Biopolymer-basedFormulations:BiomedicalandFood Applications1stEditionKunalPal(Editor)
https://ebookmass.com/product/biopolymer-based-formulationsbiomedical-and-food-applications-1st-edition-kunal-paleditor/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Biopolymer-Based Food Packaging: Innovations and Technology Santosh Kumar
https://ebookmass.com/product/biopolymer-based-food-packaginginnovations-and-technology-santosh-kumar/
ebookmass.com
Biopolymer Membranes and Films: Health, Food, Environment, and Energy Applications 1st Edition Mariana Agostini De Moraes (Editor)
https://ebookmass.com/product/biopolymer-membranes-and-films-healthfood-environment-and-energy-applications-1st-edition-mariana-agostinide-moraes-editor/
ebookmass.com
Vibrational Spectroscopy Applications in Biomedical, Pharmaceutical and Food Sciences 1st Edition Andrei A. Bunaciu
https://ebookmass.com/product/vibrational-spectroscopy-applicationsin-biomedical-pharmaceutical-and-food-sciences-1st-edition-andrei-abunaciu/
ebookmass.com
Opera in the Tropics: Music and Theater in Early Modern Brazil Rogerio Budasz
https://ebookmass.com/product/opera-in-the-tropics-music-and-theaterin-early-modern-brazil-rogerio-budasz/
ebookmass.com
Vicarious Narratives: A Literary History of Sympathy
Jeanne M. Britton
https://ebookmass.com/product/vicarious-narratives-a-literary-historyof-sympathy-jeanne-m-britton/
ebookmass.com
The Science of Psychology: An Appreciative View 5th Edition Laura A. King
https://ebookmass.com/product/the-science-of-psychology-anappreciative-view-5th-edition-laura-a-king/
ebookmass.com
The Lean Strategy: Using Lean to Create Competitive Advantage, Unleash Innovation, and Deliver Sustainable Growth 1st Edition Daniel Jones
https://ebookmass.com/product/the-lean-strategy-using-lean-to-createcompetitive-advantage-unleash-innovation-and-deliver-sustainablegrowth-1st-edition-daniel-jones/ ebookmass.com
Classroom Management in Teacher Education Programs 1st Edition Jonathan Ryan Davis (Auth.)
https://ebookmass.com/product/classroom-management-in-teachereducation-programs-1st-edition-jonathan-ryan-davis-auth/
ebookmass.com
Textbook of Biochemistry with Clinical Correlations, 7th Edition 7th Edition, (Ebook PDF)
https://ebookmass.com/product/textbook-of-biochemistry-with-clinicalcorrelations-7th-edition-7th-edition-ebook-pdf/
ebookmass.com
The Next Step Forward in Guided Reading: An Assess Decide Guide Framework for Supporting Every Reader (Ebook PDF)
https://ebookmass.com/product/the-next-step-forward-in-guided-readingan-assess-decide-guide-framework-for-supporting-every-reader-ebookpdf/ ebookmass.com
Editedby
KunalPal
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright © 2020ElsevierInc.Allrightsreserved
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationor methodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomthey haveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeany liabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceor otherwise,orfromanyuseoroperationofanymethods,products,instructions,orideascontainedinthe materialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-816897-4
ForinformationonallElsevierpublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionEditor: EdwardPayne
EditorialProjectManager: JohnLeonard
ProductionProjectManager: R.VijayBharath
CoverDesigner: GregHarris
TypesetbyTNQTechnologies
Contributors
SaumyaAgarwal
DepartmentofFoodProcessEngineering,NITRourkela,Rourkela,Odisha,India
JasimAhmed
FoodandNutritionProgram,EnvironmentandLifeSciencesResearchCenter,KuwaitInstitute forScientificResearch,Safat,Kuwait
B.Amulyasai
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
C.Anandharamakrishnan
ComputationalModelingandNanoScaleProcessingUnit,IndianInstituteofFoodProcessing Technology(IIFPT),MinistryofFoodProcessingIndustries,GovernmentofIndia,Thanjavur, India
ArfatAnis
DepartmentofChemicalEngineering,KingSaudUniversity,Riyadh,SaudiArabia
MuhammadArshad
DepartmentofAgricultural,FoodandNutritionalScience,UniversityofAlberta,Edmonton,AB, Canada
IndranilBanerjee
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
ManavBandhuBera
DepartmentofFoodEngineeringandTechnology,SantLongowalInstituteofEngineeringand Technology,Longowal,Sangrur,Punjab,India
RakeshBhaskar
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
PravinBhattarai
DepartmentofBiomedicalEngineering,CollegeofEngineering,PekingUniversity,Beijing, Haidian,China
NisaraniBishoyi
DepartmentofChemistry,NITRourkela,Rourkela,Odisha,India
PrasantaKumarBiswas
DepartmentofFoodTechnologyandBiochemicalEngineering,JadavpurUniversity,Kolkata, WestBengal,India
RaginiGBodade
DepartmentofMicrobiology,SavitribaiPhulePuneUniversity,Pune,Maharashtra,India
AnandGBodade
DepartmentofTransfusionMedicine,SethGSMedicalCollegeandKEMHospital,Mumbai, Maharashtra,India
SubhadeepBose
DepartmentofFoodTechnologyandBiochemicalEngineering,JadavpurUniversity,Kolkata, WestBengal,India
MuhammedYusufC¸aglar
IstanbulSabahattinZaimUniversity,FacultyofEngineeringandNaturalSciences,Department ofFoodEngineering, _ Istanbul,Turkey
MustafaC¸avu¸ s
IgdırUniversity,EngineeringFaculty,DepartmentofFoodEngineering,Igdır,Turkey
JiansheChen
SchoolofFoodScience,UniversityofIdaho,Moscow,ID,UnitedStates
HarishKumarChopra
DepartmentofChemistry,SantLongowalInstituteofEngineeringandTechnology,Longowal, Sangrur,Punjab,India
DonghwaChung
FoodTechnologyMajor,GraduateSchoolofInternationalAgriculturalTechnology,Instituteof GreenBioScienceandTechnology,SeoulNationalUniversity,Pyeongchang,Gangwon, RepublicofKorea
GetaDavid
Gh.AsachiTechnicalUniversityofIasi,Iasi,Romania
RajDeb DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
MehmetDemirci
IstanbulSabahattinZaimUniversity,FacultyofEngineeringandNaturalSciences,Department ofFoodEngineering, _ Istanbul,Turkey
Win-PingDeng
SchoolofDentistry,CollegeofOralMedicine,TaipeiMedicalUniversity,Taipei,Taiwan;Stem CellResearchCenter,CollegeofOralMedicine,TaipeiMedicalUniversity,Taipei,Taiwan; GraduateInstituteofBasicScience,FuJenCatholicUniversity,NewTaipeiCity,Taiwan
ChandaVilasDhumal
DepartmentofFoodProcessEngineering,NITRourkela,Rourkela,Odisha,India
NavneetKumarDubey
SchoolofDentistry,CollegeofOralMedicine,TaipeiMedicalUniversity,Taipei,Taiwan;Stem CellResearchCenter,CollegeofOralMedicine,TaipeiMedicalUniversity,Taipei,Taiwan
RajniDubey
InstituteofFoodScienceandTechnology,NationalTaiwanUniversity,Taipei,Taiwan
SayantaniDutta
ComputationalModelingandNanoScaleProcessingUnit,IndianInstituteofFoodProcessing Technology(IIFPT),MinistryofFoodProcessingIndustries,GovernmentofIndia,Thanjavur, India
RimpiFoujdar
DepartmentofFoodEngineeringandTechnology,SantLongowalInstituteofEngineeringand Technology,Longowal,Sangrur,Punjab,India
AdvaitaGanguly
ComprehensiveTissueCentre,UAHTransplantServices,AlbertaHealthServices,Edmonton, AB,Canada;HealthSciencesEducationandResearchCommons,UniversityofAlberta, Edmonton,AB,Canada
AnnMaryGeorge
DepartmentofBiomedicalEngineering,ManipalInstituteofTechnology,ManipalAcademyof HigherEducation,Manipal,Karnataka,India
MukeshKumarGupta
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
SadafHameed
DepartmentofBiomedicalEngineering,CollegeofEngineering,PekingUniversity,Beijing, Haidian,China
ThiThanhHanhNguyen
TheInstituteofFoodIndustrialization,InstitutesofGreenBioScience & Technology,Seoul NationalUniversity,Pyeongchang-gun,Gangwon-do,RepublicofKorea;Departmentof InternationalAgriculturalTechnology & InstituteofGreenBioScienceandTechnology,Seoul NationalUniversity,Pyeongchang,Gangwon-do,RepublicofKorea
MdSaquibHasnain
DepartmentofPharmacy,ShriVenkateshwaraUniversity,Amroha,UttarPradesh,India
MonjurulHoque
DepartmentofFoodProcessEngineering,NITRourkela,Rourkela,Odisha,India
MargaretO.Ilomuanya
DepartmentofPharmaceuticsandPharmaceuticalTechnology,FacultyofPharmacy, UniversityofLagos,Surulere,Lagos,Nigeria
