https://ebookmass.com/product/benzodiazepine-based-drug-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Imidazole-Based Drug Discovery (Heterocyclic Drug Discovery) Shikha Agarwal
https://ebookmass.com/product/imidazole-based-drug-discoveryheterocyclic-drug-discovery-shikha-agarwal/
ebookmass.com
Piperidine-Based Drug Discovery Ruben Vardanyan
https://ebookmass.com/product/piperidine-based-drug-discovery-rubenvardanyan/
ebookmass.com
Peptide based Drug Discovery: Challenges and New Therapeutics (ISSN Book 59) 1st Edition, (Ebook PDF)
https://ebookmass.com/product/peptide-based-drug-discovery-challengesand-new-therapeutics-issn-book-59-1st-edition-ebook-pdf/
ebookmass.com
Biology. Concepts & Applications 10th Edition Cecie Starr
https://ebookmass.com/product/biology-concepts-applications-10thedition-cecie-starr/
ebookmass.com
The Holloway Girls Susan Bishop Crispell
https://ebookmass.com/product/the-holloway-girls-susan-bishopcrispell-3/
ebookmass.com
The Many and the One: A Philosophical Study of Plural Logic Salvatore Florio
https://ebookmass.com/product/the-many-and-the-one-a-philosophicalstudy-of-plural-logic-salvatore-florio/
ebookmass.com
eTextbook 978-1305880412 New Perspectives Microsoft Office 365 & Excel 2016: Intermediate
https://ebookmass.com/product/etextbook-978-1305880412-newperspectives-microsoft-office-365-excel-2016-intermediate/
ebookmass.com
The Ancient Celts, Second Edition Barry Cunliffe
https://ebookmass.com/product/the-ancient-celts-second-edition-barrycunliffe/
ebookmass.com
Networking Essentials: A CompTIA Network+ N10-006 Textbook 4th Edition Beasley
https://ebookmass.com/product/networking-essentials-a-comptianetwork-n10-006-textbook-4th-edition-beasley/
ebookmass.com
https://ebookmass.com/product/introduction-to-forensic-psychologyresearch-and-application-null/
ebookmass.com
BENZODIAZEPINE-BASED DRUGDISCOVERY HeterocyclicDrugDiscoverySeries BENZODIAZEPINE-BASED DRUGDISCOVERY FARZADZAMANI SANKEN,OsakaUniversity,Mihogaoka,Ibaraki-shi,Osaka,Japan
ESMAILDOUSTKHAH KoçUniversityTüpra¸sEnergyCenter(KUTEM),DepartmentofChemistry, KoçUniversity,Istanbul,Turkey
SeriesEditor
RUBENVARDANYAN
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorage andretrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowto seekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandour arrangementswithorganizationssuchastheCopyrightClearanceCenterandthe CopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyright bythePublisher(otherthanasmaybenotedherein).
Notices Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchand experiencebroadenourunderstanding,changesinresearchmethods,professionalpractices, ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgein evaluatingandusinganyinformation,methods,compounds,orexperimentsdescribed herein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirownsafety andthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatter ofproductsliability,negligenceorotherwise,orfromanyuseoroperationofanymethods, products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-12-824516-3
ForInformationonallElsevierpublicationsvisitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: GabrielaD.Capille
EditorialProjectManager: AndraeAkeh
ProductionProjectManager: BharatwajVaratharajan
CoverDesigner: MarkRogers
TypesetbyAptara,NewDelhi,India
Contributors FatemehAhmadi
DepartmentofMaterialsScience,ScienceandResearchBranch,IslamicAzadUniversity, Tehran,Iran
ElahehAkbarzadeh
DepartmentofOrganicChemistry,FacultyofChemistry,KharazmiUniversity,Tehran,Iran
FatemehM.Arlan
DepartmentofChemistry,AcademicCenterforEducation,CultureandResearch,Urmia, Iran
RonaldW.Brown
BayerAG,FrankfurtamMain,Germany
EsmailDoustkhah KoçUniversity,Tüpra¸sEnergyCenter(KUTEM),DepartmentofChemistry,Koç University,Istanbul,Turkey
MohammadHeidarizadeh
DepartmentofBiotechnology,FacultyofBiologicalScienceandTechnology,Universityof Isfahan,Isfahan,Iran
ChristopherJ.T.Hyland
SchoolofChemistryandMolecularBioscience,UniversityofWollongong,Wollongong, NSW,Australia
ArashJanaty
DepartmentofBiologicalSciencesandBiotechnology,FacultyofSciences,Universityof Kurdistan,Sanandaj,Iran
RaminJavahershenas
DepartmentofOrganicChemistry,UrmiaUniversity,Urmia,Iran
RafaelLuque
DepartamentodeQuimicaOrganica,UniversidaddeCórdoba,CampusdeRabanales, EdificioMarieCurie,Córdoba,Spain.
SaeedehMohammadi
DepartmentofPhysics,ShahidRajaeeTeacherTrainingUniversity,Lavizan,Tehran,Iran
ZahraNikfarjam
ChemistryandChemicalEngineeringResearchCenterofIran(CCERCI),Tehran,Iran
TakayoshiSuzuki SANKEN,OsakaUniversity,Mihogaoka,Ibaraki-shi,Osaka,Japan
AbtinTavakoli
DepartmentofBiologicalSciencesandBiotechnology,FacultyofSciences,Universityof Kurdistan,Sanandaj,Iran
Contributors
RajenderS.Varma
RegionalCentreofAdvancedTechnologiesandMaterials,CzechAdvancedTechnology andResearchInstitute,PalackýUniversityinOlomouc,Olomouc,CzechRepublic
MasoudYarmohammadi
FacultyofMechanicalEngineering-EnergyDivision,K.N.ToosiUniversityofTechnology, Tehran,Iran
FarzadZamani
SANKEN,OsakaUniversity,Mihogaoka,Ibaraki-shi,Osaka,Japan
NasrinZamani
GrowthandDevelopmentResearchCenter,TehranUniversityofMedicalSciences, Tehran,Iran
Contributorsix
Preface xi
Acknowledgmentxiii
1.Anintroductiontobenzodiazepinesandbenzothiazepines1
FarzadZamaniandEsmailDoustkhah
1.1 Benzodiazepines1
1.2 Benzothiazepines4 References5
2.Structuralfeaturesof1,4-benzodiazepines9
FarzadZamani,FatemehM.Arlan,RaminJavahershenas, MasoudYarmohammadi,RajenderS.VarmaandEsmailDoustkhah
2.1 Introduction9
2.2 Chemicalstructuralvariationsof1,4-benzodiazepines11
2.3 Conformationalstudiesof1,4-benzodiazepines20
2.4 Polarityandchargedistributionof1,4-benzodiazepines26
2.5 1,4-Benzothiazepines28
2.6 Conclusion29 References29
3.Synthesisof1,4-benzodiazepinesand1,4-benzothiazepines35
