresultsin anincreaseinthevolumeofsingle-bondedoxygenfunctionalgroupssuchasphenols,ethers, andlactones[43].Ithasbeendemonstratedthat oxidationofACinthegasphaseincreasesmainly theconcentrationofhydroxylandcarbonyl
surfacegroups,whileoxidationintheliquid phasecanincorporateahigheramountofoxygen intheformofcarboxylicandphenolichydroxyl groupsontothecarbonsurfaceatmuchlower temperaturescomparedwiththegasphase oxidation[65].
BasicityofACscanbeassociatedwith: (I)resonating p-electronsofcarbonaromatic ringsthatattractprotons,and(II)basicsurface functionalities(e.g.,nitrogen-containinggroups) capableofbindingwithprotons[60,66].It wasproposedthatcertainoxygen-containing surfacefunctionalitiessuchaschromene,ketone, andpyronecancontributetothecarbonbasicity (Fig.6.3A).However,thebasiccharacterofACs arisesprimarilyfromdelocalized p-electronsof graphenelayers.Itwaspointedoutthatthe p-electronsoftheselayerscouldactasLewisbases.Someresearchersstudiedthecontributionof basalplanestothecarbonbasicity[67,68].The surfacebasicityoftwoseriescarbonswasstudied,whereoxygen-freecarbonsitescanadsorb protonsfromsolution.Thesesitesarelocated in p-electronrichregionsonthebasalplaneof carboncrystallites.Therefore,basicsitesare Lewis-typeassociatedwiththecarbonstructure itself[65].Ithasbeenshownthatintroduction ofnitrogenfunctionalgroupsintothecarbon surfacecanincreasethecapacityofACsto adsorbUO2 2þ [69].Nitrogen-containingfunctionalitiescanbeintroducedthrougheitherreaction withnitrogen-containingreagents(suchasNH3, nitricacid,andamines)oractivationwith nitrogen-containingprecursors(Fig.6.3B) [70,71].Possiblestructuresofthenitrogenfunctionalitiesincludethefollowing:amidegroup, imidegroup,lactamgroup,pyrrolicgroup, andpyridinicgroup.Nitrogenfunctionalities generallyprovidebasicproperty,whichcan enhancetheinteractionbetweencarbonsurface andacidmoleculessuchas,dipole-dipole,Hbonding,covalentbonding,andsoon[61].
2.2Graphene
Graphene,anallotropeofcarbon,hastriggeredanew “goldrush” sinceitsdiscoveryby
FIGURE6.3 Proposedacidicandbasicoxygenfunctionalitiesoncarbonsurfaces(left)[60];Typesofnitrogensurfacefunctionalgroups(right): (A)pyrrole,(B)primaryamine,(C)secondaryamine,(D)pyridine,(E)imine,(F)tertiaryamine,(G)nitro,(H)nitroso,(I)amide,(J)pyridone, (K)pyridine-N-oxide,(L)quaternarynitrogen[61].
NovoselovandGeim[72,73].Asthe firsttwodimensional(2D)atomiccrystalhasattracted greatattentioninthescientificcommunity[74]. Idealgrapheneisasinglelayerofsp2-hybridized carbonatomsjoinedbycovalentbonds.Because ofthedifficultyinisolatingsinglelayersofgraphene, “few-layer” graphene(2 5layers),multilayergraphene(2 10layers)andgraphite nanoplates(2Dgraphitematerialwithathicknessand/orlateraldimensionlessthan 100nm)areallconsideredgraphene-family nanomaterials[75].Now,graphenehasbeenin thelimelightforsometimeandmanyresearchershavebeenworkingongraphenesynthesis;thus,severalmethodshavebeen reportedfortheexfoliationofgraphiteintographene[76,77].Theycanbedividedintotwo maincategories:top-downapproach(mechanicalexfoliation(scotchtape);graphiteintercalation;nanotubeslicing;pyrolysismethod; reductionofgraphiteoxide;electrochemical exfoliation;sonication;ballmilling;radiation basedmethods)andbottom-upapproach
(growthfrommetal-carbonmelts;epitaxial growthonsiliconcarbide(SiC);dryicemethod; deposition)[76,77].Atpresent,thedominant typeofgrapheneusedinadsorptionapplication ispreparedviatheGOroute(Fig.6.4)notonly becauseofitspotentialforlarge-scaleproduction,butalsobecauseitproducesafunctional formofgraphenethatisattractiveforadsorption applications[79].GOisanintermediateproduct duringsynthesisofreducedgrapheneoxide (RGO)andpreparedbyoxidativeexfoliationof graphite[78].GOisconsideredtheoxidized formofgraphene,functionalizedbyarangeof reactiveoxygenousfunctionalgroups,resulting inextendedgraphenesheetsdecoratedwith epoxyandhydroxylfunctionalgroupsinthe basalplanesandcarboxylicacidgroupsatthe edges,andtheoxygenousfunctionalgroupson GOmakeasignificantcontributiontoitshydrophilicityandhighnegativechargedensity, whichisimportantfortheheavymetaladsorption[80].Therefore,wemainlyintroducethe propertiesofGOandRGO. FIGURE6.4
6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials
GOisconventionallypreparedbychemical oxidationandsubsequentexfoliationofpristine graphitewitheithertheBrodie,Staudenmaier, Hofmann,orHummers’ methods,orsomevariationsofthesemethods.In1859,Brodie firstfound thatonlygraphitizablecarbonscontainingregionsofgraphiticstructurecouldbeoxidizedto generategraphiteoxidebypotassiumperchlorate andconcentrationnitricacidmixture[81].Then, Staudenmaierheatedthemixtureofgraphite,sulfuricacid,nitricacidandpotassiumperchlorate, andpreparedgraphiteoxidein1898[82].In 1937,Hofmannetal.[83]usedconcentratedsulfuricacidincombinationwithconcentratednitric acidandKClO3 fortheoxidationofgraphitefor thepreparationofgraphiteoxide.In1958,a convenientmethodwasintroducedtoprepared graphiteoxidewithconcentrationsulfuricacid, sodiumnitrateandpotassiumpermanganateby HummersandOffeman[84].Atpresent,there alsohadimprovedormodifiedHummers method[85,86].Allthesemethodschemically oxidizedgraphitetovariouslevels,andtheinterlayerdistanceofgraphiteoxideincreasedfrom 0.34nmto0.8 1.0nmduetotheintercalation ofoxygenousfunctionalgroups[87,88].Theincreaseininterlayerdistancecouldweakenthe vanderWaalsforcesbetweentheadjacent graphiticlayersandisbeneficialtotheexfoliation ofgraphiteoxidetoobtainGOviaultrasonic methodormechanicalstirring[89].TheconcentrationofexfoliatedGOinorganicsolventscould beupto1mgmL 1,whilethatinwatercould reachto7mgmL 1 duetothehydrophilicoxygenousfunctionalgroupsonthesurface[85]. ThereductionofGOtographenehasbeen approachedbyavarietyofmethods[90].The
simplestwayisthermalannealing,whichcauses disproportionationofGOintoCO2 andgraphene. Althoughthismethodisattractiveduetoits simplicity,perfectgrapheneisnotobtained, evenattemperaturesupto1100 C[91].Instead, arupturedcarbonframeworkisobtainedthat containsholedefectsfunctionalizedwithoxygen functionalities,suchascarbonylgroupsorethers. Temperatureshigherthan1500 Carerequired forthecompletedeoxygenationofGO,which causesreorganizationofthecarbonframework [92].Suchconditionsarenotfavorabledueto thehighenergycostortheincompatibilitywith temperature-sensitivesubstrates.Chemical reductionofgraphiteoxideisoneoftheexcellent procedurestosynthesizedRGO/graphenein largequantities.TheGOisreducedbyasuitable chemicalprocess;thereducedGOformedresemblesgraphenebutcontainsresidualoxygenand otherheteroatoms,aswellasstructuraldefects [86].Variousinorganicandorganicreducing agentssuchasphenylhydrazine[93],hydrazine hydrate[94],sodiumborohydride[95],ascorbic acid[96],hydroxylamine[97],hydroquinone [98],etc.[86]havebeenexploredforthechemical reductionofGO.Duringthereductionprocesses, mostoxygen-containingfunctionalgroupsofGO areeliminatedandthe p-electronconjugation withinthearomaticsystemofgraphiteispartially restored.Finally,theRGOgetsprecipitatedfrom thereactionmediumbecauseoftherecovered graphitedomainsofchemicallyconvertedgraphenesheetswithincreasedhydrophobicityand p-stackinginteraction[86].Themostwidely appliedtechniqueusedforpreparingchemically convertedreducedGOisthechemicalreduction ofGOasshownin Fig.6.5.
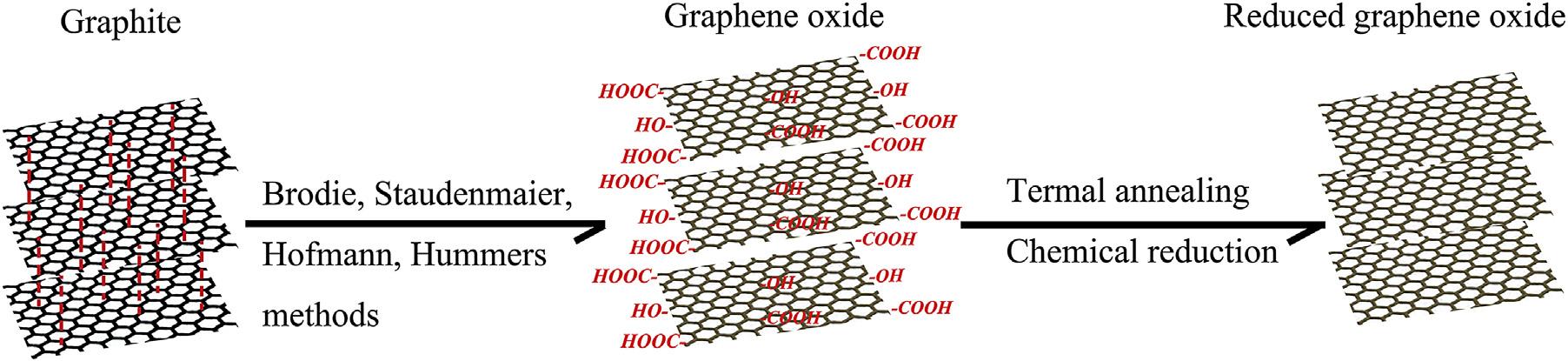
FIGURE6.5 SynthesisprocessofGOandRGOfromthepristinegraphite.
