Editor’sbiography
MaulinP.Shah iscurrentlyaresearchscientistattheEnvironmentalBiologyLab,India.Dr.ShahhasservedasanassistantprofessoratGodhra,GujaratUniversitysince2001.Hehasmorethan 160researchpublicationsinhighlyreputednationalandinternationaljournals.Heisanactiveeditorialboardmemberin75 highlyreputedjournalsinthefieldofEnvironmentalandBiologicalSciences.Hehasbeenappointedasaneditor-in-chiefof twojournals:theResearchJournalofMicrobiologyandtheJournalofBiotechnologyandBiomaterials.Hisworkhasbeenfocused onassessingtheimpactofindustrialpollutiononthemicrobial diversityofwastewaterfollowingcultivation-dependantand cultivation-independentanalysis.Maulin’sworkinvolvesisolation,screening,identification,andgeneticengineeringofthehigh impactofmicrobesforthedegradationofhazardousmaterials.
Contributors
KomalAgrawal BioprocessandBioenergyLaboratory,Department ofMicrobiology,CentralUniversityofRajasthan,Ajmer,Rajasthan,India
J.Anandkumar DepartmentofChemicalEngineering,NationalInstitute ofTechnologyRaipur,Raipur,Chhattisgarh,India
AditiBanerjee CentreforNanotechnology,IndianInstituteofTechnology Guwahati,Guwahati,Assam,India
JayBergi DepartmentofBiotechnology,ShreeRamkrishnaInstitute ofComputerEducationandAppliedSciences,Surat,Gujarat,India
NavneetaBharadvaja PlantBiotechnologyLaboratory,DelhiTechnological University,Delhi,India
Q.Q.Cai DepartmentofCivil&EnvironmentalEngineering,Faculty ofEngineering,NationalUniversityofSingapore,Singapore,Singapore
JayashankarDas SOADeemedtobeUniversity,Bhubaneswar,Odisha, India
ShivikaDatta DepartmentofZoology,DoabaCollege,Jalandhar,Punjab, India
SushmaDave JodhpurInstituteofEngineeringandTechnology,Jodhpur, Rajasthan,India
S.H.Deng DepartmentofCivil&EnvironmentalEngineering,Faculty ofEngineering,NationalUniversityofSingapore,Singapore,Singapore
DaljeetSinghDhanjal DepartmentofBiotechnology,LovelyProfessional University,Phagwara,Punjab,India
AnuragGarg EnvironmentalScienceandEngineeringDepartment,Indian InstituteofTechnologyBombay,Mumbai,India
ShashwatiGhoshSachan DepartmentofBio-Engineering,BirlaInstitute ofTechnology,Mesra,Ranchi,Jharkhand,India
J.Y.Hu DepartmentofCivil&EnvironmentalEngineering,Facultyof Engineering,NationalUniversityofSingapore,Singapore,Singapore
PylaJayasree IndianInstituteofTechnologyBhubaneswar,Argul,Odisha, India
L.Jothinathan DepartmentofCivil&EnvironmentalEngineering,Faculty ofEngineering,NationalUniversityofSingapore,Singapore,Singapore
SukhmanpreetKaur DepartmentofChemistry,RayatBahraInstitute ofEngineeringandNano-Technology,Hoshiarpur,Punjab,India
PrateekKhare DepartmentofChemicalEngineering,MadanMohan MalaviyaUniversityofTechnology,Gorakhpur,UttarPradesh,India
PrashantKumar DepartmentofBio-Engineering,BirlaInstituteof Technology,Mesra,Ranchi,Jharkhand,India
VijayKumar RegionalAyurvedaResearchInstituteforDrugDevelopment, Gwalior,MadhyaPradesh,India
VineetKumar EnvironmentalMicrobiologyandBiotechnologyLaboratory, SchoolofEnvironmentalSciences,JawaharlalNehruUniversity, NewDelhi,Delhi;DepartmentofEnvironmentalMicrobiology,Babasaheb BhimraoAmbedkar(Central)University,Lucknow,UttarPradesh,India
LalMohanKundu CentrefortheEnvironment;Department ofChemistry,IndianInstituteofTechnologyGuwahati,Guwahati, Assam,India
SandhyaMishra AppliedPhycologyandBiotechnologyDivision,CSIR— CentralSaltandMarineChemicalsResearchInstitute,Bhavnagar,India
H.Y.Ng DepartmentofCivil&EnvironmentalEngineering,Faculty ofEngineering,NationalUniversityofSingapore,Singapore,Singapore
S.L.Ong DepartmentofCivil&EnvironmentalEngineering,Faculty ofEngineering,NationalUniversityofSingapore,Singapore,Singapore
LalitM.Pandey CentrefortheEnvironment;Bio-Interface&Environmental EngineeringLab,DepartmentofBiosciencesandBioengineering,Indian InstituteofTechnologyGuwahati,Guwahati,Assam,India
RatneshKumarPatel DepartmentofChemicalEngineering,Madan MohanMalaviyaUniversityofTechnology,Gorakhpur,UttarPradesh, India
V.Prashanth IndianInstituteofTechnologyBhubaneswar,Argul,Odisha, India
ParthRajput IndianInstituteofTechnologyBhubaneswar,Argul,Odisha, India
ShristiRam AppliedPhycologyandBiotechnologyDivision,CSIR—Central SaltandMarineChemicalsResearchInstitute,Bhavnagar;Academyof ScientificandInnovativeResearch(AcSIR),Ghaziabad,India
NeelancherryRemya IndianInstituteofTechnologyBhubaneswar,Argul, Odisha,India
RominaRomero TechnologicalDevelopmentUnit(UDT),University ofConcepcion,Coronel,Chile
ArpitaRoy PlantBiotechnologyLaboratory,DelhiTechnologicalUniversity, Delhi,India
BijuPravaSahariah UniversityTeachingDepartment,ChhattisgarhSwami VivekanandTechnicalUniversity,Bhilai,Chhattisgarh,India
MonalisaSatapathy DepartmentofChemicalEngineering,National InstituteofTechnologyRaipur,Raipur,Chhattisgarh,India
MaulinP.Shah ResearchScientist,EnvironmentalMicrobiologyLab, Ankleshwar,Gujarat,India
RaviShankar DepartmentofChemicalEngineering,MadanMohan MalaviyaUniversityofTechnology,Gorakhpur,UttarPradesh,India
ShambhooSharan DepartmentofChemicalEngineering,Madan MohanMalaviyaUniversityofTechnology,Gorakhpur,UttarPradesh, India
JoginderSingh DepartmentofBiotechnology,LovelyProfessional University,Phagwara,Punjab,India
SimranjeetSingh DepartmentofBiotechnology,LovelyProfessional University,Phagwara;PunjabBiotechnologyIncubator(PBTI); RAWTL,DepartmentofWaterSupplyandSanitation,SASNagar, Punjab,India
SwatiSingh EnvironmentalScienceandEngineeringDepartment,Indian InstituteofTechnologyBombay,Mumbai,India
SushmaRaniTirkey AppliedPhycologyandBiotechnologyDivision, CSIR—CentralSaltandMarineChemicalsResearchInstitute, Bhavnagar;AcademyofScientificandInnovativeResearch(AcSIR), Ghaziabad,India
RatnaTrivedi DepartmentofEnvironmentalScience,ShreeRamkrishna InstituteofComputerEducationandAppliedSciences,Surat,Gujarat, India
AyushiVerma PlantBiotechnologyLaboratory,DelhiTechnological University,Delhi,India
PradeepVerma BioprocessandBioenergyLaboratory,Department ofMicrobiology,CentralUniversityofRajasthan,Ajmer,Rajasthan,India