JohnJeslin
DepartmentofBiotechnology,St.Joseph’sCollegeofEngineering,Chennai,TamilNadu,India
JuhuiJin
GraduateSchoolofInternationalAgriculturalTechnology,SeoulNationalUniversity, Pyeongchang-gun,Gangwon-do,RepublicofKorea
PadmajaKar
DepartmentofChemistry,NITRourkela,Rourkela,Odisha,India
HyoJinKim
GraduateSchoolofInternationalAgriculturalTechnology,SeoulNationalUniversity, Pyeongchang,Gwangwon-do,RepublicofKorea;InstitutesofGreenBioScienceand Technology,SeoulNationalUniversity,Pyeongchang,Gwangwon-do,RepublicofKorea
DomanKim
DepartmentofInternationalAgriculturalTechnology & InstitutesofGreenBioScienceand Technology,SeoulNationalUniversity,Pyeongchang,Gangwon-do,RepublicofKorea;The InstituteofFoodIndustrialization,InstitutesofGreenBioScience & Technology,SeoulNational University,Pyeongchang-gun,Gangwon-do,RepublicofKorea;GraduateSchoolof InternationalAgriculturalTechnology,SeoulNationalUniversity,Pyeongchang-gun,Gangwondo,RepublicofKorea
SanjeevKumar
DepartmentofBiotechnology,Dr.Y.S.ParmarUniversityofHorticultureandForestry,Solan, HimachalPradesh,India
Chi-ChingLee
IstanbulSabahattinZaimUniversity,FacultyofEngineeringandNaturalSciences,Department ofFoodEngineering, Istanbul,Turkey
TimothyLeeTurner
DepartmentofMicrobiology-Immunology,FeinbergSchoolofMedicine,Northwestern University,Chicago,IL,UnitedStates
L.Mahalakshmi
ComputationalModelingandNanoscaleProcessingUnit,IndianInstituteofFoodProcessing Technology(IIFPT),MinistryofFoodProcessingIndustries,GovernmentofIndia,Thanjavur, TamilNadu,India
TanushreeMaity
DefenceResearchandDevelopmentOrganization,DRDOBhawan,RajajiMarg,NewDelhi, India
SamrendraMaji
SRMResearchInstitute,SRMInstituteofScienceandTechnology,Kanchipuram,TamilNadu, India
KaustavMajumder
DepartmentofFoodScienceandTechnology,UniversityofNebraska-Lincoln,Lincoln,NE, UnitedStates
M.MariaLeena
ComputationalModelingandNanoscaleProcessingUnit,IndianInstituteofFoodProcessing Technology(IIFPT),MinistryofFoodProcessingIndustries,GovernmentofIndia,Thanjavur, TamilNadu,India
NupurMohapatra
DepartmentofFoodProcessEngineering,NITRourkela,Rourkela,Odisha,India
JeyanA.Moses
ComputationalModelingandNanoScaleProcessingUnit,IndianInstituteofFoodProcessing Technology(IIFPT),MinistryofFoodProcessingIndustries,GovernmentofIndia,Thanjavur, India
SomaMukherjee
DepartmentofVeterinaryMedicineSchool,MississippiStateUniversity,MississippiState,MS, UnitedStates
AmitKumarNayak
DepartmentofPharmaceutics,SeemantaInstituteofPharmaceuticalSciences,Mayurbhanj, Odisha,India
SurajKumarNayak
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
BhagyashreePadhan
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
DilipkumarPal
DepartmentofPharmaceuticalSciences,GuruGhasidasVishwavidyalaya,Koni,Bilaspur, Chhattisgarh,India
KunalPal
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
AshokR.Patel
GuangdongTechnionIsraelInstituteofTechnology,Shantou,China
RehanAliPradhan
DepartmentofAgricultural,FoodandNutritionalScience,UniversityofAlberta,Edmonton,AB, Canada
SushantPrajapati
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
DilshadQureshi
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
K.J.Rao
DepartmentofBiotechnology,VeltechUniversity,Chennai,TamilNadu,India
SirsenduS.Ray
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
SaiPreethamReddyPeddireddy
DepartmentofBiomedicalEngineering,ManipalInstituteofTechnology,ManipalAcademyof HigherEducation,Manipal,Karnataka,India
FionaConcyRodrigues
DepartmentofBiomedicalEngineering,ManipalInstituteofTechnology,ManipalAcademyof HigherEducation,Manipal,Karnataka,India
SaiS.Sagiri
DepartmentofChemistryandBiochemistry,TheCityCollegeofNewYork,NewYork,NY,United States
LannySapei
DepartmentofChemicalEngineering,UniversityofSurabaya,RayaKalirungkut,Surabaya,East Java,Indonesia
NandiniSarkar
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology Rourkela,Rourkela,Odisha,India
PreetamSarkar
DepartmentofFoodProcessEngineering,NITRourkela,Rourkela,Odisha,India
AnganaSarkar
DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology, Rourkela,Odisha,India
AlokSaxena
AmityInstituteofFoodTechnology,AmityUniversityUttarPradesh,Noida,UttarPradesh,India
IisSeptiana
GraduateSchoolofInternationalAgriculturalTechnology,SeoulNationalUniversity, Pyeongchang-gun,Gangwon-do,RepublicofKorea
KumakshiSharma
Health,SafetyandEnvironmentBranch,NationalResearchCouncilCanada,Edmonton,AB, Canada
LoveleenSharma
AmityInstituteofFoodTechnology,AmityUniversityUttarPradesh,Noida,UttarPradesh,India
AbhinayKumarSingh
SchoolofDentistry,CollegeofOralMedicine,TaipeiMedicalUniversity,Taipei,Taiwan;Stem CellResearchCenter,CollegeofOralMedicine,TaipeiMedicalUniversity,Taipei,Taiwan
VinayK.Singh
ResearchandDevelopment,IntasPharmaceuticalsLimited,Ahmedabad-Gujarat,India
AgustinWulanSuciDharmayanti
UniversityofJember,Jember,EastJava,Indonesia
YumewoSuzuki
DSPGokyoFood & ChemicalCo.,Ltd.,Osaka,Japan
IrshaanSyed
DepartmentofFoodProcessEngineering,NITRourkela,Rourkela,Odisha,India
AkiraTabuchi
DSPGokyoFood & ChemicalCo.,Ltd.,Osaka,Japan
GoutamThakur
DepartmentofBiomedicalEngineering,ManipalInstituteofTechnology,ManipalAcademyof HigherEducation,Manipal,Karnataka,India
AmanUllah DepartmentofAgricultural,FoodandNutritionalScience,UniversityofAlberta,Edmonton,AB, Canada
RitujaUpadhyay
SchoolofFoodScienceandBiotechnology,ZhejiangGongshangUniversity,Hangzhou,China; SchoolofFoodScience,UniversityofIdaho,Moscow,ID,UnitedStates
MadanL.Verma
CentreforChemistryandBiotechnology,DeakinUniversity,Geelong,VIC,Australia
LeiWang DepartmentofFoodScienceandTechnology,UniversityofNebraska-Lincoln,Lincoln,NE, UnitedStates
VarshaWankhade DepartmentofZoology,SavitribaiPhulePuneUniversity,Pune,Maharashtra,India
HiroyukiYamada
DSPGokyoFood & ChemicalCo.,Ltd.,Osaka,Japan
KazuhikoYamatoya
DSPGokyoFood & ChemicalCo.,Ltd.,Osaka,Japan
YueZhang
CollegeofFood & BiologyEngineering,ZhejiangGongshangUniversity,Hangzhou,Zhejiang, China;DepartmentofFoodScienceandTechnology,UniversityofNebraska-Lincoln,Lincoln, NE,UnitedStates
MuhammadZubair
DepartmentofAgricultural,FoodandNutritionalScience,UniversityofAlberta,Edmonton,AB, Canada
Introductionofbiopolymers:food andbiomedicalapplications 1
DilshadQureshi1,SurajKumarNayak1,ArfatAnis2,SirsenduS.Ray1,DomanKim3, ThiThanhHanhNguyen3,KunalPal1
1DepartmentofBiotechnologyandMedicalEngineering,NationalInstituteofTechnology,Rourkela,Odisha,India;
2DepartmentofChemicalEngineering,KingSaudUniversity,Riyadh,SaudiArabia; 3DepartmentofInternational AgriculturalTechnology & InstituteofGreenBioScienceandTechnology,SeoulNationalUniversity,Pyeongchang, Gwangwon-do,RepublicofKorea
1.Introduction
Polymersaremacromoleculeswhichusuallyhavemonomericunits(sameordifferent)thatcombinein differentways(MillsandWhite,2012).Thepolymersmaybeeitherofsyntheticornaturalorigin(Deb etal.,2019).Nowadays,humankindhasbecomemoredependentontheuseofsyntheticpolymers (Buggy,2016).Unfortunately,theuseofthesesyntheticpolymersisassociatedwithalargenumberof environmentalandhealthissues(Kaushiketal.,2016).Thiscanexplainthesearchofnaturalpolymers bytheresearchers,whichcanefficientlybeusedtoreplacethesyntheticpolymersfordifferentapplications.Thenaturaloriginpolymersarealsoregardedas“biopolymers”(NumataandKaplan, 2011).Hence,biopolymerscanbedescribedasnaturallyoccurringmacromoleculeswhichareusually producedbylivingsystemsincludingplants,animals,andmicroorganisms(Yadavetal.,2015).In recentyears,thereisagrowingtendencytousemoreofnaturalpolymersfordevelopingvariousfood andbiomedicalproducts(Babuetal.,2013;Davidenkoetal.,2014;Verbeek,2012).Itisnoteworthyto mentionoverherethatthemankindhasbeenusingbiopolymersnotonlyforfoodandbiomedical applications,whereithasfoundnumerousapplications,butalsointextile,cosmetics,pharmaceuticals, andpaperindustries(AnwunobiandEmeje,2011).