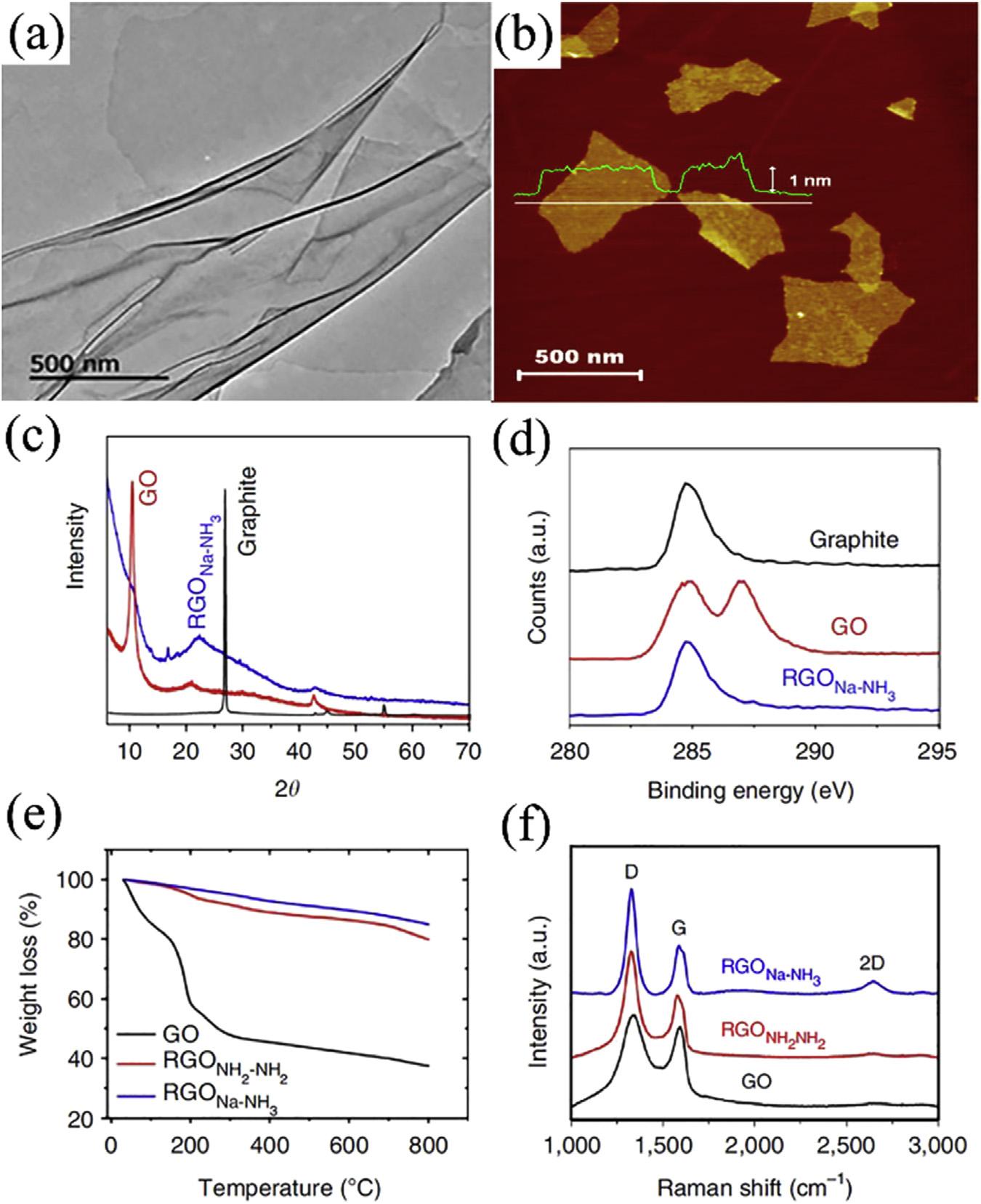
GOisamonolayergraphiteoxide,the2DGO exhibitsatypicallywrinkledandsheet-likestructure,asshownin Fig.6.6A.Thetypicalthickness ofthepreparedmonolayerGOsheets(Fig.6.6B) isabout0.7 1.2nm,whichismuchlargerthan thatoftheidealgraphene(0.335nm)duetothe existenceofepoxy,carboxylandhydroxylgroups onbothsidesoftheGOsheet[102,103].ComparisonwiththeX-raydiffractionofGOandRGO (Fig.6.6C),atypicalbroadpeaknear10.21 degrees(d-spacing~8.67A)canbeobservedfor
theGOpowder.ThepeakofRGOshows anobviousshifttohigher2q angles(25.04degrees;d-spacing~3.56A),suggestingthatRGO waswellorderedwith2Dsheetswithmorethoroughremovalofsurfacefunctionalgroups[101]. X-rayphotoemissionspectroscopy(XPS)in Fig.6.6D showstheC1spectraofgraphite,GO andRGOpowdersamples.Ingeneral,theRGO exhibitsthesimilarXPSspectrumtothatofthe naturalgraphite,C]Cbondsdominate,as shownbyonesinglepeakwithsmalltailsatthe
FIGURE6.6 (A)TEMimageofGO[99];(B)hydrazine-reducedgrapheneoxidesheets[100];(C)X-raydiffraction(XRD) patternsofgraphite,GOandRGOpowder;(D)XPSspectraofgraphite,GOandRGO;(E)TGAthermogramsforGOand RGO;(F)RamanspectraofGO,RGONH2 NH2 andRGONa NH3 powder[101].
6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials
higher-bindingenergyregion,confirmingthe goodrestorationofC]CbondsintheRGO.In contrast,theoriginalGOsignalshowstwoseparatedpeaksbecauseofthehighpercentageofoxygenfunctionalities,whichcontainedtheC C/ C]Cinthearomaticrings,theC Oofepoxy andalkoxygroupsandC]O/O C]Ogroups [101,104].Thermogravimetricanalysis(TGA) wasusedtofurtherassessthelevelofreduction inGOplatelets(Fig.6.6E).TheGOsample showedsignificantweightlosswithanonsettemperatureatslightly >10 C,whichisattributedto theeliminationofinterlamellarwater,followed bylossofoxygenfromtheGOplateletsthemselvesatslightlyhighertemperatures.RGO showedmuchhigherthermalstabilitybecause ofthemorecompletede-oxygenationandbetter graphitizationwithenhancedvanderWaals forcesbetweenlayers[93].Ramanspectroscopy providesanondestructivemethodforcharacterizinggraphene.Graphenetypicallyexhibitsthree mainRamanfeatures,G-band,D-bandand two-dimensionalmodes,eachhavingdifferent physicalorigins.RamanspectraofGOand RGOpowderareshownin Fig.6.6F.TheRaman spectrumofGOandRGOexhibitedtwointense peaksat1328and1595cm 1,whichcorrespond totheDandGbands,respectively.Theratioof
I(D)/I(G)oftheRGOincreasedwhencompared withGO,indicatingthatnumeroussmallsp2 domainswereformedduringthereduction[93,101].
2.3Carbonnanotubes
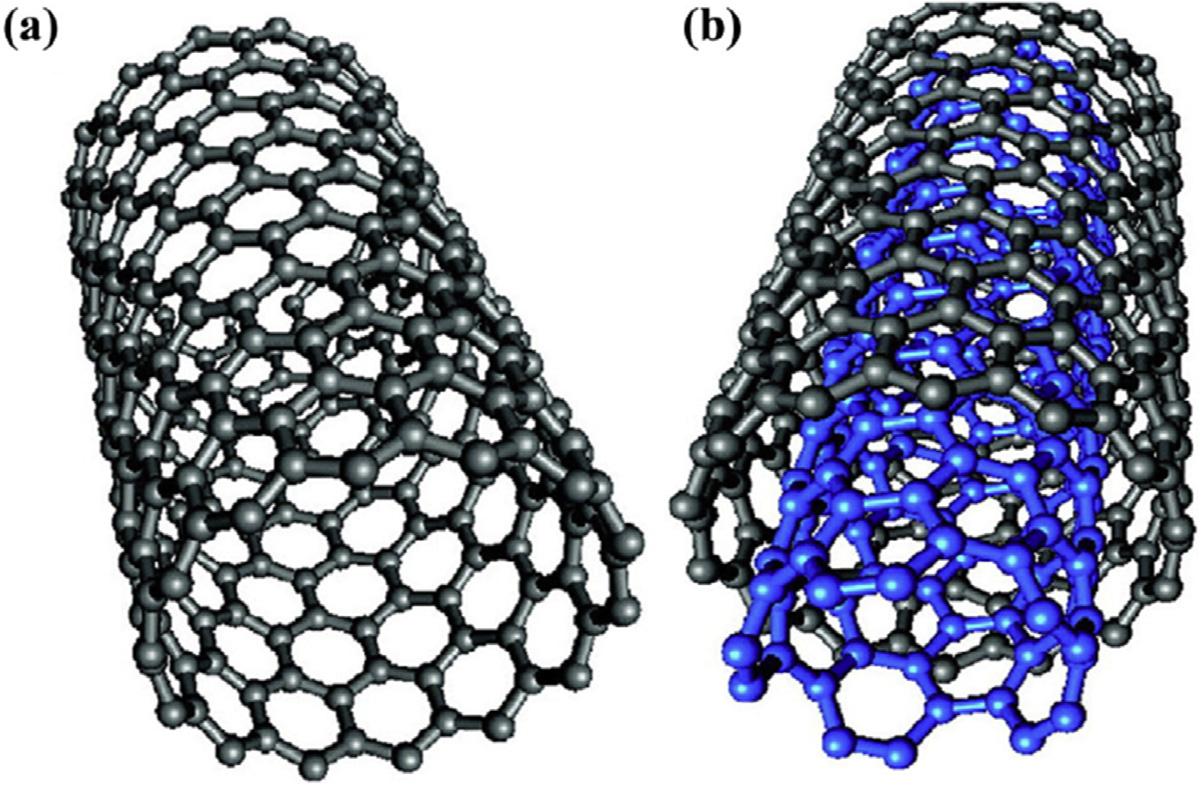
Afterthediscoveryofbuckyball(aball-like moleculemadeofpurecarbonatoms)in1985 byKrotoetal.[105],atubularformofcarbon wasreportedbyIijimain1991andnamedcarbonnanotubes(CNTs)[106].CNTshavehollow, one-dimensional(1-D)tubelikestructures,with thincarbonwalls,whichbringfascinatingmechanical,electrical,andthermalproperties [107].CNTshavenano-sizeddiameterswith highaspectratios.Theycanbethoughtofasgraphenenanosheetsrolledintocylindricaltubes, andcanbecategorizedassingle-walledCNTs (SWCNTs)andmulti-walledCNTs(MWCNTs) (Fig.6.7)[109].TheuniquestructureofCNTsoffersexcellentintrinsicproperties,includinga largeactivesurfacearea,highchemicalandmechanicalstabilities,andhighelectricalconductivity,whichprovidesimmensepotentialforuseas sensorsandelectronicsforbiomedicalapplications,ascomposites,andforadsorption[110]. Thehighsurfaceareaandporosity,thehollow, layeredarchitecture,smalldiameter,andhigh
FIGURE6.7 structurerepresentationof(A)SWCNTsand(B)MWCNTs[108].
aspectratioofCNTsmakethemeffectivefor pollutantadsorption,andtheyarewidely employedtoadsorbwatercontaminants[10].
Therearemainlyaboutthreemethodstoproducecarbonnanotube.
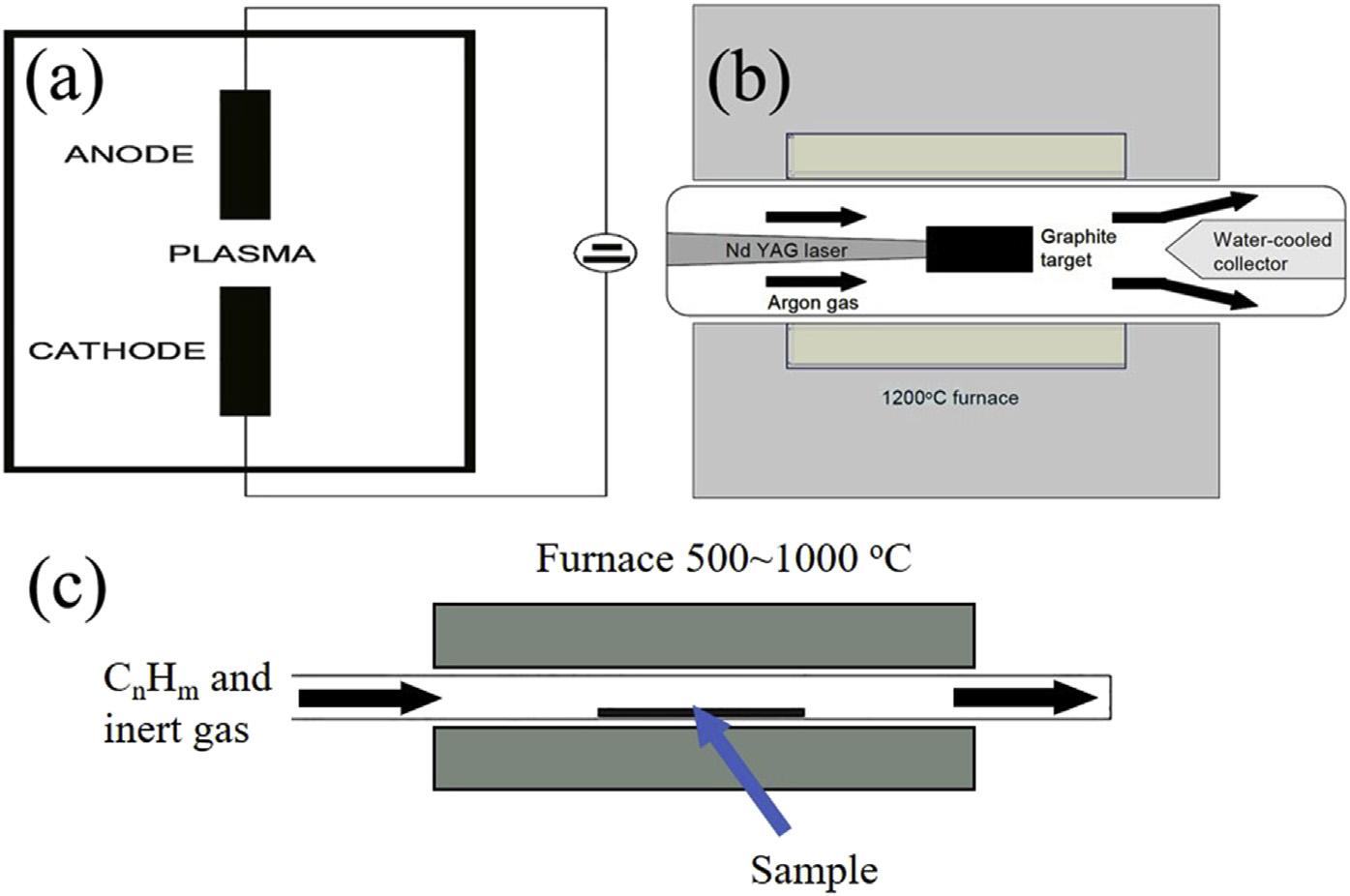
Arc-dischargeistheeasiestandmostcommon methodofproducingCNTs.Inthismethod, CNTsareformedbycreatingahotplasma dischargebetweentwographiteelectrodes whichareconnectedtothepowersupply(100 A;20V)inthepresenceofhelium(He)gas (Fig.6.8A).AccordingtotheEbbesenand Ajayan[113],asthepressureofHeinthechamberincreaseduptoacertainvalue,theyieldof nanotubesalsoincreases,butafterthatvalue, furtherincreaseinHepressureleadstothefall inCNTyields.Betterqualityofthenanotubes dependsupontheloweringofthecurrent [114].Variousgaseslike,N2,CF4 wereusedin placeofHegas[115,116].Shimotanietal.[117] reportedtheincreasedyieldofnanotubeswith theuseoforganicvapors.
