AppliedMechanics ofPolymers
Properties,Processing,andBehavior
GeorgeYoussef SanDiegoStateUniversity,SanDiego,CA,USA
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans, electronicormechanical,includingphotocopying,recording,oranyinformationstorageand retrievalsystem,withoutpermissioninwritingfromthepublisher.Detailsonhowtoseek permission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangements withorganizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,can befoundatourwebsite: www.elsevier.com/permissions
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers, includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors, assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproducts liability,negligenceorotherwise,orfromanyuseoroperationofanymethods,products, instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-821078-9
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MatthewDeans
AcquisitionsEditor: DennisMcGonagle
EditorialProjectManager: VeronicaSantosIII
ProductionProjectManager: DebasishGhosh
CoverDesigner: VictoriaPearson
TypesetbySTRAIVE,India
1.Introductionandbackground
4.Linearelasticbehaviorofpolymers
4.1Introduction
4.2Stressandequilibrium
4.2.1Planestress90
4.2.2Simpletension91
4.2.3Simpleshear91
4.2.4Hydrostaticstress91
4.3Strainandcompatibility 92
4.3.1Planestrain93
4.4Linearelasticmaterialbehavior 94
4.4.1Isotropicmaterials94
4.4.2Orthotropicmaterials99
4.4.3Transverseisotropicmaterials100
4.5Structuralcomponentdesign
5.Hyperelasticbehaviorofpolymers
5.1Introduction
5.2Theoreticalpreliminaries 118
5.2.1Displacementfield118
5.2.2Deformationgradient120
5.2.3Polardecomposition123
5.2.4Straintensors125
5.2.5Stresstensors126
5.3Stress–strainrelationships 128
5.4Hyperelasticmodels 132
5.4.1Neo-Hookeanmodel133
5.4.2Mooney-Rivlinmodel134
5.4.3Yeohmodel135
5.4.4Gentmodel136
5.4.5Ogdenmodel137
5.4.6OgdenHyper-foammodel138
5.5Applicationsofhyperelasticmodelsincomponent design 138
6.Creepbehaviorofpolymers
6.1Introduction
6.2Simplecreepmodels
6.2.1Maxwellmodel150
6.2.2Kelvinmodel152
6.2.3Four-parametersmodel154
6.2.4Zenermodel156
6.3Additionalcreepmodels
6.3.1Findleypowerlaw158
6.3.2Norton–baileylaw159
6.3.3Prandtl–Garofalolaw159
6.4Applicationsofcreepincomponentdesign
6.5AppliedFEAsimulationexample
7.Viscoelasticbehaviorofpolymers
7.1Introduction
7.2Theoreticalpreliminaries
7.2.1Boltzmannsuperpositionprinciple167
7.2.2GeneralizedMaxwellmodel168
7.2.3GeneralizedKelvinmodel170
7.3Linearviscoelasticity
7.3.1Small-strainlinearviscoelasticity172
7.3.2Large-strainlinearviscoelasticity185
7.4Applicationsoflinearviscoelasticityincomponent
7.5AppliedFEAsimulationexample
8.Electroactivepolymers
9.Hydrogels
9.1Introduction
9.2Mechanicsofhydrogels
9.3Applicationsofhydrogels
9.4AppliedFEAsimulationexample
10.Failureandfractureofpolymers
11.Characterizationofpolymers
Introductionandbackground
1.1Introduction
Polymersareoneoftheprimaryclassesofmaterialsrivalingmetalsand ceramicswhilebeinganintegralpartoftheremarkableandtransformative hybridmaterialsofpolymermatrixcomposites.Polymersarecommonly referredtoasplastics,butweoughttoformalizethisnomenclaturesincethe classificationofpolymersisimportantforthefundamentalunderstandingof theirphysicalpropertiesandmechanicalbehaviors,aswillbediscussedlater. Polymersareubiquitousinmanyapplicationsrangingfromaerospacetoautomotive,fromconsumergoodstohouseholdgoods,andfromsportsgeartobiomedicaldevices.Infact,wedailyencounterpolymers,intentionallyor unintentionally,inevensomeofthemostmundaneactivities.Forexample, anobservanteyewouldnoticepolymerseverywhereintheinteriorofanaircraft assoonasyoustepinsidethefuselagecabin,wherethestructuralcabinwindow systemandtheaccompanyingdustcoverthatweoftenareaskedtolowerdown duringtakeoffandlanding,aremadeofdifferentpolymers.Anothercommon transportationvehiclethatencompassesalargepercentageofpolymersisthe passengerautomobile,whichhasnearly30%ofallitspartsmadeofdifferent typesofpolymersdependingonthedesignrequirements.Thedashboard,instrumentpanel,interiortrim,seating,interlayerinthefrontandrearwindshields, andcarpetfibersarejustafewexamplesofinteriorpartsinthecarthataremade ofpolymers.Manyautomotiveexteriorpartsarealsomadeofpolymerswhere theyaresubjectedtoaggressiveloadingandenvironmentalconditionssuchas thetires(beingthemostobvious),thebumpers,theundercarriage,thewheel housing,theradiatorsupport,andthemanypartsinthefuelsystem,againjust tolistafewexamples.
Evidentfromthediversityoftheapplicationsmentionedaboveisthebroad rangeofoperatingandenvironmentalconditions.Polymersthatareusedunder thehoodofacararerequiredtoendurecombinedthermalandmechanicalloadingforalongduration,whilethosethatareusedinthefuelsystemarerequired toresistaggressivechemicals,e.g.,thefuel,whilesupportingaworkingpressureof 345kPa(mechanicalloads).Moreover,somepolymershavetobear substantialmechanicalloadswhilebeingabrasiveresistant,e.g.,tireshelp
supportasizableportionofthecarweight( 25%)whileenduringabrasionduringcontactwiththeroad.Inall,theclassofpolymericmaterialsitselfisas diverseastheapplicationstheyareintegratedinto.Therefore,thegoalofthis textbookistoprovidethefundamentalbackgroundtoassistengineersinperformingmeaningfulstressanalysesbasedonmaterialconstitutivemodels derivedfromthetheoryofcontinuummechanics.Thesematerialmodelsrepresentanddescribethemechanicalbehaviorofthepolymerusedinthedesign. Withthisbackgroundinmind,itisnowfittingtobrieflydefinepolymers notingthatthetermpolymeriscommonlyusedinterchangeablywiththeword macromolecule,withinthepolymersciencecommunity.Thebasicdefinitionof polymerscomesfromtheGreekrootsoftheword,wheretheoriginof‘poly’in Greekiseither“polus’ meaning“much’or‘polloi” meaning“many.”Polymers arethenmaterialsofmanyrepeatingparts,units,or“mers”(fromtheGreek word“meros”).Polymersarecompoundsconsistingofrepeatinglong-chain unitsthatareconnected,whereeachsinglechainmayhavethousandsoreven millionsoftherepeatingmers.Moreover,polymersarematerialswithinterdigitatedmoleculeswithdifferentlengthscales.Generally,polymersareeither hydrocarbons(covalently-bondedcarbon-hydrogenbackbone)orsilicones (silicon-oxygenbackbone).Thismolecularstructureisresponsiblefortheoverallpropertiesofpolymers.Polymerscaneitherbenatural(e.g.,humanDNA andhair)orengineered(suchasnylonandpolyvinylchloride).Someofthe overarchingadvantagesandlimitationsofpolymersaresuccinctlysummarized in Table1.1,whichisnotintendedtobecomprehensiveorapplicablyinclusive toalltypesofpolymers.Whiletime-dependentpropertiesareincludedinthe tableasanexampleofgeneraldisadvantage,thismightbeadesirableattributes forpolymersusedindynamicorimpactsituations.
