https://ebookmass.com/product/applications-of-nanovesicular-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Biological Macromolecules: Bioactivity and Biomedical Applications Amit Kumar Nayak
https://ebookmass.com/product/biological-macromolecules-bioactivityand-biomedical-applications-amit-kumar-nayak/
ebookmass.com
Applications of Nanotechnology in Drug Discovery and Delivery Chukwuebuka Egbuna
https://ebookmass.com/product/applications-of-nanotechnology-in-drugdiscovery-and-delivery-chukwuebuka-egbuna/
ebookmass.com
Applications of Polymers in Drug Delivery 2nd Edition Ambikanandan Misra (Editor)
https://ebookmass.com/product/applications-of-polymers-in-drugdelivery-2nd-edition-ambikanandan-misra-editor/
ebookmass.com
The Girl with the Dragonfruit Tattoo (Trouble in Paradise! 3) Carrie Doyle
https://ebookmass.com/product/the-girl-with-the-dragonfruit-tattootrouble-in-paradise-3-carrie-doyle/
ebookmass.com
Lion Brothers: Menage Sci-Fi Romance (Lion Pride Book 2)
Lilly Wilder
https://ebookmass.com/product/lion-brothers-menage-sci-fi-romancelion-pride-book-2-lilly-wilder/
ebookmass.com
The New Institutionalist Economic History of Douglass C. North: A Critical Interpretation 1st Edition Matthijs Krul
https://ebookmass.com/product/the-new-institutionalist-economichistory-of-douglass-c-north-a-critical-interpretation-1st-editionmatthijs-krul/
ebookmass.com
What Lies Adventure Mystery 02 What Lies in the Hills
Steve Kittner
https://ebookmass.com/product/what-lies-adventure-mystery-02-whatlies-in-the-hills-steve-kittner/
ebookmass.com
Test
Your Vocabulary 1 Peter Watcyn-Jones
https://ebookmass.com/product/test-your-vocabulary-1-peter-watcynjones/
ebookmass.com
Invertebrate Histology 1st Edition Elise E.B. Ladouceur (Editor)
https://ebookmass.com/product/invertebrate-histology-1st-editionelise-e-b-ladouceur-editor/
ebookmass.com
The All-Consuming Nation: Chasing the American Dream Since World War II
https://ebookmass.com/product/the-all-consuming-nation-chasing-theamerican-dream-since-world-war-ii-lytle/
ebookmass.com
ApplicationsofNanovesicularDrugDelivery
Applicationsof NanovesicularDrug Delivery
Editedby
AmitKumarNayak DepartmentofPharmaceutics,SeemantaInstituteofPharmaceuticalSciences, Mayurbhanj,Odisha,India
MdSaquibHasnain DepartmentofPharmacy,PalamauInstituteofPharmacy, Daltonganj,Jharkhand,India
TejrajM.Aminabhavi
SchoolofAdvancedSciences,KLETechnologicalUniversity, Hubballi,Karnataka,India
VladimirP.Torchilin CenterforPharmaceuticalBiotechnologyandNanomedicine,NortheasternUniversity, Boston,MA,UnitedStates
AcademicPressisanimprintofElsevier 125LondonWall,LondonEC2Y5AS,UnitedKingdom 525BStreet,Suite1650,SanDiego,CA92101,UnitedStates 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,including photocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher. Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswith organizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions .
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybe notedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding, changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation, methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheir ownsafetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjury and/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationof anymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-91865-7
ForInformationonallAcademicPresspublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: MicaH.Haley
AcquisitionsEditor: AndreG.Wolff
EditorialProjectManager: PatGonzalez
ProductionProjectManager: PunithavathyGovindaradjane
CoverDesigner: MarkRogers
TypesetbyMPSLimited,Chennai,India
1.Targetingcellularandmolecular mechanismsofnanovesicularsystems forthetreatmentofdifferentdiseases1
NatassaPippa,HectorKatifelis,MariaGazouli andStergiosPispas
1.1Introduction1
1.2Lipidnanovesicularsystems2
1.2.1Liposomes2
1.2.2Elasticliposomes:ethosomesand transferosomes2
1.2.3Niosomes5
1.2.4Ufasomes5
1.3Polymernanovesicularsystems5
1.3.1Polymersomes/polymervesicles5
1.3.2Nanovesicularsystemsfortargetingto cellularmechanisms7
1.3.3Nanovesicularsystemsfortargeting molecularmechanismsandtheeraof CRISPR/CAS910
1.3.4Nanovesicularsystemsforthe treatmentofdifferentdiseases11
1.4Conclusions13 References14
2.Nanovesiclesfordrugcodelivery21
NafiuAminu
2.1Introduction21
2.2Combinationdrugtherapy22
2.3Generaloverviewofnanovesicles23
2.3.1Liposomes24
2.3.2Niosomes25
2.3.3Exosomes26
2.3.4Spanlastics26
2.4Designandpreparationtechniquesof codeliverynanovesicles26
2.4.1Mechanicaldispersion/filmhydration method26
2.4.2Ultrasonicationmethod26
2.4.3Self-assembling27
2.4.4Solventdispersionmethods27
2.4.5Detergentremovalmethod27
2.4.6Microfluidizationmethod27
2.4.7Handjani Vilamethod28
2.5Nanodrugcodeliverysystems28
2.5.1Nanovesicles-hydrogelsfor codeliveryofdrugs28
2.5.2Nanovesiclesforcodeliveryof anticancerdrugs31
2.5.3Nanovesiclesforcodeliveryof cardiovasculardrugs33
2.5.4Nanovesiclesforcodeliveryof antibacterial/antiinflammatorydrugs33
2.6Conclusion34 References34
3.Theranosticnanovesicles39
ArnabDe,ShilpaDas,SantanuGhosh, BhaskarDas,SonalinandiniSamanta, BolayBhattacharyaandAmaleshSamanta
3.1Introduction39
3.2Imagingstrategies40
3.2.1Opticalimaging41
3.2.2Magneticresonanceimaging41
3.2.3Radionuclide-basedimaging41
3.2.4Computedtomography41
3.2.5Ultrasound42
3.3Differentnanovesiclesusedastheranostic system42
3.3.1Liposomes42
3.3.2Ethosomes44
3.3.3Transferosomes45
3.3.4Niosomes45
3.3.5Polymersomes47
3.4Conclusion48 References48
4.Nanovesiclesforoculardrugdelivery53
SophiaG.AntimisiarisandEvangelosNatsaridis
4.1Introduction53
4.2Physiology,routesofdrugadministration andocularbarriersfordrugpenetration53
4.2.1Physiologyoftheeye53
4.2.2Routesofdrugadministrationto theeyeandcorrespondingocular barriers54
4.3Oculardiseases57
4.3.1Anteriorsegmentdiseases57
4.3.2Posteriorsegmentdiseases58
4.4Nanovesiclesforoculardrugdelivery59
4.4.1Preclinicalstudies61
4.4.2Clinicalstudiesandapproved products74
4.5Conclusionsandfutureperspectives77 Acknowledgmentsandfunding77 References77
5.Nanovesiclesfornasaldrugdelivery81 GouravPaudwal,NagmaBanjareand PremN.Gupta
5.1Introduction81
5.2Intranasaldrugdeliverysystem82
5.3Dosageformsandabsorption enhancers82
5.3.1Nasaldrops82
5.3.2Nasalspray83
5.3.3Nasalgel83
5.3.4Nasalpowders83
5.4Benefitsofintranasaldrugdelivery83
5.5Barriersinnasaldistribution84
5.5.1Poorbioavailability84
5.5.2Biliaryclearance84
5.5.3Enzymedegradation84
5.6Needforintranasaldrugdelivery system85
5.7Anatomyandphysiologyofnasal route85
5.8Mechanismofabsorptionofdrugsvia nasalroute86
5.8.1Intracellularpathway86
5.8.2Transcellulartransport86
5.9Nasaldevices87
5.10Roleofnanotechnologyintranasaldrug delivery87
5.11Nanovesiclesforintranasaldrugdelivery88
5.11.1Lipidbasednanovesicles88
5.11.2Nonionicsurfactantbased nanovesicles91
5.11.3Biologicallyderivednanovesicles92
5.12Applicationsofnanovesicularintranasal deliverysystem92
5.12.1Viralinfection92
5.12.2Osteoclasticboneresorption93
5.12.3Centralnervoussystemdisorders93
5.12.4Migraine93
5.12.5Hypertension93
5.12.6Anxietydisorders93
5.12.7Antinociceptive94
5.12.8Oxytocinandinsulindelivery94
5.12.9Cancer94
5.12.10Neurodegenerative/brain inflammatorydisease94
5.12.11Cerebralarteriosclerosis, thrombosis,andvertigo disorders94
5.13Conclusion96 References96
6.Nanovesiclesfortransdermaldrug delivery103
HongdaZhu,ChaoboYangandKaiMa
6.1Introduction103
6.1.1Themechanismsofinteractions betweennanovesiclesystemsand skin104
6.2Lipid-basedvesicularnanostructuresfor transdermaldrugdelivery104
6.2.1Traditionalliposomesasskindrug deliverysystems104
6.2.2Transfersomes104
6.2.3Ethosomes107
6.2.4Invasome108
6.2.5Glycerosomes109
6.2.6Hyalurosomes110
6.3Nanovesiclesformedbynonlipidbuilding blocks110
6.3.1Niosomesastransdermaldrug deliverysystems110
6.3.2Polymersomesastransdermal drugdeliverysystems111
6.4Conclusionandfutureperspective111 References112
7.Nanovesiclesforintravenousdrug delivery115
HazalEzgiGu ¨ ltekin,EzgiOner,Miray Ilhan andMerveKarpuz
7.1Introduction115
7.2Intravenousdrugadministration116
7.3Nanovesicularsystems121
7.3.1Liposomes121
7.3.2Niosomes124
7.3.3Polymersomes125
7.3.4Transfersomes126
7.3.5Ethosomesandethosomal nanovesicles127
7.3.6Phytosomes127
7.3.7Extracellularvesicles128
7.4Intravenousnanovesiclesforimaging128
7.5Intraveneousnanovesiclesfortherapy133
7.5.1Tumortargetingandcancertherapy133
7.5.2Fungalinfections134
7.5.3Painmanagementandinflammatory diseases134
7.5.4Others134
7.6Intravenousnanovesiclesforgenetherapy135
7.6.1Intravenousnanovesicularsystems developedforgeneaugmentation136
7.6.2Intravenousnanovesicularsystems developedforgenesilencing (suppression)136
7.6.3Intravenousnanovesicularsystems developedforgenomeediting136
