inSynthesisGas:Methods,Technologies andApplications,Volume3:SyngasProductsand UsagesMohammadRezaRahimpour
https://ebookmass.com/product/advances-in-synthesis-gasmethods-technologies-and-applications-volume-3-syngasproducts-and-usages-mohammad-reza-rahimpour/
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Advances in Synthesis Gas: Methods, Technologies and Applications, Volume 1: Syngas Production and Preparation
Mohammad Reza Rahimpour
https://ebookmass.com/product/advances-in-synthesis-gas-methodstechnologies-and-applications-volume-1-syngas-production-andpreparation-mohammad-reza-rahimpour/ ebookmass.com
Advances in Synthesis Gas: Methods, Technologies and Applications, Volume 2: Syngas Purification and Separation
Mohammad Reza Rahimpour
https://ebookmass.com/product/advances-in-synthesis-gas-methodstechnologies-and-applications-volume-2-syngas-purification-andseparation-mohammad-reza-rahimpour/ ebookmass.com
Advances in Synthesis Gas: Methods, Technologies and Applications, Volume 4: Syngas Process Modelling and Apparatus Simulation Mohammad Reza Rahimpour
https://ebookmass.com/product/advances-in-synthesis-gas-methodstechnologies-and-applications-volume-4-syngas-process-modelling-andapparatus-simulation-mohammad-reza-rahimpour/ ebookmass.com
Chemistry for today Ninth ed Spencer L. Seager
https://ebookmass.com/product/chemistry-for-today-ninth-ed-spencer-lseager/
ebookmass.com
Vincent & Sien Silvia Kwon
https://ebookmass.com/product/vincent-sien-silvia-kwon/
ebookmass.com
Tall, Dark, and Dirty: A Bossy Billionaire Novel A.M. Hargrove
https://ebookmass.com/product/tall-dark-and-dirty-a-bossy-billionairenovel-a-m-hargrove/
ebookmass.com
How International Law Works in Times of Crisis George Ulrich (Editor)
https://ebookmass.com/product/how-international-law-works-in-times-ofcrisis-george-ulrich-editor/
ebookmass.com
Programming for Problem Solving E Balagurusamy
https://ebookmass.com/product/programming-for-problem-solving-ebalagurusamy/
ebookmass.com
(eTextbook PDF) for Peak Performance: Success in College and Beyond 10th Edition
https://ebookmass.com/product/etextbook-pdf-for-peak-performancesuccess-in-college-and-beyond-10th-edition/
ebookmass.com
Liquid-Liquid and Solid-Liquid Extractors 1st Edition
Jean-Paul Duroudier
https://ebookmass.com/product/liquid-liquid-and-solid-liquidextractors-1st-edition-jean-paul-duroudier/
ebookmass.com
AdvancesinSynthesisGas: Methods,Technologies andApplications
Volume3:SyngasProductsandUsages
Editedby
MohammadRezaRahimpour DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
MohammadAminMakarem MethanolInstitute,ShirazUniversity,Shiraz,Iran
MaryamMeshksar DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
Contributors
MitraAbbaspour DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
AmrAbdalla DepartmentofChemicalandPetroleumEngineering,UniversityofCalgary,Calgary, AB,Canada
WaqarAhmad DepartmentofChemicalandBiologicalEngineering,MonashUniversity,Clayton, VIC,Australia
ChayeneGonc ¸ alvesAnchieta PaulScherrerInstitut,Villigen,Switzerland
PrakashAryal DepartmentofChemicalandBiologicalEngineering,MonashUniversity,Clayton, VIC,Australia
NooshinAsadi DepartmentofChemicalandBiologicalEngineering,MonashUniversity,Clayton, VIC,Australia
KorooshAsghari DepartmentofChemicalandPetroleumEngineering,UniversityofCalgary, Calgary,AB,Canada
ElisabeteMoreiraAssaf UniversityofSa ˜ oPaulo,Sa ˜ oCarlosInstituteofChemistry,Sa ˜ oCarlos, Sa ˜ oPaulo,Brazil
JoseMansurAssaf FederalUniversityofSaoCarlos,ChemicalEngineeringDepartment,SaoCarlos, SaoPaulo,Brazil
ManuelBailera DepartmentofMechanicalEngineering,UniversidaddeZaragoza,CampusRı´o Ebro,Bldg.Betancourt,Zaragoza,Spain;GraduateSchoolofCreativeScienceandEngineering, WasedaUniversity,Tokyo,Japan
CarlosGilbertoTemoltzinCaballero MonterreyInstituteofTechnologyandHigherEducation, Puebla,Mexico
JoaoSousaCardoso PolytechnicInstituteofPortalegre,Portalegre;InstitutoSuperiorTecnico, UniversityofLisbon,Lisbon,Portugal
JoseAntonioMayoralChavando PolytechnicInstituteofPortalegre,Portalegre,Portugal
SilviodeOliveiraJunior DepartmentofMechanicalEngineering,PolytechnicSchoolofUniversity ofSa ˜ oPaulo,Sa ˜ oPaulo,Brazil
MeireEllenGoreteRibeiroDomingos DepartmentofChemicalEngineering,PolytechnicSchoolof UniversityofSa ˜ oPaulo,Sa ˜ oPaulo,Brazil
MoisesTelesdosSantos DepartmentofChemicalEngineering,PolytechnicSchoolofUniversityof Sa ˜ oPaulo,Sa ˜ oPaulo,Brazil
SwaritDwivedi DepartmentofChemicalandBiologicalEngineering,MonashUniversity,Clayton, VIC,Australia
DanielaEusebio PolytechnicInstituteofPortalegre,Portalegre,Portugal
AzharuddinFarooqui DepartmentofChemicalandPetroleumEngineering,UniversityofCalgary, Calgary,AB,Canada
CarlaFerna ´ ndez-Blanco ChemicalEngineeringLaboratory,FacultyofSciencesandCentrefor AdvancedScientificResearch(CICA),UniversityofLaCorun ˜ a,LaCorun ˜ a,Spain
DanielA.Flo ´ rez-Orrego DepartmentofMechanicalEngineering,PolytechnicSchoolofUniversity ofSa ˜ oPaulo,Sa ˜ oPaulo,Brazil;FacultyofMinas,NationalUniversityofColombia,SchoolofProcesses andEnergy,Medellin,Colombia;DepartmentofMechanicalEngineering, EcolePolytechnique FederaledeLausanne,Switzerland