Aspreviouslymentionedbiopolymerscontainrepeatingunitsofmonomersandareusuallyderived fromplants,animals,andmicroorganisms.Ingeneral,therepeatingunitsofabiopolymermayeither besugars,aminoacids,orfermentativeproductslikealiphaticpolyesters(Prameelaetal.,2018). Thesebiopolymersmayhavedifferentfunctionalgroupslikehydroxyl,amino,amide,carboxyl, phosphate,phenolic,etc.(Lietal.,2012),whichimparttotheirdifferentbiologicalactivities(Kim, 2016).Thebiopolymersareusuallybroadlyclassifiedintothreegroups,namely,polysaccharides, proteins,andpolynucleotides(Mohanetal.,2016).Polysaccharidesaregenerallymadeupofsugar moietieswhicharecovalentlybondedwitheachotherviaglycosidiclinkages(Garcı´a,2018).Removal ofonewatermoleculeoccurswiththeformationofeachglycosidicbond.Onthebasisofcharge, polysaccharidescanbeneutral(dextran,pullulan),polycationic(chitosan),andpolyanionic(alginate)
Biopolymer-BasedFormulations. https://doi.org/10.1016/B978-0-12-816897-4.00001-1 Copyright © 2020ElsevierInc.Allrightsreserved.
(Hardingetal.,2015).Polysaccharidesarehomogenous(containingonetypeofmonomerssuchas glycogen)orheterogeneous(containingdifferentsugarunits,e.g.,xanthangum,gellangum) (Johnson-Green,2002).Ontheotherhand,proteinsmaybedefinedasthepolymersthatconsistof aminoacidmoietiesasthemonomericunit.Theseaminoacidsarejoinedtogetherbyamidelinkages resultingintheformationofthree-dimensional(3D)structure(WoolandSun,2011).Thispolyamide chainisthebasiclevelinthehierarchyoftheproteinstructure.Variousmolecularinteractionssuchas hydrogenbonding,saltanddisulfidebridges,andhydrophobicandhydrophilicinteractionsare responsibleforthefoldingofthechainintosecondarystructures(e.g., a-helix, b-pleatedsheets).The structureisfurtherpackedcloselythroughtheaforementionedinteractionsintotertiarystructures. Interactionsamongdifferentproteinsubunitsresultintheformationofaquaternarystructure(Pollock, 2007).Interestingly,theotherclassofbiopolymers,i.e.,polynucleotides(suchasDNA,RNA), compriseofasmanyas13ormorenucleotidemonomers(Davidenkoetal.,2014).Thesetwoheteropolymericmoleculesexhibitsignificanceinthelivingnature.Theyhaveadistinctbiological functionregardingthestorage,replication,anddiscerningofthegeneticinformation.Thebackbone frameworkofthetwonucleicacidscomprisesofaphosphategroup,sugarmoiety,andfournitrogenousbaseswithfewdifferences(Frank-Kamenetskii,2005).
Duetotheavailabilityofthebiopolymerswithdifferentchemistries,itispossibletoformulate variousfoodandbiomedicalproductsthathavevariedstructuralandphysicochemicalproperties.This ispossiblebecauseofthepresenceofdifferentfunctionalgroupsonthepolymericchainsofthe biopolymer(SenGupta,2007).Thesefunctionalgroupshelpthebiopolymerstointeractwith thedifferentcomponentswhicharepresentintheproducts(Maleki,2008;Shishiretal.,2018).Despite theaforementionedadvantages,thebiopolymericmaterialshavebeenreportedtohaveinadequate mechanicalproperties,whichmakethemunsuitableforuseindesigningspecificproducts(Vieira etal.,2011).Tocircumventthisproblem,manyauthorshaveproposedtheuseofcross-linkers,which aremultifunctionalchemicalagents,andhavetheabilitytoformcovalentbondswiththepolymer chains(Reddyetal.,2015).Further,theemployedcross-linkingtechniquemayalsoresultinthe formationofproductswithvariedpropertiesasthechemicalreactionmaytakeplaceindifferentways whenenvironmentalconditionsofthecross-linkingreactionarechanged(AkakuruandIsiuku,2017; Thakuretal.,2017).
Consideringtheabovediscussioninmind,inthischapter,wewillbediscussingaboutthevarious cross-linkingstrategiesforthebiopolymers,thepropertiesofsomeofthecommonlyusedpolysaccharideandprotein-basedbiopolymers,andfinallytheirfoodandbiomedicalapplications.
2.Cross-linkingmethodsemployedtodesignbiopolymer-based polymericarchitectures
Biopolymersarebeinginvestigatedthoroughlytodesignmatricesforfoodandbiomedicalapplications.Someofthecommonapplicationsincludecontrolledreleaseofbioactivemolecules(e.g.,flavors,proteins,probiotics,vitamins,drugs,mammaliancellsincludingstemcellsetc.),scaffold fabricationforregenerativemedicine,gelsforfood,pharmaceuticals,andbiomedicalapplications (ShitandShah,2014;UdenniGunathilakeetal.,2017).Inmanyoftheaforesaidapplications, designedpolymericarchitecturesareexpectedtoundergodegradationundercertainconditions(Jain etal.,2017).Itisimportantthattheproductsgeneratedfromthedegradationofthesepolymeric
2. Cross-linkingmethodsemployedtodesignbiopolymer-basedpolymeric architectures
architecturesareharmlesstothehumanbody(Uleryetal.,2011).Sucharequirementisnecessaryfor ensuringgoodbiocompatibilityofthepolymericarchitectures.Inthissection,wewilldiscussabout thedifferentphysicalandchemicalcross-linkingstrategiesthatareusedfordesigningpolymeric architecturesfordifferentapplications.Itisimportanttonotethatthechemicalcross-linkingstrategies helpindesigningpolymericarchitectureswithverygoodmechanicalstability(Ozbolat,2016).Unfortunately,thecross-linkingagents(thechemicalcompoundsusedforcross-linking)havemostly beenreportedtobetoxic(Ozbolat,2016)andcanalsoreactwiththebioactiveagentswhichareloaded inthepolymericarchitectures,therebyrenderingtheminactive.Suchdisadvantagecanbeeasily avoidedifthephysicalcross-linkingstrategiesareapplied(Puoci,2014).Thecommoncross-linking strategiesemployedhavebeendiscussedinthissection.
2.1Physicallycross-linkedgels
Recentyearshasseenanincreasedinterestinemployingphysicalcross-linkingstrategiestodesign polymericarchitectures(deOliveiraetal.,2019;Ebaraetal.,2014;Montaseretal.,2019).Thisis mainlybecausethephysicalcross-linkingstrategieshelpineliminatingtheuseofchemicalcrosslinkingagents.Inthissection,variousphysicalcross-linkingstrategiesforbiopolymerswillbe discussed.
2.1.1Cross-linkingbyionicinteractions
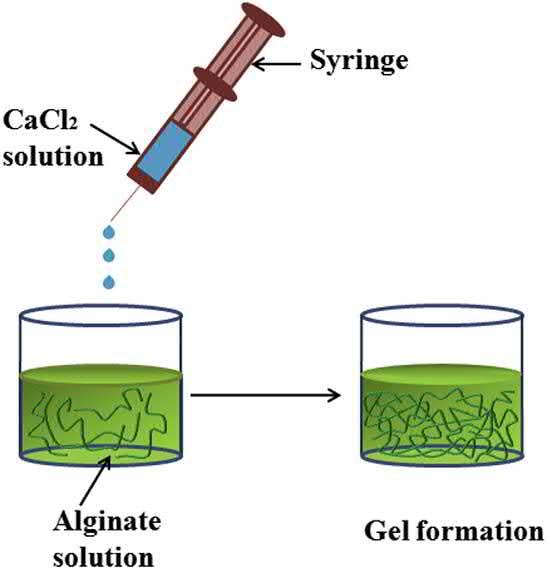
Alginateisacommonlyusedbiopolymerthatcanbeeasilycross-linkedbyionicinteractions.The biopolymerconsistsof b-D-mannuronicacidand a-L-guluronicacidresidues.Theseacidicresidues canionicallyinteractwithmultivalentcationslikecalcium(Ca2þ)ions.Guluronateacidresiduesplay aprominentroleinthecross-linkingofalginatebyinteractingwithCa2þ ionsandformingacharacteristic“egg-box”structure(BruchetandMelman,2015)(Fig.1.1).
Inmanycases,alginatebeadsarebeingpreparedbyaddingaqueoussolutionsofsodiumalginate (SA)orpotassiumalginateintoanaqueoussolutionofCa2þ ionspreparedusingcalciumchloridesalt. Thecross-linkingofthealginatepolymeroccursveryfastresultingintheformationofglobulargels. Unfortunately,thismethodresultsintheformationofgelbeadswithvaryingcross-linkingdensityand apolymerconcentrationgradient,therebyresultingintheformationofgelbeadswithdifferent physicalstabilities(KuoandMa,2001)(Fig.1.2).
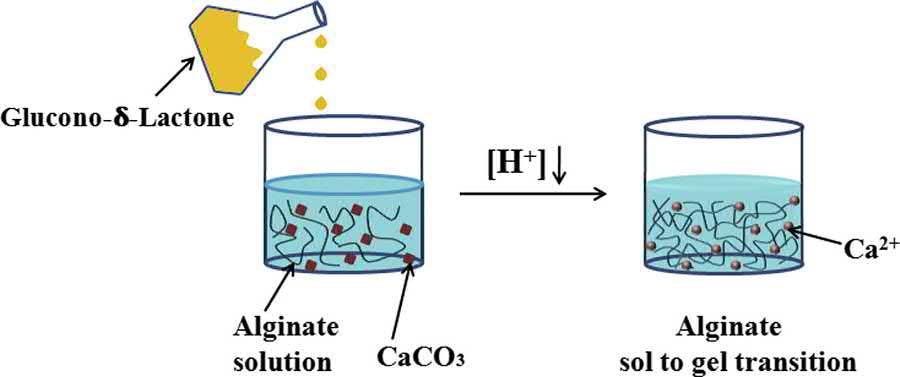
Inanalternativemethod,calciumcarbonate(CaCO3)powder(havingverylowsolubilityinwater) ishomogeneouslydispersedinthealginatesolution.Thegelationofthealginatephaseisinducedby releasingCa2þ withtheadditionofacids(e.g.,aceticacid,Glucono-a-lactone)(ConnonandHamley, 2014;Thakuretal.,2017).SincetheCaCO3 ishomogeneouslydispersedwithinthealginatesolution, thecross-linkingofthealginatematricesoccursinanearhomogeneousmanner(Rehm,2009).Hence, thegelsproducedbythismethodareusuallyuniformgels.Theuseofcalciumsulfate(CaSO4)has beenexploredforthecross-linkingofthealginatepolymerbymanyauthors.Unfortunately,itisvery difficulttocontrolthegelationkineticsofalginatephasewhenCaSO4 isusedastheionicallycrosslinkingmaterial.Intheyear2001,KuoandMareportedthatthemixturesofCaCO3 andD-glucono-dlactone,andCaCO3,D-glucono-d-lactone,andCaSO4 wereabletoreducetherateofgelation significantlywhichallowedthemtopreparealginatematriceshavingstructurallyuniformproperties (KuoandMa,2001)(Fig.1.3).
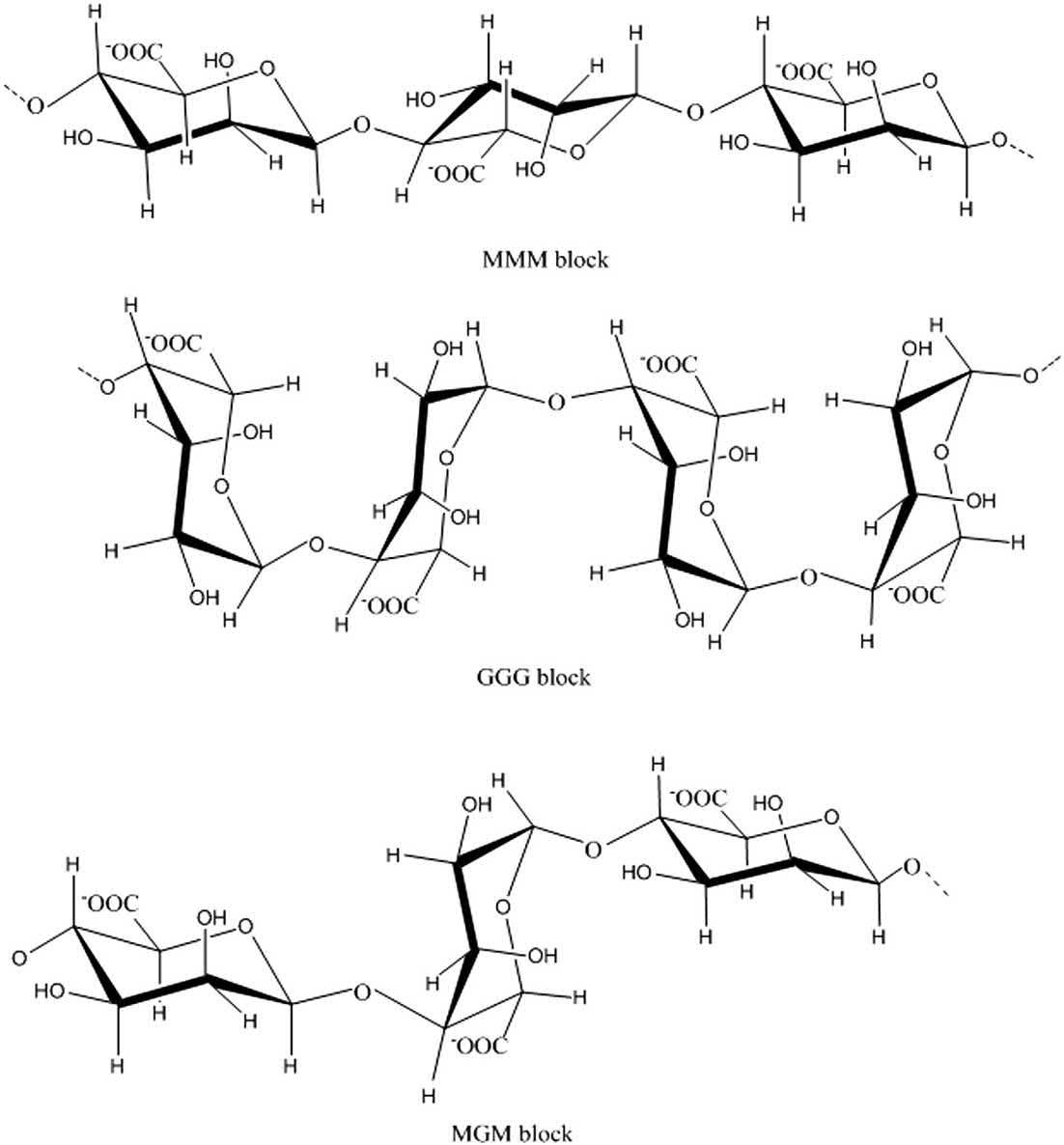
Structuralframeworkofthealginatebackbone.
Alginatehasbeenrecognizedasanimportantingredientinfoodindustriesasathickeningagent, gellingagent,filmforming,stabilizing,andemulsifyingagent(Qinetal.,2018).Alongwiththis, alginatehaswideapplicationsinpharmaceuticsforcontrolleddrugdelivery,andinbiomedicalsciencesincellculture,tissueregeneration,andwoundhealing(LeeandMooney,2012).
FIGURE1.1
2. Cross-linkingmethodsemployedtodesignbiopolymer-basedpolymeric
FIGURE1.2
Schematicdiagramoftheexternalgelationofthealginatesolutionusingcalciumchloridesaltsolution.
Inasimilarmanner,polycationicpolymerslikechitosancanbeionicallycross-linkedusingpolyanions(Wuetal.,2014).Onesuchexampleischitosan.Itconsistsof b-(1 / 4)-linkedglucosamine units.Thesolutionsofhydratedglycerolphosphatedisodiumsaltandsodiumtriphosphatearetwoof thecommonlyusedphysicalcross-linkersofthechitosan(Patil,2008;SaharanandPal,2016).Further, itisimportanttonotethatwhenthesolutionofpolymericanions(e.g.,alginate)andpolymericcations (e.g.,chitosan)aremixedtogether,theoppositelychargedionicgroupspresentinthepolymers
FIGURE1.3
Structuraldiagramoftheinternalgelationofthealginatesolutionusingcalciumcarbonateandglucono-dlactone.
ionicallyinteractwitheachothertoformagellednetwork(Kuligetal.,2016).Unfortunately,these gelsusuallyformstructurallyinhomogeneousmatrices(KuoandMa,2008).Duetothisreason, matricesformedasperthismethodologyisnotcommonlyused.Preparationofmatricesusingthis methodologyiscarriedoutverycarefully.Thispolymer-polymercomplexationprocesshasmainly beenrestrictedtothecoatingofoneofthepolymerwiththeother.Asforexample, Gandomietal. (2016) coatedporousalginatematriceswithachitosanlayer,whichresultedintheformationofa semipermeablemembraneacrossthealginatematrix(Gandomietal.,2016).Itissurprisingtonote thatmanyneutralbiopolymers(whichdonothaveanyionicgroups)canalsoresultintheformationof gelledstructuresinthepresenceofions.Onesuchexampleisdextranbiopolymer.Thispolymercan formgelledstructurethroughcrystallizationbyforminghydrogenbondsorinthepresenceofpotassium(Kþ)ions.Althoughbeingneutral,theglucoseresiduesinthepolymerchainsofthedextran moleculesformacage-likestructure.TheKþ ionsaresuitablyplacedwithinthecage.Thisphenomenonresultsintheformationofacomplexcross-linkedstructure(Mishra,2017).
Thoughioniccross-linkingallowsustoavoidtheuseofgenerallytoxicchemicalcross-linkers (Grumezescu,2018b),thesestructuresareusuallyunstableinnatureinaqueous/physiologicalenvironment(KuoandMa,2008).Theygetdestabilizedandundergostructuralbreakdownwhenplacedin theaforesaidconditions(Grumezescu,2018a).Thisphenomenonappearstobeusefulinsomefood applicationsbutinmanybiomedicalapplicationssuchphenomenonisundesirable.Hence,for biomedicalapplicationsusuallyasecond-stagecross-linkingiscarriedoutaftertheinitialioniccrosslinkingprocess(Peppas,2010;ThakurandThakur,2015).
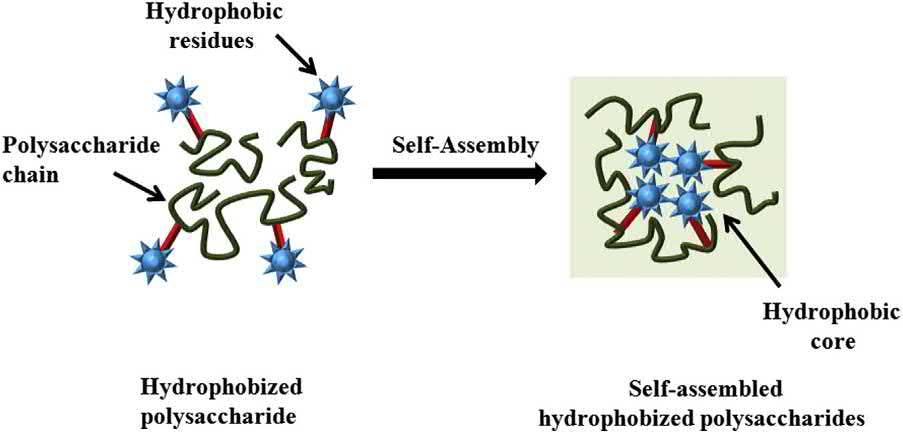
2.1.2Self-assemblyofhydrophobizedpolysaccharides
Gelationinwater-basedsystemsusingamphiphilicmoleculeshasbeenwidelystudied.Inmanyof thesesystems,theamphiphilicmoleculesundergoself-assemblytoformanetwork-likestructure, which,inturn,inducesthegelationoftheaqueousphase(MalodeMolinaandGradzielski,2017). Manyauthorshaveexploredthistechniquefordesigningpolysaccharide-basedphysicalhydrogels (Fig.1.4).Hydrophobicmodificationsofthepolysaccharidechainscanhelpinachievingthis.The
FIGURE1.4
Schematicdiagramofthecross-linkingofhydrophobizedpolysaccharidesthroughself-assembly.
hydrophobicmodificationrendersthepolysaccharideasamphiphiles.Inotherwords,thebiopolymers becomepolymeramphiphiles(Alhaiqueetal.,2015).Theself-assemblyortheaggregationofthese polymeramphiphilestoformgelledstructureshasbeengreatlyexploredforpharmaceuticaland biotechnologicalapplications(Hassanietal.,2012;Thomasetal.,2018).Someofthecommon polysaccharideswhichhavebeensuccessfullymodifiedfordesigninghydrogelsincludecarboxymethylcellulose(CMC),hyaluronicacid,guargum,chitosan(Camponeschietal.,2015),starch, alginate,agarose(Ahmed,2015),dextran,pullulan,andcarboxymethylcurdlan(MaitraandShukla, 2014).Thehydrophobizationofthepolysaccharidesareusuallyachievedbypreparingpalmitoyl, cholesterol,andpolyesterside-chain-substitutedderivativesofthepreviouslymentionedpolysaccharides(Liuetal.,2016).Modificationofthechitosanusingpolyacrylicacidandpoly-NisopropylacrylamidepolymershasalsobeenproposedfordesigningpH-andtemperature-sensitive hydrogels,respectively(Kimetal.,2000;Yazdani-Pedrametal.,2000).Further,themodification ofcarboxymethyldextranwithpoly-N-isopropylacrylamidetodesignthermosensitivehydrogelshas alsobeenproposed(Huhetal.,2000).
2.1.3Cross-linkingbythecrystallizationofthepolysaccharides
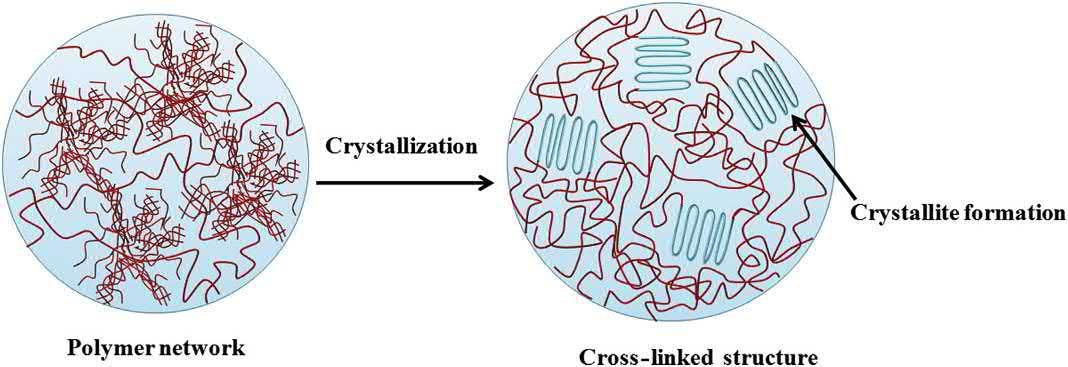
Thecrystallizationofthepolysaccharides,whichresultsintheformationofanetworkstructure,is preparedbyfreeze-thawtechnique.Thehydrogelspreparedbythismethodarealsoreportedas “physicalhydrogels.”Inthesegels,aspecifictypeofgelationisinducedbycryogenictreatmentofthe polysaccharidesolution.Itisreportedthatduringthefreezingandsubsequentstorageofthepolysaccharide,solutioninthefrozenstateresultsintheformationofside-by-sideassociationsofthe polysaccharidechainsduringthecrystallization(freezing)ofthesolvent(inthiscasewater)(Fig.1.5). Whenthisfrozenstructureisthawedthepolysaccharidenetworkwhichhasbeenformedduringthe crystallizationstepmaintainsitsstructuralintegrity(Zhangetal.,2013).Thismethodologyhasbeen usedtodesignhydrogelsusingvariouspolysaccharideincludingmaltodextrins,locusbeangum, starch,agarose,hyaluronan,andxanthan(Bhatia,2016).
FIGURE1.5
Schematicdiagramofthecross-linkingofpolysaccharidesusingfreeze-thawmethod. 2. Cross-linkingmethodsemployedtodesignbiopolymer-basedpolymeric
2.1.4Cross-linkingbyinductionofhydrogenbondsamongthepolysaccharide molecules
Hydrogenbondingamongthepolysaccharidemoleculesisoneofthemajormechanismsthatis employedforthegelationoftheaqueousphase.Thegelationoftheagarosesolutioninwaterisone suchexample.Agaroseremainsinsolubleincoldwater.Theagarosegelsareformedbydissolvingthe agarosepowderinnearboilingwater,whichissubsequentlycooleddowntoroomtemperatureto inducegelation(Zuccaetal.,2016).Thegelationisinducedbytheformationofinter-andintramolecularhydrogenbonds,whichareresponsibleformaintainingthe3Dstructureoftheagarosegels (TakoandNakamura,1988)(Fig.1.6).Thisstructurecanbedestabilizedbydisruptingthehydrogen bondsbysupplyingexternalthermalenergy.Thisresultsintheconversionofthegelledstructureintoa liquidstate(BansalandBoccaccini,2012).Anotherexampleofcross-linkingbyinductionof hydrogenbondsisthegelationofthechitosanmoleculesinapH-dependentfashion. Cheniteetal. (2000) reportedtheformationofsuchhydrogelsbyaddingglycerolphosphatesaltstoaqueoussolutionsofchitosan(Cheniteetal.,2000).Thegelswereproducedbyheatingthesolutiontobody temperature.TheyreportedthatthethermalreversibilityofgelationisdependentonthepHofthe chitosanandglycerolphosphatesolutions.IfthefinalmixturewasatapHvalueintherangeof 6.9 7.2,thenthegelsformedwerepartiallythermoreversible.Onthecontrary,completelythermoreversiblehydrogelscouldbeproducedwhenthepHvalueswereintherangeof6.5and6.9.The completethermoreversibilityofthegelswhosesolutionwashavingpHintherangeof6.5and6.9was attributedtotheinhibitionofthehydrogenbondformationwiththeconsequenthigherelectronic repulsionwithinthepolymericchainsofthechitosanmolecules(Cheniteetal.,2000).Hydrogen bondingalsoplaysanimportantroleinthepreparationoftheprotein-basedgelsincludinggelatingels (Lean,2006).Itisworthytonotethatinthegelspreparedbyhydrogenbonding,hydrophobicinteractionsalsoplayanimportantroletoacertainextent,andviceversa(Changetal.,2018b;Karino etal.,2002).
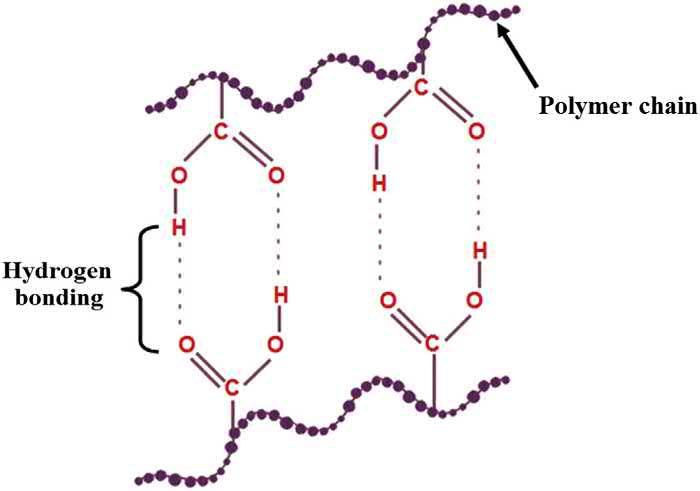
FIGURE1.6
Schematicdiagramofhydrogenbonding inducedcross-linkingofpolysaccharidechains.
2. Cross-linkingmethodsemployedtodesignbiopolymer-basedpolymeric architectures
2.1.5Cross-linkingusingproteinmolecules
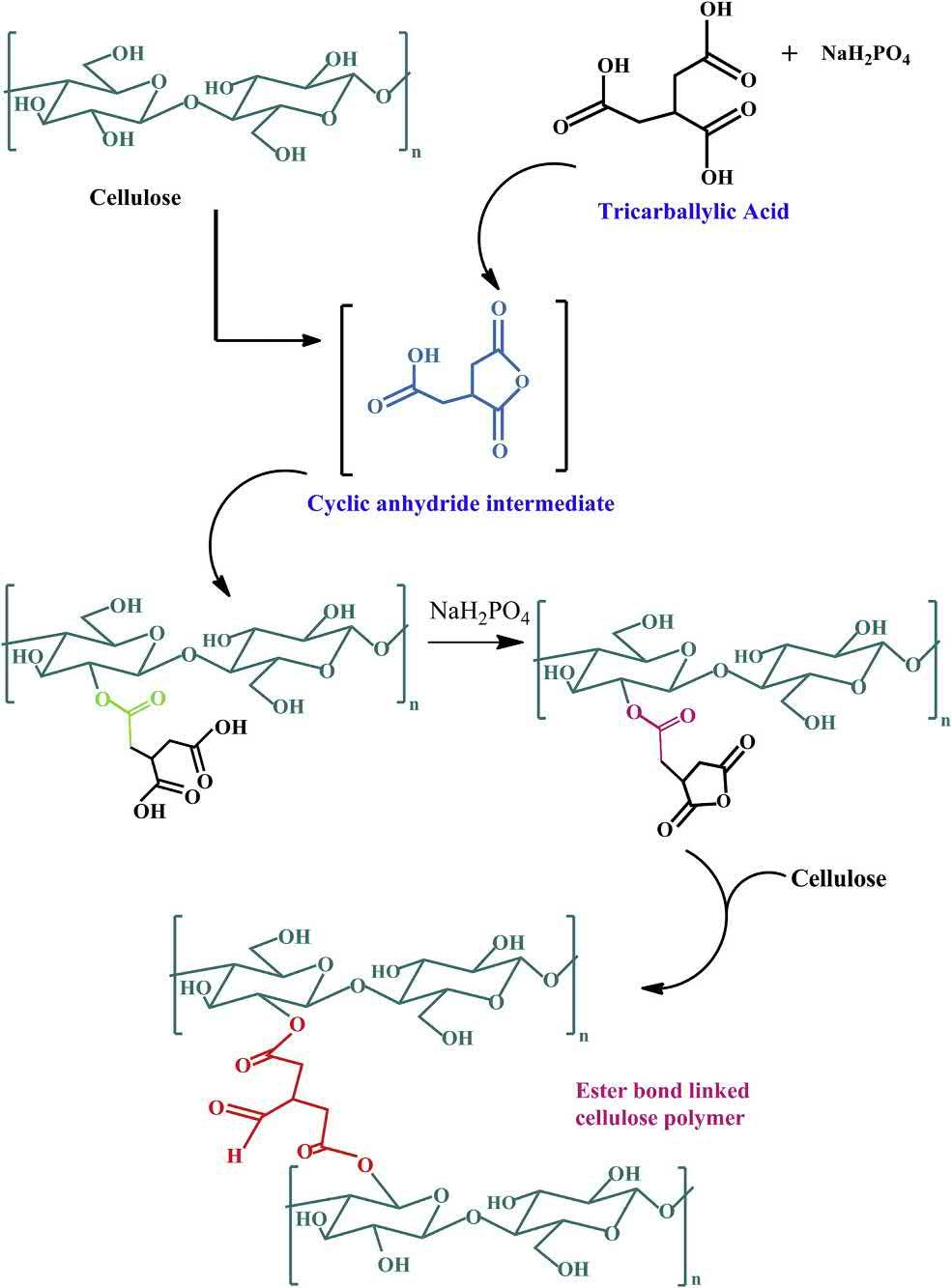
Theinteractionsbetweenthespecificpeptidedomainshavebeenreportedtosynthesizegels.Insucha method,twopeptidedomainsthatshowpositiveinteractionsareincorporatedseparatelyintononinteractingpolymericchains.Whenthesetwoprotein-engineeredpolymericsolutionsaremixed together,thepositiveinteractionsbetweenthepeptidedomainsincorporatedwithinthepolymerchains serveasthecross-linkingpoints.Thisresultsintheformationofa3Dnetworkstructurewitha capabilitytoimmobilizewatermolecules(Fig.1.7).Themainadvantageinthistypeofhydrogelsis thatthereisnoneedtoalterthepH,temperature,andionicstrengthofthepolymericsolutionduring theformationofthehydrogels.Thisisbeneficialwhenvariouslivingbiologicalentities,e.g.,microbial andmammaliancells,andbiologicallyactiveagentsareincorporatedwithinthehydrogelmatrix(Foo etal.,2009).
2.2Chemicalcross-linking
Physicalcross-linkingmethodsprovideagoodopportunitytoimprovethepropertiesofthebiopolymericstructures.Unfortunately,inmanycaseswhereanalterationintheenvironmentalconditions (e.g.,pH,temperature,andionicstrength)occursduringtheapplicationstage,thepolymericarchitecturesmaylosetheirstructuralintegrity(Saini,2017).Thoughinmanyapplications,thisproperty maybeuseful,butinmanyapplicationsthispropertyisundesirable.Hence,ithasbeenfoundthateven
FIGURE1.7
Schematicdiagramofthecross-linkingofpolymersusingproteinsascross-linkingagents.
aftercarryingoutthephysicalcross-linking,thepolymericarchitecturesarefurtherbeingchemically cross-linkedsoastoconsolidatethestructuralpropertiesofthephysicallycross-linkedpolymeric architectures(Parhi,2017).Also,manybiopolymers/biopolymermixturescannotbephysicallycrosslinked.Insuchcases,chemicalcross-linkingremainstheonlyavailablestrategyforimprovingthe structuralpropertiesofthebiopolymericarchitectures.Thechemicalcross-linkersarebasically multifunctionalchemicallyreactivemoleculeswhichcanformcovalentbondswiththefunctional groupspresentinthebiopolymers(Shenetal.,2016).Inthisway,chemicalcross-linkersform interconnectingbridgesamongthebiopolymericmolecules.Duringtheprocessofchemicalcrosslinking,thereisanincreaseinthemolecularweightwiththecorrespondingincreaseinthemechanicalandthestructuralabilityofthepolymericarchitectures(MaitraandShukla,2014).
Thoughthechemicalcross-linkerscanprovidetheaforesaidadvantages,theyarenotdevoidofthe disadvantages.Theprimaryconcernwiththeuseofcross-linkersisthetoxicityduetothepresenceof unreactivemonomerswithinthepolymericarchitectures(PatelandMequanint,2011).Hence,proper careshouldbetakentoeliminatetheunreactivemonomersafterthecross-linkingprocessisover. Further,thesitesforthedegradationofthepolymericstructurescanbesignificantlyalteredduetothe chemicalreactionoccurringduringthecross-linkingprocess.Thismaysignificantlyreducethe biodegradabilityofthebiopolymericarchitectures(AzeredoandWaldron,2016).Also,thereisa decreaseinthenumberoffunctionalgroupswithinthebiopolymericarchitectures.Thismayalterthe environment-sensitivepropertiesofsomeofthebiopolymers,which,inturn,willcompromisethe environment-sensitivepropertiesofthefinalproduct(Heetal.,2012).
Theresearchershaveexploredvariouschemicalcross-linkersandcross-linkingstrategies.Someof thecommoncross-linkersusedforthecross-linkingofbiopolymersincludeglutaraldehyde,polycarboxylicacidslikecitricacidandmaleicacid,EDC/NHS(1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide),epichlorohydrin,STMP(trisodiummetaphosphite), polysaccharidedialdehydeslikedextrandialdehydeandalginatedialdehyde,genipin,proanthocyanidin(PA),andglyoxal.Inthissection,wewillbediscussinginbriefthecross-linkingmechanismof theaforesaidcross-linkingagents.
2.2.1Glutaraldehyde
Glutaraldehydeisadialdehydeandisverycommonlyusedforitsabilitytoreactwithbothproteinand polysaccharides.Itcanstabilizeproteinandpolysaccharide-basedbiopolymericarchitectureswitha veryhighefficiency.Thiscross-linkingagentisanonspecificcross-linkerandthecross-linkingcan happenbothbetweeninter-andintramolecularfunctionalgroups.Thealdehydicgrouppresentinthe glutaraldehydemoleculeshasthecapabilitytoreactwiththefreeaminogroupspresentinthepolypeptidechain(Fanetal.,2018).Further,glutaraldehydecanalsoreactwiththehydroxylgroupswhich arepresentinbothproteinsandpolysaccharidemolecules(W. Wangetal.,2016).Themechanismof thechemicalreactionhasbeenprovidedin Fig.1.8.Itisnoteworthytomentionthatallthe glutaraldehyde-basedcross-linkingoccursatlowpH.Hence,theadditionofacidsinthereaction mixtureisanessentialstepinglutaraldehyde-basedcross-linking.
Apartfrommixingtheglutaraldehydereagentinthesolutionformtothepolymericsolutionsto inducecross-linking,manyauthorshavereportedvapor-phasecross-linkingusingglutaraldehyde (Destayeetal.,2013;Luetal.,2015).Inthisprocess,glutaraldehydesolutionsaremadeindifferent concentrations.Thegels/matricesthatneedtobecross-linkedareplacedatthetopofthecontainer, whichcontainsglutaraldehydesolutionsofdefiniteconcentrationusingsuitableattachments.
2. Cross-linkingmethodsemployedtodesignbiopolymer-basedpolymeric architectures
Schematicrepresentationforthecross-linkingofthepolymerusingglutaraldehyde.
Thereafter,thecontaineriscompletelysealed.Thisarrangementisincubatedataparticulartemperatureforadefinitetimeperiod.Duringtheincubationprocess,theglutaraldehydevaporsarereleased fromthesolution,whichtheninteractswiththesamplestobecross-linked.Ithasbeenfoundthatan alterationintheconcentrationoftheglutaraldehydesolutionsresultsinthedifferentialcross-linking density(Luetal.,2015).
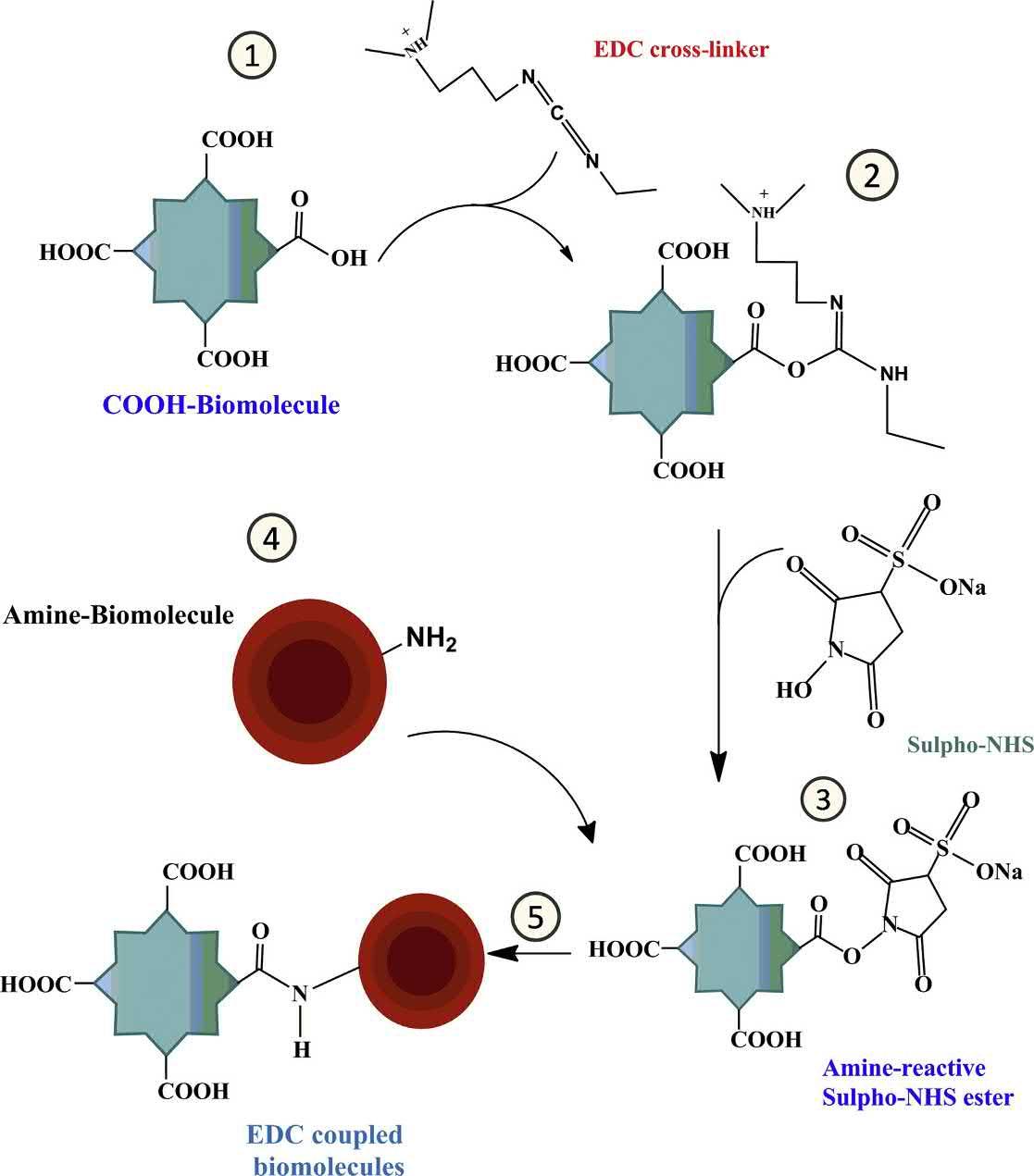
2.2.2Polycarboxylicacids
Polycarboxylicacidslikecitricacid,maleicacid,succinicacid,andtricarballylicacid,whichhave morethanthreecarboxylicgroups,havebeenusedtocross-linkcelluloseandstarchmolecules.These polycarboxylicacidsarereportedtoreactwiththehydroxylgroupspresentinthecelluloseandthe starchmolecules.Duringthecross-linkingprocess,anintermediatepolycarboxylicacidanhydrideis reportedtobeformed(FahmyandFouda,2008).Aplausiblemechanismforthereactionprocessusing polycarboxylicacidshasbeenprovidedin Fig.1.9.
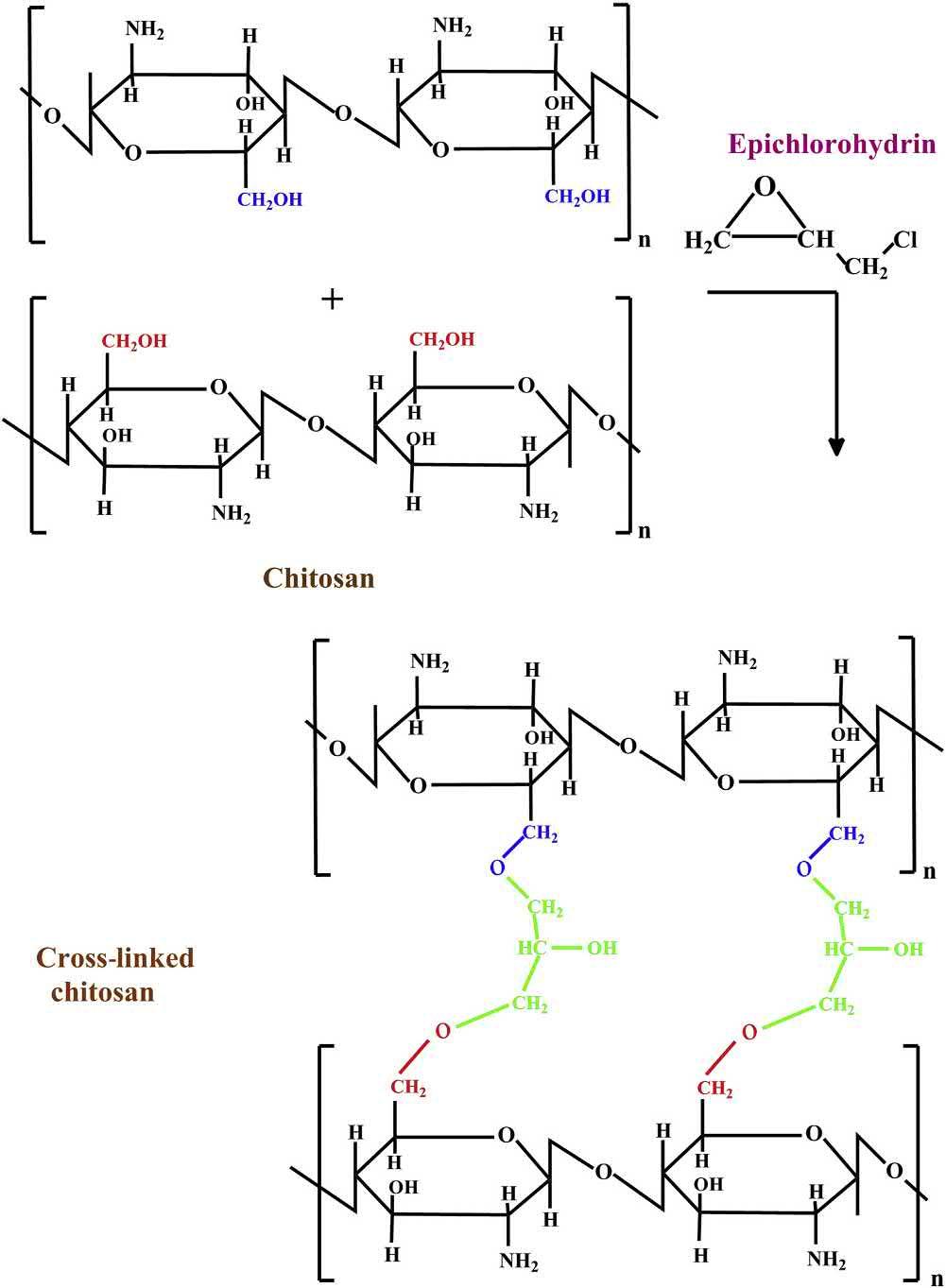
2.2.3EDCcoupling
EDC,chemicallyknownascarbodiimide,hasbeenusedtocouplecarboxylgroupstoprimaryamines. Thecouplingreactionresultsintheformationofanintermediatecompound,whichisknownasaminereactiveO-acylisourea.ThisintermediateproductisstabilizedusingNHSorsulfo-NHStoforman amine-reactiveNHSorsulfo-NHSester.Additionally,theO-acylisoureaintermediatemayalso chemicallyreactwithanotheraminegroupwhichispresentinaseparatebiopolymericchain.This leadstothecross-linkingofbiopolymershavingcarboxylandaminegroups.Ithasbeenreportedthat theremovaloftheexcessEDCmaybedoneeasilyusingdialysisandgelfiltrationtechniques.Thisis possibleduetothewater-solublenatureofEDC(Condeetal.,2014).Theprobablemechanismof EDC/NHScross-linkingmechanismhasbeenprovidedin Fig.1.10
FIGURE1.8
FIGURE1.9
Schematicrepresentationforthecross-linkingofcellulosewithtricarballylicacid.
2. Cross-linkingmethodsemployedtodesignbiopolymer-basedpolymeric architectures
FIGURE1.10
Schematicrepresentationforthecross-linkingofpolymerchainsusingEDC.
2.2.4Epichlorohydrin
Epichlorohydrinisachlorinatedcyclicetherandhasbeenusedforcross-linkingbiopolymerslike starchandchitosan.Itcovalentlyreactswiththehydroxylgroupspresentinthebiopolymerbackbone. Themainadvantageoftheepichlorohydrincross-linkingincomparisontotheglutaraldehydecrosslinking(whichalsoreactswiththehydroxylgroups)isitsabilitytoselectivelyreactwiththehydroxyl groupstoformglyceroldietherbridges(Motawieetal.,2014;Zhouetal.,2016).Thisisofimportance whilecross-linkingbiopolymerslikechitosanthathaveadditionalfunctionalgroupslikeamines. Sinceepichlorohydrinsparestheaminegroupsofchitosanbiopolymer,thecross-linkedchitosan matrixremainsascationicmatrixandaccordinglyexhibitspH-sensitivecharacter.Unlikeglutaraldehydecross-linking,theepichlorohydrincross-linkingisusuallycarriedoutunderalkalineconditions (KuniakandMarchessault,1972).Theschematicdiagramoftheepichlorohydrin-basedcross-linking mechanismhasbeenshownin Fig.1.11
2.2.5Trisodiummetaphosphite
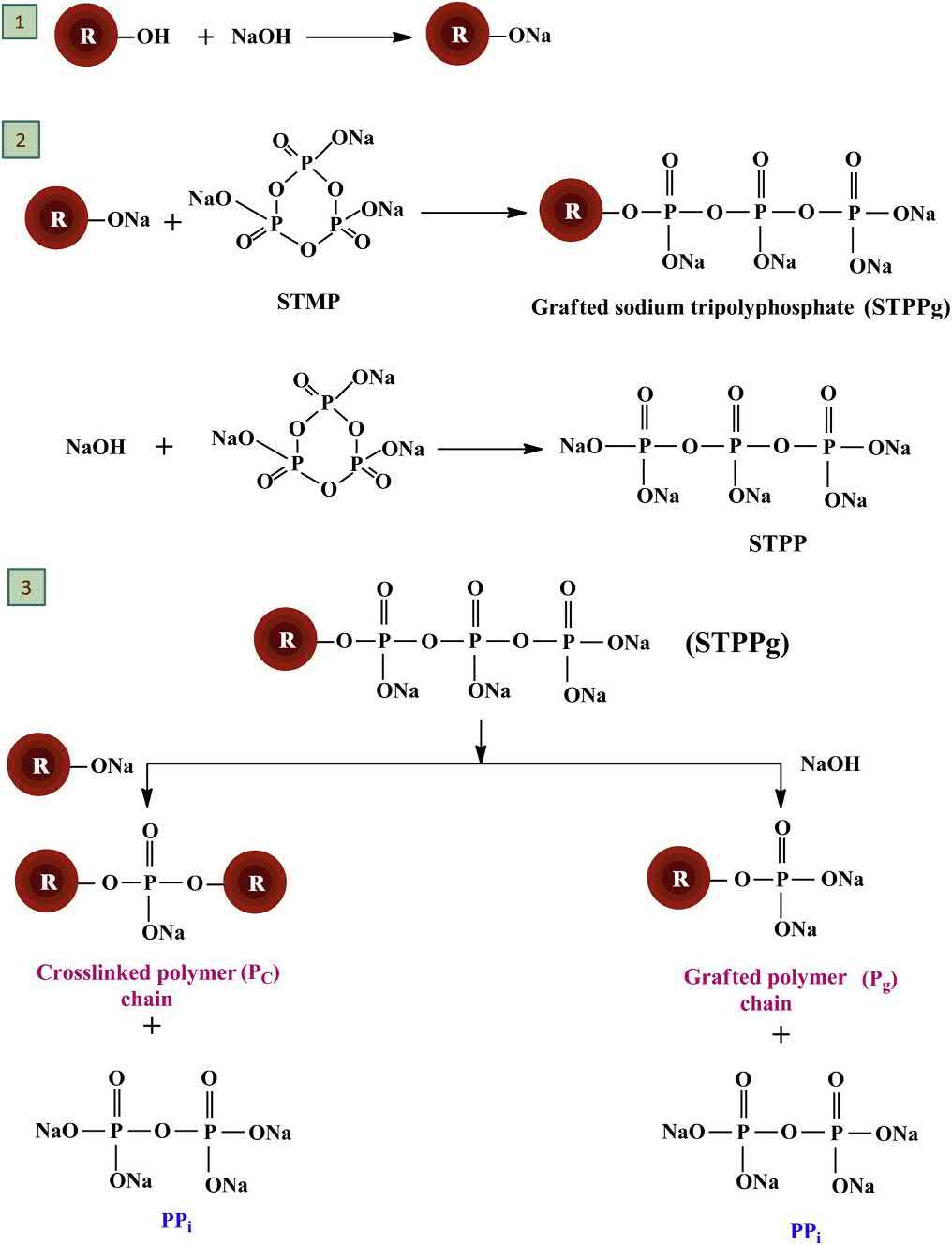
STMPisanontoxiccross-linkingagent.TheFoodandDrugAdministration(FDA)hasapprovedthis cross-linkingagent.STMPreactswiththeprimaryalcoholicgroupsofthepolysaccharidesunder strongalkalineconditions,whichhelpsinachievingthecross-linkingofthepolysaccharidemolecules. Usually,thecross-linkingofthebiopolymersusingSTMPiscarriedoutatpH > 9.5.Itisreportedthat thecross-linkingofthebiopolymersoccursasatwo-stepreaction.Inthefirststep,thepolysaccharide alkoxides,whichareformedbythedissolutionofthebiopolymersinsodiumhydroxidesolution (alkalinemedia),undergoreactionwithSTMP.Duetothisreaction,thecyclicstructureoftheSTMPis compromised,andformstripolyphosphatedpolymer.Inthesecondstep,thetripolyphosphated polymerso-formedreactswithanotherbiopolymeralkoxidemolecule,therebyresultingintheformationofacross-linkedmatrix(Dulongetal.,2011).Thecross-linkingmechanismoftheSTMPbasedcross-linkinghasbeenshownin Fig.1.12.
2.2.6Polysaccharidedialdehydes
Themechanismofthereactionofthepolysaccharidedialdehydesissimilartothatof glutaraldehyde.Thepolysaccharidedialdehydesareformedbyoxidizingthepolysaccharidesusing periodatereaction.TheoxidationprocessresultsinthecleavageoftheC2 C3bondofglucose residues.Duringtheoxidationprocesstwoaldehydeunitspermonosaccharideunitsareformed, therebyresultingintheformationof2,3-dialdehydepolysaccharides(AzeredoandWaldron,2016). Themechanismoftheformationoftheoxidizedpolysaccharideviaperiodateoxidationhasbeen providedin Fig.1.13.
2.2.7Genipin
Genipinisanaturallyoccurringcross-linkingagent.Itisaniridoidglycosidethatisextractedfromthe fruitsof Gardeniajasminoids.Thesaidcross-linkerexhibitsverylowcytotoxicityandhence,hasbeen proposedforcross-linkingofbiopolymers.Thegenipinmoleculesinteractwiththeprimaryamine groups.Thecross-linkingreactionoccursviaatwo-stepreactionmechanism.Inthefirststep,the primaryaminegroupsreactwiththegenipinmoleculesviaanucleophilicreaction.Thisresultsinthe formationofanunstableintermediatecompound,whichformsatautomericaldehyde.Thealdehydic groupthereafterformsacovalentbondwithanotheraminegroup.Thisresultsinthecross-linkingof
2. Cross-linkingmethodsemployedtodesignbiopolymer-basedpolymeric architectures
FIGURE1.11
Schematicrepresentationforthecross-linkingofchitosanusingepichlorohydrin.
FIGURE1.12
Schematicrepresentationforthecross-linkingofpolysaccharideusingtrisodiummetaphosphite(STMP).