Contributors
TehSabariahBintiAbdManan Instituteof TropicalBiodiversityandSustainableDevelopment,UniversitiMalaysiaTerengganu, KualaNerus,TerengganuDarulIman;Civil andEnvironmentalEngineeringDepartment, FacultyofEngineering,UniversitiTeknologi PETRONAS,SeriIskandar,PerakDarul Ridzuan,Malaysia
HasdiantyAbdullah InstituteofBio-ITSelangor,UniversitiSelangor,ShahAlam;Faculty ofEngineering&LifeSciences,Departmentof Science&Biotechnology,UniversitiSelangor, Selangor,Malaysia
MuhammadAfzaal SustainableDevelopment StudyCentre,GovernmentCollegeUniversity, Lahore,Pakistan
AmirrudinAhmad FacultyofScienceand MarineEnvironment,andInstituteofTropical BiodiversityandSustainableDevelopment, UniversitiMalaysiaTerengganu, KualaNerus,TerengganuDarulIman, Malaysia
AshfaqAhmad DepartmentofChemicalEngineering,KhalifaUniversityofScienceandTechnology,AbuDhabi,UnitedArabEmirates
MohdFadzliAhmad InstituteofBio-ITSelangor,UniversitiSelangor,ShahAlam;Faculty ofEngineering&LifeSciences,Departmentof Science&Biotechnology,UniversitiSelangor, Selangor,Malaysia
SahibAlam DepartmentofAgriculturalChemistryandBiochemistry,TheUniversityof AgriculturePeshawar,KhyberPakhtunkhwa, Pakistan
HigoForlanAmaral DepartmentofAgronomy, PhiladelphiaUniversityCenter,UniFil, Londrina,Parana,Brazil
DivaSouzaAndrade Parana ´ RuralDevelopmentInstitute,IAPAR-EMATER,Londrina, Parana,Brazil
JerusaSouzaAndrade NationalInstituteof AmazonResearch,INPA,Brazil
FawziBanat DepartmentofChemicalEngineering,KhalifaUniversityofScienceandTechnology, AbuDhabi,UnitedArabEmirates
JuanC.Castro UnidadEspecializadade Biotecnologı´a,CentrodeInvestigacionesde RecursosNaturalesdelaUNAP(CIRNA), UniversidadNacionaldelaAmazoniaPeruana (UNAP),Iquitos,Peru
GiovannaChianese NationalResearchCouncil, InstituteofAppliedSciencesandIntelligent Systems,UnitofNaples,Naples,Italy
MarianelaCobos LaboratoriodeBiotecnologı ´ a yBioenergetica,UniversidadCientı´ficadel Peru ´ ,Iquitos;FacultaddeCienciasBiolo ´ gicas, UniversidadNacionalSanLuisGonzagade Ica(UNICA),Ica,Peru
JorgeAlbertoVieiraCosta Departmentof BioprocessEngineeringandBiotechnology, FederalUniversityofParana ´ ,Curitiba,Parana ´ ; LaboratoryofBiochemicalEngineering,CollegeofChemistryandFoodEngineering, FederalUniversityofRioGrande,RioGrande, RioGrandedoSul,Brazil
MarianyCostaDepra ´ BioprocessIntensificationGroup,FederalUniversityof SantaMaria,UFSM,SantaMaria,RioGrande doSul,Brazil
Ros^ angelaRodriguesDias BioprocessIntensificationGroup,FederalUniversityof SantaMaria,UFSM,SantaMaria,RioGrande doSul,Brazil
DenysDutykh Univ.GrenobleAlpes,Univ.SavoieMontBlanc,CNRS,LAMA,Chambery, France
A.S.Fernandes Department ofFoodScience andTechnology,FederalUniversityof SantaMaria(UFSM),SantaMaria,RioGrande doSul,Brazil
SuchitraGaur TERI-DeakinNanobiotechnology Centre,DivisionofSustainableAgriculture, TheEnergyandResourcesInstitute(TERI), Gurugram,Haryana,India
MayurikaGoel TERI-DeakinNanobiotechnology Centre,DivisionofSustainableAgriculture,The EnergyandResourcesInstitute(TERI), Gurugram,Haryana,India
RicardoFranciGonc ¸ alves DepartmentofEnvironmentalEngineering,FederalUniversityof Espı´ritoSanto,Vito ´ ria,Espı´ritoSanto,Brazil
MostafaM.Gouda CollegeofBiosystemsEngineeringandFoodScience,ZhejiangUniversity, Hangzhou,China;DepartmentofNutrition& FoodScience,NationalResearchCentre,Dokki, Giza,Egypt
SamanHameed SustainableDevelopmentStudy Centre,GovernmentCollegeUniversity,Lahore, Pakistan
NoorHazaFazlinHashim WaterQualityLaboratory,NationalHydraulicResearchInstitute Malaysia(NAHRIM),SeriKembangan,Selangor, Malaysia
AbdulKarimRussHassan UniversitiKuala LumpurRoyalCollegeofMedicinePerak (UniKLRCMP),Ipoh,PerakDarulRidzuan, Malaysia
MohamedHasnainIsa CivilEngineering Programme,FacultyofEngineering,Universiti TeknologiBrunei,Gadong,BruneiDarussalam
MohdHafiizJaafar SchoolofIndustrialTechnology,UniversityofScienceMalaysia (USM),GeorgeTown,Penang,Malaysia
EduardoJacob-Lopes BioprocessIntensification Group;DepartmentofFoodScienceandTechnology,FederalUniversityofSantaMaria (UFSM),SantaMaria,RioGrandedoSul,Brazil
GuozhaoJi KeyLaboratoryofIndustrialEcologyandEnvironmentalEngineering,School ofEnvironmentalScience&Technology,DalianUniversityofTechnology,Dalian,China
RishuKalra TERI-DeakinNanobiotechnology Centre,DivisionofSustainableAgriculture, TheEnergyandResourcesInstitute(TERI), Gurugram,Haryana,India
MohdAsyrafKassim SchoolofIndustrialTechnology,UniversityofScienceMalaysia(USM), GeorgeTown,Penang,Malaysia
AshvinderKaur AmityInstituteofBiotechnology,AmityUniversity,Noida,UttarPradesh, India
GaganjotKaur AmityInstituteofBiotechnology, AmityUniversity,Noida,UttarPradesh,India
TaimurKhan CivilandEnvironmentalEngineeringDepartment,FacultyofEngineering, UniversitiTeknologiPETRONAS,Seri Iskandar,PerakDarulRidzuan,Malaysia
WaqasUdDinKhan SustainableDevelopment StudyCentre,GovernmentCollegeUniversity, Lahore,Pakistan
JuliaKrylova Saint-PetersburgBranchofthe FederalStateBudgetaryScientificInstitution “All-RussianResearchInstituteofFisheries andOceanography”(“GosNiorch”byL.S. Berg),SaintPetersburg,Russia
EvgenyKurashov InstituteofLimnology, aseparatesubdivisionoftheSt. PetersburgFederalResearchCenterofthe RussianAcademyofSciences,SaintPetersburg, Russia
JaparengLalung SchoolofIndustrialTechnology,UniversityofScienceMalaysia(USM), GeorgeTown,Penang,Malaysia
PaolaLasta BioprocessIntensificationGroup, FederalUniversityofSantaMaria,UFSM, SantaMaria,RioGrandedoSul,Brazil
AmirSharifuddinAbLatip CentreofStudies forSurveyingScienceandGeomatics,Faculty ofArchitecture,Planning,andSurveying, UniversitiTeknologiMARA,ShahAlam,SelangorDarulEhsan,Malaysia
SiewYoongLeong DepartmentofPetrochemicalEngineering,FacultyofEngineeringand Green Technology,UniversitiTunkuAbdul Rahman,Kampar,PerakDarulRidzuan, Malaysia
MuxuanLi KeyLaboratoryofIndustrialEcologyandEnvironmentalEngineering,School ofEnvironmentalScience&Technology,DalianUniversityofTechnology,Dalian,China
ShaoyangLiu DepartmentofChemistryand Physics,CenterforMaterialsandManufacturingSciences,TroyUniversity,Troy,AL,United States
Ba ´ rbaraFrancoLucas LaboratoryofBiochemicalEngineering,CollegeofChemistryand FoodEngineering,FederalUniversityof RioGrande,RioGrande,RioGrandedoSul, Brazil
YichaoMa BiosystemsEngineeringDepartment,AuburnUniversity,Auburn,AL,United States;ShanghaiInstituteofQualityInspection andTechnicalResearch,Shanghai,People’sRepublicofChina