ForooghMohseniGhalehGhazi DepartmentofChemicalEngineering,ShirazUniversity,Shiraz, Iran
AshwinHatwar DepartmentofChemicalandBiologicalEngineering,MonashUniversity,Clayton, VIC,Australia
FatemehKhodaparastKazeroonian DepartmentofChemicalEngineering,ShirazUniversity, Shiraz,Iran
ChristianKennes ChemicalEngineeringLaboratory,FacultyofSciencesandCentreforAdvanced ScientificResearch(CICA),UniversityofLaCorun ˜ a,LaCorun ˜ a,Spain
DavidM.Kennes-Veiga CRETUS,DepartmentofChemicalEngineering,Universityof SantiagodeCompostela,SantiagodeCompostela,Spain
HadisehKhosravani DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
AnandaValleziPaladinoLino FederalUniversityofSa ˜ oCarlos,ChemicalEngineeringDepartment, Sa ˜ oCarlos,Sa ˜ oPaulo,Brazil
PilarLisbona DepartmentofMechanicalEngineering,UniversidaddeZaragoza,CampusRı´oEbro, Bldg.Betancourt,Zaragoza,Spain
NaderMahinpey DepartmentofChemicalandPetroleumEngineering,UniversityofCalgary, Calgary,AB,Canada
MohammadAminMakarem MethanolInstitute,ShirazUniversity,Shiraz,Iran
TayebehMarzoughi DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
MaryamMeshksar DepartmentofChemicalEngineering;MethanolInstitute,ShirazUniversity, Shiraz,Iran
RafaelNogueiraNakashima DepartmentofMechanicalEngineering,PolytechnicSchoolof UniversityofSaoPaulo,SaoPaulo,Brazil
VirginiaPerez CentrefortheDevelopmentofRenewableEnergy-CentreforEnergy,Environment andTechnologyResearch(CEDER-CIEMAT),Soria,Spain
ElhamRahimpour ShirazUniversityofMedicalSciences,Shiraz,Iran
HamidRezaRahimpour DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
MohammadRezaRahimpour DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
TayebeRoostaie DepartmentofChemicalEngineering;MethanolInstitute,ShirazUniversity, Shiraz,Iran
SoniaSepahi DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
MohammadJavadShahbazi DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
NazaninAbrishamiShirazi GraduateFacultyofEnvironment,UniversityofTehran,Tehran,Iran
ValterSilva PolytechnicInstituteofPortalegre,Portalegre;CentreforEnvironmentalandMarine Studies(CESAM),DepartmentofEnvironmentandPlanning,UniversityofAveiro,Aveiro,Portugal
AnahitaSoleimani DepartmentofNano-ChemicalEngineering,FacultyofAdvancedTechnologies, ShirazUniversity,Shiraz,Iran
AkshatTanksale DepartmentofChemicalandBiologicalEngineering,MonashUniversity,Clayton, VIC,Australia
Luı´sA.C.Tarelho CentreforEnvironmentalandMarineStudies(CESAM),Departmentof EnvironmentandPlanning,UniversityofAveiro,Aveiro,Portugal
Marı´aC.Veiga ChemicalEngineeringLaboratory,FacultyofSciencesandCentreforAdvanced ScientificResearch(CICA),UniversityofLaCoruna,LaCoruna,Spain ShabnamYousefi DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands
TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright©2023ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicor mechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem,without permissioninwritingfromthepublisher.Detailsonhowtoseekpermission,furtherinformationaboutthe Publisher’spermissionspoliciesandourarrangementswithorganizationssuchastheCopyrightClearance CenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher (otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenour understanding,changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecome necessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusing anyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethods theyshouldbemindfuloftheirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliability foranyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,or fromanyuseoroperationofanymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-91878-7
ForinformationonallElsevierpublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: SusanDennis
AcquisitionsEditor: AnitaKoch
EditorialProjectManager: ZsereenaRoseMampusti
ProductionProjectManager: SruthiSatheesh
CoverDesigner: MarkRogers
TypesetbySTRAIVE,India
Preface
Vol.3:Syngasproductsandusages
Synthesisgas(syngas)anditsproductssuchashydrogenareindispensableinchemical,oil,and energyindustries.Theyareimportantbuildingblocksandserveasfeedstockfortheproduction ofmanychemicalcompoundssuchasammoniaandmethanol.Hydrogenisexpectedtobecome acommonenergycarriernolaterthanthemiddleofthe21stcenturysinceitoffersconsiderable energydensityandreleasesnegligiblepollutants.Itisalsoutilizedinpetroleumrefineriesfor producingcleantransportationfuels,anditsconsumptionisexpectedtoincreasedramatically inthenearfutureasrefineriesneedtointensifyproductioncapacities.Manypublicationshave hithertofocusedonsyngasproductionandpurificationmethods,aswellasitsapplicationsin industrialproductionunits.Despitethefactthatextendedstudieshavebeenundertaken,thereis stillroomforimprovement.Thefourvolumesofthisbookseriesexplaintheconventionaland state-of-the-arttechnologiesfortheproduction,purification,andconversionofsyngas meticulously.
Thedevelopmentofdifferenttechnologiesfortransformingsyngasorthemajoringredients intovalue-addedproductssuchashydrogen,ammonia,ethanol,andmethanolhasattractedthe attentionofresearchersinbothacademicandindustrialcommunities.Thankstotheir undeniablesignificance,manystudieshavebeenhithertodevotedtothedevelopmentofthese processesandthereisawealthofinformationontheconventionaltechnologies,whichshould becollectedinacomprehensivecontribution.However,theprogressthatisbeingmadeata breakneckpaceshouldnotbeoverlooked.Forthisgoaltobeachieved,thisengagingtext touchesonthedetailsofdifferentproductsofsyngas,aswellastheiroperatingconditionsand challenges,andwouldthusserveasaconnectionbetweenthescientistsinresearchlaboratories andtheoperatorsinindustrialplants.
Todoso,thebookisdividedintothreesectionsofchemicalandenergyproductionsfrom syngaswithrelatedchallenges.Thefirstsectionincludesapplicationsandutilizationofsyngas forproducingavastvarietyofchemicalmaterialssuchashydrogen,methanol,ethanol, methane,ammonia,aceticacid,fuelgas,andbiofuel,whilethesecondaddressespower generationinfuelcellseithersolelyorpowerandheatgenerationintandem,aswellasiron reductionindetail.Inthethirdsection,theenvironmentalchallengesofsyngasanditsfuture prospectsandindustrialoutlookarepresented.
Theeditorsfeelobligedtosincerelyappreciatetheauthorsofthechaptersfortheir contributions,hardwork,andgreatassistanceinthisproject.Furthermore,theauthors,aswell astheeditors,aregratefultoalltheElsevierstafffortheirinvaluableandirreplaceable step-by-stepassistanceinpreparingthisbook.
MohammadRezaRahimpour
MohammadAminMakarem
MaryamMeshksar
ReviewerAcknowledgments
Theeditorsfeelobligedtoappreciatethededicatedreviewers(listedbelow)whowereinvolved inreviewingandcommentingonthesubmittedchaptersandwhosecooperationandinsightful commentswereveryhelpfulinimprovingthequalityofthechaptersandbooksinthisseries.
Dr.MohammadHadiSedaghat
SchoolofMechanicalEngineering,ShirazUniversity,Shiraz,Iran
Dr.AliBakhtyari
ChemicalEngineeringDepartment,ShirazUniversity,Shiraz,Iran
Dr.JavadHekayati DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
Ms.ParvinKiani
DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
Ms.SamiraZafarnak DepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran
AbouttheEditors

Prof.MohammadRezaRahimpour isaprofessorin ChemicalEngineeringatShirazUniversity,Iran.Hereceived hisPhDinChemicalEngineeringfromShirazUniversityin cooperationwiththeUniversityofSydney,Australia,in 1988.Hestartedhisindependentcareerasassistantprofessor atShirazUniversityinSeptember1998.Prof.Rahimpourwas aresearchassociateattheUniversityofCalifornia,Davis, from2012to2017.DuringhisstintattheUniversityof California,hedevelopeddifferentreactionnetworksand catalyticprocessessuchasthermalandplasmareactorsfor upgradingligninbio-oiltobiofuelwiththecollaborationof UCDAVIS.HehasbeenachairoftheDepartmentof ChemicalEngineeringatShirazUniversityfrom2005to2009andfrom2015to2020.Prof. Rahimpourleadsaresearchgroupinfuelprocessingtechnologyfocusedonthecatalytic conversionoffossilfuelssuchasnaturalgasandrenewablefuelssuchasbio-oilsderived fromlignintovaluableenergysources.Heprovidesyoungdistinguishedscholarsfrom developingcountrieswithperfecteducationalopportunitiesinbothexperimentalmethodsand theoreticaltoolstoundertakein-depthresearchinthevariousfieldsofchemicalengineering includingcarboncapture,chemicallooping,membraneseparation,storageandutilization technologies,noveltechnologiesfornaturalgasconversion,andimprovingtheenergy efficiencyintheproductionanduseofnaturalgasinindustries.

Dr.MohammadAminMakarem isaresearchassociateat MethanolInstitute,ShirazUniversity.Hisresearchinterests arefocusedongasseparationandpurification,nanofluids, microfluidics,catalystsynthesis,reactordesign,andgreen energy.Inthegasseparationfield,hisfocusisonexperimental andtheoreticalinvestigationandoptimizationofthepressure swingadsorptionprocess,andinthegaspurificationfield,heis workingonnoveltechnologiessuchasmicrochannels. Recently,hehasinvestigatedmethodsofsynthesizing bio-templatenanomaterialsandcatalysts.Hehascollaborated inwritingandeditingvariousbooksandbookchaptersfor famouspublisherssuchasElsevier,Springer,andWiley,in additiontoguesteditingjournalspecialissues.

MaryamMeshksar isaresearchassociateatShiraz University.Herresearchisfocusedongasseparation,clean energy,andcatalystsynthesis.Inthegasseparationfield,sheis workingonmembraneseparationprocesses,andintheclean energyfield,shehasworkedondifferentreforming-based processesforsyngasproductionfrommethaneexperimentally. Shehasalsosynthesizednovelcatalystsfortheseprocesses, whichhavebeentestedforthefirsttime.Shehasreviewed noveltechnologieslikemicrochannelsforenergy production.Recently,shehaswrittenvariousbookchaptersfor famouspublisherssuchasElsevier,Springer,andWiley.
Introductiontosyngasproductsand applications
HadisehKhosravania,MaryamMeshksara,b,HamidRezaRahimpoura,and MohammadRezaRahimpoura
aDepartmentofChemicalEngineering,ShirazUniversity,Shiraz,Iran bMethanolInstitute,Shiraz University,Shiraz,Iran
1.Introduction
Theextremeuseoffossilfuelsduetothefastindustrialdevelopment,aswellaspopulation increment,hascreatedmanyenvironmentalproblemslikeincreasedglobalwarminggases concentrationintotheatmosphere(CO2,CH4,etc.) [1].Recently,theincreasedrateofCO2 concentration(1.5ppm/year)forcedtheinternationalsocietytodecreasetheconcentrationof greenhousegasesbyloweringtheirproductionsorconsumingtheminotherplants [2–4].Forthis purpose,manyapproacheshavebeenreviewedbybiologicalandchemicalresearchers [5,6].
