Effect of electrode Pt-loading and cathode flow-field plate type on the degradation of PEMFC Qu
https://ebookmass.com/product/effect-of-electrodept-loading-and-cathode-flow-field-plate-type-onthe-degradation-of-pemfc-qu/

Download more ebook from https://ebookmass.com
More products digital (pdf, epub, mobi) instant download maybe you interests ...

Interface Engineering in Organic Field-Effect Transistors Xuefeng Guo
https://ebookmass.com/product/interface-engineering-in-organicfield-effect-transistors-xuefeng-guo/

COVID-19: The Essentials of Prevention and Treatment Jie-Ming Qu
https://ebookmass.com/product/covid-19-the-essentials-ofprevention-and-treatment-jie-ming-qu/

Effect of protein on the post-ingestive response of tilapia fed encapsulated diets Leandro S. Costa
https://ebookmass.com/product/effect-of-protein-on-the-postingestive-response-of-tilapia-fed-encapsulated-diets-leandro-scosta/

COVID-19: The Essentials of Prevention and Treatment 1st Edition Jie-Ming Qu
https://ebookmass.com/product/covid-19-the-essentials-ofprevention-and-treatment-1st-edition-jie-ming-qu/

The Effect
of Fines on Critical
State and
Liquefaction
Resistance Characteristics of Non-Plastic Silty Sands
Anthi Papadopoulou & Theodora Tika
https://ebookmass.com/product/the-effect-of-fines-on-criticalstate-and-liquefaction-resistance-characteristics-of-non-plasticsilty-sands-anthi-papadopoulou-theodora-tika/

The Forms of Michael Field Palgrave
https://ebookmass.com/product/the-forms-of-michael-fieldpalgrave/

Sustainability and Financial Risks: The Impact of Climate Change, Environmental Degradation and Social Inequality on Financial Markets 1st ed. Edition Marco Migliorelli
https://ebookmass.com/product/sustainability-and-financial-risksthe-impact-of-climate-change-environmental-degradation-andsocial-inequality-on-financial-markets-1st-ed-edition-marcomigliorelli/

The Secret History of Bigfoot: Field Notes on a North American Monster John O’Connor
https://ebookmass.com/product/the-secret-history-of-bigfootfield-notes-on-a-north-american-monster-john-oconnor/

Transcriptomic profiling and novel insights into the effect of AG ablation on gonad development in Macrobrachium rosenbergii Kianann Tan
https://ebookmass.com/product/transcriptomic-profiling-and-novelinsights-into-the-effect-of-ag-ablation-on-gonad-development-inmacrobrachium-rosenbergii-kianann-tan/

Contents lists available at ScienceDirect
Journal of Energy Chemistry
journal homepage: www.elsevier.com/locate/jechem
Effect of electrode Pt-loading and cathode flow-field plate type on the degradation of PEMFC
Lijuan Qu a,b , Zhiqiang Wang a , Xiaoqian Guo a , Wei Song a , Feng Xie a , Liang He a,b , Zhigang Shao a,∗ , Baolian Yi a
a Fuel Cell System and Engineering Group, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, Liaoning, China
b University of Chinese Academy of Sciences, Beijing 10 0 049, China
a r t i c l e i n f o
Article history:
Received 27 July 2018
Revised 5 September 2018
Accepted 11 September 2018
Available online 19 September 2018
Keywords:
Proton exchange membrane fuel cell
Electrode platinum loading
Current-variation cycle
Traditional solid plate
Water transport plate
a b s t r a c t
The electrode Pt-loading has an effect on the number of active sites and the thickness of catalyst layer, which has huge influence on the mass transfer and water management during dynamic process in PEMFCs. In this study, membrane electrode assemblies with different Pt-loadings were prepared, and PEMFCs were assembled using those membrane electrode assemblies with traditional solid plate and water transport plate as cathode flow-field plates, respectively. The performance and electrochemical surface area of cells were characterized to evaluate the membrane electrode assemblies degradation after rapid currentvariation cycles. Scanning electron microscope and transmission electron microscope were used to investigate the decay of catalyst layers and Pt/C catalyst. With the increase of Pt-loading, the performance degradation of membrane electrode assemblies will be mitigated. But higher Pt-loading means thicker catalyst layer, which leads to a longer pathway of mass transfer, and it may result in carbon material corrosion in membrane electrode assemblies. The decay of Pt/C catalyst in cathode is mainly caused by the corrosion of carbon support, and the degradation of anode Pt/C catalyst is a consequence of migration and aggregation of Pt particles. And using water transport plate is beneficial to alleviating the age of cathode Pt/C catalyst.
©2018 Published by Elsevier B.V. and Science Press on behalf of Science Press and Dalian Institute of Chemical Physics, Chinese Academy of Sciences
1. Introduction
Proton exchange membrane fuel cell (PEMFC) is considered as a promising energy power [1] that possesses less pollution [2,3], high efficiency [4–6], and outstanding start-up rate [3,7–9] Lately, tremendous progresses have been made for the commercial popularization of PEMFC, particularly in the auto industry [10,11] However, the expensive membrane electrode assemblies (MEAs) [12] andthe suboptimaldurability [13] have beentheprimary problems which impede the widespread application of PEMFC.
The rate of cathode oxygen reduction reaction (ORR) is sluggish, which is an important challenge for the development of PEMFC. Until now, Pt-based electrocatalysts are the best choices in practical terms [14] However, since platinum is rare metal, its high price accounts for an important portion of the expensive material cost of MEA [12]. Decreasing Pt-loading of MEA without cell performance loss is the aim of many researches on ORR electrocatalyst. Although there are great efforts of researches on Pt-free
∗ Corresponding author.
E-mail address: zhgshao@dicp.ac.cn (Z. Shao).
ORR electrocatalyst, owing to their poor performance and unsatisfying stability, it is very difficult for Pt-free electrocatalyst to be used in practical application. Therefore, most ORR electrocatalysts used today are based on Pt, which are dispersed on the carbon black support in the form of nanoparticles. With state-of-the-art Pt/C catalysts, it is equally important to achieve platinum-loading reduction as well as enhanced catalyst activity and MEA durability [15] Thus it is necessary to understand the distinct degradation of MEAs with different Pt-loadings.
Comparing with steady-state, the dynamic operation is much more noticeable [16] When it comes to automotive applications, PEMFC will undergo many drastic current changes, and its voltage will oscillate. Since fuel is pure hydrogen and the easy nature of hydrogen oxidation reaction, the anode potential is approximate value of reversible hydrogen potential, which indicates that cathode experiences potential oscillation when the cell voltage changes [17] When voltage changes, the corrosion of carbon material can influence the long-term durability of PEMFCs [17] Besides, platinum is highly stable with both low and high cell voltage in PEMFC. However, when cathode potential changes rapidly, platinum will dissolve quickly [18,19]. Thus for a long time, the cell performance will seriously degrade.
https://doi.org/10.1016/j.jechem.2018.09.004 2095-4956/©2018PublishedbyElsevierB.V.andSciencePressonbehalfofSciencePressandDalianInstituteofChemicalPhysics,ChineseAcademyof Sciences
The degradation of MEAs during dynamic processes is highly impacted by water management. Water management is always deemed to be a significant factor for optimal performance and durability of PEMFC [20–22]. It is one of the major technical challenges to achieve proper proton exchange membrane hydration without electrode flooding in PEMFCs. One way to improve the water management is controlling the relative humidity of the reactants [23] The other promising way to improve water management is introducing water transport plates (WTPs), which is put forward by the United Technologies Corporation (UTC) [24,25]. The characteristics of WTPs have been detailed described in references [24–26]
Until now, there is no related report about the effect of electrode Pt-loading on the degradation of MEAs in the PEMFC with WTP as flow-field plates. Therefore, in this paper, MEAs with different Pt-loadings were prepared. Besides, to compare the effect of cathode flow-field plates on MEAs degradation, traditional solid plate (SP) and WTP were used as cathode flow-field plate respectively, and different PEMFCs were assembled with different MEAs. Following, the degradation degree of PEMFCs performance was compared after current-variation cycles with measurements of polarization curves and cyclic voltammetry (CV) curves. In addition, scanning electron microscope (SEM) and transmission electron microscope (TEM) were used to study the microstructure degradation of MEAs.
2. Experimental
2.1. Preparation of MEAs
MEAs, with an active area of 5 cm2 (2 cm∗ 2.5 cm), were assembled with catalyst-coated proton exchange membrane (CCM) and gas diffusion layer (GDL). The CCM and GDL were homemade. Catalyst ink, which consisted of commercial catalyst powder (70 wt% Pt/C, Johnson Matthey Corporation), Nafion solution (5 wt%, DuPont Corp.) and iso-propyl alcohol, was prepared. Then homogeneous catalyst ink was sprayed on the one side of proton exchange membrane (Nafion®212, DuPont) to prepare CCM. Each MEA included two CCMs, with the side without catalyst being sticked together. Four types of MEAs were prepared with different Pt-loadings CCMs. The Pt-loading of CCM on both anode and cathode was the same, and one side Pt-loadings were 0.1 mgPt cm 2 , 0.2 mgPt cm 2 , 0.3 mgPt cm 2 , 0.4 mgPt cm 2 , respectively. Our home-made GDL was carbon paper (Toray, TGP-H-060) as the substrate, with polytetrafluoroethylene (PTFE, 25 wt%) and carbon black impregnating it. The MEAs were prepared by placing the GDLs on the anode and cathode side of CCMs, and subsequently by hot pressing at 140 °C and 0.2 MPaabs for 2 min.
2.2. Fuel cell design
A special single cell was designed [24] For the WTPFC, the cathode flow-field plate was WTP. And for the SPFC, the cathode flow-field plate was SP. The anode flow-field plates were SPs. Circulating water flowed through the hollowed channels between flow-field plate and current collector plate, as well as saturated the WTP. All the flow-field plates were made of graphite with thickness of 1.3 mm, and parallel gas flow-field was machined with following dimensions: rib width of 0.8 mm, channel width of 0.8 mm, and channel depth of 0.8 mm.
2.3. Fuel cell test system
The PEMFC test station was home-made. The test station could control the operating parameters (such as relative humidity of reactants, cell temperature, gases flow rate and backpressure) during
tests. KFM 2030 (Kikusui, Japan) was used as the electric load in the testing processes, and it could record data automatically. The cell temperature was kept at 65 °C for every experiment. Both anode and cathode gases were humidified by bubbling gas through distilled water tanks held at an assigned temperature. Before gases were fed into, they were first humidified by passing through their corresponding humidifiers.
2.4. Degradation experiment conditions
Degraded MEAs were achieved by carrying out rapid currentvariation cycles experiments with fuel cells by employing electric load. For a single current-variation cycle, the current density evolved as the following process: maintaining at 0 mA cm 2 for 2 s, changing from 0 mA cm 2 to 600 mA cm 2 taking 1 s, maintaining at 600 mA cm 2 for 2 s, changing from 600 mA cm 2 to 0 mA cm 2 taking 1 s (Fig. 1). It took about 6 s for each degradation cycle, and there were 80,0 0 0 current-variation cycles in all for every MEA before degradation test stopped. Thus, the degradation procedure of each MEA lasted about 133.3 h. In the course of last 30,0 0 0 degradation cycles, the anode was fed with saturated humidified hydrogen (99.9%) at the flow rate of 80 mL min 1 and saturated humidified air served as oxidant at the flow rate of 120 mL min 1 . At the beginning and the end of degradation cycles, cell performance was recorded respectively, with measurements of polarization curves and CV curves.
2.5. Polarization curves
To study the performance degradation, polarization curve was obtained. For every measurement, the measuring conditions were maintained at the same. The flow rate of hydrogen (0.15 MPaabs ) was 100 mL min 1 and the air (0.15 MPaabs ) flow rate was 800 mL min 1 . The pressure of circulating water was maintained at 0.11 MPaabs . Both H2 and air were saturated humidified. Before polarization curve test, cells were fully activated to a steady-state.
2.6. Cyclic voltammetry curves
In order to calculate electrochemical surface area (ECSA) of MEAs, CV measurements were carried out. For every measurement, the test conditions were identical, cell cathode was fed with saturated humidified nitrogen (0.15 MPaabs ) and its flow rate was 120 mL min 1 Saturated humidified hydrogen (0.15 MPaabs ) was supplied to anode with the flow rate of 80 mL min 1 Before CV test, nitrogen and hydrogen purged cell until the cell open circuit voltage was 0.1 V or below. The cathode potential scanned from 0.08 V to 1.2 V (versus the reference electrode) with the scanning rate of 50 mV s 1 at 65 °C. The CV curves were measured with CHI-630C (CH Instruments, Inc.) with cathode serving as the work electrode, and anode acting as reference electrode (dynamic hydrogen electrode, DHE) and counter electrode. The ECSA was calculated from integrated hydrogen desorption area of CV curves with 0.21 mC cmPt 2 as the conversion factor.
In order to compare the performance and electrochemical characters of MEAs, electrochemical characterizations of all MEAs were conducted in an identical PEMFC with SPs as flow-field plates.
2.7. Transmission electron microscopy
In order to investigate the Pt/C catalyst degradation of different MEAs, TEM images were obtained with employing JEOL JEM20 0 0EX transmission electron microscope, operating at 120 kV. Following the sizes of Pt particles were analyzed. The size distribution of Pt particles was obtained by calculating 300 particles size on each TEM image.

2.8. Scanning electron microscopy
The cross-section of the fresh and degraded MEAs was prepared by riving MEAs in liquid nitrogen. The morphology of the cross section of MEAs was obtained with JEOL JSM-6360LV scanning electron microscope. Thus, the changes of microstructure from the pristine and degraded MEAs were observed.
3. Results and discussion
3.1. Electrochemical characterization of MEAs
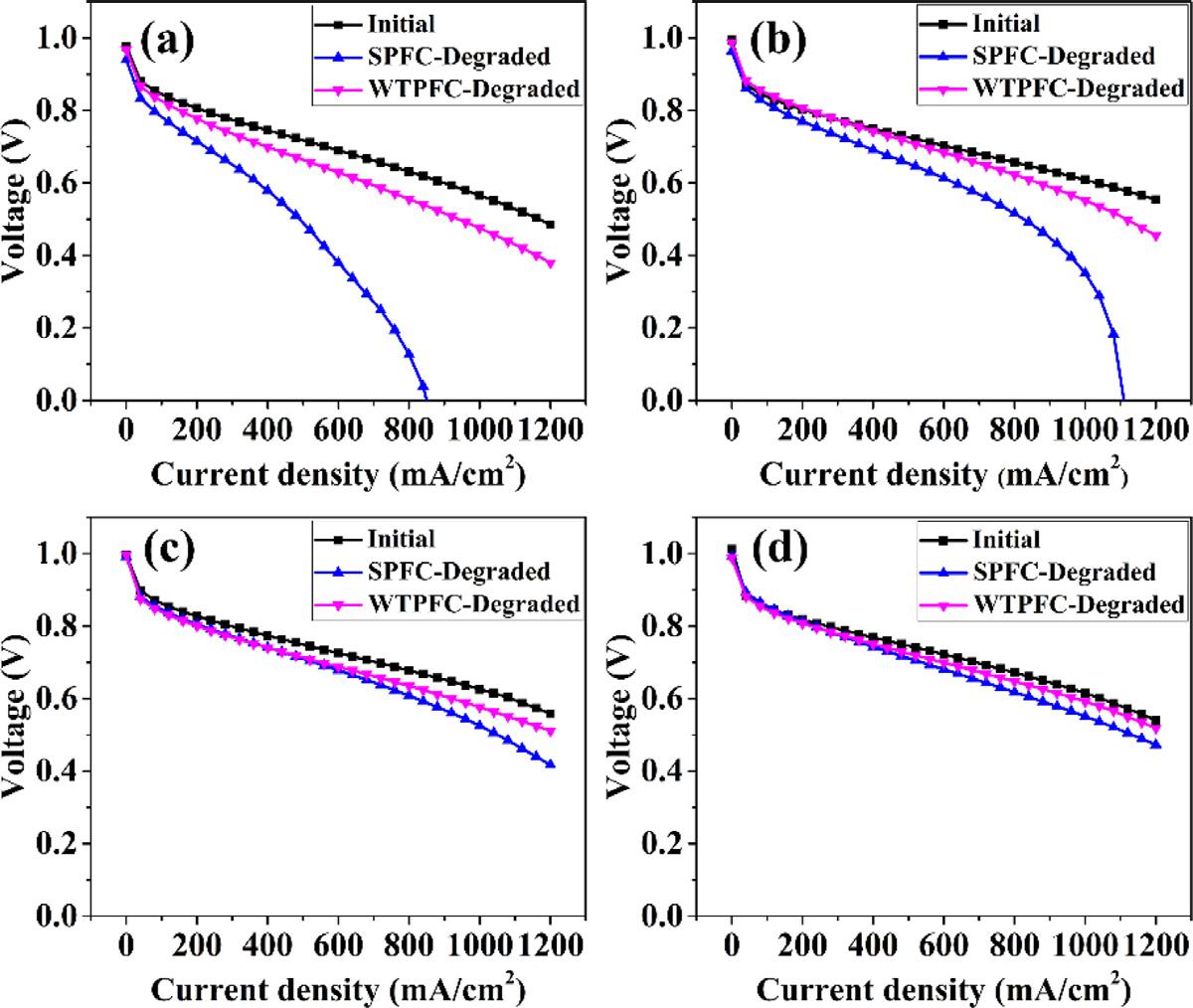
The polarization curves of MEAs before and after 80,0 0 0 current-variation cycles for various Pt-loading MEAs are shown in Fig. 2. And the corresponding voltage loss percent at 10 0 0 mA cm 2 is shown in Fig. 4(b). It is obvious that the performance loss is less when the Pt-loading increases. And the performance decline of MEAs degraded in WTPFC is obviously lower than that degraded in SPFC. The cell voltage decline percent at 10 0 0 mA cm 2 of MEAs degraded in SPFC is 42.27%, 16.13%, 10.46%, corresponding
Pt-loading of 0.2 mg cm 2 , 0.3 mg cm 2 , 0.4 mg cm 2 . After degradation cycles, the voltage at 10 0 0 mA cm 2 is 0.466 V when the Pt-loading is 0.1 mg cm 2 (Fig. 4(a)). However, when it comes to MEAs degraded in WTPFC, the voltage decrease at 10 0 0 mA cm 2 is 16.36% (0.1 mg cm 2 ), 9.09% (0.2 mg cm 2 ), 7.84% (0.3 mg cm 2 ), 3.22% (0.4 mg cm 2 ) (Fig. 4(b)). Because the repeated rapid currentvariation operation can lead to the irreversible oxidation, dissolution, migration and aggregation of the cathode Pt in entire cathode layer [27], which will cause the decay of MEAs, the performance declines.
A high-performance electrode in PEMFCs should have continuous electrolyte pathways to access the Pt surface throughout the CL. Carbon support corrosion will change the porous structure of catalyst and catalyst/ionomer interfaces, which will lead to the decrease of proton conductivity in CL [28] Thus the ohmic resistance of degraded MEAs increases, such as the result in Fig. 2(a). In addition, altering the porous structure in MEAs will lead to increasing mass transfer resistance of reactants, which also results in decreased performance.
The CV curves of different MEAs before and after degradation cycles are presented in Fig. 3 And corresponding ECSA decline percent is shown in Fig. 4(d). The ECSA decline percent of MEAs degraded in SPFC is 72.53%, 59.90%, 47.73% and 60.58% with electrode Pt-loading of 0.1 mg cm 2 , 0.2 mg cm 2 , 0.3 mg cm 2 and 0.4 mg cm 2 , respectively. And the ECSA loss of MEAs degraded in WTPFC is 48.61% (0.1 mg cm 2 ), 46.96% (0.2 mg cm 2 ), 37.27% (0.3 mg cm 2 ), 50.37% (0.4 mg cm 2 ). During the current-variation cycles, cells have undergone open circuit state, which means the high potential of cathode. It is reported that the Pt can be oxidized to Pt–O at high potential, and the Pt–O can be chemically dissolved in solution, which will cause the Pt loss or precipitation by reduction [29] The ECSA should depend on two elements, the one is Pt particles number attached on carbon support, and the other is the Pt particles size [30]. The dissolved Pt ions may diffuse into the PEM and be chemically reduced by hydrogen crossover from anode, which can lead to the decrease of Pt particles number on carbon support. Cooperating with the Ostwald ripening and
2. Comparison of polarization behavior of MEAs before and after current-variation cycles for various Pt-loading
0.4 mg
(The Pt-loadings in all figures in this paper are one side electrode platinum loading.)
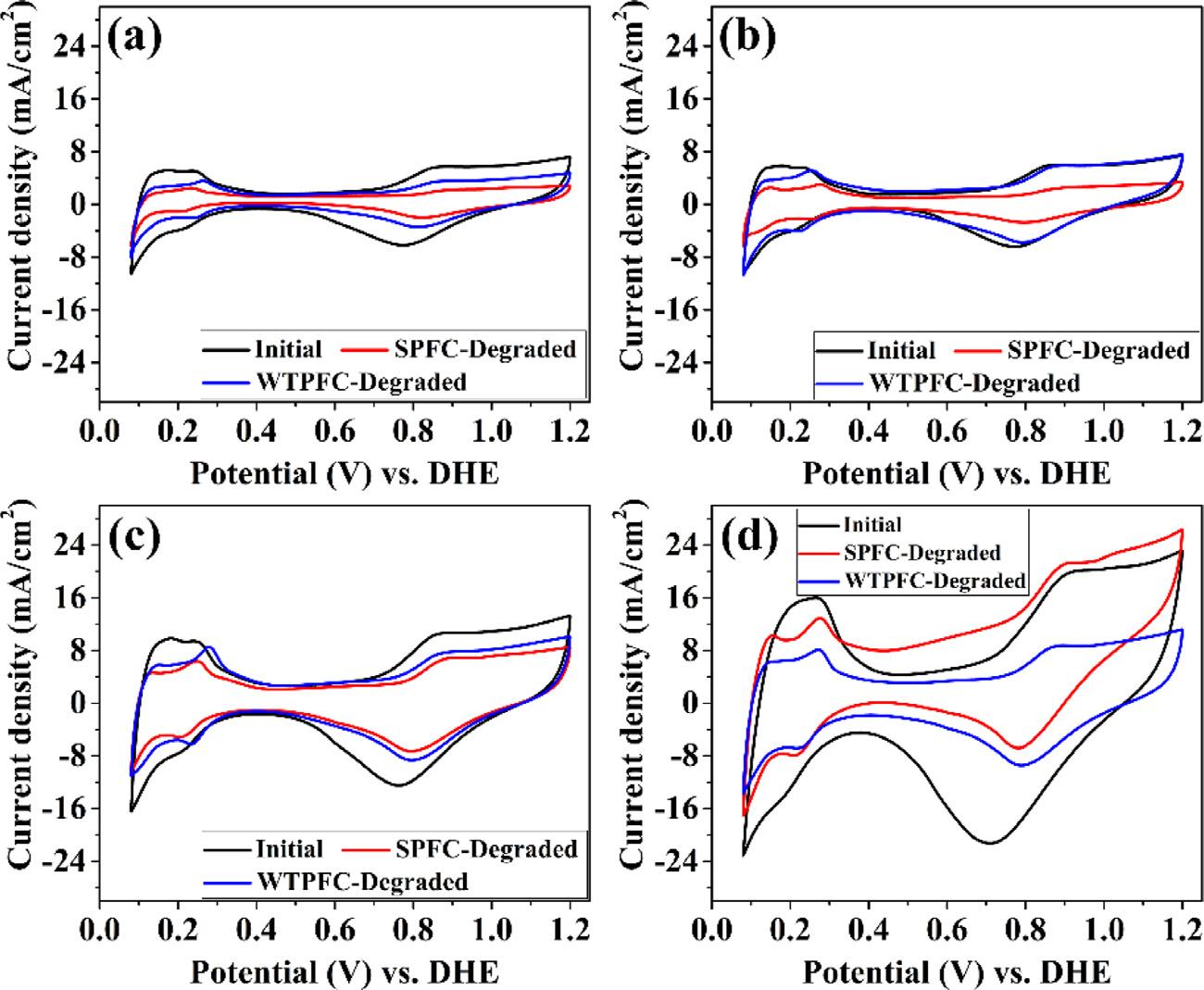
Fig. 3. Comparison of CV curves characterization of MEAs before and after current-variation cycles for various Pt-loadings MEAs: (a) 0.1 mg
0.3 mg cm 2 ; (d) 0.4 mg cm 2

Fig. 4. (a) MEAs performance and (b) their degradation percent at 10 0 0 mA cm 2 ; (c) normalized ECSA and (d) ECSA decline percent of MEAs before and after
current-variation cycles with different Pt-loadings.
aggregation of cathode Pt nanoparticles, the cell ECSA declines. ECSA loss will make a significant influence on the drop of ORR kinetics, and MEAs performance declines.
It is noted that the ECSA loss percent of MEAs with Pt-loading of 0.4 mg cm 2 is higher than that of 0.2 mg cm 2 , 0.3 mg cm 2 , and it is even higher than that of 0.1 mg cm 2 with MEAs degraded in WTPFCs. It might be owing to that the CL of MEAs with Ptloading of 0.4 mg cm 2 is too thick. Thicker CL can impede the mass transfer in MEAs. During dynamic processes, the obstructed
mass transfer may lead to local starvation of reactants, which can boost the corrosion of carbon materials. Electrochemical corrosion of catalyst carbon-support will lead to the electrical isolation of Pt particles because they are apart from the carbon-supports. These Pt particles will tend to aggregate and grow up, which might be the dominating factors that lead to the ECSA loss of Pt/C catalyst. However, although the ECSA degradation percent of MEAs with Ptloading of 0.4 mg cm 2 is the biggest, its performance loss is minimum. That can be attributed to the greater basic amount of Pt/C
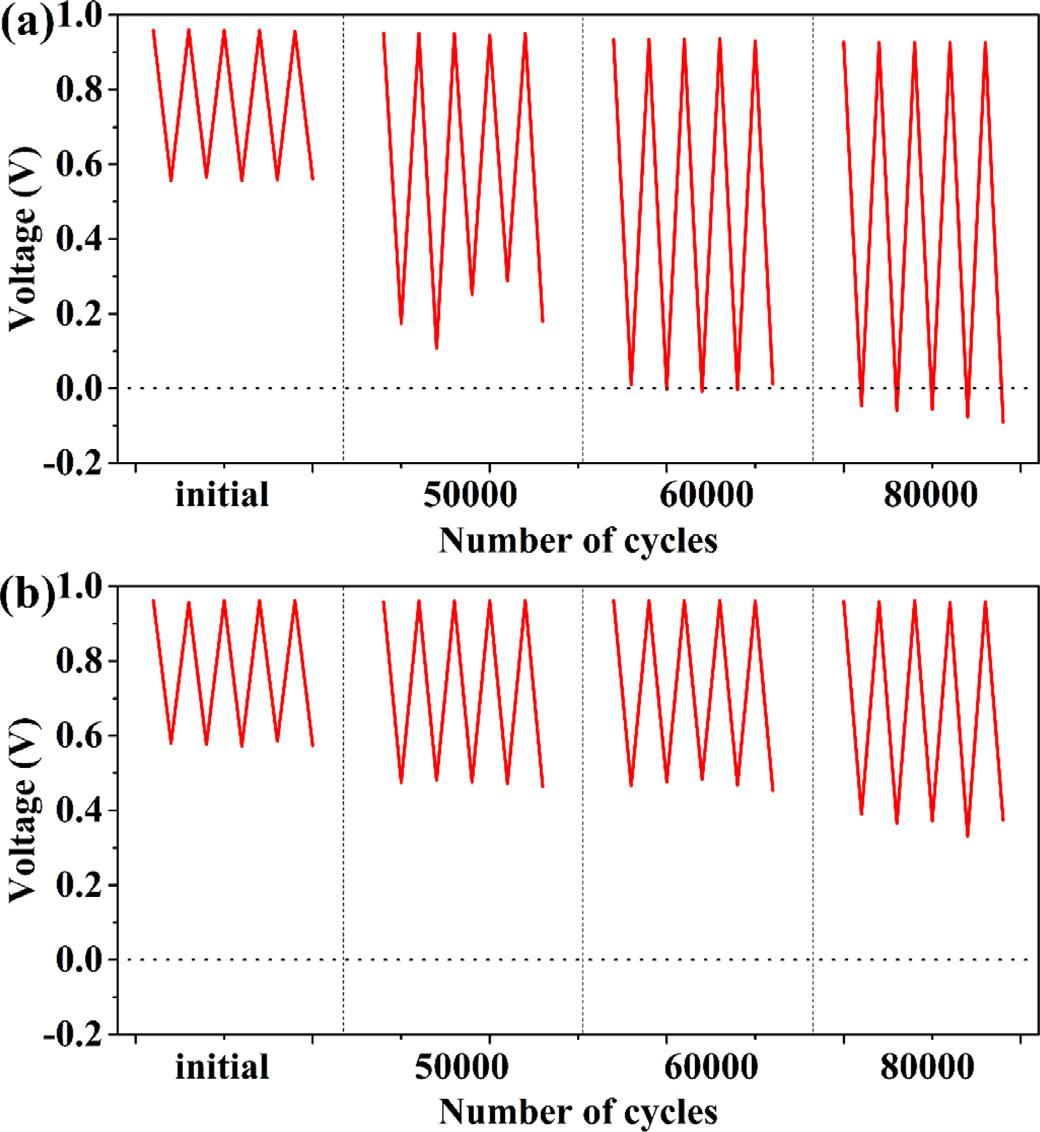
Fig. 5. Voltage evolution of SPFC (a) and WTPFC (b) with Pt-loading of 0.1 mg cm 2
catalyst for Pt-loading of 0.4 mg cm 2 Despite the largest ratio loss of ECSA, the quantity of residual healthy Pt/C after 80,0 0 0 currentvariation cycles is yet high, therefore the MEAs performance is still high and the performance loss is least.
It is obvious that the loss of performance and ECSA of MEAs degraded in WTPFC are less than MEAs degraded in SPFC. With the MEAs after current-variation cycles in SPFC, the performance at 10 0 0 mA cm 2 declines about 0.257 V, 0.101 V and 0.064 V corresponding Pt-loading of 0.2 mg cm 2 , 0.3 mg cm 2 , 0.4 mg cm 2 With regard to degraded MEA of Pt-loading 0.1 mg cm 2 , the voltage at 10 0 0 mA cm 2 is even lower than 0 V. However, when it comes to the MEAs degraded in WTPFC, performance decline is about 93 mV, 55 mV, 49 mV and 20 mV, respectively. Which is much smaller than that of MEAs degraded in SPFC, implying better durability of MEAs in WTPFC. This is owing to the ability of WTP improving water management [24,25] Because of the water drainage function of WTP, excessive water is transported from cathode flow-field channels to circulating water chamber; if there is insufficient of water in MEAs, WTP transports water from circulatory water chambers to MEAs [31].
Fig. 5 shows the voltage evolution of SPFC (Fig. 5(a)) and WTPFC (Fig. 5(b)) with Pt-loading of 0.1 mg cm 2 It is obvious that
SPFC voltage at 600 mA cm 2 fluctuates severely at about 50,0 0 0 degradation cycles, but voltage of WTPFC is more stable. And about 60,0 0 0 cycles, SPFC voltage at 600 mA cm 2 is lower than 0 V occasionally, which is influenced by inadequate water management. When it is at the end of degradation cycles, the voltage (at 600 mA cm 2 ) of SPFC is invariably under 0 V, which means that the MEA is damaged seriously. With regard to WTPFC, voltage is more stable, and at later period of degradation cycles, performance of WTPFC is much higher than that of SPFC. Therefore, the MEAs degradation caused by water management can be mitigated with WTP as cathode flow-field plate.
3.2. Physical characterization of MEAs
In order to monitor the degradation of Pt/C catalyst, TEM images of catalyst before and after current-variation cycles with different MEAs were obtained. Fig. 6 shows the TEM image of Pt/C catalysts and the distribution of Pt particles size before currentvariation cycles. The average Pt particle size of original Pt/C catalyst is about 3.22 nm.
When it comes to MEAs after 80,0 0 0 current-variation cycles, the Pt particles in cathode of all MEAs grow larger, and agglomerate can be clearly observed (Fig. 7). It is obvious that after currentvariation cycles, the size distributions of cathode Pt particles are much wider and there are long tails in the large particle size part. It is account for that under fuel cell running conditions, oxygen is adsorbed, split and converted to water on catalyst active sites, and some fundamental reaction processes are accompanied by structural changes of Pt catalyst. Repeated rapid potential cycling can lead to the mixed state of Pt catalyst with various structural conditions, which may cause the degradation of cathode Pt catalyst [32]. In addition, there is report that rapid potential variation can lead to Pt oxidation and dissolution [33,34], and for Ostwald ripening, small particles shrink and the other big particles grow [35] All these factors lead to the growing up and aggregation of Pt particles. The long tails in the large particle size part may derive from the micrometer-scale platinum dissolution-diffusion-precipitation mechanism [34].
After current-variation cycles, with regard to the MEAs degraded in SPFC, the average sizes of Pt particles in cathode are 6.30 nm, 6.73 nm, 7.08 nm and 7.79 nm, respectively, corresponding the Pt-loadings of 0.1 mg cm 2 , 0.2 mg cm 2 , 0.3 mg cm 2 and 0.4 mg cm 2 (Fig. 7(a), (c), (e) and (g)). It is obvious that the average Pt particle size of cathode catalysts becomes larger as the cathode Pt-loading increases. This is a result of more serious corrosion of carbon support with higher cathode Pt-loading, which means a thicker CL. Thicker CL results in longer distance of mass transfer, which may cause uneven reactants distribution. Uneven fuel distribution in a PEMFC causes “no fuel”regions, which will be occupied by oxygen permeating through the membrane, resulting in a potential jump of the cathode to meet the demand of current, thus carbon corrosion is accelerated [28] Because Pt


particles are anchored on carbon support, carbon corrosion can cause Pt particles detaching from it. Pt particles dissolve, diffuse and redeposit onto larger particles, thus the size of Pt particles increase [17]. So, a direct effect of thicker CL is harmful for mass transfer in MEAs, which can aggravate the carbon material decay. The corrosion of carbon support can cause the detachment of Pt particles from it and aggregation of Pt particles [30] In the case of oxidant starvation on cathode, hydrogen pump will occur and hydrogen is generated in cathode. The presence of hydrogen on
cathode could chemically generate heat on the platinum particles [36] The solubility of Pt increases with temperature [17], so local oxidant starvation can accelerate the dissolution of Pt particles. Specifically, the density of Pt particles on carbon support after dynamic cycles decreases obviously in comparison with the initial Pt/C catalyst. Additionally, there is obviously bare carbon support, and the distribution of Pt particles on carbon support is uneven, which can be contributed to that Pt particles could detach from carbon support under the potential cycling condition [37]. When it

8. Comparison of TEM images of SPFC and WTPFC anode catalysts after current-change cycling for various Pt-loading MEAs: (a) and (b) 0.1
(c) and (d) 0.2 mg cm 2 ; (e) and (f) 0.3
; (g) and (h) 0.4
(a), (c), (e) and (g) degraded in SPFC; (b), (d), (f) and ( h) degraded in WTPFC.
comes to the MEAs degraded in WTPFC, after current-variation cycles, the average Pt particle sizes in cathode are 5.84 nm, 5.88 nm, 6.04 nm and 6.01 nm, respectively, corresponding to the Pt-loadings of 0.1 mg cm 2 , 0.2 mg cm 2 , 0.3 mg cm 2 and 0.4 mg cm 2 (Fig. 7(b), (d), (f) and (h)), which are also much larger than that of the initial Pt/C catalyst. Besides, there is aggregation of Pt for all MEAs. Comparing with MEAs degraded in SPFC, after current-variation cycles, the average Pt particle size of MEAs degraded in WTPFC is less. In addition, the dispersion of Pt particles on carbon support
is better than that of MEAs degraded in SPFC. It implies that using WTP is beneficial to alleviating the degradation of cathode catalysts during the dynamic process. Moreover, the average Pt particle sizes of MEAs degraded in WTPFC after current-variation cycles are approximate, and it might be that WTP can mitigate the influence of CL thickness on mass transfer effectively because of its ability to improve water management.
Fig. 8 shows the comparison of TEM images of anode catalysts after current-variation cycles for various Pt-loading MEAs. After


dynamic cycles, the average Pt particle sizes are approximate with all MEAs. Comparing with initial Pt/C catalyst, the Pt particles grow up a little (< 0.4 nm). However, it cannot be ignored that the aggregation of anode Pt/C catalyst is more serious than that of cathode Pt/C catalyst. But the distribution of anode Pt particles on carbon support is more even than that of cathode Pt particles. It can be attributed to that the cathode has suffered high potential during current-variation cycles, and the Pt oxide layers forming and removal repeatedly, which leads to the quicker decay of cathode Pt/C catalyst [38].
Because the difference of performance degradation between MEAs degraded in SPFC and WTPFC increases as the electrode Ptloading decreases, MEAs with lower Pt-loading (0.1 mg cm 2 and 0.2 mg cm 2 ) were chosen to compare the difference of CL decay.
Fig. 9 shows the SEM images of cathode CL. It can be seen that the thickness of cathode catalyst layer from fresh MEAs with Pt-loading 0.1 mg cm 2 is 0.648 μm (Fig. 9(a)). However, after 80,0 0 0 current-variation cycles, the thickness of cathode CL for MEA degraded in SPFC is 0.5 μm (Fig. 9(b)) and that for MEA degraded in WTPFC is 0.592 μm (Fig. 9(c)). For the case of Pt-loading 0.2 mg cm 2 , the thickness of cathode CL changes from 0.592 μm (fresh) (Fig. 9(d)) to 0.889 μm (MEA degraded in SPFC) (Fig. 9(e)) and 1.246 μm (MEA degraded in WTPFC) (Fig. 9(f)). The thickness of cathode CL of MEAs degraded in WTPFC is higher than that of MEAs degraded in SPFC, and it can be contributed to the severer cathode carbon support corrosion of MEAs degraded in SPFC. Serious carbon support corrosion can bring about running offof Pt particles and attenuation of CLs. The above state further demonstrates that taking advantage of WTP as cathode flow-field plate is good for the durability of MEAs during the current-variation cycles.
Fig. 10 presents the SEM images of anode CL. It is obviously that the CL thickness of degraded MEAs is approximately the same with fresh MEAs when the Pt-loading is uniform, which also proves that the degradation of anode catalyst is a consequence of migration and aggregation of Pt particles, rather than carbon support corrosion.
4. Conclusions
The performance and ECSA of MEAs were characterized to evaluate the MEAs degradation after 80,0 0 0 rapid current-variation cycles. With the increase of Pt-loading, MEA performance degradation will be mitigated. But when the Pt-loading is 0.4 mg cm 2 , the degraded percent of ECSA is largest, which may result from the weaker mass transfer in thicker cathode CL, and blocked mass transfer can lead to degradation of carbon materials. Besides, the loss of performance and ECSA of MEAs degraded in SPFC is higher than that of MEAs degraded in WTPFC.
SEM and TEM images confirm that the WTP as cathode flowfield plate can mitigate the degradation of Pt/C catalyst caused by mass transfer in CL, because of the ability of WTP to improve the water management of PEMFC. Moreover, it is concluded that the cathode Pt/C catalyst decay is mainly caused by the corrosion of carbon support, and the degradation of anode Pt/C catalyst is a consequence of migration and aggregation of Pt particles.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (Grant no.
2016YFB0101208), NSFC-Liaoning Joint Funding (Grant no. U1508202) and the National Natural Science Foundations of China (Grant no. 61433013 and 91434131)
References
[1] G. Zhang, Z.G. Shao, W. Lu, H. Xiao, F. Xie, X. Qin, J. Li, F. Liu, B. Yi, J. Phys. Chem. C 117 (26) (2013) 13413–13423
[2] K. Isegawa, T. Nagami, S. Jomori, M. Yoshida, H. Kondoh, Phys. Chem. Chem. Phys. 18 (36) (2016) 25183–25190
[3] H. Chang, Z. Wan, X. Chen, J. Wan, L. Luo, H. Zhang, S. Shu, Z. Tu, Appl. Therm. Eng. 104 (2016) 472–478
[4] S.W. Jeon, D. Cha, H.S. Kim, Y. Kim, Appl. Energy 166 (2016) 165–173
[5] Y. Qiu, H. Zhong, M. Wang, H. Zhang, J. Power Sources 283 (2015) 171–180
[6] Q. Shen, M. Hou, X. Yan, D. Liang, Z. Zang, L. Hao, Z. Shao, Z. Hou, P. Ming, B. Yi, J. Power Sources 179 (1) (2008) 292–296
[7] P.K. Takalloo, E.S. Nia, M. Ghazikhani, Energy Convers. Manag. 114 (2016) 290–302
[8] X. Yan, M. Hou, L. Sun, D. Liang, Q. Shen, H. Xu, P. Ming, B. Yi, Int. J. Hydrogen Energy 32 (17) (2007) 4358–4364
[9] X. Guo, Z. Shao, Y. Xiao, Y. Zeng, S. Liu, X. Wang, B. Yi, Electrochem. Commun. 44 (2014) 16–18
[10] D.G. Sanchez, T. Ruiu, K.A. Friedrich, J. Sanchez-Monreal, M. Vera, J. Electrochem. Soc. 163 (3) (2016) F150–F159
[11] D.N. Ozen, B. Timurkutluk, K. Altinisik, Renew. Sustain. Energy Rev. 59 (2016) 1298–1306
[12] S.H. Ahn, S. Jeon, H.-Y. Park, S.-K. Kim, H.-J. Kim, E. Cho, D. Henkensmeier, S.J. Yoo, S.W. Nam, T.-H. Lim, J.H. Jang, Int. J. Hydrogen Energy 38 (23) (2013) 9826–9834.
[13] D.G. Sanchez, T. Ruiu, I. Biswas, M. Schulze, S. Helmly, K.A. Friedrich,J. Power Sources 352 (Suppl. C) (2017) S42–S55
[14] M.K. Debe, Nature 486 (2012) 43
[15] H.A. Gasteiger, S.S. Kocha, B. Sompalli, F.T. Wagner, Appl. Catal. B Environ. 56 (1) (2005) 9–35
[16] R. Lin, F. Xiong, W.C. Tang, L. Techer, J.M. Zhang, J.X. Ma, J. Power Sources 260 (2014) 150–158
[17] R. Borup, J. Meyers, B. Pivovar, Y.S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J.E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K.-I. Kimijima, N. Iwashita, Chem. Rev. 107 (10) (2007) 3904–3951
[18] R.M. Darling, J.P. Meyers, J. Electrochem. Soc. 152 (1) (2005) A242–A247
[19] R.M. Darling, J.P. Meyers, J. Electrochem. Soc. 150 (11) (2003) A1523–A1527
[20] I. Khazaee, H. Sabadbafan, Energy 101 (2016) 252–265
[21] M. Andersson, S.B. Beale, M. Espinoza, Z. Wu, W. Lehnert, Appl. Energy 180 (2016) 757–778
[22] K. Ketpang, S. Shanmugam, C. Suwanboon, N. Chanunpanich, D. Lee, J. Membr. Sci. 493 (2015) 285–298
[23] L. Xing, Q. Cai, C. Xu, C. Liu, K. Scott, Y. Yan, Energy 106 (2016) 631–645
[24] X. Guo, Y. Zeng, Z. Wang, Z. Shao, B. Yi, J. Power Sources 302 (2016) 84–91
[25] Z. Wang, Y. Zeng, S. Sun, Z. Shao, B. Yi, Int. J. Hydrogen Energy 42 (34) (2017) 21922–21929
[26] X. Guo, Y. Zeng, Z. Wang, L. Qu, Z. Shao, Z. Yuan, B. Yi, Electrochim. Acta 191 (2016) 116–123.
[27] H. Matsui, N. Ishiguro, T. Uruga, O. Sekizawa, K. Higashi, N. Maejima, M. Tada, Angew. Chem. Int. Ed. 56 (32) (2017) 9371–9375
[28] S. Park, Y. Shao, H. Wan, V.V. Viswanathan, S.A. Towne, P.C. Rieke, J. Liu, Y. Wang, J. Phys. Chem. C 115 (45) (2011) 22633–22639
