ADVANCESIN PARASITOLOGY
Editedby
DAVIDROLLINSON
LifeSciencesDepartment
TheNaturalHistoryMuseum, London,UnitedKingdom
RUSSELLSTOTHARD
DepartmentofTropical DiseaseBiology
LiverpoolSchoolofTropical Medicine,Liverpool,UnitedKingdom
AcademicPressisanimprintofElsevier 125LondonWall,LondonEC2Y5AS,UnitedKingdom TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates 525BStreet,Suite1650,SanDiego,CA92101,UnitedStates
Firstedition2022
Copyright©2022ElsevierLtd.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronic ormechanical,includingphotocopying,recording,oranyinformationstorageandretrievalsystem, withoutpermissioninwritingfromthepublisher.Detailsonhowtoseekpermission,further informationaboutthePublisher’spermissionspoliciesandourarrangementswithorganizationssuch astheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythe Publisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperience broadenourunderstanding,changesinresearchmethods,professionalpractices,ormedical treatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluating andusinganyinformation,methods,compounds,orexperimentsdescribedherein.Inusingsuch informationormethodstheyshouldbemindfuloftheirownsafetyandthesafetyofothers,including partiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assume anyliabilityforanyinjuryand/ordamagetopersonsorpropertyasamatterofproductsliability, negligenceorotherwise,orfromanyuseoroperationofanymethods,products,instructions,orideas containedinthematerialherein.
ISBN:978-0-323-98949-7
ISSN:0065-308X
ForinformationonallAcademicPresspublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: ZoeKruze
AcquisitionsEditor: LeticiaLima
DevelopmentalEditor: CindyAngelitaGardose
ProductionProjectManager: AbdullaSait
CoverDesigner: ChristianBilbow
TypesetbySTRAIVE,India
1.Themicroscopicfiveofthebigfive:Managingzoonoticdiseases withinandbeyondAfricanwildlifeprotectedareas1
AnyaV.Tober,DannyGovender,Isa-RitaM.Russo,andJoCable
1. Introduction2
2. The ‘MicroscopicFive’ 4
3. ChallengesofBTbcontrolatthewildlife-livestockinterface:TheSouth Africancasestudy10
4. Driversofdisease:TheKrugerNationalParkcasestudy13
5. DiseaseknowledgegapsandlessonslearntfromAfricanprotectedareas28
6. Communitiesandconservation33
7. Conclusions35 Acknowledgements37 References37
2.Improvingtranslationalpowerinantischistosomaldrug discovery47
AlexandraProbst,StefanBiendl,andJenniferKeiser
1. Fillingthedrugpipelineforschistosomiasis48
2. Evaluatingtheimportanceof S.mansoni isolateoriginforearly antischistosomaldrugdiscovery49
3. The S.mansoni mousemodelfordrugefficacytesting51
4. Infectionintensityofthepatent S.mansoni mousemodel54
5. Pharmacokinetic/pharmacodynamic(PK/PD)relationshipofselecteddrugs56
6. Concludingremarks68
Acknowledgementsandfunding69 References69
3.Uniquethiolmetabolismintrypanosomatids:Redox homeostasisanddrugresistance75
VahabAli,SachidanandaBehera,AfreenNawaz,AsifEqubal, andKrishnaPandey
1. Introduction77
2. Trypanothionemetabolism80
3. Effectorproteinsoftheantioxidantdefence:Oldandnewactors90
4. Trypanothionemetabolismislinkedtocysteine,polyamine, andpentosephosphatepathway103
5. Theroleofredoxactivecompoundsandtheirmechanism inparasitessurvival110
6. Roleofthiolmetabolismindrugresistance118
7. Anti-parasiticpotentialofmoleculestargetedagainstredoxmetabolism123
8. Unsolvedquestionsandfutureprospects131
Acknowledgements132 References132
Contributors
VahabAli
LaboratoryofMolecularBiochemistryandCellBiology,DepartmentofBiochemistry, ICMR-RajendraMemorialResearchInstituteofMedicalSciences(RMRIMS),Patna, Bihar,India
SachidanandaBehera
LaboratoryofMolecularBiochemistryandCellBiology,DepartmentofBiochemistry, ICMR-RajendraMemorialResearchInstituteofMedicalSciences(RMRIMS),Patna, Bihar,India
StefanBiendl
SwissTropicalandPublicHealthInstitute,DepartmentofMedicalParasitologyand InfectionBiology;UniversityofBasel,Basel,Switzerland
JoCable
SchoolofBiosciences,CardiffUniversity,Cardiff,Wales,UnitedKingdom
AsifEqubal
LaboratoryofMolecularBiochemistryandCellBiology,DepartmentofBiochemistry, ICMR-RajendraMemorialResearchInstituteofMedicalSciences(RMRIMS),Patna; DepartmentofBotany,ArariaCollege,PurneaUniversity,Purnia,Bihar,India
DannyGovender
SANParks,ScientificServices,SavannaandGrasslandResearchUnit,Pretoria;Department ofParaclinicalSciences,UniversityofPretoria,Onderstepoort,SouthAfrica
JenniferKeiser
SwissTropicalandPublicHealthInstitute,DepartmentofMedicalParasitologyand InfectionBiology;UniversityofBasel,Basel,Switzerland
AfreenNawaz
LaboratoryofMolecularBiochemistryandCellBiology,DepartmentofBiochemistry, ICMR-RajendraMemorialResearchInstituteofMedicalSciences(RMRIMS),Patna, Bihar,India
KrishnaPandey
DepartmentofClinicalMedicine,ICMR-RajendraMemorialResearchInstituteofMedical Sciences(RMRIMS),Patna,Bihar,India
AlexandraProbst
SwissTropicalandPublicHealthInstitute,DepartmentofMedicalParasitologyand InfectionBiology;UniversityofBasel,Basel,Switzerland
Isa-RitaM.Russo
SchoolofBiosciences,CardiffUniversity,Cardiff,Wales,UnitedKingdom
AnyaV.Tober
SchoolofBiosciences,CardiffUniversity,Cardiff,Wales,UnitedKingdom
Themicroscopicfiveofthebig five:Managingzoonoticdiseases withinandbeyondAfricanwildlife protectedareas
AnyaV.Tobera,∗,DannyGovenderb,c,Isa-RitaM.Russoa,† , andJoCablea,†
aSchoolofBiosciences,CardiffUniversity,Cardiff,Wales,UnitedKingdom bSANParks,ScientificServices,SavannaandGrasslandResearchUnit,Pretoria,SouthAfrica cDepartmentofParaclinicalSciences,UniversityofPretoria,Onderstepoort,SouthAfrica
∗Correspondingauthor:e-mailaddress:tobera@cardiff.ac.uk
Contents
1. Introduction2
2. The ‘MicroscopicFive’ 4
2.1 Bovinetuberculosis7
2.2 RiftValleyfever7
2.3 Brucellosis8
2.4 Cryptosporidiosis8
2.5 Schistosomiasis9
3. ChallengesofBTbcontrolatthewildlife-livestockinterface:TheSouthAfrican casestudy10
3.1 Controlinlivestock10
3.2 Controlinwildlife10
4. Driversofdisease:TheKrugerNationalParkcasestudy13
4.1 Pastandpresentdiseasemanagement13
4.2 KrugerNationalPark’scurrentadaptivemanagementapproach14
4.3 Environmentaldriversofdiseasetransmission15
4.4 Anthropogenicdriversofdiseasetransmission:Wildlife-livestock-human interface23
5. DiseaseknowledgegapsandlessonslearntfromAfricanprotectedareas28
6. Communitiesandconservation33
7. Conclusions35 Acknowledgements37 References37 † Authorscontributedequallytothiswork.
AdvancesinParasitology,Volume117Copyright # 2022ElsevierLtd ISSN0065-308XAllrightsreserved. https://doi.org/10.1016/bs.apar.2022.05.001
Abstract
Africanprotectedareasstrivetoconservethecontinent’ sgreatbiodiversitywitha targetedfocusontheflagship ‘ BigFive’ megafauna.Thoughoftennotconsidered, thisbiodiversityprotectionalsoextendst othelesser-knownmicrobesandparasites thataremaintainedinthesediverseecosyst ems,ofteninasilentandendemicallystablestate.Climateandanthropogenicchange,andassociateddiversityloss,however, arealteringthesedynamicsleadingtoshiftsinecologicalinteractionsandpathogen spilloverintonewnichesandhosts.AsmanyAfricanprotectedareasareborderedby gameandlivestockfarms,aswellasvillages,theyprovideanidealstudysystemto assessinfectiondynamicsatthehuman-live stock-wildlifeinterface.Herewereview fivezoonotic,multi-hostdiseases(bovinetuberculosis,brucellosis,RiftValleyfever, schistosomiasisandcryptosporidiosis) the ‘ MicroscopicFive’— anddiscussthe bioticandabioticdriversofparasitetransmissionusingtheiconicKrugerNational Park,SouthAfrica,asacasestudy.Weidentifyknowledgegapsregardingtheimpact ofthe ‘ MicroscopicFive ’ onwildlifewithinparksandhighlighttheneedformore empiricaldata,particularly forneglected(schistosomiasis)andnewlyemerging(cryptosporidiosis)diseases,aswellaszoonoticdiseaseriskfromtherisingbushmeat tradeandgamefarmindustry.Asprotectedareasstrivetobecomefurtherembedded inthesocio-economicsystemsthatsurroundthem,providingbenefitstolocalcommunities,OneHealthapproachescanhelpmaintaintheecologicalintegrityofecosystems,whileprotectinglocalcommunitiesandeconomiesfromthenegative impactsofdisease.
1.Introduction
Asweenterthesixthmassextinction,protectingtheworld’sbiodiversityhasneverbeenmorecritical.Protectedareas,includingnational parks,coverover18.8millionkm2 andareattheforefrontofaglobaleffort tosafeguardbiodiversity(Chapeetal.,2003).Managersoftheseprotected areasmuststrikeabalancebetweenprotectingtheecologicalintegrityof ecosystemsandpreventingexploitationoflocalresourceswhilepromoting theiruseineducationandrecreation(Chapeetal.,2003).Ifmanaged correctly,protectedareascanbebeneficialtowildlifeconservationand thecountry’seconomythroughpromotingecotourismandcreatinglocal employmentopportunities(Cheung,2012; Spiesetal.,2018).However, themanagementofprotectedareasischallenging,particularlyinthe Anthropoceneeraofhumanmediatedglobalchange,andincreased emergenceandre-emergenceofinfectiousdiseases(reviewedby Cable etal.,2017).Thesediseasescanreducefitness,alterwildlifepopulation
structure/sizeandevenalterecosystemfunction(Holdoetal.,2009; Prins andWeyerhaeuser,1987; Scott,1988).Therefore,toeffectivelymanage wildlifepopulationsandecosystems,itisessentialtounderstandthethreats posedbypathogensandthediseasestheycause.