Synthesisgasasanimportantintermediateorfeedstockinmanymanufacturesorprocessescan bemadefromadiversityofsourcescontainingnaturalgas(NG),coal,oxygen,carbondioxide, ornearlyeachhydrocarbonfeedstock [7].Fromsuchmentionedrawmaterials,NGisthemost commonandthelowestpricedoneforsyngasproduction [8–10].Methaneisthemost importantconstituentofNG,whichmainsourceisreservesofoilandgas,aswellaslandfill gas.Asaresultofabout20%ofglobalwarmingcausedbyCH4 emissionintotheatmosphere, thedevelopmentoftechnologiesforconvertingmethaneintovaluableproductsisessential [11,12].Differentconvectionalprocessesexistforproducingsyngasfrommethane,listedas steamreforming,dryreforming,partialoxidation,inadditiontothenewtechnologieslikethe plasmaprocess [13–16].Assyntheticgasgenerationisveryendothermic,itneedshigh temperaturesandis,therefore,costly [17].In Table1,thesummariesofdifferentsyngas producingprocessesusedinammoniaplantsregardingtheirenergyconsumptionandcosts [18].Byloweringtheactivationenergyofthedesiredreactionsinthesyngasproduction processes,thecatalystplaysakeyroleinincreasingreactionkineticsandachievingamaximum yieldofsyngasproduction.Severaleffortshavebeendonetodevelopcatalyticsystemsthat haveenhancedresilienceforcokeformationandcheaperprecursors.Theseattemptsinclude AdvancesinSynthesisGas:Methods,TechnologiesandApplications. https://doi.org/10.1016/B978-0-323-91878-7.00014-9 Copyright # 2023ElsevierInc.Allrightsreserved. 3
Table1Relativeplantinvestmentcostandenergyconsumptionforvariousfeedstockusedfor syngasproductionforammoniasynthesis [18].
aDependsontheefficiencyoftheelectricitygeneration.
Fig.1 Examplesofchemicalsmadefromsynthesisgas [7].
thealterationofsynthesisconditionsandmethodsinadditiontoapplyingmixedsupport approachesandbimetalliccatalysts [11,19].
Syntheticgasmayproduceabroadrangeofenvironmentallyfriendlychemicalsandfuels, whichconventionaluseshavebeensteadilyincreasing. Fig.1 liststhemostimportant chemicalsproducedfromsyngas.Syntheticgasisagreatsourceofhydrogenthatcanbeusedto makealmostallchemicalsfromhydrogenlikeammonia [3,4].In1910,HaberandBosch developedamethodforproducingammoniafromN2 andH2, andthefirstindustrialammonia
Fuel gas
Town gas
Fischer-Tropsch liquids
Synthesis gas
Synthetic natural
Introductiontosyngasproductsandapplications5
synthesisplantwasbuiltin1913 [20].Besides,themethanol—anotherproductofsyngas—has exhibitedtremendousdevelopmentforsynthesizingmethylethersusedasoctaneboostersin vehiclefuels,despiteremainingthesecond-largestconsumerofsynthesisgas.Fisher-Tropish synthesisisstillthethird-largestconsumerofsyntheticgasinwhichkerosene,naphtha,waxes, etc.,arethemainproducts [7].Inthefollowing,eachapplicationofsyngasisintroducedand discussedinmoredetail.
2.Chemicalsfromsynthesisgas
Asmentioned,differentchemicalsareproducedfromsyntheticgas,whichareintroducedinthe followingsectionswiththeirproductionmethodsindetail.
2.1Hydrogen
Hydrogenislargelyusedintheproductionofammoniaandmethanol,aswellasin petroleumrefining.Itcanbealsobeutilizedtogenerateenergyorbeappliedasa transportationfuel.Inadditiontovarioushydrogenapplicationslistedin Table2 ,its differentproducingmethodsarealsomentioned.Amongdifferentprocesseslisted previouslyforhydrogenproduction,80%–85%ofhydrogenneededindifferentfactories comesfromSMR(steammethanereforming),whichisshownin Fig.2[21].Different separatingmethodscanbeusedforproducinghigh-purityH2 fromsyngas,suchaspressure swingadsorption(PSA)andmembrane-basedt echnologies,eachofwhichisdiscussedin moredetailinthefollowing.
2.1.1Swingadsorptiontechnologies
PSA,themostmaturecyclicadsorptiontechnology,hasbeenwidelyappliedforgas separationandpurification.Theadsorption-desorptioncycleisachievedbythedropin solidadsorptioncapacitywithdecreasingpressure.PSAprocesseshavebeensuccessfully appliedinseveralprocessestoseparatevariousgasmixtures,suchasCO2 capture, olefin-paraffinseparation,methanerecovery,NGupgrading,andairseparation.PSAhas becomethemostwidespreadtechnologyusedtoproducehigh-purityH 2 fromagas mixture.Currently,PSAtechniquesareusedinover85%ofglobalH 2 plantsfor high-purityH 2 production [22] .
Thistechnologycanbearrangedintosingleormulticolumnarrangementsthatdependonthe numberofadsorbers.MulticolumnPSAunitshaving4–12parallelcolumnsarefrequentlyused forindustrialapplications,asforlarge-scaleplants,continuousoperationisdesired. Fig.3 showsatypicaldouble-bedPSAsetup:oneadsorbingwhiletheotherregenerates [20].
ForseparatingH2 fromsyngas,high-pressuresyngasisfedintothePSAabsorber,andits impuritiesmainlyconsistofCO,CO2,CH4,andN2 areselectivelyadsorbedonthesurfacesof
Table2Differentapplicationsandproducingmethodsofhydrogen [7].
