Chapter One: The microscopic five of the big five: Managing zoonotic diseases within and beyond Africanwildlife protected areas
Anya V. Tobera,⁎; Danny Govenderb,c; Isa-Rita M. Russoa,†; Jo Cablea,†
a School of Biosciences, Cardiff University, Cardiff, Wales, United Kingdom
b SANParks, Scientific Services, Savanna and Grassland Research Unit, Pretoria, South Africa
c Department of Paraclinical Sciences, University of Pretoria, Onderstepoort, South Africa
† Authors contributed equally to this work
⁎ Corresponding author: email address: tobera@cardiff ac uk
Abstract
African protected areas strive to conserve the continent's great biodiversity with a targeted focus on the flagship ‘Big Five’ megafauna Though often not considered, this biodiversity protection also extends to the lesser-known microbes and parasites that are maintained in these diverse ecosystems, often in a silent and endemically stable state. Climate and anthropogenic change, and associated diversity loss, however, are altering these dynamics leading to shifts in ecological interactions and pathogen spill over into new niches and hosts. As many African protected areas are bordered by game and livestock farms, as well as villages, they provide an ideal study system to assess infection dynamics at the human-livestock-wildlife interface Here we review five zoonotic, multi-host diseases (bovine tuberculosis, brucellosis, Rift Valley fever, schistosomiasis and cryptosporidiosis) the ‘Microscopic Five’ and discuss the biotic and abiotic drivers of parasite transmission using the iconic Kruger National Park, South Africa, as a case study. We identify knowledge gaps regarding the impact of the ‘Microscopic Five’ on wildlife within parks and highlight the need for more empirical data, particularly for neglected (schistosomiasis) and newly emerging (cryptosporidiosis) diseases, as well as zoonotic disease risk from the rising bush meat trade and game farm industry As protected areas strive to become further embedded in the socioeconomic systems that surround them, providing benefits to local communities, One Health approaches can help maintain the ecological integrity of ecosystems, while protecting local communities and economies from the negative impacts of disease.
Keywords
Emerging Infectious Diseases; One Health; Zoonoses; Spill over; Spill Back; Kruger National Park
1: Introduction
As we enter the sixth mass extinction, protecting the world's biodiversity has never been more critical. Protected areas, including national parks, cover over 18 8 million km2 and are at the forefront of a global effort to safeguard biodiversity (Chape et al , 2003) Managers of these protected areas must strike a balance between protecting the ecological integrity of ecosystems and preventing exploitation of local resources while promoting their use in education and recreation (Chape et al., 2003). If managed
correctly, protected areas can be beneficial to wildlife conservation and the country's economy through promoting ecotourism and creating local employment opportunities (Cheung, 2012; Spies et al., 2018). However, the management of protected areas is challenging, particularly in the Anthropocene era of human mediated global change, and increased emergence and re-emergence of infectious diseases (reviewed by Cable et al , 2017) These diseases can reduce fitness, alter wildlife population structure/size and even alter ecosystem function (Holdo et al , 2009; Prins and Weyerhaeuser, 1987; Sco�, 1988) Therefore, to effectively manage wildlife populations and ecosystems, it is essential to understand the threats posed by pathogens and the diseases they cause.
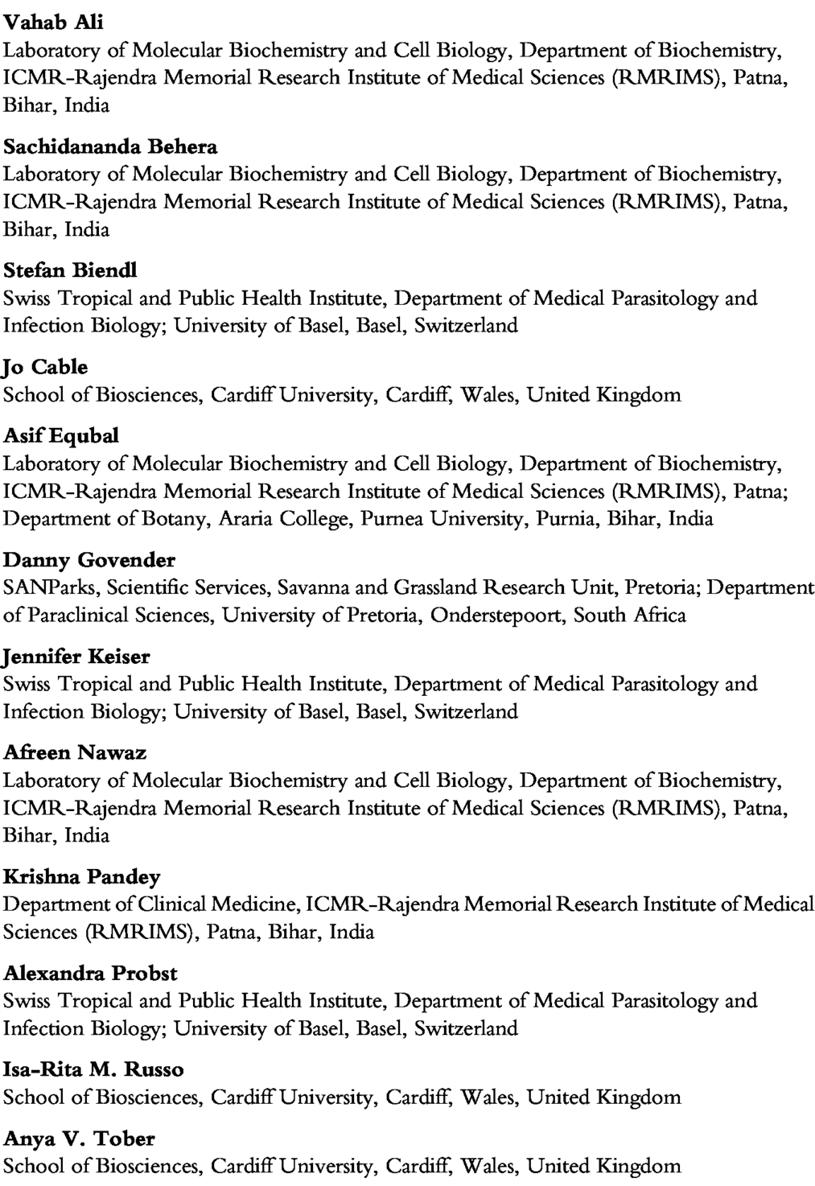
Of the 3881 terrestrial and marine national parks in the world, almost half are in sub-Saharan Africa, with terrestrial parks here covering 1 million km2 (4% of the total land area; Chape et al , 2003; Muhumuza and Balkwill, 2013). These parks aim to conserve Africa's unique and iconic ecosystems ranging from open savannas and grasslands to dense forest. This variety of habitats supports high levels of biodiversity, drawing numerous tourists who aspire to spot the ‘Big Five’ megafauna: African buffalo (hereafter referred to as buffalo), lion, African elephant (hereafter referred to as elephant), rhinoceros and leopard (Dube and Nhamo, 2019) However, hidden and often forgo�en biodiversity within protected areas includes pathogens, which modulate animal abundance, fitness and behaviour (Gómez and Nichols, 2013) It is crucial to be�er understand drivers for past and current wildlife disease outbreaks within protected areas, to find new approaches to predict and prevent future outbreaks. A review of all infectious wildlife diseases within protected areas would be too large a task. Instead, we focus on five diseases referred to here as the ‘Microscopic Five’, which are important at the humanlivestock-wildlife interface due to their broad host range and zoonotic potential These interface diseases would all benefit from a ‘One Health’ approach to management (Fawzy and Helmy, 2019; Innes et al , 2020; Webster et al , 2016) We therefore purposefully included high profile diseases (bovine tuberculosis (BTb), Rift Valley fever and brucellosis) as well as neglected diseases (cryptosporidiosis and schistosomiasis) for study. Using Kruger National Park, one of the most researched parks in Africa (van Wilgen et al., 2016), we will review the key factors that can influence outbreaks and transmission of the ‘Microscopic Five’ within and around protected areas (Fig. 1). By focusing on a select group of pathogens within a specific park our intention is to highlight drivers of disease common among many protected areas and the importance of considering all infectious diseases in wildlife management plans We will first give a brief introduction to the ‘Microscopic Five’ and then give examples of the environmental and anthropogenic factors driving the dynamics of these diseases within and around Kruger National Park. We will then discuss the key knowledge gaps and future challenges for managing the ‘Microscopic Five’ and other important diseases and touch on different management approaches followed in various parks
FIG. 1 The ‘Big Five’ and ‘Microscopic Five’, and the drivers of disease at the wildlifelivestock-human interface. Arrows represent anthropogenic drivers from beyond Kruger National Park Created with Microsoft PowerPoint (version 2109) and Adobe Photoshop (2021)
2: The ‘Microscopic Five’
