SupramolecularGels
MaterialsandEmergingApplications
Editedby TifengJiao
Editor
Prof.TifengJiao YanshanUniversity SchoolofEnvironmentalandChemical Engineering No.438WestHebeiStreet Qinhuangdao 066004HebeiProvince China
Cover
CoverImage:©Fotaro1965/Shutterstock
Allbookspublishedby WILEY-VCH arecarefullyproduced.Nevertheless,authors,editors,andpublisher donotwarranttheinformationcontainedinthesebooks,includingthis book,tobefreeoferrors.Readersare advisedtokeepinmindthatstatements,data,illustrations,procedural details,orotheritemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>
©2021WILEY-VCHGmbH,Boschstr. 12,69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc. usedinthisbook,evenwhennot specificallymarkedassuch,arenotto beconsideredunprotectedbylaw.
PrintISBN: 978-3-527-34511-3
ePDFISBN: 978-3-527-81703-0
ePubISBN: 978-3-527-81701-6
oBookISBN: 978-3-527-81700-9
Typesetting Straive,Chennai,India PrintingandBinding
Printedonacid-freepaper 10987654321
Contents
Preface ix
1MolecularGelasMediumorIntermediateinFunctional MaterialsSynthesis 1
RongMiaoandJunxiaPeng
1.1Introduction 1
1.2MolecularGelasIntermediateinSynthesizingFluorescentSensing FilmswithHighPerformance 2
1.2.1MolecularDesign 3
1.2.2MolecularGelStrategy-BasedSensingFilmforVOCVaporDetection 4
1.2.3MolecularGelStrategy-BasedFilmforChemicalsSensinginLiquid Phase 9
1.3MolecularGelasIntermediateinSynthesizingPorousMaterials 9
1.3.1PorousMaterialsforRemovalofOilonWaterSurface 11
1.3.2PorousMaterialsforVOCsAdsorption 13
1.4SummaryandPerspectives 14 References 16
2PreparationandSensingApplicationofFluorescent OrganogelsandHydrogels 21
XudongYu,LijunGeng,andJiangboGuo
2.1Introduction 21
2.2TypesofGelsthatRespondtoDifferentStimuli 22
2.2.1FluorescentGelsthatRespondtoPhysicalStimuli 22
2.2.1.1FluorescentGelsthatRespondtoHeat 22
2.2.1.2FluorescentGelsthatRespondtoLight 23
2.2.1.3FluorescentGelsthatRespondtoUltrasound 25
2.2.1.4FluorescentXerogelsthatRespondtoGrindingorPressure 28
2.2.2FluorescentGelsforVisualChemicalStimulusSensing 30
2.2.2.1FluorescentGelsforCationSensing 30
2.2.2.2FluorescentGelsforAnionSensing 35
2.2.2.3FluorescentGelsforCO2 Sensing 37
2.2.2.4FluorescentGelsforSolventandHumiditySensing 38
2.2.2.5FluorescentGelsforNitroaromaticDerivativeSensing 42
2.2.2.6FluorescentGelsforAmineSensing 42
2.3SummaryandPerspectives 45 References 46
3PreparationofSelf-AssembledCompositeHydrogelsandTheir ApplicationinBiomedicineandWastewaterTreatment 51 RanWang,JingxinZhou,LexinZhang,andTifengJiao
3.1Introduction 51
3.2PreparedCompositeHydrogelsUsedinBiomedicine 52
3.2.1Self-AssemblyandDrugReleaseCapacitiesofOrganogelsviaSome AmideCompoundswithAromaticSubstituentHeadgroups 52
3.3PreparedCompositeHydrogelsUsedinWastewaterTreatment 55
3.3.1PreparationandSelf-assemblyofSomeFunctionalizedSupramolecular Hydrogels 55
3.3.2PreparationandSelf-assemblyofSomeGrapheneOxide-Based CompositeHydrogels 57
3.4ConclusionandPerspectives 64 Acknowledgments 65 References 65
4ConductiveHydrogelsforFlexibleMechanicalSensors 71 ZhihuiQinandTifengJiao
4.1Introduction 71
4.2FabricationofConductiveHydrogels 73
4.2.1ElectronicallyConductiveHydrogel 74
4.2.2IonicallyConductiveHydrogels 78
4.3ConductiveHydrogel-BasedMechanicalSensors 80
4.3.1StrainSensors 81
4.3.2PressureSensors 85
4.4ConclusionandOutlook 89 Acknowledgments 90 References 90
5RecentProgressonHeat-SetMolecularGels 99 YuangangLi,ZonglinYang,YongChen,HuajingLi,RongYang,andChenyu Huang
5.1Introduction 99
5.2Heat-SetMolecularGels 101
5.2.1Heat-SetMolecularHydrogel 101
5.2.2Heat-SetOrganicGel 110
5.3ConclusionandPerspectives 118 Acknowledgements 120 References 120
6SupramolecularGelsfromCarbohydrateBiopolymersfor WaterRemediation 127
XuefengZhangandWeiqiLeng
6.1Introduction 127
6.2HydrogelsfromCarbohydrateBiopolymers 128
6.2.1HydrogelsfromNativeCelluloseorChitin 129
6.2.2HydrogelsfromCelluloseorChitinDerivatives 130
6.2.3HydrogelsfromBiopolymerNanomaterials 132
6.2.3.1HydrogelsfromPhysicallyCross-LinkedNCorNCh 134
6.2.3.2HydrogelsfromChemicallyCross-LinkedNCorNCh 137
6.3AerogelsfromCarbohydrateBiopolymers 138
6.4Biopolymer-DerivedGelsforWaterRemediation 140
6.4.1HeavyMetalRemoval 141
6.4.2OrganicPollutantsRemoval 146
6.5ConclusionsandPerspectives 156
References 156
7BiobasedAerogelsforOilSpillRemediation 169
WeiqiLeng,ShengHe,XuefengZhang,XiangWang,andChanakaM. Navarathna
7.1Introduction 169
7.2Aerogels:Classification,Fabrication,andProperties 172
7.2.1ClassificationofAerogels 172
7.2.2FabricationofBiobasedAerogels 173
7.2.2.1SupercriticalDrying 177
7.2.2.2Freeze-drying 179
7.2.3FunctionalizationofBiobasedAerogels 186
7.3BiobasedAerogelsforOilSpillRemediation 193
7.3.1ParametersThatAffecttheOilAbsorptionPerformance 193
7.3.2MechanismsofOilAbsorption 197
7.3.3Post-processingofAerogelAbsorbentAfterOilSpillRemediation 200
7.4ConclusionandFutureScope 201 References 201
8LuminescentSupramolecularGels 215 XueJinandPengfeiDuan
8.1Introduction 215
8.2FluorescenceinSupramolecularGels 216
8.3PhosphorescenceinSupramolecularGels 219
8.4UpconvertedLuminescenceinSupramolecularGels 224
8.5CircularlyPolarizedLuminescenceinSupramolecularGels 230
8.5.1CPL-ActiveGelBasedonChiralLuminescentGelators 234
8.5.2CPL-ActiveSupramolecularGelBasedonAchiralLuminescent Gelators 238
viii Contents
8.5.3CPL-ActiveSupramolecularGelbyUsingOrganicLuminophoresas
Guests 239
8.5.4CPL-ActiveSupramolecularGelBasedonInorganicLuminescence
Guest 245
8.6ConclusionandPerspectives 249 Acknowledgments 250 References 250 Index 257
Preface
Gelsaresoftmaterialscomposedofthree-dimensionalcross-linkednetworks filledwithaseconddispersionmedium.Virtuallyanyfluidcanbeusedasdispersionmedium,includingwater(hydrogels),anorganicsolvent(organogels), andevenair(aerogels).Gelscanbeclassifiedindifferentwaysdependingon theirconstitution,thetypesofcross-linking,andthemediatheyencompass. Supramoleculargels,relyingonnon-covalentinteractionsforself-organizationinto hierarchicalstructures,arebecomingoneofthemostattractiveresearchsubjects inthefieldofsupramolecularchemistryandmaterialsciences.Thedynamicand reversiblenatureofsupramoleculargelsendowsthemwithuniquepropertiesand makestheircharacterizationdiversified.Meanwhile,severalfunctionalitiessuchas optical,electrical,magnetic,thermal,andotherpropertiescanbealsointroduced intosupramoleculargelsbydesigningnetworkstructureandincorporatingan activegrouporareceptorunit.Thisclassofmaterialsnotonlycanbeutilizedas mediumorintermediatetemplatestosynthesizefunctionalmaterialsforpossible applicationsinseparationandinformationstorage,butalsoserveasmediafor arangeofapplications,suchasbiomaterials,industry,electronicmaterials,and personalcareproducts.
Asweallknow,variouseffortshavebeenandarebeingmadetodevelopnovel supramoleculargelsandexploretheirpotentialapplications,andthereareplentyof articlesandreviewspublished.However,therearestillfewreportsonthedetailed descriptionofsupramoleculargelpreparationandemergingapplications.Thus,this bookaimstoprovidesuchareviewaboutthepreparationandemergingapplication ofdifferenttypesandfunctionsofsupramoleculargels.Inthefollowingchapters, severalexamplesandachievementsofrecentandcurrentresearchesaregiven.Rong MiaoandYuFangreviewmoleculargelasmediumorintermediateinfunctional materialssynthesis.Yuetal.addsaperspectiveondesignofsupramolecular low-molecularmassorganogelatorswithfluorescenceandsensingabilitiestoward bothphysicalandchemicalstimuli.Preparationofself-assembledcomposite hydrogelsandtheirapplicationinbiomedicineandwastewatertreatmentare discussedbyJiaoetal.Inaddition,ZhihuiQinandTifengJiaohighlightrecent progressinthefieldofconductivehydrogelsasflexiblemechanicalsensors.Lietal. provideanoverviewofbothheat-sethydrogelsandheat-setorganicgelsanda briefdescriptionoftheirpossiblepotentialapplications.XuefengZhangandWeiqi
x Preface
Lengreviewtherecentadvancesforthesynthesisofhydrogelsandaerogelsfrom celluloseandchitinandtheirapplicationforwaterremediation.Fabricationand functionalizationofbiobasedaerogelsandtheirperformanceforoilremediation aresummarizedbyHeetal.Finally,XueJinandPengfeiDuanhighlightandput intoperspectiveafewrecentadvancesinthefieldofluminescentgels.
Iwouldliketogreatlythankallthesecolleaguesforprovidingthesedeepand illustrativeinsightsintotheirworkandthatoftheirpeers.Ibelievethatthecontent ofsupramoleculargelspresentedinthisbookcanprovidereaderswithguidanceon fundamentalunderstandingofsupramoleculargelsandpromotethedevelopment ofsupramoleculargels.
MolecularGelasMediumorIntermediateinFunctional MaterialsSynthesis
RongMiaoandJunxiaPeng
KeyLaboratoryofAppliedSurfaceandColloidChemistry,MinistryofEducation,SchoolofChemistryand ChemicalEngineering,ShaanxiNormalUniversity,Xi’an,P.R.China
1.1Introduction
Sincethe1990s,substantivedevelopmenthasbeenachievedinresearchrelated tomoleculargels[1,2].Thousandsofmoleculargelatorshavebeenreported[2]. Meanwhile,withrapiddevelopmentinsupramolecularchemistryandnanoscience, in-depthunderstandinghasbeengainedandmanynewresearchbrancheshave emerged[3,4].Bycombiningresearchresultsandtheoreticalcalculations,theories inmoleculargelstudyhavebeenbuilt.Preparationofmoleculargelhasalsobeen graduallychangedfromtheaccidentaldiscoverystagetotheaimeddesignedstage. Atypicalcharacteristicofresearchonmoleculargelsistheinterdisciplinaryfeature. Generally,researchonmoleculargelsincludesknowledgerelatedtosoftmatter, self-assembly,rheology,thermodynamics,chemicalsynthesis,theoreticalcalculation,simulation,etc.Macroscopically,moleculargelsaresolid-like.Microscopically, moleculargelsaremadeupofnon-covalentinteraction-basednetworkstructures consistingofasmallquantityofmoleculargelatorsandabundanceofsolvents[5]. Thesolventmoleculesaretrappedinthenetworkstructureviacapillarity,surface tension,andotherimmobilizationforces.Thus,moleculargelspossesstypical microheterogeneousstructure.Differentfromchemicalgelsandpolymergels, non-covalentbonding-basednetworkendowsmoleculargelsprominentthermal reversibility,shearthixotropy,andstimulusresponse[6,7].Thesepropertieslay solidfoundationforfurtherapplicationofthemoleculargels.However,pronounced applicationofmoleculargelhasnotbeenrealized.
Stabilityisanimportantfactorthataffectsthepracticalapplicationsofmolecular gels[8].Thisisbecause,theformationandexistenceofamoleculargelisbasedon anequilibriumofdissolve-aggregation,whichisbasedonavarietyofnon-covalent interactions[7].Theequilibriummaybebrokenbyanytinyexternaldisturbance, causingmeltingofphasetransitioninthemoleculargel.Inordertogetstablemoleculargels,itisnecessarytostrengthenthefactorsthatarehelpfultomaintainthe dissolve-aggregationbalance.Forexample,high-boilingpointliquidsareusedto SupramolecularGels:MaterialsandEmergingApplications, FirstEdition.EditedbyTifengJiao. ©2021WILEY-VCHGmbH.Published2021byWILEY-VCHGmbH.
1MolecularGelasMediumorIntermediateinFunctionalMaterialsSynthesis
preventgel-solphasetransitionscausedbysolventvolatilization[9].Thermalinsulationandvibrationreductionarerealizedthroughpackaging,soastoovercomethe geldamagecausedbyvibrationandheat.Infact,itisinthiswaythattheintelligent propellantbasedonmoleculargelisprepared.
Anotherwaytopromotethepracticalapplicationofmoleculargelsistousethem asanintermediatetoconstructspecialmaterialswithspecialstructuresandexcellentproperties,wheretheapplicationofmoleculargelswillbehighlighted[10,11]. Basedonthisconsideration,effortshavebeendevotedtothestudyofmoleculargels asintermediatesinfunctionalmaterialsfabrication(Scheme1.1),suchasinfluorescentsensingfilm,gelemulsions,andlow-densityporousmaterials.Withmolecular gelstrategy,aseriesofmaterialswithfavorableperformancehavebeenobtained.In thischapter,theapplicationofmoleculargelstrategyinthepreparationoffluorescencesensingthinfilmmaterialsandlow-densityporousmaterialsisdescribedin combinationwithlaboratorypractice.Onthisbasis,themainchallengesofrelated researchareproposed,andtheprospectanddevelopmenttrendofmoleculargel expansionresearchareprospected.
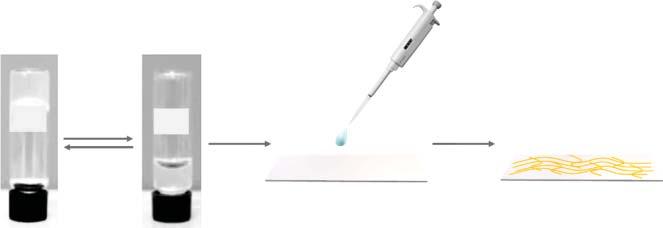
Scheme1.1 Moleculargelstrategyinfabricationoffluorescentsensingfilmandgel emulsionaswellasporousmaterials.
1.2MolecularGelasIntermediateinSynthesizing
FluorescentSensingFilmswithHighPerformance
Comparedwithhomogenouschemicalsensing,film-basedchemicalsensingowns advantagesinlittleinfluenceonthedetectionsystem,favorablereusability,andeasy tobeintegratedintodevice.[12,13]Amongchemicalsensors,fluorescentsensors havereceivedconsiderableattention,becauseoftheirmeritsinregardtohighsensitivity,multipleparameters,littleconsumptionofreagent,aswellasnoreference, orevenremotemonitoring[14,15].Forafilm-basedsensordevice,fluorescentfilm withdesiredsensingperformanceplaysapivotalrole.Thus,exploringfluorescent filmwithdesiredsensingabilitywilllayasolidfoundationinthedevelopmentof high-performancesensors.Generally,sensingperformanceofafluorescentfilmcan beevaluatedbysensitivity,selectivity,responsetime,reversibility,reusability,and stability.Sensingbehaviorofafluorescentfilmisnotonlydependentonconstitution ofthefilm,butalsoonmicrostructureofthefilm[12,16].Accordingly,rationally designedfluorescentsensingunitandsuitablefilmfabricationstrategyshouldbe twokeyissuesinfluorescentsensingfilmfabrication.Adesiredsensingfilmwould showcharacteristicsinabundanceofeffectivesensingunits,whichreactstotheanalytetocauseachangeinfluorescencesignal[17].
Fluorescent
1.2MolecularGelasIntermediateinSynthesizingFluorescentSensingFilmswithHighPerformance 3
Gel Stimuli Sol CastingDrying
Gel network-based fluorescent film
Figure1.1 Illustrationofthemoleculargelforfluorescentfilmfabrication.
Asknown,solventisthemaincomponent(morethan90%)inamoleculargel andtheself-assembledsupramoleculargelnetworkaccountsforonly ∼2%.Thus, thecorrespondingxerogelobtainedfromremovingthesolventpossessesfeatures ofporousnetworkstructure,whichisbeneficialforfluorescentsensingbecauseof thepermeabilityatthemolecularlevel.Preferablepermeabilitywilllayafoundationforfastandeffectiveinteractionbetweentheanalyteandthesensingunitsand, therefore,resultinimprovedsensingperformance,suchasfastresponseandhigh reversibilityandsensitivity.Inaddition,shearthixotropyofsomemoleculargels wouldfacilitatesensingfilmpreparation,astheycanbeeasilysprayedorspincoated ontothesolidsubstratetoformporousfilms.Accordingly,moleculargelstrategy hasbeendevelopedtofabricatedifferenttypesoffluorescentsensingfilms[17–19]. DetailsofthestrategyareillustratedinFigure1.1.Inthestrategy,fluorescentunitis introducedtothemoleculardesignofagelatorandtheachievedfluorescentmoleculargelsareusedtoconstructsensingfilmwithporousnetworkmicrostructure. Withthemethod,fluorescentfilmswithfavorablesensingperformanceforvolatile organiccompounds(VOCs),narcotics,andchemicalagenthavebeenrealizedand someofthesensingfilmshavebeenmadeintodevicesforreal-lifeapplications.
1.2.1MolecularDesign
Tomakefluorescentsensingfilmwithmoleculargelstrategy,structureofthemoleculargelatoristhebasis;moleculardesignandsynthesisplayanimportantrole.In general,therearetworequirementsthatamoleculargelatorneedstomeetwhenitis tobeusedtopreparefluorescentsensingfilmviamoleculargelstrategy:(i)fluorescentunitthatcanrespondtosometypicalanalyteshouldbeincludedand(ii)units withsupramolecularbindingsitesshouldalsobeincludedtoendowthemolecule self-assemblyability(Scheme1.2).Uptonow,varioustypesoffluorophoreshave beenreported[20–22].Basedontheircharacteristicsinmolecularstructures,differenttypesoffluorophoreshavevariedresponsetotheanalytes.Forexample,mostof theperylenebisimide(PBI)derivativesareelectron-deficientcompoundsandtheir fluorescenceiseasilyquenchedbysomeelectron-richcompounds,suchasamines andphenols.[23,24]Fluorescenceofsomepolycyclicaromatichydrocarbon(PAH) derivativesshowstypicaldifferencebetweentheirmonomerstateandaggregated
1MolecularGelasMediumorIntermediateinFunctionalMaterialsSynthesis state[23].Aftermorethan20yearsofdevelopment,abundanceofgelatorunitshave beenreported,includingcholesterolanditsderivatives,alkanes,aminoacids,and aromaticcompounds.[1].Rationalcombinationofafluorophoreandthegelator unitswillenabletheaccessoffavorablefluorescentmoleculargelator,whichlay solidfoundationforfluorescentsensingfilmfabrication.
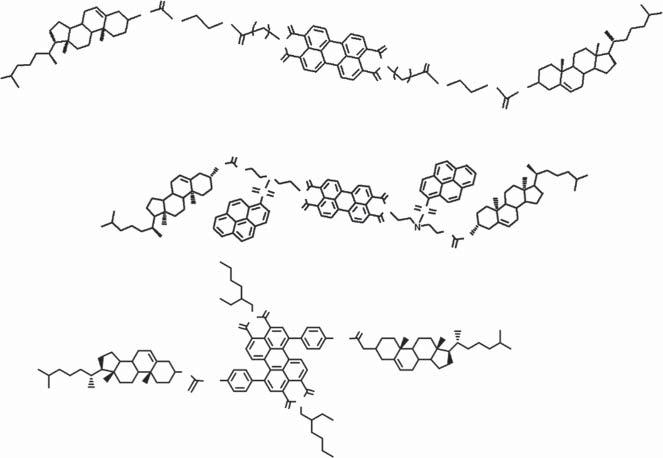
Scheme1.2 Structuredesignforgelatormoleculesusedforfluorescentfilmfabrication.
InSections1.2.2and1.2.3,fluorescentgelatorswithdifferentmolecularstructureswillbeintroduced.Basedonsystematicstudyoftheself-assemblyfeature, gelationbehavioraswellasrheologicalpropertiesofthegelators,fluorescentfilms willbeobtainedviatechniquesofdropcastingorspraycoating.Whengelatingsolventisremoved,poroussensingfilmscanbeachieved.Meanwhile,porosityand microstructureofthefilmcanbeadjustedbyvaryingtheconcentrationofthegelator,gelatingsolvent,orthedryingprocess.Onthatbasis,thephotophysicalbehavior andsensingperformanceofthefilmswillevaluated.Furthermore,filmswithoutstandingsensingperformancewillbeselectedtomakesensordevice.Themolecular gelstrategywillnotonlybroadentheapplicationofmoleculargel,butalsoprovide convenienceforsensordevelopment.
1.2.2MolecularGelStrategy-BasedSensingFilmforVOCVapor Detection
VOCsincludeavarietyofchemicals,whichareemittedasgasesfromcertainsolids orliquids[25,26].MostVOCsaretoxicandsomeofthemhaveshort-orlong-term adversehealtheffects,includingirritationofeyes,nose,andthroat,anddamageto theliver,kidneys,andcentralnervoussystem.SensorsforVOCsdetectionwouldbe ofgreathelpinreducingexposurerisk.
Amines,especiallyorganicamines,arerecognizedasimportantenvironmental pollutantsandtheyposeadirectthreattohumans[27,28].Meanwhile,active ingredientsofsomeillegalchemicalsarederivativesoforganicamines[12].Many PBIderivativeshaveshownexcellentsensingperformance,especiallyfororganic amines,duetotheirelectron-deficientnature.Inaddition,energiesofhighest occupiedmolecularorbital(HOMO)andlowestunoccupiedmolecularorbital (LUMO)canbeadjustedbyvaryingthesubstituents(boththesubstitutiontype andposition)[29–32].Inthisway,chargetransferprocessbetweensomePBI derivativesandtypicalaminescanbetunedandsensingperformanceofthesystem canbeoptimized.Nevertheless,strong �� –�� interactionbetweenthePBImolecules resultsinpoorsolubilityofthePBIderivativesandmaybringdifficultiesfortheir usethroughmoleculargelstrategy.Forthisreason,cholesterolgroupshavebeen involvedtoimprovethesolubilityofthePBImoleculesandalsoendowthem supramolecularassemblyability.
Linker
Fluorescent unitSelf-assembly unit
1.2MolecularGelasIntermediateinSynthesizingFluorescentSensingFilmswithHighPerformance 5
Compound 1: n=2
Compound 2: n=11
Compound 3
Compound 4
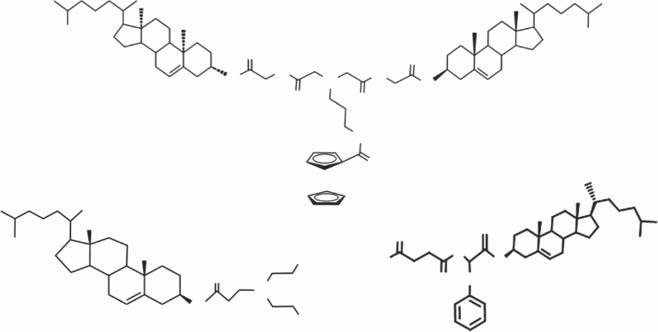
Scheme1.3 Molecularstructuresofcompound1,2,3,and4.
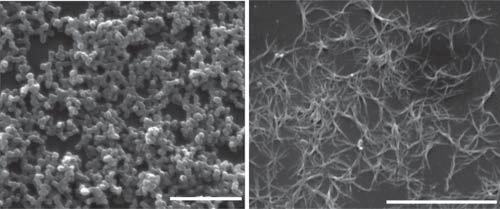
Aseriesofmolecularsystems(compounds1∼4)fororganicaminesensinghas beenachievedbythecombinationofthetwounits(Scheme1.3)[29–32].Thefour compoundsshowvariedassemblybehaviorindifferentsolventsanditwasfound thatsensingperformanceofthesystemwashighlydependentontheassembled structureofthemolecules.Themoleculargel-basedfilmwasobviouslysuperiorto thefilmobtainedbysimplecastingofthecompounds.Sensingfilmsobtainedfrom moleculargelstrategyshowfeaturesofporousnetworkstructure:film1ismade upofnanofibersof ∼80nm[29];film2ischaracterizedbystackingofnanoparticlesof ∼150nm(Figure1.2a)[30];film3containsabundanceofnanofibersof ∼100nm(Figure1.2b)[31];morphologyoffilm4issimilartothatoffilm3except forthesmallersizeofnanofibers[32].Thefourfilmsexhibiteddifferentsensing
Figure1.2 Self-assembledstructuresofcompound2(a)and3(b).(aandb)SEMimagesof film2and3throughtheassemblyofcompound2and3,respectively.Source:Adaptedwith permissionfromHeetal.[31]andShangetal.[32].©2016Elsevier.
(a)
(b)
1. MPEA 2. Xylidine 3. o-toluidine 4. 1,4-diaminobenzene
5. Aniline 6. Propylamine 7. Methyiamine 8. Hydrazine hydrate 9. Triethylamine 10. Diethylenetriamine 11. Triethylenetetramine 12. Ammonium hydroxide 13. Ethanediamine 14. Diethylamine 15. Toluene 16. Chloroform 17. Acetone 18. Dichloromethane 19. n-hexane 20. Cyclohexane 21. Methanol 22. Apple pomace 23. Ethanol 24. THF 25. Phenol 26. Banana juice 27. Benzene 28. Water 29. Acetonitrile.
11121314151617181920212223242526272829
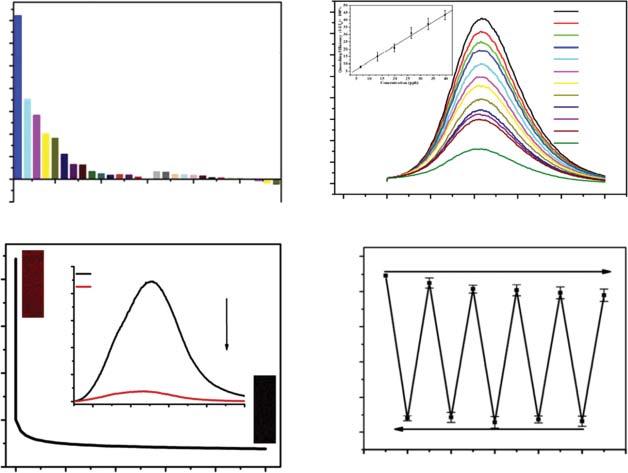
Figure1.3 Sensingperformanceofsometypicalfluorescentfilmsbasedonmoleculargel strategy.(a)Fluorescenceresponseoffilm2toaseriesofvolatilecompounds;(b) fluorescenceresponseoffilm3toaniline;(c)fluorescenceresponseoffilm3to N -methyl-phenethylamine(MEPA);(c)reversiblesensingresponseoffilm2toMEPAvapor; insetshowsthefluorescenceimagesofthefilmbefore(up)andafter(down)exposureto MEPAvapor;(d)reversiblefluorescenceresponseoffilm2toMEPAvapor.Source:Heetal. [31]/Elsevier.
performanceandshowedfavorablesensingperformance(sensitivity,responsetime, andreversibility)inorganicamineanditsanalogues,suchasmethylamphetamine detection(Figure1.3).Fluorescenceoffilmcanbequenchedbymostcommonly usedaminestestedwithquenchingefficienciesintherangeof60∼80%.Sensingperformanceoffilm2issimilartothatoffilm1,butthequenchingefficienciesaredifferent(40∼85%).Film3showedremarkablefluorescenceresponse(∼90%quenching efficiency)tomethylamphetamine,ananalogueofnarcotics.Detectionlimitoffilm 3tomethylamphetamineis5.5ppb,whichoffersgreatopportunityfornarcotics detection.Detectionlimitoffilm4toanilinevaporis15ppbandafilm4-basedsensordevicecanbeusedtodetectsimulatedexhaled(withanilinevapor)samplefor lungcancerpatients.Thedifferentsensingbehaviorsofthethreefilmsshouldbe attributedtobothvariedmolecularstructureandself-assembledmicrostructureof thefilms.
SimilartoPBI,naphthalimide(NID)isalsoanelectron-deficientfluorophore.A long-chainalkane-modifiedNIDderivative(compound5,Scheme1.4)hasshown stronggelationability,andseveralsolvents(toluene,hexane,etc.)canbegelledusing theNIDgelator[33].Xerogelsfromtolueneandhexanehavedifferentmorphologies
1.2MolecularGelasIntermediateinSynthesizingFluorescentSensingFilmswithHighPerformance 7 andthecorrespondingfilmsshowdifferentsensingperformance.Thefilmmadeup ofuniformnanofiberswasprovedtobesufficientinanilinesensingwithresponse lessthan1sandadetectionlimitof12.7mgm 3 ppm.
Scheme1.4 Molecularstructureofcompound5.
7-Nitrobenzofuranzanisawidelyusedbioimagingandbioanalysisagent, whosefluorescenceissensitivetoenvironmentalchange.Usingphenylalanineas thelinker,aderivativeof7-nitrobenzofuranzanwasconnectedwithcholesterol andcompound6wasprepared(Scheme1.5)[34].Owingtothechiralityofthe phenylalanine(D/L)group,theisomersofcompound6showeddifferentgelation behaviors(Table1.1).Systematicgellingbehaviorstudyrevealedthatthecompound wasanexcellentmoleculargelatorandafluorescentDMSOgelwasgot.Thegel wassensitivetochemicalstimulus:introductionofammoniacouldbreakdownthe gelandfurtherremovaloftheammoniabybubblingofN2 oraircouldresultin therecoveryofthegel.Meanwhile,thereversiblesol-geltransitionisaccompanied byobviousfluorescencechange:theDMSOgelishighlyfluorescent,butthe fluorescenceisquenchedwhenammoniaisintroduced;thefluorescencecanbe recoveredwhentheammoniaisremoved.Therefore,sensingfilmforammoniacan beeasilyobtainedbyspraycoatingorhigh-speedcoating.Microstructureaswell sensingperformanceofthefilmcanbeoptimizedbychangingtheconcentration ofthecompound.Underlowgelatorconcentration,thefilmischaracterized withlooselyandrandomlypackednanofibers,whilethefibersbecomedenseand uniformwhenconcentrationofthegelatorisincreased.Withthesensingfilm asthekeypart,fluorescentsensordeviceformonitoringammonialeakagewas realized.

Scheme1.5 Structureofthetypicallydesignedmoleculargelator,compound6.Source: BasedonYuetal.[34].
O2N
1MolecularGelasMediumorIntermediateinFunctionalMaterialsSynthesis
Table1.1 Gelationbehaviorofcompound6(2.5wt%).
SolventC6-LC6-DSolventC6-LC6-D
MethanolIS n-HexaneII
EthanolIG n-HeptaneII
n-PropanolIG n-OctaneII
n-ButanolIG n-NonaneII
n-PentanolIG n-DecaneII
n-HexanolIPCyclohexaneII
n-HeptanolIPAcetonitrileIG
n-OctanolIPWaterII
n-NonanolIIDMFSP
n-DecanolIIDMSOIG
AcetoneSPAceticacidII
BenzeneSSEthyletherII
TolueneSSEthylacetateSS THFSSDichloromethaneSS
PyridineSSTrichloromethaneSS
Note:Irepresentsinsoluble;Srepresentssolution;Prepresentsprecipitate;Grepresentsgel.
Hydrogenchlorideisanextremelyharmfulcorrosivegas,whicheasilycauses irritationtoeyes,skin,andrespiratorysystem.Moreover,theexistenceofhydrogen chlorideinnear-earthspacewillleadtoabnormalatmosphericprocessesoreven affecttheglobalenvironment.Thus,thedetectionofhydrogenchlorideingas stateisofgreatimportance.Bythecombinationof1,4-bis(substitutedphenylacetylenyl)benzene,glucose,andcholesterol,afluorescentmoleculewithtypical assemblyabilityisconstructed(compound7,Scheme1.6)[35].Themolecules existintheaggregatedforminchloroform,andfluorescentfilmsconsistingof numeroussphericalparticlescanbeobtainedbytransferringtheaggregatesonto asolidsurface.Hydrophilicityaswellmorphologiesofthefilmcouldbetuned bycontrollingthedryingprocess.Quickdryingproduceshydrophobicfilm,but slowdryinginhumidairleadstohydrophilicfilm.Thehydrophobicfilmismade upofparticlesofsize1∼3 μm;thehydrophilicfilmischaracterizedbyvehiclesof size ∼10 μm.Differenceinfilmhydrophilicityandmorphologycausesremarkable differenceinsensingperformance(tohydrogenchloridevapor),whichshouldbe attributedtotheinteractionbetweentheanalyteandthefilm.Fluorescenceof thehydrophilicfilmissensitivetothepresenceofhydrogenchloridevaporwitha detectionlimitof ∼0.4ppb.Butfluorescenceofthehydrophobicfilmshowedlittle changeuponhydrogenchloridevaporexposure:highconcentrationofhydrogen chloridevapor(88ppmoreven2860ppm)couldonlycause25%offluorescence quenching.
1.3MolecularGelasIntermediateinSynthesizingPorousMaterials 9
Scheme1.6 Molecularstructureofcompound7.Source:BasedonSunetal.[35].
1.2.3MolecularGelStrategy-BasedFilmforChemicalsSensing inLiquidPhase
Waterpollutionisoneofthemostchallengingproblemsfacedbyhumans.Methods thatcanbeusedforreliable,sensitive,anduser-friendlydetectionoftoxicchemicals inwaterareurgentlydemanded.Withtheadvantagesofmoleculargelstrategyin fluorescentsensingfilmfabrication,aseriesoffluorescentmoleculeswithfavorite self-assemblybehaviorhavebeensynthesized[36–38],andcorrespondingfluorescentsensingfilmwithtypicalself-assembledstructurearepreparedforthedetection oftoxicchemicalsinwatersystem.Someofthefilmscanbeusedtodetecttoxic substances,suchasnervepoison,pesticide,formaldehyde,inwater.Thedetection issensitive,selective,andtheprocesscanberealizedeitherbyspectroscopy,orby visualization.
1.3MolecularGelasIntermediateinSynthesizing PorousMaterials
Becauseoftheiradvantagesatlowdensity,highporosity,largesurfacearea,and tunableporesize,porousmaterialsareplayingincreasinglyimportantrolesin manyfields,suchasseparation,catalysis,molecularrecognition,bioengineering, andenvironmentalscience[39,40].Akeypointinporousmaterialspreparation istocontrolthepores,includingporesize,poreshape,andporedistribution. Commonlyusedmethodsforporousmaterialspreparationincludesgasfoaming,
Figure1.4 Illustrationofatypicalprocessforthepreparationofmolecular gelator-stabilizedgelemulsionsandthecorrespondingporousmaterials.Source:Basedon Chenetal.[42].
porogenadding,ortemplating[41].Amongthesemethods,templatingispreferred owingtoitsadvantageinregardtofacilecontrolofporesizeanddistribution.Gel emulsion,alsoknownashighlyconcentratedemulsionorhigh-internalphase ratioemulsion,isatypicaltemplateinporousmaterialspreparation.Similartoa gel,gelemulsionissolidlike,apparently.Butinthemicrophase,agelemulsion istotallydifferentfromagel.Thereismorethanonephaseinagelemulsion, usuallycalledcontinuousphaseanddispersedphase.Differentfromtraditional emulsions,gelemulsionshaveseveraladvantageswhenusedasreactionintermediate.Coexistenceofhydrophobicandhydrophilicdomainsenablesgoodsolubility ofthegelemulsionsysteminbothnonpolarandpolarreactants.Highviscosity ofthesystemhelpstoavoidtheprecipitationoftheproductandprovidebasisfor theformationofnetworkstructure.WateraccountsforalargeportioninW/O (waterinoil)gelemulsion,whichisbenefitforthereductionoforganicliquidfor aconsiderationofbothenvironmentalprotectionandcostsaving.Thesizeand shapeoftheproducedmaterialscanbeeasilycontrolledbyusingamold.Internal structureoftheporousmaterialscanbeeasilytunedbyvariationoftheconstitution ofthegelemulsions(Figure1.4)[42].Whenusedasreactionintermediate,gel emulsionscanserveasbothtemplateandsolvent,andtheyarewidelyusedinthe fabricationoflow-densityporousmaterials.
Formationofagelemulsionisbasedonthestabilizer,whichreducesthe interfacialenergybetweentheoilphaseandthewaterphase[43].Uptonow,the widelyusedstabilizersincludesurfactants,micro-ornanoparticles(organicand inorganic),andmoleculargelators.Usually,thestabilizeraccountsforlessthan 3%(w/v)inagelemulsionstabilizedbymoleculargelator,whichismuchlower thanwhensurfactantisusedasstabilizer.Comparedwithgelemulsionstabilized bymicro-ornanoparticles,gelemulsionsstabilizedbymoleculargelatorspossess betterstability.Inaddition,undesiredchangessuchasphaseinversionandphase separationinparticle-stabilizedgelemulsionscanbeavoidedinthemolecular gelator-stabilizedsystem.Moreover,contentofthedispersedphaseisnotlimited toamaximumof74%inmoleculargel-stabilizedgelemulsion.Meanwhile,the ratioofthetwophases(oilphaseandwaterphase)inmoleculargel-stabilizedgel emulsioncanbetunedinawiderange,whichprovidesthebasisforadjustingthe internalstructureoftheporousmaterials.Lowcontentofstabilizerisbeneficial forcostsavingandalsoforfurtherpurificationoftheobtainedporousmaterials.
1.3MolecularGelasIntermediateinSynthesizingPorousMaterials 11
Basedontheadvantagesofmoleculargelatoringelemulsionstabilization,plenty oflow-densityporousmaterialshavebeensynthesized.Detailsoftheporous materialsaswellastheirpreliminaryapplicationsinadsorption,absorption,and environmenttreatmentwillbeintroducedinSections1.3.1and1.3.2.
1.3.1PorousMaterialsforRemovalofOilonWaterSurface
Withrapiddevelopmentinchemicalengineering,thereisincreasingdemandfor petroleumextraction,refining,andapplications.Asaconsequence,leakageof petroleumortherelatedproductshasbecomeanemergingproblem,whichposes hugethreatstoecologyandhumanhealth[44,45].Sorptionisanefficientwayto reducetheriskofpetroleumleakage,wheresorbentswithhighperformanceare desired.Itisofgreatsignificancetoexploresorbentswithhighsorptioncapacity. Porousmaterialsarewidelyusedassorbents.Sorptionabilityofporousmaterialsis highlydependentontheconstitution,internalstructure,hydrophilicity,mechanical strength,aswellasstabilityofthematerials.Meanwhile,costandthecomplexity ofthefabricationprocessareimportantissuesthatneedtobeconsideredwhen thematerialsareputintoapplication.Withtypicallydesignedmoleculargelatoras stabilizer,aseriesofgelemulsionshavebeenprepared.Polymerizablemonomers, suchasstyreneandacrylicacid,areusedasthecontinuousphaseandwaterserves asthedispersedphase.Then,thegelemulsionsundergoapolymerizationanda subsequentdryingprocessandthusporousmaterialscanbeachieved.
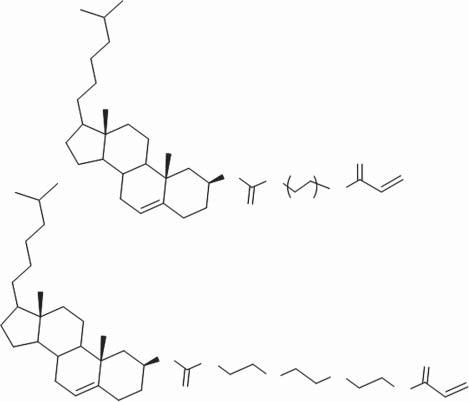
In2009,ProfessorFang’sgroupreportedthefirstmoleculargelator-basedgel emulsionstabilizer(compound8,Scheme1.7)[46].Thegelatorcontainstwo cholesterolgroupsandhasastronggellingability.Withthegelator,different typesofoilscanbegelated,including n-heptane, n-octane, n-nonane, n-decane, kerosene,diesel,andgasoline.Basedonthegels,aseriesofgelemulsions(W/O type)weremadebyintroducingwaterintothegellingsystem.Microscopicstudies andrheologicalmeasurementsuggestedthatthegelemulsionsarestableandhave typicalfoamingstructure.Gelatorsbasedonthecombinationofcholesteroland ferrocenehavealsobeenproventobecapableinthestabilizationofgelemulsion. Somecholesterol-ferrocenegelatedgelsareresponsivetomultiplestimuli.
Scheme1.7 Molecularstructureofcompound8.
MolecularstructuresofsometypicalmoleculargelatorsthatcanserveasstabilizersingelemulsionsformationareshowninScheme1.8.Asanexample,aseriesof gelemulsionsofW/Otypecanbeobtainedwheneitherofthegelatorsisusedasa
1MolecularGelasMediumorIntermediateinFunctionalMaterialsSynthesis
stabilizerandtert-butylmethacrylateisusedasthecontinuousphase[47].Polymeric porousmaterialswithvariedinternalstructureaswellasmechanicalpropertiescan beachievedbypolymerizationofthedifferentgelemulsions(Figure1.5)[48].The porousmaterialsshowhighperformanceinabsorptionoforganicliquids.Meanwhile,theabsorbedsolventscanbeeasilyrecoveredbyasqueezingprocess.Inthis way,theporousmaterialscanberecycledandtheabsorptionprocesscanberepeated formorethan10times.Exceptforoptimizationonthewatercontentinthegel emulsion,propertiesaswellabsorptionbehavioroftheporousmaterialcanalso beenhancedbytheintroductionofsometypicaladditives.Forexample,introducingsomereactivesilylreagentsishelpfultoimprovethemechanicalstrengthand absorptioncapacityoftheporousmaterials.
Scheme1.8 Molecularstructureofthegelators(compound9–11)stabilizerusedforgel emulsionandporousmaterialspreparation.
Compound10,whichisatypicalcholesterolgelatorwithtwohydroxylgroupsin thestructure,canalsoserveasastabilizerforW/Ogelemulsions[49].Usingstyrene asthecontinuousphaseandwaterasthedispersedphase,aseriesofgelemulsionswithoutstandingstabilitycanbeprepared.Afterpolymerizationanddrying, correspondingporouspolystyrenematerialsweresynthesized.Densityofthematerialscanbeadjustedbythevariationofwatercontentinthegelemulsionsand polystyrenematerialswithultralowdensitywaspresented.Thepreparationcanbe processedundermildconditions,wherenocomplexorhighenergycostdryingprocessisneeded.Nohigh-costpost-treatmentisneededasthewaterinthepolymerizedmaterialscanberemovedbysqueezingandsimpledryingat50 ∘ C.Theporous polystyrenematerialscanbeusedtoremovesomeorganicliquidsfromwater.The materialscanberecycledbysubsequentsqueeze,washingwithethanol,anddrying steps(Figure1.6)[50].
Anotherrepresentativemoleculargelatorstabilizercontainsaphenylalanine groupasalinker,andacarboxylgroupinacholesterolderivative(compound11) [51].Withthestabilizer,gelemulsionwithtunablerheologicalpropertiescanbe
Compound 9
Compound 10Compound 11
1.3MolecularGelasIntermediateinSynthesizingPorousMaterials
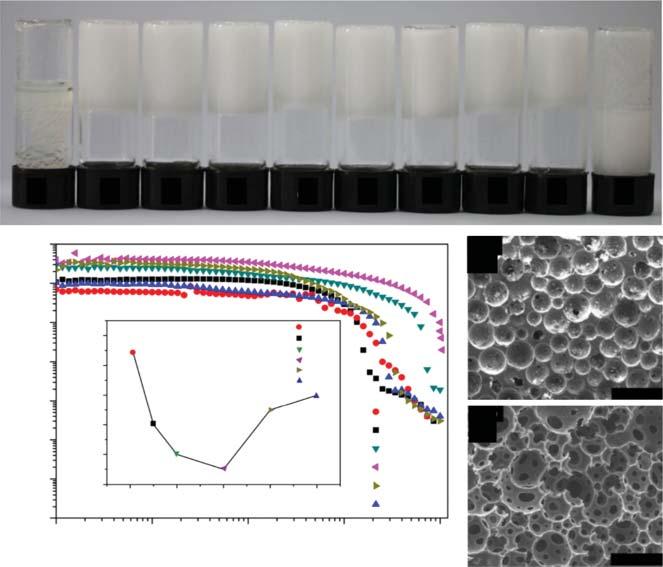
Figure1.5 Typicalgelemulsionsandporousmaterialspreparedbyusingmolecular gelatorasstabilizer.(a)Photosofthestyrenegel.Photosofgelemulsionwithdifferent amountsofstabilizer.(b)Rheologicalbehaviorsofgelemulsionswithdifferentwater contents.(candd)SEMimagesofporousmaterialspreparedfromgelemulsionswith differentamountsof n-heptaneasadditives.
prepared.Inaddition,low-densityporousmaterialswithfavorablemechanical strengthandflexibilityareobtained.Thesynthesizedmaterialswithadensityof 0.18gm 3 havegoodcompressibility;thematerialscanbefullyrecoveredwith70% compressionandthereislittlechangeaftercompression(at70%compression)for 25times.Thefavorablemechanicalstrengthlaysasolidfoundationforfurther applicationsofthematerials.
1.3.2PorousMaterialsforVOCsAdsorption
Efficientmethodforpollutedairtreatmentisagreatchallenge,especiallythe removalofVOCs.Organicchemicalsarenotonlywidelyusedinchemicalproduction,butalsousedasingredientsinhouseholdproducts,suchaspaints, detergents,disinfectors,andcosmetics.RemovalofVOCsfrompollutedairhas attractedtremendousattentionaroundtheworld[52,53].Usually,concentrations ofsomeVOCsaremuchhigherindoorsthanoutdoors,whichmaycauseshortandlong-termhealtheffects.AmongthemethodsforVOCsremoval,adsorptionis preferredbecauseofitsadvantagesintermsoflowcostandhighefficiency.
(c)
(d)
Figure1.6 Typicalapplicationofgelemulsion-templatedporousmaterialsinorganic solventremovalfromwater.(a)Maximumoiladsorptioncapacityoftheporousmaterials. (b)showsthereusabilityofthematerials.Source:Liuetal.[48]/Wiley.
Toavoidtheeffectofunreactedstabilizer(moleculargelator)tothepolymerizedporousmaterials,severalcholesterolderivativeswithpolymerizablebonds havebeendesignedandsynthesized(Scheme1.9)[54,55].Usingthegelators asstabilizers,aseriesofW/Ogelemulsions(continuousphase:styrene;dispersedphase:water)havebeenprepared.Then,thegelemulsionswereforcedto undergopolymerizationanddrying.Accordingly,porousmaterialswithdifferent constitutionandvariedinternalstructurescanbesynthesized.Owingtothe hydrophobicnatureoftheporousmaterials,theyhavetheabilitytoadsorb VOCs(includingbenzene,toluene,ethylbenzene,andethylene)andshow higheradsorptioncapacitycomparedtothecommonlyusedadsorbents,active carbon.
1.4SummaryandPerspectives
Physicalgels,especiallymoleculargelsmadefromlow-molecularweightgelators andsolventsareacombinationofsynthetictechnologyandsupramolecular self-assembly.Differentfromchemicalgels,gelnetworksofmoleculargelsrely onsupramolecularinteractions(includinghydrogenbonding, �� –�� stacking, hydrophobicinteractions,electrostaticinteractions,dipoleinteractions)between gelatormolecules,solventmolecules,andgelator-solventmolecules.Theremarkabledifferenceinstructuresmakesthetwotypeofgels(chemicalgelsandphysical
Scheme1.9 Structuresofstabilizerswithpolymerizablebonds.
gels)showdifferentresponsetoexternalstimulus.Forexample,networksinboth typesofthegelscanbebrokenundervigorousshearing,suchasstirring,pumping, orshaking,whichmayresultinphaseseparationorphasetransitionofthegel.Itis hardlyforthechemicalgelstoberecoveredafterthedisruption,asthechemically bondednetworkisreversiblybroken.Butmanymoleculargelscanberecovered whentheshearingisremoved.Thepreferablestimulus-responsivecharacteristics ofmoleculargelsendowthemgreatpotentialinapplicationsoftemplatesynthesis, controlledrelease,tissueculturing,separation,informationstorage,sensing,etc. Moreover,theconcentrationofagelatorisusuallyverylowinamoleculargel, whichisabigadvantageforfurtherapplicationsinmolecularrecognition,template synthesis,andsyntheticintermediate.Introducingthemoleculargelstrategyto synthesizehigh-performancefluorescentsensingfilmandlow-densityporousmaterialsshowssomepreliminaryapplicationofmoleculargelinsyntheticintermediate. Itisexpectedthatmoleculargelswillplaymoreimportantrolesintheresearcharea ofsyntheticmedium,whichmayincludeinnovationinliquid–liquidextraction, cultivationofhigh-qualitycrystals,stabilizationofsolid–liquidsuspensionsystem extractiontechnology.
Inaddition,thereareproblemstobesolvedwhenmoleculargelstrategyisused inthesynthesisoffluorescentsensingfilmsandlow-densityporousmaterials. Forexample,adhesionofsomefluorescentfilmsobtainedfrommoleculargel needstobeimproved.Effectofmolecularstructureofthegelatorstabilizeron thepropertiesofthegelemulsionsaswellasthelow-densityporousmaterialsneedstobegivenmoreattention.Thiswillpavewayforachievingporous materialswithexcellentmechanicalstrengthandtunableinternalmicrostructure.
1MolecularGelasMediumorIntermediateinFunctionalMaterialsSynthesis
References
1 George,M.andWeiss,R.G.(2006).Molecularorganogels.Softmattercomprised oflow-molecular-massorganicgelatorsandorganicliquids. Acc.Chem.Res. 39: 489–497.
2 Weiss,R.G.(2014).Thepast,present,andfutureofmoleculargels.Whatisthe statusofthefield,andwhereisitgoing? J.Am.Chem.Soc. 136:7519–7530.
3 Smith,D.K.(2009).Lostintranslation?Chiralityeffectsintheself-assemblyof nanostructuredgel-phasematerials. Chem.Soc.Rev. 38:684–694.
4 Yan,N.,Xu,Z.,Diehn,K.K.etal.(2013).Howdoliquidmixturessolubilize insolublegelators?Self-assemblypropertiesofpyrenyl-linker-gluconogelatorsin tetrahydrofuran-watermixtures. J.Am.Chem.Soc. 135:8989–8999.
5 Abdallah,D.J.,Sirchio,S.A.,andWeiss,R.G.(2000).Hexatriacontaneorganogels. Thefirstdeterminationoftheconformationandmolecularpackingofa low-molecular-massorganogelatorinitsgelledstate. Langmuir 16:7558–7561.
6 Estroff,L.A.andHamilton,A.D.(2004).Watergelationbysmallorganic molecules. Chem.Rev. 104:1201–1217.
7 Prost,J.,Jülicher,F.,andJoanny,J.-F.(2015).Activegelphysics. Nat.Phys. 11: 111–117.
8 Chivers,P.R.A.andSmith,D.K.(2019).Shapingandstructuringsupramolecular gels. Nat.Rev.Matter. 4:463–478.
9 Ruiz-Olles,J.,Slavik,P.,Whitelaw,N.K.,andSmith,D.K.(2019).Self-assembled gelsformedindeepeutecticsolvents:supramoleculareutectogelswithhigh ionicconductivity. Angew.Chem.Int.Ed. 58:4173–4178.
10 Hawkins,K.,Patterson,A.K.,Clarke,P.A.,andSmith,D.K.(2020).Catalyticgels foraprebioticallyrelevantasymmetricaldolreactioninwater:fromorganocatalystdesigntohydrogeldiscoveryandbackagain. J.Am.Chem.Soc. 142: 4379–4389.
11 Rizzo,C.,Arcudi,F.,Ðord-evi ´ c,L.etal.(2018).Nitrogen-dopedcarbon nanodots-ionogels:preparation,characterization,andradicalscavengingactivity. ACSNano 12:1296–1305.
12 Liu,K.,Shang,C.,Wang,Z.etal.(2018).Non-contactidentificationanddifferentiationofillicitdrugsusingfluorescentfilms. Nat.Commun. 9:1695.
13 Ding,L.andFang,Y.(2010).Chemicallyassembledmonolayersoffluorophores aschemicalsensingmaterials. Chem.Soc.Rev. 39:4258–4273.
14 Thomas,S.W.,Joly,G.D.,andSwager,T.M.(2007).Chemicalsensorsbasedon amplifyingfluorescentconjugatedpolymers. Chem.Rev. 107:1339–1386.
15 Wang,X.andWolfbeis,O.S.(2014).Opticalmethodsforsensingandimagingoxygen:materials,spectroscopiesandapplications. Chem.Soc.Rev. 43: 3666–3761.
16 Li,M.,Liu,J.,Shang,C.etal.(2019).Porousparticle-basedinkjetprintingof flexiblefluorescentfilms:enhancedsensingperformanceandadvancedencryption. Adv.Mater.Technol. 4:1900109.
17 Miao,R.,Peng,J.,andFang,Y.(2016).Recentadvancesinfluorescentfilmsensingfromtheperspectiveofbothmoleculardesignandfilmengineering. Y.Mol. Syst.Des.Eng. 1:242–257.
18 Miao,R.,Peng,J.,andFang,Y.(2017).Moleculargelsasintermediatesinthe synthesisofporousmaterialsandfluorescentfilms:conceptsandapplications. Langmuir 33:10419–10428.
19 Miao,R.andFang,Y.(2017).Extendedresearchonmoleculargels:fromthe perspectiveofdevelopmentofthreedimensionalfluorescentsensingfilmsand low-densityporousmaterials. Chin.Sci.Bull. 62:532–545.
20 Bosch,P.,Catalina,F.,Corrales,T.,andPeinado,C.(2005).Fluorescentprobes forsensingprocessesinpolymers. Chem.Eur.J. 11:4314–4325.
21 Basabe-Desmonts,L.,Reinhoudt,D.N.,andCrego-Calama,M.(2007).Designof fluorescentmaterialsforchemicalsensing. Chem.Soc.Rev. 36:993–107.
22 Thomas,S.W.,Joly,G.D.,andSwager,T.M.(2007).Chemicalsensorsbasedon amplifyingfluorescentconjugatedpolymers. Chem.Rev. 107:1339–1386.
23 Würthner,F.,Möller,C.R.S.,Fimmel,B.etal.(2016).Perylenebisimidedye assembliesasarchetypefunctionalsupramolecularmaterials. Chem.Rev. 116: 962–1052.
24 Zhan,X.,Facchetti,A.,Barlow,S.etal.(2011).Ryleneandrelateddiimidesfor organicelectronics. Adv.Mater. 23:268–284.
25 Haick,H.,Broza,Y.Y.,Mochalski,P.etal.(2014).Assessment,origin,and implementationofbreathvolatilecancermarkers. Chem.Soc.Rev. 43: 1423–1449.
26 Ramos,M.E.,Bonelli,P.R.,Cukierman,A.L.etal.(2010).Adsorptionofvolatile organiccompoundsontoactivatedcarbonclothsderivedfromanovelregeneratedcellulosicprecursor. J.Hazard.Mater. 177:175–182.
27 Zhang,S.,Yang,H.,Ma,Y.,andFang,Y.(2016).Afluorescentbis-NBDderivativeofcalix[4]arene:SwitchableresponsetoAg+ andHCHOinsolutionphase. Sens.Actuators,B:Chem. 227:271–276.
28 Chen,C.Y.,Ko,C.W.,andLee,P.I.(2007).Toxicityofsubstitutedanilinesto pseudokirchneriellasubcapitataandquantitativestructure-activityrelationship analysisforpolarnarcotics. Environ.Toxicol.Chem. 26:1158–1164.
29 Peng,H.,Ding,L.,Liu,T.etal.(2012).Anultrasensitivefluorescentsensing nanofilmfororganicaminesbasedoncholesterol-modifiedperylenebisimide. Chem.AsianJ. 7:1576–1582.
30 Wang,G.,Chang,X.,Peng,J.etal.(2015).TowardsanewFRETsystem viacombinationofpyreneandperylenebisimide:synthesis,self-assemblyand fluorescencebehavior. Phys.Chem.Chem.Phys. 17:5441–5449.
31 He,M.,Peng,H.,Wang,G.etal.(2016).Fabricationofanewfluorescentfilm anditssuperiorsensingperformanceto N -methamphetamineinvaporphase. Sens.Actuators,B:Chem. 227:255–262.
32 Shang,C.,Wang,G.,He,M.etal.(2017).Ahighperformancefluorescentarylaminesensortowardlungcancersniffing. Sens.Actuators,B 241: 1316–1323.
1MolecularGelasMediumorIntermediateinFunctionalMaterialsSynthesis
33 Fan,J.,Chang,X.,He,M.etal.(2016).Functionality-orientedderivatizationof naphthalenediimide:amoleculargelstrategy-basedfluorescentfilmforaniline vapordetection. ACSAppl.Mater.Interfaces 8:18584–18592.
34 Yu,H.,Lü,Y.,Chen,X.etal.(2014).Functionality-orientedmoleculargels: synthesisandpropertiesofnitrobenzoxadiazole(NBD)-containinglow-molecular massgelators. SoftMatter 10:9159–9166.
35 Sun,X.,Qi,Y.,Liu,H.etal.(2014).“YinandYang”tunedfluorescencesensing behaviorofbranched1,4-bis(phenylethynyl)benzene. ACSAppl.Mater.Interfaces 6:20016–20024.
36 Zhang,S.,Yang,H.,Ma,Y.,andFang,Y.(2016).Afluorescentbis-NBDderivativeofcalix[4]arene:SwitchableresponsetoAg+ andHCHOinsolutionphase. Sens.Actuators,B:Chem. 227:271–276.
37 Lü,Y.,Sun,Q.,Hu,B.etal.(2016).Synthesisandsensingapplicationsofanew fluorescentderivativeofcholesterol. NewJ.Chem. 40:1817–1824.
38 Qi,Y.,Sun,X.,Chang,X.etal.(2016).Anewtypeof1, 4-bis(phenylethynyl)benzenederivatives:opticalbehaviorandsensingapplications. ActaPhys.-Chim.Sin. 32:373–379.
39 Slater,A.G.andCooper,A.I.(2015).Function-leddesignofnewporousmaterials. Science 348:8075.
40 Oh,J.Y.,Rondeau-Gagné,S.,Chiu,Y.etal.(2015).Intrinsicallystretchableand healablesemiconductingpolymerfororganictransistors. Nature 539:411–415.
41 Georgakilas,V.,Tiwari,J.N.,Kemp,K.C.etal.(2016).Noncovalentfunctionalizationofgrapheneandgrapheneoxideforenergymaterials,biosensing, catalytic,andbiomedicalapplications. Chem.Rev. 116:5464–5519.
42 Chen,X.,Liu,K.,He,P.etal.(2012).PreparationofnovelW/Ogel-emulsions andtheirapplicationinthepreparationoflow-densitymaterials. Langmuir 28: 9275–9281.
43 Ikem,V.O.,Menner,A.,Horozov,T.S.,andBismarck,A.(2010).Highlypermeablemacroporouspolymerssynthesizedfrompickeringmediumandhigh internalphaseemulsiontemplates. Adv.Mater. 22:3588–3592.
44 Swannell,R.P.,Lee,K.,andDonagh,M.M.(1996).Fieldevaluationsofmarine oilspillbioremediation. Microbiol.Rev. 60:342–365.
45 Dowd,R.M.(1984).Leakingundergroundstoragetanks. Environ.Sci.Technol. 18:309A.
46 Peng,J.,Xia,H.,Liu,K.etal.(2009).Water-in-oilgelemulsionsfromacholesterolderivative:structureandunusualproperties. J.ColloidInterfaceSci. 336: 780–785.
47 Chen,X.,Liu,L.,Liu,K.etal.(2014).Facilepreparationofporouspolymeric compositemonolithswithsuperiorperformancesinoil-waterseparationa low-molecularmassgelators-basedgelemulsionapproach. J.Mater.Chem.A 2: 10081–10089.
48 Liu,J.,Wang,P.,He,Y.etal.(2019).Polymerizablenonconventionalgel emulsionsandtheirutilizationinthetemplatepreparationoflow-density, high-strengthpolymericmonolithsand3Dprinting. Macromolecules 52: 2456–2463.
49 Jing,P.,Fang,X.,Yan,J.etal.(2013).Ultra-lowdensityporouspolystyrene monolith:facilepreparationandsuperiorapplication. J.Mater.Chem.A 1: 10135–10141.
50 Liu,J.,Yang,H.,Liu,K.etal.(2020).Gel-emulsion-templatedpolymericaerogelsforwatertreatmentbyorganicliquidremovalandsolarvaporgeneration. ChemSusChem 13:749–755.
51 Chen,X.,Liu,L.,Liu,K.etal.(2015).Compressibleporoushybridmonoliths: preparation via alowmolecularmassgelators-basedgel-emulsionapproachand exceptionalperformances. J.Mater.Chem.A 3:24322–24332.
52 Shah,J.J.andSingh,H.B.(1988).Distributionofvolatileorganicchemicalsin outdoorandindoorair:anationalVOCsdatabase. Environ.Sci.Technol. 22: 1381–1388.
53 Hakim,M.,Broza,Y.Y.,Barash,O.etal.(2012).Volatileorganiccompoundsof lungcancerandpossiblebiochemicalpathways. Chem.Rev. 112:5949–5966.
54 Fu,X.,Wang,P.,Miao,Q.etal.(2016).Polymerizableorgano-gelator-stabilized gel-emulsionstowardthepreparationofcompressibleporouspolymericmonoliths. J.Mater.Chem.A 4:15215–15223.
55 Miao,Q.,Chen,X.,Liu,L.etal.(2014).Synergeticeffectbasedgel-emulsions andtheirutilizationforthetemplatepreparationofporouspolymericmonoliths. Langmuir 30:13680–13688.