Winterberries-like 3D network of N-doped porous carbon anchoring on N-doped carbon nanotubes for highly efficient platinum-based catalyst in methanol electrooxidation Tong Wang https://ebookmass.com/product/winterberrieslike-3d-network-of-n-doped-porous-carbonanchoring-on-n-doped-carbon-nanotubes-for-highlyefficient-platinum-based-catalyst-in-methanolelectrooxidation-tong-wang/ Download

More products digital (pdf, epub, mobi) instant download maybe you interests ...




In-situ growth of CoP wrapped by carbon nanoarray-like architecture onto nitrogen-doped Ti3C2 Pt-based catalyst for efficient methanol oxidation W. Zhan & L. Ma & M. Gan
https://ebookmass.com/product/in-situ-growth-of-cop-wrapped-bycarbon-nanoarray-like-architecture-onto-nitrogen-doped-ti3c2-ptbased-catalyst-for-efficient-methanol-oxidation-w-zhan-l-ma-mgan/
Fabrication of 3D interconnected porous MXene-based PtNPs as highly efficient electrocatalysts for methanol oxidation Qiang Zhang & Yuxuan Li & Ting Chen & Luyan Li & Shuhua Shi & Cui Jin & Bo Yang & Shifeng Hou
https://ebookmass.com/product/fabrication-of-3d-interconnectedporous-mxene-based-ptnps-as-highly-efficient-electrocatalystsfor-methanol-oxidation-qiang-zhang-yuxuan-li-ting-chen-luyan-lishuhua-shi-cui-jin-bo-yang/
Achieving uniform Pt deposition site by tuning the surface microenvironment of bamboo-like carbon nanotubes Meng Jin
https://ebookmass.com/product/achieving-uniform-pt-depositionsite-by-tuning-the-surface-microenvironment-of-bamboo-likecarbon-nanotubes-meng-jin/
Emerging Applications of Carbon Nanotubes in Drug and Gene Delivery Prashant Kesharwani
https://ebookmass.com/product/emerging-applications-of-carbonnanotubes-in-drug-and-gene-delivery-prashant-kesharwani/




Industrial Applications of Carbon Nanotubes 1st Edition
Peng Huisheng (Ed.)
https://ebookmass.com/product/industrial-applications-of-carbonnanotubes-1st-edition-peng-huisheng-ed/
Spectroscopy of Lanthanide Doped Oxide Materials Dhoble
https://ebookmass.com/product/spectroscopy-of-lanthanide-dopedoxide-materials-dhoble/
Chapter
6 - Water treatment and environmental remediation applications of carbon-based nanomaterials Xiaoli Tan & Xin Wang
https://ebookmass.com/product/chapter-6-water-treatment-andenvironmental-remediation-applications-of-carbon-basednanomaterials-xiaoli-tan-xin-wang/
Magnetism and Spintronics in Carbon and Carbon Nanostructured Materials
Sekhar Chandra Ray
https://ebookmass.com/product/magnetism-and-spintronics-incarbon-and-carbon-nanostructured-materials-sekhar-chandra-ray/

Carbon Capture Technologies for Gas-Turbine-Based Power Plants Hamidreza Gohari Darabkhani
https://ebookmass.com/product/carbon-capture-technologies-forgas-turbine-based-power-plants-hamidreza-gohari-darabkhani/

Contents lists available at ScienceDirect
journal homepage: www.elsevier.com/locate/carbon
Winterberries-like 3D network of N-doped porous carbon anchoring on N-doped carbon nanotubes for highly efficient platinum-based catalyst in methanol electrooxidation
Tong Wang , Jianting He, Qin Wu ** , Yunqi Yu, Kangcheng Chen *** , Daxin Shi , Yaoyuan Zhang , Hansheng Li *
School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, 100081, China
ARTICLE INFO
Keywords:
N-doped porous carbon N-doped carbon nanotubes 3D network
Methanol electrooxidation


ABSTRACT
A series of platinum-based catalyst Pt/Co/N-PC (N-doped porous carbon)/N-CNTs (N-doped carbon nanotubes) were prepared successfully by loading Pt nanoparticles on the winterberries-like 3D network of Co/N-PC/NCNTs, which was obtained from carbonization of in-situ grew ZIF-67/PPy (polypyrrole). The Pt nanoparticles with about 2.5 nm in diameter uniformly dispersed on the Co/N-PC/N-CNTs. The prepared electrocatalyst Pt/ Co/N-PC/N-CNTs exhibited excellent activity toward methanol electrocatalytic oxidation reaction. The mass activity (583 mA mg 1 Pt ) tested by the Cyclic voltammograms reached 2.1-fold greater than that of commercial Pt/C. The steady-state current density (42.3 mA mg 1 Pt ) tested by the chronoamperometric measurement reached 6.9-fold higher than that of commercial Pt/C. The outstanding methanol oxidation activity and stability of the Pt/Co/N-PC/N-CNTs were ascribed to the rich nitrogen-containing sites, large specific surface area and 3D conductive network of the winterberries-like carbon support Co/N-PC/N-CNTs, which displayed excellent support of Pt-based catalyst and has potential industrial applications.
1. Introduction
The severe environmental problems and growing energy crisis prompt people to exploit new clean energy devices. Researchers have more and more in-depth exploration and understanding of new clean energy devices in recent years [1–3]. Direct methanol fuel cells (DMFCs) have received increasingly extensive attention from researchers due to its environmentally friendly, excellent energy density and easy operation [4,5]. However, the problems,such as slow oxidation kinetics of methanol and CO poison, hindered the commercialization of DMFCs. Researchers have focused on developing novel catalysts to solve the problems [6–10]. Even if Pt-based catalysts show excellent electrochemical performance in a DMFC, high cost, low CO poisoning tolerance and poor interaction with support are the serious issues urgent to investigation. Therefore, developing suitable supports to reduce the amount of precious metal or replace Pt with non-precious metals attract extensive attention [11].
* Corresponding author.
** Corresponding author.
*** Corresponding author.
Carbon supports have become the preferred support due to the superior corrosion resistance and stability [12–19]. Carbon nanotubes (CNTs) are always thought as potential catalyst support for fuel cell on account of the considerable specific area, high conductivity as well as good electrochemical stability [20,21]. However, catalysts prepared with conventional CNTs as supports showed poor performance [22], Nitrogen-doped CNTs (N-CNTs) as supports enhance the methanol oxidation activity of catalysts. Zhang et al. [23]. prepared Pt-based catalysts with N-CNTs as the support, which was carbonized by polypyrrole nanotubes (PPy-NTs), and the peak current of the prepared catalyst Pt/N-CNTs for methanol electrocatalytic oxidation was 2.3 times higher than that of commercial catalyst.
The nitrogen-doped porous carbon (N-PC) prepared by carbonization of zeolite imidazole frameworks (ZIFs) has been widely studied in various electrocatalytic reactions because of its substantial nitrogen content, diverse pore structure as well as large specific surface area [24–27]. Zhang et al. [28] reported a Pt-based catalyst, with the
E-mail addresses: wuqin_bit@126.com (Q. Wu), chenkc@bit.edu.cn (K. Chen), hanshengli@bit.edu.cn (H. Li).
https://doi.org/10.1016/j.carbon.2022.08.058
Received 11 May 2022; Received in revised form 5 August 2022; Accepted 17 August 2022
Availableonline22August2022 0008-6223/©2022ElsevierLtd.Allrightsreserved.
Co@N-CNTs-PC derived from ZIF-67 as support, showing excellent electrocatalytic performance. Yang et al. [29] prepared the catalyst Pt–CoO@NPC@SnO2 using ZIF-67 as the precursor, showing good methanol oxidation performance. However, the poorly inter-connecting N-PC derived from ZIFs or ZIFs derivatives result in insufficient electrical conductivity. The conductive network structure of N-CNTs connects N-PC in series and improve the poor conductivity of individual N-PC. ZIF-67/PPy-NTs as precursors studied in methanol oxidation is barely shown in the literatures.
In this work, the winterberries-like Co/N-PC/N-CNTs with rich nitrogen content prepared by calcining ZIF-67/PPy-NTs, was synthesized by in-situ growth. The structure and properties of Co/N-PC/N-CNTs and the corresponding carbon support was characterized by various techniques. Methanol oxidation reaction activity of the electrocatalysts Pt/ Co/N-PC/N-CNTs was investigated. The correlation between electrochemical activity and the physicochemical properties for the Pt/Co/NPC/N-CNTs was studied.
2. Experimental
2.1. Materials
Cobalt nitrate hexahydrate (Co(NO3)2 6H2O), Pyrrole, 2-methylimidazole (2-MeIM), ferric chloride hexahydrate(FeCl3⋅6H2O), methyl orange, Sodium borohydride (NaBH4), methanol, ethanol were gained from Sinopharm Group, Hexachloroplatinic acid (H2PtCl6 6H2O) was purchased from Meryer Chemical Technology Co., Ltd. Nafion solution (5 wt%) was received from Alfa Aesar (China) Chemical Co., Ltd, Commercial Pt/C (20 wt%) catalyst was gained from Johnson Matthey Company. They are of analytically grade and used as received.
2.2. Preparation of Co/N-PC/N-CNTs
The PPy-NTs were prepared according to the literature [30]. The preparation of ZIF-67/PPy-NTs were described as follows. In a solution of 6 mmol Co (NO3) 6H2O in 100 mL methanol, a certain amount of PPy nanotubes was dispersed under ultrasound to form Mixture A. 100 mL methanol with 24 mmol 2-MeIM as poured into Mixture An under
stirring for 30 min and maintained in still under 25 ◦ C for 24 h. The product was washed completely with methanol, and dried at 60 ◦ C for 12 h under vacuum. The prepared ZIF-67/PPy-NTs was placed in tube furnace and calcined under Ar atmosphere at 700 ◦ C for 2 h. After cooling to room temperature naturally, the Co/N-PC/N-CNTs was obtained (see Scheme 1).
2.3. Preparation of Pt/Co/N-PC/N-CNTs
Pt nanoparticles loaded on Co/N-PC/N-CNTs were obtained by the modified NaBH4 reduction method [31–33]. In solution of 5 mL water with 30 mg sodium citrate and 149 μL H2PtCl6 6H2O (33.6 mg/mL) was drop wised into the mixture of 20 mg Co/N-PC/N-CNTs in 20 mL water, which formed a homogeneous dispersion; 3 mg NaBH4 in water (43 wt %) was added the solution described above and continuous stirring for 24 h. The Pt/Co/N-PC/N-CNTs was obtained through centrifugation, washed thoroughly with a mixture of ethanol and water five times, then dried at 60 ◦ C for 12 h under vacuum (see Scheme 1).
2.4. Characterization of Pt/Co/N-PC/N-CNTs and their intermediates
The crystalline structures of the supports and catalysts was characterized by X-ray powder diffraction (XRD) conducted on a Rigaku Ultima Ⅳ equipped with a Cu-Kα radiation. The external morphology and internal morphology and structure of the samples was observed through scanning electron microscope (SEM, Hitachi S4800). The internal morphology and structure of the samples was studied by transmission electron microscope (TEM, JEM-2100, JEOL, Japan) and the highresolution transmission electron microscopy (HRTEM). X-ray photoelectron spectrometry (XPS) is used to determine the chemical valence state of the sample near the surface area. Raman characterizes the disorder and graphitization degree of the samples, and the N2 adsorptiondesorption experiment carried out at 77K is used for analyzing the pore structure of the prepared supports (AUTOSORB IQ). The content of metal was determined by inductively coupled plasma-optical emission spectrometer (ICP-OES, Agilent725ES). TG curves was carried out under air atmosphere by thermogravimetric analysis (TGA, STA449 F5) from 30 ◦ C to 900 ◦ C.

