Contributors
MaxineP.Bonham DepartmentofNutrition,DieteticsandFood,SchoolofClinicalSciencesatMonashHealth,FacultyofMedicineNursingand HealthSciences,MonashUniversity,Clayton, VIC,Australia
FangCai SchoolofLifeSciences,GuizhouNormalUniversity,Guiyang,China
JanePei-ChenChang DepartmentofPsychiatry andMind-BodyInterfaceLaboratory(MBI-Lab), ChinaMedicalUniversityHospital,Taichung, Taiwan;DepartmentofPsychologicalMedicine, InstituteofPsychiatry,PsychologyandNeuroscience,King’sCollegeLondon,London,United Kingdom;SchoolofMedicine,CollegeofMedicine,ChinaMedicalUniversity,Taichung, Taiwan
DanielTzu-LiChen DivisionofNeuroscience, GraduateInstituteofBiomedicalSciences,China MedicalUniversity;DepartmentofPsychiatry andMind-BodyInterfaceLaboratory (MBI-Lab),ChinaMedicalUniversityHospital; SchoolofChineseMedicine,CollegeofChinese Medicine,ChinaMedicalUniversity,Taichung, Taiwan
JocelynChia-YuChen DepartmentofPsychiatry andMind-BodyInterfaceLaboratory(MBI-Lab), ChinaMedicalUniversityHospital;SchoolofChineseMedicine,CollegeofChineseMedicine,China MedicalUniversity,Taichung,Taiwan
Zhen-YuChen SchoolofLifeSciences,TheChinese UniversityofHongKong,Shatin,NewTerritories, China
KatyaM.Clark Nutrition&Dietetics,CurtinSchool ofPopulationHealth,CurtinUniversity,Bentley, WA,Australia
PermalDeo ClinicalandHealthSciences,UniversityofSouthAustralia,Adelaide,SA,Australia
VarinderpalDhillon ClinicalandHealthSciences, UniversityofSouthAustralia,Adelaide,SA, Australia
MichaelFenech ClinicalandHealthSciences,UniversityofSouthAustralia,Adelaide,SA,Australia
YuanqingFu LaboratoryofPrecisionNutritionand ComputationalMedicine,SchoolofLifeSciences, WestlakeUniversity,Hangzhou,China
Xiao-feiGuo InstituteofNutrition&Health; SchoolofPublicHealth,QingdaoUniversity,Qingdao,China
CanxiaHe SchoolofMedicine,NingboUniversity, Ningbo,China
Wen-SenHe SchoolofFoodandBiologicalEngineering,JiangsuUniversity,Zhenjiang,Jiangsu,China
DhanushkaHettiarachchi SchoolofPharmacy, CurtinHealthInnovationResearchInstitute,CurtinUniversity,Perth,WA,Australia
XiangHu DepartmentofEndocrineandMetabolic Diseases,TheFirstAffiliatedHospitalofWenzhou MedicalUniversity;InstituteofLipidsMedicine& SchoolofPublicHealthandManagement,WenzhouMedicalUniversity,Wenzhou,China
XiaojieHu CollegeofLifeScience,LinyiUniversity, Linyi,China
Jian-YingHuang DepartmentofFoodScienceand Technology,SchoolofFoodScienceandBiotechnology,ZhejiangGongshangUniversity,Hangzhou,China
TaoHuang DepartmentofEpidemiologyand Biostatistics,SchoolofPublicHealth,Peking University;KeyLaboratoryofMolecularCardiovascularSciences,PekingUniversity,Ministryof Education;CenterforIntelligentPublicHealth, InstituteforArtificialIntelligence,PekingUniversity,Beijing,China
CatherineE.Huggins DepartmentofNutrition, DieteticsandFood,SchoolofClinicalSciencesat MonashHealth,FacultyofMedicineNursingand HealthSciences,MonashUniversity,Clayton, VIC,Australia
Ling-ShenHung DepartmentofFoodScience,CollegeofChemistryandFoodScience,YulinNormal University,Yulin,Guangxi,China
AnthonyP.James Nutrition&Dietetics,Curtin SchoolofPopulationHealth,CurtinUniversity, Bentley,WA,Australia
AnuraP.Jayasooriya DepartmentofBasicVeterinarySciences,FacultyofVeterinaryMedicine andAnimalScience,UniversityofPeradeniya,Peradeniya,SriLanka
MinJia Grain&OilEngineeringLab,Instituteof Agro-foodScienceandTechnology,Shangdong AcademyofAgriculturalSciences,Jinan,China
SarahD.Lee DepartmentofNutrition,Dietetics andFood,SchoolofClinicalSciencesatMonash Health,FacultyofMedicineNursingandHealth Sciences,MonashUniversity,Clayton,VIC, Australia
DuoLi InstituteofNutritionandHealth,Qingdao University,Qingdao,Shandong;Departmentof FoodScienceandNutrition,ZhejiangUniversity, Hangzhou,Zhejiang,China;DepartmentofNutrition,DieteticsandFood,MonashUniversity, Melbourne,VIC,Australia
JiaomeiLi DepartmentofNutritionandFood Hygiene,ZhejiangChineseMedicalUniversity, Hangzhou,China
KeleiLi InstituteofNutritionandHealth;DepartmentofNutritionandFoodHygiene,SchoolofPublicHealth,QingdaoUniversity,Qingdao,China
YandiLiu SchoolofPharmacy,CurtinHealthInnovationResearchInstitute,CurtinUniversity,Perth, WA,Australia
Wen-JunMa InstituteofNutrition&Health;School ofPublicHealth,QingdaoUniversity,Qingdao, China
AmalD.Premarathna SchoolofNaturalSciences andHealth,TallinnUniversity,Tallinn,Estonia
CeQi InstituteofNutritionandHealth,Qingdao University,Qingdao,China
YanShi InstituteofNutritionandHealth;DepartmentofNutritionandFoodHygiene,Schoolof PublicHealth,QingdaoUniversity,Qingdao, China
AndrewJ.Sinclair DepartmentofNutrition,DieteticsandFood,MonashUniversity,Melbourne; FacultyofHealth,DeakinUniversity,Geelong, Australia
AnishSingh InstituteofNutrition&Health,QingdaoUniversity,Qingdao,China
Kuan-PinSu DivisionofNeuroscience,Graduate InstituteofBiomedicalSciences,ChinaMedical University;DepartmentofPsychiatryandMindBodyInterfaceLaboratory(MBI-Lab),China MedicalUniversityHospital,Taichung,Taiwan; DepartmentofPsychologicalMedicine,Institute ofPsychiatry,PsychologyandNeuroscience, King’sCollegeLondon,London,UnitedKingdom; SchoolofMedicine,CollegeofMedicine,China MedicalUniversity,Taichung;DepressionCenter, An-NanHospital,ChinaMedicalUniversity, Tainan,Taiwan
JinSun InstituteofNutritionandHealth,Qingdao University,Qingdao,China
BruceSunderland SchoolofPharmacy,Curtin HealthInnovationResearchInstitute,CurtinUniversity,Perth,WA,Australia
JunTang LaboratoryofPrecisionNutritionand ComputationalMedicine,SchoolofLifeSciences, WestlakeUniversity,Hangzhou,China
YunyiTian SchoolofLifeSciences,WestlakeUniversity,Hangzhou,China
YiWan DepartmentofFoodScienceandNutrition, ZhejiangUniversity,Hangzhou,China
LingWang DepartmentofFoodMicrology,College ofFoodScienceandTechnology,HuazhongAgriculturalUniversity,Wuhan,Hubei,China
Xiang-YangWang DepartmentofFoodScience andTechnology,SchoolofFoodScienceandBiotechnology,ZhejiangGongshangUniversity, Hangzhou,China
Li-LiXiu DepartmentofFoodScienceandTechnology,SchoolofFoodScienceandBiotechnology, ZhejiangGongshangUniversity,Hangzhou,China
TongchengXu Grain&OilEngineeringLab,InstituteofAgro-foodScienceandTechnology,ShangdongAcademyofAgriculturalSciences,Jinan,China
BoYang InstituteofLipidsMedicine&Schoolof PublicHealthandManagement,WenzhouMedical University,Wenzhou,China
RenqiangYu DepartmentofNeonatology,The AffiliatedWuxiMaternityandChildHealthCare HospitalofNanjingMedicalUniversity,Wuxi, China
GaofengYuan DepartmentofFoodScience,College ofFoodandMedicine,ZhejiangOceanUniversity, Zhoushan,China
XiaohongZhang SchoolofMedicine,NingboUniversity,Ningbo,China
Ju-ShengZheng SchoolofLifeSciences,Westlake University,Hangzhou,China
ZhenhuangZhuang DepartmentofEpidemiology andBiostatistics,SchoolofPublicHealth,Peking University,Beijing,China
ZuquanZou BeilunDistrictCenterforDisease ControlandPrevention,Ningbo,Zhejiang, China
Overviewofdietarylipidsandhuman health
DuoLi
InstituteofNutritionandHealth,QingdaoUniversity,Qingdao,Shandong,China
DepartmentofFoodScienceandNutrition,ZhejiangUniversity,Hangzhou, Zhejiang,China
DepartmentofNutrition,DieteticsandFood,MonashUniversity,Melbourne, VIC,Australia
1.1Introduction
Inthepastfourdecades,therehasbeen extensiveresearchondietarylipidsand non-communicablediseasesandtheirriskfactors.Thischaptersystematicallyreviewedthe effectsofdietaryfatintakeonhumanhealth basedontheresultsofmeta-analysisofrecent randomizedcontrolledtrialsorcohortstudies. Meta-analysiscombinesthesimilarresultsof individualstudies,enablingustomakefull useofallinformation,andimprovestheaccuracyandpowerofintervention/treatmenteffect evaluation(Moher,Liberati,Tetzlaff,&Altman, 2009).Uncommondietarylipids,lipidsinfood withaverylowcontent,suchastrans-fatty acids,conjugatedlinoleicacids,furanfatty acids,andmonoacylglycerols,and manufactory-producedstructuredlipidsare notincludedinthechapterandthebook.
