Tille, Y: Sampling and Estimation from Finite Populations (Wiley Series in Probability and Statistics) Yves Tille
https://ebookmass.com/product/tille-y-sampling-and-estimation-fromfinite-populations-wiley-series-in-probability-and-statistics-yvestille/ ebookmass.com
Field Guide to Sharks, Rays and Chimaeras of the East Coast of North America (Wild Nature Press, 21) Dando
https://ebookmass.com/product/field-guide-to-sharks-rays-andchimaeras-of-the-east-coast-of-north-america-wild-naturepress-21-dando/ ebookmass.com
Banshee: MC, Strong Woman Lead, Women's MC, Harlots MC, Royal Bastards Spin-off, RBMC, Irish Alpha (The Royal Harlots MC Book 1) K.L. Ramsey
https://ebookmass.com/product/banshee-mc-strong-woman-lead-womens-mcharlots-mc-royal-bastards-spin-off-rbmc-irish-alpha-the-royal-harlotsmc-book-1-k-l-ramsey/ ebookmass.com
Consciences and the Reformation: Scruples over Oaths and Confessions in the Era of Calvin and His Contemporaries (Oxford Studies in Historical Theology) 1st Edition
Timothy R. Scheuers
https://ebookmass.com/product/consciences-and-the-reformationscruples-over-oaths-and-confessions-in-the-era-of-calvin-and-hiscontemporaries-oxford-studies-in-historical-theology-1st-editiontimothy-r-scheuers/ ebookmass.com
Psychiatric Nursing eBook 8th Edition, (Ebook PDF)
https://ebookmass.com/product/psychiatric-nursing-ebook-8th-editionebook-pdf/ ebookmass.com
So You Want to Start a Side Hustle Carrie Bohlig
https://ebookmass.com/product/so-you-want-to-start-a-side-hustlecarrie-bohlig-2/
ebookmass.com
There are no such things as applied sciences, only applications of science.
Louis Pasteur (11 September 1871)
Dedicated to my wife, Anne, without whose unwavering support, none of this would have been possible.
First published 2016 in Great Britain and the United States by ISTE Press Ltd and Elsevier Ltd
Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the Copyright, Designs and Patents Act 1988, this publication may only be reproduced, stored or transmitted, in any form or by any means, with the prior permission in writing of the publishers, or in the case of reprographic reproduction in accordance with the terms and licenses issued by the CLA. Enquiries concerning reproduction outside these terms should be sent to the publishers at the undermentioned address:
ISTE Press Ltd
27-37 St George’s Road
Elsevier Ltd
The Boulevard, Langford Lane London SW19 4EU Kidlington, Oxford, OX5 1GB UK UK
www.iste.co.uk
www.elsevier.com
Notices
Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary.
Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility.
To the fullest extent of the law, neither the Publisher nor the authors, contributors, or editors, assume any liability for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions, or ideas contained in the material herein.
For information on all our publications visit our website at http://store.elsevier.com/
© ISTE Press Ltd 2016
The rights of Jean-Paul Duroudier to be identified as the author of this work have been asserted by him in accordance with the Copyright, Designs and Patents Act 1988.
British Library Cataloguing-in-Publication Data
A CIP record for this book is available from the British Library Library of Congress Cataloging in Publication Data
A catalog record for this book is available from the Library of Congress ISBN 978-1-78548-179-6
Printed and bound in the UK and US
gain more in-depth knowledge about each subject if he/she so desires. In a reflection of today’s multilingual world, the references to which this series points are in German, French and English.
The problems of optimization of costs have not been touched upon. However, when armed with a good knowledge of the devices’ operating parameters, there is no problem with using the method of steepest descent so as to minimize the sum of the investment and operating expenditure.
Humid Air Water Cooling Towers
1.1. Properties of humid air
1.1.1. Absolute humidity and relative humidity
We shall base our discussion here on the most common case, which is that of a mixture of air and water vapor. The conclusions drawn can easily be transposed to apply to gaseous mixtures of a different nature.
The absolute humidity of an air–water vapor mixture is written as Y. It is the mass (in kg) of water vapor associated with 1 kg of dry air.
The air is saturated with vapor when the partial pressure of the vapor PV is equal to the vapor pressure π (t) corresponding to the temperature of the mixture.
The relative humidity is the ratio:
The saturation rate is the ratio of the absolute humidity Y of a given mixture to the humidity Ys which it would have had if it were saturated.
The absolute humidity of humid air is easily calculated as a function of the partial vapor pressure V
Now consider 1 m3 of humid air. The molar fraction of the water vapor is:
T: absolute temperature: K;
P: pressure: Pa;
Y: in humid air, the ratio of the mass of water vapor to the mass of dry air.