AffianiMachmudah IndustrialEngineering, FacultyofAdvancedTechnologyand Multidiscipline,UniversitasAirlangga,Jalan Mulyorejo,KampusC,Surabaya,EastJava, Indonesia
MaegalaNallapanManiyam InstituteofBio-IT Selangor;CentreforFoundationandGeneral Studies,UniversitiSelangor,ShahAlam,Selangor,Malaysia
Ma ´ rcioFerreiraMartins LaboratoryofCombustionandCombustibleMatter(LCCm), PPGEM,FederalUniversityofEspı´ritoSanto, Vito ´ ria,Espı´ritoSanto,Brazil
HabsahMohamad InstituteofMarineBiotechnology,UniversitiMalaysiaTerengganu, KualaNerus,TerengganuDarulIman, Malaysia
ZarimahMohdHanafiah CivilEngineeringDepartment,FacultyofEngineeringand BuiltEnvironment,UniversitiKebangsaanMalaysia,Bangi,SelangorDarulEhsan,Malaysia
MicheleGrequedeMorais LaboratoryofMicrobiologyandBiochemistry,CollegeofChemistryandFoodEngineering,FederalUniversity ofRioGrande,RioGrande,RioGrandedoSul, Brazil
LuizRodrigoItoMorioka Pita ´ gorasUniversity/NorthofParana ´ ,CampusPiza,Campus Piza,Londrina,Parana,Brazil
RodrigoBragaMoruzzi UniversidadeEstadual deSaoPauloJu ´ liodeMesquitaFilho(UNESP), Sa ˜ oPaulo,Brazil
SitiFatimahZaharahMustafa InstituteofMarineBiotechnology,UniversitiMalaysiaTerengganu,KualaNerus,TerengganuDarul Iman,Malaysia
T.C.Nascimento DepartmentofFoodScience andTechnology,FederalUniversityof SantaMaria(UFSM),SantaMaria,RioGrande doSul,Brazil
P.P.Nass DepartmentofFoodScienceand Technology,FederalUniversityof SantaMaria(UFSM),SantaMaria,RioGrande doSul,Brazil
AnaMariaPereiraNeto UniversidadeFederal doABC(UFABC),SaoPaulo,Brazil
M.L.N € ornberg DepartmentofFoodScience andTechnology,FederalUniversityof SantaMaria(UFSM),SantaMaria,RioGrande doSul,Brazil
SobiaQazi DepartmentofFoundationEngineeringandPhysicalScience,Advance ManufacturingBuilding,JubileeCampus,UniversityofNottingham,Nottingham,United Kingdom
BoyuQu KeyLaboratoryofIndustrialEcology andEnvironmentalEngineering,SchoolofEnvironmentalScience&Technology,DalianUniversityofTechnology,Dalian,China
AbdulRaheem InstituteofcleancoalTechnology,EastChinaUniversityofScienceandTechnology,Shanghai,China
MonikaPrakashRai AmityInstituteofBiotechnology,AmityUniversity,Noida,Uttar Pradesh,India
RizwanRasheed SustainableDevelopment StudyCentre,GovernmentCollegeUniversity, Lahore,Pakistan
IlariaRea NationalResearchCouncil,Institute ofAppliedSciencesandIntelligentSystems, UnitofNaples,Naples,Italy
Reetu AmityInstituteofBiotechnology,Amity University,Noida,UttarPradesh,India
RafaelaBassoSartori BioprocessIntensification Group,FederalUniversityofSantaMaria, UFSM,SantaMaria,RioGrandedoSul,Brazil
AidaSorayaShamsuddin DepartmentofNutritionSciences,KulliyyahofAlliedHealthSciences,InternationalIslamicUniversity Malaysia,IIUM,KuantanCampus, KuantanDarulMakmur,Pahang,Malaysia
GustavoHenriqueRibeiroSilva Universidade EstadualdeSaoPauloJu ´ liodeMesquitaFilho (UNESP),Sa ˜ oPaulo,Brazil
FridelinaSjahrir FacultyofEngineering&Life Sciences,DepartmentofScience&Biotechnology,UniversitiSelangor,Selangor,Malaysia
RenanBarrosoSoares LaboratoryofCombustionandCombustibleMatter(LCCm),PPGEM; DepartmentofEnvironmentalEngineering, FederalUniversityofEspı´ritoSanto,Vito ´ ria, Espı´ritoSanto,Brazil
LaisGalileuSperanza UniversidadeEstadual deSa ˜ oPauloJu ´ liodeMesquitaFilho(UNESP), SaoPaulo,Brazil;Associac ¸aoOceanoVerde (GreenCoLab),Faro,Portugal
LucaDeStefano NationalResearchCouncil,InstituteofAppliedSciencesandIntelligentSystems,UnitofNaples,Naples,Italy
MusaA.Tadda CollegeofBiosystemsEngineeringandFoodScience,ZhejiangUniversity, Hangzhou,China;DepartmentofAgricultural andEnvironmentalEngineering,FacultyofEngineering,BayeroUniversity,Kano,Nigeria
HanifaTaher DepartmentofChemicalEngineering,KhalifaUniversityofScienceand Technology;ResearchandInnovationCenter onCO2 andH2 (RICH),KhalifaUniversity, AbuDhabi,UnitedArabEmirates
TiagoSantosTelles Parana ´ RuralDevelopment Institute,IAPAR-EMATER,Londrina,Parana, Brazil
MonicaTerracciano DepartmentofPharmacy, UniversityofNaplesFedericoII,Naples,Italy
RodolfoSbroliniTiburcio UniversidadeFederaldoABC(UFABC),SaoPaulo,Brazil
ChiaraTramontano NationalResearchCouncil, InstituteofAppliedSciencesandIntelligent Systems,UnitofNaples;Departmentof Pharmacy,UniversityofNaplesFedericoII, Naples,Italy
WanHannaMeliniWanMohtar CivilEngineeringDepartment,FacultyofEngineering andBuiltEnvironment,UniversitiKebangsaan Malaysia,Bangi,SelangorDarulEhsan, Malaysia
NadiahWanRasdi InstituteofTropicalBiodiversityandSustainableDevelopment;Faculty ofFisheriesandFoodScience,UniversitiMalaysiaTerengganu,KualaNerus,Terengganu DarulIman,Malaysia
YiWang BiosystemsEngineeringDepartment; CenterforBioenergyandBioproducts,Auburn University,Auburn,AL,UnitedStates
YifenWang BiosystemsEngineeringDepartment;CenterforBioenergyandBioproducts, AuburnUniversity,Auburn,AL,UnitedStates
NorSuhailaYaacob InstituteofBio-ITSelangor; CentreforFoundationandGeneralStudies, UniversitiSelangor,ShahAlam,Selangor, Malaysia
MuniseZaparoli DepartmentofBioprocessEngineeringandBiotechnology,FederalUniversityofParana ´ ,Curitiba,Parana ´ ,Brazil
LeilaQueirozZepka BioprocessIntensification Group,FederalUniversityofSantaMaria, UFSM,SantaMaria,RioGrandedoSul,Brazil
Q.Z.Zepka DepartmentofFoodScienceand Technology,FederalUniversityof SantaMaria(UFSM),SantaMaria,RioGrande doSul,Brazil
AshfaqAhmada,*,FawziBanata,andHanifaTahera,b
UnitedArabEmirates bResearchandInnovationCenteronCO2 andH2 (RICH),Khalifa University,AbuDhabi,UnitedArabEmirates
lightintensitiesandcangrowaloneorwithotherorganismsbecauseoftheirsymbioticrelationship [1,2].TheyaregenerallycategorizedasRhodophyta(redalgae),Phaeophyta(brownalgae),andChlorophyta(greenalgae).Theycanbegroupedbysizes,e.g.,macroalgae (seaweeds)aremulticellular,large,andcanbeseenwiththenakedeye.Incontrast,microalgae areunicellular,smallerinsize,andcanonlybeseenmicroscopically.Likeconventionalfood crops,algaerequirewater,sunlight,carbondioxide(CO2),andnutrientstogrow.However, theyhaveahighergrowthratethanotherplantsandprovideecologicalbenefits [3,4]. Microalgaecanbeprokaryoticsuchascyanobacteria(Chloroxybacteria),oreukaryoticsuch asgreenalgae(Chlorophyta). Fig.1.1 showsthegreenmarinemicroalgae Nannochloropsis oculata andthefreshwater/terrestrialalgaespecies Eustigmatossplendida and Eustigmatos magnus [5].
