Progress in Adhesion and Adhesives Volume 6
Edited by K.L. Mittal
This edition first published 2021 by John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA and Scrivener Publishing LLC, 100 Cummings Center, Suite 541J, Beverly, MA 01915, USA © 2021 Scrivener Publishing LLC
For more information about Scrivener publications please visit www.scrivenerpublishing.com.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at http://www.wiley.com/go/permissions.
Wiley Global Headquarters
111 River Street, Hoboken, NJ 07030, USA
For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com.
Limit of Liability/Disclaimer of Warranty
While the publisher and authors have used their best efforts in preparing this work, they make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives, written sales materials, or promotional statements for this work. The fact that an organization, website, or product is referred to in this work as a citation and/or potential source of further information does not mean that the publisher and authors endorse the information or services the organization, website, or product may provide or recommendations it may make. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for your situation. You should consult with a specialist where appropriate. Neither the publisher nor authors shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages. Further, readers should be aware that websites listed in this work may have changed or disappeared between when this work was written and when it is read.
Library of Congress Cataloging-in-Publication Data
ISBN 978-1-119-84665-9
Cover image: The Editor
Cover design by Russell Richardson
Set in size of 11pt and Minion Pro by Manila Typesetting Company, Makati, Philippines
in the USA
3
4
5
4.4
7
Aishee Dey, Proma Bhattacharya and Sudarsan Neogi
5.1
5.2
5.3
5.3.1
5.3.5
5.3.6
5.3.7
5.3.8
acid)-Based
Inderbir Singh, Gayatri Devi, Bibhuti Ranjan Barik, Anju Sharma and Loveleen Kaur 6.1
7.2 The State-of-the-Art Icephobic Coatings/Surfaces 174
7.2.1 Lotus-Leaf-Inspired Superhydrophobic Surfaces (SHS) with Micro-/Nano-Scale Surface Textures 176
7.2.2 Pitcher-Plant-Inspired Slippery Liquid-Infused Porous Surfaces (SLIPS) 177
7.3 Impact Icing Process Pertinent to Aircraft Inflight Icing Phenomena 179
7.4 Preparation of Typical SHS and SLIPS Coatings/Surfaces 181
7.5 Measurements of Ice Adhesion Strengths on Different Icephobic Coatings/Surfaces 182
7.6 Icing Tunnel Testing to Evaluate the Icephobic Coatings/Surfaces for Impact Icing Mitigation 184
7.7 Characterization of Rain Erosion Effects on the Icephobic Coatings 189
7.8 Summary and Conclusions 196
Adhesives
8.1 Overview and Challenges for Wood Adhesives Based on Natural Resources
8.1.1 Definition of Wood Adhesives Based on Natural Resources 205
8.1.2 Motivation to Use Wood Adhesives Based on Natural Resources 207
8.1.3 Combined Use of Synthetic and Naturally-Based Wood Adhesives 208
8.1.4 Review Articles on Wood Adhesives Based on Natural Resources 209
8.1.5 Motivation for this Review Article in Four Parts in the Journal “Reviews of Adhesion and Adhesives” 211
8.1.6 Overview on Wood Adhesives Based on Natural Resources 212
8.1.7 Requirements, Limitations, and Opportunities for Wood Adhesives Based on Natural Resources 214
8.1.8 Synthetic and Natural Crosslinkers 214
8.1.9 Future of Wood Adhesives Based on Natural Resources 219
8.2
8.2.3.4
9.1
9.5
10.3.9
11 Adhesion in Biocomposites: A Critical Review
Siji K. Mary, Merin Sara Thomas, Rekha Rose Koshy, Prasanth K.S. Pillai, Laly A. Pothan and Sabu Thomas
11.5
12 Vacuum UV Surface Photo-Oxidation of Polymeric and Other Materials for Improving Adhesion:
Gerald A. Takacs, Massoud J. Miri and Timothy Kovach 12.1
