FundamentalsofDrugDelivery
Editedby
HeatherA.E.Benson
CurtinUniversityofTechnology,SchoolofPharmacy,Perth,Australia
MichaelS.Roberts
UniversityofQueensland,SchoolofMedicine,Brisbane,Australia
AdrianC.Williams
UniversityofReading,SchoolofChemistry,FoodandPharmacy,Reading,UK
XiaowenLiang
UniversityofQueensland,FacultyofMedicine,BrisbaneStLucia,Australia
Thiseditionfirstpublished2022 ©2022byJohnWiley&Sons,Inc.
Allrightsreserved.Nopartofthispublicationmaybereproduced,storedinaretrievalsystem,ortransmitted,inany formorbyanymeans,electronic,mechanical,photocopying,recordingorotherwise,exceptaspermittedbylaw. Adviceonhowtoobtainpermissiontoreusematerialfromthistitleisavailableathttp://www.wiley.com/go/ permissions.
TherightofHeatherA.E.Benson,MichaelS.Roberts,AdrianC.Williams,andXiaowenLiangtobeidentifiedas theeditorsofthisworkhasbeenassertedinaccordancewithlaw.
RegisteredOffice
JohnWiley&Sons,Inc.,111RiverStreet,Hoboken,NJ07030,USA
EditorialOffice 111RiverStreet,Hoboken,NJ07030,USA
Fordetailsofourglobaleditorialoffices,customerservices,andmoreinformationaboutWileyproductsvisitusat www.wiley.com.
Wileyalsopublishesitsbooksinavarietyofelectronicformatsandbyprint-on-demand.Somecontentthatappears instandardprintversionsofthisbookmaynotbeavailableinotherformats.
LimitofLiability/DisclaimerofWarranty
Inviewofongoingresearch,equipmentmodifications,changesingovernmentalregulations,andtheconstantflow ofinformationrelatingtotheuseofexperimentalreagents,equipment,anddevices,thereaderisurgedtoreview andevaluatetheinformationprovidedinthepackageinsertorinstructionsforeachchemical,pieceofequipment, reagent,ordevicefor,amongotherthings,anychangesintheinstructionsorindicationofusageandforadded warningsandprecautions.Whilethepublisherandauthorshaveusedtheirbesteffortsinpreparingthiswork,they makenorepresentationsorwarrantieswithrespecttotheaccuracyorcompletenessofthecontentsofthisworkand specificallydisclaimallwarranties,includingwithoutlimitationanyimpliedwarrantiesofmerchantabilityor fitnessforaparticularpurpose.Nowarrantymaybecreatedorextendedbysalesrepresentatives,writtensales materialsorpromotionalstatementsforthiswork.Thefactthatanorganization,website,orproductisreferredtoin thisworkasacitationand/orpotentialsourceoffurtherinformationdoesnotmeanthatthepublisherandauthors endorsetheinformationorservicestheorganization,website,orproductmayprovideorrecommendationsitmay make.Thisworkissoldwiththeunderstandingthatthepublisherisnotengagedinrenderingprofessionalservices. Theadviceandstrategiescontainedhereinmaynotbesuitableforyoursituation.Youshouldconsultwitha specialistwhereappropriate.Further,readersshouldbeawarethatwebsiteslistedinthisworkmayhavechangedor disappearedbetweenwhenthisworkwaswrittenandwhenitisread.Neitherthepublishernorauthorsshallbe liableforanylossofprofitoranyothercommercialdamages,includingbutnotlimitedtospecial,incidental, consequential,orotherdamages.
LibraryofCongressCataloging-in-PublicationData
Names:Benson,HeatherA.E.,editor.|Roberts,MichaelS.,1949-editor. |Williams,AdrianC.,1963-editor.|Liang,Xiaowen,editor.
Title:Fundamentalsofdrugdelivery/editedbyHeatherA.E.Benson, MichaelS.Roberts,AdrianC.Williams,XiaowenLiang.
Description:Hoboken,NJ:Wiley,2022.|Includesbibliographical referencesandindex.
Identifiers:LCCN2021033395(print)|LCCN2021033396(ebook)|ISBN 9781119769606(cloth)|ISBN9781119769651(adobepdf)|ISBN 9781119769675(epub)
Subjects:MESH:DrugDeliverySystems|DrugAdministrationRoutes
Classification:LCCRS199.5(print)|LCCRS199.5(ebook)|NLMQV785| DDC615/.6–dc23
LCrecordavailableathttps://lccn.loc.gov/2021033395
LCebookrecordavailableathttps://lccn.loc.gov/2021033396
CoverDesign:Wiley
CoverImage:©shutterstock\YurchankaSiarhei
Setin9.5/12.5ptSTIXTwoTextbyStraive,Chennai,India
10987654321
Contents
Preface xvii
ListofContributors xix
PartIProductDesign,theEssenceofEffectiveTherapeutics 1
1ChallengesandInnovationsofControlledDrugDelivery 3 HeatherA.E.BensonandMichaelS.Roberts
1.1Background 3
1.2ParenteralDosageForms 3
1.2.1IntravenousRoute(IV) 4
1.2.2IntramuscularRoute(IM) 5
1.2.3SubcutaneousRoute(SC) 5
1.2.4OtherParenteralRoutes 5
1.3OralRouteandDeliverySystems 6
1.4NasalDrugDelivery 6
1.5PulmonaryDrugDelivery 7
1.6TransdermalDrugDelivery 7
1.7OcularDrugDelivery 9
1.8DrugDeliverySystemDevelopmentProcess 11
1.9Conclusion 12 References 12
2ChallengesinDesignofDrugDeliverySystems 15 S.NarasimhaMurthy,ShivakumarH.N,andSarasijaSuresh
2.1DrugPropertiestobeConsideredinDesignofControlledReleaseProducts 19
2.2PhysicochemicalFactorsthatNeedtobeConsideredinDesignofCRDDS 19
2.2.1DoseSize 19
2.2.2MolecularWeight/Size 19
2.2.3AqueousSolubility 21
2.2.4LipidSolubilityandPartitionCoefficient 25
2.2.5PhysicochemicalStability 26
2.3BiopharmaceuticalPropertiesthatDeserveConsiderationinDesignofControlled ReleaseProducts 26
2.3.1BiologicalHalf-life 26
2.3.2Absorption 27
2.3.3Metabolism 30
2.3.4PresystemicClearance 32
2.3.5MarginofSafety 32
2.3.6AdverseEffects 33
2.3.7TherapeuticNeed 33
2.3.8RoleofCircadianRhythm 34
2.4Conclusion 35 References 35
3DrugDeliveryoftheFuture(?) 39
AdrianWilliams
3.1Introduction 39
3.2TherapeuticIndicators 40
3.3DrugsoftheFuture 43
3.4DeliveringtheDrugsoftheFuture 45
3.5AViewtotheLongerTerm? 47
3.6Conclusion 50 References 50
4ThePharmaceuticalDrugDevelopmentProcess:SelectingaSuitableDrug Candidate 53
LionelTrottet
4.1TheOralDrugCandidate:HowtoGetThereandQuestionstoAnswer 53
4.2ChallengesforSelectingaTopicalDrugCandidate 55
4.3PercutaneousFluxasaSurrogateMeasurementofSkinTissueConcentration 57
4.4LearningsfromPastTopicalDrugDevelopmentofFactorsAffectingEfficacy 58
4.5DermalPharmacokinetics/Pharmacodynamics 62
4.6AssessmentofSystemicExposure 63
4.7ScreeningCascadeApproachtoSelectaDermalDrugCandidate 64
4.7.1Efficacy(LackofTargetEngagement) 64
4.7.2Developability 65
4.7.3LocalSafety 65
4.7.4SystemicSafety 65
4.8OpportunitiesforRepurposingMoleculesintoDermallyActiveTreatmentsfor CosmeceuticalorPharmaceuticalApproaches 66
4.9Conclusion 66 References 67
5PreformulationandPhysicochemicalCharacterizationUnderpinningthe DevelopmentofControlledDrugDeliverySystems 73 RonakSavlaandJulienMeissonnier
5.1WhenIsaControlledDrugDeliverySystemNeeded? 73
5.2OptimizingDrugCharacteristics 74
5.3DefiningtheProductProfile 75
5.4PreformulationandPhysicochemicalCharacterizationUnderpinningDevelopmentof CDD 77
5.4.1FeasibilityandRiskAssessment 78
5.4.2SolubilityandDissolutionRate 79
5.4.3Permeability 82
5.4.4DrugandDrugProductParticleSizes 83
5.4.5Solid-StateChemistry 84
5.4.5.1CrystallinityandPolymorphism 85
5.4.5.2Salts 85
5.4.6Stability 85
5.4.7ExcipientCompatibility 86
5.4.8BulkPowderProperties 87
5.4.9DrugMetabolismandPharmacokineticModeling 88
5.4.9.1GuidingtheDesignofCDDDosageForms 88
5.4.9.2Establishing InVitro–InVivo Correlation(IVIVC) 89
5.4.9.3PhysiologicallyBasedPharmacokinetic(PBPK)ModelingTools 89
5.5Conclusion 89 References 89
6MathematicalModelsDescribingKineticsAssociatedwithControlledDrug DeliveryAcrossMembranes 95
AnnetteL.Bunge
6.1Introduction 95
6.1.1GeneralDescription 95
6.1.2GoverningEquations 98
6.1.2.1DifferentialEquations 98
6.1.2.2DimensionlessDifferentialEquations 98
6.1.2.3InitialandBoundaryConditions 99
6.1.3OtherDerivedQuantities 100
6.1.4DimensionlessVariablesandGroups 102
6.2ModelSolutions 104
6.2.1TypeAModels–Well-StirredVehicleonOneMembrane 104
6.2.1.1ModelA1 104
6.2.1.2ModelA2 107
6.2.1.3ModelA3 112
6.2.1.4ModelA4 116
6.2.1.5ModelA5 120
6.2.1.6ModelA6 121
6.2.1.7ModelA7 123
6.2.1.8ModelA8 124
6.2.1.9ModelA9 125
6.2.1.10ModelA9a 128
6.2.1.11ModelA9b 130
6.2.1.12ModelA10 132
6.2.1.13ModelA11 136
6.2.1.14ModelA12 137
6.2.1.15ModelA13 138
6.2.2TypeBModels–UnstirredSemi-infiniteVehicleonOneMembrane 140
6.2.2.1ModelB1 140
6.2.2.2ModelB2 143
6.2.3TypeC–WellStirredVehicleonTwoMembranesinSeries 145
6.2.3.1ModelC1 145
6.3SolutionMethods 149
6.3.1SeparationofVariablesSolutions 150
6.3.1.1SeparatingthePartialDifferentialEquationof N IndependentVariablesinto N OrdinaryDifferentialEquations 150
6.3.1.2ChoosingtheSign(PositiveorNegative)ontheSeparationConstant 151
6.3.1.3FindingtheConstantsofIntegrationandtheEigenvalues 152
6.3.1.4Superposition 153
6.3.1.5FindingtheRemainingConstantsofIntegration 153
6.3.1.6GuidelinesforUsingSeparationofVariableMethodstoSolvePartialDifferential Equations 155
6.3.1.7MethodsforMakingaNonhomogeneousPartialDifferentialEquationor NonhomogeneousBoundaryConditionsHomogeneous 156
6.3.1.8ChoosingtheIndexStartingValueontheSumofAllSolutions (i.e.should n = 1or0?) 158
6.3.2LaplaceTransformSolutions 159
6.3.2.1UsingLaplaceTransformstoDetermineLagTimes,Steady-stateValuesandOther DerivedQuantities 159
6.3.2.2InversionofLaplaceTransformedFunctionstoTimeDomainFunctionsbyMethodof Residues 161
6.3.2.3ExampleA–ModelA1 161
6.3.2.4ExampleB–ModelA10 165
6.3.3UsefulIdentities 169 References 169
7UnderstandingDrugDeliveryOutcomes:ProgressinMicroscopicModeling ofSkinBarrierProperty,PermeationPathway,Dermatopharmacokinetics, andBioavailability 171 GuopingLian,TaoChen,PanayiotisKattou,SenpeiYang,LingyiLi,andLujiaHan
7.1Introduction 171
7.2GoverningEquation 172
7.2.1HomogenizedModel 172
7.2.2MicroscopicModel 174
7.2.2.1SoluteDiffusioninSCLipid 174
7.2.2.2SoluteDiffusioninSCCorneocytes 175
7.2.2.3SoluteDiffusioninAppendages 175
7.2.3NumericalMethods 175
7.3InputParameters 176
7.3.1SCMicrostructure 176
7.3.2SCLipid–WaterPartition 177
7.3.3DiffusivityinSCLipids 177
7.3.4BindingtoKeratin 179
7.3.5DiffusivityinCorneocytes 181
7.3.6SoluteDiffusivityandPartitioninSebum 181
7.4Application 183
7.4.1Steady-State 183
7.4.2Dermatopharmacokinetics 184
7.4.3SystemicPharmacokinetics 184
7.4.4ShuntPathway 185
7.5Perspective 186 References 188
8RoleofMembraneTransportersinDrugDisposition 193
HongYangandYanShu
8.1Introduction 193
8.2DistributionofMajorDrugTransportersinHumanTissues 194
8.2.1MajorDrugTransportersintheIntestine 194
8.2.1.1DrugTransportersExpressedintheApical(Luminal)Membrane 194
8.2.1.2DrugTransportersExpressedintheBasolateralMembrane 196
8.2.1.3ExpressionofDrugTransportersinDifferentIntestinalRegions 197
8.2.2MajorDrugTransportersintheLiver 197
8.2.2.1DrugTransportersExpressedintheApicalMembraneofHepatocytes 197
8.2.2.2DrugTransportersExpressedintheBasolateral(Sinusoidal)Membraneof Hepatocytes 199
8.2.2.3DrugTransportersExpressedintheBileDuctEpithelia(Cholangiocytes) 199
8.2.3MajorDrugTransportersintheKidney 199
8.2.3.1DrugTransportersExpressedintheApicalMembraneofProximalTubuleCells 200
8.2.3.2DrugTransportersExpressedintheBasolateralMembraneofProximalTubule Cells 200
8.2.4MajorDrugTransportersintheCentralNervousSystem(CNS) 201
8.2.4.1DrugTransportersExpressedintheCapillaryEndothelialCellsofBBB 201
8.2.4.2DrugTransportersExpressedintheChoroidPlexusEpithelialCellsofBCSFB 202
8.2.5MajorDrugTransportersinOtherTissues 202
8.2.5.1DrugTransportersExpressedinPlacentaVilliEpithelialCells (Syncytiotrophoblasts) 203
8.2.5.2DrugTransportersExpressedinMammaryGlands 203
8.2.5.3DrugTransportersExpressedintheBlood–Testis-Barrier(BTB) 204
8.3RoleofDrugTransportersinDrugDisposition 205
8.3.1RoleofP-gpinDrugDisposition 206
8.3.2RoleofBCRPinDrugDisposition 207
8.3.3RoleofBSEPinDrug-InducedCholestaticLiverInjury 214
8.3.4RoleofMRPs(MRP2,MRP3,andMRP4)inDrugDisposition 214
8.3.5RoleofOATPs(OATP1B1,OATP1B3,andOATP2B1)inDrugDisposition 215
8.3.6RoleofOATs(OAT1andOAT3)inDrugDisposition 216
8.3.7RoleofOCTs(OCT1andOCT2)/MATEs(MATE1andMATE2-K)inDrug Disposition 217
8.4ClosingRemarks 218 References 219
x Contents
PartIIChallengesinControlledDrugDeliveryandAdvancedDelivery Technologies 231
9AdvancedDrugDeliverySystemsforBiologics 233 MayWencheJøraholmen,SeleniaTernullo,AnnMariHolsæter,GørilEideFlaten, andNatašaŠkalko-Basnet
9.1Introduction 233
9.2ConsiderationsinBiologicsProductDevelopment 234
9.2.1ChallengesSpecifictotheRouteofAdministration 234
9.2.2ChallengesRelatedtoParenteralAdministration 234
9.2.3OptimizationofDosageRegimens 234
9.3AdministrationRoutesforBiologicsDelivery 235
9.3.1ParenteralRoute 235
9.3.2OralRoute 236
9.3.3BuccalRoute 237
9.3.4SublingualRoute 238
9.3.5PulmonaryRoute 238
9.3.5.1AdditionalConcernsinPulmonaryDelivery 239
9.3.6IntranasalRoute 239
9.3.7Trans(dermal)Delivery 240
9.3.7.1GeneDelivery 241
9.3.7.2VaccineDelivery 242
9.3.7.3ProteinDelivery 243
9.3.8DermalDeliveryofGrowthHormones 243
9.3.9VaginalRoute 247
9.4Conclusion 251 References 251
10RecentAdvancesinCell-MediatedDrugDeliverySystemsforNanomedicine andImaging 263
LiLiandZhiQi
10.1Introduction 263
10.2CellTypesandModificationforTherapeuticAgentDelivery 264
10.2.1CellTypes 264
10.2.1.1BloodCells 264
10.2.1.2StemCells 267
10.2.1.3AntigenPresentingCells(APCs) 268
10.2.1.4CellMembranes 268
10.2.2CargoLoadingMethods 269
10.3ImagingandTrackingofCell-BasedDeliverySystems 270
10.3.1MRI 271
10.3.2PET 272
10.3.3X-RayImaging 272
10.3.4MultimodalImagingTechniques 272
10.4Cell-MediatedDrugDeliverySystemsforDiseaseTreatment 272
10.4.1CancerTherapy 272
10.4.2Immunotherapy 272
10.4.3Brain-RelatedDiseases 274
10.4.4InflammatoryDiseases 274
10.4.5TheranosticApplication 275
10.4.6Others 275
10.5TheMechanismofCell-MediatedDeliverySystemsfortheCellTherapies 275
10.5.1Detoxification 276
10.5.2AdhesiveMechanism 277
10.5.3HomingMechanism 278
10.6TheAdministrationApproachofCell-AssistDrugDeliverySystem 278
10.7ClinicalApplicationofCell-BasedDeliverySystems 279
10.8ConclusionandOutlook 279 References 280
11OvercomingtheTranslationalGap–NanotechnologyinDermalDrug Delivery 285
ChristianZoschkeandMonikaSchäfer-Korting
11.1Nanotechnology–FailureorFutureinDrugDelivery? 285
11.2IdentificationoftheClinicalNeed 286
11.3NanoparticleDesignandPhysicochemicalCharacterization 289
11.4BiomedicalStudies 294
11.4.1AtopicDermatitis 294
11.4.2Psoriasis 295
11.4.3Ichthyosis 296
11.4.4WoundHealing 297
11.4.5Infections 297
11.4.6SkinCancer 298
11.4.7AlopeciaAreata 299
11.5ApproachestoFilltheTranslationalGapsinNanotechnology 299 References 303
12TheranosticNanoparticlesforImagingandTargetedDrugDeliverytothe Liver 311
HaoluWang,HaotianYang,QiRuan,MichaelS.Roberts,andXiaowenLiang
12.1Introduction 311
12.2TheTypesofTheranosticNPs 312
12.2.1Lipid-andPolymer-BasedNPs 312
12.2.2MesoporousSilicaNPs 312
12.2.3Bio-nanocapsules 313
12.2.4IronOxideNPs 313
12.3MechanismsofNPsTargetingtheLiver 313
12.3.1PassiveTargetingtotheLiver 313
12.3.2ActiveTargetingtotheLiver 314
12.3.3StrategiesforCombiningPassiveandActiveTargeting 315
12.4NPsinLiverTargetImaging 315
12.4.1NP-BasedContrastAgentsinLiverMRI 315
12.4.2NP-BasedContrastAgentsinLiverCTImaging 316
12.4.3NPsforNear-InfraredFluorescenceImaginginLiver 316
12.5NPsforTherapeuticandDrugDeliveryinLiverDisease 316
12.5.1NPDeliverySysteminHCC 316
12.5.2NPDeliverySysteminNon-tumoralLiverDisease 318
12.6TheranosticNPsinLiverDiseases 318
12.7Conclusions 322 References 323
13ToxicologyandSafetyofNanoparticlesinDrugDeliverySystem 329
KlinteanWunnapuk
13.1Introduction 329
13.2Lipid-BasedNanocarrier:Liposomes 329
13.3CellularUptakeMechanismofLiposomes 330
13.4Biodistribution,ClearanceandToxicityofLiposomes 331
13.4.1EffectofLipidCompositionsonLiposomeDistributionandBloodCirculation 331
13.4.2EffectofSurfaceChargeonLiposomeDistributionandBloodCirculation 333
13.4.3EffectofSizeonLiposomeDistributionandBloodCirculation 333
13.5ApplicationofLiposomesinDrugDelivery 334
13.6InorganicNanocarrier:CarbonNanotubes 336
13.7CellularUptakeMechanismofCarbonNanotubes 337
13.8Biodistribution,Clearance,andToxicityofCarbonNanotubes 337
13.9ApplicationofCarbonNanotubesinDrugDelivery 342
13.10Conclusion 342 References 342
14ControlledDrugDeliveryviatheOcularRoute 351 PeterW.J.MorrisonandVitaliyV.Khutoryanskiy
14.1Introduction 351
14.2PhysiologyoftheEye 352
14.2.1OcularMembranes;Conjunctiva,Cornea,andSclera 353
14.2.2InternalOcularStructures 354
14.2.3AnteriorChamber,Lens,andVitreousBody 355
14.3OcularDisorders 355
14.3.1PeriocularDisorders 355
14.3.2IntraocularDisorders 356
14.4ControlledDrugDeliverySystems 357
14.4.1FormulationStrategies 358
14.4.2MucoadhesiveSystems 358
14.4.3SolutiontoGel InSitu GellingSystems 359
14.4.4PenetrationEnhancers 361
14.4.5ContactLensesandOcularInserts 364
14.4.6IntraocularSystems(Implants,Injectables,andDegradableMicroparticles) 366
14.4.7PhonophoresisandIonophoresis 367
14.4.8TopicalProdrugs 368
14.4.9MicroneedleSystems 368
14.5Conclusions 369 References 370
15ControlledDrugDeliveryviatheOticRoute 377
JinsongHaoandS.KevinLi
15.1Introduction 377
15.2AnatomyandPhysiologyoftheOticRoute 377
15.2.1AnatomyoftheOticRoute 377
15.2.2BarriersRelevanttoInnerEarDrugDelivery 378
15.2.2.1BloodLabyrinthBarrier 378
15.2.2.2RoundWindowMembrane 380
15.2.2.3OvalWindow 380
15.2.2.4EustachianTube 380
15.2.2.5TympanicMembrane 381
15.3ControlledDrugDeliverySystems 381
15.3.1IntratympanicAdministration 381
15.3.1.1SilversteinMicroWick 382
15.3.1.2RoundWindowMicrocatheter(μCath) 383
15.3.1.3Gelfoam 383
15.3.1.4Seprapack 383
15.3.1.5OzurdexasaRWMImplant 384
15.3.1.6PropelSteroid-ElutingStent 384
15.3.2Trans-OvalWindowAdministration 384
15.3.3IntracochlearAdministration 385
15.3.3.1Drug-ElutingCochlearImplants 386
15.3.3.2MicrofluidicReciprocatingReservoir 386
15.4Conclusions 388 References 388
16ControlledDrugDeliveryviatheNasalRoute 393
BarbaraR.ConwayandMuhammadU.Ghori
16.1Introduction 393
16.2AnatomyandPhysiologyoftheNose 393
16.3AbsorptionfromtheNasalCavity 395
16.3.1TheEpithelialBarrier 395
16.3.2Absorption 395
16.4MucusandMucociliaryClearance 398
16.5DrugDeliverySystems 399
16.5.1SolutionsandSuspensions 400
16.5.2MucoadhesivePolymers 401
16.5.2.1 InSitu FormingNasalGels 401
16.5.2.2NasalInserts 409
16.5.2.3MicrospheresandNanospheres 410
16.5.2.4Liposomes 411
16.5.2.5MicroemulsionsandNanoemulsions 413
16.5.2.6Combination/HybridProductsandOthers 413
16.5.3TheNasalRouteandtheBlood–BrainBarrier 415
16.5.4TheNasalRouteforVaccinations 419
16.5.5 InVitro/inVivo ModelsforNasalAbsorption 421
16.6Conclusion 423 References 423
17ControlledDrugDeliveryviatheBuccalandSublingualRoutes 433
JavierO.Morales,ParameswaraR.Vuddanda,andSitaramVelaga
17.1Introduction 433
17.2BuccalandSublingualPhysiologyandBarrierstoDrugDelivery 434
17.2.1SalivaandMucus 434
17.2.2BuccalandSublingualEpitheliumandPermeationBarrier 434
17.3ControlledDrugDeliverySystems 436
17.3.1Tablets 436
17.3.2Films 437
17.3.3Gels,Ointments,andLiquidFormulations 438
17.3.4Spray 438
17.3.5Wafers 439
17.3.6Lozenges 439
17.3.7AdvancedandNovelDrugDeliverySystems 439
17.4FunctionalExcipientsUsedinControlledReleaseSystemstoEnhanceBuccaland SublingualDrugBioavailability 440
17.4.1PermeationEnhancers 440
17.4.2MucoadhesivePolymers 441
17.5Conclusions 442 Acknowledgments 443 References 443
18ControlledDrugDeliveryviatheLung 449
MaríaV.Ramírez-Rigo,NazarethE.Ceschan,andHughD.C.Smyth
18.1Introduction 449
18.2TheRelevantPhysiologyoftheRouteIncludingtheBarrierstoDrugDelivery 449
18.3ControlledDrugDeliverySystems 451
18.3.1Formulations 451
18.3.1.1DissolutionRateControlled 451
18.3.1.2SustainedReleaseSystems 451
18.3.1.3DrugComplexes 455
18.3.1.4Drug–ReceptorBinding 456
18.3.1.5DrugConjugates 457
18.3.1.6Drug–PolymerMatrixParticles 458
18.3.1.7LargePorousParticles 459
18.3.1.8Nanosystems 459
18.3.2Devices 459
18.3.2.1ControllingLungDepositionPatterns 459
18.3.2.2Nebulizers 460
18.3.2.3DryPowderInhalers 461
18.3.2.4PressurizedMetered-DoseInhalers 462
18.4Conclusions 464
Acknowledgments 464 References 464
19ControlledDrugDeliveryviatheVaginalandRectalRoutes 471
JosédasNevesandBrunoSarmento
19.1Introduction 471
19.2BiologicalFeaturesoftheVaginaandColorectum 472
19.2.1Vagina 472
19.2.2Colorectum 473
19.3ControlledDrugDeliverySystems 474
19.3.1VaginalRoute 476
19.3.1.1ConventionalDosageForms 476
19.3.1.2RemovableDrugDeliverySystems 483
19.3.1.3Nanotechnology-basedDrugDeliverySystems 486
19.3.2RectalRoute 489
19.3.2.1DosageForms 489
19.3.2.2Nanotechnology-basedDrugDeliverySystems 493
19.4Conclusions 494
Acknowledgments 494 References 494
20ControlledDrugDeliveryintoandThroughSkin 507
AdrianWilliams
20.1Introduction 507
20.1.1HumanSkinStructureandFunction 507
20.1.1.1BiologicalFactors 507
20.1.1.2SkinasaPhysicalBarrier 508
20.1.2DrugTransportThroughSkin 512
20.2ControlledDrugDeliveryintoandThroughSkin 513
20.2.1SkinBarrierModulation 513
20.2.1.1PenetrationEnhancers 514
20.2.1.2Ablation 515
20.2.2ControlledReleaseTransdermalandTopicalSystems 515
20.2.2.1Supersaturation 516
20.2.2.2ReservoirFormation 518
20.2.2.3FilmFormingSystems 518
20.2.2.4Vesicles 519
20.2.2.5Particles 520
20.2.3Device-BasedControlledDelivery 522
20.2.3.1Iontophoresis 523
20.2.3.2Sonophoresis 524
20.2.3.3Electroporation 525
20.2.3.4Microneedles 525
20.2.3.5Heat 526
20.2.3.6OtherDevices 527
20.3CombinationApproaches 528
20.4Conclusions 528
References 529 Index 535
Preface
Effective,controlleddrugdeliveryhasthepotentialtogreatlyimpactthetherapeuticoutcome, clinicalbenefit,andsafetyofdrugsinawiderangeofdiseasesandhealthconditions.Therearea largenumberofpotentiallyusefuldrugswithlimitedeffectivenessand/orsafetyconcernsdueto poordrugdelivery.Thismayoccurbecauseaphysiologicallyrelevantconcentrationisnotdeliveredtothetargetsite,doesnotremainincontactforasufficientperiod,orcausesadverseeffects becauseoftheresultinghighbloodconcentrationsassociatedwithindiscriminaterelease.Controlleddrugdeliverysystemsaredesignedtocarrythedrugandreleaseitatthetargetsiteina timelymanner,facilitatingitsabsorptionandoptimizingitsphysiologicalaction.Aneffectivecontrolleddrugdeliverysystemimprovesefficacyandsafetybycontrollingtherate,time,andplace ofdrugreleasewithinthebody,therebyminimizingdoserequirementsandthepotentialtointeractwithnon-targetbodysightsthatcancontributetoundesirablesideeffects.Drugdeliveryhas evolvedfromrelativelysimplesystemstomoderntechnologiesdesignedtopersonalizemedicines thathavebiologicallyprecisedrugreleaseinresponsetoreal-timemonitoringofbodyparameters.
Controlleddrugdeliverysystemdevelopmentisrapidlyevolvingwithanever-increasingfocuson advancedtechnologiesthatbringtogetherawiderangeofskilledprofessionsincludingpharmaceuticalscientists,chemical,mechanical,andelectricalengineers,chemists,physicists,andclinicians. Itisanexcitingfieldthathashelpedtoadvanceclinicaloutcomesinalmosteveryhealthcondition, rangingfromnegatingtheneedforcold-chainstoragethusallowingmedicinestobetransportedto themostremotepartsoftheworld,toprecisiontargetingofdrugsincancertreatment.Thisbookis designedtoprovideaninsightintothefundamentalsofdrugdeliveryandtheimportantprocesses inthedevelopmentofcontrolleddrugdeliverysystems.
Thebookisdividedintothreeparts.
Part1(Chapters1–8)introducestheconceptofdrugdeliveryandprovidesaperspectiveintothe challenges,opportunities,andfundamentalprocessesinvolvedinthedevelopmentofcontrolled drugdeliverysystems.Itincludesahistoricalperspectiveandapeekintothefutureofdrugdelivery.Thereisafocusonthedrugdevelopmentprocess,includingtheselectionofpharmaceutical candidatesandevaluationoftheirphysicochemicalcharacteristicswithemphasisontherelevance todosageformdesign.Theroleandapplicationofmathematicalmodelingandtheinfluenceof drugtransportersinpharmacokineticsanddrugdispositioncompletethissection.
Part2(Chapter9–13)isfocusedonparticularchallengesincontrolleddrugdeliveryand advanceddeliverytechnologies.Thisincludesdeliverysystemsforbiologicals,anincreasing drugcategorythatpresentsenormoustherapeuticopportunitiesandequallyenormousdelivery challenges.Theapplicationandrecentadvancesincell-mediateddrugdeliveryarediscussed, andthereisaseriesofchaptersonnanotechnologythatincludefundamentals,applicationsfor targeteddelivery,anddiscussionofthetoxicologicalandsafetyissues.
Part3(Chapters14–20)providesa“toptobottom”critiqueofthecommonadministrationroutes forcontrolleddrugdelivery.Eachchapterbeginswithashortintroductionandthenamoredetailed discussionofthephysiologypertinenttoeachadministrationroute,focusingonthebarriersto drugdelivery.Controlleddrugdeliverysystemsthathavebeenevaluatedforeachroutearethen discussedbeforesomeconclusionssummarizingthestate-of-the-artandpotentialfuturedevelopments.Eachchapterincludescomprehensivereferencesatthetimeofwritingforthosewishing toreadtheprimaryliterature. Controlled drugdeliverysystemsimplythatcontroloverdosing residesintheformulationwithcontroloverdrugreleaseandpredictabledrugdelivery.However, itisapparentthat,giventhecomplexitiesofthebiologicalbarrierspresentfortheadministration routes,inseveralcasescontroloverdrugdeliveryarisespredominantlyfromthebiologicalbarrier. Althoughstrategieshavebeendevelopedtoreducethesebarriers–forexampletheuseofpenetrationenhancers–itiscontentiouswhethertheseapproachestrulyallow controlled drugdelivery. However,inseekingtoprovideacomprehensivecritiqueofthecurrentliterature,suchpartially controlledsystemshavebeenconsidered.
Weexpressourthankstotheauthorswhohavecontributedtothisbook.Ineachcase,the chaptersareauthoredbywell-respectedresearchersinthefieldwhohavegenerouslyprovided theirknowledgeandexperience,andcontinuetocontributetoadvancingresearchintheirfields. WearealsogratefultoJonathanRoseandhisteamatWileywhohavebroughttheconceptsand chapterstofruition,andshownremarkablepatienceindealingwitheditorswhoagreewithAlbert Einsteinthat“timeisanillusion”.
Australia June2021
HeatherA.E.Benson
MichaelS.Roberts
AdrianC.Williams
Xiaowen(Tina)Liang
ListofContributors
HeatherA.E.Benson CurtinMedicalSchool
CurtinHealthInnovationResearchInstitute CurtinUniversity Perth,WA,Australia
AnnetteL.Bunge ChemicalandBiologicalEngineering Department ColoradoSchoolofMines Golden,CO,USA
NazarethE.Ceschan DepartamentodeBiología BioquímicayFarmacia,UNS BahíaBlanca,Argentina and
PlantaPilotodeIngenieríaQuímica (PLAPIQUI)
ConsejoNacionaldeInvestigaciones CientíficasyTécnicas(CONICET)–UniversidadNacionaldelSur(UNS) BahíaBlanca,Argentina
TaoChen DepartmentofChemicalandProcess Engineering UniversityofSurrey Guildford,UK
BarbaraR.Conway DepartmentofPharmacy UniversityofHuddersfield Huddersfield,HD13DH,UK
GørilEideFlaten DrugTransportandDeliveryResearchGroup DepartmentofPharmacy,FacultyofHealth Sciences UniversityofTromsøTheArcticUniversityof Norway Tromsø,Norway
MuhammadU.Ghori DepartmentofPharmacy UniversityofHuddersfield Huddersfield,HD13DH,UK
ShivakumarH.N DepartmentofPharmaceutics K.L.ECollegeofPharmacy Bengaluru India
LujiaHan CollegeofEngineering ChinaAgriculturalUniversity Beijing,P.R.China
JinsongHao
DepartmentofPharmaceuticalSciences CollegeofPharmacy&HealthSciences CampbellUniversity BuiesCreek,NC,USA
AnnMariHolsæter
DrugTransportandDeliveryResearchGroup DepartmentofPharmacy,FacultyofHealth Sciences
UniversityofTromsøTheArcticUniversityof Norway Tromsø,Norway
MayWencheJøraholmen
DrugTransportandDeliveryResearchGroup DepartmentofPharmacy,FacultyofHealth Sciences
UniversityofTromsøTheArcticUniversityof Norway Tromsø,Norway
PanayiotisKattou DepartmentofChemicalandProcess Engineering UniversityofSurrey Guildford,UK
VitaliyV.Khutoryanskiy ReadingSchoolofPharmacy UniversityofReading Reading,UK
LiLi AustralianInstituteforBioengineeringand Nanotechnology(AIBN) TheUniversityofQueensland Brisbane,QLD,Australia
LingyiLi CollegeofEngineering ChinaAgriculturalUniversity Beijing,P.R.China
S.KevinLi
DivisionofPharmaceuticalSciences
JamesLWinkleCollegeofPharmacy UniversityofCincinnati Cincinnati,OH,USA
GuopingLian
UnileverResearchColworth Bedford,UK and DepartmentofChemicalandProcess Engineering UniversityofSurrey Guildford,UK
XiaowenLiang TherapeuticsResearchGroup TheUniversityofQueenslandDiamantina Institute
TheUniversityofQueensland TranslationalResearchInstitute Brisbane,QLD,Australia
JulienMeissonnier ScienceandTechnology CatalentPharmaSolutions Beinheim,France
JavierO.Morales CenterofNewDrugsforHypertension (CENDHY) Santiago,8380494,Chile and AdvancedCenterforChronicDiseases (ACCDiS) Santiago,8380494,Chile and DepartmentofPharmaceuticalScienceand Technology SchoolofChemicalandPharmaceutical Sciences UniversityofChile Santiago,8380494,Chile
PeterW.J.Morrison ReadingSchoolofPharmacy UniversityofReading Reading,UK
S.NarasimhaMurthy PharmaceuticsandDrugDelivery
TheUniversityofMississippi 113FaserHall,MS,USA and
InstituteforDrugDeliveryandBiomedical Research Bengaluru,India and DepartmentofPharmaceutics
K.L.ECollegeofPharmacy Bengaluru,India and InstituteforDrugDeliveryandBiomedical Research Bengaluru,India
JosédasNeves i3S–InstituteforResearchandInnovationin Health UniversityofPorto,Portugal
ZhiQi
AustralianInstituteforBioengineeringand Nanotechnology(AIBN)
TheUniversityofQueensland Brisbane,QLD,Australia
MaríaV.Ramírez-Rigo DepartamentodeBiología BioquímicayFarmacia,UNS SanJuan670,8000BahíaBlanca,Argentina and PlantaPilotodeIngenieríaQuímica (PLAPIQUI)
ConsejoNacionaldeInvestigaciones CientíficasyTécnicas(CONICET)UniversidadNacionaldelSur(UNS) BahíaBlanca,Argentina
MichaelS.Roberts TherapeuticsResearchCentre
BasilHetzelInstituteforTranslationalMedical Research
TheQueenElizabethHospital Adelaide,SA,Australia and SchoolofPharmacyandMedicalSciences UniversityofSouthAustralia Adelaide,SA,Australia and DiamantinaInstitute
TheUniversityofQueensland,Translational ResearchInstitute Brisbane,QLD,Australia and
TherapeuticsResearchGroup
TheUniversityofQueenslandDiamantina Institute
TheUniversityofQueensland,Translational ResearchInstitute Brisbane,QLD,Australia
QiRuan TherapeuticsResearchGroup
TheUniversityofQueenslandDiamantina Institute,TheUniversityofQueensland TranslationalResearchInstitute Brisbane,QLD,Australia
BrunoSarmento
INEB–InstituteforBiomedicalEngineering UniversityofPorto,Portugal
RonakSavla ScienceandTechnology CatalentPharmaSolutions Somerset,NJ,USA
ListofContributors
MonikaSchäfer-Korting InstituteofPharmacy(Pharmacologyand Toxicology)
FreieUniversitätBerlin Berlin,Germany
YanShu
DepartmentofPharmaceuticalSciences SchoolofPharmacy,UniversityofMarylandat Baltimore Baltimore,MD,USA
NatašaŠkalko-Basnet
DrugTransportandDeliveryResearchGroup DepartmentofPharmacy,FacultyofHealth Sciences
UniversityofTromsøTheArcticUniversityof Norway Tromsø,Norway
HughD.C.Smyth CollegeofPharmacy
TheUniversityofTexasatAustin 2409WestUniversityAvenue Austin,TX,UnitedStates
SarasijaSuresh
InstituteforDrugDeliveryandBiomedical Research Bengaluru India
SeleniaTernullo
DrugTransportandDeliveryResearchGroup DepartmentofPharmacy,FacultyofHealth Sciences
UniversityofTromsøTheArcticUniversityof Norway Tromsø,Norway
LionelTrottet
DMPK(DrugMetabolismand PharmacoKinetics) Galapagos,Romainville,France
SitaramVelaga KenoxPharmaceuticalsInc MonmouthJunction NJ,08852,USA
ParameswaraR.Vuddanda ResearchCentreforTopicalDrugDeliveryand Toxicology
UniversityofHertfordshire Hertfordshire,AL109AB,UK
HaoluWang TherapeuticsResearchGroup TheUniversityofQueenslandDiamantina Institute,TheUniversityofQueensland TranslationalResearchInstitute Brisbane,QLD,Australia
AdrianWilliams UniversityofReading Reading,UK
KlinteanWunnapuk ToxicologyDivision DepartmentofForensicMedicine,Facultyof Medicine ChiangMaiUniversity ChiangMai,Thailand
HaotianYang TherapeuticsResearchGroup TheUniversityofQueenslandDiamantina Institute,TheUniversityofQueensland TranslationalResearchInstitute Brisbane,QLD,Australia
HongYang
DepartmentofPharmaceuticalSciences SchoolofPharmacy UniversityofMarylandatBaltimore Baltimore,MD,USA
SenpeiYang CollegeofEngineering ChinaAgriculturalUniversity Beijing,P.R.China
ChristianZoschke InstituteofPharmacy(Pharmacologyand Toxicology)
FreieUniversitätBerlin Berlin,Germany
PartI
ProductDesign,theEssenceofEffectiveTherapeutics
ChallengesandInnovationsofControlledDrugDelivery
HeatherA.E.Benson 1 andMichaelS.Roberts 2,3,4
1 CurtinMedicalSchool,CurtinHealthInnovationResearchInstitute,CurtinUniversity,Perth,WA,Australia
2 DiamantinaInstitute,TheUniversityofQueensland,TranslationalResearchInstitute,Brisbane,QLD,Australia
3 SchoolofPharmacyandMedicalSciences,UniversityofSouthAustralia,Adelaide,SA,Australia
4 TherapeuticsResearchCentre,BasilHetzelInstituteforTranslationalMedicalResearch,TheQueenElizabethHospital,Adelaide, SA,Australia
1.1Background
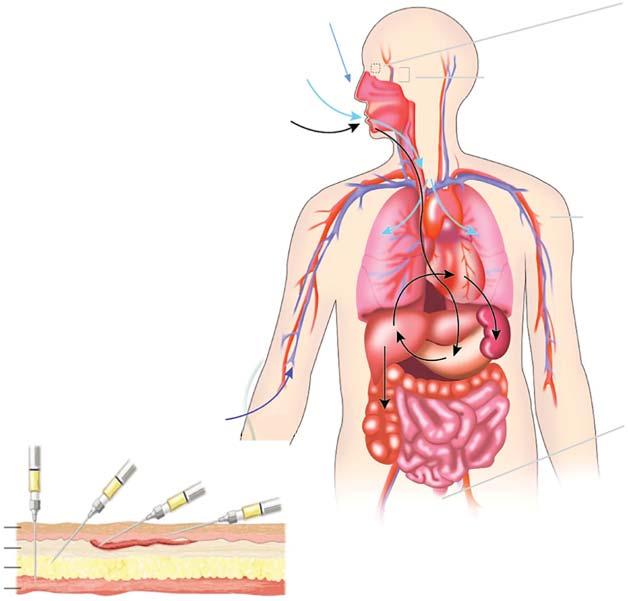
Akeyhealthcareadvanceoverthelastcenturyhasbeenthedevelopmentofdrugdeliverysystems thatnotonlyenableeffective,safe,andreproducibledeliveryforoptimaltherapeuticbenefit,but arealsochemicallyandphysicallystable,aestheticallyacceptable,convenienttouse,cost-effective andoptimizedforthemostappropriatemodeofadministrationintheirtargetpopulation.Thereis awiderangeofdrugdeliverysystemsavailableforarangeofroutesofadministration,withtheaim ofachievinglocalorsystemiceffects,asshowninFigure1.1.Themostcommonrouteofadministrationistheoralrouteduetoitsconvenience,cost,andpatientacceptance.Injectablesincludethe morecommonparenteralrouteswhichareintradermal,subcutaneous(SC),intramuscular(IM), andintravenousdelivery(Figure1.1).Inadditiontotheoralroute,themostcommonnonparenteral routesofdeliveryaretopical/transdermal,nasal,pulmonary,ocular,rectal,andvaginal(Figure1.1). Drugabsorptionprocessesfornonparenteraladministrationgenerallyinvolvepassiveand/oractive transportacrossanepithelialbarrierandcarriageawayintothesystemiccirculationbyeitherlocal bloodflowand/orviathelymphaticcirculation.Topicaldeliveryisusedforalocaleffect,suchas thetreatmentofpain,inflammation,orinfectionoftheoropharynx(includingnasal,buccal,and sublingual),eye,skin,lung,rectum,andvaginaorforsystemicdelivery.
Inthischapter,weintroducetheroutesofadministrationoftherapeuticanddiagnosticcompoundstothebody,theconsiderationsindesigningdrugdeliverysystems,andsomeexamplesof theinnovationsthathavecontributednewcontrolleddeliveryproducts.Theaimistoprovidean overviewofthetopic,withconsiderabledetailprovidedinthesubsequentchaptersinthisandother volumesinthisseries.
1.2ParenteralDosageForms
Theparenteralrouteenablesprecisedosingfordrugsthathaveanarrowtherapeuticindex, completedosingforthosewithpoororalbioavailabilitytomaintainoptimaltherapeuticconcentrations,readyaccessinpatientswithswallowingdifficulties,oranunconsciouspatient,and FundamentalsofDrugDelivery, FirstEdition. EditedbyHeatherA.E.Benson,MichaelS.Roberts,AdrianC.Williams,andXiaowenLiang. ©2022JohnWiley&Sons,Inc.Published2022byJohnWiley&Sons,Inc.
1ChallengesandInnovationsofControlledDrugDelivery
- Drops - Sprays
- Dry powders
- Liquid sprays
Oral
(Including buccal and sublingual)
- Tablets
- Capsules
- Orally disintegrating tablets
- Buccal tablets
- Sublingual tablets
- Mini tablets
- Effervescent tablet
- Thin films
- Medicated gums
- Granules
- Troches
- Lozenges
- Solutions
- Suspension
- Emulsion
- Elixir
- Buccal sprays
Epidermis
Dermis
Parenteral
- Intracochlear Inhalation
Rectal / Vaginal Ocular Otic Nasal
IntramuscularSubcutaneousIntravenousIntradermal
Subcutaneous Muscle tissue
- Topical - Intratympanic
- Solutions - Emulsions
- Suspension
- Ointments
- Contact lens
- Implants
- Inserts
- Intravitreal
Topical / Transdermal
- Ointments
- Creams
- Lotion
- Gel - Sprays
- Patches
- Suppository
- Enema
- Tablets
- Pessary
- Gel - Cream
- Foam
- Sponge
Figure1.1 Routesofdrugadministrationandassociateddosageforms.Source:KhanandRoberts[1].© 2018Elsevier.
immediatebloodlevelsofdrugsinanemergency.Itisusedtodeliverintravenousfluidreplacement andparenteralnutritionfluidsandisthepreferreddeliveryrouteforemergingnoveltherapeutic molecules,suchasproteins,peptides,andbiologics.However,parenteraladministrationrequires specializedpersonnel,andthereisariskoftissuedamage,infection,andimmunereactions. Thereisarequirementforsterilityinprocessing,storage,andadministrationmakingitmore expensivethanotherconventionaldrugproducts.Themostcommonroutesusedforsystemic deliveryofawiderangeofmoleculesincludingbiopharmaceuticals,areSC,intravenous(IV),and IM[2]asshowninFigure1.1.Whilemanyparenteralproductsaresolutions,controlledrelease fromformulationssuchasmicroparticlesorimplantscomposedofbiodegradablepolymersisalso available.
1.2.1IntravenousRoute(IV)
TheIVrouteisusedtoadministeradrugdirectlyintoavein,eitherasabolusorbyinfusion,thus allowingadministrationofrelativelylargevolumesoffluid.Itavoidsfirstpassmetabolismandis thepreferredchoiceforrapidpharmacologicalaction,especiallyinemergenciesandanesthesia. However,administrationrequiresmedical/nursingsupervision,andthereareassociatedrisksof infectionviatheinjectionsite,coagulationofinfusionlines,andthepossibilityofredbloodcell
hemolysis,pain,andphlebitis.Effectivesterilemanufactureandappropriatestorageisrequiredto preventcontaminationwithmicrobes,pyrogens,andinertparticles.
1.2.2IntramuscularRoute(IM)
IMinjectionenablessystemicdosing,highbioavailability,andarapidonsetofactionofdrugssuch asantibiotics,steroids,andnarcoticanalgesics.Itprovidesalow-costalternativetoIVdosingas demonstratedbyMilkovichandPiazza,whoreported10timeslowercostassociatedwithIMcomparedtoIVantibioticsandtheopportunityforself-administrationandearlierhospitaldischarge [3].TheIMrouteavoidsmanyoftheadministration-relatedrisksofIVadministrationandcan provideasafe,rapiddrugadministrationoptioninthemanagementofanaphylaxis,whereIMdosingofepinephrinewasassociatedwithsignificantlylesscardiovascularadverseeventscompared withIVbolusepinephrine(1.3%vs.10%)[4]andcanbeadministeredrapidlybyateacher,relative, ormemberofthepublic.IMinjectionisassociatedwithpainfromneedleinsertionandcareis requiredtoavoidpiercingarteriesorcausingnervedamage.
1.2.3SubcutaneousRoute(SC)
TheSCrouteiscost-effective,amenabletoself-injection,minimallyinvasive,andassociatedwith fewersideeffectsthantheIVroute[5].WhileadversereactionstoSCdosingareuncommon, edema,pain,inflammation,infection,abscesses,andinjection-sitereactionsmaybemorecommon intheelderly[6].TheSCrouteofferstheopportunityforplacementofimplantsthatcanprovide controlleddrugreleaseoverprolongedperiods.PortabledevicesforSCinjectionsuchasinsulin pumps,autoinjectors,andpeninjectorsallowoptimaldosedeliveryandself-administrationinthe managementofarangeofconditions.Examplesincludeinsulin,epinephrine,interferon β-1a(multiplesclerosis),andEnbrel® (autoinjectorscontainingthebiologicetanerceptforuseinarthritis) (https://www.enbrel.com/rheumatoid-arthritis/about-enbrel-for-ra,in).
1.2.4OtherParenteralRoutes
Lesscommonparenteralroutesfordrugdeliveryincludeepidural,intrathecal,intracerebroventricular(ICV),intra-arterial,intra-articular,intracavernous,intralesional,intraosseous,and intravesicalinjectionandinfusion.Epidural(outsidethedura/spinalfluidsac)andintrathecal (insidesac)routesareusedprimarilyforanesthesia/analgesia.ICVinvolvesinjectionintothe cerebrospinalfluidincerebralventriclestobypasstheblood–brainbarrier.Itcanbeusedfordrug administrationinneurodegenerativedisorderssuchasspinalmuscularatrophyorchemotherapeuticagentsingliomas.Itisalsousedasaresearchtool.Intra-articularinjections(intoajoint) ofanti-inflammatorydrugssuchassteroidsaregenerallyusedforthetreatmentofinflammatory jointconditionssuchasarthritis,tendinitis,gout,andCarpalTunnelSyndrome.Vasodilatorsand vasoconstrictorsareadministeredbyintracavernousinjection(intothepenis)totreaterectile dysfunction.Intralesionalsteroidtherapyismostcommonlyusedinthetreatmentofhypertrophic andkeloidscars,acnecysts,andalopeciaareata.Intraosseousinflusion(IO)involvesinjecting directlyintothemarrowofaboneasanalternativetoprovidedrugsorfluidwhenIVinjectionis notavailable,suchastraumapatientswithcompromisedIVaccess.Aneedleisinsertedthrough thehardbonecortexintothesoftbonemarrowtoprovideimmediateaccesstothevascular system[7].Intravesicularinjectioninvolvesadministrationintothebladderusingacatheter,used primarilytoinstilimmunotherapeuticandchemotherapeuticagentsinthetreatmentofbladder cancer.
1.3OralRouteandDeliverySystems
Morethan75%ofalldosesaregivenastabletsandcapsulesduetotheireaseandlowcostofmanufacture,stability,convenienceincarryingandstoring,easeofuse,effectivetaste-maskingstrategies, andlessdosingerrors[8].However,frequentdosingofthreetofourtimesadaymayberequiredfor drugswithshortorimmediateeliminationhalf-lives,potentiallycreatingahighpillburdenand adherenceissuesparticularlyintheolderpopulationwhomaybetakinganumberofmedications tomanagemultipleconditions.Thereisclearlyenormouspotentialforcontrolleddrugdelivery viatheoralroute,hence,wehavedevotedavolumeinthisseriestothistopic.Hereweprovidean overviewoftheformulationchallengesandopportunitiesofferedbytheoralroute.
Whilemostoralformulationsareswallowedintact,asshowninFigure1.1,immediaterelease andsolubletabletsofferquicktherapeuticeffectandareparticularlysuitableforpeoplewithswallowingproblemssuchaschildrenandtheelderly.Formulationoptionsincludetabletsthatare allowedtoeffervesceinwater,andoraldisintegratingtablets,medicatedgums,andoralfilms thatcanbesuckedorchewedbeforeswallowing.Sublingual(placedunderthetongue)andbuccal(placedbetweenthegumandcheek)tablets,sprays,andpatchesdeliverthedrugthroughthe mucosalsurfacesofthemouth,providingtheaddedadvantageofavoidingfirst-passmetabolism.
Oralmodifiedreleasedosageformsincludedelayed,extended,entericcoated,andotherdelivery systemsthatcanimprovethestability,efficacy,safety,andconvenienceforpatients.Theycanoffer reduceddosingfrequency,withimprovedadherence,clinicaloutcomes,andareductionintheneed forfurtherclinicalinterventionsuchashospitalreadmissions[9].Controlledreleasetechnologies includetheuseofbiodegradablepolymers,releaserate-controllingmembranes,microencapsulation,andbioadhesivestoenhancedrugcontacttime[10].Multiparticulatecontrolledrelease formulationsoffermoreflexibilityforpediatricorelderlypatientswhohavetroubleswallowing astheycanbefilledintosachetsorcompressedintominitablets[11].
1.4NasalDrugDelivery
Intranasaldeliverysystemsarecommonlyusedfortreatinglocalconditions(Figure1.1)suchas nasalallergy,congestion,andnasalinfections,andareincreasinglybeingusedfordeliveryofopioids,respiratorymedicines,vaccines,andothermedicinesforsystemiceffect.Thethinandhighly vascularizednasalmucosallayerenablesafastonsetofdrugactionandavoidanceofgastrointestinaltractandhepaticfirst-passmetabolism[12],andthenasal-associatedlymphoidtissue(NALT) offersopportunitiesforvaccinedelivery.Themostcommondeliverysystemsarenasalspraysand dropsadministeredtotheanteriorsiteofthenasalcavity,withtheformershowingtwofoldtothreefoldgreaterdrugbioavailability[13].Thenasalcavityisdesignedtoprotectthelungsbyfiltering inhaledairviaitshairfolliclesandmucousmembrane,thentransportingthoseparticlesdown throughtheesophagustothestomachbyamucociliaryclearanceprocess,thusexposingthemto thedegradativeprocessesofthegastro-intestinaltract.Thisclearanceprocessrepresentsachallengetonasalbioavailabilityasdrugresidencetimeinthenasooralcavityisdecreased,resultingin reducedtransportintothebloodstream.Adhesivepolymers,enzymeinhibitors,ortheuseofnovel drugdeliveryformulationssuchasliposomes,nanoparticles,microspheres,microemulsions,and penetrationenhancerscanenhancedrugretentionand/orpenetrationacrossthenasalmucosa [14].Themostfavorablecandidatesfornasaldelivery,asshowninTable1.1,aresmalllipophilic moleculessuchaspropranolol(M W = 295.8,log Po/w = 2.7),fentanyl(M W = 336.5,log Po/w = 4.1), andestradiol(M W = 296.4,log Po/w = 4.3)[15].
Table1.1 Propertiesandindicationsofnasallydelivereddrugs.
Drug M W Log P Indication
Butorphanol327.53.7Migraine
Estradiol296.44.3HRT
Fentanyl336.54.1Cancerpain
Naloxone327.40.6Opioidoverdose
Nicotine162.21.2NRT
Sumatriptan295.40.8Migraine
Zolmitriptan287.31.6Migraine
HRT,hormonereplacementtherapy;NRT,nicotinereplacementtherapy; M W ,molecularweight.
Source:Fortunaetal.[15].©2014Elsevier.
1.5PulmonaryDrugDelivery
Pulmonarydeliverytakesadvantageofthefavorablepropertiesofthelungsfordrugdelivery:large surfaceareaofthealveoli,richsupplyofbloodvessels,thin(0.1–2 μm)alveolarmembranes,limited enzymaticdegradation,andavoidanceoffirst-passhepaticmetabolism[16].Consequently,relativelyhighbioavailabilityofsmallandlargemolecules,suchashumangrowthhormone,interferon �� ,immunoglobulinG(IgG),andinsulin(M W approximately22,19,150,and5.8kDa,respectively), canbeachieved.Themostcommonlyusedinhalerdevicesarepressurizedmetered-doseinhalers (pMDIs:deliveringliquiddroplets)withorwithoutaspacer(largevolumecontainerintowhichthe aerosolisactuatedallowingregularbreathsforinhalation),drypowderinhalers(DPIs)andnebulizers(aerosolizedliquiddroplets)[17,18].Eachdevicehasadvantagesanddisadvantages,andthe utilityofeachtypecanvary,withsomepatientgroupsmorecommonlyexperiencingcoordination issuesorlungdiseasethatcanaffectbioavailabilityfromparticulardevices.Metereddoseinhalers (pMDIs)deliver1–3 μmparticlestotherespiratorysurfacesofthelungwithatypicalefficiencyof 10–20%oftotalexpelleddoseinyounghealthyadults,andevenlessinchildren,theelderlyand inthepresenceofreducedlungfunction[19].pMDIswithspacerarelessportablebutdoincrease bioavailabilitybyremovingtheneedtocoordinateactuationwithinhalation.DPIshaveanease ofuseadvantagebutstillrequireminimuminspiratoryflowandthepresenceofthedrypowder maybeirritatingtolungtissue[20].Nebulizersareparticularlyusefulforchildrenandtheelderly astheyprovideaconstantaerosolflowwithinafacemask.NewtechnologiessuchastheHailieTM (formerlyknownastheSmartinhalerTM :Adherium,SanMateo,CA)allowremotemonitoringof inhalermedicationuseanddosingreminderstoimproveadherenceandtreatmentoutcome.
1.6TransdermalDrugDelivery
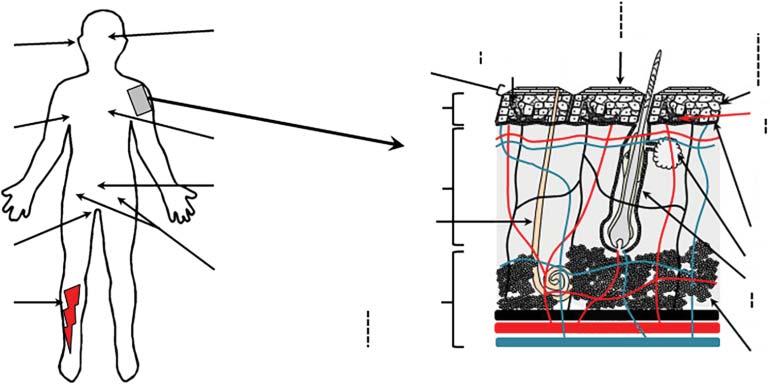
Drugsareappliedtotheskinforsuperficial(e.g.cosmetic,sunscreen,moisturization,andrepelling insects),local(e.g.skininfection,inflammation,anddisease),appendageal(e.g.acneandhairloss), deeptissue(e.g.painandinflammation)orsystemic(e.g.smokingprevention,hormonereplacement,andcontraceptivepatches)effects(Figure1.2)[21].Whiletheskinoffersalarge,readily accessiblesitefordrugadministration,italsoprovidesaformidablebarriertopermeation.The
Selegiline Rotigotine Rivastigmine Methylphenidate Nicotine Sumatriptan
Stratum corneum Epidermis Langerhans cell
Testosterone Estradiol Ethinyl estradiol Levonorgestrel Norelgestromin Norethindrone Fentanyl Buprenorphine
Keratinocyte Melanocyte
Melanostatin-5 N-pentadecanoyl Melanin L-Tyrosine Psoralen
Basal membrane Sebaceous gland Hair follicle
Minoxidil (hair) Acne treatments
Adipose tissue
Figure1.2 (a)Approvedandunderdevelopmentoftransdermaldrugsandtheirrecommendedsiteofapplication.(b)Close-upofhumanskinandvarioussites andtargetswithintheskin.Source:Bensonetal.[21].©2019EurekaScience.
Hypodermis
epidermisisaprotectivelayertopreventingressofenvironmentalcontaminantswhilealsopreventingwaterlossfromthebody.Itisinaconstantstateofrenewalwithformationofanewcell layerofkeratinocytesatthestratumbasale,lossoftheirnucleus,andotherorganellestoformdesiccated,proteinaceouscorneocytesontheirjourneytowarddesquamation,whichoccursfromthe skinsurfaceatthesamerateasformationinnormalskin(Figure1.2).Theoutermostlayer,thestratumcorneum(SC),consistsofabrickwall-likestructureofcorneocytesinamatrixofintercellular lipids,withdesmosomesactingasmolecularrivetsbetweenthecorneocytes.Itiswidelyaccepted thatpermeationacrosstheSCisprimarilyviathelipiddomains,evenforpolarcompounds;therefore,solutediffusionmodelsandpermeationenhancementtechniquesareprimarilyfocusedon thisregion.
Therangeofproductsappliedforlocaleffectsintheskinandunderlyingtissuesinclude solutions,powders,ointments,pastes,gels,foams,creams,andvesicles-basedformulations. Transdermaldosageformsareprimarilypatches,althoughothertechnologiessuchasthe“patchlesspatch,”ametered-dosespraythatformsareservoirwithintheskin,developedbyAcrux (Melbourne,Australia)offeranalternative.Theidealdrugcandidateforskindeliveryshould havehighpotencywithadailydoserequirementofafewmilligrams,smallsize(M W < 500Da), bemoderatelylipophilic(log P of1–3),anduncharged[22].Clearly,thisimposesasevere limitationonthenumberofdrugsthatcanbedevelopedasatransdermalproduct.Therehas beenamajorfocusondevelopmentofskinpermeationenhancementapproachesthatrangefrom formulation-based(includinginclusionofchemical,solvents,andnano-systems),energy-based (includingiontophoresis,sonophoresis,magnetophoresis,andstratumcorneumablation),and minimallyinvasivetechnologies(includingjetpropulsionsystemsandmicroneedles).Transdermalpatchesarenowwidelyusedforsystemictherapyofpain,angina,smokingcessation, cognitivedisorders,andhormonereplacementtherapy(HRT).Table1.2summarizesthedrug propertiesandindicationsofsomeofthekeyproductsnowinuse[22,23].Asthetransdermal routeavoidsfirst-passmetabolism-reduceddosagescanprovidethesametherapeuticeffect,thus reducingside-effectsandincreasingsafety.Transdermalproductsalsoincreasepatientacceptability,adherence,andsatisfactionaswasdemonstratedinAlzheimer’spatientswherethoseusing patcheswerereportedtocomplybetterwithtreatmentthanpatientsusingoraldosageforms overasix-monthstudyperiod[24].Themostcommonside-effectsfoundwiththeapplicationof transdermalpatchesaremildtomoderateerythemaoritchatthepatchsite.
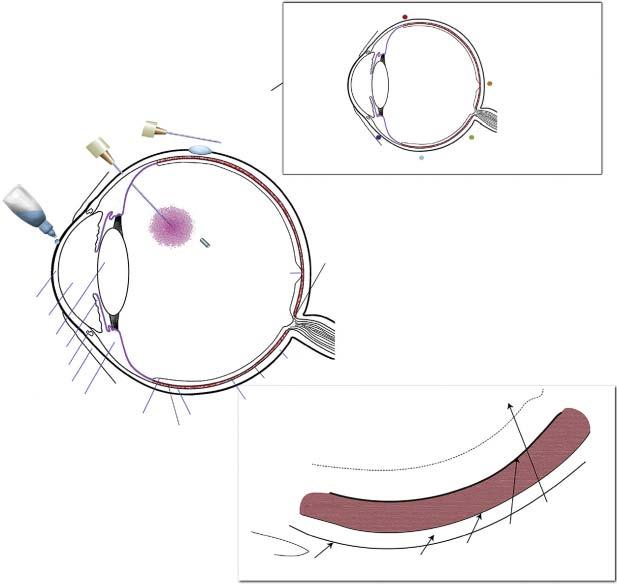
1.7OcularDrugDelivery
Topicaladministrationofeyedropsandointmentsarethemainstayofdrugdeliverytothe anteriorsegmentoftheeye;however,bioavailabilityislimitedduetowashoutbytearfluid[25]. SomedeviceshavebeendevelopedtoincreaseresidencetimesuchastheLacrisert® ophthalmic insert(Bausch + Lomb)(http://www.bausch.com/ecp/our-products/rx-pharmaceuticals/ rx-pharmaceuticals/lacrisert)whichisasterile,translucent,rod-shaped,water-soluble, preservative-free,slow-releaselubricantcomposedofhydroxypropylcellulosethatisplaced intotheinferiorcul-de-sacoftheeye.Oneinsertperdayprovidescontinuouslubrication.The Ocusertimplant(Sandoz)(https://www.drugs.com/cons/ocusert-pilo.html)isalsoplacedinthe cul-de-sacoftheeyeandreleasespilocarpineoversevendaystotreatglaucoma.Thelatterdevice isnotbiodegradableandmustberemovedandreplacedweekly(Figure1.3).
Drugdeliverytotheposteriorsegmentoftheeyeforthetreatmentofretinalandchoroid diseaseischallengingduetomultiplebarriers[26].Conjunctivalsystemicabsorptionandtear turnoverprocesseseffectivelylimittopicaldrugdeliveryandcannotbeprevented;therefore,
Table1.2 Physicochemical,pharmacokineticpropertiesandindicationsofcurrentlymarketedtransdermaldrugs.
Drug
Buprenorphine0.12–1.684682093.80.047,0.008 (32 C)
0.1–0.47.70.8ButransModeratetosevere pain
Clonidine0.1–0.32301302.70.17,13.58156–20950.2–2.03–301.2Catapres-TTSHypertension Ethinyloestradiol—296141–1464.30.039,0.0092 (25 C) 707.7p.o,17t.d.550.025–0.0751.75–5.250.07OrthoEvraFemale contraception Fentanyl0.288–2.40033783–843.90.15,0.2(30 C), 0.2(25 C) 27–753–12501–327–2252.4DuragesicChronicpain
Granisetron3.1312152–1542.60.01733–764–6603.9(t.d.Mean C max) 129–2962.5SancusoChemotherapyinduced emesis
Glyceryltrinitrate2.4–15.022713,liquid10.66,1.3(30 ∘ C)216–32700.03–0.05 < 1 0.1–54.32–130820,30Nitro-DurAngina Estradioland Levonorgesterel 0.050, 0.0007–0.015 272,312173–179 235–237 4.2 3.8 0.003(30 C) 0.0015(25 C) 0.017 600–800 5.7
Approx.1p.o. 28t.d 19.3p.o. 3–50.03–0.05 0.1–0.2 24–48 1 0.63,0.09,0.12, 0.18,0.14,0.23, 0.42,0.17 0.2 0.03 ClimaraProFemale contraception
Methylphenidate26.0–80.023374–75,liquid2.11.812(d);21(1) (children30kg) 1.5–55–205–2560–31588DaytranaADHD
Nicotine7.0–21.0162 79,liquid1.162,1085(30 C)77 2 20–455–30 385–231029,69,31,40NicodermSmokingcessation
Norelgestromin0.2 327110–1303.670.0043
Norethindrone
—0.6–1.2 0.31 OrthoEvraFemale contraception
acetate 0.125–0.250341161–1623.20.0065 20.6 6–8600.5–0.8 10.3–16.50.65 CombipatchFemaleHRT
Oxybutynin3.9 35856–584.3
0.12,0.42,0.18, 0.63,0.23,0.09 VivelleDotFemaleHRT
Rivastigmine4.6–9.5250Oilat25 C2.325 108 1.5362.5–20 270–216039 Exelon Alzheimer’sdisease Rotigotine 1.0–3.031675–774.70.017 600 5–7—0.4–2 240–12008.3 NeuproParkinson’sdisease andrestlessleg syndrome
Scopolamine0.3 30355,liquid0.81.8,75(30 ∘ C)65–121 1–54–270.04 3.25–6.055.6 TransdermscopTravelsickness Seligiline 6.0–12.0187Liquidat 25 ∘ C 2.7 0.73 84 10 4,102 16812.5 EmsamDepression
Testosterone0.3–5.02881553.60.02 41 0.17–1.7 <1,73–10.5 123–430.513.9 AndrodermHypogonadism
Notes:i.v.,intravenous;i.m.,intramuscular;p.o.,peroral;t.d.,transdermal;s.I.,sublingual;bc,buccal. Source:AdaptedfromRefs.[22,23].
Intravitreal administration (drug in solution, suspension, control release formulation, implant)
Periocular administration (injections, implants ...)
Sub-conjunctival
Topical administration (eye drops, ointments...)
Cornea
Anterior chamber
Posterior juxtascleral
Retrobulbar
Peribulbar
Systemic administration (oral, intravenous infusion...)
Suprachoroidal administration
Sub-retinal administration
Figure1.3 Routesforadministrationforretinaldrugdelivery.Source:DelAmoetal.[26].©2017Elsevier. alternativedeliverysystemshavebeendevelopedtoimprovebioavailability.Intravitrealinjection ofanti-vascularendothelialgrowthfactor(VEGF)proteins(bevacizumab,ranibizumab,and aflibercept)isthetreatmentofchoiceformanagementofwetage-relatedmaculardegeneration (AMD).However,complianceispoor,andtherefore,idealinjectionfrequencyisrarelymaintained [27].Therearetwomainfactorsinimprovingdrugdeliverytotheposteriorsegmentoftheeye: extendretentiontimeofthedeliverysystemtoincreasedrugavailability;overcomethebarriers toincreasedrugpenetrationandtargetdrugtoaspecificsitewithintheeye.Controlledrelease approacheshaveincludedbiodegradablepolymericparticlesandimplants,nonbiodegradable implants, insitu gellingformulations,andencapsulatedcelltechnology(ECT:entrapmentof geneticallyengineeredcellswithinasemipermeablematrixtoimmunologicallyisolatedthem fromthehost’sbody)[28].
1.8DrugDeliverySystemDevelopmentProcess
Thedevelopmentofanewdrugdeliverysystemtocontrolortargetdeliverytoatherapeutic siteinvolvesmanystepsfrombasicresearchthroughtoclinicalapplicationassummarized inFigure1.4[29].Theearlystepsinvolvecharacterizationofthedrugwhichmaybeasmall molecule,peptide/proteinorgene-derived,andchoiceofdeliverysystemdesignandmaterials. Thedeliverysystemisthenevaluatedfordrugreleaseanddelivery invitro and invivo priorto clinicalstudiesinvolunteersandpatientgroups.Theprocessesinvolvedindevelopinganew controlledreleasedosageformfromconcepttoconsumerareaddressedindetailinlaterchapters ofthisEncyclopedia.
Sub-conjunctival Inter-scleral
Retina
Retina
Ciliary body
Conjunctiva
Schlemm’s canal Iris
Lens
Sub-Tenon’s
Vitreous humour
Vitreous humour
Macula
Retinal pigment epithelium
Choroid
Sclera
Choroid
ScleraConjunctiva
RPE
Suprachoroidal
Sub-retinal
Intravitreal