Spilloverandemerginginfectious diseases
Lambcastrationisnotalwaystheidyllicpastimethatsomepeoplemaythinkitis.
—MarcAbrahams
1.1 Introduction
Wyoming,USA,June2011—Inaworldthatisincreasinglyurbanized,andwherefarmingispractisedby fewerandfewerpeople,youmaynotbefamiliar withanyofthetime-honouredtechniquesusedto castratelambs.Awebsearchwillsuggestthatyou canuseyourteeth,andthatthishastheadvantage ofreducingthelikelihoodofaconsequentinfection inthenewlyemasculatedlamb.However,thereare risksforthecastrator.
Atamultidayeventtocastrateanddockthetails of1600lambsonaWyomingsheepranch,twomen usedtheirteethtocastratesomeofthelambs.Ten otherpeopledidnotadoptthistechnique.Subsequently,thetwomenwerestrickenbydiarrhoea, andonealsosufferedabdominalcramps,fever, nausea,andvomiting.Onepatientwashospitalized foraday,butbothmenrecoveredfromtheirbouts ofillness(VanHoutenetal.2011).
TheWyomingDepartmentofHealthinvestigatedthesecasesandmanagedtoisolateindistinguishablebacteria—Campylobacterjejuni—from bothmen’sstoolsamples.Although Campylobacter causesanestimated1.5millionillnesseseachyear intheUnitedStates,the Campylobacterjejuni strain identifiedintheWyomingshepherdswasextremely rare(8from8817strains),suggestingacommon sourceofinfection.Thesourceturnedouttobethe lambs,someofwhichwereexperiencingtheirown boutsofdiarrhoea.
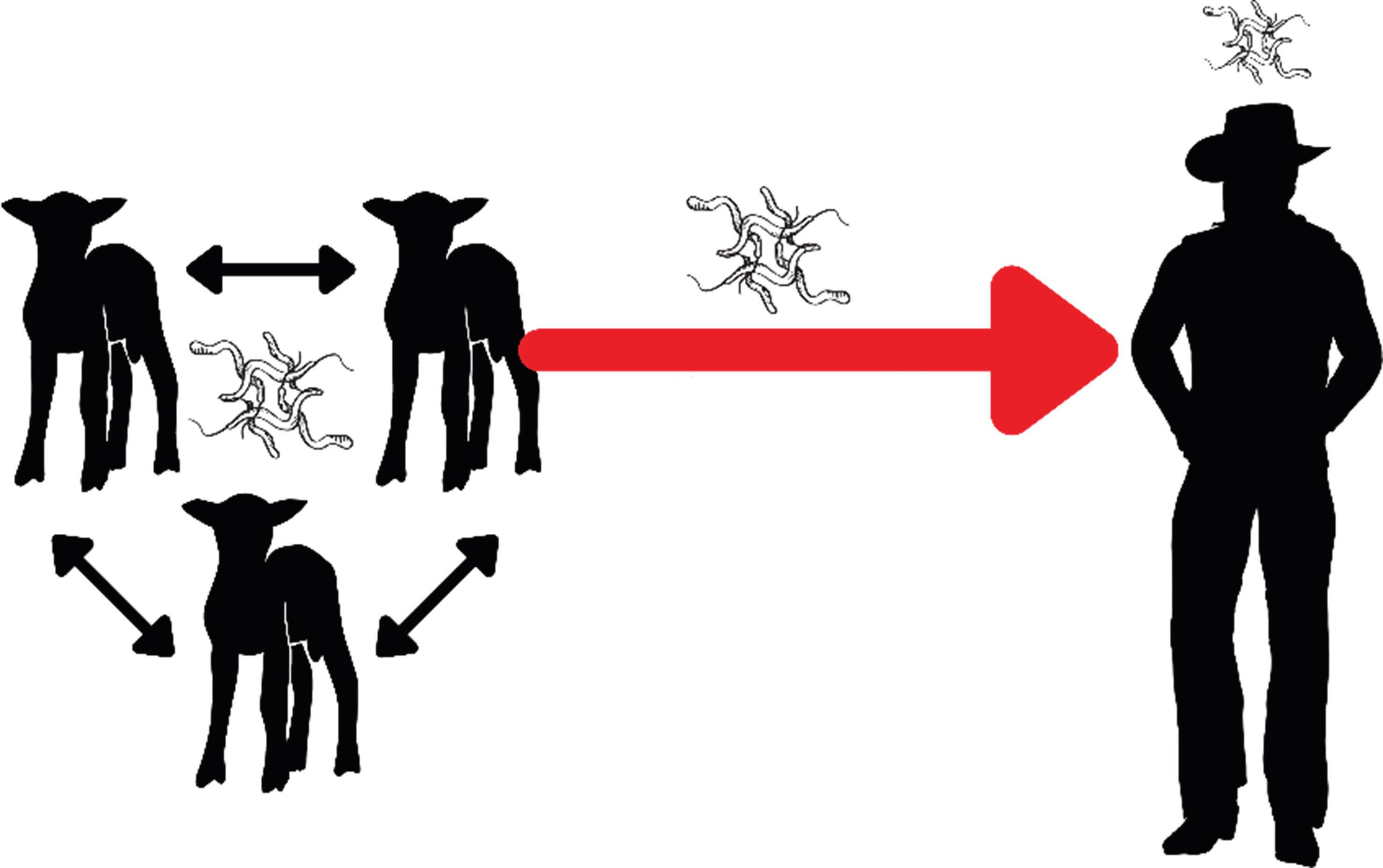
Thisisamildlyamusingcaseofa zoonosis apathogentransmittedfromnon-humananimals tohumans (Fig. 1.1). Campylobacterjejuni wascirculatinginthesheepflockandtheunusualcontactbetweenlambscrotumandhumanteethand mouthsenabledzoonoticdiseasetransmission.
Itcouldalsobeconsideredanexampleofan emerginginfectiousdisease (EID)—causedbya pathogenspreadingtonewspecies,spreadingto newgeographicareasandpopulations,orspreadingatfasterratesinplacesorpopulationswhere ithaspreviouslybeenendemic (Jonesetal.2008). Inthiscase, Campylobacterjejuni appearedtohave enteredthelocalhumanpopulation(Wyoming shepherds)forthefirsttime.
Scientistspridethemselvesonbeingobjectiveandsystematic,butwhenit’syouwithanarachnidinyournasal cavity,thatsenseofdistancegoesrightoutofthewindow.Whenyoufirstrealizeyouhaveatickupyournose, ittakesallyourwillpowernottoclawyourfaceoff.
—TonyGoldberg
Kibale,UgandaandWisconsin,USA,June2012 Exposuretowildlifezoonosesand/ortheirvectors isnotanunusualcircumstanceaffectingonlylucklessshepherds.InJune2012,DrGoldberg,aveterinaryepidemiologistwitharesearchbackground inprimatologyandemerginginfectiousdiseases, reluctantlyacknowledgedaseverepaininhisnose (Goldberg2013).Severaldayspreviously,Goldberg
CreatedbyHannaD.KirylukusingCanva.com.
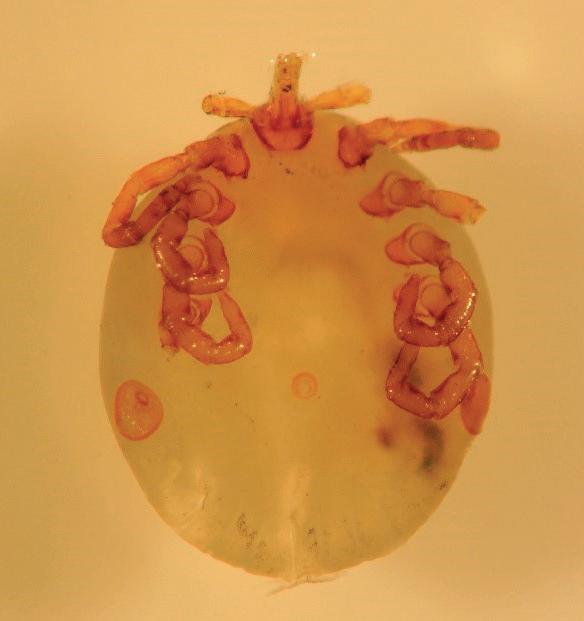
hadbeeninatropicalforestinKibale,Uganda, studyinghowanthropogenicchangestoecosystemsalterhealth-relatedoutcomesandinfectious diseasedynamicsinpeople,wildlife,anddomestic animals.NowbackathisWisconsinhome,Goldbergusedanangledmirrortoexaminehisnostril, andconfirmedhissuspicionsbyseeingthe‘smooth, roundedbacksideofafullyengorgedtick’.While rare,these‘nostrilticks’arenotunheardofinvisitorstoKibaleNationalPark.ThiswasDrGoldberg’s thirdinfestation(andyethekeptreturning!).Fortunately,hesufferednolastingharm,besidesthe mentaltraumaofhavingablood-suckingarachnid insidehisnose.
Collaboratingwithmedicalentomologistsand primatologists,Goldbergandcolleagueswereable todescribethistickaslikelyanew Amblyomma species(Fig. 1.2).Usinghighresolution photographsofchimpanzeenoses(Fig. 1.2),they showedthatroughly20%ofyoungchimpanzees hadtick-infestednostrils(Hameretal.2013).Presumably,theticksinfestthenostrilbecausechimpanzeeslovetogroomeachother,soticksneed aplacetohidefromchimps’stubbyfingers.FurtherresearchsuggeststhatsporadiccasesofnostriltickshaveoccurredinpeoplevisitingAfrica’s equatorialforestsforseveraldecades.Canthenostriltickstransmitpathogens,andcouldunwary
travellersinadvertentlycarrydiseaseagentsfrom tropicalAfricatotheirfar-flunghomes?Thatquestionremainsunanswered.
Thisincidenthasseveralpertinentlessons.First, human–wildlifeinteractionsoccuratindividual levels,andoftengounrecognized.Second,although contactisatanindividuallevel,theimpactsare nolongerlimitedtosmallgeographicallocales. Airtravelmeansthatpathogensandvectorsnow haveopportunitiestotravelgloballyandwithin infectiousperiods(Chapter9).Third,human–wildlifeinteractionshavenotalwaysbeenassiduouslydescribedorcatalogued. Amblyomma ticks haveprobablybeenexploringprimatenostrilsfor alongtime,butittookthechanceinfestationofa qualifiedveterinariantoresultinitsscientificidentificationandfurtherresearch.
1.2 Fromspillovertopandemic
Spilloverevents—theoccasionswhenapathogen jumpstonewspeciesandtherebyinfectshumans (zoonotic)oranimalsthatarenottheusualhost species—occuratthescaleoftheindividual,but theimpactsofpathogensinanewhostspecies areunpredictable.DrGoldbergdidnotreportany pathogentransmissionafterbeingbittenbyan African Amblyomma tick—perhapsbecausethetick
zoonotic transmission
Figure1.1 Zoonotic(non-humananimaltohuman)transmissionof Campylobacterjejuni fromWyominglambstohumans.
Figure1.2 A,B,C.Nostriltick, Amblyomma,extractedby,andfromthenostrilof,TonyGoldberginWisconsin,USA,aftertravelsinKibale NationalPark,Uganda.PhotosbyGabrielL.Hamer.D.Nostriltickinayoungchimpanzee,Thatcher,inKibaleNationalPark,Uganda,2012.Photo byAndrewB.Bernard/KibaleChimpanzeeProject.
Originallypublishedin Hameretal.(2013).CoincidenttickinfestationsinthenostrilsofwildchimpanzeesandahumaninUganda.AmericanJournalofTropical MedicineandHygiene,89,924–7.UsedwiththepermissionofAmericanJournalofTropicalMedicineandHygiene.
wasnotcarryingapotentiallypathogenicinfection, orbecauseanypotentialpathogenitcarriedwas notabletoinfectahumanhost,orwasunableto infectDrGoldberginparticularbecausehewasnot susceptible (see Chapter2).Thebacteriuminfecting shepherdsinWyomingdidcauseillness,butasfar asweknow,therewasnosubsequenttransmission fromthosetwohumancasestootherhumansor lambs.
Termstodescribeadisease’simpactincludeclusters,outbreaks,epidemicsandpandemics.
• A cluster isan observationofanabove-normal numberofcases,normallyaggregatedintime atalocalscale.Oftenthisissimplythetermfor thebeginningofanoutbreak,butbeforeconnectionsandtransmissionchainshavebeenofficially identified.
• An outbreak or epidemic (alsocalledan epizootic whenreferringtowildlifeordomesticanimaldisease)isan increaseofdiseasecases,often withcontinuedtransmissionandwithestablishedcausativelinksbetweencases.
• A pandemic isan outbreak/epidemicatthe internationalorglobalscale
Thesedescriptionsblurandbleedintoeachother; thereisnotnormallyanofficialthresholdforwhen aclusterbecomesanoutbreak,orforwhenanepidemicbecomesapandemic.
Howdoesonedefinean‘above-normalnumberofcases?’Thenormalnumberofcasesrefers toobservedbaselineinfectionratesofapersistent pathogeninapopulationorcommunity.Thisis referredtoasthe endemic levelofthedisease(called enzootic whenreferringtowildlifeordomestic
(d)
Box1.1Ondefinitions
...
Ihatedefinitions.
—BenjaminDisraeli,BritishPrimeMinister (1804–1881)
Definitionscanbehelpfulforestablishingcommonunderstanding(ormisunderstanding).Thisbecomesespeciallyimportantduringmultidisciplinaryconversations.For example,theabbreviationEBMsignifiesEcosystem-Based Management,ExpressedBreastMilk,orEvidence-Based Medicine,dependingonwhetheryouareaconservationist,apaediatricnurse,oraphysician.Definingyourterms isalwaysuseful.
However,definitionsmaynotbeeasilyachievable.For example,in1964,theUSSupremeCourtJusticePotter Stewartargued‘Ishallnottodayattemptfurthertodefine thekindsofmaterialIunderstandtobeembracedwithin thatshorthanddescription[‘hard-corepornography’],and perhapsIcouldneversucceedinintelligiblydoingso.But IknowitwhenIseeit ’.Wecouldn’tagreemore. Forexample,wehavedefined zoonosis asapathogen transmittedfromnon-humananimalstohumans.Other definitionssuggest‘apathogentransmittedfromanimals tohumans’;theyneglect‘non-human’.Ifyouconsider humanstobeanimals,thenthisdefinitionwouldencompasssyphilisandgonorrhoea—notourintention!Even ouradopteddefinitionhassomeissues.Typically,malaria causedby Plasmodiumfalciparum isnotconsidereda zoonosis,becauseitpossessesahuman-mosquito-human lifecycle.Butisn’tamosquitoananimal?Slipperyslopes becomeabundantasoneargueswhat kind ofanimal constitutesananimal.
Wesuggestthatthereaderdoesn’tbecomebogged downbydefinitions.Adoptthemiftheyareuseful,adapt themiftheyarenot,andbeforgiving!
animaldisease)(Box 1.1).Wheretransmissionina populationispersistentandatrelativelyhighlevels, thediseasepatterncanbereferredtoas hyperendemic.Incontrast,diseasesthatoccurinfrequently andirregularlyareoftentermed sporadic.
1.3 Zoonoses
Zoonoticpathogentransmissioncanbeclassifieddependinguponthetransmissionpatterns inhumanpopulations(Wolfeetal.2007, LloydSmithetal.2009, Woolhouseetal.2013).(Similar
classificationscanbeusedforanyinfectionentering anynewhostspecies,suchasgiraffesornewts insteadofhumans.)Importantly,pathogenscould fallintodifferentcategoriesindifferentcontexts.
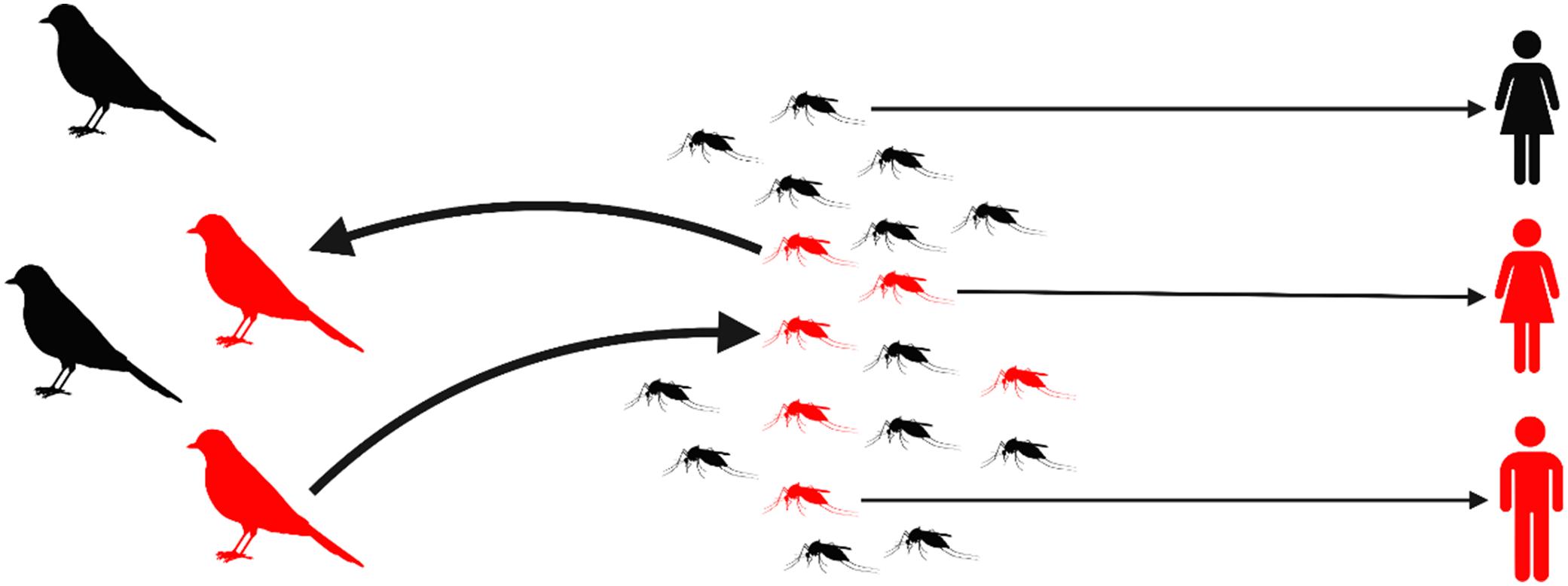
Dead-endzoonoses canbetransmittedfromanimalstohumans,butdonotsubsequentlyspread fromhuman-to-human.Forexample,WestNile viruscirculatesintheUSinbirdsandmosquitoes andcancauseillnessinpeopleafteramosquito bite.However,human-to-humantransmissiondoes notoccur(Fig. 1.3).(Althoughinfectionsacquired frombloodtransfusionsarepossible(Iwamotoetal. 2003),bloodcollectionagenciesscreendonated bloodforWestNilevirus.)Anotherdead-end zoonosisisrabiesvirus,whichistransmittedto peoplethroughbitesofrabidwildlifeordomestic species.Despitebeingvaccine-preventable,rabies killsapproximately59,000peopleeveryyear(the equivalentofonepersoneverynineminutes),40% ofwhomarechildrenlivinginAsiaandAfrica. Humansarecapableofsheddingrabiesvirusduringtheclinicalphaseofdiseasebuttherehasnot beenadocumentedcaseofhuman-to-humantransmission,exceptfromunusualcasesinvolvingorgan ortissuetransplantation(Blackburnetal.2022, Chapter2).
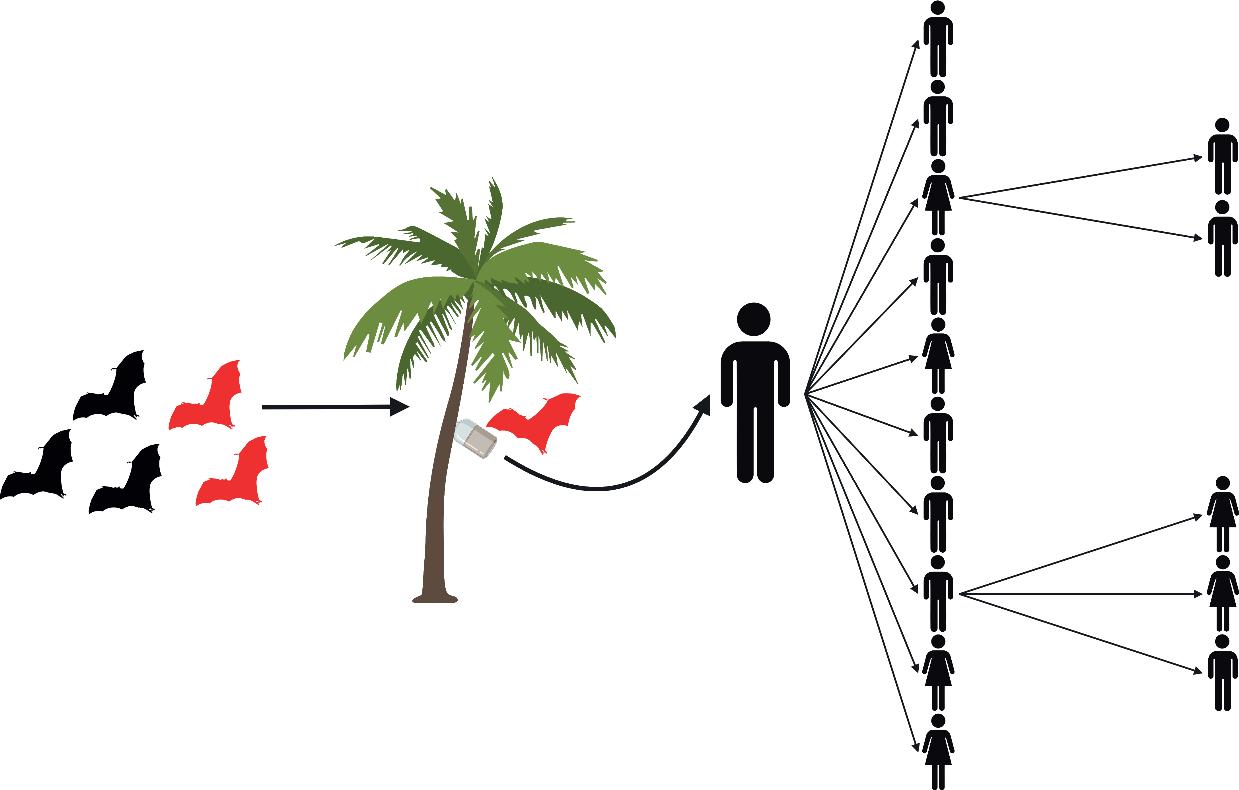
Stutteringzoonoses canjumpfromanimalsinto humans,andthen,withsomelimitedsuccess,into otherpeople.Intheseoutbreaks, human-to-human transmissionislimitedandthepathogen stutters or fadesout beforeafull-blownepidemicoccurs. Forexample,since2001,inBangladesh,therehave beenrecurrentspilloversofNipahvirustohumans fromIndianfruitbats(Pteropusmedius).Often,the spilloverresultsinanisolatedhumancase,but human-to-humantransmissioncaninfectamean of7persons(range1–22)resultinginastuttering outbreak(Lubyetal.2009)(Fig. 1.4).Inanother examplefromSouthTexas,USA,therearesuitablemosquitovectorsforZikavirus,butZikaoutbreaksinhumanshavetendedtofadeout,perhaps becausethemosquitoesarepredominantlybiting non-humanhosts(dogs,domesticanimals)(Olson etal.2020).
Sustainablezoonoses originateandpersistin animals, butonceinhumanpopulations,theycan spreadthroughhuman-to-humantransmissionto causeoutbreaksthatpersistthroughmultiple generationsoftransmissionandhumancohorts
Figure1.3 WestNilevirusistransmittedamongwildbirdsbymosquitoesintheUnitedStates.Infected(red)mosquitoesthatfeedonhumans maycauseazoonoticspilloverbyinfectingpeople(red),butthereisnofurthertransmissionfromperson-to-person;itisadead-endzoonosis.
CreatedbyHannaD.KirylukwithBioRender.com
Stuttering humanto-human transmission
Figure1.4 Stutteringzoonosesinvolvelimitedhuman-to-humantransmissionaftertheinitialzoonoticspilloverandendupfadingout.For example,inBangladesh,NipahviruspersistsinpopulationsofgreaterIndianfruitbats(Pteropusmedius)butoccasionallymakesthejumpinto humansthroughrawdatepalmsapcontaminatedbybatwaste.Theprimaryhumancasecantheninfectotherpeople.Astuttering human-to-humanNipahvirusoutbreakoccurredin2004,whentheindexpatientinfectedfourofthepeoplecaringforhim(hismother,son,aunt andaneighbour;theincubationperiodwas15–27days).Whilstinhospital,theauntwascaredforbyapopularlocalreligiousleader,who becameill13dayslater,andwasvisitedbymanyofhisrelativesandmembersofhisreligiouscommunityathishome.Twenty-twopeoplewere infectedaftercontactwiththereligiousleader.Furthertransmissionsoccurredand,intheend,thechainoftransmissioninvolvedfivegenerations andaffected34people(Lubyetal.2009).
CreatedbyHannaD.KirylukusingBioRender.comandasketchonanoldpizzabox.
Wildlife reservoir
Zoonotic spillover
Aclassicexampleofasustainablezoonosiswould beplague,causedbythebacterium Yersiniapestis, famousfortheBlackDeathinmedievalEurope. Inthemodernera, Yersiniapestis stillsporadically jumpsfromanimalpopulationsandsubsequently spreadsfromperson-to-person,e.g.in2017aplague outbreakoccurredinMadagascarwhenaman,presumablyinfectedfromananimalsource,travelled bytaxithroughthenation’scapital,Antananarivo, andtransmitted Yersiniapestis todozensofpeople.Person-to-persontransmissioncontinuedand
causedpneumonicdisease(infectionofthelungs), resultingin2348confirmed,probable,andsuspectedcases,including202deaths.
Sustainablezoonosesalsoincludepathogensnow regardedashumandiseases,buthistoricallyor evolutionarilythediseasehadazoonoticorigin, whetherthatwasfromdomesticatedanimals,livestock,orwildlife(Fig. 1.5).Recurrentintroduction fromanimaltohumanpopulationsisnolonger aconcern;thepathogensareadaptedtohuman transmission.Duringtheslowwritingofthisbook,
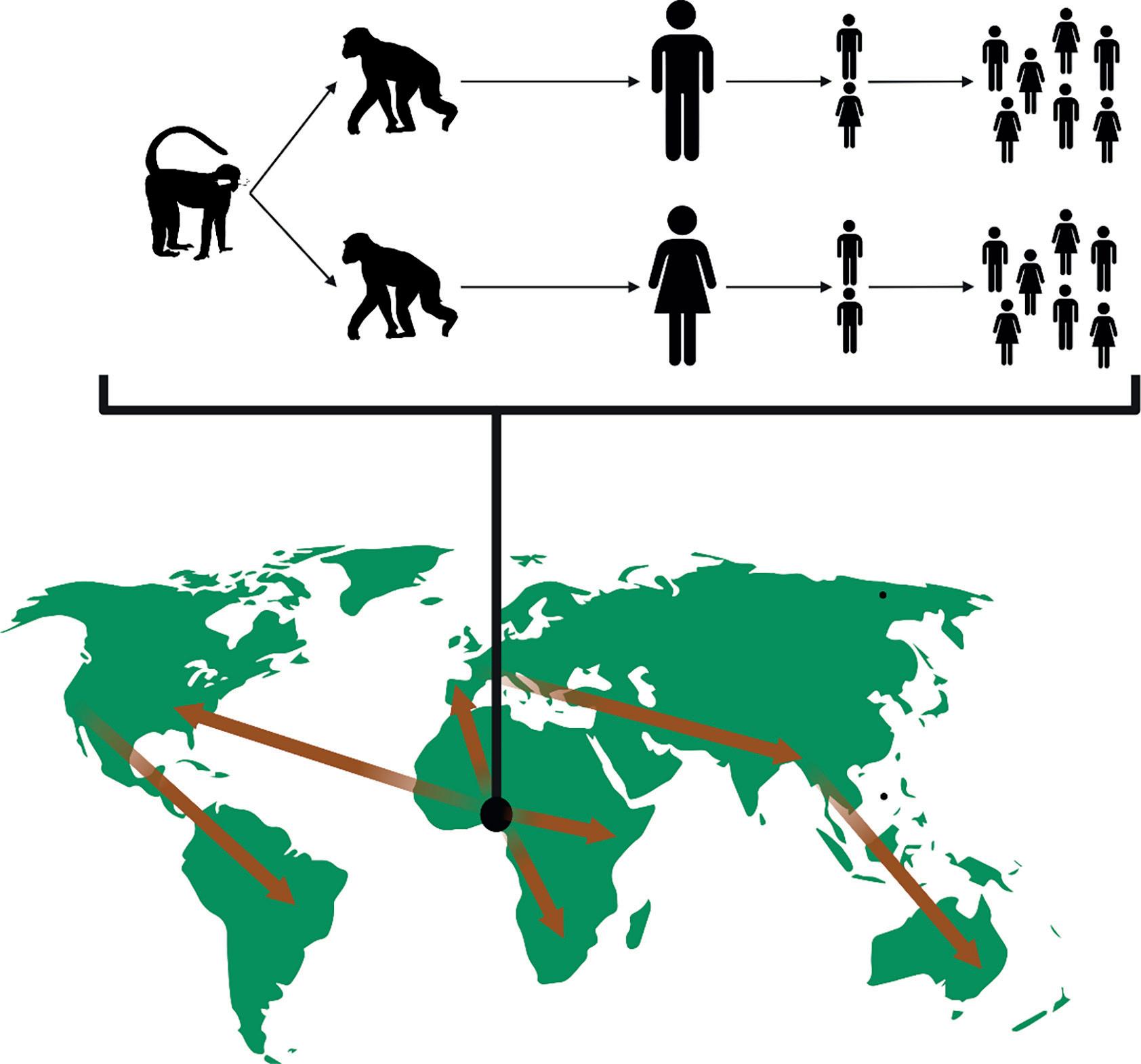
Figure1.5 Sustainablezoonosescanemergefromanimalhostsintohumanpopulations,possiblyviaa‘bridge’reservoirhost,andthenadaptto persistenttransmissionwithinhumans.Humantravelcanthenelevatethespilloverincidenttoanoutbreakandthentopandemictransmission.For example,humanimmunodeficiencyvirus-1(HIV-1)probablyjumpedfrommonkeypopulationstochimpanzeestohumans,andthechimpanzeeto-humanspilloveroccurredmorethanonce.Human-to-humantransmissionisnowresponsibleformaintainingthevirus,whichisnowpresent throughoutglobalpopulations(arrowssimplyrepresenttheconceptofglobaltravel).Similarly,SARS-CoV-2jumpedintohumanpopulationsin 2019andisnowasustainablepandemicpathogen. CreatedbyHannaD.KirylukwithBioRender.com.
Monkey (e.g. Cercocebus spp. or Cercopithecus spp.)
Chimpanzee (Pan troglodytes) x 1,000,000
SARS-CoV-2hasprovidedaprimeexampleofa novelvirus(sourcestillunknown)thathasspilled overintohumanpopulationsandcontinuedevolutionandcirculationinitsnewhumanhost (Chapter15).
1.4 Zoonoticoriginsofthe‘BigThree’
Malaria,tuberculosis,andAIDSareconsideredthe ‘BigThree’infectiousdiseases,withcatastrophic impactsuponhumanpopulations,especiallyin low-incomecountries(Rocheetal.2018).These threediseasesillustratevariedevolutionaryand zoonotichistories.
Humanmalariaiscausedbytheprotist Plasmodium,whichistransmittedbyfemale Anopheles mosquitoes.Estimatesofthepublichealthburden createdbymalariasufferfromhighuncertainty, butin2013,therewerebetween95millionand 284millionnewcasesglobally,incurring703,000–1,032,000deaths(Murrayetal.2014). Plasmodium falciparum isthedeadliestofthevariousmalariaprotozoa.Bysamplingfor Plasmodium infaecalsamples fromwildapesthatwerelivinginremoteforested areasawayfromhumans,researchersdiscovered that Plasmodiumfalciparum probablyoriginatedin gorillas.Thoughthetimingofthiseventinevolutionaryhistoryisunknown,thespillovertohuman populationsmayhavebeenasinglecross-species transmissionoccurrence(Liuetal.2010, Prugnolle etal.2011).Thereislittleevidencetosuggestthat therearerecurrentintroductionsofmalariafrom apestohumansinAfrica(Sundararamanetal.2013) (Fig. 1.6).
However,‘zoonotic’malariacasesof Plasmodium dooccur: Plasmodiumknowlesi typicallyinfectsmonkeysinsouth-eastAsiabutwasrecentlydiscovered asahumanpathogen(Singhetal.2004, Singhand Daneshvar2013, Rajaetal.2020).
isresponsibleforoveramilliondeathsayear (datafor2013; Murrayetal.2014).Thevirus jumpedfromnon-humanprimatestohumanpopulationsmultipletimes,andnowisfirmlyestablishedasahumanpathogen.Forexample,HIV-2 likelyemergedinhumanpopulationsfromasooty mangabey(Cercocebusatys)sometimeinthelatterhalfofthe20thcentury.Thisinterpretationis basedonthreepiecesofevidence:thecommon ancestor,simianimmunodeficiencyvirus(SIVsm), isprevalentinWestAfricanpopulationsofsooty mangabeys;HIV-2isendemicinWestAfrica;and thegenomesofSIVsm andHIV-2arecloselyrelated (Hirschetal.1989).HIV-1hasadifferentorigin,andphylogeneticstudiessuggestthatchimpanzeesinsouth-easternCameroonweretheoriginalreservoirsofatleasttwoHIV-1strains.These apepopulationsprobablyacquiredthevirusfrom red-cappedmangabeysor Cercopithecus speciesat atimewhenchimpanzeepopulationswereevolvingintosubspecies(Keeleetal.2006).Thespillover tohumanpopulationsprobablyoccurredearlyin the20thcentury(Keeleetal.2006, Worobeyetal. 2008).
Thesootymangabey(Cercocebusatys)isasmoky-gray creaturewithadarkfaceandhands,whiteeyebrows, andflaringwhitemuttonchops,notnearlysodecorative asmanymonkeysonthecontinentbutarrestinginits way,likeanelderlychimneysweepofdappertonsorial habits.
—DavidQuammen
Today,thehumanimmunodeficiencyvirus(HIV) thatcausesacquiredimmunodeficiencysyndrome (AIDS)infectsapproximately30millionpeopleand
Tuberculosis(TB)isoneoftheworld’sdeadliestinfectiousdiseases—theWorldHealthOrganization(WHO)estimatesthatthebacteriumkills betweentwoandthreemillionpeopleannually, 98%ofwhomliveinthedevelopingworld—even thoughacureisavailable(Arnold2007).Athirdof peopleinfectedwithHIVhaveTB,andtheevolutionofmultidrugresistanttuberculosis(MDRTB) strainsisagrowingpublichealthconcern.Tuberculosisisusuallycausedby Mycobacteriumtuberculosis, buttheWorldHealthOrganization(WHO)estimatesthat>10,000deathsweredueto‘bovine’TB globallyin2010(Olea-Popelkaetal.2017),whichis causedbytherelated Mycobacteriumbovis Mycobacteriumbovis hasabroadhostrangeincludingcattle, Europeanbadgers(Melesmeles),Australianbrushtailpossums(Trichosurusvulpecula),deer,andsoon (BritesandGagneux2015)(see Chapter14).Consequently,ithasbeenassumedthat Mycobacterium bovis wasancestralto Mycobacteriumtuberculosis, whichwouldmakehumanTB(Mycobacteriumtuberculosis)anotherexampleofasustainablezoonosis. However,geneticanalyseshavediscoveredthat certainhuman Mycobacteriumtuberculosis lineages areactuallymoreancestral(phylogeneticallymore
10 nt
n=2/20
n=3/2
n=109/43/6
n=2/3/1
n=1/2
n=2 n=11 n=2 n =2 n=3 n=3 n=3 n=3 n=4
n=115/210/47
n=2/1/1
n=3/1 n=2 n=4/1
n=45/5 n=4/4
n=1/5/4
Figure1.6 Phylogenyof Plasmodiumfalciparum strainsfromruralCameroon. Plasmodiumfalciparum isthemosquito-bornediseaseagentthat causesfalciparummalaria.SequencesfromhumanslivinginCameroon(black)arecomparedtosequencesfromGenbank(white)andtheSanger Institute(grey), Plasmodiumpraefalciparum fromgorillas(green),and Plasmodiumreichenowi fromchimpanzees(red).
From: Sundararamanetal.(2013) Plasmodiumfalciparum-likeparasitesinfectingwildapesinsouthernCameroondonotrepresentarecurrentsourceofhuman malaria.ProceedingsoftheNationalAcademyofSciencesUSA,110,7020–5.
basal)thantheanimal-adapted Mycobacteriumbovis (Arnold2007, BritesandGagneeux2015, Brosch etal.2002).Thisevolutionarystoryappearsmore complexthatthoseofmalariaandHIV/AIDS!
1.5 Barrierstoemergence
Manypathogenswithzoonoticoriginthatbecame adaptedtohumanpopulationsaretermed‘crowd diseases’,becausethepathogenscouldonlybecome establishedinhumansocietiesthathadreached highenoughdensitiestoallowsustainedtransmissionandreplacementofsusceptibleindividuals.Crowddiseasestendtobeveryinfectiousand causesomeantibody-derivedresistance.Historically,someofthesepathogenspilloversoccurred synchronouslywiththedevelopmentofagriculture anddomestication,implyingapathogenspillover doublejeopardy:increasedexposuretolivestock species,andincreasedhumandensities(Wolfeetal. 2007, Woolhouseetal.2013).
However,notallanimalpathogensspilloverand becomehuman-specializedpathogens(Wolfeetal. 2007),eveniftherearemanyhumanslivingin oneplace.Humansmightnotbesusceptibleto thepathogen,oropportunitiesforhumanexposure maybelimited,evenifapathogenispotentially capableofinfectingpeople.Behaviourcanreduce orpreventexposures.Forexample,insteadofusing yourteeth,usingalternativecastrationtechniques toreducetheriskof Campylobacterjejuni transmissionfromlambs;oravoidingpotentialexposure toprionslikebovinespongiformencephalopathy (BSE),alsoknownasmadcowdiseaseorchronic wastingdisease,bynoteatingpartsoftheanimal’s centralnervoussystem(CNS)andlymphoidtissue.
1.6 Ebolavirusasacasestudy ofspillover
Guinea,Liberia,SierraLeone,Spain,UnitedStates,etc., 2013–2016—Firstdescribedin1976fromanoutbreakintheremotevillageofYambuku,Democratic RepublicoftheCongo,andnamedaftertheEbola River,theEbolavirus(orZaireebolavirus)isa zoonoticpathogenthatcancausehighmortality amonghumancases(i.e.ahighcase-fatalityrisk or,morecommonlyknownas,casefatalityrate).
SpillovereventsforEbolavirusinhumanpopulationshavebeenlinkedtoexposurefromdiverse animalsources,e.g.carcassesofduiker(asmall forestantelope),gorillas,andchimpanzees(Rouquetetal.2005).Typically,Ebolavirusoutbreaks haveoccurredinremotelocationsinCentralAfrica: Gabon,theRepublicofCongo,andtheDemocratic RepublicofCongo.Betweendescriptionin1976 andearly2014,thelargestknownEbolavirusdiseaseoutbreaksinfectedapproximately300people, and47–89%ofcaseswerefatal.Aftertheoutbreaksended,severalyearsnormallypassedbefore anotherEbolavirusoutbreak.Thisboom-and-bust patternisoftenexhibitedbyzoonoticdiseases. Illnessstrikesmenwhentheyareexposedtochange. —Herodotus
Morerecently,though,outbreakshavefollowed verydifferenttrajectories(Fig. 1.7).InDecember 2013,an8-month-oldboyfromasmallvillagein south-easternGuineawasbelievedtobetheindex caseforanEbolavirusepidemic(Baizeetal.2014, MaríSaézetal.2015).Thoughthecauseoftheboy’s deathwentunrecognizedatthetime,theEbola viruscausedfurthercasesof‘fataldiarrhoea’and anofficialmedicalalertwasissuedtodistricthealth officialsinlateJanuary2014.Thevirusspreadto Conakry,Guinea’scapitalcity,andwasconfirmed asZaireebolavirusinMarch2014.Guineaisabout 2500kmfromthetraditionalrangeofEbolavirus inCentralAfrica.Inthiscase,althoughithasbeen recognizedinCentralAfricaforfourdecades,Ebola virusalsoconstitutesan emerginginfectiousdisease,becauseitisa pathogenthathasrecently increasedinincidenceorgeographicrange,even ifithasinfectedhumanshistorically(Jonesetal. 2008).
Ebolavirushadneverinfectedpeopleanywhere closetoWestAfricabefore,andtheregionwas blindsided.Ontopofthat,thiswasthefirsttime thatEbolaviruswasactiveinheavilypopulatedand connectedurbancentresratherthanisolated,rural areas.Acombinationofdensepopulations,inadequatepublichealthinfrastructureandresponse,and alackofpriorexperiencewiththedisease,meant thattheEbolavirusspreadamonganunprecedentednumberofpeople.ByJuly2014,thevirus wasalsopresentinMonroviaandFreetown,the
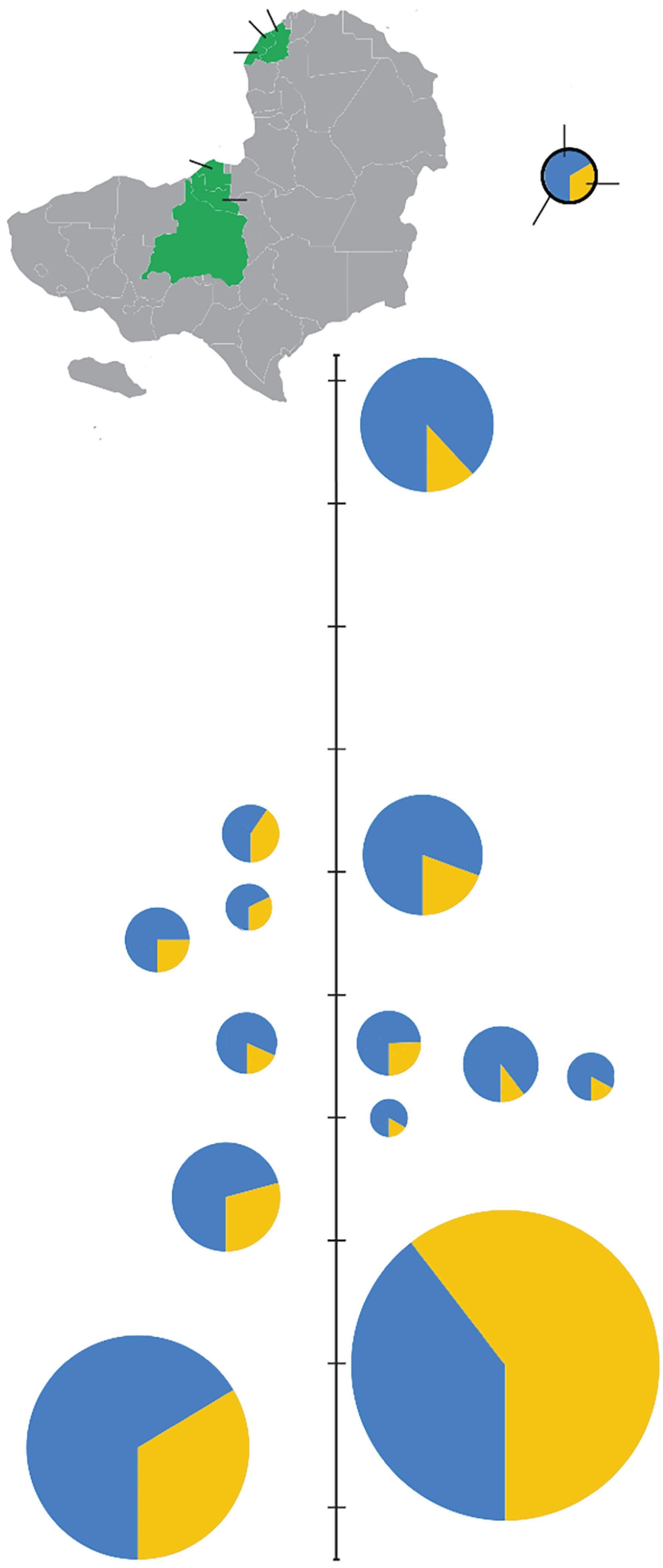
Figure1.7 EbolavirusoutbreaksinAfricafrom1976to2020,scaledbytheoutbreaksize(numberofcases;blue = deaths,yellow = survived).RecentoutbreaksinWestAfricaandtheDemocratic RepublicofCongohavebeenmuchlargerthanpreviousoutbreaksandaffectedmoreurbanizedpopulations.
CreatedbyHannaD.Kiryluk.
capitalsofGuinea’sneighbouringcountriesLiberia andSierraLeone.InfectedtravellersormedicalpersonnelwerereportedinItaly,Mali,Nigeria,Senegal,Spain,andtheUnitedStates.Theofficialtallies forthepandemicinGuinea,Liberia,andSierra Leonewere28,610reportedcases,with11,308fatal cases.
DuringtheWestAfricanEbolavirusoutbreak, speculationwasrifethattheviruswouldevolve tobecomemoredeadlyandtransmissible. Newly evolvingstrainsofpathogen areanotherclassof emerginginfectiousdisease (Jonesetal.2008).Normallycirculatinginwildlifespecies,Ebolavirus certainlymutatedduringtransmissioninthisnaive humanpopulation(e.g. Gireetal.2014).However, themutationsthataccrueddonotseemtohave affecteddiseaseprogression,atleastin invivo laboratoryanimalmodels(miceandmacaques)(Marzi etal.2018).Instead,thespreadofEbolavirusinthe WestAfricaepidemiccanbebetterexplainedbythe socialcontextofcloselyconnectedhumanpopulations,andnotastheresultofchangesinthevirus’s intrinsicbiologicalproperties(Marzietal.2018).
1.7 Improveddiagnosticsandincreasing rateofpathogendiscovery
Manyemerginginfectiousdiseasesareprobably instancesofrecentscientificdescription,rather thannovelintroductionstohumanpopulations (Gireetal.2012).Illnessesoftendisplay‘flu-like symptoms’suchasfever,headache,andnausea. Withoutproperlaboratorydiagnostics,itisdifficult todiscernwhetherthesesymptomsarearesult ofmalaria,typhoid,shigella,orevenmildcasesof viralhaemorrhagicfever.Thus,‘rare’spilloversof zoonosesintohumanpopulationsareperhapsmore frequentthanrecognized,andonlyconsideredrare becausesurveillanceanddiagnosticsarelackingor notapplied.
Diagnostictestsandourabilitytodescribe pathogensareconstantlyimproving.Considerthat whenplagueemergedacrosstheglobearound theturnofthe20thcentury—newlyinvadingthe UnitedStates,Australia,Brazil,SouthAfrica— bacteriologywasinitsinfancy,germtheory (therecognitionthatdiseasemightbecausedby microorganismsratherthanbadair)wasanideanot
evenhalfacenturyold,and,intheabsenceofantibiotics,investigativepublichealthagentsmightbeas afearedofinfectedcutsasthethreatofflea-borne bubonicplague(Chase2004).Now,moleculartechniquescandiscernpreviouslyunknownpathogens withindaysandevenhoursoftheobservationof suspiciousnewillnessesusinggenomictechnologies,e.g.thediscoveryofthecoronavirusesthat causedMiddleEastrespiratorysyndrome(MERS) in2012(Zakietal.2012)andtherelatedSARS-CoV-2 inChinain2020(Zhouetal.2020).
Itisoftensuggestedthattherateofemergence ofinfectiouszoonoticdiseaseishigherintheearly 21stcenturythanithasbeeninthemoredistant past;aresultofcombineddriversofemergencesuch ashumanpopulationgrowth,populationdensity, industrializationandchangingfarmingmethods, globalizationofgoodsandhumantransport,andso on(seeChapters9–11).Thishypothesisisadifficultonetotest(Woolhouseetal.2013)!Arguably, therehavebeenjustahandfulofgloballydevastatingEIDeventsinthepastcentury,e.g.‘Spanish’ H1N1influenza(1918–1920)andotherinfluenza outbreaks;HIV-1enteringhumanpopulationsseveraltimesinthe20thcentury;thecurrentSARSCoV-2pandemic.Otherevents,liketheSARScoronavirusoutbreakin2002–2003(see Chapter8),had substantialimpactsuponeconomies,government policies,andpublicperception,butitsimpactspale incomparisontoHIV,H1N1influenza,orSARSCoV-2.Simultaneously,otherdevastatingzoonoses likeplague,whichdevastatedmedievalEurope, representlessofacurrentthreatbecauseofantibiotics,sanitation,andurbanization.Havebetterpublichealthresponsespreventedincreasedmortalitiesfromemergingpathogens?Undoubtedly.The impression,then,ofamodernworldundergoing unprecedentedthreatsofzoonoticepidemicsisless clearcutwhenexaminedinalongerhistorical context.
Perhapslessdebatableisthepatternofincreased emergenceofzoonosesinnewpopulationsandnew geographiesasaresultofglobalizationandnew densehumanpopulations(Fig. 1.8).Still,though, advancesindiagnostictechniquesandincreased interestinEIDsmeanthatpatternsofdisease emergenceshouldbeconsideredwithnuanceand thoughtfulness.
Lassa Fever (Nigeria, Togo, UK) Yellow Fever (Kenya) Ebola (DRC) Monkeypox (Europe and North America)
Monkeypox (Nigeria) Yellow Fever (Brazil) Rat hepatitis E virus
Avian Influenza H7N9
Avian Influenza H10N8 Sosuga virus
Chikungunya (Caribbean)
Variegated Squirrel Bornavirus 1
SARS-CoV-2
Zika (Americas)
Lassa Fever (Ghana) Swine Influenza A H3N2v
SARS-CoV EBLV-2 (Scotland) Avian influenza A H5N1 Melaka (Malaysia) Lujo virus (Southern Africa)
Avian Influenza A H5N1 (Hong Kong) West Nile virus (USA) Itaya virus (Peru)
Yellow Fever (West/Central, Africa) Ebola (DRC)
199719981999 2000 200120022003 200420052006200720082009 2010 2011 2012
MERS-CoV Mojiang Paramyxovirus Ebola (West Africa) Avian Influenza H5N6 Lassa Fever (Benin) Bourbon virus CCHF (Spain) Chikungunya (Pakistan) Lassa Fever (Togo) Ntwetwe virus Avian Influenza H7N4 Monkeypox (Liberia, UK) Nipah virus (India)
Pandemic Influenza A H1N1 Heartland virus Bas-Congo virus Lassa Fever (Mali) Huaiyangshan virusSFTS virus
Human CoV HKU-1 Human retroviruses HTLV3 HTLV4 Zika (Micronesia)
Nipah virus (Malaysia) Ngari virus (East Africa) Rift Valley Fever (Saudi Arabia & Yemen) Monkeypox (USA) Avian Influenza H7N7 (Netherlands) Chapare virus (Bolivia)
Figure1.8 Atimeline(1997topresent)ofzoonoticemergenceandspilloverofsomeRNAvirusesandmonkeypoxvirus.Repeatspilloversareindicatedinred;thecountriesinvolvedarein parentheses.Threerecentemergingcoronaviruspathogensarelabelledwithlargefont(i.e.SARS-CoV,MERS-CoV,andSARS-CoV-2).Abbreviationsinclude:EBLV-2,Europeanbatlyssavirustype2; DRC,DemocraticRepublicofCongo;HKU-1,HKU-1coronavirus;HTLV3,humanT-lymphotropicvirustype3;HTLV4,humanT-lymphotropicvirustype4;SFTS,severefeverwiththrombocytopenia syndromevirus;CCHF,Crimean-Congohaemorrhagicfevervirus.
From Keuschetal.(2022)
1.8 Epidemiologymeetsdiseaseecology
Wheninfectinghumans,emerginginfectiousdiseasesaremainlytheconcernof epidemiologists whostudy thedistributionanddeterminants ofhealth-relatedstatesoreventsinspecified (human)populations,andtheapplicationofthis studytocontrolhealthproblems.Traditionally, epidemiologistshavenotoftenbeenexpertsin theanimalcomponentofcross-speciespathogen transmission.
Instead,studyofdiseasetransmissioninanimalpopulationshasbeenthepurviewof disease ecologists Broadly, ecology isthestudyofthe distributionoforganismsandtheirinteractions witheachotherandtheirenvironment,anddiseaseecologypaysspecialattentiontotheecology ofinfectiouspathogens.Inessence, diseaseecology isthe multidisciplinarystudyoftheinteractionsandcausalrelationshipsbetweenhosts, pathogens,andvectorswithinthecontextoftheir environmentandevolution,embracingmoleculartopopulationscales,andoptimisticallyprovidinginsightsintoforecastingdiseaseriskand transmission (Stapp2007, Waller2008, Kilpatrick andAltizer2010, KoprivnikarandJohnson2016) (Box 1.1).Diseaseecologyoftenbroachesexpertise fromfieldsasdiverseasparasitology,mathematics,engineering,conservation,climatescience,and publichealthpolicy.
Inthisbook,wemostlyfocusontheepidemiologyandecologyofzoonoticinfectiousdiseases andwildlifepathogens,butwealsoillustratemechanismsandphenomenausingexamplesofinfectiousdiseasesinpopulationsofdomesticanimals. Admittedly,thereisabiastowardssystemsthat wearefamiliarwithorexcitedby,e.g.plague, histoplasmosis,theSerengeti,tick-bornediseases, mange,Ebolavirus,andnorthernhairymammals.Wealsosufferbiasesbasedonourbackgrounds:weareallwhiteacademicswithrootsin thenorthernhemisphere.Wehavetriedtoinclude casesoutsideofourexpertise,butwewillhave missedthingsandwewillhavemademistakes. Wewillwelcomefeedbacktobroadenthescope ofthebook’stopicsforthesecondandthird editions.
1.9 Whystudytheecologyand epidemiologyofinfectiousdisease?
Wewouldarguethatnostrilticks,lambcastrations, sickcowboys,andfatalhaemorrhagicviruses spreadingfromunknownsourcesacrosstheworld shouldbeanswersenough!Whywouldyoustudy anythingelse?
However,thereareotherreasonsthatthisfield, andhopefullythisbook,shouldexciteyourinterest.DavidQuammen,theauthorof Spillover (which willbemuchquotedinthisbook),suggeststhat zoonosis is‘awordofthefuture,destinedforheavy useinthetwenty-firstcentury’(Quammen,2012). Zoonoticdiseasesand/ortheirvectorsarecertainly emergingandshiftingingeographicalscopeand incidence(Jonesetal.2008).Changesindistribution maybeduetohabitatchange,climatechange,globalization,shiftsinhostcommunityecology,happenstance,oracombinationofthesefactors(e.g. Chapters9,10and11).Itisanexcitingtimetobe monitoringandattemptingtoexplainthefluctuatingpatterns.Forthemoremercenaryamongus,the fieldofdiseaseecologyalsooffersjobopportunities.Understandingprocessesinfluencingzoonoses anddiseaseincidenceinhumanpopulationsisan importantareaofstudy,asistheabilitytoinnovate andimplementinterventionsthatcancombatand controlthediseases.
Theappliednatureofthephenomenacanalso berewarding.Devotingtimeandefforttounderstanding,forexample,interactionsbetweenaharmlessandobscureparasiteincharismatic-but-obscure lizardsmaybeinherentlyinterestingtosome (SalkeldandSchwarzkopf2005),butothersmay revelintheirworkhavingsomerelevancetoconservationscience,orpublichealthimpacts,orenvironmentalpolicy.Indeed,thisaddededgeofpotential implicationsoftheresearchcanaddheatandpassiontodebatedhypotheseswithindiseaseecology, whichlendsthetopicmotivationandinterest,even tothoseoutsidethefield.
Likeallgoodscience,diseaseecology should be hypothesisdriven.Becausenewpathogensare emerging,thereisthethrillofdevelopinghypothesesatthemostfundamentallevel:whatmightthe causeofthisnewdiseasebe?Wherediditcome
from?Howisittransmitted?Whyisitemerging now?Andbecausefactsanddatabegintoroll in,oneisforcedtorevisitorrejecthypothesesat aninvigoratingpace.And,thus,thefieldofdiseaseecologyisconstantlyundergoing‘Bayesian updating’—theconstantrevisingofatheory,procedure,oropinionasnewdatabecomeavailable (Schneider2005).Giventhepotentialstakes— stickingtoaparticularpethypothesisthatisfalse canhavedeleteriouseffects—diseaseecologymust be evidence-based,andtheevidencemustbetranslatableacrossdisciplines,whichideallyresultsin asciencethatembraceshumilityandtransparency. Howverynoble!
1.10 Notesonsources
Informationonlambcastrationtechniques: http://www.infovets.com/books/smrm/c/ c104.htm (accessed5/7/2018).
Informationon Campylobacterjejuni infections intheUS: https://www.cdc.gov/campy lobacter/index.html (accessed3/3/2021).
InformationonEbolavirusoutbreaks: https:// www.cdc.gov/vhf/ebola/history/chronology. html (accessed1/6/2018).
Informationonrabiescasenumbers: https:// www.who.int/health-topics/rabies#tab=tab_1 (accessed1/24/2022).
Informationonthe2017plagueoutbreakin Madagascar: www.who.int/csr/don/27november-2017-plague-madagascar/en/ (accessed6/7/2018).
1.11 References
Arnold,C.(2007).Molecularevolutionof Mycobacterim tuberculosis.ClinicalMicrobiologyandInfection,13, 120–28.
Baize,S.,Pannetier,D.,Oestereich,L.,etal.(2014).EmergenceofZaireEbolavirusdiseaseinGuinea.New EnglandJournalofMedicine,371,1418–25.
Blackburn,D.,Minhaj,F.S.,AlHammoud,R.,etal.(2022). Humanrabies—Texas,2021.MorbidityandMortality WeeklyReport(MMWR),71,1547–9.
Brites,D.,Gagneux,S.(2015).Co-evolutionof Mycobacteriumtuberculosis and Homosapiens.Immunological Reviews,264,6–24.
Brosch,R.,Gordon,S.V.,Marmiesse,M.,etal.(2002).A newevolutionaryscenarioforthe Mycobacteriumtuberculosis complex.ProceedingsoftheNationalAcademy ofSciencesUSA,99,3684–9.
Chase,M.(2004).TheBarbaryPlague:TheBlackDeathin VictorianSanFrancisco.PenguinRandomHouse. Gire,S.K.,Goba,A.,Andersen,K.G.,etal.(2014).Genomic surveillanceelucidatesEbolavirusoriginandtransmissionduringthe2014outbreak.Science,345,1369–72.
Gire,S.K.,Stremlau,M.,Andersen,K.G.,etal.(2012). Emergingdiseaseordiagnosis?Science,338,750–52. Goldberg,T.(2013).Experience:Idiscoveredanewspecies upmynose.TheGuardian: https://www.theguardian. com/lifeandstyle/2013/dec/07/experience-newspecies-up-my-nose,accessed5/7/2018.
Hamer,S.A.,Bernard,A.B.,Donovan,R.M.,etal.(2013). CoincidenttickinfestationsinthenostrilsofwildchimpanzeesandahumaninUganda.AmericanJournalof TropicalMedicineandHygiene,89,924–7.
Hirsch,V.M.,Olmsted,R.A.,Murphey-Corb,M.,etal. (1989).AnAfricanprimatelentivirus(SIVsm)closely relatedtoHIV-2.Nature,339,389–92.
Iwamoto,M.,Jernigan,D.B.,Guasch,A.,etal.(2003). TransmissionofWestNilevirusfromanorgandonor tofourtransplantrecipients.NewEnglandJournalof Medicine,348,2196–203.
Jones,K.E.,Patel,N.G.,Levy,M.A.,etal.(2008).Global trendsinemerginginfectiousdiseases.Nature,451, 990–94.
Keele,B.F.,VanHeuverswyn,F.,Li,Y.,etal.(2006). Chimpanzeereservoirsofpandemicandnonpandemic HIV-1.Science,313,523–6.
Keusch,G.T.,Amuasi,J.H.,Anderson,D.E.,etal.(2022). PandemicoriginsandaOneHealthapproachtopreparednessandprevention:solutionsbasedonSARSCoV-2andotherRNAviruses.Proceedingsofthe NationalAcademyofSciencesUSA,119,e2202871119.
Kilpatrick,A.M.,Altizer,S.(2010).Diseaseecology.Nature EducationKnowledge,3,55.
Koprivnikar,J.,Johnson,P.T.J.(2016).Theriseofdisease ecologyanditsimplicationsforparasitology—areview. JournalofParasitology,102,397–409.
Liu,W.,Li,Y.,Learn,G.H.,etal.(2010).Originofthe humanmalariaparasite Plasmodiumfalciparum ingorillas.Nature,467,420–25.
Lloyd-Smith,J.O.,George,D.,Pepin,K.M.,etal.(2009). Epidemicdynamicsatthehuman-animalinterface.Science,326,1362–7.
Luby,S.P.,Gurley,E.S.,Hossain,M.J.(2009).Transmission ofhumaninfectionwithNipahVirus.ClinicalInfectious Diseases,49,1743–8.
MaríSaéz,A.,Weiss,S.,Nowak,K.,etal.(2015).InvestigatingthezoonoticoriginoftheWestAfricanEbola epidemic.EMBOMolecularMedicine,7,17–23.
Marzi,A.,Chadinah,S.,Haddock,E.,etal.(2018). RecentlyidentifiedmutationsintheEbolaVirusMakonagenomedonotalterpathogenicityinanimal models.CellReports,23,1806–16.
Murray,C.J.L,Ortblad,K.F.,Guinovart,C.,etal.(2014). Global,regional,andnationalincidenceandmortality forHIV,tuberculosis,andmalariaduring1990–2013: asystematicanalysisfortheGlobalBurdenofDisease Study2013.TheLancet,384,1005–70.
Olea-Popelka,F.,Muwonge,A.,Perera,A.,etal.(2017). Zoonotictuberculosisinhumanbeingscausedby Mycobacteriumbovis—acallforaction.LancetInfectious Disease,17,e21–e25.
Olson,M.F.,Ndeffo-Mbah,M.L.,Juarez,J.G.,etal.(2020). Highrateofnon-humanfeedingby Aedesaegypti reducesZikavirustransmissioninSouthTexas.Viruses, 12,453.
Prugnolle,F.,Durand,P.,Ollomo,B.,etal.(2011).A freshlookattheoriginof Plasmodiumfalciparum,the mostmalignantmalariaagent.PLOSPathogens,7(2), e1001283.
Quammen,D.(2012).Spillover:AnimalInfectionsandthe NextHumanPandemic.W.W.Norton.
Raja,T.N.,Hu,T.H.,Kadir,K.A.,etal.(2020).Naturally acquiredhuman Plasmodiumcynomolgi and P.knowlesi infections,MalaysianBorneo.EmergingInfectiousDiseases,26,1801–09.
Roche,B.,Baldet,T.,Simard,F.(2018).Infectiousdiseases inlow-incomecountries:wherearewenow?1–16inB. Roche,T.Baldet,F.Simard(Eds.),EcologyandEvolutionofInfectiousDiseases:PathogenControlandPublic HealthManagementinLow-IncomeCountries,Oxford UniversityPress.
Rouquet,P.,Froment,J-M.,Bermejo,M.,etal.(2005). Wildanimalmortalitymonitoringandhuman Ebolaoutbreaks,GabonandRepublicofCongo, 2001–2003.EmergingInfectiousDiseases,11, 283–90.
Salkeld,D.J.,Schwarzkopf,L.(2005).Epizootiologyof bloodparasitesinanAustralianlizard:amarkrecapturestudyofanaturalpopulation.International JournalforParasitology,35,11–18.
Schneider,S.H.(2005).ThePatientfromHell.DaCapo Press.
Singh,B.,Daneshvar,C.(2013).Humaninfectionsand detectionof Plasmodiumknowlesi.ClinicalMicrobiology Reviews,26,165–84.
Singh,B.,KimSung,L.,Matusop,A.,etal.(2004).A largefocusofnaturallyacquired Plasmodiumknowlesi infectionsinhumanbeings.Lancet,363,1017–24.
Stapp,P.(2007).Trophiccascadesanddiseaseecology. EcoHealth,4,121–4.
Sundararaman,S.A.,Liu,W.,Keele,B.F.,etal.(2013). Plasmodiumfalciparum-likeparasitesinfectingwildapes insouthernCameroondonotrepresentarecurrent sourceofhumanmalaria.ProceedingsoftheNational AcademyofSciencesUSA,110,7020–25.
VanHouten,C.,Musgrave,K.,Weidenbach,K.,etal. (2011).Notesfromthefield: Campylobacterjejuni infectionsassociatedwithsheepcastration—Wyoming,2011. MorbidityandMortalityWeeklyReport(MMWR),60, 1654.
Waller,L.A.(2008).Statisticsindiseaseecology:introductiontoaspecialissue.EnvironmentalandEcological Statistics,15,259–63.
Wolfe,N.D.,Dunavan,C.P.,Diamond,J.(2007).Originsofmajorhumaninfectiousdiseases.Nature,447, 279–83.
Woolhouse,M.E.J.,Adair,K.,Brierley,L.(2013).RNA viruses:acasestudyofthebiologyofemerginginfectiousdiseases.MicrobiologySpectrum,1,OH-00012012.
Worobey,M.,Gemmel,M.,Teuwen,D.E.,etal.(2008). DirectevidenceofextensivediversityofHIV-1inKinshasaby1960.Nature,455,661–4.
Zaki,A.M.,vanBoheemen,S.,Bestebroer,T.M.,etal. (2012).Isolationofanovelcoronavirusfromamanwith pneumoniainSaudiArabia.NewEnglandJournalof Medicine,8,1814–20.
Zhou,P.,Yang,X.L.,Wang,X.G.,etal.(2020).Apneumonia outbreakassociatedwithanewcoronavirusofprobable batorigin.Nature,579,270–73.