The ‘Big Five’ are undoubtedly one of the biggest a�ractions for tourists visiting South Africa's protected areas (Dube and Nhamo, 2019) To conserve these and other wildlife, and to reduce transmission of infectious diseases among wildlife, domestic animals and humans, we focus on the lesser known ‘Microscopic Five’ These comprise zoonotic diseases caused by pathogens that have multiple hosts, including humans, and are of particular importance at the human-livestock-wildlife interface. Although we focus on five specific diseases, there are many more of importance within protected areas (Table 1) but by highlighting a distinct few we aim to raise the profile of all infectious diseases and possible drivers The first three of the ‘Microscopic Five’ (bovine tuberculosis, brucellosis and Rift Valley fever) are high profile or state-controlled diseases in South Africa and any outbreaks must be reported to the World Organisation of Animal Health (OIE) All three of these diseases are trade-sensitive diseases and may change the trading status of a country and its ability to trade on the global market. The remaining two (schistosomiasis and cryptosporidiosis) are neglected in comparison, particularly in wildlife. By including these in the ‘Microscopic Five’, we aim to bring greater a�ention to overlooked yet highly important diseases (see WHO, 2020). In the following account, we briefly cover each of the ‘Microscopic Five’ discussing their host specificity, transmission pathways and known impacts on wildlife, livestock and humans
Table 1
Some diseases of large herbivores within Kruger National Park which may pose a threat to livesto
Disease. Pathogen
Bacteria
Transmission modes Transmission routes Drivers Spatial distribution in KNP
Anthrax Bacillus anthracis Vector, environme ntal, direct contact, fomites
Ingestion of contamina ted vegetation or carcasses
Bovine Tb Micobacteria bovis Aerosol Respiratory tract
Virus
Foot and mouth Aphtovirus Aerosol, fomites
African horse sickness Orbivirus Mosquito vector, direct contact
Rift Valley fever Phlebovirus Mosquito vector, direct contact
Protozoa
Respiratory tract
Cutaneous penetratio n
Cutaneous penetratio n
Calcium soil content, drought Northern and central regions
Wildlife/livestock interface, host density South, central, moving north
Wildlife/livestock interface, host density North, central, south
Introduced horses, season Central
Climate change, drought, rainfall Higher in south and central regions
Bovine brucellosis Brucella abortus Direct contact Ingestion of infected discharges during birth, milk, mucus membrane s Host density North, central, south
Cryptosporidiosis Cryptosporidium spp Environmental Faecal-oral via contamina tion of food and water
Dependant on species and host range
Unknown
Disease. Pathogen Transmission modes Transmission routes Drivers Spatial distribution in KNP
Piroplasma
Corridor disease Theileria parva Tick vector
Cutaneous penetratio n
Wildlife/livestock interface North, central, south
Babesiosis Babesia spp Tick vector Cutaneous penetratio n Unknown
Digenea
Fascioliasis Fasciola spp Environmental Contact with infected water
Schistosomiasis Schistsoma spp Environmental Contact with infected water
2.1: Bovine tuberculosis
Dependant on species and host range Unknown
Dependant on species and host range Unknown
Bovine tuberculosis (BTb) is caused by the bacterium Mycobacterium bovis and predominantly infects bovines, such as African buffalo (Syncerus caffer caffer) and ca�le (Bos taurus), yet most warm-blooded animals including humans can be infected (Ayele et al , 2004) Transmission mainly occurs through inhalation of infectious particles, which is particularly problematic when livestock are kept at high densities (Ayele et al , 2004) Though thought to have spilled over from ca�le to buffalo in the early 1960s in South Africa (Bengis et al., 1996), buffalo now serve as the primary maintenance host for BTb within Kruger National Park and Hluhluwe-iMfolozi Park, spilling over into various species of wildlife and livestock (Michel et al., 2006). Although BTb is a controlled disease within South Africa, its control is becoming increasingly challenging due to the presence of wildlife reservoirs, difficulty in controlling disease in communal herds and lack of practical control options in wildlife (see Section 3) The WHO estimated 147,000 new cases of zoonotic Tb in humans in 2016 with 12,500 deaths globally but mostly in Africa (Sichewo et al , 2019b) Humans can become infected through drinking unpasteurised milk, eating undercooked meat and via aerosols inhaled from infected ca�le (DAFF, 2016; Sichewo et al., 2019a).
2.2: Rift Valley fever
Rift Valley fever (RVF) is caused by a zoonotic, vector borne virus predominantly spread by Aedes mosquitoes (Clark et al., 2018). The virus was first reported in South Africa in 1950 and subsequent outbreaks have occurred sporadically every 7–11 years infecting mainly domestic livestock but also a range of wild mammals and humans (Beechler et al , 2015a; Métras et al , 2015) Human infection occurs mainly through direct contact with blood or tissue from infected animals or through consuming unpasteurised milk but can also result from an infected mosquito bite Symptoms vary from mild, flulike to severe haemorrhagic fever that can be fatal (Clark et al., 2018). Over 4000 human cases and around 1000 deaths have been reported in the last 20 years, predominantly in Africa and Saudi Arabia (Petrova et al., 2020). Li�le is known about how the pathogen is maintained during inter-epidemic periods One suggestion is vertical transmission from mosquitoes to their ova, which has been
demonstrated with Aedes mosquitoes under laboratory-controlled conditions (Romoser et al., 2011). Another possibility is that it is maintained in wild animal populations (Beechler et al., 2015a; see Section 4.3.4). Commercial vaccines are available for livestock but there is currently no licensed human vaccine (Petrova et al., 2020).
2.3: Brucellosis
Brucellosis, caused by bacteria of the Brucella genus, is ranked among the most economically important zoonotic diseases globally Although it is an OIE notifiable disease, outbreaks are thought to be greatly under-reported in Africa (McDermo� et al , 2013) The species of medical and veterinary importance are Brucella abortus, Brucella melitensis and B suis (see Ducrotoy et al , 2017) Infection in humans can lead to a debilitating illness known as ‘Mediterranean’ or ‘undulant’ fever and is commonly misdiagnosed as malaria (Ducrotoy et al., 2017; Godfroid et al., 2011). Human infection occurs through direct contact with or consumption of an infected animal. Consumption of un-pasteurised milk causes most human infections, while human to human transmission is rare (Godfroid, 2018). Several wildlife species have been reported as seropositive for this disease and African buffalo are thought to be a reservoir for B abortus (see Godfroid et al , 2013) Infection can cause abortions in livestock reducing farm productivity, however the effects of the disease on wildlife are largely unknown and may differ between species (Gorsich et al., 2015). Vaccines are available for livestock and small ruminants but not yet for humans (Ducrotoy et al., 2017).