Laser-ablation,at firstSmalleyandhiscolleaguesreportedthesynthesisofsingle-walled nanotubeswiththismethodin1995[118].
FIGURE6.8 (A)arc-discharge[111];(B)Laser-ablation[112];(C)Chemicalvapordeposition. 2.Propertiesofcarbon-basednanomaterials
Inthismethod,thecarbonsource(graphite)is dopedwithsmallamountsofmetalliccatalyst (CoandNi)whichisthenvaporizedwiththe helpofpulsedlaserbeaminthepresenceofinert gas(typicallyargon)atveryhightemperatures (approximately1200 C)andataconstantpressureof500Torr.AstheNd:YAGlaserbeamimpregnatesthetargetmaterial,itstartsvaporizing andcondensesontheotherendwhichisat comparativelylowertemperature(Fig.6.8B). Someresearchersuseddoublepulsedlasertoincreasethevaporizationwhichalsoincreasedthe yieldofsingle-wallednanotubesupto1gday 1 [107,119].Duetotherequirementofhigh-power usageandemployabilityofexpensivelasers,this synthesisrouteisaverycostlyaffair.
Thechemicalvapordeposition(CVD)method isusedforbulkproductionandcontrolled growthofnanotubes[120].Inthismethod,as shownin Fig.6.8C,synthesisofCNTstakeplace duetodecompositionofamixtureofhydrocarbongases(methane,ethylene,etc.)orvolatile carboncompoundspresentinthechamber ontoametallicsubstrate,wheremetallicnanoparticlesbehaveascatalystandnucleationsites
6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials
inthegrowthprocessofcarbonnanotubesat temperaturesof500 1000 Candunderatmosphericpressure.Theselectionofcatalystand preparationofsubstratedecidesthetypeand qualityofthenanotubesproduced.Usually,Fe, Co,Ninanoparticlesareusedascatalyst[121]. Poroussiliconisconsideredanidealsubstrate forcontrolledgrowthofcarbonnanotubes.In 2002,GovindarajandRao[122]usedorganometalliccompoundsinplaceofhydrocarbongasfor thesynthesisofCNTs.Themainadvantageof usingorganometalliccompoundsisthatthere isnorequirementoftheremovalofthecatalyst supportafterthereactionduetothepresence ofsamephaseofcarbonsourceandcatalyst simultaneously.Furthermodificationswere doneinthistechniquelater.Plasma-enhanced CVD(PECVD)isthewidelyusedmethodfor CNTsynthesis.Thistechniquewas firstused byRenetal.[123]in1998.Inthisprocessalso, adirectcurrentplasmaisusedwhichisresponsibleforthealignmentofthecarbonnanotubes. Thistechniqueisbasicallyusedinthefabrication ofCNT-based flatpaneldisplays,solarcellsetc. Similarly,anumberofstudieshavebeendoneto synthesizeCNTsbyvariousothertypesofCVD methods[124,125].
Ingeneral,CNTsarecategorizedintothree typesbasedonthetwo-dimensionalformof theirsheets:zigzag,armchairandchiralnanotubes(Fig.6.9).Thezigzagtypicallyhasahexagonalpatternmovingaroundthebodyofthe tubule.Thearmchairformcontainsoneortwo cyclohexaneconformers,withthecarbonatom alsodescribingahexagonalpatternasitmoves aroundthebodyofthetubulebody.Thethird formofCNTsisthechiralform.Thetermchiral denotes ‘handedness,’ andindicatesthatthe tubecanbetwistedinanydirection.Thechiral shapeofSWCNTsissimilartothezigzagand armchairforms.Allthesetypesusuallyoccur withinSWCNTs[126].
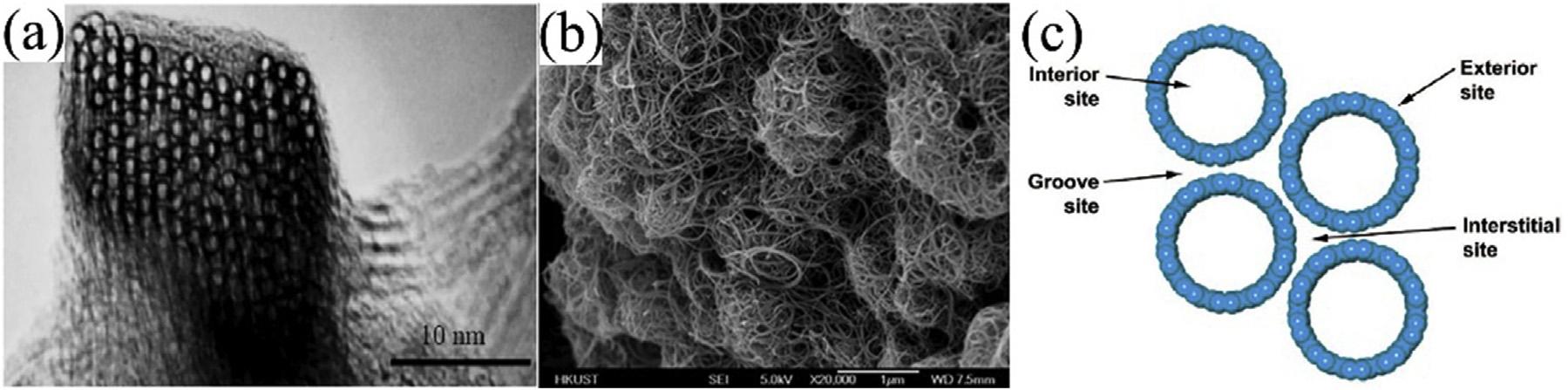
SWCNTsconsistofsheetsofsp2-hybridized carbon(graphenesheets)rolledintocylinders withdiametersrangingfrom6to~20Å (Fig.6.10A),dependingontheparticularmethod
andconditionsduringtheirsynthesis.Lengths typicallyrangeinthehundredsofnanometers ormicrometers[128].Thecontactsurfaceof SWCNTswithpollutantsismorethanMWCNTs becauseSWCNTsconsistofasinglecylindrical layer.However,SWCNTsasasorbentareused lessthanMWCNTsforremovingcontaminants becauseitssynthesisismoredifficultandmore expensivethanMWCNTs[126].
MWCNTscontainagroupofagraphenecylindersnestedtogether(Fig.6.10B).ATEMexaminationrevealsanintershellspacingofbetween 0.335and0.34nm,supplementingadiminishing tubediameter.Thesmallestdiameterandlargest spacingarefoundinthehighcover,subsequent inanunwelcomeforce,andassociatedtothe decreasingdiameterintheCNTsshell.The bulkgraphitecrystalspacingvalueof0.34nm isnearlyaslargeasthatoftheCNTsthemselves [126].Ru[129]establishedthattheinterlayer spacingmeanvalueis0.3444 0.001nm,and thatCNTsarelargerbyafewpercentthan bulkgraphitecrystal.Thereisaspacingbetween thelayers,denotedbyd ¼ 3.39Å,whichisbased ontheoreticalcomputationandisgreaterthan thatobservedforgraphite.UsingaTEMimage experimentally,MWCNTswerefoundtohave aspacingofd ¼ 3.4Å[113].ThismagicalCNT structureresultsinawesomechemicalandphysicalproperties.Becauseofthebondbetweenthe carbonatomsinthesp2 direction,CNTsareone ofthestrongestmaterialsintheworld[130]. ConcludedthatCNTshaveastrengthand Young’smodulus10 100timesgreaterthan thatofsteel[126].CNTassociatewitheachother duetoattractivedispersiveforcestoformbundles,typicallycomprisingtensorhundredsofindividualnanotubes.Adsorptionofmolecules takesplaceonthesebundles[128].Thus,to gaininsightintoadsorptiononcarbonnanotube, onemustconsiderthestructureofthebundle andtheadsorptionsitesavailabletotheadsorbatemolecules.Fourtypesofadsorptionsites canbeidentified:thenanotubeinteriorsites, thesitesontheexteriorsurface,thegroovesites, andtheinterstitialsites,asshownin Fig.6.10C.
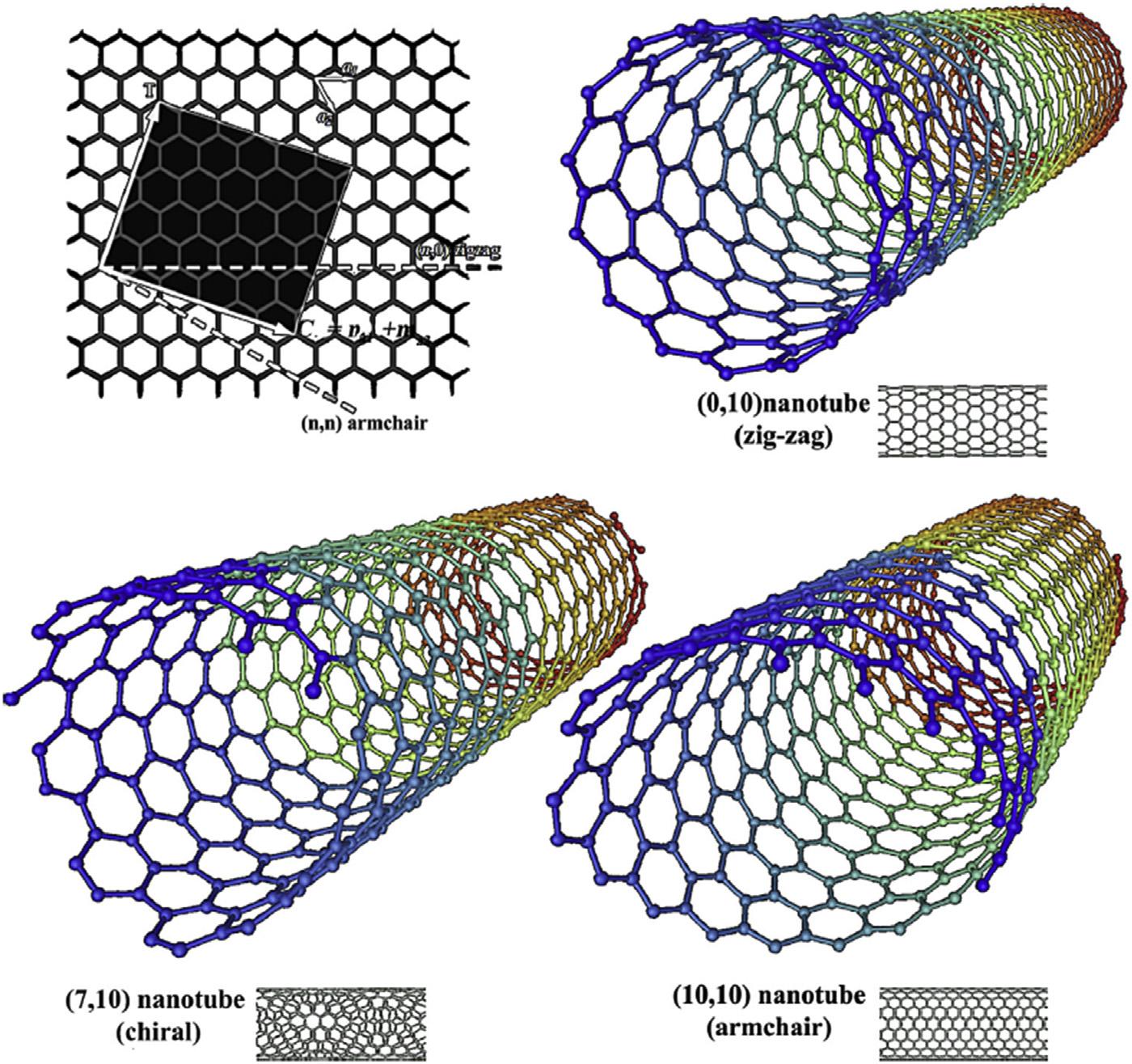
FIGURE6.9 MolecularmodelsofexhibitedbySWNTsbasedonthechirality:armchair,zigzag,andchiralbasedconformation[107].
