https://ebookmass.com/product/3d-lung-models-for-
Instant digital products (PDF, ePub, MOBI) ready for you
Download now and discover formats that fit your needs...
Developing the Digital Lung: From First Lung CT to Clinical AI John D. Newell
https://ebookmass.com/product/developing-the-digital-lung-from-firstlung-ct-to-clinical-ai-john-d-newell/
ebookmass.com
Developing the Digital Lung: From First Lung CT to Clinical AI 1st Edition John D. Newell
https://ebookmass.com/product/developing-the-digital-lung-from-firstlung-ct-to-clinical-ai-1st-edition-john-d-newell/
ebookmass.com
Lung Cancer: Standards of Care Goetz Kloecker
https://ebookmass.com/product/lung-cancer-standards-of-care-goetzkloecker/
ebookmass.com
Introductory Chemistry: A Foundation 9th Edition Steven S. Zumdahl
https://ebookmass.com/product/introductory-chemistry-a-foundation-9thedition-steven-s-zumdahl/
ebookmass.com
Vulnerability and the Corporate Immune System: An Integrated and Systemic Approach to Risk Management
Alessandro Capocchi
https://ebookmass.com/product/vulnerability-and-the-corporate-immunesystem-an-integrated-and-systemic-approach-to-risk-managementalessandro-capocchi/
ebookmass.com
The Hawk Laird (Celtic Hearts Book 1) Susan King
https://ebookmass.com/product/the-hawk-laird-celtic-heartsbook-1-susan-king/
ebookmass.com
CRAFTING & EXECUTING STRATEGY: CONCEPTS 21st Edition
Arthur Thompson
https://ebookmass.com/product/crafting-executing-strategyconcepts-21st-edition-arthur-thompson/
ebookmass.com
Sicurezza dei computer e delle reti 1st Edition W. Stalling
https://ebookmass.com/product/sicurezza-dei-computer-e-delle-reti-1stedition-w-stalling/
ebookmass.com
From Centralised to Decentralising Global Economic Architecture: The Asian Perspective Pradumna B. Rana
https://ebookmass.com/product/from-centralised-to-decentralisingglobal-economic-architecture-the-asian-perspective-pradumna-b-rana/
ebookmass.com
Food Waste Recovery: Processing Technologies, Industrial Techniques, and Applications 2nd Edition Charis M. Galanakis
https://ebookmass.com/product/food-waste-recovery-processingtechnologies-industrial-techniques-and-applications-2nd-editioncharis-m-galanakis/
ebookmass.com
3DLUNGMODELSFORREGENERATING LUNGTISSUE
Thispageintentionallyleftblank
3DLUNGMODELSFOR REGENERATINGLUNG TISSUE
Editedby
GunillaWestergren-Thorsson LungBiology,DepartmentofExperimentalMedicalScience,FacultyofMedicine, LundUniversity,Lund,Sweden
SaraRolandssonEnes LungBiology,DepartmentofExperimentalMedicalScience,FacultyofMedicine, LundUniversity,Lund,Sweden
AcademicPressisanimprintofElsevier 125LondonWall,LondonEC2Y5AS,UnitedKingdom 525BStreet,Suite1650,SanDiego,CA92101,UnitedStates 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom
Copyright©2022ElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyanymeans,electronicormechanical,including photocopying,recording,oranyinformationstorageandretrievalsystem,withoutpermissioninwritingfromthepublisher. Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’spermissionspoliciesandourarrangementswith organizationssuchastheCopyrightClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions
ThisbookandtheindividualcontributionscontainedinitareprotectedundercopyrightbythePublisher(otherthanasmaybenoted herein).
Notices
Knowledgeandbestpracticeinthisfieldareconstantlychanging.Asnewresearchandexperiencebroadenourunderstanding, changesinresearchmethods,professionalpractices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledgeinevaluatingandusinganyinformation, methods,compounds,orexperimentsdescribedherein.Inusingsuchinformationormethodstheyshouldbemindfuloftheirown safetyandthesafetyofothers,includingpartiesforwhomtheyhaveaprofessionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,oreditors,assumeanyliabilityforanyinjury and/ordamagetopersonsorpropertyasamatterofproductsliability,negligenceorotherwise,orfromanyuseoroperationof anymethods,products,instructions,orideascontainedinthematerialherein.
ISBN:978-0-323-90871-9
ForInformationonallAcademicPresspublications visitourwebsiteat https://www.elsevier.com/books-and-journals
Publisher: StacyMasucci
AcquisitionsEditor: AnaClaudiaA.Garcia
EditorialProjectManager: PatGonzalez
ProductionProjectManager: SwapnaSrinivasan
CoverDesigner: ChristianJ.Bilbow
TypesetbyMPSLimited,Chennai,India
Listofcontributorsix
Prefacexiii
1Isthelungacomplexorganto rebuild?1
ABDULLAHJABERAALTHUWAYBIAND CHRISTOPHERWARD
Overview:theform,function,andbeautyofthelung1
Themucusbarrierandlungsentinelimmunity2
Thetracheaandupperairways3
Theairwayepithelium4
Spatialorganizationofintegratedinnateandadaptive immunityinthelung7
Thelungcirculation8
Gasexchange:thecorejobofthelungs9
Bioengineeringinthelungsiskeytofunction10
Thelunglymphatics12
Testinglungfunction12
Thelungmicrobiomeinnormallunghomeostasis13
References14
I2Dcultureandthe microenvironment
2Two-dimensionalcellculturingonglass andplastic:thepast,thepresent,andthe future21
ATENAMALAKPOUR-PERMLIDANDSTINAOREDSSON
Definitionoftwo-dimensionalcellculturing21
Glass:thetwo-dimensionalsurfaceinthebeginningof cellculturing21
Glass:atwo-dimensionalsurfaceusedtoday24
Plasticfortwo-dimensionalcellculturing25
Hydrophilicity,surfacechemistry,andcell attachment27
Cellsintwodimensionsandthreedimensionsinthe bodyandrelevancefortwo-dimensionalcell culturing28
Theimpactofswitchingbetweendifferentkindsof surfacesandtheroleofmediumcomposition30
Conclusions32
References32
3Theimportanceoflung microenvironment37
ANDERSMALMSTROM
Introduction37
Collagenandfibrilformation38
Fibrillarycollagensandfibrilformation38
Nonfibrillarycollagens39
Supportiveglycoproteins40
Elastin,fibrillin,andemilin40
Lamininandthebasementmembrane40
Fibronectin41
Proteoglycans41
Chondroitinsulfate/dermatansulfateproteoglycans41
Heparansulfateproteoglycans42
Keratansulfateproteoglycan42
Glycosaminoglycanstructureandfunction42
Chondroitinsulfate/dermatansulfate42
Heparansulfate43
Hyaluronan44
Microenvironments44
Cellsinthemicroenvironmentsofthehumanlung44
Fibroblasts44
Conclusions45
References46
II
3Dlungmodels
4Theair liquidinterfacemodel51
TILLIE-LOUISEHACKETT,GWENDAF.VASSE, ANNEM.VANDERDOES,BRADYRAE, MARTIJNC.NAWIJNANDIRENEH.HEIJINK
Introduction51
Lungstructureandepitheliumcomposition51
Air liquidinterfacemodelsofthelungepithelium:what canwemeasure?52
Single-cellRNAsequencinganalysisofair liquid interface differentiatedcellsubtypesandstates:how welldotheserepresenttheairwayepithelium invivo?56
Recapitulationofepithelialphenotypeinair liquid interfaceculturesfromasthmapatients57
Recapitulationofepithelialphenotypeinair liquid interfaceculturesfromchronicobstructivepulmonary diseasepatients60
Useofair liquidinterfaceculturetostudyeffectsof environmentalinsults:respiratoryvirus61
Useofair liquidinterfaceculturetostudyeffectsof environmentalinsults:cigarettesmoke62
Useofair liquidinterfaceculturetostudyeffectsof environmentalinsults:airpollution63
Concludingremarks65
References65
5Lungorganoidmodels73
M.G.REA,T.JOHN,Y-W.CHENANDA.L.RYAN
Introduction73
Mouselungorganoids75
Humanadultlungorganoids77
Humanlungorganoids—embryonic78
iPSC-derivedlungorganoids78
Diseasemodelsusinglungorganoids:chroniclung diseases81
Fibrosis81
COPDandemphysema81
Diseasemodelsusinglungorganoids:geneticlung diseases82
Cysticfibrosis82
Hermansky-Pudlaksyndrome82
Diseasemodelsusinglungorganoids:virallung infections83
Conclusion84
Acknowledgments84
References84
6Biomaterialsforinvitromodelsinlung research91
Introductiontobiomaterials91
Briefhistoryofbiomaterials91
Propertiesandtypesofbiomaterials92
Scaffoldfabricationandmodificationstrategies94
Biomaterialsforlung-relatedapplications98
Specificfeaturesofthelung98
Biomaterialsforhealthyanddiseasedlung modeling100
Biomaterialsfororganoidformationand engraftment100
Biomaterialsinair-liquidinterphasecultures101
Newbiomaterial-basedstrategiesformodelingthelung microenvironment103
Conclusions104
Acknowledgment104
References104
7Threedimensionallungmodels-Three dimensionalextracellularmatrix models109
MEHMETNIZAMOGLU,MUGDHAM.JOGLEKAR, RODERICKH.J.DEHILSTER,MAUNICKLEFINKOLOKO NGASSIE,GRETAJ.TEITSMA,NATALIYAMIGULINA, KAJE.C.BLOKLANDANDJANETTEK.BURGESS
Introduction109
Extracellularmatrixchangesinchroniclung diseases112
Asthma112
Chronicobstructivepulmonarydisease112
Idiopathicpulmonaryfibrosis113
Two-dimensionalversusthree-dimensionalcellculture systems114
Three-dimensionalmodels—1:single-protein models116
Collagen116
Gelatinandmethacrylatedgelatin117
Otherextracellularmatrixcomponents119
Three-dimensionalmodels—2:extracellularmatrix modelswithcomplexextracellularmatrix mixtures119
Decellularizedlungscaffolds119
Decellularizedlungextracellularmatrix derived hydrogels122
Challenges123
Conclusions125
References126
8Lung-on-chip133
ANNEM.VANDERDOES,OLIVIERT.GUENAT, THOMASGEISERANDPIETERS.HIEMSTRA
Introduction133
Briefhistoricalbackgroundoforgan-onchip technology135
Alveoluslung-on-chipmodels136
Mimickinglungparenchymaldiseaseson-chip140
Airwaylung-on-chipmodels141
Applicationoflung-on-chipdiseasemodelswithafocus onregenerativemedicine143
Useoflung-on-chipmodelstostudyeffectsof environmentalinsults144
Advantagesandchallengesoflung-on-chip technology146
Concludingremarks147
References148
9Mechanicalstimuliinlung
regeneration153
JORGEOTERO,ISAACALMENDROSANDRAMONFARRE
Introduction153
Stiffnessofthecellmicroenvironment154
Cellstretch156
Cellshearstress159
Microbioreactorsformechanicallypreconditioninglung cells161
Mechanicalstimuliinwholelungbioreactors163
Conclusion164
Funding165 References165
Newdirections
10Advancedmanufacturing:threedimensionalprintingandbioprintingof modelsoflungandairways171
SINEMTAS,EMILREHNBERGANDDARCYE.WAGNER
Introduction171
Designconsiderationsformanufacturedlung scaffolds181
Biomaterialsformanufacturinglungscaffolds183
Naturalmaterials183
Syntheticmaterials183
Hybridmaterials184
Manufacturingporouslungscaffolds184
Casting185
Self-assembly185
Electrospinning185
Manufacturingmethodswithtruearchitecture control186
3Dprinting186
Bioprinting187
Four-dimensionalprintingandresponsivematerials188
Summaryandfuturedirections189
Acknowledgments190
References190
11Drugscreeningandhighthroughputin three-dimensionallungmodels197
LOESEMKISTEMAKERANDREINOUDGOSENS
Introduction197
Themaindifficultiesassociatedwiththeidentificationof newpharmacologicalapproaches198
Howthree-dimensionalmodelscanhelptoovercome theseproblems199
Targetdiscovery199
High-throughputscreeningandleadoptimization201
Phenotypicscreeninganddrugrepurposing201
Absorption,distribution,metabolismandexcretionand inhalationsafetystudies202
Concludingstatements203
References204
12Modelvisualization:frommicroto macro207
SEBASTIANWASSERSTROM,LINDAELOWSSON, SARAROLANDSSONENESANDJOHNSTEGMAYR
Modelvalidation:frommicrotomacro207
Lightversuselectronmicroscopy208
Lightmicroscopyacrossscales212
Imagingattheorganellelevel212
Imagingattheorganlevel217
Conclusion220
Acknowledgments220
References220
13Artificialintelligenceand computationalmodeling223
DANAIKHEMASUWANANDHENRIG.COLT
Introduction223
Definitionandhistoryofartificialintelligenceand machinelearning224
Whatismachinelearning?224
Whatisdeeplearning?225
Machinelearninginthoracicimaging225 Computer-aideddetection225 Radiomics227
Applicationsofmachinelearninginpulmonary diseases230
Machinelearningforlungcancerdiagnosis230
Deeplearningmodelforairwaylocalization230
Deeplearningmodelforclassificationofchronic obstructivepulmonarydisease230
DetectionofCovid-19onmedicalimaging231
Challengestofurtherimplementationofcomputer-aided diagnosisandradiomics232
Conclusion232
References233
Concludingremarksandfuture directions
14.Challengesandopportunitiesfor regeneratinglungtissueusingthreedimensionallungmodels239
GUNILLAWESTERGREN-THORSSONAND SARAROLANDSSONENES
Acknowledgments241
Index243
Listofcontributors
IsaacAlmendros UnitatdeBiofı´sicai Bioenginyeria,FacultatdeMedicinaiCie ` nciesde laSalut,UniversitatdeBarcelona,Barcelona, Spain;CIBERdeEnfermedadesRespiratorias, Madrid,Spain;Institutd’Investigacions BiomediquesAugustPiSunyer,Barcelona,Spain
AbdullahJaberAAlthuwaybi Translationaland ClinicalResearchInstitute,FacultyofMedical Sciences,NewcastleUniversity,Newcastleupon Tyne,UnitedKingdom
KajE.C.Blokland UniversityofGroningen, UniversityMedicalCenterGroningen, DepartmentofPathologyandMedicalBiology, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands; SchoolofBiomedicalSciencesandPharmacy, UniversityofNewcastle,Callaghan,NSW, Australia;NationalHealthandMedicalResearch CouncilCentreofResearchExcellencein PulmonaryFibrosis,Sydney,NSW,Australia
JanetteK.Burgess UniversityofGroningen, UniversityMedicalCenterGroningen, DepartmentofPathologyandMedicalBiology, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands; UniversityofGroningen,UniversityMedical CenterGroningen,W.J.KolffInstitutefor BiomedicalEngineeringandMaterialsScienceFB41,Groningen,TheNetherlands
Y-W.Chen DepartmentofMedicine,Divisionof Pulmonary,CriticalCareandSleepMedicine,
HastingsCenterforPulmonaryResearch, UniversityofSouthernCalifornia,LosAngeles, CA,UnitedStates;DepartmentofStemCell BiologyandRegenerativeMedicine,Universityof SouthernCalifornia,LosAngeles,CA,United States
HenriG.Colt PulmonaryandCriticalCare Medicine,UniversityofCaliforniaIrvine,Irvine, CA,UnitedStates
RoderickH.J.deHilster UniversityofGroningen, UniversityMedicalCenterGroningen, DepartmentofPathologyandMedicalBiology, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands
LindaElowsson LungBiology,Departmentof ExperimentalMedicalScience,Facultyof Medicine,LundUniversity,Lund,Sweden
RamonFarre ´ UnitatdeBiofı´sicaiBioenginyeria, FacultatdeMedicinaiCie ` nciesdelaSalut, UniversitatdeBarcelona,Barcelona,Spain; CIBERdeEnfermedadesRespiratorias,Madrid, Spain;Institutd’InvestigacionsBiomediques AugustPiSunyer,Barcelona,Spain
ThomasGeiser DepartmentofPulmonary Medicine,Inselspital,UniversityHospital, UniversityofBern,Bern,Switzerland; DepartmentforBioMedicalResearch,University ofBern,Bern,Switzerland
ReinoudGosens DepartmentofMolecular Pharmacology,GroningenResearchInstitutefor AsthmaandCOPD,UniversityofGroningen, Groningen,TheNetherlands
OlivierT.Guenat Organs-on-ChipTechnologies Laboratory,ARTORGCenter,UniversityofBern, Bern,Switzerland;DepartmentofPulmonary Medicine,Inselspital,UniversityHospital, UniversityofBern,Bern,Switzerland;Divisionof GeneralThoracicSurgery,Inselspital,University Hospital,UniversityofBern,Bern,Switzerland
Tillie-LouiseHackett Departmentof Anesthesiology,Pharmacology&Therapeutics, CentreforHeartLungInnovation,TheUniversity ofBritishColumbia,Vancouver,Canada
IreneH.Heijink DepartmentofPathologyand MedicalBiology,UniversityofGroningen, UniversityMedicalCenterGroningen, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands; DepartmentofPulmonology,Universityof Groningen,UniversityMedicalCenter Groningen,Groningen,TheNetherlands
PieterS.Hiemstra DepartmentofPulmonology, LeidenUniversityMedicalCenter,Leiden,The Netherlands
ArturoIba ´ n˜ez-Fonseca LungBiology,Department ofExperimentalMedicalScience,Facultyof Medicine,LundUniversity,Lund,Sweden
MugdhaM.Joglekar UniversityofGroningen, UniversityMedicalCenterGroningen, DepartmentofPathologyandMedicalBiology, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands
T.John DepartmentofMedicine,Divisionof Pulmonary,CriticalCareandSleepMedicine, HastingsCenterforPulmonaryResearch, UniversityofSouthernCalifornia,LosAngeles, CA,UnitedStates;DepartmentofStemCell BiologyandRegenerativeMedicine,Universityof SouthernCalifornia,LosAngeles,CA,United States
DanaiKhemasuwan PulmonaryandCriticalCare Medicine,VirginiaCommonwealthUniversity HealthSystem,Richmond,VA,UnitedStates
LoesEMKistemaker DepartmentofMolecular Pharmacology,GroningenResearchInstitutefor
AsthmaandCOPD,UniversityofGroningen, Groningen,TheNetherlands;AquiloBV, Groningen,TheNetherlands
AtenaMalakpour-Permlid FunctionalZoology, DepartmentofBiology,FacultyofScience,Lund University,Lund,Sweden
AndersMalmstrom LungBiology,Departmentof ExperimentalMedicalScience,FacultyofMedicine, LundUniversity,Lund,Sweden
NataliyaMigulina UniversityofGroningen, UniversityMedicalCenterGroningen, DepartmentofPathologyandMedicalBiology, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands
MartijnC.Nawijn DepartmentofPathologyand MedicalBiology,UniversityofGroningen, UniversityMedicalCenterGroningen, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands
MaunickLefinKolokoNgassie Universityof Groningen,UniversityMedicalCenter Groningen,DepartmentofPathologyand MedicalBiology,Groningen,TheNetherlands; UniversityofGroningen,UniversityMedical CenterGroningen,GroningenResearchInstitute forAsthmaandCOPD,Groningen,The Netherlands
MehmetNizamoglu UniversityofGroningen, UniversityMedicalCenterGroningen, DepartmentofPathologyandMedicalBiology, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands
StinaOredsson FunctionalZoology,Department ofBiology,FacultyofScience,LundUniversity, Lund,Sweden
JorgeOtero UnitatdeBiofı´sicaiBioenginyeria, FacultatdeMedicinaiCie ` nciesdelaSalut, UniversitatdeBarcelona,Barcelona,Spain; CIBERdeEnfermedadesRespiratorias,Madrid, Spain
BradyRae DepartmentofPathologyandMedical Biology,UniversityofGroningen,University MedicalCenterGroningen,Groningen,The Netherlands;UniversityofGroningen,University MedicalCenterGroningen,GroningenResearch InstituteforAsthmaandCOPD,Groningen,The Netherlands
M.G.Rea CIRMBridgestoStemCellResearch andTherapy,PasadenaCityCollege,Pasadena, CA,UnitedStates;DepartmentofMedicine, DivisionofPulmonary,CriticalCareandSleep Medicine,HastingsCenterforPulmonary Research,UniversityofSouthernCalifornia,Los Angeles,CA,UnitedStates
EmilRehnberg DepartmentofExperimental MedicalSciences,StemCellCentre,Wallenberg CenterforMolecularMedicine,LundUniversity, Lund,Sweden
SaraRolandssonEnes LungBiology,Department ofExperimentalMedicalScience,Facultyof Medicine,LundUniversity,Lund,Sweden
A.L.Ryan DepartmentofMedicine,Divisionof Pulmonary,CriticalCareandSleepMedicine, HastingsCenterforPulmonaryResearch, UniversityofSouthernCalifornia,LosAngeles, CA,UnitedStates;DepartmentofStemCell BiologyandRegenerativeMedicine,Universityof SouthernCalifornia,LosAngeles,CA,United States;DepartmentofAnatomyandCellBiology, CarverCollegeofMedicine,UniversityofIowa, IowaCity,Iowa,UnitedStates
JohnStegmayr LundUniversityBioimaging Centre,FacultyofMedicine,LundUniversity, Lund,Sweden;LungBioengineeringand Regeneration,DepartmentofExperimental MedicalSciences,WallenbergCentrefor MolecularMedicine,StemCellCenter,Facultyof Medicine,LundUniversity,Lund,Sweden
SinemTas DepartmentofExperimentalMedical Sciences,StemCellCentre,WallenbergCenterfor MolecularMedicine,LundUniversity,Lund, Sweden
GretaJ.Teitsma UniversityofGroningen, UniversityMedicalCenterGroningen, DepartmentofPathologyandMedicalBiology, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands
AnneM.vanderDoes Departmentof Pulmonology,LeidenUniversityMedicalCenter, Leiden,TheNetherlands
GwendaF.Vasse DepartmentofPathologyand MedicalBiology,UniversityofGroningen, UniversityMedicalCenterGroningen, Groningen,TheNetherlands;Universityof Groningen,UniversityMedicalCenter Groningen,GroningenResearchInstitutefor AsthmaandCOPD,Groningen,TheNetherlands
DarcyE.Wagner DepartmentofExperimental MedicalSciences,StemCellCentre,Wallenberg CenterforMolecularMedicine,LundUniversity, Lund,Sweden;NanoLund,LundUniversity, Lund,Sweden
ChristopherWard TranslationalandClinical ResearchInstitute,FacultyofMedicalSciences, NewcastleUniversity,NewcastleuponTyne, UnitedKingdom
SebastianWasserstrom LundUniversity BioimagingCentre,FacultyofMedicine,Lund University,Lund,Sweden
GunillaWestergren-Thorsson LungBiology, DepartmentofExperimentalMedicalScience, FacultyofMedicine,LundUniversity,Lund, Sweden
Thispageintentionallyleftblank
Preface
Studiesoncreatingbioartificiallungsor lungtissuepieceshaveevolvedrapidlyoverthe last10years,partlyasaresponsetotheincreasingnumberofpatientswithend-stagelung diseaseswhorequirelungtransplantation.This responseinvolvesincreasingnumbersofdifferentthree-dimensionallungmodelsandthe developmentofnewadvancedtechniquesand bioreactors.Theideaofwriting 3DLungModels forRegeneratingLungTissue grewoutofavery successfulresearchsessiontitled“From2Dto 3DModelsofHumanLungDisease:Novel DevelopmentstoRebuildtheLung,”whichwas givenatthe2019EuropeanRespiratorySociety conferenceinMadrid,Spain.
Inthistextbook,leadingresearchersshare theircollectivewisdomondifferentadvanced 3Dlungmodels,noveltechniquesincluding bioprintingandadvancedimagingtechniques, andimportantknowledgeaboutthecomplexityofthelunganditsextracellularmatrixcomposition.Theobjectiveinformulating 3DLung ModelsforRegeneratingLungTissue wastocreateahigh-qualitycollectiondescribingavailablepulmonarymodelsthatcanbeusedto
assesspathogenesisandpotentiallydevelop andtesttherapeutictargets.
Thishighlytopicalbookisstructuredas13 chaptersontopicsthatrangefromtheoriginal 2Dcellculturemodelsonplastictoadvanced cutting-edge3Dlungmodelsusingnaturalor syntheticbiomaterials.Thefirstchapterserves asanintroductiontothelunganditscomplexity.PartIfocuseson2Dcellculturemodelsand thelungmicroenvironment.PartIIdescribes different3Dlungmodelsanddiscussesadvantagesandchallengesofthedifferentmodelsystems.PartIIIcoversfuturedirectionswith chaptersdescribingnewtechniques,including 3Dand4Dprinting,bioprinting,andartificial intelligence,thatcanbeusedtodrivethefield forwardandsomefutureperspectives.PartIV providesthereaderwithsomeconcluding remarksandfuturedirections. 3DLungModels forRegeneratingLungTissue wascraftedby43 scientists,andwewishtothankeachofthem fortheircontributions.
SaraRolandssonEnes
GunillaWestergren-Thorsson
Thispageintentionallyleftblank
Isthelungacomplexorgantorebuild?
AbdullahJaberAAlthuwaybiandChristopherWard
TranslationalandClinicalResearchInstitute,FacultyofMedicalSciences,NewcastleUniversity, NewcastleuponTyne,UnitedKingdom
Lifeandrespirationarecomplementary.Thereis nothinglivingwhichdoesnotbreathenoranything whichbreathingwhichdoesnotlive.
[LecturesontheWholeofAnatomy;WilliamHarvey 1653] [1].
Overview:theform,function,andbeauty ofthelung
Breathingisremarkable.Itisessentialto existenceyetseemseffortless.Thecoreworkof therespiratorysystemistoprovideoxygen (O2)tothebodyandtoremovecarbondioxide (CO2).Todothis,theaveragepersontakes between17,000and23,000normalortidal breathsaday [2],bringingin(O2-richairon inspirationandremovingCO2)onexpiration.
Breathingcanbeconsideredintwostages. First,gasneedstobeconductedfromtheairto theperipherallung.Second,gasexchangeof O2 andCO2 needstotakeplacebetweenthe alveoliandpulmonarycirculation [3].Thelung isakeyorganofhomeostasis.Throughoutthe workofbreathing,therespiratorysystemis protectedfromexternalpathogensthrough sophisticateddefensesystems,conditionedby ahumanlungmicrobiome.Thecoordinated
activityofthesedefenseshaveformillennia providedprotectiontohumansfrominhaled externalpathogens,wellbeforethegeneral availabilityofantibioticsinthe1940s,with seamlessintegrationintothemechanicaland gasexchangecorefunctionsofthelungs.
Ventilationcanincreaserapidly,andmaximalvoluntaryventilationormaximalbreathingcapacityisabout15 20timesgreaterthan therestingminutevolumeof5L/min [4].In contrast,aresting,normalcardiacoutputof about5L/mincanincreaseto25L/minwith vigorousexercise;thisisaroundafivefold increaseincardiacoutput.
Throughoutwiderangesofrespiratoryactivity,variedworkloads,andchangesinrespiratory capacity,arterialbloodgasesandpHremain tightlyregulatedandconsistent.Amateurultramarathonrunnerscomplete100-kmruns,representingaconsiderableachievementofextreme exerciseendurance.Fingertipcapillaryblood samplesshowa96%O2 saturationaftertherun, comparedwith97%beforetherun [5],illustratingtheremarkablefunctionalityofthelung.This functionalityiscomplementedbyaconsiderable functionalreserve,illustratedbythefactthat patientswhohaverecoveredfromlobectomyor
singlelungtransplantationareabletolive,work, andexercise.
Thefundamentalrelationshipbetweenthe anatomyoftherespiratorysystem,itskey functions,andlifeitselfwererecognizedbythe GreekpolymathAristotlein350 BCE:“theair whenexpiredishot,whiletheinspiredairis cold, ... theairmakesitswayintonumerous pipesornarrowpassageswithinthelungand alongsideeachoftheserunbloodvessels. ... Theentranceoftheairistermedinspiration anditsexitiscalledexpiration. ... lifeandrespirationareinseparablyboundtogether” [6].
Theimportantrelationshipbetweenanatomy andkeyfunctionsoftherespiratorysystem recognizedin350 BCE thereforeincludedan appreciationoftheconditioningofinspiredair, consistentwithhostprotectionandthecorebusinessofgasexchange.Thisconditioningandinitialmovementofairforsubsequentgasexchange startinthenose,mouth,andupperairways.
Mostpeoplebreathethroughtheirnose whennotexercising.Thenoseisanareaofturbulentflow,accountingforhalfthetotalresistanceoftheairways [7].Thenostrilsofthenose narrowtoform3-mm2 “valves”whichgenerate highresistancetoairflow.Thenasalturbinates havealargesurfaceofaround150cm2 andare coveredbyavascularmucosa [8].Thenasal anatomyisthereforeideallysuitedtotheroleof warmingandmoisteningdryinspiredair,consistentwithsubsequentefficientgasexchange inthealveoliofthedistallung [8,9].Particles thataregreaterthan0.5 μminsizearealsoefficientlyfilteredbythenose,facilitatedbynasal hairsthatprojectintothenasallumen [7]
Themucusbarrierandlungsentinel immunity
Inadditiontofacilitatinggasexchangeinthe distallung,theconditioningofairinthenose andupperairwaysisakeyaspectofinnate immunefunctionanddefenseagainstthe
externalenvironment.ThesaturationofairfacilitatestransferofO2 fromthegaseousairintothe liquidphasecirculationbutisalsokeytotheefficientmucociliaryclearanceofmicroorganisms andparticulatesandtheefficientfunctionof mucussecretedbygobletcellsoftheupperairwayepithelium.Mucusisafundamental,evolutionaryconserveddeterminantofinnateimmune function.Mucusoriginshavebeentracedtothe Cnidaria,mostlysea-basedinvertebrates,which includecoralsandjellyfish [10].Gobletcellsthat secretemucusarepresentthroughoutthehuman respiratorytractbutareprominentintheupper airwaymucosa,with10,000cells/mm2 present inthemaxillarysinus [11].Thenumberofgoblet cellsthengenerallydecreasesfromthebronchi tothemoredistalairways [12]
Mucusactsasaformidablephysicalbarrier [13,14],augmentedbythepresenceofantimicrobialpeptidesandglycoproteins [15].Athinfilm ofepithelialliningfluidthatisaround10 μm deep,rangingfrom5to100 μm,linestheairways. Thisconsistsofthegel,amucuslayerontopofa looser,morewateryphasecoveringtheciliated epithelium.Thisallowstheciliatobeatinacoordinatedfashion,facilitatingmucociliaryclearance ofmicroorganismsandparticulates [16].
Thegelpropertiesofthemucusderivefrom theircontentofmucins [17],andthemainairwaymucinsareMUC5ACandMUC5B.Mucins aresecretedinvesiclesthatarederivedfrom theGolgiapparatuswithcompactstoragein thecytoplasm.Structurally,mucusformsatangledpolymerwithathreedimensionalstructureideallysuitedtoimmobilizinginhaled microbesandparticles [14].Thegel-forming mucinsarehighlyglycosylated,viscoelastic, disulfide-bondedglycoproteinswithhighmolecularweights(typically2 20 3 105 Da)and highcarbohydratecontentofbetween50%and 90%byweight [17] Mucinsecratagoguesinclude inflammatorycytokines,suchasinterleukin8 (IL-8)andIL-17A [18 20],whichcanalso recruitinnateimmunecellsthatcankilland phagocytosemicroorganisms.Invivo,mucin
secretionoccursinseconds [17,21],MUCgene upregulationtakesminutestohours,andmucin biosynthesisrequiresbetween6and24hours. Mucusisthenproducedbyhydrationof mucins.Thisindicatesthecapacityforacoordinated,graded,innateresponseinvolvingmucus andcellsofinnateimmunity [18 20].Acalibratedacuteorchronicdefensecantherefore bemounted,accordingtothelevelofhomeostatic challenge [17] tothefunctionalrespiratorysystem.
Thestickyprotectivenasalmucusblanketalso containsmicrobicidalagents,whichareproduced bynasalairwayepithelialcells,constitutingafurtherchemicalbarriertopathogens,conferringa broadspectrumofmicrobiologicalprotection. Microbicidalagentsincludelysozyme [22],which wasfirstdescribedandnamedbyAlexander Fleminginthe1920s.Fleming,whosubsequently discoveredpenicillin,demonstratedthat“diluted nasalmucus”addedto“athicksuspensionof bacterialcoccicausedtheircompletedisappearanceinafewminutesat37 C” [23].Lactoferrin, whichisalsoproducedbynasalepithelialcells [24],isaglycoproteinthatbindsbioavailableiron, whichmostbacteriarequiretogrow [25,26]. Lactoferrinalsohasantifungalandantiviralproperties.Broadmicrobicidalactivitieshavealsobeen shownforthesmallcationicproteindefensins. LL-37isadefensinproducedbyairwayepithelial cellsandneutrophilsthatkillsgram-positiveand gram-negativebacteria,fungi [27,28],andviruses viadisruptionofmembranes [29].
Thefundamentalimportanceofthenoseand upperairwayininnateimmunityhasbeenunderlinedintheCovid-19pandemic.Thisinfection remainsasymptomaticinmanypeople,butthe spectrumofclinicaloutcomesalsoincludesdeep lungpneumonia,intensivecareadmission,and death.AsofApril27,2022,therehadbeenover halfabillionreportedinfections,witharound6.2 milliondeathsduetoCovid-19.(JohnsHopkins UniversityCoronavirusResourceCenter. https:// coronavirus.jhu.edu/).Thecoronavirusthatis responsibleforCovid-19,SARS-CoV-2,infectstargetcellsviatheangiotensin-convertingenzyme2
receptor,whichispresentinthenasalepithelium. Wehaveusedacellmodelofhuman primarynasalepitheliumdifferentiatedatanairliquidinterface [30] tostudypatient-derived SARS-CoV-2infection [31].Patient-derived virusdemonstratedbroadnasalepithelialtropism,withviralproductiondemonstratedin bothciliatedandsecretorycells.Single-cell RNAsequencing,proteomics,andpathological assessmentsshowedthattheepithelialhost responsewasdominatedbytypeIandIIIinterferonsandinterferon-stimulatedgeneproducts. This“delayed”responseoccurred24hoursafter viralgeneexpression,notaffectingSARS-CoV-2 replicationsignificantly.Theprovisionofrecombinantinterferonbeta(IFN-β)orIFN-λ1priorto infectionaugmentedanantiviralstatethat potentiallyrestrictedSARS-CoV-2viralreplication,representingapotentialprophylactic opportunityforintervention(Fig.1.1)andillustratingtheimportanceoftheintegrateddefenses ofthenose,upperairways,andmicrobiome.
Thetracheaandupperairways
Theextrathoracicwindpipeortrachea throughwhichrespiredairflowswasdescribed byAristotle,whoobservedthat“allanimals thatevidentlyrespiredosobymeansofthe windpipe,whentheybreatheeitherthrough themouthorthroughthenostrils” [33].
Conditioned,warmed,hydrated,andfiltered [7] airflowsfromthemouthandnoseintothetracheaandbronchialtreeoninspiration.Thisfollowspressuregradients,whicharecreatedby changesinlungvolume,withtheprocessreversed onexpiration.Therateofairflowisproportional totheresistanceoftheairways,withmostresistanceintheupper,largeconductingairways.
Duringnormalswallowing,thelarynxiselevatedtothetopofthetrachea,andmovement oftheepiglottisoccurstoeffectivelysealthelarynx [34].Inspirationisinhibitedduringswallowing,andthiscomplex,interrelatedresponse
isafurtherkeydefenseoftheairway,thefailureofwhichcancauseaspirationpneumonia, withhigh,relatedmorbidityandmortality.
Thetracheaisprotectedandsupportedby horseshoe-shapedringsofcartilageandcontainssmoothmuscle.Thisanatomyallowsthe posteriorwallofthetracheatobulgeduring cough [35],withluminalnarrowing,increased resistance,andshear.Thisservestodislodge mucus,promotingclearanceandfurtherexemplifyingtheinterrelationshipbetweenanatomicalformandfunctionthatispresentthroughout thetracheobronchialsystem.
Theelegant,strikinglatexcaststhatwere producedbypathologistssuchasWeibel [36] andFrancisGreen [37] revealatracheobronchialtreeformedbyaround24airwaygenerations(Fig.1.2).Thetracheaisthefirstand largest(generation0)andsubsequentlyundergoesfractal [38] branching,witheachairway dividingintotwo.Theprinciplesoffractal geometryasobservedinthelungprovidea systemofairwaysandbloodvesselsthatare matchedasevenlyandefficientlyaspossible forgasexchange,withinanoptimallyminimizedspace [39].Theformationofsmaller daughterairwaysquicklygeneratesahuge cross-sectionalarea,withmostareacontributed tobyairwaygenerations10 24.Theincreasein cross-sectionalareameansthatairwayresistanceismuchlowerinthesmallerairways comparedtothelargeairways.Functionally, thisalsoleadstoarapidfallinthevelocityof
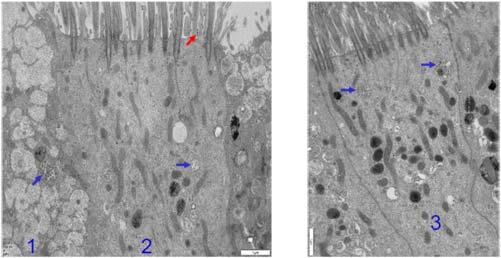
FIGURE1.1 Culturedhumannasalsecretory (cell1)andciliatedcells(cells2and3)showing SARS-CoV-2viralparticlesinsideandoutsidecells withelectronmicroscopy.Thevirusappearsassmall spheres,approximately0.1 μmindiameter(arrows). Redarrow showsvirusthathasleftthehumanhost cell,allowingspread.Scalebar:1 μm [32]
theairasitmovesintothelung.Theconducting airwaygenerations0 16continuetohumidify andwarmairmovingintothelungandlead intotherespiratoryzoneoftheairwaytree.In therespiratorybronchioles,beyondgeneration 17thereisachangeinfunction,withanincreasingnumberofthin-walledgasexchangeairsacs oralveolipresent,groupsofwhichformacini. Thealveolarductsthenleadintothefinalgenerationofairpassagesandfurtheralveoli.
Thewallsofthealveoliarethin(0.1 0.2 μm), ideallysuitedtogasexchangeandO2 uptakeof upto3L/min [12] fromtheair,warmedand humidifiedbythenoseandupperairways.In totaltherearearound3 3 106 alveoliarising fromairwaygenerations17 23,providinga massivesurfaceareaofaround70m2 tofacilitategasexchange [40].
Theairwayepithelium
Thecellsandassociatedstructuresoftheairwayareimportantcontributorstothefunctional respiratorysystemandlungs.Theseincludeairwayepithelia,associatedionchannelsandbasementmembrane,mucus-producinggobletcells, tissuemacrophages,neutrophils,eosinophils, mastcells,lymphocytes,dendriticcells(DCs), fibroblasts,smoothmusclecells,andvessels. Thematrixcomponentsofthelunginclude elastinandcollagens.
Thefulldetailsofthisarebeyondthescope ofthissinglechapter.However,thekeyroles
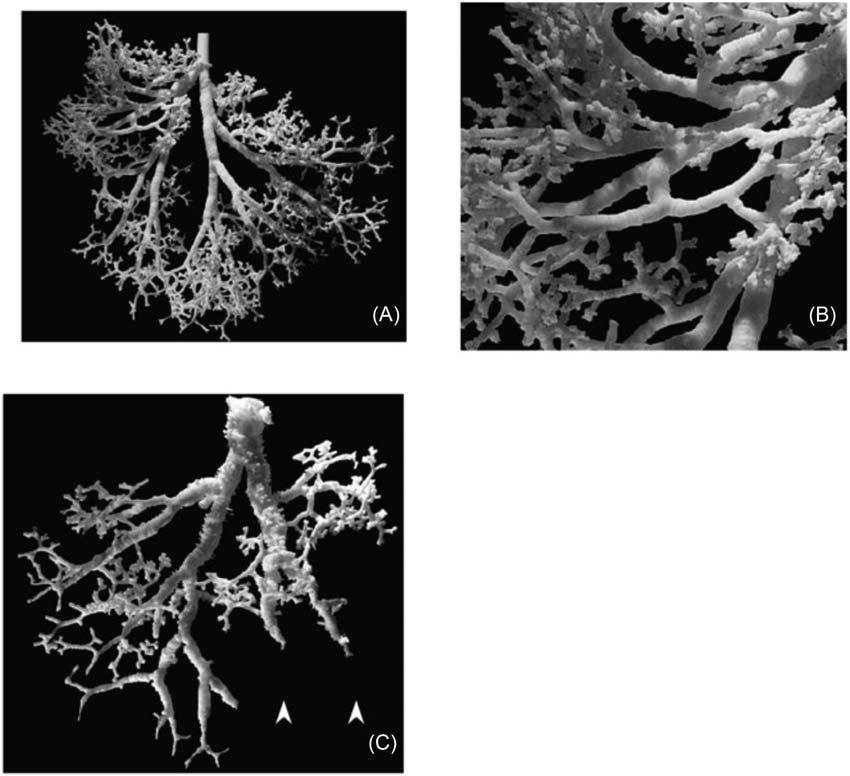
FIGURE1.2 Fractalgeometryofairwayremodelinginhumanasthma.(A)Low-powerphotographofnonasthmacontrollatex castofapicalsegmentofleftlowerlobeofhumanlung.Thecastshowscharacteristicdichotomousbranchingpatternwithsmooth parallel-sidedsegmentsandcompletefillingtothelevelofrespiratorybronchioles(showningreaterdetailin(B).(C)Castofan 18-year-oldfemalewhodiedofasthma.Thecastshowsirregularsegmentswithtapering, constrictions,andsurfaceprotrusions. Source: AdaptedwithpermissionoftheAmericanThoracicSociety.Copyright r 2022AmericanThoracicSociety.Allrightsreserved. BoserSR,ParkH,PerrySF,MenacheMG,GreenFHY,AmJRespirCritCareMed2005;172:817 823.TheAmericanJournalof RespiratoryandCriticalCareMedicineisanofficialjournalofthe AmericanThoracicSociety.Readersareencouragedtoreadtheentire articleforthecorrectcontextatAmJRespirCritCareMed.2005Oct1;172(7):817 823. https://doi.org/10.1164/rccm.200411-1463OC. Epub2005Jun23.Theauthors,editors,andTheAmericanThoracicSocietyarenotresponsibleforerrorsoromissionsinadaptations.
oftheepitheliaoftherespiratorytractinthe airwayandlungwarrantattentionandare particularlyrelevanttotheconsiderationof requirementsforafunctionallung.Thefunctionalimportanceofrespiratoryepithelia rangesfromstructural,barrier,anddefense considerationsintheairwaystotheremarkable gasexchangecapabilityoftheperipherallung. Theairwayepitheliumformsaninterface betweenthehostandthesurroundingexternal
environmentwithconstantexposuretobacteria,viruses,fungi,parasites,andpollution. Alongwiththisbarrierfunction,mediators fromtheairwayepitheliumcanactivateor downregulateimmunefunction,withapivotal roleinlungandhosthomeostasis.
Inthesurfacepseudostratifiedepitheliaof proximalairways,fourcelltypeswereclassicallyrecognizedasmakingupthesurface epithelium:ciliatedcells,mucus-secretinggobletcells,intermediarycells,andbasalcells. Ciliatedcellsoccurthroughouttherespiratory tract,includingtherespiratorybronchioles [12],butwithagreaterdensityofciliainthe proximalairways.Thebronchialepithelium containsabout6000gobletcells/mm2,andthis reemphasizesthemucociliaryescalatorasa keypartofthelung’sdefensesagainstpathogens,augmentedbysubmucosalglandspresentinlargercartilaginousairways.
Classicalcharacterizationsoftheairwayepitheliumincludeddescriptivemorphologyand traditionaltechniquessuchasmicroscopy informedbytinctorialdyestains.Hencethe gobletcellcanlooklikeacuporgoblet,while periodicacid-Schiffstainingdenotespolysaccharidestaining,consistentwithmucusproduction.Intheterminalbronchioles,highly metabolic,secretory,andstemcell likeclub cellsaredescribed.Thesenonciliatedcellsare describedasthemainpopulationofsecretory andstemcellsperipherally,wheretheairway epitheliumbecomesthinnerandcuboidalwith progressivelyfewerciliatedcells [12].
Aswellastheregenerativecapabilityofthe classicalairwaybasalandclubcells,ithasbeen increasinglyrecognizedthatcellularplasticity intheairwayexistsandpotentiallycontributes toairwayandlungphysiologyandpathophysiology.Ourgroupmadethefirstdescriptionof epithelial-mesenchymaltransition(EMT)inthe humanairway [41,42],wherebyairwayepithelialcellscanlosetheirepithelialcharacteristics andgainmesenchymalmarkers,withinvasive potentialdemonstratedthroughbioassay [41],
withawiderangeofpotentialintermediary typespossible [43].
BothEMTandendothelial-mesenchymal transitionhavebeenshowntobemodulated bymicro-RNAs [44,45].Newandcomplex insightshavealsobeenrecentlyaffordedby theadventofsingle-cellmRNAanalyses andworkembodiedwithintheHumanTissue AtlasConsortium [46].Single-cellapproaches havedescribednovelpopulationsofepithelial cells.Thisincludesarareionocytecelltype thatexpresseshighlevelsofcysticfibrosis transmembraneconductanceregulator(CFTR) activityintheairwayepithelium [32,47]. DeficienciesinCFTRareresponsibleforhigh ratesofmorbidityandmortalityinpeoplewith cysticfibrosis [47]
Deprezetal.havepublishedasingle-cell atlasofhealthyhumanairways,aconsiderable achievement,withdatafromthenose,trachea, andbronchi [48].Thisyieldeddetailedinformationfromthefirsttothe12thairwaydivisions,showingthatthegreatestchangesin cellularitywereapparentbetweennasaland tracheobronchialsamples,withincreaseddensityofglandsandsecretorycellsinnasal samples.
Thisisarapidlyevolvingfield,andwork atthetimeofwritingisalsobeingdrivenby theCovid-19pandemic [32,46].Overall,anintegrationofnewmolecularandclassicalpathologyapproachesindicatesthatthefunctional lungcontainsadynamicepithelial,resident immune,andneuronalcellcommunity.The single-cellmRNAapproachessupportacore residencyprogramforimmunecells,whichcan beaugmentedbycellsthatarerecruitedfrom theblood.Itisnotablethatmiceseemtolack approximatelyone-thirdofhumanlungcell typesthathavebeenidentifiedaspotentially maintainingmucosalimmunehomeostasisand hostdefense [49].Thisindicatestheimportance ofhumansamplestoinformtranslational researchandprovideinsightsforrebuilding thefunctionalhumanlung [50].
Spatialorganizationofintegratedinnate andadaptiveimmunityinthelung
Tocomplementitsinnateimmunedefenses, therespiratorysystemincludesevolutionary conserved,anatomicallyconstitutive,adaptive immunecapabilityrepresentedbylymphocytic aggregates,sometimeswithgerminalcenters.
Nasal [29,49] andbronchus-associatedlymphoidtissue(BALT) [51,52] hasbeendescribed, withanincreaseddensityofBALTpresent insmokers [51,53].Thereislimitedavailable research,giventhepotentialimportanceof thesesystems,butitisofinterestthatclassical andpainstakinganalysisofwholelungs showedthatBALTwasdetectedthroughout therespiratorysystemintrachea,carinae,main stembronchi,lobarbronchi,andperipheral lungcontainingbronchioles [51,53].
Thedefensesofthenonrespiratoryairway generations,togetherwiththewarmingand humidificationconditioningofatmosphericair, representintegratedexamplesoflungfunctionality.Thisfacilitatesthefirstcoreaspect ofthefunctionallung,namely,conductingthe air,whichwouldneedtobereplicatedfor rebuildingafunctionallung.
Intherespiratorybronchiolesbeyondgeneration15 [39] thereisanincrementalchange infunction,withadevelopingcapabilityfor thesecondcoretaskofthelung,thatis,gas exchange.Thisgasexchangefunctionalityis providedbyanincreasingnumberofthinwalledgasexchangeairsacsoralveoli.After aboutthreefurthergenerationsbeyondgeneration15theairwaywalliscompletelycovered withalveoli,whichformalveolarducts.In thefinalairwaygenerationthealveolarsac endsblindly.Airwaygenerations15 23thereforeformpulmonaryacini,theunitofthe gasexchangerventilatedbyonetransitional bronchiole:oninspirationoxygenatedairflows alongtheconductingairwaysintotheacinus, andaround10,000alveoliinanacinussource, acommonairsupply [39].Theacinitherefore
connecttotheairwayleadingtoandfrom theexternalatmosphere;alveolarpressureis thereforeclosetothatoftheatmospheremost ofthetime.ChangesbroughtaboutbyinspiratoryandexpiratorylungmechanicsareresponsibleforthemovementofO2-richairintothe lungandremovalofCO2 onexpiration.The alveoliallowgasexchange,withthiscoreprocessofthefunctionallungagainenabledby anatomicalform.
Thediameterofthealveoliinhealthylungs isaround200 μm,anddiffusionofairthough thegas-filledalveolioccursinafewmilliseconds [34].Theprocessofdiffusionintoliquid phasesisslowerthanthatingas,butthefunctionallungisanatomicallyoptimizedfor thisprocess.Thewallsofthealveoliareboth thin,lessthan1 μmthick,andarethesole placeinthebodywherecapillariesare exposedtotheair.Thisoccursasaresultof fusionbetweentheendotheliumofpulmonary capillariesandthethinwallsofthetype1 cells.Thehybrid,fusedbarrierofthetype1 cellsandendotheliumofthecapillaryisthereforeaminimizedbarrier,facilitatingdiffusion ofgas.Thetype1alveolarepithelialcellsare thefunctionalunitforgasexchangeandconstitutearound95%ofthealveolarcellsurface throughverythincytoplasmicprocessesthat generateahugearea,ofaround5000 μm 2 associatedwitheachnucleus [39].Type1cells areunabletodividebymitosis,butelectron micrographsofthelungstructurereveala mosaicofalveolarepitheliumthatincludes morphologicallydistinct,rounded,type2alveolarepithelialcellsatthealveolarwall.Type2 cellsproduceandstorephospholipidsurfactant.Thiscruciallydecreasesthesurfacetensionofthealveoli.Thetype2cellsalsoforma keypoolofprogenitorstemcellswithan importantroleintheregenerationoftype1 andtype2epithelialcells [39].Themillionsof alveoliofthefunctionallungprovideamassivesurfaceareaofaround70m2 tofacilitate gasexchange [40].
Thelungcirculation
Thekeyroleofthecirculationinthenormal functionalhumanlungisunderscoredbythe factthatthelungreceivesbloodsupplyfrom twodistinctcirculatorysystems.
Thelow-pressure,low-shear,andhigh-flow systemofthepulmonarycirculationcarries thetotalcardiacoutputfromtherightventricle oftheheart.Thisenablesbloodoxygenationin thelungandnutrientandliquidexchangefor thelungparenchyma.
Thebronchialcirculationisderivedfromthe aortaandsoispartofthesystemiccirculation. Itisofinterestthatthemouselungdoesnot haveaseparatebronchialcirculation [54].The bronchialcirculationconstituteslessthan1%of thelung’sbloodflow,doesnotnormallyparticipateingasexchange,butisthemainsupplier ofnutrientsandO2 fortheairways.Thetwo separatevascularbedsmergeintothepulmonaryvein,andthesmallamountofdeoxygenatedbloodfromthebronchialcirculationleaves thelungviathepulmonaryvenousnetwork. Thisbloodthereforepassesthroughthelungs withoutbeingoxygenated,representingapart ofasmallphysiologicalshuntthatispresentin thenormalfunctionallung [35].
Thelow-pressurepulmonarycirculation flowsfromtherightventricleintothepulmonaryarteryanddividesintotheleftandright pulmonaryarteries,whichthenfurtherdivide intobranchesthatfollowthebronchialtree. Thehighcapillarityofthealveolarwallsand thealveolarcirculationessentiallyprovidesa bloodfilm,coveringthealveoli,withflow occurringthroughthecapillaryinaround 0.8secondsatrest.Oxygenationofthemixed venousbloodoccursinaroundone-thirdof thistime,meaningthatthereiscapacityto accommodatetheeffectsofexercisewhenblood transittimesdecrease [55].Thepulmonary venulescontainingoxygenatedblooddrainlaterallyintofourmainpulmonaryveins,which
emptyintotheleftatriumtoprovideoxygenatedbloodtothesystemiccirculation [35].
Neithertheairofaninspiredbreathorthe distributionofthelow-pressurepulmonary circulationisevenlydistributedinthelung. Humanshaveevolvedasuprightbipeds,afeaturethatexertsgravitationaleffectsonboth thelungandtheassociatedcirculationofthe integratedrespiratorysystem.Thereisregional variationinlungcompliance,andsomeregions arethereforepreferentiallyventilatedwith inspiredair.Alongwiththeregionalvariability incomplianceofthelungs,small-scalevariationsduetotheunevenlengthofairwaysgeneratedbytheasymmetricaldichotomousand fractalbranchingpatternoftheairwaysalso occur [38,39]
Thelungscanbeconsideredtobefreely hanginginsidethethorax.Theresultantgravitationalpullonthelungsmeansthattheupper lobesofthelungarerelativelydistended whilebasallungareasarerelativelysupported bythediaphragm.Duringinspiration,inan uprightindividualthismeansthattheupper partsofthelung,whicharealreadystretched, arelesscompliantandsolessabletofillwith air.Incontrast,thelungbasesarelessstretched andabletofillmoreeasily.Asaresult,more ventilationfromaninspiredbreathtendsto occurinthelowerzonesofthelungs.Ona smallerscale,adjacentlobulesorevenalveoliin thelungmaynothavethesamecompliance, owingtotheeffectsofairwayanatomy,contributingoveralltoheterogeneityofventilation(V) inthelung [35].
Gravityalsoaffectsthelow-pressurepulmonarycirculation,whereatrest,thedriving pressureisaround15mmHg.Thisisnotsufficienttofullyperfusetheupperlung,sothe lungapicesreceivelittleperfusion(Q)fromthe pulmonarycirculation.Althoughventilationis alsodiminishedatthelungapex,ventilation stillexceedsperfusion,andtheV/Qratiois greaterthan1.Bycontrast,atthebasesofthe
lung,theV/Qratioislessthan1.Thedistributionofperfusionisalsoinfluencedbyhypoxia. LowO2 levelsinregionsofthelungexert pulmonaryarteryvasoconstriction,decreasing thebloodsupplytothatregion.Thissystemis akeyhomeostaticandadaptiveprocessthat broadlymaintainsaV/Qbalanceofventilation andperfusion.Ithasbeenshownthatinthe normallungtheoverallV/Qisaround1,and theV/Qrelationshipisacriticaldeterminant oflungphysiology [56,57].
Inhumans,afteradeepinspiration,around 80%ofthetotallungvolumeisair,and 10%isblood.Thefewhundredgramsof tissueandcellsthatmakeupthefunctional lungincludeasophisticatednetworkofconnectivetissue.Thispinnacleofdesignaccommodatesthefunctionsofthelungatdifferent volumes,associatedwithnormalinspiration andexpirationandthedemandsofexercise [5,58]
Ithasbeencalculatedthatalveolarstructuresmustcopewithbreathing-relatedalveolarstrainforupto109 cyclesduringtidal breathingduringtheirlifetime [59].Thecomplexarchitectureofthelungisnotablysupportedbylittletissue,however,with“little wastedstructureandnooverdesign” [59].This isofkeyimportanceinconceptualizingthe rebuildingafunctionallung.
Gasexchange:thecorejobofthelungs
Thelungparenchymaincludesthefunctional gas-exchangingalveoli,which,asdescribedearlier,arelinedbyepithelium,coveredbyathin liquid-phasebloodlayer.Theresultingairliquidinterfaceandsurfacetensioncontribute tothefunctionallyimportantelasticrecoilofthe lung.Thecellsofthealveolarwallscanaffect thelocaltensionoftheextracellularmatrix (ECM).Inturnmechanicalforcesassociated withmovementofthechestwallandbreathing
aretransferredtothecellsthroughintermediary ECMcomponents.
The“connectivetissue”componentsofthe ECMincludecollagenfibers.Therearearound 30differentcollagenmolecules.Theinterstitial collagensaremostlyhelicalstructuresthatconferabasicsupportfunction.Thehelicesform rigidrodlikestructuresabout300nminlength and1.5nmindiameter,whicharecapable ofspontaneousfibrilformation.Thecollagen fibrilscanfurtherorganizeintothickercrosslinkedfibers,andcollagenfibril-fibernetworks mayberandomlyarrangedorformoriented structures.Thelungparenchymainterstitium containsmostlycollagentypesIandIII,with sometypeIVcollageninthebasementmembranesofepithelia [60,61].Collagenisless deformablethanelastinandthereforeprovides astructuralscaffoldforthewallsofalveoli [62]
Variationsinparenchymalcollagencontent indevelopment [63],andfibrosis [64,65] indicatethatcollagencontentisakeydeterminant ofparenchymalbiomechanics.Thisisanincrediblydynamicsystem,andithasbeenshown thataround10%ofthetotallungcollagenin ratsissynthesizedeachday,consistentwitha continuoushigh-volumeturnoverofthelung matrix [66].
Elastinisanotherload-bearingECMcomponent,whichismoreflexiblethancollagens. Elasticfibersarecomposedofelastinandassociatedmicrofibrils.Theseoftenformafibrous outerlayeraroundthelessorganizedelastin. Elasticfibersarestructurallyvariedand mechanicallyinterconnectedwithcollagen [67].
Collagenandelastinthereforeprovidecomplementaryresilienceandbiomechanicalpropertiesoverawidestrainspectrum,accommodating changesinlungvolumeencompassingfunctional residualcapacityandtotallungcapacity,supportingsedentaryworkloads,andenabling extremesofexercise [5,58].Thecollagenand elastinfibersofconnectivetissuesarecontained inahydratedgel.Thematrixcompositionand
ratiooffibertogelvaryamongtissuesin maturationandwithpathophysiology [65]. Glycosaminoglycans(GAGs)arekeyelements ofthismatrix,andthereareseveraldifferent types.Molecularweightsvarywidelyover ordersofmagnitude,andpolymericchainscan containupto100units.Thereistherefore markedvariabilityinsizeandinsecondaryand tertiarystructures,someofwhichcanbevisualizedbyelectronmicroscopy [60].
ProteoglycansformedfromGAGsubunits haveanumberofbiologicalfunctions.These rangefromactingascellsurfacereceptors throughtothebindingofgrowthfactorsand proteins,constitutingafunctionalreservoir thatcouldaffectcellularmigrationandtissue repair.Theorganizationofcollagenandelastin fibersmaybeinfluencedbyproteoglycans [60]
Aswellascollagen,elastin,andproteoglycansthelungparenchymaalsocontainscells thatarebothaffectedbyandcontributetothe biomechanicsofthelung.Priortosingle-cell mRNAstudies,around40differentcelltypes wereidentifiedwithintheparenchyma,with potentialinteractionthroughthestructureof theECM [60].
Contractilecellsthatareknowntobepresentinthelungparenchymaincludefibroblasts andmyofibroblasts [49] andsmoothmuscle cellsthatarepresentinthesmallairways, bloodvessels,andalveolarducts.Itisofinterestthat α-actinhasbeenidentifiedinalveolar septa,consistentwithapotentiallocalized responsetoCO2 levels,promotingefficientgas exchangebymodulatinglocalventilationperfusionrelationshipsinthelung [68]
Traditionally,ithasalsobeenrecognized thatepithelialandendothelialcellsmaycontractbutthattheforcesarerelativelysmall [60] However,thisispotentiallyanoversimplification,giventherecognitionthatendothelial [44] andepithelial-mesenchymaltransition [41 43] mayoccurinthelunganddevelopinginsights fromsingle-cellmRNAstudies [49].Itisincreasinglyrecognizedthatthereisconsiderable
cellularplasticityincellsoftheparenchyma, whichmaybeadeterminantofthebiomechanicsofthefunctionallung.Animportantfunctionofparenchymalcellsistotakepartin growthandintheresponsetoinjuryand repair.Thiscanincludetheproductionof matrixcomponents,includingcollagenfrom fibroblastsandmyofibroblasts.Itisrecognized thattheproductionofkeygrowthfactors,such asthetransforminggrowthfactorbetasuperfamily,arealteredbymechanicalforcessuchas stretch,andstretchhasbeenshowntostimulate EMTintype2alveolarepithelialcells [69].The indirect,longer-termmaintenance,remodeling, andrepairfunctionsofparenchymalcellsare thereforelikelytorepresentanimportantdeterminantoflungmatrixbiomechanics.
Bioengineeringinthelungsiskeyto function
Thestructureoftherespiratorysystem, includingitscellsandmatrix,ismaintained byasupportivenetworkofinterdependent bioengineering.Thisinturncontributestothe inherentelasticpropertyofthelungsandcharacteristicsofairflowduringnormalinspiration andexpiration.
Theelastictendencyofthelung,which favorscollapseofthestructure,isheldincheck bythemechanicsofthechestwall,whichhas anopposingtendencytospringoutwards.At theendofanormaltidalbreath,thereisthereforeabalancebetweenthecontractionof thelungandtheopposingexpansionofthe chestwallwithnomusculareffortrequired. Breathingatthislungvolume,whichisdeterminedbytheintegratedbiomechanicsofthe lungs,isthereforeveryefficient,minimizing theworkofbreathing.
Thebalancedopposingforcesofthelung andchestwallmechanicsgenerateanegative pressurearoundthelung,maintainingitina stretchedstate [35].Thevitalconnections
betweenthechestwallandthelungsare maintainedbythesupportingconnectivetissue structuresofthelung.Thisremarkablethreedimensionalscaffoldisestablishedbyacontinuumoffibersthatinterconnecttheentirelung fromthechestwallandpleuratothemajorairways.Thiscanbeconsideredathree-partintegratedsystem [39].
Theairwayaxialfibersystemisanchoredto theairwaysandextendsfromthehilum,where themainbronchusentersthelung,through tothealveolarductsandacini,forminga branchedaxialscaffoldsupportingtheairways.
Thisiscomplimentedbytheperipheral fibersystem,whichoriginatesfromthevisceral pleuraconnectingthelungparenchymaand theperipheryoftheacini.
Finally,theseptalfibersystemwithinthe alveolarwallsisinterwovenwiththecapillary network,andthisisinterconnectedtoboththe axialandtheperipheralfibersystems.Overall, therefore,thisestablishesacontinuumofstructuralfibersthatphysicallyandfunctionally connectthelungtothemechanicsofthechest wallwhilealsoprovidingincrediblelevelsof structuralintegritythroughoutthemechanical distentionandrelaxationcyclesofthefunctionallung [66].Thisfibersystemisunderconstanttensionthroughtheoutwardpullofthe visceralpleura,whichistransmittedtotheseptalfiberandaxialfibersystems.Thelunghas thereforebeendescribedasatensegritystructure [39,70],inwhichthefunctionalityisintrinsicallydependentonthemechanicaltensions thatarepresentinthefibercontinuum.
Emphysema,commoninpeoplewithchronic obstructivepulmonarydisease(COPD),and recognizablewithlungimaging,isinformative whengaugingthefundamentalimportanceof thesestructurestothefunctionallung.Thecentrilobularformofemphysemaisconsistentwith thesnappingoftheaxialfibersystemofthe normalfunctionallung,leadingtocollapseof theperipheralairwaysandprofoundlydeleteriousairflowlimitation.
Theexquisitebalanceofthissystemandthe importanceofappropriatelyconsideringand researchingtheroleofbiomechanicsinthe functionalhumanlungarefurtherillustrated byotherpathophysiologicalstudiesinpeople withCOPD.Theseindicatethatarelativeloss oftissueelasticityintheabsenceoffrank emphysemacanoccurwithoutcompletefiber breakdown.Carefulmorphometricanalysesof tissueinwhichelasticfiberswerevisualized byusingvanGiesonstaininghaveshoweda quantitativelossofelastinvolumefraction inthesmallairwaysandalveoliofpeople withCOPDandthatthevolumefractionof elastinfiberscorrelatedwithlungfunction. Complementaryconfocalmicroscopyataqualitativelevelshowfibersincontrollungparenchymathatmarkedtherimsofalveoli.In COPDtheseweregenerallyfewer,withthinner elasticfibers [71]
Thestructuralpropertiesofthecollagen andelastinfibernetworkarethereforekey mechanicaldeterminantsofacinarandalveolar mechanicsandthefunctionallung [58].Afurtherimportantcomponentofthisoverall structure-functionrelationshipisrepresented bysurfacetensioninthealveolarspacewhich wouldfavorcollapseofthealveolarcomplex. Thesupportingarchitectureofthecollagen andelastinfibersdoesnotfullyprotectagainst thesurfacetensionsthatformwhenairfilled alveoliaresurroundedbyaliquidfilmof bloodwiththealveoliconnectedtotheairway systemfullofair.Thetendencyforalveolito collapseparticularlyatlowlungvolumesis preventedbythesecretionofanalveolarlining fluid,whichisabilayerofawateryphase toppedbyphospholipidsurfactantsecreted fromtype2alveolarepithelialcells [39,72].The biophysicalpropertiesofsurfactantmeansthat surfacetensionisdecreaseddynamicallyasit becomescompressedatlowlungvolumes [73]. Whenitisneededmostfromthepointofview oflungmechanics,thatis,whenthealveoliare attheirmostvulnerabletocollapse,surface