Algaecanpotentiallybeusedtoproducebiofuel,bioproducts,medicines,andcosmeticsas theyarearichsourceofcarboncompounds [6].Bioproductsproducedbyalgaearepolysaccharides,lipids,pigments,proteins,vitamins,bioactivecompounds,andantioxidantsthat canbeusedforvariouspurposes.Algaehaveextensiveapplicationsinindustrialwastewater treatmentandCO2 sequestration [7].Algaefeedstockisdeemedrenewableandsustainablefor biofuels,whichhasencouragedsettingupbiorefineries.Integratedalgalengineeringapproachesforimprovingtheirgrowthrateandgeneticmodificationcanenhancetheirfuture applicationsforproducingrenewablebioproducts.Rapidclimatechangeisbeingcaused duetotheburningoffossilfuels,thereleaseofanthropogenicCO2,andtheincreasingpopulationworldwide.Microalgaeandcyanobacteriacanbepromisingbiologicaltoolstotackle thesepersistentproblems [8,9].Algalbiotechnologyaimstoproducesustainablebiofuelswith zeroCO2 emissions.Analgalstraincanbemodifiedthroughgeneticengineeringtoenhance biofuelproductionbytargetingeitherasinglegeneormultiplegenes [10]. Fig.1.1 presents
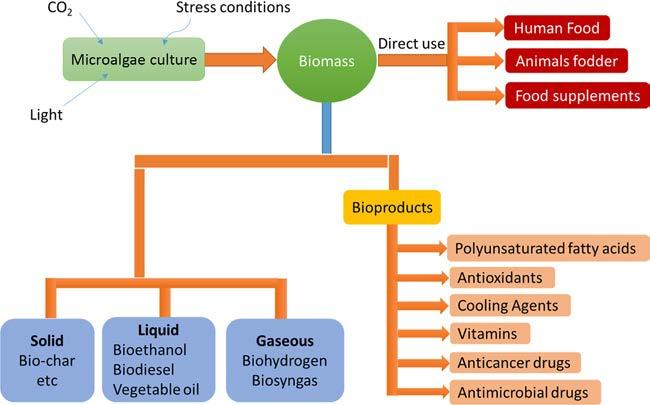
FIG.1.1 MicroalgaeconvertCO2 intocarbohydrates,lipids,andothervaluedbioproductsbyusingsunlight. From M.I.Khan,J.H.Shin,J.D.Kim,Thepromisingfutureofmicroalgae:currentstatus,challenges,andoptimizationofasustainable andrenewableindustryforbiofuels,feed,andotherproducts,Microb.CellFact.17(1)(2018)36.
theconversionofCO2 intocarbohydrates,lipids,andothervaluedbioproductsbyusing sunlight.
Commercialalgaecultivationforgeneratingbiofuelsandbioproductshassignificantlyincreasedrecently [4].Anenormousamountofalgaeisbeingproducedandsoldfordifferent purposes,suchastheproductionoffoodandnutrientsupplements.Algalextractsand by-productscanbeusedinthepharmaceuticalandcosmeticindustries [5–7].Algaefeedstocks areproficientanddesirableforbiofuelproduction.Theydonotrequirevastlandsforcultivationandcanquicklygrowinindustrialwastewater.Algaedonotcontesthumanandanimal foodchainsandmitigateatmosphericCO2 [11–13].Microalgaedonothavelignocellulosicmaterialsinthecellwall.Thisfacilitatesthepretreatmentmethodandreducesproductioncosts. Algaecangrowinindustrialwastewaterandrequirelessenergyfortheircultivationthanthe energytheycanproduce [14–16].Productionofsecond-generationbiofuelsfromterrestrial plantsisanimmenselydebatedissuebecausebiofuels’productionfromsuchfoodcropsisexpensiveandcompeteswithfoodandfeedrequirements.Moreover,cropfoodsneedarableland andanenormousquantityofwater,makingbiofuelproductionunsustainable.Therefore,liquidfuelsfromalgaeareanincomputablealternative [17,18].Biofuelgenerationfrom microalgaeisstillinthedevelopingphase,andasignificantimprovementisessentialforits commercialapplicationandattractinginvestorsandconsumers.
2Industrialwastewatertreatment
Severalphysical,chemical,andbiologicaltreatmenttechniqueshavebeenusedforindustrialwastewatertreatment.Conventionalmethodsforwastewatertreatmentinvolveintensiveaerationfortheoxidationoforganiccarbonandremovalofothercontaminantsusing microorganisms.Anenormousamountofenergyisrequiredfortheaerationofwastewater treatmentplants,accountingfor50%ormoreofthetotalenergycosts [19–21].Numerous studieshavesuggestedthatalgaecanusevarioustypesofwastewatersuchasindustrial,domestic,municipal,oragriculturalwastewater.Combiningsewagewiththefluegas(atmosphericCO2)enhancesmicroalgaebiomassproductivity [22,23].Organiccarbonoxidation directlyemitsCO2 intotheatmosphere,whereastheenergyusedfortheaerationoftreatment plantscanindirectlyemitCO2 [24,25].
Additionally,substantialquantitiesofpotentgreenhousegases,suchasnitrousoxide (N2O),arealsodischargedinthelattercase.OneofthemainconstraintsfortraditionalwastewatertreatmentistherecoveryofNandPafterthetreatment [24,25].Therefore,thealgal wastewatertreatmentapproachcanbeeconomicalandecologicallyfriendly,mainlyforremovingandrecoveringNandP.Algaecanproduceoxygen(O2)throughphotosynthesisand assimilateCO2 duringthephotosyntheticprocess.Theyhaveasymbioticrelationshipwith bacteria.Duringtheoxidationoforganiccarbon,thebacteriautilizetheO2 producedbyphotosyntheticalgae,andthealgaesimultaneouslyassimilatetheCO2 generatedbybacterialrespiration.Therefore,theintegrationofalgaeinwastewatertreatmentcandecreaseaeration requirementsandCO2 emissions.AlgaeabsorbNandPandphotosyntheticallyfixcarbon duringtheirgrowth.ThisreducesthebacterialrequirementsforNandPremovalandthe associatedaerationdemandsandN2Oemissions [21].Anotherstudyhasreportedthatalgae

FIG.1.2 Anintegratedapproachofmicroalgaecultivationindifferentwastewatersforbioproductsapplication. FromR.K.Goswami,etal.,Microalgae-basedbiorefineriesforsustainableresourcerecoveryfromwastewater,J.WaterProcess. Eng.(2020)101747.
couldbeculturedviaanintegratedcultivationsystemusingwastewaterfromthefoodindustryandCO2 fromtheatmosphere.Further,biomasscanbeusedtoproducebioenergyand bioactivecompounds [22].
Moreover,algaebiomassproducedinthewastewatertreatmentprocesscouldberecycled fordiverseapplications,asshownin Fig.1.2.Algalbiomasscontainslipids,carbohydrates, andproteinswithhighnutritiousandcalorificvalue.Afteritsharvesting,itcanbeusedas animalfeed [26],slow-releasefertilizer [27],orbiofuel [28,29],thusturningwasteintovaluableresources.Biogas,suchasbiomethaneandbiohydrogen,canbeproducedthroughanaerobicdigestionfromwastewater.Thebiomasscanalsobeutilizedassupplementaryfeed foraquacultureandanimalsandasfertilizerforcrops.
2.1Removaloftotalnitrogen(TN)andtotalphosphorus(TP)
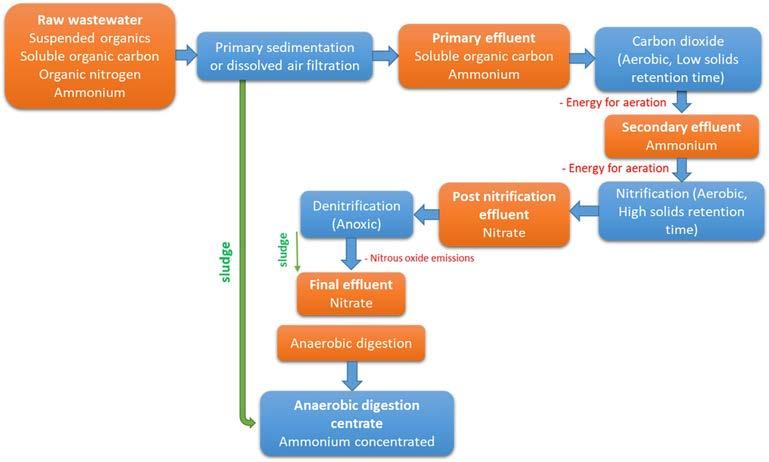
Fig.1.3 explainsthestandardwastewatertreatmentprocess,includingprimary,secondary,andfinalprocessingstages.RawwastewaterusuallycomprisesorganicNthatcan quicklydegradeintoammonium.OrganicNandinorganicN,includingurea,andsuch wastewatercouldbeusedtogrowfilamentousalgae.Putatively,itisfavorabletocultivate freelysuspendedcellsoffilamentousalgaeinrawwastewater.However,theexistenceof asuspendedsubstanceinwastewatermakesittooturbidandblockslightpenetration, inhibitingalgae’sphotosyntheticactivity.Thesolidwastespresentinthewastewatercan betypicallyremovedthroughtheprimaryprocessofsedimentationordissolvedairflotation togetarelativelyperfecteffluentthathassolubleorganiccarbonandammonium [21].
FIG.1.3 Wastewatertreatmentprocess (orangeboxes,grayinprintversion) andthedifferentwastewaterstreams (blueboxes,darkgrayinprintversion) inwhichalgaecouldbecultivated. FromJ.Liu,etal.,Wastewatertreatmentusing filamentousalgae—areview,Bioresour.Technol.298(2020)122556.
Wastewatercontainsorganic,andinorganicP.Algaeutilizeorthophosphate,polyphosphate, pyrophosphate,andmetaphosphatefortheirgrowth [30].NandPareimportantnutrientsrequiredforalgalgrowthandareassimilatedinstantaneously. Table1.1 presentsthewastewater contaminationremovalratefordifferentspeciesofmicroalgae.AnNtoPratio(N:Pratio)isusuallydefinedtoidentifywhethertheyarethelimitingnutrientsforalgalgrowthincertainwastewater.Microalgae Pantanalinema hasbeenreportedforNandPremovalfromwastewaterunder thedark-lightcondition.Around86%ofPwasremovedwithinadark-lightcycleof6h.Cellular andpolyphosphatemechanismsofmicroalgal-bacterialgranuleswereresponsibleforaccumulatingP.Approximately70%ofsolublePremovalwasreportedduetopolyphosphatedevelopmentin Pantanalinema algalcells [31].Thealgal-bacterialsymbiosis(ABS)systemhasbeen reportedtoimprovetheremovalofnutrientsusingasequencingbatchbiofilmreactor (SBBR).ThetotalN’seliminationefficienciesincreasedfrom38.5%to65.8%andPfrom31.9% to89.3%usingthealgae-assistedSBBR.Moreover,chlorophyll-a (3.59mg/g)productionincreasedatastablestageandwas4.07-foldhigherthanthatoffreelysuspendedcells.Ananalysis ofthemechanismsproposedthatthehighremovalofNandPismostlyduetoenhancingboth algalbiomassandtotalbiomassinthebiofilm [32]. Chlorellavulgaris and Neochlorisoleoabundans havebeenreportedtoremovetheChemicalOxygenDemand(COD),inorganicN,andtotal dissolvedPat36°Cfromprimaryandsecondaryeffluentsandcentrate(CEN).Theefficiency ofCOD’sremovalachievedwith C.vulgaris was51%fromprimaryeffluent,55%fromsecondary effluent,and80%fromCEN.Incontrast,theefficiencyofCOD’sremovalachievedwith N.oleoabundans was63%fromprimaryeffluent,47%fromsecondaryeffluent,and72%from CEN.Simultaneously,ammoniaremovalefficiencies(70%–84%)wereobtainedwithboth
TABLE1.1 Microalgaespeciesandtheirremovalrateofcontaminationfromdifferentwastewater.
FromN.S.M.Aron,K.S.Khoo,K.W.Chew,A.Veeramuthu,J.S.Chang,P.L.Show,Microalgaecultivationinwastewaterandpotentialprocessing strategiesusingsolventandmembraneseparationtechnologies, J.WaterProcessEng.39(2021) 101701.
speciesindifferentwastewaters.HighPconcentrationsthatwereremovedfromprimaryeffluentwere >84%.TheseweremoderateinCEN(>22%)andlessinsecondaryeffluent(<15%). Thesestudiesconfirmedthatalgaecouldgrowinwastewatersatahottemperatureof36°C andremovecontaminantssuchasorganiccarbon,N,andP [33].Algae-basedmembranebioreactor(A-MBR)hasbeenreportedtocultivatealgaewithhighcelldensitytoremoveP.Theconcentrationofalgaecellswasincreasedfrom385to4840mg/L,andtheaveragesolidsyield productionrateof32.5g 3/daywasattained.TotalPremovalof66%wasachievedfromwastewaterinA-MBR.Thisstudysuggestedthatalgae-inducedphosphateprecipitationisthekeyto removingP.Thehigh-celldensityofalgalcultivationcanproduceP-richbiomasswithbrilliant harvestingproperties [34].ThedischargeofexcessivePcausesextensiveeutrophicationandwaterpollutionthatthreatensbothecologicalandhumanhealth.However,Pisanimportantcomponentforalllivingmicroorganisms,butitisnonrenewable.Further,itsnaturalreservesare depletingrapidly.AlgaecansustainablyreusePfromwastewaterfortheirgrowth.Ultramembrane-treatedlandfillleachatecanbeutilizedasanutrientmediumforculturingindigenousalgalspecieswithimmediateeliminationofPandN.MaximumNremovalof69%and Premovalof100%wereachievedfrom100mg/LP-PO4 3 -supplementedmedium.Algae canbegrownandusedtosustainPandNfromlandfillleachate [35].Astudyhasrecommended naturalalgaegranulationinopensequencingbatchreactors(SBRs)fortreatingsyntheticwastewatertoovercomethehighseparationcostofalgae.HighremovalofPcontent(33mg-P/g-TSS) withhigherPbioavailability(92%)wasachievedwithalgaegranulesascomparedtoseedalgae (20mg-P/g-TSS).ThealgaegranuleshavearichperspectiveforPrescueandreuse [36].The anaerobic-aerobic-anoxicsequencingbatchreactor(AOA-SBR)systemhasbeensuggestedfor instantaneouscarbon,N,andPremoval.HighremovalproficienciesofCOD(97%),TN (96%),andTP(94%)wereachievedwiththeAOA-SBRsystemin6-hcycles [21].Algae
immobilizationhasgreatpotentialtoeliminatenutrientsfromwastewater.However,itscommercialapplicationischallengedbythehighcostandthemaintenanceofmanyviableandactive microalgalcells.Agar-immobilizedmicroalgalcellsefficientlyremovedNH4 + -N(96%)and PO4 3 -P(99%)inbothbatchandcontinuousmodes.Theimmobilizedalgalcellswerestillactive andcouldeliminate94%ofNH4 + -Nand66%ofPO4 3 -Pafterbeingrecycledfor8cycles.Further, theirnutrientremovalefficiencywasstillmorethan60%evenaftertheirpreservationfor 120daysundernormalconditions.Thisstudysuggestedthatalgaeimmobilizationissimple,less costly,andpracticableinpreservingthemicroalgaeatroomtemperatureforalongtime.Applyingalgaetoremovenutrientsinwastewatertreatmentismoresuitableandadvantageous [37]. Anotherstudyreportedremovingmorethan94%ammonium-nitrogenandphosphatephosphoruswiththeuseof Chlorellasorokiniana cultivatedin10-Lflat-panelbioreactors.This studyindicatedthatcontrolledpHandhighhydraulicretentiontime(HRT)couldmaximize thealgaeyieldandimprovetheuptakeofnutrientswithoutmagnesium(Mg)enrichment throughcontinuouscultivation [38].Outdoorcultivationof C.vulgaris inathin-filmflatplate photobioreactor(PBR)usingdigestedpiggerywastewaterastheculturemediumhasalsobeen reported.HighlevelsofTN(72.48%),TP(86.93%),andCOD(85.94%)removalwereachieved with C.vulgaris.Thisstudysuggestedthatalgalcellscanadaptquicklytowastewaterinoutdoor conditions [39]
2.2Heavymetal(HM)removalbyalgae
HMcontaminantsareincreasinginwaterbodiesduetourbanization,industrialization, andnaturalearthprocesses.HMsaccumulateinthehumanbodybyconsumingpollutedwaterandfood.Theconventionalmethodsreportedfortheirremovalareelectrolysis,ionexchange,precipitation,chemicalextraction,hydrolysis,polymermicroencapsulation,and leaching.Severalalgalspeciesaresaidtobeusefulbiosorbentmaterialsforremoving HMs [50–55].However,theseapproachesarenoteconomical,especiallyonalargescale, duetocontinuousmonitoringandcontrolrequiredandbecauseoftheirlower HM-removalefficiency.FilamentousalgaecanadsorbHMsfromwastewater.AlgaecanremoveHMsbybiosorptionandbioaccumulation,aswellasthroughphysicalandchemical mechanisms.HMremovalbydriedalgalcellsiscalled“biosorption,”andtheuseofaccumulatingabilitiesofalivealgalcellsiscalled“bioaccumulation.”Amajorityofthestudiesonthis subjecthavefocusedonthebiosorptionofHMsfromwastewaterbyutilizingthedrymassof filamentousalgaeasbio-adsorbentmaterial.HMremovalbyinactiveanddeadbiomassdependsonthemetalionsandthebiomass’shighaffinities. Fig.1.4 illustratesthecomplex mechanismsofmetalionbinding.Moreprecisely,thepropertiesofalgalcellwallconstituents,suchasalginateandfucoidan,arespecificallyresponsibleformetalions’sequestration. Themainfunctionalgroupsexistinginthebrownandgreenalgae,suchascarboxyl,hydroxyl,sulfate,phosphate,andaminegroups,playanessentialpartinmetalbinding [50,56] Thealginatesinthecellwallandtheintercellularsubstanceofbrownalgaehaveagreater uptakefordivalentcationssuchasPb2+,Cu2+,Cd2+,andZn2+ [50].Inthiscase,theuseof driedbiomassof Oedogonium, Spirogyra,and Cladophora forthebiosorptionoflead(Pb),cadmium(Cd),nickel(Ni),andmercury(Hg)wasreported [51–54].Filamentousalgaebiomass performsthedualfunctionofremovingNfromwastewaterandsubsequentlybeingusefulas

FIG.1.4 Thecomplexmechanismsofmetalionbinding. FromJ.He,J.P.Chen,Acomprehensivereviewonbiosorption ofheavymetalsbyalgalbiomass:materials,performances,chemistry,andmodelingsimulationtools,Bioresour.Technol.160 (2014)67–78.
abiosorbentforremovingHMsfromothercontaminatedwater.Algaecanofferanunconventional,sustainable,andenvironment-friendlyapproachforthebioremediationofHMs [57,58].Numerousstudieshavereportedtheuseofdiversefilamentousalgaespecies,including Oedogonium, Rhizoclonium,and Hydrodictyon,formaximumremovalofHMs.Thecultivationofthesealgalspeciesinashdamwaterconfirmedmorethan60mg/gofHMs’ accumulationfromwastewater [59].Itwasobservedthatthecultivationof Cladophora insyntheticwastewaterremovedmorethan80%ofCdinbatchandsemibatchsystems [60].
Thealga Distigmaproteus,isolatedfromindustrialwastewater,hasbeenreportedtohavea hightoleranceagainstHMssuchasCd2+,chromium(Cr6+),Pb2+,andcopper(Cu2+).The growthrateofthealgaewasfoundtoslowdownbyday8againstthemetalstressofCu2+ (90%),Cd2+(84%),Cr6+ (71%),andPb2+ (63%).ThehighestCd2+ removalrateof90%was achievedfromthemediumafter8dayswiththeuseofthe Distigma algae.Themetalremoval capabilityof Distigma canbeexploredformetaldetoxificationandenvironmentalclean-up [61].Theuseoffreelysuspendedandimmobilizedcellsof Anabaenadoliolum and C.vulgaris hasbeenreportedforCuandiron(Fe)removal.Theimmobilizedalgalcells showedahighremovalrateforbothCuandFe.Thismeansthatimmobilizationcanprotect thealgalcellsfromtoxicmetalscomparedtothefreelysuspendedcells.Theimmobilization technologycanalsoeasetheharvestingprocessandcanpotentiallybeusedduringrepeated cycles [62].Immobilized Microcystis hasbeenusedinthepackedcolumnforCu2+ removalat differentflowratesandmetalionconcentrations.ThehighestreductionofCu2+ (54%)was reachedataflowrate(0.75mL/min)withaninitialmetalionconcentrationof30 μg/mL andbiomassdosageof0.016g.TheeliminationofCu2+ wasfoundtobeinfluencedbyinlet metalionconcentrationandbiomassdensity.Increasingthebiomassdosagefrom0.016to 0.128gincreasedtheremovalpercentage,andtheCu2+ adsorbedperunitdryweight
dropped [63].Theuseofbrownseaweeds,suchas Hizikiafusiformis, Laminariajaponica,and Undariapinnatifida,forthebiosorptionofheavymetalions(Pb2+,Cd2+,Mn2+,Cu2+,and Cr2 O7 2 )hasbeenreported [64].Driedbiomassofbrownmarinealgae Eckloniaradiata has beenobservedtouptakeCdfromanaqueoussolution.ThemaximumremovalofCd (1634mg/g)drybiomassatanoptimumpHof4and50°Ctemperaturehasbeenreportedly achieved.AdsorptiontemperaturesandpHlevelscanplayanessentialroleinCduptake [65]. Thebiomassofthebrownmarinealgae Sargassumfluitans hasbeenreportedtomaximizeCr, Cu,andAlremoval [66].
Anativecyanobacterialspecies Nostocmuscorum hasbeenreportedlyusedforCu [67],Zn [67],Pb [67],andCd [67] biosorptionfromanaqueoussolution.ThehighestbiosorptionofPb (96.3%) [67] andZn(71.3%) [67] wereobtainedat60hofincubationwiththealgae.The biosorptionofmetalswasattributedtopassivebiosorptionandaccumulationbytheactively growing N.muscorum biomass.Thisstudydemonstratedthatcyanobacteriacouldbeusedto removemetalsfromamulticomponentsystem [68].Anotherstudyreportedtheuseofdried biomassofthemostcommonfilamentousalgaeforthebiosorptionofHMsinthebatchprocess.TheremovalofPb2+ by97%and89%werereportedwith Pithophoraoedogonia and Spirogyraneglecta,respectively,in30minfromaninitialconcentrationof5mg/Lmetalions. TheremovalefficiencyofPb2+ decreasedastheinitialconcentrationofmetalionsincreased. ReductionofPb2+ 70%wasobtainedwith S.neglecta ataninitialconcentrationof75mg/Lof Pb2+,whilemorethan75%ofPb2+ andCu2+ reductionwereachievedwith S.neglecta and P.oedogonia frommixed-metalionssolutions.Somealgalspecies,suchas Hydrodictyon reticulatum, Cladophoracallicoma,and Aulosirafertilissima,couldnotefficientlyremovemetal ionsfrommixedsolutions [69].
Conversely,highconcentrationsofHMscancausetoxiceffectsonalgalcellsandhinder theirgrowthandcellularmetabolism.TheabsorptionofHMsthroughalgaecouldbedeterminedbythelatter’sgrowthphaseandotherenvironmentalsettings.Numerousstudieshave reportedthattheuseofdeadalgaeshowsmoresignificantadvantagesthantheuseofliving cells.Deadalgaebiomasscomprisescellulose,glycoprotein,andpectinsthatactas biosorbentsandadsorbentsofHMsviatheextracellularprocess [70].
3AlgaeforCO2 sequestration
CO2 iscontinuouslyincreasingintheatmosphereduetovariousanthropogenicactivities. Theburningoffossilfuelshascontributedaround87%ofCO2;deforestationcontributes9% ofit,whereas4%isgeneratedbyindustrial,manufacturing,andhumanactivities [71].Ingeneral,thethreesectorsthatcontributetoCO2 emissionsaretransport,industrialproduction, andfuelcombustionforsomeotheractivities [72,73].Recentstatisticshaverevealedthat transportationandindustrialproductionaccountforabout80%offuelcombustion,while theelectricitysectoraccountsfortheremaining20% [74].Therapidincreaseinatmospheric CO2 levelsisakeycontributoryfactorforglobalwarming,whichisoneoftheleadingchallengesthreateningenvironmentalsustainability [75,76].Severalconventionaltechnologies havebeendevelopedtodealwiththischallengethatinvolvesthesequestrationofCO2,particularlyalongwithenergyretrieval,whichhasbecomeanurgentneed.Theprocesses
involvingthemitigationofCO2 bychemicalabsorption,membraneseparation,andphysical adsorptionandthecryogenicprocessesareallexpensiveandcausesecondarypollution [75,77].Inthenaturalenvironment,CO2 fixationcanbeconceivablydoneviaphotosynthetic landplantsandmicroorganisms.
AlgaeandcyanobacteriahavebeenreportedforCO2 fixationduetotheirfastgrowthrate andgreaterfixationefficiencythanlandplants [78].Hence,thesequestrationofCO2 viaphotosyntheticalgaeandcyanobacteriaisanidealapproach.Themicroalgae Spirulinaplatensis hasbeenreportedasafavoritestrainforthefixationofCO2 duetoitsfastgrowthrate,high resistancetohighCO2 concentrationandtemperature,nutrientdeficiency,andpHflocculation.Additionally,itcanproducepreciousbioactivecompoundstoimproveimmunity [79] andpreventaging [80].BiofixationofCO2 throughalgaeisconsideredfavorableasitcanfix CO2 andinstantaneouslyyieldvalue-addedchemicalstoo [81].
Algae-basedCO2 sequestrationoffersanencouragingprospecttodecreaseCO2 andconvertcarbonintobioproducts.Further,thisprocesshasfewersafetyrequirementsthanthatof thestorageofCO2.Algae’shighphotosyntheticabilitymakestheirCO2 utilization10–50 timesgreaterthananyotherterrestrialplantsusingsunlight [82].Thebubbling-typephotosyntheticalgaemicrobialfuelcell(B-PAMFC)hasbeenreportedforwastewatertreatment, anditfacilitatesCO2 sequestrationalongwithinstantaneouspowerproduction.Thehighest fixationrateofCO2 wasachievedwith C.vulgaris at2.8g/LureaintheB-PAMFC.TheabsorptionefficiencyofCO2 andlipidproductivity(105.9mg/L/day)wasenhanceddueto urea’sapplication.Thehighestnetenergyof1.824kWhm 3 wasproduced.ThisstudydemonstratedthatB-PAMFCwithureaastheNsourceofferedabeneficialmethodforinstantaneousCO2 mitigationandbioenergyproduction [83].Microalgae Phormidiumvalderianum BDU20041reportedhightoleranceat15%CO2,thusprovingtobeasuitablecontestant forcarboncapture.AnincreasedamountoflipidatanelevatedCO2 levelwasachievedwith actualfluegasconditionsthanthatachievedinambientair [84].