FIGURE6.10 ElectronicmicroscopeimagesofdifferentCNTs:(A)TEMimageofSWCNTbundle;(B)SEMimageof entangledMWCNTagglomerates[127];(C)Fourtypesofadsorptionsitesofcarbonnanotubes[128].
Internalsites:thesesitesarefoundwithinthe hollowstructureoftubesandavailableonly whenendsofthetubeareopen.Interstitialchannels:thesesitesareeasilyaccessibleforthe adsorbatespeciesandfoundintheinteriorspace ofthebundlebetweenindividualnanotubes. Externalgrooves:thegroovespresentontheperipheryofananotubebundleandtheexterior surfaceoftheoutermostnanotubes,wheretwo adjacentparalleltubesmeet.Exposedsurface siteoroutsidesurface:outsidesurfacesiteis highlyaccessiblefortheadsorbate(externalsurfaceadsorption)andfoundonthecurvedsurfaceofindividualnanotubesontheoutsidethe nanotubebundles[131,132].
2.4Carbonnanofiber
Carbonnanofiber(CNF)was firstreportedina patent filedmorethan120yearsago;carbon filamentsaregrownfromcarbon-containinggases usingametalliccrucible.In1991,Iijima[106] foundcarbonnanotubesandotherfullerenesduringarcdischarge,whichtriggeredanoutburstof interestincarbonnanotubeandnanofiber.Nanometerscalecarbon fibers(carbonnanofibers) showhighsurfacearea-to-volumeratio,nanoscalediameterandmechanicalproperties,allof whichareofgreatpotentialapplicationinmaterialsscience,compositeproduction,energystorageandthechemicalindustry.Thehydrocarbon
gasesandpolymersarewidelyemployedascarbonprecursorsfortheproductionofcarbonnanofibers[133 135].Synthesisroutesofcarbon nanofiberaremainlyCVD,electrospinning,and templating.Thecarbonnanofibersderivedfrom differentroutesembodydifferentcarbonstructuresandmorphologies[136].
TheCVDmethodisnotpreparedfroma fibrousprecursor,butratherfromhydrocarbon gas,usingacatalyticgrowthprocess,involving acomplicatedchemicalandphysicalprocess;ultrafinetransitionmetalparticles,suchasironor nickelparticleswithdiameterlessthan10nm, aredispersedonaceramicsubstrate,andahydrocarbon,suchas,benzenedilutedwithhydrogen gas,isintroducedatatemperatureofabout 1100 C.Hydrocarbondecompositiontakesplace onthecatalyticparticle,leadingtocontinuous carbonuptakebythecatalyticparticleand continuousoutputbytheparticlesofwellorganizedtubular fi lamentsofhexagonalsp2carbon.Thus,theassociatedcostsareinevitably high.TheCVDmethodisonlycapableofproducingrelativelyshort fi bersthataredif fi cult toalign,assemble,andprocessintoapplications.CNFsfromvapor-growthcarbonnanofi bershaveaveryspecialstructurelike annular-rings;thenano fi bergrowthandstructureareshownin Fig.6.11 .Theadvantageof vapor-growthcarbonnano fi berstructureis sp 2 graphite.Thethicknessofthe fi berscanbe FIGURE6.11
2.Propertiesofcarbon-basednanomaterials
adjustedbythemetalparticlesizeandthe orientationofthegraphiteplanecanbesteered bythegrowthtemperatureand/orthenature ofthemetal,suchas,iron,whichtendstogive toparallel fi ber,whilenickeloftenleadsto fi shbone-type fi bers.However,itrequiredthe carbonprecursorstobegasorlowboilingpoint organicmolecules,hightemperature,andthe presenceofnanometalparticles,orelse,the yieldofnano fi berislower[ 136].
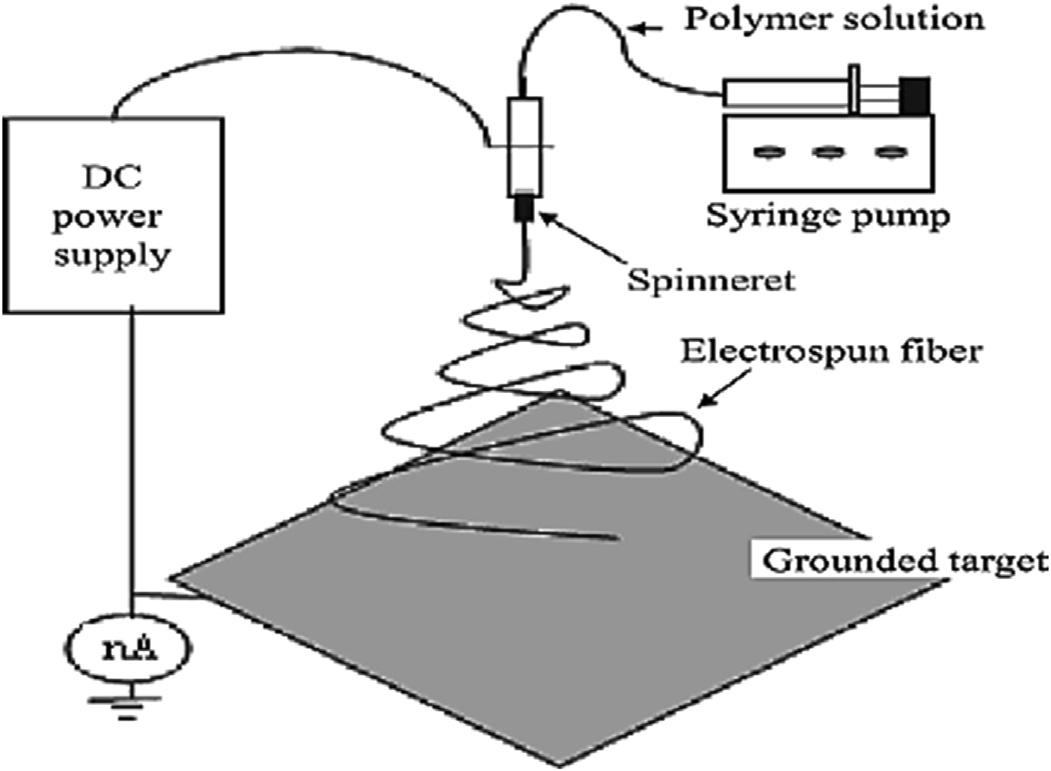
Electrospinningisaneffectivetechniqueto producepolymericnanofibers.Therapidlydevelopingtechniqueofelectrospinningprovidesa straightforwardandcost-effectiveapproachto produce fiberswithdiametersrangingfromsubmicronstonanometerswithdiametersofapproximately300nm.Itoffersmanyopportunitiesto tailorthe fibermorphology,chemicalcomposition, fibrousarchitecture,andfunctionality.Duringelectrospinning,apolymersolutionis stretchedunderahighelectricalvoltageinto fine filaments,whichdepositrandomlyonan electrodecollectorformingarandomlyoriented nanofiberweb.Atypicalelectrospinningsetup consistsofametallicspinneret,asyringepump, ahigh-voltagepowersupply,andagrounded collectorinahumidity-controlledchamber (Fig.6.12).Apolymersolution,polymermelt,or
asol-gelsolutioniscontinuouslypumpedthrough thespinneretataconstantrate,whileahighvoltagegradientisappliedbetweenthespinneret tipandthecollectorsubstrate.Thesolventcontinuouslyandrapidlyevaporates,whilethejet streamiswhippedandstretchedbyelectrostatic repulsionformingsolidifiedcontinuousnanofibers(diameters50 500nm)onthegroundedcollector.Improvedelectrospinningtechniques havebeenabletoproducealignednanofiber arrays,nanofiberswithporoussurfaces,and bicomponentcross-sectionalconfigurations(e.g., coresheathandside-by-sidenanofibers),andto generatenanofibersonalargescale[137].
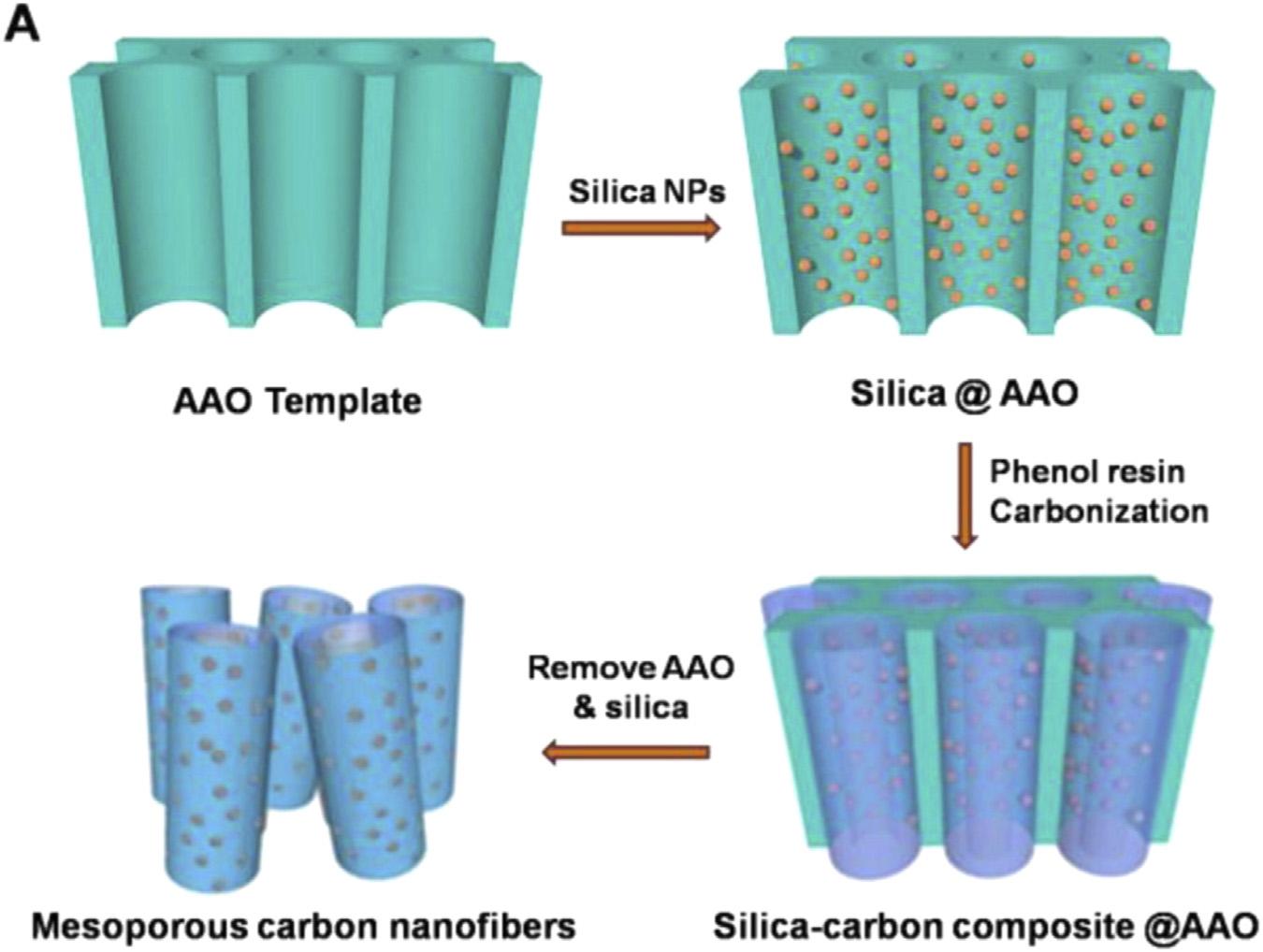
InadditiontoelectrospinningandCVDsynthesis,templatedsynthesiswithporoussubstratesandnanowiresprovidesalternative waysfortheproductionofCNFs.Forinstance, Xingetal.[138]demonstratedthetemplated synthesisofCNFsbyusinganodicaluminum oxide(AAO)membranesandcommercially availablecolloidalsilicananoparticles(SiNPs) ascotemplatesandphenolresinasacarbon source.Asindicatedin Fig.6.13,SiNPswere firstlyincorporatedintotheinnerwallofAAO membranetoformSi@AAO,andthenphenol resinwasaddedintothedualtemplatedfor carbonizationat973KunderN2 gas flowto
schemeoffundamentalsetupforelectrospinning[137].
