Organic
Visit to download the full and correct content document: https://ebookmass.com/product/organic-electronics-for-electrochromic-materials-anddevices-1st-edition-hong-meng/

More products digital (pdf, epub, mobi) instant download maybe you interests ...

Composite Materials It Meng Low
https://ebookmass.com/product/composite-materials-it-meng-low/

Thermoelectric Materials and Devices Lidong Chen
https://ebookmass.com/product/thermoelectric-materials-anddevices-lidong-chen/


Quantum Materials, Devices, and Applications Mohamed Henini
https://ebookmass.com/product/quantum-materials-devices-andapplications-mohamed-henini/ Terahertz Biomedical and Healthcare Technologies: Materials to Devices 1st Edition Amit Banerjee (Editor)
https://ebookmass.com/product/terahertz-biomedical-andhealthcare-technologies-materials-to-devices-1st-edition-amitbanerjee-editor/

Quantum Physics of Semiconductor Materials and Devices
Debdeep Jena
https://ebookmass.com/product/quantum-physics-of-semiconductormaterials-and-devices-debdeep-jena/

Novel Electrochemical Energy Storage Devices: Materials, Architectures, and Future Trends 1st Edition Feng Li
https://ebookmass.com/product/novel-electrochemical-energystorage-devices-materials-architectures-and-future-trends-1stedition-feng-li/

Go Programming Language For Dummies 1st Edition WeiMeng Lee
https://ebookmass.com/product/go-programming-language-fordummies-1st-edition-wei-meng-lee/

Principles of Electronic Materials and Devices 4th
Edition Safa O. Kasap
https://ebookmass.com/product/principles-of-electronic-materialsand-devices-4th-edition-safa-o-kasap/

Lectures on Digital Design Principles (River Publishers
Electronic Materials, Circuits and Devices) 1st Edition
Pinaki Mazumder
https://ebookmass.com/product/lectures-on-digital-designprinciples-river-publishers-electronic-materials-circuits-anddevices-1st-edition-pinaki-mazumder/