RahulVerma CentrefortheEnvironment,IndianInstituteofTechnology Guwahati,Guwahati,Assam,India
AparnaYadu DepartmentofChemicalEngineering,NationalInstitute ofTechnologyRaipur,Raipur,Chhattisgarh,India
Preface
Watertreatmenttechnologiessuchasadsorptionormembranefiltration,advancedoxidationprocessesofferthepossibility ofthecompleteconversionofhazardoussubstancestocarbon dioxide,water,andsalt-freeproducingresidues.However,one challengeisthecombinationofadvancedoxidationprocesses withotherunitoperationstoenhancetheoverallprocessefficiency.Themostsignificantdisadvantagesofadvancedoxidation processesarethehighenergyconsumptionandtheproductionof criticalintermediates.Newknowledgeofoxidantproductionwith higheryields,reactionspaths,reactordesign,acombinationof processes,thedeterminationofdangerousintermediatessuch asgenetictestmethods,andwaterapplicationshasbeenreused. Alltheseparametersareconsideredkeystoexploitingthepotentialofadvancedoxidationprocessesforwatertreatmentand reuse.Advancedoxidationprocessesarepurificationtechnologies aimedatdegradingandmineralizingunsurpassedorganicmatter fromwastewaterbyreactionwithhydroxylradicals.Recently, thesetechnologieswereproposedasasolutionforthetreatment ofemergingcontaminants,inparticularpharmaceuticalsand personalcareproducts.Theadvancedoxidationprocessreactions canfurtherbesupportedbyironcatalystsandUVradiation, resultingintheformationofphotopentonesystems.Theoptimumdesign,operation,andcontroloftheseprocessescanbe drivenbysophisticatedprocesssystemdesigntoolsthatcombine advancedoxidationprocessscience,photo-Fentonchemistry, andcutting-edgetechnologywithmodel-basedoptimization strategies.However,theuseofoptimizationtoolsrequiresthe availabilityofreliablemodels.
Thefollowingcontentsarediscussed:
Thebasicprinciplesofoxidationreactionsarefundamentalfor thesuccessfulapplicationofadvancedoxidationprocessesin complexmatricessuchaswaterandwastewater.Theinvestigationsofreactionpathsandmodelingpromoteunderstanding thebasics.TheFentonreactionisoneoftheoldestinducediron oxidationprocessesandisusedworldwideinitsentirety.However,therearesomedrawbackssuchashighsludgeproduction, ironleaching,acidicpH,etc.Wetrytoovercomethesedrawbacks throughabetterunderstandingoftheprocess,usingreaction promotersandheterogeneouscatalysts.Photocatalysiswas
discoveredmorethan30yearsagobyJapanesescientists. Althoughahugenumberofinvestigationshavebeencarried outtoachieveabetterunderstandingoftheprocessandthereactionpathways,nocommercialapplicationisavailableworldwide. Othercatalystsunderdevelopmentwithanemphasisonhigher photonicsefficiency,ashiftoftheabsorptionspectrumtothevisiblelightrange,andhigherphotoreactorefficiencycouldhelp overcomeconstraints.Oxidationprocessesareusedinmany plantsaroundtheworldtoremoveinadequatesubstances.New applicationssuggestopportunitiesforfurtherdevelopment.Integratingadvancedoxidationprocessesintoprocessesoffersother economicbenefits.Combinationswithbiologicalprocessesand inparticularmembranebioreactorsarecontinuallyinvestigated andsupportedbytoxicologicaltests.
Thisworkisausefulreferenceforresearchersandstudents involvedinwastewatertechnologies,includinganalyticaland environmentalchemistry,chemicalandenvironmentalengineering,toxicology,biotechnology,biochemicalengineering,liquid wastemanagement,andrelatedfields.Itisintendedtoencourage industrialandpublic-healthscientistsaswellasdecision-makers toacceleratetheapplicationofAOPsastechnologicalalternatives fortheimprovementofwastewatertreatmentplants.
Advancedoxidationprocesses forcomplexwastewater treatment
VineetKumara,b,MaulinP.Shahc
aEnvironmentalMicrobiologyandBiotechnologyLaboratory,Schoolof EnvironmentalSciences,JawaharlalNehruUniversity,NewDelhi,Delhi,India. bDepartmentofEnvironmentalMicrobiology,BabasahebBhimraoAmbedkar (Central)University,Lucknow,UttarPradesh,India. cResearchScientist, EnvironmentalMicrobiologyLab,Ankleshwar,Gujarat,India
1Introduction
ComplexwastewaterwithhighCOD,BOD,andcolorisdischargedfromvariousindustriessuchasdistilleries,breweries, tanning,leathermanufacturing,pulp-paper,etc.(Chandraand Kumar,2015,2018).Amongthem,thealcoholdistilleriesare oneofthemajorsourcesgeneratinghigh-strengthcomplex wastewater(Natarajetal.,2006 ).Amedium-scaledistilleryusing sugarcanemolasses,aby-productofsugarproductionthatisthe mostcommonlyusedrawmaterial,cangenerateanaverageof 12–15Lofcomplexwastewaterpe rliterofalcoholproduced (DeVriezeetal.,2014; KumarandChandra,2020 ).Thishighstrengthcomplexwastewaterisalsoknownasspentwash, stillage,raweffluent,orvinasses.Distilleryspentwashisan undesirableviscous,hydrophilic,andresidualbrowncoloredliquidwaste,whichcontainshighlevelsofbiologicaloxygen demand(40,000–60,000mgL 1),chemicaloxygendemand (90,000–190,000mgL 1),andtotaldissolvedsolids(90,000–150,000mgL 1)withanacidicpH(3.0–4.07)(Acharyaetal., 2011; SinghandDikshit,2011; ChandraandKumar,2017a ).Spent washalsocontainsresidualreducingsugars,phenolics,lipids, proteins,aminoacids,andvolatileorganicacidsgeneratedby
theyeastsduringfermentationprocess.Besidestheorganiccontent,ahighmineralloadofsulfateSO4 2 ,potassium(K+),phosphorousPO4 2 ,calcium(Ca2+),andsodium(Na+)hasbeen reportedingeneratedspentwash( JainandSrivastava,2012; ChandraandKumar,2017a).Moreover,italsocontainslargeconcentrationsofheavymetalsviz.iron(Fe),zinc(Zn),nickel(Ni), manganese(Mn),lead(Pb),mercury(Hg),copper(Cu),andchromium(Cr)( Jacketal.,2014; Maharetal.,2013).Recently,spent washwasreportedtocontainhighamountsofbutanedioicacid bis(TMS)ester;2-hydroxysocaproicacid;benzenepropanoicacid, α-[(TMS)oxy],TMSester;vanillylpropionicacid,bis(TMS),and otherrecalcitrantorganicpollutantssuchas2-furancarboxylic acid,5-[[(TMS)oxy]methyl],TMSester;benzoicacid 3-methoxy-4-[(TMS)oxy],TMSester;andtricarballylicacid 3TMS(ChandraandKumar,2017a).Accordingtothe USEPA (2012),mostoftheseidentifiedorganiccompoundsaretoxic,carcinogenic,mutagenic,andendocrinedisruptorsinnaturewhile