PREFACE
Diversityofcatalysts,forexample,catalysisbymineralacids,complexesoftransition metals,organicmolecules,enzymes,andalumosilicates,resultedinfragmentationof thediscipline.Separatecoursesaregivenbasedontextbookscoveringseparately,despite allsimilarities,homogeneous,heterogeneous,andenzymaticcatalysis.Moregeneral booksonphysicalchemistry,whilediscussingsomeaspectsofcatalytickinetics,cannot gointodetailstotheextentthatisoftenneeded.
Althoughinthechemicalindustry,themainfocusiscurrentlyonheterogeneous catalysis,otherfields(homogeneous,enzymatic,photochemical,electrochemical catalysis)shouldnotbeoverlooked,asveryoftentheindustrialaimistoproduceacertain chemicalinthemostoptimalway,butnotwithaparticulartypeofcatalyst.Theindustrial importanceofcatalysisalsodictatesanecessitytohaveabookdevotedtothefundamental aspectsofcatalytickinetics,aswellascatalyticengineeringand,morespecifically, transportphenomena.
Suchanintegratedapproachtokineticsandtransportphenomenaincatalysis,while stillrecognizingthefundamentaldifferencesamongdifferenttypesofcatalysis,was appliedinthefirsteditionofthisbook,publishedin2005.
Theauthorshavereceivedanumberofencouragingcommentsaswellascritiqueabout whatwasmissingandsuggestionsforhowthebookcouldbeimproved.Wewerealsovery happytolearnthatduetoapositiveresponse,Elsevierdecidedtoproceedwiththesecond editionofthisbookoncatalytickinetics.Theauthorscarefullydiscussedthematerialtobe includedintherevisedversion.Materialforchapters10.1–10.2,10.4,and11.3–11.6was preparedbyTapioSalmi,whiletheremainingtextwaswrittenbyDmitryYu.Murzin, whoalsooversawthesecondeditionandimplementationofallchanges.
Inthisedition,thecontentwasexpandedtocoverinmoredepthseveralareas,suchas organocatalysis;enzymatickinetics;nonlineardynamics;solventeffects;nanokinetics (structuresensitivity);kineticisotopeeffects;andpolynomialkinetics,tonameafew. Inaddition,aseparatechapteroncascadecatalysishasbeenwritten.
Eachandeverychapterinthesecondeditionalsocontainsexamplesandexercises, whichfacilitatesunderstandingandmasteringoftheunderlyingconcepts.
Inordertostaymorefocusedonthecoreofcatalytickinetics,asectionongas-liquid masstransfer,whichwaspresentinthefirstedition,wasnotincluded.
Inadditiontothesechanges,unfortunatemisprintswerecorrectedandgeneraleditingofthetext,introducingnewfiguresandexamples,wasperformed.
isnotconsumedbythereaction.Thisdefin itionallowsforthepossibilitythatsmall amountsofthecatalystarelostinthereaction orthatthecatalyticactivityisslowlylost.
1.2.CATALYSIS
Alreadyfromthesedefinitions,itisclearthatthereisadirectlinkbetweenchemical kineticsandcatalysis;accordingtotheverydefinitionofcatalysis,itisakineticprocess. Therearedifferentviews,however,ontheinterrelationbetweenkineticsandcatalysis. Whilesomeauthorsstatethatcatalysisisapartofkinetics,otherstreatkineticsasapartof abroaderphenomenonofcatalysis.
Despitethefactthatcatalysisisakineticphenomenon,therearemanyissuesincatalysiswhicharenotrelatedtokinetics.Mechanismsofcatalyticreactions,elementary reactions,surfacereactivity,adsorptionofreactantsonth esolidsurfaces,synthesis andstructureofsolidmaterials,enzymesororganometalliccomplexes,notto mentionengineeringaspectsofcatalysis,areobviouslyoutsidethescopeofchemical kinetics.
Somediscrepancyexistswhetherchemicalkineticsincludealsothemechanismsof reactions.Infact,ifreactionmechanismsareincludedinthedefinitionofcatalytickinetics,itwillbeanunnecessarygeneralization,ascatalysisshouldcovermechanisms.
