https://ebookmass.com/product/cancer-biomarkers-clinicalaspects-and-laboratory-determination-1st-edition-lakshmi-v-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Biomarkers in Inborn Errors of Metabolism. Clinical Aspects and Laboratory Determination 1st Edition Edition Uttam Garg And Laurie D. Smith (Auth.)
https://ebookmass.com/product/biomarkers-in-inborn-errors-ofmetabolism-clinical-aspects-and-laboratory-determination-1st-editionedition-uttam-garg-and-laurie-d-smith-auth/ ebookmass.com
Contemporary Clinical Immunology and Serology (Pearson Clinical Laboratory Science)
https://ebookmass.com/product/contemporary-clinical-immunology-andserology-pearson-clinical-laboratory-science/
ebookmass.com
Prostate Cancer, Second Edition: Science and Clinical Practice Jack H. Mydlo Md Facs
https://ebookmass.com/product/prostate-cancer-second-edition-scienceand-clinical-practice-jack-h-mydlo-md-facs/ ebookmass.com
Green Dumb Guide to Houseplants : 45 Unfussy Plants That Are Easy to Grow and Hard to Kill Holly Theisen-Jones
https://ebookmass.com/product/green-dumb-guide-tohouseplants-45-unfussy-plants-that-are-easy-to-grow-and-hard-to-killholly-theisen-jones/ ebookmass.com
National Kidney Foundation's primer on kidney diseases 7th Edition Scott J. Gilbert (Editor)
https://ebookmass.com/product/national-kidney-foundations-primer-onkidney-diseases-7th-edition-scott-j-gilbert-editor/
ebookmass.com
Molds, Mushrooms, and Medicines: Our Lifelong Relationship with Fungi 1st Edition Money
https://ebookmass.com/product/molds-mushrooms-and-medicines-ourlifelong-relationship-with-fungi-1st-edition-money/
ebookmass.com
Chemically Modified Minds: Substance Use for Cognitive Enhancement 1st Edition Matthew Hall
https://ebookmass.com/product/chemically-modified-minds-substance-usefor-cognitive-enhancement-1st-edition-matthew-hall/
ebookmass.com
Breakthrough Supply Chains: How Companies and Nations Can Thrive and Prosper in an Uncertain World Christopher Gopal
https://ebookmass.com/product/breakthrough-supply-chains-howcompanies-and-nations-can-thrive-and-prosper-in-an-uncertain-worldchristopher-gopal/ ebookmass.com
Objection: Disgust, Morality, and the Law Debra Lieberman
https://ebookmass.com/product/objection-disgust-morality-and-the-lawdebra-lieberman/
ebookmass.com
The
Sophia Vasalou
https://ebookmass.com/product/the-measure-of-greatness-philosopherson-magnanimity-sophia-vasalou/
ebookmass.com
CancerBiomarkers: ClinicalAspectsand Laboratory Determination
ClinicalAspectsandLaboratoryDeterminationofBiomarkersSeries
SeriesEditor:AmitavaDasgupta
Volume1
AlcoholanditsBiomarkers:ClinicalAspectsandLaboratoryDetermination
Volume2
BiomarkersinInbornErrorsofMetabolism:ClinicalAspectsandLaboratory Determination
Volume3
EndocrineBiomarkers:ClinicalAspectsandLaboratoryDetermination
Volume4
KidneyBiomarkers:ClinicalAspectsandLaboratoryDetermination
Volume5
CancerBiomarkers:ClinicalAspectsandLaboratoryDetermination
CancerBiomarkers: ClinicalAspectsand Laboratory Determination
Editedby
LakshmiV.Ramanathan
DepartmentofPathologyandLaboratoryMedicine,MemorialSloan KetteringCancerCenter,NewYork,NY,UnitedStates
MartinFleisher
DepartmentofPathologyandLaboratoryMedicine,MemorialSloan KetteringCancerCenter,NewYork,NY,UnitedStates
MichaelJ.Duffy
UCDSchoolofMedicine,ConwayInstituteofBiomolecularand BiomedicalResearch,UniversityCollegeDublin,Dublin,Ireland; UCDClinicalResearchCentre,St.Vincent’sUniversityHospital, Dublin,Ireland
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
ISBN:978-0-12-824302-2
ForInformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: StacyMasucci
AcquisitionsEditor: PatriciaM.Osborn
EditorialProjectManager: MatthewMapes
ProductionProjectManager: SelvarajRaviraj
CoverDesigner: BridgetHoette
TypesetbyMPSLimited,Chennai,India
Listofcontributors..................................................................................................xv
Preface....................................................................................................................xix
Acknowledgments..................................................................................................xxi
CHAPTER1Overviewoftraditionalandnontraditionaltumor markers.......................................................................... 1 JieliLi,QingH.MengandLakshmiV.Ramanathan Objectives......................................................................................1
1.1 Introduction....................................................................................1
1.2 Definitionandcharacteristicsoftumormarkers...........................2
1.3 Utilizationofserumtumormarkersinmalignantand benigntumors.................................................................................4
1.3.1Alphafetoprotein................................................................5
1.3.2CEA.....................................................................................5
1.3.3CA19-9................................................................................5
1.3.4CA15-3/CA27-29................................................................6
1.3.5CA125,HE4,ROMAscore(riskofovarian malignancyalgorithm)........................................................6
1.3.6Prostate-specificantigenandprostate-specific antigenderivatives..............................................................6
1.4 UnderstandFDAapprovedandnon-FDAapprovedtumor markers...........................................................................................7
