Syngas Purification Processes for Coal Gasification Systems Makoto Kobayashi
https://ebookmass.com/product/dry-syngaspurification-processes-for-coal-gasificationsystems-makoto-kobayashi/
More products digital (pdf, epub, mobi) instant download maybe you interests ...
Handbook of Gasification Technology: Science, Processes, and Applications James G. Speight
https://ebookmass.com/product/handbook-of-gasificationtechnology-science-processes-and-applications-james-g-speight/
Advances in Synthesis Gas: Methods, Technologies and Applications, Volume 2: Syngas Purification and Separation Mohammad Reza Rahimpour
https://ebookmass.com/product/advances-in-synthesis-gas-methodstechnologies-and-applications-volume-2-syngas-purification-andseparation-mohammad-reza-rahimpour/
Marine Ecology: Processes, Systems, and Impacts 2nd Edition
https://ebookmass.com/product/marine-ecology-processes-systemsand-impacts-2nd-edition/
Advancing Information Systems Theories: Rationale and Processes 1st Edition Nik Rushdi Hassan
https://ebookmass.com/product/advancing-information-systemstheories-rationale-and-processes-1st-edition-nik-rushdi-hassan/
Entropy of Complex Processes and Systems Eugene Barsky
https://ebookmass.com/product/entropy-of-complex-processes-andsystems-eugene-barsky/
Plasma Etching Processes for CMOS Devices Realization 1st Edition Nicolas Posseme
https://ebookmass.com/product/plasma-etching-processes-for-cmosdevices-realization-1st-edition-nicolas-posseme/
Dry Eye Disease Anat Galor
https://ebookmass.com/product/dry-eye-disease-anat-galor/
Coal Geology 3rd Edition Larry Thomas
https://ebookmass.com/product/coal-geology-3rd-edition-larrythomas/
Application, Purification, and Recovery of Ionic Liquids 1st Edition Olga Kuzmina
https://ebookmass.com/product/application-purification-andrecovery-of-ionic-liquids-1st-edition-olga-kuzmina/
DrySyngasPurificationProcesses forCoalGasificationSystems
MAKOTOKOBAYASHI
AssociateVicePresident, EnergyEngineeringResearchLaboratory, CentralResearchInstituteofElectricPowerIndustry, Yokosuka,Japan
Elsevier
Radarweg29,POBox211,1000AEAmsterdam,Netherlands TheBoulevard,LangfordLane,Kidlington,OxfordOX51GB,UnitedKingdom 50HampshireStreet,5thFloor,Cambridge,MA02139,UnitedStates
Copyright © 2021MakotoKobayashi.PublishedbyElsevierInc.Allrightsreserved.
Nopartofthispublicationmaybereproducedortransmittedinanyformorbyany means,electronicormechanical,includingphotocopying,recording,orany informationstorageandretrievalsystem,withoutpermissioninwritingfromthe publisher.Detailsonhowtoseekpermission,furtherinformationaboutthePublisher’ s permissionspoliciesandourarrangementswithorganizationssuchastheCopyright ClearanceCenterandtheCopyrightLicensingAgency,canbefoundatourwebsite: www.elsevier.com/permissions.
Thisbookandtheindividualcontributionscontainedinitareprotectedunder copyrightbythePublisher(otherthanasmaybenotedherein).
Notices
Knowledgeandbestpracticeinthis fieldareconstantlychanging.Asnewresearch andexperiencebroadenourunderstanding,changesinresearchmethods,professional practices,ormedicaltreatmentmaybecomenecessary.
Practitionersandresearchersmustalwaysrelyontheirownexperienceandknowledge inevaluatingandusinganyinformation,methods,compounds,orexperiments describedherein.Inusingsuchinformationormethodstheyshouldbemindfulof theirownsafetyandthesafetyofothers,includingpartiesforwhomtheyhavea professionalresponsibility.
Tothefullestextentofthelaw,neitherthePublishernortheauthors,contributors,or editors,assumeanyliabilityforanyinjuryand/ordamagetopersonsorpropertyasa matterofproductsliability,negligenceorotherwise,orfromanyuseoroperationof anymethods,products,instructions,orideascontainedinthematerialherein.
