Complete Physics
Fourth Edition
Stephen Pople
Anna Harris
Naseemunissa Azam
Elliot Sarkodie-Addo
Helen Roff
O XF ORD
\.'NTVBRSITY PRESS
(,ff;\l ( l.1JYJll1on ~ln: l O~onl OXl 601' lJnltt'd Kingdom
Oxlunl lh11\·1•"1 Pn", 1, tlr11.u111w•111 c>i lh\' l1'111-.,-n.1ry or O\lun1 II furth,•~ [ht' ll111\'t•l'\1I) ,. UlJJt'tll\'f' uf f').( >Jk'Otl' bi rl'".l".lh ll -.<ho t.mlup ;md Nlui: ¥Ion by publblalng wurlctwklc O>.Jonl h Jt'~lf'Jt'd tn <k' m;u>. ol OXlllfd llru\' l"T'aty ...-n, ml~ llK iWl1 m «'rt.UU otlt<•-rcountri 0 O\lonl Umv,•r.11)' Pw-., 202 l lbC" moral rigllh 01 lhl' a.-JJOI\ b.1\' t' l>ttn , 'o('ftOO I '"' 1mbl """' 111 2021
AU light, ft' rwd '- 0 p,ut 01 tl1.h pubbc:ulnn OU)' br rroc1Ul't"(l ,ton'<I U1 n'tril"'\•.tl \o) 'll°lll or lr.\mIDJllC'd. in ,wy form T by ;wy D)t',\U\ , Wllhoul Cltc• pnnr Jk'nlll\\lCJU III wn1111y,01 (hlc•nll1111\1'"''Y Pn,, o,,.,,•x11n ,ty l"'m> 1J1t'tl hy t.n,, , I>}' 1it1"11t1" 01 1111tlt"1 IN 1n, ~""''tl v, 11 h , l1 .11>11n>1>rur,• ft"pt'\~plllt'> ~•.Lili. orr,.mlutJon.. [llilpJ[J;e,,, (00 maog n,inxlllctlOO otmklf' u ,... OJ ll .bot, ,boul<I br s.t'nl totbl' R.lglll'\. l>-'p.1ttmt'nt Olll lln1 llau\ l'l"lt)" f'n•-,., , .-.t UM" Mlrtn, •,1)()\'t'
You n,m, 110C cm uLtlf' 1 lili v,01 l.. u1 .iu,y O(Jlf'r 1,, m1-' 1x1 )'t"1 uHl'll 1u1 po'lt' Ltu" ~mlf' CllllditJOll 00.lny .-.n,iln·r
flnt"h IJhr,11) c~ratnr,111_,,. 111 l\1hh, ..aic111 n.1r,1
0.tl,\ .lV,111.lblt•
97&- l • 200W.t_,. 1,u111c1,utl)
10 9 8 7 6 3 •l J 2 I
•, 13- , J.<lt ,,mo •'>, 1 4t"lllu,l('\"C'ft
10 9 A 7 <• :; I l '2 I
11,lJk'f u I lo lb pnnictftl() 0, Lilli boo o a ~mral r«)dlbl proclU<t m.vlr lrom "'ooctgrowu Jnsu~.unilbk- l•'lff~, lbr- ru.\uu~cturiug pro<',") ni11lcim1, 10 tl t'IIV1nrn11w •11ul n •p,1bhm1, of thf' n11111try of PTiY,111 , Pnutt'tl Ln 11,11)' b)' Lt G O Sp,\ At knowlt'()~•111eut,
IC C'\ I (I) "'t 11 n,.~,h'll'll lr,1d1.•1i1,ul: ,,1 C.u11bnl1,-• hllC' m.Ut1l1 ul l:\.a 111111Jctt1m
AU ;\IL"-"l' 1' II.IV(' ~o wrtttl'D b)• tu , lt~ lll f'X.1lllln.tlx)l) , ll:u• w;\y ru.u~ Ml" ,l\l,,Udrd m;q be- dlllt'n'llt , SI•• 1•lt1• 11 Pupk• wuuk1 hu• 10 llun._ ~11"'111 h 111h• 1111 J)r 0MT1'11 IA-WI'- aur 1lt1•ar IW'lp , WIii\ 'Pf'(a.\l lll.lJII.., ;\MIO Z.ll. ll.ll)' ,\lldl Ii ii lklpk,y too 1:N.>piu~: lll, ,plrill> up
111 p,.,h,l111•r ,wl .mthn" \tot11ild m,1• tn 1l1.111l.; tltt• foll<Mm:,,. lor tH•m1i-.,,n11 [O ll~ phou~:, .11>lt, .u111tlll11•r (ll IJ)' 11Y,lll 1ll-'h'1'1.l I:
OVt'r: Will.).im Bratl> 11) 1 Jruttt'NOCL
Photo : pt 1 : Vac t.J\•llilJCbtcbJ ltull c mod.:: p M It J.2 : ~l'UC('pUola./ AL1111~• !.I (X I: Photo: Jl H, : !lo('~('\• 1 1 pan,. h11l h~.-.ioc t , {l I K! ,A \A , p.LH: j.1-lll)"'-lV)'i &toctphoto; pl t Jl'lJl lniVC'f'\jty of Arl7on; '\A~A; pl:!bt Ti\•U,oll<Lt &.~tod;photo p2.lh EJ~•UK'llUlhU,\g&Hf,/l tud, photo: vi1 : fldvicl 11,llk'lt/ ~t • 1ng., /ArP,'(;c,uy In•~· plK: KNIil KNH / lt'lk i• r'llC•l0 tibral)-: p32~ Dl~egu Pk~ lu c/Al.uuy tocl Photo: pJ4 ; ( Lt.uk~ \I Dull" JrJ~\A: p 39; n y F,bl/t5todplloto· p40 : Ell lo( lod,,,1 -.0: p42; ' J~tC"'ihi r.tk.1-h.1DJ!,tic11cc Photo Ll1>r.uy. p1:.U : l'lXbox77/ ~hll11C'NOlt.: p-131: I'( N PbotOf.i,jphy/.i\laoay ~tocli. fllaoto; p13r. Trub,wiltJ llullc~loct : p4K! 1m.1gt• IUtOICI AL11U) MOtl.: Pli to, p54 : Kt}l\11~!.hnltC'r-., 1:: p:;'J: 7.t'br.10.l09J~hu11,·ruoct · p(,:1: ;i1"foto...toct1AL1my ~toct l 1hoto; p(•7! c~ug u,~S1111tll•Nn<l. : p(,9J : Anc ln-w Bud,m./ h11tll'Noe l.· 11169111 : ,urlato C,111lt;ll).lil l «)loll,1 : pC,9 PNt•r Gca1lc 'OllP: p70: O:\RJ'-lt 10 11,11 Uudc~~ea R~e,ud1 Program \\\xxls llok O~aiiograpluc bot J '°"''° p72· \~AJ)PI.NC DJ " p74 ; Phil1p1,c.• Pl.ully/ Ol"tw-t• Photo Ubracy. plCO: Action Pba, ~ports )O).lf.~/Al,uuy ~· I. ('11oto: pMt:Jmi B~jmcr/l)n-a.unuruc p88b: \lidL.tt'Jl939 Stockpholo: p90 : c.;.uy V,Ul.:t' SOt'UO' l'hOlu I lhr.uy. p9 I : I).; Id It l "r.17. r l'hotolihr.1 • ln r Al.101y !.tock Photo; P'JY : l'm1l Urabo(J~hnllM'\t t.: p 100 : 1'ttti10lkb) l)l<>d1>1J~o: pt 07: JtAfl fr.UK'O~ \lmu..• r,St nugl•rfAJllf(,('ll y IU14'K( pi 01}: \lilt• IHI Llw~., A'l.\tlC 1;\l t IPlkio,IN 1'<,t'lt)' llll "&C'' pint: Wotlcl lmlory Anlun.·/Al.tmy l k Ph~o: pt 1 w : \' tr;wcJ bt1lll·ntock: pl IJl,- "ngt•Dl bulll'nloc:l.: p114: Pt'll•rGuu)f~otJP: pt l -1: Ju\lm Pu01frl'yfll1c llll.i\f.{' lwlk.i(.t'lty IDl.lf.l"; pl r;na: KcrtU 'i~t L7>hoto; pl 1:;r. lli~t'ji~ kpboto: pi J'J : Auc1cr.cu-Ra. Getty lmag!.."~ pl24 : ~l.lri 07/ ~h11llt'NOt Ii: p l ZK : J.lnant' Wli'tk-1 Ph tolibl ry/AL: "''' !.toe Ii:
l'l1olo; plltJI: Antony Jon ,'< Olllributor,c:t-tty lo1.1r. pl.!'!Jr. A, pk'-.J AJ..\my \le IC t.. Photo. pJ :m: ,oAi-\ n•ulr.l ] I 1br.uy: 0 ,\RJFRIJ\~l lOIM_l !\c•vt'n~ \•uma-.. l..:ahor.uo t'-~~I OM l'hu,o I 1b1 p1 : w : \t t•tll11 TutUl" , llt'UC\.' Photo libraJ)': pl35 ; Monkey Bu.~lll , ; F toliA ; p139; lrtJf>IC""111~tug,q)Ol'\'1JStc.x I.J)boto: pl IOC · en,y1>lX/iSlod,pboto: pMOb: (\'tcr \kni:c~~CClC(' Pholo Libr.uy: pl41: \~ icnn- Plaoto lJbrary: pl42: Br.mli.lo lmllc~lcxt.: pt 46: Pl'tl"rGwk~' OUP: pM9r. Da, id ~I. \{, nm , :\ll )i\Ol ' lltt' l'IKIIO I tbr.uy pM9l ltl 16' \. l.>.wkl P.ITi:t'r, !\c "110" l11oto Libr.uy: p 1S 1: Royc1('('/ ~ loct phueo p 160: Joriwo)~lnrttc r.1ock : p1<.ll! Kr'-) ~11 , no•AnNmll' , pl<,th : Tc!Ul,IJ' 1\IO( l)h11co: pl(. l : A l)t ·-.V••: ~111111C'"IO(l.. p ,,., : (.r,IIV'' Alu hb.on/ 1ulOI&.:. p 1(,11 : P~•t•r \klll c\ CX'llCl" Pllolo Libr.uy. pl70&172: P :tcr ~ll'lll'-'~So,•tan• PltolO LJ.br.uy: p174 p17?: l\•ll"T Goul(~<)llP; pJ84; Aki~ 8.llocltJll.l'llll'r..: pl4J'.l: hdJoij.lnJ bulll'~loct ; pl '>J : ('l'lcrC..wk~Ol ': pl tJ7 : l.o'i Abmos Nauoual LaboralOl)'I ClC.'UC\.' Photo Library: pl 9-J : ScicuCt'pbot Al.lmy ''°' t l'hOCo p-Z00: ·~•er c.oul(~ )I IV- pl0 I ! nry;m t, C lK-11)' .Ak-x.rndcr l •h~or,raph)'/ Arctkphoco; p'.l.O:J: ~cit-u c cphoco,, /1\L1-my ~lock Photct p204 : Nl}',UM \\•11\Ur-'!'ft •l'UY lm..lgt • p:Z05: P1.•ll"r(.c,11J(I/ OUP; p207 !.c1,•1k i'pl1 hl-lo /A l;m• y !.Hk i.. Phurn: 1-,209 Pt••rr C.oukV OUP: p21 l ; ,\j.ty B~ktlJI lmttt•~tock p213; d c deb\fiSlod.,phcto : .,:z 1;,.r: .1uh ,...,J}' ¥Ll,l)' "'Dn•.un"w11.•: p21 ;1 b p216t · Pl•lt'I' C.ouJ,\OUP: p216r.1) lcr OlsortJ hultc~toct : p1 llC: J\ID71 tol~ · pUl : ~tcphm Mmk)crjl)rean"llnK': p'.l:JO: David Pa.rtcr, :i.c-k'u~ Photo Library: p231 : !.Cil'(l<(' Photo lihr.i ': p-Z: 17: ,1.man llon(~~irn,t' l'llOCO I 1hr;i • pl UC : u~ lkp.1rt01cnt or1:.t,c'Y.,V/~<'lln' l'hOCo l lbrary: p:Ll'J : (.c lp ¥ SI111lll""'101 p240: B.." P111u1~"(•NI llU~(•, C:ro111•!C •uy hU ~"\ ; p24 I : ,i.11n t)ul.l.:;i1 ~!.huHt·"u>tl::, t"l4:J : n-.1 K11hm~n , ~ ,rn n· ~c•an.-. l !.c11•1w ,-. Plloto LibraJ)•: p244: 0 2007 { at.'t p249; Ev(.'Ul lloriLou Te l••~c roll,dlC.lr,.tluu · pl51 ; julil•uTcllt 1~0t l..J>ht.Co · p?:>4t· ,A \; · 'IK'\' Photn Library. p2:>-1t ~C'UC"t' l 1bo10 Library • Victor Habbict VL'iion, 'Brancl X PKtun- Gl'lly ln•~~l..._~ p2jjl;Jcny l odrigu J:i.c-icuCt' Photo Libr.uy: p:Zur. l);ivill l);u-1.t-r/ k'll<'t' Ph~ o I lhr.uy: p:C.7 : !.c. tt'llC'r Photo llhr.t I) ': p15Hr: Audmb Utrl>il~ ~IUlllt"N t.: p£,7b: lll\'ld l~rt t' ~<'Irr Photo L1b1 .uy. pl :,9: C t'k"~I Ml h1 t.1gt• ( <¥S( k'U('(' Pl10111 Uhr,11 , p2',2 : [l.lv1d N11nt1~1!.rif.•1w ,, PIMIIO I 1b1 ;\fV {lU17: unLbn1hf 1S,oc 1...ph.oto: p2<)1ll l.dwn'uC\.'Sa"-)'\'ijl l kphotn; p2~8b: Tr,11r.port oC Dcbgblµ\l~IU) locl Plt w : p26C)t · Alo111Mlon",'Dn·,llmlJD) : Jl269h · m r, bulll'nlllC'l.: p:.t?O: SpulmtJ ClCUCt' P1lot.o Libr.tlj': p:011: Prol. Pt-ll"r fowler, l<'llCt' Photo LibraJ)•: p271b: a:R., IICUC\.' Pll~o library: p272l : 81}' .lll It C huty Alt'X,10C lt'r l'htAOf.l~phv/ Arct c photo, p:Z72b ~Wit~ \locli.:phot~ p:Z7.it L>orU nr. Kmd('r.lcy/l nc, /~ii.'ncc l'hot o lJbr.uy. p.l? Jb: Roo1.1n K1ucia11 ~h11UtT'\lo1 ._ p:Z74: 1,• 111; \' Pholo I al>rAI) , p275l Roy 1 A<ill'01.--n11c ;\l K IV/!.C' ac•aw i• PIMIIO I ihr.uy p:Z7"ih ,A.\A p277 : P,•tN \ll'UL c\ Ol'UCl" Photo LihrM)-: p278 & p279: 1\-ler GoukV<XJP
AltW o ct.: by: GrN'oG ll' l'Ublbhlug ~rvt Q.B~ l.L'.mllog t.i °' . f , IV I ISi~ l'l. l'N'n DlMlr en ('(1111 ¥"I, ori,Tf.111 ,•• •111-f', 41' OUI rnal n-pm.lllf'\"CI 1n lli~ l••IL Au nal6,i•~ ~Ul l~ h' (llllNI "dl1il't41J(1)l rnuat~ U OICJn• l, rJ\rO lo thf' p1hlhl1"r.
11\ ou an: tudying ph, ic~ forCJmbridge JGC E > , then lhi book i de\igned for\ ou. IC cxplai ns the concepts that you will meet, and should help \'OU \\ il h \'our practical work. It i mo!'lth \\Titten in double -page uni~ which we htl\e a lied !ipre ad s. Th~c are group--d into sections.
S e cti o n s I to J 1 The main areas or ph) ic arc covered here. t the end of each ol thi..: e cction there i a 1-c,i ion un1n1at') g ivin g the main topic!-. covered in each spread.
H is tory o f key id eas ection 12 describe ' how scicnti t have devdoped 1heir unden,ttmding or phy~i cs on:1· the \'ean,
Practi ca l ph ys i cs ection 13 te11 )OU how to p]an and ca1T)' out c,pcriments and interpret th,. 1-c uh It include uggc lion for invc tigations, and guidance on taking prncti ca l te t~ l\1 a th e m a ti c fo r ph ys ics ection 14 un1mmi:1~ the mnthcnialical kill you wilJ need \\hen stud\ ing ph)sics tor Cambridge IG E.
Exa min a ti o n qu es ti o n s The1-e are practice examination questions at the end ot each ection (1 to 11 ). Jn addition, ·ction 15 conlain a collection ol ome altetnatiYcto-practical que tion
R efere n ce sec ti o n ection 16 incJude e ential equation , unit of mea urement, ircuit )mhols, answers to questions, and an index
Core s y llabus content
Suppl ement s y llabus content 11you ttrc following the Con ·~) llabu:,; contl·nt.) ou can ignun.- ..my matc1ial with a rc<l Ii nc hl·sidt· it.
For thb, you n-..·cd all 1h-..· ma1crial on the while pages, indu<ling tht.• supplement matt·1ial markL-d ,, ith a red line.
The..• E nhanced Online Book supports thb student book h • olTc..•ring hii!h-quality digital resources that help lo hui]d scientific an<l l'xamination skills in prl.'par~uion fur thl' high-slakes !GCSE assessml.'nl. H you purchasca1:ccss to the digital cou1~c.~c>U will find a \\l.'ahh ofac.Jdi1ional rl.'soun:c..•s 10 hdp)OU with )O\ff stu<.Hc., ;m<l n.:\·ision:
• A \\ork...,fu:l.'t anc.l intcracti\t.' quiz frn·L'\L'I)' unil
• On Your Mark:-, activitic to hdp )<>U ~chic,c )OUI" ~st
• Glossary quizzes to consolid:ne , ow · und..:1 landing of scientific 1tcrmino]ogy
• Full prac tkc papc1'.S \\ it h mark schemes
Each pe~on ha their own way of working, hul the lollo,, ing tip might help you to ge l the most from 1his book:
• U e the co ntent · page - thi will pro\'ide infonnation on large topic
• u~e lhe index - thi wiJI ollow you to u~e a single woa-d to dkect vou to page \\ here you can find out more.
• Use the que~tions - thi · is the be t way of checking,, hether you ha\'e le~u-ned nnd understood Lhe n1aLe1ial on ~jch spread.
Oucslions arc to be lound on mosl units and wiLhin or at the end of each seccion.
Harder que lion are identified by the blue circle.