Beforedelvingintomoredetails,therearethreequestionsthatweneedto askandanswertonotonlymotivatethewholefieldofstudyofpolymer mechanicsbutalsotogainaninsightfulperspectiveaboutthisinterestingclass
Time-dependentproperties 2 Appliedmechanicsofpolymers
TABLE1.1 Generaladvantagesandlimitationsofengineeringpolymers.
Generaladvantages
Generallimitations
Lowdensityrelativetometals LowYoung’smodulus(stiffness)
Goodstrength-to-weightratio Lowstrengthrelativetometals
Highcorrosionresistance Limited-servicetemperature
LowelectricalandthermalconductivitySusceptibilitytoultravioletandother radiation
Moisture,abrasion,andimpact resistance
ofmaterials.Thisperspectivewillbearunningthemethroughoutthebook.Itis worthnotingthatthesequestionsarelistedfromtheperspectiveofaskeptical engineerwhomaybeconcernedaboutleaningtowardsapolymerforthedesign ofanewpartorcomponent.
1. WouldamaterialwitharelativelylowYoung’smodulus,mediocre strength,andlowthermalandelectricconductivitybeusefulin mechanicaldesignapplications?
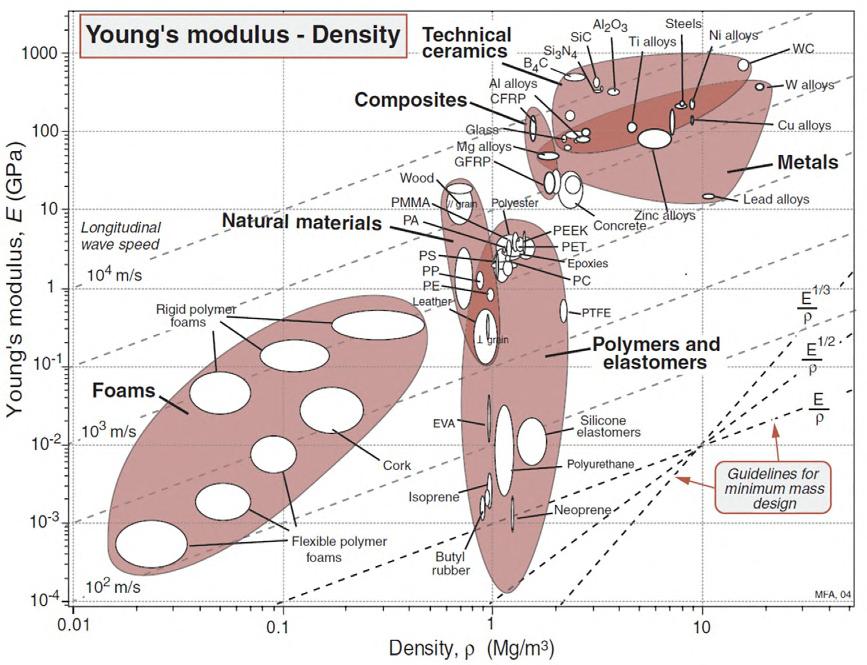
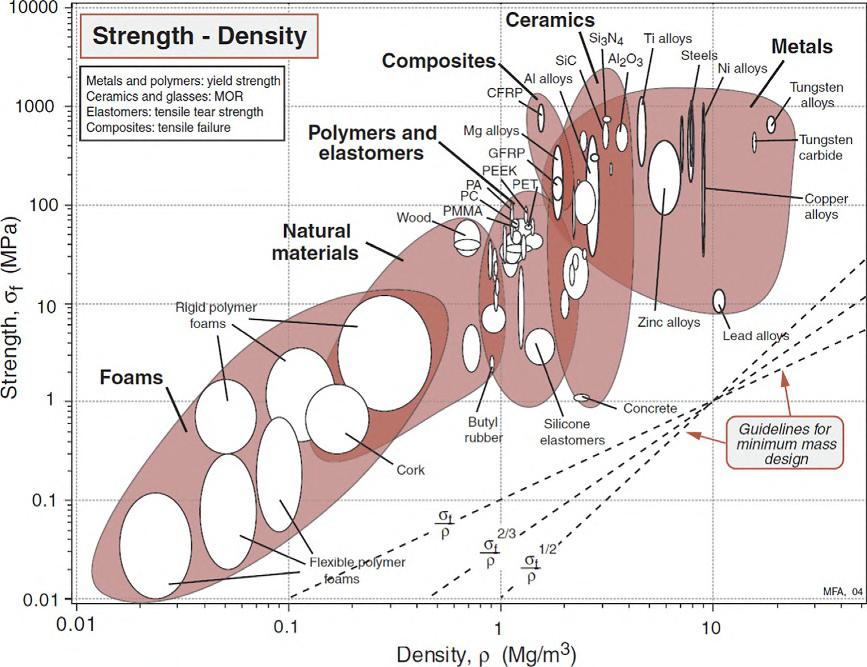
Incomparisontothepropertiesofmetalsandceramics(see [1,2] tobenchmark theproperties),unreinforced,commonengineeringpolymershaveanelastic modulusthatcanrangefromafewkPatolessthan10GPa,afailurestrength between10and100MPa,andlowthermalconductivityintheorderof0.1W/m. K [1].However,thedensityofpolymersissubstantiallylowerthanotherclasses ofmaterialsandaveragesaround1000kg/m3.Therefore,theutilityofpolymers inengineering,load-bearingapplicationsissubstantiatedbasedonthespecificproperty(i.e.,propertyperunitdensity)asexplicatedbyAshby’sselectionplots shownin Figs.1.1and1.2,whereYoung’smodulusvs.densityandthefailure strengthasafunctionofdensityaredefined,respectively [1].Collectively, polymerscanbeselectedandtakenintoconsiderationtomeetcertaindesign requirements(definedbythedotteddesignlinesonAshby’splotsbasedon
FIG.1.1 Ashby’smaterialselectionchartofYoung’smodulusasafunctionofdensityforthe majorclassesofmaterialsincludingpolymersandelastomers [1]
FIG.1.2 SpecificstrengthAshby’sselectionchartshowingrelativepropertiesofpolymerswith respecttootherclassesofmaterials [1] 4 Appliedmechanicsofpolymers
theobjectiveofminimummass)andarefoundtobetechnicallycompetitive,on aperunitmassbasis,comparedtoothertraditionalcounterparts.Awordofcautionis,however,warrantedhereabouttheboundariesofthedesignenvelope thatisinformedbyotherrequirementsthatextendbeyondlightload-bearing toinclude,forexample,thermalandhygrothermalstability,corrosion-resistant, andhigh-cyclefatigue,tonameafewotherpossibleandcommonmechanical designrequirements [3,4].Inshort,polymersconstituteacompetitiveclassof materialsformanyapplicationsuponcorrectsynthesisofthedesignrequirements.Theremainingchaptersofthisbookwillillustratethishypothesis throughmanyanalyticalandcomputationalexamples.