The mass of dry air occupying the volume V at the pressure P is:
The density of that air is, as we have just seen:
Now consider 1 m3 of humid air. The partial pressure of the dry air in that mixture at the total pressure P is P () V 1y , and the mass of dry air present is:
The mass of humid air making up this mixture occupying 1 m3 is:
Thus, we can write:
As the molecular mass of a gas is proportional to its density, the mean molecular mass of the humid air is:
The volume VGH of humid air corresponding to 1 kg of dry air is:
1.1.4. Vapor pressure of water
The vapor pressure of water is given by:
π (t): pressure of the water vapor: Pa;
t: temperature of the water: °C.
1.1.5. Transfer coefficients and Lewis number
Remember that the Prandtl number Pr is Cμ λ
C: specific heat capacity of the fluid: J. o 11 kg.C;
µ: dynamic viscosity of the fluid: Pa.s. = kg.m-1.s-1;
J: unit of work: 2 kg.m².; s
The equation of the wet-bulb thermometer can be written thus, according to equation [1.6].
However, according to equation [1.5b]:
CGH: specific heat capacity of the humid gas: J.kg-1.°C-1 or indeed, by setting ()
This equation is known as the wet-bulb thermometer equation
The cooling of the air is compensated by its enrichment in water. The enthalpy of the air in contact with the wet-bulb thermometer does not vary.
1.2. Mollier diagram
In Europe, there is a tradition of representing the properties of humid air using the Mollier diagram [MOL 23].
On this diagram, established at atmospheric pressure, the ordinate axis shows the enthalpy of the humid air and the abscissa axis the absolute humidity Y of the air.
In addition, we plot the lines corresponding to constant values of the properties of the air:
1) Lines ε const. =
We know that () PV ε π t =
Conversely: ()()HV t t π f P/ ε ==
However, V P Y P BY = +
Thus:
2) Lines PV = const.
V P Y P BY = +
These lines, therefore, are vertical.
3) Lines t = const.
The enthalpy of the humid air expressed in relation to the dry air is written, in accordance with equation [1.2]:
HGH = HG + YHV
These lines are therefore straight lines whose ordinate at the origin is HG and whose slope is HV. As the enthalpy of the vapor includes its latent heat, that slope is steep.
Remember that:
For the air–water system, the coefficient σ is very close to 1, on condition that the Chilton–Colburn analogy is verified, meaning that we are in the turbulent regime.
Lu: Luikov number. This number is very close to 1 for water vapor in air. The Luikov number is none other than the Lewis number.
H π : vapor pressure of water at tH: Pa.
L: latent heat of vaporization of water from the liquid state (at temperature tH) to the vapor state (at temperature tG): J.kg-1.
A psychrometer is a device made up of two temperature probes: – one measures the dry temperature GH t;
Figure 1.1. Shape of the Mollier diagram
– the other measures the humid temperature H t.
In order for the measurement of tH to be correct, the Chilton–Coburn analogy must be valid, meaning that we must be in the turbulent regime around the probe. It is recommended that the gaseous flow rate be greater than 2 m.s-1.
1.4. The cooling tower
1.4.1.
Description of the tower
As regards the internal packing of the tower, in the past, stacks of horizontal planks of wood were used. Each layer was laid at a right angle from the layers above and below it. However, this device was rather heavy, and the surface usefully wetted by the water did not surpassed 30% of the total surface area of the planks.
Today, plates of PVC (polyvinyl chloride) are used, arranged vertically and parallel to one another on a given level. On a level, the vertical plates are at a right angle to the plates on the levels above and below it.
Figure 1.2. Wet-bulb thermometer
Thus, at least 70% of each face of a plate is wetted by the liquid. In addition, these plates are significantly lighter than the wooden planks, which produces a saving in terms of the construction of the tower.
The tower is often in the form of a hyperbolic paraboloid. Any vertical section passing through the axis is a hyperbola. That surface is engendered by a straight line inclined in relation to an axis, situated at a certain distance from that axis and rotating around it. This configuration ensures the best possible mechanical strength to support the internal packing, as well as the weight of the water which runs off.
As regards the partial coefficient αL of heat transfer on the side of the liquid, readers can refer to the discussion in section 3.3.5 of [DUR 16b]. We would do the same for the coefficient G β of material transfer on the side of the air.
1.4.2. Calculation of the tower
The equilibrium curve, also known as the saturation curve, is calculated as follows.