OrganicElectronicsforElectrochromicMaterialsandDevices
HongMeng
Author
Prof.HongMeng PekingUniversity ShenzhenGraduateSchool
BuildingG306
LishuiRoad,NanshanDisctrict 518055Shenzhen China
CoverDesign:Wiley
CoverImage:©Andrew Goodsell/Shutterstock
Allbookspublishedby Wiley-VCH arecarefullyproduced.Nevertheless, authors,editors,andpublisherdonot warranttheinformationcontainedin thesebooks,includingthisbook,to befreeoferrors.Readersareadvised tokeepinmindthatstatements,data, illustrations,proceduraldetailsorother itemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>
©2021WILEY-VCHGmbH, Boschstr.12,69469Weinheim, Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc. usedinthisbook,evenwhennot specificallymarkedassuch,arenotto beconsideredunprotectedbylaw.
PrintISBN: 978-3-527-34871-8
ePDFISBN: 978-3-527-83061-9
ePubISBN: 978-3-527-83062-6
oBookISBN: 978-3-527-83063-3
Typesetting SPiGlobal,Chennai,India PrintingandBinding
Printedonacid-freepaper
10987654321
Contents
Preface xiii AbouttheAuthor xiv
1Introduction 1
1.1GeneralIntroduction 1
1.2TheHistoryofElectrochromicMaterials 3
1.3TheKeyParametersofElectrochromism 5
1.3.1ElectrochromicContrast 5
1.3.2SwitchingTime 8
1.3.3ColorationEfficiency 9
1.3.4OpticalMemory 11
1.3.5Stability 12
1.4Conclusion 14 References 14
2AdvancesinPolymerElectrolytesforElectrochromic Applications 17
2.1Introduction 17
2.2RequirementsofPolymerElectrolytesinElectrochromic Applications 18
2.3TypesofPolymerElectrolytes 20
2.3.1GelPolymerElectrolytes(GPEs) 20
2.3.1.1PEO-/PEG-BasedElectrolytes 21
2.3.1.2PMMA-BasedPolymerElectrolytes 21
2.3.1.3PVDF-BasedPolymerElectrolytes 22
2.3.2Self-HealingPolymerElectrolytes 24
2.3.3Cross-LinkingPolymerElectrolytes(CPEs) 26
2.3.4CeramicPolymerElectrolytes 27
2.3.5IonicLiquidPolymerElectrolytes 30
2.3.6Gelatin-BasedPolymerElectrolytes 32
2.4ConclusionandFutureOutlook 33 References 40
3ElectrochromicSmallMolecules 49
3.1BackgroundofSmallMoleculeElectrochromic 49
3.2TechnologyDevelopmentofSmallMoleculeElectrochromic Materials 50
3.3Violene–CyanineHybrids(AIEPLOEC) 50
3.4TerephthalateDerivatives(MulticolorOEC) 56
3.4.1Conclusion 63
3.5IsophthalateDerivatives 64
3.5.1Conclusion 79
3.6MethylKetoneDerivatives 79
3.6.1Conclusion 84
3.7Diphenylacetylenes 84
3.8FluoranDyeDerivatives 85
3.9PH-ResponsiveMoleculeDerivatives 92
3.10TPADyeDerivatives 95
3.11HydrocarbonDerivatives-NIR-OEC 99
3.12ConclusionsandPerspective 101 References 101
4ViologenOEC 105
4.1TheIntroductionofOECandViologen 105
4.1.1GeneralIntroduction 105
4.1.2ResearchHistoryofViologen 105
4.1.2.1FirstStage(1930s–1970s) 107
4.1.2.2SecondStage(1970s–2000s) 107
4.1.2.3ThirdStage(2000s–2010s) 107
4.1.2.4FourthStage(2010s–Present) 108
4.1.3ElectrochromismandElectrochemistryofViologensandTheir Device 109
4.2DifferentStructuresofViologen-BasedElectrochromicMaterials 110
4.2.1SynthesisofViologens 110
4.2.1.1DirectSubstitutionReaction 110
4.2.1.2ZinckeReaction 110
4.2.1.3MethodsforSynthesizingBipyridine 110
4.2.2The1,1′ -SubstitutedViologen 111
4.2.2.1SimpleAlkyl 111
4.2.2.2AcidGroup 111
4.2.2.3EsterandNitrogenHeterocycle 112
4.2.2.4AsymmetricSubstitution 113
4.2.3ConjugateRingSystemExpansion 113
4.2.3.1Thiazolothiazole(TTz)Unit 113
4.2.3.2Perylenediimide(PDI)Unit 115
4.2.3.3PBEDOTPh 115
4.2.3.4HeteroatomsBridged 115
4.2.3.5BithiopheneBridged 118
4.2.4Viologen-BasedPolymer 119
4.2.4.1ViologenintheSideChain 120
4.2.4.2ViologenintheMainChain 122
4.3ViologenElectrochromicDevice 124
4.3.1DeviceStructure 124
4.3.1.1Five-LayerClassicStructure 124
4.3.1.2SimpleSandwichStructure 125
4.3.1.3CathodicAnodeSeparationStructure 125
4.3.1.4ReflectiveDeviceStructure 126
4.3.2Electrolyte 126
4.3.3RedoxMediator 126
4.3.4ConductiveMedium 128
4.3.5ProblemswithViologenCompound 128
4.3.5.1Dimerization 128
4.3.5.2AggregationandSolubility 131
4.3.5.3ResponseTime 131
4.3.5.4DrivingVoltage 131
4.3.5.5Conclusion 131
4.3.6ExamplesofViologen-BasedECD 132
4.4CompaniesOperatingintheFieldofViologenElectrochromism 132
4.4.1Gentex 132
4.4.2Essilor 134
4.4.3Haoruo 134
4.5Conclusion 134 References 135
5Metallohexacyanates 143
5.1Background 143
5.2TechnologyDevelopmentofPB 144
5.3CrystalStructure 144
5.4ElectrochromicMechanism 145
5.5Synthesis 147
5.6ElectrochromicDevices(ECDs) 150
5.7Nanocomposites 154
5.8PBAnalogs 160
5.9MultifunctionalApplications 170 References 175
6ElectrochromicConjugatedPolymers(ECPs) 183
6.1Introduction 183
6.1.1CommonCategoriesandOperationMechanism 183
6.1.2SyntheticMethods 186
6.2Thiophene-BasedConjugatedElectrochromicPolymers 190
6.2.1Introduction 190
6.2.2Color-TuningStrategiesforThiophene-BasedPolymers 191
6.2.2.1StericEffects 192
6.2.2.2SubstituentandElectronicEffects 193
6.2.3TypicalColoredPolymers 195
6.2.3.1YellowandOrange 196
6.2.3.2Red 198
6.2.3.3MagentaandPurple 199
6.2.3.4Black 202
6.2.3.5Multicolored 203
6.2.3.6AnodicallyColoringPolymers 205
6.2.4Water-or“GreenSolvents”-SolubleECPs 208
6.3Polypyrroles-BasedConjugatedElectrochromicPolymers 216
6.3.1Introduction 216
6.3.2ElectrochromicPropertiesofPolypyrroles(PPy) 218
6.3.3TuningofElectrochromicPropertiesofPolypyrrole(PPy) 218
6.3.3.1StructuralModification 218
6.3.3.23-and3,4-SubstitutedPolypyrroles 235
6.3.3.3Donor–AcceptorApproach 236
6.3.3.4TeraryleneSystems 237
6.4Polycarbazole-BasedConjugatedElectrochromicPolymers 237
6.4.1Introduction 237
6.4.2ElectrochromicPropertiesofPolycarbazoles(PCARB) 238
6.4.3ElectrochromicPropertiesofPolycarbazolesDerivatives 238
6.4.3.1LinearPolycarbazoleDerivatives 241
6.4.3.2Cross-LinkedPolycarbazolesDerivatives 249 References 260
7TA-BasedElectrochromicPolyimidesandPolyamides 269
7.1Introduction 269
7.1.1AromaticPolyimidesandPolyamides 269
7.1.2Triarylamine-BasedAromaticPolymers 270
7.1.3ElectrochemicalandElectrochromicBehaviorsofMVTriarylamine Systems 272
7.2DevelopmentofTA-BasedElectrochromicPolyimidesand Polyamides 272
7.2.1SideGroupEngineering 276
7.2.1.1IntroductionofProtectingGroups 276
7.2.1.2IntroductionofElectroactiveGroupstoAchieveColorTuningofEC Material 277
7.2.1.3IntroductionofSideGroupstoAchieveElectrofluorochromic Materials 278
7.2.1.4IntroductionofOtherFunctionalSideGroupstoAchieveMultiple FunctionsECMaterial 281
7.2.2BackboneModulation 283
7.2.2.1ExtendingthePolymerBackbonebyIntroducingMoreElectroactive Groups 283
7.2.2.2IntroductionofAmideLinkageintoPolyimideBackbone 285
7.2.2.3IntroductionofEtherLinkageintoPIs/PAsBackbone 285
7.2.2.4IntroductionofAlicyclicStructuresintoPIs/PAsBackbone 288
7.3Conclusions 290 References 290
8Metallo-SupermolecularPolymers 295
8.1Introduction 295
8.2SingleMetallicSystem 296
8.2.1Fe(II)-andRu(II)-BasedMetallo-SupramolecularPolymers 296
8.2.2CoII -BasedMetallo-SupramolecularPolymers 299
8.2.3ZnII -BasedMetallo-SupramolecularPolymers 301
8.2.4Cu-BasedMetallo-SupramolecularPolymers 305
8.2.5EuIII -BasedMetallo-SupramolecularPolymers 308
8.3Hetero-MetallicSystem 311
8.4TheFabricationMethodofMetallopolymerFilm 314
8.4.1Layer-by-LayerSelf-AssemblyandDip-CoatingMethods 314
8.4.2ElectropolymerizedConductingMetallopolymers 315
8.5Conclusion 323 References 323
9Metal-OrganicFramework(MOF)-andCovalentOrganic Framework(COF)-BasedElectrochromism(EC) 327
9.1Introduction 327
9.2CurrentStudiesinECMOFs 327
9.2.1TheOrganicLinkersinECMOFs 328
9.2.1.1NDI-BasedOrganicLinkers 328
9.2.1.2OtherOrganicLinkers 332
9.2.2TheTransportofElectrolyteIonsinECMOFs 335
9.2.3SpecialECMOFs 338
9.2.3.1PhotochromicandElectrochromicMulti-ResponsiveMOF 338
9.2.3.2MOF-BasedDouble-SidedECDeviceandOtherColor-Switching Mechanisms 339
9.2.3.3ECBaseon“Guest@MOF”CompositeSystem 340
9.3CurrentStudiesinECCOFs 341
9.4ConclusionandProspect 348 References 348
10Nanostructure-BasedElectrochromism 353
10.1Introduction 353
10.2CurrentStudiesofNanostructureinElectrochromism 354
10.2.1Non-ElectrochromicActiveMaterialsasaTemplateforECs 354
10.2.1.1PhotonicCrystalsasTemplatesforECs 354
10.2.1.2PlasmonicStructuresasTemplatesforECs 359
x Contents
10.2.2NanostructuredElectrochromicMaterialsinECs 365 10.3ConclusionandProspect 369 References 369
11OrganicElectroluminochromicMaterials 373
11.1Introduction 373
11.2ConventionalMechanismsofElectroluminochromism 375
11.2.1IntrinsicMechanism 375
11.2.2ElectronTransfer(ET)Mechanism 376
11.2.3EnergyTransfer(EnT)Mechanism 376
11.3ElectroluminochromicPerformanceParameters 376
11.3.1EmissionContrast 376
11.3.2SwitchingTime 377
11.3.3Long-TermStability/CycleLife 377
11.4ClassicalMaterials 378
11.4.1SmallMolecules 378
11.4.1.1SmallMolecularDyads 378
11.4.1.2Redox-ActiveMoietyandLuminophoresSystem 380
11.4.1.3ElectroactiveLuminophores 382
11.4.2TransitionMetalComplexes 386
11.4.3Polymers 387
11.4.3.1Non-ConjugatedPolymers 387
11.4.3.2ConjugatedPolymers 396
11.4.4NanocompositeFilms 407
11.5FuturePerspectivesandConclusion 408 References 408
12OrganicPhotoelectrochromicDevices 415
12.1Introduction 415
12.2StructureDesignofPECDs 417
12.2.1PowerSupplyforPECD 417
12.2.1.1DSSC-BasedPECD 418
12.2.1.2PSC-BasedPECD 423
12.2.1.3OPV-BasedPECD 423
12.2.2ElectrochromicMaterialsinPECD 425
12.2.2.1SmallMolecule 425
12.2.2.2ConductingPolymers 427
12.2.2.3Near-Infrared(NIR)ElectrochromicMaterials 433
12.2.3ElectrolytesinPECD 435
12.2.4SubstratesinPECD 435
12.3FuturePerspectivesandConclusion 436 References 436
13ApplicationofOECDevices 445
13.1SmartWindow 445
13.1.1TheStructureandWorkingMechanismofSmartWindows 445
13.1.2TheMaterialsforElectrochromicWindows 446
13.1.3Prospects 450
13.2DimmableRearviewMirror 450
13.3Sensors 451
13.3.1ApplicationofElectrochromicSensorsonFoodPreservation 451
13.3.2ApplicationinBio-Sensing 454
13.4TheApplicationofElectrochromicDeviceinDisplay 460
13.5OtherApplicationsofOEC 462 References 469
14CommercializedOECMaterialsandRelatedAnalysisof CompanyPatents 471
14.1GeneralIntroduction 471
14.2GentexCorporation 471
14.3RicohCompany,Ltd. 475
14.4CanonInc. 476
14.5BOETechnologyGroupCo.,Ltd.andOPPOGuangdongMobile CommunicationsCo.,Ltd. 477
14.6OtherImportantEnterprises 481
14.6.1NinboNinuoElectronicTechnologyCo.,Ltd. 481
14.6.2AmbilightInc. 483
14.6.3FurciferInc. 483
14.6.4ChangzhouSpectrumNewMaterialCo.Ltd. 484
14.7Conclusion 485 References 485
15MainChallengesfortheCommercializationofOEC 491
15.1Introduction 491
15.2TheLong-TermStabilityofOECMaterials 491
15.3TheMechanicalStabilityofOECDevices(Encapsulation Technology) 495
15.4Large-AreaProcessTechnology:SprayCoatingandRoll-to-Roll Processes 498
15.4.1InkjetPrinting 498
15.4.2SprayCoating 500
15.4.3Slot-DieCoating 500
15.4.4ScreenPrinting 501
15.5ConclusionsandPerspective 501 References 502
Index 505
Preface
Inrecentyears,withthedevelopmentofartificialintelligence,moreandmore industriesstrivetobe“smart.”Asanewgenerationofdisplaytechnology,organic electrochromic(OEC)devicesoffernumerousadvantagessuchasflexibility,full colors,wideoriginsofmaterials,fastswitchingtime,lowdrivingvoltage,and simpleconfiguration.Inaddition,thesedevicespossess“smart”characteristicsof multi-stimulationandmulti-response.Therefore,theOECindustryisemergingas apotentialdisplaycompetitorinthefieldofelectronicinformation.
Thisbookcoversmajortopicsrelatedtothephenomenonofelectrochromism, includingthehistoryoforganicelectrochromism,fundamentalprinciples,different typesofelectrochromicmaterials,developmentofdevicestructures,multifunctional devices,theircharacterizationsandapplications,andfutureprospectsofOECtechnology.Italsospotlightsrecentresearchprogressreportedbyacademicinstitutes andenterprises,anddiscussestheexistingchallengesinfurtherdevelopmentofthis area.
ThisbookprovidesacomprehensivereviewofOECmaterialsanddevices,and canbeusedasateachingreferenceforundergraduateandgraduatestudentsaswell asteachersinthefieldsoforganicchemistryandpolymerscienceetc.Also,this bookcanbeadoptedasacomprehensivereferenceforresearchersengagedinthe developmentofOECtechnologyenterpriseinthefieldofelectrochromism.
Shenzhen,PRChina 11November2020
HongMeng
AbouttheAuthor