Hydrogenapplications
Naphthahydrotreater:
• Hydrogenisusedtodesulfurizenaphthafromatmosphericdistillation;thenaphthamustbehydrotreated beforebeingsenttoacatalyticreformerunit.
Distillatehydrotreater:
• Afteratmosphericorvacuumdistillation,desulfurizesthedistillates;insomeunits,aromaticsare hydrogenatedtocycloparaffinsoralkanes.
Hydrodesulfurization:
• AsafeedforClausplants,sulfurcompoundsarehydrogenatedtohydrogensulphideH2S.
Hydroisomerization:
• Toimprovetheproduct’scharacteristics,normal(straight-chain)paraffinistransformedintoisoparaffins (e.g.,octanenumber).
Hydrocracker:
• Hydrogenisusedtoconvertheavyfractionsintohighervalue,lowerboilingproducts.
Hydrogenproduction
Catalyticreformer:
• Hydrogenisproducedasaby-productoftheconversionofnaphtha-boilingrangemoleculesintohigher octanereformate.
Steam-methanereformer:
• Hydrogenisproducedforthehydrocrackerorthehydrotreaters.
Steamreformingofhydrocarboncompoundswithagreatermolecularweight:
• Otherthanmethane,itproduceshydrogenfromlow-boilinghydrocarbonderivatives.
Off-gasesfromrefineriescanberecovered:
• Hydrogenintherangeof50%(v/v)iscommoninprocessgas.
Gasificationofpetroleumresidua:
• Gasificationunitsproducesynthesisgas,whichcanberecovered.
Gasificationofpetroleumcoke:
• Ingasificationunits,synthesisgasisrecovered.
Partialoxidationprocesses:
• Producesynthesisgasfromwhichhydrogencanbeextracted,similartothegasificationprocess.
theadsorbingbed.Therefore,theproductofthefirstcolumnishighpurityH2.Adsorption typicallyoccursatrelativelyhighpressure(20–50bar)andupto50–60°Ctemperature [23].In continuousadsorption,thesyngasfeedstreamisswitchedtoanotherbedbyreachingthe adsorbentscapacitytoitssaturationamount.Then,thesaturatedadsorbentsarerecoveredvia depressurizationaidedthroughapurge-gassupplyforreleasingthetrappedimpuritiesinto
adsorbents.Thesereleasedgasesarecalledoff-gas.PartoftheH2 feed-stream(usuallyupto 20%)maybelostintheoff-gas,dependingontheoperatingcondition.Off-gasiscommonly partiallyburntinthereformerorappliedasaheatsource,avoidingunnecessaryemissions.The COcontentinthetail-gasimprovesthereformerflamestabilityandenhancesthereforming equilibrium [14].
DespitegoodadvantagesofPSAtechnology,includingrelativelysimpleoperation,notable performanceatambienttemperatures,highregenerationefficiency,equipmentcompactness, andlowenergyintensity,ithassomedrawbackslistedashighsyngaslossesinthebedafter purginginthedepressurizationstep,lowH 2 recoveries,andlimitedimpuritiessorptioninto theadsorbents [2].Therefore,acompleterecoveryofH2 bycouplingPSAwithother separationtechnologiesishighlydemanded.Membranetechnologiesoffersignificant advantagesforH 2 purificationwiththeirtypicallyhighrecoveryrates,facileoperation,and energyefficiency [4].Therefore,byusingmembranereactorsinthesyngasproducingunit, thenumberofstageswillbereducedasbothpurificationandproductionstepsoccur simultaneouslyatthesametime.
2.1.2Membrane-basedtechnologies
Membranesactasaphysicalbarrierthatallowmassspeciesselectivetransport,whichare widelyappliedforpurificationandseparationinmanyindustries.Membranesaredividedinto threecategories:inorganic,organic,andhybridsoforganic/inorganicmembranes.Inorganic membranescanbeclassifiedintoceramicandmetallicconstituents,whileorganiconesare
Pre-heater Syngas (H2, CO, CO2)
Fig.2
SchematicofSMRforH2 production [20]
Fig.3
TypicalPSAdouble-bedarrangementforH2 recovery [22]
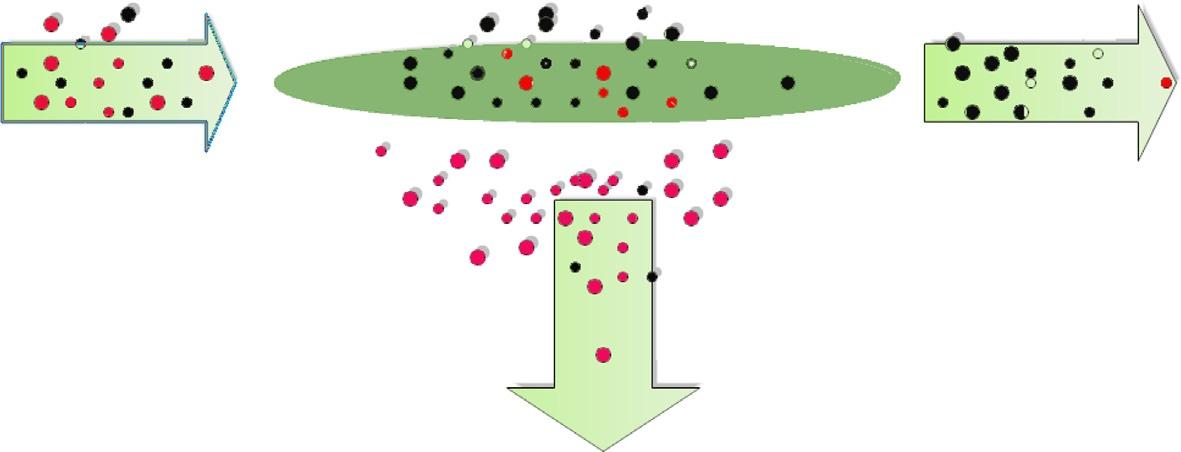
dividedintobiologicalandpolymericmembranes.Membraneperformanceintheseparation processisshownin Fig.4,inwhichthedrivingforceistheconcentrationoffeedstockspeciesor pressuregradient [24].