someorganiccompoundsinspentwasharerecalcitranttobiodegradation(Chowdharyetal.,2018).Thepollutantspresentingeneratedspentwasharereactingtoeachotherandmakethe wastewatermoretoxicandcomplex(Arimietal.,2014; Kumar andSharma,2019).Dependingonthesugarcaneoriginandopted industrialprocessforalcoholproduction,theintrinsiccompositionofspentwashcanvarysignificantly.TheacidicpH(from3.8 to5)ofspentwashisassociatedmainlywiththepresenceof organicacidsproducedbytheyeastsduringthealcoholicfermentationprocess.Theacidityofthespentwashcausesthedissolution ofmetalsinwaterbodies.Moreover,thedarkbrowncolorofspent washisimpartedbycomplexcompoundssuchasmelanoidin,caramel,hexosealkalinedegradationproducts(HADP),furfurans (fromacidhydrolysis),lignin,andpolyphenolsaswellasplantpigmentssuchascarotenoids,chlorophyll,anthocyanins,andtannins,whichmakespentwashmorecomplex(Hatanoetal.,2008; DaiandMumper,2010; Arimietal.,2014; Zhangetal.,2017).These colorantsareconcentratedinmolassesafterthecrystallizationof sugarandarefurthertransferredtothespentslurryduringmolassesfermentation.Amongthecolor-causingpigments,melanoidin isthemajordarkbrowncolorpigmentofspentwash,anditis producedthroughnonenzymaticbrowningreactionssuchasthe Maillardreaction,alkalinedegradationreactions,orsugardegradationoccurredatmediumtemperature( >50 °C)andinabasic pHmedium(Hodge,1953; Cocaetal.,2004; Kumarand Chandra,2018a).Melanoidinpolymersaregenerallyregardedto beheterogeneousandacidicwithahighmolecularweight(5000 and40,000Da)alongwithsimilarchemicalpropertiestohumic
substances(i.e.,humicandfulvicacids)(Liangetal.,2009; Liuetal., 2013; KumarandChandra,2020).Theyarecomposedofhighlydispersedcolloids,whicharenegativelychargedduetothedissociationofcarboxylicacidsandphenolicgroups.Melanoidinsare difficulttocharacterizeduetotheirvaryingsizesandthetypes ofreducingsugarsandaminoacidsinvolvedintheirformation (Chandraetal.,2008; Arimietal.,2014; HatanoandYamatsu, 2018).Ithasbeenreportedthatmelanoidinshavenetnegative charges.Therefore,differentheavymetalssuchasCu,Cr,Cd,Fe, Zn,Ni,andPbstronglybindwithmelanoidinstoformorganometalliccomplexesindistilleryspentwash(Migoetal.,1997; Hatanoetal.,2013,2016; Chandraetal.,2018a,b,c).
Overthelastdecadesandduetoitshighinorganicloads,spent washhasbeenwidelyusedasaliquidfertilizerforsustainable agriculture(KumarandChopra,2012; JainandSrivastava,2012; Kumarietal.,2015).However,regulationshavemadespreading spentwashmoredifficultbecauseofitslowpHandhighorganic andinorganiccontent,whichmayberesponsibleforgroundwater contaminationandsoilcompaction(Ansari,2014).Somestudies haveindicatedthatspentwashnegativelyaffectsthephysical propertiesofsoil,suchashydraulicconductivityandredoxpotential(Alvesetal.,2015).Thus,thesafedisposalofspentwashis becomingaseriousproblemthroughouttheworld(Chowdhary etal.,2018).Differentconventionalprocessessuchasanaerobic digestion(biomethanation),anaerobiclagoons,andactivated sludgeareavailabletotreatspentwash(KumarandSharma, 2019; Kumaretal.,2020).Amongthem,biomethanationisapopular,cost-effective,first-stepconventionalbiologicaltreatmentof spentwashthatproducesmethanetomeetpartofthepower requirementindistilleries(Khairnaretal.,2013; Sankaranetal., 2017).Thismethanegasismainlyutilizedforrunningsteam boilerstogenerateelectricity.Onaverage,1m3 ofspentwashproduces38–40m3 ofbiogas(Sankaranetal.,2014).Moreover,in mostinstancesduringbiomethanation,themelanoidincompoundsrepolymerize,therebyintensifyingthecolorofspentwash andmakingthedecolorizationofwastewaterevenmoredifficult (Zhangetal.,2017).Thepolymerizationmayalsoextendtodifferentlevels,anditoccursincomplexways.Afterbiomethanation, thedistillerywastewaterstillretainsahighCOD(40,000–52,000mgL 1),BOD(8000–12,000mgL 1),andsubstantialcolor (Saneretal.,2014; NaveenandPremalatha,2016).Mostofthedistilleriesemployadirecttwo-stageaerobicprocessforfurther treatmentofthebiomethanatedspentwash.Often,itbecomes recalcitrant[biodegradabilityindex(BI) <0.2])tofurthereffective treatmentbybiologicalaerobicprocesses.Theantioxidizing
propertiesofmelanoidinsandplantphenolicsmakethewastewatertoxictolivingorganisms.Therefore,theprimarybiologicalaerobicandanaerobictreatmentswerefoundtobeineffectiveto degradecolorcompounds(Caderbyetal.,2013; Moraesetal., 2015; Kaushiketal.,2018).Themajorchallengesofthispartially treatedoruntreatedcoloreddistillerywastewaterbeforeitcan besafelydisposedintotheenvironmentaretheremovalanddegradationofcolor-contributingcompoundssuchasmalanoidins, caramel,andphenolicsandtheirmetabolicproductsaswellas inorganiccompounds(RayandGhangrekar,2015).Theconventionalwastewatertreatmentprocessesaredesignedtoreduce solidsinsuspension,biodegradableorganicproducts,microorganisms,andnutrientsfromwastewater,butnottoreducethe chemicalpollutantsandmuchlessemergingchemicalpollutants (Shahetal.,1989; Ghoshetal.,2004; Chandraetal.,2018a).However,forthetreatmentofspentwashbyconventionalmethods suchasaerobicandnonaerobicdigestion,theratioofBODto CODshouldbe40.6(ChainandDewalle,1977).DistillerywastewaterafterbiomethanationhasahighBOD/COD,whichwould causethedestructionofmicroorganismsthatareusefulin biodegradationprocesses.Conventionalanaerobic-aerobictreatmentprocessescanremoveamaximumofonly6%–7%oftheinitialconcentrationofmelanoidinsfromspentwash(Blonskajaand Zub,2009).Hence,thebiomethanatedspentwashstillhasahigh organicandinorganicload,whichrequiresfurthertreatment beforeitssafedisposalintotheenvironment(Chandraetal., 2015,2018b; ChandraandKumar,2017; Sanchez-Galvanand Bolanos-Santiago,2018).Theshortfallsofexistingtreatmenttechnologiesincludethehugefuelconsumptionforairdiffusionfor theaerobicoxidationoforganicmatter,andthepollutionof groundwaterandsurroundinglandsbytheleachingofpollutants fromaeratedlagoons(Padoleyetal.,2012a,b; Moraesetal.,2015; Chowdharyetal.,2018).Therefore,thesemethodsareexpensive andnoteco-friendlyastheygeneratelargeamountsofsludge, whichalsorequiressafedisposalandcancausesecondarypollution(Liangetal.,2009; LiuandZhang,2014; Liakosand Lazaridis,2014).
Whenuntreatedorpartiallytreatedcomplexwastewaterisdischargedintoawaterresource(rivers,lakes,andlagoons),melanoidinsreducesunlightpenetration,whichinturndecreases boththedissolvedoxygenconcentrationandthephotosynthetic activityofmarineplants;thiscreatesanaerobicconditionsthat killmostoftheaquaticlife(Ramakritinanetal.,2005; Kumar andGopal,2001).Additionally,thedischargeofspentwashinto waterbodiesreleasesanunpleasantodorandalsocontributes
todisseminatingendemicdiseasesintheabsenceofnaturalpredators.Thedisposalofspentwashonlandisequallyhazardous;it inhibitsseedgerminationanddepletesvegetationbyreducingthe soilalkalinityandmanganeseavailability(Pandeyetal.,2008; BharagavaandChandra,2010).Thedarkbrowncoloredwastewaterafterbiomethanationandfurtherdilutionisusedforirrigation, causinggradualsoildarkeningthataffectsthephysicochemical propertiesofsoilwhilealsobeingharmfulforbeneficialsoil microorganisms(ChidankumarandChandraju,2008; Juwarkar andDutta,1989).Sometimes,theleachingofspentwashinto thegroundwatertableresultsinseveregroundwatercontamination( Jainetal.,2005; ChauhanandRai,2010).Thedirectdumpingofcoloredwastewaterposestherisksofsoilsalinization (Kaushiketal.,2005)andcontaminationofgroundwaterbyheavy metals(Maharetal.,2013).Hence,untreatedspentwashisan ecotoxiceffluentandisunsafeforuseinanyformforhumans andtheenvironmentsuchasagriculturalandaquaticecosystems (Kaushiketal.,2005; Ayyasamyetal.,2008; Alvesetal.,2015).The wastewaterdischargesitesandtheiradverseeffectsonhumans andanimalsareshownin Fig.1.Thereisanurgentneedtotreat

Fig.1 Aviewofcomplexcoloredwastewaterdischargedfromalcoholdistilleriesanditsimpactinenvironment: (A)spentwash,(B)biomethanatedspentwash,(C)aquaticpollution,and(D,E)soilpollution.
(A)
(B) (E)
theseindustrialeffluentstoremovecontaminantspriortodischargeintothesurroundingsoilandwaterbodies(Kaushik etal.,2018).Recentadvancesinnewlydevelopedprocessesto treatdistillerywastewaterincludedifferentphysical,chemical, biological,andintegratedtechniques.Nowadays,“advancedoxidationprocesses”(AOPs)havereceivedgreatattentionforthe removaloforganicpollutantspresentinvariousindustrialeffluents( WangandXu,2011; DengandZhao,2015).AOPshavebeen usedasalternativeandeffectiveoptionsfortreatinguntreatedor partiallytreatedDSW.
Thischapterofferslatestandadvancedinformationaboutthe variousAOPsandtheirdegradationmechanismsofpollutants thatarebeingusedforthetreatmentofcomplexdistillerywastewater.Further,theauthorsalsodiscussedissuesassociatedwith practicalwastewatertreatmentandofferedsuggestionsforthe directionoffutureresearch.
2Advancedoxidationprocesses(AOPs)
AOPsareconsideredhighlyeffectivewastewatertreatment technologiesforremovinglowbiodegradability,refractory,inhibitory,orhighchemicalstabilitypollutants.TheAOPmechanisms relyontheinsituformationofhighlyreactiveoxidantspecies, mainlyhydroxylradicals( OH).The OHradicalsarehighlyeffective,powerful,ubiquitousinnature,andnonselectivewith electrophilicbehaviorandaredoxpotentialof2.8V,whichacceleratetheoxidationanddegradationofawiderangeofcontaminantsinwastewaterbyabstractingahydrogenatomfrom aliphaticcarbon,oraddingahydrogenatomtothedoublebonds andaromaticrings( WangandXu,2011; OturanandAaron,2014). The OHiscompoundstodestroyandevenmineralizetheminto carbondioxide(CO2),water(H2O),andinorganicsaltstosome extent.
Organicspecies+ HO ! CO2 +H2 O+inorganicions(1)
Theorganiccompoundscontainingcarbon-carbondoublebonds intheirstructurearemorereactivetoward OH.However,thelifetimeof OHisextremelyshort(t 10 3 s),andoncethe OHhas formed,itcangiverisetoseveralelementaryreactions.TheAOPs arealsooperatedatambienttemperature.Otherradicalsand activeoxygenspeciesgeneratedinAOPsaresuperoxideradicals O2 ðÞ,hydroperoxylradicals HO2 ðÞ,sulfateradicals( SO4 ), andorganicperoxylradicals( ROO)(Manickavachagametal., 2014; OturanandAaron,2014).Theseradicalsareformedfrom