1.2Dietarylipids
Dietarylipidsareimportantsubstancesfound infood.Theyplayamajorroleintaste,texture, color,andflavor.Inthehumanbody,theymaintaintheintegrityandfunctionofcellsandserve asenergystorage.Dietarylipidsareinsolublein waterandsolubleinmanyorganicsolvents.This propertyofnonpolarityandhydrophobicityis largelycontrolledbyfattyacids.Mostdietary lipidsaremoleculesthatcontainorarederived fromfattyacids.Thelipidinformationrequired bythefooddatabaseforcalculatingenergycontentandcomparisonbetweenfoodsincludes contentandcompositionoftotalfat,fattyacids (includingsaturates,cis-andtransmonounsaturates,n-6andn-3polyunsaturates), andsterols.Fat-solublevitaminswillnotbediscussedinthisbook,eventhoughthesevitamins canbeclassifiedaslipids.
Dietarylipidscanbedividedintotwocategories:thenonpolarlipids(suchastriacylglycerol andcholesterylesters)andthepolarlipids (amphiphilic,becausetheycontainbothhydrophobicandhydrophilicdomainsinthesame molecule,suchasphospholipids).Morethan 95%ofdietarylipidsareacylglycerols,andtriacylglycerol(TG)ismostpredominantone,in whichthethreehydroxylgroupsofglycerol areesterifiedwithonefattyacid.Theycontain fattyacidswithdifferentchainlengthsandsaturationlevels.Vegetableoils,suchassoybean andsunfloweroil,aremainlycomposedofTG withunsaturatedfattyacids,sotheyareliquid atroomtemperature.TGcontainsonlysaturatedfattyacids,suchastristearin,themain componentofbeeffat,whichisawhitegreasy solidatroomtemperature.Monoacylglycerols, diacyglycerols,andfreefattyacidsarepresent onlyinrelativelyminoramountsandoccur largelyasmetabolicintermediatesinthebiosynthesisanddegradationofglycerol-containing lipids.
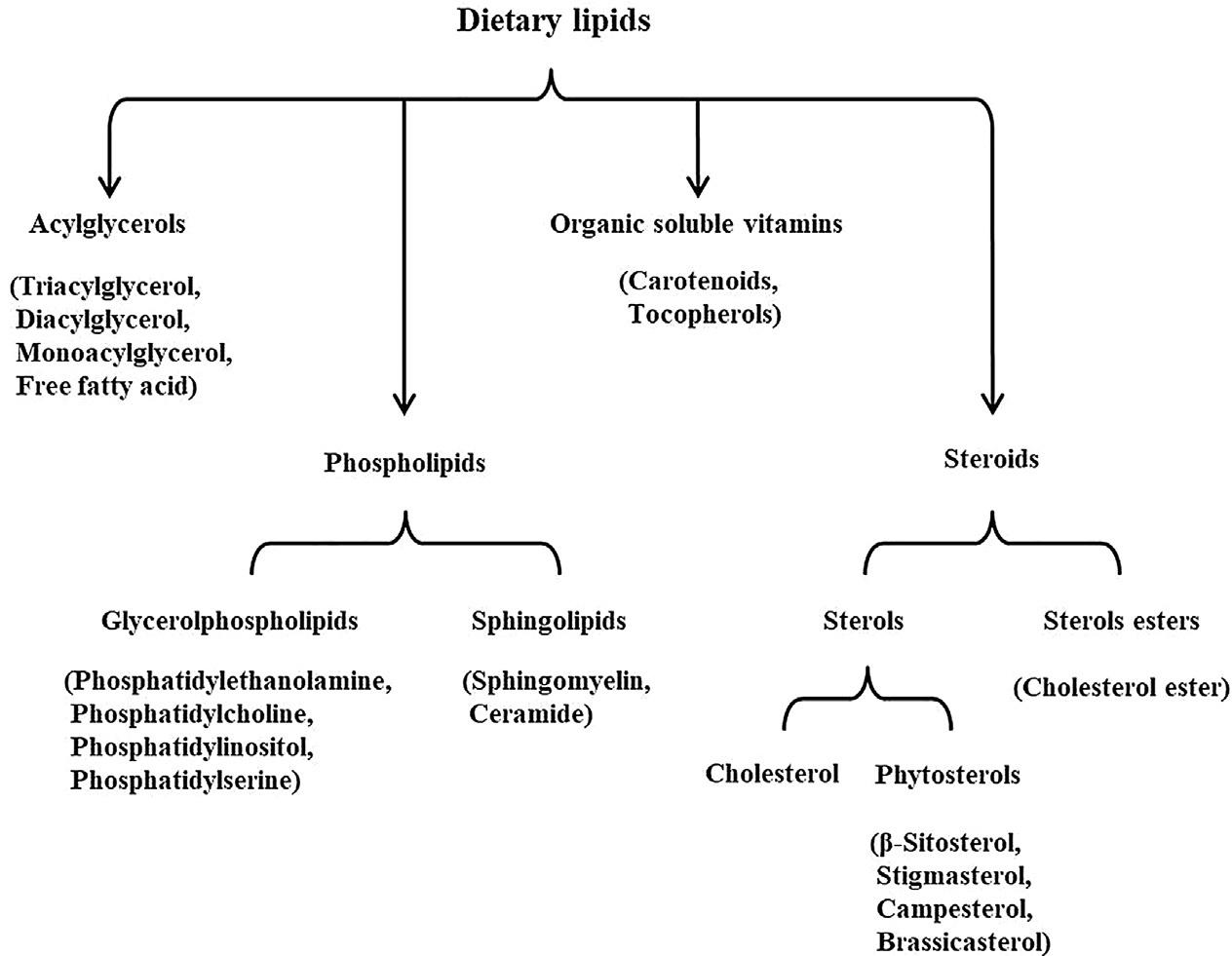
Allanimalfoods,includingseafood,contain differentamountsofcholesterol,becauseallanimalcellsmakecholesterol.Themaindietary sourcesofcholesterolincludeyolkandwhole egg,redmeat,offal,seafood,anddairyproducts.Humanbreastmilkalsocontainsgenerous cholesterol.Cholesterolissynthesizedbyplant cellsonlyasanintermediate(precursor)ofother compounds,suchasphytosterols.Therefore, thereislittleornocholesterolinplantfood.Vegetableoilisamainsourceofdietaryphytosterols.Themostcommondietaryphytosterols are β-sitosterol,campesterol,stigmasterol,and stigmastanol.Phospholipidsaredividedinto twomainclasses:glycerophospholipidsand phosphosphingolipids,dependingonwhether theycontainaglycerolorasphingosinebackbone.Themaindietaryglycerophospholipids arephosphatidylethanolamine(cephalin,PE), phosphatidylcholine(lecithin,PC),phosphatidylserine(PS),andphosphatidylinositol(PI).
Themainphosphosphingolipidsareceramides andsphingomyelins.Majorsourcesofdietary phospholipidsaresoya,rapeseed,sunflower, eggs,milk,seafood,eggs,etc.(Fig.1.1).
Plant/vegetableseedoilandanimalfatare themostpredominantdietaryfatforhuman beings.Fattyacidsarethefunctionalgroupof mostdietarylipids.Naturaldietaryfattyacids canbeclassifiedassaturated,monounsaturated fattyacids(MUFA),andpolyunsaturated fattyacids(PUFA).Animalfatsareamajor sourceofsaturatedfattyacids,aswellascoconutoilandcertaintropicalplantoilsuchaspalm oil.Oleicacid(C18:1)isthemostabundant monounsaturatedfattyacidinthediet,andolive oilisprobablythemostwell-knownsourceof C18:1.
PUFAhasfourfamilies,thatis,n-7,n-9,n-6, andn-3,derivedfromthefourparentfattyacids, palmitoleicacid(C16:1n-7),oleicacid(C18:1n9),linoleicacid(C18:2n-6,LA),anda-linolenic acid(C18:3n-3,ALA).C18:2n-6andC18:3n-3 areessentialfattyacidsforhumans.C18:2n-6 isthepredominantPUFAintheglobalfood chain,whichiscommonlyfoundinplant/vegetableseedoils.C18:3n-3islessabundantthan C18:2n-6,andisthepredominantn-3PUFA foundinplantfoodandcommonlyfoundin someplant/vegetableoilssuchasflaxseed, perilla,chiaseed,canola,soybean,andwalnut oils.C18:3n-3andC18:2n-6canbeconverted invivotoC20andC22longchain(LC)PUFA inhumans(Li,2015).Eicosapentaenoicacid (C20:5n-3,EPA)isapredominantLCn-3PUFA inmostfishandfish/marineoils,docosahexaenoicacid(C22:6n-3,DHA)ismostabundantLC n-3PUFAintunaandtunaoil(Sinclair,Oon, Lim,Li,&Mann,1998),whiledocosapentaenoic acid(C22:5n-3,DPA)isamajorLCn-3PUFAin certainseafoodsuchasabalone(Su,Antonas,& Li,2004),leanmeat,andmeatproducts(Li,Ng, Mann,&Sinclair,1998).Stearidonicacid(18:4n3,SDA)isfoundinRibesberriesandBoraginaceaefamilies(Li&Hu,2011)(Fig.1.2).
1.3Dietarytotalfatintakeandhuman health
FAO/WHOrecommendedthatthedietary intakeoftotalfatshouldnotexceed30%oftotal energyforthegeneralpopulation,and35%for heavyphysicalpersonnel(FAO,2010).Comparedwithconventionalorguidelinerecommendeddietaryfatintake,increasingtotal fatintakecansignificantlyincreasebodyweight, bodymassindex(BMI),waistcircumference, serumtotalcholesterol(TC),andlowdensity lipoproteincholesterol(LDL-C)undertheconditionofequalcalories.
Inasystematicreviewandmeta-analysis, whichincluded32RCTs,31studiesinhealthy adults,and1inchildren,involvingapproximately54,000participantswithanintervention periodof6monthsto >8years,excludingthe studieswithonlysubjectBMI >25kg/m2,all includedstudieswerefromdevelopedcountries
(21fromNorthAmerica,9fromEurope,and1 fromNewZealand),andthereisnostudyfrom developingortransitionalcountries.Withthe isocaloricdiets,thestudyfoundthatreducing thedietaryproportionoffat(30%energy) resultedinaslightbutsignificantdecreasein bodyweight,bodymassindex,andwaistcircumferencecomparedwithhabitualdietaryfat intake.Thiseffecthasbeenfoundinbothadults andchildren.Theeffectdidnotchangeover time.Comparedwithhabitualormodifiedfat diets,thelow-fatdietcouldreduceserumconcentrationsoftotalcholesterol(TC)andlowdensitylipoproteincholesterol(LDL-C),but hadnosignificanteffectonhigh-densitylipoproteincholesterol(HDL-C)andTG.Thereis noevidencethatalow-fatdietisharmfulto humanhealth(Hooperetal.,2015).
