BiomassValorization
SustainableMethodsfortheProductionofChemicals
Editedby DavideRavelli ChiaraSamorì
Editors
Prof.DavideRavelli
UniversityofPavia DepartmentofChemistry vialeTaramelli12 27100Pavia
Italy
Prof.ChiaraSamorì UniversityofBologna DepartmentofChemistry “GiacomoCiamician” viaS.Alberto163 48123Ravenna Italy
Cover ©TBstudio/Shutterstock
Allbookspublishedby WILEY-VCH arecarefullyproduced.Nevertheless, authors,editors,andpublisherdonot warranttheinformationcontainedin thesebooks,includingthisbook,to befreeoferrors.Readersareadvised tokeepinmindthatstatements,data, illustrations,proceduraldetailsorother itemsmayinadvertentlybeinaccurate. LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek
TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>
©2021WILEY-VCHGmbH,Boschstr. 12,69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc. usedinthisbook,evenwhennot specificallymarkedassuch,arenotto beconsideredunprotectedbylaw.
PrintISBN: 978-3-527-34717-9
ePDFISBN: 978-3-527-82501-1
ePubISBN: 978-3-527-82503-5
oBookISBN: 978-3-527-82502-8
Typesetting SPiGlobal,Chennai,India
Printedonacid-freepaper 10987654321
Contents
Foreword xi
Preface xiii
1RoleofBiomassintheProductionofChemicals 1 LaylaFiliciotto,EvanPfabandRafaelLuque
1.1Introduction 1
1.2BiomassValorization 3
1.3LignocellulosicBiomass 5
1.4KeyBiomolecules 6
1.5Solvents 10
1.6PretreatmentofLignocelluloses 12
1.7ConclusionsandPerspectives 15 References 15
SectionICatalyticStrategies 23
2BiomassProcessingviaAcidCatalysis 25 IuriiBodachivskyi,UnnikrishnanKuzhiumparambilandD.BradleyG. Williams
2.1Introduction 25
2.1.1IsanAcidtheBestCatalyst? 26
2.2Acid-CatalyzedProcessingofCellulosicPolysaccharides 29
2.3Acid-CatalyzedProcessingofLignin 44
2.4ConclusionsandPerspectives 47 References 47
3BiomassProcessingviaBaseCatalysis 57 LichenLiu,MariaJ.ClimentandSaraIborra
3.1Introduction 57
3.2AldolCondensation 60
3.2.1AldolCondensationofFuranicAldehydes 60
3.2.2Self-AldolCondensationofAcetone 63
3.2.3AldolCondensationBetweenAlcohols:GuerbetCouplingReaction 64
3.3KetonizationReactionofCarboxylicAcids 65
3.4TransesterificationReaction 68
3.4.1BiodieselProduction 68
3.4.2HighValue-AddedChemicalsfromTransesterificationReactions 70
3.5ConclusionsandPerspectives 73 References 74
4BiomassProcessingviaMetalCatalysis 81 SofiaCapelliandAlbertoVilla
4.1Introduction 81
4.2SyntheticStrategiesforSupportedMetalNanoparticles 83
4.2.1Impregnation 83
4.2.2Precipitation 84
4.2.3SolImmobilization 85
4.3Furfural 86
4.3.1FurfuralHydrogenation 87
4.3.1.1FurfuraltoFurfurylAlcohol 87
4.3.1.2FurfuraltoTetrahydrofurfurylAlcohol 88
4.3.1.3FurfuraltoPentanediols 89
4.3.1.4Furfuralto2-Methylfuran 90
4.3.2FurfuralOxidation 92
4.3.2.1FurfuraltoFuroates 92
4.45-Hydroxymethylfurfural(HMF) 92
4.4.1HMFHydrogenation 93
4.4.1.1HMFto2,5-Dimethylfuran(DMF) 94
4.4.1.2HMFto2,5-Dihydroxymethyltetrahydrofuran(DHMTHF) 95
4.4.2HMFOxidation 96
4.4.2.1HMFto2,5-FurandicarboxylicAcid(FDCA)UsingMonometallic Systems 96
4.4.2.2HMFOxidationoverBimetallicCatalysts 100
4.5ConclusionsandPerspectives 103 References 103
5BiomassProcessingwithBiocatalysis 113
RogerA.Sheldon
5.1Introduction 113
5.2GenerationsofRenewableBiomass:AdvantagesandLimitations 113
5.3AdvantagesandLimitationsofBiocatalysis 116
5.4EnzymeDiscoveryandOptimizationofEnzymePerformance 117
5.5EnzymeImmobilization 118
5.5.1EnzymeImmobilizationbyCross-linkingEnzymeMolecules 119
5.5.2AdvantagesandLimitationsofCross-LinkedEnzymeAggregates (CLEAs) 120
5.5.3MagneticallySeparableImmobilizedEnzymes 120
5.6EnzymaticHydrolysisofStarchtoGlucose 121
5.7EnzymaticDepolymerizationofLignocellulose 122
5.8EnzymaticHydrolysisofCelluloseandHemicellulose 123
5.8.1MagnetizableImmobilizedEnzymesinLignocelluloseConversion 124
5.9EnzymaticHydrolysisof3rdGeneration(3G)Polysaccharides 124
5.10CommodityChemicalsfromCarbohydrates(Monosaccharides) 126
5.10.1FermentativeProductionofCommodityChemicals 126
5.10.2DeoxygenationviaDehydrationofCarbohydratestoFuran Derivatives 129
5.10.3PolyethyleneFurandicarboxylate(PEF)asaRenewableAlternativeto PET 129
5.10.4EnzymaticSynthesisofBio-basedPolyesters 131
5.11EnzymaticConversionsofTriglycerides:ProductionofBiodieseland BulkChemicals 132
5.12ConclusionsandPerspectives 133 References 133
SectionIIThermalStrategies 147
6BiomassProcessingviaPyrolysis 149
DanieleFabbri,YunchaoLiandShurongWang
6.1BriefIntroduction 149
6.2ChemicalsfromCellulosePyrolysis 151
6.2.1GeneralAspects 151
6.2.2Levoglucosan 154
6.2.3Levoglucosenone 156
6.2.4LAC, (1R,5S)-1-Hydroxy-3,6-Dioxabicydioxabicyclo-[3.2.1]octan-2-one 157
6.3ChemicalsfromLigninPyrolysis 160
6.4PyrolysisofBiomass 161
6.4.1Levoglucosan 161
6.4.1.1EffectsofMetalOxides 162
6.4.1.2EffectsofAlkaliandAlkalineEarthMetals 162
6.4.1.3EffectsofAcidImpregnation 162
6.4.1.4EffectsofOtherComponents 163
6.4.2Levoglucosenone 163
6.4.2.1EffectsofMetalChlorides 163
6.4.2.2EffectsofAcidCatalysts 163
6.4.2.3Others 164
6.4.3Furfural 164
6.4.4AromaticHydrocarbons 167
6.4.5PhenolicCompounds 169
6.5ConclusionsandPerspectives 170 References 171
7BiomassProcessingviaThermochemical–BiologicalHybrid Processes 181 CristianTorri,AlessandroGirolamoRombolà,AlisarKiwanandDaniele Fabbri
7.1Introduction 181
7.1.1HybridThermochemical/BiologicalProcessingwithSingle-Strain Microorganisms 183
7.1.2HybridThermochemical/BiologicalProcessingwithMicrobialMixed Consortia(MMC) 183
7.2PyrolysisProducts(PyP)fromtheMicroorganism’sStandpoint 185
7.2.1WhatPyrolysisCanDoforMicroorganisms:YieldsandBioavailabilityof PyP 186
7.2.2ViablePathwaysAccordingtoThermodynamicsLaws 188
7.2.3RateofMMCBiologicalConversionsinRelationshipwithPyP Treatment 191
7.2.4ToxicityofPyPTowardMMC 193
7.3ConversionofPyPwithMMC:SurveyofExperimentalEvidence 198
7.3.1SyngasConversiontoMethane 203
7.3.2SyngasConversiontoH2 ,VolatileFattyAcids(VFA),andAlcohols 203
7.3.3ConversionofCondensablePyPtoMethane 205
7.3.4ConversionofCondensablePyPtoVFAandOtherIntermediates 206
7.4FeasiblePathwaysforProducingChemicalsfromPyPwithMMC 207
7.4.1HybridPyrolysisFermentationandExtractionofMixed VFA/Alcohols 207
7.4.2AlkalineFermentationofPyrolysisProductstoVFASalts,Ketonization, andHydrogenationtoC3–C6MixedAlcohols 209
7.4.3AlkalineFermentationofPyrolysisProductstoVFASaltsand Polyhydroxyalkanoates(PHA)ProductionviaAerobicMMC 211
7.4.4DirectAlcoholProductionbyMeansofFermentationofPyPunderHigh HydrogenPressure 213
7.5ConclusionsandPerspectives 215 References 216
SectionIIIAdvanced/UnconventionalStrategies 225
8BiomassProcessingviaElectrochemicalMeans 227 RomanLatsuzbaia,RoelJohannesMartinusBisselink,MarcCrockatt,Jan CornelisvanderWaalandEarlLawrenceVincentGoetheer
8.1Introduction 227
8.2ElectrochemicalConversionofBio-BasedMolecules 228
8.3ConversionofSugars 230
8.4ConversionofFuranics 234
8.4.15-(Hydroxymethyl)furfural(5-HMF) 234
8.4.1.15-HMFOxidation 235
8.4.1.25-HMFReduction 238
8.4.2Furfural 240
8.5ConversionofLevulinicAcid 244
8.6ConversionofGlycerol 246
8.7LigninDepolymerization 248
8.8Scale-upofElectrosynthesisofBiomass-DerivedChemicals 248
8.9ConclusionsandPerspectives 254 References 254
9BiomassProcessingviaPhotochemicalMeans 265 AndreyShatskiyandMarkusD.Kärkäs
9.1Introduction 265
9.2FundamentalAspectsofPhotoredoxCatalysis 266
9.3PhotochemicalValorizationofLignin 267
9.3.1StrategiesforCα —Cβ BondCleavage 268
9.3.2StrategiesforLigninOxidationandCβ —OBondCleavage 272
9.3.3StrategiesforAr—OBondCleavage 278
9.4ConclusionsandPerspectives 281 References 282
10BiomassProcessingviaMicrowaveTreatment 289 RobertoRosa,GiancarloCravottoandCristinaLeonelli
10.1Introduction 289
10.2Microwave–MatterInteraction:AdvantagesandLimitationsinthe ProcessingofBiomass 291
10.3MicrowavePyrolysis 296
10.4Microwave-assistedHydrolysis 299
10.5Microwave-assistedExtractionofPhytochemicalCompounds 303
10.6ConclusionsandPerspectives 306 References 307
11BiomassProcessingAssistedbyUltrasound 315 CezarA.Bizzi,DanielSantos,GabrielleD.IopandEricoM.M.Flores
11.1Introduction 315
11.2UltrasoundBackground 316
11.3Ultrasound-AssistedBiomassPretreatments 319
11.4Ultrasound-AssistedBiomassConversion 322
11.4.1ThermochemicalConversionAssistedbyUltrasound 323
11.4.2BiochemicalConversionAssistedbyUltrasound 324
11.4.3ChemicalConversion(Synthesis)AssistedbyUltrasound 325
11.5Ultrasound-AssistedExtractionofValue-AddedCompounds 326
11.5.1UltrasoundContributiontoBiomassExtractionProcesses 326
11.5.2UsesofAlternativeApproachesforBiomassExtractionsAssistedby Ultrasound 328
11.6AlternativeSolvents 331
x Contents
11.7ConclusionsandPerspectives 332 References 333
12BiomassProcessingviaMechanochemicalMeans 343 GeorgeMargoutidisandFrancescaM.Kerton
12.1OverviewandIntroduction 343
12.1.1BackgroundtotheMethod 343
12.1.2PropertiesofaTypicalLaboratoryMixer/Mill 346
12.2CrystallinityReductioninBiopolymersviaMechanochemistry 348
12.3MechanochemicalTransformationsofPolysaccharides 352
12.3.1CelluloseDepolymerization 352
12.3.2CelluloseModificationTowardCompositeMaterials 355
12.3.3TransformationsofChitin 355
12.4MechanochemicalTransformationsofAminoAcids,Nucleotides,and RelatedMaterials 357
12.5MechanochemicalTreatmentofLignin 359
12.6BiomineralsfromMechanochemicalProcessingofBiomass 360
12.7ConclusionsandPerspectives 361 References 361
SectionIVClosingRemarks 367
13IndustrialPerspectivesofBiomassProcessing 369 TommasoTabanelliandFabrizioCavani
13.1ReplacingExistingPetrochemicalswithAlternativesfromBiomass:An Introduction 369
13.2OleochemicalBiorefinery:AConsolidatedandMultifacetedExampleof BiomassProcessing 371
13.2.1BiofuelsandCoproducedChemicalsfromOilsandFats 371
13.2.2SkeletalIsomerizationofUnsaturatedFattyAcidsforIsostearicAcid Production 379
13.2.3Bio-basedSynthesisofAzelaicandPelargonicAcids:ARenewableRoute TowardBio-basedPolyestersandCosmetics 382
13.3FromSugartoBio-monomers:TheCaseof2,5-FurandicarboxylicAcid (FDCA) 385
13.4FromBioethanoltoRubber:TheSynthesisofBio-butadiene 388
13.5ConclusionsandPerspectives 391 References 391
Index 411
Foreword
Whybotherwithbiomassconversion?Itseemssodifficultcomparedtoconverting hydrocarbonsintoproducts.Peopleusedtothinkthatwewereabouttorunoutof fossilfuels,butthatwasaredherring–thereisenoughleftinthegroundtoserve usforhundredsofyears.So,ifrunningoutofhydrocarbonsisnottheproblem,then whyarewetryingtoconvertbiomass?Theproblemisinthe consequences ofhydrocarbonusage,notinthedepletionofhydrocarbons.Thoseconsequencesaresevere. Fossilfuelresourcesareheavilycontaminatedwithsulfur,mercury,andotherpollutants.Manyproductsfromsuchfeedstocksarepersistent,leadingtoproblemssuch asplasticsintheoceans.However,mostimportantly,burningfossil-basedfuelsor incineratingfossil-basedproductsgeneratescarbondioxidewhiletheproductionof thefeedstocksconsumesnoCO2 .
Now,imagineasustainablefuture–afutureinwhichallofourneedsaremetwith productsmadefromrenewableresources,andthosesameproductsarethemselves recycledintonewproducts.Thefeedstocksarenontoxic,theproductsarebiodegradable,andgreenhousegasemissionsarecompletelyoffsetbyCO2 consumption. Everyonewouldagreewiththatasadesirablegoal,butwearealongwayfromthat future.Thereissomuchworktodobeforewegetthere.Weasasocietyaremarried toourpetrochemicalpastinsomanyways,fromtheproductswechoosetomake, thewaysinwhichwemakethoseproducts,andevenwhatweteachourfuture chemistsandchemicalengineersattheuniversities.Weneedtodivorceourselves fromourpetrochemicalpastinordertobringaboutthatsustainablefuture.
Makenomistakeaboutit.Thatdivorceisgoingtobemessy,butitisstillworth doing.Atfirst,wethoughtthatitwouldbeeasy.Justmakeexistingproductsfrom biomassinsteadoffossilfuels.Itsoundssosimple,butstudyafterstudyhasshown thattheenvironmentalimpactoftransformingbiomassintotraditionalchemical productsisusually moreharmful thanmakingthesameproductsfromfossilfuels. So,whatarewegoingtodo?Wehavetobesmarteraboutit.Thosetraditional chemicalproductswerechoseninthepastbecausetheywereeasytomakefrom hydrocarbons.Whycannotwechoosenewchemicalproducts?Letuschoose chemicalproductsthatareeasytomakefrom biomass!
ChoosingnewproductsisnottheonlytaskonourToDolist.Thereisalonglist oftasksaheadofus.Wehaveto
● increaseourknowledgeofbiomassconversions.
● evaluatenewconversionprocessesandproductsintermsofpotentialenvironmentalimpact,notjustasanafterthoughtbutduringthedesignstage,
● useonlyrenewableenergyinthebiomassconversionprocesses,
● developlessenergeticallycostlywaysofremovingorganicproductsfromwater, giventhatwaterisoftenthesolventforfermentationsandotherstandardtechniquesforbiomasstransformations,
● buildupsupplychainsfromproducersofrawbiomass,throughtransportationand conversiontoplatformchemicals,allthewaytomanufacturefinishedproducts,
● seeknewsustainablefeedstocksandnewplatformchemicals,keepinginmind theirpotentialavailabilityatindustrialscaleandtheenvironmentalimpactof usingsuchfeedstocksatscale,
● designournewproductssothattheycanberecycledefficiently,canbiodegradeif discarded,anddonotthemselvesleadtoenvironmentalcrises,
● changehoworganicchemistryistaughtattheuniversitiessothatbiomassfeedstocksbecomethenorm.
Thatsoundslikealotofwork,butdonotdespair.Therearesomanytalentedpeopleworkingonbiomassconversion,suchastheauthorsandeditorsofthisvolume, thateachoftheseitemsonour“ToDo”listcanbeachieved.Thesheervarietyof approachesdescribedinthefollowingchaptersassuresmethatthereisgreathope forthefutureofbiomassconversion.Manyofyou,thereaders,arealsodevelopingnewtechnologiesforsustainablechemicalmanufacturing. Wewillattainthat sustainablefuture,andthisbookdemonstratesthatwearemakingprogresstoward thatgoal.
MycommendationstoChiaraSamorìandDavideRavelliforputtingthiswork together,totheauthorsfortheirmanycontributionstothebookandthefield,and indeedtoeveryoneinthegreenandsustainablechemistryresearchcommunityfor theireffortsindevelopingthechemistrythatwillmakesustainablelivingareality.
Preface
Itisnowadaysapparentthatthechemistryofthefuturewillinvolvetheexploitationofbiomass-basedrenewablematerials,thecurrentlyavailablestockoffossil resourcesbeingdoomedtoexhaustion.Thistransitionmayindeedbringaboutseveralbenefitsbecausehavingrecoursetorenewableresourcesshouldlimittheimpact humanactivitiesarehavingonclimatechange.
Currently,theuseofbiomassbymankindislimitedtoaddressingafewspecific needs,notablyfulfillingthefeeddemandandsupplementingenergyproductionin additiontothefossilfuelportfolio.Theimpactoftheseactivitiesonthenetprimary production(NPP)ofterrestrialandmarinebiomasscanbeaccountedforbyconsideringthehumanappropriationofnetprimaryproduction(HANPP)parameter, whichcorrespondstoallthehumanalterationsofphotosyntheticproductioninthe ecosystems.ThisconstantHANPPrepresentsasignificantfractionoftheNPPand hasahugeimpactonecosystemsbecauseitreducestheamountofenergyavailable tootherspecies,influencesbiodiversity,andalterswater,energy,andcarbonflows withinfoodwebs,alsomodifyingthedistributionofresources.Intheprospected futurescenarioofamassiveuseinindustry,biomasswillatsomepointbecome ascarceresource,anditsutilizationshouldbeconsideredwisely,accordingly.In particular,theentiresubstitutionoffossilfuelswithbiomassforenergyproductionpurposesisunrealisticbecauseofthehugeamountofbiomassthatshouldbe devotedatthisend.Furthermore,onemayargueifthiskindofapplicationisthe bestusepossibleofbiomass,fullyexploitingitscharacteristicsintermsofchemical composition.
Throughhistory,avarietyofbiomassconstituentshavebeenemployedinthe preparationofvaluabledrugs,flavors,andfragrances,ortoprovide,especiallyin thesecondhalfofthenineteenthcentury,commoditymaterialssuchascellulose esters(nitrateandacetate)andoxidizedlinseedoil(linoleum).Indeed,thereexist differentoptionsofusingbiomasstoproducechemicals.Nowadays,letapartthe useofwoodinthepaperindustry,bio-basedsurfactants,lubricants,coatings/dyes, additivesforplasticsandsolvents(mostlybasedonvegetableoils/animalfats, sugar,orstarch)arethemostimportantapplicationsofbiomassinchemistry.As forfutureapplications,thequestionisstillopen;however,itcanbeanticipated thattheuseofbiomassforchemicalsproductionisamuchmoresustainable optionthanhavingrecoursetoitforenergeticpurposes.Furthermore,inaddition
tomerelyduplicatingexistingproductsderivingfromfossilresources,thechemistry ofbiomassopenstheopportunitytodevelopanewportfolioofproducts,having noequivalenceamongthosepresentlymanufacturedbyclassicalsyntheticroutes fromhydrocarbons.Asubsidiaryadvantageisthatthedevelopmentofbioproducts requiresfewerlegislativeconstraints.
Accordingly,thereisanincreasinginterestindevelopingsuitabletechniquesto tacklethevalorizationofbiomasstoproducechemicalsandthisareaisexpectedto furtherexpandinthefuture.Alongthesameline,bio-basedwastematerials,tobe includedinacirculareconomyperspective,canlikewisecontributesignificantly. Independentlyfromtheactualbiomassemployed,thisisachallengingareabecause inhomogeneousmaterialswithvariablecompositionmustbeprocessedwith tailoredtechnologies.Thebook“BiomassValorization:SustainableMethodsfor theProductionofChemicals”isintendedtopresentthestateoftheartofthe differentstrategiesavailablenowadaystoconvertbiomassintousefulbuilding blocks/commoditychemicals.
Eachchapterfeaturesanintroductorysection,detailingthecoredetailsofthe describedtechnologyandshowcasingthetypicalchemicalpathwaysthatcanbe activatedbyhavingrecoursetoit.Next,peculiaradvantagesandlimitationsof thedescribedstrategyintheprocessingofbiomassaredescribed.Finally,relevant examplesfromtherecentliteraturearereported,withattentiontotheorganic chemistryperspective,alsoindicatinghowthedifferentapproachescanmodifyand valorizethenativefunctionalitiespresentinthestartingbiomass.
Afteranintroductorysection(Chapter1),intendedtosetthestageanddescribe howbiomasscancontributetotheproductionofchemicals,therestofthebookhas beenorganizedaccordingtothediverseapproachesthatcanbeexploited,alsohighlightingthepotential,challenges,andinnovativesolutionsassociatedwiththem. Biomassvalorizationprocesseshavebeenexploredusingcatalyticroutes,including acidcatalysis(Chapter2),basecatalysis(Chapter3),metalcatalysis(Chapter4),and biocatalysis(Chapter5).Variousthermalstrategiesthatcanbeappliedforthevalorizationofbiomassinvolvepyrolysis(Chapter6)andthermochemical–biological hybridprocesses(Chapter7).Differentadvanced/unconventionalstrategieshave alsoshowngreatpromise,suchasthoseinvolvingelectrochemical(Chapter 8)andphotochemical(Chapter9)means,microwavetreatment(Chapter10), ultrasound-assistedapproaches(Chapter11),andmechanochemicalapproaches (Chapter12).Asafinalcontribution,biomassprocessingfromanindustrial perspectiveisassessed(Chapter13).
Thereisnodoubtthatinthefuture,theproductionofchemicalswillbebased ontheexploitationofbiomassandthetimehascometofindthebestmethodsto addressthischallengeandputitintopractice.
November2020ChiaraSamorì,UniversityofBologna,Italy DavideRavelli,UniversityofPavia,Italy
RoleofBiomassintheProductionofChemicals
LaylaFiliciotto,EvanPfab,andRafaelLuque
UniversidaddeCórdoba,CampusdeRabanales,DepartamentodeQuimicaOrganica,EdificioMarieCurie (C-3),CtraNnalIV-A,Km396,Córdoba,Spain
1.1Introduction
Chemistryisafundamentalpartofeverythingaroundus.Natureislargelyresponsibleforallofthechemistrythatoccursandhasbeensofromthedawnoftime.However,societaladvancesandtechnologicaldevelopmentsinrecentyearshaveallowed ustocontributefarmorechemistrythaninthepast.Quality-of-lifeimprovements formajorpartsoftheworldwithbetterfooddistribution,clothing,technological devices,andmedicaltreatmentshaverequiredthechemistrytoprogressfurtherwith detrimentalunknowneffectsontheenvironment.Policiesandscientistsworldwide arenowstrivingtowardthedevelopmentofatrulysustainablesociety,culminatingintotheimplementationoftheUN’s17SustainableDevelopmentGoalsthat tacklevariousissuesincludinginfrastructures,education,equality,peace,andenvironmentalprotection[1].Intheactivesearchforsolutions,biomassvalorizationhas emergedasthemostviableoptionforamoresustainablechemicalindustry.
Theimpactthatsustainabilitycouldhaveonthechemicalindustryisbestreflected inthemagnitudeofthechemicalindustryitself.Today,thechemicalindustrygeneratesapproximately$4trillioninglobalsaleswiththeproductionofmorethan95% ofallcommodities[2].Oneofthebiggestturningpointsinthechemicalindustry, andwhatarguablyledittosuchheights,wastheadventofcatalyticcrackinginthe nineteenthcenturyfortherefiningoffossilresources.Catalyticcrackingallowedfor mostoftheproductsweusedailytobeeasilysourcedfrompetroleum[3].Biomass valorizationprocesseswerealsobeingexploredaroundthesametime.However,the complexnatureofbiomassandthewideavailabilityoffossilresourcesgainedall ofsociety’sattentionontheuseofthelatter[4].Assuch,petroleumprocesseshave beenthemajorfocusofscientistsandengineersforthepasttwocenturies.Although significantdevelopmentshavebeenachievedconsideringthiswithhigherresource efficiencyandcleanertechnologies,theresultingenvironmentalconcernsdrivenby theemissionsandspillshaveledmuchattentionbacktorenewableprocessessuch asbiomassvalorization.
BiomassValorization:SustainableMethodsfortheProductionofChemicals, FirstEdition. EditedbyDavideRavelliandChiaraSamorì. ©2021WILEY-VCHGmbH.Published2021byWILEY-VCHGmbH.
Biomassvalorizationandmoresustainablepracticesareimportantstepsforoverturningthe“disposablesociety”mindsetwhereresourcesareviewedasinfinite, cheap,andharmless.Oneofthemoststraightforwardexamplesofthiscanbeseen withplastic.Advancesinchemistrynotonlycreatedplasticsbutalsohelpednotice thealarmingconsequences.
Plasticsweredevelopedinconjunctionwiththeadventofpetroleumprocesses. Plasticproductspossessdesirablecharacteristics(lightweight,durable,etc.)that allowforendlessapplicationsatalowmanufacturingcost.Plasticsfoundtheir wayintodailyusewiththingssuchasclothingandpackaging.However,we wereunequippedtoproperlyhandlethisnewtechnology.Thecharacteristicsthat makeplasticsoappealingforawidevarietyofapplications(i.e.durableandheat resistant)arethesamethatmakeplasticsodifficulttodealwith.Itsinherent non-degradability,alongwithextremelycarelesshandlingandlittering,createda plasticwastecrisiswiththenowwidespreadproblemofmicroplasticsinouroceans [5,6].Biomassisamoreattractivefeedstockthatcancreatebio-basedand/or biodegradableplasticstohelpoverturnthedrasticimpactfrompetroleum-based products.Muchinitialresearchhasfocusedonusingbiomassfor drop-in solutions, i.e.plasticswiththesamecompositionandpropertiesasthetraditionalones(e.g. polyethylene[PE]andpolyethyleneterephthalate[PET]).However,theprocess chemistrylimitstheefficiencytosugars.Ontheotherhand,otherbio-based plasticswithnewpropertieshavebeendeveloped,e.g.polyethylenefuranoate (PEF)orpoly-lactate(PLA).Theformerisadurableplasticbasedonfuranandthe latteracompostableplastic.Developingbio-basedplasticsthatarealsobiodegradable–afundamentalchallengeinbiomassvalorization–canensureahigher sustainabilityatthewastemanagementstage,astheirwasteislessdangerous toanimalsandhumans(microplastics,trappedinfishingnets).However,differentiationinthelifetimesofplasticswillalsorequirethedevelopmentofdurable bioplastics.
Accumulatingplasticwasteisjustoneofthemanyconcernsthatishelpingto drivesustainablepracticesforward.Otherconcernsfromthefossil-drivenindustrial revolutionincludethefollowing:
● Irreversibledepletionoffossilfuels(i.e.oilandgas)andtheirdetrimentalenvironmentalissues[7,8].
● Higheraveragetemperaturesandaggravationofweatherconditionsworldwide (e.g.heavierrains)fromanincreaseofgreenhousegasesandrecordlevelsofCO2 intheatmosphere[9].
● Globalpopulationgrowth(>9billionprojectedby2050)leadingtohigherenergy, food,andchemicaldemands[10].
Theseconcernsrequireasustainablechemicalindustrythatembracestheconceptsof greenchemistry [11], circular [12]and low-carboneconomies [13],andhigh resourceefficiency [14].Assuch,biomassvalorizationandconversionofrenewable feedstocksthroughgreenprocessesareadvancingtofullyshifttowardasaferand sustainablechemicalindustry.
1.2BiomassValorization
Thesustainableproductionofchemicalsandproductscanbeachievedfrom conversionofbiomass,aninherentlyrenewablesource.Biomasscoversawide rangeofbio-basedresourcesfromplantsoranimals.Theseresourcesinclude plant-basedmaterials,biowastes,andaquaticorganisms.Valorizingrenewable biomassfeedstockscanofferenvironmentalbenefitsthatincludereducedemissions,saferfeedstocks,bettergeographicdistributionofresources,andachievement ofacirculareconomy[12,15–18].
Inacirculareconomy,resources–suchascarbon,nitrogen,andphosphorous compounds–areusedwithacircular“take–make–reuse/recycle”approach,as opposedtoalinear“take–make–dispose”approach[12].Aclosedcyclecanbe achievedwithbiomassvalorizationprocessesbyrecyclingthegeneratedCO2 throughnaturalphotosyntheticprocesses[19,20].Thisprocesshappensparticularlywithbiodegradableplastics.Further,theexistenceofnonedibleand rapidlygrowingplantsparalleltothedevelopmentofhigh-throughputagricultural technologiescanleadtoacarbon-neutralcycleinshortperiodsoftime,readjusting theincreasedlevelsofCO2 emissiongivenbythefossilindustries[21].
Inthecontextofbiofuels,biomasshasbeensubdividedinthreecategoriesgiven asfollowsalongwiththemajorevidenceddrawbacks:
(1) First-generationbiomass: Thisincludesallediblebiomasses(e.g.sugarcane, corn,whey,barley,andsugarbeet)thatarecomposedofsucroseorstarchy carbohydrates,hencerelativelysimplemacromoleculeswithlowrecalcitrance. Biologicalfermentationofsaidsugarpolymersyieldsbioethanol,oneof themoststudieddrop-inbiofuelswithcurrentindustrialproduction[22]. Food-derivedvegetableoilsarealsoconsideredasfirst-generationbiomassand theyyieldbiodieselthroughtransesterification[23].Themainissueofthis typeofbiomassistheclearcompetitionwithfoodresources(whichwillbe continuouslymoreprecious,giventheincreaseofworldpopulation)aswellas theintensiveuseofwaterandlandforthegrowthofsaidcrops[24].
(2) Second-generationbiomass:Nonfoodrawmaterials,includingby-products andwastematerials.Generally,second-generationbiofuelsareproducedfrom lignocelluloses(e.g.grasses,softorhardwood,andforestryresidues)orvarious wastes/by-products(e.g. agricultural:stover,wheatstraw,corncob,ricehusk, andsugarcanebagasse; industrial:glycerol,grainsfromdistilleries,andpaper sludge;or urban:householdandmunicipalsolidwastes).Giventhestructural compositionofthesefeedstocks(mixturesofcellulose,hemicellulose,and lignin),pretreatmentisusuallyrequiredforfermentationtobiofuelsand biochemicals,andtheprocesseconomicsarehinderedbytheuseofmultiple steps,leadingtoloweroverallconversions[25–30].Themaintechnological challengeofthesefeedstocksis,infact,thestructuralcomplexitythathinders theefficientuseofthelignocellulosesasawhole,callingforpretreatmentsthat inturnpossessdrawbacksdependingonthemethod(videinfra).
(3) Third-generationbiomass:Thisincludesnonediblefeedstocksthatdonot requireagriculturallandsfortheircultivation,namely,aquaticbiomass,such asalgaeandothermicroorganisms(e.g.cyanobacteria).Dependingonthe strain,thesefeedstocksmaycontainmono/polyunsaturatedhydrocarbonsto producegasoline-likefuelsviacrackingorhigherlipidcontentforbiodiesel applicationsviatransesterification.Whenconsideringalgae,themainissueis correlatedwiththehighwatercontentthathinderstransportationorrequires significantenergyinputsorlongtimestodrythem,whereasmicroorganisms requirespecificoperatingconditions.Furthermore,theeconomicchallenges ofthesefeedstockslimittheirindustrialapplication,giventhelowcultivation volumesandresourceefficiencyinprocessing[31–33].
Afourthgenerationofbiomassisalsocontemplatedandexemplifiedasmodifiedmicroorganismsconsideredinthethirdgeneration,finallyusedtoharvestsolar energythroughphotosyntheticprocesses[34,35].However,thesemicrobialspecies requireimprovementsofgenomics-basedbreedingandcarrytheusualconcernsof modifiedorganisms,suchasunexpectedmicrobialresistance.
Theavailablevolumesofthesetypesofbiomasswillplayamajorroleinidentifyingthebiggestdriverforchemicalsustainability.Accordingtoa2018report fromtheEuropeanUnion(EU),theannualproductionofagriculturalbiomass (i.e.firstgeneration)wasestimatedat956milliontonnes(Mt)ofdrymatterof which54%directlyusedforfoodconsumptionand46%ofresidues(e.g.leaves andstems)partiallyusedforanimalbeddingorbioenergyproduction.Infact, 80%oftheagriculturalbiomassisusedasfoodandfeed,showingthelimited potentialofusingfirst-generationbiomassforchemicalsandenergyproduction. Asitconcernsthird-generationbiomass,inparticularalgae(includingmacroand micro),only0.23Mtofwetmatterwasestimated,correspondingtoamere0.027Mt ofdrymass.Ontheotherhand,thetotalwoodybiomass(aboveground,second generation)wasestimatedat18600Mtofdryweight[36].Lookingatthequantities ofthedifferentbiomasses,thehighavailabilityoflignocellulosesinEuropemakes themthemostattractive.The >18000MtofwoodyresourcescanmakeEurope competitiveworldwideandsupportsustainableprocesses.Particularly,theefficient useoflignocellulosesandresidueswouldimprovethelong-termsustainabilityof thechemicalindustry,giventhevolumesandlittleimpactonthefoodresources, althoughthesefeedstocksstillrelyonforestmanagementconstraints.Otherwaste materials(e.g.foodandmunicipal)areincreasinginvolumes,giventheconcomitantincreaseofworldpopulationandimprovementoftheirlivingconditions.For example,61MtoffoodwasteareproducedyearlyintheEUalone[37].However, themajorchallengesoftheseproductsarethevariableseasonalcompositionas wellastheimplementationofapropersupplychainoftheseanthropologicalside streamstobiorefineries[38].
Conversionstrategiesofbiomass,however,generallycomewithlowresource efficiency,causinghigherproductioncostsandlimitedcompetitivenesswiththe well-establishedpetroleummarket.Thus,foreconomicadvantage,highvolumes, easeofproduction,andlimitedcompetitionwithothermarkets(e.g.food)are required.Inthissense,theuseoflignocellulosicbiomassmayagainoffera
1.3LignocellulosicBiomass 5 promisingalternativetothefossil-basedindustry.Fromanenergeticperspective, lignocellulosesandotherwastematerialspossesslowerenergydensitiescompared tononrenewableresourcessuchascoal,oil,andnaturalgas.However,biopower possessesnegativeemissionsthankstothephotosyntheticprocess,whereasfossil fuelscausesignificantlifecyclegreenhousegasemissions[39].Also,conversionof biomasstokeymolecules(e.g.ethanol,2-methylfuran,andhydrogenatedethers andfattyacids)canofferbiofueldiversificationwithvariousenergycontentsfor differenttransportapplications,includingaviation;theseprocessesrelyonthe separationofthedifferentbiomasscomponents[21,40].Fromachemicalpoint ofview,theuseoflignocellulosescanofferawidevarietyofplatformchemicals forthesynthesisofnotonlytraditionalbutalsonewproductstosatisfydifferent areasinthechemicalindustry(pharmaceuticals,textiles,andmaterials),which arediscussedinthefollowingparagraph.Aseparationofbio-componentswillbe requiredandexplainedtherein.
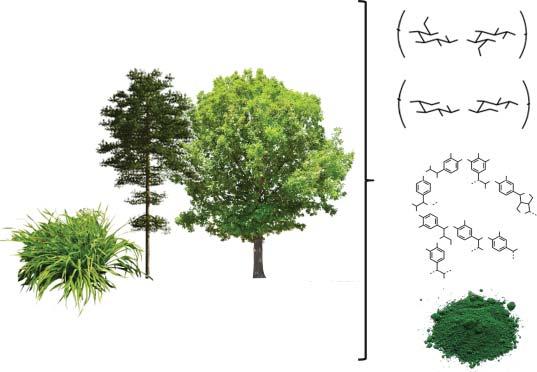
1.3LignocellulosicBiomass
Ofalltypesofbiomass,lignocellulosesarethemostavailableontheplanet, rangingfromwoodandforestrywastetostrawandagriculturalwaste.Lignocellulosicbiomassiscomposedofcellulose(40–50%),hemicellulose(15–20%),lignin (25–35%),andotherelements(Figure1.1).Bothcelluloseandhemicelluloseare carbohydrate-basedpolymers,whileligninisanaromaticpolymer.Celluloseis alinear,glucose-basedpolymer,makingitagoodsourceofthisC6-sugar.Cellulosecross-linkswithhemicellulose,abranchedpolymercomposedofdifferent C5-carbohydrates,uronicacids,andC6-sugars.Lignin,perhapsthemostirregular componentoflignocellulose,isapolyaromaticmacromoleculecomposedofphenylpropanederivatives.Ligninismostlyresponsibleforstructuralrigiditywithinthe
Grasses
Softwood Hardwood
Hemicellulose
Figure1.1 Schematicrepresentationofthecomponentsoflignocelluloses.
1RoleofBiomassintheProductionofChemicals
lignocellulose.Further,lignocellulosicbio-feedstocksincludevariablequantitiesof pigments;terpenes;inorganicelementssuchasMn,K,P,Cl,Ca,Mg,andNa,as wellasAl,C,Fe,N,S,Si,andTitoasmallerextent;andvariousextractives,e.g. carbohydrates,proteins,lipids,waxes,chlorophyll,terpenes,tannins,anduronic acids.
Anextensiveandsystematicreviewonthecompositionofvarioustypesofbiomass showsthesignificantchangesinthecompositionoftheseelementsdependingon thetypeofbiomass[41].
Overall,lignocellulosesaremadeofhighlyoxygenatedC5-andC6-derivatives. Theoxygenfunctionalitiesmakelignocellulosesamuchdifferentfeedstockto petroleumsourcesthataremainlyhydrocarbons.Theoxygenfunctionalitiesinlignocellulosesareinsomecasesadvantageousbecausetheycanminimizeoxidation reactions,whichusuallyhaveanegativeenvironmentalimpact,andfavorreduction reactions,whicharetypicallymilderprocessesandhavelessenvironmentalimpact. Further,thepropensitytoproducecoke/huminsandashobligestheuseofmild temperaturesfortheseby-products’minimization,asopposedtothetraditional catalyticcracking/reformingoffossils.Infact,thepresenceofplentyofoxygen functionalitiesandlowvolatilitytendtoleadtothemolecules’decompositionat hightemperatures,generatingcarbonaceousresidues.
Lignocelluloseshavevariablecompositionintheirsingularcomponentsdependingontheplantorigin.Waterandinorganicresiduecontentsalsovarysignificantly fromgrasstowood.Althoughcompositiondoesvarysignificantly,biomasscan sourceseveralusefulcompounds,includingcarbohydrates,aromatics,terpene,and fattyesters.Thesedifferentcomponentscanbeisolatedandconvertedforusein manyapplicationsincludingpharmaceutical,cosmetics/perfumes,plastics,textiles, andspecialtychemicals.Forthis,severaldifferentbiomassvalorizationroutescan beenvisionedwithawiderangeofobtainableproducts.
1.4KeyBiomolecules
Duringthefirstattemptsofbiomassvalorization, drop-in energysolutionshave beeninvestigatedastheycoulddirectlysubstitutetheuseoffossilresourcesfor transportationvehicles.Themostcommonexamplesaretheuseofbioethanoland biodieselasadditivestocommonautomotivefuels.Bioethanolismostlyproduced inindustryusingyeastfermentationofC6-sugars.Withanincreaseof25billion gallons(roughly75Mt)worldwide,bioethanolisoneofthemostmass-produced bio-basedmolecules.However,starchyfeedstocks(i.e.firstgeneration)aremostly usedintheproductionofbioethanol,causingdirectcompetitionwiththefood market,widespreaddeforestation,andconcernsonthepresenceofenoughfood sourcesforbothhumansandanimals[42].Also,bioethanolhaslimitedcompetitivenesswithpetroleumoptionsbecauseoflowproductvalueandrelativelyhigh price,especiallywhenconsideringfoodsustainability.Toaddperspective,theprice ofoilwouldhavetobeabove$70–80perbarrelforbioethanoltobecompetitive fromacoststandpoint,whiletoday,oilisat <$40perbarrel[43].
Alternatively,anotherapproachistoobtaindifferentplatformmoleculesfrom biomassthatcanbeusedforproductionofawidevarietyofchemicals.Withashift onhowweperceiveplatformmolecules,newchemical(andbiological)pathways canbeenvisioned.Inordertoinducethisshift,severalimportantbio-products uniquefrompetrol-basedoneswereidentifiedina2004USDepartmentofEnergy report[44]laterupdatedbyBozellandPetersen[45]andfurtherreviewedby Gallezot[46].Bio-basedplatformchemicalfamiliesandtheirrespectiveprocesses, industrialapplicationsandcurrenttechnicalchallenges,aresummarizedin Table1.1[44–47].Formoreinformationontheindustrialchallengesforbiomass valorization,thereaderisreferredtoChapter13ofthisbook.
Table1.1representsonlyasmallfractionofallthemoleculesthatcouldbeidentifiedasvaluableplatformchemicals,openingasignificantnumberofpossibilitiesfor thesynthesisofpetrol-likeornewmolecules.However,apartfromestablishedprocessessuchasthoseofsorbitolandglycerol,allotherbiomoleculesgenerallysuffer fromhighproductioncoststhatmightbecausedby (i)highpriceoffeedstocks(dependingontherequiredsugarpurity).
(ii)lowresourceefficiency(e.g.synthesisofby-productsthatlowerconversions andintensifypurification/separationprocesses).
(iii)highinvestmentandoperationalcostrequiredforthereactorvolumesor design,orneedtomaintainsterileconditionsduringproduction.
(iv)inefficientcatalysts,whichcouldbe (a)biological (enzymeandbacteria),which requiremetabolicengineeringforhigherefficiencyanddurability; (b)homogeneous,whichtendtobecorrosive,toxic,ordifficulttoreuseandrecycle;or (c) heterogeneous,whichhavelowerconversionseveniftheycanberecoveredand reused,butarepronetoirreversibleadsorptionoforganicmolecules,leading tocokeandthusreactorfouling.
Particularly,whencomparedtopetrol-likecompounds,thedisadvantagesof chemicalsfrombiomassprocessingbecomeincreasinglyapparentintermsof overallcosts.Evenwhenonlyconsideringfeedstocktransportation,theadvantage goestopetroleum,asitisafluidthatcanbepumped(ornaturalgasthrough pipelines).Biomasstendstooccupylargervolumes,givenitsphysicalnature,and ismuchmoredifficulttotransportasaresult.Nevertheless,themostnotable differencethatgivespetrol-likecompoundstheslightedgeistheabsenceofoxygen functionalities(aliphatics/aromatics/olefins),whichreducestheirreactivitybut yieldslargerproductionvolumesbytheadditionofheteroatoms.Infact,although fossilcompoundsaremodifiedviaoxidation,bio-derivedcompoundsoftenrequire oxygenremoval.Inthissense,largerinitialvolumesarerequiredforbiomassto reachthesamefinalproductvolume,makingiteconomicallyinefficient.Moreover, thereactivityofoxygengroupsinbiomassgivesinefficientprocesses,especiallyif targetingpetrol-likecompounds.Inthisregard,abetterrouteistobuildoffthese differentfunctionalitiesandexplorenewplatformchemicalsthatarespecificfor biomassproducts.Mostoftheadvanceshavebeenachievedlargelybecauseof catalyticpathwaysthatallowforlowerenergyrequirementsandhigherresource efficiency.
Table1.1 Keyexamplesofthepossiblebio-basedproducts,state-of-the-artprocesses,and challenges[44–47].
Bio-product platform(example)Process
1,4-Diacid(succinic acid)
Anaerobic fermentation
Furanics(HMF)Acid-catalyzed dehydrationofC-5 andC-6 sugars/oxidation
3-Hydroxypropionic acid(acrylicacid)
Aminoacid(aspartic andglutamicacids)
Gluconicacid (methylglucoside)
Itaconicacid(itaconic anhydride)
Glycerol (dihydroxyacetone)
Aerobic fermentation
Industrial application
Pharmaceutical, food,polymers, solvents
Food/cosmetics, polymers, construction, textiles,fuels
Technological challenge
Separation/purificationof products
Lowresourceefficiency
Polymers,textilesLowresourceefficiency
Undermetabolicengineering research
Levulinates (γ-valerolactone)
MicrobialprocessBiodegradable polymers, pharmaceuticals
Aerobicfermentation/catalytic oxidation
Aerobic fermentation
By-productof biodiesel/soap manufacture
Food, pharmaceuticals
Specialtypolymers (including biodegradable)
Cosmetics,food, pharmaceuticals, lubricants, polymers,Li batteries
Acid-catalyzed dehydrationofC-6 sugars
Sorbitol(isosorbide)Hydrogenationof C-6sugars
Lactones(3-hydroxybutyrolactone)
Lacticacid (oxalicacid)
Oxidative degradationofC-5 andC-6sugars
Anaerobic fermentation
Fragrances,food, fuels,solvents, pharmaceuticals, polymers
Food, pharmaceuticals
Pharmaceuticals, chiralbuilding block,polymers
Cosmetics, pharmaceutical, biodegradable polymers
Needofsterileconditions
Complexseparation
Undermetabolicengineering research
Lowresource efficiency/catalyst deactivation
Lowresourceefficiency
Undermetabolicengineering research
Lowmarketprice
Expensivepurification
Catalyst separation/deactivationin upgrade
Lowresourceefficiency
Establishedtechnology
Lowmarketprice
Inefficientoxidation
Lowresourceefficiency unlessstarchisused
Inhibitoryeffectofbiomass
Highfeedstockcost (high-puritylignocellulosic sugarsorfoodderived)
Separation/purificationof products
(continued)
Table1.1 (Continued)
Bio-product platform(example)Process
Industrial application
Technological challenge
Biohydrocarbons (isoprene) Aerobic fermentation PolymersInvestmentcost(reactors) Highfeedstockcost (high-puritylignocellulosic sugarsorfoodderived)
ABE(acetone, butanol,ethanol)
ABEfermentationFuels,solventsHighfeedstockcost
LigninCatalytic decomposition Polymers,food, pharmaceuticals, fuels
Lowresourceefficiency
Lowresourceefficiency
Sources:Werpyetal.[44],Bozelletal.[45],Gallezot[46],Isikgoretal.[47].
Forinstance,bytakingthecasestudyofplasticproductionfrombiomass,a varietyofoptionscanbeimagined[47],givingstrongenvironmentalbenefits. Iftraditionalplastics(e.g.PE,polyamides,andPET)areproducedstartingfrom biomass,apossiblereductionofca.310MtofCO2 -equivperyearcouldbeachieved withthesubstitutionoflessthan66%ofthecurrentfossil-basedplastics[48].This footprintreductionissolelybasedontheprocessandnotontheproductasthe degradationcharacteristicsoftheseplastics(i.e.nonbiodegradable)arethesame regardlessofthefeedstocktype(e.g.biomassandpetroleum).Atthesametime, newandinnovativeplatformchemicalscanbeproducedwithfewerchemical steps(e.g.furanicsasopposedtoaromatics),openingnewopportunitiesinthe productionofbio-basedplastics.Forinstance,afuran-basedplasticwassynthesized viathepolymerizationof2,5-furandicarboxylicacid(FDCA),anoxidizedproduct of5-hydroxymethylfurfural(HMF)(seeTable1.1),andethyleneglycol[49,50]. Thisplastic,knownaspolyethylenefuranoate(PEF),canbeconceivedasthe bio-basedparallelismofthecommonPET,wheretheC-6aromaticfunctionalityis substitutedbyafuranring.Further,thefuran-basedpolymerwasfoundtoperform bettercomparedtoPETintermsofphysicalpropertiessuchasgasbarrier[49]. TheproductionofPEFreliesontheacid-catalyzeddehydrationoflignocellulosic biomassand/orsugars(e.g.glucose)intoHMFwithsubsequentoxidationtoFDCA (Figure1.2)(seefurtherChapter13).
However,thisprocessishinderedbythecoproductionofsubstancesknownas humins(attimes,referredtoascoke)inthedehydrationstep[51–53].Thelower resourceefficiencyofthefirststepincreasestheoverallcostofPEF,limitingitscompetitionwithfossil-basedPETregardlessoftheimprovedproperties.Thislowatom economyiscommontomanybio-basedprocesses.
Generally,whenproducingchemicalsfromwholelignocelluloses,theyieldsof conversionprocessesarelowercomparedtothesamesynthesisstartingfromthe sugar(e.g.fructose,xylose,andglucose);hence,processcoststendtoincrease. Predominantly,thedifferencesoflignincontentandfeedstockdensitydepending ontheconsideredbiomass(e.g.grassesvs.softwoodvs.hardwood)causevariation ontheprocessyieldsaswellastheamountofvolumestobeprocessed(e.g.grasses
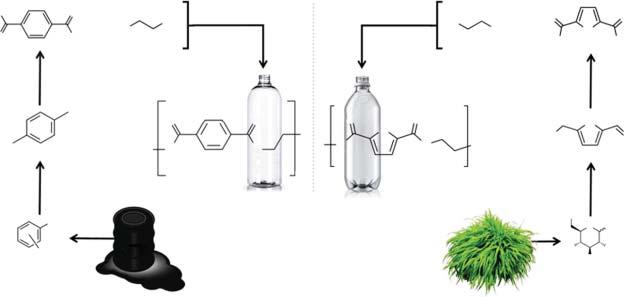
Figure1.2 ParallelismbetweentheproductionofPET(left)andPEF(right). requirebiggervolumes).Inparticular,thehydrogenbondingbetweenthedifferent components(i.e.lignin,hemicellulose,andcellulose)reducestheavailablesurface toprocessing,increasingthestructuralcomplexityandtherecalcitrantnatureof lignocellulosicfeedstocks.Furthermore,theinorganicmetals(e.g.MgandCa) naturallypresentinplantsmayinducereactorfoulingbyinducedprecipitationof saltsorheterogeneouscatalyst(e.g.zeolites)deactivationbyionexchange[54,55]. Aboveall,theaforementionedlargepresenceofheteroatoms(particularlyoxygen) increasesthemoieties’reactivity,leadingtolowatomefficiencyandoccurrence ofundesiredsidereactions,suchasthesynthesisofhuminsthatactasacatalyst deactivator(inasimilarwaytocoke)andpromotereactorfouling.
Inordertoovercomethesechallenges,strategiesincludetheuseofunconventionalsolvents,milderconditions,andvariouspretreatmentmethodsinordertoseparatethesinglecomponents(e.g.decomposecellulosetoglucose)andallowtargeted valorization.
1.5Solvents
Solventselectionisanimportantfactorintheviabilityofbiomassprocesses.Aqueoussolventsareadvantageousforhighsolubilitywithmostbiomassfeedstocks. Otherdesirablefactorsofaqueoussolventsarelowcostandlowenvironmental impact,especiallywhencomparedtoothersolvents.Water,forinstance,isthe idealsolventforbiocatalyticroutesastheyhavefewerchancestodenaturethe usedmicroorganism[56].However,adownsideofusingwaterisusuallythehigher energy-intensiveseparationprocesses,hencehighercost[57].Fromthechemical pointofview,aqueoussolventstypicallyfacilitatemanysidereactionsthatlower yields,includingdecompositionandpolymerization[58–61].Infact,manyof themechanismsofhuminformation(i.e.sugarplatformchemicalpolymerwith lowcurrentmarketvalue)areattributedtoring-openinghydrolysis[62].Theuse
C6-sugars
Crude
1.5Solvents 11
oforganicsolventscanovercomethistypeofmechanismandmaintainalow environmentalimpactiftheprocessisdesignedtorecyclesaidsolvents.Polar aproticsolventscanofferhigheryieldsofthedesiredproductscomparedtoaqueous systemsthankstohighsugarsolubilityandsuppressionofsidereactions[63,64]. However,mostpolaraproticsolvents,suchasdimethylsulfoxide(DMSO),have highboilingpointsthatmakeproductseparationmoredifficultandsignificantly increaseproductioncosts.
Otherconventionalsolventshavecertainlybeenemployedinbiomassapplicationsaswell,butfocushasbeenplacedoncouplingbiomassprocesseswith bio-basedorgreensolvents.Theseincludebio-basedconventionalsolvents,terpenes,ionicliquids,switchablesolvents,fattyacid/glycerol-basedsolvents,and liquidcarbondioxide.
Conventionalbio-basedsolventscanmostlybeusedasa drop-in replacement. Commonexamplesofthisareglycerol,ethyllactate,and2-methyltetrahydrofuran. Inaddition,acetoneandvariouslinearalcohols(e.g.methanol,ethanol,and butanol)canbederivedfrombio-basedsources,butcurrenttechnologyisnot themostefficient[65].Theuseofthesesolventsmayofferanadvantage,given thechemicalaffinitytothedesiredplatformbiomolecules.Thesesolventsalone, however,havealsoinducedtheformationofhumins.
Terpenescanbeextractedfromvariousmaterialsinnatureandsubsequentlyused asabio-basedsolvent. Cis-richpinanecanbeextractedfrompinetreeby-products andusedasasuitablereplacementfor n-hexane[66].Anotherexample, D-limonene, hassimilarpropertiesandcomesfromorangepeels.Ithasbeensafelydesignatedby theUSFDAforuseinhomeandpersonalproducts[65].However,thesmallvolumes ofthesepotentialsolventslimittheirusetospecialtyapplications(e.g.cosmetics).
Ionicliquids(ILs)arealsobeingexploredassolventsforbiomassprocesses. Recently,theyhavebeenamajorfocusinbiomassapplicationsfortheirpotential toovercomeothersolventlimitationsbecauseoftheirversatility(i.e.largeworking temperaturerange,acidicorbasiccapabilities,andcompatibilitywithdifferent materials)andnon-volatility.Initialstudiesshowthationicliquidscanoffer satisfactoryproductyieldswhencombinedwithmetalhalides.Infact,whereas theionicliquidprovidesastablemediumforsugarconversion,thehalideactsas Brønstedacidcatalyzingthesystem.Anotheradvantageofusingionicliquidsis thattheyaregenerallyconsideredtohavehighstability.However,applicationof ionicliquidsisstillarelativelynewfieldandthephysicochemicalpropertiesarenot properlydefined,intheendcausingsafetyconcerns[67].Themajorissuerelatedto theuseofionicliquids,however,isthehinderedseparationofoxygenatedproducts suchasfuranics,giventhehigherstabilityofthesemoleculesinchargedmedia[68].
Switchablesolventsareattractiveforcouplingdesirablesolventpropertiesthat wouldotherwisebeontheoppositeendsofthespectrum.Switchablesolvents areabletoquicklyundergoreversiblepropertyswitchesthatareactivatedbyan externalchange.TheexternaltriggeristypicallyachangeinpH,temperatureor concentration[65].Switchablesolventsarelikebiphasicsolventsinthattheyboth canpossesstwodifferentsolventpropertiesinjustonesystem.Onehighlydesirable solventpropertyformanipulationishydrophiliccharacter[69].Aswitchable
1RoleofBiomassintheProductionofChemicals
hydrophilicsolvent(SHS)hasbeenusedforextractingdifferentfractionssuch asproteins,lipids,andcarbohydratesfrommicroalgalbiomass[69]andalsofor extractingphenolsfromligninpyrolysisoil[70].TheSHSemployedinbothof thesestudieswas N,N -dimethylcyclohexylamine[69,70].However,limitationsare presentwithswitchablesolventsasitisanewerfieldlackinginresearch.
Othersolventsworthnotingarefattyacid/glycerol-basedsolvents,advantageous forchemicalinertness,andliquid/supercriticalCO2 ,advantageousforwideavailability,goodsolubility,andlowtoxicity[65].Thehighboilingpointoftheglycerol mixtures(higherthanDMSO)maybeusedasanadvantageforseparatingvolatile moleculesbuthinderstheirrecyclabilityifnonvolatilemoleculesarecoproduced (e.g.humins).Alternatively,employingliquid/supercriticalCO2 asasolventindicatescostlyhigh-pressuresystems/vesselsandadditionalsafetyrequirements.
Anothersolventapproachistheuseofbiphasicsystems.Withtwodifferent phases,reactionstakeplaceintheaqueousphasebutextractionandseparation intheorganicphase[59,71–74].Hydrophobicextractingphases(e.g.cyclopentyl methylether,CPME)inthepresenceofachloridesalt(e.g.NaCl)canenhancethe partitioningcoefficientoftheorganicsolvent,favoringextraction[71].Otherwise, polarsolventswithlowwatersolubility(e.g.methylisobutylketone,MIBK)canbe usedinthesugarconversions.Evenso,theadditionofmultiplesolventsincreases theproductioncostsevenifrecycled(smalllossofsolvents,specialtymolecules) andreducesthe greenness oftheoverallprocess.
Byusingthese(mixturesof)solvents,one-pottransformationoflignocelluloses viadifferentmethodologieshasbeenattempted[50].However,one-stepprocedures aredifficulttoachievewithbiomassprocessesbecauseoftheintricaciesassociated withsolventselection,catalyst,andotheroperatingconditions.Withthisinmind, biomassprocessesthatfocusonindividualbio-componentsasopposedtoentiresystemscouldbemoreeffective.
1.6PretreatmentofLignocelluloses
Pretreatmentisanecessarymeasureforhandlingbiomassonanindividualcomponentbasis.Oneofthemainfunctionsforpretreatmentistofacilitateseparation andallowforimprovedaccessofthedifferentbiomassfractions,particularlyfrom therigidcomponentsthatmakeuptheplantwall[75].Inlignocellulose,theserigid componentsthatsignificantlyhindersolubilizationareligninandcellulose.Peculiarly,theseparationofeachbio-componentwithoutfurtherdecomposition(e.g. toby-products)couldgreatlycontributetothedevelopmentofefficientconversion strategies,improvingthecompetitivenessofabio-economy.
Biomasspretreatmentscanbeclassifiedasphysical,chemical,physicochemical, orbiological[75–77].Physicalpretreatmentisreservedmainlyforlesscomplex applicationswhereonlyanincreasedsurfaceareaismostlydesired.Somecommonphysicalpretreatmentmethodsaresheermixing,milling,andgrindingthat physicallybreakaparttheplantwallcomponents[76].Chemicalpretreatment iswidelyusedforitsabilitytogreatlyimprovesolubilizationinordertomake subsequentbiomassprocessespossible.Chemicalmethodsincludeacid/alkali
1.6PretreatmentofLignocelluloses 13 treatment,ozonolysis,andorganosolv[77].Manyresearchershavebeenutilizing acidtreatmentasasimplechemicaltransformationroutethatisparticularly usefulforreleasingsomeofthebio-sugarsthatarelockedbehindthemorerigid components.Furthermore,ifthetargetedreactionisacid-catalyzeddehydration ofthesugarstofuranicsorlevulinics,theplausibleresidualpresenceofacids mayonlyenhancetherateofsaidreaction.Ontheotherhand,mostbiological treatmentsaresafeandgreenprocessesthatutilizefungiorothermicroorganisms. Theenzymesbreakdownhemicelluloseandligninratherwell,whileleaving intactcellulose.However,biologicalprocessesproceedatratherslowratesand themicroorganismstypicallyonlythriveinafine-tunedaqueousenvironment. Amajorityoftheinnovativepretreatmentmethodsfallunderphysicochemical, asmanybenefitsfromthecombinatoryapproach.Thesecombinationtreatments includesteamexplosion,ammoniafiberexpansion,carbondioxideexplosion,and wetoxidationwithsteamexplosionbeingoneofthemostused.Steamexplosion useshigh-pressuresteamthatcreatesaself-reactingautohydrolysisenvironment fortransformingbiomassmechanicallyandchemically[25].
Withcurrentprogress,pretreatmentisanecessarymeasureforprocessinglignocellulosicbiomass.Thelignincontentislargelyresponsibleforcomplicatingthe heterogeneousnatureoflignocellulosesandforcontributingrecalcitrantproperties thatmakeitdifficulttohandle.Withoutpretreatment,mostvalorizationapproaches arenotcosteffective[78].
Ideally,apretreatmentstepwouldefficientlyseparatelignocelluloseintoitssingle components.Ifthisisachieved,biomassprocessescouldbestandardized(basedon eachcomponent)togreatlyalleviatetheissuesofbiomassinconsistency.Itwould alsocontributetooptimizedprocessesthatmaximizeprocessconditionssuchas yieldsandoverallcosts.Whencontemplatingbiocatalyticconversionofbiomass (e.g.yeastfermentation),typicalyieldsarelow(<20%)withoutpretreatment.This phenomenonisgivenbythebarriereffectoflignintoenzyme,physicallyhindering thehydrolysisofthedigestiblecomponents(i.e.sugars)[79,80].Improvements ofproductyieldshavebeenobtainedwitheitherbiological[81],physical[82], andchemicalsteps[78,83]orphysicochemical[84,85]pretreatments,thusgiving higherresourceefficiency.Cost-effectivesolutionswouldideallyremovelignin withoutaffectingthedesiredcarbohydrates,hencebeingenergyeffectivewhile havingasimplereactordesignandlowproductionofwastecompounds(including solvents)[86].Nevertheless,improvementofthecurrentpretreatmenttechnologies isstillrequiredtoobtaineconomicalsolutions.Variouspretreatmentstrategiesand theiradvantagesanddisadvantagesareillustratedinTable1.2.
Overall,differentpretreatmentmethodswillbepreferredfordifferentapplications.Forexample,applicationsrequiringlowtoxicitywouldbebettersuitedwith microbialconversions.Alternatively,applicationsrequiringhighsugaryieldswould probablyutilizechemicalconversions.Themainchallengeforselectivebiomass processesisachievingareasonablebalancebetweencostconsiderationsandefficientseparationofeachcomponent.Inaddition,pretreatmentrequirementswith currenttechnologiesfurthercomplicatetheprocesses[87].Innovativesolutions
Table1.2 Advantagesanddisadvantagesofpretreatmentmethodsforlignocelluloses.
MethodPretreatmentAdvantagesDisadvantages
BiologicalFungiEnergyeffectiveLowhydrolysisrate Degrades lignin/hemicellulose network
PhysicalMillingReducescellulose crystallinity Energyintensive
ChemicalOzonolysisLigninreductionCostineffective(ozone)
Lowmicrobialinhibitors
OrganosolvLigninandhemicellulose hydrolysis
Bigsolventvolumes Requiressolventrecycle
AlkaliLigninremovalInefficientforsoftwoods Largeamountsofwater
Reducescellulose crystallinity Longpretreatmenttimes
Limitedhemicellulose degradation Baserecycle
Concentrated acid HighglucoseyieldLargeamountsofacids EnergyeffectiveRequiresacidrecycle
Reactorcorrosion
DilutedacidLowmicrobialinhibitorsLowsugaryields LowercorrosionissuesDegradationproducts
IonicliquidsReducescellulose crystallinity
Costineffective(ionic liquids)
Higheraccessiblesurface area Difficult recovery/separationof desiredproducts
LigninremovalPotentialtoxicityand thermalinstabilityofionic liquids Degrades lignin/hemicellulose network
PhysicochemicalSteamexplosionLigninremovalHighmicrobialinhibitors
Hemicellulose solubilization
Ammoniafiber expansion (AFEX)
FairsugaryieldsPartialhemicellulose degradation
Economical
Higheraccessiblesurface area Inefficientwithlignin-rich biomass
LowmicrobialinhibitorsBigammoniavolumes (cost) (continued)
Table1.2 (Continued)
MethodPretreatmentAdvantagesDisadvantages
CO2 explosionHigheraccessiblesurface area Noeffecton lignin/hemicelluloses network
LowmicrobialinhibitorsHighpressure(cost, reactor)
Economical
WetoxidationLigninremovalCostineffective(oxygen andalkalinecatalyst)
Lowmicrobialinhibitors
Energyeffective
thataddressthesechallengeswillhelppushbiomassprocessesclosertopractical implementation.
1.7ConclusionsandPerspectives
Thesearchofatruesustainablechemicalindustryisdrivenbythedevelopment ofprocessesthatrelyonnotonlyrenewablefeedstocksassociatedwith low environmentalimpact techniquesbutalsoeconomicviabilitytocompetewith thewell-establishedoilandgasmarkets.Torecedethedependencyonpolluting resources,creativesolutionsfollowinga green designinthemostrestringingwayare required.Thefollowingchaptersinthisbookdiscussvariousmethodsofbiomass valorization,alongwiththeirrespectivechallengesandinnovativesolutions,as meanstoprogresstowardchemicalsustainability.
References
1 UnitedNationsSustainableDevelopmentKnowledgePlatform.(2019).Sustainabledevelopmentgoals.https://sustainabledevelopment.un.org/sdgs(accessed20 November2019).
2 InternationalCouncilofChemicalAssociations.(2019).Sustainabledevelopment. https://www.icca-chem.org/sustainable-development/(accessed10September 2019).
3 Speight,J.G.(1999). TheChemistryandTechnologyofPetroleum.NewYork:MarcelDekker.
4 Filiciotto,L.andLuque,R.(2018).Nanocatalysisforgreenchemistry.In: EncyclopediaofSustainabilityScienceandTechnology (ed.R.A.Meyers),1–28.New York:Springer.
5 Eerkes-Medrano,D.,Thompson,R.C.,andAldridge,D.C.(2015).Microplasticsin freshwatersystems:areviewoftheemergingthreats,identificationofknowledge
1RoleofBiomassintheProductionofChemicals
gapsandprioritisationofresearchneeds. WaterResearch 75:63–82.https://doi .org/10.1016/j.watres.2015.02.012.
6 Law,K.L.andThompson,R.C.(2014).Microplasticsintheseas. Science 345 (6193):144–145.https://doi.org/10.1126/science.1254065.
7 Aboudah,M.(2015).DealingwitheconomicsustainabilitychallengesevolvingfromdecliningoilproductioninSaudiArabia.Masterthesis.Michigan TechnologicalUniversity.
8 Campbell,C.J.(2013). CampbellsAtlasofOilandGasDepletion.NewYork,NY: Springer.
9 ClimateChange:VitalSignsofthePlanet.(2018).https://climate.nasa.gov/ (accessed14November2019).
10 Worldpopulationprojectedtoreach9.8billionin2050,and11.2billionin2100 |UNDESADepartmentofEconomicandSocialAffairs.(2017).https://www .un.org/development/desa/en/news/population/world-population-prospects-2017 .html(accessed14November2019).
11 Anastas,P.T.andWarner,J.C.(1998). GreenChemistryTheoryandPractice.New York:OxfordUniversityPress.
12 Keijer,T.,Bakker,V.,andSlootweg,J.C.(2019).Circularchemistrytoenable acirculareconomy. NatureChemistry 11(3):190–195.https://doi.org/10.1038/ s41557-019-0226-9.
13 Lanzafame,P.,Centi,G.,andPerathoner,S.(2014).Catalysisforbiomassand CO2 usethroughsolarenergy:openingnewscenariosforasustainableand low-carbonchemicalproduction. ChemicalSocietyReviews 43(22):7562–7580. https://doi.org/10.1039/C3CS60396B.
14 Sheldon,R.A.(2016).Greenchemistryandresourceefficiency:towardsa greeneconomy. GreenChemistry 18(11):3180–3183.https://doi.org/10.1039/ c6gc90040b.
15 Cong,W.-F.,Jing,J.,Rasmussen,J.etal.(2017).Forbsenhanceproductivityof unfertilisedgrass-cloverleysandsupportlow-carbonbioenergy. ScientificReports 7(1):1–10,ArticleID1422.doi:https://doi.org/10.1038/s41598-017-01632-4.
16 Mauser,W.,Klepper,G.,Zabel,F.etal.(2015).Globalbiomassproduction potentialsexceedexpectedfuturedemandwithouttheneedforcroplandexpansion. NatureCommunications 6(1):1–11,ArticleID8946.doi:https://doi.org/10 .1038/ncomms9946.
17 Woodward,F.I.,Lomas,M.R.,andKelly,C.K.(2004).Globalclimateandthe distributionofplantbiomes. PhilosophicalTransactionsoftheRoyalSocietyof London.SeriesB:BiologicalSciences 359(1450):1465–1476.https://doi.org/10 .1098/rstb.2004.1525.
18 Abdel-Shafy,H.I.andMansour,M.S.M.(2018).Solidwasteissue:sources,composition,disposal,recycling,andvalorization. EgyptianJournalofPetroleum 27 (4):1275–1290.
19 EuropeanCommission(2019).RenewableEnergy:Bioenergy.http://ec.europa .eu/research/energy/index.cfm?pg=area&areaname=renewable_bio(accessed10 November2019).
20 Obermeier,W.A.,Lehnert,L.W.,Kammann,C.I.etal.(2016).ReducedCO2 fertilizationeffectintemperateC3grasslandsundermoreextremeweather conditions. NatureClimateChange 7(2):137–141.https://doi.org/10.1038/ nclimate3191.
21 Wu,L.,Moteki,T.,Gokhale,A.A.etal.(2016).Productionoffuelsandchemicals frombiomass:condensationreactionsandbeyond. Chem 1(1):32–58.https://doi .org/10.1016/j.chempr.2016.05.002.
22 Mousdale,D.M.(2008). Biofuels:Biotechnology,ChemistryandSustainableDevelopment.BocaRaton,FL:CRCPress.
23 Ho,D.P.,Ngo,H.H.,andGuo,W.(2014).Aminireviewonrenewablesources forbiofuel. BioresourceTechnology 169:742–749.https://doi.org/10.1016/j .biortech.2014.07.022.
24 Rulli,M.C.,Bellomi,D.,Cazzoli,A.etal.(2016).Thewater-land-foodnexusof first-generationbiofuels. ScientificReports 6(1)https://doi.org/10.1038/srep22521.
25 Achinas,S.andEuverink,G.J.W.(2016).Consolidatedbriefingofbiochemical ethanolproductionfromlignocellulosicbiomass. ElectronicJournalofBiotechnology 23:44–53.https://doi.org/10.1016/j.ejbt.2016.07.006.
26 Lee,W.C.andKuan,W.C.(2015).Miscanthusascellulosicbiomassforbioethanol production. BiotechnologyJournal 10(6):840–854.https://doi.org/10.1002/biot .201400704.
27 Stoffel,R.B.,Neves,P.V.,Felissia,F.E.etal.(2017).Hemicelluloseextractionfrom slashpinesawdustbysteamexplosionwithsulfuricacid. BiomassandBioenergy 107:93–101.https://doi.org/10.1016/j.biombioe.2017.09.019.
28 Wilkinson,S.,Smart,K.A.,James,S.etal.(2016).Bioethanolproduction frombrewersspentgrainsusingafungalconsolidatedbioprocessing(CBP) approach. BioEnergyResearch 10(1):146–157.https://doi.org/10.1007/s12155016-9782-7.
29 Prasetyo,J.,Naruse,K.,Kato,T.etal.(2011).Bioconversionofpapersludge tobiofuelbysimultaneoussaccharificationandfermentationusingacellulaseofpapersludgeoriginandthermotolerant Saccharomycescerevisiae TJ14. BiotechnologyforBiofuels 4(1):35.https://doi.org/10.1186/1754-6834-4-35.
30 Sims,R.E.,Mabee,W.,Saddler,J.N.etal.(2010).Anoverviewofsecondgenerationbiofueltechnologies. BioresourceTechnology 101(6):1570–1580.https://doi .org/10.1016/j.biortech.2009.11.046.
31 Pierobon,S.C.,Cheng,X.,Graham,P.J.etal.(2018).Emergingmicroalgaetechnology:areview. SustainableEnergyFuels 2:13–38.https://doi.org/10.1039/ C7SE00236J.
32 Koller,M.,Salerno,A.,Tuffner,P.etal.(2012).Characteristicsandpotentialof microalgalcultivationstrategies:areview. JournalofCleanerProduction 37: 377–388.https://doi.org/10.1016/j.jclepro.2012.07.044.
33 Singh,A.andOlsen,S.I.(2011).Acriticalreviewofbiochemicalconversion, sustainabilityandlifecycleassessmentofalgalbiofuels. AppliedEnergy 88(10): 3548–3555.https://doi.org/10.1016/j.apenergy.2010.12.012.
34 Aro,E.-M.(2015).Fromfirstgenerationbiofuelstoadvancedsolarbiofuels. AMBIO 45(S1):24–31.https://doi.org/10.1007/s13280-015-0730-0.