Ofthe3881terrestrialandmarinenationalparksintheworld,almosthalf areinsub-SaharanAfrica,withterrestrialparksherecovering1million km2 (4%ofthetotallandarea; Chapeetal.,2003; Muhumuzaand Balkwill,2013).TheseparksaimtoconserveAfrica’suniqueandiconicecosystemsrangingfromopensavannasandgrasslandstodenseforest.Thisvarietyofhabitatssupportshighlevelsofbiodiversity,drawingnumerous touristswhoaspiretospotthe‘BigFive’megafauna:Africanbuffalo(hereafterreferredtoasbuffalo),lion,Africanelephant(hereafterreferredtoas elephant),rhinocerosandleopard(DubeandNhamo,2019).However, hiddenandoftenforgottenbiodiversitywithinprotectedareasincludes pathogens,whichmodulateanimalabundance,fitnessandbehaviour (Go ´ mezandNichols,2013).Itiscrucialtobetterunderstanddriversforpast andcurrentwildlifediseaseoutbreakswithinprotectedareas,tofindnew approachestopredictandpreventfutureoutbreaks.Areviewofallinfectiouswildlifediseaseswithinprotectedareaswouldbetoolargeatask. Instead,wefocusonfivediseasesreferredtohereasthe‘Microscopic Five’,whichareimportantatthehuman-livestock-wildlifeinterfacedue totheirbroadhostrangeandzoonoticpotential.Theseinterfacediseases wouldallbenefitfroma‘OneHealth’approachtomanagement(Fawzy andHelmy,2019; Innesetal.,2020; Websteretal.,2016).Wetherefore purposefullyincludedhighprofilediseases(bovinetuberculosis(BTb), RiftValleyfeverandbrucellosis)aswellasneglecteddiseases(cryptosporidiosisandschistosomiasis)forstudy.UsingKrugerNationalPark,oneofthe mostresearchedparksinAfrica(vanWilgenetal.,2016),wewillreviewthe keyfactorsthatcaninfluenceoutbreaksandtransmissionofthe‘Microscopic Five’withinandaroundprotectedareas(Fig.1).Byfocusingonaselect groupofpathogenswithinaspecificparkourintentionistohighlightdrivers ofdiseasecommonamongmanyprotectedareasandtheimportanceof consideringallinfectiousdiseasesinwildlifemanagementplans.Wewillfirst giveabriefintroductiontothe‘MicroscopicFive’andthengiveexamplesof theenvironmentalandanthropogenicfactorsdrivingthedynamicsofthese diseaseswithinandaroundKrugerNationalPark.Wewillthendiscussthe keyknowledgegapsandfuturechallengesformanagingthe‘Microscopic Five’andotherimportantdiseasesandtouchondifferentmanagement approachesfollowedinvariousparks.
Co-infections
Reservoir hosts
Mixing at waterholes
Fig.1 The ‘BigFive’ and ‘MicroscopicFive’,andthedriversofdiseaseatthewildlifelivestock-humaninterface.Arrowsrepresentanthropogenicdriversfrombeyond KrugerNationalPark. CreatedwithMicrosoftPowerPoint(version2109)andAdobe Photoshop(2021).
2.The ‘MicroscopicFive’
The‘BigFive’areundoubtedlyoneofthebiggestattractionsfortouristsvisitingSouthAfrica’sprotectedareas(DubeandNhamo,2019).To conservetheseandotherwildlife,andtoreducetransmissionofinfectious diseasesamongwildlife,domesticanimalsandhumans,wefocusonthe lesserknown‘MicroscopicFive’.Thesecomprisezoonoticdiseasescaused bypathogensthathavemultiplehosts,includinghumans,andareofparticularimportanceatthehuman-livestock-wildlifeinterface.Althoughwe focusonfivespecificdiseases,therearemanymoreofimportancewithin protectedareas(Table1)butbyhighlightingadistinctfewweaimtoraise theprofileofallinfectiousdiseasesandpossibledrivers.Thefirstthreeofthe ‘MicroscopicFive’(bovinetuberculosis,brucellosisandRiftValleyfever) arehighprofileorstate-controlleddiseasesinSouthAfricaandanyoutbreaksmustbereportedtotheWorldOrganisationofAnimalHealth (OIE).Allthreeofthesediseasesaretrade-sensitivediseasesandmaychange thetradingstatusofacountryanditsabilitytotradeontheglobalmarket. Theremainingtwo(schistosomiasisandcryptosporidiosis)areneglectedin
Microscopic Five
Big Five
trade
Table1 SomediseasesoflargeherbivoreswithinKrugerNationalParkwhichmayposeathreattolivestockand/orhumans.
Disease.Pathogen
Bacteria
Transmission modes Transmission routesDrivers
Spatial distributionin KNP Known susceptible hostsReservoirhosts
Anthrax Bacillusanthracis Vector, environmental, directcontact, fomites Ingestionof contaminated vegetationor carcasses Calciumsoil content,drought Northernand centralregions Most mammals including humans Maintained inenvironment
BovineTb Micobacteriabovis AerosolRespiratorytractWildlife/livestock interface,host density South,central, movingnorth Cattle, buffalo, humans Capebuffalo
Virus
Footandmouth Aphtovirus Aerosol, fomites RespiratorytractWildlife/livestock interface,host density North,central, south Cloven hooved animals Capebuffalo
Africanhorse sickness Orbivirus Mosquito vector,direct contact Cutaneous penetration Introducedhorses, season CentralZebra, domestic horses Culicoides mosquito, possiblyzebra
RiftValleyfever Phlebovirus Mosquito vector,direct contact Cutaneous penetration Climatechange, drought,rainfall Higherin southand centralregions Cattle, buffalo Aedes mosquito Capebuffalo
Continued
Table1 SomediseasesoflargeherbivoreswithinKrugerNationalParkwhichmayposeathreattolivestockand/orhumans.—cont’d
Disease.Pathogen
Protozoa
Transmission modes Transmission routesDrivers Spatial distributionin KNP Known susceptible hostsReservoirhosts
Bovine brucellosis Brucellaabortus DirectcontactIngestionof infected dischargesduring birth,milk,mucus membranes
Cryptosporidiosis Cryptosporidium spp.
HostdensityNorth,central, south Cattle, buffalo,wild animals, humans Capebuffalo
EnvironmentalFaecal-oralvia contaminationof foodandwater Dependanton speciesandhost range UnknownWild animals, humans, domestic animals Unknown
Piroplasma
Corridordisease Theileriaparva TickvectorCutaneous penetration Wildlife/livestock interface North,central, south CattleCapeBuffalo
Babesiosis Babesiaspp. TickvectorCutaneous penetration
Digenea
Fascioliasis Fasciola spp.EnvironmentalContactwith infectedwater Dependanton speciesandhost range
Schistosomiasis Schistsoma spp.EnvironmentalContactwith infectedwater Dependanton speciesandhost range
UnknownRhinoceros, lions Unknown
UnknownRuminantsUnknown
UnknownWild animals, humans Unknown
comparison,particularlyinwildlife.Byincludingtheseinthe‘Microscopic Five’,weaimtobringgreaterattentiontooverlookedyethighlyimportant diseases(see WHO,2020).Inthefollowingaccount,webrieflycovereach ofthe‘MicroscopicFive’discussingtheirhostspecificity,transmission pathwaysandknownimpactsonwildlife,livestockandhumans.