1.5 Overviewofmethodologiesformeasurementof tumormarkers................................................................................8
1.5.1Clinicalsensitivityandspecificityoflaboratory tests....................................................................................12
1.6 Laboratorytechnologiesfortumormarkermeasurement...........14
1.6.1Immunoassaymethodologies............................................14
1.6.2Othertypesofimmunoassays...........................................15
1.7 Technicallimitationsofimmunoassays.......................................17
1.7.1Standardizationoftumormarkerassays..........................17
1.7.2Interferencesinimmunoassays.........................................19
1.8 High-pressureliquidchromatographymassspectrometry..........21
1.9 Methodsfordetectingnontraditionalandemergingtumor markers.........................................................................................23
1.9.1Metabolomicsandproteomics..........................................23
1.9.2Molecularandcompaniondiagnostics.............................24
3.3 Interferencesproducedbycancer................................................74
3.3.1Monoclonalproteins.........................................................74
3.3.2Choriogonadotropin..........................................................76
3.4 Physiologicchangesduetocancertreatment..............................78
3.4.1Menopause........................................................................78
3.4.2Stemcelltransplant...........................................................79
3.4.3Bonemarrowrecovery......................................................79
3.4.4Filgrastim..........................................................................80
3.5 Preanalyticalissues......................................................................81
3.5.1Pseudohyperkalemia.........................................................81
3.5.2Lactatedehydrogenase......................................................82
3.5.3Hemolysis..........................................................................83
3.5.4Contamination...................................................................83
3.6 Analyticalissues...........................................................................84
3.6.1Glucose-6-phosphatedehydrogenase................................84
3.6.2Therapeuticantibodies......................................................85
3.6.3Analyticsensitivitylowerrange.......................................86
3.6.4Analyticsensitivityhigherrange......................................87
3.7 Postanalyticalissues.....................................................................88
3.7.1Criticalvalues...................................................................88
3.8 Conclusionandfuturedirections.................................................89
3.9 Summarypoints............................................................................90 References....................................................................................90
CHAPTER4Thyroglobulinandthyroidcancer .............................. 93 WilliamS.Phipps,AndrewN.Hoofnagle, MaraY.RothandChristopherM.Shuford Objectives....................................................................................93
4.1 Introduction..................................................................................93
4.2 Thyroidnoduleevaluationandthelimitedroleof preoperativeserumthyroglobulinmeasurements........................94
4.3 Postoperativeevaluationofdifferentiatedthyroid cancer............................................................................................99
4.4 Thyroglobulininthemonitoringofdifferentiatedthyroid cancer..........................................................................................101
4.5 Utilizationofthyroglobulinautoantibodiesassurrogate marker.........................................................................................104
4.6 Themeasurementofthyroglobulinbyimmunoassaysand thechallengeofautoantibodies.................................................106
4.7 Thyroglobulinmeasurementbymassspectrometry usingpeptideimmunoaffinityenrichment.................................108
4.8 Thyroglobulinmeasurementbymassspectrometry—toward harmonization.............................................................................111
4.9 Thyroglobulinmeasurementbymassspectrometry—other challenges...................................................................................114
4.10 Practicalapplicationofthyroglobulinmeasurementbymass spectrometry:asuggestedapproach..........................................114
4.11 Thyroglobulinmeasurement—futuredirections........................117
4.12 Conclusion..................................................................................117
4.13 Summarypoints..........................................................................118
SigridV.Carlsson,KazunoriMurata,DanielC.Danila andHansLilja Objectives..................................................................................131
5.1 Introduction................................................................................132
5.2 PSA:physiologicalfunctionandanalyticalassay.....................132
5.3 PSAscreeningtrials...................................................................135
5.4 BaselinePSAstudiesandlong-termriskoflethalprostate cancer..........................................................................................139