2.5. Electrochemical measurements
We conducted the electrochemical measurements at the CHI604E workstation by a standard three-electrode system, and Pt foil, saturated calomel electrode and glassy carbon electrode with 5 mm in diameter covered with a catalyst layer were respectively employed as the counter, reference and working electrodes. The preparation method of the electrocatalyst slurry was as follow: A catalyst slurry was formed by adding 2 mg the prepared electrocatalyst into 1 mL mixed solution consisting of 500 μL of ethanol, 480 μL of deionized water and 20 μL of Nafion solution (5 wt%) under ultrasonication for 30 min. After that, 10 μL electrocatalyst slurry was dripped on the glassy carbon electrode and dried naturally. The test temperature was 25 ◦ C.
The electrochemical surface area (ECSA) calculated with the formula ECSA = QH/(0.21MPt) was determined by the hydrogen adsorption/ desorption peaks of the cyclic voltammograms (CV) curves in acidic medium. In the formula above, QH and MPt respectively represented the charge of hydrogen adsorption/desorption (mC cm 2) and the loading of Pt (mg cm 2) of the working electrode. In addition, 0.21 denoted the charge which was connected with monolayer adsorption of hydrogen on bright Pt [6].
3. Results and discussion
3.1. Morphology and structure of ZIF-67/PPy-NTs
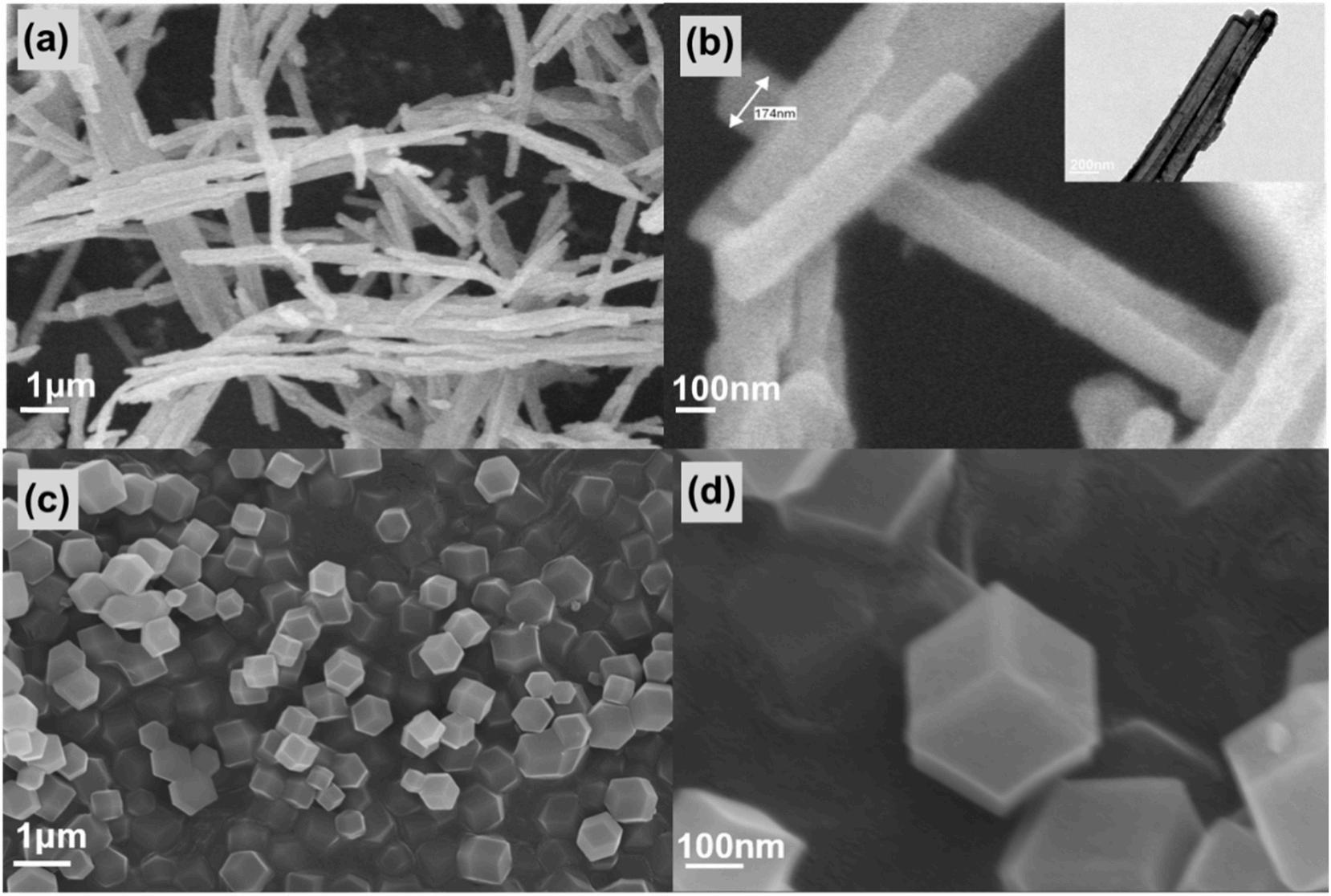
The morphology of PPy-NTs and ZIF-67 were investigated by SEM and TEM (Fig. 1). Average diameter of about 200 nm, hollow structure, length of several micrometers and interconnect 3D conductive network was observed for the PPy-NTs in Fig. 1(a) (b). The structure of synthesized ZIF-67 in Fig. 1(c) (d) have a rhombohedral dodecahedron morphology with a particle size of about 400 nm [34–36].
The morphology of ZIF-67/PPy-NTs were investigated by SEM and TEM (Fig. 2). The SEM images for ZIF-67/PPy-NTs in Fig. 2(a) (b) displayed that the ZIF-67 with the similar size and morphology to that of Fig. 1 were grown on the external surface of PPy-NTs. It was identified in the TEM images shown in Fig. 2(c) (d) that PPy-NTs and ZIF-67