2.1Bovinetuberculosis
Bovinetuberculosis(BTb)iscausedbythebacterium Mycobacteriumbovis andpredominantlyinfectsbovines,suchasAfricanbuffalo(Synceruscaffer caffer)andcattle(Bostaurus),yetmostwarm-bloodedanimalsincluding humanscanbeinfected(Ayeleetal.,2004).Transmissionmainlyoccurs throughinhalationofinfectiousparticles,whichisparticularlyproblematic whenlivestockarekeptathighdensities(Ayeleetal.,2004).Though thoughttohavespilledoverfromcattletobuffalointheearly1960sin SouthAfrica(Bengisetal.,1996),buffalonowserveastheprimarymaintenancehostforBTbwithinKrugerNationalParkandHluhluwe-iMfolozi Park,spillingoverintovariousspeciesofwildlifeandlivestock(Micheletal., 2006).AlthoughBTbisacontrolleddiseasewithinSouthAfrica,itscontrol isbecomingincreasinglychallengingduetothepresenceofwildlifereservoirs,difficultyincontrollingdiseaseincommunalherdsandlackofpracticalcontroloptionsinwildlife(see Section3).TheWHOestimated 147,000newcasesofzoonoticTbinhumansin2016with12,500deaths globallybutmostlyinAfrica(Sichewoetal.,2019b).Humanscanbecome infectedthroughdrinkingunpasteurisedmilk,eatingundercookedmeatand viaaerosolsinhaledfrominfectedcattle(DAFF,2016; Sichewo etal.,2019a).
2.2RiftValleyfever
RiftValleyfever(RVF)iscausedbyazoonotic,vectorborneviruspredominantlyspreadby Aedes mosquitoes(Clarketal.,2018).Theviruswasfirst reportedinSouthAfricain1950andsubsequentoutbreakshaveoccurred sporadicallyevery7–11yearsinfectingmainlydomesticlivestockbutalso arangeofwildmammalsandhumans(Beechleretal.,2015a; Metras etal.,2015).Humaninfectionoccursmainlythroughdirectcontactwith bloodortissuefrominfectedanimalsorthroughconsumingunpasteurised milkbutcanalsoresultfromaninfectedmosquitobite.Symptomsvaryfrom mild,flu-liketoseverehaemorrhagicfeverthatcanbefatal(Clarketal., 2018).Over4000humancasesandaround1000deathshavebeenreported
inthelast20years,predominantlyinAfricaandSaudiArabia(Petrovaetal., 2020).Littleisknownabouthowthepathogenismaintainedduring inter-epidemicperiods.Onesuggestionisverticaltransmissionfrommosquitoestotheirova,whichhasbeendemonstratedwith Aedes mosquitoes underlaboratory-controlledconditions(Romoseretal.,2011).Another possibilityisthatitismaintainedinwildanimalpopulations(Beechler etal.,2015a;see Section4.3.4).Commercialvaccinesareavailableforlivestockbutthereiscurrentlynolicensedhumanvaccine(Petrovaetal.,2020).
2.3Brucellosis
Brucellosis,causedbybacteriaofthe Brucella genus,isrankedamongthe mosteconomicallyimportantzoonoticdiseasesglobally.Althoughitisan OIEnotifiabledisease,outbreaksarethoughttobegreatlyunder-reported inAfrica(McDermottetal.,2013).Thespeciesofmedicalandveterinary importanceare Brucellaabortus, Brucellamelitensis and B.suis (see Ducrotoy etal.,2017).Infectioninhumanscanleadtoadebilitatingillnessknown as‘Mediterranean’or‘undulant’feverandiscommonlymisdiagnosedas malaria(Ducrotoyetal.,2017; Godfroidetal.,2011).Humaninfection occursthroughdirectcontactwithorconsumptionofaninfectedanimal. Consumptionofun-pasteurisedmilkcausesmosthumaninfections,while humantohumantransmissionisrare(Godfroid,2018).SeveralwildlifespecieshavebeenreportedasseropositiveforthisdiseaseandAfricanbuffaloare thoughttobeareservoirfor B.abortus (see Godfroidetal.,2013).Infection cancauseabortionsinlivestockreducingfarmproductivity,howeverthe effectsofthediseaseonwildlifearelargelyunknownandmaydifferbetween species(Gorsichetal.,2015).Vaccinesareavailableforlivestockandsmall ruminantsbutnotyetforhumans(Ducrotoyetal.,2017).
2.4Cryptosporidiosis
Cryptosporidiosis,causedbyseveralspeciesoftheprotozoan Cryptosporidium genus, canleadtoseverediarrhoeainhumansandanimals globally.Infectiousdiarrhoeaisamajorcauseofdeathinchildrenunderfive inAfricaand Cryptosporidium issecondonlytorotavirusasacontributorto thisdisease(Kotloffetal.,2013; SquireandRyan,2017).Transmission occursthroughthefaecaloralrouteviaclosecontactwithinfectedhumans, animalsorcontaminatedfoodandwater(Innesetal.,2020).Currentlythere areatleast40recognisedspecieswithvaryinghostspecificitiesbutthemost importanttwospeciesinfectinghumansandlivestockare C.hominus and
C.parvum. Thelatteristhepredominantcauseofdiarrhoeainyoungcalves andisthemostimportantzoonoticspecies. Cryptosporidiumparvum ismore geneticallydiversethan C.hominus withseveralsubtypeswithdifferinghost specificities,thereforeanintegratedgenotypingapproachhasbeenadvocatedtodifferentiatethesesubtypes(Innesetal.,2020). Cryptosporidium specieshavebeenidentifiedinarangeofwildlife,yetmoststudiesfocus onhumansandlivestock(Zahedietal.,2016). C.parvum,C.ubiquitum andC.bovis wererecentlyidentifiedinwildlifewithinKrugerNational Parkinelephant(Loxodontaafricana),buffalo(Synceruscaffer)andimpala (Aepycerosmelampus;see Samraetal.,2011).Oocystsof Cryptosporidium spp.havealsobeendetectedinzebra(Equuszebra),buffaloandwildebeest (Connochaetesgnou)faecesinMikumiNationalPark,Tanzania(Mtambo etal.,1997).Thereiscurrentlynoavailablevaccineforcryptosporidiosis yetthereispotentialtodeveloponeforcattle(Innesetal.,2020).