5.4.1PSAvariation..................................................................139
5.4.2PSAasaprognosticmarker...........................................141
5.5 PSAasatumormarkerandscreeningtoolforprostate cancerandrecommendationsfromnationalguidelinegroups..146
5.6 PSA-relatedbloodbiomarkerstoimprovethespecificity ofPSAandruleouttheneedforprostatebiopsy.....................148
5.6.1PSAforms.......................................................................148
5.6.2BiomarkerstoimprovethespecificityofPSA..............149
5.6.34-Kallikreinpanel...........................................................150
5.7 MethodsofdetectingPSA.........................................................155
5.8 RoleofPSAinmonitoringdiseaserecurrenceand progression.................................................................................157
5.9 Conclusionsandfuturedirections..............................................159 Conflictofinterestdeclaration..................................................160 References..................................................................................160
CHAPTER6Monoclonalgammopathydetectionandcurrent technologies .............................................................. 173
ShelbyM.HutchersonandKatieL.Thoren Objectives..................................................................................173
6.1 Introduction................................................................................173
6.2 Monoclonalgammopathies........................................................174
6.3 CurrenttechnologiesforM-proteindetection...........................178
6.3.1Serumproteinelectrophoresis........................................178
6.3.2Immunofixationandimmunosubtraction.......................180
6.3.3Urineproteinelectrophoresisandimmunofixation........182
6.3.4Freelightchainquantitation...........................................185
6.3.5Testingguidelines...........................................................186
6.4 NewandemergingtechnologiesforM-proteindetection.........187
6.4.1Shiftassays......................................................................189
6.4.2Massspectrometry..........................................................191
6.4.3Currentstatusandpotentialroleofmass spectrometryforM-proteindetection.............................195
6.5 SummaryPoints.........................................................................197
6.6 Futuredirections.........................................................................197 References..................................................................................198
CHAPTER7Liquidbiopsyasacancerbiomarker-potential, andchallenges .......................................................... 203
DanielC.Danila
Objectives..................................................................................203
7.1 Introduction................................................................................203
7.2 Clinicalutilityofliquidbiomarkers..........................................204
7.3 Clinicaldevelopmentandvalidationofliquidbiomarkers.......205
7.3.1Contextofuse.................................................................205
7.3.2Analyticalvalidation.......................................................206
7.3.3Clinicalqualification.......................................................206
7.4 Circulatingtumorcells...............................................................207
7.4.1Themorphologyandcharacteristicsofcirculating tumorcells.......................................................................208
7.4.2Detectionmethods..........................................................210
7.5 CirculatingtumorDNA.............................................................221
7.6 Conclusion..................................................................................229
7.7 Summarypointsandfuturedirections.......................................229 Furtherreadings.........................................................................230 References..................................................................................230
CHAPTER8ChimericantigenreceptorTcellsand managementoftoxicities:implicationsof biomarkers ................................................................ 245 KitsadaWudhikarn,AnaAlarco´nToma ´ s, KazunoriMurataandMiguel-AngelPerales Objectives..................................................................................245
8.1 Introduction................................................................................246
8.2 ConstructandmanufacturingprocessofCD19chimeric antigenreceptorTcells..............................................................247
8.2.1Relapsed/refractorylargeB-celllymphoma...................247
8.2.2Relapsed/refractoryB-cellacutelymphoblastic leukemia..........................................................................249
8.2.3Relapsed/refractorymantlecelllymphoma....................249
8.2.4Relapsed/refractoryfollicularlymphoma.......................250
8.2.5Relapsed/refractorychroniclymphocytic leukemia..........................................................................250
8.3 Immune-mediatedtoxicitiesofCD19chimericantigen receptorT-celltherapy...............................................................250
8.3.1Cytokinereleasesyndrome.............................................251
8.3.2Immuneeffectorcell-associatedneurotoxicity syndrome.........................................................................254