MixedalgalculturesinbatchmodewithanexternalsupplyofCO 2 fromwheatstraw fermentationhavebeenreported.HighsequestrationofCO 2 (287mg/L/day)hasbeenobservedwithmixedculturesofalgae.Remo valof87%ammonium,78%phosphate,68% COD,and65%nitratewasalsoachieved.About12.29%oflipidswereproducedwith thehelpofenrichedCO2 andwastewaterforthesupplyof nutrients.Thetotalamount ofchlorophyllandproteincontentachievedwas14.3and12.3 μg/Land0.13and 0.15mg/L,respectively.Thisstudyindicatedthatalgaeconsortiacouldbepotentiallyused forCO2 mitigation,wastewatertreatment,andbioenergyproduction [85].Freshwateralgae C.vulgaris wascultivatedinPBRswithlowdosesofsugarstoenhanceCO 2 mitigationunder thelight-emittingdiode’sillumination.Gluc oseadditionatlowconcentrationimprovedthe photoautotrophicgrowthandbiomassgenerationandCO2 captureby10%.Atechnoeconomicanalysis(TEA)suggestedthatLED-basedPBRsareafeasibleapproachfor transformingCO 2 intovalue-addedalgalbiomass [86].Anotherstudyreportedtheuse of Chlorococcumhumicola , C.vulgaris ,and Scenedesmusquadricauda fortotalCO 2 fixation andchlorophyll,protein,andcarbohydratepro duction.Sodiumbicarbonateataconcentrationof0.2%– 1%wasusedintheboldbasalmediumfortheuptakeofcarbon,anditactedas asubstitutesourceofatmosphericCO2 .AhighamountoflipidcontentandCO2 fixation rateof0.4%wereachieved,whereasthemax imumamountofchlorophyllcontentwas obtainedat0.6%ofsodiumbicarbonatec oncentration.Thisstudyshowedthat
I.Environmentalsector
S.quadricauda couldbesuitableforCO2 mitigationandthehighproductionofpolyunsaturatedfattyacid [87] . Microalgaecultivationhasbeenreportedinanoutdoorracewaypondtoproducebiomass andforCO2 mitigationfromthefluegasunderdiverseconditions.Algalgrowthisaffectedby numerousphysiochemicalparameters,predominantlytemperature,solarirradiance,CO2, andcross-contamination.Loweralgalbiomassproductivityinbatchcultivationwasachieved withouttheadditionofexternalcarbonsupplementation.Incontrast,feedingoffluegasata concentrationof10%CO2 v/vimprovedthedissolvedmediumcarbonconcentration,which enhancedtherateofCO2 fixation.Thesemicontinuousmethodhasbeenadoptedto strengthentheperformanceofthesystemfurther.Thehighestbiomassdensityof0.42g/L anda3.5-foldimprovementinarealproductivityof11.488g/m2 wereachieved [88].TheeffectofN:PratiosonthegrowthandCO2 fixationof Chlorella sp.wasevaluatedinthebubble columnPBR.Themaximumbiomassyield(3568mg/L)wasachievedattheN:Pratioof15:1. Thehighestalgaecelldensityof105 106 cells/mLandsequestrationofCO2 at28%was obtainedafter92h.Thisstudydemonstratedthatorganicandinorganiccarboncouldinfluencealgalcultivation [89].Themarinealgae Nannochloropsissalina hasbeenreportedlyused forCO2 fixationandhighlipidproduction.Theoptimumgrowthrateof N.salina was reportedattheconcentrationof6%CO2,whilesomecellswerefoundtogrowwellunder theCO2 concentrationof20%.IncreasingthelevelofCO2 causedacidificationinthemedium, whichreducedthepigmentandinhibitedthecells’growth [90].Conversely,CO2 fixationand theproductionofspecificlipidsincreasedwiththeremovalofO2 fromtheinletgas.IncreasingtheconcentrationofCO2 from25%to100%causedinhibitedcellgrowthentirely.These findingssuggestthat,inthefuture,amoreefficientapproachtoalgalbiotechnologycanbe developedandappliedforbothCO2 mitigationandbiofuelproduction [90]. Chlorella minutissima cultivationinanindoorPBRforCO2 absorptionfromtheanaerobicdigestionsystemhasbeenreported.TheintakeofCO2 by C.minutissima wasfoundtobeintherangeof 75%–85%atalightintensityof1296 μmolm2/sandgasflowsat0.33vvm [91].
TheregenerationofdifferentsolventsusedintheabsorptionofCO2 isasignificantchallenge.AhybridsystemofammoniaandmicroalgaeforcapturingCO2 hasbeenreported.The fixationofcarbonover85%wasobtainedwith Chlorella sp.L166,L38,andUTEX1602 suggestedthatalgaecouldbeusedinachemical-biologicalhybridsystemtocaptureCO2. Thisstudydemonstratedthatthenewabsorption-algaehybridprocesscouldreplacetheconventionalmethodforCO2 absorption [92].However,itwasalsofoundthatslowdiffusionof CO2 inwaterforashortresidenttimecouldlimitmicroalgaegrowth.Thus,polyethyleneglycol(PEG)200enhancedthetransferofCO2 fromthegaseousphasetotheliquidphase.The sequestrationofCO2 increasedthegrowthof Nannochloropsisoceanica withtheCO2 bubbling of15%vol.Themaximumspecificgrowthrateof N.oceanica (1.41/day)wasachievedwith 1mmolofPEG200intheculturemedium.Thealgaebiomassincreasedbyabout79%with increasedTICbecauseofmoreCO2 dissolutionintheculturemedium.Thus,asaCO2 absorbent,PEG200canefficientlycaptureCO2 fromfluegastogrowalgae [93].Asubstantial amountofnaturalgasesaregeneratedintheprocessofoilextraction.Thecompositionofnaturalgasesgeneratedduringtheprocessvaried,butthemostdominantonesweremethane (CH4)(80%–95%)andCO2 [92].
Conversely,thereleaseofboththesegasesisconsideredtobetheprimarycauseofglobal warmingandclimatechange.Therefore,itisnecessarytoconvertnaturalgasintoother
valuableproductsbytheprocessofmitigation.Amicrobialconsortiumofalgaeandbacteria hasbeensuggestedforquickmetabolizationofthehighlevelsofCO2 andCH4 andtheir transformationintovalue-addedbioproducts.Theconsortium(algaeandbacteria)isolated frommangrovescansurvivein70%ofCH4 and30%ofCO2 [94].Anovelairliftphotosyntheticmicrobialfuelcell(AL-PMFC)with C.vulgaris hasbeenreportedforthebiofixation ofCO2 andbioenergy.ThemaximumCO2 fixationrateof835.7mg/L/daywasachievedwith AL-PMFC.ThehighestCO2 fixationrateof1292.8mg/L/day,lipidproductivityof 234.3mg/L/day,andpowerdensityof5.94Wm3 havebeenattainedattheoptimized C.vulgaris inoculumsize,levelofCO2,andaerationrate.Thus,AL-PMFCcanprovideanattractiveapproachforCO2 fixationandbioenergyproduction [95].Cultivationof N.oculata in semibatchPBRsforthebioconversionofCO2,wastewatertreatment,andbiomassproduction hasbeenreported.Themaximumgrowthrateof N.oculata withtheproductivityof 0.088g/L/daywasobtainedat18%ofCO2 andtheoptimalpHrangeof5.5–6.5 [96].
4Bioenergyfromalgae
Withagrowingpopulationworldwide,bioenergydemandsareincreasingglobally.Fossil fuelsarereducingworldwideandareclosetotheirexhaustionpointduetohighand unsustainableconsumptionandduetotheirnonrenewablenature.Therefore,biofuelsare gainingmuchattentiongloballyasanalternativetofossilfuels.Biofuels,includingbiodiesel andbioethanol,arenowbeingproducedcommerciallyinseveraldevelopedcountries.Alternativebiofuelscanbeproducedfromnumerousrenewablesubstratessuchasfoodcrops, croporfruitwastes,woodypartsofplants,garbage,andalgae [97,98].Thekeybenefitofproducingandusingbiofuelsisthattheyarerenewableandsustainableandcanconsiderably reduceenvironmentalpollutionandglobalwarming.Aleadingsourceofglobalwarming istheemissionofgreenhousegases,suchasCO2,generatedduetotheburningoffossilfuels. Approximately29gigatonsofCO2 aregeneratedannually,andatotalamountof35.3billion tonsofithasbeenproduceduntilnowdueonlytotheburningoffossilfuels [99].Algal biofuelscouldbeanalternativetofossilfuelsastheyhave10%–45%ofO2 andfewersulfur emissions [100].