Thecriterionforselectingamembraneisbasedonitsapplication.Importantpropertiesof membranesincludingmechanicalintegrityanddurability,aswellasseparationselectivityand productivity,mustbebalancedagainsttheircost [25].However,permeance(permeationrate) andselectivityarethemainpropertiesofmembranes.
Pt-basedmembranes,denseceramicmembranes,andmicroporousmembranesarethreemajor typesofH2 separatingmembranesinwhichtheresearchershaveprimarilyfocusedonsolving
Syngas
Column Adsorbing
Pure Gas
Valve close
Column Regenaration
Open
Membraneperformanceforseparatinggasescomponents [24]
theirmajorchallenges,includingmaximizinghydrogenfluxandselectivity,aswellas minimizingmembranecostandmembranefailurecreatedbythechemicalinteractionsor thermalcycling [26].Thecharacteristicsandoperatingconditionsofthesethreemembranesare summarizedin Table3.Thegenericdrawbackofthesemembranesisrecoveryofhydrogenat lowpressure,whichisappropriateforfuelcellapplications,forprocesseswherehigh-pressure hydrogenisrequired,H2 needstobecompressedbeforebeingapplied.However,the pressurizationofhydrogenisdifficultandneedshighcostsasithassmallmolecularsizes.
MorecompleteCOtoH2 shiftisrequiredinasinglereactorforremovinghydrogenina membranereactor.Thisreactionisexothermic,soH2-membranesneedheatremovaltoprevent catalystdeactivationcausedbycokeformationorsinteringathightemperatures.Thisisa significantchallengeoffixed-bedreactorsasinthesesystems,hotspotsareformedbecauseof gasesmixinglessthanidealandnaturalheatflowintothereactor [20].
2.2Methanolproduction
Methanol,alsoknownasmethylalcoholormethylhydratewiththeformulaCH3OH,isthe simplestalcohol.Itisacolorless,light,volatile,flammable,andtoxicmaterial.Methanol consistsofamethylgrouplinkedtoapolarhydroxylgroupandisapolarchemical,thusis completelysolubleinwaterandorganicsolventsandonlyslightlysolubleinfatandoil [27]. Methanolhasamolecularweightof32.04,anoctanenumberof113,anditsdensityisabout halfthatofgasoline.Itcanbeusedasatransportationfuelintheroleofadditivetogasoline. Combining10%methanolwith90%gasolinecancauseanoctanenumberupto130.Any additionalpercentageofmethanoltofueladvancestheperformanceofinternalcombustion engines(ICE)andreducesthepollutantssuchashydrocarbons,NOx,SOx,and particulates [23].
Methanol,asthesecondmostcommonsynthesisgasproductafterhydrogen,canbeconsumed asasolvent,chemicalfuel,aswellasbeingappliedasarawmaterialindifferentindustrieslike formaldehyde,methyltert-butylether(MTBE),aceticacid,dimethylether(DME),methyl,
Fig.4
Table3CharacteristicsofH2 membranes [20]. Dense ceramic membrane Ceramic membraneMicroporousmembraneMetallicmembrane
Materials Silica, perovskite MixedofPd withdense ceramic Zeolites,silicaonceramicPd,Pd-Ag,orPd-Cuon ceramicorstainlesssteel
Operating tempeature (°C)
H2 flux (ft3H2/ ft2membrane × 100psi)
Chemical stability HighMedium tolerantto feedstream impurities
Hightoleranttosulfur, carbonatesathigh temperaturesareformedby thereactionofsome membraneswithCO2
Low,reductioninH2 flux athightemperatures,Pd alloyismoreefiicient
Low,COandsulfurare twomajorpoisonsofPd, sidereactionsand defectionsareoccurred byalloys
Cost LowMediumLowHigh(forpurePd)to medium(foralloys)
vinylacetates,methylmethacrylate(MMA),methylamines,melamineresin,ethylene,and propyleneproduction.Asmethanolhasanimportanteffectonenergy-relatedconversionand therateofenergyrequirementhasbeenincreased,thedevelopmentofmethanol-synthesizing industrieswithlowercostsisanurgentnecessityinthefuture.
Methanolsynthesisprocedurecanbesummarizedinthreestepsasfollows,whichdetailsare illustratedin Fig.5:
• Synthesisgasproduction
• Methanolsynthesis
• Productseparation/purification
Aspreviouslymentioned,synthesisgascanbeobtainedbyNGorliquidhydrocarbons reformingorcoalorbiomassgasification.Themostwidelyusedrouteforsyngasproductionis steamreformingofNG,commonlyreferredtomethanesteamreforming(MSR),bywhich morethan75%ofsyngasareproduced [29].Inthefollowing,methanolsynthesisplantsare discussedinmoredetail.