Catalysisisofcrucialimportanceforthechemicalindustry.Thenumberofcatalysts appliedinindustryisverylarge,andcatalystscomeinmanydifferentforms,fromheterogeneouscatalystsintheformofporoussolidstohomogeneouscatalystsdissolvedin theliquidreactionmixturetobiologicalcatalystsintheformofenzymes.Catalysisisa multidisciplinaryfieldrequiringeffortsofspecialistsindifferentfieldsofchemistry,
WilhelmOstwaldandSvanteArrhenius
JönsJakobBerzelius
physicsandbiologytoworktogethertoachievethegoalssetbymankind.Knowledgeof inorganic,organometallic,organicchemistry,materialsandsurfacescience,solidstate physics,spectroscopy,reactionengineeringandenzymologyisrequiredfortheadvancementsofthedisciplineofcatalysis.
Despitethefundamentaldifferencesbetweenelementarystepsincatalyticprocesson surfaces,therearestrikingsimilaritiesalsointermsofchemicalkineticswithenzymesor homogeneousorganometalliccomplexes.Althoughsuperficiallyitisdifficulttofind somethingincommonbetweenthereactionofnitrogenandhydrogenformingammoniaonasurfaceofiron,D-fructose6-phosphatereactionwithATPinvolvinganenzyme phosphofructokinaseorozonedecompositionintheatmosphereinthepresenceofNOx, allthesetransformationsrequirethatthecatalystformsbondswiththereactingmolecules. Suchacomplexthenreactstoproducts,leavingthecatalystunalteredandreadyfortaking partinanextcatalyticcycle.
Fig.1.1 isanexampleofacatalyticreactionbetweentwomoleculesAandBwiththe involvementofacatalyst.
Inordertounderstandhowacatalystcanaccelerateareaction,apotentialenergy diagramshouldbeconsidered.
Fig.1.2 representsaconceptforanon-catalyticreaction.Arrheniussuggestedthat reactionsshouldovercomeacertainbarrierbeforeareactioncanproceed.
ThechangeintheGibbsfreeenergybetweenthereactantsandtheproducts ΔG does notchangeincaseofacatalyticreaction;however,thecatalystprovidesanalternative pathforthereaction(Fig.1.3).
Fig.1.1 Catalyticcycle.
Fig.1.5 Potentialenergydiagramofanenzymaticreaction. (From https://upload.wikimedia.org/ wikipedia/commons/thumb/c/c0/Enzyme_catalysis_energy_levels_2.svg/1280px-Enzyme_catalysis_ energy_levels_2.svg.png?1450038342767 ).
catalystandareactantshouldnotbetoostrong.Alternatively,ifitistooweak,thenthe catalyticcyclecouldnotproceed.
Chemicalkineticsasadisciplineaddresseshowthereactionratesdependonreactant concentration,temperature,natureofcatalysts,pH,andsolvent,tonameafewreaction parameters.
Chemicalkinetics,togetherwithothermeansofstudyingcatalyticreactionslikespectroscopyofcatalystsandcatalystmodels,quantum-chemicalcalculationsforreactants, intermediatesandproducts,calculationofthethermodynamicsofreactants,intermediatesandproductsfrommeasuredspectraandquantum-chemicalcalculations,formthe modernbasisforunderstandingcatalysis.
Kineticinvestigationsareoneofthewaystorevealreactionmechanisms.Thefollowingproblemscanbesolvedusingthekineticmodel:
•choosingthecatalystandcomparingtheselectivityandactivityofcatalystsandtheir performanceunderoptimumconditionsforeachcatalyst;
•thedeterminationoftheoptimumsizesandstructureofcatalystgrainsandthenecessaryamountofthecatalysttoachievethespecifiedvaluesoftheselectivityoftheprocessandconversionofthestartingproducts;
•thedeterminationofthecompositionofallbyproductsformedduringtheprocess;
•thedeterminationofthestabilityofsteadystatesandparametricsensitivity;thatis,the influenceofdeviationsofallparametersonthesteady-stateregimeandthebehaviorof thereactorunderunsteadystateconditions;
•thestudyofthedynamicsoftheprocessanddecidingiftheprocessshouldbecarried outunderunsteady-stateconditions;
•thestudyoftheinfluenceofmassandheattransferprocessesonthechemicalreaction rateandthedeterminationofthekineticregionoftheprocess;
•selectionofthereactortypeandstructureofthecontactunitprovidingthebest approximationstotheoptimumconditions.
Veryoften,theratesofchemicaltransformationsareaffectedbytheratesofotherprocesses,suchasheatandmasstransfer.Theprocessshouldbetreatedasapartofkinetics. Thegas/liquidmasstransferinmultiphaseheterogeneousandhomogeneouscatalytic reactionscouldbetreatedinasimilarway.Themathematicalframeworkformodeling diffusioninsidesolidcatalystparticlesofsupportedmetalcatalystsorimmobilized enzymesdoesnotdifferthatmuch,butpropercareshouldbetakenofthereaction kinetics.
Theimmenseimportanceofcatalysisinchemicalindustryismanifestedbythefact thatroughly85–90%ofallchemicalproductshaveseenacatalystduringthecourse ofproduction.
Fig.1.6 demonstratesapplicationsofcatalysisinindustry.Inthelastfewyears,thereis anincreaseofcatalyticapplicationsalsofornon-chemicalindustries,includingtreatment ofexhaustgasesfromcarsandothermobilesources,aswellaspowerplants(Fig.1.7).
Worldwidecatalystmarket.
Fig.1.7 CatalytictreatmentofNOx in(A)mobileand(B)stationarysources.
The catalyst group: The intelligence report: global shifts in the catalyst industry
Fig.1.6
(A)
(B)
Acomparisonbetweenhomogeneousandheterogeneouscatalystsfromtheviewpointofahomogeneouscatalysisexpertispresentedbelow:
Selectivity High Variable
Conditionsofreaction Mild Harsh
Lifetimeofcatalyst Variable Long
Sensitivitytodeactivation Low High
Problemsduetodiffusion None Difficulttosolve
Recyclingofcatalyst Usuallydifficult Caneasilybedone
StericandelectronicpropertiesEasilychanged Novariationpossible Mechanism RealisticmodelsexistNotobvious
Thetopicsaddressedabovewillbediscussedinmoredetailinthesubsequentchapters.
Agreatvarietyofhomogeneouscatalystsareknown,includingmetalcomplexesand ions,BrønstedandLewisacids,andenzymes.Homogeneoustransitionmetalsareusedin severalindustrialprocesses,afewofthemaregivenbelow: Process
Acetaldehyde 2.5 Pd/Cu375–4053–8 Aceticacid 4.0 Rh425–47530–60
Oxo-alcohols 7 CoorRh335–470200/30
Dimethylterephthalate3.3 Co415–4454–8
Terephthalicacid9.4 Co450–50515–30
Metalcomplexescanhaveaverysophisticatedstructurewithavarietyofligands.An exampleofsuchligandsforRhcatalyzedhydroformylationisgivenbelow(Fig.1.8) alongwithsomeimagesofheterogeneouscatalysts(Fig.1.9).
Enzymesrepresentaspecialtypeofhomogeneouscatalyst.Theyarelargeproteins (Fig.1.10)capableofincreasingthereactionratesbyafactorof106 atmildreactionconditionsanddisplayingveryhighspecificityandcapabilityofregulation.
Specificity(Fig.1.11)iscontrolledbytheenzymestructure;moreprecisely,aunique fitofsubstratewiththeenzymethatcontrolstheselectivityforthesubstrateandtheproductyield.
Superficially,thereisnotthatmuchincommonbetweenalargeproteinandaPt/ Al2O3 heterogeneouscatalyst.Atthesametime,thechemicalreactionswhichoccurwith bothtypesofcatalystsinvolvecertainactivesites;forexample,regionswherecatalysis occurs.Whateverthespecificreaction,itcanbeschematicallyrepresentedby
where A and B arereactants, C and D products,and a, b, c,and d arestoichiometriccoefficients.Anequationforachemicalreactioniswritteninsuchawaythatallthemolecules participatinginthereactionarebalanced.
Veryofteninchemicalreactionengineering,thestoichiometriccoefficient νi is definedastheamountofproductproducedafteronerunofthereaction.Itimpliesthat thestoichiometriccoefficientispositiveforaproductandnegativeforareactant.
Thus,forthereaction:
Thefollowingstoichiometriccoefficientshold:
Anextensivequantitydescribingtheprogressofachemicalreactionequaltothenumber ofchemicaltransformations(thetotalnumberofreactionruns)dividedbyAvogadro’s number(itisessentiallythe amount ofchemicaltransformations)iscalledtheextentof reaction.Thechangeintheextentofreactionisgivenbydξ ¼ dni/νi,where νi isthe stoichiometricnumber ofanyreactionentity i (reactantorproduct)and ni isthecorrespondingamountinmoles.