2.4: Cryptosporidiosis
Cryptosporidiosis, caused by several species of the protozoan Cryptosporidium genus, can lead to severe diarrhoea in humans and animals globally Infectious diarrhoea is a major cause of death in children under five in Africa and Cryptosporidium is second only to rotavirus as a contributor to this disease (Kotloff et al., 2013; Squire and Ryan, 2017). Transmission occurs through the faecal oral route via close contact with infected humans, animals or contaminated food and water (Innes et al., 2020). Currently there are at least 40 recognised species with varying host specificities but the most important two species infecting humans and livestock are C hominus and C parvum The la�er is the predominant cause of diarrhoea in young calves and is the most important zoonotic species Cryptosporidium parvum is more genetically diverse than C hominus with several subtypes with differing host specificities, therefore an integrated genotyping approach has been advocated to differentiate these subtypes (Innes et al., 2020). Cryptosporidium species have been identified in a range of wildlife, yet most studies focus on humans and livestock (Zahedi et al., 2016). C. parvum, C. ubiquitum and C. bovis were recently identified in wildlife within Kruger National Park in elephant (Loxodonta africana), buffalo (Syncerus caffer) and impala (Aepyceros melampus; see Samra et al , 2011) Oocysts of Cryptosporidium spp have also been detected in zebra (Equus zebra), buffalo and wildebeest (Connochaetes gnou) faeces in Mikumi National Park, Tanzania (Mtambo et al., 1997). There is currently no available vaccine for cryptosporidiosis yet there is potential to develop one for ca�le (Innes et al., 2020).
2.5: Schistosomiasis
Schistosomiasis is a waterborne, zoonotic disease of veterinary and medical importance, caused by digenean parasites of the genus Schistosoma. Schistosomiasis is a major public health threat with an estimated 207 million people infected and 779 million people at risk globally, with 90% of these infections in Africa (Steinmann et al , 2006) Like all digeneans, schistosomes have an indirect lifecycle They require an intermediate freshwater snail host within which they reproduce asexually ultimately producing cercariae, which are free-swimming larval stages that subsequently infect a definitive mammalian host (Cribb et al., 2003). Definitive animal or human hosts can become infected with schistosomiasis by entering infested waters the water-borne larvae burrow through the skin of the new host (Cribb et al., 2003). There are at least 12 known schistosome species in Africa of which 5 are known to infect humans (S. haematobium, Schistosoma mansoni, S. intercalatum, S. guineensis and S. ma�heei). Schistosoma ma�heei is of note as although predominantly a parasite of ca�le, it has also been found in wildlife and humans where it is known to hybridise with S haematobium (see Pitchford, 1961) The other
species infect a wide range of domestic and wild animals including ca�le, horses, buffalo, baboons, zebra, hippopotamus and rodents (Standley et al., 2012). Traditionally, malacological monitoring programmes have only targeted snail species known to harbour human infecting schistosomes, but a wider approach is clearly needed as we become aware of wider host ranges (Pennance et al., 2021) that are likely to shift with increasing environmental stressors There is currently no vaccine for schistosomiasis and the main control strategy for humans is preventative chemotherapy, improved water, sanitation and hygiene and snail control (WHO, 2022)
3: Challenges of BTb control at the wildlife-livestock interface: The South African case study
South Africa has been challenged with the control of BTb since the disease was first reported in the country in 1880, initially focusing on livestock, and now including control in wildlife (DAFF, 2016)
3.1: Control in livestock
Early BTb surveillance included the introduction of tuberculin skin testing in ca�le in 1905, followed by its declaration as a notifiable disease in 1911 and the initiation of the Division of Veterinary Services BTb scheme in 1969 (DAFF, 2016; Michel et al., 2019). This scheme focused on compulsory testing of commercial ca�le herds suspected to be infected, with slaughter of positive individuals, quarantine and disinfection of farms Initially, great progress was made, reducing prevalence to 0 04% by 1991 (1 1 million ca�le tested); however, the number of tests have since declined due to budget cuts and a decreased workforce (DAFF, 2016; Michel et al., 2019). Current prevalence in communal livestock is variable (< 0.5% to > 15%) (Musoke et al., 2015; Sichewo et al., 2019b).
In 2021 the national ca�le herd was estimated at 12 million, consisting of commercial dairy herds (20%) and beef and dual-purpose herds (80%) (DAFF, 2021) Testing of ca�le is no longer compulsory and current control of BTb is guided by the Interim BTb Manual from the Department of Agriculture, Forestry and Fisheries (DAFF), South Africa, which proposes the use of four testing programmes (Table 2; DAFF, 2016) All programmes are voluntary apart from the infected herd program, which can be enforced by the Animal Diseases Act, 1984 (Act No. 35 of 1984) (DAFF, 2016). The approved test is the cervical intradermal tuberculin (CIT) test (DAFF, 2016).
Table 2
Four levels of Bovine Tb surveillance programmes in South Africa.
Surveillance herd programme One off survey used by state officials to determine the prevalence of BTb within an area or by a stock owner conducting a self-assessment
Maintenance herd programme
Infected herd programme
To join this programme, herds are required to undergo two consecutive tests with 100% negative results at least 3 months apart. These BTb free herds are then tested every 2 years If an individual tests positive, then the entire herd is moved to the infected herd programme
Compulsory programme for herds that have tested positive with the CIT test, as well as those detected from meat and milk inspection, post-mortems or clinical cases. These herds are placed under quarantine and kept under supervision of a state veterinarian, who will order the slaughter of infected animals The rest of the herd is tested every 3 months and is only let out of quarantine once the herd has undergone two consecutive negative tests
Diagnostic testing programme (individuals)
Individual ca�le destined to be imported or exported. Imported ca�le are kept in quarantine and must undergo a compulsory CIT test. Before export, ca�le must also receive a comparative CIT test a requirement for many importing countries
3.2: Control in wildlife
The control of BTb in wildlife is becoming increasingly important as many farms switch from livestock to game farming, and wild buffalo reservoirs hinder control efforts in ca�le (Michel et al , 2019) Bovine Tb has been identified in 21 different wildlife species in South Africa, including most recently giraffe (Hlokwe et al , 2019) The current control scheme is focused on domestic ca�le and although some tests have been adjusted for use in buffalo, this is not the case for other wildlife species The Buffalo Veterinary Procedural Notice (VPN) was published in 2017 outlining the procedures for disease testing, movement and contingency planning for disease outbreaks in buffalo (DAFF, 2017). The buffalo VPN states that for movement purposes, buffalo must have a negative CIT test as outlined in the manual for ca�le Importantly, the interpretation of CIT has been based on ca�le thresholds due to the lack of species-specific cut-off values for African buffaloes The gamma interferon test is also an effective diagnostic tool for buffalo but is not approved by DAFF for movement purposes There is currently no guidance on control of BTb in other wildlife species and there are limited verified diagnostic tests in these species (DAFF, 2017).