Prof.Dr.HongMengobtainedhisBSinChemistryat SichuanUniversityin1988andMSdegreeinOrganic ChemistryfromPekingUniversityin1995.Hethenstudied PolymerScienceandEngineeringandacquiredhissecond MSdegreefromtheNationalUniversityofSingaporein 1997.AfterworkingattheInstituteofMaterialsScience andEngineering(IMRE)inSingaporefortwoyears,he wenttotheUnitedStatesin1999andcompletedhisPhD underthesupervisionofProf.FredWudlattheUniversity ofCalifornia,LosAngeles(UCLA),in2002.Prof.Dr.Mengworkedasaresearch consultantatBellLabs,LucentTechnologies,withDr.ZhenanBaoforoneyear.He thenjoinedDuPontExperimentalStation,CentralResearchandDevelopment,asa seniorresearchchemistin2002.In2012,hemovedbacktoChinaandworkedasthe CEOofalaserprintingindustryatLeputaiTechnologyCompany.In2014,hejoined theSchoolofAdvancedMaterialsatPekingUniversity,Shenzhenasachairprofessor.Hehasbeenengagedintheresearchanddevelopmentofsolid-stateorganic synthesis,organicsemiconductordeviceengineering,organicelectronics,andother relevantfields,especiallyorganiclight-emittingdiodes,organicelectrochromics, organicthin-filmtransistors,organicconductivepolymers,andnanotechnology.He haspublishedmorethan200articlesininternationallyrenownedjournals,participatedinthewritingofthreebookchaptersandco-editedtwobooksinthefieldof organicoptoelectronictechnology.Hehasobtainedmorethan150patentsforinventionsintheUnitedStatesandChina,amongwhich,severalpatentedproductshave beencommercialized.
Introduction
1.1GeneralIntroduction
Electrochromismisthephenomenonthatdescribestheoptical(absorbance/ transmittance/reflectance)changeinmaterialviaaredoxprocessinducedbyan externalvoltageorcurrent[1].Usuallytheelectrochromic(EC)materialsexhibit colorchangebetweenacoloredstateandcolorlessstateorbetweentwocolors, evenmulticolor.Innature,itsoriginisfromthechangeofoccupationnumberof material’sinternalelectronicstates.AsthecoreofECtechnology,theECmaterials havebuiltupmanycategoriesduringdecadesofdevelopment,forexample,accordingtothecolorationtype,itcouldbeclassifiedasanodicallycoloringmaterials (colorationuponoxidation)orcathodicallycoloringmaterials(colorationupon reduction)[2].Basedonthelightabsorptionregioninthesolarradiation,which consistsofthesethreeparts:ultraviolet(UV),visible(Vis),andnear-infrared radiation(NIR)lights(Figure1.1),itcouldbedividedintovisibleECmaterials (wavelength:380–780nm),whichcanbeseenbythehumaneyeandtherefore aresuitableforsmartwindowandindicatorapplications,andNIRECmaterials (wavelength:780–2500nm),whichhavegreatpotentialforthermalregulation technologiesandeveninnationaldefense-relatedapplications[3].Andonthe basisofmaterialsspecies,therearemainlyinorganic,organic,andhybridECmaterials[4,5](https://commons.wikimedia.org/wiki/File:Solar_spectrum_en.svg). InorganicECmaterialsaretransitionmetaloxides(TMOs)(WO3 ,NiO,TiO2 ,and PrussianBlue[PB]),organicECmaterialsincludingsmallmolecules(e.g.viologen), conjugatedpolymers(e.g.poly(pyrrole),poly(thiophene),andpoly(carbazole))and aromaticpolymers(e.g.polyimides[PIs]andpolyamides[PAs]),organic–inorganic hybridmaterialsreferringtometallo-supermolecularpolymers,andmetal–organic framework(MOF).Amongthem,inorganicmaterialsexhibitexcellentlong-term stabilitycomparedwithorganicones;however,consideringthestructurevariety, flexibility,andlow-costsolutionprocessability,organicECmaterialsaresuperior toinorganicmaterials.Theorganic–inorganichybridmaterialsaredesignedto combineadvantagesofbothorganicandinorganicmaterials.
ECmaterialsexhibitcolorchangesduringtheredoxprocess;thereforethe electrochromicdevices(ECDs)generallyconsistofthreeelements:electrodes,EC materials,andelectrolytesolution.Theelectrodesofferaconstantsupplyofelectric OrganicElectronicsforElectrochromicMaterialsandDevices, FirstEdition.HongMeng. ©2021WILEY-VCHGmbH.Published2021byWILEY-VCHGmbH.

Figure1.1 SolarirradiancespectrumaboveatmosphereandatthesurfaceoftheEarth. Source:Nick84:https://commons.wikimedia.org/wiki/File:Solar_spectrum_en.svg,Licensed underCCBY-SA3.0.
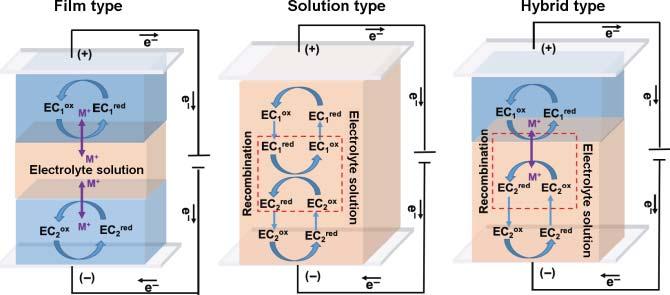
Figure1.2 Theschemeofthreetypesofelectrochromicdevices. current,andionsareconductedbytheelectrolytesolution.ThentheECmaterials undergoelectrochemicaloxidationand/orreduction,whichresultsinchangesin theopticalbandgapandcolors.AsshowninFigure1.2,atypicalECDhasfivelayers: twotransparentconductingoxide(TCO)layers,EClayer,ion-conductinglayer (electrolytesolution),ionstoragelayer.Particularly,theionstoragelayeractsasthe “counterelectrode”tostoreionsandkeepelectricchargebalance.Andaccordingto theexactstateofECmaterials,therearethreetypesofECD:filmtype(I),solution type(II),andhybridtype(III).TheTypeIECDisthemostcommon;manykindsof ECmaterialsaresuitableforthistypeincludingTMOs,conjugated/non-conjugated polymers,metallo-supermolecularpolymers,andMOF/covalentorganicframework(COF)materials,whichusingspin-coating,spray-coating,anddip-coating processestoformuniformfilms;thesefilmswon’tdissoluteinelectrolytesolutions. TypeIIECDrequiresthattheECmaterialshavegoodsolubilityinelectrolyte
1.2TheHistoryofElectrochromicMaterials 3 solutions.Thereforemanyorganicsmallmoleculessuchasviologen,terephthalate derivatives,andisophthalatederivativesareappropriateforthistypeofdevice. Meanwhilethefabricationmethodforthistypeofdeviceisthemostsimpleone. ItjustneedstodissolvetheelectrolyteandECmaterialinaspecificsolventand injectintothepreparedconductingcell.TypeIIIECDusesfilm-typeECmaterials asworkingelectrodeandsolution-typeECmaterialsasionstoragelayer.
1.2TheHistoryofElectrochromicMaterials
Theword“electrochromism”wasinventedbyJohnR.Plattin1960[6],inanalogy to“thermochromism”and“photochromism.”However,theECphenomenoncould betracedtothenineteenthcentury,asearlyas1815.Berzeliusobservedthecolor changeofpuretungstentrioxide(WO3 )duringthereductionwhenwarmedunder aflowofdryhydrogengas.Thenfrom1913to1957,somepatentsdescribedthe earliestformofECDbasedonWO3 [7,8].Thereforetheoriginsofelectrochromism arethenineteenthandtwentiethcenturies.Afterthen,electrochromismtechnology begantoundergorapiddevelopment,especiallytheexplorationofmanyclassesof ECmaterials.Asshowedinthetechnologyroadmap(Figure1.3),wesummarized severalgenerationsofECmaterialsduringlong-termdevelopment.
Thefirst-generationECmaterialisTMOs(e.g.WO3 ,NiO,andPB).Amongthem, WO3 playsanimportantroleintheelectrochromismfield;asthefirstfoundedEC material,ithasalreadyrealizedcommercializationinsmartwindowsapplication. PBwasdiscoveredasadyebyDiesbachin1704,andthentheelectrochemicalbehaviorandECperformanceofPBwasfirstlyreportedbyNeffat1978[9].Benefitted fromthestructurestabilityandreversibleredoxprocessofthoseinorganicTMOs, theelectrochromismbasedonthethin-filmTMOsarewidelyinvestigated,including