2. Whyarepolymersprolificinseveralindustrialapplications(e.g., automotive)?
Theintroductorypreamblestatedaboveillustratesthebroadintegrationofpolymersinmanyindustrialdomains.Itcanbesaidthatthereareseveralunique advantagesofpolymersthatmarktheirindustrialimportanceandsubstantiate theirproliferationintheseapplicationsdiscussedattheonsetofthischapter. First,polymersarelightweight,hencereducingtheoverallweightoftheproductand,inthecaseofpoweredvehicles,improvingfuelefficiencybyincreasing the fuel-to-weight ratio.Second,somepolymersexhibitsuperiorimpactmitigationpropertiesexemplifyingtheirsignificantcontributionstothesafetyof
passengercarsbyimprovingcrashworthiness.Forexample,apolymer-based bumpercanabsorbuptofourtimes(conservativeestimate)moreenergyduring animpactduetoacarcrashthanalternativematerials.Finally,polymersare competitivewithmetalsfromaproductionandmanufacturingpointsofview, wherepolymersrequirelowerenergytoproducethanmetalssincethemelting (orsoftening)temperatureoftheformercanonlybeacoupleofhundred degreescentigradefromtheambienttemperature.Atthesametime,themelting temperatureofthelattercanexceed1000°C.Thisadvantagepositionspolymers ascostcompetitivematerialsincomparisontometals.Anotherdesirableattributeofpolymersformanufacturingistheirsuitabilityfornet-shapeprocesses, wheretheycanbemoldedintointricatepartswithlittletonopostprocessingor finishingsteps.Aninterestingexamplethatclearlydemonstratestherelevance ofpolymersforcomplex-shapeandnet-shapeprocessingistheall-plasticpatio chair,whichiscommonlyproducedbyinjectionmodeling.Otherexamples includegears,pulleys,cams,andtimingbeltsthatareusedinroboticsand RChobbyistcars,boats,andplanes.Asubtle,butimportant,byproductof theeaseofmoldingofpolymerproductsisthedesignflexibilityitprovides tomechanicaldesignersinrealizingcomplexandconvolutedshapestohelp indistributingtheloadsthroughoutthestructure,inadditiontoimprovingaesthetics.Infact,therationaleforadoptingpolymersasaviableandcompetitive classofengineeringmaterialsismultifaceted;astheapplicationsthemselves areexpansive.
3. Whatarethemajorcontributorstotheoverallmechanicalbehaviorof polymers?
Itmaynotcomeasasurprisethatthestructure– property-processingperformancenexusthatbroadlyappliestoallmaterialsisalsoatplayin thecaseofpolymers.Theoverarching objectiveofthisbookisemphasize thestructure-property-processing-performanceinterrelationshipinthisfascinatingclassofmaterials.Hence,thereareafewpointstokeepinmindwhen analyzingpolymer-basedparts.First, themolecularstructureplaysamajor roleindifferentiatingthemechanicalresponseofdifferentpolymersdependingonthemicrostructuralclassification(discussedlater).Second,polymers aresensitivetothetemporalaspectsofloadingencompassingbothduration andstrainrate,inwhatwillbediscussedlaterinthebookastheviscoelastic responseofpolymers.Finally,environmentalconditio ns,specificallytemperature,dictatewhetherornotthepolymer canbetreatedaslinearornonlinear elasticsolid.Inshort,microstructur e,time,andtemperaturearecrucial parameterstokeepinmindastheywillap pearrepeatedlythroughoutthistextbook.Inlightofthisdiscussion,thereadermaythinkthedependenceofpolymer’sresponseontimeandtemperatureisanotherreasontostayawayfrom theminpracticalapplications.Thiscouldnotbefurtherfromthetruthsince thecontinuummaterialmodelspres entedthroughoutthebookscansuccessfullyaccountforbothoftheseparameters ,makingdesigningwithpolymeran intellectuallyenjoyabletask.
1.2Historicalperspective
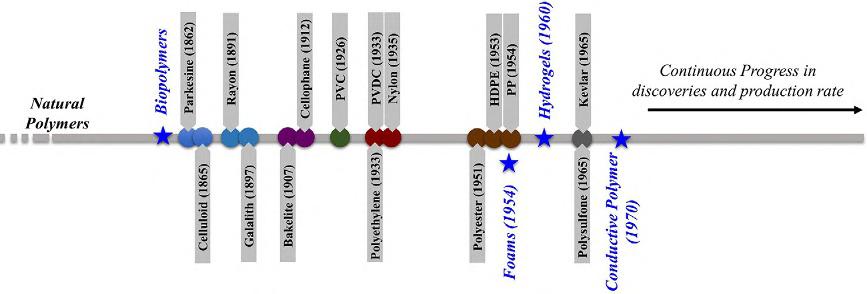
Tracingbacktothebeginningofpolymersinpracticalapplicationsisadaunting tasksincetheexistenceofnaturalpolymers,likewoodandproteins,isparallel tolifeformingonearth.Therefore,thefollowingtimelineofpolymerdiscoveriesisbriefandonlyfocusesonmajormilestonesthatweretransformationalto subsequentdiscoveries.Anallinclusivehistoricalaccountofthepolymerwas nottheintentionnorwasitattemptedsincetheobjectiveofthistextbookison themechanicsofpolymersandnotontheirhistory.Yet,thefollowingtimeline (Fig.1.3)providesthelinkbetweenthecallofindustrialorsocietalneedsand theinceptionofpolymerstoanswerthesechallenges.