displayed clearly position relationship. The novel structure of ZIF-67/ PPy-NTs was similar to winterberries, which were named as winterberries-like ZIF-67/PPy-NTs. The formation of the winterberrieslike structure is attributed to that Co2+ were adsorbed on the external surface of PPy-NTs by electrostatic attraction [37,38].
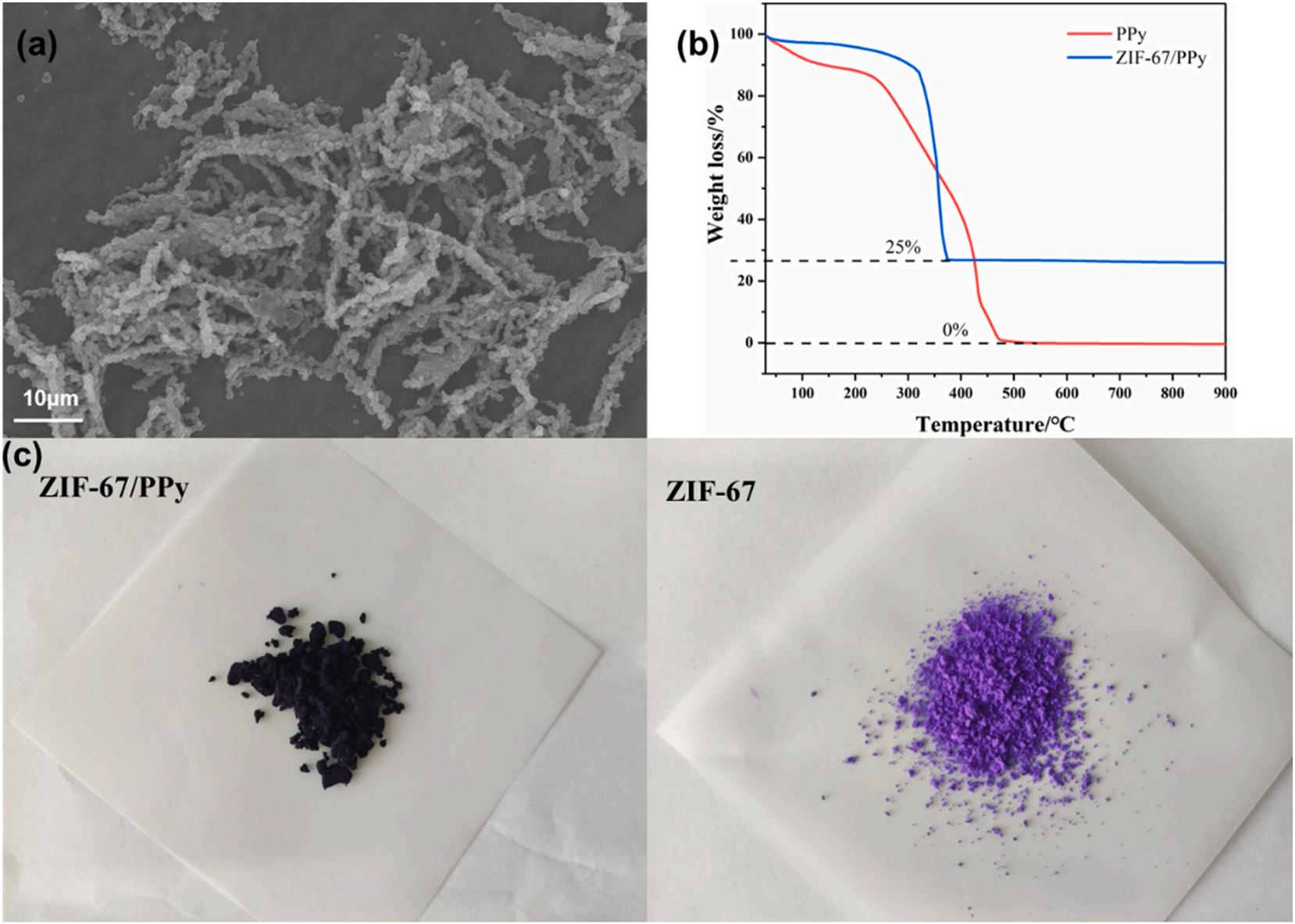
As can be seen in Fig. 3(a), ZIF-67 grew almost entirely on the surface of PPy nanotubes at a scale of 10 μm, and almost no individual ZIF-67 appeared. The color contrast image of ZIF-67/PPy and PPy was shown in Fig. 3(c). The color of ZIF-67/PPy, which showed purple black, was different from that of ZIF-67, which was bright purple. In addition, the abundant nitrogen sites on PPy surface provided a lot of nucleation sites for growth of ZIF-67. So ZIF-67 preferentially grew on the surface of PPy rather than bulk liquid phase. Thermogravimetric analysis of ZIF-67/ PPy and PPy was done to calculate the yield of ZIF-67 in ZIF-67/PPy (Fig. 3(b)). PPy was decomposed completely and ZIF-67/PPy was completely converted into Co3O4 during pyrolysis in air atmosphere, the content of which was 25%.
The XRD patterns of the PPy-NTs, ZIF-67/PPy-NTs and ZIF-67 for their crystal structures were shown in Fig. 4. PPy-NTs had a large broad peak between 20 and 30◦ due to the amorphous properties of PPy with the repeating unit of the pyrrole rings [39]. The XRD spectra of ZIF-67 in Fig. 4 was highly consistent with the typical diffraction peaks of the standard ZIF-67 [40]. Compared with PPy-NTs and ZIF-67, the diffraction peaks of ZIF-67/PPy-NTs were mostly from that of ZIF-67, and it indicated the ZIF-67/PPy-NTs were prepared successfully.
3.2.
Morphology and structure of Co/N-PC/N-CNTs
The morphology of N-CNTs and Co/N-PC were studied by TEM (Fig. 5). The morphology of N-CNTs still presented hollow structure, indicating the stability of the hollow PPy-NTs (Fig. 5(a)). Fig. 5(b) showed that the surface of N-CNTs was smooth and pure. The TEM images of Fig. 5(c) (d) for Co/N-PC showed that Co/N-PC boundary slightly shrinks due to the increase of stress caused by outward diffusion of organic ligand during carbonization process. In addition, the SEM of Fig. 5(c) showed that the Co/N-PC became rough due to the accumulation of Co nanoparticles.