FarzadZamani,RaminJavahershenas,FatemehM.Arlan, ChristopherJ.T.HylandandEsmailDoustkhah
3.1 Introduction35
3.2 Synthesisof1,4-benzodiazepines37
3.3 Synthesisoffused1,4-benzodiazepines50
3.4 Synthesisof1,4-benzothiazepines65
3.5 Conclusionandfuturedirections70 References70
4.Biologicalbehaviorof1,4-benzodiazepines and1,4-benzothiazepines77
FarzadZamani,NasrinZamani,TakayoshiSuzukiandEsmailDoustkhah
4.1 Introduction77
4.2 Metabolismof1,4-benzodiazepinesand1,4-benzothiazepines78
4.3 GABAmetabolismandGABAreceptors80
4.4 Mechanismofactionof1,4-benzodiazepinesonGABAA receptors85
4.5 Moleculareffectsofchronic1,4-benzodiazepinesexposure86
4.6 Effectof1,4-benzodiazepinesoncholecystokininreceptors100
4.7 Effectof1,4-benzodiazepinesonopioidreceptors106
4.8 Effectof1,4-benzothiazepinesoncellularreceptors108
4.9 Conclusion111
References111
5.Pharmaceuticalapplicationsof1,4-benzodiazepines125 ZahraNikfarjam,EsmailDoustkhah,FarzadZamaniandRonaldW.Brown
5.1 Introduction125
5.2 Short-acting1,4-benzodiazepines126
5.3 Intermediate-acting1,4-benzodiazepines133
5.4 Long-acting1,4-benzodiazepines147
References172
6.Structuralfeaturesof1,5-benzodiazepines and1,5-benzothiazepines183 FarzadZamani,EsmailDoustkhahandRafaelLuque
6.1 Introduction183
6.2 Chemicalpropertiesof1,5-benzodiazepines185
6.3 Chemicalpropertiesof1,5-benzodiazepinones188
6.4 Conformationalstudiesof1,5-benzodiazepines and1,5-benzodiazepinones191
6.5 Chemicalpropertiesof1,5-benzothiazepines193
6.6 Conclusion196
References196
7.Synthesisof1,5-benzodiazepinesand1,5-benzothiazepines199 FarzadZamani,EsmailDoustkhah,FatemehAhmadi andRajenderS.Varma
7.1 Introduction199
7.2 Synthesisof1,5-benzodiazepines201
7.3 Synthesisof1,5-benzothiazepines225
7.4 Futuredirections244
References244
8.Biologicalbehaviorof1,5-benzodiazepines and1,5-benzothiazepines249 MohammadHeidarizadeh,SaeedehMohammadi,ArashJanaty, AbtinTavakoli,NasrinZamani,EsmailDoustkhahandFarzadZamani
8.1 Introduction250
8.2 Interactionof1,5-benzodiazepinesand1,5-benzothiazepines withcellreceptors250
8.3 Interactionof1,5-benzodiazepinesand1,5-benzothiazepines withenzymes261
8.4 Interactionof1,5-benzodiazepinesand1,5-benzothiazepines withionchannels270
8.5 Interactionof1,5-benzodiazepinesand1,5-benzothiazepines withplatelets274
8.6 Conclusion275 References276
9.Pharmaceuticalapplicationsof1,5-benzodiazepines283 FarzadZamani,EsmailDoustkhahandRonaldW.Brown
9.1 Introduction283
9.2 Clobazam284 References290
10.Pharmaceuticalapplicationsof1,5-benzothiazepines295 ElahehAkbarzadeh
10.1 Introduction296
10.2 Diltiazem296
10.3 Clentiazem303
10.4 Thiazesim306
10.5 Quetiapine307
10.6 Clotiapine313 References316 Index 321
Preface Benzodiazepinesandbenzothiazepinesaretwopivotalclassesofheterocycliccompoundswidelyusedascorestructuresofvariousdrugsto treatdepression,epilepsy,seizures,andmusclespasms.Accordingly,these versatileskeletonsareinacontinualstreamtoreceiveup-to-dateadvances inmedicinalresearchbysynthesizingandscreeningtheiranalogsfornovel applicationsindrugdiscovery.Despitethehighimportanceofbenzodiazepinesandbenzothiazepinesinpsychoactivedrugsinthemarket,there isnoupdatedreferenceresourcewithcomprehensivecoverageanddetails inthecaseofbenzodiazepine/benzothiazepine-baseddrugs.Severalmedical bookshavebeenrecentlywritten,focusingsolelyontheadverseeffectsof benzodiazepinesaspartofadiscussionoftheproblemswithpsychotropic drugs.Furthermore,someothershaveexclusivelydiscussedthepharmaceuticalaspectsofbenzodiazepines.Thisbookisthefirsttodemonstrate detailedchemical–pharmaceuticalfeaturesofbothbenzodiazepinesand benzothiazepines,includingtheirsyntheticprocedures,structuralfeatures, pharmacokineticsandpharmacodynamics,clinicaluses,andadverseeffects. Webelievethatthisbookwouldbeanall-inclusiveresourceforscientists interestedinthedesignanddevelopmentofinnovativedrugsbasedonbenzodiazepinesandbenzothiazepines,researchersandundergraduate/graduate studentsengagedinorganicandmedicinalchemistry,andR&Dexpertsin thepharmaceuticalindustry.
First,wewishtoexpressoursincerethankstoProfessorRubenVardanyan(UniversityofArizona)forhisvaluablesupportandencouragement. Second,wethankallourcontributorswhohelpedusinwritingthisbook. Specialappreciationalsogoestothepublishinghouse,Elsevier,fortheir exemplarycollaborationandsupport.Lastbutnotleast,wewouldliketo thankourfamilies.Thisbookwouldneverhavebeenachievedwithouttheir warmencouragementandsteadysupport.
FarzadZamani
Acknowledgment FarzadZamaniacknowledgestheJapanSocietyforthePromotion ofScience(JSPS)forprovidingtheJSPSstandardpostdoctoralfellowship. EsmailDoustkhahacknowledgestheTÜBITAKandHorizon-2020Marie SkłodowskaCurieforprovidingthefinancialsupportinCo-FundedBrain CirculationProgram(ProjectNo.120C057)framework.
Anintroductionto benzodiazepinesand benzothiazepines FarzadZamani a andEsmailDoustkhah b
a SANKEN,OsakaUniversity,Mihogaoka,Ibaraki-shi,Osaka,Japan
b KoçUniversityTüpra¸sEnergyCenter(KUTEM),DepartmentofChemistry,KoçUniversity,Istanbul, Turkey
Chapteroutline 1.1
1.1Benzodiazepines Benzodiazepinesaspharmaceuticalcompoundshaveemergedasa powerfultoolforcuringanxietydisorders.Accordingtoanational12-month surveyconductedbytheUSNationalComorbiditySurveyReplication (NCS-R)andtheNationalInstituteofMentalHealth(NIMH),approximately40%ofpeopleintheUSsufferfromgeneralizedanxietydisorder (GAD),panicdisorder,post-traumaticstressdisorder(PTSD),obsessivecompulsivedisorder(OCD),oranyothertypesofmentalailments(Kessler, Chiu,Demler,&Walters,2005).Traditionalclinicaltreatmentsforanxiety comprisedofconsuminggeneralsedativessuchasopiates,alcohol,chloral hydrate,andlithiumbromide.Theseanxiolyticshoweverdisplayedlimited efficacyalongwithseveraldrawbackssuchasdizziness,impairedcognitive functions,sexualdysfunction,death,andotheradverseeffects.Inthisregard, thedevelopmentofefficienttreatmentsforanxietydisordershasalwaysbeen ahottopicinmedicinalresearch.