12.2.1
12.2.2.1
12.2.2.3
12.2.4 Penetration Depths of VUV Radiation in Polymers 565
12.2.5 Analytical Methods for Surface Analysis 565
12.2.6 VUV Photochemistry of Oxygen 565
12.2.7 Reaction of O Atoms and Ozone with a Polymer Surface 566
12.3 Adhesion to VUV Surface Photo-Oxidized Polymers 567
12.3.1 Fluoropolymers
12.3.2 Nafion®
12.3.3 Polyimides
12.3.4 Metal-Containing Polymers
12.3.5 Polyethylene (PE)
12.3.6 Polystyrene
12.3.7 Other Polymers
12.3.7.1 Polypropylene (PP)
12.3.7.2 Poly(ethylene terephthalate) (PET)
12.3.7.3 Poly(ethylene 2,6-naphthalate) (PEN)
12.3.7.4
12.4 Applications of VUV Surface Photo-Oxidation to Other Materials
12.4.1 Carbon Nanotubes and Diamond
12.5 Prospects
13 Bio- and Water-Based Reversible Covalent Bonds Containing Polymers (Vitrimers) and Their Relevance
Natanel Jarach, Racheli Zuckerman, Naum Naveh, Hanna Dodiuk and Samuel Kenig
13.1 Introduction
13.1.1 RCBPs Classification
13.1.2.1 General Reversible Covalent Bonds
13.1.3
13.1.3.3 Shape-Memory
13.1.3.4
13.1.3.5 Adhesives
13.2 Bio-Based RCBPs
13.2.1.1
13.2.1.2
13.2.2
13.2.2.2 Bio-Based AcylhydrazoneContaining RCBPs
13.2.2.3 Bio-Based Imine (Schiff-Base)Containing RCBPs
13.2.2.4 Bio-Based β-Hydroxy Ester Containing RCBPs
13.2.2.5 Bio-Based Disulfide-Containing
13.3.2.1 Acylhydrazone-Containing Water-Based RCBPs
13.3.2.2 Schiff-Base-Containing Water-Based RCBPs
14.2
14.1.2.1
D.A.
14.4.1
15.2.2
15.2.3
15.2.4
15.3
15.4.1
15.4.2
15.4.3 Additives Modifying the Curing
15.5 Comparison of the Properties of SAAs and Other Reactive Adhesives
15.6 Summary and Outlook
16 Current Progress in Mechanically Durable Water-Repellent Surfaces: A Critical Review
Philip Brown and Prantik Mazumder
16.1 Introduction
16.2 Fundamentals of Superhydrophobicity and SLIPs
16.2.5 Slippery Liquid-Infused Porous Surfaces (SLIPs)
16.3 Techniques to Achieve Water-Repellent Surfaces
16.3.1
17 Mussel-Inspired Underwater Adhesives- from Adhesion Mechanisms to Engineering Applications: A Critical Review 739
Yanfei Ma, Bozhen Zhang, Imri Frenkel, Zhizhi Zhang, Xiaowei Pei, Feng Zhou and Ximin He
17.1 Introduction
17.2 Adhesion Mechanisms of Mussel and the Catechol Chemistry
17.2.1
17.2.2
17.2.4 The Flexibility of the Molecular Chain
17.3 Catechol-Functionalized Adhesive Materials
17.3.1 Permanent/High-Strength Adhesives
17.3.2 Temporary/Smart Adhesives 748
17.3.2.1
18 Wood Adhesives Based on Natural Resources: A Critical Review
18.6 Poly(hydroxyalkanoate)s (PHAs)
18.7
18.7.1
18.8 Cellulose Nanocrystals (CNCs) and Cellulose Nanofibrils (CNFs)
18.8.1 Cellulose Nanofibrils (CNFs) as Sole Adhesives 810
18.8.2 Cellulose Nanofibrils as Components of Adhesives 812
18.9 Cashew Nut Shell Liquid (CNSL) 812
18.10 Summary 819
General Literature (Overview and Review Articles) for Wood Adhesives Based on Natural Resources 820 References
19 Cold Atmospheric Pressure Plasma Technology for Modifying Polymers to Enhance Adhesion: A Critical Review
Hom Bahadur Baniya, Rajesh Prakash Guragain and Deepak Prasad Subedi
19.1 Introduction 842
19.2 Atmospheric Pressure Plasma Discharge 844
19.2.2 Dielectric Barrier Discharge (DBD) 845
19.2.3 Cold Atmospheric Pressure Plasma Jet (CAPPJ) 845
19.2.4 Polymer Surface Modification by CAPPJ 845
19.3 Experimental Setup for the Generation of Cold Atmospheric Pressure Plasma Jet 846
19.4 Methods and Materials for Surface Modification of Polymers 847
19.5 Direct Method for the Determination of Temperature of Cold Atmospheric Pressure Plasma Jet (CAPPJ) 848
19.6 Results and Discussion 848
19.6.1 Temperature Determination of Cold Atmospheric Pressure Plasma Jet (CAPPJ) 848
19.6.2 Electrical Characterization of the CAPPJ 849
19.6.2.1 Power Balance Method 849