TEM images of Co/N-PC/N-CNTs were shown in Fig. 6. Fig. 6(a)(b) exhibited that the structure of Co/N-PC/N-CNTs was the same as before calcination, indicating the stability of the structure of ZIF-67/PPy-NTs. The enlarged images of Co/N-PC/N-CNTs from Fig. 6(c) (d) showed that Co nanoparticles existed on the surface of N-CNTs and Co/N-PC.
Due to the high-temperature carbonization process, Co nanoparticles of Co/N-PC migrated, causing the existence of Co nanoparticles on the surface of N-CNTs.
The XRD patterns of the series supports mentioned above for the crystal structure were shown in Fig. 7(a). Strong diffraction peaks of Co/

N-PC at 44.2◦ , 51.5◦ and 75.8◦ are correspond to (111), (200) and (220) crystal planes of Co (JCPDS, No. 15–0806). At about 25◦ , the broad diffraction peak of Co/N-PC was assigned to the graphitic plane (002) of carbon. N-CNTs have only broad diffraction peak at about 25◦ , which was assigned to the graphitic plane (002) of carbon. The diffraction peaks of Co/N-PC/N-CNTs are basically the same as that of Co/N-PC, indicating that ZIF-67/PPy-NTs was successfully transformed into Co/ N-PC/N-CNTs.
The Raman spectrum of the series supports mentioned above in Fig. 7 (b) showed that they all have sharp peaks at 1350 cm 1 and 1580 cm 1 Generally speaking, the two typical peaks of 1580 cm 1 and 1350 cm 1 corresponded to the stretching of all sp2 carbon domains (G peak) and
the sp3 defect sites (D peak), respectively. The peak intensities ratio value of D peak to G peak (ID/IG) represented the level of disorder of carbon materials [41]. The value of ID/IG for Co/N-PC/N-CNTs was 1.06, larger than that of N-CNTs (1.0013) and Co/N-PC (0.99), suggesting that the disorder and defect sites of Co/N-PC/N-CNTs were increased. It was beneficial to the dispersion and anchoring of nano-sized Pt particles.
The pore structure of the supports mentioned above were investigated and the pore size distribution was calculated by the DFT method (Fig. 8). The isotherms of Co/N-PC showed type-I curve (Fig. 8(a)), indicating the existence of micropores, and the pore size distribution curve showed the presence of mesopores (Fig. 8(b)). The isotherms of NCNTs in Fig. 8(a) were type-IV curves, indicating the coexistence of micropores and mesoporous, which was in accordance to the pore distribution curve in Fig. 8(b). Co/N-PC/N-CNTs showed typical type-IV isotherm in Fig. 8(a), the pore distribution curve in Fig. 8(b) indicated mesopores as the main pore structure. Table 1 showed the data of pore structure for the different supports. The specific surface area of Co/NPC/N-CNTs was 336.7 m2 g 1 , much larger than that of N-CNTs (81.8 m2 g 1) and was near to that of Co/N-PC (361.1 m2 g 1). It was shown that the specific surface area of Co/N-PC/N-CNTs was improved by the introduction of Co/N-PC. The pore volume of Co/N-PC/N-CNTs was 0.386 cm3 g 1 , larger than that of Co/N-PC (0.113 cm3 g 1) and N-CNTs (0.295 cm3 g 1), indicating that Co/N-PC/N-CNTs had rich pore structure. The large specific surface area of Co/N-PC/N-CNTs played a vital role in the dispersion and deposition of Pt nanoparticles, and the abundant pore structure of Co/N-PC/N-CNTs promoted mass transfer process.
3.3. Morphology and structure of Pt/Co/N-PC/N-CNTs
Fig. 9 showed TEM images of the series Pt-based catalysts that we prepared by loading Pt nanoparticles on the obtained supports. Compared with transparent hollow N-CNTs in Fig. 5(b), Dense distributed Pt nanoparticles were anchored to the surface of N-CNTs (Fig. 9(a)).


6. TEM images of Co/N-PC/N-CNTs.

Fig. 7. Co/N-PC/N-CNTs, N-CNTs and Co/N-PC (a) XRD patterns, (b) Raman spectrum.
There were Co nanoparticles besides the dense distributed Pt nanoparticles on Pt/Co/N-PC (Fig. 9(b)). As can be seen in Fig. 9(c)(d), Pt/ Co/N-PC/N-CNTs showed that it maintained winterberries-like structure, indicating that loading process of Pt did not damage the morphology of Co/N-PC/N-CNTs. Compared with Co/N-PC/N-CNTs in Fig. 6(b), the Pt nanoparticles were densely deposition on the Co/N-PC/ N-CNTs (Fig. 9(c)). Pt/Co/N-PC/N-CNTs was investigated by HRTEM as shown in Fig. 9(e)(f) to verify the existence of Pt nanoparticles, the lattice fringes were clear, and the crystalline interplanar spacing were 0.225 nm、0.207 nm and 0.34 nm, and they corresponded to the (111) plane of Pt nanoparticles, (111) plane of Co nanoparticles and (002) plane of graphitic carbon. The scattered white dotted circles in Fig. 9(e) were dispersed Pt nanoparticles, and the diameter of Pt nanoparticles was about 2.5 nm. It was clarified that Pt/Co/N-PC/N-CNTs was formed
by loading uniformly distributed Pt nanoparticles on Co/N-PC/N-CNTs in Fig. 9
The distribution of nitrogen element of Pt/Co/N-PC/N-CNTs was characterized by the EDS elemental mapping and the corresponding figure is shown in Fig. 10. It can be seen that nitrogen element was uniformly distributed on the catalyst Pt/Co/N-PC/N-CNTs. It is worth noting that Pt elements were also uniformly distributed on the catalyst Pt/Co/N-PC/N-CNTs, and the distribution of Pt element is consistent with that of nitrogen element, indicating that the successful loading of Pt was closely related to the distribution of Nitrogen element.
The XRD patterns of the series Pt-based catalysts mentioned above were displayed in Fig. 11 They all exhibited typical diffraction peaks of Pt, including the peaks at 81.3◦ ,67.4◦ , 46.2◦ and 39.7◦ , which corresponded to the (311), (220), (200) and (111) planes of Pt (JCPDS, No.
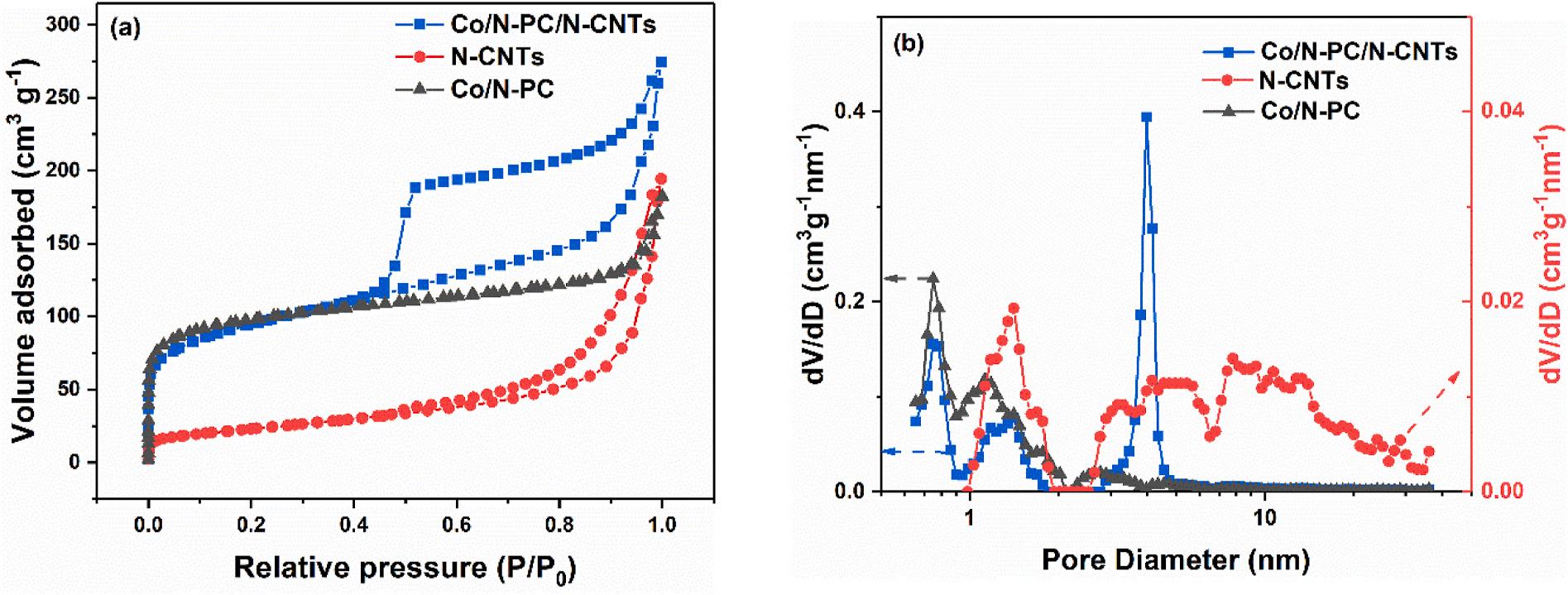
isotherms (b) pore distribution curves.
Table 1
BET surface area and pore volume for different supports.
Sample
04–0802), respectively. In addition, the peak at 25◦ was belonged to the (002) plane of graphitic carbon. Compared with Pt/N-CNTs, Pt/Co/NPC/N-CNTs and Pt/Co/N-PC showed typical diffraction peaks of Co (JCPDS, No. 15–0806) due to the Co element in ZIF-67. The results of XRD corresponded to the HRTEM of Pt/Co/N-PC/N-CNTs. Moreover, ICP of the Pt/Co/N-PC/N-CNTs showed that the content of Pt is 17.32 wt %, which was nearly consistent with the theoretical content (20 wt%), indicating the feasible method of reducing Pt.
XPS survey and High resolution XPS survey spectrum were displayed for interrogating the surface element valence states and chemical composition of as prepared catalysts (Fig. 12). N1s peaks appeared except commercial Pt/C, indicating the presence of N element in the other three Pt-based catalysts, and it was beneficial to the dispersion of Pt nanoparticles [42]. The Co2p peak appeared in Pt/Co/N-PC and
Pt/Co/N-PC/N-CNTs, and the content was 1.49 wt% and 1.54 wt%, respectively. The O1s peak may be caused by the surface oxidation due to exposure to air. For the purpose of exploring the composition of Pt of the obtained catalysts and the change of binding energy, the high-resolution Pt 4f spectra of the series Pt-based catalysts as above mentioned and commercial Pt/C were shown in Fig.12 (b), Fig.12 (c), Fig. 12(d) and (e), respectively. All Pt 4f spectra of the above catalysts were deconvoluted into two pairs of peaks, and they belonged to Pt2+ and Pt0. The binding energies of Pt2+ and Pt0 of the above catalysts were shown in Table 2 The binding energies of Pt0 in Pt/Co/N-PC/N-CNTs, Pt/Co/N-PC, Pt/N-CNTs and commercial Pt/C were 71.33 eV, 71.55 eV, 71.25 eV and 71.69 eV, respectively. Different with commercial Pt/C, the binding energy of Pt0 in the other three Pt-based catalysts all shifts negatively, the deviation of Pt/N-CNTs (0.44 eV) was higher than Pt/Co/N-PC/N-CNTs (0.36 eV) and Pt/Co/N-PC (0.14 eV), the deviation of Pt/Co/N-PC/N-CNTs was moderate, indicating that the interaction between Pt and adsorption species was moderate. In addition, the slight change of binding energies was because of the change of Pt electronic structure, owing to the presence of N element [43].
High resolution N1s spectra of Pt/Co/N-PC/N-CNTs was displayed in Fig. 12 (f), it was deconvoluted into three peaks located on 402.19 eV (graphitic N), 400.05 eV (pyrrolic N) and 398.66 eV (pyridinic N), respectively [44–46]. Undoubtedly, Reasonable distribution of different



and Pt/Co/N-PC
types of N elements was an important factor to achieve excellent electrocatalytic performance. Table 3 showed the atomic percentages distribution of different nitrogen types of the three prepared catalysts. the N content of Pt/Co/N-PC/N-CNTs is as high as 11.25 at. %, which is greater than that of Pt/Co/N-PC (8.57 at. %) and Pt/N-CNTs (9.82 at. %)). From the perspective of pyridine N, Pt/Co/N-PC (45.9 at.%) is greater than Pt/N-CNTs (39 at.%) and Pt/Co/N-PC/N-CNTs (38.67 at. %); From the perspective of pyrrole N, Pt/N-CNTs (45.33 at.%) has the highest content, which is greater than Pt/Co/N-PC/N-CNTs (30.71 at.%) and Pt/Co/N-PC (29.2 at.%); From the point of view of graphite N, Pt/Co/N-PC/N-CNTs (30.62 at.%) is greater than Pt/Co/N-PC (24.9 at. %), much greater than Pt/N-CNTs(15.67 at.%). From the distribution of the three types of N elements, it was shown that Co nanoparticles migrating to CNTs (Fig. 6(c)) promotes that the Pyridine N of N-CNTs converted to graphite N, so the graphite N content of Pt/Co/N-PC/N-CNTs increased. the electrical conductivity of the catalyst can be enhanced because of the graphite N [47], and pyridine N and pyrrole N can offer the primary nucleation sites for deposition of Pt nanoparticles [48]. Therefore, the N distribution of
Pt/Co/N-PC/N-CNTs was optimal, and it has good electrical conductivity and certain nucleation sites for Pt deposition.
3.4. Electrochemical performance
Methanol electrooxidation consists of the following three steps [49, 50]. The electrocatalytic oxidation process of the three steps is shown in Equations (1.1) - (1.3). The total reaction of the anodic reaction is shown in Equation (1.4). Firstly, methanol adsorbs on Pt and deprotonates gradually to generate carbon contained species and electron. Secondly, Pt dissociates water to produce oxygenated species, H+ and electron. Lastly, oxygenated species oxidize the carbon contained species to produce carbon dioxide, H+ and electron. Simultaneously, Pt is released. The proton and electron transfer to the catalyst/electrolyte interface and external circuit, respectively. Therefore, the number of active center Pt and the electrical conductivity of catalyst play a vital role in the methanol electrooxidation.
The electrochemical activity of the synthesized electrocatalysts were investigated by CV test. The CV curves of the series Pt-based catalysts as above mentioned and commercial Pt/C in acidic solution were exhibited in Fig. 13 (a). All those synthesized Pt-based catalysts exhibited the typical peaks of Pt involving peaks of hydrogen adsorption and desorption in CV curves [51], and it can determine the electrochemically active surface area (ECSA). The ECSA of Pt/Co/N-PC/N-CNTs was 76.79 m2 g 1 , and it was obviously greater than commercial Pt/C catalyst (33.10 m2 g 1), Pt/N-CNTs (60.67 m2 g 1) and Pt/Co/N-PC (66.95 m2 g 1). The large ECSA further demonstrated that more intrinsic catalytic active sites were exposed on the surface of Pt/Co/N-PC/N-CNTs, consistent with the TEM images in Fig. 9 Methanol oxidation activity most intuitively showed the electrochemical activity of the prepared catalysts. The CV curves in the mixed acidic solution in Fig. 13 (b) suggested that the forward peak current density for Pt/Co/N-PC/N-CNTs was 583 mA⋅mg 1 Pt in methanol oxidation reaction, significantly greater than that of Pt/N-CNTs (366 mA mg 1 Pt ) and

Fig. 12. (a) XPS survey spectra of Pt/Co/N-PC/N-CNTs, Pt/Co/N-PC, Pt/N-CNTs and commercial Pt/C; High resolution XPS survey spectra of Pt 4f for(b) Pt/Co/NPC/N-CNTs, (c) Pt/Co/N-PC, (d) Pt/N-CNTs, (e) commercial Pt/C; High resolution XPS survey spectra of N 1s for(f) Pt/Co/N-PC/N-CNTs, (g) Pt/Co/N-PC, (h) Pt/ N-CNTs.
Table 2
The binding energies of Pt0 and Pt2+ of the catalysts.
Table 3
Atomic percentage distribution of different N types for different catalysts.
large specific surface area, abundant N sites, high pyridine N and pyrrole N content of Co/N-PC/N-CNTs facilitate the formation of high-density and well-distributed Pt nanoparticles, resulting in improving the mass activity of Pt/Co/N-PC/N-CNTs. Thus, Pt/Co/N-PC/N-CNTs exhibited the largest ECSA and highest mass activity.
Note: “N” means the content of nitrogen element.
Pt/Co/N-PC (363 mA mg 1 Pt ), and its peak current density was 2.1-fold higher than that of commercial Pt/C (277 mA⋅mg 1 Pt ). The methanol oxidation activity of the above catalysts were corresponding to its ECSA (Fig. 13(a)). The uniform distribution of nitrogen element of Pt/Co/N-PC/N-CNTs was better helpful for anchoring Pt in section 3.3 (Fig. 10), which can be distributed evenly on the support. Therefore, it increased the number of active centers and electrochemical active surface area. In addition, the appropriate nitrogen distribution improved the conductivity of the support and provided a large number of nucleation sites for Pt deposition combined with XPS analysis (Fig. 12). The
Electrochemical impedance spectroscopy (EIS) was regarded as a helpful characterization to reflect charge transfer properties towards methanol oxidation reaction. The Nyquist plots of the Pt-based catalysts as above mentioned and commercial Pt/C were shown in Fig. 13 (c). The diameters of the capacitive reactance arc reflected the charge transfer resistance (Rct) in the methanol oxidation process. The smaller the value of Rct, the faster the electrochemical reaction rate, resulting in the higher methanol oxidation activity [52,53]. Compared to the other three catalysts, Pt/Co/N-PC/N-CNTs had the smallest diameters of the capacitive reactance arc, revealing that Pt/Co/N-PC/N-CNTs had lower Rct of methanol oxidation reaction. Based on the analysis of section 3.2, the hollow structure of N-CNTs is very favorable for the mass transport in the electrochemical reaction. The N-CNTs overlapped with each other as the base and Co/N-PC as the hub formed three-dimensional conductive network structure of Co/N-PC/N-CNTs. The interesting structure undoubtedly made a great contribution to the conductivity of the catalysts. The micropore structure reduced the exposure of accessible active sites. The pore structure of Co/N-PC/N-CNTs was dominated by mesoporous structure, which increased the exposure of more accessible Pt sites. In a word, owing to the hollow morphology of N-CNTs, the abundant pore of N-PC, and the winterberries-like conductive network structure obtained by in-situ growth, a highway-like channel for electron and ion transport in methanol oxidation was formed. In addition, the high content of graphite N in Co/N-PC/N-CNTs improved the conductivity, which was in accordance to the results of XPS (Fig. 12). Therefore, Pt/Co/N-PC/N-CNTs had lower Rct of methanol oxidation reaction.

Fig. 13. Cyclic voltammetry curves of Pt/Co/N-PC/N-CNTs, Pt/Co/N-PC, Pt/N-CNTs and commercial Pt/C(a) in N2 saturated 0.5 M H2SO4 and (b) in 0.5 M H2SO4 + 0.5 M CH3OH at a scan rate of 50 mv/s; (c) Nyquist plots of Pt/Co/N-PC/N-CNTs, Pt/Co/N-PC, Pt/N-CNTs and commercial Pt/C; (d) I-t curves of Pt/Co/N-PC/NCNTs, Pt/Co/N-PC, Pt/N-CNTs and commercial Pt/C in 0.5 M H2SO4 + 0.5 M CH3OH. (A colour version of this figure can be viewed online.)
The stability and anti-poisoning ability of the catalysts were investigated through the chronoamperometric measurements. Fig. 13 (d) showed the I-t curve of the series Pt-based catalysts as above mentioned and commercial Pt/C at 0.4 V. The steady-state current density of Pt/Co/ N-PC/N-CNTs, Pt/N-CNTs, Pt/Co/N-PC and commercial Pt/C were 42.3 mA mg 1 Pt , 14.4 mA mg 1 Pt ,12.6 mA mg 1 Pt and 6.1 mA mg 1 Pt respectively after 7200 s, and the Pt/Co/N-PC/N-CNTs was 6.9-fold larger than commercial Pt/C. The above data highlighted the remarkable long-term stability of Pt/Co/N-PC/N-CNTs. Based on the analysis in Fig. 10 and Table 3 of Section 3.3, uniform and abundant nitrogen sites of Co/N-PC/ N-CNTs played an important role in the dispersion and anchoring of Pt. It was not easy for Pt to agglomerate and fall off during chronoamperometric measurements. Then the particle size of Pt did not change greatly, resulting in the higher current density of the Pt/Co/NPC/N-CNTs. Moreover, the electron-accepting ability of nitrogen enabled it to polarize adjacent carbon atoms, enhancing the binding ability between carbon atoms and adsorbed water molecules. Therefore, more oxygen-containing species are generated in methanol oxidation reaction, which improved the anti-poisoning ability of the catalyst [54].
4. Conclusion
In summary, the winterberries-like 3D network of Pt/Co/N-PC/NCNTs with abundant nitrogen content have prepared successfully. Fine Pt nanoparticles with about 2.5 nm in diameter uniformly dispersed on Co/N-PC/N-CNTs, which was prepared by carbonizing the ZIF-67/PPyNTs obtained by in-situ growth method. The Pt/Co/N-PC/N-CNTs exhibited excellent electrocatalytic activity for the methanol oxidation reaction, including the amazing mass activity (583 mA mg 1 Pt ), which reached 2.1-fold greater than that of commercial Pt/C, and good stability with the steady-state current density of 42.3 mA⋅mg 1 Pt shown by the chronoamperometric measurements, which was 6.9-fold higher than that of commercial Pt/C. Abundant nitrogen content and large specific surface area of the winterberries-like Co/N-PC/N-CNTs as support for the Pt/Co/N-PC/N-CNTs catalyst, which was conducive to the anchoring and dispersion of Pt nanoparticles to make it difficult to agglomerate and fall off, lead to improving electrochemical performance for methanol oxidation. Winterberries-like Co/N-PC/N-CNTs with Co/N-PC as the connecting node formed the 3D conductive network with abundant pore structure, which provide a highway transport path for ions and electrons. Pt/Co/N-PC/N-CNTs is a promising electrocatalyst in direct methanol fuel cells.
CRediT authorship contribution statement
Tong Wang: Data curation, Writing - original draft. Qin Wu: Software. Kangcheng Chen: Methodology. Daxin Shi: Investigation. Yaoyuan Zhang: Investigation. Hansheng Li: Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors sincerely acknowledged the financial support from the State Key Program of National Natural Science Foundation of China (No. 21736001). Furthermore, the authors thanked the technical support from Analysis & Testing Center (Beijing Institute of Technology).
References
[1] D. Liu, N. Yang, Q. Zeng, H. Liu, D. Chen, P. Cui, L. Xu, C. Hu, J. Yang, Core-shell Ag–Pt nanoparticles: a versatile platform for the synthesis of heterogeneous
nanostructures towards catalyzing electrochemical reactions, Chin. Chem. Lett. 32 (11) (2021) 3288–3297, https://doi.org/10.1016/j.cclet.2021.04.053
[2] B. Zhou, N. Zhang, Y. Wu, W. Yang, Y. Lu, Y. Wang, S. Wang, An option for green and sustainable future: electrochemical conversion of ammonia into nitrogen, J. Energy Chem. 60 (2021) 384–402, https://doi.org/10.1016/j. jechem.2021.01.011
[3] J. Liu, H. Yuan, H. Liu, C.Z. Zhao, Y. Lu, X.B. Cheng, J.Q. Huang, Q. Zhang, Unlocking the failure mechanism of solid state lithium metal batteries, Adv. Energy Mater. 12 (4) (2021), https://doi.org/10.1002/aenm.202100748
[4] H. Liu, C. Song, L. Zhang, J. Zhang, H. Wang, D.P. Wilkinson, A review of anode catalysis in the direct methanol fuel cell, J. Power Sources 155 (2) (2006) 95–110, https://doi.org/10.1016/j.jpowsour.2006.01.030.
[5] M. Winter, R.J. Brodd, What are batteries, fuel cells, and supercapacitors? Chem. Rev. 105 (3) (2005) https://doi.org/10.1021/cr040110e, 1021-1021.
[6] Y. Sun, C. Du, G. Han, Y. Qu, L. Du, Y. Wang, G. Chen, Y. Gao, G. Yin, Boron, nitrogen co-doped graphene: a superior electrocatalyst support and enhancing mechanism for methanol electrooxidation, Electrochim. Acta 212 (2016) 313–321, https://doi.org/10.1016/j.electacta.2016.06.168
[7] Y. Sun, C. Du, M. An, L. Du, Q. Tan, C. Liu, Y. Gao, G. Yin, Boron-doped graphene as promising support for platinum catalyst with superior activity towards the methanol electrooxidation reaction, J. Power Sources 300 (2015) 245–253, https://doi.org/10.1016/j.jpowsour.2015.09.046
[8] Q. Zhou, Z. Pan, D. Wu, G. Hu, S. Wu, C. Chen, L. Lin, Y. Lin, Pt-CeO2/TiN NTs derived from metal organic frameworks as high-performance electrocatalyst for methanol electrooxidation, Int. J. Hydrogen Energy 44 (21) (2019) 10646–10652, https://doi.org/10.1016/j.ijhydene.2019.01.231
[9] Z. Li, S. Ji, B.G. Pollet, P.K. Shen, Supported 3-D Pt nanostructures: the straightforward synthesis and enhanced electrochemical performance for methanol oxidation in an acidic medium, J. Nanoparticle Res. 15 (10) (2013), https://doi. org/10.1007/s11051-013-1959-9
[10] H. Huang, Y. Wei, Y. Yang, M. Yan, H. He, Q. Jiang, X. Yang, J. Zhu, Controllable synthesis of grain boundary-enriched Pt nanoworms decorated on graphitic carbon nanosheets for ultrahigh methanol oxidation catalytic activity, J. Energy Chem. 57 (2021) 601–609, https://doi.org/10.1016/j.jechem.2020.08.063
[11] A. Serov, C. Kwak, Review of non-platinum anode catalysts for DMFC and PEMFC application, Appl. Catal. B Environ. 90 (3–4) (2009) 313–320, https://doi.org/ 10.1016/j.apcatb.2009.03.030
[12] L. Zhao, X.L. Sui, J.L. Li, J.J. Zhang, L.M. Zhang, Z.B. Wang, 3D hierarchical PtNitrogen-Doped-Graphene-Carbonized commercially available sponge as a superior electrocatalyst for low-temperature fuel cells, ACS Appl. Mater. Interfaces 8 (25) (2016) 16026–16034, https://doi.org/10.1021/acsami.6b03520
[13] W. Yuan, Y. Zhang, N. Zhang, C. Yin, X. Zhang, X. Liu, Carbon riveted Pt-MnO2/ reduced graphene oxide anode catalyst for DMFC, Catal. Commun. 100 (2017) 66–70, https://doi.org/10.1016/j.catcom.2017.06.030
[14] X. Li, L. Luo, F. Peng, H. Wang, H. Yu, Enhanced activity of Pt/CNTs anode catalyst for direct methanol fuel cells using Ni2P as co-catalyst, Appl. Surf. Sci. 434 (2018) 534–539, https://doi.org/10.1016/j.apsusc.2017.10.218
[15] K. Wang, Z. Pan, F. Tzorbatzoglou, Y. Zhang, Y. Wang, T. Panagiotis, S. Song, An investigation of WC stability during the preparation of Pt@WC/OMC via a pulse microwave assisted polyol method, Appl. Catal. B Environ. 166-167 (2015) 224–230, https://doi.org/10.1016/j.apcatb.2014.11.025
[16] M. Huang, J. Zhang, C. Wu, L. Guan, Networks of connected Pt nanoparticles supported on carbon nanotubes as superior catalysts for methanol electrooxidation, J. Power Sources 342 (2017) 273–278, https://doi.org/10.1016/j. jpowsour.2016.12.054
[17] H. Huang, S. Yang, R. Vajtai, X. Wang, P.M. Ajayan, Pt-decorated 3D architectures built from graphene and graphitic carbon nitride nanosheets as efficient methanol oxidation catalysts, Adv. Mater. 26 (30) (2014) 5160–5165, https://doi.org/ 10.1002/adma.201401877
[18] K. Cheng, K. Zhu, S. Liu, M. Li, J. Huang, L. Yu, Z. Xia, C. Zhu, X. Liu, W. Li, W. Lu, F. Wei, Y. Zhou, W. Zheng, S. Mu, A spatially confined gC3N4-Pt electrocatalyst with robust stability, ACS Appl. Mater. Interfaces 10 (25) (2018) 21306–21312, https://doi.org/10.1021/acsami.8b03832.
[19] M. Li, Q. Jiang, M. Yan, Y. Wei, J. Zong, J. Zhang, Y. Wu, H. Huang, Threedimensional boron- and nitrogen-codoped graphene aerogel-supported Pt nanoparticles as highly active electrocatalysts for methanol oxidation reaction, ACS Sustain. Chem. Eng. 6 (5) (2018) 6644–6653, https://doi.org/10.1021/ acssuschemeng.8b00425
[20] W. Yang, X. Wang, F. Yang, C. Yang, X. Yang, Carbon nanotubes decorated with Pt nanocubes by a noncovalent functionalization method and their role in oxygen reduction, Adv. Mater. 20 (13) (2008) 2579–2587, https://doi.org/10.1002/ adma.200702949
[21] R. Chetty, W. Xia, S. Kundu, M. Bron, T. Reinecke, W. Schuhmann, M. Muhler, Effect of reduction temperature on the preparation and characterization of Pt-Ru nanoparticles on multiwalled carbon nanotubes, Langmuir 25 (6) (2009) 3853–3860, https://doi.org/10.1021/la804039w
[22] W. Li, C. Liang, W. Zhou, J. Qiu, H. Li, G. Sun, Q. Xin, Homogeneous and controllable Pt particles deposited on multi-wall carbon nanotubes as cathode catalyst for direct methanol fuel cells, Carbon 42 (2) (2004) 436–439, https://doi. org/10.1016/j.carbon.2003.10.033
[23] L.M. Zhang, X.L. Sui, L. Zhao, J.J. Zhang, D.M. Gu, Z.B. Wang, Nitrogen-doped carbon nanotubes for high-performance platinum-based catalysts in methanol oxidation reaction, Carbon 108 (2016) 561–567, https://doi.org/10.1016/j. carbon.2016.07.059
[24] G. Li, C. Zhang, Z. Wang, H. Huang, H. Peng, X. Li, Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene
oxidation, Appl. Catal. Gen. 550 (2018) 67–76, https://doi.org/10.1016/j. apcata.2017.11.003
[25] J. Wei, Y. Hu, Y. Liang, B. Kong, Z. Zheng, J. Zhang, S.P. Jiang, Y. Zhao, H. Wang, Graphene oxide/core–shell structured metal–organic framework nano-sandwiches and their derived cobalt/N-doped carbon nanosheets for oxygen reduction reactions, J. Mater. Chem. 5 (21) (2017) 10182–10189, https://doi.org/10.1039/ c7ta00276a
[26] D. Ji, L. Fan, L. Tao, Y. Sun, M. Li, G. Yang, T.Q. Tran, S. Ramakrishna, S. Guo, The Kirkendall effect for engineering oxygen vacancy of hollow Co3 O4 nanoparticles toward high-performance portable Zinc-air batteries, Angew Chem. Int. Ed. Engl. (2019), https://doi.org/10.1002/anie.201908736
[27] Z. Wu, J. Wang, L. Han, R. Lin, H. Liu, H.L. Xin, D. Wang, Supramolecular gelassisted synthesis of double shelled Co@CoO@N-C/C nanoparticles with synergistic electrocatalytic activity for the oxygen reduction reaction, Nanoscale 8 (8) (2016) 4681–4687, https://doi.org/10.1039/c5nr07929b.
[28] J. Zhang, R. Wang, X. Hu, Z. Sun, X. Wang, Y. Guo, L. Yang, M. Lou, P. Wen, MOFderived Co embedded into N-doped nanotube decorated mesoporous carbon as a robust support of Pt catalyst for methanol electrooxidation, Appl. Surf. Sci. 533 (2020), https://doi.org/10.1016/j.apsusc.2020.147319
[29] Y. Yu, S. You, J. Du, Z. Xing, Y. Dai, H. Chen, Z. Cai, N. Ren, J. Zou, ZIF-67-derived CoO (tetrahedral Co2+)@nitrogen-doped porous carbon protected by oxygen vacancies-enriched SnO2 as highly active catalyst for oxygen reduction and Pt cocatalyst for methanol oxidation, Appl. Catal. B Environ. 259 (2019), https://doi. org/10.1016/j.apcatb.2019.118043
[30] Y. Li, X. Liu, H. Li, D. Shi, Q. Jiao, Y. Zhao, C. Feng, X. Bai, H. Wang, Q. Wu, Rational design of metal organic framework derived hierarchical structural nitrogen doped porous carbon coated CoSe/nitrogen doped carbon nanotubes composites as a robust Pt-free electrocatalyst for dye-sensitized solar cells, J. Power Sources 422 (2019) 122–130, https://doi.org/10.1016/j.jpowsour.2019.03.041
[31] Y. Wang, C. Du, Y. Sun, G. Han, F. Kong, G. Yin, Y. Gao, Y. Song, The enhanced CO tolerance of platinum supported on FeP nanosheet for superior catalytic activity toward methanol oxidation, Electrochim. Acta 254 (2017) 36–43, https://doi.org/ 10.1016/j.electacta.2017.09.099
[32] J. Ding, L. Ma, M. Gan, W. Zhan, C. Zhou, D. Wei, S. Han, J. Shen, F. Xie, X. Zhong, Facile fabrication of ultrafine Pt nanoparticles supported on 3D macro-/oversized mesoporous N-doped carbon for efficient methanol oxidation, Int. J. Hydrogen Energy 44 (57) (2019) 30388–30400, https://doi.org/10.1016/j. ijhydene.2019.09.182.
[33] M.H. Jiang, L. Ma, M.Y. Gan, L.Q. Hu, H.M. He, F. Xie, H.H. Zhang, Worm-like PtP nanocrystals supported on NiCo2Px/C composites for enhanced methanol electrooxidation performance, Electrochim. Acta 293 (2019) 30–39, https://doi. org/10.1016/j.electacta.2018.10.022
[34] D. Shao, C. Wang, L. Wang, X. Guo, J. Guo, S. Zhang, Y. Lu, A N-doped Cobalt@ graphitized carbon material derived from ZIF-67 assisted polyvinylidene fluoride hollow fiber membrane for supercapacitors, J. Alloys Compd. 863 (2021), https:// doi.org/10.1016/j.jallcom.2021.158682
[35] P. Dai, Y. Yao, E. Hu, D. Xu, Z. Li, C. Wang, Self-assembled ZIF-67@graphene oxide as a cobalt-based catalyst precursor with enhanced catalytic activity toward methanolysis of sodium borohydride, Appl. Surf. Sci. 546 (2021), https://doi.org/ 10.1016/j.apsusc.2021.149128
[36] Y. Tao, W. Zeng, ZIF-67 MOF-derived Co3O4/NiCo2O4/CC unique layered structure with excellent gas performances, Ceram. Int. 47 (6) (2021) 8441–8446, https://doi.org/10.1016/j.ceramint.2020.11.209
[37] L.-H. Xu, S.-H. Li, H. Mao, A.-S. Zhang, W.-W. Cai, T. Wang, Z.-P. Zhao, An advanced necklace-like metal organic framework with an ultrahighly continuous structure in the membrane for superior butanol/water separation, J. Mater. Chem. 9 (19) (2021) 11853–11862, https://doi.org/10.1039/d1ta01736e
[38] Q. Zhou, Z. Zhang, J. Cai, B. Liu, Y. Zhang, X. Gong, X. Sui, A. Yu, L. Zhao, Z. Wang, Z. Chen, Template-guided synthesis of Co nanoparticles embedded in hollow nitrogen doped carbon tubes as a highly efficient catalyst for rechargeable Zn-air batteries, Nano Energy 71 (2020), https://doi.org/10.1016/j. nanoen.2020.104592
[39] X. Du, T. Yang, J. Lin, T. Feng, J. Zhu, L. Lu, Y. Xu, J. Wang, Microwave-Assisted synthesis of SnO2@polypyrrole nanotubes and their pyrolyzed composite as anode for lithium-ion batteries, ACS Appl. Mater. Interfaces 8 (24) (2016) 15598–15606, https://doi.org/10.1021/acsami.6b03332
[40] W. Xia, J. Zhu, W. Guo, L. An, D. Xia, R. Zou, Well-defined carbon polyhedrons prepared from nano metal–organic frameworks for oxygen reduction, J. Mater. Chem. 2 (30) (2014) 11606–11613, https://doi.org/10.1039/c4ta01656d
[41] X.X. Yang, J.W. Li, Z.F. Zhou, Y. Wang, L.W. Yang, W.T. Zheng, C.Q. Sun, Raman spectroscopic determination of the length, strength, compressibility, Debye temperature, elasticity, and force constant of the C-C bond in graphene, Nanoscale 4 (2) (2012) 502–510, https://doi.org/10.1039/c1nr11280e
[42] Y. Zhou, K. Neyerlin, T.S. Olson, S. Pylypenko, J. Bult, H.N. Dinh, T. Gennett, Z. Shao, R. O’Hayre, Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports, Energy Environ. Sci. 3 (10) (2010), https://doi.org/10.1039/c003710a
[43] X. Zhao, J. Zhu, L. Liang, C. Li, C. Liu, J. Liao, W. Xing, Biomass-derived N-doped carbon and its application in electrocatalysis, Appl. Catal. B Environ. 154-155 (2014) 177–182, https://doi.org/10.1016/j.apcatb.2014.02.027
[44] H. Park, S. Oh, S. Lee, S. Choi, M. Oh, Cobalt- and nitrogen-codoped porous carbon catalyst made from core–shell type hybrid metal–organic framework (ZIF-L@ZIF67) and its efficient oxygen reduction reaction (ORR) activity, Appl. Catal. B Environ. 246 (2019) 322–329, https://doi.org/10.1016/j.apcatb.2019.01.083
[45] Y. Zhao, H.-M. Zhang, Y. Zhang, M. Zhang, J. Yu, H. Liu, Z. Yu, B. Yang, C. Zhu, J. Xu, Leaf-like 2D nanosheet as efficient oxygen reduction reaction catalyst for Znair battery, J. Power Sources 434 (2019), https://doi.org/10.1016/j. jpowsour.2019.226717
[46] J. Ou, C. Gong, J. Xiang, J. Liu, Noble metal-free Co@N-doped carbon nanotubes as efficient counter electrode in dye-sensitized solar cells, Sol. Energy 174 (2018) 225–230, https://doi.org/10.1016/j.solener.2018.09.018
[47] S.H. Lim, R. Li, W. Ji, J. Lin, Effects of nitrogenation on single-walled carbon nanotubes within density functional theory, Phys. Rev. B 76 (19) (2007), https:// doi.org/10.1103/PhysRevB.76.195406
[48] S.C. Roy, A.W. Harding, A.E. Russell, K.M. Thomas, Erratum: spectroelectrochemical study of the role played by carbon functionality in fuel cell electrodes [J. Electrochem. Soc., 144, 2323 (1997).], J. Electrochem. Soc. 158 (2) (2011), https://doi.org/10.1149/1.3529355
[49] T. Iwasita, Electrocatalysis of methanol oxidation, Electrochim. Acta 47 (22–23) (2002) 3663–3674, https://doi.org/10.1016/s0013-4686(02)00336-5
[50] A. Hamnett, Mechanism and electrocatalysis in the direct methanol fuel cell, Catal. Today 38 (4) (1997) 445–457, https://doi.org/10.1016/s0920-5861(97)00054-0
[51] S. Gilman, Anion adsorption on platinum by the multipulse potentio-dynamic method, Nature 203 (4950) (1964) 1130–1131, https://doi.org/10.1038/ 2031130a0
[52] I.M. Hsing, X. Wang, Y.-J. Leng, Electrochemical impedance studies of methanol electro-oxidation on Pt/C thin film electrode, J. Electrochem. Soc. 149 (5) (2002), https://doi.org/10.1149/1.1467940
[53] J.T. Müller, P.M. Urban, W.F. Holderich, Impedance studies on direct methanol fuel cell anodes, J. Power Sources 84 (2) (1999) 157–160, https://doi.org/ 10.1016/s0378-7753(99)00331-6
[54] G. Yang, Y. Li, R.K. Rana, J.-J. Zhu, Pt–Au/nitrogen-doped graphene nanocomposites for enhanced electrochemical activities, J. Mater. Chem. 1 (5) (2013) 1754–1762, https://doi.org/10.1039/c2ta00776b
Another random document with no related content on Scribd:


The Project Gutenberg eBook of The English provincial printers, stationers and bookbinders to 1557
This ebook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this ebook or online at www.gutenberg.org. If you are not located in the United States, you will have to check the laws of the country where you are located before using this eBook.
Title: The English provincial printers, stationers and bookbinders to 1557
Author: E. Gordon Duff
Release date: April 29, 2024 [eBook #73493]
Language: English
Original publication: Cambridge: University Press, 1912
Credits: Alan, deaurider and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive)
*** START OF THE PROJECT GUTENBERG EBOOK THE ENGLISH PROVINCIAL PRINTERS, STATIONERS AND BOOKBINDERS TO 1557