6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials
FIGURE6.13 SchematicdiagramforthesynthesisofMCNFs(AAO,anodicaluminumoxide; NPs,nanoparticles)[138].
formSi C@AAOsubstrate.Finally,freestandingCNFswithmesoporousstructure wereproducedbyetchingtheAAOandSiNPs inthealkalinesolution.Inasimilarstudy, CNFswithuniformstructurehavebeenproducedbyusingtubularporousAl2O3 substrates andCVDgrowthofcarbon[139].Carbon nano fiberscontaininglinearmesocagearrays werepreparedviaevaporation-inducedselfassemblymethodwithinAAOtemplatewith anaveragechanneldiameterofabout25nm. Themesocageshaveanelongatedshapeinthe transversaldirectionandpossessalargepore sizeofabout18nm.Sparkplasmasintering andhotextrusionprocesseshavebeenemployed forfabricatingcarbonnano fiber-aluminummatrixbulkmaterials.Thealuminumpowderand thecarbonnano fibersweremixedinamixing mediumofnaturalrubber.Thecarbonnanofiberswerewelldispersedontothealuminum particles.Thecarbonnano fiberswerefound tobelocatedoneverygrainboundaryand alignedwiththeextrusiondirectionofthe
aluminum-carbonnano fiberbulkmaterials. Thecarbonnano fibersandtheformationof Al4C3 playanimportantroleintheenhancement ofthemechanicalpropertiesofthealuminumcarbonnano fiberbulkmaterial[136].
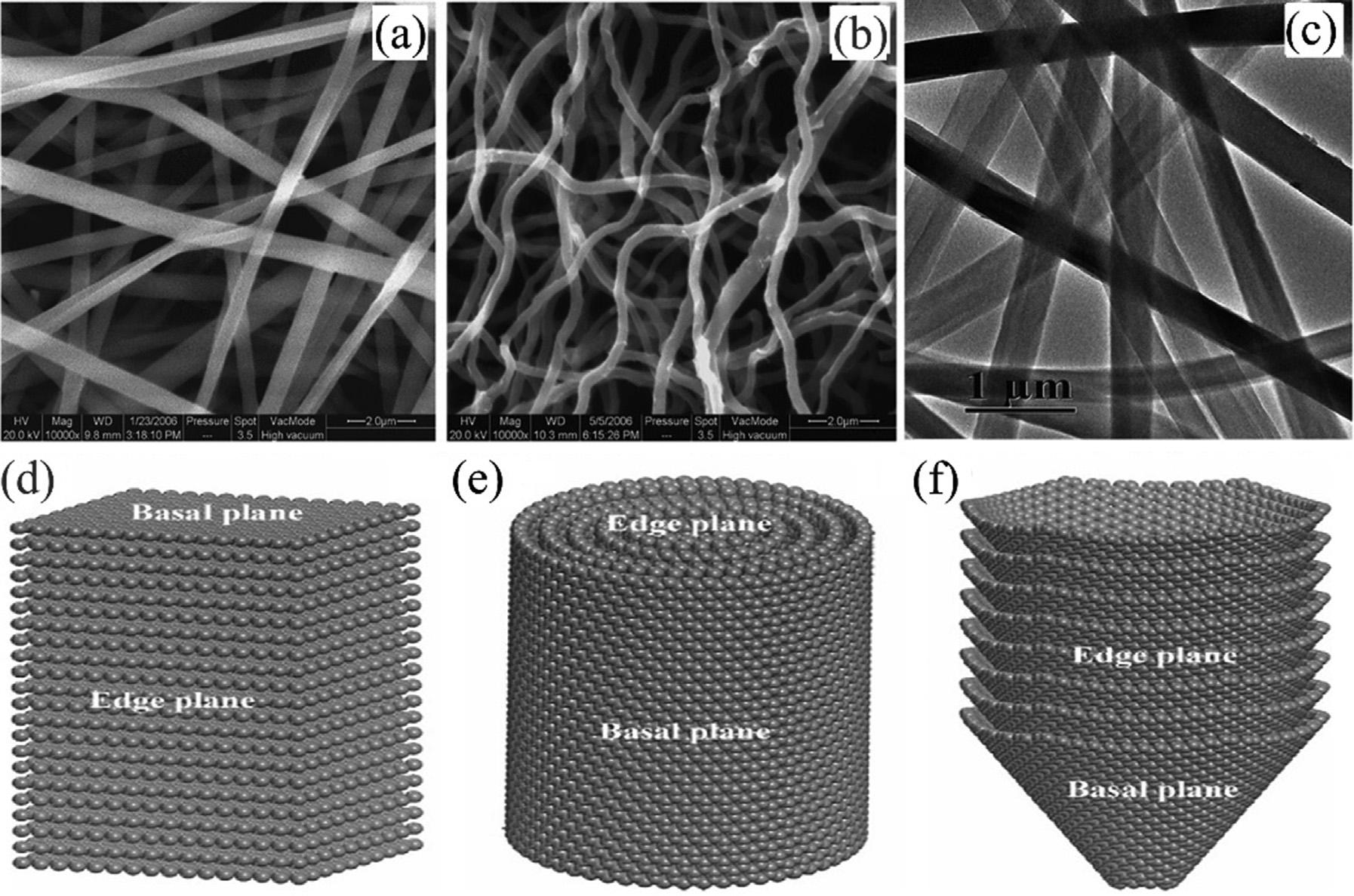
Carbonnano fibersaredescribedasanoncontinuous1Dcarbonnano-allotropeofcylindrical orconicalshape(Fig.6.14A C),consistingof stackedandcurvedgraphenesheetsarranged invariousways.Carbonnano fiberscanshow differentshapes,asdepictedin Fig.6.14D F, basedonthecriterionoftheangleofthegraphenelayersthatcomposethe filament.Besides platelets(Fig.6.14D),tubularorribboncarbon nano fibers(i.e.,carbonnanotubes, Fig.6.14E, so-called fishbonecarbonnano fibers)existin whichthegraphenelayersareorientedunder ananglebetweenprincipalandperpendicular axis[141].Thedistinctivearrangementsofthe graphenelayersdependonthegeometricaspectsofthemetallicnanoparticlecatalystand thefeedstockofthegaseouscarbon(COorhydrocarbongas)whichisintroducedduringthe
3.Removalofheavymetalionsbycarbon-basednanomaterials
FIGURE6.14 SEM(A,B)andTEM(C)imagesofcarbonizednanofibers[140];Schematicrepresentationsofthethreetypesof CNFswithdifferentbasal-to-edgesurfacearearatios:(D)platelet-typeCNF,(E)tubular-typeCNF,and(F) fishbone-typeCNF[141].
synthesisprocessing[142].Itwasfoundthatthe differentgraphenecontactareas(edgeplanes andbasal)playdistinctroleswithinthephysical andchemicalbehaviorofCNFs.Theyare frequentlydescribedassp2-basedlinear filamentswithadiameterrangingfrom50to 200nmandahighaspectratioexceeding100. Ingeneral,thecarbonnano fiberderivedfrom CVDorcarbonizationofelectrospinningnanofibershowsundevelopedornonporestructure andpoorfunctionalgroupsbythesemethods, whichinhibitsitseffectiveapplicationinadsorption/separation,catalystsupports,andelectronicmaterials fields.Inadsorption filed, chemicalactivation,enhancingporestructure andgraftwithpolymerandmetaloxidesare usefulwaytoimprovetheirpotential[143 145].
3.Removalofheavymetalionsby carbon-basednanomaterials
Fordecades,carbonnanomaterialshave attractedintenseattentionfromscientistsand beenemployedinaprodigiousnumberofapplications[26,146].Carbonnanomaterialsarethe mostcommonnanoadsorbents,duetotheirabundantavailability,excellentadsorptioncapacities, cost-effectiveness,highchemicalandthermalstabilities,highactivesurfaceareas,andenvironmentallyfriendlynaturesandthuscontributeto wastewatermanagement[147].Beinghighly porouswithalargesurfacearea,AC[34]has beenthemostcommonlyusedadsorbentfor years.Anddifferentformsofcarbonorfunctionalizedcarbonsuchasgraphene[77,148],CNT
[149],CNF[145]alsohavebeenimplementedfor adsorption.Researchontheadsorptionofwater contaminantsusingcarbon-basednanomaterials isproliferating[26,150].Inthischapter,itisvital toreviewthecurrentstateofthearticle,forthe useofcarbon-basednanomaterialsforadsorption ofheavymetalandradionuclides,andtoexplore newdimensionsandemergingtrendswhich couldhelptoprovidenewdirectionsinthisactive researcharea.
3.1Activatedcarbon-based nanomaterials
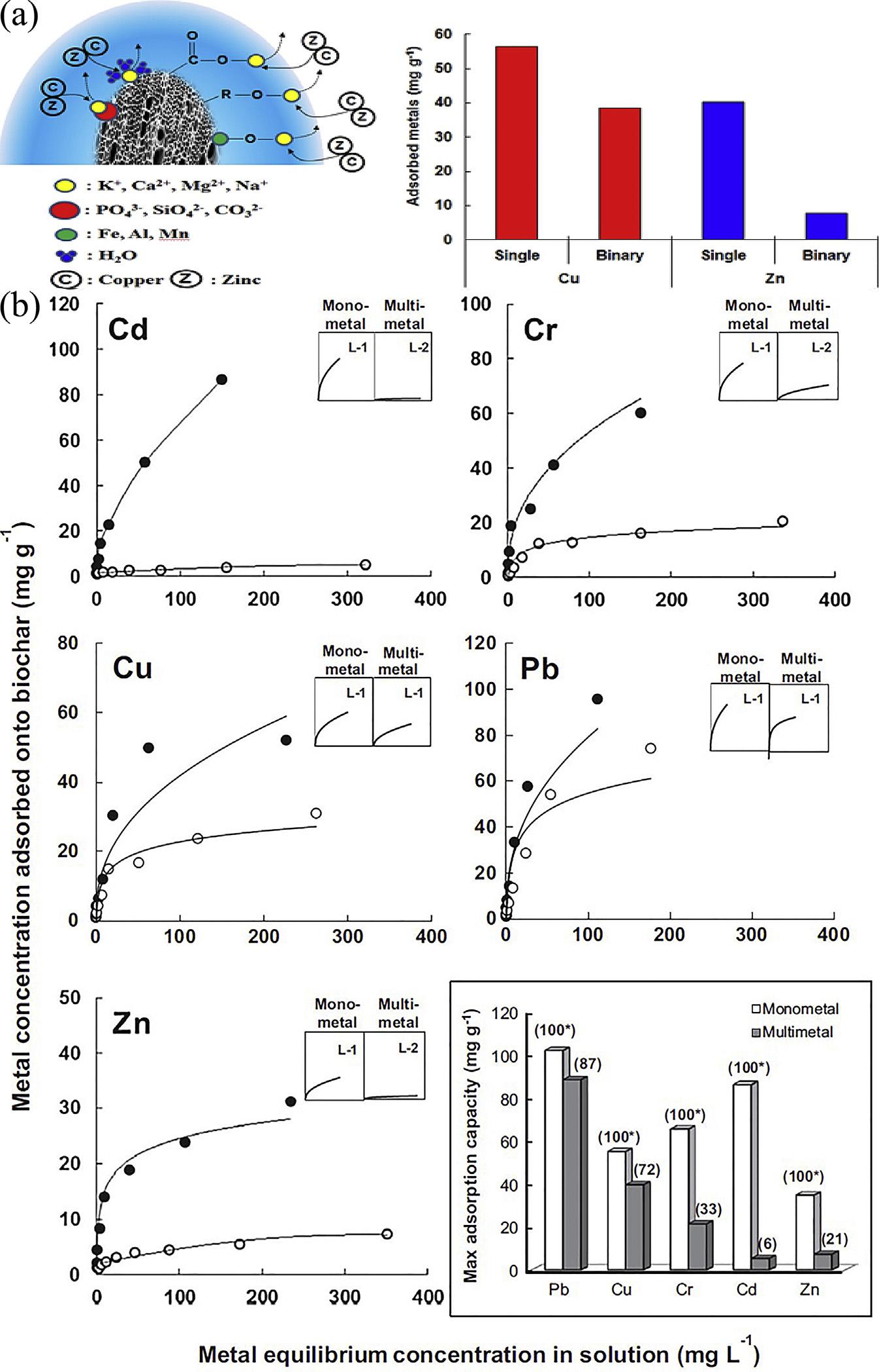
ACisconsidereduniversaladsorbentbecause ofitsinherentphysicalpropertiessuchaslarge surfacearea,porousstructure,highadsorption capacityandlargereactivesurface.Extensive researchonheavymetaladsorptionfromindustrialwastewaterusingACfromagriculturalor industrialproductswasteshavebeencarried out.Forinstance,Shresthaetal.[151]purchased theligniteandcoconutshell-basedACsfor Zn(II)adsorption.ThemaximumadsorptioncapacityforZn(II)ionswasfoundtobe 9.43mg$g 1.Parketal.[152]preparedthe porousbiocharfromricestrawat600 Cto removetheCu(II)andZn(II).TheadsorptioncapacitiesofricestrawbiocharcalculatedbyLangmuirmodelforCu(II)andZn(II)were56.5and 38.6mg$g 1 insingle-metalsystemand40.2 and7.9mg$g 1 inbinary-metalsystem,respectively.TheresultsdemonstratethattheCu(II) andZn(II)adsorptionbyricestrawbiochar wastheexchangeablecationandcomplexation, asshownin Fig.6.15A.Xuetal.[37]usedthe dairymanuretoproducedtwokindsofchars (DM200andDM350)at200and350 C.ACpyrolyzedatthehightemperatureshowbetteraffinityforCu(II),Zn(II)andCd(II),whichis attributedtoitshighercontentofCO3 2 .The maximumsorptioncapacitiesofCu,Zn,and CdbyDM350were54.4,32.8,and51.4mg$g 1 , respectively.Parketal.[153]alsocollected
sesamestrawfromalocalagricultural fieldand usedtoproducebiocarbonbypyrolyzingat 700 Cfor4h.Theyhadtestedthecompetitive sorptionofmetalsbytheadsorbents.The maximumadsorptioncapacities(mg g 1)of metalsbyACdeterminedfromtheLangmuir isothermswereintheorderofPb(102) > Cd (86) > Cr(65) > Cu(55) > Zn(34)inthemonometaladsorptionisothermandPb(88) > Cu (40) > Cr(21) > Zn(7) > Cd(5)inthemultimetaladsorptionisotherm,asshownin Fig.6.15B. ACshowsthepotentialfortheremediationof heavymetal.However,theadsorptioncapacity needstobefurtherimproved.