19.6.2.2 Current Density Method 850
19.6.2.3 Determination of Energy Dissipation in the Cold Plasma Discharge per Cycle by the Lissajous Figure Method 851
19.6.3 Optical Characterization of CAPPJ 852
19.6.3.1 Line Intensity Ratio Method 852
19.6.3.2 Stark Broadening Method 856
19.6.3.3 Boltzmann Plot Method 858
19.6.3.4 Determination of the Rotational Temperature 859
19.6.3.5 Determination of the Vibrational Temperature 860
19.7 Surface Characterization/Adhesion Property of Polymers 862
19.7.1 Contact Angle Measurements and Surface Free Energy Determination 862
19.7.1.1 Poly (ethylene terephthalate) (PET) 862
19.7.1.2 Polypropylene (PP) 864
19.7.1.3 Polyamide (PA) 867
19.7.1.4 Polycarbonate (PC) 869
19.7.2 FTIR Analysis 871
19.7.2.1 Fourier Transform Infrared (FTIR) Analysis of PET 871
19.7.2.2 Fourier Transform Infrared (FTIR) Analysis of PP 872
19.7.3
Preface
The present volume constitutes Volume 6 in the series “Progress in Adhesion and Adhesives” which made its debut in 2015. Volume 5 (published in 2020) was comprised of 13 review articles initially published in the journal Reviews of Adhesion and Adhesives (RAA) in 2019. Volume 4 (published in 2019) consolidated the 9 review articles published in RAA in 2018. Volume 3 (published in 2018) was based on the 12 review articles initially published in RAA in 2017. Volume 2 (published in 2017) documented the 14 review articles which initially appeared in RAA in 2016. The premier volume (published in 2015) in this vein was based on the 13 review articles originally published in RAA in 2014.
It should be recorded for posterity that the inaugural volume in this series was not designated as Volume 1, as at that time we did not have the crystal ball to foretell the future of this series. But now we can gladly report that this series of books have been warmly and enthusiastically received by those with vested interest in Adhesion Science and Adhesive Technology. We feel fully vindicated in developing these books and that they have served their intended mission.
The success of the first five volumes in this series has provided us the impetus to keep the series going. It should be recorded here that the authors of the review articles initially published in RAA have wholeheartedly endorsed this Volume 6.
The sole purpose of RAA has been to publish concise, critical, illuminating, and thought-provoking review articles on any topic within the broad scope of Adhesion Science and Adhesive Technology. The review articles for RAA have been commissioned from internationally-renowned researchers who have been invited to write articles in their specialties. So, the review articles published in RAA have been of the highest caliber.
The rationale for bringing out Volume 6 remains the same as for its predecessors: RAA had limited circulation so these books will provide broad exposure and wide dissemination of very valuable information published in the excellent review articles summarizing the latest status of many
topics of contemporary interest, with commentary on future prospects and potential.
Yours truly strongly feels that the present Volume 6 will be very warmly received by the Adhesion & Adhesive community and will serve the intended purpose: to provide a resource for valuable information for both seasoned researchers and the budding scientists who wish to delve into the wonderful world of adhesion and adhesives. This particular volume, as well as its predecessors in this series, should be of interest to a broad range of adhesionists, adhesive technologists, polymer scientists, materials scientists, packaging technologists, and those with keen or peripheral interest in the pervasive wettability phenomena.