Benzodiazepines(BDZs)areafamilyofbicyclicheterocyclesconsisting ofabenzeneringfusedtoadiazepineunit.AccordingtoHantzsch–WidmansystematicchemicalnomenclaturerecommendedbyTheInternationalUnionofPureandAppliedChemistry(IUPAC),the“benzo” prefixaccountsforthebenzenering,andthediazepinereferstothesevenmemberedheterocyclewithtwonitrogenandfivecarbonatomsforming
Benzodiazepine-BasedDrugDiscovery. Copyright c 2022ElsevierInc. DOI: https://doi.org/10.1016/B978-0-12-824516-3.00007-0
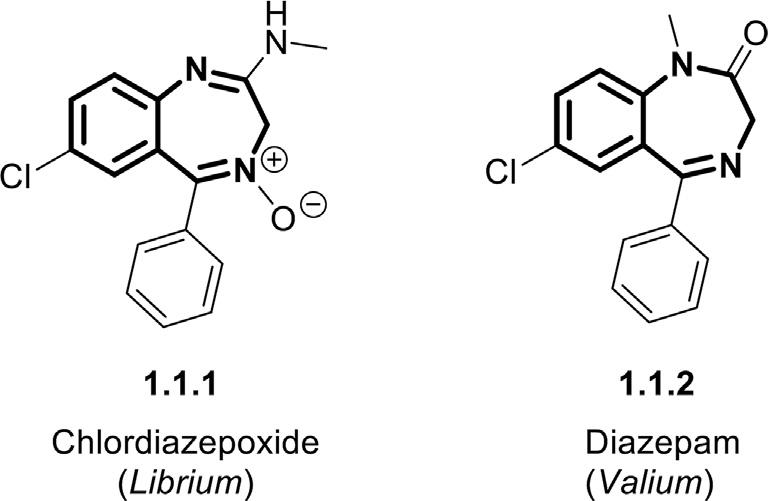
Figure1.1 Chemicalstructuresoffirstmarketedbenzodiazepines,chlordiazepoxide (Librium),anddiazepam(Valium).
themaximumnumberofcontiguousdoublebonds(Samardzic&Strac, 2016; Schütz,1982).Changingthepositionofthenitrogenatomsresults intheformationofdifferentclassesofthesebenzo-fuseddiazepines.Inthis regard,therearefourmaingroupsofBDZsdependinguponthenitrogen positions:(1,4)-,(1,5)-,(2,4)-,and(2,5)-BDZs.Amongthem,theisomers (1,4)and(1,5)arethemostcommonbenzodiazepinesinbothchemical andmedicinalresearch.TheiminebondintheBDZsskeleton,especially in1,4-benzodiazepines,isprovedtobeabio-reactivesite,whichinitiates thebiologicalprocessestothesemoleculesaftermetabolism(Kuch,1979). Thefirstbenzodiazepinecompound,chlordiazepoxide(1.1.1, Fig.1.1),was serendipitouslydiscoveredbyanAustralianchemist,Dr.LeoSternbach,and hisresearchgroupinHoffmann-LaRochelaboratoriesin1957,wherethey wereworkingonthesynthesisofsomenewquinazoline-basedtranquilizers (Sternbach,1979; Sternbach&Reeder,1961).Duringthisresearchproject,a nicelypurewater-solublecrystalwasunexpectedlyformedasaby-product. Itwassoonrealizedthatthiscompounddisplaysoutstandingsedativeandanticonvulsanteffectsinmice.Afterthisinitialdiscovery,Hoffmann-LaRoche initiatedclinicalstudiesonthechlordiazepoxide,wherealargenumberof patientswerebeingtreatedwiththisdrug.Theseexceptionalstudiesled tointroducethecompound 1.1.1 [7-chloro-2-(methylamino)-5-phenyl3H-l,4-benzodiazepine4-oxidel]asananxiolyticunderthetrademarkof Librium in1960.Comparedtotheconventionalantidepressantsandsedatives, Librium provedtobeapromisingnewtypeofeffectiveanxiolyticwithfewer adverseeffectsinshort-termtreatment.Bypursuingthestructuralalteration ofthechlordiazepoxideinordertoimproveitsbiologicalperformance, diazepam(Valium, 1.1.2)wassynthesizedinRoche’slaboratoriesin1963 asamorepotentbenzodiazepine(Fig.1.1).Installingacarbonylgroupon
the2-positionofthequinoxalineringresultedinsignificantlyimproved bioactivityof Valium,whichquicklybecamethemostfrequentlyprescribed seductivedrugforthetreatmentofnotonlyanxietybutalsoepilepsyand musclespasmsinthe1970s(Calcaterra&Barrow,2014),reachedapeakof 2.3billiontabletssoldintheUSin1978.
Followingthesuccessof Valium,morethan20benzodiazepineanalogs wereapprovedforhumanuseundervariousauthorities(Lader,1991).Since the1960s,BZDsquicklyhavegainedgreatprevalenceandtheirconsumption asdrugshavebeendramaticallyboostinguntiltoday.Despitemanyhelpful applicationsofBZDsinthetreatmentofanxietydisorders,theextraordinary growthofBDZsusagehasraisedconcernsindifferentcountriesdueto theirhighprescriptionrateandthepotentialincompensableconsequences associatedwiththeirlong-termconsumptionsuchasaddiction,cognitive impairments,paradoxicalreactions,depression,physicaldependence,dementia,andcancer(Penninkilampi&Eslick,2018; Schmitz,2016).Benzodiazepinesconsumptionisrelativelysafeforamaximumdurationoftwoto fourweeks,andseveralphysicalandpsychologicalhealthrisksmayappearin approximately50%ofthosepatientswhousebenzodiazepinesformorethan onemonth(Lader,2011).Nevertheless,benzodiazepineshavestillcontinued tobeoneofthehighlyprescribeddrugsofalltimeinmanycountries. Forexample,arecentstudydisclosedthat30.6millionadultshaveused benzodiazepinesintheUSin2018,including25.3millionareprescribed and5.3millionhavemisusecases(Agarwal&Landon,2019).
Althoughbenzodiazepinesquicklybecameoneofthetop-sellingdrugs inmedicaltreatment,ittookabout15yearstodiscovertheirmechanismof action.TheearliestreportbyRochein1974demonstratedthatdiazepam (1.1.2)mightalterthebehavioroftheGABA(gamma-aminobutyricacid) receptorsinthespinalcord(Polcetal.,1974).Tounderstandthemechanism ofactionofbenzodiazepines,itisnecessarytounderstandthefunctionof GABAreceptors.GABAisoneofthemaininhibitoryneurotransmittersin thenervecellsreleasedbythebrain.Theyplayapivotalroleincontrolling neuronalexcitabilityviabindingtotheirreceptors,GABAA andGABAB (Wangetal.,2015).TheGABAA receptorsarechloride-permeablechannels, whicharesusceptibletogetmodifiedbyvariousligandsthroughallosteric sitesinthereceptor.UponbindingtheGABAtothereceptors,transferring thechlorideions(Cl¯)throughneuronalcellmembranesisfacilitated, causingneuronalinhibition.Disruptionofthesereceptorsinananxiety situationleadstodifferentneurologicaldisorders(Nemeroff,2003).In thiscase,benzodiazepinesareperfecttherapeuticagentsthatcouldserve
aspositiveallostericregulatorsoftheGABAreceptorsviahavingseveral interactionswiththespecificsitesonthe α -γ subunitinterface.Uponthese interactions,theGABAcomplexundergoesaconformationalmodification thatincreasesthechlorideioninfluxinneurons,leadingtohyperpolarizing postsynapticmembranesandsubsequentlyimprovingtheresponseofthe centralnervoussystem(CNS)depressiontoendogenousGABA(Nutt& Malizia,2001).Theseeventsoccurredinthelimbicsystemfinallycause anxiolyticeffectsthatendthestateofanxiety(Zakusovetal.,1977). Substitutionpatternsonthecorestructureofbenzodiazepinescangenerally affecttheirbindingcapabilitytotheGABAreceptors,modulatingtheir therapeuticandpharmacologicaleffectssuchasthepotencyandduration oftheeffect.Forexample,thebenzo-fusedringwithelectron-withdrawing groupsatthe7-positionand/orthependantphenylgroupwitheither nosubstitutionor ortho-halosubstitutionresultsinsignificantlyimproved anxiolyticperformanceinbenzodiazepines(Sternbach,1979).Furthermore, removalofthependantphenylgroupfrombenzodiazepinescanchangetheir mechanismofaction,inwhichtheybecomeneutralallostericmodulators provedtobevaluableradiotracersforGABAreceptors(Brogden&Goa, 1988; Lassenetal.,1995).