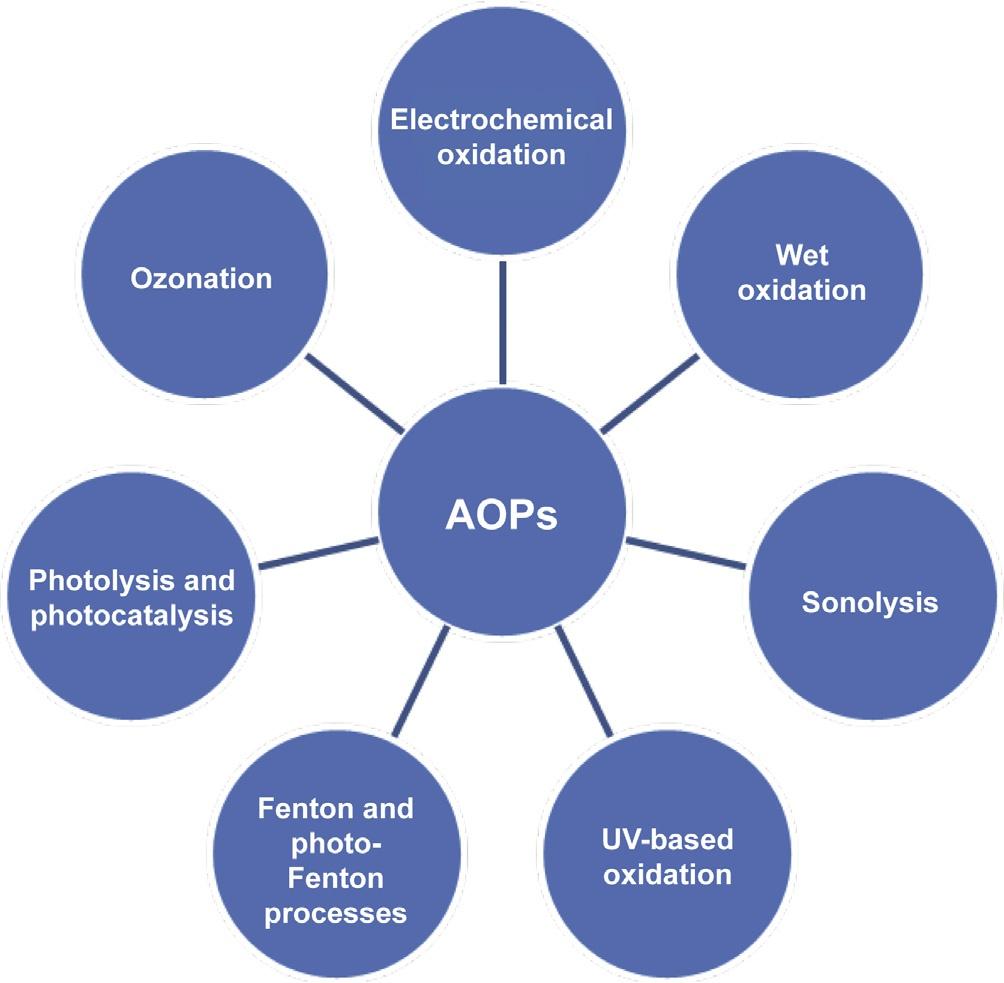
alessreactiveprimaryoxidantsuchashydrogenperoxide(H2O2) orozone(O3).Thegenerationofthesereactiveagentscanbe achievedbymeansofseveralprocesses,includingsonolysis, ozone-basedprocesses(ozonation),ultraviolet(UV)irradiation, Fentonoxidation,photo-Fentonoxidation,ultrasound,photocatalysis,andvariouscombinationsofthesetechnologiessuchas Fenton(H2O2/Fe2+),photo-Fenton(H2O2/UV/Fe2+),peroxidation combinedwithultravioletlight(H2O2/UV),peroxone(O3/H2O2), peroxonecombinedwithultravioletlight(O3/H2O2/UV),theO3/ UVsystem,O3/TiO2/H2O2,andO3/TiO2/electronbeamirradiation(Fig.2).
2.1Fentonandphoto-Fentonoxidationtreatment
Fentonoxidationisaveryeffectiveandadvancedtechnology totreatcomplexdistillerywastewater(Hadavifaretal.,2009).In additiontoimprovingthebiodegradabilityoftheeffluent,the FentonprocessremovestheremnantCOD,toxicity,andcolorof thewastewater(BhoiteandVaidya,2019).IntheFentontreatment process,theFentonreagent,amixtureofH2O2 andferrousiron salts(Fe2+),generateshighlyreactiveradical( OH),whichinvolves
Fig.2 Differentcategoriesof advancedoxidationprocesses (AOPs)usedforcomplex wastewatertreatment.
acomplexreactionsequenceandultimatelyleadstoorganic removalofthewastewater(Zhangetal.,2019).TheFe2+ initiates andcatalyzesthedecompositionofH2O2 inacidicconditions, resultinginthegenerationof OH.TheFentonprocessmechanismispresentedbythefollowingreactions:
TheFentonoxidationandadsorptionpretreatmentofbiomethanatedspentwashimprovesthebiogasrecovery(Bhoiteand Vaidya,2019).Usingaferroussulfatecatalystfordilutedwastewater,a54%reductioninCODwasachievedwithin1hinacidic medium(pH3)atambienttemperature(30°C).Afterpostoxidation,theBIvaluewasimproved(0.33).Aftersubsequentadsorptionoveractivatedcarbon(loading5%)for1h,theCODreduction (70%),andBIvalue(0.43)improvedfurther.Uponanaerobictreatmentwith1%acclimatizedbiomass,1Nm3 ofbiogas(47%CH4) wasadditionallyformedperm3 oftreatedwastewater;without pretreatment,thisvaluewas0.9Nm3 (just11%CH4).Lastly,aerobictreatmentwasperformedandtheresultswereencouraging: theBIwasimprovedto0.51andtheCODreductionwasactivated 94%.AlthoughtheoxidationprocessesbasedontheFentonreactionarechemicallyveryeffectivetotreatawiderangeoforganic pollutantsinwastewater,theyhaveseverallimitationsthat includehighsludgeformation;operationatadversepHvalues (2–3);theadditionofrelativelylargeamountsofFe2+;theconcentrationofH2O2;slowingdownoftheFentonreactionaftertheinitialconversionofFe2+ toferricions(Fe3+);thepresenceofother ionssuchasphosphate,sulfate,fluoride,bromide,andchloride; theinactivationofheavymetalionsinthesludgebytheformation ofhydroxidecomplexes;andtheneedtoseparatethecatalyst aftertheprocess( WangandXu,2011; OturanandAaron,2014). Moreover,anotherlimitingfeatureoftheFentonreaction-based oxidationprocessisthegenerationofrecalcitrantintermediates, whichcanconstrainthecompletemineralizationofpollutants. Evenwiththeexistenceoftheselatentlimitations,conventional Fentonreactionprocesseshavebeenextensivelyusedinsmallscaleindustrialwastewatertreatmentorspecialpollutant removal.
InpursuitofthelimitationsoftheclassicalFentonprocess, severalotherprocesseshavebeendevelopedfromthatprocess.
Fe2+ +H2 O2 ! Fe3+ +OH + OH (2)
Fe2+
(3)
(4)
(5)
ThefirstprocesswastocouplelightenergyfromUVsourcesor emissionsfromthesuninaprocesscalledphoto-Fentonoxidation.Thephoto-Fentonprocess(H2O2/Fe2+/UV)involves OHformationthroughthephotolysisofH2O2 inthepresenceofUV.In thepresenceofUVirradiation,theFe3+ producedinEq. (3) is photocatalyticallyconvertedtoFe2+,withtheformationofan additionalequivalentof OH.
