Achieving uniform Pt deposition site by tuning the surface microenvironment of bamboo-like carbon nanotubes Meng Jin
https://ebookmass.com/product/achieving-uniformpt-deposition-site-by-tuning-the-surfacemicroenvironment-of-bamboo-like-carbon-nanotubesmeng-jin/

Download more ebook from https://ebookmass.com
More products digital (pdf, epub, mobi) instant download maybe you interests ...

Industrial Applications of Carbon Nanotubes 1st Edition
Peng Huisheng (Ed.)
https://ebookmass.com/product/industrial-applications-of-carbonnanotubes-1st-edition-peng-huisheng-ed/



Winterberries-like 3D network of N-doped porous carbon anchoring on N-doped carbon nanotubes for highly efficient platinum-based catalyst in methanol electrooxidation Tong Wang
https://ebookmass.com/product/winterberries-like-3d-network-of-ndoped-porous-carbon-anchoring-on-n-doped-carbon-nanotubes-forhighly-efficient-platinum-based-catalyst-in-methanolelectrooxidation-tong-wang/
In-situ growth of CoP wrapped by carbon nanoarray-like architecture onto nitrogen-doped Ti3C2 Pt-based catalyst for efficient methanol oxidation W. Zhan & L. Ma & M. Gan
https://ebookmass.com/product/in-situ-growth-of-cop-wrapped-bycarbon-nanoarray-like-architecture-onto-nitrogen-doped-ti3c2-ptbased-catalyst-for-efficient-methanol-oxidation-w-zhan-l-ma-mgan/
Emerging Applications of Carbon Nanotubes in Drug and Gene Delivery Prashant Kesharwani
https://ebookmass.com/product/emerging-applications-of-carbonnanotubes-in-drug-and-gene-delivery-prashant-kesharwani/

Mechanical Behaviors of Carbon Nanotubes. Theoretical and Numerical Approaches. A volume in Micro and Nano Technologies 1st Edition Edition Kim Meow Liew
https://ebookmass.com/product/mechanical-behaviors-of-carbonnanotubes-theoretical-and-numerical-approaches-a-volume-in-microand-nano-technologies-1st-edition-edition-kim-meow-liew/

National Uniform Legislation Guzyal Hill
https://ebookmass.com/product/national-uniform-legislationguzyal-hill/



Structure of the in situ produced polyethylene based composites modified with multi-walled carbon nanotubes: In situ synchrotron X-ray diffraction and differential scanning calorimetry study Coll.
https://ebookmass.com/product/structure-of-the-in-situ-producedpolyethylene-based-composites-modified-with-multi-walled-carbonnanotubes-in-situ-synchrotron-x-ray-diffraction-and-differentialscanning-calorimetry-study-coll/
The Law of Contracts and the Uniform Commercial Code 3rd Edition, (Ebook PDF)
https://ebookmass.com/product/the-law-of-contracts-and-theuniform-commercial-code-3rd-edition-ebook-pdf/
Composite
Materials It Meng Low
https://ebookmass.com/product/composite-materials-it-meng-low/

Full Length Article
Contents lists available at ScienceDirect
Applied Surface Science
Achieving uniform Pt deposition site by tuning the surface microenvironment of bamboo-like carbon nanotubes
Meng Jin a, c, * , Rong Wang a , Bi Jia a , Jun Zhang a , Hui Liu d , Shi-Yu Lu a, b, *
a College of Metallurgy and Materials Engineering, Chongqing University of Science and Technology, Chongqing 401331, China
b BIC-ESAT & School of Materials Science and Engineering, Peking University, Beijing, 100871, China
c Department of Chemistry, Tsinghua University, 30 Shuangqing Rd, Haidian District, Beijing 100084, China
d Key Laboratory of Luminescence Analysis and Molecular Sensing (Southwest University), Ministry of Education, School of Materials and Energy, Southwest University, Chongqing, 400715, China
ARTICLE INFO
Keywords:
Pt deposition
bamboo-like CNTs
Surface microenvironment
Methanol oxidation
ABSTRACT
1. Introduction
With gaining much considerable attention to the increasing the energy crisis and environmental pollution, the utilization and development of various energy sources with more environmentally friendly, renewable, and efficient are becoming an urgent problem to be solved [1–3]
Direct methanol fuel cells (DMFCs), due to the property of low operating temperature, high energy density, and energy conversion efficiency, have been widely deemed as the ideal energy conversion devices for portable electronic devices and electric vehicles [4–8]
Currently, commercial catalysts utilized in fuel cell anode for MOR are mainly Pt-based materials, which means sluggish reaction kinetics, exorbitant costs, inferior CO tolerance and poor stability. Reasonable and valid modulation of Pt NPs on the supports are an essential approach to reduce the usage and promote utilization efficiency of Pt in anode catalysts, further improving its catalytic activities towards to MOR [9–13] As everyone knows, the size distribution, interaction force, morphology, and coordinate form of Pt NPs on supports are crucial factors relative to the surface state of the carrier [14]. Nitrogen site,
* Corresponding authors.
journal homepage: www.elsevier.com/locate/apsusc https://doi.org/10.1016/j.apsusc.2022.153201
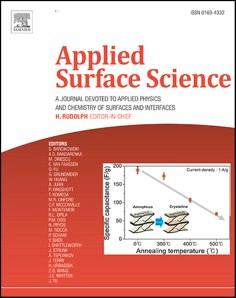

The surface microenvironment of supports, including surface state and constitution, are crucial adjective factors to the dispersibility and particle size of Pt nanoparticles (NPs) on carrier, follow-up affecting their catalytic activities for methanol oxidation reaction (MOR). Here an in-situ pyrolysis approach is reported for preparing bamboo-like N doped carbon nanotubes coupled with fewer metallic particles (M@N-CNTs (M = Co, Ni and Fe3C)), enabled tunable surface state and constitution of carbon layers. Combined experimental and theoretical investigation, as fabricated Co@N-CNTs provide optimal surface of backbone for Pt deposition with narrowest particle size distribution and minimum size by virtue of dominated pyridinic-N, enriched defect, abundant wrinkle and fewer number of carbon layer, which exhibits superior MOR performance as well as long-lifespan. Our findings not only exhibit some new insights for accurate modulation and optimization of carbon skelecton at the atomic scale in fundamental, but also reveal the structural and compositive origin of the enhanced catalytic activity towards to MOR.
intrinsic defects and structure in carbon skeleton can be severed as eminent traps to help to absorb Pt ion and restrict it migrating on the carrier, further contributing to nucleation and Ostwald ripening of Pt NPs [15–17] The nitrogen dopants not only break electroneutrality of the original carbon skeleton, avoiding aggregation of Pt NPs, but also provide strong interaction between Pt NPs and support, enhancing charge transfer and catalytic activity of catalyst [19–21]. Besides, abundant OH species offer from the N dopants are benefited to eliminate the absorbed byproducts during the MOR process [22] The surface morphology of supports is highly determined by the nitrogen configuration and content, such as graphitic N, pyridinic N, and pyrrolic N. Up the to date, the role of nitrogen configuration in carbon materials including by way of experiment and theory needs to deeper understanding. Furthermore, it is also highly raring to develop an electrocatalyst with fine tunability in microenvironment for regulating Pt deposition, as well as improving the catalytic activities towards MOR.
Herein, we elaborately design an in-situ pyrolysis approach to synthesize bamboo-like N doped carbon nanotubes coupled with fewer metallic particles (M@N-CNTs (M = Co, Ni and Fe3C)), enabled tunable
E-mail addresses: jinmeng0807@mail.tsinghua.edu.cn (M. Jin), lushiyu@cqust.edu.cn, lushiyu1990@pku.edu.cn (S.-Y. Lu).
Received 2 January 2022; Received in revised form 20 March 2022; Accepted 24 March 2022
Availableonline26March2022
0169-4332/©2022PublishedbyElsevierB.V.
surface state and constitution of carbon layers. The nitrogen doping state, thickness and defect degree of the carbon layer in bamboo-like M@N-CNTs (M = Co, Ni and Fe3C) could be effectively tuned by using dicyandiamide and different acetylacetone salt (Co, Ni, Fe) as the precursor. The distribution and particle size of Pt NPs on M@N-CNTs (M = Co, Ni and Fe3C) subsequently affected by surface state and constitution of carbon layers in N-CNTs using a simple microwave-assisted glycol process, which further influences its MOR activity, CO tolerance ability and durability in alkaline media. Co@N-CNTs with a high content of pyridinic-N, enriched defect, abundant wrinkle and fewer number of carbon layer provide excellent landing sites for Pt NPs, causing uniformly narrowest and minimum particle size distribution for Pt deposition, which greatly enhance the activity of MOR and reduce the usage of Pt. Pt/Co@N-CNTs exhibits high mass activity and specific activity (1652 mA mg-1Pt and 4.82 mA cm-2Pt) with outstanding CO tolerance ability and stability.
2. Results and discussion
The synthesis route of the Pt/M@N-CNTs (M = Co, Ni, Fe3C) is illustrated in Fig. 1a The bamboo-liked M@N-CNTs (M = Co, Ni, Fe3C) were first synthesized by using direct pyrolysis of dicyandiamide under high temperature with the assist of acetylacetone salt. Then, Pt/M@NCNTs (M = Co, Ni, Fe3C) were obtained by microwave-assisted reduction process in ethylene glycol contained hexachloroplatinic acid. As shown in Fig. 1b, there are several step in dicyandiamide decomposition, which release NH3 molecules and successively form melamine, melem, melon, g-C3N4 and finally N-CNTs. Melem bands in products well formed at temperature range of 400 ~ 500 ℃ Melon products appear at ~ 500 ℃ and grows rapidly up to 600 ℃. g-C3N4 derived from melon thermal polymerizate at the temperature range of 550 ~ 700 ℃ while translate N doped carbon higher than 700 ℃ [23] Internal stresses created from M metal (M = Co, Ni, Fe3C) cause a curling of the layered g-C3N4 [24] A typical scanning electron microscopy (SEM)
images of M@N-CNTs (M = Co, Ni and Fe3C) presents uniformed hollow cavity with average tubular diameters of 15–30 nm (Fig. S1). The intrinsic microstructure of M@N-CNTs (M = Co, Ni and Fe3C) are analyzed by transmission electron microscopy (TEM). As revealed in Fig. 1c to 1e, bamboo-like N-CNTs are all multiwalled and encapsulated fewer M metal nanoparticles (M = Co, Ni and Fe3C) in part of their cavities, which clearly originate from dicyandiamide and metal precursor by growth mechanism at high temperature [25,26]. The gaseous state of hydrocarbon derived from dicyandiamide decomposition mutually dissolute with metal NPs reduced from metallic precursor at high temperature, which metals and hydrocarbons continuously accumulate as time goes by. When reaching saturation of carbon on the metal surface, the carbon separated out and crystallizes to form graphite cylinders. High-resolution TEM (HRTEM) images (Fig. 1f to 1 h) exhibit increase in the carbon layer thickness when nickel acetylacetonate and ferric acetylacetonate are used. The carbon layer thickness increased in the following order: Co@N-CNTs<Ni@N-CNTs<Fe3C@N-CNTs. Furthermore, the lattice of carbon layer in Co@N-CNTs and Ni@N-CNTs are discontinuous compared with that of Fe3C@N-CNTs, revealing existence of defect in Co@N-CNTs and Ni@N-CNTs. Obviously, the carbon layers in Co@N-CNTs is highly curved-evidence of its superthin character.
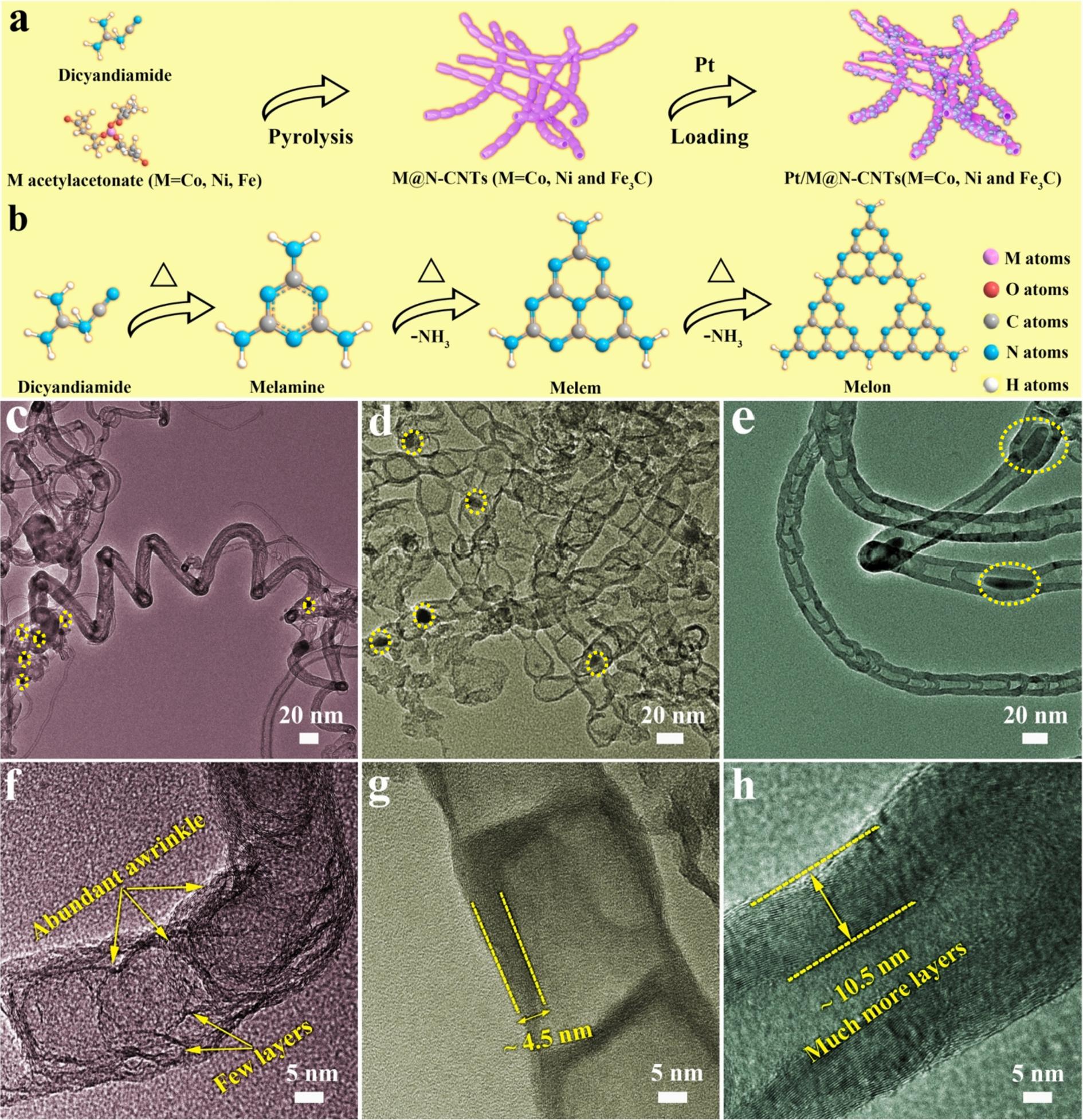
Fig. 2a shows the powder X-ray diffraction (XRD) pattern of the assynthesized M@N-CNTs (M = Co, Ni and Fe3C). An obvious sharp diffraction peak at about 2θ = 26◦ is observed in Co@N-CNTs, Ni@NCNTs and Fe3C@N-CNTs, which is corresponded to the (0 0 2) plane of graphite. The other diffraction peaks can be ascribed to the cobalt metal (PDF#15–0806), nickel metal (PDF#04–0850) and iron carbide (PDF#85–0871) for Co@N-CNTs, Ni@N-CNTs and Fe3C@N-CNTs, respectively. The XRD patterns of Pt/M@N-CNTs (M = Co, Ni and Fe3C)
are presented in Fig. 2b The location of diffraction peaks for Pt/M@NCNTs (M = Co, Ni and Fe3C) are similar with their original M@N-CNTs (M = Co, Ni and Fe3C), but relative intensity of carbon and metallic phase are totally different, indicated partly dissolution of metallic phase under Pt loading process. The diffraction peak located at ~ 39.8◦ relate to the (1 1 1) plane of Pt (PDF#04–0802). The ratio of carbon peak strength and metal peak strength of Pt/Co@N-CNTs and Pt/Ni@N-CNTs are significantly enhanced, revealed that partial dissolution of metal under Pt loading process, while only the diffraction peaks of carbon and Pt can be discerned in Pt/Fe3C@N-CNTs, attributing to the greatly dissolution of Fe3C under Pt loading process. The texture specific surface area and pore size distribution of M@N-CNTs (M = Co, Ni and Fe3C) are investigated by using N2 adsorption–desorption isothermal analysis. The N2 adsorption–desorption isotherm measured for all samples are typical IV-type with H3-type hysteresis loops (Fig. S2), indicating the presence of mesopores in three supports. Noticeably, the Brunauer-Emmett-Teller (BET) specific surface area of Co@N-CNTs, Ni@N-CNTs and Fe3C@NCNTs are 48.1, 136.1 and 87.7 m2 g 1 , respectively. Remarkably, in the region with high relative pressure (P/P0), N2 adsorption–desorption isotherm loops of Co@N-CNTs is higher than that of Ni@N-CNTs and Fe3C@N-CNTs, indicated that a large number of mesoporous exist in Co@N-CNTs [27,28] The pore size distribution curve calculated based on the BJH method further illustrates the existence of the mesoporous structure of the support materials. It also proves that the pore size of Co@N-CNTs is larger, which is conducive to the transport of methanol molecules [29]
In Fourier Transform Infrared (FTIR) spectroscopy (Fig. 2c), the three obvious peaks located at 890, 1070 and 1600–1650 cm 1 reflect vibrations of N-H bonds, C-N bonds and C = N bonds, which are assigned to pyrrolic nitrogen, graphitic nitrogen and pyridinic nitrogen in M@N-
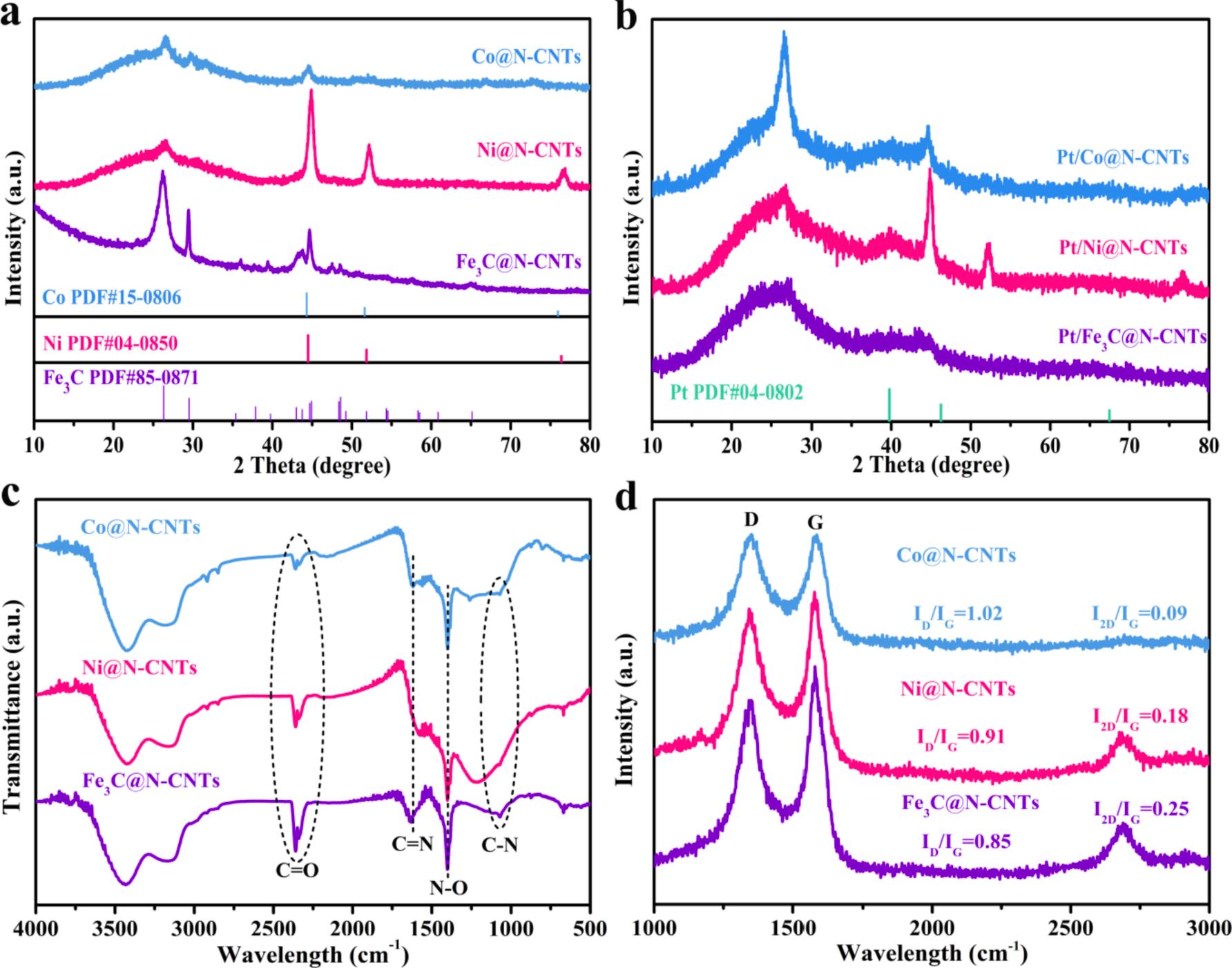
CNTs, respectively [30,31] In addition, the peak at ~ 1392 cm 1 is corresponding to the N-O (pyridinic oxide) bonds while the two peaks at ~ 2360 cm 1 correspond to C = O bonds. Furthermore, Co@N-CNTs and Ni@N-CNTs show two new small peaks at ~ 2917 cm 1 , which are related to the C-H stretching. The formation of C-H bonds may improve the hydrophilicity of M@CNTs (M = Co, Ni and Fe3C). Raman spectra are obtained for M@N-CNTs (M = Co, Ni and Fe3C) in the frequency range from 1000 to 3000 cm 1 using 514 nm excitation (Fig. 2d). Two characteristic peaks at ~1350 and ~1590 cm 1 are renowned to D band and G bands in the carbon structure, respectively. The D band reflect to disordered sp2 carbon and the G band represent ordered graphitic sp2 carbon. By comparison of spectra, in Co@N-CNTs nanocomposite the intensity ratio of ID/IG (~1.02) is higher than that of Ni@N-CNTs (~0.91) and Fe3C@N-CNTs (~0.85), which may be attributed to the domination of disordered structure of Co@N-CNTs. Moreover, the order peak at ~ 2680 cm 1 is renowned to 2D band. The intensity ratio of 2D band to G band (I2D/IG) has been reported to a valuable approach to characterize the number of carbon layer in carbon nanotubes and graphene [32,33]. Hence, lowest values of I2D/IG suggest that Co@N-CNTs (~0.09) forms N-CNTs with the relatively small thickness of walls followed by Ni@CNTs (~0.18) and Fe3C@N-CNTs (~0.25), which also is consistent with directly observation in HRTEM images (Fig. 1f to 1 h).
The TEM images of Pt/M@N-CNTs (M = Co, Ni and Fe3C) are depicted in Fig. 3a-3c to analysis the size and distribution of Pt NPs on M@N-CNTs (M = Co, Ni and Fe3C). The Pt NPs uniformly disperse both in Pt/Co@N-CNTs and Pt/Ni@N-CNTs, while greatly aggregate in Pt/ Fe3C@N-CNTs. High-resolution TEM (HRTEM) (Fig. 3d to 3f) reveals that well-crystalline structure of Pt NPs with smaller particle size uniformly monodisperse in Pt/Co@N-CNTs. Poor distribution of Pt NPs in Pt/Fe3C@N-CNTs reveals relatively perfect carbon layer of Fe3C@NCNTs is not suitable to help to the nucleation and anchoring of Pt NPs. The HAADF-STEM images of Pt/Co@N-CNTs catalyst and the corresponding EDS elemental mapping displays the homogeneous distribution of C, N, Co and Pt elements in the whole nanotubes (Fig. S3). The lattice pattern with interplanar spacing of ~ 0.221 nm attribute to (1 1 1) plane of the face-centered cubic Pt (Fig. 3d and 3e). The average particle size of Pt NPs (Fig. S4a to 4c) in Pt/Co@N-CNTs, Pt/Ni@N-CNTs
and Pt/Fe3C@N-CNTs are 1.65, 1.73 and 9.27 nm, respectively. Pt NPs with smaller size and well uniformly distribution in Pt/Co@N-CNTs improve the utilization rate of Pt, reduce unnecessary side reactions, and enhance the catalytic activity towards MOR [14,34]
The composition and nature of coordination environment of M@NCNTs and Pt/M@N-CNTs (M = Co, Ni and Fe3C) are also characterized by using X-ray photoelectron spectroscopy (XPS). Fig. 4a exhibits Co 2p, Ni 2p and Fe 2p core level spectra of Co@N-CNTs, Ni@N-CNTs and Fe3C@N-CNTs, respectively. For spectra of Co 2p, the centered peaks at binding energy of 779.0 eV and 794.3 eV are accorded with Co 2p3/2 and Co 2p1/2, respectively [35] The doublet peaks at 780.8 eV and 795.8 eV are associated to Co (II) state, while the peaks located at higher binding energy of 783.5 eV and 802.7 eV correspond to satellite peaks of Co 2p [36]. As displayed in the spectra of Ni 2p, the two peaks observed at 853.7 eV and 871.4 eV can be attributed to Ni 2p3/2 and Ni 2p1/2, respectively, indicating that primarily exist Ni (0) [37,38] Meanwhile, the peaks at 855.3 eV and 873.6 eV represent Ni (II). The spectra of Fe 2p shows the peaks around at 707.8 eV and 720.3 eV corresponding to the Fe 2p3/2 and Fe 2p1/2, respectively, demonstrating the presence of Fe (0). In addition, the peaks at 710.8 eV and 723.8 eV are discovered, which are consistent with iron carbide [39] The XPS spectra of Co 2p of Pt/Co@N-CNTs, Ni 2p of Pt/Ni@N-CNTs and Fe 2p of Pt/Fe3C@N-CNTs are separately exhibited in Fig. 4b The peak intensity of Co 2p in Pt/ Co@N-CNTs, Ni 2p in Pt/Ni@N-CNTs and Fe 2p in Pt/Fe3C@N-CNTs weaker than that of fresh M@N-CNTs, which is consistent with XRD pattern (Fig. 2b). Above results indicate that a vast of metal species in M@N-CNTs (M = Co, Ni and Fe3C) will dissolve after Pt deposition process while a small amount of metallic matter remained in Pt/M@NCNTs (M = Co, Ni and Fe3C).
Pt 4f spectra of Pt/M@N-CNTs (M = Co, Ni and Fe3C) are shown in Fig. 4c. The Pt 4f spectra mainly shows two peaks at 70.9 eV and 74.1 eV, which are consistent with Pt 4f5/2 and Pt 4f7/2, respectively. Moreover, the peaks located at 72.1 eV and 76.0 eV are also observed, corresponding to Pt (II) [40] The Pt 4f peak of Pt/Co@N-CNTs have significantly negative shift relative to Pt/Ni@N-CNTs and Pt/Fe3C@NCNTs, manifesting the stronger interaction between Pt NPs and support [19] At the same time, the negative shift of Pt 4f also means that Pt NPs
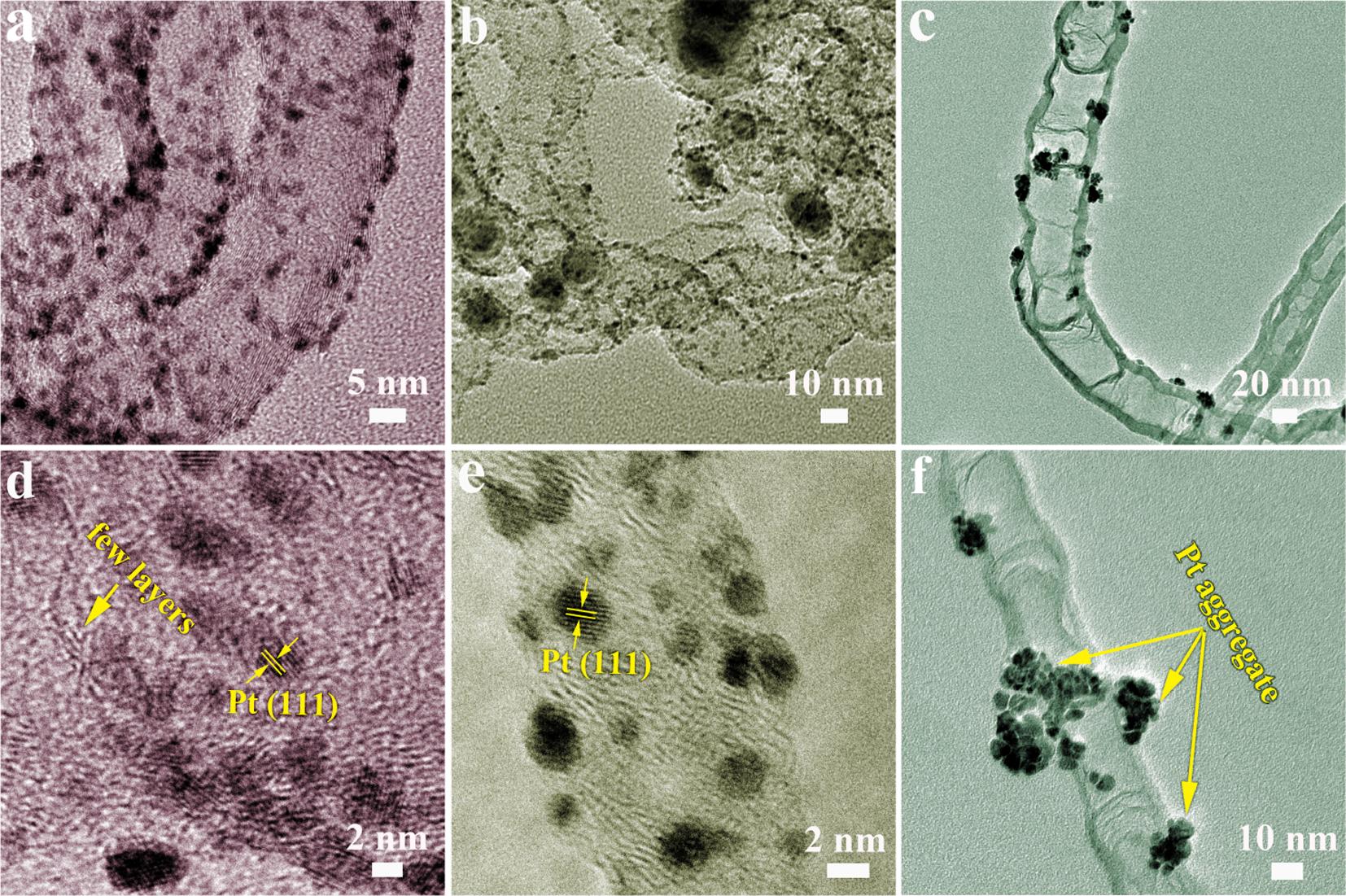
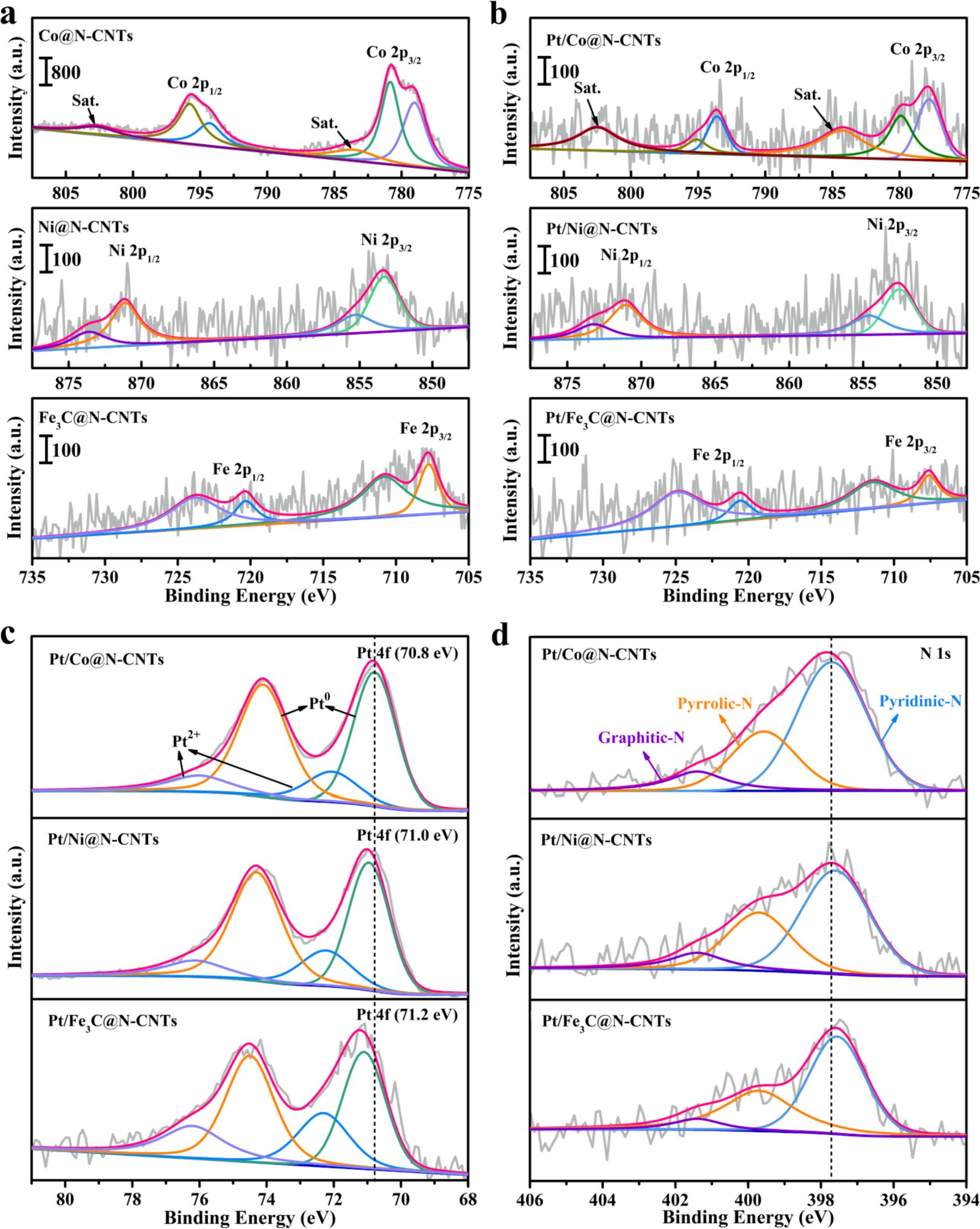
Fig. 4. (a) The XPS spectra of Co 2p of Co@N-CNTs, Ni 2p of Ni@N-CNTs and Fe 2p of Fe3C@N-CNTs. (b) The XPS spectra of Co 2p of Pt/Co@N-CNTs, Ni 2p of Pt/ Ni@N-CNTs and Fe 2p of Pt/Fe3C@N-CNTs. The XPS spectra of (c) Pt 4f and (d) N 1 s of Pt/M@N-CNTs (M = Co, Ni and Fe3C).
can obtain electrons from the Co@N-CNTs support, thus changing the dband center of Pt. According to the d-band center theory, the CO adsorption energy of Pt would weaken with the decrease of the d-band center, which is also advantageous to the removal of COads intermediates on the catalyst surface [41,42]. As is well known, Pt (0) is deemed to provide more active sites for MOR than Pt (2 + ), which is crucial for the realization of high catalytic performance [18,43] The content of Pt (0) in Pt/Co@N-CNTs is obviously higher than that of Pt/Ni@N-CNTs and Pt/Fe3C@N-CNTs (Table S1), indicating that the Pt/Co@N-CNTs could provide more active sites for methanol oxidation. The high-resolution XPS spectra of the N1s (Fig. 4d) confirms the presence of three
nitrogen functional groups in Pt/M@N-CNTs, including pyridinic N (397.5 eV), pyrrolic N (399.7 eV) and graphitic N (401.4 eV). Notably, the positive shift of N1s in Pt/Co@N-CNTs reveal the occurrence of electron transfer from N to Pt NPs. Generally speaking, pyridinic N is recognized as promote electron transfer, meanwhile, graphitic N can improve the conductivity of the catalyst [44,45]. The percentage of pyridinic N and graphitic N in Pt/Co@N-CNTs are highest among all catalysts (Table S2), which might be vital to enhance the catalytic activity. The C 1 s spectrum (Fig. S5) could be fitted into four peaks at 284.3 eV (sp2-sp2 C), 285.5 eV (N-sp2 C), 286.2 eV (N-sp3 C) and 288.4 eV (π-π*) [42]. The deconvoluted XPS spectra of O 1 s (Fig. S6)
demonstrated existence of carboxyls (530.8 eV) and hydroxyls (532.5 eV) in electrocatalyst.
Fig. 5a displays the cyclic voltammograms (CVs) of Pt/M@N-CNTs (M = Co, Ni and Fe3C) and commercial Pt/C in 1 M KOH. The electrochemically surface area (ECSA) can be calculated by the hydrogen desorption region (-0.8 V ~ -0.5 V) of CVs. The Pt/Co@N-CNTs has the largest ECSA (34.3 m2 g 1) in all catalysts (Fig. 5d), indicating that Pt/ Co@N-CNTs provides more catalytic active sites in electrocatalytic process. The catalytic properties of Pt/M@N-CNTs (M = Co, Ni and Fe3C) towards methanol oxidation are investigated in 1 M KOH and 1 M CH3OH (Fig. 5b). The mass activity of Pt/Co@N-CNTs is 1652 mA mg1 Pt, which is 1.16, 1.97 and 2.19-fold larger than that of Pt/Ni@N-CNTs, Pt/Fe3C@N-CNTs and commercial Pt/C, respectively (Fig. 5e). Meanwhile, the specific activity of Pt/Co@N-CNTs (4.82 mA cm-2Pt) is 1.13, 1.24 and 1.41 times higher than that of Pt/Ni@N-CNTs, Pt/Fe3C@NCNTs and commercial Pt/C, respectively. The ratio of the peak current density for forward (If) and backward (Ib) scans (If/Ib) can be used to evaluate the CO tolerance of the catalysts (Table S3). Traditionally, a lower If/Ib value reveals weaker tolerance to intermediate carbon species, which means incompletion of methanol oxidation [46,47]. The If/Ib ratios of Pt/Co@N-CNTs, Pt/Ni@N-CNTs, Pt/Fe3C@N-CNTs and Pt/C calculated from Fig. 6b are 5.51, 5.24, 4.32 and 5.12, respectively. It is demonstrated that Pt/Co@N-CNTs possess the enhanced anti-poisoning ability, which might be attributed to the uniform and dense dispersion of Pt NPs on the surface of Co@N-CNTs. The dense and uniform distribution of Pt NPs mean that the distance between Pt and Pt is relatively close, which is facilitating the removal of the COads intermediate by OH adsorbing on adjacent Pt. Obviously, Pt/Co@N-CNTs also displays the much lower oxidation potential in MOR at the same oxidation current density (Fig. 5c) than other catalysts (Fig. 5f), implying that methanol can be more easily oxidized on the Pt/Co@N-CNTs.
We used DFT calculations of models to optimal anchoring sites of Pt4 cluster on the N-CNTs supports. As presented in Fig. 6a, the substitution of carbon atom with a nitrogen atom in hexatomic ring can form three nitrogen bonding configurations, which are graphitic N, pyridinic N and pyrrolic N, respectively. To understand the effect of N on the stability of Pt NPs, the adhesion energy of Pt, Eadh was calculated with the following equation:.
Where Eslab+Pt4, Eslab and EPt4 are the total energy of Pt4 cluster with substrate, the energy of free-standing substrate, the energy of Pt4 cluster. As shown in Fig. 6b, N doped can significantly decrease the adhesion energy of each Pt atom, from 0.57 eV in Pt4@C system to 0.68 eV in Pt4@graphitic N and then to 1.39 eV in Pt4@pyridinic N (Fig. S7-S8, Fig. 6c-6e, Table S4-S5 and Table 1). Analyzing the results of the calculations, indicating the formation of Pt-N bonds, in agreement with the XPS spectra. Thus, the systematic binding energies simulation in DFT methods revealed that Pt-group metals preferentially interacts with pyridinic nitrogen (Npyr) [48] As listed in Fig. 6c-6e and Table 1, the Pt atom prefers to form a bridge in the centers of three pyridinic nitrogen. It can be attributed to the number and stability of generated bonds interaction between Pt-group metals and vacancy Npyr atoms, which is not only break C-C bonds in the hexatomic ring and symmetry, but also can further improve catalytic activity and stability of MOR. We also calculated the charge density differences (Fig. S9) and DOS (density of states, see Fig. S10) of Pt4/C, Pt4/graphitic N, and Pt4/pyridinic N models, respectively. The d band center of Pt moves upwards after bonding with N atoms because of the stronger electronic accumulation of Pt4 clusters after interacting with pyridinic nitrogen (Npyr).
The steady-state chronoamperometry of Pt/M@N-CNTs (M = Co, Ni and Fe3C) and commercial Pt/C are measured in 1 M KOH and 1 M CH3OH (Fig. S11). Conspicuously, the Pt/Co@N-CNTs exhibits the highest initial current density and slowest deterioration rate during the entire test time in comparison with other catalysts, indicating an excellent CO tolerance during the methanol oxidation processes. Continuous CV tests of all catalysts are conducted in 1 M KOH (Fig. S12). After 1000 cycling, the ECSA of Pt/Co@N-CNTs remained at 53 % relative to the initial ECSA, which is highest retention rate among all catalysts. The CO stripping measurement of Pt/M@N-CNTs (M = Co, Ni and Fe3C) and commercial Pt/C are conducted (Fig. S13). The onset potential of Pt/Co@N-CNTs (-0.475 V) is more negative than that of Pt/ Ni@N-CNTs (-0.399 V), Pt/Fe3C@N-CNTs (-0.352 V) and commercial Pt/C (-0.363 V). Specifically, the Pt/Co@N-CNTs also displays the smallest peak potential (-0.114 V) among all catalysts. Above results illustrate superior CO tolerance of Pt/Co@N-CNTs. All of Pt/M@N-CNTs (M = Co, Ni and Fe3C) exhibit three peaks at the potential ranging from 0.4 to 0 V, compared with commercial Pt/C, revealing that the CO oxidation occurs on different active sites [49,50] All of these
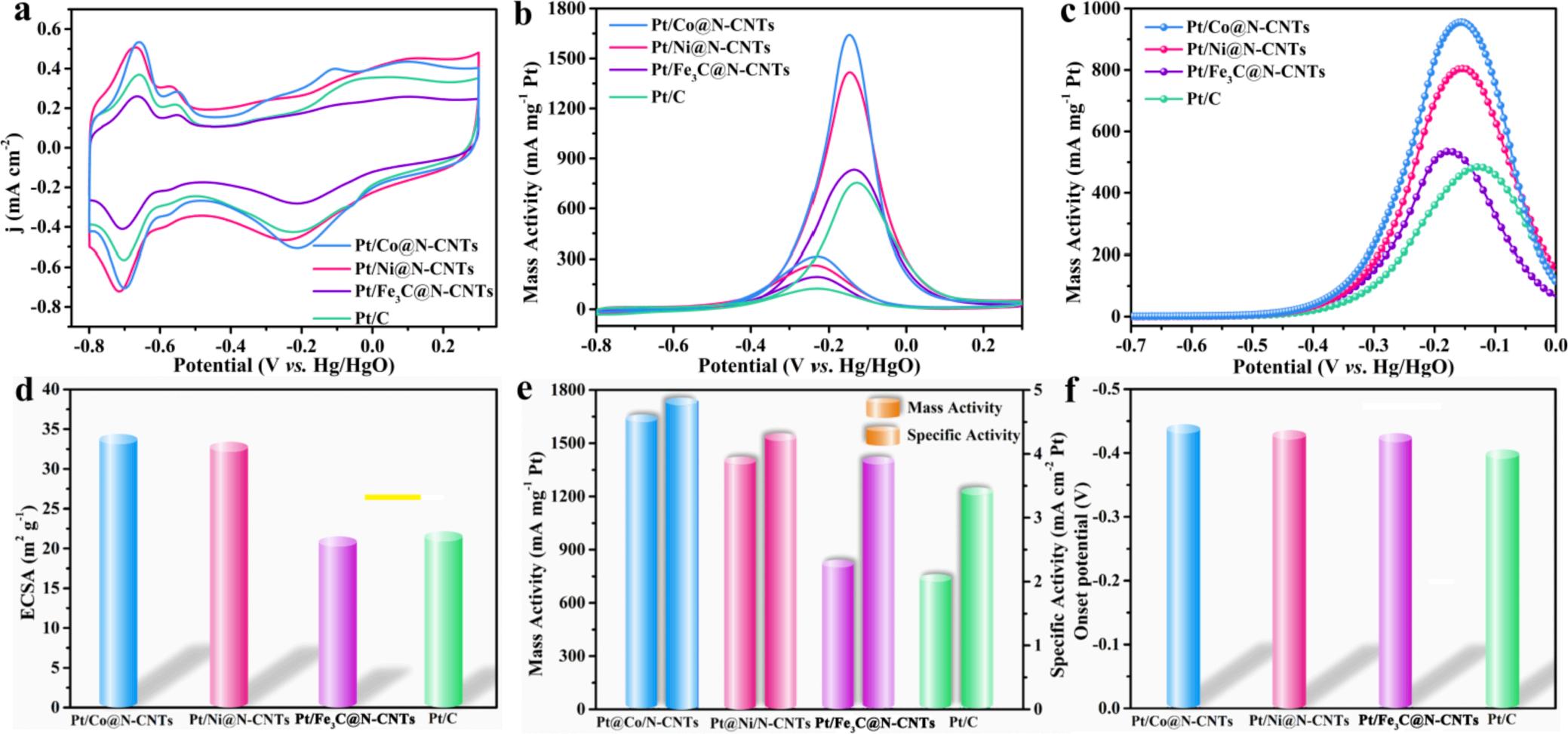
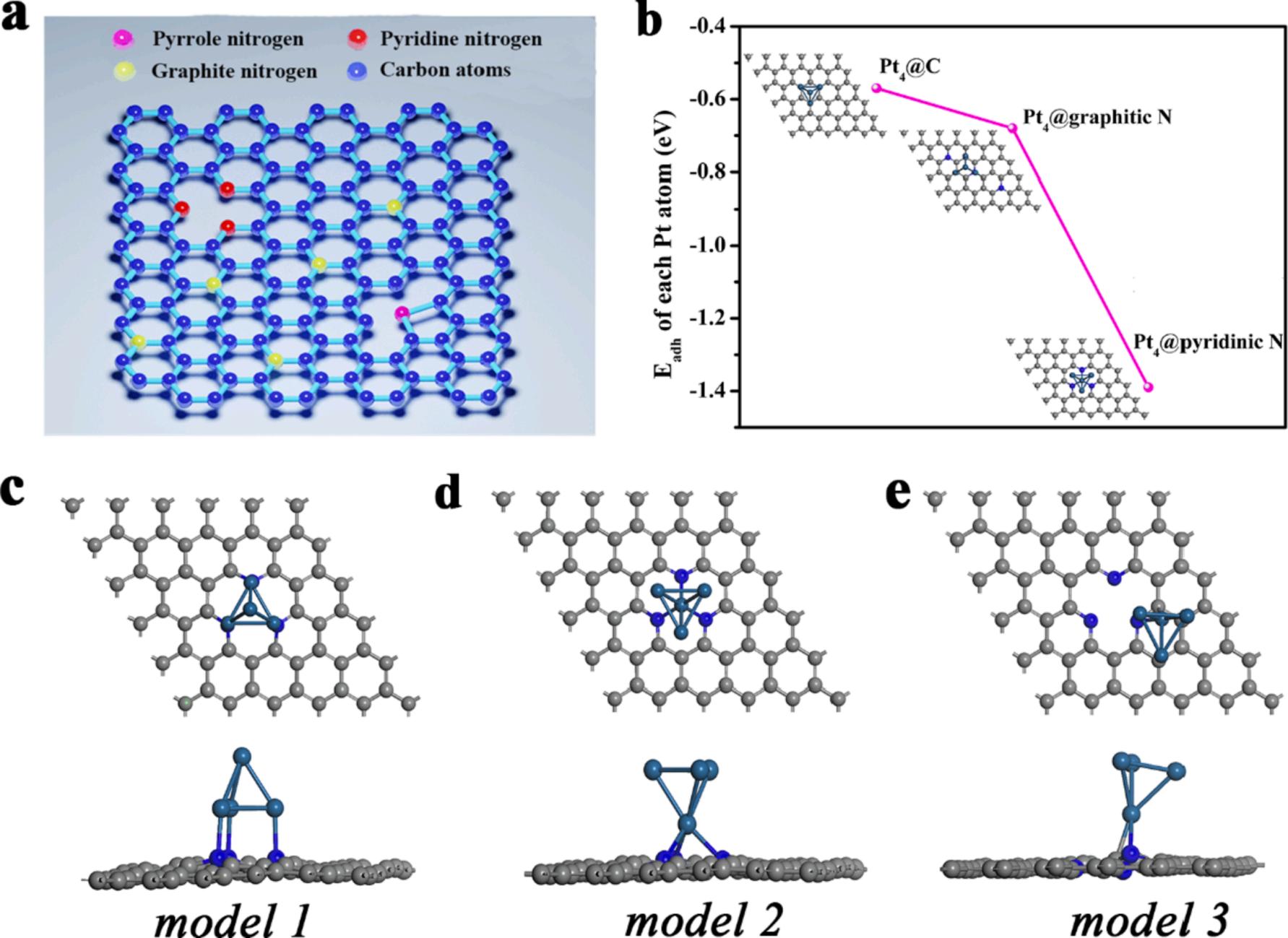
Table 1
The adhesion energy for Pt cluster anchored to pyridinic N-doped CNTs (Model 1 and 3).
1 13.09
2 13.09 450.29 468.93 5.55 1.39
3 13.09 450.29 465.86 2.48 0.62
consequences provide clear evidence of the remarkable electrocatalytic activity and good catalytic stability of Pt/Co@N-CNTs for MOR. The Pt/ Co@N-CNTs is more competitory than those of recent methanol oxidation electrocatalysts in alkaline medium (Table S6).
More importantly, electrochemical impedance spectroscopy (EIS) can be used to estimate the activity of catalysts. The Nyquist plots of Pt/ M@N-CNTs (M = Co, Ni and Fe3C) and commercial Pt/C are depicted in Fig. 7a The smaller semicircle diameter of Nyquist plots indicates the lower charge transfer resistance [51] Pt/Co@N-CNTs has the smallest semicircle diameter, and hence Pt/Co@N-CNTs has the fastest rate in methanol oxidation. To further investigate the kinetics of methanol oxidation in Pt/Co@N-CNTs, the Nyquist plots at different potentials are shown in Fig. 7b to 7d Manifestation of approximatively straight lines in EIS spectra below 0.4 V reveals no occurrence of methanol oxidation lower 0.4 V, agreeing with the CV data [43] From 0.4 V to 0.2 V, an obvious clockwise loop at low frequencies centered on the fourth quadrant reveals behavior of pseudocapacitance and the semicircle diameter of Nyquist plots decreases with potential increasing. Above results illustrates the process that adsorbed CO intermediate species dehydrogenized from methanol (Equation (1) to (4)) oxidize to CO2 to removal from catalyst (Equation (5)). More active site freshly exposing due to oxidation removal of COads from Pt/Co@N-CNTs, the kinetics of the methanol oxidation reaction facilitate [52].
+ OH– → Pt-CHO + H2O + e -
+ OH– → Pt-CO + H2O + e -
+ Pt-OH + OH– → 2Pt + CO2 + H2O + e -
As the potential goes by, the semicircle diameter of EIS first increases at potential from 0.2 V to 0.15 V. Then the impedance profile suddenly exhibits negative values at 0.1 V, turned to the second quadrant (Fig. 7c). Owing to formation of the chemisorbed OH species, the ratedetermining step of MOR turned into the oxidation removal of COads intermediates from methanol dehydrogenation [53,54] As shown in Fig. 7d, further increase the potential beyond 0.1 V, the impedance arc tries back to the first quadrant with large resistance. Over-high potential caused excessively strong adsorption of OH species on the surface of Pt/ Co@N-CNTs, which inhibit happening of methanol oxidation reaction.
3. Conclusion
In summary, we have successfully tuned microstructure of carbon layer in M@N-CNTs (M = Co, Ni and Fe3C) via acetylacetone salt. After Pt loading, the distribution of Pt NPs in Pt/M@N-CNTs are strongly depend on surface state and constitution of carbon layer in M@N-CNTs, which further influences their catalytic ability and stability towards to MOR. The state-of-art Pt/Co@N-CNTs exhibits superior both mass and specific activity towards to MOR in alkaline medium than that of commercial Pt/C. Proper tuning of N configuration, thickness and defect of carbon layer not only endow more active site to well- disperse Pt NPs deposition, but also provide strong interaction to prevent migrating and
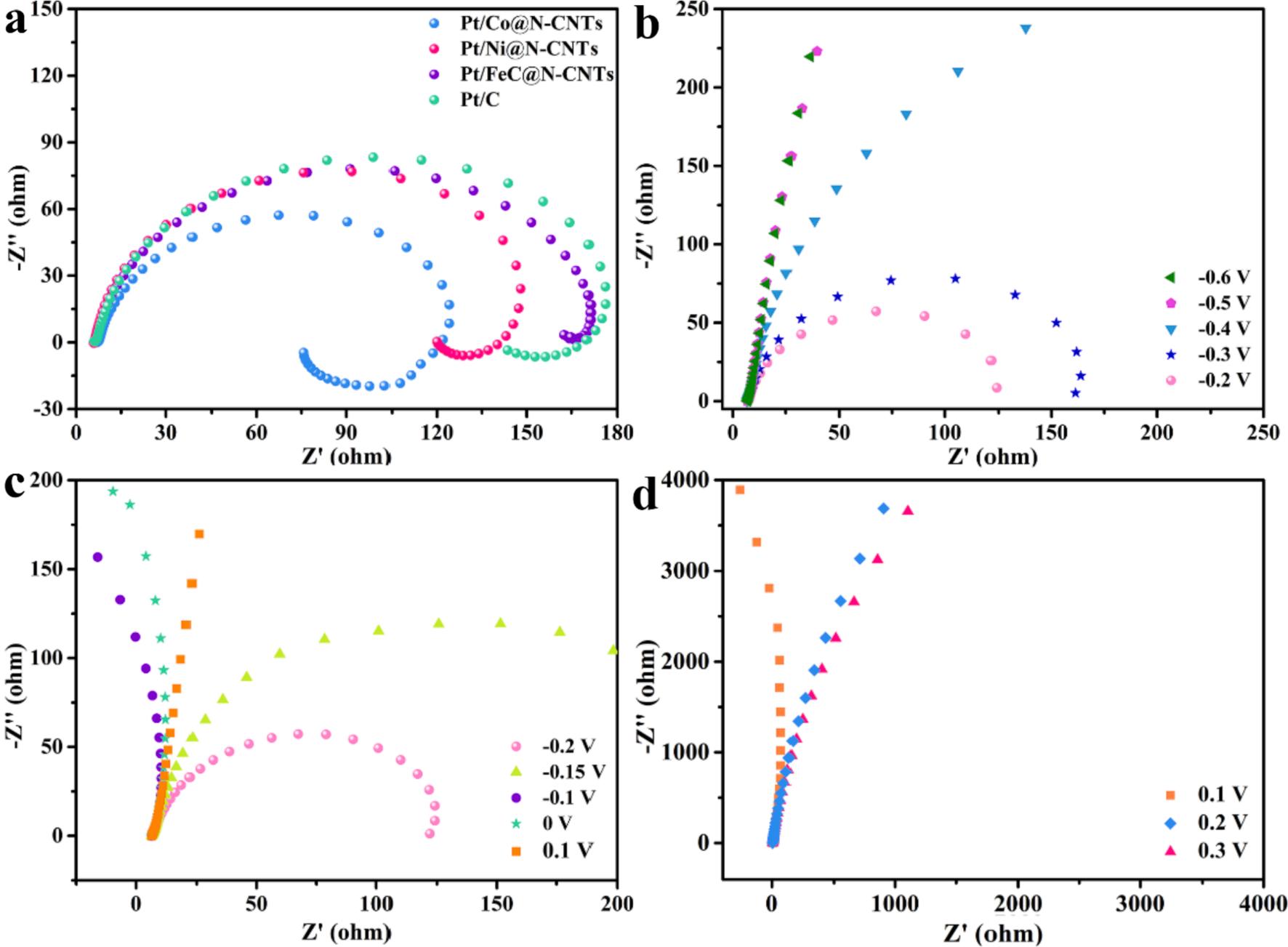
Fig. 7. Nyquist plots of (a) Pt/M@N-CNTs (M = Co, Ni and Fe3C) at 0.2 V in 1 M KOH + 1 M CH3OH. Nyquist plots of (b-d) Pt/Co@N-CNTs in 1 M KOH + 1 M CH3OH solution at electrode potentials from 0.6 V to 0.3 V.
excellent charge/electron transport ability, further improving stability and catalytic activity in electrochemical process. This work not only provide a new viewpoint to develop novel carbon/metal oxide composite with desired surface state and constitution, but also open an approach for design and construct of nanomaterials for energy storage and conversation.
CRediT authorship contribution statement
Meng Jin: Writing – original draft, Investigation, Methodology. Rong Wang: Formal analysis, Software. Bi Jia: Investigation. Jun Zhang: Investigation. Hui Liu: Methodology. Shi-Yu Lu: Conceptualization, Funding acquisition, Writing – review & editing, Investigation, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was financially supported by Talent Introduction of Chongqing University of Science and Technology (No. ckrc2021050), “Young scholar in engineering science” post-doctoral project of BICEAST (Peking University) and Project funded by China Postdoctoral Science Foundation (No.2020M670021). The authors acknowledge Beijng PARATERA Tech CO.,Ltd. for providing HPC resources that have contributed to the research results reported within this paper. URL: https://paratera.com/
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi. org/10.1016/j.apsusc.2022.153201.
References
[1] M. Luo, Z. Zhao, Y. Zhang, Y. Sun, Y.i. Xing, F. Lv, Y. Yang, X.u. Zhang, S. Hwang, Y. Qin, J.-Y. Ma, F. Lin, D. Su, G. Lu, S. Guo, PdMo bimetallene for oxygen reduction catalysis, Nature 574 (7776) (2019) 81–85
[2] A. Kudo, Y. Miseki, Heterogeneous photocatalyst materials for water splitting, Chem. Soc. Rev. 38 (1) (2009) 253–278
[3] S.U.M. Khan, M. Al-Shahry, W.B. Ingler, Efficient photochemical water splitting by a chemically modified n-TiO2, Science 297 (5590) (2002) 2243–2245
[4] S.S. Munjewar, S.B. Thombre, R.K. Mallick, Approaches to overcome the barrier issues of passive direct methanol fuel cell-Review, Renew. Sust. Energy Rev. 67 (2017) 1087–1104
[5] Y. Tong, X. Yan, J.i. Liang, S.X. Dou, Metal-Based Electrocatalysts for Methanol Electro-Oxidation: Progress, Opportunities, and Challenges, Small 17 (9) (2021) 1904126.
[6] X.L. Tian, L. Wang, P. Deng, Y.u. Chen, B.Y. Xia, Research advances in unsupported Pt-based catalysts for electrochemical methanol oxidation, J. Energy Chem. 26 (6) (2017) 1067–1076
[7] J. Zhang, X. Qu, Y.u. Han, L. Shen, S. Yin, G. Li, Y. Jiang, S. Sun, Engineering PtRu bimetallic nanoparticles with adjustable alloying degree for methanol electrooxidation: Enhanced catalytic performance, Appl. Catal. B: Environ. 263 (2020) 118345
[8] X. Zhao, M. Yin, L. Ma, L. Liang, C. Liu, J. Liao, T. Lu, W. Xing, Recent advances in catalysts for direct methanol fuel cells, Energy Environ. Sci. 4 (8) (2011) 2736
[9] S. Guo, S. Dong, E. Wang, Constructing carbon nanotube/Pt nanoparticle hybrids using an imidazolium-salt-based ionic liquid as a linker, Adv. Mater. 22 (11) (2010) 1269–1272
[10] J.-J. Fan, Y.-J. Fan, R.-X. Wang, S. Xiang, H.-G. Tang, S.-G. Sun, A novel strategy for the synthesis of sulfur-doped carbon nanotubes as a highly efficient Pt catalyst support toward the methanol oxidation reaction, J. Materials Chem. A 5 (36) (2017) 19467–19475
[11] R.-X. Wang, Y.-J. Fan, L. Wang, L.-N. Wu, S.-N. Sun, S.-G. Sun, Pt nanocatalysts on a polyindole-functionalized carbon nanotube composite with high performance for methanol electrooxidation, Journal of Power Sources 287 (2015) 341–348
[12] J. Zhong, L. Li, M. Waqas, X. Wang, Y. Fan, J. Qi, B. Yang, C. Rong, W. Chen, S. Sun, Deep eutectic solvent-assisted synthesis of highly efficient PtCu alloy nanoclusters on carbon nanotubes for methanol oxidation reaction, Electrochimica Acta 322 (2019), 134677
[13] J.-P. Zhong, C. Hou, L. Li, M. Waqas, Y.-J. Fan, X.-C. Shen, W. Chen, L.-Y. Wan, H.G. Liao, S.-G. Sun, A novel strategy for synthesizing Fe, N, and S tridoped graphenesupported Pt nanodendrites toward highly efficient methanol oxidation, Journal of Catalysis 381 (2020) 275–284
[14] Y. Chen, S. Ji, C. Chen, Q. Peng, D. Wang, Y. Li, Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications, Joule 2 (2018) 1242–1264
[15] L. Liang, M. Xiao, J. Zhu, J. Ge, C. Liu, W. Xing, Low-temperature synthesis of nitrogen doped carbon nanotubes as promising catalyst support for methanol oxidation, J. Energy Chem. 28 (2019) 118–122
[16] H. Sun, J. Liu, C. Zhang, Q. Yuan, Y. Ye, W. Yan, Z. Tian, C. Liang, S, N dual-doped carbon nanotubes as substrate to enhance the methanol oxidation performance of NiO nanoparticles, Carbon 152 (2019) 114–119
[17] I. Fampiou, A. Ramasubramaniam, Binding of Pt Nanoclusters to Point Defects in Graphene: Adsorption, Morphology, and Electronic Structure, J. Phys. Chem. C 116 (2012) 6543–6555
[18] J. Zhang, Y. Liu, C. Sun, P. Xi, S. Peng, D. Gao, D. Xue, Accelerated Hydrogen Evolution Reaction in CoS2 by Transition-Metal Doping, ACS Energy Lett. 3 (4) (2018) 779–786
[19] Z. Tan, K. Ni, G. Chen, W. Zeng, Z. Tao, M. Ikram, Q. Zhang, H. Wang, L. Sun, X. Zhu, X. Wu, H. Ji, R.S. Ruoff, Y. Zhu, Incorporating Pyrrolic and Pyridinic Nitrogen into a Porous Carbon made from C60 Molecules to Obtain Superior Energy Storage, Adv. Mater. 29 (2017) 1603414
[20] C. Tang, H.-F. Wang, X. Chen, B.-Q. Li, T.-Z. Hou, B. Zhang, Q. Zhang, M.M. Titirici, F. Wei, Topological Defects in Metal-Free Nanocarbon for Oxygen Electrocatalysis, Adv. Mater. 28 (32) (2016) 6845–6851
[21] M. Jin, S.-Y. Lu, X. Zhong, H. Liu, H. Liu, M. Gan, L. Ma, Spindle-like MOFs derived TiO2@ NC-NCNTs composite with modulating defect site and graphitization nanoconfined Pt NPs as Superior Bifunctional Fuel cell electrocatalysts, ACS Sustain. Chem. Eng. 8 (2020) 1933–1942
[22] X. Zhang, J. Zhu, C.S. Tiwary, Z. Ma, H. Huang, J. Zhang, Z. Lu, W. Huang, Y. Wu, Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation, ACS Appl. Mater. Inter. 8 (17) (2016) 10858–10865
[23] E. Groppo, F. Bonino, F. Cesano, A. Damin, M. Manzoli, . Raman, IR and INS Characterization of Functionalized Carbon Materials, (2018).
[24] M. Ning, J. Li, B. Kuang, C. Wang, D. Su, Y. Zhao, H. Jin, M. Cao, One-step fabrication of N-doped CNTs encapsulating M nanoparticles (M Fe Co, Ni) for efficient microwave absorption, Appl. Surf. Sci. 447 (2018) 244–253
[25] S. Ghosh, S. Ramaprabhu, Green synthesis of transition metal nanocrystals encapsulated into nitrogen-doped carbon nanotubes for efficient carbon dioxide capture, Carbon 141 (2019) 692–703
[26] G. Rajeshkhanna, G. Ranga Rao, Micro and nano-architectures of Co3O4 on Ni foam for electro-oxidation of methanol, Int. J. Hydrogen Energ. 43 (9) (2018) 4706–4715
[27] M. Jin, S. Lu, L. Ma, M. Gan, One-step synthesis of in situ reduced metal Bi decorated bismuth molybdate hollow microspheres with enhancing photocatalytic activity, Appl. Surf. Sci. 396 (2017) 438–443
[28] S.-Y. Lu, Y.-N. Yu, S.-J. Bao, S.-H. Liao, In situ synthesis and excellent photocatalytic activity of tiny Bi decorated bismuth tungstate nanorods, RSC Adv. 5 (104) (2015) 85500–85505
[29] A. Corma, P. Concepci ´ on, M. Boronat, M.J. Sabater, J. Navas, M.J. Yacaman, E. Larios, A. Posadas, M.A. L ´ opez-Quintela, D. Buceta, Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity, Nat. Chem. 5 (2013) 775
[30] S.S. Meshkat, A. Rashidi, O. Tavakoli, Removal of mercaptan from natural gas condensate using N-doped carbon nanotube adsorbents: Kinetic and DFT study, J. Nat. Gas Sci. Eng. 55 (2018) 288–297
[31] E. Ghasemy, H.B. Motejadded, A. rashidi, T. Hamzehlouyan, Z. Yousefian, N-doped CNT nanocatalyst prepared from camphor and urea for gas phase desulfurization: Experimental and DFT study, J. Taiwan Inst. Chem. E. 85 (2018) 121–131
[32] D. Liu, W. Lei, Y. Chen, Scalable production of wrinkled and few-layered graphene sheets and their use for oil and organic solvent absorption, Phys. Chem. Chem. Phys. 17 (10) (2015) 6913–6918
[33] S. Gupta, L. Qiao, S. Zhao, H. Xu, Y. Lin, S.V. Devaguptapu, X. Wang, M.T. Swihart, G. Wu, Highly active and stable graphene tubes decorated with FeCoNi alloy
nanoparticles via a template-free graphitization for bifunctional oxygen reduction and evolution, Adv. Energy Mater. 6 (2016) 1601198
[34] Y. Liu, H. Tsunoyama, T. Akita, S. Xie, T. Tsukuda, Aerobic oxidation of cyclohexane catalyzed by size-controlled Au clusters on hydroxyapatite: size effect in the sub-2 nm regime, ACS Catal. 1 (2011) 2–6
[35] Y. Dong, J. Zhang, Z. Ma, H. Xu, H. Yang, L. Yang, L. Bai, D. Wei, W. Wang, H. Chen, Preparation of Co-Mo-O ultrathin nanosheets with outstanding catalytic performance in aerobic oxidative desulfurization, Chem. Commun. 55 (93) (2019) 13995–13998
[36] Q. Zhang, J. Zhang, H. Yang, Y. Dong, Y.u. Liu, L. Yang, D. Wei, W. Wang, L. Bai, H. Chen, Efficient aerobic oxidative desulfurization over Co-Mo-O bimetallic oxide catalysts, Catal, Sci. Technol. 9 (11) (2019) 2915–2922
[37] S.-Y. Lu, S. Li, M. Jin, J. Gao, Y. Zhang, Greatly boosting electrochemical hydrogen evolution reaction over Ni3S2 nanosheets rationally decorated by Ni3Sn2S2 quantum dots, Appl. Catal. B: Environ. 267 (2020), 118675.
[38] J. Gao, T. Meng, S. Lu, X. Ma, Y. Zhang, D. Fu, Z. Lu, C.M. Li, Manganese-doped tremella-like nickel oxide as biomimetic sensors toward highly sensitive detection of glucose in human serum, J. Electroanal. Chem. 863 (2020), 114071
[39] Q. Yang, S. Lu, B. Shen, S. Bao, Y. Liu, An iron hydroxyl phosphate microoctahedron catalyst as an efficient peroxidase mimic for sensitive and colorimetric quantification of H2O2 and glucose, New J. Chem. 42 (9) (2018) 6803–6809
[40] Y.-N. Yu, S.-Y. Lu, S.-J. Bao, Photocatalytic activity of Pt-modified Bi2WO6 nanoporous wall under sunlight, J. Nanopart. Res. 17 (2015) 323
[41] D. Wang, S. Lu, Y. Xiang, S.P. Jiang, Self-assembly of HPW on Pt/C nanoparticles with enhanced electrocatalysis activity for fuel cell applications, Appl. Catal. B: Environ. 103 (2011) 311–317
[42] S. Deng, Y. Zhong, Y. Zeng, Y. Wang, X. Wang, X. Lu, X. Xia, J. Tu, Hollow TiO2@ Co9S8 Core-Branch Arrays as Bifunctional Electrocatalysts for Efficient Oxygen/ Hydrogen Production, Adv. Sci. (Weinh) 5 (2018) 1700772
[43] Z.-B. Wang, G.-P. Yin, Y.-Y. Shao, B.-Q. Yang, P.-F. Shi, P.-X. Feng, Electrochemical impedance studies on carbon supported PtRuNi and PtRu anode catalysts in acid medium for direct methanol fuel cell, J. Power Sources 165 (1) (2007) 9–15
[44] S.Y. Lu, M. Jin, Y. Zhang, Y.B. Niu, J.C. Gao, C.M. Li, Chemically Exfoliating Biomass into a Graphene-like Porous Active Carbon with Rational Pore Structure, Good Conductivity, and Large Surface Area for High-Performance Supercapacitors, Adv. Energy Mater. 8 (2018) 1702545
[45] J. Zhang, L. Ma, M. Gan, S. Fu, Y. Zhao, TiN@ nitrogen-doped carbon supported Pt nanoparticles as high-performance anode catalyst for methanol electrooxidation, J. Power Sources 324 (2016) 199–207
[46] C.-T. Hsieh, J.-Y. Lin, Fabrication of bimetallic P-M (M Fe Co, and Ni) nanoparticle/carbon nanotube electrocatalysts for direct methanol fuel cells, J. Power Sources 188 (2009) 347–352
[47] L.-X. Ding, A.-L. Wang, G.-R. Li, Z.-Q. Liu, W.-X. Zhao, C.-Y. Su, Y.-X. Tong, Porous Pt-Ni-P composite nanotube arrays: highly electroactive and durable catalysts for methanol electrooxidation, J. Am. Chem. Soc. 134 (2012) 5730–5733
[48] D.A. Bulushev, M. Zacharska, E.V. Shlyakhova, A.L. Chuvilin, Y. Guo, S. Beloshapkin, A.V. Okotrub, L.G. Bulusheva, Single isolated Pd2+ cations supported on N-doped carbon as active sites for hydrogen production from formic acid decomposition, ACS Catal. 6 (2) (2016) 681–691
[49] D. Gao, S. Li, G. Song, P. Zha, C. Li, Q. Wei, Y. Lv, G. Chen, One-pot synthesis of PtCu bimetallic nanocrystals with different structures and their enhanced electrocatalytic properties, Nano Res. 11 (5) (2018) 2612–2624
[50] C.-S. Chen, F.-M. Pan, H.-J. Yu, Electrocatalytic activity of Pt nanoparticles on a karst-like Ni thin film toward methanol oxidation in alkaline solutions, Appl. Catal. B: Environ. 104 (2011) 382–389
[51] M. Jin, S.-Y. Lu, L. Ma, M.-Y. Gan, Y. Lei, X.-L. Zhang, G. Fu, P.-S. Yang, M.-F. Yan, Different distribution of in-situ thin carbon layer in hollow cobalt sulfide nanocages and their application for supercapacitors, J. Power Sources 341 (2017) 294–301
[52] M. Zhu, C. Zhai, M. Sun, Y. Hu, B. Yan, Y. Du, Ultrathin graphitic C3N4 nanosheet as a promising visible-light-activated support for boosting photoelectrocatalytic methanol oxidation, Appl. Catal. B: Environ. 203 (2017) 108–115.
[53] C. Wang, H. Wang, C. Zhai, F. Ren, M. Zhu, P. Yang, Y. Du, Three-dimensional Au0.5/reduced graphene oxide/Au0.5/reduced graphene oxide/carbon fiber electrode and its high catalytic performance toward ethanol electrooxidation in alkaline media, J. Mater. Chem. A 3 (8) (2015) 4389–4398
[54] S. Shahrokhian, S. Rezaee, Vertically standing Cu2O nanosheets promoted flowerlike PtPd nanostructures supported on reduced graphene oxide for methanol electro-oxidation, Electrochim. Acta 259 (2018) 36–47
Another random document with no related content on Scribd:
Esteazutcán. Tíz óra, annyit mutat a hirdető-oszlop tetejére szerelt nagy kerek óra, amelynek a cifferblattját villanynyal világítják. Az ember azt hiszi egy pillanatra, hogy valamelyik külföldi nagyvárosban bolyong, mert ezeket az órákat nemrégen honosították meg Budapesten. A boltokat bezárták, a kirakatok nagy tarka üvegszemei lezárultak s alusznak a rollók unalmasan vonalzott sötét vaspillái alatt. A rollók bánatát egy-egy világos helyiség vidítja, utcai buffet, cukorkabolt, cukrászat, amelyet még nem zártak be. A Rákóczi-úton állok. Egy lépésnyire, a járda meg a villamos sínje közt gyümölcsös asszony piszmog az asztalkája mellett, oly kicsi asszony, mint egy gyermek, semmi formája nincsen, derékig egy rakás fakó szoknya, mint egy rőzsecsomó, derékon felül egy nagykendő, amibe belekötöttek valamit. Az ember ráér, belenéz a nagykendőbe, egy kis háromszögletű nyílásból, hátul valami olyan látszik kifelé, mint egy ember arca. Mily öreg arc, mily szűk, mily kicsi és élettelen. Ez a meg-megmozduló batyu egy öreg asszony, volt javakorbeli asszony is, azelőtt meg menyecske, valamikor leány és apró leányka… borzasztó. Az asztalkán alma, körte, olasz mogyoró, szilva meg malagaszőlő-foszlányok s néhány elzüllött cukorka. Az öreg asszony papirost hajlít, úgynevezett staniclit csinál belőle. A villamosok csengetnek s tűnnek egymás után, nem sokan ülnek most a villamosokon, ilyenkor már otthon vannak, vagy még a színházban ülnek. A kávéház teli van, telisteli, úgy ülnek a népek a nagy ablakok mögött a fényben, mintha zsúfolt villamoson ülnének. Az ember szinte várja, hogy a kávéház elinduljon, s elvigye valahová a benne ülőket. Vagy ez volna a céljuk, így ülni, ülni a kávéházban?
A kávéház hátsó sarkában a cigányok játszanak, a régi szép magyar élet vídám kísértetei. A járdán emberek vonulnak, családok, párok, magánosok. Kerepesi-úti arcok. A kerepesi-úti emberek nagyon lehangolók. Egy nagy kórház, egy nagy mozi, meg egy nagy színház áll egymáshoz közel, mint szövetségesek, vagy mint ellenségek. A színház körül sok a kocsi, néhány autó is áll itt, ilyenkor Budapesten
premiére van. Mily szépek a színház előtt álló lámpaoszlopok, amelyek az ünnepélyes, tejfényű lámpalabdákat markolják a magasban. A nagy mozi ragyog, mint valami pillangószörnyeteg. A nagy kórház sápadt nyugalomban áll ijesztő fölényben. Odafenn az égen a rengeteg csillag leskelődik le az utcára. Vagy nem érdeklődnek irántunk? Nem mulattatja őket a mi világunk és a járáskelés idelenn? Tán csak a hiúság vonzotta őket az ég ablakaiba, hogy mutogassák magukat minékünk? De tán nem okoz örömet nékik a mi bámulatunk. Nem hiszem, hogy ilyen nevetséges érzéseik volnának, mint ragyogni, tetszeni vágyni, hódítani. Bizonyosan csupa jóságból jelennek meg nekünk esténkint, hogy gyönyörködhessünk bennük. A jóság a legelőkelőbb érzés, s azt hiszem, az isten birodalmában odafenn a jóság az egyetlen udvarképes tulajdonság. Eszembe jut egy valamikor kedves könyvem írója, Xavier de Maistre, aki azt mondja egy helyen, hogyha ő király lenne, rendeletben parancsolná meg minden alattvalójának, hogy minden este, mikor az ég csillagos, legalább egy félóra hosszat felfelé nézzenek az égre. Őröket állítana a legkisebb faluba is, hogy ellenőrizzék az embereket és szigoruan megbüntetné, aki egy este nem nézte a csillagokat. Milyen bolondság. Ez a francia író régen élt s nagyon rosszaknak tartotta az embereket.
Velence. Mit csináltál már megint, Halál? Ó Halál, vén barátom, magas, szikár, fekete agglegény, akit oly szívesen üdvözlök, mikor magányos sétáimon szembekerülsz velem, megemeled fénytelen, meredek cilinderedet és hozzám szegődöl. Milyen jól ki tudom magam diskurálni veled a világról, mennyi türelmet, elnézést, jóságot tanultam tőled, mennyi sóhajt hessegettél el ajkaimról s láthatatlanul sétálván mellettem fák közt vagy zajos utcákon s hallhatatlanul beszélvén hozzám, bölcsebb vagy Tolsztojnál, nagyobb költő vagy Göthénél s szellemesebb vagy Chamfortnál, Heine-nél és Wide-nál. Nekem csak te tudod megmagyarázni az életet s felőle megnyugtatni, a tiszta életöröm ópiumát is te adod nekem s olykor csókom fölé hajoltál és megszázszoroztad. Barátom lettél s bizalmasodnak érzem magamat… de kérlek, mit csináltál most Velencében? Ötven embert, akik a vídám fehér vaporettón sütkéreztek, beléfullasztottál a vízbe. A Lidóról Velencébe akartak menni. Egy pár percre szálltak a kis hajóra. Kirándulók voltak, vendégek, üdülők, látogatók. Mi köze ilyen kis vaporettónak tehozzád, nagyszerű Halál, se a nagyságában, se a szerkezetében, se útja hosszúságában és céljában semmi ambició, erőszakoskodás, semmi nyugtalanság, semmi különösebb vágy nem volt, ami a végzetet ingerelhetné. Szerény, kiváncsi, egyszerű, nyugodt teremtmények könyököltek a fedélzet korlátaira, akik egy pár napocskát vagy hetecskét akartak pihenni, üdülni, szórakozni Velencében, ők is szórni akartak egy kevés morzsát a Márk-téri galamboknak, föl akarták emelni a fejüket a Campanilét méltányolni és csudálni akarták a Canale Grande palotáinak renaissance ékeit s el akartak merengeni a falak alján, amelyet a víz oly zöldre mosott, mint a sötétzöld selyem. Belé akartak ülni a gondolába, amelynek eleje bölcsőre, hátulja koporsóra emlékeztet, s a közepén az élet kurta boldogsága vonul, a boldogság, amely ott szorul két jobbkéz egymásba fonódott ujjai közt, s meglapul két egymáshoz hajló fej
hajzata között és ott ragad a szemek összeért pillái közt, mikor lecsukódnak.
Ó Halál, én is rejtegettem még egy ifjonti ábrándot a gondoláról, amelyben egy nő szivére téve homlokomat, érzem Velencében a sűrű édes semmit, a boldogságot. Már nem lehet, mindíg eszembe fog jutni, amit most csináltál Velencében, Halál, az ötven kiránduló, akiket megfullasztottál Velencében. Nem értelek, Halál, nem értelek… nem néztél a lábod alá? Mért bántottad ezt az ártatlan, könnyű vaporettót, amely a szegény földi szórakozást szállította. Ugy kell egy kis Velence e kedves, jóhiszemű közönségeseknek. Ezt észre se kéne venned, el kéne nézned, helyeselned kéne s elszórakoznod rajta. Nem ismerek rád, Halál, jó barátom. Oktalan gonoszságnak látszik, amit most műveltél, lelketlen csínynek. Izetlennek, neveletlennek érezlek ma. Utálatosnak.
Sütanap. Az omnibusz oldalán felragyognak a reklámtáblák betüi, az ablakok szikráznak, a házak sárga falai mosolyognak, szinte világítanak, ajkaink mohón nyílnak lélekzetet venni, az égnek az az olcsó, boldog kék szine van, mint a kirakatban a selyempaplannak… csak egy pár napja, hogy korcsolyáztam, meg a hídra könyököltem nézni a befagyott Dunát, meg az alkonyat hideg homályában jártam a lemondás hízelgő fájdalmával lelkemben és csudálkozva nézek körül a szép időben, képzeletemet szédíti az örök idő körhintája, amelynek édes és keserves zenéjű forgásában megint a tavasz képe jön a szemem elé. Milyen szép idő van, a rikkancs gyorsabban szalad és hangosabban kántálja a lapokat, a kocsis emelgeti az ostort, suhogtatja és csettintget a nyelvével a lónak, a ló szalad, mert jól esik neki, a fáradt kocsi is könnyebben gurul, a hordárok sapkáján az életöröm piroslik. A neuraszténiások rosszul érzik magukat, türelmetlenek, éjszaka sokat sóhajtanak és sírnak is egy kicsit, ha tudnak. Oh, én nem akarok magammal foglalkozni, szégyellem eltünő életem gondjait, kínjait, kényességeit a föléje boruló végtelen kék ég alatt és a nap sugarainak édes zápora alatt, magamat, mint egy véletlen pelyhet fuvom el lelkemről és mélységes megindultságban érzek együtt egy szegény világgal, amelynek a jó isten megint engedélyezi a tavaszt.
Hogy szeretlek, szegény földi öröm, mely fölserkensz a tavaszt harsonázó napsugaraktól, áldottak legyetek ápolatlan és mosdatlan kezek, amelyek az utcasarkon az ibolyacsokrot nyujtogatjátok, áldottak legyetek mennyországba vágyakozó piros és kék luftballonok, áldottak legyetek elkínzott, ráncos arcok, amelyek az utcán zölden mosolyogtok a rátok eső napfényben, áldottak legyetek kapualjban sütkérező nyomorékok, akiknek a lábaszára csomóban végződik, áldottak legyetek napszámosok, akik a fának támasztott létrán álltok és vagdaljátok a fölösleges ágacskákat, áldottak legyetek elmélyedt kereskedősegédek, akik a nyitott kirakat előtt a nyakkendőket aggatjátok a vékony rudakra, áldottak legyetek
nikkelnyelű olcsó sétapálcák és hiú lilanyakkendők és neveletlen
ízlésű tarka mellények, áldottak legyetek irodába siető kis szüzek gyönge mellecskéi és vékony lábacskái, amelyeknek tündéri vonala most hajlik, áldottak legyetek zokogó verklik oldalára pingált álomvárak és elfoszlott rococcoidyllek, áldottak legyetek pesztrák, akik kizörgetitek a gyerekkocsit a sétatérre, áldottak legyetek sáros labdák, melyeket üres telken a nyomorúság gyermekei rugdosnak, áldottak legyetek kis padok a kórházak udvarán, áldottak legyetek olajos papirral ragasztott ablakok, amelyeket ki lehet nyitni, áldottak legyetek fehérsapkás kávéfőzőlegények, akik éjjel kiállotok az utcára cigarettázni, áldottak legyetek árva szerelmes párok, akik estefelé kimentek a ligetbe sétálni, derékon ölelve egymást. Áldottak legyetek vén koldusok és fiatal csavargók, akik az országuton mentek és leültök az alacsony mértföldjelzőre a csizmátokat lehúzni.
Áldottak legyetek gyámoltalan juhok, akik fölemelitek a fejetek és egymás felé bégettek és loncsos kutyák, akik marakodni kezdtek, áldottak legyetek pondrók a nyirkos pincében a hordók alatt, kik megérzitek, hogy a poros pinceablakon lesüt a nap. Áldottak legyetek fagyott magocskák a föld alatt, akik puhultok, dagadtok, kínlódtok és fölhasadtok és szűzi zöld füvecskét hoztok világra. Áldott légy mindenféle dudva veteményes kertben, szántóföldön, árokparton és szemétdombon, melyeket kiszurkálnak, kitaposnak, elpusztítanak mindig, mégis kijönnek a földre, egy kicsit élni akarnak ők is, mert süt a nap.