Asix-monthrandomizedcontrolledfeeding trialintwocenters,threearms,fromChina,found consistentresultswiththeabovemeta-analysis,
FIG.1.1 Classificationofmaindietarylipid. Nopermissionrequired.
inwhich,307healthyyoungadults,aged 18–35yearsandwithbodymassindexlessthan 28,wererandomlyassignedtothethreeisocaloric diets:thelowerfatdiet(carbohydrate66%,fat 20%energy),themoderatefatdiet(carbohydrate 56%,fat30%energy),andthehigherfatdiet (carbohydrate46%,fat40%energy)withsame protein(14%energy).Thebodyweight,BMI, andwaistcircumferenceofthesubjectsinthe
low-fatandhigh-carbohydratedietintervention groupweresignificantlylowerthanthoseinthe othertwogroups.SerumconcentrationsofTC, LDL-C,non-HDL-C,HDL-C,andplasmaproinflammatoryfactorssuchasleukotrieneB4, thromboxaneB2, prostaglandinE2, andhighsensitivityC-reactiveproteinweresignificantly lowerinthelow-fatandhigh-carbohydratediet interventiongroupthanthoseintheothertwo
FIG.1.2 Metabolismofessentialfattyacidsinvivo. Nopermissionrequired.
groups(Wanetal.,2019; Wanetal.,2017).Based ontheresults,theauthorsinterpretedthatthe possiblebenefitsofahigh-fatdietinEuropean andNorthAmericanpopulationsarenotapplicabletootherethnicgroups,atleastnottoChinese people.
Thereareseveralrandomizedcontrolled trials(RCTs)conductedbyEuropeanandAmericanresearchersonpatientswithobesityormetabolicdiseasesusingketogenicandAtkinsdiets comparedwithtraditionaldietsordietsrecommendedbydietaryguidelines.Theyfoundthat short-term,high-fat,andlow-carbohydratediets causedweightlossandserumTGreductionin obeseormetabolicdiseasesubjects(Gardner etal.,2007; Moreno,Crujeiras,Bellido, Sajoux,&Casanueva,2016).Basedonthesefindings,somepeoplesuggestthattherestrictionof carbohydrates,ratherthanthereductionoffat, shouldberecommendedasthemostappropriate nutritionalmethodtocontrolobesityandreduce theriskofcardiovascularandmetabolicdiseases. However,thegenesencodingfat,carbohydrate, andmetabolism-relatedenzymesinpatientswith obesityormetabolicdiseasesaredifferentfrom thoseinhealthypeople,andtheresponseofthese volunteerstofatandcarbohydrateisalsodifferentfromthatinhealthypeople,whichmaybe ignored.
1.4Dietarysaturatedfatintakeand humanhealth
Animalfat,butter,palmoil,andcoconutoil havebeenconsideredassaturatedfat.FAO/ WHOrecommendsthatthedietaryintakeofsaturatedfatshouldnotexceed10%oftotalenergy. ReplacementofMUFAandPUFAwithisocaloricSFAsignificantlyincreasedserumTCand LDL-C,resultinginasignificantlyincreasedrisk ofCVDevents.
Inasystematicreviewandmeta-analysisof dietarysaturatedfatintakeandcardiovascular diseaseincluded15RCTswithapproximately
59,000participants.Resultsfrom11long-term trials( 2years)with53,3000subjectsshowed thatreducingdietaryintakeofsaturatedfatacid (SFA)significantlyreducedtheriskofcombined cardiovasculareventsby21%.Themetaregressionshowedthatagreaterreductionin dietaryintakeofSFAledtoatremendousreductioninserumTC,resultinginagreaterreduction intheriskofcardiovasculardisease(CVD) events.SubstitutingthedietaryenergyinSFA withPUFAorcarbohydrateseemstobeauseful strategy,whiletheeffectofreplacingSFAwith MUFAisunclear.Thereductionincardiovasculareventsduetoreduceddietaryintakeofsaturatedfatwasnotaffectedbystudyduration, gender,orbaselinelevelofcardiovascularrisk. ReducingdietaryintakeofSFAcanbenefit peoplewhoarecurrentlyhealthy,whohave anincreasedriskofheartdiseaseorstroke (e.g.,peoplewithhighbloodpressure,high serumTC,andLDL-Cordiabetes),andwho alreadyhaveheartdiseaseorstroke(Hooper etal.,2020).Anothersystematicreviewand meta-analysisofRCTsfoundwhenthedietary intakeofSFAwasreducedandreplacedwith PUFA,serumglucose,glycosylatedhemoglobin A1c(HbA1c),C-peptideandhomeostasismodel assessmentforinsulinresistance(HOMA-IR) weresignificantlydecreased(Imamuraetal., 2016).
Palmoilisoneofthemostusedsaturatedfats globally.Itiscomposedofapproximately50% palmiticacid,40%oleicacid,and10%linoleic acid.Ameta-analysisofRCTsfoundthatpalm oilsignificantlyincreasedserumTC,LDL-C, andHDL-C,butnotTGcomparedwithMUFA andPUFAsuchasoliveoilandsafflower/sunfloweroil(Hisham,Aziz,Huin,Haur,& Jamil,2020).
ComparedwithC18:0,MUFAsandPUFAs enrichdiets,apartfromTC,LDL-C,andHDL-C, palmoilalsosignificantlyincreasedapolipoproteinBandapolipoproteinA-I.However,when comparedwithmyristic/lauricacid-enriched diet,palmoilshowedasignificantlylower
serumconcentrationsofTC,HDL-C,and apolipoproteinA-I(Fattore,Bosetti,Brighenti, Agostoni,&Fattore,2014).
AWHOpublicationentitled“Effectsofsaturatedfattyacidsonserumlipidsandlipoproteins:asystematicreviewandregression analysis”summarizedthatthemixtureofSFA wasreplacedbycis-PUFA(mainlylinoleicacid and α-linolenicacid)orcis-MUFA(mainlyoleic acid),whichwasmorebeneficialonserum lipidsandlipoproteinsprofilethanreplacing SFAwithamixtureofcarbohydrates.Especially forTC,LDL-C,andTG,PUFAshavethebest effect.Comparedwiththemixtureofcarbohydrates,increasingdietaryintakeofindividual C12:0,C14:0,orC16:0increasedserumconcentrationsofTC,LDL-C,andHDL-C,and decreasedTG,whileincreasingC18:0intake didnotseemtohaveasignificanteffecton theseserumconcentrationsoflipidsandlipoproteinsascomparedwithcarbohydrate. Comparedwithcarbohydrate,C12:0decreased theratiosofTC:HDL-CandLDL-C:HDL-C ( Mensink,2016 ).
1.5Dietarymonounsaturatedfatintake andhumanhealth
ThereisnorecommendedacceptablemacronutrientdistributionrangeforMUFA.Onthe premisethatthetotalfatdoesnotexceed30% ofthetotalenergy,subtractingSFAandPUFA istheintakeofMUFAforthegeneralpopulation.IsocaloricallysubstitutingSFAandcarbohydratewithMUFAhasabeneficialeffecton metabolicriskfactorsinhealthyadultsubjects andtype2diabeticpatients.
Inasystematicreviewandmeta-analysisof RCTs,themetaboliceffectsofhigh-MUFAand high-carbohydratedietswerecomparedin 24studieswith1460participants,andhighMUFAandhigh-PUFAdietsin4studieswith 44participants.Comparingahigh-MUFAdiet withahigh-carbohydratediet,therewas
significantreductioninfastingplasmaglucose, TAG,bodyweight,andsystolicbloodpressure, andsignificantincreasesinHDL-C.Comparison ofhigh-MUFAdietwithhigh-PUFAdiets showedsignificantlylowerfastingplasmaglucose(Qian,Korat,Malik,&Hu,2016).
Inanothersystematicreviewandmetaanalysisofrandomizedcontrolledfeedingtrials, itincluded102trials,239dietarms,and4220 adults.Thedose-responseeffectofisocaloric substitution,adjusteddietaryintakesofprotein, trans-fat,anddietaryfibershowedthatHbA1c, 2-hpostchallengeinsulin,andHOMA-IRwere significantlyloweredwhendietarycarbohydratewasreplacedbyMUFA(Imamuraetal., 2016).
1.6Dietarypolyunsaturatedfatintake andhumanhealth
Therecommendedacceptablemacronutrient distributionrangeforPUFAis6%–11%oftotal energy.IsocaloricreplacementofSFAwith PUFAhasabeneficialeffectonreductionof theriskofcardiovascularandmetabolicdiseases.Theeffectofn-3PUFAonNCDshasbeen extensivelystudiedduringthepastthree decades.Increasingdietaryintakeofn-3PUFA canreducetheriskofcardiovasculardisease, type2diabetes,breastcancer,acuterespiratory distresssyndrome,nonalcoholicfattyliverdisease(NAFLD),andchronickidneydisease, reduceserumconcentrationoftriacylglycerol andbloodpressure,andimprovecognitive anddepressionsymptoms.
1.6.1Dietarypolyunsaturatedfatintake andcardiovasculardiseasesandriskfactors
N-3PUFAshavebeneficialeffectsoncardioprotection,andreducingcardiovascularmortality,CHDmortality,andevents.Inthemost extensivesystematicreviewandmeta-analysis ofn-3PUFAfortheprimaryandsecondar
preventionofcardiovasculardisease,which included86RCTsand162,796participants, studylengthwasbetween12and88months. MosttrialsusedLCn-3PUFAcapsules,some adaptedLCn-3PUFAorC18:3n-3-richor enrichedfoodsordietaryadvicecomparedwith placebocontrolorhabitualdiet.Dosageranged from0.5gto >5gdaily(19RCTsgaveatleast3g LCn-3PUFAaday).Resultsindicatedthat increasedLCn-3PUFAreducedcardiovascular mortality(RR ¼ 0.92,95%CI:0.86–0.99),coronaryheartdiseasemortality(RR ¼ 0.90,95% CI:0.81–1.00),coronaryheartdiseaseevents (RR ¼ 0.91,95%CI:0.85–0.97),andserumtriacylglycerolby 15%inadose-dependentmanner.IncreaseddietaryintakeofALAreduced riskofarrhythmia(RR ¼ 0.73,95%CI: 0.55–0.97)andlightlyreducedriskofCVD events(RR ¼ 0.91,95%CI:0.79–1.04) (Abdelhamidetal.,2020).