Thephoto-Fentonreactiongivesfasterratesandhigherdegreesof mineralizationcomparedtotheconventionalFentonprocess. Thisreactioncanbedrivenbylowenergyphotons,anditcanalso beachievedusingsolarirradiation.Thisfactcansignificantly reducetheoperationalcostofthetreatment(Ebrahiemetal., 2017).Besides,thisprocesshasbeeninstrumentalinimproving thekineticsandtheperformanceoftheclassicalFentonprocess, includingdecreasingthedemandforthecatalystandimproving colorremoval.However,thephoto-Fentonprocessdoesnot addresstheotherlimitations,especiallythelowpHoperation andtheproblemofhighheavymetalionsinthefinalwastewater (Ahmedetal.,2011).TherearealsoreportsofenhancedperformanceinCODremoval,anincreaseinbiodegradability,andthe detoxificationofcomplexwastewateraftertreatmentwith photo-Fentonoxidation(DerakhshanandFazeli,2018).ThetoxicityofH2O2 toseveralmicroorganismsandtheuseofexcess amountsofH2O2 couldpossiblydeterioratetheoveralldegradationefficiencyforcaseswheretheFentonprocessisfollowedby biologicaloxidation.Additionally,thenecessityofwastewater acidificationduetotheoptimumlowpHrange(pH3)forthe photo-Fentonreactionoccurrence,andtheinevitableneedfor catalystremovalattheendofthereactionareadditionallimitationsoftheseAOPs.AnotherkeymodificationtotheclassicFentonprocessaimsateliminatingthelimitationsoflowpH operationandtherecoveryofspentcatalystsfromwastewater bytheuseofheterogeneousFentonprocesses.Itentailsembeddingtheferrouscatalystandtheacidgrouponacarriermaterial. Bylimitingthecatalystsupplyintheeffluent,themethodensures thatminimalsludgeisformedbythecoagulationprocess.Inaddition,theamountofheavymetalionsinthesludgeandtreated effluentisminimized.Thecatalystionsaresupposedtobeslowly releasedfromtheembedmentwheretheyreactwiththeH2O2 to formtheradicals.TheuseofmodifiednaturalzeoliteasaheterogeneousFentoncatalystinthetreatmentofrecalcitrantindustrial wastewaterwasreportedby Arimi(2017).Inthisstudy,thezeolite pelletsweremodifiedbypretreatmentwithsulfuricacid(H2SO4),
Fe3+ +H2 O ! Fe2+ +H + + OH (6)
nitricacid(HNO3),andhydrochloricacid(HCl),beforeembeddingontheFe2+ ions.TheH2SO4-Fe2+-modifiedcatalystsshowed thehighestactivity,whichachieved90%colorand60%TOC(total organiccarbon)removalat150g/Lpelletcatalystdosage,2g/L H2O2 at25°C.TheheterogeneousFentonwiththesamecatalyst improvedthebiodegradabilityoftheanaerobiceffluentfrom 0.07to0.55.Inadditiontoposttreatment,thecatalystwasalso appliedtopretreattherawmolassesdistillerywastewaterand increaseditsbiodegradabilityby4%.Subsequently,thecolorof theresultantanaerobiceffluentwasalsoreduced.Theauthor recommendedthatthemodifiedzeolitecanbeusedasaheterogeneousFentoncatalystinthepretreatmentoftherawmolasses distillerywastewaterbeforeanaerobicdigestionwithminimal CODloss.However,theUVandphotocatalysisprocessesarealso costlybecauseofthehighenergyrequirements.
2.2Ozone-basedoxidationtreatment
Theozonation/ozonolysisprocessseemstobeapromising oxidationtreatmenttechnologyfortheremovalofcolorantfrom molasses-derivedwastewaterorsyntheticdistillerywastewater (Otienoetal.,2019; Kimetal.,1985).O3 isusuallygeneratedinsitu byelectricdischargeinastreamofoxygen(O2)orair,leavingneitherodorsnorresidualtastes.Itiswidelyusedfordisinfectiondue toitsstrongcelllyticactivitythatcankillthemicroorganisms foundinthewastewater(Martı´nezetal.,2011).Intheozonation process,O3 isusedasapowerfuloxidizingagent(E0 ¼ 2.07V)that isabletotransformtheorganicpollutantsintosmallercompoundsorCO2,H2O,andsulfateandnitrateanionswhenSand Natomsarepresent( WangandXu,2011; DengandZhao, 2015).OnceO3 isdissolvedinH2O,itreactswithalargenumber oforganiccompoundsintwopossibleways:directoxidationas molecularO3,oranindirectreactionthroughtheformationof secondaryoxidantssuchasfreeradicals,thatis, OH.BothO3 and OHarestrongoxidantsandarecapableofoxidizinganumberoforganicpollutants,finallyreducingthevalueofCODin wastewater(Gulyasetal.,1995; Rice,1995).Differentdetailed mechanismshavebeenproposedtoexplainthe OHgeneration, andtheoverallreactioninvolving OHgenerationisexpressedas follows( WangandXu,2011; DengandZhao,2015):
O3 +H2 O ! 2 OH+4O2 Ozonation ðÞ (7)
Inthepresenceofotheroxidantsorirradiation,the OHyieldcan besignificantlyimproved.Forexample,intheperoxone (O3/H2O2)system,theO3 decompositionand OHproduction
areenhancedbyhydroperoxideHO2ðÞ producedfromH2O2 decomposition.H2O2 producesHO2 underbasicconditions. HO2 inducedfromH2O2 reactswithO3 andproducessomefree radicals.
H2 O2 ! HO2 +H + O3 =H2 O2 ðÞ (8)
HO2 +O3 ! OH+O2 +O2 O3 =H2 O2 ðÞ (9)
InO3/UVirradiation,H2O2 isgeneratedasanadditionalprimary oxidantthroughphotolysisoftheO3,whichproducestheformationof OH.H2O2 furthergenerates OHradicals,asshowninthe followingreaction(DengandZhao,2015):
O3 +H2 O+ hv ! H2 O2 +O2 O3 =UV ðÞ (10)
H2 O2 + hv ! 2OHPhotolysisofH2 O2 ðÞ
H2 O2 +OH ! HO2 +H2 OPhotolysisofH2 O2 ðÞ (11)
Theaboveoptimizestheapplicationofozone,andtheconcentrationsof OH(E0 ¼ 2.80V)thatareproducedbyO3 decomposition inaqueoussolutionsproduceasignificantincreaseinthedecompositionrateofpollutants(Gulyasetal.,1995).Inaddition,H2O2 is aweakacidwitharelativelyhighoxidantpotential(E0 ¼ 1.77V), whichalonedoesnotleadtothegenerationofhydroxylradicals. Theslowreactionsratesmaketheprocessineffectivewhenoxidizingmorerefractoryandrecalcitrantpollutants.Therefore, theadditionofH2O2 withO3 increasestherateofO3 oxidation byallowinganenhancementinthequantumyieldoftheformationofthe OH(Rice,1995).
