
AnovelTiO2nanotubearrays/MgTixOymultiphaseheterojunctionfilmwithhighefficiencyfor photoelectrochemicalcathodicprotectionChang Feng&ZhuoyuanChen&JiangpingJing&Mengmeng Sun&GuiyingLu&JingTian&JianHou https://ebookmass.com/product/a-novel-tio2-nanotube-arraysmgtixoy-multiphase-heterojunction-film-with-high-efficiencyfor-photoelectrochemical-cathodic-protection-chang-fengzhuoyuan-chen-jiangping-jing-mengmeng-sun-guiyi/

Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
High-efficiency photoelectrochemical cathodic protection performance of the TiO2/AgInSe2/In2Se3 multijunction nanosheet array Xuhong Jiang & Mengmeng Sun & Zhuoyuan Chen & Jiangping Jing & Chang Feng
https://ebookmass.com/product/high-efficiency-photoelectrochemicalcathodic-protection-performance-of-thetio2-aginse2-in2se3-multijunction-nanosheet-array-xuhong-jiangmengmeng-sun-zhuoyuan-chen-jiangping-jing-chang-feng/ ebookmass.com
Elsevier Weekblad - Week 26 - 2022 Gebruiker
https://ebookmass.com/product/elsevier-weekbladweek-26-2022-gebruiker/
ebookmass.com
Jock Seeks Geek: The Holidates Series Book #26 Jill Brashear
https://ebookmass.com/product/jock-seeks-geek-the-holidates-seriesbook-26-jill-brashear/
ebookmass.com
Population Genetics and Microevolutionary Theory 2nd Edition Alan R. Templeton
https://ebookmass.com/product/population-genetics-andmicroevolutionary-theory-2nd-edition-alan-r-templeton/
ebookmass.com




Transitioning from the Top : Personal Continuity Planning for the Retiring Family Business Leader 1st Edition
Stephanie Brun De Pontet
https://ebookmass.com/product/transitioning-from-the-top-personalcontinuity-planning-for-the-retiring-family-business-leader-1stedition-stephanie-brun-de-pontet/ ebookmass.com

Patterning and Cell Type Specification in the Developing CNS and PNS: Comprehensive Developmental Neuroscience 2nd Edition John Rubenstein (Editor)
https://ebookmass.com/product/patterning-and-cell-type-specificationin-the-developing-cns-and-pns-comprehensive-developmentalneuroscience-2nd-edition-john-rubenstein-editor/ ebookmass.com
Hegemonic Transformation: The State, Laws, and Labour Relations in Post-Socialist China 1st Edition Elaine SioIeng Hui (Auth.)
https://ebookmass.com/product/hegemonic-transformation-the-state-lawsand-labour-relations-in-post-socialist-china-1st-edition-elaine-sioieng-hui-auth/ ebookmass.com
The R Book, 3rd Edition Elinor Jones
https://ebookmass.com/product/the-r-book-3rd-edition-elinor-jones/



ebookmass.com
Alphahole (Billionaire Boss Girl Book 3) Ann Grech
https://ebookmass.com/product/alphahole-billionaire-boss-girlbook-3-ann-grech/
ebookmass.com

The Unfinished Nation: A Concise History of the American People
https://ebookmass.com/product/the-unfinished-nation-a-concise-historyof-the-american-people/
ebookmass.com


CorrosionScience
journalhomepage: www.elsevier.com/locate/corsci
AnovelTiO2 nanotubearrays/MgTixOy multiphase-heterojunctionfilmwith highefficiencyforphotoelectrochemicalcathodicprotection
ChangFenga,b,c,d,e,ZhuoyuanChena,c,d,e,*,JiangpingJinga,d,e,MengmengSuna,d,e, GuiyingLua,b,d,e,JingTiana,b,d,e,JianHouc
a KeyLaboratoryofMarineEnvironmentalCorrosionandBio-fouling,InstituteofOceanology,ChineseAcademyofSciences,7NanhaiRoad,Qingdao266071,China
b UniversityofChineseAcademyofSciences,19(Jia)YuquanRoad,Beijing100049,China
c StateKeyLaboratoryforMarineCorrosionandProtection,LuoyangShipMaterialResearchInstitute,WenhaiRoad,Qingdao266237,China
d CenterforOceanMega-Science,ChineseAcademyofSciences,7NanhaiRoad,Qingdao266071,China
e OpenStudioforMarineCorrosionandProtection,PilotNationalLaboratoryforMarineScienceandTechnology(Qingdao),No.1WenhaiRoad,Qingdao,266237,China
ARTICLEINFO
Keywords:
A.TiO2/MgTixOy
A.Multiphase-heterojunctionfilm
B.SKP
C.Photoelectrochemicalcathodicprotection
C.Stability


ABSTRACT
Heterojunctionengineering,asarisingstarinthephotoelectrochemicalcathodicprotection(PECCP)field, contributestopromotetheseparationofthephotoinducedelectronsandholes.Inthispaper,theTiO2/MgTixOy multiphase-heterojunctionfilmwaspreparedanditsPECCPperformancewasstudied.Duetoagoodenergy bandalignmentgradientformedbetweenthemultiphases,theseparationefficiencyofthephotoinducedelectronsandholesgeneratedbyTiO2/MgTixOy aredramaticallyenhanced,leadingtoitsgoodPECCPperformance andhighstability.SKPis,forthefirsttime,usedtostudythesurfaceworkfunctionoftheTiO2/MgTixOy multiphase-heterojunctionfilmforcharacterizingitsPECCPperformance.
1.Introduction
Asapromisinggreentechnology,photocatalysisandphotoelectrochemistryareextensivelystudiedandusedinthefieldsofenvironmentandenergy[1–6].Asanimportantbranchofphotocatalysisand photoelectrochemistry,thephotoelectrochemicalcathodicprotection (PECCP)technologyusestheseparatedphotoinducedelectronsgeneratedbysemiconductormaterialsandtransfersthemtothecoupled metaltoprovidecathodicprotection.Thisisaneffectivewaytoprotect metallicmaterialsusingsolarenergy.DuringthePECCPprocess,the photoelectricconversionsemiconductormaterialwillnotbeconsumed andthusthistechnologywillnotpollutetheenvironment.Meanwhile, thecontrolsynthesisofthephotoelectrodeisrelativelysimpleandthe costislow[7,8].Therefore,thePECCPtechnologyisapromising,green andenvironmentallyfriendlycorrosionprotectiontechnologywitha greatapplicationpotential[9,10].TiO2 [11,12],ZnO[13,14],g-C3N4 [15],SrTiO3 [16,17]etc.arecommonsemiconductormaterialswith goodPECCPperformance.However,asinglephotoelectricconversion semiconductormaterialtendstohaveafastrecombinationrateofthe photogeneratedelectronsandholes,enablinglessphotoinducedelectronstobeeffectivelyutilizedforPECCP.Therefore,furthermodificationsofthephotoelectricconversionsemiconductormaterialisofgreat significancetorealizethesustainableandeffectiveutilizationofthe
⁎ Correspondingauthor.
E-mailaddress: zychen@qdio.ac.cn (Z.Chen).
https://doi.org/10.1016/j.corsci.2020.108441
excitedphotogeneratedelectronsforPECCP.
Asaneffectivewaytosignificantlyinhibittherecombinationofthe photogeneratedelectronsandholes,heterojunctionengineeringhas beenreportedtoachieveeffectiveseparationofthephotogenerated electronsandholes[18–20],andtoefficientlytransferthephotogeneratedelectronstothecoupledmetals,therebyenhancingthe PECCPperformanceofsemiconductormaterials.Thereportedheterojunctionsystems,suchasSrTiO3/TiO2 [21,22],Bi2X3/TiO2 (XisSorO) [23,24],In2O3/TiO2 [25],SnO2/TiO2 [26],WO3/TiO2 [27,28],Ag2S/ TiO2 [29],Ni2S3/TiO2 [30],N-dopedTiO2/TiO2 [31],BiVO4/TiO2 [32],ZnInS/TiO2 [33],Co3O4/ZnO[34],ZnxMg1-xO/ZnO[35],g-C3N4/ ZnO[36],TiO2/ZnO[37],g-C3N4/In2O3 [38],etc.,showsignificant improvementsinthePECCPperformance.Althoughtheestablishment ofheterojunctionscansignificantlyimprovethePECCPperformanceof compositeheterojunctionmaterials,itslong-termstabilityisstillabig challenge.Buetal.havesystematicallyreportedthePECCPmechanism forsteelusingtheSrTiO3/TiO2 compositephotoelectrodes[21].However,thestabilityofthephotoelectrodesappliedforthePECCPshowed obviousshortcomings[21].Sunetal.haveinvestigatedtheenhanced PECCPperformanceoftheIn2O3/TiO2 composite[25].Thestability testingwasstillanimportantparttobefurtherverified.Kuangetal. havedesignedadual-functionalZnxMg1-xOsolidsolutionnanolayer modifiedZnOtussock-likenanorodstoapplyforthePECCP[35].The
Received18October2019;Receivedinrevisedform31December2019;Accepted4January2020
Availableonline08January2020
0010-938X/©2020ElsevierLtd.Allrightsreserved.
stabilityoftheperformancewasanimportantfactorthatbothersthem inchoosingthebestphotoelectrode.Accordingly,howtodesigna photoelectrodewithhighPECCPactivityandstabilityisstillanurgent issuetobesolved.Ascanbewellknown,theestablishmentofmultiphaseheterojunctionscaneffectivelyacceleratetheseparationofthe photogeneratedchargecarriers[39–43].Awellbandalignmentformed amongthemulti-heterojunctionscanprovidealargerchargecarrier transfergradient[39],whichcansignificantlyinhibitthesecondary recombinationofthephotogeneratedelectronsandholes.And,this providesanideatodesignanefficientandstablephotoelectrodeappliedforPECCP.
Inaddition,thescanningkelvinprobe(SKP)testingsystemisconsideredtobeanovelmicro-electrochemicaltechnologywhichcan measurethesurfaceworkfunction(WF)ofamaterial.Ithasbeen widelyusedinthefieldsofmetalliccorrosion,coatings,solarcellsand photocatalysis[44–51].Ononehand,SKPtechnologycanbeusedto recordthesurfacepotentialofthematerialandobservethepotential distributionofdifferentcomponentsinthemicro-region.Ontheother hand,thesurfacepotentialdistributionmeasuredbySKPtechnology canbetransformedintosurfaceWF,whichcanbeusedtoanalyzethe capabilityofthesurfaceelectronsescapingfromthematerialandstudy theapplicationinelectrochemistryandphotoelectrochemistry.Hua etal.haveusedtheSKPtechnologytoprovethatthediffusionrateof hydrogenin(001)and(101)grainsof304SSisfasterthanthatin(111) grains,andhydrogenisprovedtobetrappedatthephaseboundary betweenausteniteandmartensite[52,53].Lietal.exploredthereasons fortheimprovementofthephotocatalyticperformanceofAg-modified TiO2 throughSKPtechnology[44].TheirresultsshowedthatlowWF madetheAg-modifiedTiO2 beeasiertoescapeelectrons,andthus acceleratedtheseparationofphotogeneratedchargecarriers,leadingto theenhancementofthephotocatalyticperformanceoftheAg-modified TiO2.AlthoughSKPtechnologyhasbeenappliedinvariousresearch fields,thereisnoreportconcerningabouttheSKPanalysisinthefield ofthePECCP.Inviewofthis,theintroductionoftheSKPtechnology intotheareaofthePECCPwillplayapositiveroleforcomprehensively andprofoundlyunderstandingtheeffectofthephotoelectrodesonthe PECCPprocess.
MgTixOy isconsideredtobeawidebandgapsemiconductormaterialwithgoodphotocatalyticperformance[40,54].Thewideband gapcanmakethephotoinducedelectronsandholesgeneratedby MgTixOy bedifficulttorecombine,therefore,thephotogenerated chargecarrierscanefficientlytransferintheprocessofthephotocatalyticreactions.Furthermore,MgTixOy hasamorenegativeconductionbandpotentialthanTiO2,whichisbeneficialtoPECCP. Meanwhile,awellbandalignmentcanbeformedbetweenTiO2 and MgTixOy,therefore,theTiO2/MgTixOy multiphaseheterojunctionsenablethephotogeneratedchargecarrierstomigratedirectionally.Inthis way,morephotogeneratedelectronscanbeseparatedandparticipate inthePECCPreactions,therebygreatlyimprovingthePECCPperformanceandstabilityoftheTiO2/MgTixOy multiphaseheterojunction system.
Inthepresentpaper,anovelTiO2/MgTixOy multiphase-heterojunctionfilmwaspreparedandreportedforthefirsttime,andits PECCPperformanceandstabilityfor304SSwerestudied.SKPtechniquewasusedforthefirsttimetostudythesignificantlyenhanced PECCPperformanceofthepreparedTiO2/MgTixOy multiphase-heterojunctionfilm.ThelowestsurfaceWFoftheTiO2/MgTixOy multiphase-heterojunctionfilmmakesitbeeasiertoescapetheelectrons, andhigheramountofphotogeneratedelectronscanbeproducedfor protecting304SSundersimulatedsolarlightillumination.Inaddition, theestablishmentofmultiphaseheterojunctionsmakestheTiO2/ MgTixOy filmshavedurablestabilityforthePECCP.Thisstudyfurther enrichesthemeansofcharacterizingthephotoelectrodes,andprovides animportanttheoreticalbasisforunderstandingthepromotionofthe PECCPperformanceandthestabilityofthephotoelectrodes.
2.Experimentalsection
2.1.Preparationofthephotoelectrodes
Thereagentsusedintheexperimentswereallpurchasedfrom SinopharmChemicalReagentCo.,Ltdwithoutfurtherpurification.The anataseTiO2 nanotubearrays(NTAs)(TiO2(A))photoelectrodewas preparedbyatwo-stepanodizationmethodaccordingtotheprevious reports[55,56].Acleanedtitanium(Ti)sheetwasusedastheanode andaPtelectrodewasusedasthecathode,whichwasplacedinparallel intheelectrolyteofethyleneglycol(0.35wt%NH4F,10wt%H2O).A constantpotential(60V)wasappliedforanodicoxidationfor1hat roomtemperature.After10minofultrasoniccleaningin10wt%HCl solution,theobtainedTisheetwasrinsedwithdeionizedwaterfor severaltimes.Theabove-mentionedprocessofanodicoxidationwas repeatedonemoretime.Afterthat,thesamplewasputintodeionized waterandultrasonicallycleanedfor30S.Thesamplewasdried,and thenwasannealedat450°Cfor3.5htopreparetheTiO2(A)photoelectrode.
TheTiO2/MgTixOy photoelectrodewaspreparedasfollows.The magnesiumhydroxidewasfirstlydepositedontothesurfaceofthe TiO2(A)photoelectrodebyamulti-potentialstepmethodusinga CHI660Delectrochemicalworkstation(ShanghaiChenhuaInstrument Co.,Ltd.,Shanghai,China).Athree-electrodeconfigurationwasapplied inthedepositionprocess,inwhichthepreparedTiO2(A)wasusedas theworkingelectrode,thePtelectrodeworkedasthecounterelectrode, andthesaturatedcalomelelectrode(SCE)actedasthereferenceelectrode,respectively.0.1MMg(NO3)2 solutionwasusedastheelectrolyte.Duringthedepositionprocess,thesteppotentialswere−1.5and −1.2V,respectively,andthecorrespondingtimewas5sand0.5s. After8cyclesofdeposition,theobtainedsamplewaswashedwith deionizedwaterandalcohol.Subsequently,thepreparedsamplewas thermallytreatedinamufflefurnaceat700°Cfor2htoobtainthe TiO2/MgTixOy photoelectrode.Ascanbewellknown,thephase transformationofTiO2 occursduringhightemperaturetreatment(≥ 550°C),resultinginthecoexistenceofanataseandrutileTiO2 (TiO2(R))[57–59].Therefore,asacomparison,thepreparedTiO2(A) photoelectrodewasalsothermallytreatedinamufflefurnaceat700°C for2htoobtaintheTiO2(A)/TiO2(R)photoelectrode.
2.2.Characterizationofthepreparedphotoelectrodes
X-raydiffractometer(XRD,D/max-500,RigakuCo.,Tokyo,Japan) wasusedtocharacterizethecrystalstructuresofthepreparedphotoelectrode.Fouriertransforminfrared(FT-IR)spectraweretestedusing aFouriertransforminfraredspectroscopy(FT-IR,Thermo-Nicolet8700, ThermoElectronScientificInc.,USA)atroomtemperature.UV–vis diffusereflectancespectrophotometer(U-41000;HITACHI,Tokyo, Japan)wasusedtoanalyzetheopticalabsorptionpropertiesofthe preparedTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodes.Photoluminescence(PL)spectraofthepreparedphotoelectrodes weremeasuredwithafluorescencespectrometer(PL,Microconfocal RamanSpectrometer,HoribaJobinYvonLabRAMHR800,325nm, France).Fieldemissionscanningelectronmicroscopy(FE-SEM,ZEISS, ULTRA55,Germany)wasusedtoanalyzethemicromorphologiesof thepreparedTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodes.TheelementalcompositionandmappingofthepreparedphotoelectrodewereanalyzedbyanX-rayenergydispersivespectrometer (EDS,Oxford,UK).ThemicrostructuresofthepreparedTiO2/MgTixOy photoelectrodeandinterfacialinformationofdifferentphasecomponentswereobservedbyfieldemissiontransmissionelectronmicroscope (FE-TEM,TecnaiG2F20,FEICompany,USA).
2.3.Photoelectrochemical(PEC)performance,PECCPperformanceand SKPmeasurements
ThelightsourceusedforthePECandPECCPperformancemeasurementsis150-WXelamp(PLSSXE300,ChangtuoCo.Ltd.,Beijing, China).AsimulatedsolarilluminationisobtainedbyaddinganAM1.5 Gfilterandadjustingthelightintensityofthislightsourceto100mW cm−2.ThePECperformancewastestedinathree-electrodecellsystem usingtheCHI660Delectrochemicalworkstation.Thevariationsofthe currentdensitiesoftheTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodesweremeasuredusingthepreparedphotoelectrodeas theworkingelectrode,theplatinumelectrodeasthecounterelectrode, andAg/AgCl(saturatedKCl)asthereferenceelectrode.Thebiasvoltagewassetas0V(vsAg/AgCl)andtheelectrolytewas0.1MNa2SO4 solution.Thevariationsinthecurrentdensitiesandthemixedpotentials,andthepolarizationcurvesofthegalvaniccoupleofthe304SS electrodeandthepreparedphotoelectrodesweremeasuredtocharacterizethePECCPperformance.Thegalvaniccoupleofthe304SS electrodeandthepreparedphotoelectrodeswasusedastheworking electrode.TheAg/AgCl(saturatedKCl)electrodeandtheplatinum electrodeservedasthereferenceandcounterelectrode,respectively. Boththe304SSelectrodeandthepreparedphotoelectrodesareplaced in3.5wt%NaClsolutionandthebiasvoltagewassetas0V(vsAg/ AgCl)duringthetestingprocess.Intermittentsimulatedsolarlight(100 mW,AM1.5G)wasilluminatedonthesurfaceofthephotoelectrodes. Electrochemicalimpedancespectroscopy(EIS)testswereperformedat opencircuitpotentialoverthefrequencyrangebetween105 and10-1 Hz,withanACvoltagemagnitudeof5mV.Thepolarizationcurves weremeasuredusingtheCHI660Delectrochemicalworkstationwitha scanrateof1mV s-1 from-400to400mV(vsopencircuitpotential).
ThesurfaceWFsofthepreparedphotoelectrodeswereanalyzed usingSKP(VersaSCAN,Ametek).Thetungstenprobewiththediameter of250μmwasactedasthereferenceanddetectionprobe.Thetesting areaonthesurfaceofthepreparedphotoelectrodeswas1×1mm2 and thescanningratewassetas50μm s−1 withsensitivityof500μV.The surfaceWFsofthephotoelectrodescanbecalculatedbasedonthe followingformula[60–62]:WF(Sample)=WF(Tungsten)+ΔW (photoelectrode)/1000.Amongthem,WF(Tungsten)istheWFofthe referenceelectrodewithstandardvalueof4.55eVfortungsten;WF (Sample)isthesurfaceWFforthepreparedphotoelectrode,ΔW (photoelectrode)isthesurfacepotentialofthephotoelectrodeobtained fromtheSKPmeasurement.
3.Resultsanddiscussion
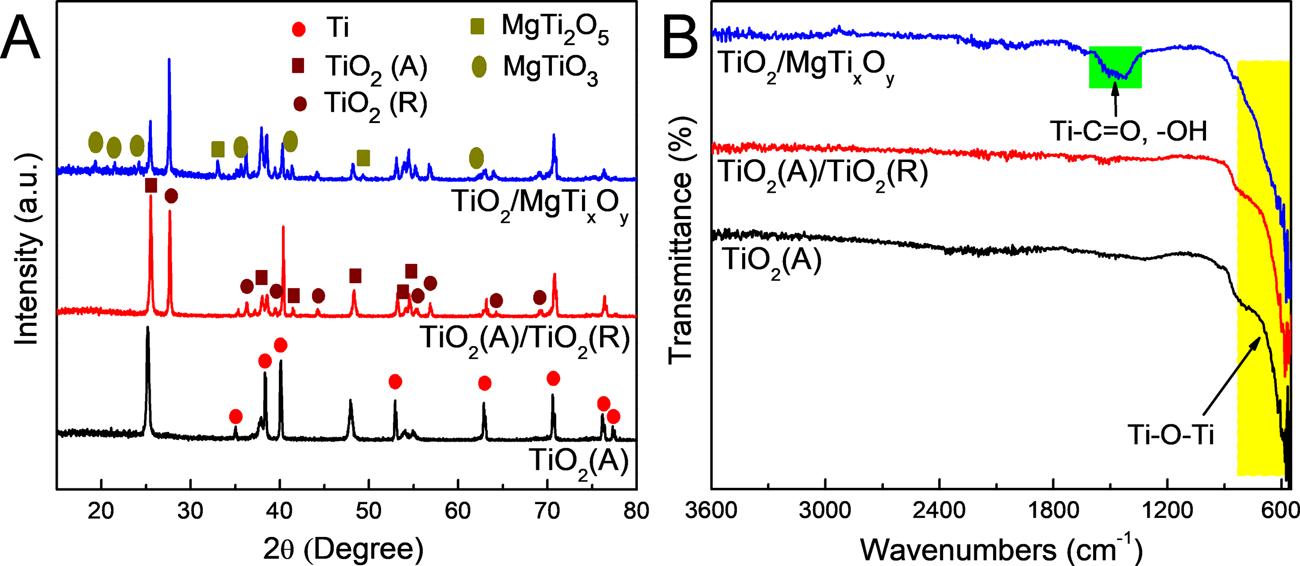
Fig.1AshowstheXRDpatternsofthepreparedTiO2(A),TiO2(A)/ TiO2(R)andTiO2/MgTixOy.FortheXRDpatternofTiO2(A),thediffractionpeaksat25.5°,37.9°,48.4°,54.2°and55.3°areobserved,correspondingtothe(101),(004),(200),(105)and(211)crystalplanesof standardanataseTiO2 (JCPDSNo.21-1272),respectively.Theother
diffractionpeaksintheXRDpatternofTiO2(A)areattributedtothe characteristiconesoftheTisubstrate[55,56].FortheXRDpatternof TiO2(A)/TiO2(R),thecoexistenceofanataseandrutilephasesofTiO2 canbeclearlyobservedafterhightemperaturetreatmentat700°C.In additiontothediffractionpeaksofTisubstrateandanataseTiO2,the correspondingrutilephasesofTiO2 (JCPDSNo.21-1276)areobserved at27.6°,36.1°,41.2°,44.1,54.3°,56.7°,64.1°and68.9°,whichareassignedtothe(110),(101),(111),(210),(211),(220),(310)and(301) crystalplanes,respectively.FortheXRDpatternofTiO2/MgTixOy,the weakcharacteristicpeaksofMgTiO3 andMgTi2O5 canbeobserved.It maybeduetotherelativelysmallamountofMgTixOy depositedonthe surfaceofTiO2 tubeorifice.Althoughtheintensitiesofthediffraction peaksareweak,itcanbeclearlyseenthatthediffractionpeaksare locatedat19.1°,21.2°,23.9°,35.5°,40.6°and63.8°,respectively.These diffractionpeakscorrespondwelltothe(003),(101),(012),(104), (110)and(214)crystalplanesofrhombohedralMgTiO3 (JCPDSno.060494).Inaddition,thediffractionpeaksat32.7°and48.6°areobserved,whichareassignedtothe(230)and(331)crystalplanesof orthorhombicstructureofMgTi2O5 (JCPDSno.35-0792).TheXRD resultssuggestthatMgTixOy wassuccessfullymodifiedonthesurfaceof TiO2 Fig.1BdisplaystheFT-IRspectraofthepreparedTiO2(A), TiO2(A)/TiO2(R)andTiO2/MgTixOy.TheFT-IRspectraofTiO2(A)and TiO2(A)/TiO2(R)showahighdegreeofconsistencyandthebroadband below750cm−1 ismainlyfromthestretchingvibrationmodeofTi-OTiinTiO2 [63,64].Noothercharacteristicpeakswereobserved,indicatingthehighpurityofTiO2(A)andTiO2(A)/TiO2(R).FortheFT-IR spectrumofTiO2/MgTixOy,anotherbroadregionatapproximately 1400−1550cm−1 canbeobserved,whichcomesfromtheTi–carboxyliccomplexesandhydroxylgroup[65–67].TheFT-IRresults indicatethatthecarboxylandhydroxylgroupscanbeeasilyadsorbed onthesurfaceofTiO2(A)/TiO2(R),whichfacilitatesthecontactbetweenthephotoelectrodeandtheelectrolytesolution.
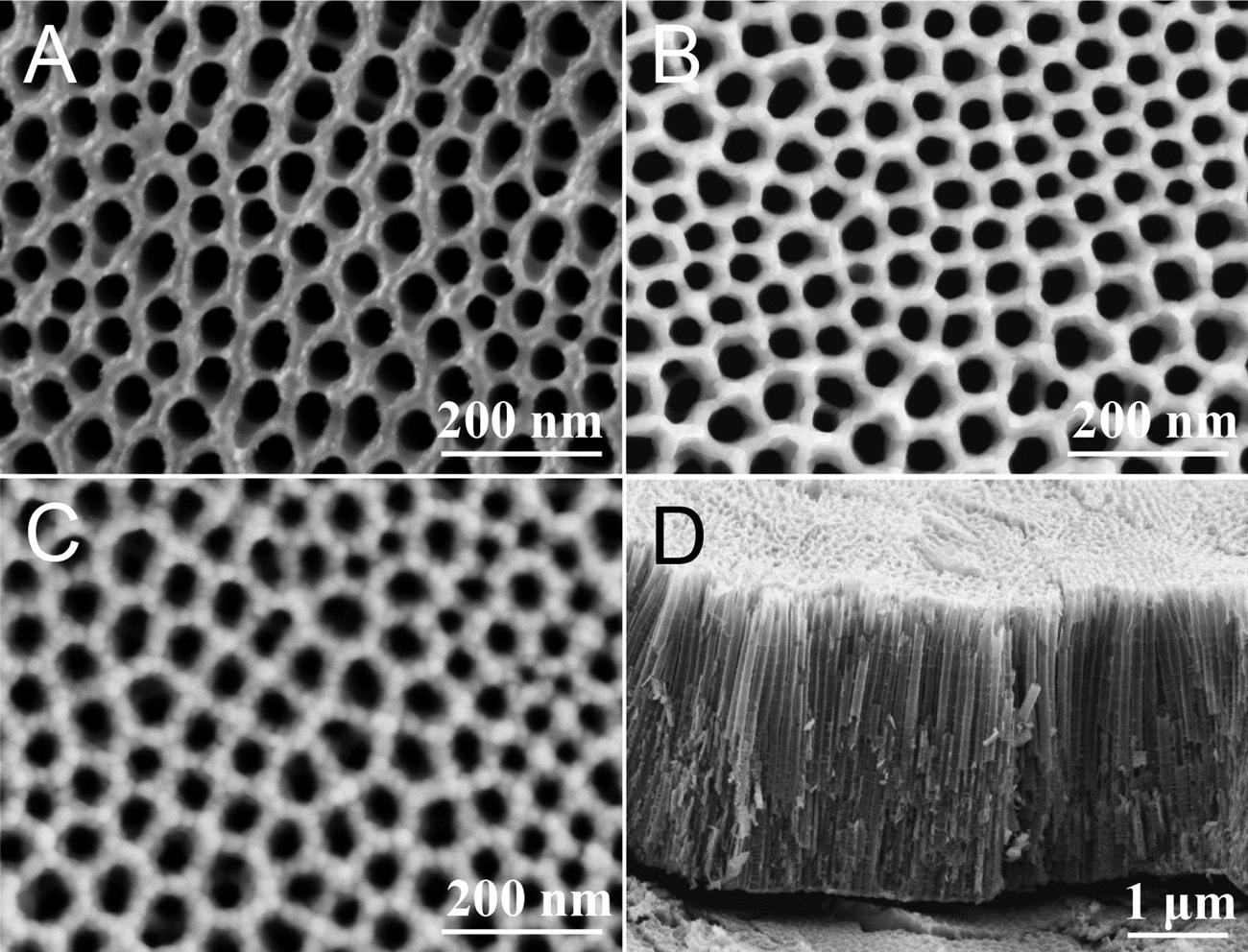
Fig.2 showstheSEMimagesofTiO2(A),TiO2(A)/TiO2(R)and TiO2/MgTixOy,respectively.Asshownin Fig.2A,TiO2(A)exhibitsa goodNTAstructure.Theorificesarecloselyarrangedwithadiameter ofapproximately80nm. Fig.2BshowstheSEMimageoftheTiO2(A)/ TiO2(R)photoelectrode.ThesurfaceofTiO2(A)/TiO2(R)ismuch smootherthanthatofTiO2(A),whichmaybeduetotheformationof anataseandrutileTiO2 afterthehightemperaturetreatment.Forthe SEMimageofTiO2/MgTixOy displayedin Fig.2C,somenanoparticles obviouslyappearattheorificesandarewellcoatedonthesurfaceof theorificeofTiO2 NTAs.Thismaybethesurface-modifiedMgTixOy nanoparticles. Fig.2DshowstheSEMimageofthecrosssectionof TiO2/MgTixOy.Theheightofthenanotubescanbemeasuredasapproximately4μm.Thebasicstructureofthenanotubeshasnotbeen destroyedafterhightemperaturetreatmentat700°C,andthetubular structureremainsintact.
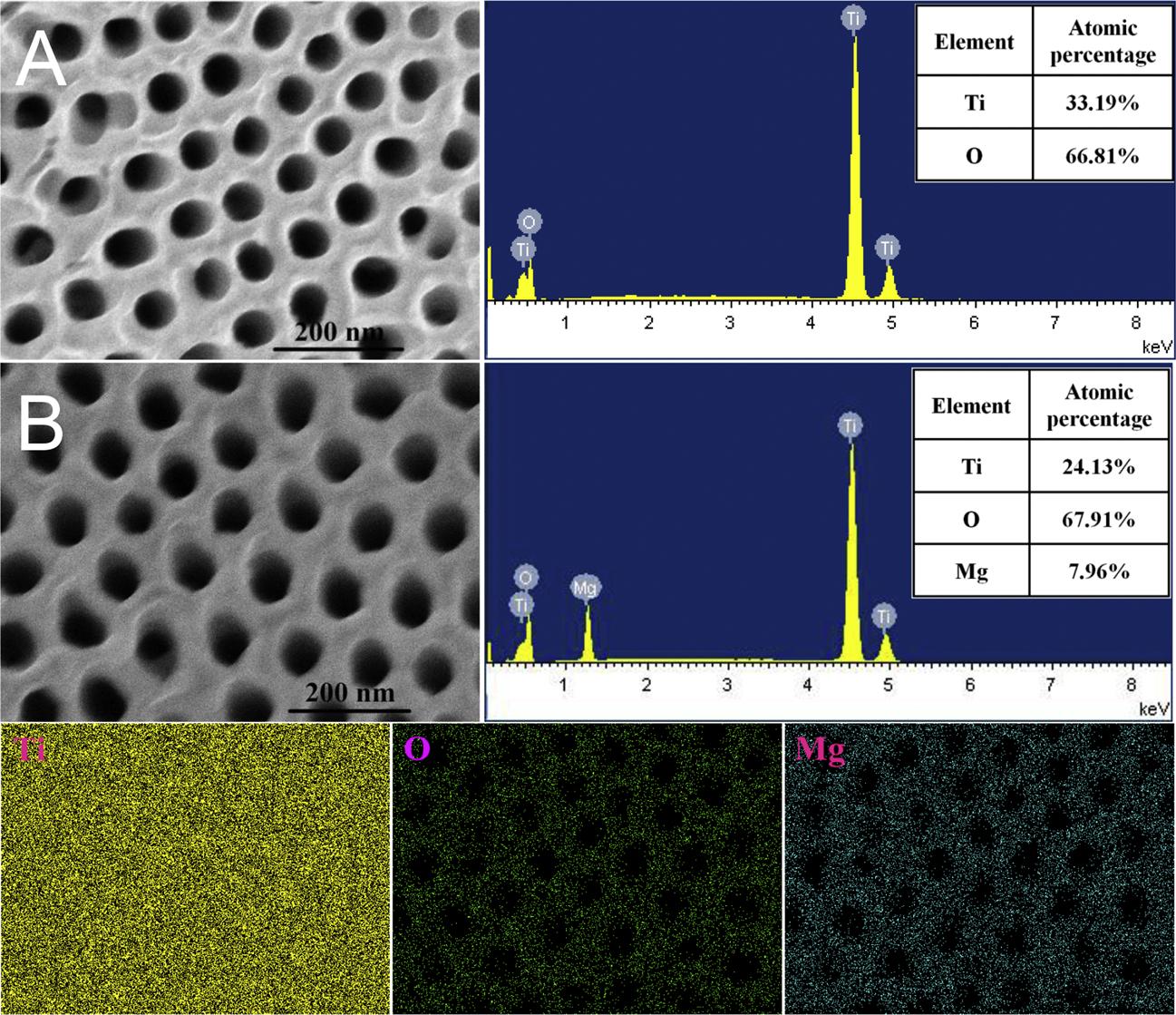
Fig.3AshowstheSEMimageandthecorrespondingEDSspectrum ofTiO2(A).InadditiontoTiandOelements,nootherimpurityelementsareobserved.TheatomicmasspercentagesofTiandOwere
33.19%and66.81%,whichfitswellwiththeelementcompositionof TiO2. Fig.3BshowstheSEMimageandthecorrespondingEDSresults ofTiO2/MgTixOy.Ti,OandMgelementsareclearlyobservedwiththe atomicpercentagesof24.13%,67.91%and7.96%,respectively.As showninthecorrespondingEDSmappingresults,Tielementcoversthe wholescanningareaduetotheTisubstrate.While,OandMgelements correspondwelltotheSEMimageshownin Fig.3B.Thetubeorificeis clearlyobservedintheEDSMgandOmappings,indicatingthatMgand OelementsarewelldispersedonthesurfaceoftheTiO2 nanotubeorifices.ThedifferencebetweentheSEMimagesin Fig.3 andthosein Fig.2 ismainlyduetothefactthatEDSistestedunderhighexcitation
voltages,whichwillbreakdownthesurfacecomponentsandmakethe surfacemorphologybedifficulttobeobserved.
ThecompositionandinterfacialinformationoftheTiO2/MgTixOy multiphaseheterojunctionsarefurtherstudiedbyHRTEM. Fig.4A presentstheTEMimageobservedatlowmagnification.Thestructureof nanotubescanbeclearlyobserved,whichcorrespondswelltotheSEM imageofTiO2/MgTixOy shownin Fig.2.Theedgeofthenanotubeswas furtherenlarged,asshownin Fig.4B.Thenanostructureswithdifferent latticefringesarefoundandformtheheterojunctionsonthesurfaceof thenanotube.Theobservationresultsarefurthermagnifiedattheselectedtworectangularareas. Fig.4Ccorrespondstotherectangular
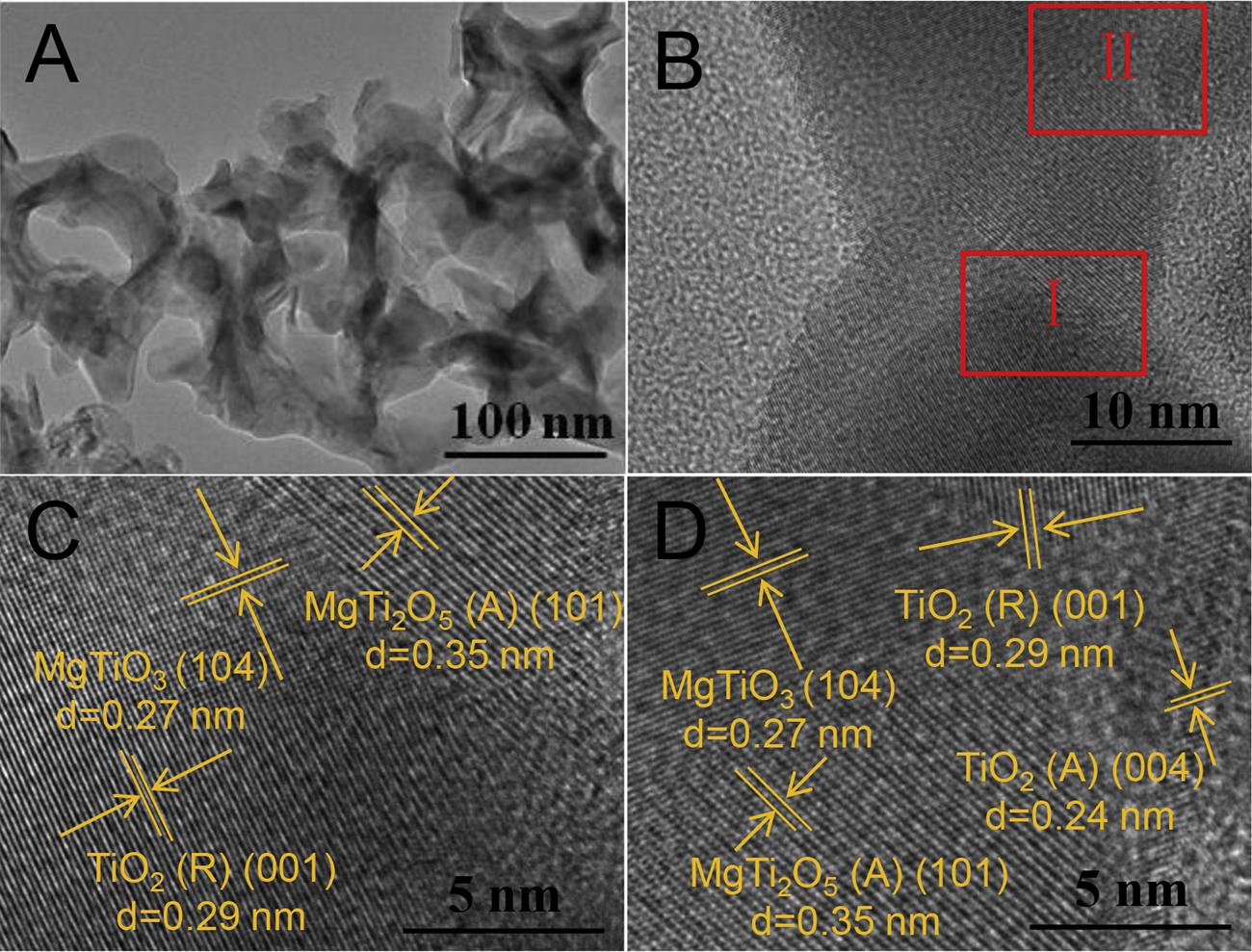
areaI.ThelatticefringesofTiO2(R),MgTiO3 andMgTi2O5 canbe observedwiththevaluesof0.29,0.27and0.33nm,respectively. Furthermore,thelatticefringesofTiO2(A),TiO2(R),MgTiO3 and MgTi2O5 canbeclearlyobservedcorrespondingtotherectangulararea II,asshownin Fig.4D.Combiningwiththephysicalcharacterization resultsshownin Figs.1–4,itcanbeprovedthatMgTixOy issuccessfully coatedonthesurfaceofTiO2 NTAs,andthemultiphaseheterojunctions areformed.
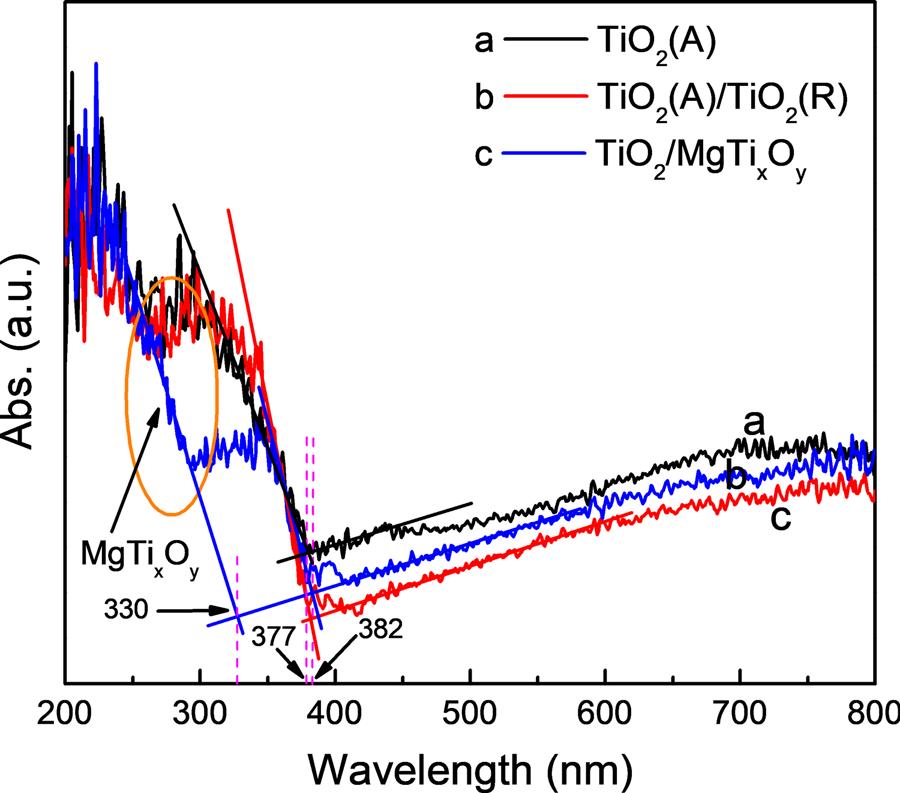
Fig.5 showstheUV–visabsorptionspectraoftheTiO2(A),TiO2(A)/ TiO2(R)andTiO2/MgTixOy photoelectrodes.Ascanbefound,thelight absorptionthresholdofTiO2(A)isobservedat382nm.Forthe TiO2(A)/TiO2(R)photoelectrode,thelightabsorptionthresholdhasa slightredshiftcomparedwiththatofTiO2(A),whichmaybecausedby theformationofTiO2(R)withanarrowerbandgap.Inadditionto havingthesameabsorptionthresholdasTiO2(A)/TiO2(R),theTiO2/ MgTixOy photoelectrodehasanadditionalabsorptionthresholdatapproximately330nm,indicatingthesuccessfulsynthesisofMgTixOy on thesurfaceofTiO2 NTAs.
ThePECCPperformanceofthepreparedphotoelectrodesarecharacterizedbymeasuringthephotoinducedcurrentdensitiesandthe photoinducedpotentialdrops,andtheresultsareshownin Fig.6 Fig.6Ashowsthevariationsinthegalvaniccurrentdensitiesbetween the304SSelectrodeandthepreparedphotoelectrodesin3.5wt%NaCl solutionunderintermittentsimulatedsolarlightillumination.Positive excitationcurrentdensitiesareobtainedunderlightillumination,indicatingthatthephotoinducedelectronsgeneratedbythephotoelectrodestransfertothecoupled304SSelectrodeandprovidethePECCP forit.Thephotoinducedcurrentdensityofapreparedphotoelectrode, whichisprovidedforprotectingthecoupledmetallicelectrode,isobtainedbysubtractingthestablegalvaniccurrentdensitybetweenthe protectedmetalelectrodeandthephotoelectrodeinthedarkfromthat underlightillumination.Asshownin Fig.6A,thephotoinducedcurrent densityofTiO2(A)is9μA·cm−2 andthatofTiO2(A)/TiO2(R)is23 μA·cm−2.While,thatofTiO2/MgTixOy is49μA·cm−2,whichis5.4and 2.1timesofthatofTiO2(A)andTiO2(A)/TiO2(R).Theresultsshownin Fig.6AindicatethatTiO2/MgTixOy canproducethemostphotogeneratedelectronsandcanprovidethemostelectronsneededforthe cathodicprotectionofthecoupled304SS. Fig.6Bshowsthevariations ofthemixedpotentialofthe304SSelectrodecoupledwiththepreparedphotoelectrodesin3.5wt%NaClsolutionunderintermittentsimulatedsolarlightillumination.Themixedpotentialsofthe304SS electrodecoupledwiththepreparedphotoelectrodesimmediatelyshift tonegativedirectiononcethelightisswitchedon,demonstratingthat thepreparedphotoelectrodescanprovidethePECCPforthecoupled 304SSelectrode.Thephotoinducedmixedpotentialdropisthemixed potentialofthe304SSelectrodecoupledwiththepreparedphotoelectrodeunderlightilluminationminusthatinthedark.Asshownin Fig.6B,thephotoinducedmixedpotentialdropsofTiO2(A),TiO2(A)/ TiO2(R)andTiO2/MgTixOy are-230,-250and−320mV,respectively. TheTiO2/MgTixOy exhibitsthemaximumphotoinducedmixedpotentialdrop,indicatingitsexcellentPECCPperformance.Thephotoinducedmixedpotentialdropresultsshownin Fig.6Baresimilartothe photoinducedcurrentdensityresultsshownin Fig.6A,andbothof themprovethatTiO2/MgTixOy hasthebestPECCPperformance. Meanwhile,in Fig.6B,thestabilityofthepreparedphotoelectrodesis evaluatedbymeasuringthepotentialvariationsduringthelongdurationoflightillumination.Asshownin Fig.6B,themixedpotentialof the304SSelectrodecoupledwiththepreparedphotoelectrodesis
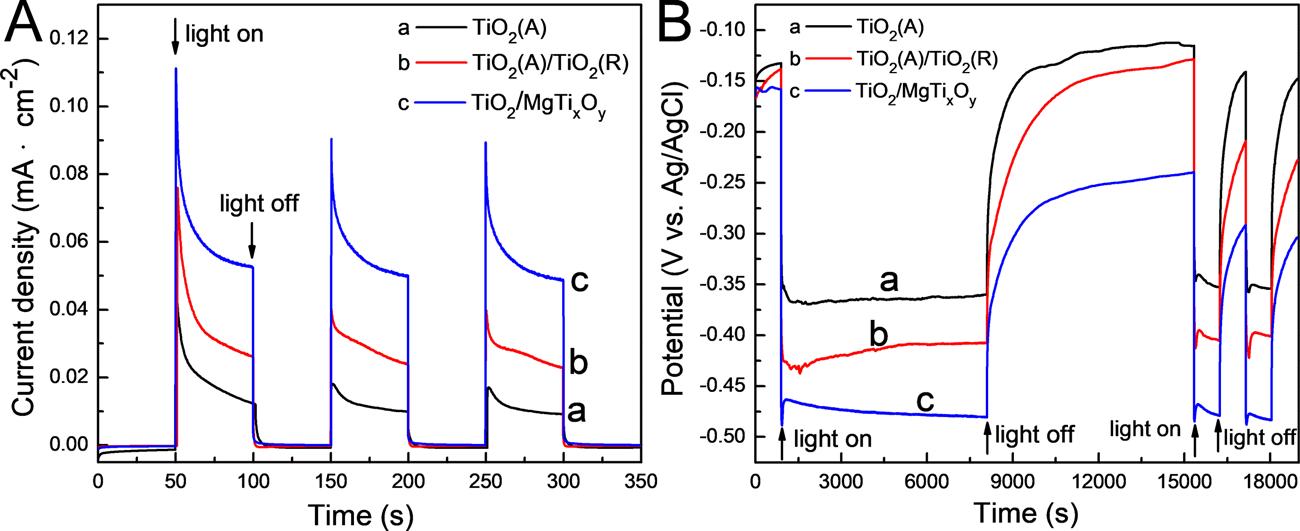
Fig.6. (A)Thevariationsinthegalvaniccurrentdensitiesbetweenthe304SSelectrodeandthepreparedphotoelectrodesand(B)thevariationofthemixed potentialsofthegalvaniccoupleofthe304SSelectrodeandthepreparedphotoelectrodesin3.5wt%NaClsolutionunderintermittentsimulatedsolarillumination.
stabilizedfor900sinthedark.Subsequently,thelightisswitchedon andilluminatedfor2honthephotoelectrodes.Themixedpotentialsof the304SSelectrodecoupledwiththeTiO2(A)andTiO2(A)/TiO2(R) photoelectrodeschangefrom-0.37and-0.42Vto-0.33and-0.39V, showingaslightdecreaseintheirPECCPperformanceduringthelight illumination.However,forTiO2/MgTixOy,themixedpotentialofthe 304SSelectrodecoupledwiththeTiO2/MgTixOy photoelectrodedoes nothavesignificantchangeduringthe2-hlightillumination.Theresultsshownin Fig.6Bindicatethatthereisnoobviousattenuationof thePECCPperformanceoftheTiO2/MgTixOy photoelectrodeduringthe longdurationoflightillumination,demonstratingthattheTiO2/ MgTixOy photoelectrodepossesseshighstabilityandhasgoodapplicationprospectsinthefieldofPECCP.
Thepolarizationcurvesof304SSelectrodeand304SSelectrode coupledwiththeTiO2,TiO2(A)/TiO2 andTiO2/MgTixOy photoelectrodesintheabsenceandpresenceofsimulatedsolarlightillumination areshownin Fig.7.Thecorrosionpotentialofthe304SSwasabout −154mV(vs.Ag/AgCl)beforecouplingwiththepreparedphotoelectrodesin3.5wt%NaClsolution,however,thoseof304SSpositivelyshiftto-102,-101and−113mVaftercouplingwithTiO2, TiO2(A)/TiO2 andTiO2/MgTixOy photoelectrodesinthedark,respectively.Undersimulatedsolarlightillumination,significantnegative potentialshiftsareobtainedfor304SScouplingwithdifferentphotoelectrodes,indicatingthegoodPECCPperformanceoftheprepared photoelectrodes.Underthesimulatedsolarlightillumination,thecorrosionpotentialsof304SScouplingwithTiO2,TiO2(A)/TiO2 andTiO2/ MgTixOy are-353,-426and−486mV,respectively.Theseresultsarein
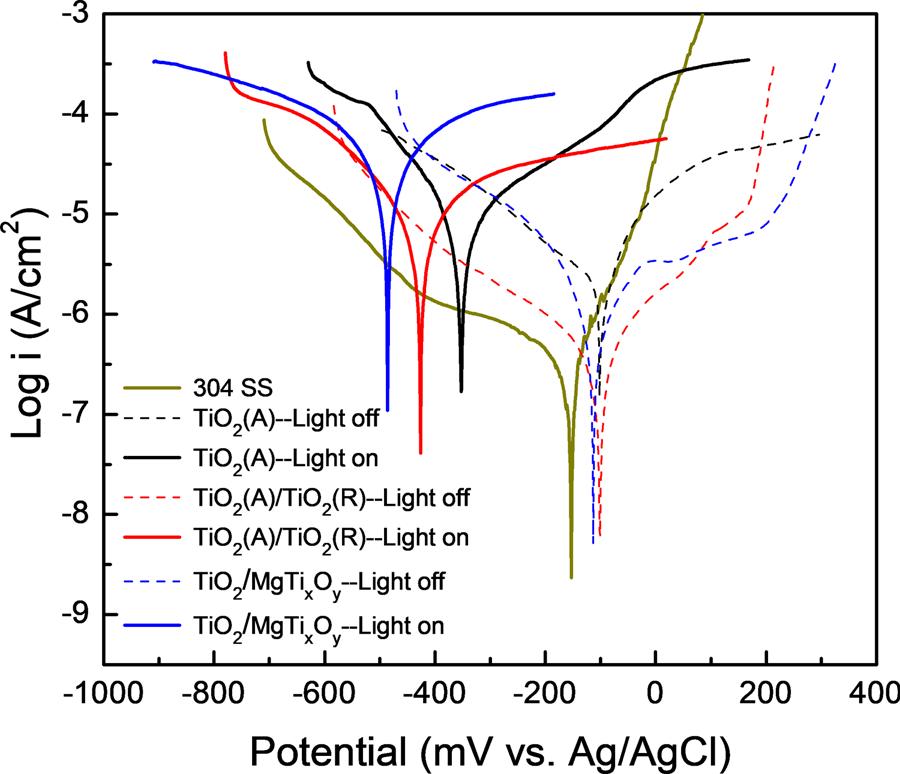
Fig.7. Thepolarizationcurvesofthe304SSelectrodeandthe304SSelectrode coupledwiththeTiO2,TiO2(A)/TiO2 andTiO2/MgTixOy photoelectrodesinthe absenceandpresenceofsimulatedsolarlightillumination.
goodagreementwiththepreviousPECCPtestsshownin Fig.6,which furtherindicatestheexcellentPECCPperformanceoftheTiO2/MgTixOy photoelectrode.
SKPtechnologyisusedtofurthercharacterizethecapabilityofthe electronsescapingfromthesurfaceofthepreparedphotoelectrodes. ThesurfaceWF,whichreferstotheminimumenergyrequiredtoescape anelectronfromthesurfaceofthephotoelectrode,canbeeasilycalculatedfromthesurfacepotentialofthephotoelectrodesmeasuredby SKP[68,69].ThesmallertheWFis,theeasieritisforelectrontoescape,i.e.forelectronstoflowoutofthephotoelectrodeandparticipate inthereactions[70]. Fig.8Adisplaysthesurfacepotentialdistributions ofdifferentphotoelectrodesmeasuredbySKP.Ascanbefoundin Fig.8A,thepotentialfluctuationofthepreparedindividualphotoelectrodeissmallerthan±50mVaroundthemedianvalue,suggesting thatthesurfaceofwhichisfairlyflatanduniform.Bycomparingthe surfacepotentialsofTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodes,TiO2(A)hasthemostpositivesurfacepotentialdistributionwithanaveragevalueofapproximately990mV.TiO2(A)/ TiO2(R)hasasurfacepotentialofapproximately830mV.While,TiO2/ MgTixOy hasthemostnegativepotentialofapproximately690mV, whichis300and140mVlowerthanthoseofTiO2(A)andTiO2(A)/ TiO2(R),respectively. Fig.8BshowsthesurfaceWFsoftheprepared photoelectrodesobtainedfromtheresultsshownin Fig.8A.Thesurface WFsofTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodes are5.54,5.38and5.24eV,respectively.Thelowestsurfacepotential distributionandsurfaceWFofTiO2/MgTixOy indicatethatthemultiphaseheterojunctionscaneasilyexcitetheelectronsandtransferthem tothesurfaceof304SStoprovideeffectiveprotectionforit.
Fig.9AshowsthevariationsinthecurrentdensitiesoftheTiO2(A), TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodesin0.1MNa2SO4 solutionunderintermittentsimulatedsolarillumination.Asshownin Fig.9A,allofthepreparedphotoelectrodesshowspositiveexcitation currentdensitiesunderlightillumination.Thephotoinducedcurrent densityoftheTiO2(A)photoelectrodeis0.12mA cm−2,andthatofthe TiO2(A)/TiO2(R)is0.16mA cm−2.However,thephotoinducedcurrent densityoftheTiO2/MgTixOy photoelectrodeis0.29mA⋅ cm−2,whichis approximately2.4timesand1.8timesoftheTiO2(A)andTiO2(A)/ TiO2(R)photoelectrodes,respectively.Thegenerationofphotoinduced currentisakeyfactorthataffectsthePECCPperformanceofthephotoelectrode.TheTiO2/MgTixOy photoelectrodehasthehighestphotoinducedcurrentdensity,indicatingthatithasthebestPECCPperformance.
Theelectrochemicalimpedancespectroscopyandthephotoluminescencespectroscopyofthepreparedphotoelectrodeswere measuredtoevaluatethephotogeneratedchargecarriermigration abilityandtherecombinationabilityofthephotogeneratedelectrons andholes.Therelevantresultsareshownin Figs.9Band9C.Asshown in Fig.9B,theTiO2/MgTixOy photoelectrodehasthesmallestsurface resistancethanTiO2(A)andTiO2(A)/TiO2(R),indicatingthattheTiO2/ MgTixOy hasthefastestelectronmobility.Inaddition,asshownin
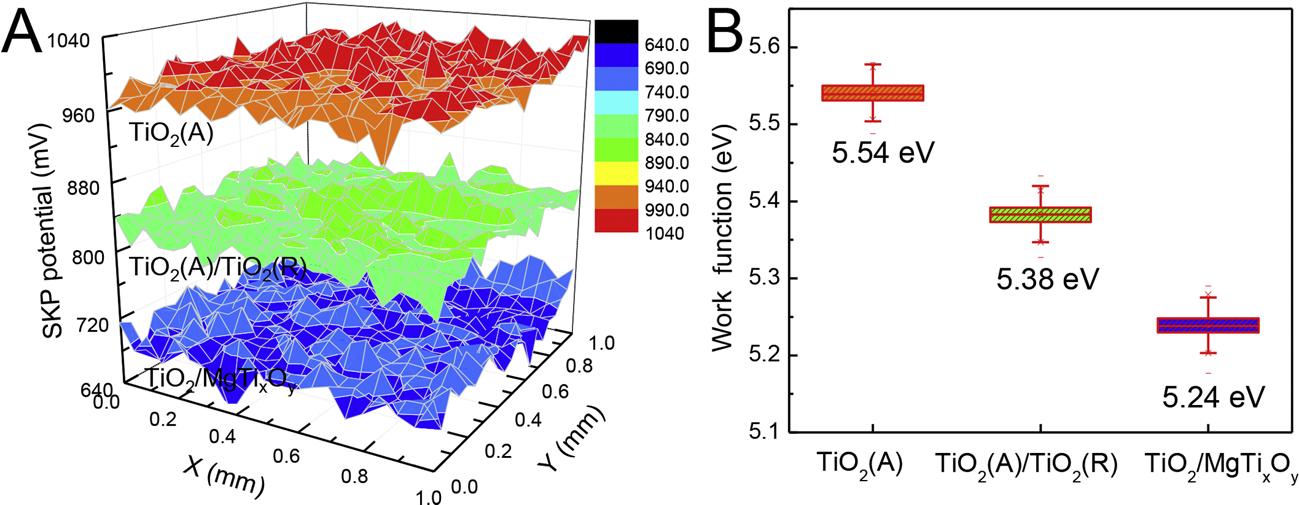
Fig.8. (A)ThesurfacepotentialdistributionsofTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodesmeasuredbySKPtechnique;and(B)thesurfaceWFs ofthepreparedphotoelectrodestransformedfromtheSKPresultsin Fig.8A.
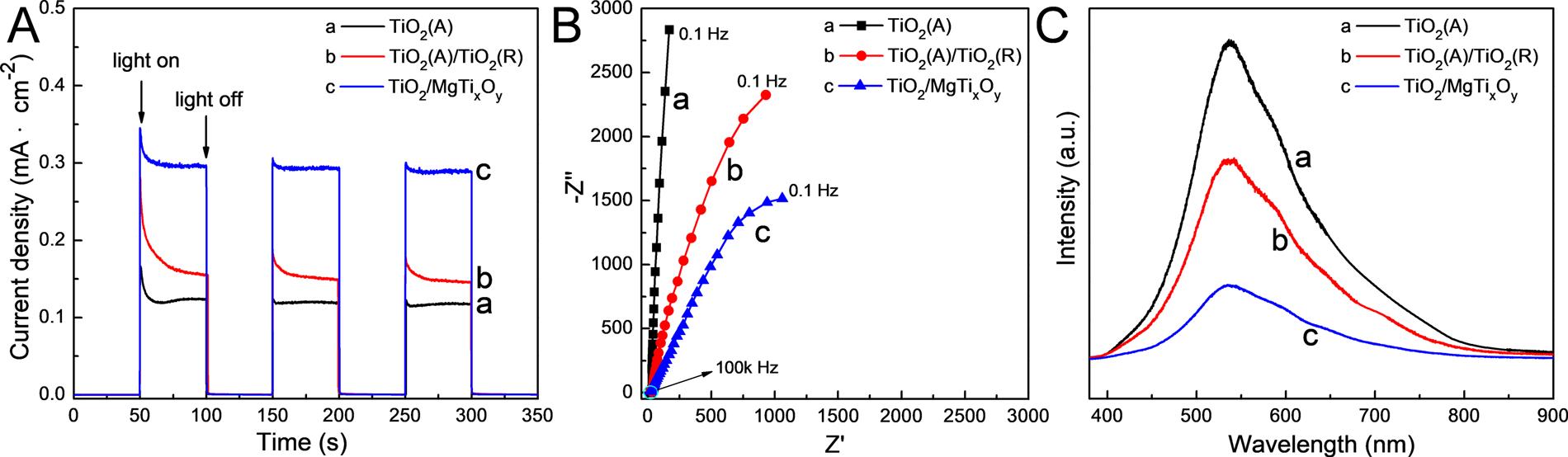
Fig.9. (A)ThevariationsinthecurrentdensitiesoftheTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodesunderintermittentlightillumination,(B) Electrochemicalimpedancespectra(EIS)and(C)photoluminescencespectraoftheTiO2(A),TiO2(A)/TiO2(R)andTiO2/MgTixOy photoelectrodes.
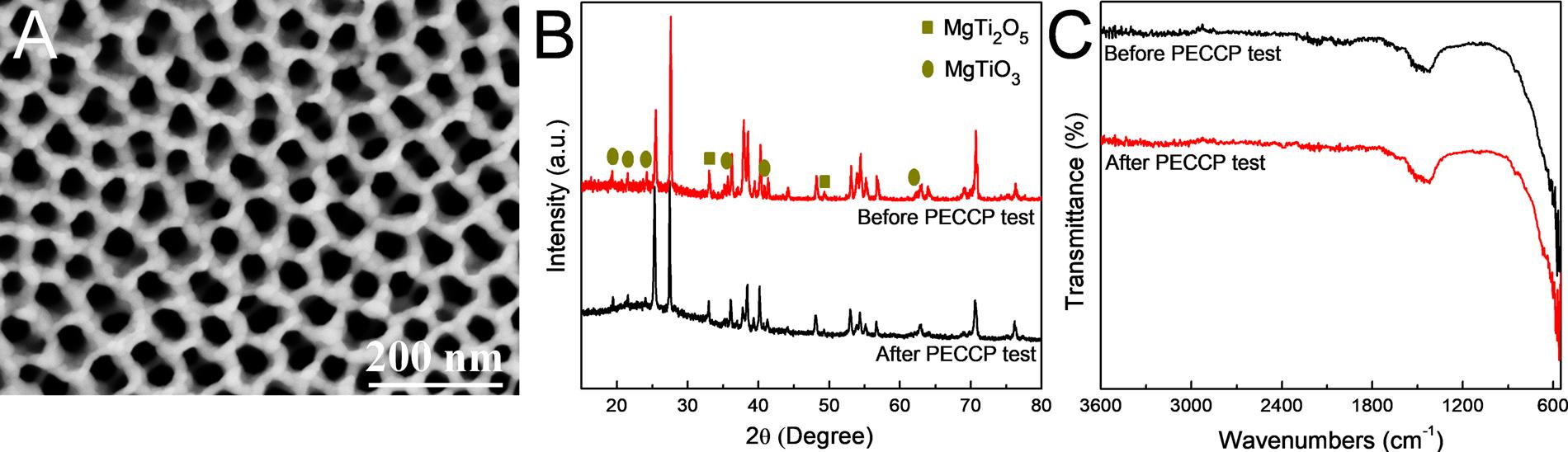
Fig.10. SEMimageoftheTiO2/MgTixOy photoelectrodeafterthePECCPtestandtheXRDandFT-IRspectraoftheTiO2/MgTixOy photoelectrodebeforeandafter thePECCPtests.
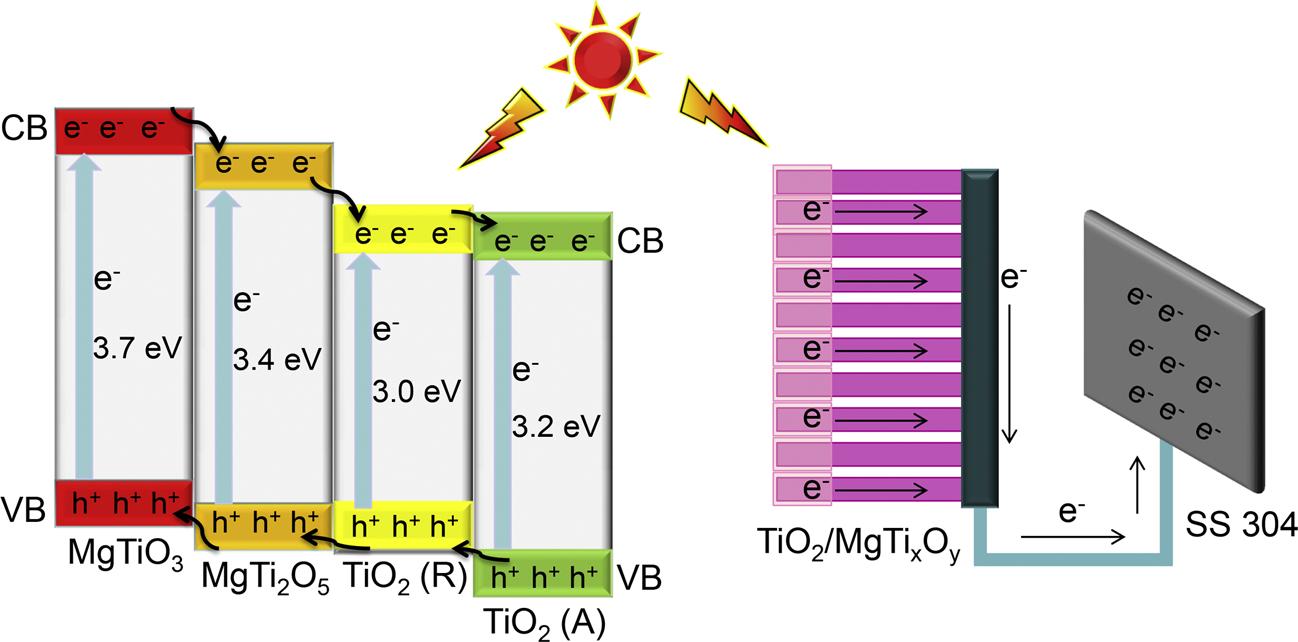
Fig.11. TheproposedtransfermechanismofthephotogeneratedchargecarriersandtheschematicdiagramofthePECCPoftheTiO2/MgTixOy multiphaseheterojunctions.
Fig.9C,thephotoluminescenceintensityofTiO2/MgTixOy hasasignificantdecreasecomparedwiththatofTiO2(A)andTiO2(A)/TiO2(R), indicatingthatthesecondaryrecombinationofthephotogenerated electronsandholeissignificantlyinhibitedfortheTiO2/MgTixOy photoelectrode.TheseresultsdisplaythattheTiO2/MgTixOy photoelectrodecanrealizethefasttransmissionofthephotogeneratedcharge carriers,caneffectivelyinhibitthesecondaryrecombinationofthe photogeneratedelectronsandholes,andcangeneratethehighest photoinducedcurrentcomparedwiththeTiO2(A)andTiO2(A)/TiO2(R) photoelectrodes,therefore,achievetheexcellentPECCPperformanceof theTiO2/MgTixOy photoelectrode.
TheSEMimageofTiO2/MgTixOy afterthePECCPtestsandtheXRD andFT-IRanalysesoftheTiO2/MgTixOy photoelectrodebeforeand afterthePECCPtestsareshownin Fig.10.Asshownin Fig.10A,the micromorphologyofTiO2/MgTixOy doesnothavesignificantchanges afterthePECCPtests.Furthermore,theXRDandFT-IRcurvesofthe TiO2/MgTixOy photoelectrodeafterPECCPtestsarehighlyconsistent withthosebeforePECCPtests,asshownin Fig.10BandC.Theseresults demonstratethatTiO2/MgTixOy photoelectrodehasahighstabilityin theprocessofPECCP.
Fig.11 showstheproposedtransferprocessesofthephotogenerated chargecarriersintheTiO2/MgTixOy multiphaseheterojunctionsandits PECCPmechanism.TheTiO2/MgTixOy photoelectrodehassignificantly enhancedphotogeneratedcurrentdensityandphotoinducedmixedpotentialdrop,indicatingthattheenergybandstructuresofTiO2 and MgTixOy matchwellandtheTiO2/MgTixOy multiphaseheterojunctions withagoodenergybandalignmentgradientareformed.Thephotogeneratedchargecarrierscanmigratedirectionallyundertheactionofthe multiphaseheterojunctions,makingthephotogeneratedchargecarriersbe welltransferred.ItwasreportedthatthebandgapsofMgTiO3,MgTi2O5, TiO2(R)andTiO2(A)are3.7eV,3.4eV,3.0eVand3.2eV,respectively, andtheconductionbandpotentialsofthemarearrangedinthefollowing order:MgTiO3 <MgTi2O5 <TiO2(R)<TiO2(A)[40,54,71,72].ThephotogeneratedelectronscanbetransferredtoTiO2(A)throughtheconductionbandgradientformedbytheTiO2/MgTixOy multiphaseheterojunctions,andeventuallybetransferredto304SSthroughtheTisubstrateto provideeffectiveprotectionforit.Meanwhile,thephotogeneratedholes willbefurthertransferredtotheTiO2/MgTixOy multiphaseheterojunctionsundertheactionoftheheterojunctionelectricfield,andthecorrespondingoxidationreactionswilloccurwiththeelectrolyte.Furthermore, thefabricationoftheTiO2/MgTixOy multiphaseheterojunctionsgreatly increasestheseparationefficiencyofthephotogeneratedchargecarriers andeffectivelysuppressestherecombinationofthephotogeneratedelectronsandholes.Inthisway,alargenumberofphotogeneratedelectrons willcontinuouslyflowfromtheTiO2/MgTixOy photoelectrodetothe coupled304SSelectrodeunderlightillumination,thussignificantlyenhancingthelong-termPECCPperformance.
4.Conclusions
Inthepresentpaper,ahighlyefficientandstableTiO2/MgTixOy multiphase-heterojunctionfilmwaspreparedanditsPECCPperformancefor304SSwasstudied.MgTixOy areuniformlydispersedonthe surfaceofTiO2 NTAs.MgTiO3,MgTi2O5,TiO2(R)andTiO2(A)contact wellandformamultiphase-heterojunctionsystem.Thephotoinduced currentdensityoftheTiO2/MgTixOy multiphase-heterojunctionfilmis 49μA·cm−2 underthesimulatedsolarlightillumination,whichis5.4 and2.1timesofthatofTiO2(A)andTiO2(A)/TiO2(R).ThephotoinducedmixedpotentialdropsoftheTiO2/MgTixOy multiphase-heterojunctionfilmis−320mV,while,thoseofTiO2(A)andTiO2(A)/ TiO2(R)are-230and-250mV,respectively.Meanwhile,theTiO2/ MgTixOy multiphase-heterojunctionfilmexhibitsextremelyhigh PECCPstability.Duringthe2hoflightillumination,thePECCPofthe TiO2/MgTixOy multiphase-heterojunctionfilmshowsnoweakening trend.ThelowersurfaceWFmakestheTiO2/MgTixOy multiphaseheterojunctionfilmbemucheasiertoescapeelectrons,whichisofgreat
benefittotheimprovementofthePECCPperformanceoftheTiO2/ MgTixOy multiphaseheterojunctions.Furthermore,thegoodPECCP performanceoftheTiO2/MgTixOy multiphaseheterojunctionsisattributedtotheestablishmentofmultiphaseheterojunctionsystem, whicheffectivelyinhibitsthesecondaryrecombinationofthephotogeneratedelectronsandholes,andacceleratesthemigrationofthe photogeneratedchargecarriers.SKPtechnologyhasbeenusedforthe firsttimetostudythePECCPperformanceoftheTiO2/MgTixOy multiphase-heterojunctionfilm.TheintroductionofSKPtechnologyinto thePECCPresearchhasgreatlyenrichedthetestmethodsinthefieldof PECCP.Atthesametime,theestablishmentofmultiphaseheterojunctionsprovidesanewideaforthedesignofthePECCPfilm.
Dataavailability
Alldataincludedinthisstudyareavailableuponrequestbycontact withthecorrespondingauthors.
DeclarationofCompetingInterest
None.
Acknowledgements
ThisworkwasfinanciallysupportedbytheNationalNaturalScience FoundationofChina(GrantNos.41576114,41676069),KeyResearch andDevelopmentProgramofShandongProvince(GrantNos. 2019GHY112066,2019GHY112085),StateKeyLaboratoryforMarine CorrosionandProtection,LuoyangShipMaterialResearchInstitute, China(ProjectNo.614290101011703),andQingdaoInnovative LeadingTalentFoundation(GrantNo.15-10-3-15-(39)-zch).
References
[1] K.Sivula,R.VanDeKrol,Semiconductingmaterialsforphotoelectrochemicalenergyconversion,Nat.Rev.Mater.1(2016)15010
[2] T.Hisatomi,J.Kubota,K.Domen,Recentadvancesinsemiconductorsforphotocatalyticandphotoelectrochemicalwatersplitting,Chem.Soc.Rev.43(2014) 7520–7535
[3] M.M.Momeni,M.Mahvari,Y.Ghayeb,PhotoelectrochemicalpropertiesofironcobaltWTiO2 nanotubephotoanodesforwatersplittingandphotocathodicprotectionofstainlesssteel,J.Electroanal.Chem.832(2019)7–23
[4] S.Chandrasekaran,L.Yao,L.Deng,C.Bowen,Y.Zhang,S.Chen,Z.Lin,F.Peng, P.Zhang,Recentadvancesinmetalsulfides:fromcontrolledfabricationtoelectrocatalytic,photocatalyticandphotoelectrochemicalwatersplittingandbeyond, Chem.Soc.Rev.48(2019)4178–4280
[5] M.M.Momeni,M.Taghinejad,Y.Ghayeb,R.Bagheri,Z.Song,Preparationof variousboron-dopedTiO2 nanostructuresbyinsituanodizingmethodandinvestigationoftheirphotoelectrochemicalandphotocathodicprotectionproperties, J.Iran.Chem.Soc.16(2019)1839–1851
[6] Y.Zhang,Y.Li,D.Ni,Z.Chen,X.Wang,Y.Bu,J.P.Ao,ImprovementofBiVO4 photoanodeperformanceduringwaterphoto‐oxidationusingRh‐DopedSrTiO3 perovskiteasaCo‐catalyst,Adv.Funct.Mater.29(2019)1902101
[7] M.M.Momeni,S.H.Khansari-Zadeh,H.Farrokhpour,Fabricationoftungsten-irondopedTiO2 nanotubesviaanodization:newphotoelectrodesforphotoelectrochemicalcathodicprotectionundervisiblelight,SNAppliedSciences1(2019) 1160
[8] M.M.Momeni,Y.Ghayeb,N.Moosavi,PreparationofNi–Pt/Fe–TiO2 nanotube filmsforphotoelectrochemicalcathodicprotectionof403stainlesssteel, Nanotechnology29(2018)425701
[9] Y.Bu,J.P.Ao,Areviewonphotoelectrochemicalcathodicprotectionsemiconductorthinfilmsformetals,GreenEnergyEnviron.2(2017)331–362
[10] J.Jing,M.Sun,Z.Chen,J.Li,F.Xu,L.Xu,Enhancedphotoelectrochemicalcathodic protectionperformanceofthesecondaryreducedgrapheneoxidemodifiedgraphiticcarbonnitride,J.Electrochem.Soc.164(2017)C822–C830.
[11] J.Li,C.J.Lin,C.G.Lin,AphotoelectrochemicalstudyofhighlyorderedTiO2 nanotubearraysasthephotoanodesforcathodicprotectionof304stainlesssteel,J. Electrochem.Soc.158(2011)C55–C62
[12] C.Lei,H.Zhou,Z.Feng,Y.Zhu,R.Du,Low-temperatureliquidphasedeposited TiO2 filmsonstainlesssteelforphotogeneratedcathodicprotectionapplications, Appl.Surf.Sci.257(2011)7330–7334
[13] Y.Yang,Y.F.Cheng,One-stepfacilepreparationofZnOnanorodsashigh-performancephotoanodesforphotoelectrochemicalcathodicprotection,Electrochim. Acta276(2018)311–318
[14] M.Sun,Z.Chen,Y.Bu,J.Yu,B.Hou,EffectofZnOonthecorrosionofzinc,Q235 carbonsteeland304stainlesssteelunderwhitelightillumination,Corros.Sci.82 (2014)77–84
[15] Y.Bu,Z.Chen,J.Yu,W.Li,Anovelapplicationofg-C3N4 thinfilminphotoelectrochemicalanticorrosion,Electrochim.Acta88(2013)294–300
[16] Y.Yang,Y.F.Cheng,FactorsaffectingtheperformanceandapplicabilityofSrTiO3 photoelectrodesforphotoinducedcathodicprotection,J.Electrochem.Soc.164 (2017)C1067–C1075
[17] J.Jing,Z.Chen,Y.Bu,M.Sun,W.Zheng,W.Li,SignificantlyenhancedphotoelectrochemicalcathodicprotectionperformanceofhydrogentreatedCr-doped SrTiO3 byCr6+ reductionandoxygenvacancymodification,Electrochim.Acta304 (2019)386–395
[18] S.J.Moniz,S.A.Shevlin,D.J.Martin,Z.-X.Guo,J.Tang,Visible-lightdrivenheterojunctionphotocatalystsforwatersplitting-acriticalreview,EnergyEnviron.Sci. 8(2015)731–759
[19] H.Wang,L.Zhang,Z.Chen,J.Hu,S.Li,Z.Wang,J.Liu,X.Wang,Semiconductor heterojunctionphotocatalysts:design,construction,andphotocatalyticperformances,Chem.Soc.Rev.43(2014)5234–5244
[20] J.Low,J.Yu,M.Jaroniec,S.Wageh,A.A.Al‐Ghamdi,Heterojunctionphotocatalysts,Adv.Mater.29(2017)1601694
[21] Y.Bu,Z.Chen,J.Ao,J.Hou,M.Sun,Studyofthephotoelectrochemicalcathodic protectionmechanismforsteelbasedontheSrTiO3-TiO2 composite,J.Alloys. Compd.731(2018)1214–1224
[22] Y.F.Zhu,L.Xu,J.Hu,J.Zhang,R.G.Du,C.J.Lin,Fabricationofheterostructured SrTiO3/TiO2 nanotubearrayfilmsandtheiruseinphotocathodicprotectionof stainlesssteel,Electrochim.Acta121(2014)361–368
[23] J.Hu,Z.C.Guan,Y.Liang,J.Z.Zhou,Q.Liu,H.P.Wang,H.Zhang,R.G.Du,Bi2S3 modifiedsinglecrystallinerutileTiO2 nanorodarrayfilmsforphotoelectrochemical cathodicprotection,Corros.Sci.125(2017)59–67
[24] Z.C.Guan,H.P.Wang,X.Wang,J.Hu,R.G.Du,FabricationofheterostructuredβBi2O3-TiO2 nanotubearraycompositefilmforphotoelectrochemicalcathodicprotectionapplications,Corros.Sci.136(2018)60–69
[25] M.Sun,Z.Chen,Enhancedphotoelectrochemicalcathodicprotectionperformance oftheIn2O3/TiO2 composite,J.Electrochem.Soc.162(2015)C96–C104
[26] J.Zhang,Z.U.Rahman,Y.Zheng,C.Zhu,M.Tian,D.Wang,NanoflowerlikeSnO2TiO2 nanotubescompositephotoelectrodeforefficientphotocathodicprotectionof 304stainlesssteel,Appl.Surf.Sci.457(2018)516–521.
[27] Y.Liang,Z.C.Guan,H.P.Wang,R.G.Du,EnhancedphotoelectrochemicalanticorrosionperformanceofWO3/TiO2 nanotubecompositefilmsformedbyanodizationandelectrodeposition,Electrochem.Commun.77(2017)120–123
[28] W.Sun,S.Cui,N.Wei,S.Chen,Y.Liu,D.Wang,HierarchicalWO3/TiO2 nanotube nanocompositesforefficientphotocathodicprotectionof304stainlesssteelunder visiblelight,J.Alloys.Compd.749(2018)741–749
[29] Y.Yang,W.Zhang,Y.Xu,H.Sun,X.Wang,Ag2SdecoratedTiO2 nanosheetsgrown oncarbonfibersforphotoelectrochemicalprotectionof304stainlesssteel,Appl. Surf.Sci.494(2019)841–849
[30] Y.Nan,X.Wang,X.Ning,J.Lei,S.Guo,Y.Huang,J.Duan,FabricationofNi3S2/ TiO2 photoanodematerialfor304stainlesssteelphotocathodicprotectionunder visiblelight,Surf.Coat.Technol.377(2019)124935
[31] P.Qiu,X.Sun,Y.Lai,P.Gao,C.Chen,L.Ge,N-dopedTiO2@TiO2 visiblelight activefilmwithstableandefficientphotocathodicprotectionperformance,J. Electroanal.Chem.844(2019)91–98
[32] X.Wang,Z.C.Guan,P.Jin,Y.-Y.Tang,G.L.Song,G.K.Liu,R.G.Du,FacilefabricationofBiVO4 modifiedTiO2 nanotubefilmphotoanodeanditsphotocathodic protectioneffectonstainlesssteel,Corros.Sci.157(2019)247–255
[33] H.Li,Y.Li,X.Wang,B.Hou,3DZnIn2S4 nanosheets/TiO2 nanotubesasphotoanodesforphotocathodicprotectionofQ235CSwithhighefficiencyundervisible light,J.Alloys.Compd.771(2019)892–899
[34] Y.Yang,W.Cheng,Y.F.Cheng,PreparationofCo3O4@ZnOcore-shellnanocompositeswithintrinsicpnjunctionashigh-performancephotoelectrodesfor photoelectrochemicalcathodicprotectionundervisiblelight,Appl.Surf.Sci.476 (2019)815–821
[35] S.Kuang,W.Zheng,Y.Gu,Z.Sun,Z.Yang,W.Li,C.Feng,Dual-functionalZnxMg1xOsolidsolutionnanolayermodifiedZnOtussock-likenanorodswithimproved photoelectrochemicalanti-corrosionperformance,J.Electroanal.Chem.815(2018) 175–182
[36] Y.Bu,Z.Chen,Highlyefficientphotoelectrochemicalanticorrosionperformanceof C3N4@ZnOcompositewithquasi-shell–corestructureon304stainlesssteel,RSC Adv.4(2014)45397–45406
[37] H.Xu,W.Liu,L.Cao,G.Su,R.Duan,PreparationofporousTiO2/ZnOcomposite filmanditsphotocathodicprotectionpropertiesfor304stainlesssteel,Appl.Surf. Sci.301(2014)508–514
[38] M.Sun,Z.Chen,Y.Bu,EnhancedphotoelectrochemicalcathodicprotectionperformanceoftheC3N4@In2O3 nanocompositewithquasi-shell–corestructureunder visiblelight,J.Alloys.Compd.618(2015)734–741
[39] C.Gao,Z.Zhang,X.Li,L.Chen,Y.Wang,Y.He,F.Teng,J.Zhou,W.Han,E.Xie, Synergisticeffectsinthree-dimensionalSnO2/TiO2/CdSmulti-heterojunction structureforhighlyefficientphotoelectrochemicalhydrogenproduction,Sol. EnergyMater.Sol.Cells141(2015)101–107
[40] L.Meng,Z.Ren,W.Zhou,Y.Qu,G.Wang,MgTiO3/MgTi2O5/TiO2 heterogeneous belt-junctionswithhighphotocatalytichydrogenproductionactivity,NanoRes.10 (2017)295–304
[41] Z.Zhang,K.Liu,Y.Bao,B.Dong,Photo-assistedself-optimizingofcharge-carriers transportchannelintherecrystallizedmulti-heterojunctionnanofibersforhighly efficientphotocatalyticH2 generation,Appl.Catal.B203(2017)599–606
[42] R.Wang,S.Chen,Y.H.Ng,Q.Gao,S.Yang,S.Zhang,F.Peng,Y.Fang,S.Zhang, ZnO/CdS/PbSnanotubearrayswithmulti-heterojunctionsforefficientvisible-lightdrivenphotoelectrochemicalhydrogenevolution,Chem.Eng.J.362(2019) 658–666
[43] Q.Wang,X.Shi,E.Liu,J.C.Crittenden,X.Ma,Y.Zhang,Y.Cong,Facilesynthesisof AgI/BiOI-Bi2O3 multi-heterojunctionswithhighvisiblelightactivityforCr(VI) reduction,J.Hazard.Mater.317(2016)8–16
[44] H.Li,L.Shen,K.Zhang,B.Sun,L.Ren,P.Qiao,K.Pan,L.Wang,W.Zhou,Surface plasmonresonance-enhancedsolar-drivenphotocatalyticperformancefromAg nanoparticle-decoratedself-floatingporousblackTiO2 foams,Appl.Catal.B220 (2018)111–117
[45] M.Wicinski,W.Burgstaller,A.W.Hassel,LateralresolutioninscanningKelvin probemicroscopy,Corros.Sci.104(2016)1–8
[46] A.Nazarov,V.Vivier,D.Thierry,F.Vucko,B.Tribollet,Effectofmechanicalstress onthepropertiesofsteelsurfaces:scanningKelvinprobeandlocalelectrochemical impedancestudy,J.Electrochem.Soc.164(2017)C66–C74
[47] X.Ma,L.Xu,W.Wang,Z.Lin,X.Li,Synthesisandcharacterisationofcomposite nanoparticlesofmesoporoussilicaloadedwithinhibitorforcorrosionprotectionof Cu-Znalloy,Corros.Sci.120(2017)139–147
[48] Y.Hu,V.Pecunia,L.Jiang,C.A.Di,X.Gao,H.Sirringhaus,ScanningKelvinprobe microscopyinvestigationoftheroleofminoritycarriersontheswitchingcharacteristicsoforganicfield‐effecttransistors,Adv.Mater.28(2016)4713–4719
[49] Z.Zhao,X.Chen,H.Wu,X.Wu,G.Cao,Probingthephotovoltageandphotocurrent inperovskitesolarcellswithnanoscaleresolution,Adv.Funct.Mater.26(2016) 3048–3058.
[50] Q.Song,Y.Zheng,D.Ni,Z.Ma,Studiesofthenobilityofphasesusingscanning KelvinprobemicroscopyanditsrelationshiptocorrosionbehaviourofNi–Al bronzeinchloridemedia,Corros.Sci.92(2015)95–103
[51] W.Hu,W.Zhou,K.Zhang,X.Zhang,L.Wang,B.Jiang,G.Tian,D.Zhao,H.Fu, FacilestrategyforcontrollablesynthesisofstablemesoporousblackTiO2 hollow sphereswithefficientsolar-drivenphotocatalytichydrogenevolution,J.Mater. Chem.A4(2016)7495–7502
[52] Z.Hua,B.An,T.Iijima,C.Gu,J.Zheng,Thefindingofcrystallographicorientation dependenceofhydrogendiffusioninausteniticstainlesssteelbyscanningKelvin probeforcemicroscopy,Scr.Mater.131(2017)47–50
[53] Z.Hua,S.Zhu,B.An,T.Iijima,C.Gu,J.Zheng,Thefindingofhydrogentrappingat phaseboundaryinausteniticstainlesssteelbyscanningKelvinprobeforcemicroscopy,Scr.Mater.162(2019)219–222.
[54] Y.Qu,W.Zhou,Y.Xie,L.Jiang,J.Wang,G.Tian,Z.Ren,C.Tian,H.Fu,Anovel phase-mixedMgTiO3-MgTi2O5 heterogeneousnanorodforhighefficiencyphotocatalytichydrogenproduction,Chem.Commun.49(2013)8510–8512
[55] S.Yan,T.Liu,Y.Zhang,D.Sun,X.Li,G.Xie,C.Feng,L.Xu,Enhancedphotoelectrochemicalperformanceofhydrogen-treatedSrTiO3/TiO2 nanotubearrays heterojunctioncomposite,J.Electroanal.Chem.807(2017)213–219
[56] K.Sun,S.Yan,T.Yu,K.Wang,C.Feng,L.Xu,G.Xie,Highlyenhancedphotoelectrochemicalcathodicprotectionperformanceofthepreparationofmagnesium oxidesmodifiedTiO2 nanotubearrays,J.Electroanal.Chem.834(2019)138–144
[57] A.Mazare,G.Totea,C.Burnei,P.Schmuki,I.Demetrescu,D.Ionita,Corrosion, antibacterialactivityandhaemocompatibilityofTiO2 nanotubesasafunctionof theirannealingtemperature,Corros.Sci.103(2016)215–222
[58] X.Wang,S.Shen,Z.Feng,C.Li,Time-resolvedphotoluminescenceofanatase/rutile TiO2 phasejunctionrevealingchargeseparationdynamics,ChineseJ.Catal.37 (2016)2059–2068
[59] Q.Chen,H.Liu,Y.Xin,X.Cheng,TiO2 nanobelts–effectofcalcinationtemperature onoptical,photoelectrochemicalandphotocatalyticproperties,Electrochim.Acta 111(2013)284–291
[60] Q.Meng,C.Lv,J.Sun,W.Hong,W.Xing,L.Qiang,G.Chen,X.Jin,High-efficiency Fe-MediatedBi2MoO6 nitrogen-fixingphotocatalyst:reducedsurfaceworkfunction andamelioratedsurfacereaction,Appl.Catal.B256(2019)117781
[61] V.Hasannaeimi,S.Mukherjee,Noble-MetalbasedMetallicGlassesasHighlycatalyticMaterialsforHydrogenoxidationReactioninfuelcells,Sci.Rep.9 (2019)1–8
[62] Y.Jiang,H.Ning,C.Tian,B.Jiang,Q.Li,H.Yan,X.Zhang,J.Wang,L.Jing,H.Fu, Single-crystalTiO2 nanorodsassemblyforefficientandstablecocatalyst-freephotocatalytichydrogenevolution,Appl.Catal.B229(2018)1–7
[63] M.Humayun,A.Zada,Z.Li,M.Xie,X.Zhang,Y.Qu,F.Raziq,L.Jing,Enhanced visible-lightactivitiesofporousBiFeO3 bycouplingwithnanocrystallineTiO2 and mechanism,Appl.Catal.B180(2016)219–226
[64] Z.Lu,L.Zeng,W.Song,Z.Qin,D.Zeng,C.Xie,InsitusynthesisofC-TiO2/g-C3N4 heterojunctionnanocompositeashighlyvisiblelightactivephotocatalystoriginated fromeffectiveinterfacialchargetransfer,Appl.Catal.B202(2017)489–499
[65] V.Hiremath,R.Shavi,J.G.Seo,ControlledoxidationstateofTiinMgO-TiO2 compositeforCO2 capture,Chem.Eng.J.308(2017)177–183
[66] J.Zhang,B.Shen,J.Zhai,X.Yao,Microwavedielectricpropertiesandlowsintering temperatureofBa0.5Sr0.5TiO3–Mg2TiO4 compositessynthesizedinsitubythehydrothermalmethod,Ceram.Int.39(2013)5943–5948
[67] X.Li,Z.Jiang,Y.Wu,H.Zhang,Y.Cheng,R.Guo,H.Wu,High-performance compositemembranesincorporatedwithcarboxylicacidnanogelsforCO2 separation,J.Memb.Sci.495(2015)72–80
[68] A.Kahn,Fermilevel,workfunctionandvacuumlevel,Mater.Horiz.3(2016)7–10
[69] L.Guo,I.B.Obot,X.Zheng,X.Shen,Y.Qiang,S.Kaya,C.Kaya,Theoreticalinsight intoanempiricalruleaboutorganiccorrosioninhibitorscontainingnitrogen, oxygen,andsulfuratoms,Appl.Surf.Sci.406(2017)301–306
[70] S.D.Tilley,Recentadvancesandemergingtrendsinphoto‐electrochemicalsolar energyconversion,Adv.EnergyMater.9(2019)1802877
[71] C.Ai,P.Xie,X.Zhang,X.Zheng,J.Li,A.Kafizas,S.Lin,Explainingtheenhanced photoelectrochemicalbehaviorofhighlyorderedTiO2 nanotubearrays:anatase/ rutilephasejunction,ACSSustain.Chem.Eng.7(2019)5274–5282.
[72] Y.Nosaka,A.Y.Nosaka,ReconsiderationofintrinsicbandalignmentswithinanataseandrutileTiO2,J.Phys.Chem.Lett.7(2016)431–434
