Bio-basedFlameRetardant Technologyfor PolymericMaterials
Editedby YuanHu
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina, Hefei,Anhui,People’sRepublicofChina
HafezehNabipour
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina, Hefei,Anhui,People’sRepublicofChina
XinWang
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina, Hefei,Anhui,People’sRepublicofChina
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,or fromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-90771-2
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: EdwardPayne
EditorialProjectManager: EmilyThomson
ProductionProjectManager:NirmalaArumugam
CoverDesigner: ChristianJ.Bilbow
TypesetbySTRAIVE,India
Contributors
K.S.Anjumol
DepartmentofMaterialsEngineering,CzechTechnicalUniversity,Prague,Czechia;Schoolof EnergyMaterials,MahatmaGandhiUniversity,Kottayam,Kerala,India
BinYu
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina,Hefei,PR China
Cheng-FeiCao
CentreforFutureMaterials,UniversityofSouthernQueensland,SpringfieldCentral,QLD,Australia
FarihaAnwar
DepartmentofEnvironmentalSciences,InternationalIslamicUniversity,Islamabad,Pakistan
SadiaBatool
DepartmentofEnvironmentalSciences,FatimaJinnahWomenUniversity;Departmentof Chemistry,RawalpindiWomenUniversity,Rawalpindi,Pakistan
GillesBoni
ICMUBInstitute,UMR6302CNRS-UB,UniversitedeBourgogneFranche-Comte,Dijon,France
P.Y.Borse
DepartmentofPolymerandSurfaceEngineering,InstituteofChemicalTechnology,Mumbai,India
HongliangDing
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina,Hefei,Anhui, PRChina
WeifuDong
KeyLaboratoryofSyntheticandBiologicalColloids,MinistryofEducation,SchoolofChemicaland MaterialEngineering,JiangnanUniversity,Wuxi,China
RohamaGill
DepartmentofEnvironmentalSciences,FatimaJinnahWomenUniversity,Rawalpindi,Pakistan
BoranHao
SchoolofMaterialsScienceandEngineering,JiangsuUniversity,Zhenjiang,People’sRepublicof China
BobA.Howell
ScienceofAdvancedMaterials,CenterforApplicationsinPolymerScience,Departmentof ChemistryandBiochemistry,CentralMichiganUniversity,Mt.Pleasant,MI,UnitedStates
YuanHu
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina,Hefei,Anhui, People’sRepublicofChina
ImranaIftekharKabir
SchoolofMechanicalandManufacturingEngineering,UNSWSydney,Sydney,NSW,Australia xiii
YinLu
SchoolofMaterialsScienceandEngineering,JiangsuUniversity,Zhenjiang,People’sRepublicof China
GiulioMalucelli
PolitecnicodiTorino,DepartmentofAppliedScienceandTechnology,andLocalINSTMUnit, Alessandria,Italy
HannaJ.Maria
SchoolofEnergyMaterials,MahatmaGandhiUniversity,Kottayam,Kerala,India;Departmentof ChemicalSciences,UniversityofJohannesburg,Doornfontein,SouthAfrica
SnehaSabuMathew
SchoolofEnergyMaterials,MahatmaGandhiUniversity,Kottayam,Kerala,India
S.U.Mestry
DepartmentofPolymerandSurfaceEngineering,InstituteofChemicalTechnology,Mumbai,India
S.T.Mhaske
DepartmentofPolymerandSurfaceEngineering,InstituteofChemicalTechnology,Mumbai,India
IrrumMushtaq
DepartmentofChemistry,Quaid-i-AzamUniversity,Islamabad,Pakistan
HafezehNabipour
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina,Hefei,Anhui, People’sRepublicofChina
ClaireNegrell
ICGM,UnivMontpellier,CNRS,ENSCM,Montpellier,France
LarsP.Passauer
DresdenInstituteofWoodTechnology—InstitutfuerHolztechnologieDresdengemeinnuetzige GmbH,Dresden,Germany
SylviePourchet
ICMUBInstitute,UMR6302CNRS-UB,UniversitedeBourgogneFranche-Comte,Dijon,France
ErikJ.Price
DepartmentofMacromolecularScienceandEngineering,CaseWesternReserveUniversity, Cleveland,OH,UnitedStates
GustavoSchinazi
DepartmentofMacromolecularScienceandEngineering,CaseWesternReserveUniversity, Cleveland,OH,UnitedStates;DepartmentofMechanicalEngineering,Pontifı´ciaUniversidade Cato ´ licadoRiodeJaneiro,RiodeJaneiro,Brazil
DavidA.Schiraldi
DepartmentofMacromolecularScienceandEngineering,CaseWesternReserveUniversity, Cleveland,OH,UnitedStates
RodolpheSonnier
IMTMinesAles,PolymersHybridsandComposites(PCH),AlesCedex,France
PetrSpatenka
DepartmentofMaterialsEngineering,CzechTechnicalUniversity,Prague,Czechia
S.N.Sreenivasan
DepartmentofPolymerChemistry,GdanskUniversityofTechnology,Gdansk,Poland; DepartmentofMechanicalEngineering,AdiShankaraInstituteofEngineeringandTechnology, Kalady,Kerala,India
SabuThomas
SchoolofEnergyMaterials;SchoolofChemicalSciencesMahatmaGandhiUniversity;International andInterUniversityCentreforNanoscienceandNanotechnology,MahatmaGandhiUniversity, Kottayam,Kerala,India;DepartmentofChemicalSciences,UniversityofJohannesburg, Doornfontein,SouthAfrica
TharaTom
SchoolofEnergyMaterials,MahatmaGandhiUniversity,Kottayam,Kerala,India
ChengWang
SchoolofMechanicalandManufacturingEngineering,UNSWSydney,Sydney,NSW,Australia
DongWang
KeyLaboratoryofSyntheticandBiologicalColloids,MinistryofEducation,SchoolofChemicaland MaterialEngineering,JiangnanUniversity,Wuxi,China
XinWang
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina,Hefei,Anhui, PRChina
ZhihuanWeng
StateKeyLaboratoryofFineChemicals,DepartmentofPolymerScience&Engineering,Dalian UniversityofTechnology,Dalian,Liaoning,PRChina
GuanHengYeoh
SchoolofMechanicalandManufacturingEngineering,UNSWSydney,Sydney,NSW,Australia
AnthonyChunYinYuen
SchoolofMechanicalandManufacturingEngineering,UNSWSydney,Sydney,NSW,Australia
RichardK.K.Yuen
DepartmentofArchitectureandCivilEngineering,CityUniversityofHongKong,HongKong,PR China
KanZhang
SchoolofMaterialsScienceandEngineering,JiangsuUniversity,Zhenjiang,People’sRepublicof China
MengZhang
KeyLabofBiomassEnergyandMaterials,InstituteofChemicalIndustryofForestProducts, ChineseAcademyofForestry(CAF),Nanjing,Jiangsu,China
YuanZhang
SchoolofMaterialsScienceandEngineering,JiangsuUniversity,Zhenjiang,People’sRepublicof China
QiZhao
KeyLabofBiomassEnergyandMaterials,InstituteofChemicalIndustryofForestProducts, ChineseAcademyofForestry(CAF),Nanjing,Jiangsu,China
YonghongZhou
KeyLabofBiomassEnergyandMaterials,InstituteofChemicalIndustryofForestProducts, ChineseAcademyofForestry(CAF),Nanjing,Jiangsu,China
Preface
Inthemodernage,theunprecedentedapplicationsofpolymersbringextensiveconveniencestoour dailylifebecauseoftheirnumerousadvantages.However,polymerspossessonefatalshortcoming, i.e.,highflammability.Polymer-relatedfireaccidentscauseseverepropertylossandcasualtiesannuallyworldwide.Therefore,manycountrieshaveformulatedanincreasingnumberofstrictlawsand regulationstoenhancetheflame-retardantpropertiesofpolymers.Currently,mostflameretardants arehighlydependentuponpetroleum-basedresources,resultinginincreasinggreenhousegasemissions.Renewablebio-basedresourcesareapromisingsolutiontotheproblem,astheycanbeconsideredasuitablealternativetopetroleumintheproductionofflameretardants.Theiravailability,safety, andenvironmentallyfriendlyfeatureshavemadethema“greener”choiceoverpetro-basedmaterials. Overthepastdecade,awidevarietyofbio-basedflameretardantshavebeenfabricatedfromcellulose, lignin,cardanol,chitosan,eugenol,isosorbide,vanillin,furan,alginate,starch,vegetableoils,etc.The rapidgrowthinbio-basedflame-retardantpolymericmaterialshascreatedademandforanup-to-date bookthatintroducesthelatestscientificdevelopmentsandtechnologicaladvancesinthisrealm.
Thisbookpresentsacomprehensivetreatiseonbio-basedflameretardantsforpolymericmaterials, first,coveringthekeyfundamentalsrelatedtotheirsyntheticapproaches,firetestingmethods,and flame-retardationmechanisms.Subsequently,thisbookalsodiscussesthesynthesis,properties,and commercialpotentialofawiderangeofbio-basedflameretardantsforpolymericmaterials.Finally, thisbookreflectsontheperspectivesandchallengesofusingbio-basedflameretardants.
Thisbookwillappealtoundergraduateandpostgraduatestudentsinpolymerchemistryandfire engineeringdisciplines,firescienceresearcherswhoarenonspecialistsinchemistryandmaterialsscience,scholarsworkingonthedevelopmentofcommercialandenvironmentallybenignflame-retardant polymers,andthoseinvolvedinthefiresafetyindustry,withcontributionsfromanarrayofinternationallyrenownedindustrialandacademicexperts.Thisgoalwouldhavebeenimpossibletoachieve withoutthechapterauthors’detailedandinsightfulcontributions,whichwegratefullyacknowledge. Wearealsothankfulforthewonderfulandproductiverelationshipwehavehadwiththechapterauthors.WerespectfullyacknowledgethesupportfromtheNationalNaturalScienceFoundationof China(Grantnos.22075265,22050410269)andtheChineseAcademyofSciences(Grantnos. 2021459,2019PE0014).WealsoexpressourgratitudetothestaffatElsevierforalltheirassistance andsupport.
Introductiontoflameretardants forpolymericmaterials 1
HafezehNabipourandYuanHu
StateKeyLaboratoryofFireScience,UniversityofScienceandTechnologyofChina,Hefei,Anhui, People’sRepublicofChina
1. Introduction
Apolymer’slowdensity,superiorrecyclability,andversatilitymakeitidealforawiderangeofpurposes.It’snowonderthatpolymersareubiquitousinallfacetsofourdailylife.Theirflammability, nevertheless,isoneofthemaindifficultiesofusingthem.Asflameretardantshavebeenusedfor decadestoinhibit,decrease,and/orpostponetheignitionofpolymers,significantadvancementshave beenachievedinthedevelopmentofflame-retardantadditivesthatmaybeutilizedinthemanufacturingprocess.Halogenatedflameretardantsareamongthemostwidelyusedandmostefficientkinds offlameretardants [1,2].Ecologicalissueshavebeenexpressedabouttheirusagenotwithstandingtheir effectiveness.Suchflameretardantsmayleakoutofcommongoodsthroughouttheirlifespan,pollutingpeople,livestock,andthesurroundingswithhazardouschemicals.
Whenexposedtofire,halogenatedflameretardantsgenerateharmfulandecologicallylastingchemicals,andtheburningordumpingofpolymersintodumpsattheendoftheirusefullifeharmsthe environmentandlivingcreaturesevenmore [1].People,creatures,sediments,water,andairhavebeen foundtocontainsuchsubstances [3–12].Agrowingnumberofcountriesarecontrollingorprohibiting halogenatedflameretardantsasaconsequence [10,13].Anenvironmental-friendly,low-toxicityflame retardantthatcanbeutilizedeffectivelyandsafelyinpolymericmaterialsisneededinlightofthetoxicologicalconsiderationsandlimitationsassociatedwiththeuseofhalogenatedflameretardants. Throughoutthelast2decades,theusageofrenewablematerialsasflameretardantsforpolymers hasincreasedrapidlyinresponsetothissocialdemandandagrowingworldwideemphasisonsustainability.Eversinceseveralothernaturalelementshavebeenutilizedasflameretardantsinpolymeric materials.Asaresultofenvironmentalconsiderationssuchasairandwaterpollutionandtherisein carbonemissionsanddisposalpollutants,severalinvestigatorshavelatelypresentedthoroughstudies ontheusageofbio-basedflameretardantsinthepolymermatrix [14–17].Tannicacid(TA),phyticacid (PA),deoxyribonucleicacid(DNA),andfishgelatin(FG)areseveralinstancesofbio-basedcompoundsthathavebeeninvestigatedasflameretardantsduringthelast2decadesandaredetailedin respectiveevaluations.Synergisticeffectsmaybeachievedbyusingacombinationofnature-derived andlow-toxicityflameretardants.Bio-based,environmentallysustainableflame-retardantsolutions thatenhanceflammabilitywithoutcompromisingphysicalqualitieshavebeendevelopedviathis approach.Wheneveremployedalone,mineralhydroxidesareamongthemostfrequentlyutilizedof
Bio-basedFlameRetardantTechnologyforPolymericMaterials. https://doi.org/10.1016/B978-0-323-90771-2.00018-3 Copyright # 2022ElsevierInc.Allrightsreserved.
allflameretardants,althoughtheirquantitiesaretypicallyhigherthan50wt% [2].Thisfamilyalso includesaluminatrihydrate(ATH)andmagnesiumhydroxide(MH),whichhavebeenusedincombinationwithTAtoflamedelayvariouspolymers [18,19].Melamine(ME),whichhasbeenused synergisticallywithTAandFGinpolyolefins [18];melaminepoly(magnesiumphosphate)(Safire 600,S6),acommercialflameretardantandsmokesuppressantrecentlyusedinpolystyrene(PS) [18];andammoniumpolyphosphate(APP),ausualelementofintumescentsystems [20–23],arealso goodcandidatestobeusedincombinationwithbio-basedflameretardants.Numerousresearcheshave alsobeendevotedtodiscoveringoptionstoreplacethetraditionalplastic-basedsubstances,whichare widelyutilizedintheconstructionandmanufacturingindustries.Severalnewbiopolymerproducts havebeencommercializedinrecentyears,whicharesubstitutingpetroleumbiomass.Asaresult,biopolymersincludepetroleum-basedpolymercompoundsthatincludebiomass-basedingredients.The utilizationofrenewablesuppliesnotonlyhelpstothecreationofinnovativebio-basedgoods,butalso areductioninrelianceonpetroleumproducts,andthereforeproducesbeneficialecologicaleffectsfor theregenerationofatmosphericcarbondioxide.Asaresult,biopolymersmaybeemployedasflame retardantsbychemicallyalteringtheirarchitecture.Thisbusinessissearchingforpractical,costeffective,andenvironmentallyacceptableflame-retardantsolutions.
Thischapter,whicheffectivelyaddressesmarketingflameretardantsaswellastechnologicaltrends intheeconomizationofbio-basedasflame-retardantingredientsforpolymercomposites,offersa demonstrationoftheprimarybiomasscomponentscapableofproducingcharthroughoutthermal degradationaswellastechnologicaladvancementsthroughoutbio-basedflame-retardantingredients.
2. Commercialflameretardants
Theconceptofflameretardationandtheuseofflame-retardantchemicalstorestrictorpostponethe spreadoffiredatesbacktothehistoricalera.Withtheadventofsyntheticpolymersinthe20thcentury andthegrowingusageofcompositematerialinawidevarietyofapplications,flameretardantsstarted tobestudiedandusedmoreextensivelyinthelatterpartofthatcentury.Thesixmajortypesofcommercialflameretardantsbeingusednowadaysareasfollows:halogenatedorganic,organophosphorus, inorganic,nitrogen,silicon-based,andintumescentsystems [2]
2.1 Halogenatedflameretardants
Approximately25%oftheglobalflame-retardantoutputcomesfromhalogenatedflameretardants [2] Flammablematerialstaysconstant;however,theheatproducedduringcombustiondiminishes,dueto chemicalinterventionwithradicalchainprocessingasphasethroughoutignition.Halogenradicals producedbyflameretardantsscavengethehigh-energyhydroxylradicalsandhydrogenradicalscreatedthroughoutignition.Mosthalogenatedflameretardantsarefoundedonchlorineorbrominebecausethecarbon–bromine/chlorineconnectionisveryfragileandthermallyunstable.Asaresult, fluorine-basedsubstancesarethermallyresistanttotheformationoffluorineradicalsatthebreakdown temperaturesatwhichpolymersusuallydegrade.Atmostpolymermanufacturingtemperatures, iodine-basedmoleculesarehighlyunsustainable.
Becauseoftheirexcellentperformance,fiveformulationsbasedonbrominatedflameretardantsare extensivelyutilized,includingtetrabromobisphenolA,hexabromocyclododecane,pentabromodiphenyl
ethers,octabromodiphenylethers,anddecabromodiphenylethers.TetrabromobisphenolAismostcommonlyfoundthroughoutepoxyresinsforprintedcircuitboards,acrylonitrilebutadienestyrene(ABS), andeffectivepolystyrene.InadditiontoABS,polyolefins,andpoly(vinylchloride)(PVC),decabromodiphenylethermaybeutilizedinalmosteverykindofpolymer.High-impactpolystyreneenclosuresfor televisionsandcomputermonitorsareoftenmadefromthismaterial.Thereareseveralapplicationsfor hexabromocyclododecane,includingpolystyrenefoamforinfrastructuredesignandtextiles [2,10]. Theyareveryeffectiveasflameretardants,yettheycreateenormousriskstotheenvironmentand publichealth.Thesetoxichalogenatedcompoundsmaybeproducedthroughoutthelifespan,ignition, and/ordecompositionofcommonitemsasdetailedinthefollowingsections.Thereleaseofpolybrominateddibenzofuransandpolybrominateddibenzodioxinsuponheatingorburningisoneofthebiggestissues.Inadditiontotheirchlorinatedequivalents,severaloftheirisomersaresimilarlyhazardous. Halogenatedchemicalscanemitlargequantitiesofabrasivevapors,erodingmetalparts,andtriggering damagetosensitiveelectronicsinclosedareas,includingaconventionalairplanefuselageormarine hullchamber [2]
2.2 Organophosphorusflameretardants
Flameretardantscomprisingphosphorusarealsofrequentlyutilized.Organicphosphorus-basedflame retardantsencompassphosphateesters,phosphinates,phosphonates,etc. [24].Severalotherinorganic P-basedflameretardantsarealsoutilized,includingredphosphorusandammoniumpolyphosphate (APP).Asflameretardants,phosphorus–nitrogenandphosphorus–halogenmixturesarealsoused. Intheinstanceoforganophosphoruscompounds,thePgroupsgivetheflameretardancy,whereas theorganiccomponentofferscompliancewiththepolymer.Asaconsequence,phosphorus-based flameretardantsmayimpedeignitioninboththecondensedandgasphases.Itshouldbenotedthat thephosphorusindifferentvalencestatesrevealsdifferentflame-retardantmechanisms.The+5phosphorusdemonstratesacondensed-phaseflame-retardantmechanism,suchasAPP.Thephosphorusin lowvalencestatesdemonstratesgas-phaseflame-retardantmechanism,suchas9,10-dihydro-9-oxa10-phosphaphenanthrene-10-oxide(DOPO).Thesemoleculesacceleratecrosslinkingofpolymers inthecondensedphase,whichreducesvolatile-fragmentproduction,andleadstocyclizationandincreasedcharproduction.Charpreventsthecomponentfromignitingbylimitingtheoxygendiffusion intoandoffuelparticlesoutofthepolymerandbyloweringtheheattransfercoefficientintothedisintegratingcomponent.Asaradical-scavengerthroughoutthegasphase,Pinhibitsexothermicoxidativeprocesses.Theireffectivenessisfrequentlyduetotheirdual-action.Itisimportanttonote, however,thatphosphorus-basedflameretardantsarealsohighlydangerous.Forinstance,theprevious studyhasdemonstratedthattri-ortho-cresylphosphate(TOCP)causespostponedneurotoxicitywithin individuals [25].Triphenylphosphatecontaminationinsediment-dwellingspeciessurrounding manufacturingfacilitiesmayhavebeensufficienttocauseharmfulconsequences [26].
2.3 Inorganicflameretardants
Flameretardantsmadefrominorganicmaterialscomesinawidevarietyofkindsandconcentrations. Metalhydroxidesarethemostextensivelyutilizedcategoryinthebusinesssector.Antimony,boron, phosphorus,andmolybdenumareamongtheotherinorganicflameretardants.Basedonthecomponentsemployed,thesubstanceshavevariousmechanisms [2].Aluminumtrihydrateandmagnesium
hydroxidearetheprimarymetalhydroxidesinuse.Intermsofvolume,aluminumtrihydrateisoneof themostfrequentlyemployedflameretardants.Endothermically,thesechemicalsbreakdown,producingwatermoleculesthatactasflameretardant.Thisactivityinterruptstheignitionprocessintwodifferentapproaches,includingtheendothermicprocessabsorbingheat,gettingcolderthepolymerandthe flame(heatsink),andtheproducedwatervapordilutingthecombustiblegaseousfuel.Mostofthese chemicalsmustbeemployedinextremelyhighconcentrations(50–80wt%)inordertobeeffective, whichmayhavenegativeeffectsonthematerial’sphysicalandmechanicalcharacteristics [2].Although otherantimonycompoundsandoxidesaresometimesemployed,antimonytrioxide(Sb2O3)isthemost frequentlyutilizedantimonycompound.Severalofthesesubstancesareusedinconjunctionwithhalogenatedflameretardants,increasingtheireffectivenessanddecreasingtheloadingofhalogenatedflame retardantsneeded [2].Someoftheseproceduresdemandhightemperatures,includingtheproductionof high-impactpolystyreneusedintelevisionandcomputer-monitorenclosures [10].Anionsreactwith antimony-containingmoleculestoproduceantimony-halogencombinationsingeneral.Inboththe compressedandgaseousphases,thesecompoundsoperateinavarietyofwaystoinhibitflamespread. Antimonycompoundsareoftenpresentinquantitiesof2–10wt%inwater [2].Regardingboricacidand sodiumborate,themostcommonlyusedmaterialsareborax(Na2B4O7 10H2O)andzincborate.Incellulosicproducts,likecottonandpaper,boricacid,andboraxareprimarilyutilized.Asasubstitutefor antimonytrioxide,zincborateisutilizedinnumerousplasticandrubbergoods.Itservesasaflame retardantandsmokesuppressorinthecompressedform [2].
RedphosphorusandAPPareamongthemajorkindsofinorganicphosphorus-basedflameretardants.Whenelementalphosphorusundergoesthermo-oxidativebreakdown,itislikelyconvertedinto eitherphosphoricacidorphosphoruspentoxide,whichsubsequentlyplaysamajorpartinboththe compressedandgasphases.Itisprimarilyutilizedinpolyamidesandphenolicproducts.Asasmoke deterrent,molybdenumflameretardantsoperatemostofteninthecompressedphase [27].Woolcontainscertaintitaniumandzirconiumelements.Zincstannateandzincborateareemployedassubstitutesfororsynergistswithantimonytrioxide.Manymorechemicalsareemployedinwoodland firefightingandcellulose-basedgoods,includingammoniumbromideandammoniumsulfamate [2].
Theprimarydisadvantageofthebiggestcategoryofinorganicflameretardants,metalhydroxides, isthehighquantityofadditivesrequiredtoachieveefficacy,whichmayresultindegradationofthe item’smechanicalcharacteristics [2].Asaresult,theusageofcertaininorganicflameretardantshas beencurtailed.Antimony-basedflameretardantsareanotherexampleofchemicalsthatmaybeharmfultohumanhealthiftheyemergefromcommercialgoods.Therearehealthconcernsassociatedwith redphosphorusmanufacturing,andphosphorus-containinggoodshaveapotentiallyharmfulimpacton individualsandtheplanet.Consequently,whileinorganicflameretardantsaregenerallylessharmful thanhalogenatedflameretardants,mostofthemareeithermoderatelytoxicormustbeemployedat extremelyhighconcentrationsinmostsituations.Thesedisadvantagessuggestpracticalapplications fornontoxicflameretardantsthatmaybeemployedatloweramountsand/orcollaborativelywithinorganicflameretardants,thusdecreasingthequantityofthelatter.Asanontoxicflameretardant,biobasedflameretardantsaregoodoptions.
2.4 Nitrogen-basedflameretardants
Inadditiontotheirlowtoxicity,minimalsmokegenerationthroughoutburning,andadaptabilityfor reprocessing,nitrogen-basedflameretardantsmayalsobeextremelyappealing [24].Theyare,nevertheless,ineffectiveforalargevarietyofpolymers.Theyaremainlyfoundinnitrogenouspolymerslike
polyurethanesandpolyamides;however,theymayalsobefoundinpolyolefinslikepolypropylene (PP),polymethylpentene(PMP),andpolyisobutylene(PIB) [2].NandPhaveastrongsynergisticimpact,contributingtotheadoptionofnumerousflameretardantsdependingonthismixture [24].Flame retardantsinthiscategorymayoperateonboththecompressedandthegaseousphases.Asaconsequence,theymayacceleratetheproductionofcharinthecondensedphaseofthereaction.Atthispoint, nitrogen-containinggases,includingN2 andNH3 areproduced,dilutingoxygenandvolatile-fuel levels,whilecreatinganinertgasshieldthatisolatesthefuelfromoxygenintheatmosphereandallows ittoburnmoreefficiently [24].Sincechemicals,likemelamineorureamayfunctionasbubbleagents owingtotheemissionofN-containinggases,theyarealsooftenemployedinintumescenttechnologies. Ammoniumpolyphosphate,melamine,melaminecyanurate,ammoniumpolyphosphate,poly(zinc/ magnesiumpyrophosphate),urea,andguanidineareamongthenitrogen-basedflameretardantsthat arecommonlyused [2,18,24].AfewoftheseexamplesdemonstratetheuseofNandPinconjunction witheachother.Themajorproblemwithflameretardantsbasedonnitrogenisthattheyarenotvery effectivewhenappliedalone,accordingtosomeexperts.Attributedtothesynergisticproperties,they maybeextremelysuccessfulwhenusedinconjunctionwithotherelements,likephosphorus [24].It alsohasthedisadvantageofbeingrestrictedtocertaintypesofpolymers [2].Nitrogen-basedflame retardantsaccountforonlyaround5%oftheglobaloutputofflameretardantsbecauseofthese limitations [10].
2.5 Silicon-basedflameretardants
Eco-friendlysubstances,silicon-basedflameretardantsweremadeinresponsetoademandfornonhalogenatedflameretardants [28,29].Flameretardantsbasedonsiliconhavebeenaroundforawhile, butrecentadvancesinpolycarbonateshavebroughtattentionbacktothem.Siliconehasbeenshownto substantiallyenhanceflamebackwardnessinpolymericmaterials,accordingtothepreviousstudies [28,30].Silicone-basedflameretardantsoperateviacompactedandvaporphaseprocesses [31].Construction,electricaltransmission,textiles,andcivilandaeronauticalengineeringareamongthefields wheretheyareutilized [32].Silicones,silica,silicateminerals(suchasmontmorilloniteandkaolinite), silanes,andsilsesquioxanesareamongthesilicon-basedflameretardantsthatareavailable.Silicais regardedasaneco-friendlyflameretardantbecauseithasalimitedenvironmentaleffect.Polydimethylsiloxanepolymersoffergoodthermaldurability,highheatemission,andminimalemission ofharmfulgasesthroughoutthermalbreakdown.Becauseoftheirgoodscatteringinpolymericmatrix andmovementtowardthematerialsurfaceduringburning,thesecompoundshavebetterflameretardancy.Silicaalsocomesinavarietyofforms,includingsilicagel,silicafumed,andfusedsilica. Itsefficacyreliesonmanyvariables,includingthesizeoftheorifices,theparticlesizedistribution, theconcentrationofsilanolonthesurface,thesurfacearea,thethickness,andtheviscosity.Flame retardantscontainingsilicahaveverylowratesofheatproductionandmasslossbecauseofsilica’s physicalproperties [32,33].
2.6 Intumescentsystems
Asaresultoftheircomparativelyhigheffectiveness,minimalsmoke,andlowtoxicity,intumescent flameretardantsareregardedoneofthemostattractiveeco-friendlyflameretardants.Therearethree maincomponentstotypicalintumescentflameretardants,includinganacidsource,acarbonizing source,andafoamingorblowingsource.Flammableflameretardantsshoulddegradeattemperatures
lowerthanthepolymermatrix’sthermaldegradationtemperature.Avarietyofacids,includingphosphoricacid,sulfuricacid,boricacid,andtheirequivalents,maybeusedastheacidorigin.Manytypes ofcompoundsmaybeusedascarbonizingagents,includingpentaerythritol(monomer,dimer,and trimer),sorbitol,mannitol,dextrins,starch,phenol-formaldehyderesins,andcharringpolymerssuch asnylon6,polyurethane,andpolycarbonates.Nitrogen-containingchemicals,includingurea,ureaformaldehyderesin,melamine,dicyandiamide,andpolyamides,aretheprimarysourcesofthegases thatcausetheblasting.Tocreatethecarbonaceouslayer,theacidsourcedisintegratesathightemperaturesandproducesaninorganicstrongacid.Dehydrationfrequencyisdeterminedbythenumberof carbonatomsinthecarbonlayer,whereastheproportionofreactivehydroxylgroups(OH)determines thequalityofthecarbonlayer.Asaresult,theblowingagentdegradesandproducesinflammable gases,whichmayextendthecarbonaceouslayerandcauseittocreateanexpandedmulticellular layer.Acarbonaceouslayerisformedwhenagasisreleasedfromasourcethatisdehydrating. Thegasisreleasedduringthedehydrationprocessofthecarbonizingsupply.Asaconsequenceof theinterplaybetweenthesethreecomponents,charproductionisincreased,contributingtohigher flameretardancy [34,35].
3. Bio-sourcedflameretardants
Numeroustypesofflame-retardantchemicalsoccurandareusedeconomically,eventhoughmosthave undesirableconsequences,primarilylinkedtotheirtoxicology.Flameretardantsthatareharmlessfor peopleandtheenvironmenthavebeenmoresoughtafterasaresultofagrowingworldwideconcernfor theenvironment,aswellasagrowingglobalenvironmentalconsciousness.Flame-retardantcompoundsthatarenontoxic,biodegradable,andbasedonrenewableresourceshavebeenthefocusof increasedstudyinthelastdecade.Sincethebeginningofthe21stcentury,scientistshavebeenstudying theeffectsofnaturalflameretardantsonhumanhealth.Thesecompoundsarehighlightedinboldin eachofthefollowinginstances.
3.1 Tannins
Tannins,whicharenaturallyoccurringphenoliccompoundsfoundinavarietyofplants,possessa numberofuniquecharacteristics,includingantibacterialandantioxidantactivity,thecapacitytosedimentproteins,andareducingcapacityasanelectrondonor(Fig.1) [36].Additionally,sincetannins areabundantinthebarkoftrees,theyareinherentlyresistanttofire—throughoutaconflagration. Tanninsalsoeffectivelydecreaseoxidantsandradicalstomaximizetheamountofunburnedsolid andcharredmaterialthatremains,assistingtreesinsurviving [37].Theyhavebeencoupledwithstiffeningagentstocreateusefulpolymers,includingsealantsandresinsbecauseoftheirheatstabilityand similaritytophenol [38] andhavebeenutilizedintheproductionofporoussubstances,includingaerogelsandfoams [39].Comparedtofossil-basedchemicals,thesetannin-basedproductshavelowheat conductivityandlowflammability.Accordingly,theapplicationoftanninsinthecreationofthermal insulationor/andfire-resistantadditivesandconstructionmaterialsseemstobebasedonanovel bio-inspiredfoundation.
Thethermalcharacteristicsoftanninshavebeeninvestigatedinconsiderabledetail.Dependingon thecontent,architecture,extentofpolymerization,andtypeofinterflavonoidlinkages,tanninsare
susceptibletoheatdisintegration [40].Therewasanincreaseinthermalstabilitieswithlowercarbohydratecontentsandgreatertanninpurity,forinstance [40].Tannincompactedat140 °Cunderwenta glasstransition,andacetylationofthehydroxylgroupsloweredtheglasstransitiontemperature [41] Theheatbreakdownofcondensedtanninisdifferentfromthatofhydrolysabletannin(i.e.,tannicacid). Decompositionofcompressedtanninstookplaceintwosectionswiththeproductionof55%char, althoughthebreakdownoftannicacidoccurredthroughoutfivestepswiththegenerationof28%char [42].Whentanninswerepyrolyzedat600 °C,catecholwasthepredominantgaseousoutput.Itwasalso foundthatwhentheoutergallicacidlayerdisintegrated,theouterlayerofgallicacidunitsproduced 1,2-benzenedioland1,2,3-benzenetriolasmajorcomponents.Whenexposedtotemperaturesabove 700 °C,theinnerlayeriscross-linkedtoproduceintumescentcarbonaceouschar [43].
Tannincompoundswereusedtoimprovethethermaldurabilityandfireresistanceofthermoplastic polyesterresinsattheNikkeshiinvestigation.Tannicacidcross-linkedwithpoly(vinylalcohol)(PVA) andpoly(ethyleneglycol)wascombinedwithpolyethyleneterephthalate(PET),polybutyleneterephthalate(PBT),polycarbonate(PC),ABS,and/ormixturesofthesematerialsatproportionsranging from2to20,000ppm.Thereisanimprovementinthermalstabilityandadecreaseorinhibitionof ignitionofthethermoplasticpolymer [44].Usingbarksandtanninsasacombination,Tributsch andFiechter [45] showedthattheABSpolymer’scharoutputwassignificantlyenhanced.Moreover, theinclusionoftanninenhancedtheLOIindexandcontributedtoestablishingnondrippingperformanceinUL94measurements.Theinvestigatorsshowedhowessentialtanninisinmakingbarks fire-resistant,andalsoillustratedhowtousetanninasachar-formingingredientwithinABS.Tannic acid-ABSpolymersmaybeenhancedbytheinclusionofCaCO3 (derivedfromseashelldebris)asa bio-filler,asstatedbyMoustafaandcolleagues [46].ConecalorimetryrevealedthatABScomprising
FIG.1
Structureoftannin.
5%tannicacidhadatimetoignition(TTI)of22sandapeakheatreleaserate(PHRR)of696kW/m2, whereasincorporating25%seashelldebrisintothepolymerresultedinaTTIof46sanda45%reductioninPHRR.Althoughitwasnotexaminedtoseehowmuchtannicacidimpactedtheflame-retardant qualities.Foamsmadefromtanninsweretestedin2008and2009byTondiandPizzi [47] intermsof fireresistancecomparedwithcommercialphenolicfoams.Scientistshavedevelopedanewclassof renewabletannin-basedfoamsthatareself-extinguishingandcanrivalsyntheticphenolicfoams. Tannin-basedformulationshavebeeninseminatedintowoodsamples,accordingtoTondietal. [48].Thetanninsenhancedthewood’sfire-resistanceconcerningtheamountoftimerequiredtostart afire,thelengthoftimethefireburnsitselfout,andtheamountofweightthefireburnswhileflaming.
3.2 Lignin
Naturalligninismorecommonthancellulose(Fig.2) [49,50].Inwoodyplants,ligninisanessential componentofthecellwall [51,52].Annually,thepulpindustrygeneratessignificantquantitiesof lignin-containingwastematerial.Itmaybeutilizedinawiderangeofindustries,thankstoitsproperty. Ligninisabiopolymermadeupofthreetypesofphenylpropaneunitslinkedbyetherlinkagesand carbon–carboninteractionstogeneratethree-dimensionalnetworkarchitecture.Itcontainsahighconcentrationofaromaticringconfigurations,aliphaticandaromatichydroxylgroups,aswellasactive groups,likequinoneorganizations [53].Thechemicalstructureprovidesligninwithseveraladditional roles.Itmaybeintroducedtoplasticizersorsolventsorflameretardantsorsurfactantsoranynumberof
FIG.2
Structureoflignin.
othersubstances.Duetothesignificantcarbonoutputfollowingthebreakdownofthearomaticstructureoflignin,bio-basedflameretardantsmadefromligninhaveattractedmuchinterestinrecentyears. Followingpyrolysisoflignin,approximately35%–40%ofcarbonmaybegenerated.BrebuandVasile investigatedtheheatdegradationoflignin [54].Lignindestroysacrossawidertemperaturerange around200and500 °Cincomparedwithotherconstituentsofbiomass.Thethermogravimetricgraph usuallydisplaysaninitialweightlossbetween100and180 °C,whichcorrelateswiththedischargeof watermechanicallyboundintotherawmaterials.Degradationbeginsatabout200 °C,accordingto cleardefinitions.At200–260 °C,propionicside-chainbreakagereleaseslow-molecular-weightcompounds.Asaconsequence,themajordegradativeprocess(275–450 °C)correlatestoeitherC Cand β-scissionoraryl-etherfragmentationofthemainchain.Atthismoment,asignificantamountofmethaneisemittedintotheatmosphere.Inaddition,at500 °C,additionaltransformationsandcompression ofthearomaticstructuredevelop,resultingintheproductionoflargeamountsofchar(57.7wt%)and theemissionofdihydrogenmostlyinthegaseousstate.
GaniandNaruse [55] studiedtheimpactofcelluloseandligninconcentrationonthepyrolysisand ignitionofvariouskindsofplantsandflora.Theresearchersconcludedthatgreaterligninconcentrationlowerstherateofpyrolysis,whereasgrowingcellulosecontentenhancesit.Thus,ligninmaybe utilizedasaflame-retardantcomponentinpolymersbecauseofitsfunctioninpostponingtheignition ofgrassesandfilament.Usinglignintoenhancethethermalandflame-retardantcharacteristicsofABS wasstudiedbySongetal. [56].Inaddition,theydiscoveredthatligninmaydelaythebreakdownprocessofthepolymerandraisethequantityofcharthatwasproduced.Moreover,lignindecreasedthe polymer’sheatreleaserateanddecelerateditscompleteignition.Thiswasconfirmedbyexamination ofthecharredremnant,whichshowedthatthedevelopmentofanantiflamecoveringofligninwas accountablefortheflame-retardantproperties.Inwood–plasticcomposites(WPC),Liuetal. [57] utilizedbiocompatiblelignin(F-lignin)asaflame-retardantingredient.TocreatetheF-lignin,scientists addedcomponentsofphosphorusandnitrogentolignin.WPCswiththisbio-basedflame-retardant ingredientweremuchmoreflameresistantthanthosewithligninalone.F-lignin,whenaddedto WPCatthesamerateaslignin,increasesthethermaldurabilityofpolypropylene/WPpolymers andimprovestheflameretardancycharacteristics,withPHRRandTHRreducedby9%and25%, respectively.Anenhancedflame-retardantpropertywasduetotheinclusionofP,N,andCu2+ catalyzingtheformationofacompressedchar(Fig.3.).
Inawarmpaddingtechnique,Shuklaetal. [58] investigatedtheuseofsodiumligninsulfonate (SLS)asaflame-retardantfinalcompoundoncottontextiles.Cottonfabricimpregnatedwith30% (w/v)SLShasanLOIlevelof28.5%withthelowestcharsizeof4cmandaminimalafterglow for9s,whereasthereferenceburnsoutwithflameandafterglowaround1min.At500 °C,theimpregnatedcottonclothcontains35wt%residues,comparedtojust8%forareferencefabricatthesame temperature.Ligninanditsvariantsweredocumentedtobeutilizedasflameretardantsinpolyurethanesandothersubstancesinalargenumberofpublications.Usinglignosulfonatetoreplacepart ofdiethyleneglycol(DEG)incopolymerizationwithisocyanate,Luetal.producedlignosulfonate centeredonrobustpolyurethane(LRPU)foams.InordertoenhancetheflameretardancyofLRPU, lignosulfonatewasusedasacarbonizationingredientincombinationwithAPP [59].Afterdissolving calciumlignosulfonateintoapolyhydroxyalcoholmixture,Gaoetal.producedpolyolbasedonlignin liquefaction.Polyol,polyurethanemicroencapsulatedammoniumpolyphosphateandorganically transformedlayereddoublehydroxidewereconcurrentlyintegratedintostiffpolyurethanefoam,along withconventionalflame-retardantpolyol(RPUF).ResearchersfoundthatRPUFpolymershadbetter mechanicalproperties,thermalconductivityaswellasflameretardancyandfireactivity [60].
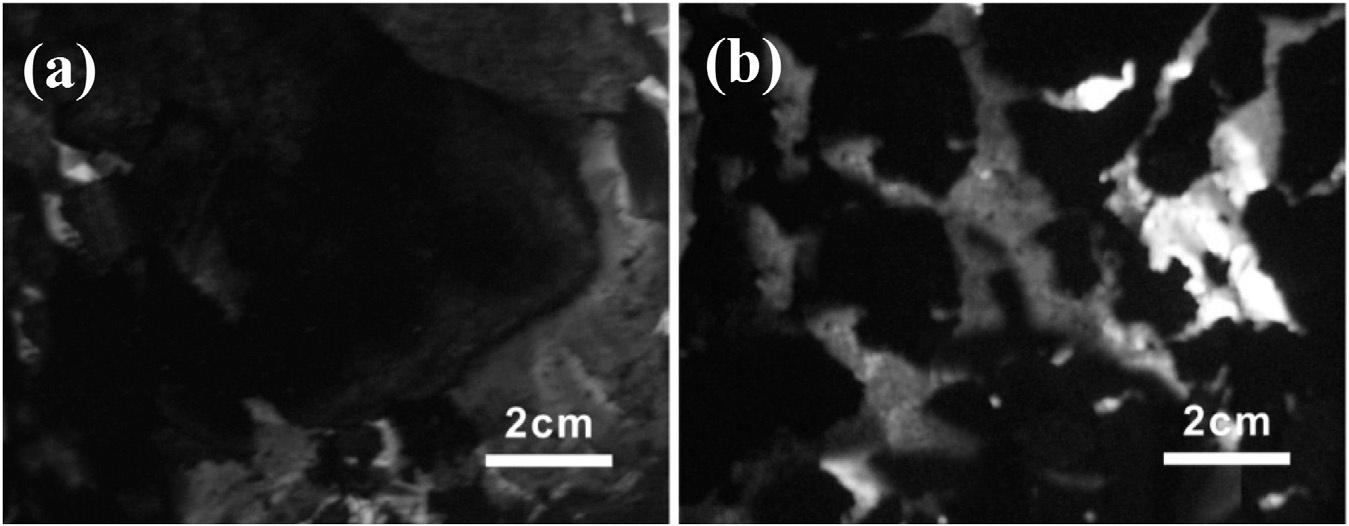
Digitalphotosofcompositesfor(A)PP/WPand(B)PP/WP/10-F-lignin [57].
3.3 Chitin
Anaturalpolysaccharideproducedbymanylivingspecies,chitin(poly(β-(1–4)-N-acetyl-D-glucosamine)),existsintheenvironmentasstructuralelementsintheexoskeletonofarthropodsorthecell wallsofyeastandfungus(Fig.4).Itisthemostabundantbiopolymerontheplanetfollowingcellulose becausealargequantityofchitinisproducedannuallyworldwide.Manyspeciesthroughouttheanimal andlowerplantkingdomsproduceitaswell,anditservesavarietyoffunctionsinwhichreinforcing androbustnessareimportant.Chitosan,themostwell-knownderivativeofchitin,isproducedviaincompletedeacetylationofchitininthesolidform.Chitinisarecyclable,harmless,andadaptablepolymer [61].Itscontemporaryusagesareprimarilyfocusedonwater-solublechitosanpolymers (deacetylatedchitin)producedfromchitin,whichisutilizedincosmetics,watertreatment,anddiagnosticsettings [62].Temperaturesbetween200and400 °Caretheoptimalrangeforchitindecomposition.Firstweightlossoccursbetween60and70 °C,whichcorrelatewiththedischargeofwaterthat hasbeenmechanicallylinkedtothecrudematerial.Therewasalsoanincreaseintherateofbreakdown ofsugarringsintheunitsoftheacetylatedchitinattemperaturesbetween250and400 °C.Duetoits aminoandhydroxylfunctionalities,thispolysaccharideisagoodcandidateforchemicalmodification, accordingtotheauthors.Severalchemicalprocessesmaybecarriedoutonchitinaswithcellulose, includingetherification [63],esterification [64],graftcopolymerization [65],etc.Basedonthetype
FIG.4 Chitinstructure.
FIG.3
ofthegroupadded,chitinwouldacquiredifferentcharacteristicsfollowingthechemicalalteration. Duetoitsintriguingbiologicalandchemicalcharacteristics,phosphorylatedchitinhasbeenextensivelydocumentedinrecentyears:biocompatible [66],bioabsorbable [67],andmetalchelatingproperties.Chitinwithphosphategroupsmaybeapolyanionicpolyelectrolyte,duetothepresenceof phosphategroups.Duetotheadditionofflame-retardantphosphoruselements,phosphorylatedchitin mayalsoexhibitflame-retardantproperties.Anumberofstudieshaveshownthattheflame-retardant propertiesofphosphorus-containingcompounds,andinspecific,theconjunctionofphosphorusand amines,maybesignificantlyimproved.Gaseousandcondensedformsofphosphorusarebothactive. Duringrecombinationwithhighlyreactiveradicalsinthegasphase,POradicalsmaydelaytheoxidationofhydrocarbons,makingthemlesshazardousbreakdownbyproducts.Phosphor-containing flameretardantscauseendothermicdehydrationandchardevelopment,owingtotheproductionof powerfulphosphoricacid.Theyarealsotoxictohumansandanimals.
Inaresearchpaper,Huetal. [68] describeamethodformanufacturingflamecottonfabricina scientificreport.Throughlayer-by-layerself-assembledtechnique,cottonfabricwascoatedwitha multilayeredcoveringcomprised ofthepositivelychargedchitosan(CH)andnegativelycharged phosphorylatedchitin(PT)(Fig.5A).Cottonfabricwith20bilayersproducedatasubstantial
FIG.5
(CH0.5/PT0.5)5
(CH0.5/PT0.5)10 (CH0.5/PT0.5)20 (CH0.5/PT2)5 (CH0.5/PT2)10 (CH0.5/PT2)20
(CH0.5/PT0.5)5 (CH0.5/PT0.5)10 (CH0.5/PT0.5)20 (CH0.5/PT2)5 (CH0.5/PT2)10 (CH0.5/PT2)20
(A)TheschemeoflayerbylayerassemblyusingCHandPT;(B)HRR;and(C)THRforpureand(CH/PT)coated cottonfabrics [68]
Rinse
phosphorylated-chitincontent(2wt%)quenchedtheflameintheverticalflameexamination.Microcombustioncalorimetry(MCC)wasusedtoassesstheburningperformanceofuntreatedandtreated cottonfabrics.Thelevelsofheatdischargeandoverallheatemissionweredecreasedby74%and 86%,respectively,using20bilayersCH/PT2%covering( Fig.5BandC).Cottontextilesmaydehydrateatlowertemperaturesduetothecatalyticac tivityofphosphoruscompounds,whichpromotes theproductionofcharand decreasestheemissionofflammableg as.Polyelectrolytemultilayerfilms comprisingphosphorusmaydecreasethefiredangerofcottonfabric, basedonthereducedtotalheat releaserate(THR)ratios.
Phosphorylatedchitinnanofibrils(ChNFs)wereidentifiedbyZhangetal. [69] asaveryapplicable anddurable,halogen-free,andheavymetal-freefamilyofnanofibrilofflame-retardantpolysaccharide. Regardingcellulosepaper,aflameretardantbasedonChNFswasshowntobeefficacious.Toexhibita lesspronouncedLOIof30%andanadditional62%lowerpeakheatrelease,the30%retardant-loaded paperdemonstratedself-extinguishingcharacteristics,too.Ontheotherhand,itreallyshouldbeemphasizedthatdeacetylatedChNFsofferasignificantoperationaladvantagewhenitcomestointrinsic fireretardancyowingtotheproductionofammoniafollowingignition,whichimprovesthethermooxidativeendurance [70].Becausethesurface-deacetylatedChNFspossessahigherconcentrationof surfaceaminegroups,whicharereadilychangedintheircounterionconstitutionusingsuitableacidsin thelatestagesoftheirproduction,thissynthesismaybedescribedasfavorablyfromachemicaland metallurgicalviewpoint.SinceChNFsmayofferaremarkableandyetundiscovereddurablematerials foundationforhighlyrequiredhalogen-anddensemetal-freeflame-retardantsubstances,thereisamotivatingfactortostudyandcomprehendthecapabilityofChNFsforfirebarrieragents.Considering heavymetalsandhalogensareexpectedtobeprohibitedinthecomingyearsandaredifficulttorecover, findingalternativeflame-retardantmaterialstoreplacethemwillbeacrucialelementoffosteringabright tomorrow.Inastudy,thephosphate-typecounterionsthatWaltheretal. [71] deliveredtothesurfacedeacetylatedChNFswerecreatedthroughelectrostaticinteractions(ChNF-P).Toprotectpersonnel frompossibleflashfireandspontaneouscombustion,thephosphate-coordinatedChNFsareextremely flameretardant,rapidlyself-extinguishing,andsuperiortoChNFsthathavenoionicgroups.ChNF-Phad thelowestoverallheatreleaseof1900J/gandamaximumheatdischargeof49W/g,representing decreasesof66%and40%,respectively,whencontrastedtoChNFs.Asphosphate-typecounterions acceleratethedehydrationofoxygen-containingbiopolymerswhentheyaretransformedintopotent acidsundercombustion,thisisclearevidenceoftheirbeneficialimpactonthermosoxidativedurability. Beginningdecompositionisrapidandbrief,althoughresiduesarethermallydurable.Theyalsoserveas thermalshieldsthroughoutburningandpreventO2 penetration,decreasingtherateofpyrolysisathigh temperatures.
3.4 Chitosan
Chitosan(comprisingof β-(1–4)-linked D-glucosamineand N-acetyl-D-glucosaminecontingentlyscatteredinsidethepolymer(Fig.6))canbederivedbyincompletedeacetylationofchitin(i.e.,poly(β-(1–4)-N-acetyl-D-glucosamine)),acrucialpolysaccharideandtheglobe’ssecondmostpopularnatural polymerfollowingcellulose.Chitosanisproducedfromnaturalsources,includingcrustaceanshells, shellfish,shrimp,crabs,andprawns,cephalopodspeckers,funguscellwalls,andbugexoskeletons [72].Biotechnologistspreferchitosanoverchitinbecauseofitsgreatersolubilityinwaterandorganic
solvents [73].AbiodegradableandbiocompatiblepolymerandNH2 groupspresentinthepolymer’s structureallowittobemechanicallyandchemicallymodifiedtogeneratenewfunctionalitiesandcharacteristics [72].Withinamotionlessenvironment,theheatbreakdownofchitosanhappensinthree stages.Itisthedischargeoflooselyboundwaterthatisresponsibleforweightlossunder140 °C [73].Dehydration,deacetylation,anddecompositionofchitosanconstitutethesecondandmajorstage ofdisintegration,whichoccursbetween250and350 °C.Asaresultofresidualdisintegration,arelativelylowmasslossisseenat400 °C.Infact,at500 °C,charcontentmayreach40wt%.Itisconsiderablylower(approximately20wt%)wheneverdecompositionhappensintheairenvironment, accordingtoMoussoutetal. [74].Thisworkhasshownthatboththehygroscopicityandthermal durabilityofthecarbohydratewasgreatlyinfluencedbytheextentofdeacetylation.
Duetothedecreasedquantityof N-acetylatedgroupsanditsmajoraminogroups(witha pKa of6.3) whichbecomeprotonated,chitosanisinsolubleinneutralwateranditssolubilityisalleviatedwithacid solutions,includinglactic,acetic,glutamic,andhydrochloricacidsolutions(pHupto6.5),resultingin apositivelychargedpolymerwiththepropertiesofapotentbase.WheneverthepHexceeds6.0,the polysaccharideremainsinsolubleanddropletsowingtothedeprotonationoftheamines.Nevertheless, theprotonationofchitosanrendersitflameresistantinLbLcoveringsonvariouskindsofpolymers [75].Becauseofitscarbonandnitrogencontent,chitosanmaybeusedtocreateflameretardantswith minimalenvironmentaleffect;additionalreactivegroups,likeNH2 andOHmaybeeffectivelyusedto providephosphorus-containingfunctionsthroughchemicalmodificationofthebiomacromolecule.In thismanner,chitosanmaybecombinedwithotheringredientstocreatehigh-performanceflameretardants,oritcanbeconvertedintoequivalentsthatcanbeusedimmediatelytoformulatepowerful flameretardants.However,chitosanalonedoesnothaveinherentflame-retardantproperties.However, itmaybeusedinconjunctionwithothercarbonsourcesinordertocreateastablecharonasubstrate, includingdensepolymer,textile,foam,wood;whencombinedwithflame-retardantadditives;itis possibletocreateflame-retardantformulationsappropriateforavarietyofpolymersubstrates,as describedinthefollowingsections.
CCD(Fig.7)isaflame-retardantadditivebasedonchitosanandDOPOdevelopedbyZhouetal. [76].CCDsignificantlyenhancedtheflameretardancyofepoxyresinsafteritsdebut.EP/10%CCD’s flammabilitycharacteristicsevaluationresultedinaUL94V-0gradingandaLOIscoreof31.6%, whichis7.6%greaterthanthatofthestraightepoxyresin.Findingsoftheconecalorimeteranalysis provethatCCDsubstantiallyreducedtheemissionofheat.
LbLassemblagewasusedbyFangetal. [77] tocreateanintumescentnanocoatingcomprisingchitosan/phyticacidtodecreasetheflammabilityofpolyester/cottonmixtextiles.AnLbLassembly
FIG.6
Structureofchitosan.
SyntheticmethodofCCD [76]
formulationforflame-retardantchitosan-montmorilloniteandchitosan-phyticacidnanocoatingshas beenconfirmed,usedonpolyurethanefoamandpoly(lacticacid)film,aswellasforflame-retardant chitosan-phyticacidintumescentandflame-retardantchitosan-montmorillonitenanocoatingsimplementedwithcottonfiber [78,79].NanocoatingsconsistingofAPPinconjunctionwithchitosan and/orsilicananoparticleswerealsoappliedtopolyester–cottonmixturesusingtheLbLassembly methodin2007 [80].Ignition,consistentcharring,andgeneralfire-retardantcharacteristicswere allenhancedbythepartiallyrecyclableAPP–chitosancovering.Anenvironmentallyfriendlycarbonizationagentbasedonnickel–chitosanphosphateforPVAwassynthesizedbyHuandcoworkers [81]. AstheMCCanalysisshowed,nickelchitosanphosphatemaylowertheheatreleaserateandtotalheat emission,suggestingthatPVA’sflammabilityhasbeensignificantlydecreased.Nickelacceleratedthe productionofchar,whichmaybeutilizedtorestrictthemovementofbulkandenergy,accordingto scientists.Becauseofthis,thethermaldecompositionofPVAwasslowedbynickelchitosanphosphate,aswellastheoverallseverityofthevolatilizedcompounds.Asaresult,thethermalstability ofsubstanceswasfurtherenhancedbythenickelthroughoutthisarrangement.
Cinnamaldehyde
FIG.7
3.5 Starch
Polymericcarbohydratessuchasstarch(ST)aremadeupofmanyglucoseunitsbondedtogetherby glycosidiclinkages(Fig.8A).Numerousgreenplantssynthesizethispolysaccharideforstoringenergy. Duetoitsavailability,flexibility,andbiodegradability,starchisoneofthemostoftenusedrecyclable andbiodegradablepolysaccharides [82].Itsbiologicalandecologicalapplicationshavereceivedalot ofinterestinrecentyears.Intheexistenceofaplasticizer,likewater,indigenousstarchmaybetransformedintothermoplasticstarch.Throughoutthefoodstuff,clothing,papermanufacturingandother sectors,thisparticularcharacteristicmakesitaviableoption [83].STdegradesthermallyduringthree phases.Inthefirstphase,absorbedwaterisreleasedfromthebody,resultinginphysicaldehydration. Thesecondstep,whichbeginsatapproximately300 °C,involveschemicaldehydrationandthermal breakdown,withthermalcompressionamonghydroxylgroupsresultingintheproductionofetherportionsandwaterrelease.TheestablishmentofC]Cconnectionsorringcleavagealsohappenswhen adjacenthydroxylgroupsthroughouttheglucoseringaredehydrated.Underhighertemperatures, aromaticrings,includingthelinkedbenzeneandfuranstructures,areproduced.Attemperaturesover 500 °C,carbonizationprocessesoccur,resultingintheproductionofmassivearomaticcompounds. Thecarbonsource,particularlyforintumescentflame-retardantcompounds,hasbeenmostly employedasstarch.Toenhanceitssolubilityandswellingcapacity,STmaybechemicallytreatedwith phosphatetoproducedistarchphosphate(DSP)orhydroxypropyldistarchphosphate(HPDSP) [84]. Itisalsopossibletousestarchanditsvariantsascarbonizationagentsowingtotheirmultihydroxyl configuration.Forflame-retardantsystems,APPandcarbonsourcesareoftenemployedtogetherasan intumescentflame-retardantsystemthatpromoteschargenerationwhenheated.Itisthuspossiblefor APP,ST,andtheirvariantstoactasintumescentflameretardantsduringimplementation.Polylactic acid(PLA)foamswithaphosphorus-containingflameretardantandstarchasanorganiccharringingredientwerestillbeingproducedin2014,accordingtostudiesdonebyWangetal. [85].Byincreasing theamountofPpresentintheflameretardant,thepolymer’sflammabilityqualitieswereenhanced, butbyaddingstarch,thepolymer’scapabilitieswereenhancedsignificantly.Inastudyconductedby MaqsoodandSeide [86],cornstarchwasusedasabiodegradablecarbonizationingredientinintumescentcompositionsasapossiblerecyclablereplacementforpentaerythritolandPLA/starchmixtures. Catalyticphosphorylationproducedphosphateesters,whichdriedthestarchandcreatedachar frameworkcomprisingresidueupto43%,whichwastheprocessofintumescence(Fig.8B).
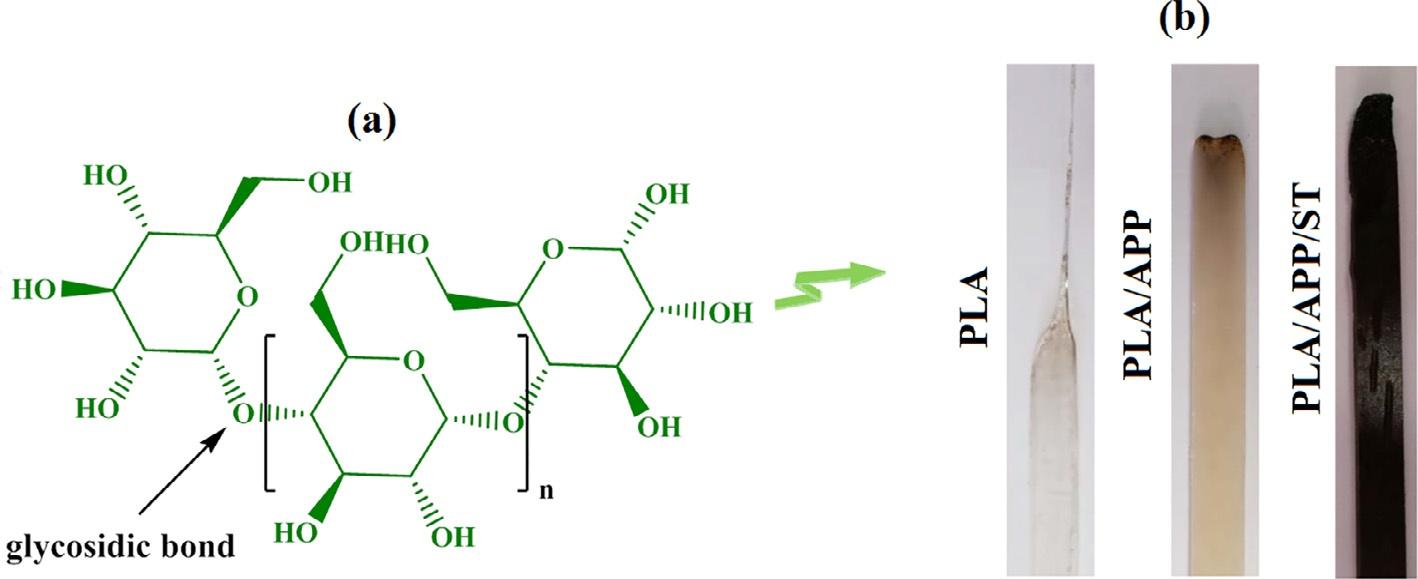
FIG.8
(A)Starchstructureand(B)photographsofpurePLA,PLA/APP,PLA/APP/STcompositesafterUL-94test [86]
3.6 Phyticacid
Duetoitsbiocompatibility,nontoxicity,eco-friendliness,andaccessibility,phyticacid(PA)istheonly onethatmeetstheabovecriteria.Beans,legumes,oilseeds,andcerealgrainsareusedtomakethecompound [87].Inositolhexakisphosphate(IHP)isanothernameforPA.Itcomprises6phosphateand12 hydroxylmoieties,whichmayinteractwithvirtuallyallmetalionsandpositivelychargedmolecules. AvarietyofadvantageshavebeenfoundforPAoritssalts,includingtumorpreventionandcorrosion dissuasionasphytatelayeronmetals.Itsuseasaflameretardanthasalsoattractedresearch,owingto itsuniquestructure,thehighphosphoruscontentof28wt%withrespecttoitsmolecularweight [88], anditsabilitytobindcellulose,proteins,anddifferentfabricssuchascotton,wool,nylon,silk,and poly(lacticacid)nonwovenfabrics,thedemandforPAutilizationasflameretardanthasincreased [89].Asacomponentofalayer-by-layercoatingoncottoncloth,itwasinitiallycombinedwithchitosan.MaterialshavingahighconcentrationofPAareself-extinguishable,andtheirfireefficiencyis superiortothatofmaterialsthatincludemorechitosan.Thesephosphonate–chitosancomplexeshave alsobeenintegratedintoethylene–vinylacetatepolymerstoproduceafire-retardantmaterial.These polymersexhibitincreasedexpansionandstabilityasaresultofphosphorus,nitrogen,andcarbonbeingcombinedwithinthesameframework [90].Aspreviouslyreported,Costesetal. [91] studiedthe applicationofbio-sourcedphytatesasP-comprisingflame-retardantcompoundsforPLA.Theygeneratedaluminum,iron,andlanthanumphytatesfromplant-derivedsodiumphytateoriginatingfrom naturalsources.Theywereabletodothisexperimentbyseparatelyandsimultaneouslyapplying phytatemetalandindigenoussodiumphytatetoPLA.Aluminumphytateincombinationwithsodium phytatehadthegreatestoutcomes,accordingtotheresultsfromtheinvestigation.Asubstantialflameretardantperformancewasobservedbyusingtheformer,whereasthelatterwasneededtoensurethermalstabilitythroughoutmanufacturing.Withthiscomposition,themaximumheatreleaselevelwas substantiallyreducedandaV-2UL94certificationwasobtained.Asaneffectiveflameretardantfor polypropylene,abio-based,phosphorus-containingPAsaltwasproducedbycombiningPAandpiperazine(PHYPI)inanaqueoussolution.WhentestedwithUL94V-0,thepolypropylene/PHYPIspecimenscored25.0%.Comparativelytopurepolypropylene,thepolypropylene/PHYPIexhibited significantlylowerHRRandPHRRvaluesaswellaslowerFGR(firegrowthrate)values [92].Based onPAandmelamine(PA-melamine),Wangandcolleagues [93] developedanadditivetypephyticacid saltintumescentflameretardantthatprovidedepoxywithexcellentflame-retardantandsmokemitigationproperties.Inaddition,theUL94V-0gradewasobtainedusingepoxyincluding6wt% (PA-melamine).PHRR,THR,andtotalsmokeproduction(TSP)weredecreasedby62.3%,9.9%, and62.16%,respectively,comparedtothoseofthepristineEP(Fig.9).
3.7 DNA
Biochemically,alllivingcreaturescontaindeoxyribonucleicacid.Itcontainsgeneticinformation,and thereforeitisseenasconstitutingeachindividual’spersonality.Nucleotiderepeatingunitsareorganizedinadoublehelixinthislengthypolymer.Thenucleotidesincludenitrogen-containingnucleobases(i.e.,cytosine,guanine,adenine,andthymine),togetherwithafive-carbonsugar(deoxyribose), andaphosphategroup(Fig.10).
Consideringitssimpleseparationfromherringspermandroesacs,thedilutedDNAmoleculeis nowaprominentmoleculeforfireretardancy.DNAoperatesasasingle-biomolecularintumescent system,havingalltherequiredingredientsofanefficientintumescentsystem.Bywayofphosphoric