7.7Intravenousnanovesiclesfortheranostic136
7.8Conclusion138 References139
8.Nanovesiclesfortargetspecificdrug delivery149
AmnaZafar,Asim-ur-Rehmanand NaveedAhmed
8.1Introduction149
8.2Liposomesasdrugdeliveryvesicles150
8.2.1Typesofliposomes150
8.2.2Applications151
8.3Polymericmicellesasdrugdelivery vehicles152
8.3.1Applications153
8.4Exosomesasdrugdeliveryvesicles153
8.4.1Applications155
8.5Niosomes—drugdeliveryvesicles155
8.5.1Applications155
8.6Neweraofvesiculardrugdeliverysystems155
8.6.1Transferosomes155
8.6.2Ethosomes157
8.6.3Sphingosomes157
8.6.4Cubosomes157
8.6.5Ufasomes158
8.6.6Colloidosomes158
8.6.7Aquasomes159
8.6.8Polymerosomes159
8.6.9Emulsomes159
8.6.10Virosomes160
8.6.11Enzymosomes160
8.6.12Pharmacosomes160
8.7Conclusions163 References163
9.Blood brainbarrierandnanovesicles forbrain-targetingdrugdelivery167 YadollahOmidi,HosseinOmidian, YoungKwonandAnaCastejon
9.1Introduction167
9.2Neurovascularunit169
9.2.1Blood brainbarrierandbloodcerebrospinalfluidbarrierroles169
9.2.2Immunosurveillancein neurovascularunit170
9.2.3Tightjunctionalmolecular machinery170
9.2.4Blood brainbarriermodels170
9.2.5Blood brainbarriertransport machinery172
9.2.6Endocytosis,transcytosis,and vesiculartrafficking172
9.2.7Nanovesiclesdeliverymechanisms174
9.3Issueswiththetargetedtherapyofbrain diseases174
9.4Nanoscalebrain-targetingdelivery systems176
9.5Nanovesicles176
9.5.1Lipid-basednanovesiclesforbrain targeting177
9.5.2Translationofbrain-targeting lipid-basednanovesicles185
9.5.3Polymer-basednanovesicles188 9.6Concludingremarks190 References190
10.Nanovesiclesforhepatic-targeted drugdelivery201
ManishKumar,AbhishekJha,KanchanBharti andBrahmeshwarMishra
10.1Introduction201
10.2Nanovesicularsystemsfordrugdelivery toliver201
10.3Mechanismofnanovesicles-targeted delivery202
10.3.1Passivetargeting202
10.3.2Activetargeting202
10.4Roleinimprovingthedrugdistribution andpharmacokineticparameters205 10.5Applications205
10.5.1Nanovesiclesforhepatocellular carcinoma205
10.5.2Nanovesiclesforhepatic infections211
10.5.3Nanovesiclesforhepatoprotective effect211
10.6Conclusion213
References214
11.Nanovesiclesfortumor-targeted drugdelivery219
MerveKarpuz,Miray ˙ Ilhan, HazalEzgiGultekin,EmreOzgenc, Zeynep¸Senyi ˘ gitandEvrenAtlihan-Gundogdu
11.1Introduction219
11.2Nanovesicles221
11.2.1Liposomes222
11.2.2Niosomes223
11.2.3Phytosomes224
11.2.4Ethosomes224
11.2.5Polymersomes225
11.2.6Exosomes225
11.2.7Transfersomes226
11.3Targetingmechanismsofnanovesicles fortumor226
11.3.1Passivetargeting226
11.3.2Activetargeting228
11.4Nanovesiclesfortumorimaging232
11.4.1Magneticresonanceimaging232
11.4.2Computedtomographyimaging232
11.4.3Nuclearmedicineimaging233
11.5Nanovesiclesfortumortreatment233
11.6Nanovesiclesfortheranosticapproach234
11.7Conclusion235 References236
12.Tumormicroenvironment-responsive nanovesiculardrugdeliverysystems245
MoniraGhoniem,KholoudK.Arafaand IbrahimM.El-Sherbiny
12.1Introduction245
12.1.1Doxorubicin-basednanovesiculars246
12.1.2Paclitaxel-basednanovesiculars246
12.1.3Physiologicaltumor microenvironmentcharacteristics247
12.1.4Tumorbiochemicalcharacteristics247
12.1.5Immunemicroenvironment characteristics250
12.2Conclusion251 References251
13.Nanovesiclesforcolon-targeted drugdelivery253
PoojaDasBidla,PritishK.Panda,AmitVerma, SarjanaRaikwarandSanjayK.Jain
13.1Introduction253
13.2Factorsaffectingcolonicdrugdelivery253
13.3Advantagesandlimitationsof colon-targeteddrugdeliverysystems255
13.4Applicationofnanocarriersotherthan nanovesiclesforcolon-targeteddrug delivery256
13.5Applicationsofnanovesiclesforthe treatmentofcolonicdisease257 13.5.1Liposomes257 13.5.2Niosomes259 13.5.3Phytosomes260 13.5.4Cubosomes260 13.5.5Emulsosome260
13.6Applicationsofnanovesiclesinthe detectionofcolonicdisease262
13.7Conclusionandfutureprospects263 References263
14.Nanovesiclesfordeliveryof anticancerdrugs267
JithuJoseph
14.1Introduction267
14.2Classificationanddevelopmentofthe nanovesicles267 14.2.1Classificationofthe nanovesicles267
14.3Applicationsofthenanovesiclesforthe deliveryofanticancerdrugs269
14.4Conclusionandfutureprospects278 References278
15.Nanovesiclesforthetreatmentof skindisorders285
AyeshaWaheed,AbdulAhad, DipakKumarGupta,Mohd.Aqil, FahadI.Al-Jenoobiand AbdullahM.Al-Mohizea
15.1Introduction285
15.1.1Skinpermeationpathways285 15.2Typesofnanovesicles286 15.2.1Liposomes288 15.2.2Ethosomes288 15.2.3Niosomes289 15.2.4Transfersomes289 15.2.5Cubosomes290 15.2.6Solidlipidnanoparticles290 15.2.7Nanostructuredlipidcarriers290 15.2.8Nanoemulsion290 15.2.9Polymericnanoparticles290 15.2.10Nanofibers290 15.2.11Dendrimers291
15.3Skindisorders291
15.3.1Skincancer291
15.3.2Psoriasis292
15.3.3Acne293
15.3.4Alopecia295
15.3.5Fungalinfections295
15.3.6Atopicdermatitis296
15.4Conclusion297 References297
16.Nanovesiclesforthedeliveryof nonsteroidalanti-inflammatorydrugs303
ShohrehFahimirad
16.1Introductionofnonsteroidal anti-inflammatorydrug303
16.2Nonsteroidalanti-inflammatoryagents303
16.3Nanotechnologyandnonsteroidal anti-inflammatorydrugsdelivery304
16.4Liposomes305
16.5Nonliposomallipid-basednanovesicles306
16.5.1Niosomes306
16.5.2Transfersomes306
16.5.3Ethosomes306
16.5.4Sphingosomes306
16.5.5Ufasomes307
16.5.6Pharmacosomes307
16.5.7Virosomes307
16.5.8Quatsomes307
16.6Methodsofpreparation307
16.6.1Conventionalpreparation methods307
16.6.2Thinlipidfilmhydrationmethod308
16.6.3Solventinjectiontechnique311
16.7Novelpreparationmethods311
16.7.1Supercriticalfluidsmethods311
16.7.2Recentapplicationof nanovesiclesfordeliveryof nonsteroidalanti-inflammatory drugs311
16.8Conclusion311 References311
17.Nanovesiclesfordeliveryofcentral nervoussystemdrugs315
ReshuVirmani,TarunVirmaniandKamlaPathak
17.1Introduction315
17.2Nanovesicles315
17.3Categoriesofnanovesicles315
17.3.1Liposomes315
17.3.2Virosomes316
17.3.3Niosomes316
17.3.4Proniosomes316
17.3.5Transferosomes316
17.3.6Proteasomes317 17.3.7Sphingosomes317
17.3.8Archaesomes317 17.3.9Ethosomes317 17.3.10Polymersomes317 17.4Nanovesiclesforcentralnervous systemdisorders317 17.4.1NanovesiclesforAlzheimer’s disease317 17.4.2NanovesiclesforParkinson’s disease320
17.4.3Nanovesiclesformigraine321 17.4.4Nanovesiclesforepilepsy322 17.4.5Nanovesiclesforpsychosis324 17.4.6Nanovesiclesforcentralnervous systeminfection325 17.4.7Nanovesiclesfordepression326 17.4.8Nanovesiclesforbraintumors327 17.4.9Nanovesiclesforneuroprotection328 17.4.10Nanovesiclesformultiplesclerosis andamyotrophiclateralsclerosis330 17.4.11Nanovesiclesforcerebral ischemia331 17.5Currentchallengesandfutureprospects333 17.6Conclusion334 Conflictsofinterest334 References335
18.Nanovesiclesforthedeliveryof cardiovasculardrugs341
DomenicoMarson,SuzanaAulic, AliceFermeglia,ErikLauriniandSabrinaPricl
18.1Introduction341
18.2Aprimerofcardiovasculardiseases342 18.2.1Atherosclerosisand hyperlipidemia342
18.2.2Venousthromboembolism343
18.2.3Acutemyocardialinfarction343 18.2.4Hypertension344
18.2.5Pulmonaryhypertension344 18.2.6Stroke345
18.3Nanovesiclesforthedeliveryof cardiovasculardrugs345
18.3.1Nanovesiclesforthetreatmentof atherosclerosisand hyperlipidemia345
18.3.2Nanovesiclesforthetreatmentof venousthromboembolism349
18.3.3Nanovesiclesforthetreatmentof hypertension351
18.3.4Nanovesiclesforthetreatmentof pulmonaryhypertension353
18.3.5Nanovesiclesforthetreatmentof acutemyocardialinfarction355
18.3.6Nanovesiclesforthetreatmentof stroke358
18.4Futureoutlook361 Acknowledgments362 References362
19.Nanovesiclesforthedeliveryof antibiotics371
Quratulain,NazimHussain, SyedAwaisAttiqueandMuhammadBilal
19.1Introduction371
19.2Nanovesiclesaspotentialantibioticdrug deliveryand/ortargetingsystems371
19.3Nanoparticlebacterialresistance372
19.4Antimicrobialresistancemechanisms372
19.5Theimpactofnanoparticleson microbialstrength373
19.5.1Treatmenttechniquesasan effectivedefenseagainst microbialresistance373
19.5.2Overcomingthecurrent mechanismsofantibiotic resistance373
19.6Usingnumerouswaystocombat microorganismsatthesametime373
19.7Assistinginthetransportofantibiotics373
19.8Negativeside:asadrugresistance promoter374
19.9Nanoparticlesantibacterialapplication375
19.10Dressingsofwound375
19.11Bonefortification375
19.12Dentalequipment375
19.13Themechanismfordrugdelivery376
19.14Typesofnanovesiclesusedforthe drugdelivery376
19.15Efficiencyofdifferentnanovesiclesfor drugdeliverysystem376
19.16Roleofnanovesiclesinthedeliveryof antibiotics378
19.17Summaryandfutureperspectives380 Acknowledgment380 Disclosurestatement380 References380
20.Nanovesiclesfordeliveryof antifungaldrugs383
BiswarupDas,AmitKumarNayakand SubrataMallick
20.1Introduction383
20.2Vesiculardeliverysystems384 20.2.1Liposomes384 20.2.2Niosomes386 20.2.3Transfersomes388 20.2.4Ethosomes390 20.2.5Transethosomes392 20.2.6Cubosomes392 20.3Conclusion392 References393