[29] Y. Shao-Horn, W.C. Sheng, S. Chen, P.J. Ferreira, E.F. Holby, D. Morgan, Top. Catal. 46 (3) (2007) 285–305
[30] Z.-M. Zhou, Z.-G. Shao, X.-P. Qin, X.-G. Chen, Z.-D. Wei, B.-L. Yi, Int. J. Hydrogen Energy 35 (4) (2010) 1719–1726
[31] Z. Wang, L. Qu, Y. Zeng, X. Guo, Z. Shao, B. Yi, RSC Adv. 8 (3) (2018) 1503–1510
[32] N. Ishiguro, T. Saida, T. Uruga, O. Sekizawa, K. Nagasawa, K. Nitta, T. Yamamoto, S.-I. Ohkoshi, T. Yokoyama, M. Tada, Phys. Chem. Chem. Phys. 15 (43) (2013) 18827–18834
[33] S. Chen, H.A. Gasteiger, K. Hayakawa, T. Tada, Y. Shao-Horn, J. Electrochem. Soc. 157 (1) (2010) A82–A97
[34] P.J. Ferreira, G.J. la O’, Y. Shao-Horn, D. Morgan, R. Makharia, S. Kocha, H.A. Gasteiger, J. Electrochem. Soc. 152 (11) (2005) A2256–A2271
[35] M. Moein-Jahromi, M.J. Kermani, S. Movahed, J. Power Sources 359 (Suppl. C) (2017) S611–S625
[36] S. Qu, X. Li, M. Hou, Z. Shao, B. Yi, J. Power Sources 185 (1) (2008) 302–310
[37] K.J.J. Mayrhofer, J.C. Meier, S.J. Ashton, G.K.H. Wiberg, F. Kraus, M. Hanzlik, M. Arenz, Electrochem. Commun. 10 (8) (2008) 1144–1147
[38] M. Darab, P.K. Dahlstrøm, M.S. Thomassen, F. Seland, S. Sunde, J. Power Sources 242 (Suppl. C) (2013) S447–S454
Another random document with no related content on Scribd:
Ennen kuin tulen noutamahan, Yheksällä orihilla, Yhen tamman kantamalla.
Sinne sinua on itkettynnä, Ikäkausi kaivattunna.
Mene tuonne kunne käsken, Ryönä kosken partahaseen, Virran korvahan kovahan, Perin siellä vieryvät petäjät, Kokonansa kokka hongat, Tyvin syösten suuret hongat, Latvoin laikka-päät petäjät.
Pakkaselle. Pakkanen puhurin poika, Älä kylmää kynsiäni, Älä päätäni palele!
Tultas tungen sukkahani, Kekälettä kenkähäni, Pakkasen palelemata, Kovan ilman koskemata, Kyllä sull on kylmäämistä, Kylmää soita, kylmää maita, Kylmää puita ja pehuja, Kylmää kylmiä kiviä!
Merimiesten rukous Ilmariselle.
Ilmarinen ilo lintu.
Lennä tuonne kunkan käsken, Iän ikuisen perähän, Päivän koitonnon kotohon!
Pane poskes pussuksihin.
Puhalla iloinen ilma,
Minulle myötäinen myry, Minnekkin nyt mennäkseni!
Rukous jolla Ahto (kalain haltia) kutsuttiin.
Anna Ahto antimesi,
Veä vilialta väleen,
Majan märjän asuvita, Kalat karulta kokoele,
Hauit hajalta hajeskele, Ahvenet myös kyrmyniskat!
Riennä ruoholle veäksen, Soitantota kuulemahan, Väännäntötä Väinämöisen.
Päivän koittaissa.
Terve kasvos näyttämästä,
Päivä kulta koittamasi», Aurinko ylenemästä!
Pääse ylös aaltoin alta, Yli männistön matkusta, Juokse kaares kaunihisti,
Pääse illalla ilohos.
Tuo tuliaiset tullessasi, Tuo meill' täyttä terveyttä, Siirrä saama saatavihin, Onni onkeemme ohaitse, Pyytö päähän peukalomme!
Nykyisempiä Runoja ja Lauluja.
Lystillinen Runo-laulu, siitä Kummasta Kala-Kukosta lookisti kokoonpantu, Henrikki Väänäseltä.
[Henrikki Väänänen myöskin kutsuttu Konsa Heikki oli muinen Oulusa köyhä työmies, joka kulkein ympärin kyliä elätti ittensä viulun soitolla ja omatekemäin runoin veisaamisella. Jotenkin viinaan menevä oli hän aina saapuilla pidoissa ja tanssi paikoissa, aina nöyrä ja aina ilonen. Hänellä oli ankara muisto ja erinomainen sukkeluus kokoonpanna veisuja aivan valmistamatonna ja mistä aineesta tahtonsa. — Viimmein kuoli tämä runo-niekka lämpöisesä uunisa,
johonka hän oli viinapäissä kontannut. Ne paremmat hänen jälkeen
säilyneistä runoista ovat: Kala-kukosta, Kaffen juomingista, Oulun orja-väestä ja Kellon kylän Mariasta. Kala-kukko on jo ennen präntätty Vaasasa v. 1801, ehkä nyt harvasa löytty.]
Jop' on laulu laitettuna, Sanat somat solmettuna,
Nytkin ruasta rumasta
Kummasta kala-kukosta,
Johonk' oli pantu Paltamossa,
Katti karvanen sisähän, Isossa itä-kylässä, Paltamossa mainiossa.
Kerran keskensä isännät, Rannin miehissä rupeisit,
Pyhä iltana puhuhun,
Julkisesti juttelehen, Kuin on Oulusa pahoja, Tullisakin turkasia;
Syövät syökärit rahata
Ilman työtä tullin miehet, Kuin vievät välistä reestä
Evähiä matkamiesten.
Talon vaari taitavasti
Kysyypi kylän väeltä: "Kusta nyt saisin kumpanita
Kusta matkallen toverit?
Lähtisin minäkin kerran
Käpäsemään kaupungissa; On mulla talia taasen,
Vielä voitakin vähäsen, Vaik' on huono heinä-vuosi, Muret muustakin ruasta".
Miehet yksi-äänisesti
Siihen vastaten sanovat:
"Meill' on miehillä samoilla
Matka pitkä mielesämme, Lähtiä sitä pitäisi
Ala mahan marsimahan, Kohen Oulua kokehen".
Kohta yksi koiran silmä
Sanoopi väen seassa:
"Jopa kotua kyllä
Joulun eellä jouvetahan;
Olis mielessä minulla
Lähtö poies Pohjan maalle;
Lähtisin minäkin muuten
Käpäsehen kaupungissa,
Vaan ompi vähän vikoa, Joka on kotona kauon
Mulla muistissa pysynyt, Poviani pureskellut.
Vietihin minulta viimein,
Väkisin vasikan paisti, Kuin näki olevan reessä,
Sitä syökäri syleili.
Sitä ano ahkerasti, Pyysi pystösä käsinni.
Minä mies sanon hänelle
Puheskelin puolestani:
'En saata evästä panna
Tyköäni, ystäväni!
Poies matkan pitkän päässä.
Kylläpä täällä tarvittoovi Pureskella porvarissa'.
Veipä sitten väkisin;
Sen kanssa meni sisälle, Vielä kiitti kinttujansa, Hyvin kosti kynsiänsä.
Kuin ovat viekkaat viriät, Omin mielin ottamahan, Talon poikain evästä!
Sitte kuin tulin kotia, Heti sai kylässä tietä
Akkani saman asian, Josta akka aikalailla, Minua toru tolvanaksi,
Kun en minä kukkoa kätehen,
Sysännynnä syökärille.
Anto hälle lemmon lintu,
Omalle tulli nälkäiselle, Kuuluupi kyllä puhuvan, Kieli-lakkarin latovan, Ett' on muutamat monasti, Kotonahan nivuiset koiran, Pannullansa paistanehet, Rasvan kanssa raskinehet.
Voilla sieviäksi silattu,
Mennesänsä on antanehet,
Tull-miehelle kätehen,
Sysäänehet syökärille.
Tuon konstin minä kotona, Saatan vielä viisahammin, Tehä toisehen tapahan".
Ottipa kissan kiiruhusti, Tarttuupi takajaloista,
Päätä pankkohon sukasi, Oikian olan takoa.
Uunin nurkkahan nutisti,
Siinä hirtti hiiren syöjän, Pani pirtin lämmitessä, Leivän sisähän leviän.
Rupeis estähän emäntä, Etteipä isäntä saisi
Kattia kakun sisähän, Panna paljaan karvan kanssa.
Emäntä isännällensä,
Sanoopi sanalla tällä:
"Kosk' on herjä herjennynnä, Naaras kissa naukumasta,
Niin nyljen nahattomaksi, Siitäpä tulisit sievät,
Turkin puuhkat pulskiammat".
Ukko uunilta puhuupi, Parta-vaari paukuttaapi:
"Kylläpä sinä siivo muori, Oikein hyvä olisit, Syökärille syölähille, Kuin nyt siitä kuoren poies, Veisikkin nahan väkisin,
Käden kesken paistumisen, Siitäpä syökärit rupiais, Arvelehen aivostansa,
Et on tähän sisähän, Jänes pantu Paltamossa, Siitä mielehen menisi, Talon-poikain tavarat, Vastakin repis rekiä, Evähiä ettiskelis.
Anna olla katti karvonensa, Että tulee tuntemahan,
Mik' on pantu Paltamossa, Koottu kuorien sisähän, Taherrettu taikinahan".
Samasta sanasta tästä, Kävi kärryksi emäntä, Rupiaapi rohkiasti,
Koukulla kopasemahan,
Ukon päätä uunin päällä; Vaan ei toki tohtinunna,
Ett' oli vaari vahvallainen; Kyll' olis muori muuten lyönyt, Takan vierestä takonut, Äijän päätä oiva lailla.
Isäntä ihasteleepi, Lieden vierehen lipuupi.
Pani kissan paistumahan, Katin karvat kärtymähän.
Vielä se vinku uunissakin, Kurasi kukon sisällä.
Sitte kuin kuori päälle kuivi,
Taikina tuli kovaksi,
Katin päälle kankiaksi.
Ulos uunista nykäsi, Paneepi säkin sisähän.
Sitte lähteepi kotua, Maanantaina marsimahan, Astuhun alusta viikon, Kohen Oulua kokehen.
Kuin tuli tykö Muhoksen, Löysi kohta kumppaninsa, Kumppanit heti kysyvät:
Joko nyt jokia menemmä, Linnan tullista livumme,
Käymmä kautta köyhän miehen?
Juuruksen [1] tykö tulivat, Kestikiivarin kedolle.
Silta-vouvi Simpermanni.
Oli juossu Juuruksehen, Tukkihin hakoin kanssa
Tietä kiinni kiiruhusti, Ettei joutuisi joelle.
Sieltä kulkisi kukana.
Siin' oli kielly Simpermanni, Talonpoille puhunut:
"Ei sa mennä Pielis-miehet, Eikä muu Muhoksen väki, Eikä käyä Paltamosta
Oulun suuhun ollenkana, Kura tullihin kukana,
Siihen siikoja isoja, Kuljettavat Kuusamosta, Punasta poron lihoa, Vaikka sentähen vähäsen, Norforssi välistä saapi, Tutummilta tuvan luona, Kuusamon mätimahoilta.
Ne jotka jokia aivot,
Mylly-tullista tulehen, Simpermannin käskyn kautta Kangas-tullihin kävivät.
Pistit ensin pienen leivän, Tulli-herralle kätehen, Tästäpä herra herjemmäksi, Kovin koiraksi rupesi,
Sano herra hilpiästi:
'Ketä sinä tällä kerjulaista,
Pilaat pila-isäntä, Ompa sulla suurempia, Kala-kukkoja komeita, Anna mulle muuankahan Teiän maanne maistiksista".
Mies se mielellä hyvällä.
Sysäsi kukon kätehen, Johonk oli sisään kissa, — Pantu katti karvonensa;
Hetipä käski tulli-herra, Tämän miehen tupahan, Kuttu Rytsi ruukostille, Anto kaffet, anto punsit.
Vielä viinatkin lisäksi.
Kuin oli sarikat saanut, Ryypyt suuhunsa suloiset,
Mies heti meni kadulle, Sieltä poikkes porvariinsa.
Tämä on laulu laitettuna, Sanat väätty Väänäseltä, Sille palkasta tulisi.
Tahtoisi jalan takasen,
Kuin olis ollut saatavilla Silloin tullisa tykönä.
Kuin Rytsi rualle rupesi, Iltaselle ilkiälle.
Haukkasi palasen päästä,
Toukaseepi toisen kerran,
Jo sattu käpälä suuhun,
Kynnet kielehen rupeisit.
Luuli hauin hampahiksi
Eli lahnan leuka-luuksi.
Eipä usko ensinkänä
Kuin ei viillä veittellähän, Näkeepi olevan siellä, Karvasen katin sisällä;
Hetipä kirosi herra, Kesken syönnin synkiästi, Sanoo sanalla tällä:
"Eipä tuota turkastakaan, Luullu nyt minä polonen, Perkelettä petturiksi.
Kuin on ilkiä isäntä,
Pilannut parahan viljan, Tuolla lailla tuhrannunna.
Ei sitä tiedä ihmis rukka, Mitä syödä synnis paran, Vasta vanhana pitääpi, Kuin ei nuorra näitä nähty, Nuita kummia rumia".
Jop' on laulu laskettuna, Loppu lookisti rakettu, Somahammilla sanoilla.
[1] Juurus on se kaikkein likimmäinen Kestikiivari Oulusta
Paltamoon päin, yksi penikulma kaupungista. Juuruksesta kuljetaan talvella Ouluun sekä jokia, että maantietä myöden. Jokia myöden tullaan sisälle kaupunkiin linna-tullin kautta, joka myös toisella nimellä kutsutaan mylly-tulliksi; haukkuvat ne sitä muutamat myöskin kura-tulliksi. Siinä linna-tullisa edes seisot yksi syökäri
Norforssi nimeltä, joka jo on kuollut, kauvan aikaa tulli-herran viran. Ne kyläkunnat, Juuruksen ja Oulun välillä, jotka juuri joen partahalla molemmin puolin jokia ovat, kutsutaan yhdellä yhteisellä nimellä Oulun suuksi. Ne matkamiehet taas, jotka Juuruksesta maantietä myöden kaupunkiin kulkevat, tulevat kangas-tullin kautta sisälle, josa Rytsi tulli-herrana oli. Syy, minkätähe Simpermanni esteli matkamiehiä jokia myöden Juuruksesta Ouluun kulkemaan, oli yksi tästä kulusta silloin ulostullut Maanherran kielto.
Sinä 24 päivänä Kesäkuuta 1791.
Vaikka nälkä nähtävästi,
Suuri surkeus ruasta,
Aivan kovin ahdistaapi
Paljo ihmis parkaisia;
Vaikka tauti tarttuvainen, Kauheasti kaateleva,
Tänä vuonna on temunnut, Pitkältä liki pitänyt;
Vaikka talvi tasamatoin, Tulo tukala keväinen,
Tahto tallit puhdistella, Laasta laattiat ladoissa, Että karja kaikellainen, Että elävät usiat,
Rupeisit ruan hädäsä, Puolen vihon puutoksesa, Äkeästi ääntämähän,
Inumahan ilkiästi;
Aika kuitenni kulunut, Nyt on siihen siirtynnynnä,
Että kesä on käsissä, Mainihtava Mehtumaari, Päivä julkea Juhanin, Ihmis paroille iloisin.
Joska jouduttas sanoman, Tämä tuottas tultuansa, Että kesä kelvollinen, Olis hyvin onnellinen, Joka jouduttas eloa, Kaikellaista kasvattelis,
Että terveys terästys, Pysyis rauha ratkomata.
Se on säännösä Jumalan, Tahdosa Taivahan Herran.
Hän on ajan alkanunna, Vuodet, päivät perustanut, Hän on vaka vallihtia, Yksinäns ylöspitäjä.
Anna siis armos Jumala!
Luoja suuri luotuitten, Ajat vielä virkistyä, Kostua koko hyväksi;
Anna armos avarasti, Laskeua laupeudes, Kansan ylitse katalan, Mailmasa matavaisen!
Herra jonka joutununna,
Nyt on oiva nimi-päivä, Sulle toivotus totinen, Altihiksi annetahan:
Onni sua ohjatkohon, Sekä hyvin seuratkohon, Kaikissa kahtottavissa, Asioisasi alati!
Papin virka painavainen, Kai raskas kannettava, Yli-paimenen parahan, Armon ja avun ohesa, Keviäksi kääntyköhön, Hupaiseksi huojetkohon!
Ikä illalle kulunut, Harmajaksi haalistunut, Uuden aamun alkakohon, Onnellisimman olennon, Autuitten asumisa, Taivahan Ilo-Talosa!
Henric Ackrenius.
Tämän toivotuksen sai entinen Kirkkoherra Pyhäjoesa Tohtori Johan Westzynthius.
Idensalmen Kirkko Herran
Herr Mag. Joh. Laguksen ja korkiasti kunnioitettavan Neitsyn Benedicta Lovisa Kraftmannin iloisisa hääpidoisa Borgosa.
Lyra lauloi lystin vuoksi, Koska mieli sinne juoksi, Tämän nöyrän naima-virren:
1. Koska luonnon Luoja Herra, antoi kerran, Koko luonnoll' lujat lait, Kaikki käyvät muuttumata, puuttumata, Niin kuin aluus käskyn sait.
2.
Vaan kosk' Herra se jalompi itse ompi, Sula puhdas rakkaus, Niin nyt rakkauden laki luoduisakin, Vahva on ja aina uus'.
3. Kosk' on kaikki kaksin luotu, heille suotu, Kumppalinsa sugustans, Rota toinen toista kahtoo, halull'kahtoo, Omistella vertaistans.
4. Ihminen se järjellinen, mielellinen, Seas Luojan luotujen, Rakkautt' ei taida salat', vaan se alat', Sytyttääpi sydämmen.
5. Eikä sääty, eikä ikä, eikä mikään, Täsä muutost' tehdä saa, Nuoret miehet ovat orjat, neidot korjat, Myyvät vapaudensa.
6.
Ei voi miehuus tätä välttää, koska jälttää
Luonnon laki landeitans, Sankarinkin sapso laulaa, tuli taula, Koska palaa povesans.
7.
Mene viisas kirjas luokse, kiiruust' juokse, Nouda vettä tulehes, Löytyy kuitenk' vihdoin syttö, kaunis tyttö, Joka johtu mielehes.
8.
Paavi sull' on suuri sappi, koskas Pappii Tahdot raket' rautaisiks, Pane pois se pyhä lakki, riisu takki, Tunnusta meit' ihmisiks'.
9. Ei ol' varaa väkevyydes, eik' pyhyydes, Luojan laki voiton saa: Kasvakaat ja lisäntykäät ja täyttäkäät Minull' kunniaks' kaikki maa.
10.
Voitto vahva rakkauden, hartauden, Kirkko-Herran tykön' nyt,
Selväst' nähdään pidois näisä, jalois häisä, Johon ompi yhtynyt.
11.
Sinä yksi yhteydes ja pyhyydes, Kolminainen Herra suur',
Joka kaikki hyvin laitat, omas naitat, Kaksi yhdeks' liität juur'.
12.
Parikunnall' tällen anna, tykö kanna, Kaikkein suurin siunaus, Että alla rakkauden, rinnauden, Heille vuotais armo uus'!
Ajan käyttämisestä, Ruotsista käännetty.
Aika aivan tärkiks' näyttää, Mikä rientää kiiruummast'?
Onnellinen joka käyttää, Muiston siitä sopivast';
Ystäväni vaarii ota, Ajast nuorra ollesas, Että ilon mahdat koota, Ikäs illall' tullesas.
Meri Miehet, Kerran mentyä merelle,
Lainehille laivan kanssa, Meri miehet melkiähän, Tulit ilmahan isohon;
Aallot alusta ajelit, Uhkasit upottamahan.
Miehet vaarasa vapisit, Kovin pelkäsit peräti, Tulevansa tuonelahan, Veden vetelän läpihte.
Mutta väen väkevästi
Sitä suuresti sureissa,
Tuuli tympeä tyveni, Ilma ankara asettu, Josta ilo joutumalla,
Väen sekahan vetäy.
Silloin hahden hallitsia,
Pulska miehille puheli:
Ei pidä pitkin surea,
Eikä iloita isosti;
Sillä maja mailmassa, Tämä vaivanen vaellus, Ompi heikko, hälyväinen, Muutos ilon ja murehen.
Koska onni oikaseepi, Kätensä kelpo hyvästi, Silloin pitäävi surua
Olla valmis ottamahan;
Mutta tultua murehen, Vastoin-käymisen käsihin, Olla toivosa totisen, Kelvokseksi kärsiväisen.
Neion valitus.
Eipä mene mielestäni, Eikä muistosta murene, Armias ihana aika, Jona laulon, ollen lassa, Pikku piikana visersin, Ilolla ihanan linnun, Leipojaisen leikihtevän, Tuolla pilvein povilla, Vapaana vaivatonna.
Vapa vaivoista syämmen, Tuuvin ennen tuulen lailla, Kiiätin kipunan lailla, Lennin lehtenä lehossa, Perhosena pyörtänöillä; Mehun maistelin makean, Kukan kullaisen kupista, Hopealta hohtavaisen.
Muukalainen murehille.
Istuin ilona aholla, Mehu-miellä mättähallä,
Istun kukkana keolla, Lempeästi leikitellen, Suloisten sisarten kanssa, Tyvenesti tuuvitettu.
Tuulen hengeltä tulovan, Mesisestä mantereesta.
Levon kuvana levolla.
Nukun nurmi-linnun lailla.
Rauha rakkahin rakensi, Sian sivuuni suloisen, Eikä uhannu uneni
Vaivalla valkenevaisen
Päivän, pahoilla suruilla, Syämmen nyt suitsevaisen.
Empä tiiä enkä taia,
Selkeästi selvitellä,
Mikä juoksi mieleheni, Mikä aivooni osasi,
Aivan ankara ajatus,
Mikä sytty syämmeeni, Tuli ennen tuntematon, Äitin mullen äännettyä:
Jo nyt vuotta viisitoista, Kohta pikku piikaseni
Olet jättänyt jälelle.
Nousi nousulla nisäini, Suihtu outo syämmeeni,
Nousi nousulla nisäini, Huoli uusi huivin alle, Polvelleni pullistuvan.
Niin on tukala tuvassa,
Mieli raskas mantereessa,
Ei ole lepoa lehossa,
Eikä onni oksapuihen
Asu mustan varjon alla.
Vaiva vaivuttaa levolle,
Vaiva vaivaapi uneni,
Vaiva herättää valolle,
Uuen päivän paisteelle.
Tuolla sytöövi syämmen
Peitetyissä pahjukoissa, Toivon tuli tuntematon,
Tuli sytöövi tukala,
Jot' en saata sammutella,
Enkä raski raiskailla.
Tuonne kiiruhtaa kivasti,
Kaikki kieleni tarinat,
Yksinäni ollessani,
Tuonne aivoni ajatus,
Tuonne suosio syämmen,
Taivon poluille pimeän,
Ahtahille aavistuksen,
Syämmelleni suruisten, Syämmelleni suloisten.
Kaikki kaikissa ajatus, Yks' on aina arvollinen,
Täytten tarvetten seassa, Yks' on tarve toivottava, Syämmelleni suruinen, Syämmelleni suloinen.
Koira joka kuljetti lihamurua suusansa joen poikki. Satu.
Kerran koira kohdattua
Lihamurun murkinaksi, Lähti uimahan uhea, Saalis suussa sutkimahan, Veden vetelän ylitse, Poikki joen joutumalla: Näki uidessa uhean
Varjonsa veden sisällä, Luuli koiran kohdanneensa, Vievän saalista samati, Jota tahto kadehtien, Kyllä hädällä häristen, Tahto taamuta äkisti
Saada saalista sitäkin.
Mutta vaivanen varisti, Suustansa palan suloisen, Ettei ollut ensinkänä, Syömistä syystä hyvästä.
Niin on ahneus alati, Peri juurin petollinen; Jok' ei suovu ensinkänä, Tydy onnehen omahan, Kadehtien kadottaavi
Senkin vähäisen välistä.
Olin ennen ollin mallin, Ollin mallista matikka, Matikasta maitopyörä, Maitopyörästä pytikkä, Pytikästä pöytä risti, Pöytä ristästä ripukka, Ripukasta rintasolki, Rinta soljesta sopukka, Sopukasta sormikintas, Sormi kinttaasta kipinä, Kipinästä kirjan merkki, Kirjan merkistä meteli, Metelistä meiän vouvi, Meiän vouvista votikka, Votikasta voltermanni,
Voltermannista matikka, Matikasta maitopyörä, Maitopyörästä j.n.e.
Tämä joutava ja loppumaton loru veisataan monesa paikasa Pohjan maalla kätkyt- ja lasten veisuna. Erinomaisuudensa vuoksi saakon se myös siansa täsä kokouksesa.
*** END OF THE PROJECT GUTENBERG EBOOK SUOMEN KANSAN
VANHOJA
RUNOJA YNNÄ MYÖS NYKYISEMPIÄ LAULUJA 2 ***
Updated editions will replace the previous one—the old editions will be renamed.
Creating the works from print editions not protected by U.S. copyright law means that no one owns a United States copyright in these works, so the Foundation (and you!) can copy and distribute it in the United States without permission and without paying copyright royalties. Special rules, set forth in the General Terms of Use part of this license, apply to copying and distributing Project Gutenberg™ electronic works to protect the PROJECT GUTENBERG™ concept and trademark. Project Gutenberg is a registered trademark, and may not be used if you charge for an eBook, except by following the terms of the trademark license, including paying royalties for use of the Project Gutenberg trademark. If you do not charge anything for copies of this eBook, complying with the trademark license is very easy. You may use this eBook for nearly any purpose such as creation of derivative works, reports, performances and research. Project Gutenberg eBooks may be modified and printed and given away—you may do practically ANYTHING in the United States with eBooks not protected by U.S. copyright law. Redistribution is subject to the trademark license, especially commercial redistribution.