• \ Vatch for this~, mbol, bclo,, and throughout the book. It indical<.. spreads or parts of spn:a<ls that ha\'c been included lo pro\'ide cxlen~ion material to set phy~ics in a broader context.
For inionnation about the link bct\\' ccn prcad and the \llnbu , ·cc page vii - x.
Syllabus and spreads
Measurements and units
1.1 Numbers and units
1.2 A system of units
1.3 Measuring Length and time
1.5 Measuring volume and density
Waves and sounds
Magnets and currents
Below, is an outline ot the Cambridge l GC Es~ llabu~ as il tood at the time of puhJi ca tion, alon g ,,ith detail · of where each topi c i~ co vered in the hook Befo re con tructing a tea c hing or rcvi ion pro g ran1mc, pica c c heck with the late t ver ion of the s, llabu s/~pccificat ion fo1 · any changes.
Assessment for IGCSE
The ICC E exam i nati o n will induck questions that Lesl you in lh1~c diITcn:nt ,, a, s . Thes e a rc c alled A scssmcnt Objec ti,c s ( AO for short ). Ho\\ these difkl'ent AOs are te ted in the c:..aminati o n i · c,pl~,ined in the table belo w:
and prob·
Experi mental sblls and investi· gations
Questions which mainly test your recall (and unde.-standing) of what you ha\'e learned About 50% of the marks in the examination are fOJ AOl.
Using what you have learned in unfamiliar situations. These questions often ask )'OU to exarrune data in graphs oc tables, or to carry out cakulations. About 30% of the ma s are for A02.
These are tested on the Practical Paper or the Alternative to Practi cal (20% of the total marks) However. the skills you develop in practising for these papers may be valuable in handling questions on the theory papers
The c n<l -o f-M~c ti o n qucMi o ns in thi s b o ok include c'\'.amplcs of th ose testin g AO I , A02 an<l A03 . Yo ur LL'a c hcr will help yo u lo attempt quC!,lion~ of all t~ pc Yo u c an cc fro m the ab o \'c table that it will n o t be en o u g h t o try o nl) ' re c all ' QUL~ti o n
All c andidate~ take lhrcc papers
The niak c -up o f e a c h m, c m c nt progr a mnic i ho wn below :
Core assessment
Quc ti o n arc ba ed o n Co re co ntent
Paper 1: Multiple Choice (Core), 45 mins
There are a total of 40 marts available, worth 30% of yout iGCSE. The paper consists of multiple-choke questions.
Extended assessment
Paper 3: Theory (Core), I hour 15 mins
There are a total of 80 marh avaiable, worth 50% of your IGCSE. The paper consists of compu~ory short-answer and suuctured questions.
Questi o ns arc based on the Core and upplemcnl s ub j ec t c ontent.
Paper 2. Multiple Cno:ce (Ell.tended). 45 mIns
There are a total of 40 marls available, worth 30% of your IGCSE. The paper consists of multiple-choice questions.
Practical assessment
Pape, 4 lheory (b:tended). I hour 1~ m,ns
There are a total of 80 marls avaiable, worth 50% of your IGCSE. The paper consists of compu~ory shon-aM•Ner and structured questions.
Lu dents take ei the,- Paper - or Paper 6.
Paper 5: Practical Tests. 1 hour 15 mins
There are a total of 40 marls available, worth 20% of your IGCSE. You will be required 10 do e:q:,eriments in a lab as part of the assessment.
Paper ,6: Ahemative to Practical, I hoUJ 15 mins
lhere are a total of 40 mar ks avaiable, worth 20% of your IGCSE. You v.;o NOT be required to do experments in a lab as part of the assessment.
An a~tronomical clock in Prague, in the C.1.ech Republic. A ,veil a · giving the tin1c, the clock al o sho\\ the position ~ of the Sun and Moon relat i\'e to the con tellation~ of the Lodiac. Until about fifty year ago. ' Cienti t had to reh on n1~chanical locks, ~uch a , the on above, to n1ea ur tit11e. Today, they have accc s to ato1nic clock ,vho ' c tin1ekeeping varie ~ by less than a s cond in a n1illion \Cal
- 10 m-------------
/ \ ourr ber uni t (m 1s the ~yrnbol for metre)
\Vhcn )OU make a 1ncasurcmcnt, you mighr gd a n..~ull like the one abo,c: a di~tancc of 10 m. The complete mca~urcmcnt is called a physical quantit 1L i n1adc up o f two part : a number and a w1it.
10 m really mean · 10 x 111 (ten time · metre). just a in algebra, 1Q\· n1cans 10 x x (ten tim~x) You can treat them ju t like a , ,nbol in an algebraic equation. Thi is important when combining unit
Advanced units
5 m/s 1s a space-saving way of writing 5 ~ . m 1 But 5 sequals S ms.
Also, { can be wntten as s- 1 • So the speed can be written as S ms- 1 • This method of showing units is mo,e common in advanced work
Combining units
In l he diagram abon?, the girl cycle!-. 10 met n!!-. in 2 s. So she l r..1.vcls 5 mctn.:s c, c~ second. Her .\p.!ed L') S metres ~r second. To work out the spet.·d, you divide the <liMancc Lr'Jvclkd by Lhc time taken, like Lhis:
spcl.'<-1 : 1O m ( · is the s~ rnbol (or econd) 2 s
A · m and · can be treated as algebraic ymbols: peed = .!Q . m - S m , s...,. - n, . II . - / ,o save space, - 1s u ua y wnttcn as ms.
o m /s is Lhc unic of spcc<l.
Rights and wrongs
Tables and graphs
You mc1y see table headings or graph axes labelled hke this: cfiS t aoce or distance/m m
That is because the values shown are just numbers, Vv'ithout units So If distance - 1O m Then distance _ 10 m
Thi equation i correct:
Thi equation b incorn:ct: pcedspeed : 10 m _ 5 m / 2 s 10 - m / 2
It h, incorr~ct because th e m and h,wl.! been left out. I 0 divided hy 2 equals, and noL - m /s.
t,;crly ~peaking, units should be indu<lcd at all stages of a calculat ion, nol just al the end. However, in thi~ book, the ·incorn..-ct' t~ pc of cquatjon "iU somL•Cimes be used so Lhat )OU can follow Lhe arithmetic without unit \\hich make the calcu lation l ook more complicated.
Bigger and smaUler
You can mak .. a unit bigger or mallcr bv putting an e-..:tra vmbol, caJlcd a prefh, in front. (Below, \ \' tand for watt, a unit of powc1: ) prefix
G (919a) 1000000000 (109) GW (gigawatt)
M (mega) 1 000000 (10 6) MW (megawatt)
k (kao) 1000 (10 3) km (kilometre)
d (dec1) 1 ( 10 1) dm (deci metre)10
c (ceot1) 1 (10 2) cm (centimetre) 100
m (milli) 1 ( 10 3) mm ( rrnllimetre) 1000
p (rrucro) 1 (10 6> µW (micrO\vatt) 1000000
n ( nano) 1 ( 10 9) nm (nanometre) 1 000000000
Scientific notation
A n ada~ says that the population of Jee land i~ this : 320000
There ill'\: two probh:ms "ith gi\ing the number in this form \ \'riling lots of LCl'O i n't \Cl"_\ con,enient. Al o, )OU don't knO\\ \\hich Lero arc accurate. Mo l arc onh there to ho\\ ,ou that it i a ix-figure number: Thc~c prob]cn1~ arc avoided if t lr" nu mbcr i \\Ti Hen u ing powc, of ten:
3 2x 10 i; (10~ ::; t 0x I0xl0x l 0x 10 = 100000)
'3 2 x I Or;, tells~ ou that the figurl.!S 3 an<l 2 are in1portanl. The number i being given to 1wo ~ig ni{icmll figure:... If the population \\Cl~ kno\\n rno1 accuratch, to three ignificant figure , it might be \\rittc-n like d1i :
3.20 X l Or;
Kun1be1 wriuen u in g powcl'~ of ten arc in cicntific notation or standard form The cxamp1c!'I on the ,;ght arc to one ~ignificant figure.
'm1lh' means 'thousandth', not ·m1lhonth'
• You would not normally be tested on micro, nano or g1ga 1n d Cambridge IGCSE exarrunat,on (see also yellow pa, el at the start of the next spread. 1 2)
1 How many grams are there in 1 kilogram?
2 How many millimetres are there in l metre?
3 How many microseconds are there in 1 second?
4 This equation is used to work out the area of a rectangle : area - length x width . If a rectangle measures x m by 2 m, calculate its area, and include tt e units in your calculation .
5 Write down the following in km :
m 200 m 2 x 10 4 rn
6 Wnte dov\fn the following in s: 5000 ms S x 107µs
7 Using scientific notation. write down the following to two signifacant figures :
A lme down the side of the text means that the material as only required for Extended Level
An asterisk 1nd cates extension material. proVlded to set physKs in a broader context. You v,ould not normally be tested oo this an a CA E !GCSE examination
.& The mass of an object can be found using a b al ance hke t his. The balance really detects the gravi tational pull on the object on the p an, but t he scale 1s ma rk ed to show t he mass
There are many different unils inducling those above . Bue in s c ientific work , life b much ca':)ic1· if C\'cryonc u c~ a common ' ) ·tcm o l units.
SI units
Mo t ' dcnti ts u c S I unit s ( full name: u: y teme lnte1national d'Uniles)
Thl" ba~ic l unib for inca ·udng mass, time , and len gth arc the kilo g ram, the econd , and the metre. Fro n1 th~c base uni t c ome a whole range of unit for measurin g \ 'Olumc , peed, fo1 e , ene1'S', and other quantitie
Other f base unit · include the amp~rc ( for measuring electric current ) and the kch in ( for measurin g tcmpcr~llure)
Mass
Ma i a mclli>urc of the quantity o f maucr in an object. ll h~ two cficc t
• 11 object ar ~ attra c te d to the art h The g reater the n1a of an objec t , the stro ng er i the a11h ' gra\'itutional pull on it.
• All o biec ts resisl being made to go lru,tcr, slower~ or in a different din: lion . The g rt!attc>r the mass, Lhc g l"\!aler the n.-si..,.lanc c lo c han g L' in motion
The I base unit of mas · is the k'il ogrn n1 ( ;ymbol k g ). At one time , the !-.tanc.lard kilogram was a hloc k of platinum allo~ s tored in Pad~ However. Lhcn: is now a more m.: curatc buL ,non: compli atcd dl:finition invoh in g an electromagnetic balance. Other unit baM:<l on the IJlog ram arc ·ho\\ n below.
com_pa rison ,with scientific base unit notation approximate size
bag of ~ugar
Time
The Iba c unit of time i the ccond ( \ n1bol ). Here at~ on1c shorter unit ba~cd on the ccond: l milli~ccond ( m~) =
l microsL-cond (µs) =
l nanosecond (ns) -
To keep Liml!, clocks and watches need somcLhing that beats at a stead~ rate. Some old dock.-.. used Lhe s\\ ing · of a pendulum. Modern di gital \\~1tchc count the \'ibration · made b) a Liny quartz C l') tal.
Length
The l ba.-;c unit o f len gth is the metre ( ·ymbol m ). At one time, the tandard met,~ was the di tance bctwcc:n t\\o mark on a metal bar kept at the Office of \\ 'eight and Mca tu in Pari~ A mo1 accurate tandard i now u ed, ba c.'Ci on the pced of light, a c"plaincd on the right.
There are la rger and smaller uniL~ of length based cm the metre: distance comparison w i th base unit scientific notation approximate s i2e
The second wa s originally
defined as 60 x 6~ x 24 of a day, one dill being the t ime i t takes the Earth to rotate once. But the Earth4s rotat ion is not quite constant. So, for accuracy, the second is now defined in terms of something that never changes: the frequency of an osdlatt00 which can occur in the nucleus of a caesium atom.
By definition. one metre is the distance travelled by light in a vacuum in 1
299 792 458 of a secood .
1 What is the SI unit of length?
2 What is the SI unit of mass?
3 What is the SI unit of time?
4 What do the following symbols stand for? g mg t µm ms
5 Write down the value of a 1564 mm in m b 1750 g in kg
c 26 t in kg d 62 in s
e 3 65 x 104 gin g f 6 16 x 10 7 mm in m
6 The 500 pages of a book have a mass of 2 50 kg
What is the ma~ of each page a in kg b i n mg? 7 km pg µm t nm kg rn ms s mg ns µs g mrn
Arrange the above units in three columns as below The units in each column should be in order, with the largest at the top mass time
• If the rule cannot be placed next to the object being measured, calipers can be used
Measuring length
Lc-n g th from a few mill metre up t o a metre can be mea ured u ing a rule , n~ ~hown abo\'e. \ hen t1 in g the rule, the , ca le :-.hould be placed right next to the ohje t bci ng mca~un:d. If thi~ i~ not po ~ihlc, calipers can he u:-,ed, a:-, sho\\n on lhc left. he calipcrs arc ~et so that their point:-, c:\m::tl) mat c h 1he enc.ls of the ohjc<.:l. Then the~ arc moved across to a rule to make the measurement.
E Lt!n gl hs of sc\·crc1l mctn.""i ca n he nlcasun."<.I u~ing a tape with a :i,,calc on it.
Acc: uratd, nw.._, uring 1nall objl·Cts is mo1\..' difficult, but thct'-! arc wa)S around the problem. a,, for example, \OU wanted 10 find the thicknt: ol a heet of A4 paper.
l"sc a ruh:1- to mca~urc the 1hickncs~ of a -oo sheet pa ck: 49 mm
Di, idin g 49 n1m by 500 g i\ l.~ the thic:kne~~ of one sheet: 0.09 mm
Measuring Length with light
0Surveyors don't need a tape to mea5ure the dimensions of a room They can ~e a laser tape measure instead . Despite its name, no tape is involved . The surveyor places the instrument against one wall, points it at the opposate wall, presses a button, and reads the distance on the display
There are various systems, but in one type, the instrument frres a pulse of laser light at the opposite wall, picks up the reflection, measures the time delay between the outgoing and returning pulses and uses this to calculate the distance light travels at a speed dose to 300 000 000 metres p~r second. So. for example. if the pulse had to travel 30 metres out and back, it would take 100 nanoseconds If this were the time measured, the display would show a distance of 15 metres (In this example, the numbers have been simplified Typically, the instrument is accurate to within 3 mm.)
Measuring time
Tin1c int e r va l o f m a n ) cco nd or minut e ca n b e n 1ea ~u re d u in g a
topclock o r a stopwatch . So n1 e in tn1m c nt h a \'C a n analogue di s pl a ~ wilh a n eedl e (' h a nd ') m o \ in g r o und a ci 1c ul ar ~ca lc. Oth e r~
ha\'C a digital di s pl a ~ . whi c h s h o w s a numbe r. Ther e an.: butto n~ fo r s ta r lin g the timin g, s to ppin g it , a nc..l n :~e llin g th e im,trum e nt to z e ro.
\ ith a h a nd -o pera ted topdo k or to p wa tc h , ma kin g acc u r at e
m ca~ure m c nt o f ho n tin1 c int c 1, a l!- (a re,\ ~eco n ds or le- ) ca n be diffi c ult. Thi i!-. bt.'Ca u ~ of th e tim e it ta ke · vo u t o 1 "ac t wh e n , ·ou ha ve to p n,;.~._. th e hult o n . F ortun a ld~ . in so m e ex perime nt""', th en: is a ~impl e w a ~ o f o verco min g th e p ro blem. H ere i ~ a n e xa mpl e:
ngio support _____
A pen du lum can be set up to invest igate the time taken for a si ngl e swi ng
!>tnng--------1 simple pendulum bob--(sma!I m~~s) 0 one complete swing
Measurin g t he t ime t i t tak es for a steel ball to fa ll a d ista nce h
Th e p c ndulun1 a b o ve t a k e a b o ut tw o eco nd t o n1 a k e o n e co mpl e t e
J,. win g. Pr o dd e d th e ~win gs a n: J,. m a lJ , eve ~ s win g t a k e~ th e sa m e tim e . Thi~ tim e i ~ c all e d its period You ca n find it ac ur a tcl~ b y m ca,uring the tinlc fo r 2- ~win g ~ . a nd th e n di, ·ic..lin g the r es ult b} 2 5. Fo 1· e x a mpl e:
Tim e rO J' 25 ' \\ in g · • s seco nd l)
o: tim e for 1 win g - 512 5 e o nd i,. - 2 2 cco nd ~
An o th er m e thod o f imp n >\ in g acc u rac , i~ to u.,c a ut o m a ti c t iming, a, s ho wn in the x a mpl c o n th e rig ht. H e~. th e tim e La ken for a ~mall o bjec l t o fall a s ho rt <lb La.nce i · be ing n1e ~t..') Ut ~ d The lim e r j ~ s tait cd a ut o m a ti c ally ,, h e n the ball c ut o ne Ji g ht bea m a nd ·to pp..-d when it c ut a no thet:
1 On the opposite page, there is a diagram of a rule .
a What is the ,eading on its scale?
b The rule has not be drawn to ,ts true s,ze What is the length of the red line as printed?
2 A student measures the time taken for 20 swings of a pendulum. He finds that the tune taken in 46 seconds
a What trme does the pendulum take for one swmg?
b How could t e student have found the time for one
Zero error
You have to allow for th is on many measu ri ng instruments
For example, bathroom scal es might give a read ing of 46 .2 kg when someone stands on them. but 0 1 g Vi/hen they step off and the expected read ing is zero In th is case, the zero error is 0 1 g and the cOC'rected measurement is 46 1 kg h
3 A student wants to find the thickness of one page of this book
a Explain how she might do this accurately
b Measure this book and then hod your own value for the thickness of one page .
4 a What ,s meant by zero ,error?
b Grve an example of when you would have lo allow '°' it. swing more accurately?
Volume
The qunntity of space an object take · up i · called it volume.
The l unit of , ·o]umc is the cubi c m e tre ( m 3 ) Howe\'cr, thi~ i~ rather large tor evcr)da. work, ~o other units an~ often used for con,l:nicnce, a ho,, n in the diagram below:
Litre (1 or L)
Note: the symbol I for Mre can be confused ,...,th a 1 (one)
- 1000 cubic centimetres (c.m3> 1000 m1lhl1tres (ml)
H>OO litres <I)
1 rubic ll'lClt(I (m ) ts 1e volume of a cube measur119 1 m 1 m x I m
Density
1 ~tie 1s the same vo ume as t <ub c deometre (d m3)
A The glowmg gas in the ta il of a comet stretches for millions of kilometres behind the comet's core. The density of the gas is less than a ilogram per cubic kilometre.
1 cubic cent11T1etre (cm 3) 1s t he v01\Jme of a cube measur ng 1 cm 1 cm It ~ the same vol 1me as 1 mBlihtre (ml)
1 lead heavier than ,,atcr? l\ot ncces aaily. It dcpcn~ on rhc voluml: oJ lead and water being compa1\.=-d. I loweYe1; lead i n1ore dense than water: it ha!-, more kilograms packed into e, ery cubic metre.
The dens i ty of a material is cakulated like thi l ma-..-.. <. l•ll~ll\'n>)umt.:
In the case of ,,ater:
a ma~s of I 000 kg of water has a \'Olumc ot I m 3
a ma~~ of 2000 kg of water has a volume of 2 n1'
a mlli> ol 3000 kg of water htU> a \'olumc oi 3 n1', an<l o on.
sing any of Lhc c set~ of figu1 ·c in the above equation, the den ·ity o water\\ ork out to I 000 kg / m 3 •
H are n1easured in gran,~ (g) and volume in cubic centimetre (c m3 ), it i simple1· Lo calcula1c den iti~ in g/c m '. Con\ ~rti ng to kg/m is ea!-.):
1 g/ m 3 = I 000 kgtin 3
The dtmsily of water is l g/c m ' . This simple \aluc is no accident. The kilogram ( l 000 g) was originaU) supposed to be the ma ·s of J 000 cn1 1 of
walc r ( pure , and at 4 °C ). H O\\ t.:\ 'CC a \'Cl''\' li g ht ctTo r ,, a n1adc in lhc carh mca~ure m c nt , o t h i i n o lo nge r ttM!d a~
1,vood (beech)
pe t rol
(0 °C)
thene
water (4 °C)
t e
(varies)
Density calculations
0.92 lead
0 95 mercury
1.0 gold
T he cquali o n linking density, mas ~ and vo lume can be wrill c n in ~)mbol s: I' Ill \I dcn_s ity, 111 = ma s.s. and V = \' olun l c
Thi equatio n can be rcan~1 gl-d l o g ive : Ill I' and Ill
The:,c an: u dul if the den il~ b kn o \\n , but the , o lumc or rna · · i 10 be c alculated On the 1i g h t i a n1eth o d o l findin g all t h ree equati o n
Emmpll.' u~i ng dc n ~i tv d a t~• from th e t~, b lc a b o , ·c. caku kt tc th e nmss o l ~t.:"-•I hav in g th e s.un c , o lu mc as~ 400 kg of a lum i n ium .
Fin-it , c alcul a te the \ ·olumc of - 400 k g o f a lun1inium . l n this c a s e ,
p i s 2700 k glm ', m i s 5400 kg, a nd Vi ~ t o be fo und o :
V = !E. 5 400 kg , P 2700 kg/ m ' · - m ·
Thi i al o the v o lum e o f th e t c el. Th e r e fo re , fo r the tccl , p i 78 00 kg/ n1 1, \/ i~ 2 111 '. and 111 i t o b ' fo und . So:
m = V p 7 800 kg/ m ' x 2 m ' I - 600 kg
S o the m a ss o f ~led is 15 600 k g.
1 How many cm 3 are there in l m3 ?
2 How many cm 3 are there in 1 litre?
3 How many ml are t here in l m3 ?
4 A tankful of liqui d has a volume of 0 2 m3 • Wha t i s
t he volume in a li tr es b cm 3 c ml?
5 Alum inium has a density of 2700 kgtm 3
a Wha t is the density in g/cm 3?
b Wha t is t he mass of 20 cm 3 o f aluminium?
c Wha t is the volume of 27 g o f aluminium?
Related topics: pressure 1n liquids 3 6
The densities of solids and liquids vary slightly with temperature Most substances get a httle bigger when heated lhe increase 1n volume reduces the density. lhe densities of gases can vary enormously depending on how compressed they are
The rare metal osmium is the densest substance found on Earth. If this book were made of osmium, it would weigh as much as a heavy suitcase.
A Cover V in the triangle and you can see what Vis equal to. It works form and /I as well.
In the density equa tion,
t he symbol p is the Greek le tter 'rho'
Use the informa tion in t he table of densi ties at t ~e top of the page t o answer t e following :
6 What ma terial, of mass 39 g. has a volume of 5 cm 3?
7 Wha t is the mass o f air in a room measuring 5 m x 2 m x 3 m?
8 Wha t is t he volume of a stOJage tank which w ill hol d 3200 kg of pe t rol?
9 What mass of lead has the same volume as 1600 kg of pe t rol?
<; 1000 cm> measuring cyt1nde,
- - 1-- levEI Of'I sca1e g,...es volume of hqutd .
.& tvteasuring th e volume of a liquid
c:; 1000 cml IOOOcnr .
asuring volumP
Liquid A \' olume of about a litre or ·o can be mca ·urcd u i ng a measurin g cylinder. \ \'hen the liquid i._, poured into the cy linder, the le\ don the s c ale g ives the volume.
1o t mca::,urin g cy linch:-1"' have ' Cale- · rnarkcd in millilitres ( ml), or c ubi centimctr~ (c m ').
Regular s olid Han object ha!> a simple hapc, it · volume ca n be calculated. For example:
volume of a re c tan g ular block len g th x width x hei g ht volume of a c~ tinder n X radius 2 X hei g ht
Irreg ular solid l f the s hape is 100 awkward for the \ ·olume to be calc ulated, the olid ca n bl" lowered into a parll~ filled mca udn g cy linder a ho\\ n o n the lclt. The ri e in level o n the ,olume calc gh c the volume o f the ~olid.
If the ~olid noaL,, it can be wei g hl.!d down with a lump of metal. Thi.! total volume is found The volun1c of the metal is n1casured in a separate ex periment and then ubtra c ted lrom this total.
.& Measurmg the volume of a small solid
Usin g a displ a cement can lf the sol id is too bi g for a measurin g cy linder, its \'Olun1c can be lound using a di~pla cmcnt can, shO\\ n below left First. the c an i~ filled up to the lc\'cl of 1hc spout (thi · is done b · o, criilling it, and then waiting for the urplu water t o run out) Then the olid i lowl) lowered into the wate1: The o lid i n ow takin g up pace once occupied by th e water - in other w ord~. it has di~pla ced it · own volume of water. The di s pla ced water i co llected in a be~1ker and emptied into a mca~urin g c..-y lin<lcr·.
The displa cc mcnl method , so the story goc , was di sco \C1\:d by accident, b, A1 himcdc . You ca n find out how on the oppo itc pa g e .
Measuring dens ·ty
The density of a material ca n be found by calc ulation, once thl.! volume and mass han! been measured The ma~s of a ~mall solid or of a liquid can be mca-;urcd usin g a balance. Howcve1: in the case of a liquid, you must remember to allow for the ma of it~ co 1uainl·1:
.& Using a dispfacement can
Provided the can is filled to the spout at the start. the volume of water collected in the beaker 1s equal to the voJume of the object lowered into the can.
Here arc some r~adin gs from an experiment to find the density of a liquid : vol«Y".,...! of Liqt-tid t.'I. ~as1,o L,~ Ct:jlt~~r = 00 c:,1.. ( A ) ~• Q.SS of '"""-a.;v.ri~ er;li ,-..der == 2 ,,; o 0 ( B ) w.nss of ..~o.~.ot.v..g c~ti ,W.!r Vitt 1 LUli.tt.0. tv.. = r;w !3 (C)
The r efore: ma s. of liquid - 560 g 240 g - 320 g ( C - B ) Therefore density of liquid = ma320 g - 0 g/c m 3 volume · 400 c n1 ' - ·
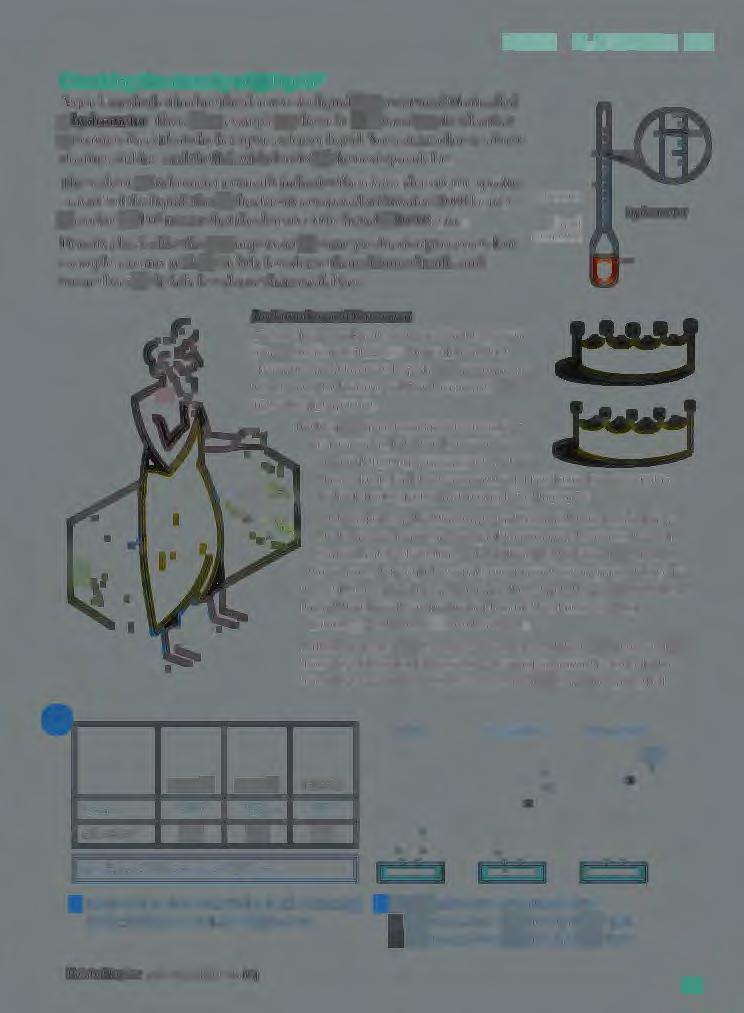
Checking the density of a t·quid*
A q uic k m e tho d o f findin g th e d e n it\ o t a liquid it lo me a mall fl oa t ca lled a h ydrometer. Th e r e b a n e '- a n1p lc o n th e ri g ht. It i ~ bn~ o n th e id ea th a t
a Ro utin g o b ject fl oa ts hi gh e r up in a chm sc r liquid Yo u ca n • c a d m ore ab o uL
fl o alin g, inkin g, a nc.l thl' link \\ilh dcnsil\ in th e n l! xt sprt!ad, 1.6 .
Th e c.: alc o n a h~ dro md e l' no n ,1all) indicate the relmit-11 d e ns it y (o r ' ·pcci [k
g r a , it\ ') o f the liquid: thar i th e d e n H, co mpar e<l \\1th wat er ( I 0 00 kg/ m ').
A r ea din g of l.0 5 mea n th a l th e d ~1i.s it , o f th e liquid i 10·0 kg/m 3 •
D e n ity c hec k lik e thi s a r e im po rt a nt in so m l! pro du c ti o n proces e~. Fo r
c xa mpl t!, c rt! a m~ milk is !--.li g htl, l~s den s e Lhan s kimm e d milk , and s lro n g b e e r is slig hth le ~~ d c n st.· th a n ,, e ak bee r
Archimedes and the crown
Archimedes, a Greek mathemat.cian. lived in Syracuse (now 1n Sicily) around 250 BCE. He made important d1scovenes aboul levers and liquids. but 1s probabty best remembered for his clever solution to a problem set him by the King of Syracuse.
The K1"9 had given his goldsmith some gold to make a crown. But when the aown was delivered, the King was suspicious Perhaps the goldsmith had stolen some of the gold and mixed in cheaper silver instead. The King asked Archimedes to test the crown . Archimedes knew that the crown was the correct mass. He also knew that silver ""as less dense than gold. So a cro..,,m with silver in i t \l\'Ould have a greater volume than it should have. But hO\v could he measure the volume? Stepping in to his bath one day. so the story goes, Archimedes noticed the rise 1n water level. Here was the answer! He was so excited that he lept from his bath and ran naked through the streets, shouting "Eureka!", which means "I have found rt!".
Later. Archimedes put the crown in a container of water and measured the rise in level. Then he d id the same with an equal mass of pure gold . The rise m level was different. So the crown could not have been pure gold.
1 Use the information above to decide which crO"Nn 1s gold, which as salver, and which 1s a mixture
2
Density essentials
. mass density = volume
A simple beam balance