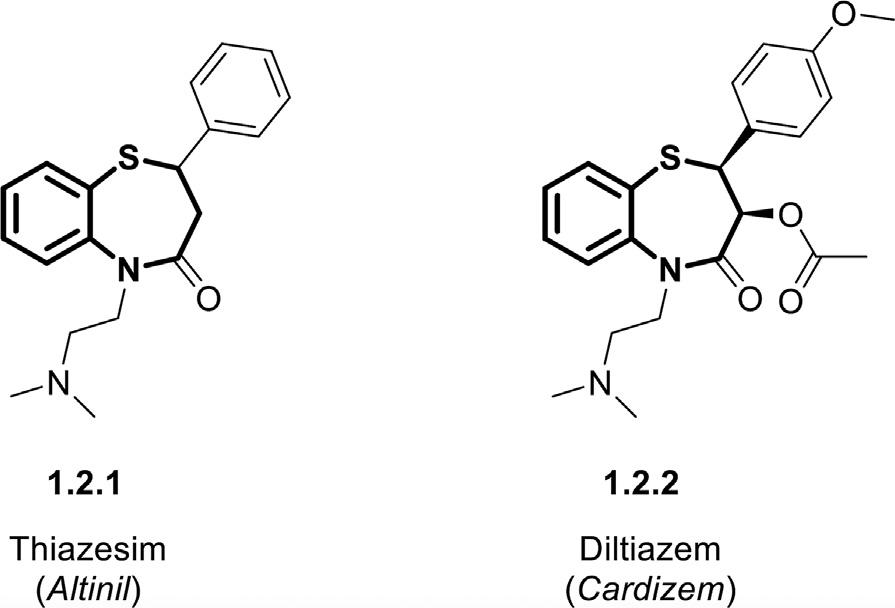
1.2Benzothiazepines Benzothiazepinesstructurallyresemblebenzodiazepines,composed ofbicyclicheterocyclesconsistingofabenzeneunitfusedtoathiazepinering.Dependingontheheteroatom’spositiononthering,differentnomenclaturescanpossess.Amongthem,1,4-benzothiazepinesand 1,5-benzothiazepinesarethemostcommonstructuralisomersforthese heterocyclicscaffolds.Thelast50yearshavewitnessedsubstantialprogress inthechemistryofbenzothiazepinesledtothesynthesisofvariousanalogs andthediscoveryoftheirbiologicalactivities.Theseversatileskeletonshave representedawiderangeoftherapeuticfunctionssuchasCNSdepressant, antimicrobial,antifungal,antiplateletaggregation,anti-HIV,Ca+2 antagonist, calmodulinantagonist,andbradykininreceptorantagonistactivities(Bariwal etal.,2008; Sahaetal.,2015).Thefirstexampleofbenzodiazepinesintroducedintothepharmaceuticalmarketwasthiazesim(Altinil, 1.2.1)as anantipsychoticagentforCNSdisorders(Geyeretal.,1970),followedby diltiazem(Cardizem, 1.2.2)developedasacardiovasculardrugofthisfamily (Fig.1.2)(Nagaoetal.,1972).Benzothiazepines,whicharebioisostersof
Figure1.2 Chemicalstructuresoffirstmarketedbenzothiazepines,thiazesim(Altinil), anddiltiazem(Cardizem).
benzodiazepines,representasimilarmechanismofactionasCNSdepressants andanticonvulsantagents(Nikaljeetal.,2016; Parjaneetal.,2020; Sarroet al.,1995).TheyactaspositiveallostericregulatorsviabindingtotheGABAA receptors,whichfacilitatestheGABA-mediatedCl– channelopeningin theneuronalcellmembranes.Thisphenomenonincreasesthechlorideions influxinthecells,leadingtoinducingsedativeandhypnoticproperties.
Thepresentbookaimstoevidentlyunitebothsynthesisandpharmaceuticalaspectsofbenzodiazepine-andbenzothiazepine-basedstructures.Here, weareprovidingacomprehensivevisionofchemicalstructuralproperties, recentandgeneralsyntheticroutes,mechanismofbiologicalmechanisms, andpharmaceuticaleffects.Althoughalargenumberofefficientsynthetic proceduresandbiologicalactivityprofilesforbenzodiazepinesandbenzothiazepinearealreadydemonstrated,weexpectthatthecompilingmedicinal researchfindingsalongwiththerecentprogressinorganicsynthesisof benzodiazepine-andbenzothiazepine-basedcompoundswillopenawindowtonewaspectsoftheseinterestingheterocyclesinthedrugdiscovery process.
References Agarwal,S.D.,&Landon,B.E.(2019).Patternsinoutpatientbenzodiazepineprescribing intheUnitedStates. JAMANetworkOpen,2(1),e187399. https://doi.org/10.1001/ jamanetworkopen.2018.7399.
Bariwal,J.B.,Upadhyay,K.D.,Manvar,A.T.,Trivedi,J.C.,Singh,J.S.,Jain,K.S.,& Shah,A.K.(2008).1,5-Benzothiazepine,aversatilepharmacophore:areview. EuropeanJournalofMedicinalChemistry,43(11),2279–2290. https://doi.org/10.1016/j.ejmech. 2008.05.035
Brogden,R.N.,&Goa,K.L.(1988).Flumazenil.Apreliminaryreviewofitsbenzodiazepine antagonistproperties,intrinsicactivityandtherapeuticuse. Drugs,35(4),448–467. https://doi.org/10.2165/00003495-198835040-00004
Calcaterra,N.E.,&Barrow,J.C.(2014).Classicsinchemicalneuroscience:Diazepam(valium). ACSChemicalNeuroscience,5(4),253–260. https://doi.org/10.1021/cn5000056
Geyer,H.M.,Watzman,N.,&Buckley,J.P.(1970).Effectsofatranquilizerandtwo antidepressantsonlearnedandunlearnedbehaviors. JournalofPharmaceuticalSciences, 59(7),964–968. https://doi.org/10.1002/jps.2600590709
Kessler,R.C.,Chiu,W.T.,Demler,O.,&Walters,E.E.(2005).Prevalence,severity,andcomorbidityof12-monthDSM-IVdisordersintheNationalComorbiditySurveyReplication. ArchivesofGeneralPsychiatry,62(6),617 627. https://doi.org/10.1001/archpsyc.62.6.617 Kuch,H.(1979).Clobazam:Chemicalaspectsofthe1,4-and1,5-benzodiazepines. BritishJournalofClinicalPharmacology,7(1),17S–21S. https://doi.org/10.1111/j.1365-2125. 1979.tb04659.x.