21.Nanovesiclesinantiviraldrug delivery399
MehvishMumtaz,ZulqarnainBaqar, NazimHussainandMuhammadBilal
21.1Introduction399 21.2Whatarenanovesicles?400 21.3Compositionofnanovesicles400 21.4Developmentofnanovesicles401 21.4.1Thinfilmhydration401 21.4.2Nonshakenmethod402 21.4.3Proliposomes402 21.4.4Methodoffreeze-drying402 21.4.5Ethanolinjectionmethod402 21.4.6Etherinjectionmethod402 21.4.7Hotmethod402 21.4.8Coldmethod402 21.4.9Reverse-phaseevaporation technique403
21.4.10Ultrasonication403 21.5Nanovesiclescharacterization403 21.5.1Efficiencyofentrapment403 21.5.2Visualrepresentationof morphology403
21.5.3Zetapotentialandvesiclesize403 21.5.4Temperatureoftransition404 21.5.5Evaluationofsurfacetension behavior404
21.5.6Sustainabilityofvesicles404 21.5.7Deformabilityorelasticity research404
21.5.8Drugcontent404 21.5.9Drugrelease404 21.5.10Permeabilityandabsorption research404
21.6Applicationofnanovesicles404 21.6.1Challengesofnanovesicles404 21.7Antiviraldrugs405 21.8Medicalapplicationsofantiviral drugs405
21.9Designingofantiviraldrugs406 21.9.1Targetingantivirals406 21.9.2Methodologiesbasedonthe pointofthevirus’slifephase406
21.9.3Priortoenteringintoacell406
21.9.4Inhibitorofentry407
21.9.5Inhibitorofuncoating407
21.9.6Asduringthepropagationof viral407
21.9.7Reversetranscription407
21.9.8Integrase407
21.9.9Translation/antisense408
21.9.10Translation/ribozymes408
21.9.11Proteintargetingand processing408
21.9.12Inhibitorsofproteases408
21.9.13LongdsRNAhelixtargeting408
21.9.14Structure409
21.9.15Stepofrelease409
21.10Approvedantiviraldrugs409
21.10.1Acyclovir409
21.10.2Valacyclovir410
21.10.3Ganciclovir410
21.10.4Penciclovir410
21.10.5Famciclovir411
21.10.6Foscarnet411
21.10.7Ribavirin411
21.10.8Lamivudine411
21.10.9Amantadineand Rimantadine411
21.10.10Interferonalfa412
21.11Nanovesiclesinantiviraldrugdelivery412
21.11.1Liposomes412
21.11.2Solidlipidnanovesicles412
21.11.3Nanoemulsions412
21.11.4Self-nanoemulsifydrugdelivery systems413
21.11.5Lipidbasednanovesiclesfor smallinterferingRNAdelivery413
21.11.6Polymer-basednanovesicles413
21.11.7Nanovesiclesmadeof polymers414
21.12Conclusion414 References414 Furtherreading419
22.Nanovesiclesfortargeting autoimmunediseases421
RahatAndleeb,MuhammadUmarIjaz, AsmaAshraf,RidaRafi,DeryaKarata¸sYeni, ShabanaNaz,TayyabaAliand MuhammadAsadSajid
22.1Introduction421
22.2Sourcesofextracellularnanovesicles423
22.2.1Tumorcells423
22.2.2Redbloodcells423
22.2.3Dendriticcells423 22.2.4Mesenchymalstemcells423 22.2.5Milk423
22.2.6Plant424 22.3Biologicalfunctions424 22.4Immunesystemresponsetogeneric nanovesicles424 22.4.1T/Bcellsformationand nanovesicles425 22.5Nanovesicleproduction,cellular communication,andautoimmunity425 22.6Nanovesiclesandautoimmune diseases426
22.6.1Systemiclupuserythematosus426 22.6.2Diabetes427 22.6.3Rheumatoidarthritis428 22.6.4Vitiligo428 22.6.5Preeclampsia429 22.6.6Multiplesclerosis429 22.6.7Sjogren’ssyndrome429 22.6.8Autoimmunethyroiddisease430 22.7Nanovesicle-facilitatedautoimmune diseasetreatmenttherapies430 22.7.1Autoimmunediseasestreatment formesenchymalstem cell-derivednanovesicles430 22.7.2Autoimmunediseasetherapyfor dendriticcell-derived nanovesicles430
22.8Modificationsforthetargeted deliveryofextracellularnanovesicles431 22.8.1Nanovesicledonorcells manipulation431
22.8.2Extracellularnanovesiclesdirect surfacemodification431
22.9Utilizationofnanovesiclesin autoimmuneclinicaltrials432 22.10Conclusionandfutureoutlook433 References433
23.Nanovesicularsystemsforprotein andpeptidedelivery441
TheodoreSentoukas,AthanasiosSkandalis andStergiosPispas
23.1Introduction441
23.2Liposomes442
23.3Polymersomes444
23.4Exosomes447
23.5Nonionicvesicles(niosomes)448
23.6Organic inorganichybridnanovesicles449 23.7Conclusions451 References451
24.Nanovesiclesforthedeliveryof siRNA457
SamuelEshorameSanni,IfiFavourand
AdedayoAdeyanju
24.1Introduction457
24.2Preparationofnanovesiclesandsmall interferingRNA-loadednanovesicles459
24.2.1Nanovesiclepreparation459
24.2.2Loadingnanovesicleswith smallinterferingRNAs459
24.2.3Preparingawesternblot459
24.2.4SmallinterferingRNA quantification460
24.2.5Sizeandzetapotential measurementofnanovesicles460
24.2.6ShortharpinRNAtransduction, PKH67labelingandnanovesicleuptake460
24.2.7Invitrotreatmentofnanovesicles inhumanumbilicalvein endothelialcellsand λ820cells460
24.2.8Thequantitativerealtime-PCR kitfornanovesicle-smallinterfering RNAsgenedetection/recognition461
24.2.9Cellcountandproliferation assays461
24.3Someapplicationsofnanovesiclesfor thedeliveryofsmallinterferingRNA intargetcells/drugdelivery462
24.4Conclusion463 References464
25.Clinicaltrialsofnanovesiclesfor drugdeliveryapplications467 MourelatouElena,GalatouEleftheria, SarigiannisYiannis,ZachariaC.Lefteris, PlioukasMichael,AislaitnerGeorgiosand PetrouC.Christos
25.1Introduction467
25.2Thelegalframeworkforclinicaltrials467 25.3Regulatorychallengesinclinicaltrials inthefieldofnanovesicles468
25.4Liposomes469
25.5Peptide-basednanovesicles476
25.6Exosomes480
25.7Phytosomes482
25.8Niosomes483
25.9Conclusions484 References484
Index487
Listofcontributors
AdedayoAdeyanju DepartmentofChemical Engineering,CovenantUniversity,Ota,OgunState, Nigeria
AbdulAhad DepartmentofPharmaceutics,Collegeof Pharmacy,KingSaudUniversity,Riyadh,SaudiArabia
NaveedAhmed DepartmentofPharmacy,Facultyof BiologicalSciences,Quaid-i-AzamUniversity, Islamabad,Pakistan
TayyabaAli DepartmentofZoology,Government CollegeUniversityFaisalabad,Faisalabad,Pakistan
FahadI.Al-Jenoobi DepartmentofPharmaceutics, CollegeofPharmacy,KingSaudUniversity,Riyadh, SaudiArabia
AbdullahM.Al-Mohizea DepartmentofPharmaceutics, CollegeofPharmacy,KingSaudUniversity,Riyadh, SaudiArabia
NafiuAminu DepartmentofPharmaceuticsand PharmaceuticalMicrobiology,Facultyof PharmaceuticalSciences,UsmanuDanfodiyo University,Sokoto,Nigeria
RahatAndleeb DepartmentofZoology,Government CollegeUniversityFaisalabad,Faisalabad,Pakistan
SophiaG.Antimisiaris LaboratoryofPharmaceutical Technology,DepartmentofPharmacy,Schoolof HealthSciences,UniversityofPatras,RioPatras, Greece;InstituteofChemicalEngineeringSciences, FoundationforResearchandTechnologyHellas (FORTH/ICE-HT),RioPatras,Greece
Mohd.Aqil DepartmentofPharmaceutics,Schoolof PharmaceuticalEducationandResearch,Jamia Hamdard(DeemedUniversity),NewDelhi,India
KholoudK.Arafa NanomedicineResearch Laboratories,CenterforMaterialsScience,Zewail CityofScienceandTechnology,Giza,Egypt
AsmaAshraf DepartmentofZoology,Government CollegeUniversityFaisalabad,Faisalabad,Pakistan
Asim-ur-Rehman DepartmentofPharmacy,Facultyof BiologicalSciences,Quaid-i-AzamUniversity, Islamabad,Pakistan
EvrenAtlihan-Gundogdu DepartmentofRadiopharmacy, FacultyofPharmacy,EgeUniversity,I ˙ zmir,Turkey
SyedAwaisAttique SchoolofInterdisciplinary Engineering&Science(SINES),NUST,Islamabad, Pakistan
SuzanaAulic MolecularBiologyandNanotechnology Laboratory(MolBNL@UniTS)—DEA,Universityof Trieste,Trieste,Italy
NagmaBanjare PK-PDTox&FormulationDivision, CSIR-IndianInstituteofIntegrativeMedicine,Jammu, JammuandKashmir,India;AcademyofScientific andInnovativeResearch(AcSIR),Ghaziabad,Uttar Pradesh,India
ZulqarnainBaqar CentreforAppliedMolecular Biology(CAMB),UniversityofthePunjab,Lahore, Pakistan
KanchanBharti DepartmentofPharmaceutical Engineering&Technology,IndianInstituteof Technology(BHU),Varanasi,UttarPradesh,India
BolayBhattacharya SchoolofPharmacy,Sister NiveditaUniversity,Kolkata,WestBengal,India
MuhammadBilal SchoolofLifeScienceandFood Engineering,HuaiyinInstituteofTechnology,Huaian, China
AnaCastejon DepartmentofPharmaceuticalSciences, CollegeofPharmacy,NovaSoutheasternUniversity, FortLauderdale,FL,UnitedStates
PetrouC.Christos DepartmentofLifeandHealth Sciences,UniversityofNicosia,Nicosia,Cyprus
BhaskarDas DirectorateofDrugsControl,Department ofHealth&FamilyWelfare,GovernmentofWest Bengal,Kolkata,WestBengal,India
BiswarupDas DepartmentofPharmaceutics,Seemanta InstituteofPharmaceuticalSciences,Mayurbhanj, Odisha,India
ShilpaDas DepartmentofPharmaceuticalTechnology, JadavpurUniversity,Kolkata,WestBengal,India
PoojaDasBidla PharmaceuticsResearchProjects Laboratory,DepartmentofPharmaceuticalSciences,
Dr.HarisinghGourVishwavidyalaya,Sagar,Madhya Pradesh,India
ArnabDe SchoolofPharmacy,SisterNivedita University,Kolkata,WestBengal,India
GalatouEleftheria DepartmentofLifeandHealth Sciences,UniversityofNicosia,Nicosia,Cyprus
MourelatouElena DepartmentofLifeandHealth Sciences,UniversityofNicosia,Nicosia,Cyprus
IbrahimM.El-Sherbiny NanomedicineResearch Laboratories,CenterforMaterialsScience,Zewail CityofScienceandTechnology,Giza,Egypt
ShohrehFahimirad MolecularandMedicineResearch Center,ArakUniversityofMedicalSciences,Arak, Iran