8.3.3OtherchimericantigenreceptorTcell-associated toxicities..........................................................................255
8.4 ClinicalapplicationofbiomarkersinCD19chimeric antigenreceptorT-celltherapy..................................................255
8.5 Predictivebiomarkersofseverechimericantigen receptorandimmuneeffectorcell-associatedneurotoxicity syndrome....................................................................................256
8.5.1ChimericantigenreceptorT-celldose...........................256
8.5.2Tumorburden..................................................................257
8.5.3Inflammatorycytokines..................................................258
8.5.4SeruminflammatorymarkersincludingC-reactive proteinandferritin..........................................................260
8.5.5Preexistingendothelialactivation...................................261
8.6 Predictivebiomarkersforhematologictoxicities......................261
8.7 Predictivebiomarkersforhemophagocytic lymphohistiocytosisandmacrophageactivation syndrome....................................................................................262
8.8 Predictivemodelsforchimericantigenreceptorand immuneeffectorcell-associatedneurotoxicitysyndrome.........263
8.9 BiomarkersofchimericantigenreceptorT-cellresponse, outcome,andtreatmentfailure..................................................265
8.9.1ChimericantigenreceptorTcelland host-immune-basedbiomarkers......................................265
8.9.2Tumor-basedbiomarkers................................................267
8.10 Summarypointsandfuturedirections.......................................269 Disclosures.................................................................................270
Acknowledgment.......................................................................271 References..................................................................................271
CHAPTER9Cerebrospinalfluidcirculatingtumorcellsfor diagnosis,responseevaluation,andmolecular profilingofleptomeningealmetastasesfrom solidtumors .............................................................. 283 MariaDiaz,MartinFleisherandElenaI.Pentsova
Objective....................................................................................283
9.1 Introduction................................................................................283
9.2 Rarecellcapturetechnologyasadiagnostictoolfor leptomeningealmetastases.........................................................285
9.3 ClinicalLaboratoryImprovementActcertification..................288
9.3.1Definitionofacirculatingtumorcellusing theCellSearchcirculatingtumorcelltest......................289
9.3.2Specimenneededforcirculatingtumorcells enumerationincerebrospinalfluid.................................290
9.3.3Interpretationofresults...................................................290
9.3.4Qualitycontrolofcirculatingtumorcellsin cerebrospinalfluid..........................................................290
9.3.5Reportingresults.............................................................291
9.3.6Referencerange..............................................................291
9.4 Additionalapplicationsofcerebrospinalfluidcirculating tumorcellsandothertechniques...............................................291
9.5 LimitationsoftheCellSearchplatform.....................................293
9.6 SummaryPoints.........................................................................293
9.7 Futuredirections.........................................................................294 References..................................................................................294
CHAPTER10Salivaasamatrixformeasurementofcancer biomarkers ................................................................ 297 LucasTrevisanFranc¸adeLima,JulianaMu¨llerBark, MohammadRasheduzzaman,ChameeraEkanayake WeeramangeandChamindiePunyadeera
Objective....................................................................................297
10.1 Introduction................................................................................297
10.2 Salivaproductionandsecretion.................................................300
10.3 Transportofbiomoleculesfromthebloodintosaliva..............301
10.4 Salivacollectionmethodsandsalivatypes...............................303
10.5 Salivarybiomarkersforcancer..................................................305
10.5.1Saliva-basednucleicacidbiomarkersforcancer detection........................................................................305
10.5.2Protein-basedcancerbiomarkersinsaliva...................313
10.5.3Glycansandglycoproteomicbiomarkersforcancer....317
CHAPTER11Emergingtechnologiesincancerdetection
ZviYaari,ChristopherP.Horoszko, MeravAntman-Passig,MijinKim,FreddyT.Nguyen andDanielA.Heller
11.2.4Transducertrends:low-costpaperdevices..................358
11.5.2Precisionmedicine........................................................369
11.7 Dataanalytics.............................................................................375
11.7.1Facilitatingconventionalbiomarkerdiscovery............375 11.7.2Artificialintelligenceincancerdiagnosis....................376
11.7.3Potentialpitfallsinartificialintelligence based sensortechnology..........................................................377
11.8 SummaryPoints.........................................................................378
11.9 Conclusionsandfuturedirections..............................................378 References..................................................................................379
CHAPTER12Oncometabolitesandtheirroleincancer ............... 393 EmilyL.Gill,KhushbuPatelandDineshRakheja
Objective....................................................................................393
12.1 Introduction................................................................................393
12.2 Oncometabolitesandtheirassociatedcancers..........................395
12.3 Invitroprofilingandmeasurementofoncometabolites...........396
12.4 Omicsapproachestometaboliteprofilingincancers...............397
12.5 Imagingforoncometabolites.....................................................399
12.6 Recentinnovationsinoncometabolomics.................................400
12.7 MeasuringoncometabolitesD-2-hydroxyglutarateand L-2-hydroxyglutarateintheclinicallaboratory........................402
12.8 Conclusionandfuturedirections...............................................404
12.9 Summarypoints..........................................................................405 References..................................................................................405
CHAPTER13Circulatingcancerbiomarkers:currentstatusand futureprospects ........................................................ 409 MichaelJ.Duffy
Objectives..................................................................................409
13.1 Introduction................................................................................409
13.2 Clinicalusesofestablishedcirculatingproteinbiomarkers......410
13.2.1Screeningforearlycancer............................................410
13.2.2Evaluatingprognosisfollowingdiagnosisofa primarycancer..............................................................417
13.2.3Surveillancefollowingcurativesurgeryfor primarycancers.............................................................418
13.2.4Monitoringtheeffectivenessofsystemictherapyin advancedcancer............................................................420
13.3 Futuretrendsincancerbiomarkerapplicationsandongoing research.......................................................................................421
13.3.1CirculatingtumorDNA................................................422
13.3.2Circulatingtumorcells.................................................431
13.3.3Micro-RNA...................................................................433
Listofcontributors
MeravAntman-Passig
MemorialSloanKetteringCancerCenter,NewYork,NY,UnitedStates
SigridV.Carlsson
DepartmentsofSurgery(UrologyService)andEpidemiologyandBiostatistics, MemorialSloanKetteringCancerCenter,NewYork,NY,UnitedStates; DepartmentofUrology,InstituteofClinicalSciences,SahlgrenskaAcademyat UniversityofGothenburg,Gothenburg,Sweden
DanielC.Danila
DepartmentofMedicine,MemorialSloanKetteringCancerCenter,NewYork, NY,UnitedStates;DepartmentofMedicine,WeillCornellMedicalCollege, NewYork,NY,UnitedStates
MariaDiaz
DepartmentofNeurology,MemorialSloanKetteringCancerCenter,NewYork, NY,UnitedStates
MichaelJ.Duffy
UCDSchoolofMedicine,ConwayInstituteofBiomolecularandBiomedical Research,UniversityCollegeDublin,Dublin,Ireland;UCDClinicalResearch Centre,St.Vincent’sUniversityHospital,Dublin,Ireland
ChameeraEkanayakeWeeramange
SalivaandLiquidBiopsyTranslationalLaboratory,CentreforBiomedical Technologies,SchoolofBiomedicalSciences,FacultyofHealth,Queensland UniversityofTechnology,Brisbane,QLD,Australia;TranslationalResearch Institute,Brisbane,QLD,Australia;GriffithInstituteforDrugDiscovery (GRIDD),GriffithUniversity,Brisbane,Australia;DepartmentofMedical LaboratorySciences,FacultyofHealthSciences,TheOpenUniversityof SriLanka,Nugegoda,SriLanka
MartinFleisher
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates
EmilyL.Gill
DepartmentofPathologyandLaboratoryMedicine,Children’sHospitalof Philadelphia,Philadelphia,PA,UnitedStates;DepartmentofPathologyand LaboratoryMedicine,UniversityofPennsylvania,Philadelphia,PA, UnitedStates
DanielA.Heller
MemorialSloanKetteringCancerCenter,NewYork,NY,UnitedStates; DepartmentofPharmacology,WeillCornellMedicine,NewYork,NY, UnitedStates;DepartmentofPhysiologyandBiophysics,WeillCornell Medicine,NewYork,NY,UnitedStates
AndrewN.Hoofnagle
DepartmentofLaboratoryMedicineandPathology,UniversityofWashington, Seattle,WA,UnitedStates;DepartmentofMedicine,UniversityofWashington, Seattle,WA,UnitedStates
ChristopherP.Horoszko
MemorialSloanKetteringCancerCenter,NewYork,NY,UnitedStates; DepartmentofPharmacology,WeillCornellMedicine,NewYork,NY,UnitedStates
ShelbyM.Hutcherson
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates
MijinKim
MemorialSloanKetteringCancerCenter,NewYork,NY,UnitedStates
JieliLi
DepartmentofPathology,WexnerMedicalCenter,TheOhioStateUniversity, Columbus,OH,UnitedStates
HansLilja
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates;DepartmentsofSurgery(Urology Service)andMedicine,MemorialSloanKetteringCancerCenter,NewYork, NY,UnitedStates;DepartmentofTranslationalMedicine,LundUniversity, Malmo ¨ ,Sweden
HungS.Luu
Children’sHealthSystemofTexas,UniversityofTexasSouthwesternMedical Center,Dallas,TX,UnitedStates
SamuelI.McCash
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates
QingH.Meng
DepartmentofLaboratoryMedicine,DivisionofPathologyandLaboratory Medicine,TheUniversityofTexasMDAndersonCancerCenter,Houston,TX, UnitedStates
JulianaMullerBark
SalivaandLiquidBiopsyTranslationalLaboratory,CentreforBiomedical Technologies,SchoolofBiomedicalSciences,FacultyofHealth,Queensland UniversityofTechnology,Brisbane,QLD,Australia;TranslationalResearch Institute,Brisbane,QLD,Australia;GriffithInstituteforDrugDiscovery (GRIDD),GriffithUniversity,Brisbane,Australia
KazunoriMurata
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates
FreddyT.Nguyen
InstituteforMedicalEngineeringandScience,MassachusettsInstituteof Technology,Cambridge,MA,UnitedStates;DepartmentofPathology, MolecularandCell-BasedMedicine,IcahnSchoolofMedicineatMountSinai, NewYork,NY,UnitedStates
KhushbuPatel
DepartmentofPathologyandLaboratoryMedicine,Children’sHospitalof Philadelphia,Philadelphia,PA,UnitedStates;DepartmentofPathologyand LaboratoryMedicine,UniversityofPennsylvania,Philadelphia,PA,UnitedStates
ElenaI.Pentsova
DepartmentofNeurology,MemorialSloanKetteringCancerCenter,NewYork, NY,UnitedStates
Miguel-AngelPerales
AdultBoneMarrowTransplantation,DepartmentofMedicine,MemorialSloan KetteringCancerCenter,NewYork,NY,UnitedStates;Departmentof Medicine,WeilCornellCollegeofMedicine,NewYork,NY,UnitedStates
MelissaS.Pessin
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates
WilliamS.Phipps
DepartmentofLaboratoryMedicineandPathology,UniversityofWashington, Seattle,WA,UnitedStates
ChamindiePunyadeera
SalivaandLiquidBiopsyTranslationalLaboratory,CentreforBiomedical Technologies,SchoolofBiomedicalSciences,FacultyofHealth,Queensland UniversityofTechnology,Brisbane,QLD,Australia;TranslationalResearch Institute,Brisbane,QLD,Australia;GriffithInstituteforDrugDiscovery (GRIDD),GriffithUniversity,Brisbane,Australia;MenziesHealthInstitute Queensland(MIHQ),GriffithUniversity,Queensland,Australia
DineshRakheja
DepartmentofPathologyandLaboratoryMedicine,Children’sHealth,Dallas, TX,UnitedStates;DepartmentofPathology,UniversityofTexasSouthwestern MedicalCenter,Dallas,TX,UnitedStates
LakshmiV.Ramanathan
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates
MohammadRasheduzzaman
SalivaandLiquidBiopsyTranslationalLaboratory,CentreforBiomedical Technologies,SchoolofBiomedicalSciences,FacultyofHealth,Queensland UniversityofTechnology,Brisbane,QLD,Australia;TranslationalResearch Institute,Brisbane,QLD,Australia;GriffithInstituteforDrugDiscovery (GRIDD),GriffithUniversity,Brisbane,Australia
MaraY.Roth
DepartmentofMedicine,UniversityofWashington,Seattle,WA,UnitedStates
ChristopherM.Shuford
LaboratoryCorporationofAmericaHoldings,Burlington,NC,UnitedStates
KatieL.Thoren
DepartmentofPathologyandLaboratoryMedicine,MemorialSloanKettering CancerCenter,NewYork,NY,UnitedStates
AnaAlarco ´ nToma ´ s
AdultBoneMarrowTransplantation,DepartmentofMedicine,MemorialSloan KetteringCancerCenter,NewYork,NY,UnitedStates;PhDPrograminSignals IntegrationandModulationinBiomedicine,CellTherapyandTranslational Medicine,UniversityofMurcia,Murcia,Spain
LucasTrevisanFranc¸adeLima
SalivaandLiquidBiopsyTranslationalLaboratory,CentreforBiomedical Technologies,SchoolofBiomedicalSciences,FacultyofHealth,Queensland UniversityofTechnology,Brisbane,QLD,Australia;TranslationalResearch Institute,Brisbane,QLD,Australia;GriffithInstituteforDrugDiscovery (GRIDD),GriffithUniversity,Brisbane,Australia;GallipoliMedicalResearch Institute,GreenslopesPrivateHospital,Brisbane,QLD,Australia
KitsadaWudhikarn
AdultBoneMarrowTransplantation,DepartmentofMedicine,MemorialSloan KetteringCancerCenter,NewYork,NY,UnitedStates;DivisionofHematology andResearchUnitinTranslationalHematology,DepartmentofMedicine, ChulalongkornUniversity,Bangkok,Thailand
ZviYaari
MemorialSloanKetteringCancerCenter,NewYork,NY,UnitedStates;School ofPharmacy,FacultyofMedicine,TheHebrewUniversityofJerusalem,Israel
Preface
Cancerisaleadingcauseofdeathworldwidewithalmost10million casesin2020perestimatesoftheWHO.In2020themostcommon causesofcancerdeathswereattributedtomalignanciesofthelung, colonandrectum,liver,stomach,andbreast.Approximately, 400,000childrenarediagnosedeveryyear,worldwide.
Giventheenormousmagnitudeandcomplexityofthisdisease process,therehavebeenremarkableachievementsovertheyears fordetecting,monitoring,andtreatmentofcancer.Theearlierthe detection,thebetterarethechancesforsuccessfultreatment. However,despitedecadesofbasicresearchinvolvingbiomarkers, fewerthan25havebeenapprovedbytheUSFoodandDrug Administrationasthejourneyofabiomarkerfromthebenchtothe bedsideiscomplexandchallenging.Thereasonforthisisthat extensivevalidationstudiesarerequiredtobedoneforanybiomarkertobeclinicallyimplemented.
Inthisbook,thatispartoftheseriesonbiomarkerspublishedby Elsevier,thefocusisoncancerbiomarkers,clinicalaspects,and laboratorydetermination.Asthebookistargetedformedicalstudents,physicians,oncologists,pathologists,clinicalchemists,and medicaltechnologists,wehavefocusedonfourareas.Thefirstsection,Chapters1 3,discusstheoverviewoftraditionalandnontraditionalbiomarkersfollowedbylaboratorytestinginpediatricaswell asadultcancerpatients.Emphasisisplacedontheuniquechallengesinthemanagementofthesetwosegmentsofthecancer patientpopulation.ThisisfollowedbyChapters4 6thatfocuson prostate-specificantigen,thyroglobulin,andmonoclonalproteins. Chapters7 9discussliquidbiopsy,chimericantigenreceptorTcelltherapies,andthevalidationofcirculatingtumorcellsandcellfreeDNAincerebrospinalfluid.Thefourthsectionthatincludes Chapters10 12assessesemergingtechnologiesaswellasdifferent
platformsinbiomarkertestingwithsummary,conclusions,andfurtherdirection.Finally,Chapter13broadlysummarizesthecurrent statusofstandardcancerbiomarkersanddiscusseslikelyfuture developmentswithcirculatingtumorDNA,circulatingtumorcells, andextracellularvesicles.
Editors
LakshmiV.Ramanathan
MartinFleisher
MichaelJ.Duffy
Acknowledgments
Ourapproachtothisbookdedicatedtomedicalstudents,oncologists,physicians,clinicalchemists,andmedicaltechnologistsisto givethemanideaofchallengesfacedwithadultandpediatric patientsininterpretinglaboratorytestresults,stepsrequiredforthe completevalidationofabiomarkerbeforeitisintroducedinclinical practice,acomprehensivediscussiononselectedbiomarkersand emergingplatforms,andtestingforbiomarkers.
Keepingouroverallperspectivesofthebookinmind,ithasbeen asheerpleasureforustoworkwithourauthorsbothwithinand outsideMemorialSloanKetteringCancerCenter.Theirsupportand enthusiasmforourendeavorisreallyappreciatedaswellastheir timelysubmissionofchaptersandresponsetoourquestions.Weare gratefultoourpublisherswhohavebeenamazinglypatientand helpfulthroughoutthisjourney.
Notenoughcanbesaidregardingourcolleaguesinclinical chemistrywhohaveworkedinourlaboratoriesformanyyearsvalidatinglaboratorydevelopedtests,performingrequiredroutinetasks, andalwaysgivingtheir100%totheirwork.Theseincludeourlaboratoryassistants,benchtechnologists,supervisors,managers,and fellowclinicalchemists.Theirhardworkandsupportcannotbe measuredaswellastheirdedicationtoourcancerpatients.Ourclinicalcolleagueshavebeenamazingandsupportiveasisevidentfrom theircontributionstoourbook.Wewouldespeciallyliketo acknowledgeDrs.PeterMaslakandMikeTuttlewhoprovidedvaluablefeedbackandcommentsforChapters2and4.Aspecialvoteof thankstoouradministratorEvelinPoncewhoworkedwithour schedulesandarrangedourweeklyzoomcalls.
Finally,oursupportfromourfamiliesovertheyearscannotbe measuredandwearetrulygratefultothem.Thiswasatrulycollaborativeteameffortamongthethreeofus,anditwasanhonortobe associatedwiththisventure.
LakshmiV.Ramanathan MartinFleisher MichaelJ.Duffy
Overviewoftraditional andnontraditionaltumor markers
1
JieliLi1,QingH.Meng2 andLakshmiV.Ramanathan3
1DepartmentofPathology,WexnerMedicalCenter,TheOhioState University,Columbus,OH,UnitedStates
2DepartmentofLaboratoryMedicine,DivisionofPathologyand LaboratoryMedicine,TheUniversityofTexasMDAndersonCancer Center,Houston,TX,UnitedStates
3DepartmentofPathologyandLaboratoryMedicine,MemorialSloan KetteringCancerCenter,NewYork,NY,UnitedStates
Objectives
•Reviewlaboratorytestsusedincancerdiagnosis.
•Definecharacteristicsoftumormarkers.
•ListFDAapprovedversusnon-FDAapprovedtumormarkers.
•Understandoverviewofdifferentmethodologiesusedfortumor markermeasurement.
•Explainroleofnontraditionaltumormarkersandtheirpotential scope.
•Discussfuturedirections.
1.1 Introduction
About10milliondeathswerereportedin2020worldwidedueto cancerorcancer-relatedconditions [1].Ithasbeenshownthatearly diagnosiscandramaticallyimprove5-yearsurvivalfrom21%to 89%forallcancersand5%to56%forlungcancer [2].Thisis mainlyduetotechnologicaladvancementsinlaboratorymedicine
withthemeasurementofbiomarkers,inpathologywithexamination oftissueandinradiologywithimagingstudies.
Cancerbiomarkersinvolvemeasurementofproteins,peptides,biochemicals,DNA,andRNA.Theycanbefurthercategorizedaspredictive,diagnostic,orprognostic.Theseincludegenetic,transcriptive, epigenetic,andproteomicbiomarkers.Theycanbedetectedinbiologicalfluidsincludingwholeblood,serum,plasma,fluids,urine,stool, sputum,andnippledischarge [3].Identifyingbiomarkerscanpredict responsetotreatmentthatisvitalforthesuccessofprecisionandtargetedtherapyincancerpatients.CurrentlytheUSFoodandDrug Administration(FDA)hasapproved23proteincancerbiomarkers,of whichonly16aredetectableinblood [4].However,thesebiomarkers donotdemonstratehighsensitivityandspecificityforclinicaldiagnosis whenemployedalone.
Diagnostictestingfortumorsi snotonlylimitedtoclinical chemistrybutalsoincludesimmunology;hematology;hematopathology;thepathologicaland histologicalexaminationof biopsy,genetic,genomic,andmolec ulartesting(liquidbiopsy); imagingtests(X-ray,computerizedtomography,ultrasound,magneticresonanceimaging,single-photonemissioncomputerized tomography,andpositronemissi ontomography);andmolecular imaging.Blood,tissues,andbodyfluidsarethemostcommon specimensinclinicallaboratorytesting.Bodyfluidsfortumor markertestingincludecerebrospin alfluid(CSF),saliva,effusions,andurine.
1.2 Definitionandcharacteristicsoftumormarkers
Tumormarkersaredefinedassubstancesfoundintissue,blood, bonemarrow,orotherbodyfluids thatmaybeindicativeofcancerorcertainbenign(noncance r)conditions.Manytumormarkersareproteinsmadebybothnormalcellsandcancercells,but theyaremadeinhigheramountsb ycancercells.Geneticchanges intumortissue,suchasgenemutations,patternsofgeneexpression,andotherchangesintumorDNAorRNA,arealsobeing usedastumormarkers.Atumormarkermaybeusedwithother teststohelpdiagnosecancer.Itmayalsobeusedtohelpplan
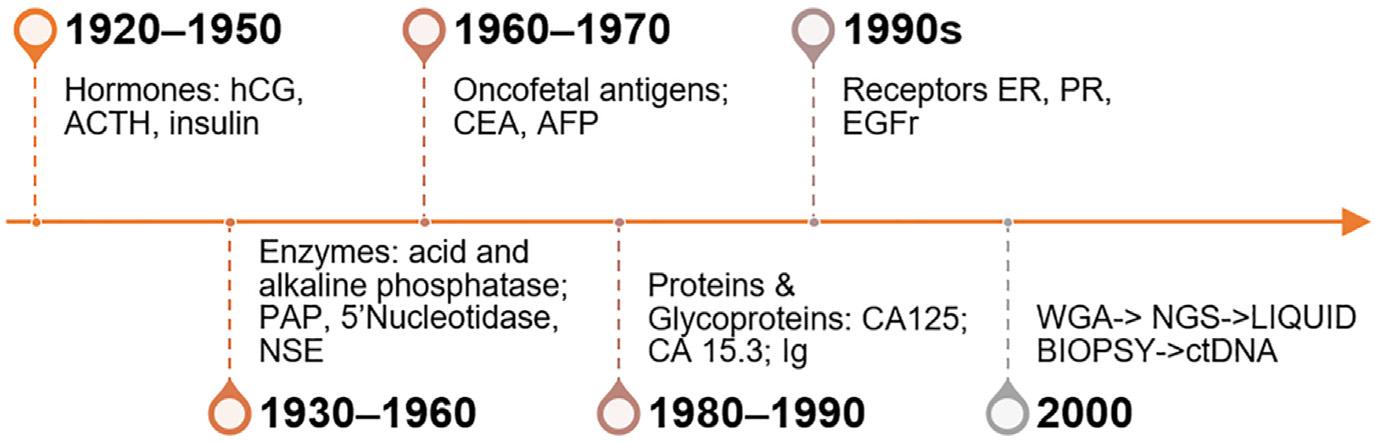
FIGURE1.1
100yearsoftumormarkerdevelopment. ACTH,adrenocorticotropic hormone; AFP,alphafetoprotein; CA,carbohydrateantigen; CEA, carcinoembryonicantigen; ct,circulatingtumor; EGFr,humanepidermal growthfactorreceptor; ER,estrogenreceptor; hCG,humanchorionic gonadotropin; NGS,nextgenerationsequencing; NSE,neuronspecific enolase; PAP,prostaticacidphosphatase; PR,progesteronereceptor; WGA,wholegenomeanalysis.
treatment,givealikely prognosis,andmonitorthepatient’s responsetotherapy.
Traditionaltumormarkerdevelopmentoverthelastfewdecades hasbeenrelativelyslowandchallengingwiththetimelineshownin Fig.1.1.
Tumormarkersinclude:
•Enzymes
•Hormonesandhormonemetabolites
•Oncofetalantigens
•Carbohydratemarkers
•Bloodgroupantigens
•Proteinssuchas β2-microglobulinandimmunoglobulins
•Receptors
•RNAs
•DNA
Table1.1 presentsasummaryoftumormarkersthathaveadequatespecificityandsensitivityforuseinprognosis,detectionof recurrence,andmonitoringresponsetotreatment.
Table1.1 Themajortumormarkersusedforprognosis, treatmentmonitoring,andrecurrencedetection.
UtilityTumormarkers
Prognosis
β2-Microglobulin
CA125
AFP,PSA,CEA
Lactatedehydrogenase
HER2/neu
Estrogenreceptor
Progesteronereceptor
MonitoringtreatmentCA125
CA19-9 CEA
AFP hCG PSA
DetectionofrecurrenceCA15-3 CA125 CEA
AFP hCG PSA
1.3 Utilizationofserumtumormarkersin malignantandbenigntumors
Despitethechallengesandinterferenceswithimmunoassaymethodologiesfortumormarkers,theirmeasurementsarereadilyavailableonautomatedplatformsthatarerelativelyinexpensiveand easytouse.Althoughtheirpotentialutilityinscreeningthegeneralpopulationislow,theyarewidelyusedforthedifferential diagnosisofsymptomaticpati entsandmonitoringtherapy. Overall,theyhavelimiteduseforearlydetection,clinicalstaging ofcancer,andestimatingtumorvolume.Althoughspecifictumor markersarediscussedindetailin otherchaptersinthisbook,we