Synthetic(artificialorhuman-made)polymersareubiquitousinliterally everyindustrialsector;however,theirintegrationinonesectormayfollowa differenttimelinethanitsadoptioninanothersector.Nonetheless,mosthistoricalaccountssetforthbydifferentorganizationsandsocietiespointtowardsthe secondhalfofthenineteenthcenturyasthebeginningofhuman-made,syntheticpolymers.ThesehistoricalaccountsarealignedwiththeinventionParkesinein1862asanalternativetonaturalrubber,Celluloid(animproved versionofParkesine)in1865asareplacementofivoryandtortoiseshell,Rayon (giventhatnamebyitsinventorsinceitemittedraysoflight)in1891asa substitutionofsilk,andGalalith(madeofcaseinandformaldehyde)in1897 asanonflammableanderasablechalkboardmaterial.Remarkably,thefirst human-madepolymerswerebio-based,i.e.,sustainableandrecyclable,butthis changedveryquicklyasdesignguidelinesandmanufacturingtechniquesprogressed.WhileParkesinewasnotacommerciallyviablediscovery,itmarked thebeginningofaneweraofsyntheticpolymersthatbloomedattheturnofthe twentiethcentury.
Despitethekeeneffortsinthe19thcentury,theinventionofBakelite(polyoxybenzylmethylenglycolanhydride)in1907trulymarkedtheinceptionof fullysyntheticpolymerssinceitwasformedbythereactionbetweenphenol andformaldehyde.Bakelitequicklyoccupiedaheftymarketsharesinceit wasfoundtobesuitableforamyriadofapplicationssuchaspiecesofjewelry,
FIG.1.3 Brieftimelineincludingthemajormilestonesinthedevelopmentofpolymers.
billiardballs,automotiveparts,electricinsulators,andevenhouseholdgoods (e.g.,pothandles).TheusesofBakelitewereinfiltratingeveryaspectofdaily lifetothepointitpromptedTimemagazinein1924tolabelita“materialwitha thousanduses,”andthatwasnotanexaggeratedsupposition.Whileitmayhave beenovershadowedbythefameofBakelite,Cellophanewascreatedin1912 andquicklysoaredinthefoodpackagingindustryespeciallyin1927when DuPontwasabletomakeitamoisture-resistantmaterial.Aroundthesametime, in1926,aformalprocesswasinventedtoplasticizedpolyvinylchloride(PVC, initiallysynthesizedin1872butwasunusableduetoprocessingissues),leading toitscommercialdominanceinmanyapplicationsincludingtheconstruction industryinplumbingcomponentssuchaspipesandtubes.
Inthe1930s,threesyntheticpolymerswereproducedandarestillinproductiontodaywithsignificantannualtonnage.Thesepolymersarepolyethylene, usedintheproductionofliquidcontainers,polyvinylidenechloride(PVDC, Saranwrap),andNylon(firstintroducedinthefashionindustryasstockings). WhiletheusefulnessofNylonwasquicklyrecognizedbytheUnitedStatesmilitaryforparachutesandrobes,polyethylenerosetofamewhenitwasutilizedto developTupperwarein1946.Ofcourse,oneshouldnotignorethefactthatthe worldwasraginginawarbetween1939and1945,hencetheslightgapinpolymerdiscoveries.Inthemid-twentiethcentury(the1950s),threeadditionalcommerciallyvaluablepolymerswereintroduced,whicharepolyester(first synthesizedin1939),polypropylene(PP),andhigh-densitypolyethylene (HDPE).Thesepolymersremainasimportanttodayastheywereinthe 1950s.Itisalsoimportanttonotetheindustrialprevalenceofpolypropylene asitbecamethenewBakeliteofthelatterendofthetwentiethcenturyand thebeginningofthe21stcentury.Thiseraofgreatdiscoveriesinsyntheticpolymerswasnotoveryet;in1965,polysulfoneandKevlarwereintroduced.The secondhalfofthelastcenturywasthepioneeringeraforaplethoraofother syntheticpolymersthatwerebasedonpetroleumoil.Petroleum-basedpolymersareaversatilegroupofmaterialsthatarefoundinnumerousapplications.
Thishastyandbriefhistoricalbackgroundwouldnotbecompletewithout embarkingonthehistoryoffourvitalsubclassesofpolymers:(1)biopolymers, (2)hydrogels,(3)conductivepolymers,and(4)polymericfoams.Wewilldiscuss,atlength,themechanicalbehaviorofsomeofthesesubclassesinlater chapters,butheretheiremergenceintothetechnicalstageisbrieflyintroduced. Biopolymersareavitalsubclassofpolymersfortheentireplasticindustryand theirrootsaretracedbacktotheinceptionofthefirsthuman-made,synthetic polymersinthelate1800ssincemostofthesepolymerswerederivedfromnaturalandrenewablesources.Theyalsoincludethoseproducedbylivingorganisms,includingpolyesters,polynucleotides,proteins(e.g.,collagenwas discoveredin1938),polysaccharides(frombacteria,fungi,plants/algal,oranimals),lipids/surfactants,andpolyphenols.Ontheotherhand,hydrogelswere introducedin1960asahydrophilicsyntheticpolymerthatundergoesasignificantvolumephasetransitionwhenstimulatedphysically(e.g.,temperature)or
8 Appliedmechanicsofpolymers
chemically(e.g.,pH).Hydrogelsareubiquitousinmanyapplicationsincluding drugdeliverysystems,heavymetalionsremovalinenvironmentalapplications, scaffoldingintissueengineering,andcontactlenses,tonameafewofthemost importantindustrialandmedicalapplications.Next,conductivepolymers (molecularelectronics)areaburgeoningsubclassofpolymerssincefirsttheorizedinthe1940s,butbecameascientificandtechnologicalmainstreamin early1970withthebreakthroughdiscoveriesbyForrestL.CarteroftheU.S. NavalResearchLaboratory.Oneprominentapplicationofconductivepolymers,whilemanyotherexist,isorganiclight-emittingdiodes.Finally,polymericfoamsaresymbiotictoprotectivepadsinthepackagingandsports gearindustries.Polymericfoamsarealsousedintheconstructionforinsulation. In1954,Styrofoam(polystyrenefoam)wasintroducedbyDowChemicaland quicklybecomeamainstreammaterialforfoodandprotectivepackaging.The recenthistoryisfullofotherfoaminnovationsthatliterallytransformedour livesandimprovedthequalityoflife.
Reviewingthistimeline,itisincomprehensiblethatsyntheticpolymershave beenaroundfornearlyacenturygiventheimpacttheyhavemadeonmany aspectsofourdailylives.Asmentioned,manytimesbefore,polymersare becominganessentialclassofmaterialsthatareintegratedandwillcontinue tobeintegrated,inmanyapplicationsfortheirdesirableattributes.Thepolymersindustryhasamultibilliondollarsannualeconomicimpactproducingbillionsofpoundsusedinpackaging,buildingandconstruction,transportation, etc.Laterinthischapter,asectionisincludedtoelaborateontheintegration ofpolymersindifferentmarketsegments.Beforetransitioningawayfromhistory,weshould,however,remarktheimpactpolymershaveontheenvironment.Indeed,someimpactsarenotasglamorousastheymayhavebeen portrayedabove.Moreeffortshouldbededicatedtorecyclingsyntheticpolymers,creatingmoreenvironmentallyfriendlyandsustainablepolymers,minimizingthecarbonfootprintofmanufacturingpolymers,andreducingthe negativeenvironmentalimpactsofnonbiodegradableplasticsfloatinginthe oceanandstackingupinlandfills.
1.3Typeofpolymers
Thereareseveralwaystoclassifyandcategorizepolymersintodifferentgroups dependingontheoriginofthematerial,themolecularstructure,andtheoverall thermomechanicalresponseaswehavebrieflyeludedtobefore.Itisimportant tonotethatapolymercanbe(andmostlikelywillbe)classifiedinmultiple waysacrossthesecategories.Asmentionedpreviously,theoverallmechanical behaviorofthepolymer(theobjectiveofthistextbook)hingesonthemolecular structureandtheprocessingconditions,amongmanyotherfactors,including environmental,operating,andloadingscenariosduringdeployment,where theinfluenceofthemolecularstructureisdiscussedbelow.Incontrast,the
effectofprocessingandtheoverallmechanicalresponseofpolymersare deferredtofuturechapters.
Polymersmaybeclassifiedaseithernaturalorsynthetic.Naturalpolymers arebasedonmatterorsubstancesthatexistinnature.Examplesofnaturalpolymersincludestarch,proteins,lignin,cellulose,collagen,naturalsilk,natural rubber,etc.Theycanbeusedintherawformormayrequireadditionalprocessingtobecomeanengineeringmaterial.Itmaynotcomeasasurprisethatmany ofthefoodthatweconsume(e.g.,chicken,meat,vegetables,etc.),aswellas partsofourbody(suchashair,skin,andDNA),areclassifiedasnaturalpolymers.Ontheotherhand,syntheticpolymersthatareman-madeinthelaboratory areusuallyderivedfrompetroleumderivatives,i.e.,oilproducts.Nylon, epoxies,phenolic,polypropylene,polyethylene,polycarbonate,andsynthetic rubberarejustafewcommonexamplesofsyntheticpolymersthatweencounter everyday.Insimple,thefirstclassificationofpolymerisbasedonwhetheritis foundinnature(naturalpolymers)ormadeinthelaboratory(synthetic polymers).
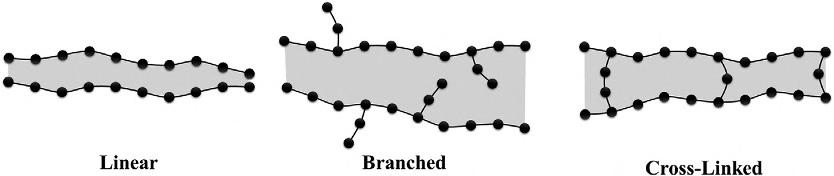
Polymersmayalsobeclassifiedbasedontheorderoftheirmolecularstructure,dependingonthedegreeofcrystallinity.Asmentionedearlier,polymers arecompoundsoflong-chainmoleculesconsistingofrepeatingunits(mersor monomers),wherethechainisalongstringofcovalentlybondedcarbonatoms. Thesynthesisprocessofjoiningmonomerstocreatethemacromoleculeis calledpolymerization.Therefore,thedegreeofpolymerizationreferstothe numberofmonomerunitsinachain.Theresultingmolecularstructurecanthen bedividedinto(1)linearstructure(chain-likestructure, Fig.1.4A),(2)branched structure(chain-likewithsidebranches, Fig.1.4B),and(3)cross-linkedstructure(connectedbranchedchains, Fig.1.4C).Thearrangementofthechainsfor oneanotheriswhatwebasethisparadigmofclassificationon.
Apolymeristhensaidtobeamorphous(Fig.1.5A)ifthemolecularstructureislackinganypositionalorder.Togetavisualofthestructureofanamorphouspolymer,onemayimagineabowlfullofflexiblestringscutintoafew centimetersinlengthandrandomlymixed.Anotheranalogyofthemolecule structureofamorphouspolymersisabowlofspaghettipastamixedwithagenerousamountofmarinarasauce.Thepastahererepresentsthelong-chainmolecules,andthesaucerepresentsthesecondaryintermolecularbonds.Ifyoucan delaydevouringthisdeliciousmealforafewminutesandobservethe
FIG.1.4 Schematicrepresentationofbasicpolymerstructure(solidlinesrepresentprimarycovalentbondswhileshadedareasindicatesecondarybonding).
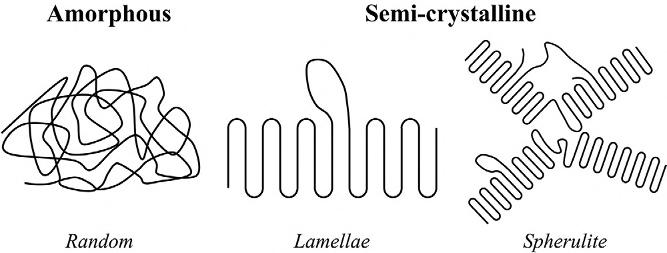
FIG.1.5 Schematicsofpolymercrystallizationshowingrandomamorphousandorderedsemicrystallinestructures(perturbationsinthelamellaeandspheruliteemphasizethebarriertocomplete crystallization).
arrangementofthestrands,youwillnoticethelackofanyspatialorder.Examplesofamorphouspolymersarepolystyrene,polymethylmethacrylate (PMMA),polycarbonate,polyvinylchloride,andnaturalrubber.
Ontheotherhand,whenthechainsmanagetoovercometheenergycostto formpositionalorder(crystallineregions),thepolymeristhensaidtobe semicrystalline.Asemicrystallinepolymercontainsadjacentcrystallineand amorphousregionswithinthesamesample.Sincepolymerscannotachieve completecrystallinityatthemacroscale,i.e.,thedegreeofcrystallinityisless than100%,theprefix‘semi’isusuallyusedtodescribethecrystallinityofpolymers.Thedegreeofcrystallinity(DoC)isthendefinedastheproportionofthe crystallizedphaseinthemass.Thesecrystallinephasesareformedbythechains foldingtogether,creatingorderedlamellaeregions(Fig.1.5B),whichcanselfarrangeintospherulites(radiallyspreadingtentaclesmadeoflamellaeandseparatedbyamorphousregions)asshownin Fig.1.5C.TheDoCisaffectedbythe spatialarrangementofatomsandgroupofatomsinthemonomer(stereoregularity),chainbranching,andwhetherthehydrocarbonbackboneisaliphatic (linear)oraromatic(ring)structure.Itisalsoinfluencedbytheconditionssurroundingthepolymerizationprocess,e.g.,theevaporationrateofsolventand thecoolingrate.Examplesofsemicrystallinepolymersincludepolyethylene, polypropylene,polyamide(Nylon),andpolytetrafluoroethylene(Teflon).In general,thedegreeofcrystallizationaffectsthephysicalpropertiesofthematerialsuchthatagreaterproportionofcrystallizedregionsisassociatedwithan overallhigherelasticmodulusandhighermeltingtemperature.Forexample, low-densitypolyethylenewith55%DoChasadensityof920kg/m3,anelastic modulusof140MPa,andameltingtemperatureof115°C,whereasthesame propertiesofhigh-densitypolyethylenewith92%DoCarefoundtobe 960kg/m3,700MPa,and135°C,respectively.Additionally,somepolymers areonlytransparentintheamorphousphasewithinthevisiblerangeoftheelectromagneticspectrum,andtheybecomeopaquewhenpartiallycrystallized.The opticalpropertiesofpolymersatotherwavelengthsarealsoassociatedwith changesinthedegreeofcrystallinity.Generally,thecorrespondingincrease
inthephysicalpropertiesasthedegreeofcrystallinityincreasesisconsistent withthebackgroundknowledgeofmetals,wheretheyexhibithighlycrystalline structuresandhavehigherdensity,modulus,andprocessingtemperatures.
Thefinalmethodofclassificationdiscussedhereisbasedonthethermomechanicalbehaviorofpolymers,whicharedividedintothermoplastics,thermosets,andelastomers.Thermoplastic(TP)polymersaresolidmaterialsatroom temperaturebutatransitiontoaviscousliquidwhenheatedtoafewhundred degrees.TPscanbesubjectedtorepeatedheatingandcoolingcycleswithout sufferingsignificantdegradation.Theyinheritseveralcharacteristicproperties fromtheirgeneralclassofpolymersincludinglowdensity,highcoefficientof thermalexpansion(5 thevaluesformetalsand10 valuesofceramics),low meltingtemperature,highspecificheat,andelectricalinsulation.Common examplesofthermoplasticsarepolyethylene,polypropylene,polyvinylchloride,andpolystyrene.Ontheotherhand,thermoset(TS)polymersareunable totoleraterepeatedheatingcyclessincetheydegradeandcharwhenheated beyondacriticaltemperature,whichisincontrasttothesofteningbehavior ofTPsathighertemperatures.Thermosetscannotberemelted,hencelimiting theirrecyclability.Particularly,whenTSpolymersareinitiallyheated,they softenformoldingandshaping.Additionalincreaseintemperatureresultsin achemicalreactionthathardensthematerialintoaninfusiblesolid.TSpolymersaredistinguishedbytheirhighlycross-linked,three-dimensional, covalently-bondedstructure.Generalpropertiesofthermosetpolymersinclude amodulusofelasticitythatistwotothreetimeshigher(i.e.,morerigid)than theirthermoplasticcounterparts,lowtonoductility(brittleness),lowersolubilityincommonsolventsthanthermoplastics,andatolerancetosustainhigher servicetemperaturesincomparisontothermoplastics.Countertops,plywood adhesives,paints,moldedparts,printedcircuitboards,andotherfiberreinforcedplasticsarejustafewexamplesofthermoset-basedproducts.Importantcommercialthermosettingpolymersincludeepoxy,phenol-formaldehyde, unsaturatedpolyester,polyimides,andpolyurethanes.
Elastomersundergolargeelasticdeformation(extensibility)whensubjected torelativelylowstresses.Someelastomersarecapableofextensionofupto 500%ormorebutstillreturntotheiroriginalshapeuponloadremoval.Despite thisremarkableelasticresponseofelastomers,whichisquitedifferentfromTS polymers,themolecularstructureoftheformerissimilartothelatter.Elastomersconsistofcross-linked,long-chainmolecules,however,thedegreeof cross-linkingissubstantiallylowerthanthermosets.Intheabsenceofmechanicalloading,i.e.,theunstretchedstate,themoleculesaretightlykinked.However,themoleculesareforcedtouncoilandstraightenwhenstretched, providingtheinitialelasticmodulusofthematerialduetothenaturalresistance touncoiling.Asstretchingcontinues,thecovalentbondsofthecross-linked moleculesbegintoplayanincreasingroleinthemodulus.Apparentexamples ofelastomersarenaturalandsyntheticrubbers.Naturalrubbersareahigh molecular-weightisoprenepolymerthatisderivedfromlatex(amilky
Appliedmechanicsofpolymers
substanceproducedbyvariousplantsthatgrowintropicalclimates).Thedevelopmentofsyntheticrubberswasprimarilymotivatedbytheworldwarswhen thetradeofnaturalrubberswasinterrupted.Thetonnageofsyntheticrubbersis currentlyafewtimesmorethantheirnaturalcounterparts.Commerciallysignificantsyntheticrubbersincludeethylene-propylene-diene(EPDM),nitrile, syntheticisoprene,andchloroprene.Thebasicpropertiesofsomeoftheessentialpolymersineachclassarelistedin Table1.2
1.4Areasofstudyinpolymerscience
Evidentfromtheprecedingbackgroundistheinterdisciplinarynatureofpolymerscience,whichencompassesknowledgefromchemistry,physics,andengineeringtoexplainthesynthesisprocessandthemolecularstructureabout mechanicalbehavior.Thegeneralareaofpolymersciencecanthenbedivided intothreeinterconnectedsubfields,namelypolymer chemistry,polymer physics,andpolymer mechanics (includingpolymercharacterization).Eachofthese areashasbeen,andcontinuetobe,exhaustivelystudied.Therefore,thereare
TABLE1.2 Shortlistofthedensity,elasticmodulus(E),andultimatetensile strength(UTS)ofcommonthermoplastic(TP),thermosets(TS),and syntheticrubbers(SR).
Acrylonitrilebutadiene styrene ABSTP1060210050
Poly(methylmethacrylate)PMMATP1200280055
PolycarbonatePCTP1200250065
PolytetrafluoroethylenePTFETP220042520
Polypropylene PPTP900140035
Vinylesterresin VETS14903720a 30a
Polyesterresin PETS18888700b 55b
Polyurethaneresin PUTS108582.7a 16a
Polychloroprene CRSR1230725
Isobutylene/isoprene PIBSR920720
Polyisoprene IRSR9301725
aPropertylowerlimit. bBasedononeformulation.
numerousbooksandacademicjournalsthatannuallypublishthousandsof papersonthesesubfields,whereeachsubfieldisalsobrokendowntoseveral differenttopicsthatareinvestigatedusinganalytical,computational,andexperimentalapproaches.Scientistsandengineerspursueadvanceddegreesonasubtopicsuchasaspecificmaterial,modelingofmonomer-to-monomer interaction,oranovelcharacterizationtechnique.Abriefoverviewisthenpresentedtoacquaintthereaderwiththegeneralaspectsofthesesubfieldswhile highlightingtheimportanceofpolymermechanics(themaintopicofthistextbook)inthedevelopmentanddesignofpolymer-basedcomponents,parts,and structures.Thebrevityofthefollowingoverviewofthesubfieldswithinpolymerscienceismeanttodirectthereader’sattentiontotheimportanceofthe interconnectivityoftheknowledgewhileencouragingfurtherresearchbased oneachreader’sinterest.Thisshortoverviewisnotmeanttobecomprehensive orinclusive,butratherexploratory.
1.4.1Polymerchemistry
Theprimaryemphasisofpolymerchemistryisthesynthesisprocess,thechemicalstructure,andtheresultingproperties [5,6].Inthehistoricperspectivesection,allthediscussedpolymerswereinventedtoreplacepreciousorsacred naturalmaterials(e.g.,ivory)whilebeingsuitableformassproduction.Inother words,therelevanttakeawayfromthehistoricaltimelineistheabilityofchemiststocreatecompoundsinthelaboratory-basedonthedesiredphysicaland mechanicalpropertiesandeventheappearanceandtexture.Scientistscan creatematerialsbypolymerizingonemonomer,twomonomers,orthree monomersduringthepolymerizationprocess.Hence,theresultingpolymers aretermedhomopolymer(e.g.,polypropylene),copolymer(suchasstyrenebutadiene),andterpolymer(oneprominentexampleisABS),respectively. Theselectionofthedifferentmonomersleadstodifferentpolymercharacteristicsinthepursuitofapropertyprofilethatincludesspecificmechanical, thermal,andchemicalproperties.Ofcourse,thechoiceofmonomersto co-polymerize,theirrespectiveweightratio,aswellastheenvironmental conditionssurroundingthepolymerizationprocessarenotarbitrary;ratherit isdefinedbasedonthereactivityofthesemonomerswitheachotherandwith anycatalystthatmaybeusedtoinitiatetheprocess.Themixing,heating,or coolingratesarejustafewexamplesofenvironmentalconditionsthatcontributetothedegreeofpolymerizationandthedegreeofcrystallinity.Forexample, rapidcoolingofapolymermeltyieldsanamorphousstructure,whileslow coolingresultsinasemicrystallinestructure.
Asdiscussedbefore,thepolymersynthesisprocessiscalledpolymerization, wherethechemicaljoiningofmonomers(regardlessofthenumberofdifferent mers)isdonethroughadditionorsteppolymerization.Eitheroftheseprocesses canbeendothermal(e.g.,themixingpotfeelscoldduringpolymerization)or exothermal(thecontainercangetverywarmandmayrequireexternalcooling).
Additionpolymerization(alsotermed“chainpolymerization”)isarapidsynthesisprocessinitiatedbyachemicalcatalyst(alsoreferredtoasinitiator andcommonlyknownasahardener),whichradicalizestheterminalbonds ofthemonomercausingthemtojointogethertodevelopalongmacromolecule chain.Polyethylene,polypropylene,polyvinylchloride,andnaturalrubber (poly-isoprene)arejustconventionalpolymersthataresynthesizedusingadditionpolymerization.Ontheotherhand,condensation(orstep)polymerization isarelativelyslowprocess,wherelongmoleculesformafterasufficienttime lapse.Waterorammoniaareusuallybyproductsofsteppolymerization,hence thename“condensation.”Inthisprocess,thereactingmonomersjointogether forminganewmoleculeandthisstepisrepeated,wherethenewlyformedmoleculesjointogethertocreatethelong-chainmacromolecule.Henceforth,two chemicalreactionsarepartofsteppolymerization,namely,singleandcombiningreactionsthatareongoingsimultaneously.
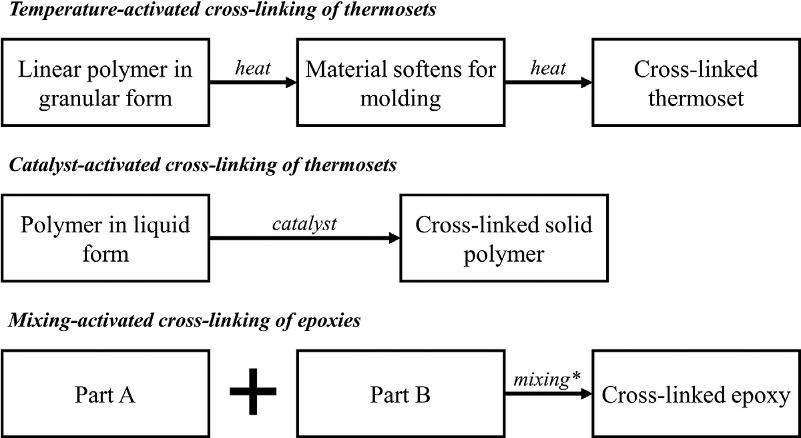
Forthermosetpolymers,theprocessofcovalentlybondingmonomers togethertocreateathree-dimensionalnetworkiscalledcross-linking,which iscommonlyreferredtoasthecuringprocessofthermosets.Inotherwords, thestagesofprocessingthermosetsarecuring,setting,andhardening.That is,whensomeoneissaying:“waitingforthepolymertodry,”thispersonisactuallyreferringtothetimerequiredtoreachthehardeningstage,wherethepolymeris“dry”ordoesnotexhibittackiness.Thecross-linkingofthermosetscan bedividedintothreecategories:temperature-activated,catalyst-activated,and mixing-activated(orkinetically-activated).Thedifferencebetweenandthe detailofeachofthecross-linkingcategoriesisshownschematicallyin Fig.1.6.Itisworthnotingthatthecrossinglinkingprocessofrubbersisusually termed vulcanization.
FIG.1.6 Categoriesofcross-linkingthermosetpolymers(* ¼ heatmaybeaddedtoacceleratethe reaction).
Giventheintendedreadershipofthistextbookbeingpredominantlyengineeringstudentsandpractitioners,itiscrucialtogoonaslighttangenthere todiscussthedifferencebetweenpolymersynthesisandpolymermanufacturing.Thelatterisusedheretorefertotheprocessingofpolymersintothefinal productor,inotherwords,“shaping”thepolymerintoausefulform.Shaping polymerscanbedonethroughextrusion,injectionmolding,thermoforming, casting,stretching,dieforming,andcalendaring,whichareaverysmallsubset ofpolymerprocessingtechniques.ThereaderisreferredtoGroover’sPrinciplesofModernManufacturing:Materials,Processes,andSystemsaswellas thePrinciplesofPolymerProcessingbyTadmorandGogosforadditional detaileddiscussionsabouttheseprocessesandmore [7,8].Alaterchapterof thistextbookisdedicatedtothecommonmanufacturingintheplasticsindustry.
1.4.2Polymerphysics
Thenextsubfield,polymerphysics,mergesknowledgeofphysicalchemistry andstatisticalphysicstoelucidatetheunderlyingphysicsandchemistryassociatedwithpolymerpropertiesindifferentforms,includingmelt,solution,and solid [9].Polymerphysicistsareconcernedwiththearrangementswithinthe molecularstructure,i.e.,linearvs.branchedvs.cross-linked,andtheenergy requiredtoachieveadegreeofcrystallinity.Earlierinourdiscussion,thesuppositionofthelackoffullycrystallinepolymerwasintroduced,wherethereis anenergybarrierthatlimitsthestateoforderlyfoldingthechainsthroughout thestructure.Polymerphysicistscanprovidescientificevidenceastowhysuch asuppositionisvalidbasedonthethermodynamicsofpolymersindifferent phases.Anotherimportanttopicthatpolymerphysicistsstudyisconformational changesandtheirevolutionunderdifferentloading,environmental,andoperatingconditions.Dr.EiseleintheprefaceoftheIntroductiontoPolymerPhysicsbooksummarizedthecontributionofpolymerphysicsinvolvingthe “investigationofmolecularmobilityanddeformation,phasetransitions,molecularinteractions,andtheresultingsupra-molecularstructures,thedevelopmentofanunderstanding,fromamolecularstandpoint,ofthephysicaland technologicalpropertiesofpolymericmaterials.” [9].Therefore,polymerphysics,atitsessence,isimperativeforprovidingthescientificreasonsleadingtoa givenpolymerarchitecture.Itisthenatthecenterofthestructure–property-processing-performancenexussinceitrelatesthestructuretothematerial properties.
1.4.3Polymermechanics
Finally,polymermechanics,thereasonyouarereadingthistextbookinthefirst place,emphasizesthemechanicalbehaviorofsolidpolymersfromtheexperimental,analytical,andcomputationalpointsofview [10–16].Inotherwords,a polymermechanician(sometimesisalsoreferredtoas“mechanist”)seeksto
explicatetheresponseofpolymerstoappliedloadsunderdifferentloading rates,temperatures,anddurations.Usingthecomplexityofthemicrostructure todescribethemechanicalresponseofpolymerscanbemorechallengingthan thatofothermaterialsgiventheextendedelasticityandinherenttimedependenceresponse.Somepolymers,suchaselastomers,canexperiencea fewhundredpercentagesofreversibleextensibility,e.g.,extendedelasticity ofarubberband,whileatthesametimesuffercontinuousdeformationifthe loadiskeptconstant,i.e.,creep.Therefore,theremustbemathematicalexpressionsalsocalledmaterialsmodelsorconstitutivemodelsthatdescribethe stress–strainrelationshipasafunctionoftimeandstretchratio.The stress–strainresponseandtheaccompanyingchangesinthepropertiesare probedusingnumerousexperimentaltechniques,someofwhichwillbediscussedlaterinthebook.
Apolymer-basedpartoracomponentthatwasdesignedtomeetspecific requirementsispossiblysubjectedtomanymodesofappliedloadsthataresustainedbasedonthematerialcapacity.Ingeneral,theappliedstressescanbe classifiedaseithernormalorshear.Normallyappliedstressescanbedue, forexample,toaxial(compressiveortensile)loading,bending,bearing,orpressureontheinternalorexternalwallsofacylinder.Ontheotherhand,applied shearstressesareattributedtodirectshear(singleordouble),transverseshear, ortorsion.Theeffectivestressduetobothnormalandshearstressestestthe materialcapacity,whichisdeterminedexperimentallywherethematerialis loadeduntilfailure.Thesetestsincludecreep,relaxation,fatigue,tensile/compression,impact,wear,fracture,andenvironmentaldegradation.Theremaining chaptersofthetextbookexhaustivelytreatthesetopicsofmechanicalresponse atadifferenttimeanddeformationscales.
1.5Industrialapplicationsofpolymers
Theproliferationofpolymersineveryindustrialandcommercialsector,assporadicallydiscussedintheprevioussections,impedesthecreationofacomprehensivelistofallpolymer-basedpartsandcomponentsthataresynthesized, manufactured,andusedwithineachindustry.Inthecommercialaircraftindustry,forexample,thereareseveralairplanemanufacturers(e.g.,Boeing,Airbus, Bombardier,etc.)withanextensivelistofsupplychainnetworksoforiginal equipmentmanufacturers(OEMs)toproduceseveralplaneswithdifferent passenger-capacityincludingdifferentconfigurationsofeachairplanegeneration.Eachtypeofaircrafthasthousandsofpartsandcomponentsmadeof polymers.Asimilarclaimwasstatedearlierinthischapterabouttheautomotive industry.Thisistruefortheelectronics,consumergoods,householdgoods,furniture,biomedical,transportation,energy,andconstructionindustries. Table1.3 includesexamplesoftheintegrationandapplicationsofpolymers invariousindustrialsectors.Theremainingsectorsareleftasanexerciseto sharpenthereader’sresearchskills(seePracticeProblemssection).
TABLE1.3 Shortlistofexamplesofpolymersinvariousindustrialsectors. Industrial sectorExampleapplications
AerospaceSeats,cabinwindow,windowshades,storagebins,lightingfixtures, severallavatorycomponents,sidewallpanels,etc.
AutomotiveInteriortrimmings,dashboard,instrumentpanel,lights,bumpers, spoilers,undercarriagecovers,wheelhousing,airintakeparts,etc.
ElectronicsIntegratedcircuitboards,chipencapsulation,keyboards,external housing,etc.
MechanicalVibrationisolationpaddings,cranewheels,reels,etc.
HouseholdKitchencountertops,bathroomvanities,lightfixtures,patiofurniture, storagecontainsgardenhoses,partsofappliances/housewares,etc.
ConstructionWindowframes,plumbingpipes,electricalconduitandcomponents, wireinsulations,thermalinsulation,crownmoldings,etc.
1.6Closingremarks
Beforeclosingthischapter,fourpracticaltidbitsabouthandlingpolymersare listedbelowtoassistnoviceengineersintheirintegrationintothisexciting community.First,donottouchtheexternalsurfacesofmixingpotsorcontainersduringthepolymersynthesisprocesssincetheycouldbeveryhot becausesomepolymerizationprocessesareexothermal.Thiscouldresultin severebodilyinjury.Second,donothandlepolymerswithoutproperprotective equipment(e.g.,gloves)becauseyoumayleavepermanentimprintsandoilresiduesthatarehardtoremove.Anentirestockpiececanbescrappedbecauseof poorhandling.Third,donotcleanthesurfacepolymerswithanysolventor solutionbeforeunderstandingthechemicalcompatibilitysincesomeorganic solvents(forexampleacetoneanddimethylformamide)areveryaggressive andwilldestroythepolymerbycrazingorevendissolution.Everychemical manufacturer(e.g.,DOWindustriesandDuPont)providestablesandlistsof solventcompatibility,whichcanbedownloadedforfreefromthecompanies’ websites.Finally,carefullyandmethodicallyreadthemixingandhandling instructionbeforestartingtheprocesssincethepolymerizationprocessishighly dependentonthemixingtemperature,mixingtime,andmixingratios.
Practiceproblems
1. Listallthepolymersusedinaproduct(e.g.,cellularphoneorcalculator) withinanindustrythatinterestsyou.Downloadingproductdescriptions, patents,anddatasheetsmightbehelpful.