thedevelopmentofnewTMOsmaterials,introductionofnewnanostructures,and differentelementdoping.
Followingthefirst-generationTMOECmaterials,organicsmallmoleculeEC materialshaveemergedsince1970.Amongthem,viologenasthemostrepresentativesmallmoleculewasfirstdiscoveredbyMichaelisandHillin1932[10],and becauseofthevioletonthereduction,these1,1′ -disubstituted-4,4′ -bipyridinecompoundswerenamed“viologen.”Thenin1973,Shootmadeanewflatalphanumeric displayusingheptylviologen;thiscanberegardedasthebeginningoftheuseof viologenforelectrochromism[11].Afteracentury’sdevelopment,viologenalready hasbeensuccessfullycommercialized.Besidestheviologen,othersmallmolecules ECmaterialssuchasterephthalatederivatives,isophthalatederivatives,methyl ketonederivatives,andsomedyemoleculeshavealsoattractedmuchattentions fromscientistsduetotheirsimplestructureandlowcost.
Thethird-generationECmaterialsareconjugatedpolymers.In1983,Francis GarnierandcoworkersfirstlycharacterizedtheECpropertiesofaseriesof five-memberedheterocyclicpolymersincludingpoly(pyrrole),poly(thiophene), poly(3-methylthiophene),poly(3,4-dimethylthiophene),andpoly(2,2′ -dithiophene).
Sincethen,conjugatedpolymersweregivenrisetotherapidemergeasanewclass ofelectrochromism[12].Fiveyearslater,BertholdSchreckobservedtheelectrochromismphenomenonofpoly(carbazole),whichshowedacolorchange frompaleyellowishtogreentogetherwiththeconductivityenhancement[13]. Todate,theconjugatedpolymerECsystemhasbeenwelldeveloped,frombetterunderstandingsonmechanismstocompletedcolorpallettewithsolubleor electro-depositedpolymers,andevenfull-colordisplaysamplesorroll-to-roll fabricatedflexibledevices.
Later,inearly2000,triarylamine(TA)-basedaromaticpolymersespeciallythe PIsandPAshavedrawnconsiderableattentionfromtheresearchcommunity asthefourth-generationECmaterials.Thecorrelationbetweenelectrochemical propertiesandchemicalstructuresofdifferentaromaticPIswasfirstlydescribed in1990.Tenyearslater,ZhiyuanWangandcoworkers[14]reportedthefirstEC behaviorofpoly(ethernaphthalimide)s,whichshowedstepwisecolorationprocess, fromcolorlesstoredandtodarkbluecorrespondingtotheneutral,radicalanion, anddianionspecies,respectively.However,duetothehighrigidityofthePIs/PAs backboneandstrongintermolecularinteractions,thepoorprocessabilitylimitedthe developmentofPIsorPAsECmaterials.ThereforetheTAgroupswereintroduced tothePIs/PAsbackbonetoimprovethesolubilityofaromaticpolymers.Thefirst TA-basedpolyamidePAwassynthesizedin1990[15],andthefirstaromaticpolyimidesintegratinginterestingECpropertiescontainingTAgroupsweredisclosed in2005[16].Sincethen,Liou,Hsiaoand,othergroupshavedevelopednumerous TA-basedECPIs/PAs.MostofthePIs/PAsweresolutionprocessibleandthermally stablewithexcellentadhesionwithindiumtinoxide-coatedglasselectrodeandhad goodelectrochemicalstability.NowtheTPA-basedPIs/PAsareconsideredasgreat anodicECmaterialsduetoproperoxidationpotentials,electrochemicalstability, andthin-filmformability.
1.3TheKeyParametersofElectrochromism 5
Benefitingfromthebloomandrevolutionoforganicpolymers,metallo-supermolecularpolymersweredevelopedbyincorporatingmetalcentersintosynthetic polymerchains,asthefifth-generationECmaterials.Thefirstmetal-containing polymer,poly(vinyl-ferrocene),wasreportedinasearlyas1955[17].However, duetotheinsolubilityofthosemacromoleculesandthelimitationofcharacteristic technologiesintheearlyyears,themetallo-supermolecularpolymershaven’tbeen rapidlydevelopeduntilthemid-1990s[18].Sincethen,metallo-supermolecular polymersbegantobewidelyexploredinECfieldwiththeadvantagesofbeneficial propertiesofbothorganicandinorganicmaterials.Especially,becausethetransitionmetalcomplexesoftenexhibitwell-definedredoxeventsandintensecharge transfertransitionsintheNIRregion,metallo-supermolecularpolymersoftenshow potentialsinNIRECapplication.
Morerecently,withtheactiveresearchesonthecrystallineandporousMOFs andCOFs,thesixth-generationMOFs/COFsECmaterialshaveemerged.In2013, thefirstECpropertiesofMOFsusingnaphthalenediimide(NDI)asorganiclinker werereportedbyProfessorM.Dinca’sgroup[19].AndthefirstCOFsECmaterial usingtheTPAasbuildingblockwasrevealedbyYuwuZhongandDongWang andcoworkersin2019[20].Allinall,someessentialfeaturesofMOFs/COFsgive themadvantagesinEC,includingdesignableandprecisemolecularstructure, simpleself-assemblysynthesis,andporousstructurethatfacilitatetheelectrolyte ionstransport.However,thesenewECmaterialshaven’tbeenfullyrevealed;many effortsshouldbetakentoimprovethedeviceperformanceofMOFs/COFs-based electrochromism.
1.3TheKeyParametersofElectrochromism
InordertoelucidatetheECpropertiesofECmaterials,insituUV–Vis–NIRspectroelectrochemistry(SEC)measurementswereperformedonaspectrophotometer, combiningwithanelectrochemicalworkstationtoapplyandcontrolthepotential intheSECcell.ThisSECspectrumdynamicallyrecordstheabsorptionchangeof ECmaterialsduringdifferentappliedvoltage,whichreflectsthecolorchangeduringthewholeredoxprocess.Asanexample,theSECofablack-to-transmissiveEC materialisshowninFigure1.4.Bothabsorbancemodelandtransmittancemodel couldbeusedtocarryouttheSECmeasurement.
1.3.1ElectrochromicContrast
ECcontrast(Δ%T )ismeasuredasthepercenttransmittancechangeoftheEC materialataspecificwavelength[22]itisaprimaryparameterforcharacterizing theECmaterials.Itiscalculatedfromthedifferenceoflighttransmission(TM)in thebleachedandcoloredstateTMb andTMc atthespecifiedwavelength.Andthe transmittancevaluesaregenerallyrecordedupontheapplicationofsquare-wave potentialstepstotheelectroactivefilmplacedinthebeamofaspectrophotometer. EachcolorhasacharacteristicwavelengthasshowninFigure1.5b,suchasthe


(b)
Figure1.4 Thespectroelectrochemistry(SEC)ofablack-to-transmissiveECmaterial.(a) Absorbancemodeland(b)transmittancemodel.Source:ReproducedbypermissionLietal. [21].©2018,RoyalSocietyofChemistry.
wavelengthofbluecolorisrangingfrom455to490nm.Therefore,inmostcases, thecontrastinthecharacteristicwavelengthischosentoevaluatethedegreesof colorchange.Usuallytheabsorptionofthischaracteristicwavelengthalsoreaches itsmaximumvalue(��max ).Moreover,thereisevidencethathumaneyesaremost sensitivetogreenlightwithawavelengthof555nm[25].It’salsorecommendedto calculatethecontrastin555nmforcomparisonindifferentpublications.Specifically,acontrasttestexampleisshowninFigure1.5a,theTMb andTMc of ��max 425nmare1%and99%,respectively;thereforethe Δ%T iscalculatedas98%.For applicationssuchassmartwindows,inwhichthedifferencebetweenthebleached andcoloredstatesisexpectedtobethehighest,the Δ%T shouldbehigherthan 80%.ManyinorganicECmaterials,organicsmallmolecules,andPEDOTseries polymers,whichhaveahightransmittanceinthebleachedstate,canachievethis index.Especially,somereportedsmallmoleculeECmaterialsevenshow Δ%T exceed95%[23].

Figure1.5 (a)TheelectrochromiccontrastofasmallmoleculeECmaterial.Source:Jiang etal.[23],(b)sensitivityfunctionofthehumaneye V (��)andluminousefficacyvs wavelength.Source:FredSchubert[24].©2006,CambridgeUniversityPress.(c)The changeofthelightnessvaluesfromtheneutraltotheoxidizedstates.Source:Lietal.[21]. ©2018,RoyalSocietyofChemistry.
Meanwhile,forsomebroadabsorptionorcolor-to-colorlessECmaterials, measurementsontherelativeluminancechange(Δ%Y )duringanECswitch aremorerealisticforexhibitingtheoverallECcontrast,whichconveysmore informationontheperceptionoftransmittancetothehumaneye.Asanexample, aluminancechangecurveduringtheredoxprocessofblack-to-transmissiveEC materialsisshowninFigure1.5c.Thelightness L* value(from0(black)to100 (white))of37.5(blackstate)increasesto60(bleachstate);thereforethe Δ%Y is calculatedas22.5%.
Exceptfortheaforementionedmethodforelectrochemicalcontrastmeasurements,aphotopicallyweightedvaluecalledphotopiccontrastwasproposedby JavierPadillaetal.[26].Thephotopiccontrastalsoreflectsanoverallcontrast duringthewholevisibleregion,whichismoreconsistentwiththerealapplication condition.Itcanbecalculatedusingthefollowingequation:
where T photopic isthephotopictransmittance, T (��)isthespectraltransmittanceof thedevice, S(��)isthenormalizedspectralemittanceofa6000Kblackbody,and P(��)
isthenormalizedspectralresponseoftheeye. ��min and ��max definetheconsidered rangeofwavelengths.
1.3.2SwitchingTime
Inthecontextofelectrochromism,theswitchingtime(t)canbedefinedasthetime neededforECmaterialstoswitchfromoneredoxstatetotheother.Itisgenerallyfollowedbyasquarewavepotentialstepmethodcoupledwithopticalspectroscopy.Switchingtimedependsonseveralparameters,suchastheabilityofthe electrolytetoconductionsaswellastheeaseofintercalationanddeintercalationof ionsacrosstheECactivelayer,theelectricalresistancesofelectrolytes,andthetransparentconductingfilms.Usuallytheliquidelectrolytehasalowerresistancethan thesolidelectrolyte;thereforethehalfdeviceandtheliquidelectrolyteECDwill exhibitarapidswitchingthansolidECD.Meanwhile,thelargeareaECDsuchasthe largesmartwindowswillshowalowerswitchingcomparedwiththesmalllaboratorysamplesduetothelargerelectricalresistancesoftransparentconductingfilms. However,fastswitchingisnotrequiredinallapplications,suchastheswitchable windowtechnologies;theobviouscolorchangeprocesswillincreasethefunofuser experience.Conversely,thesub-secondmagnituderapidswitchingisparticularly desiredfordisplayapplications.
Usuallytheswitchingtimesareevaluatedatthe ��max or555nmtogetherwith theECcontrast.Thereforetherearetwokindsofswitchingtime.Oneiselectrochemicalswitchingtime,asshowninFigure1.6a,whichisthetimerequiredfor thecurrentdensitytochangeby90%or95%betweentwoconstantvoltages.Meanwhiletheswitchingtimeofoxidization(toxidization )andreduction(treduction )process canbeestimatedfromthiscurve.Theotherisopticalswitchingtime(Figure1.6b), whichdefinesthetimeneededforthetransmittancetochangeby90%or95%.Correspondingly,thecolorationswitchingtime(tcoloration )andbleachingswitchingtime (tbleaching )arerecordedinthismeasurement.Itisworthnotingthatthepulselength ofpotentialstephasinfluenceintransmittance.Ashorterpotentialstepwillachieve asmallercontrast,andlongerpotentialwillallowECmaterialstoreachstationary transmittancevalueinbothcolorationandbleachingstate.Butafteracertainlength, continuingincreasethepulselengthwon’tboostthecontrast.Thereforethepulse lengththatjustreachedthehighestcontrastareappliedtoswitchingtimeaswellas contrasttests.
However,theaforementionedmethodofswitchingtimeisanexperientialmeasurement,whichhasadifferenceinvariedresearchgroups,suchasthedifferent percentageoftransmittancechange(90%or95%).Therefore,itisdifficulttocompare switchingtimedatabetweendifferentresearchgroups.Inrecentyears,JavierPadilla andcoworkersproposedastandardmethodforcalculatingECswitchingtimes.They fittedthecontrastvaluesasafunctionofpulselengthtothefollowingexponential increasefunction:
��TM (t)= ��T Mmax (1 e t∕�� )
where ΔT max representsthefull-switchcontrastobtainedforlongpulselengths and �� isthetimeconstant.Ifswitchingtime t isequalto �� ,63.2%ofthemaximum
Figure1.6 Theswitching timeofECmaterials.
(a)Electrochemical switchingtime.Source: Lietal.[21].©2018,Royal SocietyofChemistry(b) Opticalswitchingtime. Source:Hsiaoetal.[27].
1.3TheKeyParametersofElectrochromism 9


transmittancechangeisreached.Atatimeof2.3�� ,90% ΔTMmax isswitched, identically95%and99%of ΔTMmax correspondingto3�� and4.6�� . Therefore,fornewECmaterials,thesamechronoabsorptometricresponses[28] shouldbemeasuredandfittedtotheaforementionedfunction.Fromthefittings,the maxvaluesof ΔTMmax (thecontrastcorrespondingtoafullswitch),thetimeconstant �� ,andthecorrespondingregressioncoefficient r 2 willbeobtained.Afterwards theswitchingtime t90% or t95% willbeeasilycalculated.Thismethodallowsaneasy directcomparisonbetweendifferentreportedvalues.
1.3.3ColorationEfficiency
Colorationefficiency(CE)playsafundamentalroleintheevaluationoftheefficiencyofchargeutilizationduringtheECprocesses.Itrelatestheoptical absorbancechangeofanECmaterialatagivenwavelength(ΔA)tothedensity ofinjected/ejectedelectrochemicalchargenecessarytoinduceafullswitch(Qd ). ThehigherCEvalueindicatesalargetransmittancechangewithasmallamount ofcharge,whichmakesmoreeffectiveuseoftheinjectedcharge.CEvaluecanbe
Figure1.7 Thecalculationof Qd .Source:Hsiaoetal.[27].
calculatedusingthefollowingequation:
= ��A Qd = log ( Tox Tneut ) Qd
where T ox and T neut arethetransmittancesintheoxidizedandneutralstates,respectively,and Qd representstheinjected/ejectedchargeperunitarea,whichcouldbe obtainedfromtheintegralareaofthecurrentdensitycurveduringvoltageswitching (Figure1.7).
Verysimilartothepreviousparameterscontrastandswitchingtime,theCEvalues arealsodifferentdependingontheselectivewavelength,asshowninFigure1.8. SeveralkindsofCEvalueofthesameECmaterialsareexhibited:inmostreportedliterature,theCEofcharacteristicwavelengthreachingthemaximumcontrast(��max ) iscalculated,whichisthemaximumCEvalue(CEmax ).Also,theCEvalueat555nm

TMVIS, bleached = 77% CEmax, at 767 nm = 188 cm2/C
cm2/C TMVIS, colored = 8%
at 550 nm = 66 cm2/C
=
Figure1.8 DifferenttypesofCEvalueofthesameECmaterials.Source:Kraft[25].
1.3TheKeyParametersofElectrochromism 11 iscalculatedforcomparisonindifferentpublishesbecauseofthehighestsensitivity ofhumaneyesat555nmaswellasthephotopicCEbasedonthephotopictransmittancementionedinthecontrastpart,whichconsideredthelighttransmission overthewavelengthrangebetween380and780nmnormalizedwiththespectral sensitivityofthehumaneye.Therefore,moreplotofCEvaluesshouldbeobtained ratherthansingle-valuedCEvalues,togivemoreinformationabouttheperformanceofECmaterials.
Inaddition,wheninsightfullyconsideringtheinjected/ejectedcharge Qd ,we canfinditinfacttoconsistofthreepart:faradaiccharge QF associatedwith doping/de-doping,capacitivecharge QC duetothecapacitivenatureoftheECD, andparasiticcharge QP associatedwithelectrolyte/impurityreactions.Among them,thefaradaicchargeisthesourceofredoxactivityleadingtochromicchange actually.Therefore,Fabrettoetal.reportedanewtechniqueformeasuringCEby extractingthefaradaicchargefromthetotalchargeandcalculatedtheonlyfaradaic charge-basedCEvalue[29].Aswediscussed,thetotalchargeflowissimplythe additionofthethreeindividualchargeflowsandisgivenby
Qd = QF + QC + QP
wheretheparasiticcurrentwasasmallcomponent(approximately <2%)compared withtheothertwoandthereforecanbeignored.Thenthetime–evolutiontotal currentflowcanbedescribedasfollowing:
where n isthenumberofelectronstransferredpermolecule, F istheFaradayconstant(96500C/mol), A istheelectrodearea(cm2 ), C0 istheconcentrationofspecies inthebulksolution(mol/cm3 ), D istheapparentdiffusioncoefficient(cm2 /s), t is timeinseconds, I 0 isthemaximumcurrentflowat t = 0, R isthecellresistance,and C isthedoublelayercapacitance.Thenfittingtheexperimentaldatatothisequation andsubstitutingtheconstant k,atlast,aplotofthetime–evolutionfaradaiccurrent willbeobtained,andthecorrespondingfaradic-correctedCEscanbecalculated. Usually,thefaradic-correctedCEsarelargerthantheuncorrectedresults,because thetotalchargeingress/egress(i.e. Qd )islargerthanthefaradiccharge(i.e.QF).
1.3.4OpticalMemory
TheopticalorECmemory(alsocalledopen-circuitmemory)ofanECmaterial canbedefinedasthepropensityofthematerialtoretainitsredox/coloredstate uponremovingtheexternalbias.Usually,thememoryeffectareoftenobserved infilm-stateECmaterialssuchasconjugatedpolymers,whichwelladheredonto theelectrode,andhencerestrictthemovementoftheelectrons.Incontrast,some solution-basedECDs(e.g.viologens)willexhibitaself-erasingeffect,whichmeans thecoloredstatedisappearedrapidlyintheabsenceofappliedvoltagebecausethe electronsdiffusefreelyinthistypeofdevice.Thememoryeffectisusefulforthe energy-savingdevicesandalsocanbeappliedfordatastorage.Figure1.9shows


Figure1.9 Open-circuitmemory testsofPBOTT-BTDspraycoated onanITO-coatedglassslidein 0.1MTBAPF6/ACNat423nm: (a)short-and(b)long-term performance.Source:Lietal.[21].
anopticalmemorytest.Theshort-termmemorywasinvestigatedbyapplyinga potentialpulsefor2secondspriortoformingtheopen-circuitstatefor100seconds; thetransmittancechangeat423nmwasmonitoredsimultaneously(Figure1.9a). Thenalong-termmemoryisalsostudiedbyapplyingapotentialfortwoseconds andremovingthebiasforonehour(Figure1.9b).TheECconjugatedpolymers remainintheinitialtransmittancecontrastwellintheabsenceofanapplied voltage,whichexhibitsagoodopticalmemory.
1.3.5Stability
Inmostcasesoflaboratorystudy,researchersrecordthenumberofredoxcycles thatanECmaterialstandwithoutsignificantlossintheperformanceasthe electrochemicalstability,irreversibleoxidationorreductionatextremepotentials,sidereactionswithwateroroxygen,andheatreleaseinthesystemduring switchesmaycausethedegradationofelectrochemicalstability.Usually,the chargedensity Qd recordedunderelectrochemicalcyclingisupto104 –106 ,as showninFigure1.10a.ThechargedensityofaTi-dopedV2 O5 ECfilmhaven’t changedthrough2 × 106 cycles;meanwhilethetransmittancechangeatacertain wavelengthduringcontinuouscyclingisalsoimportanttodescribethestability
Figure1.10 Chargedensity (a)andtransmittance (b)variationcurvesofECD withthecyclenumber K :1000.Source:Wei etal.[30].

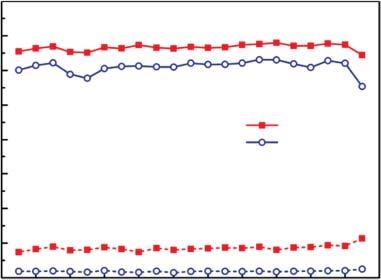
Bleached solid line Colored dot line
ofanECmaterial.SuchasshowninFigure1.10b,thetransmittanceoftheECD remainsstablethrough200000cycles.Actually,theCEchangeafternumerous cyclesalsocanbeusedtoevaluatethelong-termstabilityofECmaterials,because itcontainsinformationofbothtransmittanceandchargedensity.
However,ifweconsidertherealapplicationofECDinbuildingwindows,there aremorestrictconditionsfordurabilityandreliability.Forinstance,alifetime over20yearswithmorethan106 switchingcyclesisnecessary.Extremeweather conditionssuchastemperaturesbelow 20 ∘ Candabove +40 ∘ Carehugechallenge forbothECmaterialsandelectrolytes,aswellasotherdegradationfactorssuch ashighsolarirradiationlevels,fasttemperaturechanges,uneventemperature distributionandadditionalstresses,rain,humidity,mechanicalshock,anddrying. Therefore,in1998,CarlM.Lampertproposedastandardtestguidelineforindustry applicationofEC[31],asshowninFigure1.11.Recently,theInternationalOrganizationforStandardization(ISO)alsohaslaunchedaninternationalstandard:Glass inbuilding–Electrochromicglazings–acceleratedagingtestandrequirements (ISO18543)forECuseinbuildings.
EC color/bleach cycling
UVa stability
Heat storage
Low temperature storage
Humidity/temperature storage
Thermal cycling
Thermal shock
1. 25 000 cycles at 25 °C
2. 10 000 cycles at – 20 °C
3. 25 000–100 000b cycles with UVa at 65 °C
Prolonged cycles (12 h colored, 12 h bleach) for a total exposure of 6000 MJ/m2, UV intensity integrated between 300 and 400 nm
500 h at 90 °C
1000 h at 20 °C to – 30 °C
1000 h at 70 °C, 90% humidity
85 °C for 4 h, followed by – 40° C for 4 h and followed by 37 °C for 16 h at 100% R.H. (4 h of ramp between each condition) (repeat four times)
One hour colored and one hour bleached. Test in a UV chamber, with UVa lamp on during coloration. Spray with 25 °C water in bleached state (24 cycles)
aBlack panel temperature during the bright periods of 60 °C, and 25 °C in the dark periods.
bAsahi has used up to 100 000 cycles.
Figure1.11 RecommendedtestingguidelinesforECwindowsforexteriorarchitectural applications.Source:Lampertetal.[31].
1.4Conclusion
Inthischapter,abroadoverviewofelectrochromism,ECmaterials,devicestructure,developmenthistory,andkeyparametersofelectrochromismhavebeen introducedbriefly.Moredetaileddescriptionsofeachareawillbediscussedin Chapters2–15.Insummary,researchinECtechnologieshasachievedsignificant breakthroughsoverthedecades.ManygenerationsofECmaterialshavebeen developed,rangingfromtraditionalmetaloxidestomorerecentorganicpolymers, smallmolecules,andhybridmaterials.Moreover,benefitfromtheECDdesignand structuraloptimization,flexiblesubstrate-baseddeviceswerefabricatedwiththe low-priceroll-to-rollprocess,whichmakestheECtechnologyhavelargescope applications,suchassmartwindowsforreducingbuildingenergyconsumption, self-poweredECwindowusingorganicphotovoltaiccellsaspowersupplement,car rear-viewmirrorsforgreatersafety,andsmartsunglassesforbetterUV-radiation protection.Manyofthesetechnologiesandapplicationshavebeencommercialized andareavailableonthemarket.Withtheconcertedeffortsofresearchersand engineers,webelievethatthenewECmaterialsandadvancedtechnologieswill constantlydevelopandmoreadvancedECDwithlowmanufacturingcostwillbe exploitedtorealizepracticalapplications.
References
1 Fletcher,S.(2015).Thedefinitionofelectrochromism. JournalofSolidStateElectrochemistry 19(11):3305–3308.
2 Camurlu,P.(2014).Polypyrrolederivativesforelectrochromicapplications. RSCAdvances 4(99):55832–55845.
3 Wang,Z.,Wang,X.,Cong,S.etal.(2020).Fusingelectrochromictechnology withotheradvancedtechnologies:anewroadmapforfuturedevelopment. MaterialsScience&EngineeringR:Reports 140.
4 Wu,W.,Wang,M.,Ma,J.etal.(2018).Electrochromicmetaloxides:recent progressandprospect. AdvancedElectronicMaterials 4(8).
5 Mortimer,R.J.(2011).Electrochromicmaterials. AnnualReviewofMaterials Research 41(1):241–268.
6 Platt,J.R.(1961).Electrochromism,apossiblechangeofcolorproducibleindyes byanelectricfield. TheJournalofChemicalPhysics 34(3):862–863.
7 Hutchison,M.R.(1913).Electrographicdisplayapparatusandmethod.USPatent 1,068,774,filed29July1913.
8 LehovecK.(1957).Photonmodulationinsemiconductors.USPatent2,776,367, filed1January1957.
9 Neff,V.D.(1978).Electrochemicaloxidationandreductionofthin-filmsof prussianblue. JournaloftheElectrochemicalSociety 125(6):886–887.
10 Michaelis,L.andHill,E.S.(1933).Theviologenindicators. TheJournalof GeneralPhysiology 16(6):859–873.
11 Schoot,C.J.,Ponjee,J.J.,vanDam,H.T.etal.(1973).Newelectrochromic memorydisplay. AppliedPhysicsLetters 23(2):64–65.
12 Garnier,F.,Tourillon,G.,Garzard,M.,andDubois,J.C.(1983).Organicconductingpolymersderivedfromsubstitutedthiophenesaselectrochromicmaterial. JournalofElectroanalyticalChemistry 148:299–303.
13 Mengoli,G.,Musiani,M.M.,Schreck,B.,andZecchin,S.(1988).Electrochemical synthesisandpropertiesofpolycarbazolefilmsinproticacidmedia. Journalof ElectroanalyticalChemistryandInterfacialElectrochemistry 246(1):73–86.
14 Zheng,H.B.,Lu,W.,andWang,Z.Y.(2001).Electrochemicalandelectrochromic propertiesofpoly(ethernaphthalimide)sandrelatedmodelcompounds. Polymer 42(8):3745–3750.
15 Oishi,Y.,Takado,H.,Yoneyama,M.etal.(1990).Preparationandproperties ofnewaromaticpolyamidesfrom4,4′ -diaminotriphenylamineandaromatic dicarboxylicacids. JournalofPolymerSciencePartA:PolymerChemistry 28(7): 1763–1769.
16 Cheng,S.-H.,Hsiao,S.-H.,Su,T.-H.,andLiou,G.-S.(2005).Novelaromatic poly(amine-imide)sbearingapendenttriphenylaminegroup:synthesis,thermal, photophysical,electrochemical,andelectrochromiccharacteristics. Macromolecules 38(2):307–316.
17 Arimoto,F.S.andHaven,A.C.(1955).Derivativesofdicyclopentadienyliron. JournaloftheAmericanChemicalSociety 77(23):6295–6297.
18 Whittell,G.R.andManners,I.(2007).Metallopolymers:newmultifunctional materials. AdvancedMaterials 19(21):3439–3468.
19 Wade,C.R.,Li,M.,andDinc ˘ a,M.(2013).Faciledepositionofmulticolored electrochromicmetal–organicframeworkthinfilms. AngewandteChemieInternationalEdition 52(50):13377–13381.
20 Hao,Q.,Li,Z.-J.,Lu,C.etal.(2019).Orientedtwo-dimensionalcovalentorganic frameworkfilmsfornear-infraredelectrochromicapplication. Journalofthe AmericanChemicalSociety 141(50):19831–19838.
21 Li,W.,Ning,J.,Yin,Y.etal.(2018).Thieno[3,2-b]thiophene-basedconjugated copolymersforsolution-processableneutralblackelectrochromism. Polymer Chemistry 9(47):5608–5616.
22 Beaujuge,P.M.andReynolds,J.R.(2010).Colorcontrolin π-conjugatedorganic polymersforuseinelectrochromicdevices. ChemicalReviews 110(1):268–320.
23 Jiang,M.,Sun,Y.,Ning,J.etal.(2020).Diphenylsulfonebasedmulticolored cathodicallycoloringelectrochromicmaterialswithhighcontrast. Organic Electronics 83:105741.
24 FredSchubert,E.,Chapter16. HumanEyeSensitivityandPhotometricQuantities inLight-EmittingDiodes,2e.CambridgeUniversityPress.
25 Kraft,A.(2018).Electrochromism:afascinatingbranchofelectrochemistry. ChemTexts 5(1):1–18.
26 Padilla,J.,Seshadri,V.,Filloramo,J.etal.(2007).Highcontrastsolid-stateelectrochromicdevicesfromsubstituted3,4-propylenedioxythiophenesusingthedual conjugatedpolymerapproach. SyntheticMetals 157(6–7):261–268.
27 Hsiao,S.-H.andLin,S.-W.(2016).Electrochemicalsynthesisofelectrochromic polycarbazolefilmsfrom N -phenyl-3,6-bis(N -carbazolyl)carbazoles. Polymer Chemistry 7(1):198–211.
28 Hassab,S.,Shen,D.E.,Österholm,A.M.etal.(2018).Anewstandardmethodto calculateelectrochromicswitchingtime. SolarEnergyMaterialsandSolarCells 185:54–60.https://doi.org/10.1016/j.solmat.2018.04.031.
29 Fabretto,M.,Vaithianathan,T.,Hall,C.etal.(2008).Faradaicchargecorrected colourationefficiencymeasurementsforelectrochromicdevices. Electrochimica Acta 53(5):2250–2257.
30 Wei,Y.,Zhou,J.,Zheng,J.,andXu,C.(2015).ImprovedstabilityofelectrochromicdevicesusingTi-dopedV2 O5 film. ElectrochimicaActa 166:277–284.
31 Lampert,C.M.,Agrawal,A.,Baertlien,C.,andNagai,J.(1999).Durabilityevaluationofelectrochromicdevices–anindustryperspective. SolarEnergyMaterials andSolarCells 56(3):449–463.