Fig.5
Stepsintheconventionalmethanolproductionprocess [28]
2.2.1Methanolsynthesisplant
Methanolsynthesisisoriginallyahigh-pressureprocess,initiallyworkedundertemperature 320–450°Candpressure250–350bar,whichwasfirstdevelopedin1923 [30].Intheprocess, ZnO/Cr2O3 catalystswerewidelyusedduetotheiruniquecharacteristics,includinghigh temperatureandmechanicalresistance.Thehigh-operatingpressurebalancesoutthe decrementinmoles.Ashigh-pressureprocessesrequirehighinvestmentinoperatingcostsand factorydesign,low-pressureprocessesreceivedmuchattention.Thelow-pressureprocess worksattemperatureof200–300°Candpressureof50–100bar.Despitetheadvantagesof workingatequilibriumconditionswhichoperateatlowtemperatures,theactivityofthe catalystsdecreasesintensively.Byincreasingthereactiontemperature,thecatalystactivityis enhanced,resultinginacatalystdeactivationbycokedeposition [31].Therefore,the optimizationofreactiontemperatureandalsoitspressureisanimportantissueinthisprocess. Thelow-pressureprocesswastheonlyprocessemployedinthemarketby1999.Accordingto thistechnologyemployment,syngasshouldbewashed,compressed,andheatedbeforebeing usedasarawmaterialofthemethanolsynthesisprocess.Thisfreshfeedismixedwithrecycled unreactedsyngasandsenttoamethanolconverterwherethefollowingreactionsaretaken place [32]:
COreduction(Eq. 1)isanexothermalprocesslimitedbyequilibriumandfavoredatlow temperaturesinthegasphase.Toachieveareasonableindustrialconversionrate,theuseofan appropriatecatalystandoperatingconditionisessential [33].Theappliedcatalystinthe low-pressuremethanolsynthesisprocessisbasedoncopperoxide,zincoxide,andalumina (CuO/ZnO/Al2O3).Theselectivityofthiscatalysttowardmethanolisquitehigh.However,the selectivityperpassconversionislowforcommercialplants,necessitatingproductrecycling. Fig.6 showsatypicalmethanolsynthesissection.
2.2.2Separationandpurificationsection
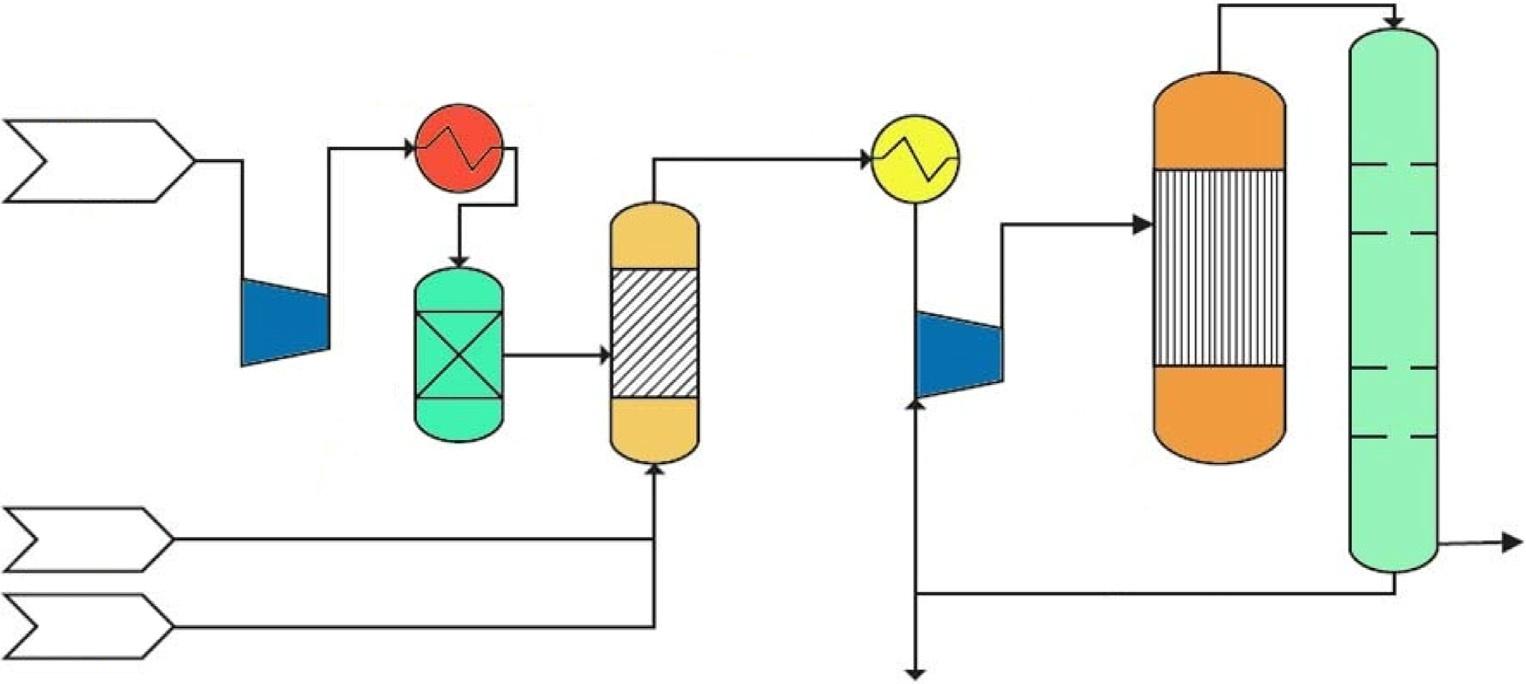
Theproducedmethanolisnotpure,andtheimpurities,includingdissolvedgases,hydrogen, andwater,mustberemovedbeforetheproducedmethanolisapplied.However,theamountof lateralcurrentpresentinthesynthesizedmethanolislowundernormaloperatingconditions. Therefore,severaldistillationcolumnsareneededtoseparatelow-boilingimpuritiesfrom methanol.Thephysicallydissolvedgasesareflashedoffinaflashvessel [34].Aftertheflash vesselisdistilledinatwo-stagesystem,thestabilizedmethanolisfirstunderpressureand secondatatmosphericpressuretoobtainaspecificpurification.Afterward,thehigherboiling pointcomponentsareremovedinadistillationcolumn.Theprocessflowsheetofsucha methanolpurificationplantisshownin Fig.7.
Steam/Reformed
Gas Reboiler
Steam/Reformed
Gas Reboiler Crude Methanol
Atwo-stagemethanoldistillationprocessflowdiagram [35].
2.2.3Methanoltogasoline
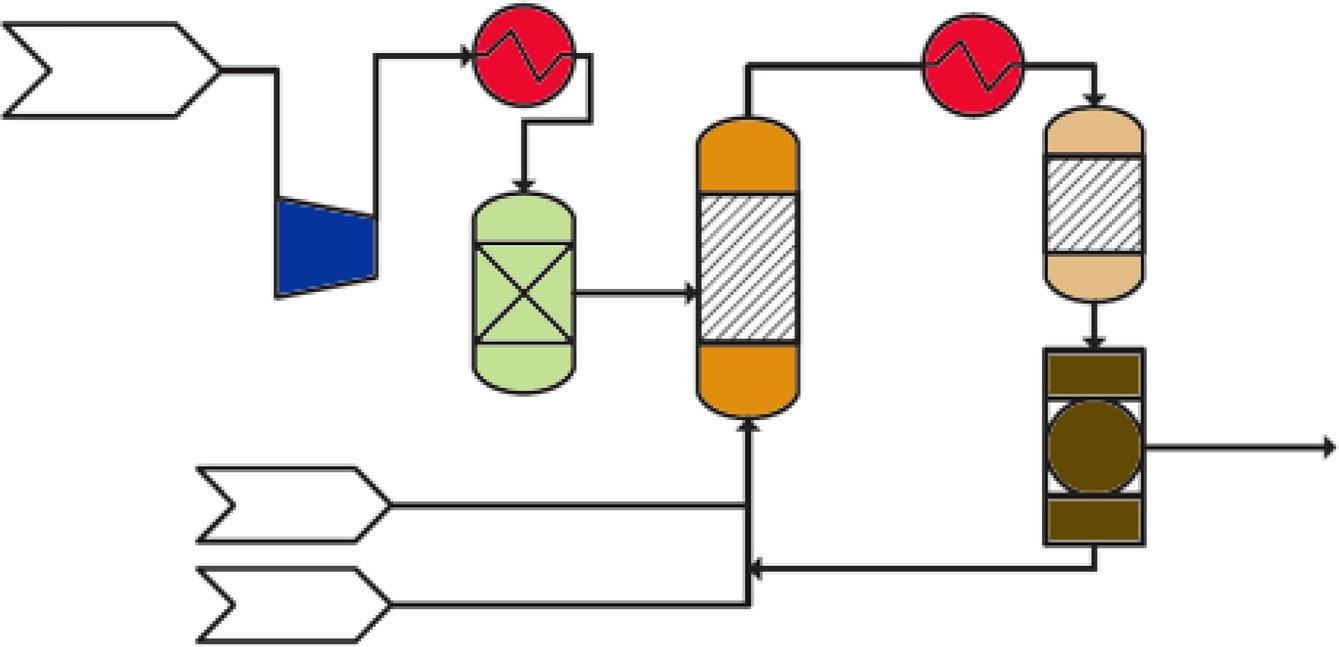
Althoughmethanolcanbeusedasatransportationfuel,currentneeds(withoutengine modifications)dictateconvertingthemethanoltoimmediatelyusablegasoline [36].The conversionofmethanoltogasoline(MTG)isbasedonappropriatecatalysts,including zeolite-basedones.Thetechnologyofproducinggasolinefromcoalviamethanolinterstage productioninvolvescoalgasificationbythereactionofoxygenandsteamunderpressurein whichthecompositionofthesynthesisgasproducedisadjustedbyashiftreaction(which convertscarbonmonoxideandsteamtohydrogenandcarbondioxide)(Fig.8).Afterward,the producedsyngasispurifiedandthenconvertedtomethanol.Thecrudeintermediatemethanol, whichcontainsabout15%water,isthenfedtotheM-Gasolineunitandconvertedtothe gasolineend-productinatwo-stagefixed-bedconversionprocess.
Inordertoconvertmethanoldirectlytohigh-octanegasoline,theMobilMTGprocesshasbeen developedinwhichbothfluidized-bedandfixed-bedreactorshavebeenused(Fig.9).Inthis process,methanolisfirstdewateredtoproducedimethylether,andthenfurtherdewatering withaZSM-5catalystendstoaseriesofC5+ hydrocarbonproduction.Zeolitecatalystshavean importantroleincontrollingthedistributionofhydrocarbonproductsduetotheirspecific geometryandsizepores.Therefore,moleculeswithlargersizesthanzeoliteporescannotbe madeinthisprocess.Aftervaporization,themethanolfeedstockisfedtothefirstconverterfor
Fig.7
Senthesis
Fig.8 M-Gasolineprocess [37].
Fig.9 TheMobilMTGprocess [36].
convertingtodimethylether,H2O,andunreactedmethanolmixtureatoperatingconditionsof 300psiand300°C.ThisreactioncanbeseeninEq. (4)[37]
Afterthat,thefirst-stageproductismixedwitharecycledstreamandsenttoasecond-stage converter.Whilethetemperatureattheentranceisaround345°C,theoutflowtemperatureis around400°C,whichisfilledwithacatalystforgasolinecomponentsproduction.Severalchain stepsoccurherefractionationofarawproduct,alkylationoflightends,hydrationofa recovered,andblendingproductsforproducinggasoline. Fig.9 illustratesthemethanolto gasolineprocessinwhichvaporizedmethanolfirstmovesupwardfromacatalyticbedata pressureof25psiandatemperatureof415°C,andthenconvertedtowaterandhydrocarbons. Afterthereaction,thecatalystisremovedfromtheproductsatthetopofthereactor,thenthe overheadproductiscondensedandseparatedfromwaterandsmallquantitiesofcoke(Fig.10). Thecatalyst’sselectivityisanessentialfactorintheM-gasolineprocessforhavinghigh-octane gasoline.ThisselectivityishigherthanthatofthetraditionalFTcatalystsastheselectivityof Mobilcatalystsisabout85%,whereasFTcatalystshavea50%selectivity [37].
Methanol + ether (0.2)
Gasoline(CS+)(60)