IfiFavour DepartmentofChemicalEngineering, CovenantUniversity,Ota,OgunState,Nigeria
AliceFermeglia MolecularBiologyandNanotechnology Laboratory(MolBNL@UniTS)—DEA,Universityof Trieste,Trieste,Italy
MariaGazouli SchoolofMedicine,Laboratoryof Biology,DepartmentofBasicMedicalScience, NationalandKapodistrianUniversityofAthens, Athens,Greece;SchoolofMedicine,2ndDepartment ofRadiology,NationalandKapodistrianUniversityof Athens,Athens,Greece
AislaitnerGeorgios DepartmentofLifeandHealth Sciences,UniversityofNicosia,Nicosia,Cyprus; FederalInstituteforDrugsandMedicalDevices (BfArM),Bonn,Germany
MoniraGhoniem DepartmentofChemistry,Collegeof Science,ImamMohammadIbnSaudIslamic University(IMSIU),Riyadh,SaudiArabia
SantanuGhosh DepartmentofPharmaceutical Technology,JISUniversity,Kolkata,WestBengal, India
HazalEzgiGultekin DepartmentofPharmaceutical Technology,FacultyofPharmacy,IzmirKatipC¸elebi University,Izmir,Turkey
DipakKumarGupta DepartmentofPharmaceutics, SchoolofPharmaceuticalEducationandResearch, JamiaHamdard(DeemedUniversity),NewDelhi, India
PremN.Gupta PK-PDTox&FormulationDivision, CSIR-IndianInstituteofIntegrativeMedicine,Jammu, JammuandKashmir,India;AcademyofScientific andInnovativeResearch(AcSIR),Ghaziabad,Uttar Pradesh,India
NazimHussain CentreforAppliedMolecularBiology (CAMB),UniversityofthePunjab,Lahore,Pakistan
MuhammadUmarIjaz DepartmentofZoology, WildlifeandFisheries,UniversityofAgriculture, Faisalabad,Pakistan
MirayI ˙ lhan DepartmentofPharmaceuticalTechnology, FacultyofPharmacy,IzmirKatipC¸elebiUniversity, Izmir,Turkey;DepartmentofPharmaceutical Technology,FacultyofPharmacy,DuzceUniversity, Du ¨ zce,Turkey
SanjayK.Jain PharmaceuticsResearchProjects Laboratory,DepartmentofPharmaceuticalSciences, Dr.HarisinghGourVishwavidyalaya,Sagar,Madhya Pradesh,India
AbhishekJha DepartmentofPharmaceutical Engineering&Technology,IndianInstituteof Technology(BHU),Varanasi,UttarPradesh,India
JithuJoseph DepartmentofAppliedChemistry,Cochin UniversityofScienceandTechnology,Kochi,Kerala, India
MerveKarpuz DepartmentofRadiopharmacy,Faculty ofPharmacy,IzmirKatipC¸elebiUniversity,Izmir, Turkey
HectorKatifelis SchoolofMedicine,Laboratoryof Biology,DepartmentofBasicMedicalScience, NationalandKapodistrianUniversityofAthens, Athens,Greece
ManishKumar DepartmentofPharmaceutical Engineering&Technology,IndianInstituteof Technology(BHU),Varanasi,UttarPradesh,India
YoungKwon DepartmentofPharmaceuticalSciences, CollegeofPharmacy,NovaSoutheasternUniversity, FortLauderdale,FL,UnitedStates
ErikLaurini MolecularBiologyandNanotechnology Laboratory(MolBNL@UniTS)—DEA,Universityof Trieste,Trieste,Italy
ZachariaC.Lefteris DepartmentofLifeandHealth Sciences,UniversityofNicosia,Nicosia,Cyprus
KaiMa DepartmentofPharmaceuticalEngineering, HubeiUniversityofTechnology,Wuhan,China
SubrataMallick DepartmentofPharmaceutics,School ofPharmaceuticalSciences,Siksha“O”Anusandhan (DeemedtobeUniversity),Bhubaneswar,Odisha, India
DomenicoMarson MolecularBiologyand NanotechnologyLaboratory(MolBNL@UniTS)— DEA,UniversityofTrieste,Trieste,Italy
PlioukasMichael DepartmentofLifeandHealth Sciences,UniversityofNicosia,Nicosia,Cyprus
BrahmeshwarMishra DepartmentofPharmaceutical Engineering&Technology,IndianInstituteof Technology(BHU),Varanasi,UttarPradesh,India
MehvishMumtaz CentreforAppliedMolecular Biology(CAMB),UniversityofthePunjab,Lahore, Pakistan
EvangelosNatsaridis LaboratoryofPharmaceutical Technology,DepartmentofPharmacy,Schoolof HealthSciences,UniversityofPatras,RioPatras, Greece;InstituteofChemicalEngineeringSciences, FoundationforResearchandTechnologyHellas (FORTH/ICE-HT),RioPatras,Greece
AmitKumarNayak DepartmentofPharmaceutics, SeemantaInstituteofPharmaceuticalSciences, Mayurbhanj,Odisha,India
ShabanaNaz DepartmentofZoology,Government CollegeUniversityFaisalabad,Faisalabad,Pakistan
YadollahOmidi DepartmentofPharmaceutical Sciences,CollegeofPharmacy,NovaSoutheastern University,FortLauderdale,FL,UnitedStates
HosseinOmidian DepartmentofPharmaceutical Sciences,CollegeofPharmacy,NovaSoutheastern University,FortLauderdale,FL,UnitedStates
EzgiOner DepartmentofPharmaceuticalBiotechnology, FacultyofPharmacy,IzmirKatipC¸elebiUniversity, I ˙ zmir,Turkey
EmreOzgenc DepartmentofRadiopharmacy,Facultyof Pharmacy,EgeUniversity,Izmir,Turkey
PritishK.Panda PharmaceuticsResearchProjects Laboratory,DepartmentofPharmaceuticalSciences, Dr.HarisinghGourVishwavidyalaya,Sagar,Madhya Pradesh,India
KamlaPathak FacultyofPharmacy,UttarPradesh UniversityofMedicalSciences,Etawah,Uttar Pradesh,India
GouravPaudwal PK-PDTox&FormulationDivision, CSIR-IndianInstituteofIntegrativeMedicine,Jammu, JammuandKashmir,India;AcademyofScientific andInnovativeResearch(AcSIR),Ghaziabad,Uttar Pradesh,India
NatassaPippa FacultyofPharmacy,Departmentof PharmaceuticalTechnology,Nationaland KapodistrianUniversityofAthens,Athens,Greece; TheoreticalandPhysicalChemistryInstitute,National HellenicResearchFoundation,Athens,Greece
StergiosPispas TheoreticalandPhysicalChemistry Institute,NationalHellenicResearchFoundation, Athens,Greece
SabrinaPricl MolecularBiologyandNanotechnology Laboratory(MolBNL@UniTS)—DEA,Universityof Trieste,Trieste,Italy;DepartmentofGeneral Biophysics,FacultyofBiologyandEnvironmental Protection,UniversityofLodz,Lodz,Poland
RidaRafi DepartmentofAppliedChemistry, GovernmentCollegeUniversityFaisalabad, Faisalabad,Pakistan
SarjanaRaikwar PharmaceuticsResearchProjects Laboratory,DepartmentofPharmaceuticalSciences, Dr.HarisinghGourVishwavidyalaya,Sagar,Madhya Pradesh,India
MuhammadAsadSajid DepartmentofZoology, GovernmentCollegeUniversityFaisalabad, Faisalabad,Pakistan
AmaleshSamanta DepartmentofPharmaceutical Technology,JadavpurUniversity,Kolkata,West Bengal,India
SonalinandiniSamanta ESI-PGIMSR,ESICMedical CollegeandHospital,Joka,Kolkata,WestBengal, India
SamuelEshorameSanni DepartmentofChemical Engineering,CovenantUniversity,Ota,OgunState, Nigeria
TheodoreSentoukas CenterofPolymerandCarbon Materials,PolishAcademyofSciences,Zabrze, Poland
AthanasiosSkandalis DepartmentofMaterials,Imperial CollegeLondon,London,UnitedKingdom
Quratulain CentreforAppliedMolecularBiology (CAMB),UniversityofthePunjab,Lahore,Pakistan
AmitVerma BabulalTarabaiInstituteofPharmaceutical Sciences,Sagar,MadhyaPradesh,India
ReshuVirmani SchoolofPharmaceuticalSciences, MVNUniversity,Palwal,Haryana,India
TarunVirmani SchoolofPharmaceuticalSciences, MVNUniversity,Palwal,Haryana,India
AyeshaWaheed DepartmentofPharmaceutics,School ofPharmaceuticalEducationandResearch,Jamia Hamdard(DeemedUniversity),NewDelhi,India
ChaoboYang DepartmentofPharmaceutical Engineering,HubeiUniversityofTechnology,Wuhan, China
DeryaKaratas¸Yeni VeterinaryControlCentral ResearchInstitute,BacterialDiseaseLaboratory, Ankara,Turkey
SarigiannisYiannis DepartmentofLifeandHealth Sciences,UniversityofNicosia,Nicosia,Cyprus
AmnaZafar DepartmentofPharmacy,Facultyof BiologicalSciences,Quaid-i-AzamUniversity, Islamabad,Pakistan
HongdaZhu DepartmentofPharmaceutical Engineering,HubeiUniversityofTechnology,Wuhan, China
ZeynepS¸enyig ˘ it DepartmentofPharmaceutical Technology,FacultyofPharmacy,IzmirKatipC¸elebi University,I ˙ zmir,Turkey
Preface
Nanotechnologyhasrecentlyprovenitsimportanceandusefulnessforguidingtherevolutionarychangesinthefields ofnanomedicines,wherenanovesicleshaveextensivelybeenstudiedandwellrecognizedforthediagnosisandtherapy. Nanovesiclesarethenanoscalecolloidalcarriersystemscomposedofanaqueouscorewithlipidcoating.Overthepast fewdecades,differentnanovesicularcarrierforms(suchasnanoemulsions,self-nanoemulsifyingsystems,nanoliposomes,transferosomes,proniosomesandniosomes,exosomes,polymerosomes,aquasomes,ethosomes,cubosomes, phytosomes,hyalurosomes,glycerosomes,andnanobubbles)havebeenreportedforthedeliveryofvariousdrugs(such asnonsteroidalantiinflammatorydrugs,centralnervoussystemdrugs,cardiovasculardrugs,antibiotics,anticancer drugs,antiviraldrugs,andproteinandpeptidedrugs)throughdifferentroutesofadministrations(suchasoral,nasal, ocular,transdermal,andintravenous).Thesehavebeenemployedforthetarget-specificdrugdeliveryapplications (suchasbraintargeting,colontargeting,livertargeting,andcancertargeting).Inaddition,someadvancedtriggerassistedsystems(suchasiontophoresisandultrasoundtriggering)havebeenusedforimproveddrugdeliverybythe variousnanovesicles.Inthiscontextthecurrentbook“ApplicationsofNanovesicularDrugDelivery”aimstopresenta thoroughinsightintothecompleteandup-to-datediscussionsaboutthevariousmultifunctionalapplicationsofnanovesiculardrugdeliverywithacollectionof 25authoritativechapters bytheleadingacademiciansandresearchersacross theworld.Aconciseaccountonthecontentsofeachchapterhasbeendescribedtoprovideaglimpseofthebookto thereaders.
Chapter1presentsaninsightintotheroleoftargetingcellularandmolecularmechanismsofnanovesicularsystems totreatdifferentdiseases.Inthefirstpart,anoverviewofthemaincategoriesofnanovesicles,formulationprocesses, andcharacterizationtechniquesisdescribed.Inthesecondpart,thenanovesicularsystemsfortargetingtocellularand molecularmechanismshavebeenanalyzedindepth.Acomprehensiveupdateabouttheirapplicationindifferentdiseases,marketedproducts,andpotentialchallengeshasalsobeendiscussed.
Chapter2highlightstheapplicationsofvariousnanovesiclesfordrugdelivery,specificallycodeliveryofmultiple drugs.Thechapteralsoprovidesanoverviewofdrugcombinationtherapyanddifferenttechniquesusedinpreparing nanovesiclesforthecodeliveryofmultipledrugs.Acomprehensiverecentupdateoftheapplicationsofvariousdrug codeliverynanovesiclesincancers,infectious,cardiovasculardiseases,andinflammatorydiseaseshasalsobeen discussed.
Chapter3providesacomprehensivereviewontheranosticapplicationsofvariousnanovesicles(suchasliposomes, ethosomes,transferosomes,niosomes,andpolymersomes).
Chapter4focusesonvariousstrategiesdevelopedinthefieldofnanovesicle-assistedoculardrugdeliveryoverthe pastdecadewithanaimtoovercomethelimitationsofoculardrugdelivery.
Chapter5discussesaboutthepotentialofnanovesicularsystemsandtheirusesinnasaldrugdelivery.Inaddition, dosageformsandabsorptionenhancers,benefitsofintranasaldrugdelivery,barriersinnasaldistribution,anatomyand physiologyofthenasalroute,needforintranasaldrugdeliverysystems,mechanismofabsorptionofdrugsviathenasal route,nasaldevicesandroleofnanotechnologyinintranasaldrugdeliveryhavebeenaddressed.
Chapter6presentsacomprehensivereviewonthevariousnanovesicularsystemsasexcellenttransdermaldrug deliverycarrierstoovercomethestratumcorneumbarrierinintactskin.
Chapter7describestherecentdevelopmentsintheintravenousadministrationofnanovesicles,theiradministrations, andcommerciallyavailableproducts.Inaddition,thischaptercoversthepotentialuseofintravenousnanovesiclesin thetreatmentofdifferentdiseasegroups,genetherapy,imaging,andtheranosticdeliverybyinvestigatingtheiradvantages/disadvantagesandtheuseofthementionedareas.
Chapter8providesadetailedreviewofvariousnaturalandsyntheticnanovesicularsystemswiththeirtherapeutic applicationsforthetarget-orienteddrugdelivery.
Chapter9impartsdeepinsightsintovarioustypesofmultifunctionalnanovesiclescapableofcrossingthe blood brainbarrierforactivetargetingandimagingintreatingtheunhealthycellspresentinthebrain.
Chapter10offersacomprehensiveinsightofnanonovesiclesfortargeteddrugdeliverytotheliverandtheirapplicationsinthemanagementofvariousliverdisorders.Inaddition,mechanismsofnanovesiclesforpassiveandactive hepatictargeting,theroleofnanovesiclesinimprovingdrugdistributionandpharmacokineticparametershavebeen welladdressed.
Chapter11reviewsdifferenttypesofnanovesiclesandtheirtargetingmechanismforcancerimagingandtreatment. Inaddition,somestudiesperformedtodevelopnanovesiclesasimaging,treatment,ortheranosticsystemsforcancers havealsobeensummarized.
Chapter12spotlightsontherecentadvancesinthedesignoftumormicroenvironment-responsivenanovesiclesfor theeffectivedeliveryofchemotherapeuticdrugsandtacklingthechallengeshinderingtheirwidescaleclinical translation.
Chapter13coverstherecentadvancementsinnanovesiclestodesigneffectivecolon-targeteddrugdeliverycarrier systemsforthetreatmentofcolonicdiseases.
Chapter14overviewstheusesofvariousnanovesiclesforthedeliveryofanticancerdrugsinafacilemannerand ultimatelyhelpstotreatvarioustypesofcancers.
Chapter15dealswiththeusesofnanovesiclesfortheeffectivetreatment/managementofsomeimportantskindisorderssuchasskincancer,psoriasis,acne,alopecia,fungalinfections,andatopicdermatitis.Differentnanovesiclesas excellentalternativestoconventionaldosageformsforefficientdermaldeliveryhavealsobeenaddressed.
Chapter16describesthepotentialapplicationsofdifferentnanovesiclesforefficientdeliveryofnonsteroidalantiinflammatorydrugs.
Chapter17providesthecurrentprogressinapplicationsofnanovesiclesforeffectivedrugdeliveryagainstcentral nervoussystemdiseasessuchasAlzheimerdisease,Parkinsondisease,psychosis,migraine,depression,epilepsy,and braintumor.
Chapter18focusesonthemostrecentadvancementsinnanovesiclesasnanodeliverysystemsforcardiovascular drugsforthetreatmentofvariouscardiovasculardiseasessuchasatherosclerosis,hyperlipidemia,thromboembolism, hypertension,acutemyocardialinfarction,andischemicstroke.
Chapter19presentsthepotentialapplicationsofdifferentnanovesiclesfortheefficientdeliveryofantibiotics.In addition,mechanismsofantibioticresistanceandovercomingstrategiesbyantibiotics-loadednanovesicleshavealso beendiscussed.
Chapter20discussestheusesofvesicularnanocarriers(nanovesicles)inthecontextofdeliveryofantifungalagents throughsystemicandtopicalroutestotreatfungalinfections.
Chapter21overviewsthepotentialapplicationsofdifferentnanovesiclesforefficientdeliveryofantiviraldrugsfor thetreatmentofvariousviralinfections.Thecomposition,preparation,andcharacterizationofnanovesiclesarediscussedinthischapter.Inaddition,thedesigningofantiviraldrugsandvariousapprovedantiviraldrugshavealsobeen presented.
Chapter22summarizestheexistingliteratureregardingtheproduction,efficacy,andpotentialtherapeuticusesof nanovesiclesinthecontextsoftargetingautoimmunediseases,fromthediseasepathologytodiagnosisaswellas treatment.
Chapter23describesthebasiccharacteristicsofeachgroupofnanovesicularstructures,providingsomeofthelatest studiesthatshowpotentialapplicationsofthesenanovesicularcarriersystemsforthedeliveryoftherapeuticproteins andpeptidesforthetreatment/managementofseveraldiseasesandconditionssuchasParkinson’sdisease,woundhealing,diabetes,inflammation,cysticfibrosis,ischemicstrokes,andseveraltypesofcancers.
Chapter24focusesontheroleofnanovesiclesforsiRNAdelivery.Othermedicalapplicationsthatbotheron approachesforsiRNAdeliveryinrelationtonanoparticlesinchemicallymodifiedsiRNAshavealsobeendiscussed.
Chapter25givesaninsightintotheregulatoryframeworkgoverningtheclinicaltrialsofvariousnanovesiclesthat arecurrentlyunderclinicaldevelopmentbasedonbibliographicsourcesandclinicaltrialdatabases.
Aseditors,wewouldliketoconveyourspecialthankstoallthecontributingauthorsfordeliveringtheirinvaluable chaptercontributionsinatimelymanner,allowingustopublishthisbookintime.Wewouldalsoliketoexpressour heartfeltgratitudetotheElsevierInc.,AndreGerhardWolff,EmmaHayes,andPatriciaGonzalez(SeniorEditorial ProjectManager)fortheirinvaluableassistanceandkindsupportduringtheeditingprocessofthisbook.Wewould liketoexpressoursincerethankstoPraveenAnandS.(CopyrightCoordinator)forhisoutstandingsupportinobtaining thecopyrightpermissionsandPunithavathyGovindaradjane(ProductionManager)forthedevelopmentandproduction ofthefinalbook.Allthecopyrightcontentsandreprintinglicensesfromdifferentcopyrightsourceshavedulybeen gratefullyacknowledged.
Finally,weappreciateourfamilymembers,alltherespectedteachers,friends,colleagues,andstudentsfortheircontinuousencouragement,inspiration,andsupportduringthepreparationofthisvoluminousbook.Wehope,alongwith ourcontributingauthorsandpublishers,thatoureffortsmeetthedemandsofstudents,academicians,researchers,drug deliveryformulators,pharmaceuticalspecialists,polymerengineers,andbiomedicalexperts.
AmitKumarNayak MdSaquibHasnain TejrajM.Aminabhavi VladimirP.Torchilin
Targetingcellularandmolecular mechanismsofnanovesicularsystemsfor thetreatmentofdifferentdiseases
NatassaPippa1,2,HectorKatifelis3,MariaGazouli3,4 andStergiosPispas2 1FacultyofPharmacy,DepartmentofPharmaceuticalTechnology,NationalandKapodistrianUniversityofAthens,Athens,Greece, 2Theoreticaland PhysicalChemistryInstitute,NationalHellenicResearchFoundation,Athens,Greece, 3SchoolofMedicine,LaboratoryofBiology,Departmentof BasicMedicalScience,NationalandKapodistrianUniversityofAthens,Athens,Greece, 4SchoolofMedicine,2ndDepartmentofRadiology,National andKapodistrianUniversityofAthens,Athens,Greece
1.1Introduction
Nowadays,oneofthekeychallengesinpharmaceuticalnanotechnologyishowtoformulateplatformsthatselectively deliverincorporatedactivesubstancestospecificcellsortissues,whenpassivetargetingisnotpossible,andthereductionofadversereactionsisrequiredforpatientcompliance.1 10 Nanovesicles,suchaslipid-based(liposomes,niosomes,transferosomes,ethosomes,etc.)andpolymer-based(polymersomes,polymernanovesicularstructures,etc.),are nowwellrecognizedaspotentialcandidatesforlow-molecular-weightdrugandantigendeliveryanddiagnostic applications.1 25 Someofthenanovesicularsystemshavebeenalreadyusedinclinicalpracticeasnanomedicineswith addedvalueforthepatientsincomparisontotheclassicalformulations.1,9
Theseconsistofbiocompatibleandbiodegradablematerials(i.e.,lipids,surfactants,andamphiphilicpolymers),and theycanself-assembleinaqueousmediaintovesicularstructureswithvariousphysicochemicalcharacteristicsandbiologicalbehaviors.Theiradvantageoverothermoreconventionalnanostructuresisthepropertytoencapsulatehydrophilicactivepharmaceuticalingredients(APIs)intotheircavityandincorporatethelipophiliconesintheirbilayers. Theycanbealsocharacterizedasbio-inspiredsystemsbecausetheymimicthestructuralpropertiesandthemorphology ofcellularmembranesandsubcellularorganelles.6 Therecenttrendinthisdirectionofresearchisthefabricationof nanovesicularsystemscomposedofdifferentmaterials(i.e.,lipids/polymersandpolymers/surfactants).20,21 Another recenttrendistheplant-derivednanovesiclesthatareaclassofnanovesiclesisolatedfromdietaryvegetablesandfruits andexhibitedseveraladvantagesandnewproperties.16 Alltheabovenanovesicularsystemscancontrolthereleaseof theencapsulatedAPIsatthespecifictargettissue.Thedesignandthedevelopmentofstimuli-responsivenanovesicles forspatiallyandtemporallycontrolledreleaseofAPIsinresponsetointracellularstimuli,suchaspH,redoxpotential, reactiveoxygenspecies,enzymesandtemperatureisveryusefulforthedecreaseofadversereactionsoftheencapsulatedAPI.22 25
NanovesiclescanbeusedfortheencapsulationofdifferentcategoriesofAPIs,andtheycanbeadministered throughdifferentroutes.Forexample,exosomes(versatile,cell-derivednanovesiclesnaturallyendowedwithexquisite target-homingspecificityandtheabilitytosurmountinvivobiologicalbarriers)holdsubstantialpromisefordeveloping excitingapproachesindrugdelivery,cancerimmunotherapy,andasnanoscalecancervaccines.12,13 Furthermore,nanovesiclesareusedasvaccinedeliveryplatformsandasadjuvantsystems.Theadjuvantpropertiesofnanovesiclesare veryimportantbecausetheycanenhancetheimmuneresponseindifferentmolecularpathwaysandinparallel,they cancontrolthereleaseoftheantigen.17,18
Thischapterprovidesaninsightintotheroleoftargetingcellularandmolecularmechanismsofnanovesicularsystemsforthetreatmentofdifferentdiseases.Inthefirstpart,wepresentanoverviewofthemaincategoriesofnanovesicles,theformulationprocessesandcharacterizationtechniquesthatareusedintherecentliterature.Inthesecondpart, 1 ApplicationsofNanovesicularDrugDelivery.DOI: https://doi.org/10.1016/B978-0-323-91865-7.00006-7 © 2022ElsevierInc.Allrightsreserved.
thenanovesicularsystemsfortargetingtocellularandmolecularmechanismsareanalyzedindepthandexamplesfrom recentlypublishedstudiesarecited.Acomprehensiveupdateabouttheirapplicationindifferentdiseasesandthemarketedproducts,aswellaspotentialchallengeswillbealsodiscussed.Systematicsearchandreviewofpapersregarding thetargetingcellularandmolecularmechanismsofnanovesicularsystemsforthetreatmentofdifferentdiseaseswere performedviaMedLine,ScopusandWebofScienceplatforms.
1.2Lipidnanovesicularsystems
1.2.1Liposomes
Liposomesarevesicularsystemscomposedofphospholipidbilayers,andtheirsizerangesfromafewtensofnanometersupto1000nm.26 36 Theyarebiocompatibleandbiodegradabledeliverynanosystems.26 36 Liposomaldrugs aremarketedmedicineswithgreatpotentialinthefieldofcancertherapyaswellasotherdiseases.30 31,34 According torecentliterature,patentpublicationsonliposomalformulationshaveexpandedinnovativeareasinpharmaceutical nanotechnology.30 31,34 Thecompositionoftheliposomaldispersionandtheformulationprocessesarethefocuspoints ofmostofthepublishedpatents.Specialattentionisgiventoformulateliposomalsuspensionswithgreatstabilityover timeandwithcontrolledreleasepropertiesoftheencapsulatedAPI.30,34 TheQuality-by-Designandthescale-upof liposomalformulationsgaintheinterestofboththeacademicandindustrialcommunity.30,34 Liposomesareadministeredthroughdifferentroutesincludingtheoralroute.TheycanimprovetheoralbioavailabilityofavarietyofAPIs includingpeptidesandproteins.29,33 Sometimes,thesurfacemodificationismandatoryfortheoraladministrationof thesedeliveryplatforms.33 Additionally,thedesignofeffectiveliposomalformsforinhalationisalsoanimportant researchsubjectanddependshighlyonthecompositionofthevesicles,withtheaimofreducingthedetrimentaleffect ofshearingonliposomestabilityandmaximizingtheirdepositioninthelungtree.32 Finally,stealthliposomesarea subcategoryofliposomeswithbiocompatibleandlowproteinbindingpolymercoatingsexhibitinghighercirculation timeinthehumanbloodavoidingthedecompositionfromtheplasmaproteins.36 Stealthliposomesarealsomarketed medicinesforcancertherapy.31,36
1.2.2Elasticliposomes:ethosomesandtransferosomes
Elasticliposomesareacategoryofliposomesforcutaneousadministration,includingdermalandtransdermaldelivery oftheAPIs.37 Inthisclassofvesicularnanosystem,ethosomesandtransferosomesarecategorized.15,38 55 Ethosomes consistofphospholipids,ethanolathighpercentages(upto40% 50%)andaqueousmedium.15,42 46 Thefluidityof ethosomalmembraneisquitefluidduetothepresenceofethanol.Additionally,theethanolhighpercentageinethosomalformulationsallowsthevesiclestopenetratedeeperintotheskin.15,42 46 Accordingtotherecentliterature,glycerosomesandtrans-ethosomesarephospholipidvesiclescontainingglyceroloramixtureofethanolandedge activators.Theedgeactivatorsalsoenhancetheskinpenetration.49 Thesevesicularsystemshavebeenalreadyusedfor thetreatmentofskinbacterialandfungalinfections,acne,skincancersandinflammation.15,42 55 Forexample,fisetin loadedbinaryethosomesformulationisapotentialdermaldeliverysystemforthemanagementofskincancer.47 Ethosomeshavepotentialapplicationsinthedevelopmentofphytomedicines,forthetreatmentofchallengingdiseases. 54 Forexample,fluorouracilethosomeshavebeendevelopedforskindepositionandmelanomatreatmentinmice models.Stabilizationandeffectiveapplicationinskinofantioxidantsandnanocosmeceuticalcompounds,likecaffeic acidandrosmarinicacid,havebeenachievedbytheirencapsulationintoethosomes.53 55 Transfersomesofferaversatiledeliveryconceptforfacingtheinstabilitylimitationsaswellasthepotentialtobeusedwithawiderangeof APIs.56 61 Comparativestudyofliposomes,ethosomesandtransfersomesascarriersforenhancingthetransdermal deliveryofdifferentAPIsinvitroandinvivohaveappearedintheliterature,showingtheaddedvalueoftransfersomes indermalandethosomesintransdermaldelivery.Theyare(quasi)metastable,whichmakesthemembraneultra-flexible andarecharacterizedas“softvesicles.”Forthisreason,theycaneasilysqueezethroughporesinthestratumcorneum. Surfactants/edgeactivators(i.e.,sodiumcholates;Tween-80andSpan-80)arealsocomponentsoftransferosomesas flexibilityagents.Thepercentageofsurfactants/edgeactivatorsisbetween10%and25%,whilethepercentageofethanolrangesbetween3%and10%.Thisisthemaindifferencebetweenethosomesandtransferosomes(thepercentageof ethanolintheformulation).49 59 Lyophilizedtransfersomalgelcontainingoleicacidwasconsideredasapromising nanosystemforhydrophilicAPIslikebuspironehydrochloride.58 Transferosomesarepromisingdeliveryplatformsfor anticanceragentforthetreatmentofskincancersaswell.59 61 In Table1.1,examplesofresearchreportsonusing transfersomesascarriersforthedeliveryofanticanceragentsarepresented.
TABLE1.1 Examplesofresearchreportsonusingtransfersomesascarriersforthedeliveryofanticanceragents.
Anticancer drugs Conventional topical available formulation (market)
InvestigationLipidandsurfactantusedObservations/conclusions
Doxorubicin hydrochloride (DOX) DOXILAnovelhyaluronicacid-modified transfersomewaspreparedto deliverdrugstolymphatics throughthetransdermalroute. Hyaluronicacideffectively improvedtheuptakeofdrugloadednanocarriersbytumorcells
5-FluorouracilFluroplexDifferentformulationof transfersomewaspreparedusing Tween-80andSpan-80asedge activators.5-FUcontaining transfersomeloaded1%Carbopol 940usedfordeeperpenetration intoskintumorsandtocompare itsanticancerefficacywithits marketedformulationforthe treatmentofskincancer
GemcitabineGemzarInvestigatedassupramolecular vesicularaggregates(SVAs) preparedbyself-assembling liposomesandpolyasparthy drazidecopolymersconjugatedto folicacidmoleculesaspotential activetargetingformulationfor anticancerdrugdelivery
EfudexCreamInthisstudy,constructed transfersomes,liposomes,and niosomesof5-FUfortopical applicationforthetreatmentof actinickeratosisand nonmelanomaskincancer. Transfersomeswerepreparedby thesolventevaporationmethod, whereasliposomesandniosomes wereconstructedbythereversephaseevaporationmethod
Cytotoxicitystudywascarriedout onHaCaTcells
Sodiumdeoxycholate,lecithinResultsrevealedthatDOX-loadedHA-GMS-Twasableto penetrateefficientlyintothedeepskintissue,leadingto enhancedabsorptionbylymphaticsanddecreasedorgan toxicity.Thisstudyprovidesanewanglefortumor metastasistherapythroughlymphaticdrugdeliverywith transdermalnanomedicine
Tween-80,Span-80,edgeactivatorsTheresultsshowedthatTween-80seemstobeabetter edgeactivatorthanSpan-80onthebasisofvesiclesize andentrapmentefficiency.Thetransfersomalgelwasable toimprovebothinvitroskinpermeationandskin depositionof5-FUcomparedwiththemarketed formulation.Transfersomesshowedmaximumskin deposition(81.3%)andcomparabletransdermalfluxof 21.46mg/cm2/h
1,2-dipalmitoyl-sn-glycero-3phosphocholinemonohydrate(DPPC)and N-(carbonyl-methoxypolyethyleneglycol2000)-1,2-distearoyl-sn-glycero-3phosphoethanolamine(DSPE-MPEG2000), cholesterol
Dimiristoylphosphatidylcholine, dipalmitoylphosphatidylcholine,cholesterol, sodiumcholate
Theresultsshowedthatchemotherapeuticactivityof gemcitabinewasincreasedextensivelyduringinvivo experimentsonNOD-SCIDmicebearingMCF-7human xenograftmodelsafteritsentrapmentinsidethefolatetargetedSVAs.Boththevolumeandweightofthetumor massesweredecreasedifcomparedwiththoseobtained bytreatinganimalmodelswithgemcitabine-loaded mPEG-SUVsandthefreeformofgemcitabine
TheIC50valueoftransfersomes(1.02 μmol/L),liposomes (6.83 μmol/:),andniosomes(9.91 μmol/L)wasfoundtobe farlessthan5-FU(15.89 μmol/l)at72h.5-FU-loaded transfersomeswerefoundtobemostcytotoxiconthe HaCaTcelllineincomparisonwithliposomesand niosomes.Theresultsconcludedthatvesiculizationof5FUnotonlyimprovesthetopicaldelivery,butalso enhancesthecytotoxiceffectof5-FU
(Continued )
5-Fluorouracil (5-FU)
TABLE1.1 (Continued)
Anticancer drugs Conventional topical available formulation (market)
Raloxifene hydrochloride
InvestigationLipidandsurfactantusedObservations/conclusions
CelecoxibCelecoxib topical
Inthisstudy,aresearcher developedandoptimized raloxifenehydrochlorideloaded nanotransfersomesfortransdermal delivery,toovercomethepoor bioavailabilityofthedrug.A responsesurfacemethodology experimentaldesignwasapplied fortheoptimizationof transfersomes,using Box Behnkenexperimental design
Threekindsofcelecoxib-loaded vesicularformulationshavebeen investigatedasdrugcarriers, liposomescontainingasurfactant, ortransfersomesandethosomes containingsuitableedgeactivators
Phospholipon90G,sodiumdeoxycholateRaloxifenehydrochloride-loadedtransfersomesproved significantlysuperiorintermsofamountofdrug permeatedanddepositedintheskin,withenhancement ratiosof6.25 6 1.50and9.25 6 2.40,respectively,when comparedwithconventionalliposomes,andanethanolic solution.Differentialscanningcalorimetrystudyrevealed agreaterchangeinskinstructure,comparedwitha controlsample,duringtheexvivodrugdiffusionstudy. Further,confocallaserscanningmicroscopyprovedan enhancedpermeationofcoumarin-6-loaded transfersomes,toadepthofapproximately160 μM,as comparedwithrigidliposomes
Tween-20,ethanolAllvesicularformulationsmarkedly(p , 0.001)improved thedrugamountthatpenetratedintotheskinwithrespect toanaqueoussuspension,from2.0to6.5,upto9.0-fold forliposomes,transfersomes,andethosomes,respectively. Inparticular,ethosomescontainingTween-20asedge activatorenabledthehighestincreaseindrugpenetration throughtheskin,probablyduetothesimultaneous presenceintheircompositionofethanolandTween-20, bothactingaspermeationenhancers
Vinblastine
Inthisstudyvinblastineliposomes werepreparedfromlipids dimiristoylphosphatidylcholineand dipalmitoylphosphatidylcholine withcholesterolandtransfersomes withsodiumcholatewereprepared bythethin-filmhydrationmethod. Thedrugencapsulation,stability, drugreleaseandinvitrohuman celllineswereperformed
Dimiristoylphosphatidylcholine, dipalmitoylphosphatidylcholine,cholesterol, sodiumcholate
Theresultsshowedthatencapsulationofvinblastineinto liposomeswashigherthan98%atadrug/phospholipid molarratiofrom0.17to0.18,whileencapsulationof vinblastineintotransfersomesvariedfrom50%to80%at adrug/phospholipidmolarratiofrom0.05to0.09.The retentionofdruginliposomesandintransfersomeswas foundtobetime-dependent.Theresultsofcelllinestudy showedthattheliposomeswerefoundtoexhibit20-fold lessactivityascomparedwiththefreevinblastine
Source:AdaptedfromRaiS,PandeyV,RaiG.Transfersomesasversatileandflexiblenano-vesicularcarriersinskincancertherapy:thestateoftheart. NanoRevExp.2017;8(1):1325708. https://doi.org/ 10.1080/20022727.2017.1325708
1.2.3Niosomes
Niosomesareself-assembledvesicularsystemsinnanoscaleformedbythehydrationofnonionicsurfactant(s)(i.e., alkylethers,alkylesters,fattyacids,andaminoacids),cholesterolorotheramphiphilicmolecules,mainlycharged,that serveasaversatileplatformwithavarietyofapplicationsrangingfromtransdermaldeliverytobrain-targeteddelivery, aswellasfororaladministrationandcosmeceuticalpurposes.62 77 Niosomesarehighlykineticallyandthermodynamicallystableincomparisontootherlipidicsystems(i.e.,liposomes).63 Theapplicationsofniosomesindifferentareasof medicineandpharmaceuticsaresummarizedin Table1.2.Furthermore,themainobjectiveofthedesignandthedevelopmentofthisvesicularsystemistocontrolthereleaseofAPIinasustainedway,alterationofitsADMEprofileand fortargetingtothespecifictissuesandorgans.67,68 Niosomescanbeusedfornuclearimagingandradiolabelling. Accordingtorecentpublications,theformulated 99mTc-labeledniosomespossessedhighradiolabellingefficacy,good stabilityinvitro,andshowgoodpromiseforpotentialuseinnuclearimaginginthefuture.66,70 SurfacemodifiedniosomesbyhyaluronicacidcomposedofTween60andSpan60asnonionicsurfactants,andcholesterol,wereusedfor thedeliveryof Centellaasiatica extractwithgreatresultsintransdermaladministration.74 TheoraldeliveryoftelmisartanwasachievedbytheniosomescomposedofSpan60andcholesterol.76 Inthefinaltwoexamplesthestudiedniosomeswerepreparedbythethin-filmhydrationmethod,asimpleandlow-costtechnique.
1.2.4Ufasomes
Ufasomesappearedintheliteratureatearly1970s.78 88 Ufasomesarestableparticlessurroundedbyunsaturatedfatty acidmembranesaccordingtoGebickiandHicks.78 Long-chainfattyacids,thatis,oleicandlinoleicacids,selfassembleintovesiclesbymechanicalagitationofthin-filmsinthepresenceofaqueousdispersions.78 85 Thesizeof ufasomesisstronglydependentontheircomposition.78 88 TheincreasedintestinalabsorptionofpoorlysolubleAPIs suchascarboxyfluoresceinmakesufasomescarrieswithgreatpotentialfororaladministrationofpharmaceutics.80 The semipermeablemembranesofufasomesareidealfordrugdeliveryandforavoidingtheenzymeattack.78 80 Ufasomes wereusedforthedeliveryofoleuropein,whichisabiologicalphenoliccompoundofoliveoil,andtheresultsshowed improvedantioxidantactivityandincreasedpotentialfornutraceuticalapplications.83 Ufasomesloadedwithcinnarizine werelyophilizedandincorporatedinhydrogelsforintranasaladministration.84 Exvivoconfocallaserimagingconfirmedtheaddedvalueofthepreparedvesicularcarrierstopenetratedeepthroughnasalmucosalayers.84 Theencapsulationefficiencyofantifungalagentsintoufasomesisapproachingto50%,whichispromisingforfurtherstudiesfor invitroandinvivoevaluation.85,86 Skinpermeationandskinretentionstudiesshowedthattheufasomesoffernumerousadvantagesforthedeliveryofantifungalagents,thatis,clotrimazoleandfluconazole.85,86 Thetopicaldeliveryof methotrexateisalsoachievedbyufasomesforthetreatmentofinflammationinrheumatoidarthritis.87
1.3Polymernanovesicularsystems
1.3.1Polymersomes/polymervesicles
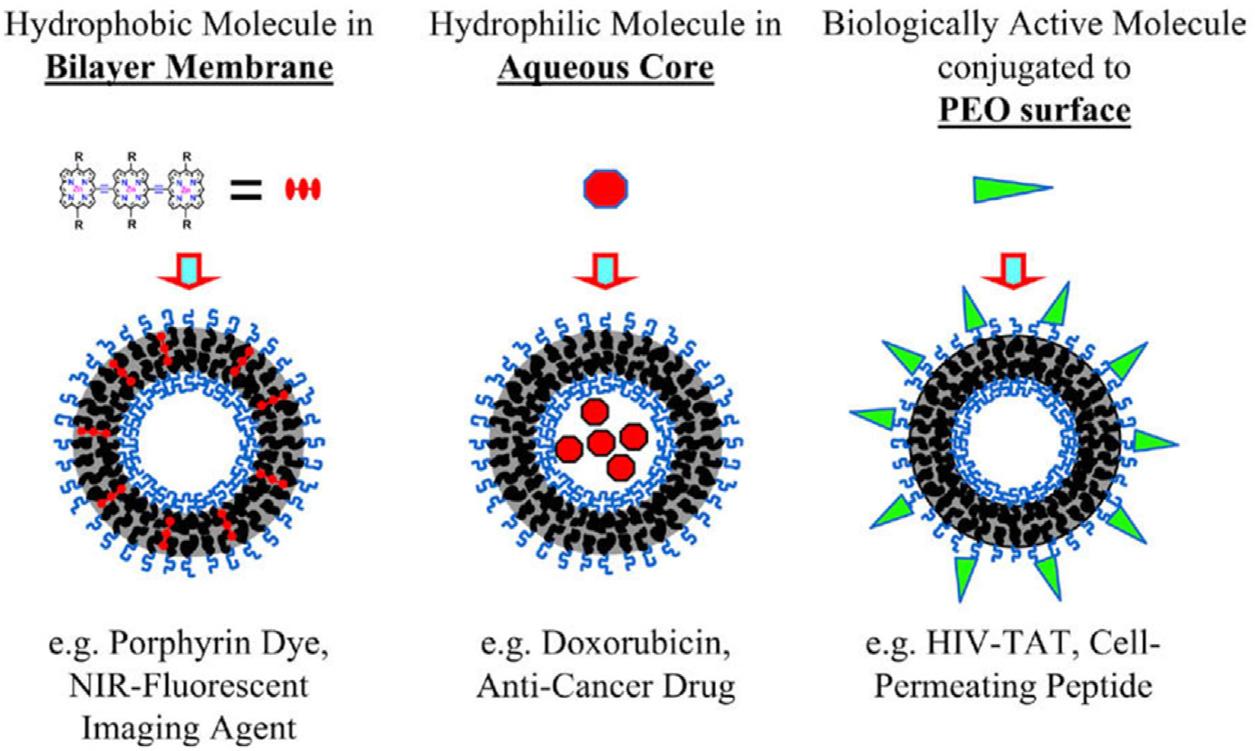
Inthelasttwodecades,polymersomeshaveattractedincreased attentioninthescientificcommunitybecausetheyexhibitsignificantadvantagesinthefieldofpharmaceuticalnanotechnologyandnanomedicine.89 107 Polymersomesarecomposedof amphiphilicblockcopolymers,whichself-assembleinto vesicularstructuresinaqueousandbuffersolutions.89 97 Thesize andtheshapeofpolymersomescouldbecontrolledbychanging themolecularweightandthefunctionalgroupsoftheblock copolymers,whicharethecrucialfactorsforthepackingofself-assemblingamphiphilicpolymerchainsintotheformed nanostructures.98 Theincreasedcolloidalstabilityofpolymersomes,thelowfluidityandthetunablemembraneproperties overcomethelimitationsoflipidvesicularsystemsfordrugdeliveryapplications,especiallyliposomes.95,98 Polymersomes havebeenalreadyusedformedicalimagingandtheranosticpurposes.100 107 In Fig.1.1 aschematicrepresentationofpolymersomeassemblyillustratingthreepossibleapplications,opticalimaging,drugdelivery,andtargetedtherapy,ispresented. Fig.1.2 illustratesthepolymersomeassembliesandthecellularuptakeandrelease.Severalexampleshaveappearedintheliteratureusingstimuli-sensitiveandstimuli-responsivepolymersomesforcontrolledreleaseandtargetingofAPIsincancer therapy.102 104 Thestimuli-responsiveAPIsreleasemayresultinsignificantlyimprovedtherapeutic effectivenessandminimizedadversedrugreactions.102 104 Polymersomesarealsousefulfortheloading ofproteins/peptideswithimprovedcirculationduration.Namely,endosomolyticpolymersomesincreasetheactivityofcyclicdinucleotidestimulatorofinterferongenes agoniststoenhancecancerimmunotherapyinhumanmelanoma.105 Simplesurfacefunctionalizationofpolymersomesusing nonantibacterialpeptideanchorshavebeenachievedfornanobiotechnologyapplications.106 Finally,itshouldbenotedthat polymersomesarethemostcommonterminologyforpolymervesicles.4,108 113 Accordingtotheliterature,polymervesicles
TABLE1.2 Applicationsofniosomes.
No.ApplicationsComponentsMethodusedDrugused
1..Asadrugdelivery carrier a, ω-Hexadecyl-bis-(1-aza)18-crown-6(bola), Span-80,Cholesterol
2Toincrease bioavailability
Cholesterol,Sorbitanmonostearate(span60), Dicetylphosphate(DCP)
Thinlayerevaporation technique 5-Fluorouracil (5-FU)
FilmhydrationmethodAcyclovir
Span20,Span40,Span60,cholesterol,DCPThin-filmmethod,Ether injectionmethod Griseofulvin
3..Forbraintargeting N-Palmitoylglucosamine(NPG),Span60, Cholesterol,SolulanC24
4..Toprolongthe releasetime
ProbesonicationmethodVasoactive Intestinal Peptide
SorbitanestersReverse-phaseevaporation method Rifampicin
5FordrugtargetingPalmiticacid N-Hydroxysuccinimide, Glucosamine,Sorbitanmonostearate(Span 60)
Cholesterol,Glycolchitosan,Sorbitan monostearate(Span60)
SonicationmethodTransferrin
Reverse-phaseevaporation method Methotrexate
6InleishmaniasisSpan40,Cholesterol,DCPSolventevaporationmethod14-Deoxy-11oxoandograph slide
Span20,Cholesterol,PhosphatidicacidMechanicalshakingmethod withoutsonication Amarogentin
7..For antiinflammatory effect
8Inanticancer therapy
9..Inlocalized psoriasis
Cholesterol(CH),Dihexadecylphosphate (DCP),Surfactants(Tween85,PluronicF108)
Reverse-phaseevaporation method Diclofenac sodium
C16MonoalkylglyceroletherSonicationmethodDoxorubicin
Span60,Cholesterol,DCPLipidlayerhydrationmethodBleomycin Span20,Span60,Span40,Tween20, Tween60,Brij76,Brij78,Brij72
ThinlayerhydrationmethodPaclitaxel
Span40,cholesterolTransmembranepHgradient (insideacidic)druguptake process(remoteloading method)
Vincristine
ChitosanLipidlayerhydrationmethodMethotrexate
10.Inoraldeliveryof peptidedrug Brij52,Brij72,Brij92,Brij76,Brij97,Brij58, Brij35,DCP,Cholesterol
11.Indiagnostic imaging N-Palmitoyl-glucosamine(NPG),Polyethylene glycol(PEG)-4400
12.Intransdermaldrug deliverysystem a, ω-Hexadecyl-bis-(1-aza)18-crown-6(bola), Span-80,Cholesterol
13.Inophthalmicdrug deliverysystem
FilmhydrationmethodInsulin
EtherinjectionmethodGadobenate
FilmhydrationmethodAmmonium glycyrrhizinate
Span20,Span60,CholesterolReverse-phaseevaporation method,Thinlayerhydration method Acetazolamide
14.ForlungtargetingSpan85,cholesterolHandshakingmethod,Ether injectionmethod Rifampicin
15.Inthromboembolic disease Hexadecylpoly(3)glycerol,DCP,CholesterolFilmmethodUrokinase (Continued )
TABLE1.2 (Continued) No.ApplicationsComponentsMethodusedDrugused 16.Forstability improvement Span60,CholesterolEtherinjectionmethodFluconazole
17.Toincreaseimmune responseand immunological selectivity
Dimethyldioctadecylammoniumbromide (DDA)anda,a-trehalose-6,6-dibehenate (TDB),1-Monopalmitoylglycerol(MP), Cholesterol
Dehydration rehydration method Ag85B-ESAT6,MSP1or GLURP
18.Forsustained antiplateleteffect Cholesterol,Tween60,StearylamineLipidhydrationmethodIndomethacin
19.ForlivertargetingSpan60,Cholesterol,DCPThin-filmhydrationmethodRibavirin 20.Forenhancementof therapeuticindex SpanandTween(20and/or60),cholesterolReverse-phaseevaporation method a-Lipoicacid
21.Toincrease entrapment efficiency Span60,Cholesterol,DCPThin-filmhydrationmethodKetoprofen
22.ToreducetoxicitySpan20,Span40,Span60,cholesterolThin-filmhydrationmethodCefpodoxime Proxetil
Source:AdaptedfromRajeraR,NagpalK,SinghSK,MishraDN.Niosomes:acontrolledandnoveldrugdeliverysystem. BiolPharmBull.2011;34 (7):945 953. https://doi.org/10.1248/bpb.34.945
FIGURE1.1 Generalapplicationofpolymersomearchitectureintherapeutics.Schematicofpolymersome assemblyillustratingthreepossibleapplications, namelyopticalimaging,drugdelivery,andtargeted-therapy. AdaptedfromLevineDH,GhoroghchianPP,FreudenbergJ,etal.Polymersomes:anew multi-functionaltoolforcancerdiagnosisandtherapy.Methods.2008;46(1):25 32. https://doi.org/10.1016/j.ymeth.2008.05.006
aregenerallyfabricatedthroughtheself-assemblyprocessofamphiphilicpolymers withalineararchitectureinaqueousor bufferdispersionmedia.4,108 113 Polymervesiclesmimicthepropertiesofbiologicalmembranesandareusedfordrugdeliverypurposes,aspolymersomes.Polymervesiclesarealsocharacterizedassmartnanocontainersduetotheirtargetedpropertiesforintracellulardelivery,especiallyincancertherapeutics.112,113
1.3.2Nanovesicularsystemsfortargetingtocellularmechanisms
Inthissection,wediscussnanovesicularsystemsformodifyingimportantcellularmechanismswhichcontrolthecell cycle,theapoptoticpathwaysandautophagy.Thecellcycleisacomplicatedprocesscomposedoftheinterphase(in whichDNAisreplicatedwhilecellgrowthispromoted)andtheMphase(inwhichthereplicatedchromosomesare separatedcelldivisionfollows).Givenitscrucialroleinhealthanddiseaseofhumans,severalnanovesicleshave