2.5Schistosomiasis
Schistosomiasisisawaterborne,zoonoticdiseaseofveterinaryandmedical importance,causedbydigeneanparasitesofthegenus Schistosoma. Schistosomiasisisamajorpublichealththreatwithanestimated207million peopleinfectedand779millionpeopleatriskglobally,with90%ofthese infectionsinAfrica(Steinmannetal.,2006).Likealldigeneans,schistosomes haveanindirectlifecycle.Theyrequireanintermediatefreshwatersnailhost withinwhichtheyreproduceasexuallyultimatelyproducingcercariae, whicharefree-swimminglarvalstagesthatsubsequentlyinfectadefinitive mammalianhost(Cribbetal.,2003).Definitiveanimalorhumanhostscan becomeinfectedwithschistosomiasisbyenteringinfestedwaters—the water-bornelarvaeburrowthroughtheskinofthenewhost(Cribb etal.,2003).Thereareatleast12knownschistosomespeciesinAfricaof which5areknowntoinfecthumans(S.haematobium,Schistosoma mansoni,S.intercalatum,S.guineensis and S.mattheei). Schistosomamattheei isofnoteasalthoughpredominantlyaparasiteofcattle,ithasalsobeen foundinwildlifeandhumanswhereitisknowntohybridisewith S.haematobium (see Pitchford,1961).Theotherspeciesinfectawiderange ofdomesticandwildanimalsincludingcattle,horses,buffalo,baboons, zebra,hippopotamusandrodents(Standleyetal.,2012).Traditionally,malacologicalmonitoringprogrammeshaveonlytargetedsnailspeciesknown toharbourhumaninfectingschistosomes,butawiderapproachisclearly neededaswebecomeawareofwiderhostranges(Pennanceetal.,2021)
thatarelikelytoshiftwithincreasingenvironmentalstressors.Thereis currentlynovaccineforschistosomiasisandthemaincontrolstrategyfor humansispreventativechemotherapy,improvedwater,sanitationand hygieneandsnailcontrol(WHO,2022).
3.ChallengesofBTbcontrolatthewildlife-livestock
interface:TheSouthAfricancasestudy
SouthAfricahasbeenchallengedwiththecontrolofBTbsincethe diseasewasfirstreportedinthecountryin1880,initiallyfocusingonlivestock,andnowincludingcontrolinwildlife(DAFF,2016).
3.1Controlinlivestock
EarlyBTbsurveillanceincludedtheintroductionoftuberculinskintesting incattlein1905,followedbyitsdeclarationasanotifiablediseasein1911 andtheinitiationoftheDivisionofVeterinaryServicesBTbschemein1969 (DAFF,2016; Micheletal.,2019).Thisschemefocusedoncompulsory testingofcommercialcattleherdssuspectedtobeinfected,withslaughter ofpositiveindividuals,quarantineanddisinfectionoffarms.Initially,great progresswasmade,reducingprevalenceto0.04%by1991(1.1millioncattle tested);however,thenumberoftestshavesincedeclinedduetobudgetcuts andadecreasedworkforce(DAFF,2016; Micheletal.,2019).Currentprevalenceincommunallivestockisvariable(<0.5%to >15%)(Musokeetal., 2015; Sichewoetal.,2019b).
In2021thenationalcattleherdwasestimatedat12million,consistingof commercialdairyherds(20%)andbeefanddual-purposeherds(80%) (DAFF,2021).Testingofcattleisnolongercompulsoryandcurrentcontrol ofBTbisguidedbytheInterimBTbManualfromtheDepartmentof Agriculture,ForestryandFisheries(DAFF),SouthAfrica,whichproposes theuseoffourtestingprogrammes(Table2; DAFF,2016).Allprogrammes arevoluntaryapartfromtheinfectedherdprogram,whichcanbeenforced bytheAnimalDiseasesAct,1984(ActNo.35of1984)(DAFF,2016).The approvedtestisthecervicalintradermaltuberculin(CIT)test(DAFF,2016).
3.2Controlinwildlife
ThecontrolofBTbinwildlifeisbecomingincreasinglyimportantasmany farmsswitchfromlivestocktogamefarming,andwildbuffaloreservoirs hindercontroleffortsincattle(Micheletal.,2019).BovineTbhasbeen
Table2 FourlevelsofBovineTbsurveillanceprogrammesinSouthAfrica.
Surveillanceherd programme
Maintenanceherd programme
Oneoffsurveyusedbystateofficialstodeterminethe prevalenceofBTbwithinanareaorbyastockowner conductingaself-assessment
Tojointhisprogramme,herdsarerequiredto undergotwoconsecutivetestswith100%negative resultsatleast3monthsapart.TheseBTbfreeherds arethentestedevery2years.Ifanindividualtests positive,thentheentireherdismovedtotheinfected herdprogramme
InfectedherdprogrammeCompulsoryprogrammeforherdsthathavetested positivewiththeCITtest,aswellasthosedetected frommeatandmilkinspection,post-mortemsor clinicalcases.Theseherdsareplacedunder quarantineandkeptundersupervisionofastate veterinarian,whowillordertheslaughterofinfected animals.Therestoftheherdistestedevery3months andisonlyletoutofquarantineoncetheherdhas undergonetwoconsecutivenegativetests
Diagnostictesting programme(individuals)
Individualcattledestinedtobeimportedorexported. Importedcattlearekeptinquarantineandmust undergoacompulsoryCITtest.Beforeexport,cattle mustalsoreceiveacomparativeCITtest—a requirementformanyimportingcountries
identifiedin21differentwildlifespeciesinSouthAfrica,includingmost recentlygiraffe(Hlokweetal.,2019).Thecurrentcontrolschemeisfocused ondomesticcattleandalthoughsometestshavebeenadjustedforuseinbuffalo,thisisnotthecaseforotherwildlifespecies.TheBuffaloVeterinary ProceduralNotice(VPN)waspublishedin2017outliningtheprocedures fordiseasetesting,movementandcontingencyplanningfordiseaseoutbreaksinbuffalo(DAFF,2017).ThebuffaloVPNstatesthatformovement purposes,buffalomusthaveanegativeCITtestasoutlinedinthemanualfor cattle.Importantly,theinterpretationofCIThasbeenbasedoncattle thresholdsduetothelackofspecies-specificcut-offvaluesforAfricanbuffaloes.ThegammainterferontestisalsoaneffectivediagnostictoolforbuffalobutisnotapprovedbyDAFFformovementpurposes.Thereis currentlynoguidanceoncontrolofBTbinotherwildlifespeciesandthere arelimitedverifieddiagnostictestsinthesespecies(DAFF,2017).