The 19 chapters in this Volume 6 follow the same order as the review articles originally published in RAA in the year 2020 and up to June 2021. The subjects of these 19 chapters fall in the following areas:
1. Adhesives and adhesive joints
2. Contact angle
3. Reinforced polymer composites
4. Bioadhesives
5. Icephobic coatings
6. Adhesives based on natural resources
7. Polymer surface modification
8. Superhydrophobic surfaces
The topics covered include: hot-melt adhesives; adhesively-bonded spar-wingskin joints; contact angle hysteresis; fiber/matrix adhesion in reinforced thermoplastic composites; bioadhesives in biomedical applications; mucoadhesive pellets for drug delivery applications; bio-inspired icephobic coatings; wood adhesives based on natural resources; adhesion in biocomposites; vacuum UV surface photo-oxidation of polymers and other materials; vitrimers and their relevance to adhesives; superhydrophobic surfaces by microtexturing; structural acrylic adhesives; mechanicallydurable water-repellent surfaces; mussel-inspired underwater adhesives; and cold atmospheric pressure plasma technology for modifying polymers.
Now comes the pleasant task of thanking all those who materialized this book. Naturally, first and foremost, I would like to profusely thank the authors of the review articles originally published in RAA in 2020 and in the first half of 2021 for their wholehearted endorsement of the idea of bringing out this volume. The rationale being that this series of books provide an alternative and easily-accessible resource for a wider dissemination of excellent review articles by top-notch researchers in the field published
Preface xxiii in RAA. I will be remiss if I fail to thank Martin Scrivener (publisher) for conceiving the idea of publishing this series of books, which proved to be opportune and much needed.
Kash Mittal Hopewell Junction, NY email: RAAReviews@gmail.com June 30, 2021
Hot-Melt Adhesives: Fundamentals, Formulations, and Applications: A Critical Review
Swaroop Gharde1, Gaurav Sharma2 and Balasubramanian Kandasubramanian1*
1Nano Surface Texturing Lab, Department of Metallurgical and Materials Engineering, Defence Institute of Advanced Technology (DU), Ministry of Defence, Girinagar, Pune-411025, India
2Nano Fabrication and Characterization Lab, Department of Nanotechnology Centre for Converging Technologies, University of Rajasthan JLN Marg, Jaipur-302004, India
Abstract: Hot-Melt Adhesives (HMAs) are typically used in applications where instant sealing is critically required. HMAs are generally preferred for those applications where processing speed is critical. These materials are widely used in various engineering applications, mainly as sealants in leakages and crack filling of walls and roofs. The industrial use of HMAs is most common in glassware and automobiles for gluing glasses in buildings and bonding heavy motor parts. The formulation of HMAs contains a polymer of suitable nature that makes the base for a strong adhesive, and waxes are added to increase the settling time of adhesive. The tackifiers are used to dilute the polymer to adjust the Glass Transition Temperature (Tg) and to reduce the viscosity for proper flow of hot-melt. This review intends to comprehensively discuss the preparation and formulations of HMAs using various polymer matrices, along with their applications and mechanics. The designing of green HMAs has been discussed in the literature and have been promoted over conventional solvent-based HMAs due to their functionality without Volatile Organic Compounds (VOCs). Various measures, challenges, and resolutions for making hazard-free HMAs have been discussed in the present review.
Keywords: Hot-melt adhesives, formulations, applications, challenges, engineering and industrial sectors
*Corresponding author: meetkbs@gmail.com
K.L. Mittal (ed.) Progress in Adhesion and Adhesives, Volume 6, (1–28) © 2021 Scrivener Publishing LLC
1.1 Introduction to Hot-Melt Adhesives (HMAs)
HMAs are solvent-free thermoplastic materials that are applied in molten state, which upon cooling become solid. An HMA is generally composed of a high molecular weight polymer which is a synthetic elastomer with enhanced viscoelasticity, and a resin for enhancing the properties like gluiness and wetting [1–3]. Here, the polymer provides an appropriate melt viscosity and cohesiveness upon cooling. The precise HMA formulation is dictated by its bonding properties, and application conditions such as time, pressure and temperature. The most common additives for HMAs are rosins, alkyds, and phenol-formaldehyde (PF) resins. Various ranges of suitable polymers are used to make HMAs such as PVAc [poly(vinylacetate)], PBA [poly (butyl acrylate)], polystyrenes, polyesters, EVA (ethylenevinyl acetate), polyethylene (PE), polyamides, amorphous polypropylene and others. A classic HMA is composed of four core constituents: polymer (~33%), resin (~33%), wax (~32%), and antioxidant (~1%). Here, the resin defines the tackiness of the HMA and also maintains the wetting of an HMA (i.e., for how long the HMA remains in liquid state after its application on the substrate). Relatively, resins have low molecular weights than the polymers but it can be said that a resin is an uncured polymer as most of the available HMAs in the market usually come in two components: resins and hardener. When these parts are mixed, the polymerization starts and the resultant product is a cured polymer. The choice of resin is always determined by its affinity with the main polymer. The resin is insoluble in water, but soluble in alcohol. Resin can also be added directly to the polymer to enhance the mechanical and chemical properties of the particular polymer. Due to the fact that the resins are usually tri- or tetra- functional molecules, and when mixed with difunctional polymers give weak mechanical properties. But, when these materials are allowed to mix with a compatible polymer, they form a crosslinked structure which is mechanically stronger than the polymer itself [4]. With higher quantity of resin, tough and strong hot-melts can be produced, while with less resin, soft and fast settling HMAs can be produced. The variety of primary constituents for a typical HMA are shown in Table 1.1 [3, 5]. HMAs generally exist in granules, powders, films, and blocks forms, where these can be applied to a surface as a solution or emulsion to be heat activated later after evaporation of solvent [6].
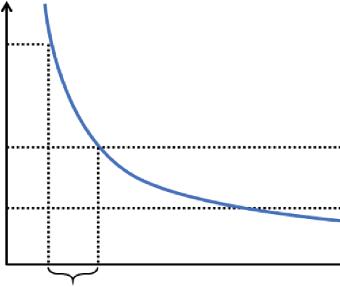
For the liquification of Hot-Melt Films (HMFs), thermal activation and solvents are used, and their solidification depends on their open time and set time, where open time is the time interval where the applicable surface remains tacky for pressure-sensitive adhesives (pressure-sensitive adhesives are a type of nonreactive adhesives which make a bond when pressure is exerted to affix the adhesive with the adherend). On the other hand, the set time is defined as the time interval required to obtain bond strength between the surface and adhesive in a temperature range of 20°C to 30°C [1, 5] (Figure 1.1).
Table 1.1 Primary Constituents for Designing a Typical HMA.
Primary Constituent Physical Properties Function in HMA
Polymer
Resin
Diluent
Wax
High molecular weight (>10000) Provides adhesive strength
T g (Glass Transition Temperature) < Room Temperature Keeps the tackifiers hot
Cross-linking upon cooling Reduces the peel force of adhesive
Low molecular weight (<5000) Lowers viscosity
T g Usually > Room Temperature Improves HMA wetting
Low molecular weight (<1000) Adjusts Tg of the system
T g < Room Temperature Dilutes polymer network
Low molecular weight (<2000) Increases setting speed of HMA
T g < Room Temperature Provides heat resistance, lowers viscosity
Figure 1.1 Graph showing temperature vs. time curve for a typical HMA.
HMAs have outstanding mechanical and physical properties in comparison to conventional solvent-based adhesives. Generally, HMAs are insensitive to aqueous media which means their bond strengths can rarely be influenced by water or moisture. If HMAs are used on a moist or wet surface then their bonding with the adherend may grow weak with time. Additionally, the technology of HMAs
exhibits various unique advantages in comparison to other types of adhesives. The prime benefits of HMAs are their low-cost, elimination of VOCs (Volatile Organic Compounds) discharge in manufacturing, no risk of heat burst (mainly occurs due to the chemical reaction processes in mixing two or more solvents in solvent-based adhesives), no need for adhesive dryers which are conventionally needed to dry the applied adhesive for good settling and maintaining it for long time, and modest application cost, i.e., mainly using a hot glue gun [7].
HMAs are widely used in consumer goods, and engineering sectors for manufacturing pressure- sensitive adhesive tapes, grooved boards, laminated panels of wood, etc. For instance, HMAs with trademarks KR-16-20, Krol, Krok, Krus-2, Stek, and Teplager provided by Jowat Adhesives are being widely used in metalnonmetal bonding along with sealing of polymer composites. For a number of bonding applications, a variety of HMAs perform their function adequately. HMAs are generally preferred for applications requiring critical speeds (critical speed is the theoretical angular velocity of a rotating object such as shaft, leadscrew, gear, etc.). Hot-melts are carrier-free and solidify swiftly upon cooling, and for this reason, they have limited capability to: (i) penetrate the substrates of low-porosity, (ii) dissolve or absorb contaminants on the surface, and (iii) wet-out metals. HMAs are good choices in automated production due to their fast speed of setting. A large variety of HMAs are chemically inert and remain thermoplastic during their use. In this review, we discuss the formulations, design principles, mechanical behavior, HMA tests, and applications, along with the current challenges of HMAs and how to meet them.
1.2 Formulation of Hot-Melt Adhesives
1.2.1
Theories or Mechanisms of Adhesion
Adhesion usually is classified by physical, chemical, and/or mechanical bonding processes, which give the interfacial strength to the joint. The adhesion bonding occurs between the adherend and the adhesive. Over the years, various theories have been established to describe the phenomenon of adhesion. Mechanical bonding is a highly efficient technique for creating joints, but it fails to join smooth surfaces. Chemical bonding is strong but it is difficult to produce in an efficient manner [8–12]. Each theory of adhesion for bonding of materials is discussed in more detail below.
1.2.1.1
Mechanical Interlocking Theory
Mechanical theory is the common and the oldest theory of adhesion. It involves penetration of adhesive in pores, cavities, and irregularities on the surface of adherend and was proposed by McBain and Hopkins in 1925 [13]. It displaces the trapped air at the interface, which determines the adhesive penetration into both
adherends. It was also found that adhesives form good bonds with porous and irregular surfaces as compared to smooth surfaces [8–11].
1.2.1.2
Electrostatic Theory
According to this theory, adhesion takes place at the adhesive-adherend interface due to exchange or transfer of electrons across the interface. Such transfer forms an electrical double layer at the adhesive-adherend boundary and results in Coulomb attraction forces between the mating partners [8–11].
1.2.1.3
Diffusion Theory
According to this theory, diffusion is only valid when both the adhesive and the adherend are polymers with relatively long chain-like structures, which deliver mobility, and permit the possibility of the chains to have molecular movements in the sub-molecular range. The diffused interfacial layer has a thickness in the range of 1–100 nm, and it is dependent on various parameters such as pressure, time, temperature, molecular size, and mutual solubility [8–11].
1.2.1.4
Physical Adsorption or Wetting Theory
According to adsorption theory, the forces responsible for adhesion between two materials are secondary valence or van der Waals forces. The process of forming contact between the adhesive and the adherend is known as “wetting”. For an adhesive to wet a solid surface, the adhesive should have a surface tension lower than the critical surface tension of the solid. This is the reason for surface treatment of plastics, which increases their surface free energy and polarity [8–11].
1.2.1.5
Chemical Bonding
This mechanism is quite similar to the physical adsorption but it involves the formation of hydrogen, covalent, and ionic bonds between the adhesive and the adherends. In general, there are four types of interactions during bonding such as covalent, hydrogen, Lifshitz-van der Waals forces, and acid-base interactions, but the exact nature of bond depends on the chemical structure of the adherend (substrate). The fabrication of adhesives based on chemical bonding theory has been adopted for decades by various industries, but the results have been disappointing due to their contamination with external environmental factors like air, moisture, temperature, etc. which made the adhesion unstable and weak [8–11].
1.2.2 Intermolecular Forces between Adhesives and Adherend
The numerous adhesion theories imply the existence of physico-chemical interactions across the interface of adherend and adhesive. The development of an adhesion bond relies on increasing the intermolecular attraction between the two
surfaces, both in the polymer bulk and between the adhesive and adherend. Among the different forces accountable for intermolecular attraction, primary forces are dispersion forces acting in all atoms and are responsible for all of the molecular attraction or cohesion in all molecules, excluding the polar molecules. Dispersion forces are short-range interaction forces that are effective at a distance of 4 Å, and rapidly decrease with the inverse 6th power of the intermolecular distance. Hence, the molecules of adhesive polymer must be sufficiently flexible to come within this interaction range with a rigid adherend surface under bond formation conditions. Additional interaction arises between dipoles in molecules, where dipoles arise when the chemically bonded electrons among atoms are shared unequally, thus creating positive and negative charge centres in the molecule. The force of interaction due to permanent dipoles of polar molecules depends on strength of the two dipoles, and is reduced with the inverse 6th power of the distance. Evidently, the dipolar interaction of adhesives will be strong when they possess polar chemical groups [14]. A strong dipolar attraction occurs when the positive centre is connected with the H-atom attached to an electronegative atom, generally N or O, as shown in the following examples
This sharing of proton between electronegative atoms is called the hydrogen bond. It arises in polymers carrying amide (-CONH-), carboxyl (-COOH), or hydroxyl (-OH) groups, and is responsible for adhesion of materials such as proteins, starch, poly(vinyl alcohol), epoxy resins, phenolics to polar substrates, especially to wood which has an abundance of hydroxyl groups [15, 16].
1.2.3 Thermodynamic Model of Adhesion
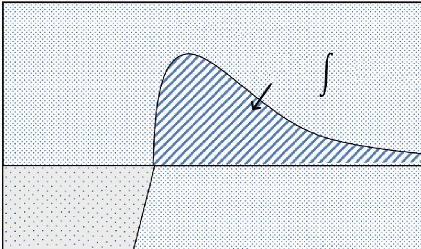
The thermodynamic work of adhesion, wetting surface tension, solubility parameter or interfacial free energy are responsible parameters for the strength of adhesive bonds. Adhesive bonding is controlled by intermolecular forces, where the attraction forces between adhesive and adherend are defined by an energy interaction that can be characterized by the Lennard-Jones potential [17].
where, F denotes the force of attraction, r denotes the separation distance between an adhesive and adherend with the variables A and C as adhesion constants. The Force of Attraction vs Distance of Separation graph is illustrated in Figure 1.2.
orce of Attra
Equation
Distance of Separation (r)
Figure 1.2 Force of attraction vs distance of separation between adhesive and adherend.
The basic equations provided by Dupre and Young-Dupre for the thermodynamics of adhesion are as follows:
(I) Dupre Equation: WA
(II) Young-Dupre Equation:
where, θLS is the equilibrium contact angle made by L (liquid) phase in contact with S (solid) phase.
The maximum strength and flexibility of an HMA bond are obtained when the interfacial free energy is minimum [18]. The interfacial free energy of the system is obtained by reorganization of Equations (1.2) and (1.3) as:
1.2.4 Bonded Joints
The lap-shear tests determine the various HMA designs based on the strength of joints and Length of Overlapping (LO). There is an approach known as IAI (Incorrect Assumption Inherent) which defines that the stress in hot-melt joints is uniform; therefore, doubling the LO can bear double the loads in an HMA. The fundamental principle for designing an HMA bond is to formulate the bond in such a way that the adhesive strength is always higher than the strength of the adherends. This approach facilitates the formation of stronger HMA joints in comparison to the parent material from which the HMA is formulated. The facility to design bonded HMA joints occurs in such a way that the adhesive part can never be the weakest portion of the joint. If the HMA will not fail due to the applied