Thus,dξ/dt isanextensiveproperty,whichismeasuredinmolesandcannotbeconsideredareactionrate,asitisproportionaltothesizeofthereactor.
Ingeneral,forahomogeneousreactionforwhichboththereactionratechangeswith timeandisnotuniformoveravolumeofareactor,thereactionrateis:
where V standsforthereactorvolume.Ifthereactorvolumeisconstant,thenthereactionrateissimply:
where i isthereactantorproductwithcorrespondingstoichiometriccoefficient νi,and Ci istheconcentrationofcomponent i.Forareaction:
therateofconsumptionofreactant A isthen:
where nA isthenumberofmolesof A inthereactorand[A]istheconcentrationof A. Similarly,forthereaction:
1.4.ACQUISITIONOFKINETICDATA
Kineticdataforachemicalreactionaregatheredindifferenttypeofreactors,andwewill brieflymentionsomerequirementsforchemicalreactorsfromtheviewpointofkinetic analysis.Ahighprecisionofthedataisneededaslargedeviationsinthevaluesofthe experimentallymeasuredrateswillbeaseriousobstacleforquantitativeconsiderations. Reproducibilityofratemeasurementsoverabroadrangeofparametersisalsoofimportance.Anothernecessaryfeatureisthepossibilitytoreachagoalofobtainingthemaximumamountofkineticinformationinminimumtime.Analysisofproductsaswellas reactorlayoutshouldpreferablybeaseasyaspossible.
Essentialfeaturesforcatalyticreactionsarethereadinessinreduction/activationof heterogeneouscatalystsandapossibilitytoutilizethemintheneededgeometricalform. Despitethestrictdefinitionofcatalysis,whichstatesthatthecatalystdoesnotchange duringthecatalyticreactions,someactivitydeteriorationtakesplace;therefore,measurementsofcatalytickineticsshouldalwaysmonitorthecatalystactivity.
Differenttypesofreactorsareappliedinpractice(Fig.1.14).Stirredtankreactors (STR),veryoftenappliedforhomogeneous,enzymaticandmultiphaseheterogeneous catalyticreactions,canbeoperatedbatch-wise(batchreactor,BR),semi-batch-wise (semi-batchreactor,SBR)orcontinuously(continuousstirredtankreactor,CSTR).
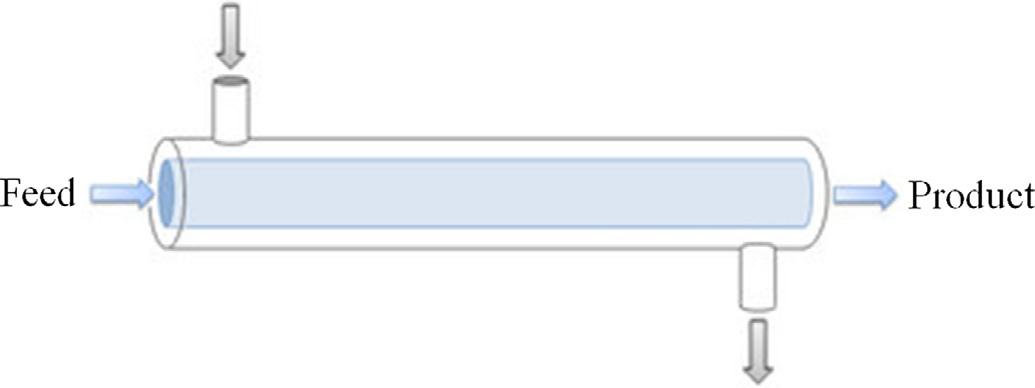
Alternatively,tubularreactorswithplugflow(pistonflow)(PFR)areusedandoperateincontinuousmode(Fig.1.15).
Fig.1.14 Differenttypesofstirredtankreactors.
Fig.1.15 Tubularreactors.
Fig.1.18 Avolumeelement.
Foraninfinitesimalvolumeelement ΔV in Fig.1.18,themassbalancecouldbewritteninaform:
IN+GENERATION ¼ OUT+ACCUMULATION
leadingtoafollowingequationintermsofmoles:
where n 0A and n A arethemolefluxes, ηA isthecatalysteffectivenessfactor(Chapter10) takingintoaccountmasstransfer.
ForabatchreactoritholdsthatIN ¼ 0,OUT ¼ 0;therefore:
Ifthevolumeisconstant,onegets:
FromEqs. (1.23) and (1.24):
makinguseoftherelationshipbetweenconcentrationandconversion α:
Assumingthatthecatalysteffectivenessfactor ηA isequalto1andtakingintoaccount boundaryconditions(t ¼ 0, α ¼ 0),wearriveat:
Infact,treatmentofheterogeneous,homogeneousandenzymaticreactionsisbasically thesamewiththeonlydifferenceintheexpressionsofreactionrates,whichreflectdifferentreactionmechanisms.Somespecificcaseswillbediscussedin Chapters5–7.Here wepresentfewexamples:
reactorsareefficientforcatalystscreening,especiallywhentheyarearrangedinaparallel mode(Fig.1.21).
Theapparentdrawbackisthatoneexperimentleadstoonlyonedatapoint.Onthe otherhand,catalystdeactivationwithtimeonstreamcouldbeeasilyseen(Fig.1.22).
Quantitativetreatmentofplugflowreactorsissomewhatcumbersome;therefore, severalassumptionsareusuallymade.Thefluidcompositionisconsideredtobeuniform alongthereactorcrosssection(ie,thereisnoradialdispersion).Thisisvalidonlywhen:
Fig.1.20 Atubularreactor.
Fig.1.21 Multitubularreactor.
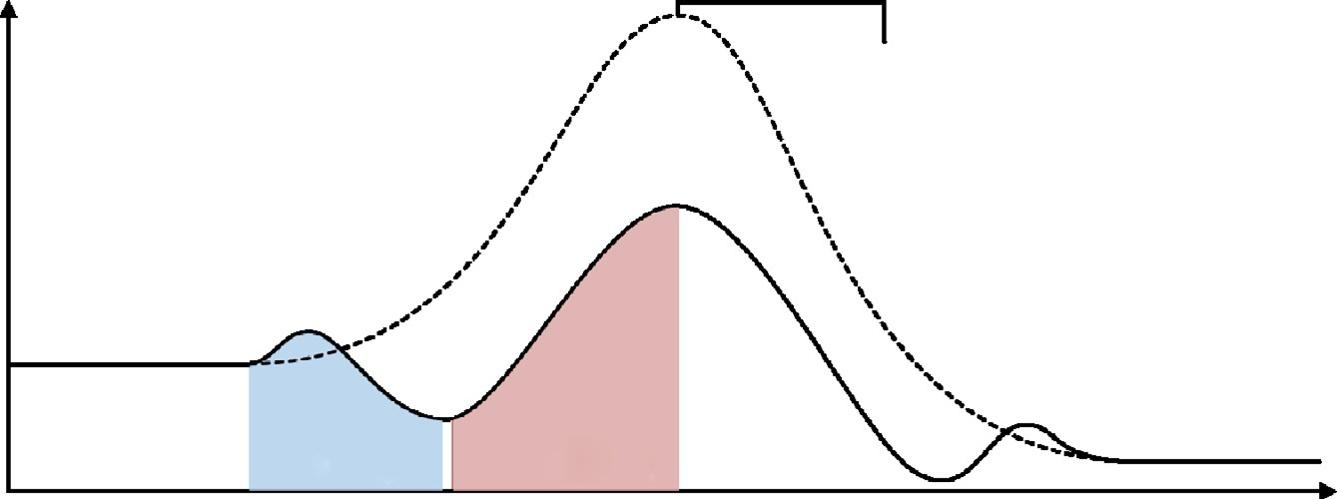
Forlargercatalystparticles,somegasbypassclosetothereactorwallsispossible.When thereactantsmovealongthereactor,thecompositionofthemixturechangesandaxial diffusioncanbecomeprominent.Toavoidthepossibleimpactofaxialdiffusionin kineticexperiments,thelevelofconversionshouldbebelow10–15%.
Ingeneral,non-isothermalbehaviorofatubularreactorshouldalsobetakeninto account,sinceso-calledisothermalreactorsareseldomisothermal(Fig.1.23);however, forthesakeofsimplicityitwillnotbeconsideredbelow.
Themassbalanceforaplugflowreactor(Fig.1.23)isthengivenby:
Fig.1.22 Catalystdeactivationwithtime-on-streamatdifferentconditions.
Fig.1.23 Temperatureprofileinatubularreactor.