KrugerNationalParkandHluhluwe-iMfoloziParkaretheonlytwo parkswithinSouthAfricathatcontainbuffaloherdsmaintainingBTbyet theyhaveadopteddifferentcontrolapproaches.BovineTbwasfirst detectedinHluhluwe-iMfoloziParkin1986andatestandculldiseaseprogrammewasinitiatedin1999.Thisprogrammeinvolvedamobilecapture unittocorralbuffaloindifferentareasofthepark,testthembymeansof theCITtestandcullingpositiveindividuals.Between1991and2006, 4733buffaloweretested,withherdprevalencerangingfrom2.3%to 54.7%.Subsequent,dataanalysissuggestedthattheprogrammewaseffective atreducingBTbprevalence,particularlyinareaswithintensivetestandcullingoperations(LeRoexetal.,2016).KrugerNationalParktookadifferent approachtomanagingBTbinitsbuffalopopulationafterthediseasewas detectedinthishostspeciesin1990.Theyaimedtobreeddiseasefreebuffalo fromFootandMouthDiseaseinfectedparentswithintheparkinorderto conservethegeneticpoolofKrugerbuffaloinanex-situpopulation (LaubscherandHoffman,2012).Thisapproach,whichuseddairycows asfosterparentsforbuffalocalvesinitially,andlaterswitchedtohaving thebuffalomothersreartheiryoung,washighlysuccessfulandalsopopular withfarmers,eventuallyshiftingfromafewgovernmentfundedprojectsto hundredsofprivatebuffalobreedingfarms(LaubscherandHoffman,2012). Additionally,KrugerNationalParkdidextensiveBTbmonitoringsurveys between1993and2007,toassessthespreadandimpactofBTbinherds,and determineifthediseasewashavingpopulationleveleffects.Sinceitentered thepark,BTbhasbeendetectedin12spill-overspecies(Micheletal.,2006) andremainsaconcerninlowdensityspecies,suchaswilddogandblack rhinoceros(Higgittetal.,2019).
Withthediseasecurrentlynotshowntobeaffectingpopulationrecruitmentorgrowthinbuffalo,therealconcernbecomesspill-overtoother hostsandthereforefindinganeffectivevaccinethatlimitsdiseaseseverity andspill-overisapriority.Currentlythereisonlyoneregisteredvaccine forBTbcontrol.TheBCGvaccineispredominantlyusedinhumansbut hasyieldedpromisingresultsforuseindomesticcattle(Arnotand Michel,2020).However,whentrialledinwildbuffalowithintheKruger NationalPark,theBCGvaccineprotectionwasinsufficientanddidnot limitbacterialshedding(DeKlerketal.,2010).Thiswasthoughttohave resultedfromprimingwithenvironmentalnon-TBmycobacteria,which hasbeenshowntoreducetheprotectiveefficacyoftheBCGvaccine (Brandtetal.,2002;DeKlerketal.,2010).Importantlysimilarstudiesin badgersintheUKfoundtheBCGvaccinetobeeffectiveinlimitingdisease
severity(andthereforebacterialload; Chambersetal.,2011),meaning thatdefiningtheclinicalendpointforvaccineefficacytrialsisimportant. Anothervaccinationtrialinbuffaloiscurrentlyunderway,testingboth BCGandDNA-sub-unitvaccines.
4.Driversofdisease:TheKrugerNationalPark
casestudy
4.1Pastandpresentdiseasemanagement
KrugerNationalParkfirstopenedastheSabiGameReservein1898 (10,364km2)asaresponsetocampaignsfortheconservationofwildanimals subjectedtouncontrolledhuntingandtothe1896rinderpestepidemic (Mabundaetal.,2003).In1926,theSabiGameReservewascombinedwith theSingwitsiReserve(5000km2 regionnamedaftertheShingwedziRiver) andlaterrenamedKrugerNationalPark.JamesStevenson-Hamilton,who wasappointedwardenin1902,wastaskedwithmanagingtheaftermathof therinderpestepidemicwhich,alongwithprevioushuntingactivities,decimatedthegamepopulation,leavingelephantandwhiterhinoceros (Ceratotheriumsimum)locallyextinct(Mabundaetal.,2003).Therinderpest epidemicalsoseverelyaffectedbuffalo,eland(Tragelaphusoryx)andgreater kudu(Tragelaphusstrepsiceros; hereafterreferredtoaskudu),whereaswildebeestandzebrawereunaffected(Stevenson-Hamilton,1957).Thefirst 60yearsofparkmanagement(1900–1960)focusedonprotecting,preserving,andpropagating,aimingtoincreasegamenumbersthroughintroductionsoflargeherbivores,provisionofwatersourcesandcullingofpredators (Venteretal.,2008).