Ozonecanselectivelyattackthedoublebonds(e.g.,C]C, N^C)andfunctionalgroups(e.g., CH3, OH,and CH3)in acidorneutralconditionswithlimitedconcentrations (MantzavinosandPsillakis,2004).Melanoidins,polyphenols, andothercontaminantsofdistillerywastewaterhavedouble bonds(C^NandC]C)andotherfunctionalgroupsintheir structurecanbeselectivelybrokendownbyO3 withouttheextensiveoxidationofthevastorganicmatter(OMs)(Naiketal.,2010). In1985,Kimandhiscolleaguesinvestigatedthedecolorization anddegradationproductsofmelanoidinspreparedfroma glucose-glycinesystemwiththeuseofO3.Inthisstudy,melanoidinsweredecolorizedtodegreesof84%and97%afterozonolysis at 1°Cfor10and90min,respectively;themeanmolecular weightofmelanoidinsdecreasedfrom7000to3000Daafterozonolysisfor40min.TheselectivityofO3 wasclearlydemonstrated inthestudyof MartinSantosetal.(2003),where80%ofphenolics and16%oftheorganicloadwereremovedaftertheozonationof molasses-baseddistillerywastewateratanacidcondition.Thus,
oxidationbyO3 couldachieve80%decolorizationforbiologically treatedspentwashwithasimultaneous15%–25%CODreduction. Italsoresultedintheimprovedbiodegradabilityoftheeffluent.In general,ozonationhasadvantagesoverAOPsbecauseitcanbe usedineitherthepretreatmentstep(Battimellietal.,2010)or inthefinaltreatmentstep(Beltranetal.,2001).Preozonation showedamorethan99.5%settlingabilityoftotalsolublesolids (TSS),presentinhighlyviscouswheatandsweetpotatodistillery wastewater( Tatedaetal.,2004).Itwasalsoobservedthatozone oxidationaftertheaerobictreatmentofwinedistillerywastewater eliminatesmostofthebiodegradableOMs.Therefractoryand undecomposedphenoliccompoundsandOMsremainingafter theaerobictreatmentcanbecompletelyoxidizedbyO3 (Benitezetal.,1999; Beltra ´ netal.,1999).Preozonationwasalso foundeffectivenotonlyforthereductionofCODandTOC,but alsoforenhancingthenitrogenremovalandsettlingproperties ofsludgeduringasubsequentaerobicbiologicalprocess (Beltra ´ netal.,2000). Sangaveetal.(2007b) conducted laboratory-scaleexperimentstoinvestigatetheeffectofozone asapreaerobictreatmentandapostaerobictreatmentforthe treatmentofdistillerywastewater.OzonewasfoundtobeeffectiveinbringingdowntheCOD(upto27%)duringthepretreatmentstepitself.Inthecombinedprocess,thepretreatmentof wastewaterwithvariousprocessesledtoenhancedratesofsubsequentbiologicaloxidationstep,almost2.5timesincreasein theinitialoxidationratehasbeenobserved.PostaerobictreatmentwithozoneledtothefurtherremovalofCODalongwith thecompletediscolorationoftheeffluent.Thepre-andpostozonationcombinedwithconventionalozone-aerobicand oxidation-ozoneofdistillerywastewaterachieved79%COD reductionalongwithalmostcompletedecolorizationofwastewater(MartinsandQuinta-Ferreira,2014; Sangaveetal.,2007b). Tsioptsiasetal.(2016) studiedthedegradationofmolasseswastewaterbybiologicaltreatmentcombinedwithozonation.They foundthecombinationofozonepretreatmentfollowedbybiologicaltreatmentresultedin66.5%CODremovalandtheproduction ofeffluentwith240mgL 1 COD.Therefore,itwasconcludedthat ozonepretreatmentmightcontributetotheenhancementofthe biodegradabilityoftheinfluent.Ozonepretreatmentofmolassesbasedbiomethanateddistillerywastewaterenhanceditsbiocomposting(Maliketal.,2019).Ozonolysisposttreatmentofanaerobicallydigesteddistillerywastewaterwasalsostudiedby Otieno etal.(2019).Inthisstudy,theozonolysisofrealanaerobically digesteddistillerywastewaterwasfoundtobeafeasibleposttreatmenttothebiomethanation.Theoptimalconditionsachieved
adequateozoneutilizationefficiency >95%andcolorremoval >80%.Thisstudyhasshownthatthroughozonolysis,thecomplex aromaticcompoundsarebrokendownintosimplealiphaticcompounds,resultinginanincreasedoxidationstateandimproved biodegradability.Apartfromcolorabatement,theozonolysisprocessalsoachieved88%sludgesolubilization.Theuseofozonation asaposttreatmenthasanadvantageoflowsludgeformationbut itsapplicationislimitedbythehighinstallationandoperation costs.Theproblembecomesevenmorecomplicatediftheprocessinvolveshighdailyvolumesofeffluents.Anotherlimitation oftheozonationprocessisthelowCODremoval,especiallywhere theinfluenthasreasonablyhighCOD(Cocaetal.,2005).However, itisstillinterestingtostudythechemicalpretreatment(partial oxidation)toincreasethemicrobialbiodegradability(posttreatment)ofmolassesdistillerywastewatercolorantstofindtheoptimalbalanceofcostandperformance.
2.3Photocatalytictreatment
Photocatalyticdegradationisanattractive,efficient,andcosteffectivetreatmenttechnologytoenhancethebiodegradabilityof hazardousandnonbiodegradablecontaminants,suchaspersistentorganicpollutants(Mabuzaetal.,2017; Takleetal.,2018). Thisprocessallowsthetransformationofchemicalpollutants intolesstoxicsubstancesand/orwithstructuralfeaturesthat aremorereadilybiodegradable.Photocatalytictreatmentprocess isbasedonthecombinationofoxidizingagentswithanappropriatedcatalystand/orlight.Theseprocessesmaybeofparticular interestfortreatingeffluentcontaininghighlytoxiccompounds, andforwhichthebiologicalprocessesmightnotbepertinent (CatalkayaandSengul,2006).Thebasisofphotocatalysisisthe photoexcitationofasemiconductorthatissolidasaresultof theabsorptionofelectromagneticradiation,oftenbutnotexclusivelyinthenear-UVspectrum.Undernear-UVirradiation,asuitablesemiconductormaterialmaybeexcitedbyphotons possessingenergiesofsufficientmagnitudetoproduceconductionbandelectrons(e )andvalencebandholes(h+).Thee andtheh+ migratefromtheirrespectivebandstothesurfaceof thesemiconductor.Theyreactwithasuitableredoxspeciesin theenvironment,whichcouldleadtoH2Osplitting(H2 andO2 generation)andtheformationof OHand O2 radicals.TheseradicalsareabletodegradeawiderangeofrecalcitrantorganiccompoundsanddetoxifyHMions(e.g.,Cr6+ andAs3+).Theresults achievedinrecentyearsshowthattechnologiesbasedonphotochemicalandphotocatalyticapproachesseemtobevery
promisingasviablealternativesforwastewatertreatments (Matthews,1991; Poulopoulosetal.,2019; Takleetal.,2018).The photocatalyticdegradationprocessusingsolarlightasanirradiationsourceshowedpotentialapplicationfortheremovalofthe colorand/orCODfromdistilleryeffluent(Vineethaetal.,2013). Themaximumcolorremovalofthedistillerywastewaterachieved was79%atanH2O2 concentrationof0.3M,apHof6,aneffluent CODconcentrationof500ppm,andacatalystdosageof0.1g/L. TheTiO2/H2O2 systemseemstobemoreefficientincomparison tothesynergeticactionthatappearswhenusingH2O2 andTiO2 ( Vineethaetal.,2013).Recently,avanadium-dopedTiO2 (V-TiO2)photocatalystwasstudiedviathedegradationofspent washandJakofixreddye(HE8BN)undernaturalsunlightaswell asastandardartificialsolarenergysource(Xelamp)( Takleetal., 2018).Thehighestactivitywasobservedfora1%V-TiO2photocatalystforthedegradationofspentwashandJakofixreddyeunder naturalsunlight.Further,thedegradationofcoloredcompounds inthespentwashwasmonitoredbygelpermeationchromatography,whichshowedthedegradationofhighmolecularweightcompoundsintolowmolecularweightfractions.Thecatalyst decomposed90%oftheJakofixreddye(HE8BN)in3.5hand 65%ofthespentwashin5hunderirradiationwithnaturalsunlight.Acyclingstabilitytestshowedthehighstabilityandreusabilityofthephotocatalystfordegradationreactions,witharecovery ofaround94%–96%. Davidetal.(2015a) reportedthedecolorizationanddegradationofspentwashusinganano-Al2O3/kaolin photocatalyst.Inthisstudy,thedegradationoforganicpollutants intheformofcolorwasperformedusingananophotocatalystpreparedusinganaluminumoxide(Al2O3)nanoparticleandkaolin clay.Theprocessparameterssuchasdosage,pH,temperature, andagitationwereoptimizedusingaTaguchiorthogonalarray designtoattainthemaximumdecolorizationefficiency.Optimizationoftheprocessparametersresultedinamaximumof80% spentwashdecolorization.Usinganartificialneuralnetwork (ANN),atwo-layeredfeed-forwardbackpropagationmodel offeredthebestperformanceandpredictivemodelforspentwash decolorization.TheexperimentaldatawerefoundtobeinexcellentagreementwiththepredictedresultsfromtheANNmodel. Thephotocatalyticdegradationofmolasseswastewaterhasbeen reportedtobeeffectiveforthecompleteremovaloftherecalcitrant melanoidinscontainedinmolasseswastewater,leadingtothe colordisappearance.Thisprocess,however,isineffectiveforthe removalofthehighorganiccontentofmolasseswastewater. Therefore,ithasbeenrecommendedforapplicationasaplausible posttreatmenttoanaerobicdigestion(Otienoetal.,2016).
2.4UV-basedoxidationtreatment
InUVdirectphotolysis,thedegradationprocessthroughthe absorptionofincidentradiationfromtheUVlightisthemain removalmechanism.Therefore,theapplicationusuallyfocuses onthecontaminantsthatstronglyabsorbUVradiation.Most UVlightabsorberscontaindoublebondsorconjugateddouble bonds,whichincludescarbon,nitrogenoroxygenatomsandis characterizedbydelocalized π-electrons(DengandZhao,2015). UVphotolysiscanselectivelyreducesomeorganiccompounds, butitaloneisnotefficientenoughforpollutantremoval.However,manyresearchershavefoundthatUVphotolysiscan enhancetheoxidationpotentialofsomeoxidationprocesses. WhenUVphotolysisiscombinedwithozonation(UV/O3 oxidation),more OHisproducedviathephotolysisofH2O2 asareactionintermediate,andorganicpollutantsaredecomposedmore completely.Thegenerationof OHisfundamentaltotheprocess, asthe OHislargelyresponsibleforthesuccessofthisprocess. ThisprocesscombinesbothH2O2 andUVlightinasynergistic effecttodegradethecontaminantsandpathogenicmicroorganismsinthecontaminatedwater.Inwastewatertreatmentprocesses,UV/O3 oxidationcanbeusedasapretreatmentfor biologicalprocessesinwhichcomplexpollutantsaredecomposedintomorebiodegradablesubstances.Therefore,more organiccompoundscanberemovedinthesubsequentbiological treatmentprocess.Thedegradationofdissolvedorganicnitrogen (DON)-associatedcolorfromwastewatercontainingmelanoidins byusingaUV/H2O2 AOPwasinvestigatedby JasonDwyer(2008). Theoxidationprocesswasmuchmorecapableofremoving99% color,50%dissolvedorganiccarbon(DOC),and25%DONatthe optimalapplieddoseofUV/H2O2 forthesystem(3300mgL 1). ThisindicatedthatcolorandDONremovalweredecoupledproblemsforthepurposeoftreatingmelanoidinbyanAOP;therefore, colorremovalcannotbeusedasanindicationofDONremoval. Colorwascausedbyorganicmoleculeswithmolecularweights greaterthan10kDa.Oxidationcausedapartialreductionofthe DON(41%–15%ofthetotaldissolvednitrogen)andDOC(29%–14%oftheDOC)associatedwiththelargemolecularweightfraction(>10kDa)aswellasalmostcompletecolorremoval.The degradedDONwasmostlyaccountedforbytheformationof ammonia(31%ofthenitrogenremovedfromthelargefraction) andsmallmolecularweightcompounds(66%ofthenitrogen removedfromthelargefraction).ThedegradedDOCappeared tobemostlymineralized(toCO2)withonly20%ofthedegraded compoundsappearingassmallmolecularweightDOC(Dwyer
etal.,2008).UVphotodegradation,ontheotherhand,can degradethecolor-causingmelanoidins,leadingtocompletecolor removalafterashortdurationofirradiation.However,theUVprocessisenergy-intensiveandincapableofeffectiveTOCreduction. Foreffectivemolasseswastewatertreatment,thesetwoprocesses canbeintegratedsuchthatUVphotodegradationisappliedasa posttreatmenttoanaerobicdigestion.Inthissystem,theanaerobicdigestionfirstremovesthehighorganiccontent,followedby colorremovalbytheUVprocess.Desk-scaleanaerobicdigestion andphotodegradationprocesseswerecarriedoutinbatchreactors.Ahybridphotocatalystconsistingoftitaniumdioxide (TiO2)andzincoxide(ZnO)wasusedforphotocatalyticdegradation.Biodegradationofwastewaterduringanaerobicdigestionat thermophilicconditionsinthebioreactorachievedhighTOCand CODreductionsof80%and90%,respectively,butwithincreased colorintensity.Contrastingly,UVphotodegradationachieveda highcolorreductionof92%withaninsignificant6%TOCreductionafter30minofirradiation.Duringphotodegradation,the mineralizationoftherecalcitrantorganiccompoundsledtocolor disappearance.TheauthorsuggestedthattheUVprocesswas onlysuitableforcolorreduction.Therefore,thereisapossible synergywhenthetwoprocessesareintegratedwithanaerobic digestionprecedingUV,whereanaerobicdigestionremovesthe highCOD/TOCwhileUVremovestherecalcitrantcolorata reducedcost(Mabuzaetal.,2017).Themaindrawbackofusing theabove-mentionedoxidationprocessesistheirhigh operationalcost.
2.5Sonication/sonolysis
Ultrasoundisincreasinglybeingseenashavingpotentialinthe treatmentofwater,wastewater,andsewagesludge.Sonochemical oxidationemploystheuseofultrasound,resultinginthecavitationphenomenon.Thatphenomenonisdefinedastheformation, growth,andsubsequentcollapseofmicrobubblesorcavities occurringinextremelysmallintervalsoftime(microseconds) andreleasinglargemagnitudesofenergyatmillionsofsuchlocationsinthereactor.Sonochemicalreactionsareinducedupon high-intensityacousticirradiationofliquidsatfrequenciesthat producecavitation(typicallyintherangeof20–1000kHz).Itis generallybelievedthattherearethreepotentialsitesforchemical reactionsinultrasonicallyirradiatedliquids:thecavitationbubble itself,theinterfacialregionbetweenthebubbleandthesurroundingliquid,andthesolutionbulk.Ultrasoundirradiationeffectivelydestroysthecontaminantsinwaterbecauseoflocalized