
Carbonquantumdots(CQDs)modifiedTiO2nanorods photoelectrodeforenhancedphotocathodic protectionofQ235carbonsteelMinFeng&Ying Liu&SainanZhang&YupengLiu&NingLuo&Daoai Wang https://ebookmass.com/product/carbon-quantum-dots-cqdsmodified-tio2-nanorods-photoelectrode-for-enhancedphotocathodic-protection-of-q235-carbon-steel-min-feng-yingliu-sainan-zhang-yupeng-liu-ning-luo-daoai-wang/

Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Elsevier Weekblad - Week 26 - 2022 Gebruiker
https://ebookmass.com/product/elsevier-weekbladweek-26-2022-gebruiker/
ebookmass.com

Facile fabrication of SnO2 modified TiO2 nanorods film for efficient photocathodic protection of 304 stainless steel under simulated solar light Juantao Zhang & Hualong Yang & Yuan Wang & Xiaohu Cui & Zhang Wen & Yunpeng Liu & Lei Fan & Jiangtao Feng
https://ebookmass.com/product/facile-fabrication-of-sno2-modifiedtio2-nanorods-film-for-efficient-photocathodic-protectionof-304-stainless-steel-under-simulated-solar-light-juantao-zhanghualong-yang-yuan-wang-xiaohu-cui/ ebookmass.com
Jock Seeks Geek: The Holidates Series Book #26 Jill Brashear
https://ebookmass.com/product/jock-seeks-geek-the-holidates-seriesbook-26-jill-brashear/ ebookmass.com
Train Me Daddy: An Age Play Mafia Daddy Romance (Mafia Daddies NYC Book 1) Zack Wish
https://ebookmass.com/product/train-me-daddy-an-age-play-mafia-daddyromance-mafia-daddies-nyc-book-1-zack-wish/ ebookmass.com



Guarded by the Marshal: A Small Town Romantic Suspense (In Clear Sight Book 1) Kennedy L. Mitchell
https://ebookmass.com/product/guarded-by-the-marshal-a-small-townromantic-suspense-in-clear-sight-book-1-kennedy-l-mitchell/
ebookmass.com
The Rise of the Middle Class in Contemporary China Hainan Su
https://ebookmass.com/product/the-rise-of-the-middle-class-incontemporary-china-hainan-su/
ebookmass.com
The Strange Appeal of Dougie Neil Keith A Pearson


https://ebookmass.com/product/the-strange-appeal-of-dougie-neil-keitha-pearson/
ebookmass.com
Chemistry & Chemical Reactivity 9th Edition, (Ebook PDF)

https://ebookmass.com/product/chemistry-chemical-reactivity-9thedition-ebook-pdf/
ebookmass.com
The Book of Minds: How to Understand Ourselves and Other Beings, From Animals to Aliens Ball
https://ebookmass.com/product/the-book-of-minds-how-to-understandourselves-and-other-beings-from-animals-to-aliens-ball/
ebookmass.com


9th Edition 9th Edition, (Ebook PDF)
https://ebookmass.com/product/environment-9th-edition-9th-editionebook-pdf/
ebookmass.com


CorrosionScience
journalhomepage: www.elsevier.com/locate/corsci
Carbonquantumdots(CQDs)modifiedTiO2 nanorodsphotoelectrodefor enhancedphotocathodicprotectionofQ235carbonsteel
MinFenga,b,YingLiub,**,SainanZhangb,YupengLiua,c,*,NingLuoc,DaoaiWanga,c,*
a StateKeyLaboratoryofSolidLubrication,LanzhouInstituteofChemicalPhysics,ChineseAcademyofSciences,Lanzhou730000,China
b InstituteofMaterialsScienceandEngineering,OceanUniversityofChina,Qingdao266100,China
c QingdaoCenterofResourceChemistryandNewMaterials,Qingdao266100,China
ARTICLEINFO
Keywords:
A.Titanium
A.Carbonsteel
B.XPS
B.EIS
C.Cathodicprotection


ABSTRACT
AnewphotoanodeofAl2O3 anchoredcarbonquantumdots/TiO2 nanorods(Al/C/TNRs)wasconstructedfor efficientphotocathodicprotectionofQ235carbonsteel(CS)byhydrothermaltreatmentandALDprocess.The Al/C/TNRsphotoanodeachievedaphotocurrentdensityof2.28mA/cm2 undersimulatedsunlight(AM1.5G), andcouldmaintainfor7daysnearlywithoutdecay.PotentialofQ235CSwasnegativelyshiftedby620mVafter couplingwithAl/C/TNRsin3.5wt%NaClsolution,andcouldmaintainmorethan7h.Thehighandstable photoelectrochemicalperformancesofAl/C/TNRsindicatepotentialphotocathodicprotectionforQ235CSin marineenvironment.
1.Introduction
Asatypicalengineeringmaterialinmarinesystem,carbonsteel (CS)hasbeenwidelyappliedinshipping,oilrecovery,offshoreplatformandminingindustriesovertheyears[1,2].Whilethecorrosion problemofCShasalwaysbeenanimportantchallenge,andwasusually inhibitedorsloweddownbyanti-corrosioncoating,cathodicprotection,corrosioninhibitorandothertechnologies[3].Asoneofthemost frequentlyusedtechnologiesformetalanticorrosion,cathodicprotectionhasmainlytwotypesofimpressedcurrentcathodicprotectionand sacrificialanodicprotection[4,5].Theimpressedcurrentcathodic protectionneedsanexternalpowersourcetoprovideelectronstothe protectedmetal,thisundoubtedlyincreasestheelectricenergyconsumption.Thesacrificialanodeprotectionintroducesanactivemetal (mostlyZn,Mg,Al)withtheself-corrosionpotentialmorenegativethan theprotectedmetaltopolarizetheprotectedmetaltocorrosionstable region,whileusuallybringssacrificialmetalanodewastingandenvironmentalpollution.Differentfromthetraditionalcathodicprotection,thenewphotocathodicprotectiontechnologyutilizesphotoelectricpropertiesofsemiconductorsandsolarenergytoprovide electronstotheprotectedmetal,whichisenvironmentallyfriendlyand willnotconsumeotherelectricenergiesduringthisprocess[6,7].It seemstobeanidealtechnologythatphotocathodicprotectioncan achieveprotectionofmetalssimplybyconsumingsunlightinnature.
However,atpresenttheefficiencyofphotocathodicprotectionisstill relativelylowbyusingonlytheultravioletpartofsunlightandthere areonlyafewsuitablematerialsforphotocathode,whichhighlylimits itspracticalapplication[8].
Inprinciple,thephotocathodicprotectionofmetalsbasedona photocatalystsemiconductormaterialoccursinthreesteps,similarto thephotoelectrochemical(PEC)processes[9].Firstly,thephotoelectrodesemiconductormaterialabsorbslightenergygreaterthanitsband gaptogenerateelectronandholepairs(hν ≥ Eg).Then,thephotoexcitedchargesseparateandtransmittotheexternalcircuitwithout recombination.Lastly,thephotogeneratedelectronsenrichedonthe surfaceoftheprotectedmetal,whichmakesthepotentialoftheprotectedmetalmorenegativethanitsself-corrosionpotentialtoform photocathodicprotection.Therefore,thephotoelectrodesemiconductor materialisthekeyfactor,whichdeterminesthefollowingefficiencyof photocathodicprotection.TakeTiO2 photoelectricsemiconductormaterialasanexample,itwasthe firstandthemostwidelystudiedmaterialinthe fieldofphotocathodicprotection,whilethelowefficiency ofphotocathodeprotectionstilllimitsitspracticalapplicationatpresent[10].Generally,theapplicationsofpureTiO2 inphotocathodic protectionareusuallyhinderedbythefollowingthreefactors.Firstly, theintrinsicwidebandgapconfinesitslightabsorptionrangewithin ultravioletlight(UV)regionofthesolarspectrum[11].Secondly,the surfacedefectstructuresandotherimpuritiesinTiO2 causehigh
⁎ Correspondingauthorsat:StateKeyLaboratoryofSolidLubrication,LanzhouInstituteofChemicalPhysics,ChineseAcademyofSciences,Lanzhou730000, China.
⁎⁎ Correspondingauthor.
E-mailaddresses: liuyingwda@ouc.edu.cn (Y.Liu), yupengliu@licp.cas.cn (Y.Liu), wangda@licp.cas.cn (D.Wang).
https://doi.org/10.1016/j.corsci.2020.108919
Received24March2020;Receivedinrevisedform29July2020;Accepted30July2020
Availableonline03August2020
0010-938X/©2020ElsevierLtd.Allrightsreserved.
photoinducedchargerecombinationrateandbringlowquantumefficiencyinPECmeasurements[12].Inaddition,noelectronsareproducedunderdarkconditions,accompaniedbyswiftphotogenerated electronsannihilation,makeithardtoprovidevalidall-weatherprotectionofmetals[6,13].
Tosolvetheaboveproblems,numerousmethodshavebeencarried outtoenhancethephotoelectricactivityofTiO2 materials. ConstructinghighlypureTiO2 withorderedstructureisaneffective waytofacilitatetheseparationandtransportationofphotogenerated electronsandholes[9],suchasTiO2 nanowires,nanotubesandnanobelts.Moreover,iftheseone-dimensionalmaterialsassembletomore orderednanoarraystructure,theefficiencyofchargeseparationand transportationcanbefurtherimproved,whichhasbeenwidelyusedin photocatalyticwatersplitting,solarcellsandchemicalsensors[14,15]. Bandmodulationisanotherfrequently-usedstrategytoimprovethe photoelectricactivityofTiO2 materialsbybroadeningtheabsorption rangeofTiO2,suchasdopingofotherelements(C,N,F,Ag, etc) [16–19],couplingwithnarrowband-gapsemiconductors(CeO2,V2O5, WO3, etc)[20–22]andabsorbingorganicdyes[23]toextendtheabsorptionedgefromultravioletlighttovisiblelightregion.Inthese strategies,thestabilityofthenewdopedorcompositematerials,the shortlifespanofsensitizersunderilluminationandtheinstabilityof nanocompositesoftendecreasetheefficiencyandrestrictpracticalapplicationinphotocatalysisandphotocathodeprotection[24,25].Recently,asanewtypeofphotocatalyticandphotoelectricmaterials,the quantumdots(QDs)havealsoattractedattentionforitsquantum confinementeffectdefinedthatbandgapandopticalpropertiesofQDs canbetunedbychangingtheirsizes[13].GiventhatQDscanbeutilizedtoabsorbhotelectronsandgenerateplentifulelectron-holepairs withasinglephotonunderlightirradiation[26],carbonquantumdot (CQD)hasbeenusedasanewkindofefficientandgreensensitizerto enhancethephotocatalyticpropertiesofTiO2 inphotocatalysisandPEC watersplitting fieldsowingtoitsstrongabsorptionbandwithinUV regionwithalongstreakingundervisiblelight[27].Meanwhile,the lowestunoccupiedmolecularorbital(LUMO)energylevelofCQDslies higherthantheconductionband(CB)ofTiO2,sotheelectronsexcited byvisiblelightcanbeeasilytransferredfromLUMOenergyleveltoCB ofTiO2,andthengreatlybroadentheopticalabsorptionrangeofTiO2 [28].However,CQDsareeasytofalloff fromphotoanodesduetothe flowandvibrationofthesolutionduringmeasurements,whichhighly limitstheapplicationsofthecompositematerials.Newtechnologyis requiredtoanchorcarbonquantumdotsonthesurfaceofphotoelectrodes.Therefore,designandpreparationofastableelectrodematerial withhighlyorderedstructureandvisiblelightabsorptionperformance isthekeytoachievehighefficiencyphotocathodeprotection.