ColonelJ.A.BSanderbergtookoverfromStevenson-Hamiltonas Wardenin1946and8yearslaterthefirstcaseofanthraxwasconfirmed inthenorthofthepark(Mabundaetal.,2003).Thiswasfollowedby repeatedoutbreaksin1959–60,1970and1990–91,andoutbreaksinthe centralpartoftheparkin1993and1999(Bengisetal.,2003; DeVos andBryden,1996).The1959–60outbreaklastedjust4monthsandyet withinthistimeover1000mammalsdied:kudu,waterbuck(Kobusellipsiprymnus)androan(Hippotragusequinus)beingthemostaffected(Pienaar, 1961).Simultaneously,BTblikelyenteredthepark,transmittedfromcattle tobuffaloonthesouthernborder,althoughitwasnotdetectedinthepark until30yearslater(Bengisetal.,1996).Atthistime,parkmanagement shiftedtoa‘managementbyintervention’approachandthenext30years (1960–1990)focusedonmeasuring,monitoringandmanipulation
(Mabundaetal.,2003).FencingoftheparkwasorderedbytheNational DepartmentofAgricultureinordertopreventthespreadofdiseaseto surroundinglivestock,suchasfootandmouth(FMD)endemicinbuffalo (Bengisetal.,2003).Fenceconstructionstartedintheearly1960swith thewesternboundaryfollowedbytheeasternboundaryinthelate1960s, by1980allboundariesoftheparkwereenclosed.Thefences(over 360kminlengthand65kminwidth)restrictedmovementofwildlifeleadingtoincreasednumbersoflargeherbivores,suchaselephantandbuffalo, whichweresubsequentlycontrolledbycullingoperationsandintheearly 1970s,acertifiedabattoirwasbuiltwithintheparktooptimiseuseofthe culledmeat(Mabundaetal.,2003).From1990to2010management shiftedagaintofocusonintegration,innovationandinternationalisation. Theseveredroughtof1992–93followedbytheFebruaryfloodsin2000 aswellasthecatastrophicwildfireinSeptember2001,whichkilledboth peopleandanimalswithinthepark,wereindicativeoftheneedformanagementtobecomemoreadaptivetotheincreasinglyunpredictableenvironment(Mabundaetal.,2003).Since1995,Krugerhasusedastrategic adaptivemanagementapproach,whichinvolvesmanagementdecisions andactionsguidedbyresearchandmonitoringwhilelearningfromunexpectedeventsoroutcomes.Thisapproachalsoaimstomaximiseheterogeneityoftheparkandledtoitsexpansionacrossnationalboundariescreating theGreaterLimpopotransfrontierconservationarea(GLTFCA)spanning theLimpopo(Mozambique),Kruger(SouthAfrica)andGonarezhou (Zimbabwe)NationalParks.Aportionoffencesofapproximately45km wasremovedbetweenLimpopoandKrugerin2002(Caronetal.,2016; Venteretal.,2008).
4.2KrugerNationalPark’scurrentadaptivemanagement approach
Inthepast,mostmanagementissuesinKrugerNationalParkwerefocused withintheparkboundaries;however,sincetherecognitionthatthreatsand driverstobiodiversityconservationoftenoccuroutsideofthefootprintof theNationalParks,managementissuesareextendingbeyondthepark boundariesandbecomingmoresocio-economicinnature(Venteretal., 2008).ThecreationoftheGreaterLimpopotransfrontierconservationarea shiftedtheparkfrombeingsingleuseforwildlifetoamulti-usepark,sharing itslandwithcommunitiesandtheirlivestock.Thepark’scurrentstrategic adaptivemanagementaimstoincreaseunderstandingofcomplexecosystems andbroadersocietalneedsoflocalcommunities.Thisprocessisguidedby
settingappropriatethresholdsofpotentialconcern(TPC),asetofadaptive managementgoalsandendpointsthatdefineupperandlowerlevelsof acceptablechange,enablingmanagementtodeterminehowmuchasystem canbeallowedtofluctuatebeforeitbecomesaconcernandrequiresmanagementaction.AlthoughTPCsproveusefulforsimplemetricslikeinvasive plantsandriverflows,theyhaveprovenmorechallengingforcomplexsystemssuchasdiseasewheredriversandrespondersarenotalwaysknown (GaylardandFerreira,2011; Venteretal.,2008).
KrugerNationalPark’s2018–28managementplanincludesadisease managementprogrammeasasupportingobjectivetothehigher-level objectiveofbiodiversityconservation.Thisprogrammeacknowledges endemicwildlifediseaseswithintheparkasakeycomponentofbiodiversity yethighlightstheneedtopreventandmitigatethespreadofdiseaseatthe wildlife-livestock-humaninterfaceandlimittheintroductionorimpactof novelinfectiousdiseases(Spiesetal.,2018).
4.3Environmentaldriversofdiseasetransmission