Kruger National Park and Hluhluwe-iMfolozi Park are the only two parks within South Africa that contain buffalo herds maintaining BTb yet they have adopted different control approaches Bovine Tb was first detected in Hluhluwe-iMfolozi Park in 1986 and a test and cull disease programme was initiated in 1999 This programme involved a mobile capture unit to corral buffalo in different areas of the park, test them by means of the CIT test and culling positive individuals Between 1991 and 2006, 4733 buffalo were tested, with herd prevalence ranging from 2.3% to 54.7%. Subsequent, data analysis suggested that the programme was effective at reducing BTb prevalence, particularly in areas with intensive test and culling operations (Le Roex et al., 2016). Kruger National Park took a different approach to managing BTb in its buffalo population after the disease was detected in this host species in 1990 They aimed to breed disease free buffalo from Foot and Mouth Disease infected parents within the park in order to conserve the genetic pool of Kruger buffalo in an ex-situ population (Laubscher and Hoffman, 2012) This approach, which used dairy cows as foster parents for buffalo calves initially, and later switched to having the buffalo mothers rear their young, was highly successful and also popular with farmers, eventually shifting from a few government funded projects to hundreds of private buffalo breeding farms (Laubscher and Hoffman, 2012). Additionally, Kruger National Park did extensive BTb monitoring surveys between 1993 and 2007, to assess the spread and impact of BTb in herds, and determine if the disease was having population level effects Since it entered the park, BTb has been detected in 12 spill-over species (Michel et al , 2006) and remains a concern in low density species, such as wild dog and black rhinoceros (Higgi� et al., 2019).
With the disease currently not shown to be affecting population recruitment or growth in buffalo, the real concern becomes spill-over to other hosts and therefore finding an effective vaccine that limits disease severity and spill-over is a priority Currently there is only one registered vaccine for BTb control The BCG vaccine is predominantly used in humans but has yielded promising results for use in domestic ca�le (Arnot and Michel, 2020) However, when trialled in wild buffalo within the Kruger National Park, the BCG vaccine protection was insufficient and did not limit bacterial shedding (De Klerk et al., 2010). This was thought to have resulted from priming with environmental non-TB mycobacteria, which has been shown to reduce the protective efficacy of the BCG vaccine (Brandt et al., 2002; De Klerk et al., 2010). Importantly similar studies in badgers in the UK found the BCG vaccine to be effective in limiting disease severity (and therefore bacterial load; Chambers et al , 2011), meaning that defining the clinical end point for vaccine efficacy trials is important Another vaccination trial in buffalo is currently underway, testing both BCG and DNA-sub-unit vaccines
4: Drivers of disease: The Kruger National Park case study
4.1: Past and present disease management
Kruger National Park first opened as the Sabi Game Reserve in 1898 (10,364 km2) as a response to campaigns for the conservation of wild animals subjected to uncontrolled hunting and to the 1896 rinderpest epidemic (Mabunda et al , 2003) In 1926, the Sabi Game Reserve was combined with the
Singwitsi Reserve (5000 km2 region named after the Shingwedzi River) and later renamed Kruger National Park James Stevenson-Hamilton, who was appointed warden in 1902, was tasked with managing the aftermath of the rinderpest epidemic which, along with previous hunting activities, decimated the game population, leaving elephant and white rhinoceros (Ceratotherium simum) locally extinct (Mabunda et al., 2003). The rinderpest epidemic also severely affected buffalo, eland (Tragelaphus oryx) and greater kudu (Tragelaphus strepsiceros; hereafter referred to as kudu), whereas wildebeest and zebra were unaffected (Stevenson-Hamilton, 1957). The first 60 years of park management (1900–1960) focused on protecting, preserving, and propagating, aiming to increase game numbers through introductions of large herbivores, provision of water sources and culling of predators (Venter et al , 2008)
Colonel J.A.B Sanderberg took over from Stevenson-Hamilton as Warden in 1946 and 8 years later the first case of anthrax was confirmed in the north of the park (Mabunda et al., 2003). This was followed by repeated outbreaks in 1959–60, 1970 and 1990–91, and outbreaks in the central part of the park in 1993 and 1999 (Bengis et al , 2003; De Vos and Bryden, 1996) The 1959–60 outbreak lasted just 4 months and yet within this time over 1000 mammals died: kudu, waterbuck (Kobus ellipsiprymnus) and roan (Hippotragus equinus) being the most affected (Pienaar, 1961) Simultaneously, BTb likely entered the park, transmi�ed from ca�le to buffalo on the southern border, although it was not detected in the park until 30 years later (Bengis et al., 1996). At this time, park management shifted to a ‘management by intervention’ approach and the next 30 years (1960–1990) focused on measuring, monitoring and manipulation (Mabunda et al., 2003). Fencing of the park was ordered by the National Department of Agriculture in order to prevent the spread of disease to surrounding livestock, such as foot and mouth (FMD) endemic in buffalo (Bengis et al , 2003) Fence construction started in the early 1960s with the western boundary followed by the eastern boundary in the late 1960s, by 1980 all boundaries of the park were enclosed. The fences (over 360 km in length and 65 km in width) restricted movement of wildlife leading to increased numbers of large herbivores, such as elephant and buffalo, which were subsequently controlled by culling operations and in the early 1970s, a certified aba�oir was built within the park to optimise use of the culled meat (Mabunda et al., 2003). From 1990 to 2010 management shifted again to focus on integration, innovation and internationalisation The severe drought of 1992–93 followed by the February floods in 2000 as well as the catastrophic wildfire in September 2001, which killed both people and animals within the park, were indicative of the need for management to become more adaptive to the increasingly unpredictable environment (Mabunda et al., 2003). Since 1995, Kruger has used a strategic adaptive management approach, which involves management decisions and actions guided by research and monitoring while learning from unexpected events or outcomes. This approach also aims to maximise heterogeneity of the park and led to its expansion across national boundaries creating the Greater Limpopo transfrontier conservation area (GLTFCA) spanning the Limpopo (Mozambique), Kruger (South Africa) and Gonarezhou (Zimbabwe) National Parks A portion of fences of approximately 45 km was removed between Limpopo and Kruger in 2002 (Caron et al , 2016; Venter et al., 2008).
4.2: Kruger National Park's current adaptive management approach
In the past, most management issues in Kruger National Park were focused within the park boundaries; however, since the recognition that threats and drivers to biodiversity conservation often occur outside of the footprint of the National Parks, management issues are extending beyond the park boundaries and becoming more socio-economic in nature (Venter et al , 2008) The creation of the Greater Limpopo transfrontier conservation area shifted the park from being single use for wildlife to a multi-use park, sharing its land with communities and their livestock The park's current strategic adaptive management aims to increase understanding of complex ecosystems and broader societal needs of local communities. This process is guided by se�ing appropriate thresholds of potential concern (TPC), a set of adaptive management goals and endpoints that define upper and lower levels of acceptable change, enabling management to determine how much a system can be allowed to fluctuate before it becomes a concern and requires management action Although TPCs prove useful for simple metrics like invasive plants and river flows, they have proven more challenging for complex systems such as disease where drivers and responders are not always known (Gaylard and Ferreira, 2011; Venter et al , 2008)
Kruger National Park's 2018–28 management plan includes a disease management programme as a supporting objective to the higher-level objective of biodiversity conservation. This programme acknowledges endemic wildlife diseases within the park as a key component of biodiversity yet highlights the need to prevent and mitigate the spread of disease at the wildlife-livestock-human interface and limit the introduction or impact of novel infectious diseases (Spies et al , 2018)
4.3: Environmental drivers of disease transmission
4.3.1: Spatial heterogeneity and the north/south divide Topography, climate, geology and the associated soil and vegetation pa�erns can exert a bo�om-up control on ecosystems The combination of these abiotic factors can influence fire pa�erns and animal behaviours, as well as disease dynamics (Venter et al., 2003).
Kruger National Park lies within part of the north-eastern South African lowveld, which generally has plains of low to moderate relief with some low mountains and hills. The geology of the park can be crudely divided into granite plains on the west and basalt plains on the east, separated by a north-south strip of sedimentary rock (Venter et al , 2003) Rainfall in the park increases along a north to south gradient with annual mean rainfall of 350 mm in the northeast to 750 mm in the southwest Geology and rainfall have influenced the difference in soil and vegetation types between the north and the south of the park. The south generally consists of deeper and more diverse soil types with predominantly open canopy acacia tree bushveld and savannah with a well wooded area in the southeast. In contrast, the north tends to have less diverse, thinner soils with a higher calcium content Vegetation is dominated by mopane trees with rare lowveld riverine forest occurring along the rivers in the northeast and sandveld vegetation type in the northwest (Gertenbach, 1983; Spies et al , 2018) The northern most section of the park is unique as it contains a varied assemblage of rock formations with associated soil and vegetation types. It also contains the only true floodplain in Kruger (Venter et al., 2003). For management purposes, Kruger National Park has been partitioned into 35 landscapes depending on geomorphology, vegetation, soil, climate types and associated fauna (Gertenbach, 1983; Venter et al., 2003). A socialeconomic gradient exists along the northern and southern boundaries of the park Dense peri-urban to urban developments lie along the southwestern border, including sugarcane plantations, forestry and the nearby city of Mbombela (previously known as Nelspruit; Fig 2) The central and north-western boundaries are buffered by private nature reserves and community subsistence farming, and further north becomes more rural with large agricultural areas and poor villages with limited economic opportunities (Spies et al., 2018). Wildlife densities also differ across the park with megaherbivore (elephant and buffalo) densities higher in the north than the south (Fig 2)
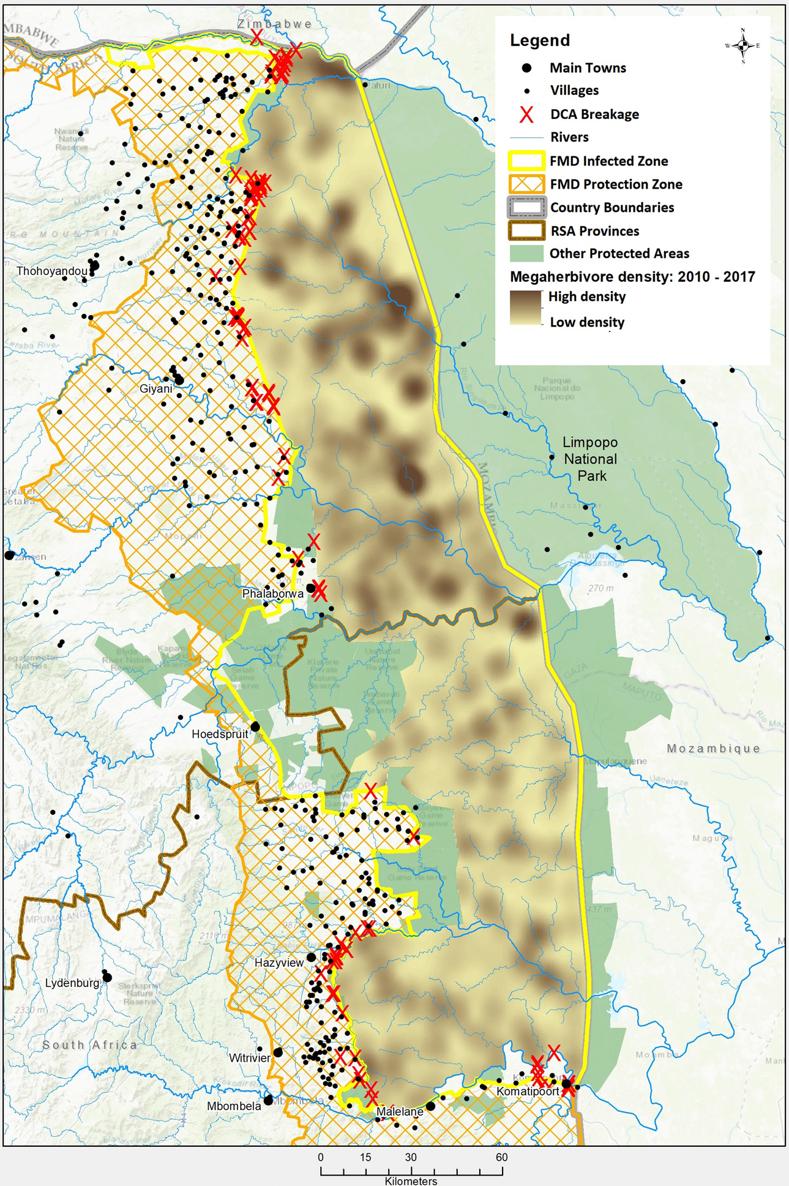
FIG. 2 Megaherbivore (African buffalo and elephant) density across Kruger National Park and fence breakages (red cross) from damage causing animals (DCAs) Elephant cause most breakages enabling diseased buffalo to escape Foot and mouth (FMD) veterinary control zones and nearby villages are also shown Map produced by the Skukuza GIS Office
This ecological heterogeneity within the park can create spatial heterogeneity in disease dynamics A park wide survey of RVF in buffalo in 1998 showed significantly higher seroprevalence of buffalo herds in the south and central regions of the park compared to the north (Beechler et al , 2015a) This was a�ributed to lower rainfall and different vegetation in the north leading to less suitable breeding habitats for mosquito vectors (Beechler et al., 2015a). Brucellosis prevalence in buffalo was significantly associated with park section and soil type (Gorsich et al., 2015). Buffalo captured on the resource poor granitic soils were twice as likely to be seropositive for brucellosis compared to those on the resource rich basaltic soils (Gorsich et al , 2015) Moreover, buffalo on granitic soils had higher prevalence in the southern section of the park compared to the central section (Gorsich et al , 2015) This was a�ributed to nutrient poor vegetation in the southwestern granitic soils and general lower body condition of buffalo in the south of Kruger National Park (Caron et al., 2003; Gorsich et al., 2015). The effect of brucellosis
infection was also dependant on the seasonal heterogeneity of the park, brucellosis infection was significantly associated with lower body condition but only in the dry season (Gorsich et al., 2015). Knowledge of this heterogeneity of different disease dynamics and how the landscape and environment affect this is of great importance and can help target monitoring and management of diseases within the park
4.3.2: Climate change and severe weather events
Africa is considered one of the most vulnerable areas to global climate change (Serdeczny et al , 2017) Average temperature readings from the Skukuza weather station in Kruger National Park have shown a 2 °C increase from 1977 to 2018 with a maximum temperature increase of 0 5 °C per decade (Dube and Nhamo, 2019). Kruger National Park has suffered numerous droughts in 1966–67, 1982–83, 1991–92, 1995–96 and most recently in 2015–17 (Staver et al., 2019). The most severe drought on record in 1991–92 had a mean total rainfall of 235.6 mm compared to the 534 mm long-term mean annual rainfall and the number of days with rain within this period (24 2) was significantly less than the mean annual total (48 3; Zambatis and Biggs, 1995) This was followed by a severe flood event in 1996 Certain disease outbreaks within the Kruger National Park, such as anthrax and foot and mouth disease (FMD) have occurred after a dry period (Pienaar, 1961).
Drought not only affects resource availability and body condition, but also the behaviour and movement of animals, including buffalo, which in turn can alter disease transmission (Cross et al., 2004; Staver et al , 2019) Combining buffalo behavioural association data with disease models predicted that dry conditions facilitate increased spread of BTb within buffalo populations due to increased herd switching (Cross et al , 2004) The 2015–17 drought forced buffalo to move north to areas where the drought was less severe (Staver et al , 2019) Movements like this could lead to the transmission of diseases into new areas of the park and is likely to have played a role in the spread of BTb northwards through the park (Michel et al., 2006).