Herein,anewAl2O3 layer-anchoredCQDs-loadedTiO2 nanorods array(Al/C/TNRs)photoanodewasconstructedforefficientphotocathodicprotectionofQ235carbonsteel(CS)byhydrothermaland atomiclayerdeposition(ALD)methods.TheAl/C/TNRselectrodewas obtainedbyloadingCQDsonorderedrutileTNRsthroughimpregnationmethodanddepositingultrathinAl2O3 filmonC/TNRsthrough thermalALDtechnique.ThemodificationofCQDshighlyextendsthe lightabsorptionarrayofTiO2 fromultravioletregiontovisibleregion. Moreover,theCQDscouldalsoactasanelectrontrapthatstores photoinducedelectronsandreleasesthemtothesurfaceofmetalsto maintainphotocathodicprotectionperformancesintheabsenceof sunlightillumination[29].ThehighorderedstructureofTiO2 nanorods arrayacceleratestheseparationandtransmissionofphotogenerated electron-holepairs.TheAl2O3 ultrathin filmonC/TNRscanhighly stabilizethesurfacestateofthenanocompositetopreventthefallof CQDsfromTNRs,anditcanalsodepresstherecombinationofphotoinducedchargesowingtothelowinterfacedefectdensityofAl2O3 layer byALDmethod[30].Thesecompositephotoanodematerialsexhibited highandstablephotoelectrochemicalandphotocathodicprotection performances,whichprovideanewwaytopreventmetalsfromcorrosionbyphotocathodicprotectioneffectsinmarineenvironment.
2.Materialsandmethods
2.1.Materials
Hydrochloricacid(HCl,37%),Tetra-n-butyltitanate(TNBT)and trimethyl-aluminum(TMA,99.99%)werepurchasedfromSinopharm ChemicalReagentCo.,Ltd.Formamideandacetonewereobtainedfrom localsupplierswiththehighestpurity.Citricacidwaspurchasedfrom Sigma.Allofthechemicalswereofanalyticalreagentgradesandused withoutfurtherpurification.Fluorine-dopedtinoxide(FTO)conductingglasses(10mm×50mm×1.1mm)werepurchasedfrom QingdaoAmyCommerce&TradingCo.,Ltd.Q235CSwasobtained fromBeijingHanlongdaTechnologyDevelopmentCo.,Ltd.
2.2.PreparationofTNRsandCQDs
TherutileTiO2 nanorodsarrayswere insitu grownonthesurfaceof FTOconductingglassesbyamodifiedhydrothermalmethod[31]. Firstly,apieceofFTOconductingglasssequentiallyultrasoniccleaned byacetone,ethylalcoholanddeionized(DI)waterwassetobliquely againsttheinnerwallofTeflon-linedstainlesssteelautoclave(100mL) withtheconductivesidefacingup.Andthenamixedsolutionconsistingof25mLHCl,25mLDIwaterand720 μLTNBTwastransferred intotheaboveautoclaveaftermagneticallystirringfor20min,and thenreactedinanovenat180°Cfor6h.Aftertheautoclavecooled downtoroomtemperaturenaturallyinair,theTNRswererinsedbyDI waterandannealedinamufflefurnace(KSL-1200X)at450°Cfor2hin airtogetcrystallizedmonocrystallineTNRs.CQDsweresynthesized throughamodifiedmethodbasedontheestablishedprocedures [32,33].Typically,asolutionofcitricacidinformamide(0.3g/mL) wasrefluxedfor15minandcooleddowntoroomtemperaturenaturally,thentheacetonewiththevolumeofequivalentto5timesofthe initialvolumewasaddedtothesolution.Finally,theprecipitateswere successively filtratedanddialysedwitha1000Dadialysistube,andthe obtainedsolutioncontainingCQDswaslyophilizedforfurtheruse.
2.3.PreparationofCQDs/TNRsphotoelectrode
Theas-preparedTNRswereimpregnatedintheaqueoussolutionof CQDswiththeconcentrationof0.5mg/mLat60 ℃ for20hinan electricthermostaticwaterchamber(H.SWX-600BS)underdarkcondition.Thenthesamplesweretakenoutanddriedinthevacuumdrying chamber(DZ-1BC Ⅱ)at80 ℃ overnightandannealedinatubefurnace (OTF-1200X)under50sccmofN2 carrier(Airgas,UltraHighPurity)at 200 ℃ for20min.
2.4.PreparationofAl2O3 anchoredCQDs/TNRsphotoelectrode
Al2O3 filmof5nmwasgrownontheas-preparedC/TNRsina commercialALDreactorat150 ℃ afterdepositionof50cyclesbyusing TMAandDIwaterasprecursorcompounds[34].Onecompletedepositioncyclewasproceededwith1sTMApulse,8sTMAexposure, 20spurgewithN2 followedwith0.1sDIwaterpulse,8sDIwater exposureand finally25spurgewithN2.TMAandDIwaterrespectively reactedwith OHgroupsand CH3 groupsonthesurface.ThenTMA waspumpedawaybypumpingintoN2 carriergasstreamwhenthe surfacereaction finished.BasedonthemeasurementontheSisubstrate byusingellipsometry,ithadbeenprovedthatoneintactcyclethat comprisedofsequentialexposuretoTMAandDIwaterobtaineda growthrateof1.1ÅAl2O3 layerpercycleundertheoptimalreaction conditions.[35]Finally,Al/C/TNRscompositeswerefabricatedcompletely.
2.5.Characterizations
ThemorphologiesofTNRs,C/TNRsandAl/C/TNRswereobserved
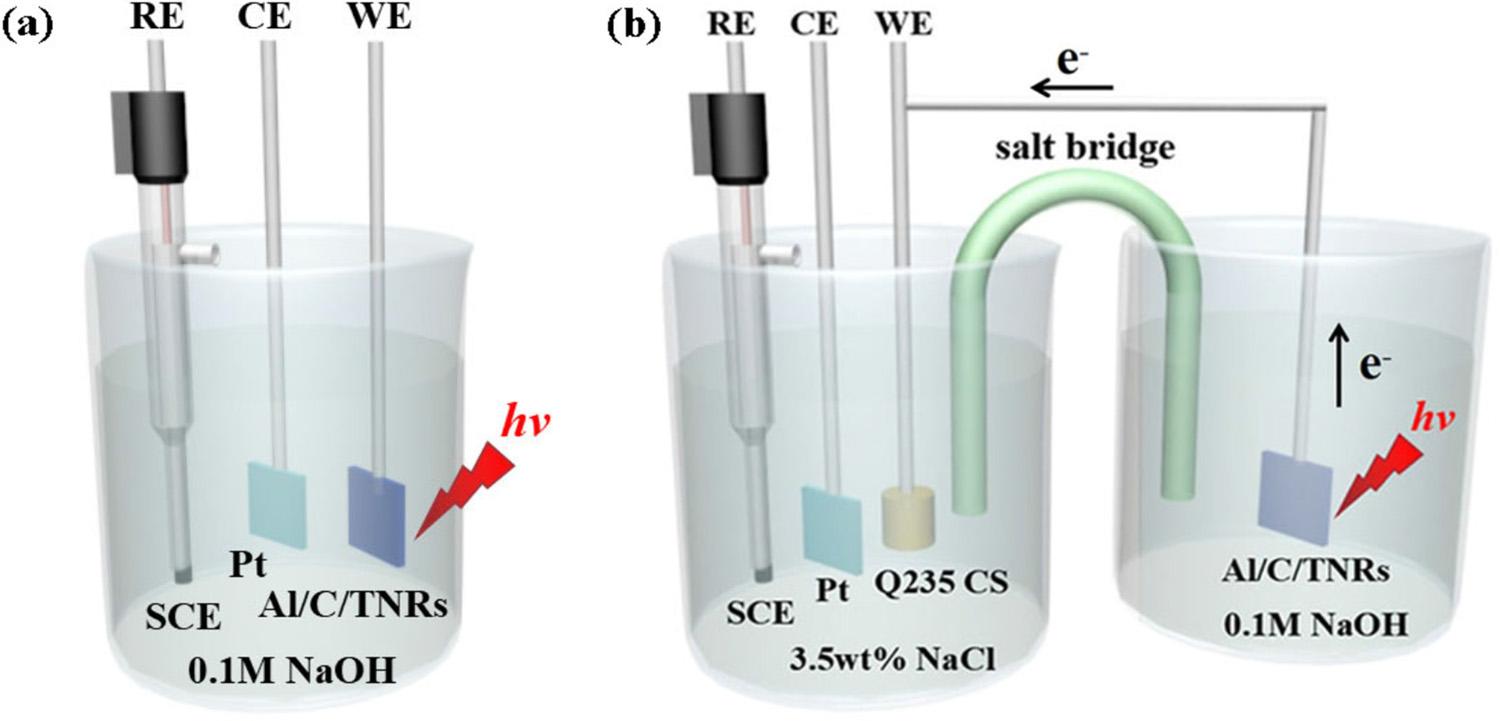
Fig.1. Schematicsketchesoftheexperimentaldevicesforphotoelectrochemical(a)andphotocathodicprotection(b)characterizations.TheCE,REandWErepresentthecounterelectrode,referenceelectrodeandworkingelectrode,respectively.
ona fieldemissionscanningelectronmicroscope(FE-SEM,S-4800, Hitachi).Highresolutiontransmissionelectronmicroscopy(HRTEM) imageswereobtainedfromaFEITecnaiG2TF20transmissionelectron microscopeoperatingattheaccelerationvoltageof200kV.ThecompositionsofsamplesweremeasuredthroughX-rayphotoelectron spectroscope(XPS)withanESCALAB250Xisystemandbindingenergieswerecorrectedbasedoncontainmentcarbon(C1s284.6eV). RamanspectrawerecollectedonaDXRRamanMicroscopewith+12V DCvoltage.UV–visabsorptionspectraweremeasuredbyU-4100 Spectrophotometer(Solid).
2.6.Electrochemicalmeasurements
Alltheelectrochemicalmeasurementsareconductedonanelectrochemicalworkstation(CHI660E,Shanghai)byusingatypicalthreeelectrodecellatroomtemperature.Theexperimentaldevicesfor measuringPECpropertiesandphotogeneratedcathodicprotection performancesareillustratedin Fig.1aandb,respectively.Asshownin Fig.1a,PECpropertiesofthesamplesweremeasuredinasingleelectrochemicalcellwiththeelectrolyteof0.1MNaOHsolution(PH13) afterpurgingwithargongasfor15mintoremovedissolvedoxygen,in whichthePtfoil,saturatedcalomelelectrode(SCE)andAl/C/TNRs compositephotoelectrodeservedascounterelectrode(CE),reference electrode(RE)andworkingelectrode(WE),respectively.Theactive illuminationareaofthesampleswascontrolledtobe1×1cm2,and thephotoanodeswereilluminatedbya300WXelamp(PLS-SXE300 UV,Beijing)coupledwithanAM1.5G filtertodemarcatethelight intensityto100mW/cm2.Photocurrentdensity vs. timecurvesand incident-photon-to-currentefficiencies(IPCE)weremeasuredat0.23V
vs. SCEundertheilluminationofsimulatedsunlight(100mW/cm2). Thestabilityofphotocurrentdensitymeasurementswasconductedfor acertaintimeat0.23V vs. SCE.PhotoinducedOCPdatawerecollected byusingtheabovethree-electrodecellundersimulatedsunlightillumination(100mW/cm2).Toevaluatephotocathodicprotectionperformancesofthecoupledmetals,adouble-cellsystemwasintroduced shownas Fig.1b.Q235CSelectrodewaspolishedbymetallographic polishingmachine(MP-2A),ultrasonicallycleanedwithacetone,ethyl alcoholandDIwaterrespectively,andthendriedbyN2.Finally,the Q235CSelectrodewassealedupwithsettingonesquarecentimeter (1cm×1cm)asidebyepoxyresinforlatermeasurements.Thephotoanodes(TNRs,C/TNRs,Al/C/TNRs)andQ235CSelectrodecoupled byaconductingwirewereisolatedlyplacedintheelectrochemicalcell andthecorrosioncell,respectively.Thesaltbridge(1MKClinagar,Utypeglasstube)providedconnectionandcompletedthecircuitbetween thetwocells.Thecorrosioncellconsistingof3.5wt%NaClsolution possessesthree-electrodestructurewithQ235CSelectrode,SCEandPt foilactingasWE,REandCE,respectively.BeforeEISmeasurement, electrodeswereimmersedin3.5wt%NaClsolutionfor30minto achieveOCPandenvironmentalstabilizationinthiscondition.EIStests wereconductedatfrequenciesbetween105 and10 2 Hzwith10mV ACamplitude,andtheconstantOCPwasusedasinitialpotential. DZsimDemosoftwarewasusedtoanalyzethecorrespondingEISresults.Tafelcurvesweremeasuredatthescanrateof1mV/sfrom 300mVto300mV vs. OCP.Thecalculated Ecorr,corrosioncurrent density(jcorr),cathodicTafelslope(βc)andanodicTafelslope(βa)valueswereanalyzedbyaCHI660Esoftware.
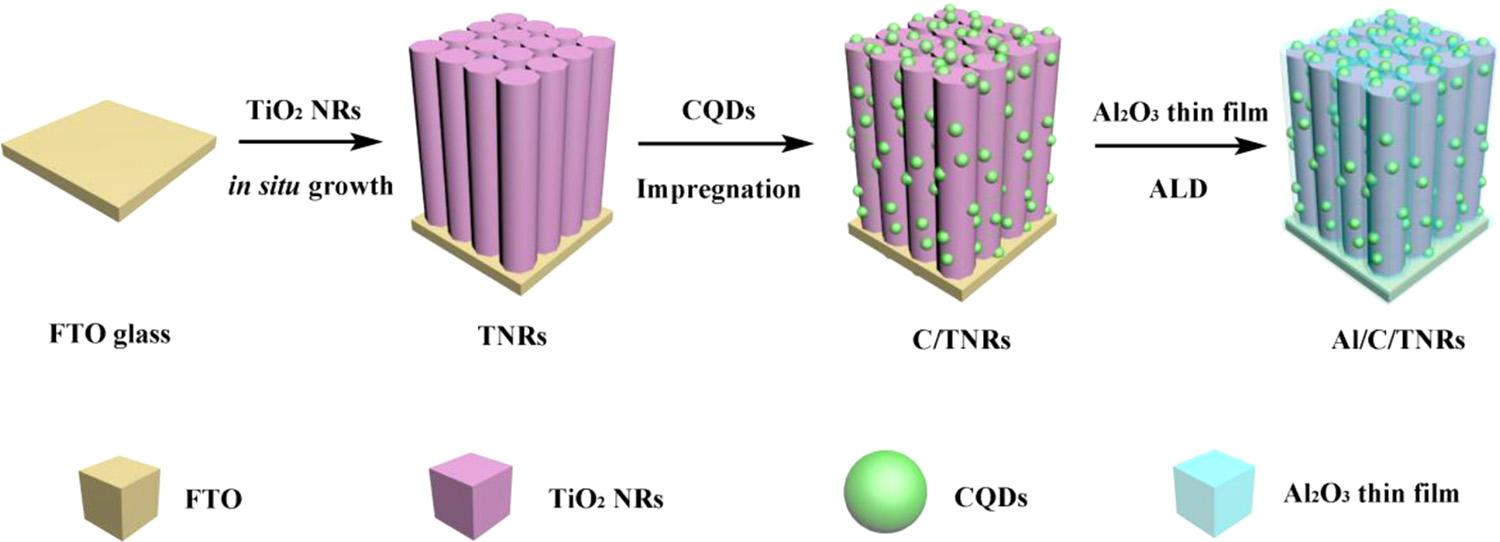
Fig.2. SchematicdiagramoftheproceduresofpreparingTiO2 NRs(TNRs),CQDs-loadedTiO2 NRs(C/TNRs)andAl2O3 anchoredCQDs-loadedTiO2 NRsbyALD method(Al/C/TNRs).
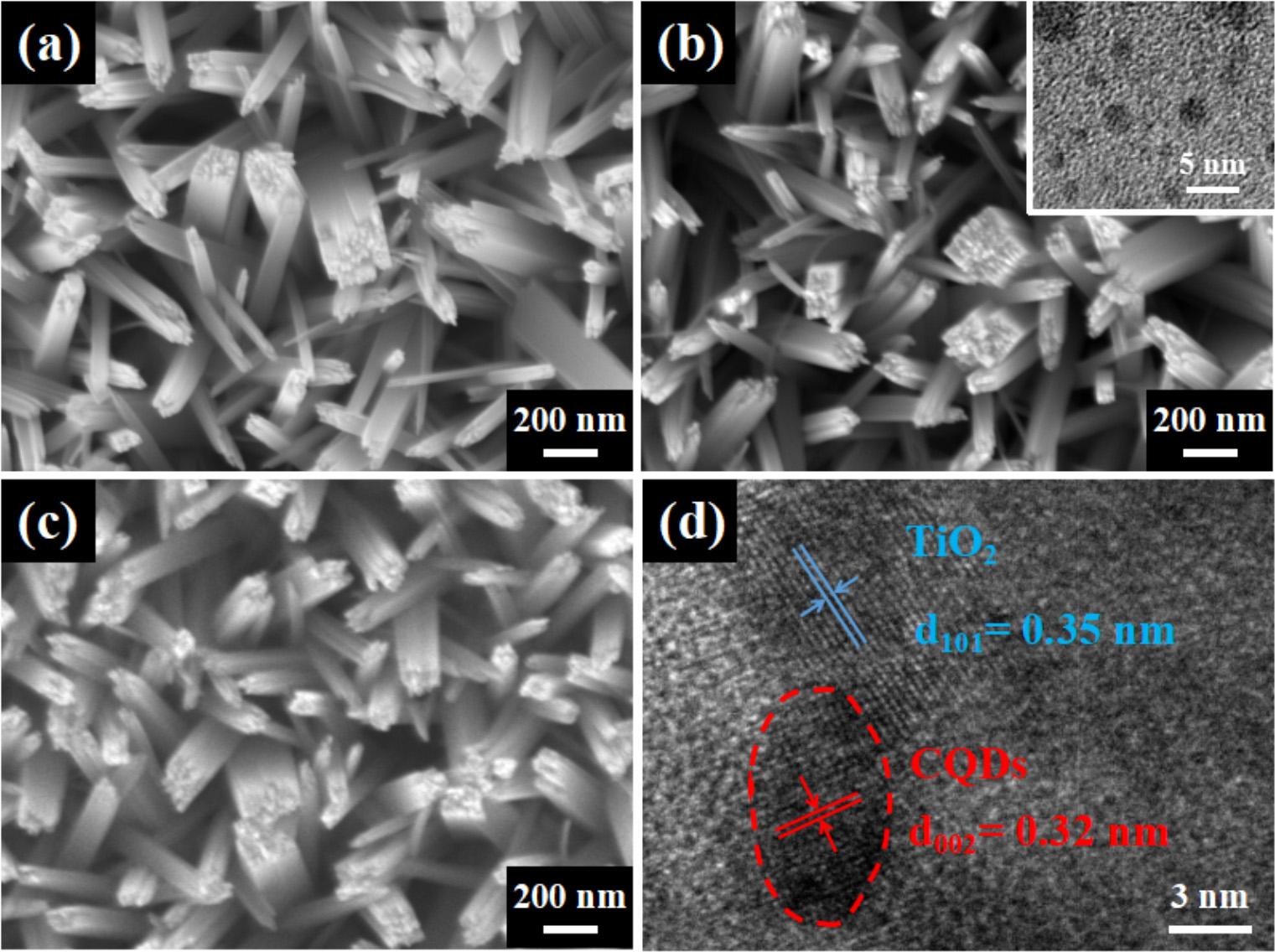
Fig.3. SEMimagesofTiO2 NRs(a),CQDs-loadedTiO2 NRs(b),CQDs-loadedTiO2 NRscoveredwith5nmAl2O3 layer(c)andHRTEMimageofCQDs-loadedTiO2 NRs(d).Theinsetof(b)showstheTEMimageofCQDs.
3.Resultsanddiscussion
Fig.2 showsthepreparationprocessofAl/C/TNRselectrodefabricatedthroughthreesteps.Firstly,orderedTiO2 nanorodsarrayswere insitu grownonFTOconductiveglassesbyhydrothermalmethod,and thenimmersedintoCQDssolutionforseveralhoursunderdarkcondition.Theuniqueone-dimensionalstructureofTNRswithahighaspectratioandgoodoxidationresistanceoffersunidirectionalchannels beneficialforthetransportationofphotogeneratedchargesandpossesseshigherPECperformancesthanthatinbulkmaterials.Thissimple insitu hydrothermalgrowthofTNRsonFTOconductiveglassesimprovestheinterfacebondingforcebetweenthem[36].Finally,toimprovetheadhesionbetweenCQDsandTNRs,the finalAl/C/TNRswere obtainedbydepositingthinAl2O3 filmonCQDs/TNRsthroughthermal ALDtechnique.
ThemorphologiesofTNRs,C/TNRsandAl/C/TNRsarraysare shownin Fig.3a–c.In Fig.3a,thediametersofdensely-arrangedTNRs distributewithintherangeof50 100nm. Fig.3bshowsthemorphologyofTiO2 NRsafterloadingCQDs.Theinsetof Fig.3bshowsthe TEMimageofCQDswiththesizeofabout2 5nm.AfterloadingCQDs anddepositing5nmAl2O3 layer,thenanorodsshowslightchangesona largescaleasshownin Fig.3bandc.WhiletheHRTEMimageshownin Fig.3dcanprovidemoredetailsonasmallerscalewhichensuresthe successfulloadingofCQDs,fromwhichtheamorphouscarbonparticles withblurrylatticefringesandhighly-crystallinerutileTiO2 withclear latticefringescanbeclearlydetected[37].Thelatticespacingsof 0.32nmand0.35nmcorrespondtothe(002)and(101)latticeplaneof carbonandTiO2,respectively[38].