ImprovingtheadsorptionpropertyofAC,itis akeyparametertoimprovetheporositystructure.Theactivatedagentsuchasacid,alkali andZnCl2 andsooncanenhancetheporosity structure.Xuetal.[33]producedACsfrom reedygrassleavesbychemicalactivationwith H3PO4 inN2 atmosphere,thesurfacearea oftheACsproducedat500 Cfor2hwere 1474m2 g;Anisuzzamanetal.[154]produced thebestACusingphysicalandchemicalactivationphosphoricacid(H3PO4),whichwasfound tobearound1238m2 g 1 oftheSBET.Mohammadietal.[155]preparedhigh-surface-areaAC bychemicalactivationofglycyrrhizaglabra residuewithZnCl2 asactiveagent.ZnCl2 modifiedAChadhigherSBET ¼ 1836.46m2 g 1 are largerthanthatofHCl(3.56m2 g 1)andKOH (20.98m2 g 1)modifiedactivatedcarbonatthe pyrolysistemperatureof700 C,anditsadsorptioncapacitycanachieve398.4mgg 1 for Cr(VI).Andthemaximumadsorptioncapacity ofPb(II)andNi(II)ionswerefoundtobe200 and166.7mgg 1 .
ImprovingtheadsorptionpropertyofACcan beexpectedtoenhancetheadsorptionsitesor surfacefunctionalgroups.Lianetal.[156]preparedACbychemicalmodificationusing HNO 3 andH3PO4 toenhancePb(II)adsorption. Batchadsorptionexperimentsrevealedthe improvementofadsorptioncapacityby39times overtheunmodi fiedAC.Themaximum
FIGURE6.15 (A)Adsorptionmechanismofricestrawbiocharandsingle-andbinary-metaladsorptionisothermsforCuand Znbyricestrawbiochar[152];(B)Monometalandmultimetaladsorptionisothermsforthe fivemetals(Cd,Cr,Cu,PbandZn)by sesamestrawinthebatchexperiment.(C)Monometaladsorptionisotherm,(B)Multimetaladsorptionisotherm. Squarebox: Comparisonofthemaximumadsorptioncapacityoftheheavymetalsinmonometalandmultimetaladsorptionisotherms[153].
adsorptioncapacitiescalculatedfromLangmuir modelswere120.6,123.9,and131.6mgg 1 , respectively,at298,308,318K.Pb(II)was mainlycombinedwiththecarboxyl,hydroxyl, andphosphatefunctionalgroupsviaelectrostaticinteractionsandhydrogenbonding.Yunus etal.[157]preparedtwotypesofhoneydewpeel activatedcarbonwith10%,20%and30%of H2SO4 (HDP-ACS)andH3PO4 (HDP-ACP). SBET israngedfrom706.35to950.09m2 g 1 . Themaximumadsorptioncapacity(Qmax)was 834.94mgforCr3þ and326.19mgforZn2þ per gramofHDP-ACS.Onthecontrary,HDP-ACP had888.85mgforCr3þ and336.51mgforZn2þ g 1.Kasnejadetal.[158]introducednitrogen functionalgroupssuchasamine,pyridinic,and pyrrolicontothesurfaceofadsorbentstoreplace oxygengroupsforproducingstrongadsorbents towardheavymetalslikeCu(II)ion.Heattreatmentofthepreoxidizedadsorbentsundertheatmosphereofammoniaat800 Cfor3h.The nitrogenfunctionalgroups fixedonthesurface ofadsorbent,gaveabasiccharacteristictoit. Thisbasicnaturecouldstrengthenthereaction ofACsurfacewithCu2þ ionsaccordingtothe acid baseLewistheory.TheCu2þ adsorption testsshowedthattheaminatedcarboncould adsorbCu(II)withahigherinitialrateand greatercapacitythanthevirginadsorbent andtheaminatedonewithoutpreoxidation. Thenitrogenfunctionalgroupsoftheperoxideaminatedsamplewerefoundastheresponsible sitesfortheenhancedmetalionadsorptioncapacityandrate.
ACalsocombinewithotherchemicalcompoundtoimproveitsperformance.Anovelengineeredbiocharpreparedthroughmodification withchitosanandpyromelliticdianhydride (PMDA)wasinvestigatedasanadsorbentfor theremovalofheavymetalionsfromsinglemetalandmixed-metalsolutions(Cd,Cuand Pb).TheLangmuirmodel fitbetterthanthe Freundlichmodel,implyingthatthemonolayer adsorptionplayedasigni ficantroleinthemetal removalmechanism(Fig.6.16A C).The
chitosan-PMDAmodifiedbiocharhadstrongselectiveadsorptionofCu(II).Thetypesofeffectivefunctionalgroupfortheseheavymetal removalsweredifferent.TheN C]Ogroup playedadominantroleintheprocessofPb(II) removal,whileseveralN-containingfunctional groupsandC]Cgroupsparticipatedinthe adsorptionofCd(II).Thenovelengineeredbiocharhadselectiveadsorptioncapacityforcopper duetotheN-containingfunctionalgroups, meanwhileabundantcarbonylgroupsalso participatedintheremovalofcopper,andmay reduceCu(II)toCu(I)[159].Poly(acrylicacid) modifiedactivatedcarbonnanocomposite (PAA-AC)wassynthesizedforCd(II)removal. Themaximumcapacityandequilibriumtime foradsorptionofCd(II)byPAA-ACwere 473.2mg$g 1 and15min (Fig.6.16DandE). Moreover,theremovalofCd(II)forrealelectroplatingwastewaterbyPAA-ACcouldreach 98.5%.ThesemeantthattheremovalofCd(II) byPAA-ACwashighlyef ficientandfast.The adsorptionmainlywasachemicalprocessby chelation[160].ACmodifiedbylinearpolyethyleneimine(PEI)wastopreparepolyethyleneiminemodifiedAC(PEI/AC)adsorbent,which wasappliedintheCd(II)ionsadsorptionin wastewater.TheadsorptionofCd(II)byPEI/ ACwasapH-dependentprocesswitha maximumadsorptioncapacityof45mgg 1 at aninitialsolutionpHof6 7[163].Atwo-inoneattemptfortheremovalofmetalionson AChasbeendeveloped.Themethodwasbased onthemodificationofACwithtartrazinethen itsapplicationfortheremovalofPb(II),Cd(II), andCr(III)ionsatdifferentpHvalues.The Qmax were121.3,67and56.7mg$g 1 atinitial pHvaluesof1.0,6.0and10,respectively.The adsorptioncapacityforPb(II),Cd(II),andCr(III) ionshasbeenimprovedwithrespecttononmodifiedcarbonreachingamaximumof140% [164].ForeasyseparationofACfromsolution, magneticactivatedcarbon/chitosancomposite (MAC/CS)fromspentcoffeegroundsand shrimpshellsweresynthesizedusinggreentea
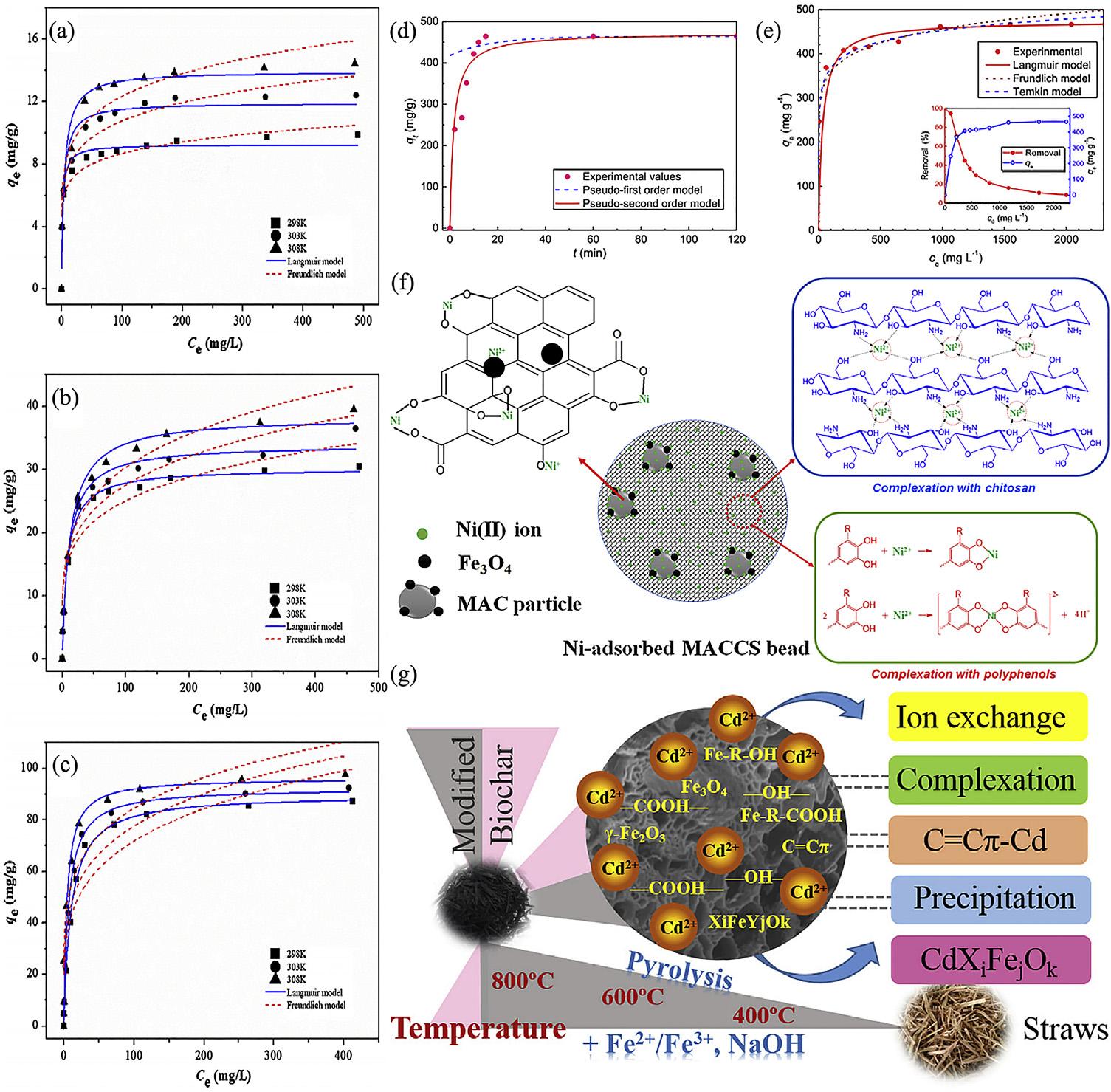
FIGURE6.16 Adsorptionisothermsoflead(A),cadmium(B)andcopper(C)ontochitosanandpyromelliticdianhydride (PMDA)modifiedbiochar(CPMB)[159];(D)AdsorptionkineticsofCd(II)onpoly(acrylicacid)modifiedactivatedcarbon nanocomposite(PAA-AC);(E)AdsorptionisothermsandremovalofCd(II)onPAA-AC[160];(F)Schematicillustrationof thepossiblemechanismfortheadsorptionofNi(II)ionsontoMACCSbeads[161];(G)adsorptionmechanismofCd2þ onto Fe2þ/Fe3þ andNaOHmodifiedbiochar[162].
extractasacross-linker[161].ApossibleadsorptionmechanismsofNi(II)ionsontotheMAC/ CSbeadsisproposed,wheretheadsorptionprocessmainlytookplacethroughionexchange, complexationandelectrostaticattraction (Fig.6.16F).Thebiocharwasmodifiedby Fe2þ/Fe3þ andNaOH,andafurtheranalysisof theadsorptionofCd(II)onthenewbiochar wasconducted.Theadsorptioncapacityfor cadmiumofthemodifiedbiocharwas
406.46mgg 1,whichwas16timesthatofthe originalbiochar;theC O Festructurethat formedonitssurfacewasthemainreasonfor thesharpincreaseinadsorption.Amongthe ironcomponents,ironoxides(Fe3O4, g-Fe2O3 andFe O Fe),iron-containingfunctional groups(-Fe-R-COOHandFe-R-OH,etc.)and themineralcrystalreactedwiththeCd(II)ion inaqueoussolutiontoexchange,formcomplexesandprecipitate,achievingthepurpose
of fixingtheheavymetal.Inaddition,thearomaticstructureC¼Cp canalsoadsorbCd2þ to generateC¼Cp-Cd,asshownin Fig.6.16G [162]. Nowadays,nanomineralmodi fi edACshow apromisingadsorptioncapacityforpollutants removalsbycombiningtheadvantagesof porousstructureofcharanduniqueproperty ofnanominerals.Thezincoxideloadingto granular-ACswasusedforPb(II)adsorption. ThezincoxideloadingtoACwasfoundtobe effectivelyusedforthePb(II)adsorption. Fromtheexperimentalresults,thesurface functionalgroupsresp onsibleforthePb(II) adsorptiononthezincoxideloadedACwere consideredhydroxylgroupsthatformedon theoxide,whilethoseontheoxidizedAC wereconsideredcarboxylicgroups[ 49 ].In addition,nanoZnO/ZnSmodi fi edbiochar wassynthesizedfromslowpyrolysisofthe zinccontaminatedcornstoverobtainedfrom abiosorptionprocess. Thecharacterization resultsindicatedthatthezincmineralmodi fi ed biocharhadabetterporousstructure (BET ¼ 397.4m2 $g 1)thanthecommonbiochar (BET ¼ 102.9m 2 g 1 ),andzincmineralswere evenlyanchoredonthebiocharsurfaceas nanoZnO/ZnS.Theenhancementofthemetals removalbythenanoZnO/ZnSmodi fi edbiocharweremainlyattributedtothehydroxyl groupsonthesurfaceofnanoZnO/ZnSparticlesandwell-developedporousstructurecatalyzedbyzincsaltduringpyrolysisprocess [50 ].Inaddition,othernewtechniquewas developedtofunctionateAC.Highlyconcentratedaminogroup-modi fi edbiocharsderived from12biomassresidualsareachievedusing atwo-stepradio-frequencyAr/NH 3 plasma. TheuptakecapacitiesofPb(II)(123.1mg $g 1 ), Cu(II)(98.5mg $g 1 ),andCd(II)(61.2mg $g 1) onaminatedbiocharsare5.42,5.38and7.56 timeshigherthanthoseonrawbiochars. Thisstudyshowsattractiveprospectsfor thefundamentalresearchinpowdermodi fi cationaswellaspotentialsinenvironmental management[ 165 ].
3.2Graphene-basednanomaterials
Graphenehastremendouspotentialin adsorption.ItsmainderivationshaveGOand RGO,whichcontainhydroxyl,carboxylic,and epoxidefunctionalgroupsonthesurfaces. Consideringthehighsurfaceareaandabundant functionalgroupsontheGOsurfaces,thematerialshowedhighadsorptioncapacityinthe removalofheavymetalionsfromaqueoussolutions.Zhaoetal.[166]preparedfew-layeredGO (FGO)bymodifiedHummers’ method.Thecaxisspacingofgraphiteincreasedfrom0.34to 0.87nmduringtheoxidationprocess,which wasattributedtotheformationoftheoxygencontainingfunctionalgroups(e.g.,C Oand C]O)onthesurfacesofFGO.Themaximum removalcapacityofPb(II)onFGOat293K was842mg$g 1.Wangetal.[167]prepared RGObyreducingGOwithethylenediamine. TheadsorptionbehaviorofRGOforPb(II), Cd(II),Cu(II),andMn(II)wasstudied.The adsorptioncapacitiesofRGOwerefoundto be413.22,162.33,55.34and42.46mgg 1 for Pb(II),Cd(II),Cu(II),andMn(II),respectively, andadsorptionreachesequilibriumwithin 60min.
Graphenehasshownexcellentpropertiesin wastewatertreatment,butstillmanydrawbacks existwhichlimitsthepracticalapplicationsof thegraphene-basedmaterials.Forinstance,graphene/RGOsheetswithahydrophobicgraphitic latticetendtoundergolayerto-layeraggregation inwaterduetohydrophobicforces,whereasGO sheetswithcarboxyl,hydroxyl,andepoxygroups onthesurfacecanformrelativelystablesuspensions[168,169].Inordertoenhanceitsdispersibilityandtoreducetheaggregationofgraphene/ GO,allkindsofsurface-modifiedgraphene/GO weresynthesizedviachemicalmodificationor attachingastabilizersuchasasolublepolymer orasurfactanttograftdifferentfunctionalgroups onthesurfaces.VariousorganicmoleculesfunctionalizedGOmaterialshavealreadyfabricated andappliedinadsorptionareas[170 176]. 6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials
Sitkoetal.[170]preparedtheaminosilanized grapheneoxide(GO-NH2)waspreparedfor selectiveadsorptionofPb(II)ions,asshownin Fig.6.17A.Theadsorptioniscontrolledbya chemicalprocessinvolvingthesurfacecomplexationofPb(II)ionswiththenitrogen-containing groupsonthesurfaceofGO-NH2.Suchfeatures ofGO-NH2 nanosheetsaswrinkledstructure, softness, flexibility,andexcellentdispersibility inwaterallowachievingverygoodcontact withanalyzedsolution,andadsorptionofPb(II) ionsisveryfast.Tanetal.[171]enhancedsorptioncapacitiesofGOforcopper(II)andlead(II) underweaklyacidicconditionsby L-tryptophan-functionalization(GO/l-Trp).Thenumber of-COONaincreasesafterGOreactedwith 1-Trpand-COONaonGO/1-Trpplaysasubstantialroleinenhancingsorptioncapacities. The OH, NH2,and COOHgroupsonGO/ 1-TrparenegativelychargedwiththepH increase,asshownin Fig.6.17B,anditssorption capacitytowardCu(II)andPb(II)isstrengthened. Carpioetal.[172]producedgrapheneoxidefunctionalizedwithethylenediaminetriaceticacid (GO EDTA)forheavymetaladsorption.The maximumadsorptioncapacityoftheGO EDTA wasdeterminedtobe454.6and108.7mg g 1 for Pb2þ andCu2þ.Lietal.[173]synthesizedthe diethylenetriaminepentaaceticacid(DTPA)modifiedmagneticgrapheneoxide(MGO)for removalofCu(II),Pb(II),andCd(II)ionsfrom acidicaqueoussolutions.DTPA/MGOcompositesexhibitedexcellentadsorptionpropertyin acidicaqueoussolutions.ThemaximumadsorptioncapacitiesatpH3.0forCu(II),Pb(II),and Cd(II)ionswere131.4,387.6,and286.5mgg 1 , respectively(Fig.6.17C).Yapetal.[174]designed cysteaminefunctionalizedRGO(Cyst-prGO) bythiol-eneclickreactionandappliedtothe removalofHg(II).Thehigheliminationcapacity (169 19mg$g 1),highselectivityandhigh regenerationabilitywereachieved(Fig.6.17D).
Nodoubtthatchemicalmodificationof GOwithdifferentfunctionalgroupshaveshown highadsorptioncapacity,fastadsorption
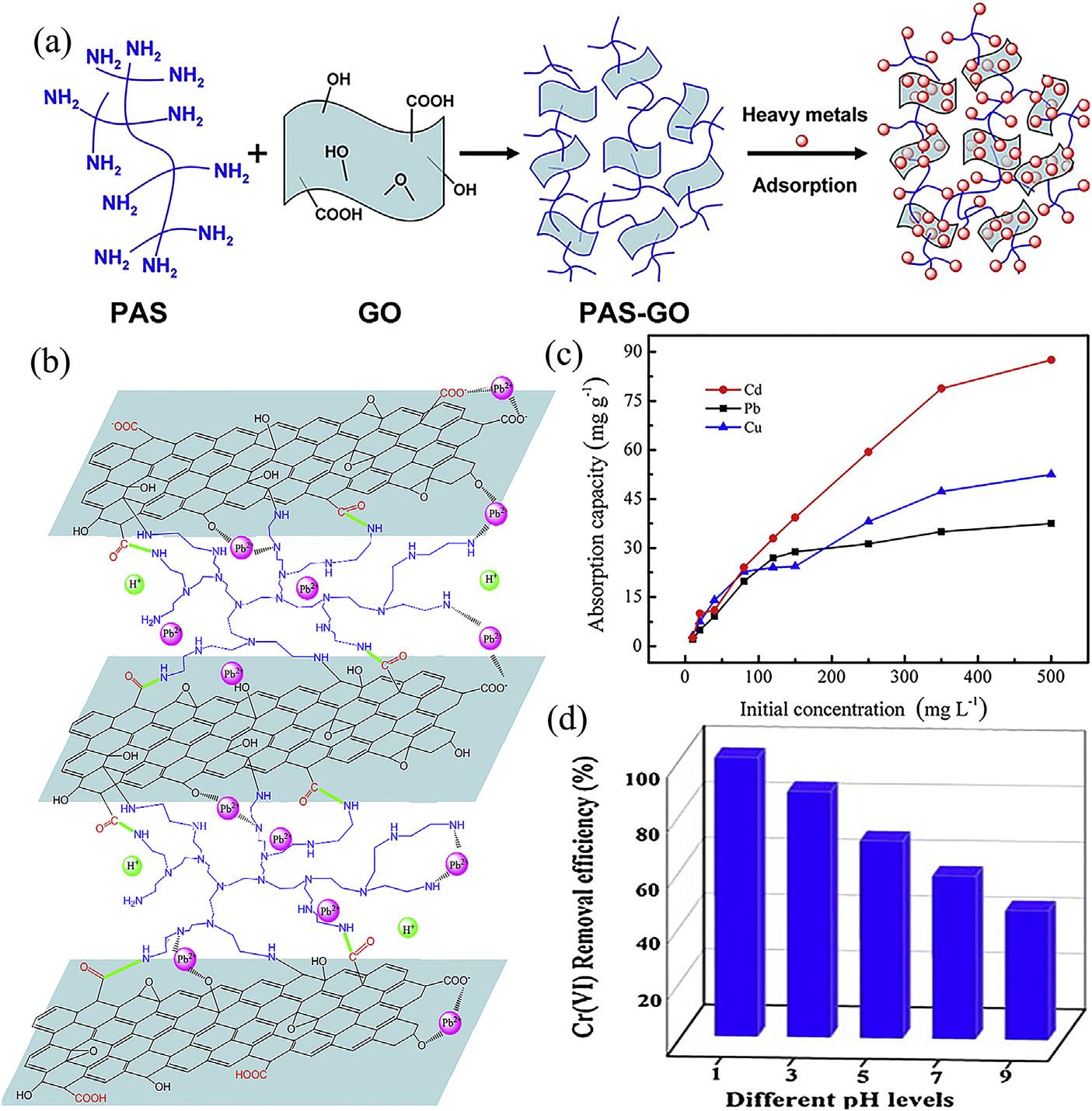
kinetics,andsuperiorselectivityinthepreconcentrationofheavymetalionsfromwastewater. GO/polymercompositeshaveexcellentadsorptionpropertiesforheavymetalions[177 182]. TheactivefunctionalgroupsfromGOandpolymercaninteractwithheavymetalionsthrough ionexchange,surfacecomplexation,andchelation,thehighspecificsurfaceareacanalsoprovideenoughadsorptionsitesandthereby enhancestheadsorptioncapacityofGO/polymer compositesforthebindingofheavymetals.Luo etal.[177]fabricatedanefficientadsorbentbya simplecross-linkingreactionbetweenGOand oligomericpoly3-aminopropyltriethoxysilane (PAS)oligomer,asshownin Fig.6.18A.PASGOhasaprioritytendencytoadsorbPband Cufromamixedsolutionofmetalions,especially frompracticalindustrialeffluent.Liuetal.[178] studiedthegrapheneoxidescross-linkedwith hyperbranchedpolyethylenimine(GO-HPEI)on thePb(II)ions,asshownin Fig.6.18B.TheGOHPEIgelexhibitedanadsorptioncapacityas highas438.6mg$g 1 forPb(II)ions,anditsinsolubilityinwatermakestheseparationfromwastewatereasy.Musicoetal.[179]usedpoly(Nvinylcarbazole)(PVK)toblendwithGOtoform aPVK GOpolymernanocompositeforremoval ofPb(II)fromwater.ThehighestadsorptioncapacityofthePVK GOnanocompositeforPb2þ was887.98mg g 1 and fitswelltheLangmuir model.Lietal.[180]successfullysynthesizedchitosan/sulfydryl-functionalizedGOcomposite (CS/GO-SH)viacovalentmodificationandelectrostaticself-assembly.Intheternarymetalion system,theresultsdemonstratedthatCS/GOSHisaneffectiveadsorbentwhenvariousions existsimultaneously.Theorderofadsorption competitivecapacityisCd(II) > Cu(II) > Pb(II), asshownin Fig.6.18C.Yakoutetal.[181]have studiedtheextractionofCu(II)andPb(II)ions fromdifferenttypesofaqueoussolutionbynovel cross-linkedGOsheetsbymodifiedextracted cellulose.Thecross-linkedGOsheetsshowed maximumadsorptioncapacity46.39and 186.48mg$g 1 forCu(II)andPb(II),respectively.
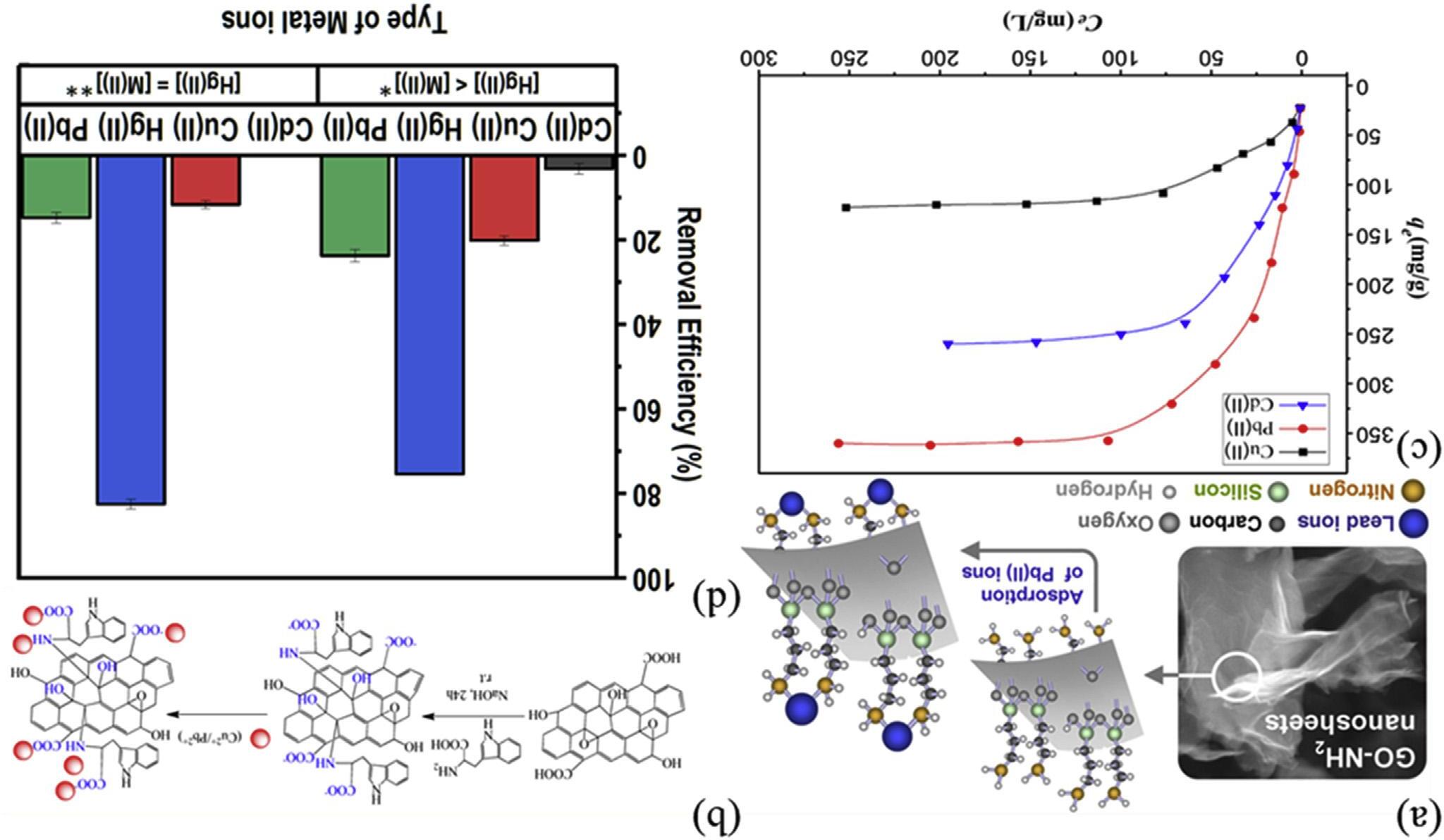
FIGURE6.17 (A)Adsorptionmechanismofaminosilanizedgrapheneoxidenanosheets(GO-NH2)forlead[170];(B)Adsorptionmechanismof Ltryptophan-functionalizedgrapheneoxideforcopper(II)andlead(II)[171];(C)AdsorptionisothermsofCu(II),Pb(II),andCd(II)ionsonDTPA/MGO (pH ¼ 3.0,mV 1 ¼ 0.4mgmL 1,T ¼ 293K,t ¼ 24h)[173];(D)Influenceofco-ionsonHg(II)adsorptionbyCyst-prGO(*[Hg(II)] ¼ 30 mg$L 1,[M (II)] ¼ 1000 mg$L 1;**[Hg(II) ¼ [M(II)] ¼ 1000 mgL 1)[174].
FIGURE6.18 (A)SynthesizeprocessandadsorptionmechanismofPAS-GOforPb(II)[177];(B)Adsorptionmechanismof GO-HPEIforPb(II)[178];(C)AdsorptionisothermsofCd(II),Pb(II),andCu(II)adsorbedbyCS/GO-SHinternarymetalion systems(dosageofCS/GO-SH1.0mg mL 1 at293K,initialsolutionpHof5,contacttime90min)[180];(D)Cr(VI)removal efficiencyofG-PDAPatdifferentpHlevelswith500mg L 1 initialCr(VI)concentration[182]. 3.Removalofheavymetalionsbycarbon-basednanomaterials
Dindaetal.[182]synthesizedsulfuricaciddoped diaminopyridinepolymersinsituonGOsurface (G-PDAP)viamutualoxidation-reductiontechnique.Itshowsveryhighadsorptioncapacity (609.76mg$g 1)duringremovalprocess.The compositetakesonly100mintoremovethe highconcentrationof500mg L 1 Cr(VI)from water.Interestingfeaturesforthismaterialare theenhancementofremovalefficiencyatlower acidicconditionduetotheformationofacid
dopedemeraldinesaltduringpolymerization, asshownin Fig.6.18D.Thepreparationofthe GO-organiccompoundnanocompositeswasnot complicated,andthesurfacegraftedorganiccompoundcouldprovidemoreactivesitesand oxygen-containingfunctionalgroups,which werefavorablefortheformationofsurfacecomplexeswithmetalionsandtherebyenhanced thesorptioncapacityofthecomposites.Another advantageofsuchcompositesisthatthesurface
graftedorganiccompoundscouldpreventtheaggregationoftheGOinaqueoussolutions,which isalsofavorabletoprovidemorefunctional groupsandactivesitesforthebindingofmetal ionstothecomposites.
However,agreatchallengeisthatthehighwatersolubilityandsmallsizeofthesefunctionalizedGOmaterialsmakethemextremelydifficulttobeseparatedfromwastewaterafter heavymetalionsadsorption.Toovercomethis deficiency,Fe3O4 wascompoundedwithGOto formmagneticgraphene/ironoxidecomposites (Fe3O4/GO)whichshowedtheadvantagesof bothFe3O4 andGOnanomaterialswithhigh sorptioncapacityandseparationefficiency. Harijanetal.[183]synthesizedmagnetitegraphenecomposite(MGC)forCr(VI)removal. ThemaximumsorptioncapacityforCr(VI)on MGCwasmuchhigherthanthatofFe3O4 nanoparticles.Thegraphenesheetscouldnotonly preventtheagglomerationofFe3O4 nanoparticlesandenableagooddispersionofthisoxide butalsosubstantiallyenhancethespecificsurfaceareaofthecomposite.Kumaretal.[184] synthesizedgrapheneoxide-MnFe 2O4 magnetic nanohybrids(GONH)foref ficientremovalof PbandAsfromwater.Thisexceptionaladsorptionpropertyisduetothecombinationofthe uniquelayerednature(allowingmaximumsurfacearea)ofthehybridsystemandthegood adsorptioncapabilitiesofboththeGOandNP. Bhuniaetal.[185]reportedthatthemaximum adsorptioncapacityofRGO-Fe(0)-Fe 3O4 for As(III)isashighas44mg$g 1,followedby RGO-Fe(0)(37mg$g 1),thismaterialcanalso adsorbotherheavymetalionslikeCr(VI), Cd(II),Pb(II),andHg(II)ionsfromwastewater. ResearchersalsotriedtosynthesizedmultifunctionalMGO[186 190].Sunetal.[186]prepared magnetictriethylenetetramine-grapheneoxide ternarynanocomposite(CoFe2O4-TETA-GO) andapplicationforCr(VI)removal.ThesaturatedadsorptioncapacityofCr(VI)wasabout 180.12mgg 1 ontheCoFe2O4-TETA-GOat pH ¼ 2.Lietal.[187]synthesizedmagnetic
chitosanandgrapheneoxide-ionicliquid (MCGO-IL)compositesasbiodegradablebiosorbentsbyimpregnatingMCGOwithionic liquid.Thestrongerintermolecularhydrogen bondbetweenMCGO-ILandCr(VI)andthehydroxylandaminegroupswerebelievedtobe themetalionbindingsites.Cuietal.[188]used EDTAfunctionalizedMGO(EDTA-mGO),a goodadsorbentforPb(II),Hg(II)andCu(II),was synthesizedinthepresentwork.Themaximum adsorptioncapacitywas508.4mgg 1 forPb(II), 268.4mg$g 1 forHg(II)and301.2mg$g 1 for Cu(II)fromLangmuirisotherm.Inaddition,there areresearcherfurtherfunctionatedthemagnetic graphene/GO.
Inaddition,GOcanalsointeractwithother commonlyusedmetaloxidessuchasMnO2 SiO2,ZnO,andZrO2 andtherebycanform compositesfortheapplicationinwastewater treatment.Wangetal.[191]fabricatednovelgrapheneoxide-orderedmesoporoussilicamaterialswith2Dmesoporousstructureandlarge surfaceareathroughsol-gelandself-assembly methods.Theresultsshowedthatthematerials exhibitedsuperioradsorptioncapacity,the removalefficienciesforAs,Cd,Cr,Hg,andPb reached97.7%,96.9%,96.0%,98.5%,and78.7%, respectively.Lingamdinneetal.[192]synthesizedahybridgrapheneoxidebasedinverse spinelnickelferrite(GONF)nanocompositematerialandsubsequentutilizationinheavymetal removalfromaqueoussolution.Santhoshetal. [193]synthesizedcobaltferritesdecorated2D porousgraphenenanocomposites(PG-C)for thecationicandanionicmetalionsremoval. TheadsorptioncapacityofPG-CforPb(II)ions wasfoundtobe131.40and68.85mg$g 1 for Cr(VI).Luoetal.[194]preparedGO-ZrO(OH)2 byhydrothermalcoprecipitationandtheadsorptioncapacitiesforAs(V)andAs(III)were84.89 and95.15mgg 1,respectively,whichwere 4.64and3.54timeshigherthanthatofZrO(OH)2 nanoparticles.Accordingtotheresultsreported byRenetal.[195],graphene/d-MnO2,which wassynthesizedbyoxidation-reduction 6.Watertreatmentandenvironmentalremediationapplicationsofcarbon-basednanomaterials