4.3.1Spatialheterogeneityandthenorth/southdivide Topography,climate,geologyandtheassociatedsoilandvegetationpatterns canexertabottom-upcontrolonecosystems.Thecombinationofthese abioticfactorscaninfluencefirepatternsandanimalbehaviours,aswellas diseasedynamics(Venteretal.,2003).
KrugerNationalParklieswithinpartofthenorth-easternSouthAfrican lowveld,whichgenerallyhasplainsoflowtomoderatereliefwithsomelow mountainsandhills.Thegeologyoftheparkcanbecrudelydividedinto graniteplainsonthewestandbasaltplainsontheeast,separatedbya north-southstripofsedimentaryrock(Venteretal.,2003).Rainfallin theparkincreasesalonganorthtosouthgradientwithannualmeanrainfall of350mminthenortheastto750mminthesouthwest.Geologyandrainfall haveinfluencedthedifferenceinsoilandvegetationtypesbetweenthe northandthesouthofthepark.Thesouthgenerallyconsistsofdeeper andmorediversesoiltypeswithpredominantlyopencanopyacaciatree bushveldandsavannahwithawellwoodedareainthesoutheast.Incontrast, thenorthtendstohavelessdiverse,thinnersoilswithahighercalciumcontent.Vegetationisdominatedbymopanetreeswithrarelowveldriverine forestoccurringalongtheriversinthenortheastandsandveldvegetation typeinthenorthwest(Gertenbach,1983; Spiesetal.,2018).Thenorthern mostsectionoftheparkisuniqueasitcontainsavariedassemblageofrock formationswithassociatedsoilandvegetationtypes.Italsocontainstheonly
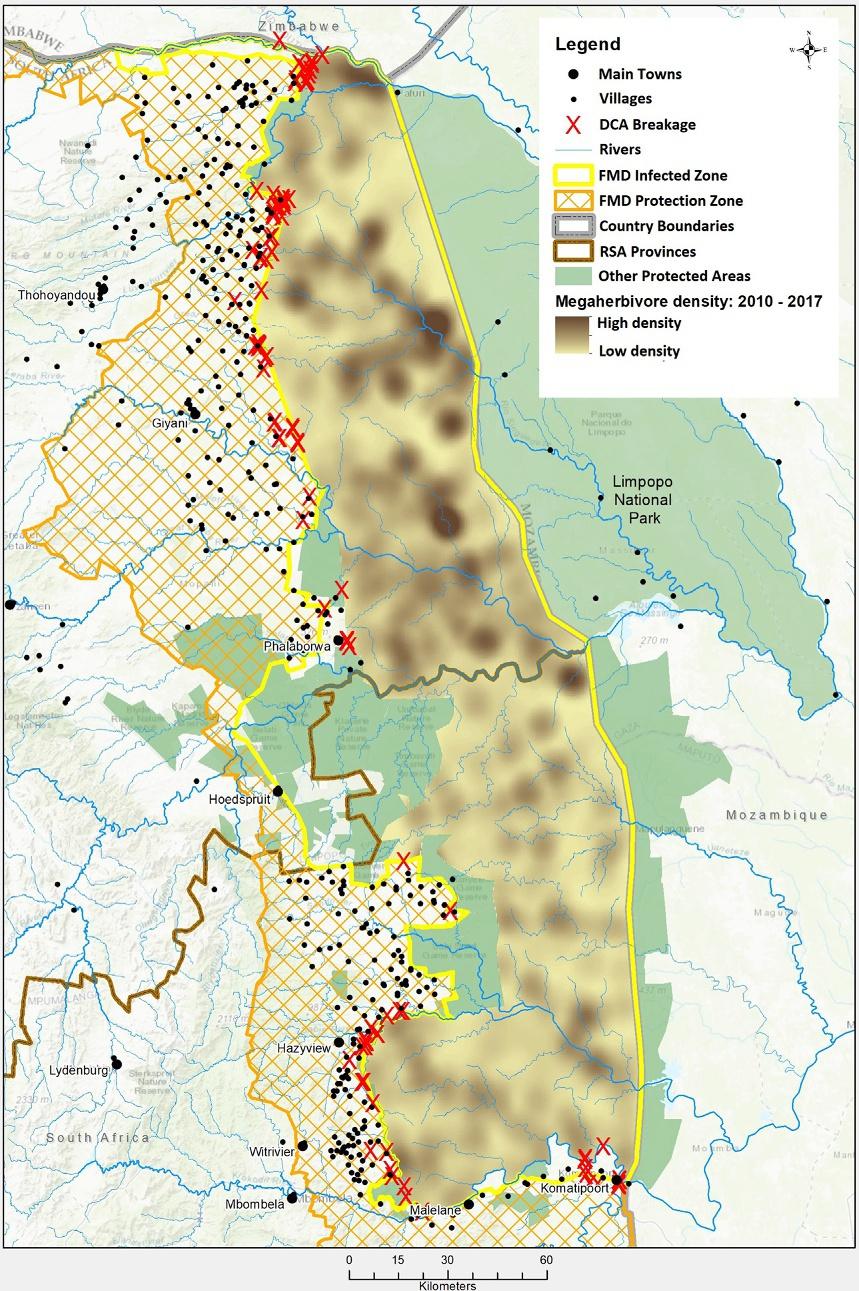
truefloodplaininKruger(Venteretal.,2003).Formanagementpurposes, KrugerNationalParkhasbeenpartitionedinto35landscapesdepending ongeomorphology,vegetation,soil,climatetypesandassociatedfauna (Gertenbach,1983; Venteretal.,2003).Asocial-economicgradientexists alongthenorthernandsouthernboundariesofthepark.Denseperi-urban tourbandevelopmentsliealongthesouthwesternborder,includingsugarcaneplantations,forestryandthenearbycityofMbombela(previously knownasNelspruit; Fig.2).Thecentralandnorth-westernboundaries arebufferedbyprivatenaturereservesandcommunitysubsistencefarming, andfurthernorthbecomesmoreruralwithlargeagriculturalareasandpoor villageswithlimitedeconomicopportunities(Spiesetal.,2018).Wildlife densitiesalsodifferacrosstheparkwithmegaherbivore(elephantand buffalo)densitieshigherinthenorththanthesouth(Fig.2).
Thisecologicalheterogeneitywithintheparkcancreatespatialheterogeneityindiseasedynamics.AparkwidesurveyofRVFinbuffaloin1998 showedsignificantlyhigherseroprevalenceofbuffaloherdsinthesouthand centralregionsoftheparkcomparedtothenorth(Beechleretal.,2015a). Thiswasattributedtolowerrainfallanddifferentvegetationinthenorth leadingtolesssuitablebreedinghabitatsformosquitovectors(Beechler etal.,2015a).Brucellosisprevalenceinbuffalowassignificantlyassociated withparksectionandsoiltype(Gorsichetal.,2015).Buffalocapturedon theresourcepoorgraniticsoilsweretwiceaslikelytobeseropositivefor brucellosiscomparedtothoseontheresourcerichbasalticsoils(Gorsich etal.,2015).Moreover,buffaloongraniticsoilshadhigherprevalencein thesouthernsectionoftheparkcomparedtothecentralsection(Gorsich etal.,2015).Thiswasattributedtonutrientpoorvegetationinthesouthwesterngraniticsoilsandgenerallowerbodyconditionofbuffalointhe southofKrugerNationalPark(Caronetal.,2003; Gorsichetal.,2015). Theeffectofbrucellosisinfectionwasalsodependantontheseasonalheterogeneityofthepark,brucellosisinfectionwassignificantlyassociatedwith lowerbodyconditionbutonlyinthedryseason(Gorsichetal.,2015). Knowledgeofthisheterogeneityofdifferentdiseasedynamicsandhow thelandscapeandenvironmentaffectthisisofgreatimportanceandcanhelp targetmonitoringandmanagementofdiseaseswithinthepark.
4.3.2Climatechangeandsevereweatherevents
Africaisconsideredoneofthemostvulnerableareastoglobalclimate change(Serdecznyetal.,2017).Averagetemperaturereadingsfromthe SkukuzaweatherstationinKrugerNationalParkhaveshowna2 °C
Fig.2 Megaherbivore(Africanbuffaloandelephant)densityacrossKrugerNational Parkandfencebreakages(redcross)fromdamagecausinganimals(DCAs).Elephant causemostbreakagesenablingdiseasedbuffalotoescape.Footandmouth(FMD)veterinarycontrolzonesandnearbyvillagesarealsoshown. MapproducedbytheSkukuza GISOffice.