Incontrast,petroleum-basedfuelsemitahighlevelofsulfuranddonothaveO2.Biofuel doesnotcreateenvironmentalpollution.Moreover,itisareadilyavailable,sustainable,and reliablefuelproducedfrombioresources.Biofuelfromalgaeisenvironment-friendlyand nontoxic,anditisconsideredtobeastrongproductforfixingCO2 worldwide.Itissaidthat 1.83kgofCO2 canbefixedperkgofalgae.Moreover,severalalgalspeciesconsumeflue gases,suchasSOx andNOx,togetherwithCO2 asnutrients [101].CO2 constitutes50%of thedryweightofalgaebiomass.Theassortmentofalgalbiomassisvitalforregulatingbiofuel productioncostsandoptimizingtheenergystructure.Thevarietyofalgalbiomassusedinthe generationofbiofuelsisdirectlyassociatedwiththeemissionofgreenhousegasandfurther withenvironmentalandeconomicsustainability [102].Algaearecurrentlythemostfavored rawmaterialforbioenergy,andtheycancatertothegrowingdemandsforbiofuels,food, feed,andvaluedcompounds [98,99].SeveralcountriesinAsia,Europe,andAmericaare startingtoproducebioenergyfromalgaecommercially [103].
4.1Biodieselproduction
Mostofthemicroalgalspeciesarepromisingwithregardtobiodieselproductiondueto theirhighlipidcontent(50%–70%).Thebiomassofseveralalgalspecies,includingmicroalga Botryococcusbraunii,canproducearound80%ofoil,makingbiodieselproductionappropriate [104–106].Algaecanyieldupto58,700L/haofoil,fromwhich121,104L/habiodieselcanbe produced [101,107,108].Alkaliphilicgreenalga C.vulgaris hasbeenreportedtobeappropriateforbiodieselproduction.Thehighestproductivityofbiomassat28–31mg/L/dayalong withlipidcontentof38%wereachievedatalowlightintensity(60–90 μmol/m2/s).Themain fractionsofthefattyacidsC16:0,C18:1,andC18:2wereobservedafterlipidtransesterification.Biodieselproductionfromthesamestrainofalgaecanalsobeenergyefficient [109].Economicalalgalbiodieselproductionisdirectlyassociatedwithhighoperational, maintenance,harvesting,andconversioncosts.Wastewaterhasbeenusedasacost-effective mediumforculturingalgaeandbiodieselproductionbyusingmagneticnanocatalyst SO4 2 /Fe3O4-Al2O3 [110].Theeffectofstoragetemperatureandtimeontheincreaseof thelipidcompositionof Scenedesmus sp.hasbeenstudied.Thisstudyfoundthatthefreefatty acidcontentinwetalgalbiomassincreasedfromatraceto62%whenstoredat4°C.Preesterificationandtransesterificationwereusedforatwo-stepcatalyticconversionofalgaloilhavinghighfreefattyacidcontentintobiodiesel.Ahighconversionrateoftriacylglycerolswas obtainedatamethanol-to-oilmolarratioof12:1duringcatalysiswith2%potassiumhydroxideat65°Cfor30min.AccordingtoChineseNationalStandards,abiodieselanalysisconfirmedthestandardlevelafterpurificationbybleachingearth [111].Dairyfarm wastewatershavebeenreportedaspotentialresourcesforalgaecultivationforbiodieselproduction.Aconsortiumofnativestrainsremovesmorethan98%ofnutrientsfromthetreated wastewater.Biomassproductionof153.54tha/yearandlipidcontentof16.89%were achievedfromthecultivatedconsortiumintreatedwastewater.Algallipidsof72.70% obtainedfromtheconsortiumcanbeconvertedintobiodiesel [112].Afreshwatermicroalgae C.zofingiensis wascultivatedinpilot-scalePBRsbyusingartificialwastewaterasamedium fortheirgrowthandlipidandbiodieselproductionandtreatment.Themaximumremoval rateofTN(92%)andTP(100%)wasattainedforamixotrophicculturewithaceticacidas apHregulator.Ahighproductivityofbiomass(66.94mg/L/day)andaspecificgrowthrate of0.260mg/daywereachievedatcontrolledpH.Higherproductivityoflipidcontent (37.48mg/L/day)wasachievedattheoptimalcondition.Abiodieselyieldof19.44%, presentingamassive16–18carboncompositionofFAME,wasobtained.Thisstudy suggestedthatpHregulationwithaceticacidisthemostusefulmethodforthegrowthof Chlorellazofingiensis inwastewaterduringwinterforbiodieselproductionandtheremoval ofnutrients [113].Anotherstudyproposedasingle-stepsubcriticalmethanolextraction (SCM)processforbiodieselproductionfrom C.pyrenoidosa.Thehighestyieldofcrudebiodiesel(7.1wt%)wasachievedat160°Cina3-minreactiontimealongwithanoptimal (7wt%)methanolratiotoalgae.ThisstudysuggestedthattheSCMprocessdoesnotrequire anypretreatmentsteporacatalystmakingiteconomicalandpracticalforlarge-scalebiodieselproductionfromalgae [114].Astudyexperimentedwithmono-orcocultivationof C.vulgaris and Scenedesmusdimorphus inmediacontainingdifferentsourcesofNforgrowth, lipidcontent,biodieselproduction,andnutrientelimination.Thisstudyconfirmedthatalgae cultivationinthemediacontainingvarioussourcesofNindicatednotonlyahighremoval
efficiencybutalsoincreasedbiomass,lipidproductivities,andbiodieselproduction.Atrend ofhighlipidcontentwasobservedinthemixedculture(asagainsttheirmono-culturing)of C.vulgaris and S.dimorphus inthemediathathadthesameNsource.Themainfattyacidsof C16:0,C16:1,C18:0,C18:2,andC18:3,accountingfor79.6%–90.6%ofthetotal,wereachieved. Further,biodieselproductionintherangeof8.5–11.2gbiodiesel/100gdryweightwas achieved,whichdemonstratesthattheNsourcecanaffectthenutrientremovalefficiency andbiodieselproductionofbothmono-andmix-culturedmicroalgae [115].
Biodieselproductionfromalgaloilviatransesterificationusingvariousacids,base catalysts,andsupercriticalfluidshasbeenreported.Thesecatalystsaretoxicandposemany challengesrelatedtoenvironmentalcontamination.Alipase-basedenzymeextractedfroma novelfungalstrain Cladosporiumtenuissimum withamolecularweightof 46kDaandspecificactivityof37.2U/mghasbeenreportedforbiodieselproduction.Apurifiedlipaseasa biologicalcatalystwassuccessfullyusedfortransesterificationof Nitzschiapunctata oilinto biodiesel.Thehighestconversionefficiencyof87.2%wasachievedwithlipaseasbiocatalyst, anditwas83.02%inthecaseofaconventionalacidcatalyst.Lipasehasthepotentialforextensivescaleapplicationstoincreasethetransesterificationprocess’conversionefficiency [116].Aquickalgalgrowthrate,biofixationofCO2,andnocompetitionwithfoodandhigh lipidcontentmakethemanappropriatefeedstockforbiodieselproduction.Conversely,the highcostassociatedwithdehydration,extraction,andbiodieselconversioncanlimittheirapplicationattheindustriallevel.Directbiodieselproductionfrompretreatedwetalgae C.vulgaris throughesterificationandtransesterificationhasbeenreported.AhighFattyAcid MethylEster(FAME)yieldof80%wasachievedwithasmallvolumeofmethanolandcatalyst(eitherHCLorNaOH)assistedbyRFheatingat55°Cfor20min [117].Theabilityof microalgalstraingrowthinvariouswastewaterstoremovenutrientsandaccumulatelipid isdemonstratedin Table1.2.
TABLE1.2 Removalofnutrientandlipidaccumulationpotentialofdifferentmicroalgaefromwastewater.
removalofcolorto increaselight availability.
sp.52.210068–742.04g/L55.4Theeffluentwas decolorizedwitha weakelectricfieldand titaniumdioxide (TiO2)andpulse intensepulsedlight(TIPL).
TABLE1.2 Removalofnutrientandlipidaccumulation potentialofdifferentmicroalgaefrom wastewater—cont’d
WastewaterAlgae
Municipal wastewater
Scenedesmus obliquus
85.4380.3095.72 – 46.92Thelipidcontentwas increasedto24%with N+ ionimplantation formutagenesis.
Scenedesmus sp. 909079–884.65g/L – Combinedmunicipal wastewaterandcattle manuredigested effluentwasusedasa nutrientsmedium.
Scenedesmus sp.HXY2
Palmmill effluent
Chlorella minutissima
Chlorella sorokiniana CY-1
Chlorella vulgaris
Textile wastewater
9696.694.5 – 15.56Algaewereableto growathigh concentrationof organiccarbonand ammonia [122]
89.492900.995g/L/day14Astainlesssteelphoto cavityreactorwasused tostopthelight scattering. [123]
93.798.6965.74g/L14.43Atriangular photobioreactorwas usedtomakealgae harvestingeasyby sedimentation.
53.755.677.32.04g/L16Coculturedwith Pseudomonas sp.to decolorizetheeffluent. [124]
Chlorella sp. WuG23 7575 – 58mg/L/day16.6HighcontentofFAME wasproducedby culturingalgaeinthe pHrangeof9–11 underaeration. [125]
Anabaena ambigua
Pharmaceutical wastewater Tetraselmis indica BDU 123
5052.9563.0511.61mg/L/ day – Mostlystudiedalgae havethepotentialto growin100%oftextile wastewater [126]
66.30677046.85mg/L/ day 16.40Anaerobictreatmentof wastewaterwas performedwithMFC beforeaerobic treatmentwithalgal [127]
FromS.K.Bhatia,S.Mehariya,R.K.Bhatia,M.Kumar,A.Pugazhendhi,M.K.Awasthi,A.E.Atabani,G.Kumar,W.Kim,S.O.Seo,Y.H.Yang, Wastewaterbasedmicroalgalbiorefineryforbioenergyproduction:progressandchallenges, Sci.TotalEnviron.751(2021) 141599.
4.2Bioethanolproduction
Bioethanolisasignificantandcleanbiofuelthatcanbeutilizedfortransport.Thereare severalbenefitsofbioethanoloverfossilfuels.Bioethanolhasahighoctanenumberthatcan preventcylindersfromknockingintheengines,anditproducesfewergreenhousegases duetoitshighO 2 content.Bioethanolcanbeutilizeddirectlyinthepresentautomotiveindustrywithoutanyalterationsandblendedwithoil [128– 130].Biofuelproductionincreased from4.8to16billiongallonsfrom2000to2007 [131] .Presently,theUnitedStatesandBrazil accountforabout75% – 80%oftheworld’sbioethanolproduction,andthesecountriesare consideredtobeworldleadersonthisfront [129,131] .TheUnitedStatesproduces bioethanolcommerciallyfr omcorngrainin187plants [132].In2013,Brazilproduced37billionlitersofbioethanolwithsugarcaneastheprimaryfeedstock.TheEuropeanUnion(EU) useswheatandsugarbeetastheidealfeedst ockandhasbeenproducing2billiongallonsof bioethanolannually [128,129].TheUnitedStates’RenewableFuelStandard(RFS)is expectedtoachieve36billiongallonsofbiofuelfromalgaeby2022 [133] .Biofuelsproduced fromrenewableandsustainablefeedstockwillprogressivelybecomethefutureenergy sourcesinplaceofliquidfossilfuels.Bioethanolproductionfromcornandsugarcane sugarsisnowshiftingtoalgalcarbohydratesaspotentialfeedstock [134,135].Bioethanol productionhasrecentlyimprovedfrom1to39 billiongallonsandisestimatedtoreach 100billiongallonsquickly [136].Algaecontainmanydiversecarbohydrates,suchasglycogen,starch,agar,andcellulose,whichcanefficientlybeconvertedintofermentablesugars forbioethanolproduction [137]
Theproductionofbioethanolfromalgaeisauniquestruggletodevelopsustainable biofuels.Therearemanychallengesrelatedtopilot-scaleproductionforitscommercialutilizationasacleanbiofuel.Anassortmentofsuitablealgalbiomass,pretreatmentmethods,and aneffectivefermentationprocessneedtobedevelopedforbioethanolproductionforindustrialapplications.Bioethanol’sfermentativeyieldsignificantlydependsontheperformanceof potentialmicroorganisms.Thefermentationprocessneedstobecarriedoutinsterileconditionstoavoidcontaminationandincreasethefinalproductionofbioethanol [138].Algae’s potentialtobeasuitablefeedstockrequiresconstanteffortstoovercometheexistinglimitationsregardingalgalcultivationandthecarbohydrate-richbiomassproductionfromthem inadditiontotheirharvestingandpretreatmentforthehighestyieldproduction.Aneconomicalalgalculturingsystemcanbeestablishedbydevelopingandoptimizingspecificprocess parameters.Algalcellbiomassandcarbohydrateproductivitymustbeimprovedforeconomicallyfeasiblebioethanolproduction [139].Severalcarbohydrates-richalgae,suchas Chlamydomonasreinhardtii and C.vulgaris,havebeenconsideredaspotentialoptionsfora TEAofbioethanolproduction [140].ATEAofcommercialbioethanolproductionfromalgae hassuggestedtheplant’ssuitabilityconcerningtotalinvestment,cost,andnetrevenue [141].
Anotherstudyreportedalgae’seconomicfeasibilityforhighbioethanolproductionviathe fermentationprocessbyconsideringnumerousparameters.Acriticalfactor,inthiscase,isto maximizethealgalbiomassproductionandreducetheoperationalandmaintenancecosts [142].Itisestimatedthatachievingalgalbiofuels’economicfeasibilityneedsmorethan 10yearsofresearchfordevelopingastableposition [101].Thoughalgalbiofuelsarenot yeteconomicallypractical,severalcompaniesintheUnitedStates,Europe,andotherregions producethemonacommercialscale [101,143].AsperaTEA,biodieselproductionfromalgae thatcostslessthan $5/galloniscomparabletoproducinggasoline,whilebioethanol
productionatthecostof $2.95/gallonisfinanciallyfeasible [144].Severalstudieshaveproposedtheproductionofbiofuelsfromalgaepricedat $1/Ltobepracticalandeconomical whencomparedtoproducingotherfuels [11].Manycompanies,suchasAlgenol,Sapphire Energy,andSeambiotic,areworkingonbioethanol’scommercial-scaleproductionwithan outputof1billiongallons/yearthatwouldcost85cents/L [145].
4.3Biogasproduction
Algaebiomasscanbepotentiallyusedasrawmaterialforbiogasproduction.However, duetotheircellwalls,theinterestinusingalgalbiomassasanalternativebiodegradableorganicmatterforbiogasproductionhasdeclined [146].Celluloseorhemicellulosecompounds inalgalcellwallshavehighresistancetodegradationunderanaerobicdigestion.Severalalgal strainsproducetoxiccompoundsthatcanharmanaerobicbacteriaduringdigestion.AnimproperC:Nratioofbiomasscanalsodisturbthefermentationprocesses [147,148].Nickel nanoparticles(Ni-NPs)wereintroducedtoincreasebiogasproductionfromthegreenalgae Enteromorpha throughanaerobicdigestion.Somecriticalparameters,suchastemperaturein therangeof25–45°C,initialpH(5–9),andNi-NPsintheconcentrationof0.5–2mg/L,were optimizedinthebatchmode.Ahighconcentrationofbiogasyieldof346mLwasachievedat 1mg/LofNi-NPscomparedtootherconcentrationsandcontrols.Thisstudyconfirmedthat aninitialconcentrationofNi-NPsat1mg/L,atemperatureof35°C,andaninitialpHof7had abettereffectontheanaerobicdigestionofgreenalgae [149].Severalpretreatmentmethods foralgalbiomassdegradationthatrequirehighenergyandaffectbiogasproduction’soverall efficiencyhavebeenreported [150].Microwave [150] pretreatmentofthebiomassofalgae Enteromorpha combinedwithmetalnanoparticles(NPs)hasbeenusedforthealgae’sdegradation.Thehighesttotalbiogasproduction(54mL/g-TS)wasattainedwhereCo-NPsplus MWpretreatmentwasused.Incontrast,Ni-NPsplusMWpretreatmentproducedamaximumbiohydrogenof60%(v/v).Further,theenergyanalysisconductedduringthestudy confirmedthatthecombinedprocessesofMWpretreatmentwithmetalNPsconsumedless energywhencomparedtotheMWpretreatmentalone [151].Anaerobicdigestionofpurealgaebiomass,suchas Chlorophyta, Cyanoprokaryota,and Bacillariophyceaephylum,hasbeenused forbiogasproductionundercontrolledconditions.Anaveragebiogasproductionyieldof 396dm3/kgatthereactionrateof r ¼ 54.3cm3/daywasachievedfrom Chlorophyta.The CH4 contentinthebiogasaccountedfor59.7%.Incontrast,abiogasyieldof382.5dm3/kg, biogasrateof r ¼ 97cm3/day,and63.1%ofCH4 wereachievedfrom Cyanoprokaryota.On theotherhand,alowconcentrationofbiogasproductionof357dm3/kg,ameanrateofbiogas at r ¼ 51cm3/day,and58%ofCH4 wereachievedwhen Bacillariophyceae wasusedasthe feedstock.Thisstudyconcludedthatlowbiogasproductionwith Bacillariophyceae algae wasduetothelatter’scomplexcellwallcomposition [152]
AddingalgalbiomasstoasubstratemixtureincreasestheC:Nratioforanaerobicdigestion [153].YenandBrune [154] observedsubstantialbiomethaneproductionduetocellulose wastes’codigestionwithalgalbiomass.Comparedtothefermentationprocesssolelyusing algae,theCH4 productionincreasedfrom0.57to1.17dm3/m3 dayafteradjustingtheratioof organicwastestoalgalbiomass(1:1).Vergara-Ferna ´ ndez [155] examinedtheanaerobicdigestionofalgalbiomass Macrocystispyrifera and Durvillaeaantarctica withothersubstrates.The highestbiogasproductionyieldof180.4dm3/kgd.m.d.wasachievedwhenallthesubstrates
I.Environmentalsector