Inasystematicreviewwithoutametaanalysis,theauthorsconcludedthatEPAand DHAsignificantlyreducedserumtriglycerides infiveofthesixrandomizedcontrolledtrials comparedwithplacebo.Thereductionranged from12%to26%comparedwithbaseline.There isevidencethatDHAmaybemoreeffectivein reducingserumtriglyceridesthanEPA (Innes&Calder,2018).
TheexistingevidenceprovidedbyRCT showsthatsupplementationofLCn-3PUFA canreducesystolicbloodpressure,and diastolicbloodpressurecanbereducedwhen thesupplementdoseisgreaterthan2g.Ina meta-analysisof70RCTs,supplementation ofEPA+DHAreducedbothsystolic ( 1.52mmHg;95%CI ¼ 2.25to 0.79)and diastolicbloodpressure( 0.99mmHg;95% CI ¼ 1.54to 0.44)comparedwithplacebo. ThestrongesteffectofEPA+DHAsupplementationwasobservedinuntreatedhypertensive subjectswithreductionofsystolicbloodpressure4.51mmHg(95%CI ¼ 6.12to 2.8)and diastolicbloodpressure3.05mmHg(95% CI ¼ 4.35to 1.74),althoughbothsystolic
bloodpressure( 1.25mmHg,95%CI ¼ 2.05 to 0.46)anddiastolicbloodpressure ( 0.62mmHg,95%CI ¼ 1.22to 0.02)also decreasedinnormotensivesubjects(Miller, VanElswyk,&Alexander,2014).
1.6.2Dietarypolyunsaturatedfatintake anddiabetesmellitus
IncreaseddietaryintakeoftotalPUFAand decreasedratioofn-6/n-3PUFAhavebeneficial effectsonreducingriskofdiabetes.Replacing carbohydratewithPUFAsignificantlylowered HbA1c( 0.11%; 0.17, 0.05)andfastinginsulin( 1.6pmol/L; 2.8, 0.4).ReplacingSFA withPUFAsignificantlyloweredglucose, HbA1c,C-peptide,andHOMA.Basedongoldstandardacuteinsulinresponsein10trials, PUFAsignificantlyimprovedinsulinsecretion capacity(+0.5pmol/L/min;0.2,0.8)whether replacingSFA,carbohydrate,orevenMUFA (Imamuraetal.,2016).
Ameta-analysisofdietaryPUFAintakeand type2diabetesincluded11RCTsandatotal of458participants(229ineachtreatmentand controlgroup,respectively).Low-ration-6/n-3 PUFAsupplementationcandecreasetheplasma insulinlevel(WMD: 3.010mIUL 1;95%CI: 5.371to 0.648mIUL 1)andinsulinresistance index(WMD: 0.460;95%CI: 0.908to 0.012) (Lietal.,2019).
1.6.3Dietarypolyunsaturatedfatintake andcancers
DietaryintakeoftotalPUFAandindividual PUFAmayhavedifferenteffectsondifferent typesofcancer.PooledRCTsshowedthatneitherdietaryintakeoftotalPUFA,n-3,norn-6 PUFAwasassociatedwithcancerincidence. However,pooledprospectivecohortstudies suggestedthatincreaseddietaryintakeofLC n-3PUFAissignificantlyassociatedwith decreasedriskofbreastcancer.
Inasystematicreview,itincluded47RCTs with108,194participants.Increasingdietary intakeoftotalPUFAmayslightlyincrease,or noeffectontheriskofcancerdiagnosis (RR ¼ 1.19,95%CI0.99–1.42)andcancerdeath (RR ¼ 1.10,95%CI:0.48–2.49).LCn-3PUFAhas littleornoeffectoncancerdiagnosis(RR ¼ 1.02, 95%CI:0.98–1.07),cancerdeath(RR ¼ 0.97,95% CI:0.90–1.06),orbreastcancerdiagnosis (RR ¼ 1.03,95%CI:0.89–1.20).IncreasingALA haslittleornoeffectoncancerdeath(RR ¼ 1.05, 95%CI:0.74–1.49).Increasingdietaryintakeof LCn-3PUFA(RR ¼ 1.10,95%CI:0.97–1.24)and ALA(RR ¼ 1.30,95%CI:0.72–2.32)mayslightly increaseriskofprostatecancer,ornosignificant effect(Hansonetal.,2020).
Inanothermeta-analysisofn-3PUFAand breastcancer,itincluded26publicationswith 883,585participantsand20,905casesofbreast cancerfrom21independentprospectivecohort studies.LCn-3PUFAwassignificantlynegativelyassociatedwithbreastcancerrisk (RR ¼ 0.86,95%CI:0.78–0.94)in17publications from16independentcohortstudiesinvolving 527,392participantsand16,178breastcancer cases.Dose-responseanalysisshoweda5% reductionofbreastcancerriskwith0.1g/day (RR ¼ 0.95,95%CI:0.90–1.00)or0.1%energy/ day(RR ¼ 0.95,0.90–1.00)incrementofdietary intakeofLCn-3PUFA.Totaln-3PUFAintake wassignificantlynegativelyassociatedwithrisk ofbreastcancer(RR ¼ 0.77,95%CI:0.60–0.99) onlyinstudieswithoutadjustmentforBMI. ForindividualLCn-3PUFA,thesignificant inverseassociationwithriskofbreastcancer wasobservedonlyinstudieswithshorter follow-up,RR ¼ 0.82(95%CI:0.70–0.96)for C20:5n-3,and0.74(0.62–0.89)forC22:6n-3 (Zheng,Hu,Zhao,Yang,&Li,2013).
1.6.4Dietarypolyunsaturatedfatintake andrespiratorydiseases
Then-3PUFAshavebeneficialeffectson respiratorydiseases.Earlyuseofn-3PUFAin
patientswithacuterespiratorydiseasecan significantlyreducemortality.
Inasystematicreview,ameta-analysisevaluatedtheimpactofn-3PUFAonsevereacute respiratorydistresssyndromein12RCTs including1280patients.Resultsshowedthat Horowitzindex wassignificantlyincreasedwith administrationofn-3PUFAsinearlystage (WMD ¼ 49.33,95%CI:20.88–77.78, P ¼ 0.0007, I2 ¼ 69%),measuredbyPaO2-to-FiO2 ratio(ratio ofpartialpressureofoxygeninblood(mmHg) andthefractionofoxygenintheinhaledair), lastsuntilthe7thto8thday(WMD ¼ 27.87; 95%CI:0.75–54.99; P ¼ .04; I2 ¼ 57%).Continuousenteralinfusionofn-3PUFAsignificantly reducedmortality(P ¼ .02).However,theanalysislimitedtoenteraladministrationwithor withoutbolusinjectionallimprovedearlyratio ofPaO2 toFiO2 (P ¼ .001),andmechanicalventilationduration(P ¼ .03)(Langlois,D’Aragon, Hardy,&Manzanares,2019).
1.6.5Dietarypolyunsaturatedfatintake andcognition
HigherintakeofLCn-3PUFAwillincrease cognitivefunctionandimprovesymptomsof childrenandadolescentswithAttentionDeficit HyperactivityDisorder(ADHD).Theeffectof n-3PUFAoncognitivefunctiondependson thespecificstudysubjectsanddosage.Increased tissuelevelsofLCn-3PUFAarepositively correlatedwithcognitivefunction.
Themeta-analysisincluded38RCTsand 41comparisonsinvolving49,757participants. TheresultsshowedthatLCn-3PUFAhadno orlittleeffectonnewneurocognitiveimpairment(RR ¼ 0.98,95%CI:0.87–1.10),newcognitiveimpairment(RR ¼ 0.99,95%CI:0.92–1.06), oroverallcognition,whichwasassessedbymini mentalstateexamination(MD0.10,95%CI: 0.03–0.16)(Brainardetal.,2020).
However,inameta-analysisoftheeffectsof LCn-3PUFAoncognitioninsubjectsaged 4–25years,33studieswereincluded,ofwhich
21werefordevelopingsubjectsand12werefor dysfunctionalsubjects.Theresultsshowedthat inthestudyofchildrenintypicaldevelopmental stages,dailysupplementationofDHA+ EPA 450mgcansignificantlyimprovecognitivefunction.Whenthen-3index(DHA+EPA redbloodcellfattyacidcomposition)isgreater than6%,ithasapositiveimpactoncognitive indicators(vanderWurff,Meyer,&de Groot,2020).
N-3PUFAimprovessymptomsofchildren andadolescentswithAttentionDeficitHyperactivityDisorder(ADHD).Inmeta-analysisof7 RCTsinvolving534youngpeoplewithADHD, thesupplementationofn-3PUFAsimproved theclinicalsymptomscoreofADHD(g ¼ 0.38, P < .0001);inthreeRCTsinvolving214young peoplewithADHD,n-3PUFAsupplementation improvedthecognitiveindicatorsrelatedto attention(g ¼ 1.09, P < .0001).Inaddition,childrenandadolescentswithADHDhadlowertissuelevelsofDHA(7studies, n ¼ 412, g ¼ 0.76, P ¼ .0002),EPA(7studies, n ¼ 468,g ¼ 0.38, P ¼ .0008),andatotalofn-3PUFAs(6studies, n ¼ 396, g ¼ 0.58, P ¼ .0001)(Chang,Su, Mondelli,&Pariante,2018).
1.6.6Dietarypolyunsaturatedfatintake anddepression
LCn-3PUFAhassomebeneficialeffectson theriskofdepressiveoranxietysymptoms. Thesystematicreviewandmeta-analysisof RCTsevaluatedtheeffectofPUFAonpreventionofdepressionandanxietysymptoms, whichincluded31studiesofLCn-3PUFA (n ¼ 41,470),onestudyofa-linolenicacid (n ¼ 4837),andoneoftotalPUFA( n ¼ 4997).
TheresultsshowedthatLCn-3PUFAmayhave littleornoeffectontheriskofdepressivesymptoms(HR ¼ 1.01,95%CI:0.92– 1.10,median dose0.95g/d,duration12months)oranxiety symptoms(SMD ¼ 0.15,95%CI:0.05 – 0.26, mediandose1.1g/d,duration6months) (Deaneetal.,2021).
However,otherstudieshavefoundthatn-3 PUFAhasabeneficiale ffectondepression. Themeta-analysisin cluded18RCTswith 4052participants,andfoundthatn-3PUFA hadasignificantsmalleffectonperinatal depressivesymptomsco mparedwithplacebo (SMD ¼ 0.236,95%CI: 0.463to 0.009; P ¼.042)(Mockingetal.,2020).
ItseemsthatEPAhastheeffectofsuppressingdepression,whileDHAdoesnot.The meta-analysisof26RCTsincluding2160participantsshowedthatn-3PUFAwasgenerally beneficialtodepressivesymptoms (SMD ¼ 0.28, P ¼ .004).PureEPA(¼100% EPA)andEPAmainpreparation( 60%EPA) hadeffectsonSMD ¼ 0.50(P ¼ .003)and SMD ¼ 1.03(P ¼ .03),respectively,whilepure DHAandmainDHAformulahadnosuchbenefitcomparedwithplacebo(Liaoetal.,2019). Anothermeta-analysisof8RCTsinvolving638 participantsshowedthatn-3PUFAhasasignificanteffectonperinataldepression.Theratioof EPA/DHA 1.5hasasignificanteffectinmildto-moderatematernalandpostpartumdepression(Zhangetal.,2020).
InanotherRCTnetworkmeta-analysis,910 patientswithmajordepressionin10trialswere treatedwiththreeadjuvantstrategies:high-dose n-3PUFA,low-dosen-3PUFA,andplacebo.The resultsshowedthatn-3PUFAwassuperiorto placebo(SMD:1.243 0.596;95%CI: 0.060–2.414),andhigh-dosen-3PUFAwasmore effectivethanlow-dosen-3PUFA,bothofwhich werehigher(SMD:0.908 3.131;95%CI: 0.262–1.581)andlowerdose(SMD:0.601 0.286;95%CI:0.034–1.18)thanplacebo(Luo etal.,2020).
1.6.7Dietarypolyunsaturatedfatintake andnonalcoholicfattyliverdisease
Then-3PUFAshaveabeneficialeffectonthe riskofNAFLD.Inameta-analysisoffattyacid andNAFLD,11RCTswereincluded.Then-3 PUFAsupplementationsignificantlyreduced
theserumconcentrationsofALT,ASL,TAGwith weightedmeandifferences(WMDs)of 7.53 U/L;95%CI: 9.98, 5.08U/L), 7.10U/L, 95%CI: 11.67, 2.52U/L,and 36.16mg/dL, 95%CI: 49.15, 23.18mg/dL,respectively. Theliverfatcontentmarginallyreduced ( 5.11%,95%CI: 10.24,0.02%, P ¼ .051),but notfastingserumglucose.Dose-responseanalysis showedthat1gperdayincrementofEPA+DHA wasassociatedwith3.14U/LreductioninALT (95%CI: 5.25, 1.02U/L),2.43U/LinAST (95%CI: 3.90, 0.90U/L),2.74%inliverfat (95%CI: 4.32, 1.16%),and9.97mg/dLin TAG(95%CI: 14.47, 5.48mg/dL),respectively (Guo,Yang,Tang,&Li,2018).
Inanothermeta-analysisofn-3PUFA supplementationandnonalcoholicfattyliver disease,itincluded22RCTswith1366participants.Supplementationofn-3PUFAssignificantlyreducedliverfatcomparedwith placebo(RR ¼ 1.52;95%CI:1.09–2.13). Supplementationofn-3PUFAalsosignificantly reducedBMIwithpooledmeandifferenceand 95%CIwere 0.46: 0.84, 0.08;andconcentrationsofserum/plasmaTAG-28.57: 40.81, 16.33;TC-7.82: 14.86, 0.79andHDL-C 3.55:1.38–5.73,respectively(Lee,Fu,Yang,& Chi,2020).
1.6.8Dietarypolyunsaturatedfatintake andchronickidneydisease
Thedietaryintakeofn-3PUFAhasabeneficialeffectontheriskofchronickidneydisease. Inameta-analysisof60RCTs(4129participants)oftheeffectsofn-3PUFAintakeoncardiovasculardeathinpatientswithchronic kidneydisease,allofthesupplementationwith amedianfollow-upof6months.Resultssuggestedthatn-3PUFAsupplementation reducedcardiovasculardeathforpatientson hemodialysis(39events;RR ¼ 0.45,95%CI: 0.23 – 0.89),andpreventedend-stagekidneydisease(29events;RR ¼ 0.30,95%CI:0.09 –0.98)in
patientswithchronickidneydiseasenotreceivingrenalreplacementtherapy( Saglimbene etal.,2020 ).
1.6.9Dietarypolyunsaturatedfatintake andinflammation
Inflammationisassociatedwithmanydiseases. N-3PUFAcanbeinvivometabolizedinthe humanbodyintospecialpotentantiinflammatory lipidmediators,namelyresolvins,protectins,and maresins.Increaseddietaryintakeofn-3PUFAor n-3/n-6ratiowillreduceseruminflammatoryfactorssuchastumornecrosisfactor-α (TNF-α)and interleukin6(IL-6).Inameta-analysiswhich included68RCTswithatotalof4601subjects, marine-derivedLCn-3PUFAssupplementation showedaloweringeffectonTNF-a,IL-6,and CRPinthreegroupsofsubjects(subjectswith chronicnonautoimmunedisease,subjectswith chronicautoimmunediseaseandhealthysubjects).However,allresultsarewithsignificantheterogeneity, I2 70%(Lietal.,2014).
Inanothermeta-analysiswhichincluded 31RCTs,theresultsindicatedthatlowerdietary intakeofn-6/n-3PUFAratiosignificantly decreasedtheserumconcentrationsofTNF-α (SMD ¼ 0.270;95%CI: 0.433, 0.106; P ¼ .001) andIL-6(SMD ¼ 0.153;95%CI: 0.260, 0.045; P ¼ .005)(Wei,Meng,Li,Wang,&Chen,2021).
1.7Conclusion
Dietaryfatprovidesenergyandessential fattyacids,participatesinallphysiological functions,andiscloselyrelatedtohumandevelopmentandhealth.Inordertopreventnoncommunicablediseases,thetotaldietaryfat intakeofthegeneralpopulationshouldnot exceed30%ofthetotalenergy,andunsaturated fatsshouldbeusedtosubstitutesaturatedfats, increasingdietaryintakeoffoodrichinn-3 PUFA,suchasfishandotherseafood,walnuts, flax,chiaandperillaseeds,andtheiroils.
References
Abdelhamid,A.S.,Brown,T.J.,Brainard,J.S.,Biswas,P., Thorpe,G.C.,Moore,H.J.,etal.(2020).Omega-3fatty acidsfortheprimaryandsecondarypreventionofcardiovasculardisease. CochraneDatabaseofSystematic Reviews, 2020(3). https://doi.org/10.1002/14651858. CD003177.pub5
Brainard,J.S.,Jimoh,O.F.,Deane,K.H.O.,Biswas,P., Donaldson,D.,Maas,K.,etal.(2020).Omega-3,omega6,andpolyunsaturatedfatforcognition:Systematic reviewandmeta-analysisofrandomizedtrials. Journal oftheAmericanMedicalDirectorsAssociation, 21(10), 1439–1450.e21. https://doi.org/10.1016/j.jamda.2020. 02.022.
Chang,J.P.C.,Su,K.P.,Mondelli,V.,&Pariante,C.M. (2018).Omega-3polyunsaturatedfattyacidsinyouths withattentiondeficithyperactivitydisorder: Asystematicreviewandmeta-analysisofclinicaltrials andbiologicalstudies. Neuropsychopharmacology, 43(3), 534–545. https://doi.org/10.1038/npp.2017.160
Deane,K.H.O.,Jimoh,O.F.,Biswas,P.,O’Brien,A., Hanson,S.,Abdelhamid,A.S.,etal.(2021).Omega-3 andpolyunsaturatedfatforpreventionofdepression andanxietysymptoms:Systematicreviewandmetaanalysisofrandomisedtrials. BritishJournalofPsychiatry, 218(3),135–142. https://doi.org/10.1192/bjp.2019.234
FAO.(2010). Fatsandfattyacidsinhumannutrition:Reportofan expertconsultation Vol.97.FoodandAgricultureOrganizationoftheUnitedNations.
Fattore,E.,Bosetti,C.,Brighenti,F.,Agostoni,C.,& Fattore,G.(2014).Palmoilandbloodlipid-related markersofcardiovasculardisease:Asystematicreview andmeta-analysisofdietaryinterventiontrials. American JournalofClinicalNutrition, 99(6),1331–1350. https://doi. org/10.3945/ajcn.113.081190.
Gardner,C.D.,Kiazand,A.,Alhassan,S.,Kim,S., Stafford,R.S.,Balise,R.R.,etal.(2007).Comparisonof theAtkins,Zone,Ornish,andLEARNdietsforchange inweightandrelatedriskfactorsamongoverweightpremenopausalwomen:TheAtoZweightlossstudy: Arandomizedtrial. JournaloftheAmericanMedicalAssociation, 297(9),969–977. https://doi.org/10.1001/jama. 297.9.969.
Guo,X.F.,Yang,B.,Tang,J.,&Li,D.(2018).Fattyacidand non-alcoholicfattyliverdisease:Meta-analysesofcasecontrolandrandomizedcontrolledtrials. ClinicalNutrition, 37(1),113–122. https://doi.org/10.1016/j.clnu. 2017.01.003
Hanson,S.,Thorpe,G.,Winstanley,L.,Abdelhamid,A.S., Hooper,L.,Abdelhamid,A.,etal.(2020).Omega-3, omega-6andtotaldietarypolyunsaturatedfatoncancer incidence:Systematicreviewandmeta-analysisofrandomisedtrials. BritishJournalofCancer, 122(8),1260–1270. https://doi.org/10.1038/s41416-020-0761-6
Hisham,M.D.B.,Aziz,Z.,Huin,W.K.,Haur,C.,& Jamil,A.H.A.(2020).Theeffectsofpalmoilonserum lipidprofiles:Asystematicreviewandmeta-analysis. AsiaPacificJournalofClinicalNutrition, 29(3),523–536. https://doi.org/10.6133/apjcn.202009_29(3).0011
Hooper,L.,Abdelhamid,A.,Bunn,D.,Brown,T., Summerbell,C.D.,&Skeaff,C.M.(2015).Effectsoftotal fatintakeonbodyweight. CochraneDatabaseofSystematic Reviews, 2015(8). https://doi.org/10.1002/14651858. CD011834.
Hooper,L.,Martin,N.,Jimoh,O.F.,Kirk,C.,Foster,E.,& Abdelhamid,A.S.(2020).Reductioninsaturatedfat intakeforcardiovasculardisease. CochraneDatabaseof SystematicReviews, 2020(8). https://doi.org/10. 1002/14651858.CD011737.pub3.
Imamura,F.,Micha,R.,Wu,J.H.Y.,deOliveiraOtto,M.C., Otite,F.O.,Abioye,A.I.,etal.(2016).Effectsofsaturated fat,polyunsaturatedfat,monounsaturatedfat,andcarbohydrateonglucose-insulinhomeostasis:Asystematic reviewandmeta-analysisofrandomisedcontrolledfeedingtrials. PLoSMedicine, 13(7). https://doi.org/10.1371/ journal.pmed.1002087
Innes,J.K.,&Calder,P.C.(2018).Thedifferentialeffectsof eicosapentaenoicacidanddocosahexaenoicacidoncardiometabolicriskfactors:Asystematicreview. InternationalJournalofMolecularSciences, 19(2). https://doi. org/10.3390/ijms19020532
Langlois,P.L.,D’Aragon,F.,Hardy,G.,&Manzanares,W. (2019).Omega-3polyunsaturatedfattyacidsincritically illpatientswithacuterespiratorydistresssyndrome: Asystematicreviewandmeta-analysis. Nutrition, 61, 84–92. https://doi.org/10.1016/j.nut.2018.10.026
Lee,C.H.,Fu,Y.,Yang,S.J.,&Chi,C.C.(2020).Effectsof Omega-3polyunsaturatedfattyacidsupplementation onnon-alcoholicfattyliver:Asystematicreviewand meta-analysis. Nutrients, 12(9),1–20. https://doi.org/ 10.3390/nu12092769.
Li,D.(2015).Omega-3polyunsaturatedfattyacidsandnoncommunicablediseases:Meta-analysisbasedsystematic review. AsiaPacificJournalofClinicalNutrition, 24(1), 10–15. https://doi.org/10.6133/apjcn.2015.24.1.21 Li,D.,&Hu,X.(2011).Fattyacidcontentofcommonlyavailablenutsandseeds.In Nutsandseedsinhealthanddisease prevention (pp.35–42).ElsevierInc. https://doi.org/10. 1016/B978-0-12-375688-6.10004-0
Li,K.,Huang,T.,Zheng,J.,Wu,K.,Li,D.,&Schunck,W.-H. (2014).Effectofmarine-derivedn-3polyunsaturated fattyacidsonC-reactiveprotein,interleukin6andtumor necrosisfactor α:Ameta-analysis. PLoSOne, 9(2). https://doi.org/10.1371/journal.pone.0088103,e88103. Li,D.,Ng,A.,Mann,N.J.,&Sinclair,A.J.(1998).Contributionofmeatfattodietaryarachidonicacid. Lipids, 33(4), 437–440. https://doi.org/10.1007/s11745-998-0225-7
Li,N.,Yue,H.,Jia,M.,Liu,W.,Qiu,B.,Hou,H.,etal.(2019). Effectoflow-ration-6/n-3PUFAonbloodglucose:
Ameta-analysis. FoodandFunction, 10(8),4557–4565. https://doi.org/10.1039/c9fo00323a
Liao,Y.,Xie,B.,Zhang,H.,He,Q.,Guo,L., Subramaniapillai,M.,etal.(2019).Efficacyofomega-3 PUFAsindepression:Ameta-analysis. TranslationalPsychiatry, 9(1). https://doi.org/10.1038/s41398-019-0515-5
Luo,X.D.,Feng,J.S.,Yang,Z.,Huang,Q.T.,Lin,J.D., Yang,B.,etal.(2020).High-doseomega-3polyunsaturatedfattyacidsupplementationmightbemoresuperior thanlow-doseformajordepressivedisorderinearlytherapyperiod:Anetworkmeta-analysis. BMCPsychiatry, 20(1). https://doi.org/10.1186/s12888-020-02656-3. Mensink,R.P.(2016). Effectsofsaturatedfattyacidsonserum lipidsandlipoproteins:Asystematicreviewandregression analysis.Geneva:WorldHealthOrganization. Miller,P.E.,VanElswyk,M.,&Alexander,D.D.(2014). Long-chainOmega-3fattyacidseicosapentaenoicacid anddocosahexaenoicacidandbloodpressure:Ametaanalysisofrandomizedcontrolledtrials. AmericanJournal ofHypertension, 27(7),885–896. https://doi.org/10.1093/ ajh/hpu024
Mocking,R.J.T.,Steijn,K.,Roos,C.,Assies,J.,Bergink,V., Ruhe,H.G.,etal.(2020).Omega-3fattyacidsupplementationforperinataldepression:Ameta-analysis. TheJournalofClinicalPsychiatry, 81(5). https://doi.org/10.4088/ JCP.19r13106
Moher,D.,Liberati,A.,Tetzlaff,J.,&Altman,D.G.(2009). Preferredreportingitemsforsystematicreviewsand meta-analyses:ThePRISMAstatement. PLoSMedicine, 6(7). https://doi.org/10.1371/journal.pmed.1000097, e1000097.
Moreno,B.,Crujeiras,A.B.,Bellido,D.,Sajoux,I.,& Casanueva,F.F.(2016).Obesitytreatmentbyverylowcalorie-ketogenicdietattwoyears:Reductioninvisceral fatandontheburdenofdisease. Endocrine, 54(3), 681–690. https://doi.org/10.1007/s12020-016-1050-2.
Qian,F.,Korat,A.A.,Malik,V.,&Hu,F.B.(2016).Metabolic effectsofmonounsaturatedfattyacid-enricheddiets comparedwithcarbohydrateorpolyunsaturatedfatty acid-enricheddietsinpatientswithtype2diabetes: Asystematicreviewandmeta-analysisofrandomized controlledtrials. DiabetesCare, 39(8),1448–1457. https://doi.org/10.2337/dc16-0513
Saglimbene,V.M.,Wong,G.,vanZwieten,A.,Palmer,S.C., Ruospo,M.,Natale,P.,etal.(2020).Effectsofomega-3 polyunsaturatedfattyacidintakeinpatientswithchronic kidneydisease:Systematicreviewandmeta-analysisof
randomizedcontrolledtrials. ClinicalNutrition, 39(2), 358–368. https://doi.org/10.1016/j.clnu.2019.02.041 Sinclair,A.,Oon,K.,Lim,L.,Li,D.,&Mann,N.(1998).The omega-3fattyacidcontentofcanned,smokedandfresh fishinAustralia. AustralianJournalofNutritionandDietetics, 55,116–120.
Su,X.Q.,Antonas,K.N.,&Li,D.(2004).Comparisonofn-3 polyunsaturatedfattyacidcontentsofwildandcultured Australianabalone. InternationalJournalofFoodSciences andNutrition, 55(2),149–154. https://doi.org/10. 1080/09637480410001666469.
vanderWurff,I.S.M.,Meyer,B.J.,&deGroot,R.H.M. (2020).Effectofomega-3longchainpolyunsaturated fattyacids(N-3LCPUFA)supplementationoncognition inchildrenandadolescents:Asystematicliterature reviewwithafocusonn-3LCPUFAbloodvaluesand doseofDHAandEPA. Nutrients, 12(10),1–28. https:// doi.org/10.3390/nu12103115
Wan,Y.,Wang,F.,Yuan,J.,Li,J.,Jiang,D.,Zhang,J.,etal. (2017).Effectsofmacronutrientdistributiononweight andrelatedcardiometabolicprofileinhealthynon-obese Chinese:A6-month,randomizedcontrolled-feeding trial. eBioMedicine, 22,200–207. https://doi.org/10. 1016/j.ebiom.2017.06.017
Wan,Y.,Wang,F.,Yuan,J.,Li,J.,Jiang,D.,Zhang,J.,etal. (2019).Effectsofdietaryfatongutmicrobiotaandfaecal metabolites,andtheirrelationshipwithcardiometabolic riskfactors:A6-monthrandomisedcontrolled-feeding trial. Gut, 68(8),1417–1429. https://doi.org/10.1136/ gutjnl-2018-317609
Wei,Y.,Meng,Y.,Li,N.,Wang,Q.,&Chen,L.(2021).The effectsoflow-ration-6/n-3PUFAonbiomarkersof inflammation:Asystematicreviewandmeta-analysis. FoodandFunction, 12(1),30–40. https://doi.org/10. 1039/d0fo01976c.
Zhang,M.M.,Zou,Y.,Li,S.M.,Wang,L.,Sun,Y.H.,Shi,L., etal.(2020).Theefficacyandsafetyofomega-3fattyacids ondepressivesymptomsinperinatalwomen:Ametaanalysisofrandomizedplacebo-controlledtrials. TranslationalPsychiatry, 10(1). https://doi.org/10.1038/s41398020-00886-3
Zheng,J.S.,Hu,X.J.,Zhao,Y.M.,Yang,J.,&Li,D.(2013). Intakeoffishandmarinen-3polyunsaturatedfattyacids andriskofbreastcancer:Meta-analysisofdatafrom 21independentprospectivecohortstudies. BritishMedicalJournal, 347(7917). https://doi.org/10.1136/bmj.f3706 (Online).
Fattyacidsandtelomeresinhumans
MichaelFenech,PermalDeo,andVarinderpalDhillon ClinicalandHealthSciences,UniversityofSouthAustralia,Adelaide,SA,Australia
2.1Introduction
Telomereslocatedattheendsofchromosomes arehighlyconserved,longhexamer(TTAGGG) repeatDNAsequencesthatareessentialfor chromosomalstabilitybecausetheyprotectends ofthechromosomefromfusionanddegradation (Blackburn,Epel,&Lin,2015).Telomerase,an intra-nuclearenzyme,isimportantfortelomere formation,maintenance,andrestoration (Blackburn,2005,2010).Withouttelomeremaintenanceandreplication,telomericDNAwouldbe losteverytimeacelldivides.Attritionoftelomeres and/orDNAbreaksinthetelomereresultsincellularsenescence,characterizedbyalterationsin geneexpressionthatpromoteapro-inflammatory state(Allsopp&Harley,1995; Coppe,Desprez, Krtolica,&Campisi,2010).
Telomerelengthdecreasesovertimedueto thetelomereend-replicationproblemandmay serveasabiomarkeroftheagingprocess (Blasco,2005; Raynaud,Sabatier,Philipot, Olaussen,&Soria,2008; Shay,2018).Telomere lengthmaintenanceislargelyinfluencedby genetic,epigenetic,andvariousenvironmental andendogenousfactors,suchasoxidative stress,whichmayfurtherinfluencetelomere integrity(Blasco,2007; Fouquereletal.,2019; Opresko,Fan,Danzy,Wilson,&Bohr,2005).
Telomereattritionhasalsobeenassociatedwith physicalinactivity,smoking,psychological stress,bodycomposition(BMI),andlower socioeconomicstatus(Needhametal.,2013; Shalevetal.,2013; Valdesetal.,2005). Genome-wideassociationstudieshaveshown thatgeneticvariationwithinthegenecoding fortelomeraseRNAcomponent(TERC)affects telomerelengthinhumans(Coddetal.,2010; Shenetal.,2011).Theexactmechanismsdeterminingtelomereattritionremainincompletely understood.However,dietaryfactorsinvolved ascofactorsinDNAreplicationandrepairand inresponsetooxidativestressmayplayan importantroleininfluencingtherateoftelomereshortening(Dhillon,Bull,&Fenech, 2016).Evidenceisemergingthatdietisan importantvariableaffectingtherateoftelomere attrition,withthemostrobustevidencesofar indicatingaprotectiverolefortheMediterraneandietanddietarypatternswithalow inflammatoryindex(Canudasetal.,2020; Garcı´a-Calzo ´ netal.,2015; Shivappa,Wirth, Hurley,&Hebert,2017).
Thischapterisanarrativereviewofthecurrentevidencerelatingtotheassociationoftelomerelengthwithdietaryorsupplementalfatty acidintakeorfattyacidstatusinhumans. Evidencefromobservationalcross-sectional
andprospectivestudiesandrandomizedcontrolledtrialshasbeenevaluated.
2.2Dietaryfattyacidsandtelomeres
Omega-3fattyacidsimprovecardiovascular healthbyloweringcholesterolandtriglycerides inthebloodandpreventingcardiacarrhythmias and,furthermore,contributetohealthyagingby attenuatingexcessiveinflammationviatheircentralroleinthesynthesisofpro-resolvingprotectin,maresin,andresolvinmediators(Innes& Calder,2020; Serhan,2014).Becausedietaryfatty acidscaninfluencetheriskofcardiometabolic diseasesandinflammation,bothofwhichare consistentlyassociatedwithincreasedtelomere attrition(Haycocketal.,2014; Zhangetal., 2016),itisplausiblethattelomerelengthmayalso beassociatedwithdietaryfattyacidintake.Furthermore,unsaturatedfattyacidsareproneto oxidationtoformreactivelipidhydroperoxides (malondialdehyde,epoxyaldehydes,2-alkenals, and4-oxo-2-alkenals)thatformDNAadducts suchasthedeoxyguanine(dG)adductsM1dG, 1,N2-propano-dG,1,N2-etheno(ε)-dG,7-(2oxo-alkyl)-εdG,respectively(Kawai&Nuka, 2018).FormationofDNAadductsonthetelomere sequencemayleadtoDNAreplicationstress whichcanresultininefficienttelomerereplicationand/orDNAstrandbreaksinthetelomere, leadingtoacentricchromosomefragmentformationandlossofthefragmenttogetherwithits associatedtelomere.Anotherplausibleindirect mechanismistheformationoflipid-derived advancedglycationend-productsthatmay adductonthetelomereand/orpromoteinflammationandoxidativestressviatheRAGEand NFκβ axis(Fishman,Sonmez,Basman,Singh,& Poretsky,2018; Fuetal.,1996; Refsgaard, Tsai,&Stadtman,2000; Slatter,Avery,&Bailey, 2004; Slatter,Bolton,&Bailey,2000; Vistoli etal.,2013). Fig.2.1 illustratesplausiblemechanismsbywhichfattyacidsmayinfluenceloss oftelomereintegrityandpromotecellularaging.
2.3Cross-sectionalandprospective studies
Thirteenobservationalstudieshavebeen reported,12arecross-sectionalstudies,and oneisaprospectivestudy.Totalfatandsaturatedfattyacidswerefoundtobeinverselyassociatedwithtelomerelengthinmen(Tiainen etal.,2012).Inanotherstudy,monounsaturated fattyacidswerefoundtobesignificantly inverselyassociatedwithtelomerelength (Kark,Goldberger,Kimura,Sinnreich,&Aviv, 2012).Inyetanotherstudy,dietaryintakeof linoleicacidwasfoundtobeinverselyassociatedwithtelomerelength(Cassidyetal., 2010).However,thestudybySongandassociatesshowedthatintakeofshort-to-mediumchainsaturatedfattyacidsisinverselyassociatedwithtelomerelength,butnosignificant associationwasfoundwithlong-chainsaturated fattyacidsorPUFAsorMUFAs(Song etal.,2013).
In287participants(55%males,6–18years), whowererandomlyselectedfromtheGENOI study,dietaryintakeofpolyunsaturatedfatty acidswassignificantlyandinverselyassociated withtelomerelength;however,noassociation withdietaryintakeoftotalfats,saturatedfatty acids,andmonounsaturatedfattyacidswas reportedwithtelomerelength(Garcı´a-Calzo ´ n etal.,2015).IntheSeychellesChildDevelopmentStudy,prenatalPUFAstatuswasnotassociatedwithtelomerelengthofthemotheror child;however,ahigherprenataln-6:n-3PUFA ratiowassignificantlyassociatedwithlonger telomerelengthinthemothers(β ¼ 0.001, P ¼ .048)(Yeatesetal.,2017).Ithasalsobeen reportedthattelomerelengthdecreasedwith increasedtrans-fattyacids,mostsignificantly withincreasedpalmitelaidicacidandlinolelaidicacid(Mazidi,Banach,&Kengne,2018). Anotherstudy,inelderlysubjectswithrecent myocardialinfarction,reportedaweakbutsignificantpositivecorrelationbetweenlinoleic acidandtelomerelength.However,other
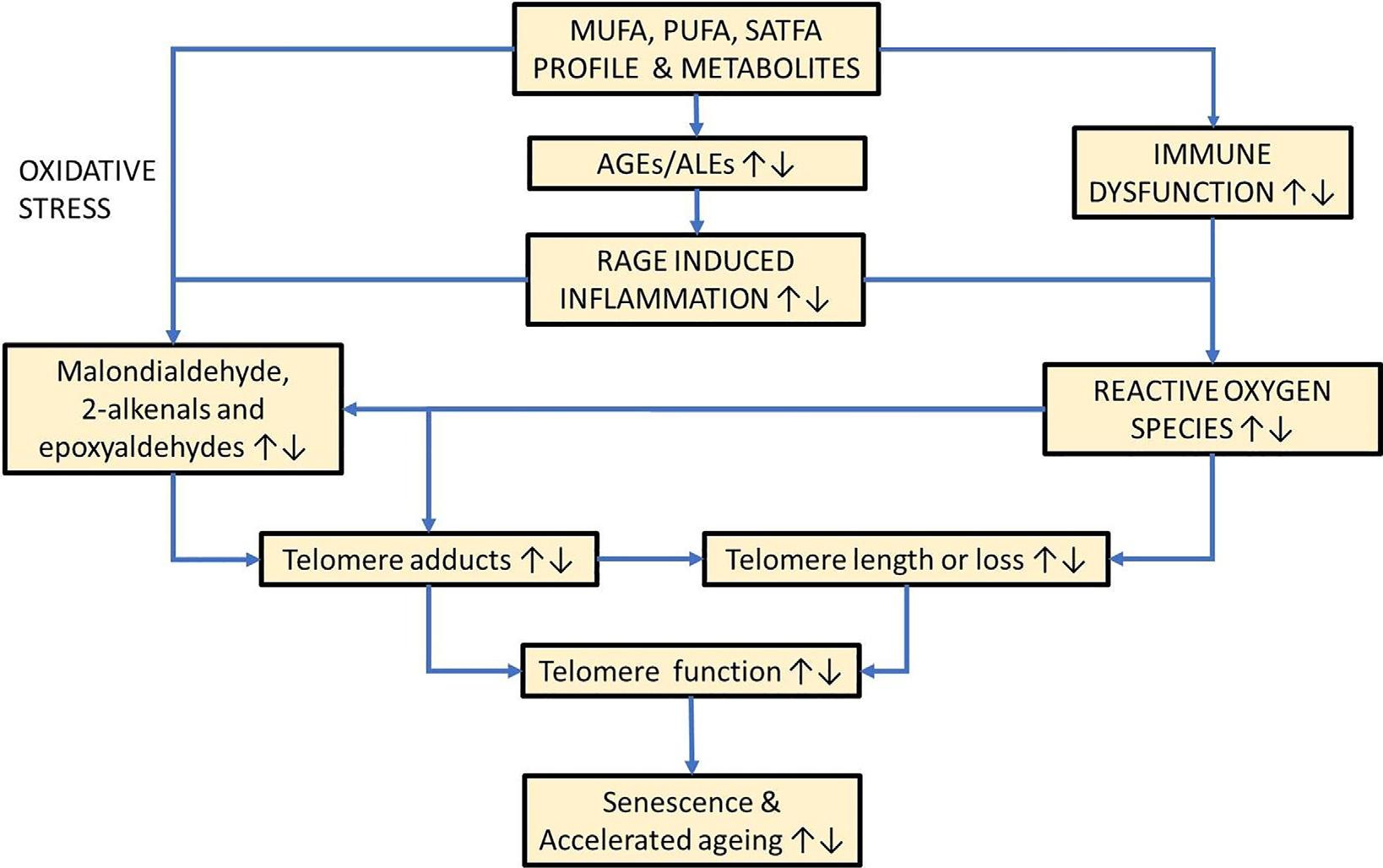
FIG.2.1 Plausiblemechanismsbywhichfattyacidsmayinfluencelossoftelomereintegrityandpromotecellularaging. No permissionrequired.
long-chainfattyacidsandthen-3/n-6ratiowere notassociatedwithtelomerelength(Kalstad etal.,2019).Inacross-sectionalstudy,involving atotalof1,246subjectsaged25–74years,elaidic acidandn-3PUFAwerenegativelyassociated withtelomerelengthandn-6PUFAwaspositivelyassociated(Zhaoetal.,2020).Recently,it hasbeenreportedthatthemajorityofsaturated andmonounsaturatedfattyacidsmeasuredin redbloodcells(RBCs)werenegativelyassociatedwithtelomerelengthinleukocyteswhereas polyunsaturatedfattyacids(morespecifically arachidonicacid)werepositivelyassociated withtelomerelength(V.S. Dhillon,Deo, Chua,Thomas,&Fenech,2021).Incontrast,an increasedconcentrationofarachidonicacidin RBCswasreportedtobesignificantlyassociated withdecreasedtelomerelengthinMediterraneanelders(Freitas-Simoesetal.,2019).Ina studyofobeseandnonobesechildren,itwas reportedthatleukocytetelomerelengthwas
positivelyassociatedwithconcentrationsof totalsaturatedfattyacidsandDHAinRBCs (P < .05),andnegativelywiththeAA/DHA ratio(P < .05)(Liu,Liu,Shi,Fan,&Qi,2021). Thereisonlyoneprospectivestudythat investigatedtheassociationoffattyacidswith telomerelength.Inthisstudy,theHeartand Soulstudy,increasedintakeofomega-3fatty acids(EPA,DHA),overa6-yearperiod,was associatedwithasignificantlyreducedrateof telomereshortening(Farzaneh-Faretal.,2010). Furtherdetailsoftheabovestudiesareprovided in Table2.1. Fig.2.2 providesacondensedlandscapeofthesevariousstudiesshowingthegreat diversityofstatisticallysignificantoutcomes involvingdifferentfattyacidclassesandspecific fattyacids.Theweightofevidencefromthis sparseobservationaldatatendstosuggestan associationbetweenshortertelomeres,and increasedsaturatedfattyacids,monounsaturatedfattyacids,andtrans-fattyacids.
TABLE2.1 Observationalcross-sectionalandprospectivestudiesonassociationoffattyacidswithtelomerelength.
ReferencesStudydesignPopulation
Cassidyetal.(2010)
Tiainenetal.(2012)
Karketal.(2012)
Cross-sectionalsubstudyfroma prospectivecohortstudy
2284femaleparticipantsfromNurses’ HealthStudyfromUSaged30–55years
Cross-sectional1942malesandfemalesaged57–70years fromtheHelsinkiBirthCohortStudy (Finland)
Cross-sectionalsubstudyfroma longitudinalobservational study
Songetal.(2013)
Cross-sectionalsubstudyfroma longitudinalobservational study
405menand204women;meanageat baseline(30.1years)andfollow-up (43.2years)fromJerusalemLRCPrevalence Study(Israel)
4029healthypostmenopausalwomenaged 58+yearsfromWHIObservationalstudy cohort(California,US)
Tissue/ methodResults
PBL/ qPCR
PBL/ qPCR
Linoleicacidintakewasinverselyassociated withtelomerelengthaftermultivariate adjustments(P ¼ .001; P fortrend ¼ 0.05)
TotalfatandSFAwereinverselyassociated withleukocytetelomerelengthinmen adjustingforageandenergyintake (b ¼ 0.001; P ¼ .04)
Buffy coat/ Southern blot MUFAwassignificantlyinversely associatedwithLTLinmenonly(b ¼ 0.104; P ¼ .042)afteradjustingforcovariates
PBL/ qPCR
Garcı´a-Calzo ´ n, Moleres,etal.(2015) and Garcı´a-Calzo ´ n, Zalba,etal.(2015)
Cross-sectionalstudy207participantsagedbetween6and18years randomlyselectedfromGENOIcohort (Spain)
Yeatesetal.(2017) Observationalstudy229expectantmotherin28-weekgestation (Seychelles)
Buffy coat/ qPCR
Intakeofshort-to-medium-chainfattyacids (SMSFAs;aliphatictailsof 12carbons)was inverselyassociatedwithtelomerelength. Nosignificantassociationswerefoundwith long-chainsaturatedfattyacids, monounsaturatedfattyacids,and polyunsaturatedfattyacids
DietaryintakeofPUFAissignificantly inverselyassociatedwithtelomerelength. However,noassociationofdietaryintakeof totalfats,SFA,andMUFAwithtelomere length
PBL/ qPCR n3andn6PUFAsareinverselybutnot significantlyassociatedwithtelomere length.However,highern6:n3ratiois significantlyassociatedwithlongertelomere length
Mazidietal.(2018) Observationalstudy5446participantswithaverageageof 47.1yearsfromNationalHealthand NutritionExaminationSurvey(NHANES) aged
PBL/ qPCR
Concentrationoftrans-fattyacidssuchas palmitelaidicacidandlinolelaidicacid decreasedwithincreasinglengthof telomeres(P ¼ .05)afteradjustmentsfor factorssuchasage,gender,ethnicity,BMI, andsmoking
Freitas-Simoesetal. (2019)
Cross-sectionalsubstudyfroma randomizedcontrolledtrial
344subjectswithmeanageof68.8yearsfrom WalnutandHealthyAgingtrial(Spain)
PBMC/ FISH
Kalstadetal.(2019) Cross-sectionalsubstudyofdata fromomega-3fattyacidsin elderlypatientswithmyocardial infarction(OMEMI)trial
299patientsagedbetween70and82years (Norway)
Zhaoetal.(2020) Cross-sectional1246participantsagedbetween25and 74years(China)
Dhillonetal.(2021) Cross-sectionalstudy174healthyelderlyparticipantswithmean ageof53.71years,fromSouthAustralian (Australia)
PBL/ qPCR
Anincreasingproportionofarachidonic acidinRBCsissignificantly(P ¼ .02) associatedwithshortertelomeresin Mediterraneanelders
Therewasaweakbutsignificantcorrelation betweenlinoleicacidandtelomerelength (r ¼ 0.139; P ¼ .017).However,otherlongchainfattyacidsandn-6/n-3ratioisnot associatedwithtelomerelength
PBL/ qPCR
Liuetal.(2021)
Farzaneh-Faretal. (2010)
Cross-sectionalStudyForty-sixpreschoolchildrenwithobesity aged3–4yearswereincludedinthestudy, withequalnumbersofage-andgendermatchedchildrenwithnormalweightas control
Prospectivecohort608outpatientsinCalifornia(US)withstable coronaryarterydisease—HeartandSoul Study.Studyduration:6years
PBL/ qPCR
Elaidicacid(C18:1)andn-3PUFAwere negativelyassociatedwithrelativetelomere lengthafteradjustmentsforage,gender, race,smoking,andexercise
Majorityofsaturatedfattyacidsand monounsaturatedfattyacidswere negativelyassociatedwithtelomerelength whereaspolyunsaturatedfattyacidswere positivelyassociatedwithtelomerelength. Multipleregressionanalysisrevealedthat arachidonicacidissignificantly, independently,positivelycorrelatedwith telomerelength(b ¼ 0.262; P ¼ 0.000).
PBMC/ qPCR
PBL/ qPCR
Correlativeanalysisshowedthatleukocyte telomerelengthhadapositiveassociation withtotalsaturatedfattyacids(r ¼ 0.49, P < .05)andDHA(r ¼ 0.44, P < .05),anda negativeassociationwiththeAA/DHA ratio(r ¼ 0.36, P < .05)
Logomega-3fattyacidswereassociated withadecreaseintelomereshortening(OR: 0.68;95%CI:0.47–0.98)
2.4Randomizedcontrolledtrials
Onlysixrandomizedcontrolledtrials(RCTs) havebeenreported,alloftheminvolvingsupplementationwithn-3fattyacids.Fourofthese RCTsinvestigatedeffectsontelomerelengthas aprimaryorsecondaryoutcomemeasure,and theremainingtwostudiedtheeffectontelomeraseactivity.Anincreaseof21and50bpintelomerelengthwasreportedamongindividuals whorespectivelyreceivedlowandhighdoses ofn-3fattyacidscomparedtotheplacebogroup whoreportedalossof43bpintelomerelength (Kiecolt-Glaseretal.,2013).Aninterventiontrial involvinglong-chainn-3fattyacidssupplementationamongpeoplewithmildcognitive impairmentdidnotshowanyassociationwith telomereattrition(O’Callaghanetal.,2014); however,increasederythrocyteDHAlevels wereassociatedwithreducedtelomereattrition (r ¼ 0.67; P ¼ .02)inthegrouptreatedwith
DHA.AnRCTonchronickidneydisease patientsreportednoeffectofn-3fattyacidsor CoQonneutrophilorPBMCtelomerelength; however,neutrophiltelomerelengthcorrected forneutrophilcountwasincreasedaftern-3 fattyacidssupplementation(P ¼ .015)(Barden etal.,2016).Inanotherstudy,maternaln-3LC PUFAsupplementationdidnotinfluenceoffspringtelomerelengthatbirthorat12y(See etal.,2016).
TwoRCTsexploredtheeffectsofn-3fatty acidsupplementationontelomeraseactivity. Thefirststudyindiabeticpatientsshowedthat supplementationwithDHAresultedinasignificantdecreaseintelomerelength(Toupchian etal.,2018).InthesecondRCTstudy,performed inschizophreniapatients,telomeraseactivityin peripheralbloodmononuclearcells(PBMCs) increasedsignificantlyinthegroupreceiving theEPA+DHAsupplementrelativetotheplacebogroup(Pawełczyketal.,2018).Telomere
FIG.2.2 Significantassociationsoffattyacidswithtelomerelength—Observationalstudies. Nopermissionrequired.