Drought particularly affects the dynamics of water borne diseases such as schistosomiasis and RVF carried by vectors (snails and mosquitoes respectively) reliant on water or moisture with populations that fluctuate depending on climate variations (Cribb et al , 2003; Romoser et al , 2011) Floods and prolonged wet periods have been associated with outbreaks of RVF South Africa has experienced three major RVF epidemics (1950–51, 1973–75 and 2008–11). Epidemiological data from the 2008 to 2011 epidemic was modelled to quantify spatial and temporal environmental factors associated with disease incidence (Métras et al., 2015). Initial years saw the incidence of RVF increase with increased vegetation density and presence of wetlands However, for 2010 and 2011, of which 2010 was the longest lasting outbreak, the strongest risk factor was temperature For 2010, the risk of RVF increased by a hazard ratio of 15 7 in areas between 25 and 32 °C and by 44 35 in areas over 35 °C compared with those below 25 °C (Métras et al , 2015) This is important for Kruger National Park as the average annual temperature in the south of the park was 32.3 °C and is set to increase (Dube and Nhamo, 2019).
Modelling the RVF outbreaks in Kruger National Park from 2008 to 2011 suggested that soil saturation index anomalies exceeding the long-term mean by 20%, followed by a sudden rainfall event could be a reliable predictor for outbreaks When tested on previous outbreaks, the model successfully predicted 90% of outbreaks more than 1 month before they occurred (Williams et al , 2016) Other factors such as vegetation density and increased temperature are also important risk factors (Métras et al , 2015)
4.3.3: Water sources
Water is often a focal point for wildlife-livestock interaction, particularly rivers which run between protected areas and communal farmlands (Kock et al., 2014). Such interaction can enable spread of diseases between wildlife within the park and livestock on the borders (Miguel et al., 2013; Pienaar, 1961) Within Kruger National Park, five perennial rivers (Sabi-Sands, Crocodile, Olifants, Letaba and Luhuvu) run through the park as well as several seasonal rivers, natural pans and wetlands (Mabunda et al , 2003; Pienaar et al , 1997) The Sabi River runs parallel to the fenced south-western border between Kruger National Park and the adjacent communal lands of Bushbuckridge Landscape resistance maps for ca�le and buffalo resource utilisation were used to model dispersal of these animals within these
two areas. Contact risk between buffalo and ca�le was significantly higher in the dry season and was concentrated along the Sabi River at the weaker parts of the fence. Contact risk was more widespread and closer to villages in the wet season, yet still highest along the river (Kaszta et al., 2018). Water sources can also increase the permeability of nearby fences to wildlife movements. Interviews with fence maintenance workers in Kruger National Park reported that fences damaged by flooding and predation were higher in areas with rivers compared to those without Furthermore, reports of kudu crossing the fences were significantly higher in areas with rivers, although this was not observed for other wildlife (elephant, buffalo, warthog (Phacochoerus africanus) and impala) in the park (Jori et al., 2011).
Contact at water sources has been a�ributed to disease outbreaks, such as FMD and anthrax (Miguel et al , 2013; Pienaar, 1961) The Limpopo River runs between the northern edge of Kruger National Park and adjacent communal land of Pezvi in Zimbabwe, both within the GLTFCA Satellite data from collared individual ca�le from Pezvi and buffalo from Kruger National Park showed that the two species shared 16 9% of habitat with most contacts occurring less than 500 m from the riverbed These contacts increased during the dry season suggesting that contact is driven by resource availability (Miguel et al., 2013). Incidence of FMD antibodies in ca�le was higher in sites with high buffalo contact suggesting spread of the infection from buffalo to ca�le (Miguel et al., 2013). Water points within protected areas are also a source for interaction between different wildlife species, particularly in dry seasons when water sources are limited The anthrax outbreaks which occurred in the northern section of Kruger National Park between 5 June and 11 October 1960 were all associated with natural and artificial water points where large numbers of animals aggregated around the remaining available water sources during the dry season (Pienaar, 1961).
Water sources also provide habitats for parasite-harbouring vectors such as freshwater snails and mosquitoes Freshwater snails harbour a huge number of digenean parasites some of which can cause diseases of veterinary and medical importance, such as schistosomiasis and fascioliasis Although freshwater snails within Kruger National Park have been surveyed several times in the past (De Kock and Wolmarans, 1998; De Kock et al , 2002; Wolmarans and De Kock, 2006), the diversity and distribution of digenean parasites hosted by these snails has not yet been studied. The original surveys identified the intermediate host snails for both schistosomes (Bulinus africanus, B. globosus, Bradyidius tropicus, B. forskali, Biompharia pfeifferi) and fasciolids (Lymnea columella, L. natalensis; see (De Kock and Wolmarans, 1998; De Kock et al , 2002), therefore it is important to explore the hidden digenean diversity within these snails Within Kruger National Park, schistosome species have been detected in several animals including baboons, zebra, warthog, giraffe, kudu, wildebeest, buffalo (S ma�heii; see Beechler et al., 2017; Pitchford et al., 1974) and hippopotamus (S. hippopotami and S. edwardiense; see Pitchford and Visser, 1981). Animals sampled near man made dams had higher S. ma�heei infection rates and egg outputs than those at natural water sources, suggesting perennial exposure and transmission at these sites (Pitchford et al., 1974).
4.3.4: Reservoir hosts
As seen with the ‘Microscopic Five’, most pathogens can infect more than one host (Cleaveland et al , 2001) This is true for 77% of known livestock pathogens and 60% of known human pathogens (Cleaveland et al , 2001; Haydon et al , 2002) Some hosts can act as reservoir hosts, also known as maintenance hosts (Ashford, 1997; Haydon et al , 2002; Swinton et al , 2002) Essentially, reservoir hosts can maintain the pathogen in the absence of cases in other species, and with a high enough prevalence that parasites can spill over into another host species. Identifying reservoir hosts is crucial to appropriately manage a disease. The failure to identify the importance of domestic dogs as a reservoir host for guinea worm in humans has led to the re-emergence of a disease on the brink of eradication (Durrant et al , 2020; Galán-Puchades, 2017)
In Africa, buffalo are well-known reservoir hosts for diseases such as BTb, FMD and corridor disease (CD; Michel and Bengis, 2012). Buffalo are a keystone species in the African savanna ecosystem and have a high economic value due to their importance for wildlife ecotourism, live game trade and hunting industries (Glanzmann et al., 2016). Buffalo's gregarious nature, tendency to form large herds, roam long distances, cross park boundaries and undergo regular fission fusion events make them ideal
hosts to maintain and transmit diseases (Caron et al., 2016; Cross et al., 2005; Wielgus et al., 2021). The population of buffalo in South Africa in 1998 was estimated at over 31,000, of which only 7.7% were disease-free (Winterbach, 1998). The two largest populations of buffalo in South Africa are found in Kruger National Park and Hluhluwe-iMfolozi Park both of which are infected with BTb and CD, with the Kruger population additionally being infected with FMD (Winterbach, 1998)
Within Kruger National Park, buffalo appear to have spread BTb to numerous wildlife species including warthogs, baboons (Papio ursinus) and lions (Panthera leo) Bovine Tb isolates from all three of these species were genetically highly similar and, in some cases, identical to buffalo strains (Keet et al., 2000; Michel et al., 2009). More recently, high sero-prevalence (83%) has been detected in endangered wild dogs (Lycaon pictus), thought to be eating infected prey such as warthogs (Higgi� et al., 2019). Although these dogs appeared to be healthy at the time of the study, li�le is known about how the disease may progress in this species (Higgi� et al , 2019) Another accepted reservoir for BTb is kudu (Michel and Mare, 2000; Renwick et al , 2007) Clinical manifestations of BTb in kudu include abscesses in the cranial lymph nodes from which infectious discharge is secreted onto thorns and leaves while the animal browses on vegetation (Palmer, 2013; Renwick et al., 2007).
Buffalo are also thought to play a part in the maintenance of RVF during the inter-epidemic periods. Between 2005 and 2008, a total of 227 buffalo seronegative for RVF were monitored within the park. During the 4 years, five of these buffalo became seropositive despite no outbreaks being detected in other species, which suggests circulation of the virus within the buffalo population (Beechler et al , 2015a)
4.3.5: Co-infections
Since over 80% of all known species are parasitic, co-occurrence of different parasites is the norm (Vaumourin et al., 2015). Such co-infections can be synergistic, by which one parasite facilitates the infection of other parasites, antagonistic where one parasite inhibits infection of other parasites, or can have no effect on each other (Hoarau et al., 2020; Vaumourin et al., 2015). A pathogen can alter the host's immune response making it more susceptible to others (Ezenwa et al , 2010) Co-infections of closely related parasite species or strains can lead to hybridisation, potentially creating more virulent pathogens as seen with certain schistosome species (Huyse et al , 2009) In South Africa the predominantly animal schistosome species S. ma�heei has become increasingly prevalent in humans, thought to be due to hybridisation with the human species S. haematobium (see Pitchford, 1961). This was confirmed experimentally, resulting in fertile first generation (F1) hybrids which were more infective and developed more quickly than the parents (Pitchford, 1961; Taylor, 1970; Wright and Ross, 1980) However, prior infection with S haematobium or S mansoni seems necessary for S ma�heei to become established in humans (Pitchford, 1961)
Over the last decade a body of research has been conducted assessing the impact of co-infections on disease dynamics within African parks and the findings are concerning (Beechler et al., 2015b, 2019; Broughton et al., 2021; Budischak et al., 2012; Ezenwa et al., 2010; Sylvester et al., 2017). In the Hluhluwe-iMfolozi Park, helminth infections have been shown to alter the immune response of wild buffalo, which could make them more susceptible to BTb infection (Ezenwa et al , 2010) Nematode infected individuals had a depressed Th1 immune response, which is important in controlling BTb infection and other intracellular microparasite infections, whereas Th1 responses were enhanced in hosts that were nematode resistant Disease modelling predicted that without these nematodes, BTb would not have established infection in the buffalo population (Ezenwa et al., 2010). In 2008, an outbreak of RVF occurred in a buffalo breeding facility close to the southern section of Kruger National Park. To determine the effect of existing BTb infection on the dynamics of RVF outbreak, BTb positive and BTb negative individuals were monitored for RVF before and during an outbreak Bovine Tb positive individuals had a twofold greater risk of RVF infection than BTb negative individuals Bovine Tb infection also worsened the clinical effects of RVF with pregnant co-infected individuals six times more likely to abort than those with just RVF infections (Beechler et al., 2015b). Scaled-up models of these data also showed that the presence of BTb increases the risk of RVF infection for the entire herd, not just those infected with BTb. These findings were mirrored in free-ranging buffalo within the park (Beechler et al , 2015b) Bovine Tb can also alter the composition of parasites within a host Buffalo
within Kruger National Park that acquired BTb infections showed significant increases in both taxonomic and functional parasite richness, as well as shifts in composition associated with the loss of nematodes and gain of schistosomes (Beechler et al., 2019). Co-infections may also reduce the efficacy of diagnostic tests. British calves experimentally infected with F. hepatica and M. bovis reacted less strongly to the single intradermal comparative cervical tuberculin test (SICCT) than those infected with M bovis alone (Claridge et al , 2012)
Another important co-infection that could threaten the conservation of another ‘Big Five’ species is BTb and Feline Immunodeficiency Virus (FIV) in lions (Sylvester et al., 2017). Feline Immunodeficiency Virus is endemic to these keystone predators, yet BTb was not reported in Kruger National Park's lions until 1996 (Keet et al., 2010). As FIV can cause lymphocyte deficiencies, infected lions may be predisposed to infection with BTb Within Kruger National Park, lions positive for FIV were more likely (although this was not significant with a sample size of 56) to be infected with M bovis than those negative for FIV (Sylvester et al , 2017) A more recent study concluded that total gastrointestinal parasite burden and richness was significantly higher in FIV positive lions (Broughton et al , 2021) Coinfections of the ‘Microscopic Five’ and other diseases in Kruger's wildlife need greater a�ention as this could greatly alter the dynamics of diseases previously thought to be benign. Co-infections leading to hybridisations of human and animal specific schistosome species could also hinder control efforts as the WHO currently focuses on treating human infection by mass drug administration to school age children and li�le is known about whether hybrids could be more resistant to preventative chemotherapy or whether it could change the age profile of infection This could delay the WHO's target to eliminate schistosomiasis as a public health problem by 2030 (Stothard et al., 2020; WHO, 2022).
4.4: Anthropogenic drivers of disease transmission: Wildlife-livestockhuman interface
Interactions between wildlife and livestock can drive the spread of diseases (Kock et al , 2014) Such interaction can be linear, across a fence, or focal, at a shared water hole, where pathogens from an infected animal or population can spill over into a vulnerable population via direct or indirect (vector) contact This spill-over can be bi-directional from livestock to wildlife or vice versa (Bengis et al , 2002) Humans can also be involved with pathogens spilling over from animals to humans known as sylvatic (from wildlife) or urban (domestic animals) zoonoses (Figueiredo, 2019). Bovine Tb likely first entered Kruger National Park via transmission from ca�le to buffalo in the south-western corner of the park (Bengis et al , 1996) An outbreak of BTb was then recorded in ca�le in the communal rangeland in Mpumalanga Province on the western border of the park in 2012, suggesting spill back of the disease from buffalo to ca�le (Musoke et al , 2015)
4.4.1: Permeability of wildlife fences
Wildlife fences are commonly used in protected areas to prevent the spread of disease, such as foot and mouth (FMD), between wildlife and livestock. A veterinary fence of note is the ‘Red Line’ in Namibia, erected in 1960 with the purpose of separating the FMD endemic north from the rest of the country. This 1250 km fence extends from east to west bisecting the entire country (Miescher, 2012) with unintended impacts on animal migration (Gadd, 2012) and socio-economic factors in the country South Africa has used a combination of fencing and zoning to improve FMD control, spli�ing the country into an infected zone, buffer zone and FMD free zone (Fig 2) This involved erecting a 750 km fence separating the western and southern boundary of Kruger from neighbouring communal land, private farms and private game reserves (Jori et al., 2011). The fences between the park and private reserves have since been removed to allow more space for wildlife and the combined area makes up the infected zone. Adjacent to this is a 10–20 km wide buffer zone which is split into two sections, one with vaccination and one without vaccination but increased surveillance (Jori et al , 2009; Kaszta et al , 2018)
Although Kruger National Park's fences were placed as a barrier between infected and buffer zones, they are highly permeable and, between 1996 and 2006, 1676 buffalo escaped across the park fence bordering the Mpumalanga Province (Jori et al., 2009). Semi-structured interviews of fence workers along 357 km of the western and southern border fence reported that higher numbers of kudu and impala were seen crossing over into communal land than buffalo (Jori et al., 2011). Impala are also
capable of transmi�ing the FMD virus, they can experience both clinical and sub-clinical infections and may maintain the South African Territory (SAT) serotype within local populations (Vosloo et al., 2009). The same workers also reported that the main causes of fence damage were elephant and humans, followed by predator and prey conflict, flooding and animals digging under the fence (Jori et al., 2011). Elephant will break fences to access water, desirable trees, notably the Marula (Sclerocarya birrea) or agricultural crops Such fence breakages occur more in the northwest of the park, likely due to greater elephant densities (Fig 2) There are also more rivers traversing from west to east, through the fences creating weak spots. These areas are also where livestock and wildlife may share water resources, particularly in the dry winter months which show peaks of fence breaking activity.
Between 2000 and 2007, five outbreaks of FMD occurred in ca�le near the western boundary of the park (Jori et al , 2009) The most recent outbreak of FMD in Kruger occurred in 2013/14 in ca�le in the Mpumalanga Province, adjacent to the park's western border within the inspection zone with vaccination Most of these outbreaks were a�ributed to contact between wildlife and livestock, mainly buffalo and ca�le (Blignaut et al , 2020) The reliance on fences to act as a barrier against diseases may exacerbate the problem as this may encourage farmers to relax vaccinating their livestock, or state officials to not conduct regular animal inspections for early detection of outbreaks.
4 4 2: Edge effects
Edge effects describe the impacts of interactions between habitat patches or fragments and the surrounding matrix (Suzán et al , 2012) In the case of Kruger National Park, the wildlife reserve is the habitat patch surrounded by a matrix of communal farms and villages Such edges can facilitate the emergence of infectious diseases into new areas
C parvum was first detected in Kruger in 2008 with low prevalence in elephant, buffalo and impala (4.2%, 1.4% and 1.9% respectively; Samra et al., 2011). For all three host animals, prevalence was significantly higher in areas close to the western park boundary than in the park centre (Samra et al., 2011). This suggests transmission from livestock or humans inhabiting the bordering farms and villages to wildlife within the park A subsequent molecular study within the same area detected a 2 8% prevalence of C ubiquitum in impala and C bovis in buffalo again near the western park boundary (Samra et al , 2013) Calves from the bordering communal farmlands of Bushbuckridge were also tested, revealing a prevalence of 4% for C. andersoni and 4% for C. bovis. Farmers from the area reported buffalo and impala as the most seen wild species outside the park boundary and 6.2% of these farmers reported bringing their ca�le into the park to drink (Samra et al., 2013). This may suggest spill over from either livestock and or humans to wildlife or vice versa Cryptosporidium was later confirmed in children from the same communal grazing area with a 5 6% prevalence, but predominantly infected with the anthroponotic species C hominus (see Samra et al , 2016)
4.4.3: Transfrontier conservation areas
Many protected areas across the globe are clustered along international borders that are usually fenced preventing the natural migration of large mammals. A management decision was taken to remove a number of these fences within southern Africa to create one large, protected area known as Transfrontier Conservation Areas (TFCA) and Transfrontier Parks (TFP) with the aim of conserving biodiversity and enabling the movement of large mammals, aiding socio-economic development, and promoting a culture of peace (Hanks, 2003) There are at least 13 TFCAs and TFPs within southern Africa, six of which include South Africa (Lunstrum, 2011) The Greater Limpopo Transfrontier Conservation Area (GLTFCA), created in 2002, spans the Limpopo (Mozambique), Kruger (South Africa) and Gonarezhou (Zimbabwe) National Parks and includes conservancies, wildlife ranches and communal farmland, covering a total area of 85,000 km2 (Caron et al , 2016; Ferreira, 2004) These areas have many conservation benefits particularly for animals with large home ranges, such as elephant, buffalo, and wild dog, that need more space to disperse or hunt (Caron et al., 2016; Cook et al., 2015; Davies-Mostert et al., 2012). However, there is also now increased potential for pathogen spread between wildlife, livestock and humans within these areas.
In 2009, a strain of BTb related to buffalo in Kruger National Park was identified in buffalo in Zimbabwe Telemetry studies revealed that sub-adult female buffalo were moving long distances
between the national parks and even out of the GLTFCA (Caron et al., 2016). One 2.5-year-old collared female walked 95 km over 6 days during which she crossed into Zimbabwe and Mozambique and visited a buffalo herd in the Limpopo National Park (Mozambique) as well as a commercial ca�le ranching area (Caron et al., 2016).
4.4.4:
Neighbouring game farms and private reserves
South Africa's economy is rapidly transitioning from livestock-based to wildlife-based agriculture and ecotourism, with the wildlife industry becoming the fastest growing agricultural sector (Saayman et al , 2018) Kruger National Park is bordered by several private game reserves and farms Certain private wildlife reserves on the western border of the park have removed their fences to allow free movement of animals, creating the Greater Kruger National Park Complex (GKNPC; Hlokwe et al., 2019). These reserves are under the jurisdiction of the state veterinary office Bushbuckridge East (Orpen) of the Mpumulanga veterinary services (Hlokwe et al., 2019). Although this creates larger areas for the wildlife to roam it also increases the likelihood of interspecific and intraspecific interaction, which can be a driver for disease transmission, particularly problematic when the disease status of some animals is unknown In 2013 a novel M bovis strain was identified in a blue wildebeest (Connochaetes taurinus) that had been culled after escaping a private game reserve in the GKNPC (Hlokwe et al., 2014). One or more translocations of untested blue wildebeest likely brought in this new strain (Hlokwe et al., 2014). In 2014 a different M. bovis strain, not previously detected in South Africa, was found in a female giraffe (Giraffa camelopardalis) within the same nature reserve (Hlokwe et al , 2019) A kudu on a game farm just south of Kruger National Park tested positive for an M bovis strain dissimilar to strains previously isolated within Kruger wildlife or ca�le in that region (Bengis et al , 2001) As kudu can easily cross fences and are a good candidate reservoir host, they could pose a threat of introducing new strains to the park The risk of transmission of M. bovis and other infectious diseases from wildlife is increased by the fact that testing, or even disease risk assessment, before translocation is only mandatory for buffalo and no other wildlife species or ca�le (Hlokwe et al., 2014, 2019). Thus, the translocation of untested animals (both wild and domestic) from these private farms and reserves could pose a threat to wildlife in Kruger National Park and surrounding livestock and humans
4.4.5: Human-wildlife conflicts and illegal wildlife trade
Protected areas may be thought of as a haven for wildlife with tourists and staff often the only human inhabitants, yet Kruger National Park, for instance, is bordered by seven municipalities in which approximately two million people reside (Swemmer et al., 2017). The Limpopo Province surrounding the north of the park is one of the poorest in the nation with a 67% poverty rate (Warchol and Johnson, 2009). It is therefore important to understand how people and their activities can influence disease dynamics within and around parks and this must be factored into management decisions A major threat to the biodiversity and conservation is the illegal wildlife trade, driven by inequality, poverty, food insecurity and increasing human populations (Bezerra-Santos et al , 2021; Lindsey et al , 2013) The illegal wildlife trade is also a significant risk factor for the spread of zoonotic pathogens (Bezerra-Santos et al., 2021). The trade directly threatens one of Kruger National Park's ‘Big Five’, the white rhinoceros, poached for their horns to be sold on the Asian market (Annecke and Masubelele, 2016).
Hunting of other game including buffalo and kudu for bush meat is also on the rise in Kruger What was once a local subsistence practice, the bush meat trade is now a commercialised, global enterprise and is no longer sustainable (Warchol and Johnson, 2009) Interviews with communities neighbouring Kruger National Park suggested that bush meat was poached from the park and readily marketed by local merchants (Warchol and Johnson, 2009). Several social and cultural factors influenced this including the affordability of bush meat and the use in traditional weddings, as well as the perception that parks were not benefiting local communities. There were also reports of game rangers in the park being complicit with poachers (Warchol and Johnson, 2009) Although there are no studies in Kruger National Park assessing the role of poaching and bush meat in the spread of zoonotic diseases to humans or their livestock, these activities have been linked to parasitic outbreaks in humans across the globe (Bezerra-Santos et al , 2021) Risk assessments are needed to determine the threat of zoonotic disease spread through the trade and consumption of bush meat from Kruger National Park. Poaching