LibraryofCongressCataloging-in-PublicationData
AcatalogrecordforthisbookisavailablefromtheLibraryofCongress
BritishLibraryCataloguing-in-PublicationData
AcataloguerecordforthisbookisavailablefromtheBritishLibrary
ISBN:978-0-12-818866-8
ForinformationonallElsevierSciencepublicationsvisitour websiteat https://www.elsevier.com/books-and-journals
Publisher: BrianRomer
AcquisitionsEditor: GrahamNisbet
EditorialProjectManager: NaomiRobertson
ProductionProjectManager: PremKumarKaliamoorthi
CoverDesigner: MarkRogers
TypesetbyTNQTechnologies
Preface
Thehighnecessityofreconcilingfossilfuelutilizationandenvironmental protectionencouragestechnicalinnovationintheenergyconversionof fossilfuel.Coalisattheheadofthetargetsofinnovativeutilization,and thuscoalenergyconversionbasedonthegasificationprocessisbeing extensivelyinvestigated.Thegasificationprocessconvertssolidcoalinto syngasthatshouldgothroughacontaminant-removalprocessbeforebeing usedinspecificapplications.Thedrysyngaspurificationprocessforcoal gasificationsystemshasahistoryofdevelopmentofnearlyahalf-century,and nowitisenteringaneweraofactualapplication.Theprocesshaslarge advantagesbothinattaininghigherthermalefficiencyoftheapplication systemsandinperformingsimultaneousremovalandseparationoftheimpuritiesinsyngas.Undertheprohibitivecircumstancesofcoalutilizationin conventionalsystems,therearevariousattemptstodevelopanadvancedcoal utilizationsystemthatreconcilestherequirementforattaininghigherthermal efficiencywiththeneedtoreducetheemissionofcarbondioxide,inwhich thedrysyngaspurificationprocessisanessentialkeytechnology.Despitethe potentialadvantagesandlonghistoryofresearchanddevelopment,the processhasnotyetresultedinapracticaltechnologyforsuchapplications.
Theintentionofauthoristoprovideessentialandpracticalinformation thatwillaidindevelopingthenewprocessofdrysyngaspurificationfora plantwiththespecificpurposeofcoalutilization.Thedevelopmentofthe drysyngaspurificationprocesshasrequiredalongstepwiseprogressionin technologyfromthelaboratoryscaletoanactualplant,thesameasistrue forotherproceduresinotherchemicalplants.Therefore,acoherentprocedurefordevelopmentisrequiredtoestablishtheprocessforapplicationin aspecificsystem.Theauthorhasconsistentexperienceindevelopingdry purificationsorbents,maneuveringvariouslaboratoryinstrumentsfor evaluatingsorbentperformance,revealingbehaviorandmechanismsofthe relevantreactionsonthesorbentsinasyngasenvironment,measuring amountsandspeciesofcontaminantsinsyngasfromagasifier,andoperatingbench-topilot-scaleplantsforthedrysyngaspurificationprocess. Thisexperienceissupportedbyacoherentdevelopmentofintegrated gasificationcombinedcycle(IGCC)powergenerationsystems.The experiencebroughtastrongmotivationtowritethisbookonthisspecific processwithawiderangeofapplications.
Theroleofthesyngaspurificationprocesscanbecomparedwiththe cookinginhousework.Theoldkitchenrangeoperatedon firewood,which isananalogyforthecoalgasifier,bakedandboiledrawfoodtoprepare dishes.Inordertoprovidedeliciousdishes,awell-organizedarrangementof rawfoodisimportant;washing,removingseeds,peeling,andlightseasoning areexamplesofthearrangement.Thetasksaresimilartotherolesofthe syngaspurificationprocess:removalofdustandimpurities,moisture retainment,compositionadjustment,etc.toensurethatthedisheswillsatisfy theappetitesifvariouspeoplewithtastesrangingfromsimpletopampered. Well-organizedsyngascanbeappliedtovarioustypesofapplications includingcombustorsandgasturbinesinthecaseofintegrated-gasification combined-cyclepowergeneration,high-temperaturefuelcells,andcatalytic synthesisprocesses.Iacquiredtheseimpressionsaboutthecourseofoperating benchorlarger-scaleplantsofthedrysyngaspurificationprocessfrommy workonthissubjectstartinginthe1980s.NowIfeelthattherolecanquite comfortablyattainasetofsolutionsfortheappropriateconfigurationand operationoftheprocess.Consequently,Ifeelthatthetimeisripefor summarizingawholesetofsystematictextbookdiscussionsofthe comprehensivetechnologyfordevelopingthedrysyngaspurification process.
Processoperabilityisakeyissueinestablishingthedrysyngaspurificationprocessasapracticaltoolforaspecificapplication.Maneuverability oftheprocessis,however,notonlyanissueintheinstrumentationofthe plant,butalsodeeplyassociatedwiththeconceptandphilosophyofthe processdevelopment.Aprocessconfigurationthatdependsondevelopmentandmanufacturingofsorbent,selectionofreactortype,samplingand measurementofimpurities,andcontrolstrategiestotallydeterminesthe maneuverabilityduringtheactualoperationofthesyngaspurification process.Thisbookprovidesconcretecasesofthoseelementsforimproving theoperabilityoftheprocess,whicharebasedontheexperienceofthe author.Readersmay findthattheintegrateddrysyngaspurificationprocess issomethinglikeacompletedjigsawpuzzlethatmaturelycombinesthe elementsofimprovement.
Readersmightfeelthatthecompletedprocessisdedicatedonlytothe specificapplicationforwhichtheauthordevelopedit.Althoughtheskillful manipulationofsorbentstructure,processconfiguration,andside-reaction controlthatprovidesthebestpracticeforestablishingthepotentialprocessesseemstonarrowitsapplicationtothespecificsystem,thecoherent conceptofprocessdevelopmentiscommonfororiginatingnewprocesses
appliedtootherspecificsystems.Therefore,alltheelementsofprocess improvementthataredeployedinthisbookwillactasversatiletoolsforthe reader’sactivityinthe fieldofsyngaspurification.
Variousindustrialapplicationswillenjoythefruitsthataredescribedin thisbook,whichisthemostprominentstatementfromtheauthor.Each elementofthisbookhasitsownfoundationinscientificpapersordoctoral thesisandrelevantliterature.Whenreadersfeelthatthecontentofthis bookistoosimpleorgeneral,theycanobtainmorespecificandtechnical informationfromtheavailableliteraturepublishedmostlysince2010. Althoughsomeoftheillustrationsorgraphsdisplayedarecitedfrompreviousworks,themajorpartofthe figuresandtablesarenewlyprepared, dedicatedforthisbook.Finally,IappreciatethatIamabletopresent comprehensiveinformationonthedrysyngaspurificationprocessforthe firsttimeinabookcomprisingasystematicandconsistentstoryofthe developmentoftheprocess.
MakotoKobayashi June2020
Acknowledgments
Whenthemaidenvoyageofthebench-scaletestplantofthedrysyngas purificationprocesstookplaceatthesiteofapressurizedcoalgasification plantinNagasaki,Japan,itfeltlikethebrideandbridegroomweregoingto openthedoortothenewlifeofcoalutilization.Thesuccessfuloperationof theirbridaljourneyencouragedmetowritethisbook,asherfosterfather, torecordandprovideinformationonalltheproceduresofherupbringing. Hergrown-uplifewasactuallyalongsequenceofeverydayaffairs,whichis alaminatedpieceofexperiencethatmakesa firmbaseofconfidenceforher activity.Thegreatandunremittingendeavorofhergrowthwascertainly supportedbysomanypeoplethatitisnotpossibletointroduceallofthem here.Iwouldliketoacknowledgeallthepersonsinvolvedinthisendeavor byexpressingmygratitudeforthespecificcontributionsofonlyfewof themhere.
Theoperationofthebench-scaleplantwasaccomplishedbythe teamworkofselectedmembersofCRIEPI,whoincludeDr.Hiroyuki Akiho,Dr.YasushiOzawa,AkiraNakajima,YoshiakiHashimoto,Kazuki Tainaka,AtsushiIkeda,andKojunSuzuki.Theymadeexceptionalefforts inpreparing,maintaining,andoperatingalloftheanalyticalinstruments, theutilityfacilities,andthemainplantofthedrysyngaspurificationprocess.Theiruntiringeffortsresultedinthevaluablesuccessofthecontinuous operationoftheplantwithoutinterruption.
Thepressurizedcoalgasificationplantcollaboratedwiththebench-scale plantinthesyngasprocessingtest.TheO2/CO2-blowngasificationtestwas directedbyKatsuhikoYokohamaandDr.RyuheiTakashima,andweekly gasifieroperationwasconductedbymanyresearchengineersatMitsubishi HeavyIndustries,Ltd.(MHI).Installation,piping,andallotherengineering fortheoperationandexperimentofthebenchplantweresupportedwith skillfulmaneuveringintheplantbyYasuoSouda,TomohiroYamauchi, GenSakashita,andYasuyukiMiyataatMHIthroughouttheproject. ThanksalsogotoKenjiKawanaka,TakanoriYamaguchi,andTomoharu NakaoofMHIST,whotookcareofthebench-scaleplantduringthe preparationandoperation.
Tremendousnumbersofexperimentswereconductedtoevaluate, compare,investigate,anddemonstratetheperformanceanddurabilityof variouskindsofsorbentsbyoperatingthe fixed-bedreactorinstrumentson
thesimulatedsyngasonanalmostdailybasisforoveradecade.Thereactor instrumentsshouldbeoperatedwithvariousnumbersofanalyticalinstrumentstogetherwithagassupplyfacilityandanexhaust-incinerating facility.ThetoughworkwasdonebyTetsuoOkabe,RyueiSato,and ChikaraNakazawaofElectricPowerEngineeringSystemsCo.,Ltd.,who exertedallpossibleeffortsothattheexperimentalinstrumentswere properlyandsafelyoperatedunderthespecifiedconditionsofsorbent reaction.
HiroakiTadaatMitsubishiHitachiPowerSystems,Ltd.,hascontributedtothisbookinprovidinghisexcellentknowledgeofintegrated gasificationcombinedcycle(IGCC)powergeneration.Hecheckedand reviewedthespecificpartsofsystemconfigurationandtheirillustrationin therelatedpapersthatwerereferredtointhisbook.
MycolleagueDr.YoshinobuNakaohassupportedmebyprovidinghis excellentknowledgeofthermalefficiencycalculationbysystemanalysisof IGCCsystems.Dr.KenjiTannoledmeinperformingcomputational simulationsonsyngas flowinthehoneycombsorbent bedreactorwithhis superiorskillincomputational fluiddynamicsmodelingandsimulation.
ProfessorShozoKanekoencouragedustoconcentrateondeveloping thedrysyngaspurificationprocessforIGCCpowergenerationsystems.He recognizedtheimportanceofdrysyngaspurificationaccordingtothe potentialefficiencyenhancementofcoalgasification basedpower generation.
Duringtheoxy-fuelIGCCproject,collaborationwithProfessorYuichi Fujiokabroughtusadeepunderstandingofthecarbondepositionphenomenoninazincferritesorbentusedforsulfurremoval.Healsosuggested thedemonstrationofthedrysyngaspurificationprocessattheresearch field ofMHIinNagasakifromhislongexperienceinthedevelopmentofcoal gasificationsystems.
IalsowouldliketoexpressspecialthankstomyseniorcolleaguesDr. HiromiShiraiandDr.ShigeoItofortheirwarmandconcentrateddirection oftheresearchactivityatCRIEPI.Finally,mysupervisors,Professor MasaruIshidaandProfessorKozoShinoda,aregreatlyacknowledgedfor theirwarminstructionthathasnurturedmystrongmotivationandfaithful positionasaresearcher,whichledmetocompletetheseriesofconsistent worksthatconvergedintothisbook.
MakotoKobayashi June2020
CHAPTER1 Introduction
Prologue:Coalgasificationisanidealprocesstoconvertsolidfueltoa gaseousfuelthathashighercompatibilitywithmodernenergysystems. Rawsyngas,however,hasthedisadvantageofcontaminationwithimpuritiesderivedbyvariouselementsinthefedcoal.Accordingtothenatureof thoseimpurities,theymustberemovedbyanefficientsyngaspurification processinordertoestablishsyngasutilizationfacilities.Requirementsfor thesyngaspurificationprocessaresummarizedasanefficientconcentrator ofimpuritiesinsyngasandareducerofby-productsofwastefromplant operations.Thenewlydevelopingdrysyngaspurificationprocesshasthe potentialtorealizeanefficientconcentratorbyminimizingconsumed materialsanddischargedwastesincontrasttotheconventionalwetprocess. Requiredtaskstoestablishthedrysyngaspurificationprocessaresummarizedinthissectionbasedonhistoricaldevelopmentofremovalsorbents forthedryprocessandmeasuresforabatementofthevarioussyngas impuritiesproducedfromthecoalgasificationprocess.Akeyissueforthe drysulfurremovalsorbent,forexample,istoreconcilethechemical equilibriumlimitationforachievablesulfurconcentrationandtheeliminationofharmfulsidereactionsbyloweringtheoperationaltemperatureof theprocessusingthesorbent.
1.1Coal-derivedsyngas
Fluidizationofsolidfuel,typicallycoal,isanessentialprocedureforefficientconversionofthefueltosyngas,chemical,liquidfuel,andcarbonbase materials.Coalcarbonization,anotherwordfordrydistillationofcoal,was anancienttechnologyforproducingcoalgastouseforheating.The carbonizationprocessdecomposescoalintogas,liquid,andsolidphases; coalgas,coaltars,andcokesareitsmainproducts.Coal-derivedsyngasisa gaseousfuelobtainedfromthecoalgasificationprocessandcanbeproducedbyvarioustypesofgasifierssuchasentrained flow, fluidizedbed,and fixedbed.Gasificationprocessesthatconvertcoalintogaseousfuelare developedtomaximizeconversionofthecalorificvalueofthefedcoalto
DrySyngasPurificationProcessesforCoalGasificationSystems
ISBN978-0-12-818866-8
https://doi.org/10.1016/B978-0-12-818866-8.00001-X
© 2021MakotoKobayashi. PublishedbyElsevierInc. Allrightsreserved. 1
thesyngas.Agasifierwithhighlyefficientperformanceachievesgreater than99%conversionofthecarboninthefedcoaltosyngas.Theresidue fromrawcoalinsuchhighlyefficientgasificationprocessisideallyglassified ashcalledslag.Amaingasifyingagentisusuallyselectedfromoxygenorair, andsecondarygasifying-agentsofsteamandcarbondioxidecanbeaddedas variationsofthegasificationprocess.Thesyngasfromtheoxygen-blown gasificationprocesscontainscarbonmonoxide,hydrogen,carbondioxide, andsteamasmajorcomponents.Anair-blowntypegasifierwillproduce syngassimilartosyngasproducedbyanoxygen-blowngasifier,butitis dilutedbyN2 suppliedtothegasifierbythemaingasifyingagent,air. Table1.1 summarizesthetypicalsyngascomponentscollectedfromthe operationsoftypicalcoalgasificationprocessesatpilotorcommercial-scale plants.Thegascompositionofthesyngasmainlydependsontheconstituentsofrawmaterials,whichincludecoal,coal-feedingmedia,andgasifyingagents.SyngasgenerallyexhibitshigherconcentrationsofCOandH2 whenitisderivedfromtheoxygen-blownanddry-coal-feedingtypeof gasifier.Thegasifierslistedinthetablecoverawiderangeoftechnology thatcombinesreactortypes,coalfeedingmethods,andgasifyingagents. Theentrained flowgasifierhasthewidestvariation;theDOWpressurized [1],Texacotypepressurized[2],Shellpressurized[3],PRENFLO[4,5], Koppers Totzec[6],andMHIpressurizedtwo-roomandtwo-stage[7] entrained flowgasifiersaresummarizedinthetable.The fixed-bedpressurizedgasifierwasdevelopedbyBritishGasandLurgi[8,9].ThehightemperatureWinklerisauniquecirculating fluidizedbedgasifier[10].In additiontothehistoricalgasifierslisted,thesimulatedsyngascompositionof arecentlydevelopedgasifierthatrealizesanewconcept,agasifyingagent usingoxygenandcarbondioxidetoenhancethegasificationreaction,has beenaddedtothetable[11].
Becausecoalconversioninagasifierislimited,atcertainlevels,toone passthroughthereactor,syngasatthegasifieroutletcontainsunreacted carbonparticles,so-calledchar.Capturingofcharanditsrecirculationto thegasifierisaroutineprocedureofamoderngasifierthatexhibitshigher conversionofcoal.Thetypicalcharrecycleprocedureisillustratedin Fig.1.1 togetherwiththebasiccoalgasificationmechanismsinreactors. Syngasisproducedbythegasificationreactionsthatconvertmajorcomponentsofcoal:carbon,hydrogen,andoxygen.Theultimateconversionof coaltosyngascalculatedbycarbonbaseexceeds99%whenthecharrecycle strategyisapplied.Thehighconversionofcoalensuressufficientcalori fic
Table1.1 Observedgascompositionsofsyngasproducedbyvariousgasifiers.
Gasifiername DOW pressurizedTexacoShell
Gasifi ertypeEntrained flowEntrained flowEntrained flow
SupplierDOWchemicalShell CoalfeedingSlurryfedSlurryfedDryfed GasifyingagentOxygenOxygenOxygen Syngascomp.Drybase,vol%Drybase,vol%Drybase,vol% CO38.541.965.1
Reference[1][2][3]
GasifiernamePlenfloKoppers TotzecMHI,air-blown
Gasifi ertypeEntrained flowEntrained flowEntrained flow
SupplierGKTEssoMHI
CoalfeedingDryfedDryfedDryfed GasifyingagentOxygenOxygen þ steamAir Syngascomp.Drybase,vol%Wetbase,vol%Wetbase,vol% CO62.337.420.0
þ
Reference[4,5][6][7]
GasifiernameBGLHTWMHI,O2/CO2blown
Gasifi ertypeFixedbedFluidizedbedEntrained flow
SupplierBritishGas, Lurgi RheinbraunAGMHI
CoalfeedingSlurryfedDryfedDryfed GasifyingagentSteam þ oxygenAir þ steamOxygen þ CO2 Syngascomp.Drybase,vol%Drybase,vol%Wetbase,vol% CO59.639.653.7
5.0 N2 þ Ar2.60.71.4
Reference[8,9][10][11]
Theindividualreferencesarelistedinthetablebybracketednumbers.
Figure1.1 Charrecycleinanair-blowntypegasifierandgasificationfunctioninthe reactor. (Citedfromhttps://www.mhps.com/products/igcc/index.html.,Copyright(2020), withpermissionfromMitsubishiHitachiPowerSystems,Ltd.)
valueofsyngasforfurtherutilizationinthegasificationplant,whichalso impliesthatimpuritiesintherawmaterialalsoconvertedtocomponentsin syngasasvariousimpurities.
Araredisadvantageofthecoalgasificationprocessisthatvariouselementsincoalarealsoconvertedtosyngasascontaminants.Sucha contaminantisaso-called “impurity” asageneraltermandisused extensivelyinthisbook.Typicalimpuritiesinsyngasarelistedin Table1.2; thelistrepresentstheminorcomponentsincoalthatareconvertedto gaseousandvolatilecomponentsinsyngasthroughthegasificationprocess. Thoseimpuritiesareconsideredtohaveharmfuleffectsduringthesyngas utilization.Therefore,theremovalofimpuritiesfromsyngasisanessential procedureforestablishingasyngasutilizationfacility.Thesyngaspurificationprocesspotentiallyplaysvariousimportantrolesintheimpurity removalprocedureforthesyngasutilizationfacility.Thesyngaspurification processcanbecategorizedintwotypicaltypesofprocesses,wetanddry. Basically,thewetsyngaspurificationprocess,whichisoperatedatambient orlowtemperature,washessyngaswithliquidsolventtoremoveimpurities.Thedrysyngaspurificationprocessistypicallyoperatedatanelevated
Table1.2 Typicalimpuritiesinsyngas.
ImpuritytypeExampleApprehendedeffects
SulfurcompoundsH2S,COS
HydrogenhalidesHCl,HF
MetalcarbonylFe(CO)5,Ni(CO)4
Nitrogen compounds
NH3,HCN,NO,N2O
AlkalimetalsNa,K
HeavymetalsHg,Cr,Mn,Zn,etc.
TarsBenzene,naphthalene
OxygenO2
OtherelementsAs,Se,B,etc.
• Corrosion,emissionto environment
• Corrosion,reactionwith ammonia(NH3)
• Depositandaccumulation downstream
• Emissiontoenvironment
• Corrosion,deposits downstream
• Depositsdownstream, emissiontoenvironment
• Coking,deposits downstream
• Oxidationofsyngas
• Effectsinherentinthe naturesoftheelements
temperaturesothatthesolidsorbentisabletoabsorbimpuritiesefficiently. Featuresandcharacteristicsofthosetwotypesofsyngaspurificationprocessesarefurtherdescribedin Sections1.3 and2.2.Letuslookintothe distinctivecharacterofimpuritiesinsyngasinthemeantime.Acidgas components,sulfurcompounds,andhydrogenhalidesaremajorimpurities thatareapprehendedtocausecorrosionoftheequipmentinsyngasutilizationfacilitiesdownstreamofthecoalgasificationprocess.Sulfurcompoundsmainlyexistintheformshydrogensulfide(H2S)andcarbonyl sulfide(COS)insyngas.Theequilibriumreactionbetweenthetwosulfur compoundsdeterminestheirratio.Roughlyfour-fifthsofsulfurcompoundsexistasH2SandtherestasCOS.Thecompositionisimportant becauseattainingalowconcentrationofCOSisusuallyadifficulttaskfor bothwetanddrysulfurremovalprocesses.Therefore,COSrequiresspecial attentiontothesufficientperformanceofthesyngassulfurremovalprocess. Hydrogenhalidesengenderconsiderablefearbecauseoftheircorrosive nature.Becausehydrogenhalidesarehighlysolubletowater,itisrather easytoremoveimpuritiesduringthewetprocess.Thedryhalideremoval processexploitstheadvantageofhigherreactivityofhalideremovalsorbents.Itisknownthatsyngascontainsquitealowlevelofmetalcarbonyls asgaseousimpurities.Becausetheyaredecomposedinaspecificcondition,
theycausedepositsdownstreamoftheplant.Compoundsofnitrogen(N)in syngasarerathercomplicatedbecausenitrogenatomtakesonvarious oxidationnumbersandproducesvariouscompounds.Majornitrogen compoundsinsyngasareammonia(NH3),nitrogengas(N2),andhydrogen cyanide(HCN).Theothernitorgencompoundsarefoundasminorspeciesin theformsnitrogenmonoxide(nitricoxide,NO)anddinitrogenmonoxide (nitrousoxide,N2O).NH3 isaprimarysourceoffuelNOx whensyngasis combustedinagasturbineforpowergeneration,forinstance.N2 isalso convertedtoNOx,so-calledthermalNOx,atextremelyhightemperatures inacombustion flameingasturbinefacilities.Althoughcountermeasuresfor NOxinacombustionprocessarepossiblewithconventionalmeasuressuchas low-NOxcombustionandcatalyticreductionofNOxfromexhaust,NH3 reductionfromsyngasisalsoanimportantissueforminimizingtheenvironmentalimpactofsyngasutilization.Alkalimetalsarevolatilewhilethe syngastemperatureissufficientlyhigh.Themetals,meanwhile,arehighly reactivetoformsaltssuchasNaClandKClwiththehalogeninthesyngas. Theyareideallyremovedwithcharcollectedatatemperaturefarbelowthe boilingpointofthemetalsandtheirproducts.Coalscontainalmostevery heavymetal,whilethebehaviorsofheavymetalsdependontheirnatures. Becausemercuryisvolatileandexistsasametalvaporinsyngas,itsabatement isanissueforsyngasutilization.Theotherheavymetalshavespecificvapor pressures,whicharenotashighasthatofmercury,tobecarrieddownstream ofthegasifier.Theymustbetreatedproperlyaccordingtotheirbehaviorin theplant.Tarssuchasbenzeneandnaphthalenearesomewhatdifferentfrom otherimpuritiesbecausetheirevolutionfromthegasifierishighlydependent onthetypeandconditionofthegasifieroperation.Astarscausecokingor depositioninsyngasutilization,theyshouldbeproperlyattendedpursuantto theirnature.Althoughtheremainingoxygeninsyngasisusuallyoflow concentration,itsoxidativenaturemayaffectthebehaviorofother components.Syngasutilizationmustaccountfortheexistenceofoxygenasa minorcomponent.Otherelementsinsyngas,whicharenotmentioned individuallyinthissection,haveinherentbehaviors.Therequiredmeasures forsuchelementsdependonthestrengthoftheirinfluenceontheplant operationandsurroundingenvironment.Utilizationofsyngasproduced throughgasificationtechnologyfacesvariousconcernsregardingimpurities. Treatingsyngaswiththedrygaspurificationprocesstoovercomeissues relatedtotheseimpuritiesisachallengingtask.Requirementsforthedry syngaspurificationprocessaredescribedinthenextsection.
1.2Requirementforsyngascleaningforcoalgasification systems
Requirementsforsyngascleaningaredefinedinthischapterasthesetof performancespecificationsforsyngaspurification.Here,therequirements aresummarizedasachievableconcentrationofimpurities,properconsumptionofchemicalsforoperation,reducedamountsofdischargedwaste, andplantsizeadequacy.Thoughotherfactorsarerequiredindesigningand constructingthesyngaspurificationprocessfromtheviewpointofengineering,theperformancespecificationsthatarethefocusofthissection illuminatethecontrastsbetweendryandwetsyngaspurificationprocesses. Comparisonoftheperformancespecificationofsyngaspurificationprocessesemphasizestheadvantageofthedrygaspurificationprocessoverthe wetprocess.Theimportanceandnecessityofthefourselectedrequirementsaredescribedinthissection.
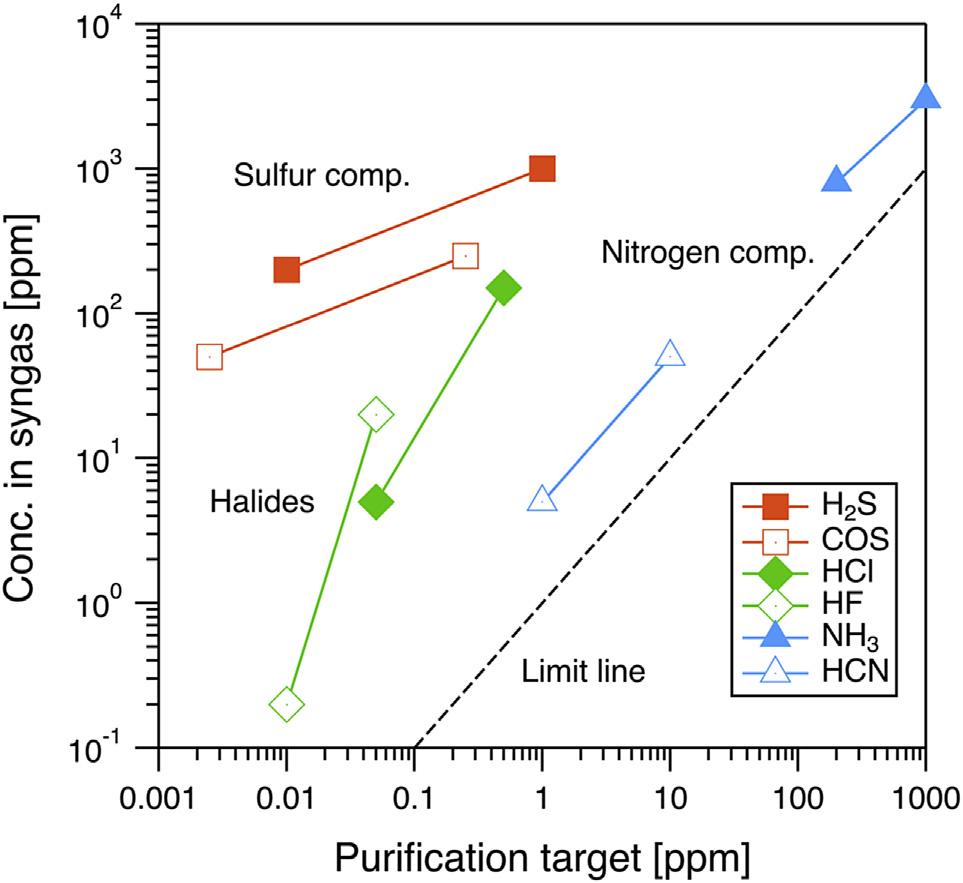
Themostimportantrequirementisanachievableconcentrationofan impurityinthepurifiedsyngas.Thisrequirementmayvaryaccordingtothe specificapplicationoftheprocess.Impuritiesinsyngasexhibitawiderange ofconcentrationsthatdependontheconditionsofsyngasproductionand natureofthefedcoal.Therelationbetweentherangesofimpurityconcentrationinsyngasandtheirtargetedvaluesinpurifiedsyngasareplotted in Fig.1.2 asanexampleofachievableconcentrationrequirements. Chemicalspecieswhoseremovaltargetisclearlydefinedareselectedfrom Table1.2.Itcanbeseenthatthepurificationprocessshouldreduceconcentrationsofsulfurcompoundsandhalidesbythreeto fiveordersof magnitude.Thisplotexpressesthetypicaltendencyinrequirementsfor syngascleaning.Sulfurcompoundswhoseconcentrationsinsyngasare relativelyhigharetobereducedstringently;theplotforsulfurisupperleft inthe figure.Nitrogencompoundsexistathigherconcentrationsthatare comparabletothoseofsulfurcompounds,whilethetargetconcentrationis notsosevere,asplottedtotheupperright.Finally,thehalidesconcentrationinsyngasislower,andstringentremovalisrequired,asshowntothe lowerleft.Thelimitlineshowsthedirectproportionlinebetweenthe syngasconcentrationandtargetedvalue.Itisobviousthattherequirement plotdoesnotgoacrossthelimitlinetothelowerrightregionbecausethe purificationtargetshouldbelowerthanthesyngasconcentration.
Regardlessofwetordry,thesyngaspurificationprocessshoulduse variouschemicalstocompletetheremovalandconcentrationofimpurities, immobilizationanddischarge,andwastetreatments.Minimizingthe
Concentrationsoftypicalimpuritiesinsyngasandtheirpurificationtargets.
consumptionofsuchchemicalsisaconsiderableissueinreducingthe operatingandlaborcostsofsyngaspurification.Thestrategiesforminimizingconsumptionofchemicalsaresummarizedintothreemethodologies:theuseofregenerableabsorbent,reductioninprocesssteps,and eliminationofby-productsorwaste. Fig.1.2 indicatesthattheimpurity concentrationinsyngasis0.1volumepercentatmost.Thevalueis significantlylow;if0.1volumepercentatsyngasprocessingconditionof 450 Cand2.7MPaingaugeisexpressedinmolarconcentration,itfallsto 4.7 10 4 mol/L thatvalueisreferredtoasthebaseconcentrationof syngaspurificationinthissection.Thesyngaspurificationprocessinherentlypossessesthefunctionalityofconcentratingimpuritiesbyremoving themandimmobilizingthecapturedimpurities.Theconcentrationofthe impurityintheimmobilizedfractionisthenmuchhigherthaninthebase concentration.Thisistheintrinsicnatureofthesyngaspurificationprocess; alltheprinciplesofthesyngaspurificationprocesssubstantiallyworkasa concentratorofimpuritiesinsyngas. Fig.1.3 illustratesthetypicalconcentrationtendencyofsulfurcompoundsthroughsyngastreatmentinadry sulfurremovalprocess.Itassumesthattherawsyngascontainssulfur compoundsat1000ppm,whichcorrespondsto4.7 10 4 mol/Las describedabove,underoperatingconditionsofadrysyngaspurification
Figure1.2
Another random document with no related content on Scribd: