Handbook on Miniaturization in Analytical Chemistry
Application of Nanotechnology
Edited by Chaudhery Mustansar Hussain
Department of Chemistry and Environmental Sciences, New Jersey Institute of Technology, Newark, NJ, United States
Elsevier
Radarweg 29, PO Box 211, 1000 AE Amsterdam, Netherlands
The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States
Copyright © 2020 Elsevier Inc. All rights reserved.
No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions
This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein).
Notices
Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary.
Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility.
To the fullest extent of the law, neither the Publisher nor the authors, contributors, or editors, assume any liability for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions, or ideas contained in the material herein.
Library of Congress Cataloging-in-Publication Data
A catalog record for this book is available from the Library of Congress
British Library Cataloguing-in-Publication Data
A catalogue record for this book is available from the British Library
ISBN: 978-0-12-819763-9
For information on all Elsevier publications visit our website at https://www.elsevier.com/books-and-journals
Publisher: Susan Dennis
Acquisitions Editor: Kathryn Eryilmaz
Editorial Project Manager: Lena Sparks
Production Project Manager: Kumar Anbazhagan
Cover Designer: Matthew Limbert
Typeset by SPi Global, India
Contributors
Numbers in parenthesis indicate the pages on which the authors’ contributions begin.
Monica Araya-Farias (35), Laboratoire Physico-Chimie Curie, CNRS UMR 168, Institut Curie, PSL Research University; Institut Pierre-Gilles de Gennes for Microfluidic (IPGG), Paris, France
Vivek B. Borse (297), NanoBioSens Laboratory, Centre for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati, Assam, India
Anirban Chowdhury (239), Metallurgical and Materials Engineering, Indian Institute of Technology Patna, Bihta, Bihar, India
Wendell K.T. Coltro (155), Instituto de Química, Universidade Federal de Goiás, Goiânia, GO; Instituto Nacional de Ciência e Tecnologia de Bioanalítica, Campinas, SP, Brazil
Amandha Kaiser da Silva (77), Institute of Chemistry, Federal University of Mato Grosso do Sul, Campo Grande, MS, Brazil
İbrahim Dolak (1), Vocational School of Technical Sciences, Dicle University, Diyarbakır, Turkey
Lucas C. Duarte (155), Instituto de Química, Universidade Federal de Goiás, Goiânia, GO, Brazil
Gerson F. Duarte-Júnior (155), Instituto de Química, Universidade Federal de Goiás, Goiânia, GO, Brazil
Szymon Dziomba (35), Department of Toxicology, Medical University of Gdansk, Gdansk, Poland
Merve Eryılmaz (139), Gazi University, Faculty of Pharmacy, Department of Analytical Chemistry, Ankara, Turkey
Vijay Kumar Garlapati (21,221), Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, Waknaghat, Himachal Pradesh, India
Fatemeh Ghorbani-Bidkorbeh (1,129,277), Department of Pharmaceutics, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Marta Gil (99), Department of Chromatographic Methods, Faculty of Chemistry, Maria Curie-Skłodowska University, Lublin, Poland
Chaudhery Mustansar Hussain (1,129,277), Department of Chemistry and Environmental Science, New Jersey Institute of Technology, Newark, NJ, United States
S.P. Jeevan Kumar (21), Agricultural & Food Engineering Department, Indian Institute of Technology Kharagpur, Kharagpur, West Bengal; ICAR-Indian Institute of Seed Science, Mau, Uttar Pradesh, India
Contributors
Rüstem Keçili (1,129,277), Department of Medical Services and Techniques, Yunus Emre Vocational School of Health Services, Anadolu University, Eskişehir, Turkey
Amogh Kodgi (221), Department of Biotechnology, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka, India
Aditya N. Konwar (297), NanoBioSens Laboratory, Centre for Nanotechnology, Indian Institute of Technology Guwahati, Guwahati, Assam, India
Kundan Kumar (239), Metallurgical and Materials Engineering, Indian Institute of Technology Patna, Bihta, Bihar, India
Fernando Mauro Lanças (77), Institute of Chemistry at São Carlos, IQSC-USP, University of São Paulo, São Carlos, SP, Brazil
Renato S. Lima (185), Brazilian Nanotechnology National Laboratory, Brazilian Center for Research in Energy and Materials; Institute of Chemistry, University of Campinas, Campinas, São Paulo, Brazil
Edvaldo Vasconcelos Soares Maciel (77), Institute of Chemistry at São Carlos, IQSCUSP, University of São Paulo, São Carlos, SP, Brazil
Naresh Kumar Mani (21,221), Department of Biotechnology, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka, India
Ninad Mehendale (21, 221), Department of Electronics, K. J. Somaiya College of Engineering, Mumbai, Maharashtra, India
Sunandan Naha (21), Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati, Assam, India
Carlos Eduardo Domingues Nazario (77), Institute of Chemistry, Federal University of Mato Grosso do Sul, Campo Grande, MS, Brazil
Caroline Y. Nakiri Nicoliche (185), Brazilian Nanotechnology National Laboratory, Brazilian Center for Research in Energy and Materials, Campinas, São Paulo, Brazil
Osvaldo N. Oliveira, Jr. (185), São Carlos Institute of Physics, University of São Paulo, São Carlos, São Paulo, Brazil
Malgorzata Olszowy (99), Department of Chromatographic Methods, Faculty of Chemistry, Maria Curie-Skłodowska University, Lublin, Poland
Kemilly M.P. Pinheiro (155), Instituto de Química, Universidade Federal de Goiás, Goiânia, GO, Brazil
Kariolanda C.A. Rezende (155), Instituto de Química, Universidade Federal de Goiás, Goiânia, GO, Brazil
Thiago Gomes Ricci (77), Institute of Chemistry, Federal University of Mato Grosso do Sul, Campo Grande, MS, Brazil
Surajbhan Sevda (21), Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati, Assam, India
Swati Sharma (21,221), Department of Biotechnology and Bioinformatics, Jaypee University of Information Technology, Waknaghat, Himachal Pradesh, India
Hardik Ramesh Singhal (221), Department of Chemical Engineering, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka, India
Contributors
Rohit Srivastava (297), NanoBios Laboratory, Department of Biosciences and Bioengineering, Indian Institute of Technology Bombay, Mumbai, Maharashtra, India
Yiğitcan Sümbelli (129), Eskişehir Technical University, Faculty of Science, Chemistry Department, Eskişehir, Turkey
Uğur Tamer (139), Gazi University, Faculty of Pharmacy, Department of Analytical Chemistry, Ankara, Turkey
Ana Lúcia de Toffoli (77), Institute of Chemistry at São Carlos, IQSC-USP, University of São Paulo, São Carlos, SP, Brazil
N. Thuy Tran (35), Université Paris-Saclay, CNRS, Institut Galien Paris-Saclay, Châtenay-Malabry, France
Özlem Biçen Ünlüer (277), Eskişehir Technical University, Faculty of Science, Chemistry Department, Eskişehir, Turkey
Dorota Wianowska (99), Department of Chromatographic Methods, Faculty of Chemistry, Maria Curie-Skłodowska University, Lublin, Poland
Ender Yıldırım (139), Middle East Technical University, Department of Mechanical Engineering, Ankara, Turkey
Era of nano-lab-on-a-chip (LOC) technology
Rüstem Keçilia, Fatemeh Ghorbani-Bidkorbehb, İbrahim Dolakc, and Chaudhery Mustansar Hussaind
aDepartment of Medical Services and Techniques, Yunus Emre Vocational School of Health Services, Anadolu University, Eskişehir, Turkey, bDepartment of Pharmaceutics, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran, cVocational School of Technical Sciences, Dicle University, Diyarbakır, Turkey, dDepartment of Chemistry and Environmental Science, New Jersey Institute of Technology, Newark, NJ, United States
1. Introduction
Lab-on-a-chip (LOC) systems are defined as miniaturized laboratories that process many specific analyses (i.e., biochemical detection or DNA sequencing) on a chip platform [1–14]. Most of the research on LOC platforms basically focuses on the design and development of diagnostic systems and analysis of DNA. Less often, researchers also make an effort to apply LOC technology to the organic synthesis of chemicals [15, 16].
In recent years, so much effort has been put into miniaturization, which is a widely applied biological and chemical process, offering various superiorities compared to macroscopic systems [17–20]. The time for mass transfer and heat is reduced in microfluidic channels because of the shorter distances in miniaturized systems. On the other hand, the high surface-to-volume ratio also provides homogeneous heat distribution. Miniaturization of various functional units (i.e., reaction, separation, detection, etc.) enables the serial processing of samples as well as the parallelization of the process for high throughput. The term “miniaturization” is not only associated with the decrease in size but is also linked to the various challenges in analytical systems, including automation, rapid analysis, operational cost, portability, etc.
This chapter provides a comprehensive overview of LOC technology. The chapter starts with a description and brief history of LOC technology. Then, the latest advancements and studies in the field of miniaturized systems based on LOC technologies are presented.
2. L ab-on-a-chip technology
In recent years, there has been great effort in the development of novel approaches for the sensitive detection and treatment of diseases and ailments. Currently, the detection of various diseases is carried out by using expensive instruments. There are various issues that should be considered when performing these experiments. First of all, bulky equipment, which can be fairly expensive to buy and/or repair, is used in the experiments. Second, because this equipment has large dimensions, it can be certain that large amounts of sample and/or reagent have to be used. The third issue is the time needed to perform each experiment. Because of these issues, innovative techniques and approaches need to be addressed. Basically, a system for sensitive detection has to be designed, which is not only light and portable, but should also be rapid in detection and must take as little fluid as possible.
On the other hand, rapid advancements in microelectronic technology since the 1960s have established semiconductor production techniques to enhance the density of transistors in integrated circuits. In the 1980s, these techniques led to the first design and fabrication of microelectromechanical systems (MEMS) [21]. Further refinement of the fabrication technologies of silicon-based MEMS made it straight-forward to design and prepare simple microfluidic platforms. The first demonstration of microfluidic systems was carried out by Terry and colleagues [22]. In their study, a microfluidic system was prepared by using a silicon wafer and the prepared system was successfully used as a miniaturized gas analysis system in terms of gas chromatography (GC) with a thermal conductivity detector. The microfluidic system was fabricated by applying the photolithography technique and chemical etching. The effort put into the design and fabrication of microfluidic platforms did not gain momentum until the early 1990s, when Manz first introduced the concept of the micro total analysis system (μTAS) [23]. According to this approach, the injection, separation, and detection systems consisted of the same interface. This research not only defined μTAS, but it was a realization at the same time for scientists to use the technique in life sciences. Since then, the developed miniaturized systems were successfully applied to different applications such as separation [24–26] and detection [27–29], or used as a reactor [30–33]. Then, scientists realized the potential of these unique systems in other fields of science besides analytical chemistry.
Microfluidic techniques can be successfully applied to the design and fabrication of LOC platforms to produce a number of microchannels on the surface of a single chip that fits in the hand. Microchannels enable the handling of fluids in quantities as low as a few picoliters, as well as the manipulation of biochemical reactions at very small volumes. In addition to microchannels, LOC platforms also contain various components such as electrodes, integrated pumps, electrical field valves, and electronics. Fig. 1.1 shows a schematic depiction of an LOC system having various electrical and fluidic connections. During the fabrication process of the system, electrodes, microfluidic structures, and various back-end processes are needed to obtain a fully functional LOC system
[34, 35]. As can be seen from Fig. 1.1, the sealing of microfluidic structures can be performed by using a cover layer that is patterned or drilled for the formation of openings for electrical/fluidic interfaces. In addition, the system can be integrated to the peripheral equipment (i.e., pumps and valves) using inlet and outlet ports.
In an interesting study, Whitesides and colleagues in 2000 developed a technique called soft lithography for the fabrication of microfluidic systems using polydimethylsiloxane (PDMS) as the substrate material [36]. This technique enabled a rapid and low-cost route for the fabrication of microfluidic cartridges. In 2002, Quake and coworkers reported the design and fabrication of a largescale integration microfluidic device with the use of a number of micropumps and microvalves [37]
Today, the concept of microfluidics is defined as the manipulation and precise control of fluids on LOC platforms [38]. The main characteristics of microfluidic devices include miniaturization and automation. Miniaturization means that bioassays can be implemented with very little reagent use in a very short time, while automation means that bioassays can be implemented requiring no skilled researchers, which eliminates the errors caused by the human operation to a large extent [39]. Microfluidics technology integrates sampling, dilution, separation, detection, and other experimental operations on a chip surface of several square centimeters or less, thereby reducing sample reagent consumption, improving detection sensitivity, shortening reaction time, and reducing the average cost. The microfluidic chips enable a very broad prospect in the fields of biomedical research [40, 41], drug synthesis screening [42], environmental monitoring or protection, quarantine, biomaterials synthesis, forensic identification, and many other fields [43]. Especially, in clinical medical diagnostic, the miniaturization, integration, and automation of microfluidics-based devices are highly relevant to the development needs of point-of-care testing (POCT), which is defined as medical diagnostic testing at the time and place of patient care.

FIG. 1.1 The schematic depiction of an LOC platform. (Reproduced with permission from Y. Temiz, R.D. Lovchik, G.V. Kaigala, E. Delamarche, Lab-on-a-chip devices: how to close and plug the lab? Microelectron. Eng. 132 (2015) 156–175.)
In recent years, microfluidics has increasingly become a research hotspot in the field of POCT [44–48]. As stated earlier, microfluidic technologies can be successfully applied for the design and fabrication of LOC platforms. With the rapid progress in the design and development of LOC systems, they can be categorized into different branches such as paper-based LOC systems, centrifugal LOC systems, droplet-based LOC systems, digital LOC systems, and surface acoustic wave-based LOC systems.
2.1 Paper-based LOC systems
In the preparation of paper-based LOC systems, hydrophobic microchannels on hydrophilic paper, which guide liquid from inlet to the designated location, are mainly printed or photoetched [49, 50]. This approach is quite cheap, disposable, facile to apply, and usually applied for environmental testing and portable glucose detection [51, 52]. However, the capillary force-driven transportation of liquid usually makes the precision of assay limited and the hydrophobic barriers may not be stable after long-time storage [53]
2.2 Centrifugal LOC systems
In centrifugal LOC systems, a centrifugal field is usually exploited to manipulate the liquid [54]. In the analyses, by using this type of miniaturized system, a cartridge including liquid reagent is usually centrifuged as a disc during the liquid process. Centrifugal LOC systems are also called “lab-on-a-disc systems,” which exhibit superiority. Centrifugal LOC systems are composed of a motor as the pumping source, which is rather simple and compact. Furthermore, analyses can be easily processed since the centrifugal force exists everywhere on the disc-shaped cartridge [55]. In addition, the centrifugal field provides the efficient removal of any disturbing bubbles or residual volumes, and inherently available density-based sample transportation and separation [56, 57]. However, due to the spin of the cartridge in this LOC system, optical detection may be difficult to implement as it is a little difficult to make the final detection chamber and photodetector focused on one line.
2.3 Droplet-based LOC systems
Droplet-based LOC systems manipulate discrete volumes of fluids with the use of immiscible phases [58]. In these miniaturized systems, a large number of droplets are used as reactors to implement bioassays. A very striking application based on droplet-based LOC systems is digital polymerase chain reaction (PCR) that exhibits great sensitivity toward nucleic acids [59, 60] However, the generation and manipulation of a large number of tiny droplets requires a careful fabrication technique and also a high-precision manipulation strategy [61].
2.4 Digital LOC systems
Similar to droplet-based LOC systems, digital LOC systems also deal with the droplets. However, during the analysis by using digital LOC systems, droplets are usually manipulated on a set of insulated electrodes, which are under software-driven electronic control [62]. In this type of LOC system, the most widely applied manipulation strategy is the electrowetting-on-dielectric phenomenon, which is based on the principle of modification of interfacial tension with an applied electric field [63].
2.5 Surface acoustic wave-based LOC systems
Surface acoustic wave-based LOC systems, in which acoustic waves having a frequency range between 10 and 1000 MHz are applied for the manipulation of microscale fluid, are extensively applied in drug delivery and point-ofcare diagnostics. This manipulation strategy consists of rapid fluid actuation, is compact, and transfers a complete microfluidics solution at the microscale. However, it still currently has some drawbacks in understanding the physics of this manipulation approach, and the deformation of the fluid interface remains an unsolved issue [64, 65]
3. Applications of LOC technology
There are a number of reported examples on the interesting applications of novel systems based on LOC technology. Various studies from the literature are briefly demonstrated in the following sections.
3.1 LOC platforms for biological applications
Over the past few years, LOC technology has received great interest from researchers for biological applications due to the superiorities of LOC platforms in biological sample processing, such as rapid analysis, high throughput, reduced reagent, sample consumption, etc. [38, 44, 66–69].
LOC technology was successfully applied to the design and fabrication of miniaturized platforms for biological applications. For example, Fan et al. reported the development of a LOC platform having an integrated blood barcode chip for the sensitive detection of various proteins in plasma samples [70]. The successful combination of a DNA-encoded barcode chip with a microfluidic system provides sensitive and reliable on-chip blood separation and in situ detection of different plasma proteins within a very short time (10 min). The results indicated that the developed LOC platform can be successfully applied as a potential miniaturized device for POCT applications since the device is a minimally invasive, low-cost, and informative clinical diagnostic platform.
In another interesting work carried out by the research group of Lee [71], a self-powered integrated microfluidic blood analysis system based on LOC
Another random document with no related content on Scribd:
these new, numerous and intricate genera and species from New Holland, made no scruple to add this plant, as another species, to the genus Pultenæa. It is a low growing bushy shrub, seldom attaining more than a foot in height; is very apt to be destroyed by damp in winter, and is not to be propagated but by seeds, which, however, it perfects in this country. The blossoms begin to appear in May, and continue in succession through the summer months of June, July, and August. It should be kept warm and dry in winter, growing with most luxuriance in light sandy peat earth, and flowering the second year from the seed. This plant was first raised in 1792, by Messrs. Lee and Kennedy, at their nursery, Hammersmith, where our drawing was taken.
PLATE CCCLII.
HELONIAS BULLATA.
Spear-leaved Helonias.
CLASS VI. ORDER III.
HEXANDRIA TRIGYNIA. Six Chives. One Pointal.
GENERIC CHARACTER.
C���� nullus.
C������. Petala sex, oblonga, æqualia, decidua.
S������. Filamenta sex, subulata, corolla paulo longiora. Antheræ incumbentes.
P��������. Germen subrotundum, trigonum. Styli tres, breves, reflexi. Stigmata obtusa.
P����������. Capsula subrotunda, trilocularis.
S����� numerosa, angulata, minima.
E���������, none.
B������. Six petals, oblong, equal, deciduous.
C�����. Six threads, awl-shaped, a little longer than the blossom. Tips lying on the threads.
P������. Seed-bud roundish, three-sided. Shafts three, short and reflexed. Summits obtuse.
S���-������. Capsule roundish, three-celled.
S���� numerous, angulated, very small.
SPECIFIC CHARACTER.
Helonias foliis lanceolatis, nervosis; bracteis cordato-lanceolatis.
Helonias with lance-shaped, nerved leaves; floral leaves between lance and heart-shaped.
REFERENCE TO THE PLATE.
1. Flower, complete.
2 The Chives and Pointal, natural size
3 The same, magnified
All the species of this genus, yet discovered, are natives of North America, and are considered as hardy herbaceous plants; the winters of this country not being too severe for them; although they are found as far south as Carolina, yet they are more plentiful in Pennsylvania, about Philadelphia. They flourish most in a shady, moist situation; and increase, freely, by parting the roots in the month of March. The flowers are produced in May, the flower-stem increasing in length, till the flowers are entirely decayed. The Helonias bullata has been an inhabitant of our gardens ever since the year 1758, when it was introduced by Mr. Ph. Miller, and cultivated by him at Chelsea. See Mill. ic. 181. t. 272.
Our drawing was made at the Nursery, Hammersmith, in June 1801.
PLATE CCCLIII.
ASPALATHUS CRASSIFOLIUS.
Thick-leaved Aspalathus.
CLASS XVII. ORDER IV.
DIADELPHIA DECANDRIA. Threads in two sets. Ten Chives.
GENERIC CHARACTER.
C����. Perianthium monophyllum, semiquinquefidum; laciniis acuminatis, æqualibus, superiore longiore.
C������ papillionacea.
Vexillum compressum, adscendens, obovatum, externe sæpius hirsutum, obtusum cum acumine.
Alæ lunulatæ, obtusæ, patulæ, vexillo breviores.
Carina bifida, alis conformis.
S������. Filamenta decem, connata in vaginam, superne dehiscentem longitudinaliter, adscendentia. Antheræ oblongæ.
P��������. Germen ovatum. Stylus simplex, adscendens. Stigma acutum.
P����������. Legumen ovatum, muticum.
S����� sæpius duo, reniformia.
O��. Singulare huic sunt folia ex eadem gemma plura in planta frutescente.
E���������. Cup one leaf, half-five-cleft; segments tapered, equal, the upper one longer.
B������ butterfly-shape.
Standard compressed, ascending, inversely egg-shaped, often hairy on the outside, obtuse with a point.
Wings half-moon-shaped, obtuse, spreading, shorter than the standard. Keel two-cleft, like the wing.
C�����. Ten threads, united into a sheath, gaping longitudinally at top, ascending. Tips oblong.
P������. Seed-bud egg-shaped. Shaft simple, ascending. Summit pointed.
S��� ������. Pod egg-shaped, beardless.
S���� frequently two, kidney-shaped.
O��. This genus is singular in having several leaves from the same bud, on a shrubby plant.
SPECIFIC CHARACTER.
Aspalathus foliis fasciculatis, carnosis, teretibus, glabris, apicibus setaceis; floribus capitatis, terminalibus; calycibus bracteatis.
Aspalathus with leaves bundled, fleshy, cylindrical, smooth and bristled at the point; flowers grow in heads at the end of the branches; cups with floral leaves.
REFERENCE TO THE PLATE.
1. The Cup.
2 The Standard
3. One of the Wings.
4. The Keel.
5. The Chives and Pointal.
6 The Chives, spread open and magnified
7. The Pointal.
From the number of specimens in the herbarium of G. Hibbert, Esq. which we should refer to this genus; it stands fair to rival, in number of species, every other, natives of the Cape of Good Hope; with the exception of Erica, Geranium, and Protea. This species has many varieties, and we are in doubt whether this is not one from the A. carnosa, or A. pinguis of Thunberg; for although, the cup, in the one, may differ, in having the segments described as pointed; and in the other, the leaves may be rather three-sided; yet, may our plant be no more than a slight variation from either of them: but this we cannot decide without living plants of each, for dried specimens would not determine the fact.
This plant was introduced to the Hibbertian Collection, in 1800, by Mr. Niven, from the Cape. It is a tender green-house plant, grows to the height of eighteen inches, very bushy, and covers itself with flowers about July; in which month, 1803, our drawing was taken. It has hitherto put the ability of Mr. Allen, which is not often the case, to a stand for a method to propagate it; but we have hopes, as most of the species do, that it will ripen its seeds in this country.
PLATE CCCLIV.
GERANIUM REVOLUTUM.
Reflex floral-leaved Geranium.
CLASS XVI. ORDER IV.
MONADELPHIA DECANDRIA. Threads united. Ten Chives.
ESSENTIAL GENERIC CHARACTER.
M�������. Stigmata quinque. Fructus rostratus; pentacoccus.
O�� P������. Five Summits. Fruit furnished with long awns; five dry berries.
SPECIFIC CHARACTER.
Geranium foliis cordatis, obtusis, nervosis, sæpe auriculatis; pedunculis multifloris; involucris polyphyllis, foliolis revolutis; floribus pentandris; radice tuberosa.
Geranium with heart-shaped leaves, obtuse, nerved and often eared; flower-stems many-flowered; fence many-leaved, leaflets rolled back; flowers with five fertile chives; root tuberous.
REFERENCE TO THE PLATE.
1. The Empalement cut open to shew its tubular structure.
2. The Chives and Pointal.
3 The Chives, spread open, magnified
4. The Pointal, magnified.
This very fine Geranium is, as yet, only in the Clapham Collection; it has no properties, which regard its culture or propagation, differing from the rest of its congeners; was sent from the Cape, by Mr. Niven, in 1800. Flowers in July. The leaves of this species have most affinity, in appearance, to those of G. melananthum, particularly in being like them frequently eared, and even sometimes winged. We have named it, specifically, from the singular revolute character of the involucrum, at the base of each bunch of flowers.
PLATE CCCLV.
LEEA PINNATA.
Winged-leaved Leea.
CLASS V. ORDER I.
PENTANDRIA MONOGYNIA. Five Chives. One Pointal.
GENERIC CHARACTER.
C����. Perianthium monophyllum, campanulatum, coriaceum, quinquedentatum, persistens.
C������ monopetala; tubus longitudine calycis; limbus quinquefidus, æqualis; laciniis saccatis.
Nectarium basi interioris corollæ adnatum, corolla dimidio brevius, erectum, urceolatum, quinquefidum; lobis emarginatis.
S������. Filamenta quinque, inserta basi exteriori nectarii. Antheræ ovatæ, versatiles.
P��������. Germen subglobosum. Stylus simplex, nectario brevior. Stigma obtusum.
P����������. Bacca orbiculata, depressa, quinquelocularis.
S����� quinque, solitaria.
E���������. Cup one leaf, bell-shaped, leathery, five-toothed, remaining.
B������ one petal; tube the length of the cup; border five-cleft, equal; segments bagged.
Honey-cup fixed to the base of the inside of the blossom, by the half shorter than the blossom, erect, pitcher-shaped, five-cleft; lobes notched at the end.
C�����. Five threads inserted into the outside of the base of the honeycup. Tips egg-shaped, versatile.
P������. Seed-bud almost globular. Shaft simple, shorter than the honeycup. Summit blunt.
S���-������. Berry orbicular, flattened, five-celled.
S���� five, solitary.
SPECIFIC CHARACTER.
Leea foliis impari-pinnatis, oppositis; caule tereti, glabro; racemis angulatis, angulis undulatis.
Leea with winged leaves terminated by an odd one and opposite; stem round and smooth; branches angled; angles waved.
REFERENCE TO THE PLATE.
1 The Cup, natural size
2 A Blossom
3. A Flower complete, magnified.
4. The Honey-cup, shewn from the inside, cut open, a little magnified.
5 A Blossom, with the Honey-cup and Chives, magnified
6. The Honey-cup and Chives shewn from the outside, magnified.
7. The Pointal, magnified.
Leea and Aquilicia have, of late, been considered as not possessing sufficient essentially differing characters to constitute two genera, and Aquilicia has been lost in Leea. Our present subject is a species of the former Aquilicias, is mentioned by Gaertner, who says, it approaches A. sambucina, now Leea sambucina; to which indeed it has much resemblance, but differs in having opposite, not alternate branches, being scentless, having the leaves rather rough and the stem smooth. It is a native of the East Indies, requiring the tan-bed to make it flower; is half shrubby, and is increased by cuttings. Flowers in August. Our figure is from a specimen communicated to us by Aylmer Bourke Lambert, Esq. who raised it from seeds received from Dr. Roxburg, in 1801, under the name of Leea crispa: which is a native of Africa, and has been long in most of our collections of hot house plants, but is in the highest state of cultivation, under the care of Mr. Hoy, in that of his Grace the Duke of Northumberland, Sion House, near Brentford.
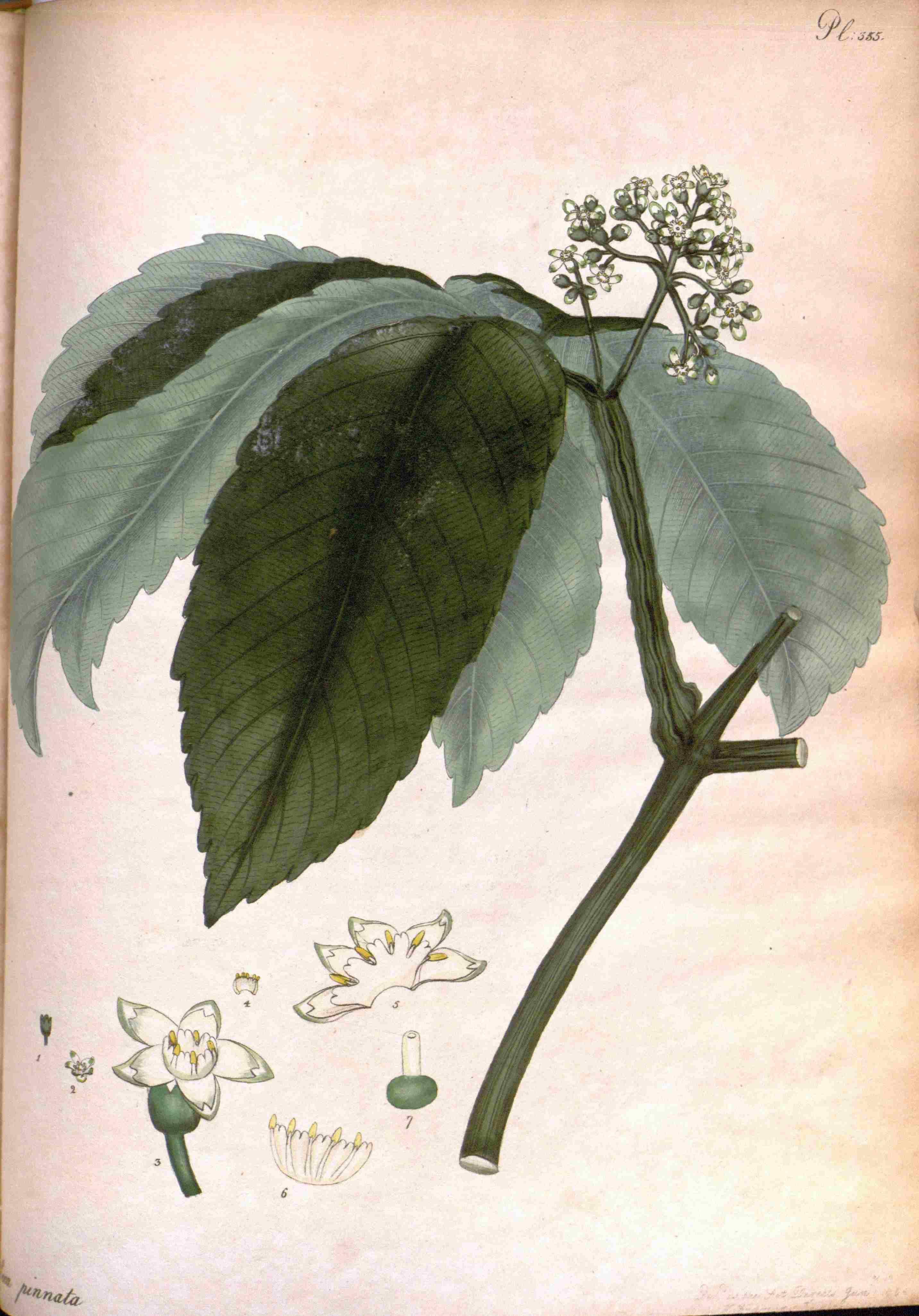
PLATE CCCLVI.
ARUM ORIXENSIS.
Orixian Cuckow-pint.
CLASS XX. ORDER XI.
GYNANDRIA POLYANDRIA. Chives on the Pointal. Many Chives.
ESSENTIAL GENERIC CHARACTER.
C����. Spatha monophylla, maxima, oblonga, basi convoluta, apice connivens, ventre compressa, interne colorata.
Spadix clavatus, simplicissimus, spatha paulo brevior, coloratus, inferne germinibus obvallatus, marcescens supra germina.
C������ nulla.
S������. Filamenta nulla, nisi nectaria basi crassa, desinentia in cirros filiformes, duorum ordinum e medio spadice egredientes. Antheræ plurimæ, sessiles, tetragonæ, cirrhorum duplici ordine interjectæ, spadici adnatæ.
P�������. Germina plurima, basin spadicis vestientia, infra stamina collocata, obovata. Styli nulli. Stigmata villis barbata.
P���������. Baccæ totidem, globosæ, uniloculares.
S����� plura, subrotunda.
E���������. Sheath one leaf, very large, oblong, convolute at the base, converging at the top, the belly compressed, coloured within.
Sheathed-Fruit-stalk club shaped quite simple, a little shorter than the sheath, coloured, set round with seed-buds on the lower part, withering above the seed-buds.
B������ none.
C�����. Threads none except the honey-cups, which are thick at the base and terminated in thread-shaped tendrils, issuing in two rows from the middle of the sheathed fruit-stalk. Tips many, sitting, four sided, fixed to the fruit-stalk, and disposed between the two rows of tendrils.
P�������. Seed-buds many, cloathing the base of the fruit-stalk, set below the chives, inversely egg-shaped. Shafts none. Summits bearded with soft hairs.
S���-�������. As many berries, globular, and one-celled.
S����. Many, roundish.
SPECIFIC CHARACTER.
Arum, acule; foliis ternatis, venosis, discoloribus; spatha declinata; flore atro-purpureo.
Cuckow-pint, stemless; leaves threefold, full of veins, two-coloured; sheath bent downward; flower of a deep purple.
REFERENCE TO THE PLATE.
1 The whole flower, shewn from the front with the sheath opened, to expose the parts of fructification.
This pretty plant is a native of the East Indies, in that country from which it derives its specific title. It has much affinity with many others of the Genus, which at first sight appear rather as varieties, than meriting to be treated as species; but, in this instance, we submit our judgment to that of Dr. Roxburg, by whom it has been introduced to us under the name it here bears, in the year 1802. Our figure was taken, in the month of October 1803, from a plant in the collection of J. Vere, Esq. Kensington Gore. The flower is scentless.
PLATE CCCLVII.
ARCTOTIS PARADOXA.
Chamomile-leaved Arctotis. CLASS XIX. ORDER IV.
SYNGENESIA POLYGAMIA NECESSARIA. Tips united. Necessary Pointals.
GENERIC CHARACTER.
C���� communis subrotundus, imbricatus; squamis inferioribus laxioribus, subulatis; mediis ovatis; intimis oblongis; apice scarioso, rotundato, concavo.
C������ composita radiata; corollulæ hermaphroditæ plurimæ in disco.
Femininæ ligulatæ, fere viginti, disci diametro longiores.
Propria hermaphroditis infundibuliformis; limbo quinquefido, apicibus reflexis, æqualibus.
Femineis ligulata, lanceolata, tenuissime tridentata, tubo brevissimo.
S������ hermaphroditis; filamenta quinque, capillaria, brevissima. Anthera cylindracea, quinquedentata, longitudine corollulæ.
P�������� Hermaphroditis; germen vix manifestum. Stylus cylindraceus, corolla paulo longior. Stigma simplex.
Femineis germen ovato-tetragonum, villosum, coronatum calyculo proprio.
Stylus filiformis. Stigmata duo, ovata-oblonga, crassiuscula, erecta.
P���������� nullum. Calyx immutatus. Semina Hermaphroditis nulla.
Femineis solitaria, subrotunda, villosa. Pappus perianthium proprium pentaphyllum; foliolis ovatis, patentibus, coronatus perianthii proprii foliolis ovatis in orbem positis.
R����������� pilosum seu paleaceum, planiusculum.
E��������� common roundish, tiled; lower scales more loose, awl shaped; middle ones egg-shaped; innermost oblong; harsh, rounded, concave at the point.
B������ compound raied; hermaphrodite florets numerous in the center. Of the Females tongue-shaped, nearly twenty, longer than the diameter of the center.
Proper of the hermaphrodites funnel-shaped; border five-cleft, ends reflexed, equal.
Of the Females tongue-shaped, lance-shaped, slightly three-toothed; tube very short.
C����� of the Hermaphrodites; five threads hair-like, very short. Tip cylindric five-toothed, the length of the floret.
P������ of the Hermaphrodites; seed-bud scarce visible. Shaft cylindric, a little longer than the blossom. Summit simple.
Of the Females seed-bud egg-shaped four-cornered, hairy, crowned by its proper cup. Shaft thread-shaped. Summits two, oblong-egg-shaped, thickish, upright.
S���-������ none. Empalement unchanged. Seeds in the Hermaphrodites none.
In the Females solitary roundish, hairy. Feather, proper cup five leaved; leaflets, egg-shaped, spreading, crowned by the proper cup with the leaflets placed in a round.
R��������� hairy or chaffy, flattish.
SPECIFIC CHARACTER.
Arctotis flosculis radiantibus sterilibus; paleis disco longioribus coloratis; foliis bipinnatis, linearibus.
Arctotis with the florets of the circumference sterile; chaffs coloured and longer than the florets of the center; leaves doubly winged; linear.
REFERENCE TO THE PLATE.
1. An inner Scale of the cup.
2. An outer Scale of the cup.
3 A Petal of the circumference, with its tubular base, which is sterile.
4. A chalky division of the florets of the center, magnified.
5. A Female floret of the center, magnified.
6 The Chives of an Hermaphrodite floret, spread open and magnified
7. The Pointal of an Hermaphrodite floret, natural size.
8. The same, magnified.
9 The Seed-bud of a female floret, natural size, with its feather
10 The same, magnified
Of all the plants, numerous as they are, composing this natural Class, we know of no one, the Virgilia (a native of Peru, and introduced to us from the
Paris gardens by Mons. Thoin, about twenty years ago, but since lost to Europe,) excepted, which can rival our present subject. It is a native of the Cape of Good Hope, and is said, in the Kew Catalogue, to have been introduced to that collection, in 1774, by Mr. Masson. Whether this is the A. paradoxa of Linn. Sp. 1307. Vol. II, we are not certain, as the chaff, dividing the florets, is there described as of nearly the length of the ray, giving the flower an appearance of doubleness, and is there likewise marked as an annual. But, there is little doubt of its being the plant intended in the Kew Catalogue, under our title; it is there made biennial, which is its true character, as it does not flower the first year from seeds. It may be propagated by cuttings of the first year’s growth; delights in a light sandy loam, and flowers in July or August. Our drawing was made in 1802, from a plant in the Hibertian Collection; which had been raised from seeds, sent the preceding year, from the Cape, by Mr. Niven.

PLATE CCCLVIII.
AMARYLLIS BRASILIENSIS.
Brasilian Lily-Daffodil.
CLASS VI. ORDER I.
HEXANDRIA MONOGYNIA. Six Chives. One Pointal.
ESSENTIAL GENERIC CHARACTER.
C������ 6-petala, campanulata, Stigma trifidum.
B������ 6-petalled, bell-shaped. Summit three-cleft.
See A�������� �������, Pl. XCV. Vol. II.
SPECIFIC CHARACTER.
Amaryllis, spatha bi-seu-triflora; petalis ovato-acuminatis, æqualibus, costatis, ad basin albidis, patentibus; genitalibus declinatis; foliis linearilanceolatis, acutis.
Lily-Daffodil, sheath two or three flowered; petals egg-shape tapered, equal, ribbed, white at the base and spreading; parts of fructification bent downwards; leaves linear-lance-shaped, pointed.
REFERENCE TO THE PLATE.
1. A petal with its Chive.
2 The Seed-bud, with part of the tube of the Blossom and partial Fruit-stalk.
This fine Lily, from the Brazils, can scarcely be thought more than a variety of A. Reginæ; to which plant, both this, and the A. equestris of Mr. Curtis, may be referred without much flexion of the original species. We have, however, preserved the title under which it has been known since its introduction; which was, in the year 1798, by the late Marchioness of Bute, from Spain. Our drawing was taken from a plant in the collection of J. Vere, Esq. Kensington Gore, in the month of October, 1803. It has all the merits of the most easily cultivated species of the genus; increasing freely by the bulb, growing with luxuriance in almost any earth, and requiring but little heat to make it flower.





