Lader,M.(1991).Historyofbenzodiazepinedependence. JournalofSubstanceAbuseTreatment, 8(1–2),53–59. https://doi.org/10.1016/0740-5472(91)90027-8
Lader,M.(2011).Benzodiazepinesrevisited-willweeverlearn? Addiction,106(12),2086–2109. https://doi.org/10.1111/j.1360-0443.2011.03563.x
Lassen,N.A.,Bartenstein,P.A.,Lammertsma,A.A.,Prevett,M.C.,Turton,D.R.,Luthra,S.K., ...Patsalos,P.N.(1995).Benzodiazepinereceptorquantificationinvivoinhumansusing [11 C]flumazenilandPET:applicationofthesteady-stateprinciple. JournalofCerebralBlood FlowandMetabolism,15(1),152–165. https://doi.org/10.1038/jcbfm.1995.17.
Nagao,T.,Sato,M.,Iwasawa,Y.,Takada,T.,&Ishida,R.(1972).Studiesonanew1,5benzothiazepinederivative(CRD-401).3.EffectsofopticalisomersofCRD-401on smoothmuscleandotherpharmacologicalproperties. JapaneseJournalofPharmacology, 22(4),467–478. https://doi.org/10.1254/jjp.22.467
Nemeroff,C.B.(2003).TheroleofGABAinthepathophysiologyandtreatmentofanxiety disorders. PsychopharmacologyBulletin,37(4),133–146.
Nikalje,A.P.G.,Ghodke,M.S.,Khan,F.A.K.,&Sangshetti,J.N.(2016).Microwaveassisted facilesynthesisandbiologicalevaluationofnovel2-indolyl-1,5-benzothiazepines. Open PharmaceuticalSciencesJournal,6,117–130. https://doi.org/10.2174/1874844901603010117
Nutt,D.J.,&Malizia,A.L.(2001).NewinsightsintotheroleoftheGABAAbenzodiazepinereceptorinpsychiatricdisorder. BritishJournalofPsychiatry,179(5),390–396. https://doi.org/10.1192/bjp.179.5.390
Parjane,S.K.,Kunkulol,R.,&Nandal,D.(2020).Synthesisofsomenovel1,5-benzothiazepine derivativesbiologicalscreeningforanticonvulsantactivity. IOSRJournalofApplied Chemistry,13(3),50–55. https://doi.org/10.9790/5736-1303015055 Penninkilampi,R.,&Eslick,G.D.(2018).Asystematicreviewandmeta-analysisoftherisk ofdementiaassociatedwithbenzodiazepineuse,aftercontrollingforprotopathicbias. CNSDrugs,32,485–497. https://doi.org/10.1007/s40263-018-0535-3 Polc,P.,Mohler,H.,&Haefely,W.(1974).Theeffectofdiazepamonspinalcordactivities: Possiblesitesandmechanismsofaction. Naunyn-Schmiedeberg’sArchivesofPharmacology, 284,319–337. https://doi.org/10.1007/BF00504702 Saha,D.,Jain,G.,&Sharma,A.(2015).Benzothiazepines:chemistryofaprivilegedscaffold. RSCAdvances,5(86),70619–70639. https://doi.org/10.1039/C5RA12422K
Samardzic,J.,&Strac,D.S.(2016).Benzodiazepinesandanxietydisorders:fromlaboratoryto clinic.InF.Durbano,&B.Marchesi(Eds.), Newdevelopmentsinanxietydisorders.London: IntechOpen https://doi.org/10.5772/64959
Sarro,G.D.,Chimirri,A.,Sarro,A.D.,Gitto,R.,Grasso,S.,&Zappalà,M.(1995). 5H-[1,2,4]Oxadiazolo[5,4-d][1,5]benzothiazepinesasanticonvulsantagentsinDBA/2 mice. EuropeanJournalofMedicinalChemistry,30(12),925–929. https://doi.org/10.1016/ 0223-5234(96)88311-5
Schmitz,A.(2016).Benzodiazepineuse,misuse,andabuse:areview. MentalHealthClinician, 6(3),120–126. https://doi.org/10.9740/mhc.2016.05.120
Schütz,H.(1982). Benzodiazepines (1sted.).BerlinHeidelberg:Springer-Verlag https:// doi.org/10.1007/978-3-642-68426-5
Sternbach,L.H.(1979).Thebenzodiazepinestory. JournalofMedicinalChemistry,22(1),1–7. https://doi.org/10.1021/jm00187a001.
Sternbach,L.H.,&Reeder,E.(1961).Quinazolinesand1,4-benzodiazepines.II.Rearrangementof6-chloro-2-chlormethyl-4-phenylquinazoline3-oxideinto2-aminoderivatives of7-chloro-5-phenyl-3H-1,4-benzodiazepine4-oxide. JournalofOrganicChemistry,26(4), 1111–1118. https://doi.org/10.1021/jo01063a034
Wang,P.,Eshaq,R.S.,Meshul,C.K.,Moore,C.,Hood,R.L.,&Leidenheimer,N.J.(2015). Neuronalgamma-aminobutyricacid(GABA)typeAreceptorsundergocognateligand chaperoningintheendoplasmicreticulumbyendogenousGABA. FrontiersinCellular Neuroscience,9,188. https://doi.org/10.3389/fncel.2015.00188. Zakusov,V.V.,Ostrovskaya,R.U.,Kozhechkin,S.N.,Markovich,V.V.,Molodavkin,G.M., &Voronina,T.A.(1977).FurtherevidenceforGABA-ergicmechanismsintheaction ofbenzodiazepines. ArchivesInternationalesDePharmacodynamieEtDeTherapie,229(2), 313–326.
Non-PrintItems Abstract
Thischapterbrieflyexplainsthehistory,mechanismofaction,andmedicalapplications ofbenzodiazepinesandbenzothiazepines.
Keywords Benzodiazepines;Benzothiazepines;GABAA receptors;CNSdrugs.
Structuralfeaturesof 1,4-benzodiazepines FarzadZamani a,FatemehM.Arlan b,RaminJavahershenas c , MasoudYarmohammadi d,RajenderS.Varma e and EsmailDoustkhah f
a SANKEN,OsakaUniversity,Mihogaoka,Ibaraki-shi,Osaka,Japan
b DepartmentofChemistry,AcademicCenterforEducation,CultureandResearch,Urmia,Iran
c DepartmentofOrganicChemistry,UrmiaUniversity,Urmia,Iran
d FacultyofMechanicalEngineering-EnergyDivision,K.N.ToosiUniversityofTechnology,Tehran,Iran
e RegionalCentreofAdvancedTechnologiesandMaterials,CzechAdvancedTechnologyandResearch Institute,PalackýUniversityinOlomouc,Olomouc,CzechRepublic
f KoçUniversityTüpra¸sEnergyCenter(KUTEM),DepartmentofChemistry,KoçUniversity,Istanbul, Turkey
Chapteroutline 2.1
2.2
2.3
2.3.1 Conformationalstatusof1,4-benzodiazepin-2-ones
2.3.2 Conformationalstatus1,4-benzodiazepine-2,5-diones
2.4
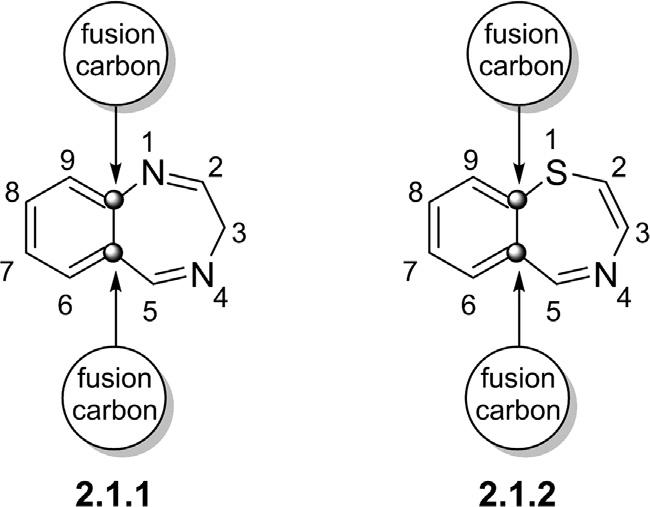
Benzodiazepinesandbenzothiazepinesaretwopharmaceuticallyimportantheterocycliccompoundswhereabenzeneringisfusedtoeithera diazepineorathiazepinering,respectively(Sahaetal.,2015).Theposition ofheteroatomsintheseven-memberedringdeterminesthetypeandthe chemistryofthesecyclo-condensedcompounds.AccordingtoIUPAC, whenaheterocyclicringisfusedwithabenzeneunit,ithastobeindicated bythe“benzo”prefix.Thenumberingofbenzodiazepinesandbenzothiazepinesshouldbestartedfromtheimmediateheteroatomadjacenttothe
Figure2.1 Thegeneralstructuresof1,4-benzodiazepine(2.1.1)and 1,4-benzothiazepine(2.1.2). fusioncarbonatomwithnogivennumbertothefusionatoms(Fig.2.1). 1,4-Benzodiazepines(1,4-BDZs) 2.1.1 and1,4-benzothiazepines 2.1.2 are themostcommonstructuralisomersofbenzodiazepinesandbenzothiazepineswiththeheteroatomsatpositions1and4(Fig.2.1).
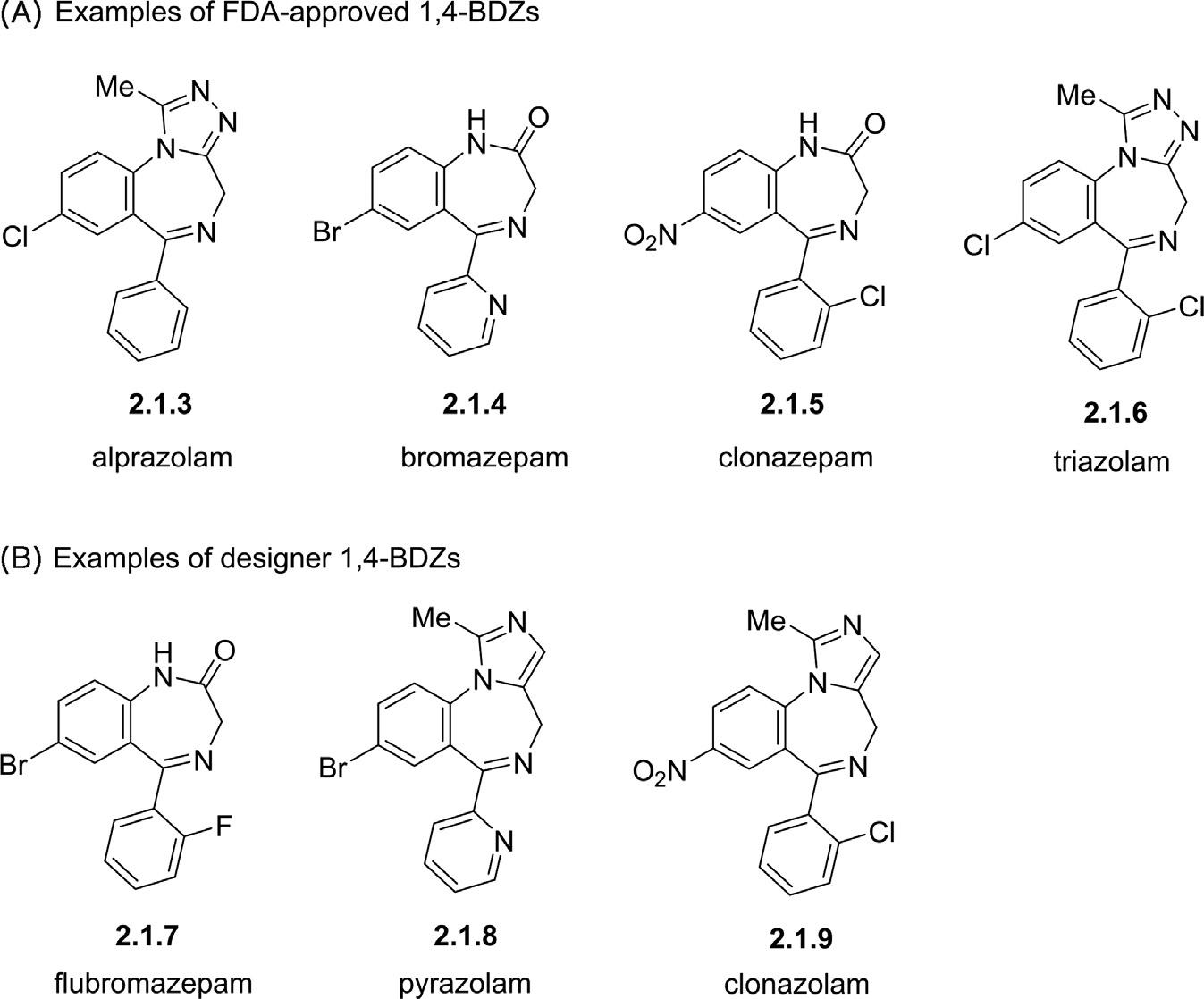
Fromthepharmaceuticalviewpoint,1,4-benzodiazepinescanbefurther dividedintotwomainclassesincludingFDA-approved1,4-BDZsand designer1,4-BDZs,alsoknownassyntheticandnon-FDA-approved1,4BDZs(Greenblatt&Greenblatt,2019).Designer1,4-benzodiazepinesare newlydevelopedcompoundshavingrelativelysimilarstructurestothe FDA-approvedcounterpartswithsomeslightdeliberatemodificationsand withnoFDAendorsementyet.Pyrazolam(2.1.8)isthefirstcompound ofdesigner1,4-BDZsfamilystructurallyderivedfromalprazolam(2.1.3) andbromazepam(2.1.4),whichbecameavailableinonlinedispensation onalargescaleinmid-2012withnomedicallicense(Fig.2.2)(Gilman etal.,1990).Althoughbeingofhighavailabilityasdrugsinmanycountries occured,pharmacologicalpropertiesandpotentialrisksforthemedicaluse ofmostdesigner1,4-benzodiazepineshavenotbeenfullyinvestigatedyet (Moosmann&Auwärter,2018).
Forobtaining1,4-benzodiazepine-and1,4-benzothiazepine-basedpharmaceuticallysignificantcompoundswithevolvedandbetterperformance, itishighlydesirabletorecognizethestructure-activityrelationships(SAR) ofthesescaffoldsfromdifferentaspects.Thischapterprincipallyillustratesthechemicalstructuralfeaturesof1,4-benzodiazepinesand1,4benzothiazepinestoprovideaclearunderstandingofthestructuralbehavior oftheseheterocyclicskeletons.
Figure2.2 Examplesof(A)FDA-approved1,4-BDZsand(B)non-FDA-approved designer1,4-BDZs.
Figure2.3 Structuralvariationsof1,4-benzodiazepines.
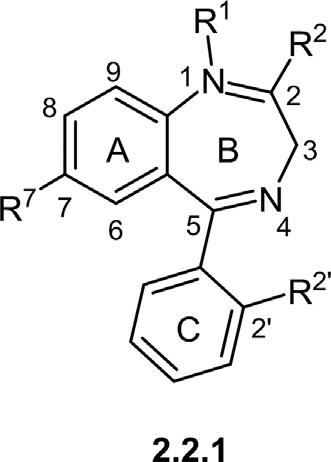
2.2Structuralvariationsof1,4-benzodiazepines Lookingmorecloselyatthestructuralvariationsof1,4-BDZs (Fig.2.3),itcanbeunderstoodthatthesemoleculesprimarilydifferin thesubstituentsatthreemajorpositions,namelyat1,2,and7onthecore
structure(Borea,1981; Meguro&Kuwada,1970).Variousfunctionalgroups orsidechainscanbeattachedtothesepositions(indicatedbyR1 ,R2 , andR7 )locatedonthebenzeneanddiazepinerings.Sometechniques suchasX-raycrystallographycanprovideaprecisemolecularstructurethat affirmsthemolecularshapeandthechemicalstructure(Chkirateetal.,2019; Hesteretal.,1971).Thechemistryofsubstitutingineachpositionof1,4benzodiazepinescanbeelaboratedasfollows.
1) Position1inclinestoaccepteitherhydrogenatomsoralkylgroups,in whichtheanxiolyticactivityofsuchstructuresisimprovedbyalkylation (Borea,1981).
2) Position2isconsideredasthekeypositiontoaddheteroatoms(Sor O)toforma(thio)ketogroup,exceptforthosebenzodiazepinesthat arefusedtoanotherringfromthisposition.Theheteroatomlinkedto position2servesasaprotonacceptor(Brönstedbase),anditisliableto getprotonatedatphysiologicalpHandconsequently,wouldaffectthe lipophilicity.
3) Position3isgenerallyunfavoredtobefunctionalizedbyasubstituent. Assuch,thepresenceofahydroxylgroupinseveral1,4-benzodiazepine derivatives,forexample,lorazepam,oxazepam,andtemazepam,significantlyenhancesthepolarityoftheseskeletons,leadingtomore glucuronidationandsubsequentlyfasterdrugeliminationfromthe body.Furthermore,ithasbeendemonstratedthatOH-substituted1,4benzodiazepinesatposition3arethermallyunstable,undergoinga thermalaFrigerio-typerearrangement(via dehydration)totransform 1,4-diazepineringtothestable1,3-diazinaneunit(Bourcieretal.,2001).
4) Position4tendstoacceptadoublebondasanunsaturationatpositions 4and5canbeobservedinallvarietiesof1,4-benzodiazepines.
5) Position5generallytendstoacceptasimplearomaticringasasubstituent,whereinallcommon1,4-benzodiazepineshaveaphenylgroup atthisposition.Notonlythepresenceofaphenylringatposition5 playsakeyrolein(bio)chemicalactivityofthefinal1,4-BDZs,butalso thesubstituentspresentonthephenylgroupcanimprovetheactivity.In thiscontext,thepresenceofelectron-withdrawinggroupsatthe ortho or di-ortho positionsofthephenylringresultsinanincreasedpotency,such asflubromazepam 2.1.7 andclonazepam 2.1.9 withFandClatposition 2ʹ,respectively(Fig.2.3).
6) Position7isacriticalsitetoimprovethebiologicalactivityof1,4-BDZs, whereintroducinganelectron-withdrawingsubstituentsuchasCF3 ,
Figure2.4 Tautomericequilibriumof1,4-benzodiazepine-2-one.
NO2 ,andhalogens(F,Cl,Br,I)wouldenhancethecompound’spotency (Sternbach,1980).
7) Otherpositions(6,8,and9)arebarelyfavorableforsubstitution,which mayreducethebiologicalactivityofbenzodiazepine(Sternbach,1980). Inthefollowingsections,1,4-benzodiazepinesarearrangedaccordingto theirstructuralvariations.
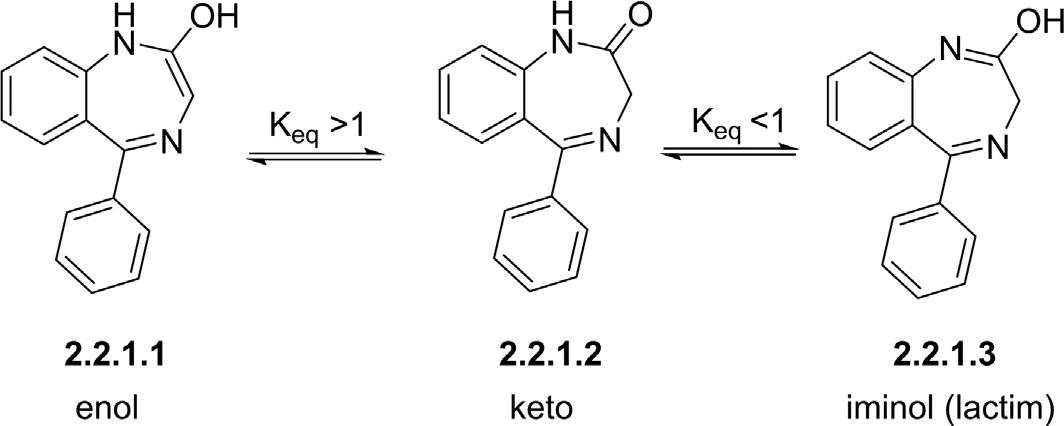
2.2.11,4-Benzodiazepinones 1,4-Benzodiazepine-2-oneand1,4-benzodiazepine-2,5-dionesareconsideredasthemostpotentmembersof1,4-BDZfamily,notonlyduetotheir wideclinicalapplicationsbutalsofortheircapabilitytoundergovarious modificationstofurnishdiversebiologicallyactivestructures(Boojamra etal.,1997; Cummingsetal.,2006; Spenceretal.,2010; Sternbach, 1971).Althoughstructuralmodificationsof1,4-benzodiazepinonesmay improvetheirphysicochemicalpropertiesandchemicalstabilities,these compoundsarepronetoundergotautomerizationduetothepresenceof thecarbonylgroup.Incontrasttoothertypesof1,4-benzodiazepines,1,4benzodiazepinonesmayexistinthreeforms,namelyketo,enol,andiminol inakineticallyfavoredequilibrium(Fig.2.4);bothiminolandenolisomers arelessstablethanthecorrespondingketoform.Computationalstudies havedemonstratedthatintroducingvariousaromaticsubstituentsattheC5 positiondisplaysminorchangesinrelativeenergiesbetweenenol/iminol andtheparentketoform(Pem&Vr ˇ cek,2017).Ontheotherhand,the presenceofacyl-typesubstituentsattheC3centerstronglyfavorsthe enoltautomercomparedtoiminolandketocounterparts(Pem&Vr ˇ cek, 2017).Theextentofenolization/iminolizationin1,4-benzodiazepinones bychoosingapropersubstituentleadstovariouspotentialbiological
Figure2.5 Thereactivityofthe1,4-benzodiazepine-2-oneand 1,4-benzodiazepine-2,5-dionesatdifferentsites.
activitiesoftheseheterocycles,whichopensanewavenueforoptimizing novel1,4-benzodiazepineswithtargetedproperties.Onthedownside,the tautomerizationof1,4-benzodiazepinonesresultsinracemizationwhenthey haveastereocenterattheC3position,whichconsequentlyreducestheir enantiomericordiastereomericpurity(Asoetal.,1988; Hoketal.,2019; Yang,1995).
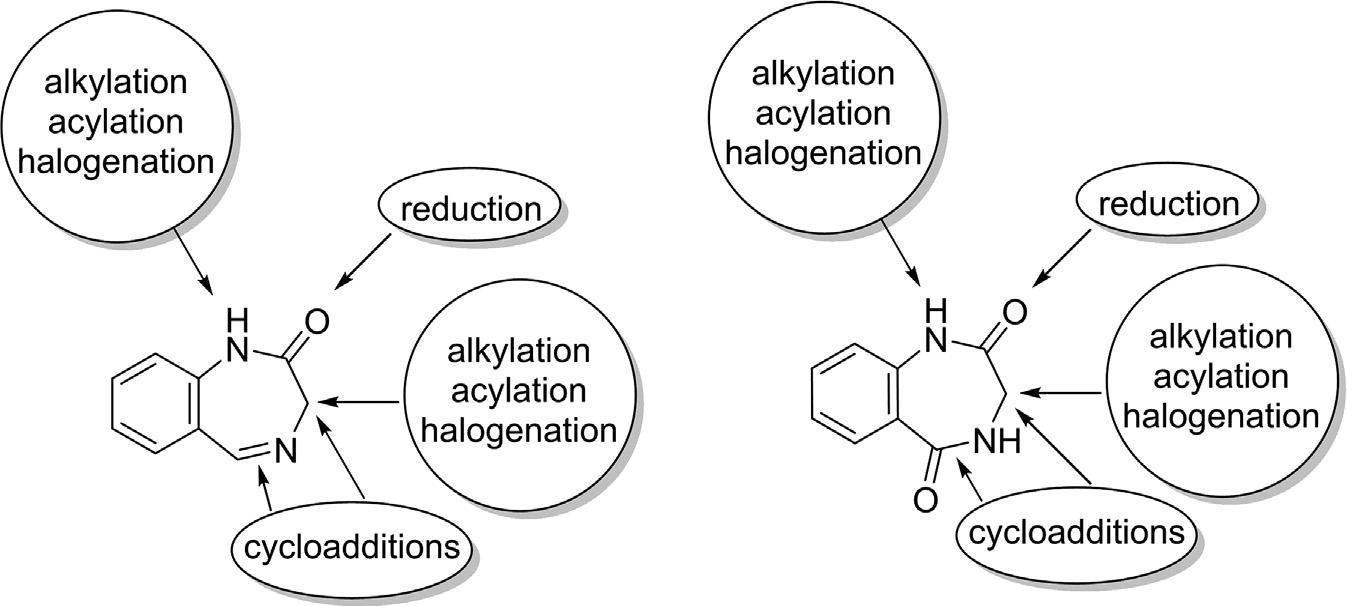
1,4-Benzodiazepine-2-oneand1,4-benzodiazepine-2,5-dioneshavea strongtendencytoparticipateinvariousorganictransformationsatdifferent reactivesites(Fig.2.5).Theamidecarbonylgroupatposition1issusceptible totheadditionofnucleophilesandthereduction via areducingagent(Batlle etal.,2019; Sharp,1984).TherearetwotypesofN1andC3anionspresent in1,4-benzodiazepinonesbecauseoftheN HandC Hdeprotonations ofamide,respectively.Thehigherstabilityoftheformerversusthelatter disclosesthehigheracidityoftheN1 Hprotoncomparedwiththe C3 H(Popovicetal.,2003).ItisalsoevidentthattheN1centerismore reactivetowardselectrophilesthantheC3position,inwhichalkylationand acylationreactionsoftenoccur(Archer&Sternbach,1968; Khanetal.,2018; Sternbach,1971).Theexcellentregioselectivityofthesereactionscanbe attributedtothehighernucleophilicityofiminolisomerincomparison totheketotautomer.Halogenationoralkylation/acylationofeitherN1or C3centersreadilyprovideusefulscaffoldsforfurthermanipulationssuchas aminationandthiation(Carlieretal.,2006; Sharp,1984).FormalcycloadditionreactionscanoccuratC3andN4 C5of1,4-benzodiazepinonesusing various1,3-dipolesincludingazomethineylidesandnitrilimines(Fanetal., 2021; Moltenietal.,2002; Sharp,1984).
Figure2.6 Schematicrepresentationofpossiblesitesof1,4-BDZsforfusinganother heterocycle.
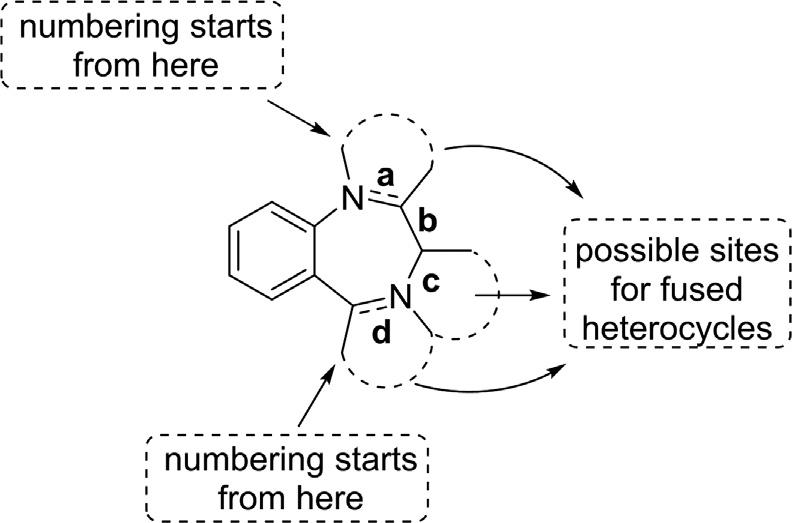
2.2.2Fused1,4-benzodiazepines Thecommercialsuccessandmedicalsignificanceof1,4-benzodiazepines haveresultedinthedevelopmentofwide-rangingsyntheticstudiesonthese scaffolds(Archer&Sternbach,1968; Meyeretal.,2017).Oneapproach thathasledtothegenerationofalargenumberofsyntheticreportsover thelastdecadesisthesynthesesof1,4-benzodiazepineswithanadditional bioactivefusedheterocyclicring.Thesefusedheterocyclicsystemsprovide aninterestingsubclassof1,4-benzodiazepinespossessingextrabioactive heterocyclicringsmainlyannulatedtothe a,c,and d facesofthecoreskeleton (Fig.2.6).Pyrrole,imidazole,triazole,andindoleareamongthemost eminentheterocyclesincorporatedintothe1,4-benzodiazepineringsystem toenhancetheirchemicalandpharmacologicalproperties.Numberingin thefusedsystemsisslightlydifferentfromthenormal1,4-BDZs,starting fromtheheteroatomthatisincommonwiththediazepineringandthe fusedring.Correlatingthechemicalactivityofthesecompoundstothetype andlocationofsubstituentsisalsoapplicableinthecaseoffused1,4-BDZs. Theassemblyofanewringon1,4-benzodiazepinesisratherstraightforward ontheir a sidesincetheseskeletonshaveanucleophilicNatposition1,which canbereadilyfunctionalizedtogenerateanelectrophiliccenteratposition2 (Sharp,1984).Accordingly,alargenumberofringsystemscanbefabricated onthe a sidebysimplychangingthestandardsyntheticprocedures.
Triazineringisaversatileheterocyclicringproventohavestrong πinteractionabilitiesandincreasedabilitytogetinvolvedinH-bondnetworks (Mooibroek&Gamez,2007).Accordingly,thefusionofthisuniquescaffold to1,4-BDZsmayimprovetheirbindingaffinitytotheGABAA receptorsin thecells,leadingtosignificantenhancementoftheirbiologicalactivities.For