The partial pressure of the water vapor is:
At the air–water interface, the equilibrium is established so that:
Referring to development 1.1.2, the equation of the equilibrium curve (E) is therefore:
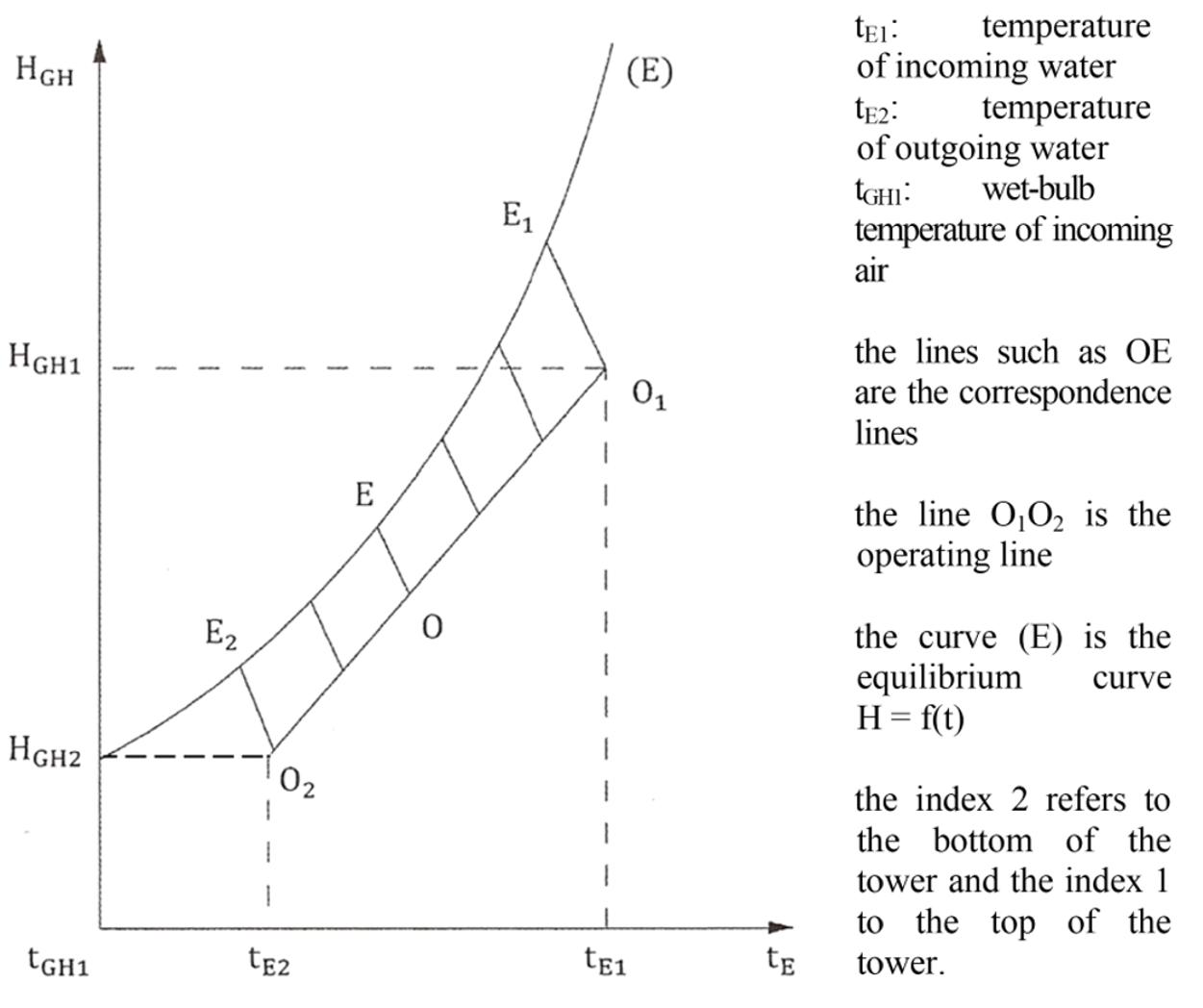
The slope of the operating line is obtained by writing that the heat lost by the water is gained by the air. WE CE d tE = WG dHGH
Figure 1.3. Graphical construction for the numerical calculation of the height of the tower
Hence: GH EE EG dH WC const. d tW ==
The temperatures of the water at inlet and outlet tE1 and tE2 are known, as is the humid temperature of the exiting air tGH1, and consequently, so too is the enthalpy HGH2 of the incoming air.
The enthalpy HGH1 of the exiting air is given by the overall heat balance of the tower.
GH1GH2 EE E1E2G HH W C ttW =
G β is then measured in m.s-1, which is the traditional unit for measuring that transfer coefficient. Therefore, it is calculated by using the usual correlations (see section 3.3.5 of [DUR 16b]).
Finally, the flux density of vapor from the liquid to the gas is:
The latent heat provided to the gas is:
L: latent heat of vaporization of the water: 1 J.kg.
The heat transfer coefficient G α is given by:
GH C : specific heat capacity of the humid gas: 1o1 J.kgC.
Finally, the heat flux density from the water to the gas is:
Note the similarity with equation [1.7].
The term in square brackets is very close to the difference in enthalpies: