CellularMigrationandFormation ofAxonsandDendrites
ComprehensiveDevelopmentalNeuroscience
SecondEdition
SeniorEditors-in-Chief
JohnRubenstein
DepartmentofPsychiatry & WeillInstituteforNeurosciences UniversityofCalifornia,SanFrancisco,SanFrancisco,CA,UnitedStates
PaskoRakic
DepartmentofNeuroscience & KavliInstituteforNeuroscience YaleSchoolofMedicine,NewHaven,CT,UnitedStates
Editors-in-Chief
BinChen
DepartmentofMolecular,Cell & DevelopmentalBiology UniversityofCalifornia,SantaCruz,SantaCruz,CA,UnitedStates
KennethY.Kwan
MichiganNeuroscienceInstitute & DepartmentofHumanGenetics UniversityofMichigan,AnnArbor,MI,UnitedStates
SectionEditors
AlexKolodkin
SolomonH.SnyderDepartmentofNeuroscience JohnsHopkinsUniversitySchoolofMedicine,Baltimore,MD,UnitedStates
EvaAnton
UNCNeuroscienceCenter & DepartmentofCellandMolecularPhysiology UniversityofNorthCarolinaSchoolofMedicine,ChapelHill,NC,UnitedStates
AcademicPressisanimprintofElsevier 125LondonWall,LondonEC2Y5AS,UnitedKingdom 525BStreet,Suite1650,SanDiego,CA92101,UnitedStates 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
Copyright © 2020ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,including photocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher. Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangements withorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmay benotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding, changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusingany information,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbe mindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforany injuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseor operationofanymethods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-814407-7
ForinformationonallAcademicPresspublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: NikkiLevy
AcquisitionsEditor: NatalieFarra
EditorialProjectManager: AndraeAkeh
ProductionProjectManager: SuryaNarayananJayachandran
CoverDesigner: MarkRogers
CoverImage: JasonKeil
TypesetbyTNQTechnologies
Contributors
FrançoisBeaubien,MontrealNeurologicalInstitute, McGillUniversity,DepartmentofNeurologyand Neurosurgery,Montréal,QC,Canada
FranckBielle,InstitutduCerveauetdelaMoelleEpinière, Paris,France
FrankBradke,LaboratoryforAxonGrowthandRegeneration,GermanCenterforNeurodegenerativeDiseases (DZNE),Bonn,Germany
FrédéricCharron,InstitutdeRecherchesCliniquesde Montréal(IRCM),Montreal,QC,Canada;Integrated PrograminNeuroscience,McGillUniversity,Montreal, QC,Canada;DepartmentofAnatomyandCellBiology, DepartmentofBiology,McGillUniversity,Montreal, QC,Canada;DepartmentofMedicine,Universityof Montreal,Montreal,QC,Canada;DivisionofExperimentalMedicine,McGillUniversity,Montreal,QC, Canada
AlainChédotal,InstitutdelaVision,SorbonneUniversité, INSERM,CNRS,Paris,France
Jean-FrançoisCloutier,MontrealNeurologicalInstitute, McGillUniversity,DepartmentofNeurologyand Neurosurgery,Montréal,QC,Canada
ChristopherL.Cunningham,SolomonH.Snyder DepartmentofNeuroscience,JohnsHopkinsUniversity SchoolofMedicine,Baltimore,MD,UnitedStates
FernandodeCastro,InstitutoCajal-CSIC,Spanish ResearchCouncil/ConsejoSuperiordeInvestigaciones Científicas-CSIC,Madrid,Spain
KevinC.Flynn,StemCellandGeneTherapy,BioTechne,Minneapolis,MN,UnitedStates
FernandoGarcía-Moreno,AchucarroBasqueCenterfor Neuroscience,ParqueCientí ficoUPV/EHUEdif.Sede, Leioa,Spain;IkerbasqueFoundation,Bilbao,Spain
SoniaGarel ,InstitutdeBiologiedel’ÉcoleNormale Supérieure(IBENS),PSLUniversité,Paris,France; InstitutNationaldelaSantéetdelaRechercheMédicale(INSERM)U1024,Paris,France;CentreNational delaRechercheScientifique(CNRS)UMR8197,Paris, France
R.J.Giger,TheUniversityofMichigan,MedicalSchool, AnnArbor,MI,UnitedStates
WesleyB.Grueber,ColumbiaUniversity,NewYork,NY, UnitedStates
K.Hayashi,KeioUniversitySchoolofMedicine,Tokyo, Japan
ZhigangHe,KirbyCenterofNeuroscience,Children’s HospitalBoston,HarvardMedicalSchool,Boston,MA, UnitedStates
HoldenHigginbotham,DepartmentofBiology,Brigham YoungUniversity,Rexburg,ID,UnitedStates
KatrineIversen,MontrealNeurologicalInstitute,McGill University,DepartmentofNeurologyandNeurosurgery,Montréal,QC,Canada
ArturKania,NeuralCircuitDevelopmentLaboratory, InstitutdeRecherchesCliniquesdeMontréal(IRCM), Montreal,QC,Canada;IntegratedPrograminNeuroscience,McGillUniversity,Montreal,QC,Canada; DepartmentofAnatomyandCellBiology,Divisionof ExperimentalMedicine,McGillUniversity,Montreal, QC,Canada
EyalKarzbrun,KavliInstituteofTheoreticalPhysicsand DepartmentofPhysics,UniversityofCalifornia,Santa Barbara,CA,UnitedStates
ArnoldR.Kriegstein ,DepartmentofNeurology,UniversityofCalifornia,SanFrancisco,CA,UnitedStates; TheEliandEdytheBroadCenterofRegeneration MedicineandStemCellResearch,UniversityofCalifornia,SanFrancisco,CA,UnitedStates
ZeljkaKrsnik ,CroatianInstituteforBrainResearch, SchoolofMedicine,UniversityofZagreb,Zagreb, Croatia
ChristopheLaumonnerie,DepartmentofDevelopmental Neurobiology,St.JudeChildren’sResearchHospital, Memphis,TN,UnitedStates
JulieL.Lefebvre,TheHospitalforSickChildren,Toronto,ON,Canada;DepartmentofMolecularGenetics, UniversityofToronto,Toronto,Canada
FannyLepiemme,GIGA-StemCells/GIGA-Neurosciences,UniversityofLiège,Liège,Belgium
GuangnanLi,DepartmentofNeurology,Universityof California,SanFrancisco,CA,UnitedStates
JosephJ.LoTurco,UniversityofConnecticut,Mans field, CT,UnitedStates
LeMa,ThomasJeffersonUniversity,Philadelphia,PA, UnitedStates
Jean-BernardManent,InmedInserm,Marseille,France
JulieMarocha,TheHospitalforSickChildren,Toronto, ON,Canada;DepartmentofMolecularGenetics,UniversityofToronto,Toronto,Canada
ZoltánMolnár,DepartmentofPhysiology,Anatomyand Genetics,UniversityofOxford,Oxford,United Kingdom
K.Nakajima,KeioUniversitySchoolofMedicine,Tokyo, Japan
LaurentNguyen,GIGA-StemCells/GIGA-Neurosciences,UniversityofLiège,Liège,Belgium
StephenC.Noctor,DepartmentofPsychiatryand BehavioralSciences,UCDavisMINDInstitute,Sacramento,CA,UnitedStates
HirofumiNoguchi,DepartmentofNeurology,University ofCalifornia,SanFrancisco,CA,UnitedStates
R.JeroenPasterkamp,DepartmentofTranslational Neuroscience,UMCUtrechtBrainCenter,University MedicalCenterUtrecht,UtrechtUniversity,Utrecht, TheNetherlands
SamuelJ.Pleasure,DepartmentofNeurology,University ofCalifornia,SanFrancisco,CA,UnitedStates
F.Polleux,ColumbiaUniversity,NewYork,NY,United States
TatianaPopovitchenko,DepartmentofNeuroscienceand CellBiology,RutgersUniversity,RobertWoodJohnsonMedicalSchool,NewBrunswick,NJ,UnitedStates
JanetE.A.Prince,MontrealNeurologicalInstitute, McGillUniversity,DepartmentofNeurologyand Neurosurgery,Montréal,QC,Canada
Mladen-RokoRasin,DepartmentofNeuroscienceand CellBiology,RutgersUniversity,RobertWoodJohnsonMedicalSchool,NewBrunswick,NJ,UnitedStates
OrlyReiner,DepartmentofMolecularGenetics,The WeizmannInstituteofScience,Rehovot,Israel
MasatoSawada,DepartmentofDevelopmentaland RegenerativeNeurobiology,InstituteofBrainScience, NagoyaCityUniversityGraduateSchoolofMedical Sciences,Nagoya,Japan
KazunobuSawamoto,DepartmentofDevelopmentaland RegenerativeNeurobiology,InstituteofBrainScience, NagoyaCityUniversityGraduateSchoolofMedical Sciences,Nagoya,Japan;DivisionofNeuralDevelopmentandRegeneration,NationalInstituteforPhysiologicalSciences,Okazaki,Japan
K.Sekine,KeioUniversitySchoolofMedicine,Tokyo, Japan
CarlaSilvaG.,GIGA-StemCells/GIGA-Neurosciences, UniversityofLiège,Liège,Belgium
DavidJ.Solecki,DepartmentofDevelopmentalNeurobiology,St.JudeChildren’sResearchHospital,Memphis,TN,UnitedStates
ConstantinoSotelo,InstitutdelaVision,SorbonneUniversité,INSERM,CNRS,Paris,France
H.Tabata,KeioUniversitySchoolofMedicine,Tokyo, Japan
MarcTessier-Lavigne,StanfordUniversity,Stanford,CA, UnitedStates
StephenR.Tymanskyj,ThomasJeffersonUniversity, Philadelphia,PA,UnitedStates
MariekeG.Verhagen,DepartmentofTranslationalNeuroscience,UMCUtrechtBrainCenter,University MedicalCenterUtrecht,UtrechtUniversity,Utrecht, TheNetherlands
FanWang,DepartmentofNeurobiology,DukeUniversity,Durham,NC,UnitedStates
FrancoWeth,KarlsruheInstituteofTechnology,ZoologicalInstitute,DepartmentofCellandNeurobiology, Karlsruhe,Germany
PatriciaT.Yam,InstitutdeRecherchesCliniquesde Montréal(IRCM),Montreal,QC,Canada
JingYang,SchoolofLifeSciences,PekingUniversity, Beijing,China
BingYe,UniversityofMichigan,AnnArbor,MI,United States
BernardZalc,UniversitéPierre & MarieCurie-Paris6, CentredeRecherchedel’InstitutduCerveauetdela Moelleépinière,UMR_S975,InsermU975,CNRS UMR7225,HôpitaldelaSalpêtrière,Paris,France
1.2Axoninitiationinvitroversusinvivo
1.2.1Axoninitiationinvitro
Historically,theadventofinvitro dissociatedneuronalculturesprovidedanexperimentaltemplateforimprovingour understandingofthecellbiologyofneuronalpolarity,includingthespecificationofaxonsanddendrites.Pioneeringwork usingtheseculturesestablishedaparadigminwhichisolatedneuronsinculturecanadoptspatiallyandfunctionallydistinct dendriticandaxonaldomains(CraigandBanker,1994;GoslinandBanker,1989).Carefulanalysisoftheseculturesledto theobservationthatculturedhippocampalneuronstransitionthroughseveralstages:fromfreshlyplatedstage1cellsbearing immatureneuritestostage5cellsthatexhibitmatureaxons,dendrites,dendriticspines,andfunctionalsynapses(Fig.1.1A) (CraigandBanker,1994;Dottietal.,1988).Itshouldbenotedthat,intheclassicalE18rathippocampalcultures,mostplated
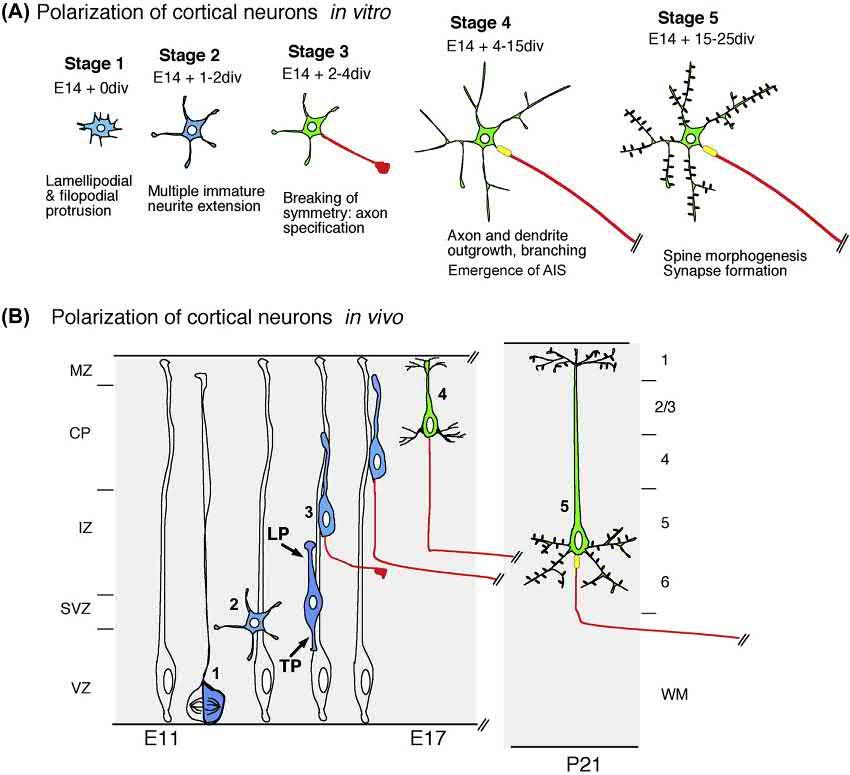
FIGURE1.1 Parallelbetweenneuronalpolarizationinvitroandinvivo.Comparisonofthesequenceofeventsleadingtothepolarizationofcortical pyramidalneuronsinvivoandinvitro.(A)Indissociatedcultures,postmitoticcorticalneuronsdisplayspecifictransitionsasclassicallydescribedfor hippocampalneuronsby Dottietal.(1988).Atstage1,immaturepostmitoticneuronsdisplayintenselamellipodialand filopodialprotrusiveactivity, whichleadstotheemergenceofmultipleimmatureneurites,stage2.Stage3representsacriticalstepwhenneuronalsymmetrybreaksandasingleneurite growsrapidlytobecometheaxon(purple)whileotherneuritesacquiredendriticidentity.Stage4ischaracterizedbyrapidaxonanddendriticoutgrowth. Finally,stage5neuronsareterminallydifferentiatedpyramidalneuronsharboringdendriticspinesandtheaxoninitiationsegment(AIS).(B)The axon dendritepolarityofpyramidalneuronsisderivedfromthepolarizedemergenceofthetrailing(TP)andleadingprocesses(LP),respectively. Invivo,pyramidalneuronsacquireotherkeyfeaturesoftheirterminalpolaritysuchastheAIS(yellowcartridge)anddendriticspines(grayprotrusions) duringthe firstpostnatalweeksofdevelopment.
cellswerepolarizedpostmitoticneuronsbeforedissociation;therefore,neuronalpolarizationusingthisinvitromodellikely correspondstothe “repolarization” ofpreviouslypolarizedneuronsinvivo.Itisthereforeimportanttokeepinmindthat molecularmanipulationsinthisinvitromodelactonpreviouslypolarizedneuronsthatmayretainsomeaspectsofpolarization,andthiscanbecriticalforinterpretingresultsfromsuchmanipulations.Recentadvancesintechniquesthatallow manipulationofgeneexpressionmorespecificallyinneuralprogenitors,suchasinuteroorexuterocorticalelectroporation (Handetal.,2005;HatanakaandMurakami,2002;SaitoandNakatsuji,2001;TabataandNakajima,2001),providea paradigmto(1)manipulategeneexpressioninprogenitors,beforeneuronalpolarizationoccursuponcellcycleexit;and(2) visualizetheearlieststagesofneuronalpolarizationinacontextualcellularenvironment,includinginorganotypicslicesor intactembryonicbrain(Barnesetal.,2007;CalderondeAndaetal.,2008;Handetal.,2005).
1.2.2Axoninitiationinvivo
Neuronalpolarizationcanbedividedintoseveralspeci ficstepsinvivo.Uponcellcycleexit,mammalianneuronsusually migrateoverlongdistancesbeforereachingtheir finaldestinations.Invivo,mostneuronsundergoaxon dendritepolarizationduringmigration.Whileinitiatingradialmigration,neocorticalPNsformaleadingprocessandatrailingprocess, eachbecomingtheaxonorthedendrite,respectively(Fig.1.1B).Carefulexaminationofthemorphologicaltransition betweenneuralprogenitorandpostmitoticneuronrevealsthatneuronscaninherittheiraxonanddendritepolaritydirectly fromtheapicobasalpolarityoftheirprogenitors.Thisisthecaseforretinalganglioncells(RGCs)andbipolarcellsinthe developingvertebrateretina(HindsandHinds,1978;Morganetal.,2006;Zolessietal.,2006,reviewedin Barnesand Polleux,2009).Inotherneuronalsubpopulationsundergoinglong-rangemigration,neuronalmorphogenesisinvolves extensivestereotypicalchanges,leadingtopolarizedoutgrowthoftheiraxonsanddendrites.Thisisthecaseforcerebellar granuleneurons(CGNs)aswellascorticalandhippocampalPNs,twoofthebest-studiedmodelsofneuronalpolarization (GaoandHatten,1993;HatanakaandMurakami,2002;Komuroetal.,2001;Noctoretal.,2004;Rakic,1971,1972; ShoukimasandHinds,1978).
BothCGNsandPNsacquiretheiraxon dendritepolarityfromthepolarizedemergenceoftheirtrailingandleading processes,respectively,duringmigration(reviewedin BarnesandPolleux,2009).Preciseexaminationoftheprocess dynamicsoccurringshortlyaftercellcycleexitindorsaltelencephalicprogenitorssuggeststhatthereisoftenaslightdelay betweenformationoftrailing(axoninitiation)andleading(dendriteelaboration)processes,withtrailingprocessformation frequentlyprecedingleadingprocessformation(CalderondeAndaetal.,2008;HandandPolleux,2011;Nambaetal., 2014).However,onethingisclearinbothPNsandCGNs:Axonformationstartsbeforeorduringradialmigration. Interestingly,differentneuronalpopulationsdisplaydistinctmodesofaxonformation,reflectingtheirmodeofmigration, lineage,andtypeofaxonprojection.Forexample,corticalinterneurons,whichwillformaxonsprojectingonlylocally withinthecortex,originatefromtheventraltelencephalonandmustmigrateoververylongdistancesbeforeinitiatingtheir axonuponreachingtheir finaldestinationinthecortex(BortoneandPolleux,2009;Cobosetal.,2007;Yamasakietal., 2010).Theprecisemechanismsunderlyingaxonemergenceincorticalinterneuronsarelargelyunknown.However,they arestrikinglydifferentfromthoseemployedbyradiallymigratingpyramidalcorticalneurons,whichinitiateaxonformationduringmigration.Thisleadstothehypothesisthattheabilityofinterneuronstoformanaxonisinhibitedduring tangentialmigrationandthataxoninitiationincorticalinterneuronsmaydependuponfactor(s)presentonlyintheirtarget environment,thecortex.
Asdiscussedlaterinthechapter,anemergingconceptfromrecentworkdoneprimarilyin Caenorhabditiselegans suggeststhat,invivo,the “symmetry-breaking” eventsthatleadtotheemergenceofthedendriteandtheaxonrequirethe abilityofpostmitoticneuronstosensegradientsofextracellularcues,leadingtotheasymmetricactivationofsignaling pathwaysunderlyingtheemergenceoftheaxon.Thesedataaresupportedbyrecentevidenceinmammalsshowingthat extracellularcuessuchasdiffusibleTGF b ormembrane-boundcelladhesionmoleculessuchasTAG-1playrolesinaxon specificationinvivobytriggeringspecificsignalingpathwaysintheneurite’strailingprocessthatbecomestheaxon(Yi etal.,2010;Nambaetal.,2014).
1.3Distinctionbetweencuesregulatingaxonspecificationversusaxongrowth
Moststudiespublishedoverthepasttwodecadesinthis fieldhavebeenperformedusinginvitroapproaches.Theclassic paradigmforconfirmingtheregulatoryroleofageneinneuronalpolarityistoshowthatdownregulationofitsexpression usingshRNAtechnologyorgeneknockouttechnologyisrequiredforaxonformation.Theseexperimentsaretypically doneusingstainingwithaxon-speci ficmakersandmeasurementofneuritelengthsincetheaxonusuallygrows5 10times fasterthanneuritis,whichwilleventuallybecomedendrites.However,thistypeofevidencemaynotbesuf ficientto
distinguishunambiguouslyaneffectorofaxonspecificationfromamoleculesimplyrequiredforaxongrowth(Jiangetal., 2005).Conversely,showingthatoverexpressionoroveractivationofacandidatemoleculeleadstotheemergenceof multipleneuritesthatdisplaythemolecularidentityofanaxonisgenerallyusedtosuggestthatthismoleculeissuf ficient toconferaxonidentity.However,thisapproachislimitedbythefactthatitreliesonoverexpression,whichcanbe confoundedbyabnormalactivationofapathwaynormallynotinvolvedinaxonspecifi cationorneuronalpolarity.Recent technicaladvancesallowforthemanipulationofgeneexpressioninvivoandincludeinuterocorticalelectroporationin rodentcortexorcerebellum(Famulskietal.,2010;SaitoandNakatsuji,2001)andalsotransgenicapproachesin Xenopus (Zolessietal.,2006).Therefore,morebiologicallyrelevantvalidationofcandidategenefunctionduringneuronalpolarizationoftenincludestestingitsrequirementusinggeneknockoutorshRNA-mediatedknockdowntechnologiesorthe analysisofconventionalorconditionalknockoutincombinationwithinuteroelectroporationallowingsinglecellresolutionanalysisofaxonformation(Barnesetal.,2007;Shellyetal.,2007;Yietal.,2010;Nambaetal.,2014).Finally,one importantcaveatoftheinvivoapproachesmentionedinthischaptertostudymammalianaxonspeci ficationisthatseveral ofthemoleculesidentifiedbyloss-of-functionanalysisnotonlyaffectaxon/dendrite(trailing/leadingprocess)generation butalsooftenwhencompromisedleadtoabnormalinitiationofradialmigration.Thiseffectonneuronalmigrationcan complicatetheinterpretationofexperimentalresultssincedefectsinaxonspecificationcouldbethedirectresultofthe molecule’sfunctioninaxonspecifi cation,orthesedefectscouldresultfromsecondaryeffectsthatarearesultofpreventing thepostmitoticneuronfromrespondingtoextracellularcuesthatarerequiredforneuronalpolarization.
1.4Extracellularcuesregulatingneuronalpolarizationandaxoninitiation
1.4.1Netrin-1andWntcontrolaxoninitiationin Caenorhabditiselegans
Isthereanyinvivoevidencefortheroleofextracellularcuesinthespecifi cationofneuronalpolarity?Signifi cantprogress inourunderstandingofthemolecularandcellularmechanismsspecifyingaxoninitiationduringneuronalpolarizationhas beenmadeusing C.elegans asamodel.Thispioneeringworkhasmarkedlyenhancedourunderstandingofhowextracellularcuesinstructaxoninitiationinvivo(reviewedby YogevandShen,2017).Theneuronsofthenematodehavea stereotypedmorphology,ascanbeseenwithrespecttospeci ficprojectionsalongthedorsoventralandanterior posterior bodyaxes.Elegantexperimentsinvolvinggeneticscreenshaveidentifi edanextracellularcue,UNC-6(Netrin),alongwith itsreceptorUNC-40(DCC),ascriticalgenesfororchestratingaxoninitiationinvivo(Adleretal.,2006).Thisworkalso identi fieddownstreamproteinsinthispathway,including(mammalianorthologsareshowninparenthesis,whenknown) AGE-1(phosphoinositide-3kinase[PI3K]),DAF-18(PTEN),UNC-34(Enabled),CED-10(Rac),UNC-115/AbLIM,and MIG-10/lamellipodin.ThecurrentmodelfortherelationshipamongthesegenesandUNC-6/netrinsignalinginvolves DAF-18’slimitationofAGE-1activityfollowingUNC-40/DCCstimulationandtheasymmetricrecruitmentofMIG-10/ lamellipodintotheplasmamembrane.ThisrecruitmentrequiresactivatedCED-10/RacbindingdirectlytoMIG-10/ lamellipodinandtheinvolvementofthePAK-likekinase,Pak-1(Adleretal.,2006).Thefunctionofakinaseinthe regulationofcytoskeletalrearrangementisconsistentwithasimilarroleplayedbyMIG-10/lamellipodinandthelikely mechanismbywhichitoperatesoncerecruitedtotheplasmamembranetostimulatedirectedneuriteoutgrowth.Another regulatorthoughttoactinconcertwithMIG-10/lamellipodintodrive filopodialformationistheEnabledhomolog,UNC34(Changetal.,2006).Finally,SLT-1(Slit)isanotherextracellularcuethatlikelyactsthroughMIG-10recruitmentto controlneuronalpolarization(Changetal.,2006).
Twootherstudieshaveidenti fiedthediffusiblesignalWntanditsreceptorascriticalregulatorsofaxonspeci fication andneuronalpolarity(HilliardandBargmann,2006;PrasadandClark,2006).Inadditiontocharacterizinglossoffunction mutantsinthegenesencodingLin-44(Wnt)anditsreceptorLin-17(Frizzled[Fzl]),ageneticscreenhasidenti fiedVPS35,acomponentofretromercomplexthatregulatesvesiculartraf ficandisrequiredforproperWntsecretion,asan importantregulatorofneuronalpolarity(Panetal.,2008;PrasadandClark,2006).
1.4.2Polarizedemergenceoftheaxoninretinalganglioncellsof Xenopus
Detailedliveimagingexperimentsof Xenopus RGCpolarizationrevealedthatpolarizedaxonoutgrowthrequiresunidenti fiedextracellularcuespresentinthebasallamina(Randlettetal.,2011;Zolessietal.,2006).Theaxonofdeveloping RGCsnormallygrowsonthebasalsideoftheneuron.Inamutantcalled Nok,characterizedbytheabsenceofretinal pigmentedepithelium,somepostmitoticRGCneuronsshowdefectivepolarizedaxonoutgrowthontheapicalsidealong thenow-exposedbasallamina.Inthiscontext,thepolarizedemergenceoftheaxononthebasalsideoftheRGCis correlatedwiththepositionofthecentrosome,Par3,andtheapicalcomplex(containingtheatypicalproteinkinaseC
[aPKC], b-catenin,andF-actin)ontheapicalsideofthecellwherethedendritewillemerge.Takentogether,thiswork stronglysuggeststhat(1)thebasallaminacontainsimportantextracellularcuesthatplayaroleinorganizingthepolarized emergenceoftheRGCaxonand(2)RGCneuronsinherittheintrinsicapicobasalpolarityoftheirprogenitoratleastwith respecttothePar3/aPKCcomponentsofthepolaritycomplex.
Recently,asignalingcascadehasbeenlinkedtopotentialextracellularcuesregulatingaxoninitiationinvivo(Barnes andPolleux,2009;Barnesetal.,2008).ConditionaldeletionofLKB1inpyramidalcorticalneurons(alsocalledPar4or STK11)demonstratedthatLKB1isrequiredforaxoninitiationincorticalneuronsbutdoesnotimpacttheirradial migration(Barnesetal.,2007).Structure/functionanalysisindicatesthatphosphorylationofLKB1atSerine431is requiredforitsfunctioninaxonspeci fication(Barnesetal.,2007).Thisphosphorylationeventhasbeenlinkedtothe abilityofextracellularcuessuchasbrain-derivedneurotrophicfactor(BDNF)tostimulatecAMPproductionandprotein kinaseA(PKA) dependentphosphorylationofLKB1onS431inthenascentaxon(seebelowfordetails; Shellyetal., 2007,2010,2011).
1.4.3Extracellularcuesunderlyingtheemergenceofaxonanddendritesinmammalianneurons
Severallinesofevidencesuggestthatextracellularcuescandirectthepolarizedemergenceoftheaxonanddendritesboth invitroandinvivo.OneparadigminvolvesdissociatedcorticalorhippocampalPNsplatedonstripedsubstratescoated withtwodifferentcelladhesionmolecules(e.g.,lamininandNgCAM,reviewedin BarnesandPolleux,2009).The first immatureneuriteofE18hippocampalneuronscontactingtheboundarybetweentwostripessystematicallybecomesthe axon.ThisoccursdespiteinitialoutgrowthofimmatureneuritesoccursonlamininorNgCAM,suggestingthatimmature neuritescandetectchangesinthenatureoftheextracellularsubstrateratherthantheabsolutenatureofthenovelsubstrate theyareencountering(Eschetal.,1999).Usingasimilarapproach,Shellyandcolleaguesshowedthatneuritesof immaturehippocampalneuronsgrowingonapatternedsubstratecandetectthepresenceofBDNF,whichplaysan instructiveroleinaxonspecificationbecausethe fi rstneuritecontactingaBDNFstripesystematicallybecomestheaxon (Shellyetal.,2007).TheeffectofBDNFonaxonspecifi cationrequirescAMP-dependentPKAactivationandphosphorylationofLKB1atposition431byPKA(Shellyetal.,2007,2010),suggestingthatLKB1phosphorylationonS431 actsasadetectorthatbreaksneuronalsymmetryfollowingencounterswithextracellularcuessuchasBDNFinthisinvitro context.
Todetecttheexistenceofextracellularcuesthatplayaroleincorticalaxonguidanceandneuronpolarization,an overlayinvitroassaywasdeveloped.Thisrathersimpleassayinvolvesplating fl uorescentlylabeleddissociatedcortical neuronsontocorticalslicestotestwhetherpolarizedaxonemergenceinvivoismainlytheresultofasymmetricactivation ofintracellulareffectors(perhapsinheritedbyprogenitors)orextracellularcuesthatdirectaxonspecifi cation.Polleuxetal. demonstratedthatthesecondscenarioismostlikelybecauseonlyacoupleofhoursafterplating,thevastmajorityof corticalneuronsdisplayedasingle,shortaxondirectedventrallytowardtheventricle(Polleuxetal.,1998),asobservedfor radiallymigratingneuronsinvivo.Theseauthorswentontodemonstratethattheclass3secretedsemaphorin,Sema3A, whichisenrichedinthemostsuper ficialpartofthecorticalwall(thetopofthecorticalplate),playsaroleinrepulsing axoninitiationventrallytowardtheventricle(Polleuxetal.,1998).Morerecently,Sema3Awasshowntoalsoregulatethe polarizedemergenceoftheleadingprocess/apicaldendritebothintheoverlayassay,thatis,independentlyofradial migrationwhereitrequirescGMPproductionandPKGactivation(Polleuxetal.,2000),andalsoinvivoduringradial migration(Chenetal.,2008).Interestingly,Sema3Acanplayaroleinthespecifi cationofdendriticidentitybyactivatinga cGMP-dependentpathwayinvolvingactivationofcGMP-gatedcalciumchannels(Nishiyamaetal.,2011)andalsoby repressingaxonalidentityinaLKB1-dependentmanner(Shellyetal.,2007,2010,2011;see Fig.1.2).
Recently,TGFb signalingwasshowntoberequiredforthepolarizedemergenceoftheaxonofradiallymigratingPN invivo(Yietal.,2010, Fig.1.3).TGFb ligandsareexpressedinthegerminalzoneofthecortex,wheretheycouldactasan instructive “ventral” cueforthepolarizedemergenceoftheaxoninmultipolarneuronsbeforeengagingradialmigration. InvitroexperimentsdemonstratedthatlocalapplicationofTGFb onasingleneuriteinimmaturestage1corticalneuronsis sufficienttotriggerfastaxonalextension(Yietal.,2010).Importantly,conditionalgeneticdeletionofTGFb receptor2 expressionleadstotheproductionofneuronswithouttrailingprocess/axoninvivo.Onenoticeabledifferencecompared withtheconditionalablationofLKB1(Barnesetal.,2007),whichalsoleadstotheabsenceoftrailingprocess/axon formationbutnotradialmigrationdefects,isthatTGF b receptor2conditionaldeletionleadstoretardationofradial migrationinasubsetofcorticalneurons(Yietal.,2010)(Fig.1.2).
TGFb receptorfunctionduringaxonspecifi cationalsorequiresthephosphorylationofPar6onS435,whichwas previouslyshowntomediatetheepithelial-to-mesenchymaltransition(EMT; Ozdamaretal.,2005).Asdiscussedlaterin thischapter,this “noncanonical” TGFb receptor dependentsignalingrepresentsanattractiveinvivosignalingpathway
Polleux et al. (2000)
Shelly et al. (2010)
Shelly et al. (2011)
Nishiyama et al. (2011)
Barnes et al. (2007)
Shelly et al. (2007)
Yi et al. (2010) LKB1
Kishi et al. (2005)
FIGURE1.2 Extracellularsignalsregulateaxonanddendritespecificationinneocorticalpyramidalneurons(PNs).Time-lapseanalysishasrevealedthat polarizationofaxodendriticpolarityinPNoccursinvivoinastepwisemanneroncellcycleexit:Followingasymmetricdivisionofradialglialcells(step 1)orintermediateprogenitorsinthesubventricularzone(SVZ)(notshown),recentlygeneratedpostmitoticneurons firstformtransient,dynamicneurites (multipolarstage;step2).Axonspecificationoccurswhenatrailingprocess(TP)isstabilized(red)eitherbefore(step3)oraftertheformationofaleading process(LP)inneuronsengagingradialtranslocation(step4).Onreachingtheir finaldestinationatthetopofthecorticalplate(CP)(step5),neurons detachfromtheradialglialscaffoldandstartanextensiveprogramofaxongrowth(see Fig.1.1)anddendriticbranchingconcomitantwithsynaptogenesis (step6).
foraxonspeci ficationsinceitisknowntoinvolverecruitmentoftheubiquitinligaseSmurf1,whichinduceslocal degradationofRhoAduringEMTandhaspreviouslybeenimplicatedinaxonspeci fication(Schwambornetal.,2007a, 2007b).Therefore,theseresultssuggestthatneuronalpolarizationmighthavecooptedsignalingpathwaysthatregulate EMTforthepurposeofaxonspeci fication.
Overall,thisworksuggeststhatthepolarizedemergenceofasingleaxon/trailingprocessandtheapicaldendrite/ leadingprocessarecontrolledatleastinpartbyextracellularcuessuchasTGFb andSema3A,respectively,expressedina gradedmanneralongtheneuron’smigratorypath(Fig.1.2).Morerecently,anothertypeofextracellularcuethatis membrane-bound(thecelladhesionmoleculeTAG-1)andexpressedalongtheaxonofpreexistingneuronshasbeen showntoregulatetheemergenceofthetrailingaxonalprocessduringthemultipolar-to-bipolartransition(Nambaetal., 2014).Thisworksuggeststhat,atdifferenttimesduringcorticalneurogenesis(whenneuronsdestinedtopopulatedifferent layersaregenerated),differenttypesofcuessuchasdiffusiblegradedTGFb ormembrane-boundaxonalCAMssuchas TAG-1playrolesinaxogenesisandmaycoordinateaxogenesisandthedirectionalityofaxonoutgrowth(forexample, projectingmediallyforcallosalprojectionsvs.laterallyforcorticofugalprojections).
Moreworkisneededtoclarifywhetherornototherextracellularcuesarerequiredforthepolarizedemergenceofaxons anddendritesindiverseneuronalcelltypesandhowtheseextracellularcuesmediatetheireffectsonthespeci ficationofthe uniquemolecularaxonalanddendriticidentity(Fig.1.2).
1.5Intracellularpathwaysunderlyingneuronalpolarization
1.5.1Roleofproteindegradationandlocaltranslationinaxonspecificationandaxongrowth
Spatialregulationofproteinexpressionbyselectivedegradationhasbeendemonstratedinseveralcontextsduringneuronal development,includingaxonalpruning(Wattsetal.,2004),variousaspectsofaxonguidancedecisions(Bloometal., 2007;CampbellandHolt,2001;DiAntonioetal.,2001;Lewcocketal.,2007),synapseformation(DiAntonioetal.,2001; Nakataetal.,2005),synapsemaintenance(AravamudanandBroadie,2003;DiAntonioetal.,2001;Ehlers,2003;Speese etal.,2003),andsynapseelimination(Dingetal.,2007,reviewedin DiAntonioandHicke,2004).Acutetreatmentwith theproteosomeinhibitorlactacystinblocksaxogenesisindorsalrootganglioncells(Klimaschewskietal.,2006). Furthermore,moreprolongedinhibitionofproteindegradationwithlactacystinleadstotheformationofmultipleaxons
Smurf1
(Par3?)-aPKC
SAD-A/B
MAP phosphorylation–microtubule dynamics? Presynaptic vesicle trafficking? Axon spcification

FIGURE1.3 Intracellularpathwaysunderlyingaxonspecificationinresponsetoextracellularcuesinvivo.Asshownin Fig.1.2,recentdata(Yietal., 2010)providedevidencethatTGFb receptoractivationisrequiredforaxonspecification/trailingprocessstabilizationinvivo.Thisworkalsosuggested thatTGFb receptorsignalinginthecontextofaxonspecificationrequireda “noncanonical” branchofTGFb involvedinEMT,whichtriggersPar6 phosphorylationbyTGFb receptor2andrecruitstheubiquitinligaseSmurf1andlocallydegradesRhoA,threestepspreviouslyshowntoberequiredfor axonspecification.AnotherkinasepreviouslyshowntoberequiredinvivoforaxonspecificationisLKB1(Par4),whichisphosphorylatedonS431 specificallyintheaxoninresponsetoseveralsignalingpathways(PKA, p90RSK,oraPKC).LKB1phosphorylatesandactivatesseveralotherkinases, includingSAD-A/Bkinases,whichhavebeenshowntoberequiredforaxonspecificationinvivo.TheactuallinkbetweenTGFb receptor/Par6/Smurf1/ RhoAandtheLKB1/SADkinasepathwayiscurrentlyunknownbutmightinvolvedirectinteractionbetweenPar6andLKB1orphosphorylationofLKB1 byaPKC,whichisknowntoformacomplexwithPar6.ThedownstreameffectorsofSADkinasesandotherLKB1-dependentkinasesarecurrently unknownbutmightinvolvelocalphosphorylationofmicrotubule-associatedproteinssuchasTauorMAP1bbutprobablyinvolvemanyothereffectors, allinvolvedinaxonspecification.Proteinsindicatedinredhavebeenshowntoberequiredinvivoforaxonspecification,whereasproteinsindicatedin orangehaveonlybeenstudiedindissociatedneuronalculturesinvitro.
(Yanetal.,2006).TheproteinkinaseAKT,whichwedescribedearlierasbeingcriticalforneuronalpolarity,appearsto undergoselectivedegradation(Yanetal.,2006).Infact,thisdegradationselectivelytargetstheinactivepoolofAKTin neurites,resultinginanetenrichmentofAKTinasingleprocessthatcontainsactiveAKT,thenascentaxon.This phenomenonisconsistentwiththenegativefeedbacksignalmodelproposedby ArimuraandKaibuchi(2007) toexplain axonspeci ficationofasingleaxonduringneuronalpolarization.Schwambornetal.showedthatthesmallGTPaseRap1b isregulatedbyasimilarschemebecausetheactiveformofRap1bissparedfromdegradationandisultimatelyenrichedin theaxon(Schwambornetal.,2007b).Inthiscase,theubiquitinligaseactingonRap1bisSmurf2,whereastherelated Smurf1appearstoaffectonlyneuriteoutgrowth.AdditionalworkdemonstratesthataninteractionbetweenSmurf2andthe polarityscaffoldPAR3mustexistforproperneuronpolarization(Schwambornetal.,2007a).This fi ndingappearstobe relatedtoPAR3targetingofSmurf2totheaxonbecauseperturbationoftheinteractionofeitherPAR3 Smurf2or PAR3 KIF3AresultsinRap1bincreaseinallneurites.TheconversesituationexistsforLIMkinase(LIMK)sincelevels ofthisproteinmustbereducedforaxoninitiationinvitro(Tursunetal.,2005).Futureexperimentsshouldclarifythe molecularmechanismsregulatingthefunctionofSmurf1/2ubiquitinligasesinaxonspeci ficationinvivoespeciallyinthe contextofTGFb signaling(Yietal.,2010)(Fig.1.2).
1.5.2Roleofcytoskeletaldynamicsinaxoninitiationandgrowth
Appropriateregulationoftheactinandmicrotubulecytoskeletoniscriticalforneuronalpolarizationandhasbeenthefocus ofnumerousstudies.Experimentsusingtheactin-destabilizingagentslatrunculinBandcytochalasinDindicatethat remodelingoftheactin-basedcytoskeletonisanimportantregulatorystepinaxonformation(BradkeandDotti,1997). TGFβ (PKA, p90RSK)?
Sema3A
Speci fically,actindepolymerizationlocalizedtoasingleneuriteinunpolarizedstage2hippocampalneuronsissuffi cientto conferaxonalidentity.Aproposedmechanismisthatlooseactin filamentsallowtheegressofmicrotubulesandleadto rapidelongationofagivenneurite,perhapsoutpacingthetransportofnegativeregulatorsofaxonalidentity.Theideaof cellularasymmetriesbeingreinforcedbylocalizedmicrotubulestabilizationandinvasionproposedby Kirschnerand Mitchison(1986) waselegantlydemonstratedusingaphotoactivatableformofthetubulin-stabilizingcompoundtaxol, whichcandirectaxonalspeci ficationtoasingleimmatureneurite(Witteetal.,2008).Collectively,theseresultssuggest thatadynamicequilibriumbetweenactindepolymerizationandmicrotubulestabilizationplaysaroleinspecifyingaxonal initiationandaxonalidentity.Futureinvestigationswillneedtoidentifytheeffectorsregulatingthisbalancelocallyand alsocharacterizehowspecifi cupstreamsignalingpathwaysmightregulatethespatialandtemporalcontrolofthese cytoskeletaldynamics.
1.5.3Majorsignalingpathwaysinvolvedinaxoninitiationandgrowth
1.5.3.1LKB1anditsdownstreamkinasesSAD-A/BandMARK1-4
ApioneeringgeneticscreenperformedbyKemphuesandcolleaguesinthelate1980sidentifiedsix Par genesencodingdistinct proteinfamilies.Manystudieshavesincedemonstratedthatinvertebrateandvertebrate Par genesplaycriticalrolesinepithelial cellpolarityduringdevelopmentaswellasinthecontextofcelltransformationandmetastasis(GoldsteinandMacara,2007; Kemphuesetal.,1988).Althoughthispathwayiscriticaltopolarityinmanyspecies,thesignallinkingthispathwaytoextracellularcueshasremainedelusive.Thefurthestupstreamcomponentknowninthiscascadeisanevolutionarilyconservedkinase namedLKB1(alsocalledPAR4).LKB1translocatesfromthenucleusandisactivatedbyheterodimerizationwithoneoftwo relatedpseudokinasesknownasStrad-a andStrad-b (DorfmanandMacara,2008).InadditiontobindingStrad,LKB1function inneuronalpolarityrequiresitsphosphorylationatS431,atargetofbothPKAand p90RSKkinases(Collinsetal.,2000;Sapkota etal.,2001).ThisphosphorylationcanbetriggeredbyextracellularcuesincludingBDNF(Shellyetal.,2007, Fig.1.2).This eventmightbemediatedinpartbycuesthatprovidechemotacticattractionofradiallymigratingneuronstowardthecorticalplate, suchasSema3A(Chenetal.,2008;Polleuxetal.,2000),orbyotherextracellularcuesincludingtheneurotrophins(NTs)BDNF/ NT4/NT3(Shellyetal.,2007),Netrin(Adleretal.,2006),FGFs,orothercuesthatcanactivatecAMP-dependentPKAor p90 RSK(RSK1-3).Atthispoint,wecanspeculatethatanyotherpresentlyuncharacterizedserine/threoninePKA,ofwhichthereare several,abletophosphorylateS431onLKB1mightmediatethepolarizingfunctionofextracellularcuesfoundindifferent developingbrainregions(BarnesandPolleux,2009).Futureinvestigationwillidentifytherelevantextracellularcuesandthe correspondingsignalingpathwaysthattriggerphosphorylationofLKB1atpositionS431,therebyspecifyingtheaxonin developingcorticalPNsinvivo(Fig.1.3).
OnceLKB1isactivatedbybindingtoitsobligatecoactivatorStrad-a andS431phosphorylationoccurs(onlyinthe neuritethatbecomestheaxon),LKB1phosphorylatesSAD-A/Bkinases(andprobablythemicrotubuleaffinity regulated kinasesMARKs1 4; MateniaandMandelkow,2009).Thesesignalingeventsarerequiredforaxonspeci ficationinpartby theresultingphosphorylationofmicrotubule-associatedproteins(MAPs)enrichedintheaxon,suchasTau.Onthebasisof knownfunctionsofSADkinasesinpresynapticvesicularclusteringin C.elegans (Crumpetal.,2001),wehypothesize thatSAD-A/Bkinasesalsospecifyaxonidentitybydirectingpresynapticvesiculartraf fickingintheneuritethatisinthe processofbecomingtheaxon.Mostimportantly,geneticdeletionofLKB1incorticalPNspreventsaxonformation, whereasoverexpressionofLKB1anditscoactivatorStradinneuralprogenitors,orLKB1aloneinpostmitoticcells,is suf ficienttoleadtotheformationofmultipleaxons(Barnesetal.,2007;Shellyetal.,2007).
Experimentsin Xenopuslaevis suggestedthatLKB1mayregulateaPKCinactivationofglycogensynthasekinase3 (GSK3) a/b (Ossipovaetal.,2003),twoproteinsinvolvedinneuronalpolarity(seelater).However,atthispoint,theexact contributionofLKB1inadenomatouspolyposiscoli(APC)/GSK3functioninneuronalpolarityispoorlyunderstood. LKB1alsophosphorylatesandactivatesafamilyof13PKAsrelatedtothe C.elegans PAR1protein(Lizcanoetal.,2004). Todate,threeofthesehavebeenimplicatedinregulatingaxonformation:SAD-AandSAD-BkinasesaswellasMARK-2 (microtubuleaffi nityregulatingkinase-2;alsocalledPar1b).RNAiknockdownofSADkinasespartiallyabrogatesthe abilityofLKB1overactivationtoinducetheformationofmultipleaxonsincorticalneurons,indicatingthatLKB1’s functioninpromotingaxogenesislargelyisderivedfromitsactivationofSAD-A/Bkinases(Barnesetal.,2007).Double knockoutmicefor SAD-A and SAD-B resultsinneuronsthatcannotformaxonsinvivo(Kishietal.,2005),andoverexpressionofSAD-A/Binducesamodestbutsignificantincreasesintheformationofmultipleaxons(Choietal.,2008). SADandMARKkinasestargetseveralMAPs,includingMAP2,MAP4,andTaubyphosphorylatingthreeK-X-G-S motifswithineachprotein,reducingtheirmicrotubulebindingaffi nityandthusdestabilizingmicrotubules(Drewes etal.,1997;Illenbergeretal.,1996).LittleisknownaboutSADkinaseregulation;however,arecentstudysuggestedthat theproteinphosphatasePP2mightdownregulateSADcatalyticactivitybyreversingLKB1-mediatedphosphorylation
(Brightetal.,2008).Anotherstudyhasrecentlyimplicatedthetuberoussclerosiscomplex(TSC)genes TSC1/2 in regulatingSADproteinabundance(Choietal.,2008).Themicrotubuleregulatoryschemeisthesameforthefour membersofMARKkinasefamily,buttodateonlyMARK2hasbeenimplicatedinneuronalpolarity(Biernatetal.,2002; Chenetal.,2006).BecauseRNAi-mediatedknockdownofMARK2inducessupernumeraryaxonsandoverexpressionof MARK2inhibitsaxonformation,itistemptingtohypothesizethatMARK2isanegativeregulatorofaxogenesis(Chen etal.,2006).Intriguingly,MARK2caninteractwiththeserine/threoninekinasePAK5,andthisinteractionisthoughtto inhibitMARK2kinaseactivitywhilesimultaneouslydestabilizingactincytoskeleton(Mateniaetal.,2005).Thus,the MARK2/PAK5dyadmightcoordinateactinandmicrotubulecytoskeletaldynamicsduringtheestablishmentand/or maintenanceofneuronalpolarity(discussedbelow).
SeverallinesofevidencerevealthatotherpotentialregulatorsofneuronalpolarityactbyregulatingMARK2.GSK3a/b caninactivateMARK2catalyticactivitythroughphosphorylation,andsimilarly,aPKCcaninhibitMARK2activity throughT595phosphorylation(Timmetal.,2008).TheplanarcellpolaritysignalingmoleculesDishevelled1(Dvl1)and Wnt5aalsoappeartobeinvolvedintheMARK2/aPKCpathwayofneuronalpolarization(Zhangetal.,2007).Inthis scenario,Wnt5aactivationofitsreceptorFzlleadstothestabilizationofaPKCthroughitsdirectinteractionwithDvl1. ThisincreaseinaPKCactivitythenleadstoanincreaseintheinhibitoryphosphorylationofMARK2.Consistentwiththis model,increasedDvl1expressionleadstomultipleaxons,andRNAiknockdowninhibitsaxonformation(Zhangetal., 2007).Furthermore,thecombinationofRNAiinhibitionofbothMARK2andDvl1resultsinnormalaxonformation.cJunN-terminalkinase(JNK)isanotherpotentialtargetforDvl1signaling(CianiandSalinas,2007)andplaysarolein neuronalpolarization,andinhibitionofJNKblocksneuronalpolarizationinareversiblemanner(Olivaetal.,2006).
1.5.3.2PAR3 PAR6 APKC
Theothercorecomponentsofthepolaritycomplexidenti fiedin C.elegans arethescaffoldingproteinsPAR-3andPAR-6. Manybindingpartnersforthiscomplexhavebeenimplicatedinregulatingthepolarityofepithelialcells.Neuroepithelial radialgliadistributethePAR3/6complextotheirapicaldomainalongtheventricularwall(Bultjeetal.,2009;Costaetal., 2008;Manabeetal.,2002).ProteinsreportedtoexistinacomplexwithPAR3/6includeaPKCandthesmallGTPase Cdc42(Jobertyetal.,2000;Linetal.,2000;Qiuetal.,2000),thekinesinmotorproteinKIF3A(Nishimuraetal.,2004), theguanineexchangefactorTiam1/STEF(ChenandMacara,2005;Nishimuraetal.,2005),thelipidandproteinphosphatasePTEN(Fengetal.,2008;vonSteinetal.,2005),theGTPase-activatingprotein(GAP) p190RhoGAP(Zhangand Macara,2008),thetumorsuppressorlethalgiantlarvae(Lgl)(Plantetal.,2003),thescaffoldproteininscuteable(Schober etal.,1999),theubiquitinligasesSmurf1(Ozdamaretal.,2005)andSmurf2(Schwambornetal.,2007b),andtheTGFb receptors1/2(TGFR1/2; Ozdamaretal.,2005).Eachoftheseproteinshasalsobeenimplicatedincontrollingpolarityin nonneuronalcellsaspartofthePAR3/6complex.
PAR3/6areenrichedinthenascentaxoninstage3hippocampalneurons,andoverexpressionofwild-typeandalso truncatedformsofeitherPAR3orPAR6perturbstheformationofasingleaxonalprocessinhippocampalneurons(Shi etal.,2003).However,in Drosophila,orthologsofPAR3(bazooka),PAR6,oraPKCdonotappeartoberequiredfor properaxon dendritespeci fication(RollsandDoe,2004).ThiscouldmeanthatPAR3/6haveacquiredafunctionin neuronalpolaritylateduringevolutioninthevertebrateradiation.Alternatively,thereissofarnogeneticloss-of-function evidenceinvertebrates(especiallyinmammals)demonstratingthatPar3andPar6arerequiredforaxonspecifi cation.This evidencewillbeclearlymorechallengingtoobtaininmammalsthanin Drosophila becauseofpotentialgeneticredundancy:TherearefourPar6genes(PAR6A-D)andtwoPar3-likegenesinmammaliangenomes(Barnesetal.,2008; GoldsteinandMacara,2007).Sofar,invivoassessmentofPar3functioninthedevelopingcortexusingshRNA-mediated knockdownhasrevealedaclearfunctionwithrespecttotheabilityofradialglialprogenitorstodivideasymmetricallyto produceneurons(Bultjeetal.,2009),butnotasyetinneuronalpolarity.FutureinvestigationofinvivoPar3/Par6 functionsinaxonspecificationandneuronalpolarizationwillbeimportant.
TheroleofaPKCinneuronalpolarityistightlylinkedtoitsabilitytoassociatewiththePAR3 PAR6complex.The activityofaPKCisgreatlyreducedwhenassociatedwithPAR6(Yamanakaetal.,2001).Thispartnershipprovidesa regulatoryschemethatrequiresadditionalsignalingeventstoproduceaspatiallylimitedpoolofactivatedaPKC,since guanosinetriphosphate(GTP) boundcdc42bindingrelievesthisinhibition.PhosphorylationtargetsofaPKCincludethe PAR3/6-bindingpartnerLgl,andthisposttranslationalmodificationisthoughttoplayacrucialroleinregulatingthe subcellularlocalizationofLglduringpolarizationinseveralcellularcontexts(Betschingeretal.,2003;Plantetal.,2003; Yamanakaetal.,2003).Asmentionedpreviously,aPKCcanalsophosphorylatethePAR1orthologMARK2onthreonine 595(Hurovetal.,2004;Suzukietal.,2004),inthiscaseinhibitingitskinaseactivity.GlobalinhibitionofaPKCactivityin polarizingneuronsclearlydemonstratesaroleforthiskinaseintheestablishmentofneuronalpolarity,atleastinvitro(Shi
etal.,2003),consistentwithobservedenrichmentofactivatedaPKCinthenascentaxon(SchwambornandPuschel,2004; Zhangetal.,2007).PAR3/6axonallocalizationlikelyoccursviaPAR3interactionwiththemicrotubuleplus-end-directed kinesinKIF3AbecauseinterferencewithKIF3AfunctionleadstothedelocalizationofPAR3andaPKCfromthenascent axontip(Nishimuraetal.,2004).Asdiscussedpreviously,inhibitionofGSK3orperturbationofAPClocalizationalso eliminatesPAR3targetingfromthetipofthenascentaxon(Shietal.,2004),suggestingahierarchicalschemethatenriches thePar3/6complexinthedevelopingaxon.
1.5.3.3Ras-andRho-familyofsmallGTPases
SmallGTPasesarecriticalregulatorsofcytoskeletalandmembranedynamicsunderlyingcellmotility,cellpolarity,and cellgrowth.SmallGTPaseproteinsaremolecularswitchesthatgenerallyactondownstreameffectorswhenboundtoGTP andareinactivewhenthisGTPishydrolyzedtoguanosinediphosphate(GDP).Rho-GTPasespossessrelativelyslow intrinsicGTPhydrolysisactivity,andtheircatalyticactivityisregulatedbyGTPase-activatingproteins(GAPs:53predictedinthehumangenome).GAPsthereforeactasnegativeregulatorsofGTPaseactivitybypromotingtheGDP-bound (inactive)state.ActivationofsmallGTPasesbyexchangingGDPforGTPiscontrolledbyguaninenucleotideexchange factors(GEFs:69predictedinthehumangenome).Notsurprisingly,bothRas-andRho-familysmallGTPaseshavebeen showntobeinvolvedinaxonspecificationandaxongrowth.
SeveralmembersoftheRas-familyofsmallGTPaseshavebeenshowntoregulateneuronalpolarityincludingH-Ras, R-Ras,K-Ras,andN-Ras.Overexpressingeitherwild-typeoraconstitutivelyactivemutant(V12orQ61L)ofH-Ras,or therelatedproteinR-RasQ87L,leadstotheproductionofmultipleaxons(Fivazetal.,2008;Oinumaetal.,2007;Yoshimuraetal.,2006).RasproteinsregulateboththeMAPkinaseandPI3Kpathways,andpharmacologicalinhibitionof eitherpathwaywassuf ficienttoinhibittheproductionofadditionalaxons;surprisingly,thisdidnotimpactaxonformation, ingeneral(Yoshimuraetal.,2006).Rasactivationiscoupledtomanycellsurfacereceptorsincludinggrowthfactor receptors,andanEGFRtyrosinekinaseinhibitor,AG1478,caninhibitaxonformation(Shietal.,2003).Elegantwork usinga fluorescentreporterofRasactivationdemonstratestherestrictednatureofRassignalinganditsrecruitmentduring axondeterminationtocontributetoapositivefeedbackloopwithPI3K(Fivazetal.,2008).Additionalworkremainsto identifywhichupstreamactivatorsmayregulateRasduringthedisruptionofneuronalsymmetrytodeterminethenascent axon;wediscussinthefollowingsomepotentialcandidatesinthischapter.
ThebeststudiesofallmammalianRho-familysmallGTPases(thereare22intotal)areCdc42,RhoA,andRac1. Expressionofdominant-negative(lockedinGDP-boundstate)orconstitutivelyactive(lockedinGTP-boundstate)mutantsofeachofthesesmallGTPasesinpolarizingneurons,ortreatmentwiththeRho-GTPaseinhibitortoxinB(Bradke andDotti,1999),demonstratesacriticalroleforbothCdc42andRac1invitroinrodentneurons(Nishimuraetal.,2005; SchwambornandPuschel,2004)andinvivointhe Drosophila CNS(Luoetal.,1994).Speci fically,theexpressionof Cdc42L28,amutantCdc42thatconstitutivelycyclesbetweenaGDP-andGTP-boundstate,leadstotheformationof multipleaxonsinrodentneurons.ThelossofCdc42expression,througheithersiRNAknockdown(Schwambornand Puschel,2004)orgeneticablation(Garvalovetal.,2007),leadstoastrongaxonspeci ficationdefect.Inthecaseof Cdc42 conditionalknockoutmice,theaxonphenotypemaybeduetoincreasedlevelsofphosphorylated,inactive,cofilin,which isaregulatorofactindynamicsenrichedindevelopingaxons(Garvalovetal.,2007).Thisphosphorylationisachievedby LIMK,anactivitystimulatedbytheCdc42effectorkinasePak1.Paradoxically,Pak1activityisgreatlyreducedin Cdc42nullmice,suggestingthatthederegulationofanotherpathwayregulatingcofilinoccursintheabsenceofCdc42,most likelytheRhoA-regulatedkinaseROCK(Maekawaetal.,1999).ThelossofPak1itselfalsoinhibitsneuronalpolarization, andconversely,constitutivelyactivePak1inducesmultipletau1-positiveprocesses(Jacobsetal.,2007).Thelattereffect canbepartiallyreducedbycoexpressionofeitheranunphosphorylatableformofco filinoraGDP-lockedRac1,suggesting thatRac1mayactdownstreamofPak1activation.Takentogether,theseresultsdemonstratearoleforactivatedCdc42in neuronalpolarizationbeyonditsassociationwiththePAR3/6complex,whichisdescribedlaterinthischapter.
RhoAisanothersmallGTPase,anditistypicallyassociatedwithdestabilizationoftheactincytoskeletonandalsowith myosin-basedcontractility.ExperimentsusingaconstitutivelyactiveformofRhoAshowthatitinhibitsneuritogenesis, whereasadominant-negativeformofRhoAenhancesneuriteoutgrowth(SchwambornandPuschel,2004).This findingis consistentwiththeregulatoryroleproposedfor p190RhoGAPandtheeffectofinhibitingtheRhoA-activatedkinase, ROCK,onaxogenesis(Bitoetal.,2000).FutureexperimentswilltestifRhoAactivationisregulatedbylocaldegradation throughSmurf1/2activityspecifi callyintheaxondownstreamoflocalTGFb receptoractivationandrecruitmentofPar6 (Yietal.,2010),aspreviouslyshownforEMT(Ozdamaretal.,2005).
TheexaminationofRac1’sroleinneuronalpolarizationhasledtosomeconfoundingresults.In Drosophila,the expressionofeitherdominant-negative(GDP-locked)Rac(Luoetal.,1994)orlossofRacexpression(Hakeda-Suzuki
etal.,2002;NgandLuo,2004;Ngetal.,2002)affectsoutgrowthbutnotpolarity.Similarly,siRNAknockdownof mammalianRac1typicallydoesnotaffectaxonidentity(Gualdonietal.,2007),althoughsomereportsdetectedunpolarizedneuronsfollowingexpressionofthedominant-negativeformofRac1(Nishimuraetal.,2005).Inculturedneurons, aconstitutivelyactiveversionofRac1doesnotaffectaxonspeci fication(SchwambornandPuschel,2004).Theseresults, whilemixed,dohintatamorecomplexregulationofRac1inneuronalpolarization.Thispointbecomesclearer,aswe discusslater,sincetheonlyGEFproteinsshowntobecrucialforaxonformationappeartocontrolRac1.
SmallGTPaseshaveaplethoraofeffectorswithincells,andproperactivationoftheseeffectors,bothspatiallyand temporally,requiresexquisitecontrolofbothactivationandinactivationbyGEFsandGAPs,respectively.Apartfrom p190RhoGAP,moststudieshavesofarfocusedonthefunctionofGEFsinneuronalpolarity.ThisincludestheGEFs Tiam1andSTEF,describedinmoredetaillater,andtheDOCK7GEF,whichhasrecentlybeenreportedtobearegulator ofaxonspeci ficationbyactivatingRac1andtherebytriggeringphosphorylationofStathmin/Op18,amicrotubuledestabilizingfactorcriticalforaxogenesis(Watabe-Uchidaetal.,2006).Anotheraxonallyenriched,unconventional Rac1regulatoryproteinisthecytoplasmicdyneinlightchainTcTEX-1(Chuangetal.,2005).IncreasedlevelsofTcTEX-1 resultinincreasesinGTP-loadedRac1,andadropinGTP-Rac1levelsisobservedfollowingTcTEX-1knockdown. MultipleaxonsresultfromoverexpressionofTcTEX-1,andthiseffectispreservedusingamutantform(T94E)thatcannot binddyneinheavychain.ConsistentwitharoleincontrollingRac1intheneuritespeci fiedtobecometheaxon,this supranumeraryaxonphenotypeissuppressedbyconstitutivelyactiveRhoAordominant-negativeRac1.Therefore,several GEFsseemtocooperateinthelocalactivationofRac1duringaxonspecification.
Rap1b,amemberoftheRassuperfamilyofGTPases,isalsorequiredforproperneuronalpolarizationinvitro (SchwambornandPuschel,2004;Schwambornetal.,2007b)andinvivo(JossinandCooper,2011).Itisfoundatthetipof thenascentaxon,anditsoverexpressionleadstohippocampalneuronsbearingmultipleaxons.ThelossofRap1b followingsiRNAknockdownabrogatesaxonformation,andexpressionofautocyclingCdc42canrescuethephenotype. ExpressionofaconstitutivelyactiveRap1bfailstoreversethelossofaxonsobservedfollowinglossofCdc42,indicating thatRap1bliesupstreamofCdc42inthisneuronpolarizationpathway.Similarly,suppressingaxogenesisviapharmacologicalinhibitionofPI3KcanbereversedbyautocyclingeitherCdc42orconstitutivelyactiveRap1b,placingbothof thesesmallGTPasesdownstreamofPI3Ksignalingduringaxonspeci fication.Inadditiontoitsroleinoneofthecanonical polaritypathways,studiesonRap1bhaveexploredanovelmechanismforproteinlocalizationduringneuronalpolarity, namely,selectiveproteindegradation(Schwambornetal.,2007a,2007b).
1.5.3.4PI3KandPTENsignalingduringaxonspecification
Thephosphatidylinositol-3kinase(PI3K)familyregulatesdiversebiologicalfunctions,includingcellpolarity,cell motility,chemotaxis,andalsoneuronalmigrationandpolarization;however,theseresultsarebasedalmostexclusivelyon theuseofpharmacologicalinhibitorssuchasWortmaninorLY294002(JossinandGof finet,2007;Polleuxetal.,2002; Shietal.,2003;Zhouetal.,2007).ThebestcharacterizedclassIaPI3-kinase(PI3KcIa)isinvolvedintheformationof phosphatidylinositol(3,4,5)-triphosphate(PIP3)andliesdownstreamofRasandupstreamofproteinkinaseB((PKB)or AKT)duringsignaltransduction.WorkfromseveralgroupshasimplicatedPI3Kinaxonspeci ficationbasedonthe observationthatpharmacologicalinhibitionofPI3KactivityusingLY294002orWortmanninpreventsaxonformation (Jiangetal.,2005;Menageretal.,2004;Shietal.,2003;Yoshimuraetal.,2006).However,thesedatahavetobe interpretedcarefullysincetheseinhibitorsarenotclassspeci fic.TheyinhibitthethreemainclassesofPI3K(Stackand Emr,1994),includingphosphatidylinositol-3kinaseclassIII(PI3Kc3alsocalledVps34)thatproducesexclusively phosphatidylinositol-3monophosphate(PI(3)P)andregulatesendocytosis,vesiculartraf ficking,trimericG-protein signaling,andthemTOR(mammaliantargetofrapamycin)pathway(Backer,2008).However,overexpressionofthe constitutivelyactivecatalyticsubunitofPI3K(p110)leadstotheformationofmultipleaxons(Yoshimuraetal.,2006), suggestingthatPI3Kactivationisbothrequiredandsuffi cientforaxonspecifi cation.Usingthepleckstrinhomology(PH) domainofAKTfusedtoGFP(PHAKT GFP)asabiosensorforPIP3formation, Menageretal.(2004) haveshownthat PIP3accumulatesselectivelywithinasingleneuritefollowinglocalapplicationoflaminintostage2hippocampalneurons. FutureinvestigationswillneedtoaddresswhenandwherePI3Kactivationoccursinvivoduringneuronalpolarizationand alsowhichclassofPI3Kisrequired(class1,2,or3),usinggeneticloss-of-functionapproaches.
TwoPI3K-interactingproteins,Shootin1(Toriyamaetal.,2006)andSingar1/2(Morietal.,2007),wererecently identi fiedaspotentialregulatorsofaxonformationusingamassspectrometryapproach.OverexpressionofShootin1leads tothegenerationofsupernumeraryaxons,andRNAiknockdowninhibitsaxonformation.Shootin1istransportedviaa myosin-dependentmechanismtoaxonalgrowthcones,anditsoverexpressionleadstoaberrantaccumulationofPI3Kin minorneurites,likelyleadingtotheobservedalterationofaxonspeci fication.Shootin1colocalizeswithactivepoolsof
PI3K,andinhibitionofPI3KactivitysignificantlyreducestheabilityofShootin1toinducemultipleaxons.Thesedata suggestaroleforShootin1inregulatingPI3Kactivity,anditsselectivetransporttothenascentaxonislikelycriticalfor establishingaxonalidentity.Singarexistsasatleasttwoproteinisoformsgeneratedbyalternativesplicing,Singar1and Singar2,andbothareexpressedindevelopingneurons.RNAiagainstbothformscausesculturedneuronstoformmultiple axons,andthiseffectispreventedwhenPI3Kactivityisinhibited.UnlikeShootin1,overexpressionofSingarisnot suf ficienttoaffectaxonformation.WhencoexpressedwithShootin1,Singar1,butnotSingar2,canreducethemultiple axonphenotypecausedbyShootin1overexpressionalone.Thisresultsuggestsanantagonisticrelationshipbetween Shootin1andSingar1andalsothatSingarproteinmayinhibitPI3Kactivity.
PTEN(phosphataseandtensinhomologdeletedonchromosome10)isalipidandproteinphosphatasethatactsin directoppositiontoPI3KactivitysincePTENdephosphorylatesPIP3intoPIP2andthuslimitsPIP3signalingboth spatiallyandtemporally.IncreasinglevelsofPTENexpressionleadtoalossofaxonformation(Jiangetal.,2005;Shi etal.,2003),whereasreductionofPTENexpressionviaRNAi-mediatedknockdownleadstoamultipleaxonphenotype (Jiangetal.,2005).Thiseffectisconsistentwiththegain-of-functionmutationofPI3Kdescribedearlierandhighlightsthe criticalneedtomaintainthedelicatebalanceofphospholipidcompositionatthemembranetoensureproperneuronal polarizationandaxonformation.Remarkably,PTENservesamajorroleinsuppressinginjury-inducedCNSaxongrowth viaregulationofthemTORpathway(Parketal.,2008;seelater).
1.5.3.5AKT/proteinkinaseB
SeveralproteinsarerecruitedviatheirPIP3-specificPHdomainstositesofmembranecreatedbyPI3KcIaactivity.The proteinkinaseAKT,alsocalledPKB,undergoessuchatranslocationtothemembraneviaitsPHdomain,asteprequired foritsdualphosphorylationonT308andS473andsubsequentactivationbythemembrane-targetedproteinkinasesPKD1 andPKD2,respectively.ThisactivatedformofAKTisenrichedingrowthconesofpolarizedneurons(Shietal.,2003). WhenamyristoylationsiteisaddedtorecombinantAKT(myr-AKT),itisconstitutivelytargetedtothemembrane,independentofPI3K,andthereforeisconstitutivelyactive.Whenoverexpressedinneurons,thisformofAKTissuf ficientto generatemultipleaxonformation(Yoshimuraetal.,2006),consistentwithaunifi edpathwayinwhichAKTacts downstreamofPI3Ktoregulateaxonformation.InadditiontoPKD,anotherPI3K-regulatedkinase,ILK(integrin-linked kinase),increasesAKTactivityviaS473phosphorylation(Delcommenneetal.,1998).SimilartootherregulatorsofAKT, hyperactiveILK(S343D)increasesmultipleaxonformationinculturedneurons,andRNAireductionorpharmacologic inhibitionofILKleadstothefailureofaxonformationwithoutaffectingthedendriticformation(Guoetal.,2007).These experimentspointtoanimportant,butnotexclusive,roleforILKinregulatingAKT.AcommontargetproteinofbothILK andAKTisGSK3b (Delcommenneetal.,1998),andphosphorylationbyeitherproteinisthoughttoinactivateGSK3 kinaseactivity(seelater).
FutureexperimentswillneedtoaddresswhichclassofPI3KisrequiredforaxonspecificationandalsoifPTENandAKT arerequiredforneuronalpolarizationinvivo.Finally,wealsoneedtoknowwhethertheseimportanteffectorsarespecifically requiredforneuronalpolarizationandaxonspecification,orrather,iftheyaremoregenerallyrequiredforneuralprogenitor polarity.ItremainspossiblethatthedefectsinpolarizationofpostmitoticneuronscausedbyalterationofPTENorAKT functionareaconsequenceofanearlierrequirementinspecifyingneuroepithelialpolarity(Barnesetal.,2008).
1.5.3.6GSK3andaxonspecification
GSK3proteinsarewell-studiedserine/threonineproteinkinasesthatfunctionintheregulationofmultipleintracellular processes,includingpathwaysdownstreamofreceptortyrosinekinasesandWnt/Fzlsignaling.Thetwogenesencoding GSK3kinases(a and b)inmammalsproduceproteinsthatperformessentiallyredundantfunctions.GSK3shavethe unusualpropertyofbeingconstitutivelyactive,astatethatisreversedfollowingphosphorylationatSer9inGSK3b or Ser21inGSK3a bymultiplekinases,includingAKT,ILK,andaPKC(Etienne-MannevilleandHall,2003).Recent invitroworkimplicatesGSK3b asacriticalregulatorofneuronalpolarity.ExperimentsusingseveraltypesofGSK3 inhibitorsindicatethatGSK3b actsasanegativeregulatorofaxonformationbecausethistreatmentleadstotheformation ofmultipleaxons(Jiangetal.,2005;Yoshimuraetal.,2005).
Gartneretal.(2006) suggestedthatthesituationisclearlymorecomplexinvivo.Usingdoubleknock-inmicebearing singlepointmutations GSK3ßS9A and GSK3a S21A,Gartneretal.reportednoobviousdeficitsinneuronalmorphogenesis invivoandinvitro(Gartneretal.,2006).Infact,thesemiceareviableanddonotshowanyobviousCNSdevelopmental phenotypes.However,usinginhibitorsofGSK3a/b suchaslithiumchlorideor,morespecifi cally,SB-415286,SB216763,orAR-A014418,Gartneretal.wereabletoreplicatethemultipleaxonphenotypeobtainedbyothers(Garrido etal.,2007;Jiangetal.,2005).TheseresultsindicatethatalthoughtheexactroleofSer9/Ser21phosphorylationinGSK3
inactivationremainstobeunderstoodandmayinvolveanalternatesite(Thorntonetal.,2008),itisclearthatthecatalytic activityGSK3sareacriticalregulatorofneuronalpolarityinstandardinvitroparadigms.
SeveraldownstreamtargetsofGSK3sarepotentialeffectorsofneuronalpolarity,andmanyinvolveregulationofthe cytoskeleton.Collapsinresponsemediatorprotein-2(CRMP-2)isonesuchmicrotubule-bindingproteinthatisenrichedin tipsofthenascentaxonandisregulatedbyGSK3b suchthatphosphorylatedCRMP-2displaysadecreasedbinding affi nityfortubulinheterodimers(Inagakietal.,2001;Yoshimuraetal.,2005;reviewedin Arimuraetal.,2004).As observedforotherpolarityregulators,overexpressionofCRMP-2issuffi cienttoinducetheformationofmultipleaxons, andtruncatedformsofCRMP-2canimpairaxonformation(Inagakietal.,2001).AlthoughtheabilityofCRMP-2to facilitatemicrotubuleassemblyisimportantinregulatingaxonformation,CRMP-2isalsoknowntoassociatewithseveral otherproteins,includingtheactinpolymerization-regulatingSra-1/WAVE1complex,whichmightcontributetoits functioninaxogenesis.Kawanoetal.showedthatCRMP-2linkstheSra-1/WAVE1complexwiththemicrotubule-based motorproteinKinesin1,andSra1/WAVEexpressionislikelyrequiredforCRMP-2’sinductionofmultipleaxons (Kawanoetal.,2005).
APCisanotherwell-establishedeffectorofGSK3a/b thatareenrichedintheneuritethatwillbecometheaxonearly duringneuronalpolarization(Shietal.,2004).MostcellbiologicalevidencesuggeststhatAPCenhancesmicrotubule stability,anditiswellestablishedthatAPCcanbindmicrotubuleplusendsviaitsEB1-bindingdomain.Phosphorylation ofAPCbyGSK3b blocksitsabilitytobindtheplusendsofmicrotubules,andinhibitionofGSK3b leadstoanaccumulationofAPCinmultipleneurites(Shietal.,2004).ExpressionoftruncatedformsofAPCissuffi cienttoinhibitaxon formationandelongation(Shietal.,2004;Zhouetal.,2004).ReductionofAPCusingshRNAhasalsobeendemonstrated tointerferewithefficientaxonelongation(Purroetal.,2008).AdditionalworkshowsthattheAPC/GSKdyadregulates targetingofanotherpolarityprotein,PAR3,sinceoverexpressionoffull-lengthortruncatedAPCdisruptsneuronalpolarization,andinhibitionofGSKb changesthedistributionofthepoolofAPCinthenascentaxon(Shietal.,2004;Zhou etal.,2004).Furthermore,growthfactor triggeredinactivationofGSK3b byPI3KsignalingactsthroughtheAPCto controlaxonelongation(Zhouetal.,2004).InvestigatorshaveobservedsimilarresultsfortwootherGSKtargets,the MAPs,MAP1b(Gonzalez-Billaultetal.,2004)andTau(Sperberetal.,1995),that,whenphosphorylatedbyGSK3b,alter microtubuledynamics.Theseresultsemphasizeakeyprinciplethatunderliesmuchofwhatisknownaboutneuronal polarization themicrotubulecytoskeletonisamajorendpointforpolarityregulators.Interestingly,PTENwasalso recentlyidenti fiedasaGSK3substrate(Maccarioetal.,2007),whichmayrepresentanegativefeedbackloopforAKT signalingfollowingactivationviastabilizationofPTEN.
Asnotedabove,thetwoGSK-3familymembers,GSK-3a andGSK-3b,havelargelyredundantfunctions.Elimination ofeitherfamilymemberalonehaslittleeffectonaxongrowtheitherinvitroorinvivo(Kimetal.,2006).However, completeinhibitionofGSK-3activityviainhibitorsorshRNAdirectedatbothfamilymembersappearstoblockaxon growthaltogetherinvitro(Garridoetal.,2007;Kimetal.,2006;Shietal.,2004).
1.6Conclusionandfuturedirections
Themechanismsthatunderlieneuronalpolarization,thespecifi cationofaxonanddendriteidentity,theirmorphology,and directedoutgrowthinvivohaveparallelswiththemechanismsdeterminedusingreductionistinvitromodels.However, thereareseveralimportantdistinctions.Theyincludethecomplexinteractionsbetweenextracellularcues,suchasgraded diffusiblecuessuchasTGFb and/orcelladhesionmoleculespresentedbypreexistingaxonssuchasTAG-1(Yietal., 2010;Nambaetal.,2014),andtheintracellularpathwaystheyactivatespeci ficallyintheneurite(trailingprocess)that becomestheaxoncanonlybestudiedinvivo.
Amongthemanyoutstandingissuesforthe fieldtoaddressrelatingtothelinkbetweentheseextracellular/intracellular signalingpathwaysandthecellulareventstheycontrolare(1)process-speci ficneuriteoutgrowth(signi ficantlyfasterfor theneuritebecomingtheaxonthanneuritesbecomingdendrites);(2)theselectionofthecargoesthatwillbespeci fically targetedandtransportedtotheaxonversusthedendrite(see,forexample,reviewsby GumyandHoogenraad,2018;Lewis etal.,2013);and(3)thestriking,compartment-speci fic,distributionandmorphologyoforganellessuchastheendoplasmic reticulumormitochondria(see,forexample, Lewisetal.,2018).Asanexcellentexampleofthislastpoint,incorticalPNs, mitochondriadisplaycompartment-speci ficmorphologies:axonalmitochondriaareshortandofastandardlength (w1 mm),whereasdendriticmitochondriaarelongandfusedand fi llalmosttheentiredendritictree(Lewisetal.,2018).
Thisobservationsuggestedthatmitochondrialfusionmustdominateover fi ssionindendritesandviceversaintheaxonof thesesameneurons.Inarecentstudy,Lewisetal.identi fiedtheDrp1receptorMffasacriticalregulatorofthehighlevels ofmitochondrial fissionobservedinaxons,butthemechanismsunderlyingthiscompartment-speci ficlevelofmitochondrial fissionarestillpoorlyunderstood.Thisstudyconstitutesanewparadigmforexploringsomeofthese
mechanismsofneuronalpolarizationfororganellesaswellasothercargoes.Theseincludeneurotransmittervesicles speci ficallyintheaxon,orpostsynapticcargoessuchasneurotransmitterreceptorsspecifi callyindendrites(Gumyand Hoogenraad,2018).Thebasicmechanismsunderlyingneuronalpolarizationarebeginningtobeunderstoodatthecellular andmolecularlevelsusinginvitromodels;however,ourunderstandingofhowthesemechanismsoperateinvivoisstill fragmentarybutwillundoubtedlybethefocusofmanyinvestigationsinthenearfuture.
References
Adler,C.E.,Fetter,R.D.,Bargmann,C.I.,2006.UNC-6/Netrininducesneuronalasymmetryanddefinesthesiteofaxonformation.Nat.Neurosci.9, 511 518.
Aravamudan,B.,Broadie,K.,2003.SynapticDrosophilaUNC-13isregulatedbyantagonisticG-proteinpathwaysviaaproteasome-dependentdegradationmechanism.J.Neurobiol.54,417 438.
Arimura,N.,Kaibuchi,K.,2007.Neuronalpolarity:fromextracellularsignalstointracellularmechanisms.Nat.Rev.Neurosci.8,194 205. Arimura,N.,Menager,C.,Fukata,Y.,Kaibuchi,K.,2004.RoleofCRMP-2inneuronalpolarity.J.Neurobiol.58,34 47.
Backer,J.M.,2008.TheregulationandfunctionofClassIIIPI3Ks:novelrolesforVps34.Biochem.J.410,1 17.
Barnes,A.P.,Polleux,F.,2009.Establishmentofaxon-dendritepolarityindevelopingneurons.Annu.Rev.Neurosci.32,347 381.
Barnes,A.P.,Lilley,B.N.,Pan,Y.A.,etal.,2007.LKB1andSADkinasesdefineapathwayrequiredforthepolarizationofcorticalneurons.Cell129, 549 563.
Barnes,A.P.,Solecki,D.,Polleux,F.,2008.Newinsightsintothemolecularmechanismsspecifyingneuronalpolarityinvivo.Curr.Opin.Neurobiol.18, 44 52.
Betschinger,J.,Mechtler,K.,Knoblich,J.A.,2003.TheParcomplexdirectsasymmetriccelldivisionbyphosphorylatingthecytoskeletalprotein LgI. Nature422,326 330.
Biernat,J.,Wu,Y.Z.,Timm,T.,etal.,2002.ProteinkinaseMARK/PAR-1isrequiredforneuriteoutgrowthandestablishmentofneuronalpolarity.Mol. Biol.Cell13,4013 4028.
Bito,H.,Furuyashiki,T.,Ishihara,H.,etal.,2000.AcriticalroleforaRho-associatedkinase,p160ROCK,indeterminingaxonoutgrowthinmammalian CNSneurons.Neuron26,431 441.
Bloom,A.J.,Miller,B.R.,Sanes,J.R.,Diantonio,A.,2007.TherequirementforPhr1inCNSaxontractformationrevealsthecorticostriatalboundaryasa choicepointforcorticalaxons.GenesDev.21,2593 2606.
Bortone,D.,Polleux,F.,2009.KCC2expressionpromotestheterminationofcorticalinterneuronmigrationinavoltage-sensitivecalcium-dependent manner.Neuron62,53 71.
Bradke,F.,Dotti,C.G.,1997.Neuronalpolarity:vectorialcytoplasmic flowprecedesaxonformation.Neuron19,1175 1186. Bradke,F.,Dotti,C.G.,1999.Theroleoflocalactininstabilityinaxonformation.Science283,1931 1934. Bright,N.J.,Carling,D.,Thornton,C.,2008.Investigatingtheregulationofbrain-specifickinases1and2byphosphorylation.J.Biol.Chem.283, 14946 14954.
Bultje,R.S.,Castaneda-Castellanos,D.R.,Jan,L.Y.,Jan,Y.N.,Kriegstein,A.R.,Shi,S.H.,2009.MammalianPar3regulatesprogenitorcellasymmetric divisionvianotchsignalinginthedevelopingneocortex.Neuron63,189 202.
CalderondeAnda,F.,Gartner,A.,Tsai,L.H.,Dotti,C.G.,2008.Pyramidalneuronpolarityaxisisdefinedatthebipolarstage.J.CellSci.121,178 185. Campbell,D.S.,Holt,C.E.,2001.Chemotropicresponsesofretinalgrowthconesmediatedbyrapidlocalproteinsynthesisanddegradation.Neuron32, 1013 1026.
Chang,C.,Adler,C.E.,Krause,M.,etal.,2006.MIG-10/lamellipodinandAGE-1/PI3Kpromoteaxonguidanceandoutgrowthinresponsetoslitand netrin.Curr.Biol.16,854 862.
Chen,X.,Macara,I.G.,2005.Par-3controlstightjunctionassemblythroughtheRacexchangefactorTiam1.Nat.CellBiol.7,262 269. Chen,Y.M.,Wang,Q.J.,Hu,H.S.,etal.,2006.Microtubuleaffinity-regulatingkinase2functionsdownstreamofthePAR-3/PAR-6/atypicalPKC complexinregulatinghippocampalneuronalpolarity.Proc.Natl.Acad.Sci.U.S.A.103,8534 8539.
Chen,G.,Sima,J.,Jin,M.,etal.,2008.Semaphorin-3Aguidesradialmigrationofcorticalneuronsduringdevelopment.Nat.Neurosci.11,36 44. Choi,Y.J.,diNardo,A.,Kramvis,I.,etal.,2008.Tuberoussclerosiscomplexproteinscontrolaxonformation.GenesDev.22,2485 2495.
Chuang,J.Z.,Yeh,T.Y.,Bollati,F.,etal.,2005.ThedyneinlightchainTctex-1hasadynein-independentroleinactinremodelingduringneurite outgrowth.Dev.Cell9,75 86.
Ciani,L.,Salinas,P.C.,2007.c-JunN-terminalkinase(JNK)cooperateswithGsk3btoregulateDishevelled-mediatedmicrotubulestability.BMCCell Biol.8,27.
Cobos,I.,Borello,U.,Rubenstein,J.L.,2007.Dlxtranscriptionfactorspromotemigrationthroughrepressionofaxonanddendritegrowth.Neuron 54, 873 888.
Collins,S.P.,Reoma,J.L.,Gamm,D.M.,Uhler,M.D.,2000.LKB1,anovelserine/threonineproteinkinaseandpotentialtumoursuppressor,isphosphorylatedbycAMP-dependentproteinkinase(PKA)andprenylatedinvivo.Biochem.J.345(Pt3),673 680.
Costa,M.R.,Wen,G.,Lepier,A.,Schroeder,T.,Gotz,M.,2008.Par-complexproteinspromoteproliferativeprogenitordivisionsinthedeveloping mouse cerebralcortex.Development135,11 22.
Craig,A.M.,Banker,G.,1994.Neuronalpolarity.Annu.Rev.Neurosci.17,267 310.
Crump,J.G.,Zhen,M.,Jin,Y.,Bargmann,C.I.,2001.TheSAD-1kinaseregulatespresynapticvesicleclusteringandaxontermination.Neuron29, 115 129.
Delcommenne,M.,Tan,C.,Gray,V.,Rue,L.,Woodgett,J.,Dedhar,S.,1998.Phosphoinositide-3-OHkinase-dependentregulationofglycogensynthase kinase3andproteinkinaseB/AKTbytheintegrin-linkedkinase.Proc.Natl.Acad.Sci.U.S.A.95,11211 11216.
DiAntonio,A.,Hicke,L.,2004.Ubiquitin-dependentregulationofthesynapse.Annu.Rev.Neurosci.27,223 246.
DiAntonio,A.,Haghighi,A.P.,Portman,S.L.,Lee,J.D.,Amaranto,A.M.,Goodman,C.S.,2001.Ubiquitination-dependentmechanismsregulatesynaptic growthandfunction.Nature412,449 452.
Ding,M.,Chao,D.,Wang,G.,Shen,K.,2007.SpatialregulationofanE3ubiquitinligasedirectsselectivesynapseelimination.Science317,947 951. Dorfman,J.,Macara,I.G.,2008.STRADaregulatesLKB1localizationbyblockingaccesstoimportin-a,andbyassociationwithCrm1andexportin-7. Mol.Biol.Cell19,1614 1626.
Dotti,C.G.,Sullivan,C.A.,Banker,G.A.,1988.Theestablishmentofpolaritybyhippocampalneuronsinculture.J.Neurosci.8,1454 1468.
Drewes,G.,Ebneth,A.,Preuss,U.,Mandelkow,E.M.,Mandelkow,E.,1997.MARK,anovelfamilyofproteinkinasesthatphosphorylatemicrotubuleassociatedproteinsandtriggermicrotubuledisruption.Cell89,297 308.
Ehlers,M.D.,2003.Activitylevelcontrolspostsynapticcompositionandsignalingviatheubiquitin-proteasomesystem.Nat.Neurosci.6,231 242.
Esch,T.,Lemmon,V.,Banker,G.,1999.Localpresentationofsubstratemoleculesdirectsaxonspecificationbyculturedhippocampalneurons. J.Neurosci.19,6417 6426.
Etienne-Manneville,S.,Hall,A.,2003.Cdc42regulatesGSK-3bandadenomatouspolyposiscolitocontrolcellpolarity.Nature421,753 756. Famulski,J.K.,Trivedi,N.,Howell,D.,etal.,2010.SiahregulationofPard3Acontrolsneuronalcelladhesionduringgerminalzoneexit.Science330, 1834 1838.
Feng,W.,Wu,H.,Chan,L.N.,Zhang,M.,2008.Par-3-mediatedjunctionallocalizationofthelipidphosphatasePTENisrequiredforcellpolarity establishment.J.Biol.Chem.283,23440 23449.
Fivaz,M.,Bandara,S.,Inoue,T.,Meyer,T.,2008.RobustneuronalsymmetrybreakingbyRas-triggeredlocalpositivefeedback.Curr.Biol.18,44 50. Gao,W.Q.,Hatten,M.E.,1993.Neuronaldifferentiationrescuedbyimplantationofweavergranulecellprecursorsintowild-typecerebellarcortex. Science260,367 369.
Garrido,J.J.,Simon,D.,Varea,O.,Wandosell,F.,2007.GSK3aandGSK3barenecessaryforaxonformation.FEBS(Fed.Eur.Biochem.Soc.)Lett. 581,1579 1586.
Gartner,A.,Huang,X.,Hall,A.,2006.Neuronalpolarityisregulatedbyglycogensynthasekinase-3(GSK-3b)independentlyofAkt/PKBserine phosphorylation.J.CellSci.119,3927 3934.
Garvalov,B.K.,Flynn,K.C.,Neukirchen,D.,etal.,2007.Cdc42regulatescofilinduringtheestablishmentofneuronalpolarity.J.Neurosci.27, 13117 13129.
Goldstein,B.,Macara,I.G.,2007.ThePARproteins:fundamentalplayersinanimalcellpolarization.Dev.Cell13,609 622.
Gonzalez-Billault,C.,Jimenez-Mateos,E.M.,Caceres,A.,Diaz-Nido,J.,Wandosell,F.,Avila,J.,2004.Microtubule-associatedprotein1Bfunction duringnormaldevelopment,regeneration,andpathologicalconditionsinthenervoussystem.J.Neurobiol.58,48 59. Goslin,K.,Banker,G.,1989.Experimentalobservationsonthedevelopmentofpolaritybyhippocampalneuronsinculture.JCB(J.CellBiol.)108, 1507 1516.
Gualdoni,S.,Albertinazzi,C.,Corbetta,S.,Valtorta,F.,deCurtis,I.,2007.NormallevelsofRac1areimportantfordendriticbutnotaxonaldevelopment inhippocampalneurons.Biol.Cell99,455 464.
Guo,W.,Jiang,H.,Gray,V.,Dedhar,S.,Rao,Y.,2007.Roleoftheintegrin-linkedkinase(ILK)indeterminingneuronalpolarity.Dev.Biol.306, 457 468.
Gumy,L.F.,Hoogenraad,C.C.,2018.Localmechanismsregulatingselectivecargoentryandlong-rangetraffickinginaxons.Curr.Opin.Neurobiol.51, 23 28.
Hakeda-Suzuki,S.,Ng,J.,Tzu,J.,etal.,2002.Racfunctionandregulationduring Drosophila development.Nature416,438 442.
Hand,R.,Polleux,F.,2011.Neurogenin2regulatestheinitialaxonguidanceofcorticalpyramidalneuronsprojectingmediallytothecorpuscallosum. NeuralDev.6,30.
Hand,R.,Bortone,D.,Mattar,P.,etal.,2005.PhosphorylationofNeurogenin2specifiesthemigrationpropertiesandthedendriticmorphologyof pyramidalneuronsintheneocortex.Neuron48,45 62.
Hatanaka,Y.,Murakami,F.,2002.Invitroanalysisoftheorigin,migratorybehavior,andmaturationofcorticalpyramidalcells.J.Comp.Neurol.454, 1 14.
Hilliard,M.A.,Bargmann,C.I.,2006.Wntsignalsandfrizzledactivityorientanterior posterioraxonoutgrowthin C.elegans.Dev.Cell10,379 390. Hinds,J.W.,Hinds,P.L.,1978.Earlydevelopmentofamacrinecellsinthemouseretina:anelectronmicroscopic,serialsectionanalysis.J.Comp.Neurol. 179,277 300.
Hurov,J.B.,Watkins,J.L.,Piwnica-Worms,H.,2004.AtypicalPKCphosphorylatesPAR-1kinasestoregulatelocalizationandactivity.Curr.Biol. 14, 736 741.
Illenberger,S.,Drewes,G.,Trinczek,B.,etal.,1996.Phosphorylationofmicrotubule-associatedproteinsMAP2andMAP4bytheproteinkinase p110mark:phosphorylationsitesandregulationofmicrotubuledynamics.J.Biol.Chem.271,10834 10843.
Inagaki,N.,Chihara,K.,Arimura,N.,etal.,2001.CRMP-2inducesaxonsinculturedhippocampalneurons.Nat.Neurosci.4,781 782.
Jacobs,T.,Causeret,F.,Nishimura,Y.V.,etal.,2007.Localizedactivationofp21-activatedkinasecontrolsneuronalpolarityandmorphology. J.Neurosci.27,8604 8615.
Jiang,H.,Guo,W.,Liang,X.,Rao,Y.,2005.Boththeestablishmentandthemaintenanceofneuronalpolarityrequireactivemechanisms:criticalrolesof GSK-3banditsupstreamregulators.Cell120,123 135.
Joberty,G.,Petersen,C.,Gao,L.,Macara,I.G.,2000.Thecell-polarityproteinPar6linksPar3andatypicalproteinkinaseCtoCdc42.Nat.CellBiol.2, 531 539.
Jossin,Y.,Cooper,J.A.,2011.Reelin,Rap1andN-cadherinorientthemigrationofmultipolarneuronsinthedevelopingneocortex.Nat.Neurosci.14, 697 703.
Jossin,Y.,Goffinet,A.M.,2007.Reelinsignalsthroughphosphatidylinositol3-kinaseandAkttocontrolcorticaldevelopmentandthroughmTorto regulatedendriticgrowth.Mol.Cell.Biol.27,7113 7124.
Kawano,Y.,Yoshimura,T.,Tsuboi,D.,etal.,2005.CRMP-2isinvolvedinkinesin-1-dependenttransportoftheSra-1/WAVE1complexandaxon formation.Mol.Cell.Biol.25,9920 9935.
Kemphues,K.J.,Priess,J.R.,Morton,D.G.,Cheng,N.S.,1988.Identificationofgenesrequiredforcytoplasmiclocalizationinearly C.elegans embryos. Cell52,311 320.
Kim,W.Y.,Zhou,F.Q.,Zhou,J.,etal.,2006.EssentialrolesforGSK-3sandGSK-3-primedsubstratesinneurotrophin-inducedandhippocampalaxon growth.Neuron52,981 996.
Kirschner,M.,Mitchison,T.,1986.Beyondself-assembly:frommicrotubulestomorphogenesis.Cell45,329 342.
Kishi,M.,Pan,Y.A.,Crump,J.G.,Sanes,J.R.,2005.MammalianSADkinasesarerequiredforneuronalpolarization.Science307,929 932. Klimaschewski,L.,Hausott,B.,Ingorokva,S.,Pfaller,K.,2006.Constitutivelyexpressedcatalyticproteasomalsubunitsareup-regulatedduringneuronal differentiationandrequiredforaxoninitiation,elongationandmaintenance.J.Neurochem.96,1708 1717.
Komuro,H.,Yacubova,E.,Rakic,P.,2001.Modeandtempooftangentialcellmigrationinthecerebellarexternalgranularlayer.J.Neurosci.21, 527 540.
Lewcock,J.W.,Genoud,N.,Lettieri,K.,Pfaff,S.L.,2007.TheubiquitinligasePhr1regulatesaxonoutgrowththroughmodulationofmicrotubule dynamics.Neuron56,604 620.
LewisJr.,T.L.,Courchet,J.,Polleux,F.,2013.Cellbiologyinneuroscience:cellularandmolecularmechanismsunderlyingaxonformation,growth,and branching.J.CellBiol.202,837 848.
LewisJr.,T.L.,Kwon,S.-K.,Lee,A.,Shaw,R.,Polleux,F.,2018.MFF-dependentmitochondrial fissionregulatespresynapticreleaseandaxonbranching bylimitingaxonalmitochondriasize.Nat.Commun.9,5008.
Lin,D.,Edwards,A.S.,Fawcett,J.P.,Mbamalu,G.,Scott,J.D.,Pawson,T.,2000.AmammalianPAR-3-PAR-6compleximplicatedinCdc42/Rac1and aPKCsignallingandcellpolarity.Nat.CellBiol.2,540 547.
Lizcano,J.M.,Goransson,O.,Toth,R.,etal.,2004.LKB1isamasterkinasethatactivates13kinasesoftheAMPKsubfamily,includingMARK/PAR-1. EMBOJ.23,833 843.
Luo,L.,Liao,Y.J.,Jan,L.Y.,Jan,Y.N.,1994.DistinctmorphogeneticfunctionsofsimilarsmallGTPases:DrosophilaDrac1isinvolvedinaxonal outgrowthandmyoblastfusion.GenesDev.8,1787 1802.
Maccario,H.,Perera,N.M.,Davidson,L.,Downes,C.P.,Leslie,N.R.,2007.PTENisdestabilizedbyphosphorylationonThr366.Biochem.J.405, 439 444.
Maekawa,M.,Ishizaki,T.,Boku,S.,etal.,1999.SignalingfromRhototheactincytoskeletonthroughproteinkinasesROCKandLIM-kinase.Science 285,895 898.
Manabe,N.,Hirai,S.,Imai,F.,Nakanishi,H.,Takai,Y.,Ohno,S.,2002.AssociationofASIP/mPAR-3withadherensjunctionsofmouseneuroepithelial cells.Dev.Dynam.225,61 69.
Matenia,D.,Mandelkow,E.M.,2009.ThetauofMARK:apolarizedviewofthecytoskeleton.TrendsBiochem.Sci.34,332 342.
Matenia,D.,Griesshaber,B.,Li,X.Y.,etal.,2005.PAK5kinaseisaninhibitorofMARK/Par-1,whichleadstostablemicrotubulesanddynamicactin. Mol.Biol.Cell16,4410 4422.
Menager,C.,Arimura,N.,Fukata,Y.,Kaibuchi,K.,2004.PIP3isinvolvedinneuronalpolarizationandaxonformation.J.Neurochem.89,109 118. Morgan,J.L.,Dhingra,A.,Vardi,N.,Wong,R.O.,2006.Axonsanddendritesoriginatefromneuroepithelial-likeprocessesofretinalbipolarcells.Nat. Neurosci.9,85 92.
Mori,T.,Wada,T.,Suzuki,T.,Kubota,Y.,Inagaki,N.,2007.Singar1,anovelRUNdomain-containingprotein,suppressesformationofsurplusaxons forneuronalpolarity.J.Biol.Chem.282,19884 19893.
Nakata,K.,Abrams,B.,Grill,B.,etal.,2005.RegulationofaDLK-1andp38MAPkinasepathwaybytheubiquitinligaseRPM-1isrequiredfor presynapticdevelopment.Cell120,407 420.
Namba,T.,Kibe,Y.,Funahashi,Y.,Nakamuta,S.,Takano,T.,Ueno,T.,Shimada,A.,Kozawa,S.,Okamoto,M.,Shimoda,Y.,etal.,2014.Pioneering axonsregulateneuronalpolarizationinthedevelopingcerebralcortex.Neuron81,814 829.
Ng,J.,Luo,L.,2004.RhoGTPasesregulateaxongrowththroughconvergentanddivergentsignalingpathways.Neuron44,779 793.
Ng,J.,Nardine,T.,Harms,M.,etal.,2002.RacGTPasescontrolaxongrowth,guidanceandbranching.Nature416,442 447.
Nishimura,T.,Kato,K.,Yamaguchi,T.,Fukata,Y.,Ohno,S.,Kaibuchi,K.,2004.RoleofthePAR-3-KIF3complexintheestablishmentofneuronal polarity.Nat.CellBiol.6,328 334.
Nishimura,T.,Yamaguchi,T.,Kato,K.,etal.,2005.PAR-6-PAR-3mediatescdc42-inducedracactivationthroughtheracGEFsSTEF/Tiam1.Nat.Cell Biol.7,270 277.
Nishiyama,M.,Togashi,K.,vonSchimmelmann,M.J.,etal.,2011.Semaphorin3AinducesCaV2.3channel-dependentconversionofaxonstodendrites. Nat.CellBiol.13,676 685.
Noctor,S.C.,Martinez-Cerdeno,V.,Ivic,L.,Kriegstein,A.R.,2004.Corticalneuronsariseinsymmetricandasymmetricdivisionzonesandmigrate throughspecificphases.Nat.Neurosci.7,136 144.
Oinuma,I.,Katoh,H.,Negishi,M.,2007.R-Rascontrolsaxonspecificationupstreamofglycogensynthasekinase-3bthroughintegrin-linkedkinase. J.Biol.Chem.282,303 318.
OlivaJr.,A.A.,Atkins,C.M.,Copenagle,L.,Banker,G.A.,2006.Activatedc-JunN-terminalkinaseisrequiredforaxonformation.J.Neurosci.26, 9462 9470.
Ossipova,O.,Bardeesy,N.,Depinho,R.A.,Green,J.B.,2003.LKB1(XEEK1)regulatesWntsignallinginvertebratedevelopment.Nat.CellBiol.5, 889 894.
Ozdamar,B.,Bose,R.,Barrios-Rodiles,M.,Wang,H.R.,Zhang,Y.,Wrana,J.L.,2005.RegulationofthepolarityproteinPar6byTGFb receptors controlsepithelialcellplasticity.Science307,1603 1609.
Pan,C.L.,Baum,P.D.,Gu,M.,Jorgensen,E.M.,Clark,S.G.,Garriga,G.,2008. C.elegans AP-2andretromercontrolWntsignalingbyregulatingmig14/Wntless.Dev.Cell14,132 139.
Park,K.K.,Liu,K.,Hu,Y.,etal.,2008.PromotingaxonregenerationintheadultCNSbymodulationofthePTEN/mTORpathway.Science322, 963 966.
Plant,P.J.,Fawcett,J.P.,Lin,D.C.,etal.,2003.ApolaritycomplexofmPar-6andatypicalPKCbinds,phosphorylatesandregulatesmammalianLgl. Nat. CellBiol.5,301 308.
Polleux,F.,Giger,R.J.,Ginty,D.D.,Kolodkin,A.L.,Ghosh,A.,1998.Patterningofcorticalefferentprojectionsbysemaphorin neuropilininteractions. Science282,1904 1906.
Polleux,F.,Morrow,T.,Ghosh,A.,2000.Semaphorin3Aisachemoattractantforcorticalapicaldendrites.Nature404,567 573.
Polleux,F.,Whitford,K.L.,Dijkhuizen,P.A.,Vitalis,T.,Ghosh,A.,2002.ControlofcorticalinterneuronmigrationbyneurotrophinsandPI3-kinase signaling.Development129,3147 3160.
Prasad,B.C.,Clark,S.G.,2006.Wntsignalingestablishesanteroposteriorneuronalpolarityandrequiresretromerin C.elegans.Development133, 1757 1766.
Purro,S.A.,Ciani,L.,Hoyos-Flight,M.,Stamatakou,E.,Siomou,E.,Salinas,P.C.,2008.Wntregulatesaxonbehaviorthroughchangesinmicrotubule growthdirectionality:anewroleforadenomatouspolyposiscoli.J.Neurosci.28,8644 8654.
Qiu,R.G.,Abo,A.,StevenMartin,G.,2000.Ahumanhomologofthe C.elegans polaritydeterminantPar-6linksRacandCdc42toPKCzetasignaling andcelltransformation.Curr.Biol.10,697 707.
Rakic,P.,1971.Neuron-gliarelationshipduringgranulecellmigrationindevelopingcerebellarcortex.J.Comp.Neurol.141,283 312. Rakic,P.,1972.Modeofcellmigrationtothesuperficiallayersoffetalmonkeyneocortex.J.Comp.Neurol.145,61 83.
Randlett,O.,Poggi,L.,Zolessi,F.R.,Harris,W.A.,2011.Theorientedemergenceofaxonsfromretinalganglioncellsisdirectedbylaminincontact invivo.Neuron70,266 280.
Rolls,M.M.,Doe,C.Q.,2004.Baz,Par-6andaPKCarenotrequiredforaxonordendritespecificationinDrosophila.Nat.Neurosci.7,1293 1295. Saito,T.,Nakatsuji,N.,2001.Efficientgenetransferintotheembryonicmousebrainusinginvivoelectroporation.Dev.Biol.240,237 246. Sapkota,G.P.,Kieloch,A.,Lizcano,J.M.,etal.,2001.PhosphorylationoftheproteinkinasemutatedinPeutz Jegherscancersyndrome,LKB1/STK11, atSer431byp90(RSK)andcAMP-dependentproteinkinase,butnotitsfarnesylationatCys(433),isessentialforLKB1tosuppresscellgrowth. J.Biol.Chem.276,19469 19482.
Schober,M.,Schaefer,M.,Knoblich,J.A.,1999.BazookarecruitsInscuteabletoorientasymmetriccelldivisionsinDrosophilaneuroblasts.Nature402, 548 551.
Schwamborn,J.C.,Puschel,A.W.,2004.ThesequentialactivityoftheGTPasesRap1BandCdc42determinesneuronalpolarity.Nat.Neurosci.7, 923 929.
Schwamborn,J.C.,Khazaei,M.R.,Puschel,A.W.,2007a.TheinteractionofmPar3withtheubiquitinligaseSmurf2isrequiredfortheestablishmentof neuronalpolarity.J.Biol.Chem.282,35259 35268.
Schwamborn,J.C.,Muller,M.,Becker,A.H.,Puschel,A.W.,2007b.UbiquitinationoftheGTPaseRap1BbytheubiquitinligaseSmurf2isrequiredfor theestablishmentofneuronalpolarity.EMBOJ.26,1410 1422.
Shelly,M.,Cancedda,L.,Heilshorn,S.,Sumbre,G.,Poo,M.M.,2007.LKB1/STRADpromotesaxoninitiationduringneuronalpolarization.Cell129, 565 577.
Shelly,M.,Lim,B.K.,Cancedda,L.,Heilshorn,S.C.,Gao,H.,Poo,M.M.,2010.Localandlong-rangereciprocalregulationofcAMPandcGMPinaxon/ dendriteformation.Science327,547 552.
Shelly,M.,Cancedda,L.,Lim,B.K.,etal.,2011.Semaphorin3Aregulatesneuronalpolarizationbysuppressingaxonformationandpromotingdendrite growth.Neuron71,433 446.
Shi,S.H.,Jan,L.Y.,Jan,Y.N.,2003.HippocampalneuronalpolarityspecifiedbyspatiallylocalizedmPar3/mPar6andPI3-kinaseactivity.Cell112, 63 75.
Shi,S.H.,Cheng,T.,Jan,L.Y.,Jan,Y.N.,2004.APCandGSK-3b areinvolvedinmPar3targetingtothenascentaxonandestablishmentofneuronal polarity.Curr.Biol.14,2025 2032.
Shoukimas,G.M.,Hinds,J.W.,1978.Thedevelopmentofthecerebralcortexintheembryonicmouse:anelectronmicroscopicserialsectionanalysis. J.Comp.Neurol.179,795 830.
Speese,S.D.,Trotta,N.,Rodesch,C.K.,Aravamudan,B.,Broadie,K.,2003.Theubiquitinproteasomesystemacutelyregulatespresynapticprotein turnoverandsynapticefficacy.Curr.Biol.13,899 910.
Sperber,B.R.,Leight,S.,Goedert,M.,Lee,V.M.,1995.Glycogensynthasekinase-3betaphosphorylatestauproteinatmultiplesitesinintactcells. Neurosci.Lett.197,149 153.
Stack,J.H.,Emr,S.D.,1994.Vps34prequiredforyeastvacuolarproteinsortingisamultiplespecificitykinasethatexhibitsbothproteinkinaseand phosphatidylinositol-specificPI3-kinaseactivities.J.Biol.Chem.269,31552 31562.
Suzuki,A.,Hirata,M.,Kamimura,K.,etal.,2004.aPKCactsupstreamofPAR-1binboththeestablishmentandmaintenanceofmammalianepithelial polarity.Curr.Biol.14,1425 1435.
Tabata,H.,Nakajima,K.,2001.Efficientinuterogenetransfersystemtothedevelopingmousebrainusingelectroporation:visualizationofneuronal migrationinthedevelopingcortex.Neuroscience103,865 872.
Thornton,T.M.,Pedraza-Alva,G.,Deng,B.,etal.,2008.Phosphorylationbyp38MAPKasanalternativepathwayforGSK3b inactivation.Science320, 667 670.
Timm,T.,Balusamy,K.,Li,X.,Biernat,J.,Mandelkow,E.,Mandelkow,E.M.,2008.Glycogensynthasekinase(GSK)3-betadirectlyphosphorylates serine212intheregulatoryloopandinhibitsmicrotubuleaffinityregulatingkinase(MARK)2.J.Biol.Chem.283,18873 18882.
Toriyama,M.,Shimada,T.,Kim,K.B.,etal.,2006.Shootin1:aproteininvolvedintheorganizationofanasymmetricsignalforneuronalpolarization. J.CellBiol.175,147 157.
Tursun,B.,Schluter,A.,Peters,M.A.,etal.,2005.TheubiquitinligaseRnf6regulateslocalLIMkinase1levelsinaxonalgrowthcones.GenesDev.19, 2307 2319.
vonStein,W.,Ramrath,A.,Grimm,A.,Muller-Borg,M.,Wodarz,A.,2005.DirectassociationofBazooka/PAR-3withthelipidphosphatasePTEN revealsalinkbetweenthePAR/aPKCcomplexandphosphoinositidesignaling.Development132,1675 1686.
Watabe-Uchida,M.,John,K.A.,Janas,J.A.,Newey,S.E.,vanAelst,L.,2006.TheRacactivatorDOCK7regulatesneuronalpolaritythroughlocal phosphorylationofstathmin/Op18.Neuron51,727 739.
Watts,R.J.,Schuldiner,O.,Perrino,J.,Larsen,C.,Luo,L.,2004.Gliaengulfdegeneratingaxonsduringdevelopmentalaxonpruning.Curr.Biol.14, 678 684.
Witte,H.,Neukirchen,D.,Bradke,F.,2008.Microtubulestabilizationspecifiesinitialneuronalpolarization.J.CellBiol.180,619 632.
Yamanaka,T.,Horikoshi,Y.,Suzuki,A.,etal.,2001.PAR-6regulatesaPKCactivityinanovelwayandmediatescell cellcontact-inducedformationof theepithelialjunctionalcomplex.GenesCells6,721 731.
Yamanaka,T.,Horikoshi,Y.,Sugiyama,Y.,etal.,2003.MammalianLglformsaproteincomplexwithPAR-6andaPKCindependentlyofPAR-3to regulateepithelialcellpolarity.Curr.Biol.13,734 743.
Yamasaki,E.,Tanaka,D.H.,Yanagawa,Y.,Murakami,F.,2010.CorticalGABAergicinterneuronstransientlyassumeaseaurchin-likenonpolarized shapebeforeaxoninitiation.J.Neurosci.30,15221 15227.
Yan,D.,Guo,L.,Wang,Y.,2006.RequirementofdendriticAktdegradationbytheubiquitin-proteasomesystemforneuronalpolarity.J.CellBiol.174, 415 424.
Yi,J.J.,Barnes,A.P.,Hand,R.,Polleux,F.,Ehlers,M.D.,2010.TGF-bsignalingspecifiesaxonsduringbraindevelopment.Cell142,144 157. Yogev,S.,Shen,K.,2017.Establishingneuronalpolaritywithenvironmentalandintrinsicmechanisms.Neuron96,638 650.
Yoshimura,T.,Kawano,Y.,Arimura,N.,Kawabata,S.,Kikuchi,A.,Kaibuchi,K.,2005.GSK-3b regulatesphosphorylationofCRMP-2andneuronal polarity.Cell120,137 149.
Yoshimura,T.,Arimura,N.,Kawano,Y.,Kawabata,S.,Wang,S.,Kaibuchi,K.,2006.RasregulatesneuronalpolarityviathePI3-kinase/Akt/GSK-3b/ CRMP-2pathway.Biochem.Biophys.Res.Commun.340,62 68.
Zhang,H.,Macara,I.G.,2008.ThePAR-6polarityproteinregulatesdendriticspinemorphogenesisthroughp190RhoGAPandtheRhoGTPase.Dev. Cell14,216 226.
Zhang,X.,Zhu,J.,Yang,G.Y.,etal.,2007.DishevelledpromotesaxondifferentiationbyregulatingatypicalproteinkinaseC.Nat.CellBiol.9, 743 754.
Zhou,F.Q.,Zhou,J.,Dedhar,S.,Wu,Y.H.,Snider,W.D.,2004.NGF-inducedaxongrowthismediatedbylocalizedinactivationofGSK-3band functionsofthemicrotubuleplusendbindingproteinAPC.Neuron42,897 912.
Zhou,P.,Porcionatto,M.,Pilapil,M.,etal.,2007.PolarizedsignalingendosomescoordinateBDNF-inducedchemotaxisofcerebellarprecursors. Neuron 55,53 68.
Zolessi,F.R.,Poggi,L.,Wilkinson,C.J.,Chien,C.B.,Harris,W.A.,2006.Polarizationandorientationofretinalganglioncellsinvivo.NeuralDev.1,2.