Tofurtherdeterminethecompositionofthesamples,TNRs,C/TNRs andAl/C/TNRswerecharacterizedbyXPSasshownin Fig.4aandb. TheC1speakforTNRsmayoriginatefromtheabsorbedhydrocarbon orcontaminationfromXPSinstruments[39],andthepeakintensity largelyincreasesafterloadingCQDs. Fig.4bexhibitsXPSspectraofC 1sforTNRsbeforeandafterloadingCQDs.AsXPSspectrumofCelementofTNRsdisplays,thepeaksat284.4eV,286.4eVand288.5eV correspondtoC C,C OHandC]Obondfromthetestsystem, respectively[40].ForC/TNRscomposite,becauseofthenitrogen constituentofCQDs,theappearanceofC Npeaklocatedat285.8eV andthenewly-formedN1speakinfullspectrumof Fig.4ademonstrate thesuccessfullyloadingofCQDsonthesurfaceofTNRs[41].As Fig.4c ofRamanspectrashows,characteristicRamanpeaksat236cm 1 , 448.4cm 1 and611.7cm 1 belongtothecompoundvibrationpeak,Eg andA1g modeofrutileTiO2 phase,respectively[42].Afterloading CQDs,thereappearsnewpeaksat1359.2cm 1 and1626.5cm 1 correspondingtoRaman-activeD-bandandG-bandofcarbon[43], indicatingthepresenceofCQDsonC/TNRsandAl/C/TNRs.Thereisno activegroupofAl2O3 forthesampleofAl/C/TNRsbecauseofthe amorphousstateofalumina.UV–visabsorptionspectraofCQDs,TNRs, C/TNRsandAl/C/TNRsareshownin Fig.4d.ForTNRs,theintersectionbetweenX-axisandthetangentlineofabsorptionbandedge(λ)is about415nm.Accordingtotheformula Eg =1240/λ [44],thecorrespondingbandgapenergy(Eg)iscalculatedtobe2.99eV.Thisresultis ingoodagreementwiththepreviouslyreportedresultsofrutileTiO2 (Eg =3.0eV,corresponding λ =413nm)[45].ComparedwithTNRs,a slightredshifttoaround447nm(calculating Eg =2.77eV)oftheopticalabsorptionedgeisobservedforC/TNRs.Thebandgapdoesnot changemuchaftermodifyingCQDsbecauseTiO2 andCQDsarenot compoundedandnonewphaseisformed.Asshownintheinsetof Fig.4d,theabsorptionbandedgereaches685nmandanindependent absorptionpeakaround530nmisobservedforsingleCQDs.TheenhancedabsorptionwithinUVandvisiblelightregionandthebulge between500and600nmforC/TNRsandAl/C/TNRsaretherefore mainlycausedbyCQDs,sinceCQDsenablesinglephotontoprovide multipleexcitonsandplayessentialrolesinutilizingsunlight,which implytheameliorativephotocatalyticperformancescomparedwith TNRs.Inaddition,ablueshiftofabsorptionbandwavelengthreaching 427nmoccursafterdepositingAl2O3 becausetheAl2O3 layernearly hasnoeffectsonvisiblelightabsorptionofphotoanodes[14].
TotestthePECpropertiesofsamplesandevaluatetheseparation efficiencyofphotogeneratedchargecarriers,linearsweepvoltammetry (LSV)andtransientphotocurrentmeasurementswithseveralon/off cycleshadbeenconducted. Fig.5aexhibitsLSVcurvesofTNRs,C/
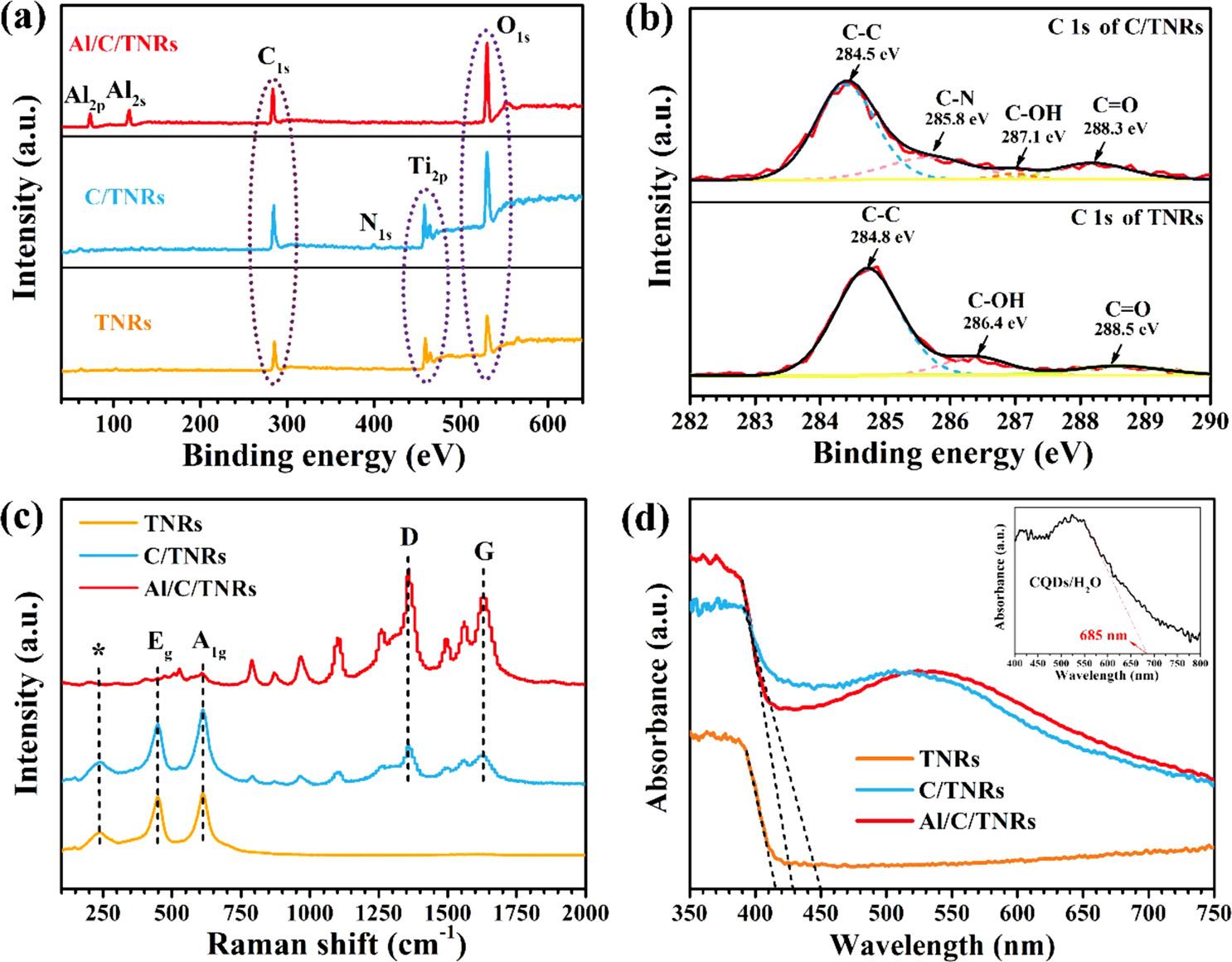
Fig.4. (a)XPSspectra,(c)Ramanspectraand(d)UV–visspectraofCQDs,TiO2 NRs(TNRs),CQDs-loadedTiO2 NRs(C/TNRs)andAl2O3 anchoredC/TNRs(Al/C/ TNRs),(b)XPSspectraofC1sinTNRsandC/TNRs.
TNRsandAl/C/TNRsmeasuredundersimulatedsunlight(100mW/ cm2).ThephotocurrentdensityofpureTNRsshowsnearly0.60mA/ cm2 becauseofthepoorvisiblelightabsorptionandhighrecombinationrateofphotoinducedelectron-holepairsinbulkTiO2.Subsequent processingsincludingCQDsmodificationandAl2O3 depositionimprove PECperformancesofthephotoanodesrespectively,andthe finalphotocurrentdensityofAl/C/TNRscompositereaches2.28mA/cm2 owing tothesynergisticeffectsofCQDsandALDAl2O3 layeronextending visiblelightabsorption,restrainingcarriersrecombinationand lengtheningthelifespanofelectrons.Asshownin Fig.5b,thetypical photocurrentresponsecurvesdisplaygoodreproducibilityofphotoanodesduringthetransientswitchingprocessfromilluminationtodark conditions.
PECperformancesofas-preparedphotoanodeswerefurtherevaluatedbyIPCEmeasurements.IPCEvaluesasafunctionofirradiation wavelengthofTNRs,C/TNRsandAl/C/TNRselectrodesareshownin Fig.5c.ThemaximalIPCEvalueofC/TNRscalculatedfromthefollowingformulareachesapproximately30.36%at385nm,whichis thricehigherthanthatofTNRs(9.58%,380nm).Theformulais:
IPCE=1240 I/(λJlight)
where I, λ and Jlight meanphotocurrentdensity,wavelengthofincident lightandirradiationintensity,respectively[46].Itcanbeseenthat highIPCEvaluesofpureTNRsonlyexistwithinUVlightregion (λ <415nm),anddecreasetoabout0.15%atwavelengthslonger than415nm.ThiscascadecurvecoincidescloselywithUV–visabsorptionspectrumofpureTNRsshownin Fig.4d.FromtheIPCEplots ofC/TNRs,wecanseethattheabsorptionedgeshifts 15nmtolonger wavelengthregioncomparedwithTNRs.Moreover,thepresenceof CQDsincreasestheIPCEvaluesto3.04%invisiblelightregion (λ >425nm)owingtotheexcellentopticalpropertiesofCQDs.The ALDAl2O3 shellnotonlygivesrisetoaremarkableenhancementof IPCEinthewholeUVregion(375 400nm)withamaximumof55.33 %at385nm,butalsohasabroaderlightharvestingrangeto435nm, andtheIPCEvalueincreasedto4.36%when λ >435nm.Thiscould
betheresultofreducedchargerecombinationandefficientcharge collection.TherelativelylowerIPCEvalueatlongerwavelengths (λ >435nm)incomparationwiththatinnearUVlightregionmaybe causedbytheexistenceofanactivationenergythresholdattheAl/C/ TNRsinterfaceandpoorlight-to-electricityconversioninvisiblelight region[47].
However,thephotocurrentdensityofC/TNRsisreducedby32.40 %comparedtotheoriginalvalueafterimmersingandmeasuringinthe electrolyteinthe first13h(shownas Fig.5d),furtherdroppedby30.10 %after7days(shownas Fig.5e),whichmeanspoorphotoresponse stabilityofthisphotoelectrodeduetotheweakphysicaladsorption forcebetweenCQDsandTNRs.Incontrast,thephotoanodeofAl/C/ TNRsrevealsexcellentlong-timestabilitywithlittledeclineofphotocurrentdensityundertheilluminationofsimulatedsunlight(100mW/ cm2)for13h.Moreimportantly,thisvaluemaintainsevenfor7days withasmalldropofonly4.23%asshownin Fig.5f,whichprovesthe stabilizationfunctionofdepositingALDAl2O3 onthesurfaceofC/ TNRs,andfurtherensurephotocathodicprotectionpropertiesofQ235 CS.Tofurtherstudythecatalyticactivityofthephotoanodes,cyclic voltammetrycurveswereanalyzedasshowninFig.S1.Theoretically, overpotential(η)inoxygenevolutionreactionmeanstheportionofthe actualvoltage(Ei)requiredtoreachacertaincurrentdensity(i, mA/ cm2)overthetheoreticalvoltage(Et)duringacatalyticreaction.Accordingtotheformula ηi = Ei - Et,wecanseethatAl/C/TNRspossesses thelowest ηi,andisthemostactivephotoanode[48].
InordertoinvestigatephotocathodicprotectioneffectsofTNRs,C/ TNRsandAl/C/TNRsonQ235CS,OCPmeasurementsandTafelcurves ofQ235CSwereconducted. Fig.6ashowsphoto-inducedOCPmeasurementresultsofTNRs,C/TNRsandAl/C/TNRsnanocomposites underintermittentsimulatedsunlight(100mW/cm2).Itcanbeseen thatthepotentialvaluesexhibitobviouspositiveshiftswhenthelightis turnedoff,andcomebacktotheoriginalvalueunderillumination.This indicatesthattheobtainednegativeshiftsofpotentialareentirelyon accountofthePECpropertyofthephotoanodes.Moreover,thepotentialchangeofAl/C/TNRselectrodeismuchmoreevidentthanthat
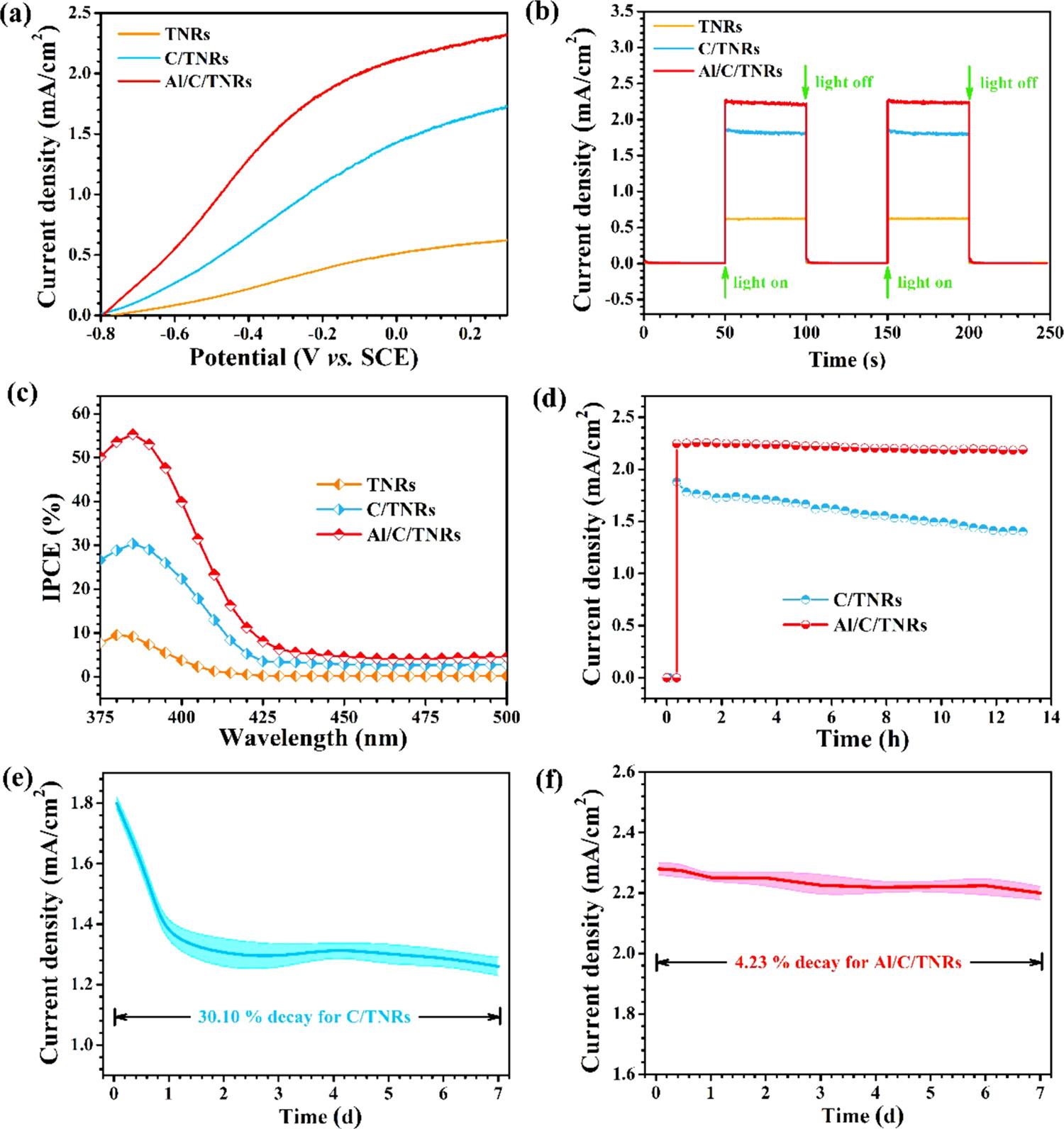
Fig.5. (a)Linearsweepvoltammetriccurves,(b)photocurrentdensity vs. timeplots,(c)IPCEspectra,(d)time-dependentphotocurrentprofilesat0.23V vs. SCE obtainedfromCQDs-loadedTiO2 NRs(C/TNRs)andAl2O3 anchoredC/TNRs(Al/C/TNRs).(e,f)Long-timestabilitycurveswitherrorbarat0.23V vs. SCEforC/ TNRsandAl/C/TNRsundersimulatedsolarlight(100mW/cm2,AM1.5G)illumination.
ofthepureTNRsandC/TNRs,suggestingamuchbetterphotogeneratedcathodicprotectionpropertyfortheAl/C/TNRs.
TheTafelcurvesofQ235CScoupledwithphotoelectrodesinthe presenceofsimulatedsolarlight(100mW/cm2)in3.5wt%NaClsolutionareshownin Fig.6b.Theelectrochemicalparametersincluding Ecorr, jcorr, βc and βa obtainedfromTafelcurvesareexhibitedin Table1 It’sclearthatthereareobviousnegativeshiftsof Ecorr whencoupling Q235CSwithTNRs,C/TNRsandAl/C/TNRs,respectively.The Ecorr of Q235CScoupledwithAl/C/TNRsexhibitsthemostnegativevalueof 0.92V vs. SCEcomparedwithQ235CScoupledwithTNRs( 0.69V vs. SCE)andQ235CScoupledwithC/TNRs( 0.76V vs. SCE).As shownin Fig.6c,suchanegativeshiftcanstillbemaintainedevenfor about9hsothattheAl/C/TNRsnanocompositecanprovidelongterm anticorrosionprotectionforQ235CS.Moreover, jcorr exhibitsincreasementcomparedwithpureTNRsaftermodifyingCQDsandAl2O3 This findingisinaccordancewiththephotocurrentspectrumshownin Fig.5a.Theenhancementof jcorr canbeascribedtotheincreaseof superficialelectrochemicalreactionsderivedfromthepolarizationof photoinducedelectrons[49].Itisworthhighlightingthatthephotoanodesremainunchangedwhengeneratingelectron-holepairsunder illumination,sotheincreasementsofanodicpolarizationcurrentinformedfromthepolarizationcurvesbringtheenhancementofphotocurrenttransfusingintoQ235CSbutnottheaggravationofcorrosion rate.Inaword,thecompositionofTNRsandCQDswhichpossess
di
fferentenergylevelfromTNRsandwideabsorptionrangeprovides moreexcitedelectroninjectiontoQ235CSsubstrate,andtheholes reactwithdissolvedoxygenundertheisolationeffectofAl2O3 layerin therustmedium[50].Therebyamorenegative Ecorr andabigger jcorr valuearematchedtothecouplingelectrodeofQ235CSandAl/C/TNRs owingtothehigherelectronstransportationrateandlowerrecombinationrateofphotogeneratedchargecarriers.
ThestabilityanddurabilityexperimentsofAl/C/TNRswerealso conductedtoinvestigatephotocathodicprotectionperformancein 3.5wt%NaClsolutionunderintermittentilluminationofsimulated sunlight(100mW/cm2).Ineachcycle,thephotoelectrodewasalternatelyilluminatedonandoff atintervalsofmorethanonehourfortwo loops,andthenitwassubsequentlyplacedindarkconditionsfor7h.As shownin Fig.6d, Ecorr ofQ235CScoupledwithAl/C/TNRsimmediatelynegativelyshiftsby620mVunderillumination,andthe potentialvalueremainsfarbelow Ecorr ofQ235CSaftertheseconduse ofAl/C/TNRsphotoanodeunderdarkcondition,whichimpliesthatAl/ C/TNRscompositesarestillhighlyactiveandhavegooddurabilityfor photocathodicprotectionperformancesofQ235CS.
Photocathodicprotectionperformancesofthesamples,thecharge transferpropertiesandinterfacialpropertiesbetweenelectrodesand electrolytewereanalyzedbyEIS.ThechargetransferprocessisreflectedfromthearchsdisplayedintypicalNyquistplotsof Fig.7a,and thehigh-frequencyarcdiametersrepresentchargetransferresistance
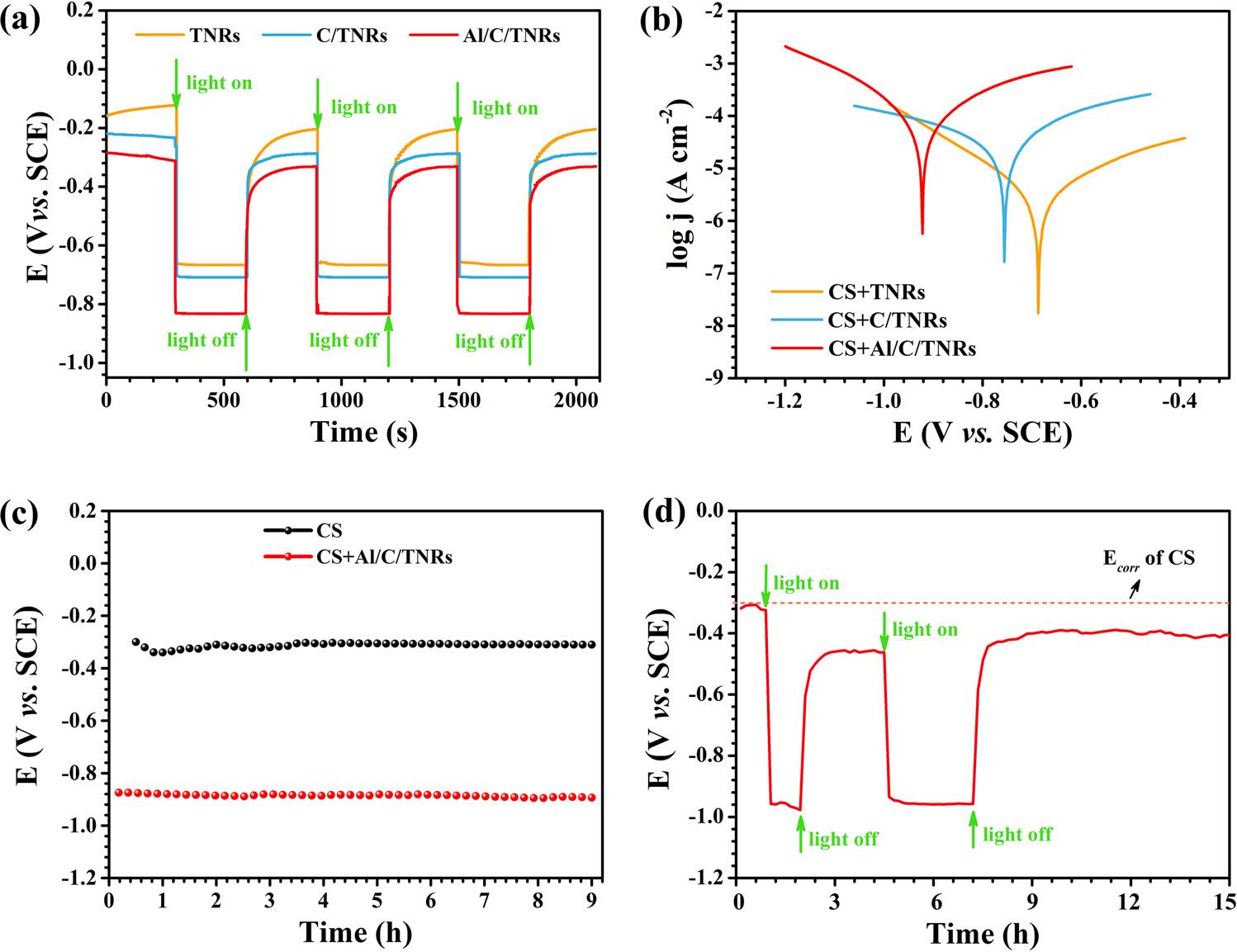
Table1
ElectrochemicalparametersobtainedbytheTafelcurvesofTiO2 NRs(TNRs), CQDs-loadedTiO2 NRs(C/TNRs)andwith5nmALDAl2O3 (Al/C/TNRs)coupledwithQ235carbonsteelin3.5wt%NaClsolutionundersimulatedsolar light(AM1.5G,100mW/cm2).
Samples
(Rct)betweenphotoelectrodesandelectrolyteduringtheelectrochemicalprocess[51].Clearly,thesemicirclediametersofimpedance arcsdecreasewiththeintroductionofCQDsandAl2O3,whichimplies
Fig.6. (a)Photo-inducedOCPvariationof TiO2 NRs(TNRs),CQDs-loadedTiO2 NRs(C/ TNRs)andAl2O3 anchoredC/TNRs(Al/C/ TNRs)photoelectrodes,(b)Tafelcurvesof Q235carbonsteel(CS)electrodeconnected withTNRs,C/TNRsandAl/C/TNRs,(c)OCPof Q235CSelectrodebeforeandafterconnecting withAl/C/TNRsand(d)OCPchangesofQ235 CSelectrodecoupledwithAl/C/TNRsin3.5wt %NaClsolutionundersimulatedsunlightillumination(AM1.5G,100mW/cm2).
thereductionofRct andinterfaceresistance.Itcanbeexplainedthatthe visible-light-excitedelectronstransferfromC/TNRstothesurfaceof Q235CS,makingelectrochemicalreactionratelargelyenhanced,and theouterALDAl2O3 layersignificantlyacceleratetheseparationof electron-holepairsthroughhole-harvestingeffect.TheACimpedance atlowfrequenciesusuallyrepresentstheFaradayreactionresistance anddouble-layercapacitance.FromtheBodeplotsin Fig.7b,itcanbe seenthatpureTNRshasonepeakwhileC/TNRsandAl/C/TNRshave twopeaks,indicatingthatpureTNRshasonetimeconstant,andbothof C/TNRsandAl/C/TNRshavetwotimeconstants.Inaddition,the characteristicpeakfrequency(fmax)inthelowfrequencyregionobtainedfromBodeplotswasusedtoestimatethelifetime(τ)ofphotogeneratedelectronsfromtheempiricalformula τ ≈ 1/2πfmax,here τ is inverselyproportionalto fmax.ForAl/C/TNRscomposite film,the
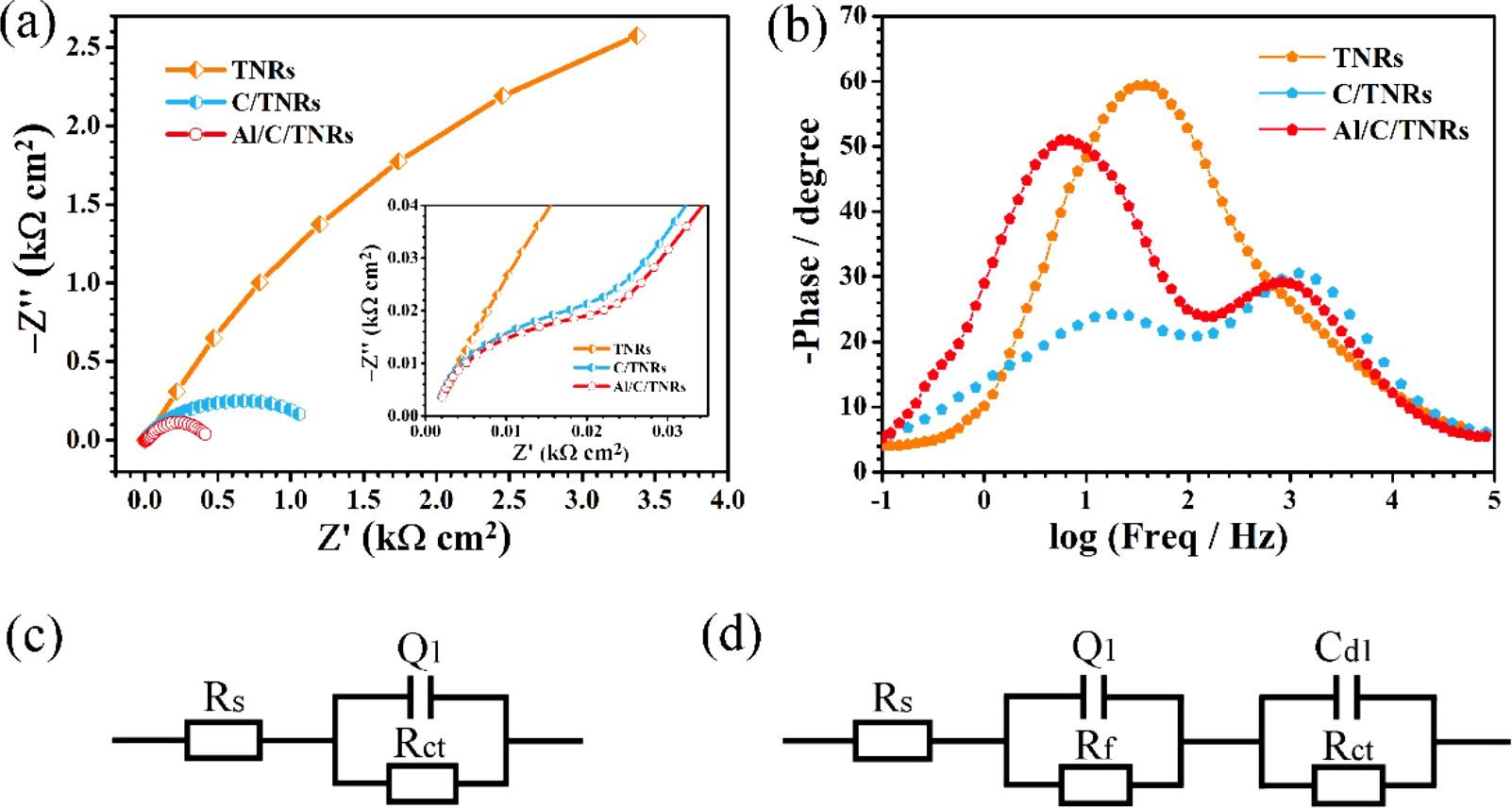
Fig.7. (a)Nyquistplots,(b)Bodeplotsand(c,d)schematicdiagramsoftheequivalentcircuitbyEISresult fittingforTiO2 NRs(TNRs),CQDs-loadedTiO2 NRs(C/ TNRs)andAl2O3 anchoredC/TNRs(Al/C/TNRs)photoanodesundersimulatedsunlight(AM1.5G,100mW/cm2)irradiationatroomtemperature.
Table2
FittingvaluesofequivalentcircuitelementsofTiO2 NRs(TNRs),CQDs-loaded TiO2 NRs(C/TNRs)andwith5nmALDAl2O3 (Al/C/TNRs)coupledwithQ235 carbonsteel.
Samples
TNRs9.7265.6380.4362
169.4
C/TNRs6.9387.7440.7924123.1186.499.66
Al/C/TNRs8.2536.0120.612558.851.7720.2298
logarithmof fmax wassmallerthanTNRsandC/TNRs,thusthelifetime ofthephotogeneratedelectronsintheAl/C/TNRscomposite film reachedthelongest,resultingfromtheinhibitedchargecarrierrecombinationandenhancedchargeseparationefficiencyinthecomposite film[52].EISresultswerealsoanalyzedthroughequivalentcircuit modelsasshownin Fig.7candd,whereQ1 representsconstantphase element(CPE)toreplacecapacitanceowingtothatpurecapacitanceis hardtorealizeinrealelectrochemicalprocess.CPEisdefinedbyadmittanceYandpowerindexnumbern,givenbyY=Y0 (jω)n.Bythis formula,CPEwouldconverttoresistance(n=0)orcapacitance (n=1).TheequivalentcircuitofTNRscanbedescribedasRs (Q1Rct), whichisatypicalcircuitforapassiveoxidelayer[6].Asexhibitedin Fig.7c,Rs andQ1 representelectrolyteresistanceandconstantphase element,respectively.TheequivalentcircuitofC/TNRsandAl/C/TNRs isinterpretedusingamodelwithtwotimeconstantsasRs (Q1Rf) (CdlRct).Asshownin Fig.7d,Rs standsfortheelectrolyteresistance,Rf andQ1 athighfrequenciesrepresenttheresistanceandconstantphase elementcorrespondingtotheCQDsandAl2O3,whileCdl atlowfrequenciesstandsforthedoublelayercapacitance. Table2 showsthe fittingvaluesofequivalentcircuitelements,wecanseethatthevalues ofRf andRct becomesmallerthanthatofTNRsaftermodifyingCQDs andAl2O3,whichindicatetheelectroderesistivityandchargetransfer resistancedecrease.CQDsmayactasthetransporterthatmakescharge transfereasierbetweentheinterfaceofsemiconductorandsolution whenCQDsattachedonthesurfaceofTNRs[27],therebyimproved PECpropertiesandphotopotentialperformancesarerealizedcombined withtheefficientchargeseparationandtransportationthroughAl2O3 layer.
Fig.8 illustratestheproposedmechanismfortheenhancedphotocathodicprotectionperformanceofAl/C/TNRscompositeforQ235CS undersimulatedsolarlightillumination.Inphotoanode,photoinduced electronstransportthroughsemiconductor filmtotheprotectedmetal, andphotogeneratedholesreactwithhole-trappingagentsonsemiconductorsurfacesimultaneously.Asshownin Fig.8a,visible-lightexcitedelectronsfromtheHighestOccupiedMolecularOrbital(HOMO)
toLUMOofCQDsaretransferredtoCBofTiO2,andtogetherwiththe photogeneratedelectronsexcitedbyUVlightfromTiO2, finallyarrive andaccumulateatthesurfaceofQ235CSsubstratethroughFTOconductingglass.Thiselectronmigrationleadstotheincreaseofelectron densityonmetalsurface,whichshowsthemacroscopicalreductionof Q235CSelectrodepotentialthatfarbelowitsnaturalcorrosionpotential,topreventQ235CSfromcorrosionundersimulatedsunlight. WhilethephotoinducedholeswillbeleftontheHOMOlevelofCQDs andtheVBofTiO2,toparticipateinoxidativedecompositionofwater ororganicactivespecies[53].Simultaneously,aportionofphotoinducedelectronsmaybestoredinCQDs,andthenreleasedunderdark conditionstorealizethedelayedcathodicprotectionofmetalsubstrate [4,29,54].Inaddition,unlikephotocorrosivematerialssuchasCdS, CQDsarestableenoughanddonotdecomposeunderlightconditions [55].
Furthermore,ALDAl2O3 ultrathinlayerplaysanactiveroleinholeharvestingeffect,whichbringsenhancedholeinjectionandblocks electroninjectionfromthegate.InAl2O3 shell,ahighdensityofnegative fixedchargesandalowinterfacedefectdensitycausetheenhanced field-effectpassivationandthedepressionofchargerecombination,respectively,thereforethesurfacetrapstatesofC/TNRs afterdepositionofALDAl2O3 showpassivationeffect[30].Asshownin Fig.8b,therearenegatively fixedchargesexistinginAl2O3 shell,by whichonlyholeswithpositivechargescanbetraversedandmigrated totheinterfacebetweenAl2O3 layerandsolution.Inthisway,theseparationefficiencyofphotogeneratedelectron-holepairsislargely enhanced,andthelifetimeofphotoinducedelectronsisprolonged,either[32].Inaddition,theALDlayercompletelycoverssubstratematerial(TiO2 andCQDs)andpreventCQDsfromfallingoff withwater flow,thusgreatlyimprovestheinterfacebondingstrength.Therefore, CQDsandALDAl2O3 takeasynergisticeffectonimprovingphotocathodicprotectionperformancesofQ235CSandprovidepossibility andanewwayforpracticalapplicationofphotocathodicprotection.
4.Conclusion
Insummary,anewAl2O3 anchoredcarbonquantumdotsmodified TiO2 nanorods(Al/C/TNRs)photoelectrodematerialwerefabricated forefficientphotocathodicprotectionofQ235carbonsteelundersolar light.FromUV–visabsorptionspectra,theintroductionofCQDsextendedlightabsorptionintovisiblelightregionsuccessfully.Theouter Al2O3 layerpromptedbetterphotogeneratedchargeseparationactivitiescomparedwithpurerutilenanorodarrays,solvedthephotoetching dissolutionofCQDsandbroughtimprovedphotocurrentdensityand photocathodicprotectionperformancesinsynergy.Theelectrochemical measurementsshowedthatphotocurrentdensityofAl/C/TNRsreached 2.28mA/cm2 at0.23V vs. SCEundersimulatedsunlightillumination
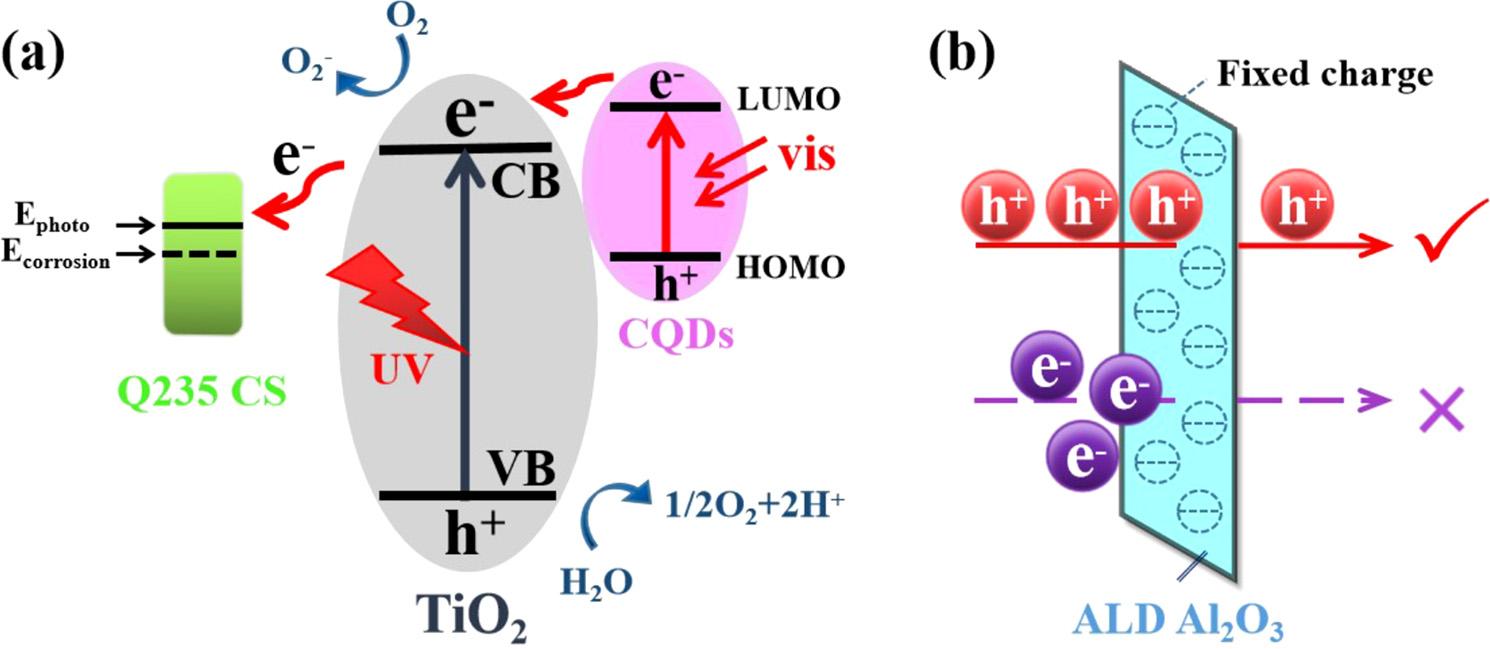
Fig.8. (a)SchematicrepresentationoftheenergybandstructureandelectrontransferprocessesinCQDs/TiO2 NRscompositeand(b)theroleofatomiclayer depositedAl2O3 layeras filtermembrane.
(100mW/cm2)andthisvaluemaintainedfor7dayswithonlyasmall dropof4.23%.ThepotentialofQ235CSin3.5wt%NaClsolution droppedby620mVwhencouplingtoAl/C/TNRsphotoanode.When lightwascutoff,thepotentialofQ235CSwas132mVlowerthanits corrosionpotentialforover7h,exhibitingprotectioneffectsoncarbon steel.TheresultsdemonstratedthattheproposedAl/C/TNRsnanocompositeispromisingformetalcorrosionprotectioninpracticalapplication.
Dataavailability
Theraw/processeddatarequiredtoreproducethese findingscannot besharedatthistimeasthedataalsoformspartofanongoingstudy.
CRediTauthorshipcontributionstatement
MinFeng: Conceptualization,Methodology,Validation, Investigation,Writing-originaldraft. YingLiu: Conceptualization, Writing-review&editing. SainanZhang: Methodology,Validation. YupengLiu: Writing-review&editing. NingLuo: Methodology. DaoaiWang: Conceptualization,Resources,Writing-review&editing, Supervision,Projectadministration,Fundingacquisition.
DeclarationofCompetingInterest
Theauthorsdeclarethattheyhavenoknowncompeting financial interestsorpersonalrelationshipsthatcouldhaveappearedtoinfluencetheworkreportedinthispaper.
Acknowledgements
Thanksforthe financialsupportoftheNSFC(No.51722510),the programforTaishanScholarsofShandongprovince(No.ts20190965), InnovationLeadingTalentsprogramofQingdao(19-3-2-23-zhc)and OpenFundofKeyLaboratoryforIntelligentNanoMaterialsand DevicesoftheMinistryofEducation(INMD-2019M01).
AppendixA.Supplementarydata
Supplementarymaterialrelatedtothisarticlecanbefound,inthe onlineversion,atdoi:https://doi.org/10.1016/j.corsci.2020.108919
References
[1] M.J.Zhou,N.Zhang,L.Zhang,J.H.Yan,Photocathodicprotectionpropertiesof NiP/TiO2 bilayercoatingsbyacombinedelectrolessplatingandsol-gelmethod, Mater.Corros.63(2015)703–706
[2] K.Xiao,C.F.Dong,X.G.Li,M.Wang,Corrosionproductsandformationmechanism duringinitialstageofatmosphericcorrosionofcarbonsteel,J.Iron.Steel.Res.Int. 15(2008)42–48
[3] L.Zhao,Q.Liu,R.Gao,J.Wang,W.L.Yang,L.H.Liu,One-stepmethodforthe fabricationofsuperhydrophobicsurfaceonmagnesiumalloyanditscorrosion protection,antifoulingperformance,Corros.Sci.80(2014)177–183
[4] H.Li,X.T.Wang,Y.Liu,B.R.Hou,AgandSnO2 co-sensitizedTiO2 photoanodesfor protectionof304SSundervisiblelight,Corros.Sci.82(2014)145–153
[5] C.Christodoulou,G.Glass,J.Webb,S.Austin,C.Goodier,Assessingthelongterm benefitsofimpressedcurrentcathodicprotection,Corros.Sci.52(2010) 2671–2679
[6] S.W.Cui,X.Y.Yin,Q.L.Yua,Y.P.Liu,D.A.Wang,F.Zhou,Polypyrrolenanowire/ TiO2 nanotubenanocompositesasphotoanodesforphotocathodicprotectionofTi substrateand304SSundervisiblelight,Corros.Sci.98(2015)471–477
[7] M.J.Zhou,Z.O.Zeng,L.Zhong,Photogeneratedcathodeprotectionpropertiesof nano-sizedTiO2/WO3 coating,Corros.Sci.51(2009)1386–1391.
[8] M.M.Momeni,M.Taghinejad,Y.Ghayeb,R.Bagheri,Z.L.Song,Preparationof variousboron dopedTiO2 nanostructuresbyinsituanodizingmethodandinvestigationoftheirphotoelectrochemicalandphotocathodicprotectionproperties, J.Iran.Chem.Soc.16(2019)1839–1851
[9] T.T.Zhang,Z.U.Rahman,N.Wei,Y.P.Liu,J.Liang,D.A.Wang,Insitugrowthof single-crystalTiO2 nanorodarraysonTisubstrate:controllablesynthesisand photoelectrochemicalwatersplitting,NanoRes.10(2017)1021–1032
[10] N.Wei,Y.Liu,T.T.Zhang,J.Liang,D.A.Wang,HydrogenatedTiO2 nanotubearrayswithenhancedphotoelectrochemicalpropertyforphotocathodicprotection
undervisiblelight,Mater.Lett.185(2016)81–84
[11] P.Roy,S.Berger,P.Schmuki,TiO2 nanotubes:synthesisandapplications,Angew. Chem.50(2011)2904–2939
[12] M.M.Momeni,S.H.Khansari Zadeh,H.Farrokhpour,Fabricationoftungsten iron dopedTiO2 nanotubesviaanodization:newphotoelectrodesforphotoelectrochemicalcathodicprotectionundervisiblelight,SNAppl.Sci.1(2019)1160
[13] Z.Q.Lin,Y.K.Lai,R.G.Hu,J.Li,R.G.Du,C.J.Lin,AhighlyefficientZnS/CdS@TiO2 photoelectrodeforphotogeneratedcathodicprotectionofmetals,Electrochim.Acta 55(2010)8717–8723
[14] M.Feng,Y.Liu,N.Wei,S.C.Ma,Z.X.Li,H.G.Li,S.G.Chen,J.Liu,D.A.Wang, AluminaanchoredCQDs/TiO2 nanorodsbyatomiclayerdepositionforefficient photoelectrochemicalwatersplittingundersolarlight,J.Mater.Chem.A6(2018) 18293–18303
[15] M.M.Momeni,M.Mahvari,Y.Ghayeb,PhotoelectrochemicalpropertiesofironcobaltWTiO2 nanotubephotoanodesforwatersplittingandphotocathodicprotectionofstainlesssteel,J.Electroanal.Chem.832(2019)7–23
[16] M.Khanmohammadi,F.Mizani,M.B.Khaleghi,A.B.Garmarudi,Optimized synthesisofpolyaniline-TiO2 compositesforcorrosionprotectionofcarbonsteel usingdesignofexperiment(DOE),Prot.Met.Phys.Chem.49(2013)662–668
[17] E.M.Samsudin,S.B.AbdHamid,J.C.Juan,W.J.Basirun,G.Centi,SynergeticeffectsinnovelhydrogenatedF-dopedTiO2 photocatalysts,Appl.Surf.Sci.370 (2016)380–393
[18] M.Nischk,P.Mazierski,Z.S.Wei,K.Siuzdak,N.A.Kouamee,E.Kowalskac, H.Remita,A.Z.Medynska,Enhancedphotocatalytic,electrochemicalandphotoelectrochemicalpropertiesofTiO2 nanotubesarraysmodifiedwithCu,AgCuand Binanoparticlesobtainedviaradiolyticreduction,Appl.Surf.Sci.387(2016) 89–102
[19] L.Wan,Y.Gao,X.H.Xia,Q.R.Deng,G.Shao,Phaseselectionandvisiblelight photo-catalyticactivityofFe-dopedTiO2 preparedbythehydrothermalmethod, Mater.Res.Bull.46(2011)442–446
[20] M.Zeng,Y.Z.Li,M.Y.Mao,J.L.Bai,L.Ren,X.J.Zhao,Synergeticeffectbetween photocatalysisonTiO2 andthermocatalysisonCeO2 forgas-phaseoxidationof benzeneonTiO2/CeO2 nanocomposites,ACSCatal.5(2015)3278–3286
[21] M.J.Zhou,N.Zhang,L.Zhang,J.H.Yan,Photocathodicprotectionpropertiesof TiO2-V2O5 compositecoatings,Mater.Corros.64(2013)996–1000
[22] T.Tatsuma,S.Satioh,Y.Ohko,A.Fujishima,TiO2-WO3 photoelectrochemicalanticorrosionsystemwithanenergystorgeability,Chem.Mater.13(2001) 2838–2842
[23] C.C.Cudia,T.Caruso,E.Maccallini,A.L.Bassi,P.Carrozzo,O.D.Luca,A.Goldoni, V.Lyamayev,K.C.Prince,F.Bondino,E.Magnano,R.G.Agostino,C.S.Casari, Chemicalbondsandcharge-transferdynamicsofadye-hierarchical-TiO2 hybrid interface,J.Phys.Chem.C119(2015)8671–8680
[24] P.Gao,H.Ma,T.Yan,D.Wu,X.Ren,J.Yang,B.Du,Q.Wei,Constructionof dentatebondedTiO2-CdSeheterostructureswithenhancedphotoelectrochemical properties:versatilelabelstowardphotoelectrochemicalandelectrochemicalsensing,DaltonTrans.44(2015)773–781
[25] M.M.Momeni,Y.Ghayeb,N.Moosavi,PreparationofNi–Pt/Fe–TiO2 nanotube filmsforphotoelectrochemicalcathodicprotectionof403stainlesssteel, Nanotechnology29(2018)425701
[26] A.J.Nozik,Excitonmultiplicationandrelaxationdynamicsinquantumdots:applicationstoultrahigh-efficiencysolarphotonconversion,Inorg.Chem.44(2005) 6893–6899
[27] M.X.Sun,X.Q.Ma,X.Chen,Y.J.Sun,X.L.Cui,Y.H.Lin,Ananocompositeofcarbon quantumdotsandTiO2 nanotubearrays:enhancingphotoelectrochemicaland photocatalyticproperties,RSCAdv.4(2014)1120–1127
[28] J.Bian,C.Huang,L.Wang,T.Hung,W.A.Daoud,R.Zhang,Carbondotloadingand TiO2 nanorodlengthdependenceofphotoelectrochemicalpropertiesincarbondot/ TiO2 nanorodarraynanocomposites,ACSAppl.Mater.Inter.6(2014)4883–4890
[29] A.Henglein,Reactionsoforganicfreeradicalsatcolloidalsilverinaqueoussolution.Electronpooleffectandwaterdecomposition,J.Phys.Chem.83(1979) 2209–2216
[30] Q.F.Gui,Z.Xu,H.F.Zhang,C.W.Cheng,X.F.Zhu,M.Yin,Y.Song,L.F.Lu, X.Y.Chen,D.D.Li,Enhancedphotoelectrochemicalwatersplittingperformanceof anodicTiO2 nanotubearraysbysurfacepassivation,ACSAppl.Mater.Inter.6 (2014)17053–17058
[31] X.B.Chen,S.S.Mao,Titaniumdioxidenanomaterials:synthesis,properties,modifications,andapplications,Chem.Rev.107(2007)2891–2959
[32] L.Pan,S.Sun,A.Zhang,K.Jiang,L.Zhang,C.Dong,Q.Huang,A.Wu,H.Lin,Truly fluorescentexcitation-dependentcarbondotsandtheirapplicationsinmulticolor cellularimagingandmultidimensionalsensing,Adv.Mater.27(2016)7782–7787
[33] S.Sun,L.Zhang,K.Jiang,A.Wu,H.Lin,Towardshigh-efficientredemissive carbondots:facilepreparation,uniqueproperties,andapplicationsasmultifunctionaltheranosticagents,Chem.Mater.28(2016)8659–8668
[34] M.D.Groner,J.W.Elam,F.H.Fabreguette,S.M.George,Electricalcharacterization ofthinAl2O3 filmsgrownbyatomiclayerdepositiononsiliconandvariousmetal substrates,ThinSolidFilms413(2002)186–197
[35] A.W.Ott,J.W.Klaus,J.M.Johnson,S.M.George,Al3O3 thin filmgrowthonSi(100) usingbinaryreactionsequencechemistry,ThinSolidFilms292(1997)135–144
[36] J.Zhang,W.X.Liu,X.W.Wang,X.Q.Wang,B.Hua,H.Liu,Enhanceddecoloration activitybyCu2O@TiO2 nanobeltsheterostructuresviaastrongadsorption-weak photodegradationprocess,Appl.Surf.Sci.282(2013)84–91
[37] J.Wang,C.F.Wang,S.Chen,Amphiphilicegg-derivedcarbondots:rapidplasma fabrication,pyrolysisprocess,andmulticolorprintingpatterns,Angew.Chem.Int. Ed.51(2012)9297–9301
[38] J.Q.Pan,Y.Z.Sheng,J.X.Zhang,J.M.Wei,P.Huang,X.Zhang,B.X.Feng, Preparationofcarbonquantumdots/TiO2 nanotubescompositesandtheirvisible
lightcatalyticapplications,J.Mater.Chem.A2(2014)18082–18086
[39] J.Haeberle,K.Henkel,H.Gargouri,F.Naumann,B.Gruska,M.Arens,M.Tallarida, D.Schmeißer,EllipsometryandXPScomparativestudiesofthermalandplasma enhancedatomiclayerdepositedAl2O3-films,BeilsteinJ.Nanotechnol.4(2013) 732–742
[40] Y.Cong,X.K.Li,Y.Qin,Z.J.Dong,G.M.Yuan,Z.W.Cui,X.J.Lai,Carbon-doped TiO2 coatingonmultiwalledcarbonnanotubeswithhighervisiblelightphotocatalyticactivity,Appl.Catal.B107(2011)128–134
[41] S.Liu,J.Tian,L.Wang,Y.Zhang,X.Qin,Y.Luo,A.M.Asiri,A.O.Al-Youbi,X.Sun, Hydrothermaltreatmentofgrass:alow-cost,greenroutetonitrogen-doped, carbon-rich,photoluminescentpolymernanodotsasaneffective fluorescentsensing platformforlabel-freedetectionofCu(II)ions,Adv.Mater.24(2012)2037–2041
[42] X.Guo,D.Xu,Z.Ding,W.Su,PreparationandRamanspectrumofrutilesingle crystalsusing floatingzonemethod,Chin.Phys.Lett.23(2006)1645–1647
[43] Y.Fang,S.Guo,D.Li,C.Zhu,W.Ren,S.Dong,E.Wang,Easysynthesisandimaging applicationsofcross-linkedgreen fluorescenthollowcarbonnanoparticles,ACS Nano6(2012)400–409
[44] Y.S.Li,F.L.Jiang,Q.Xiao,R.Li,K.Li,M.F.Zhang,A.Q.Zhang,S.F.Sun,Y.Liu, EnhancedphotocatalyticactivitiesofTiO2 nanocompositesdopedwithwater-solublemercapto-cappedCdTequantumdots,Appl.Catal.B:Environ.101(2010) 118–129.
[45] H.Li,X.T.Wang,L.Zhang,B.R.Hou,Preparationandphotocathodicprotection performanceofCdSe/reducedgrapheneoxide/TiO2 composite,Corros.Sci.94 (2015)342–349
[46] C.X.Guo,Y.Dong,H.B.Yang,C.Li,Graphenequantumdotsasagreensensitizerto functionalizeZnOnanowirearraysonF-dopedSnO2 glassforenhancedphotoelectrochemicalwatersplitting,Adv.EnergyMater.3(2013)997–1003
[47] K.Acharya,E.Khon,T.O’Connor,I.Nemitz,A.Klinkova,Heteroepitaxialgrowthof colloidalnanocrystalsontosubstrate filmsviahot-injectionroutes,ACSNano5 (2011)4953
[48] Y.M.Shia,B.Zhang,Recentadvancesintransitionmetalphosphidenanomaterials: synthesisandapplicationsinhydrogenevolutionreaction,Chem.Soc.Rev.45 (2016)1529–1541
[49] G.X.Shen,Y.C.Chen,C.J.Lin,Corrosionprotectionof316LSSbyaTiO2 nanoparticlecoatingpreparedbysol-gelmethod,ThinSolidFilms489(2005)130–136
[50] H.M.Xu,W.Liu,L.X.Cao,G.Su,R.J.Gao,PreparationofZnO/TiO2 composite film on304SSanditsphoto-cathodicprotectionproperties,J.Chin.Soc.Corros.Prot. 34(2014)507–514
[51] J.F.Ren,B.Qian,J.Z.Li,Z.W.Song,L.Hao,J.S.Shi,Highlyefficientpolypyrrole sensitizedTiO2 nanotube filmsforphotocathodicprotectionofQ235carbonsteel, Corros.Sci.111(2016)596–601
[52] C.J.Liu,Y.H.Yang,W.Z.Li,J.Li,Y.M.Li,Q.L.Shi,Q.Y.Chen,Highlyefficient photoelectrochemicalhydrogengenerationusingZnxBi2S3+x sensitizedplatelike WO3 photoelectrodes,ACSAppl.Mater.Inter.7(2015)10763–10770
[53] H.Li,X.Wang,Q.Wei,X.Liu,Z.Qian,B.Hou,Enhancedphotocathodicprotection performanceofAg/graphene/TiO2 compositefor304SSundervisiblelight, Nanotechnology28(2017)225701.
[54] J.Zhang,J.Hu,Y.F.Zhu,Q.Liu,H.Zhang,R.G.Du,C.J.Lin,FabricationofCdTe/ ZnScore/shellquantumdotssensitizedTiO2 nanotube filmsforphotocathodic protectionofstainlesssteel,Corros.Sci.99(2015)118–124
[55] L.M.Peter,K.G.U.Wijayantha,D.J.Riley,J.P.Waggett,Band-edgetuninginselfassembledlayersofBiSnanoparticlesusedtophotosensitizenanocrystallineTiO2, J.Phys.Chem.B107(2003)8378–8381


