DiaryletheneMolecularPhotoswitches
ConceptsandFunctionalities
MasahiroIrie
Author
MasahiroIrie
Professor emeritus KyushuUniversity Japan
Allbookspublishedby Wiley-VCH arecarefullyproduced.Nevertheless, authors,editors,andpublisherdonot warranttheinformationcontainedin thesebooks,includingthisbook,to befreeoferrors.Readersareadvised tokeepinmindthatstatements,data, illustrations,proceduraldetailsorother itemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>.
©2021WILEY-VCHGmbH,Boschstr. 12,69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc. usedinthisbook,evenwhennot specificallymarkedassuch,arenotto beconsideredunprotectedbylaw.
PrintISBN: 978-3-527-34640-0
ePDFISBN: 978-3-527-34642-4
ePubISBN: 978-3-527-82286-7
oBookISBN: 978-3-527-82285-0
Typesetting SPiGlobal,Chennai,India
Printedonacid-freepaper 10987654321
Contents
Preface ix
1Introduction 1
1.1GeneralIntroduction 1
1.2DiscoveryofDiaryletheneMolecularPhotoswitches 4 References 12
2ReactionMechanism 15
2.1BasicConcepts 15
2.2TheoreticalStudy 20
2.3ReactionDynamics 22
2.3.1CyclizationReaction 22
2.3.2CycloreversionReaction 27 References 29
3PhotoswitchingPerformance 31
3.1QuantumYield 31
3.1.1PhotocyclizationQuantumYield 31
3.1.2SolventEffectonCyclizationQuantumYield 42
3.1.3PhotocycloreversionQuantumYield 44
3.2ThermalStability 49
3.3FatigueResistance 53
3.4FluorescenceProperty 60
3.4.1Turn-OffModePhotoswitching 61
3.4.2Turn-OnModePhotoswitching 76
3.5ChiralProperty 80 References 86
4PhotoswitchableCrystals 93
4.1Dichroism 93
4.2X-RayCrystallographicAnalysis 97
4.3QuantumYield 101
4.4MulticoloredSystemsandNano-LayeredPeriodicStructures 106
Contents
4.5FluorescentCrystals 108
4.6PhotomechanicalResponse 110
4.6.1SurfaceMorphologyChange 112
4.6.2ReversibleShapeChange 113
4.6.3BendingResponseofMixedCrystals 116 References 121
5Memory 125
5.1Single-MoleculeMemory 125
5.2Near-FieldOpticalMemory 128
5.3Three-DimensionalOpticalMemory 130
5.4ReadoutUsingInfraredAbsorption,RamanScattering,andRefractive IndexChanges 132 References 134
6Switches 137
6.1Single-MoleculeConductancePhotoswitch 137
6.2OpticalSwitchBasedonRefractiveIndexChange 141
6.3Magnetism 141 References 146
7SurfaceProperties 149
7.1SurfaceWettability 149
7.2SelectiveMetalDeposition 151
7.3SubwavelengthNanopatterning 154 References 155
8PolymersandLiquidCrystals 157
8.1Polymers 157
8.2LiquidCrystals 175 References 178
9Applications 183
9.1OrganicField-EffectTransistors(OFETs) 183
9.2MetalOrganicFrameworks(MOFs) 185
9.3Super-ResolutionFluorescenceMicroscopy 188
9.3.1ControlofCycloreversionQuantumYield 189
9.3.2FatigueResistance 191
9.3.3PhotoswitchingwithSingle-WavelengthVisibleLight 192
9.3.4Super-ResolutionBioimaging 195
9.4ChemicalReactivityControl 197
9.5BiologicalActivity 201
9.6ColorDosimeters 204 References 209
ASynthesisProceduresofTypicalDiarylethenes 213
A.11,2-Bis(2,4-dimethyl-5-phenyl-3-thienyl)perfluorocyclopentene 213
A.21,2-Bis(2-ethyl-6-phenyl-1-benzothiophene-1,1-dioxide-3-yl)perfluorocyclopenetene 215
References 217
Index 219
Preface
Moleculescapableofreversiblephotoswitchingbetweentwoisomershaving differentabsorptionspectraarecalledphotochromicmoleculesormolecular photoswitches.Thetwoisomersdifferfromeachothernotonlyintheirabsorption andfluorescencespectra,butalsointheirrefractiveindices,dielectricconstants, oxidation/reductionpotentials,andgeometricalstructures.Thesephotoswitchable bistablemoleculesareappliedtoconstructphotonicdevices,suchaserasable opticalmemorymediaandopticalswitchelements.Althoughthefirstfindingof photoswitchablemoleculescanbetracedbacktothemiddleofnineteenthcentury, theyarestillawaitingtheirtimetogoonthestageofphotonicsdevicesinwideuse.
Digitalcamerastakephotosbyusingphysicalphenomenaofinorganicmaterials.Photodiodes,suchasCCD(charge-coupleddevice)andCMOS(complementarymetaloxidesemiconductor),detectphotonsbasedonphotovoltaiceffectsand constructphoto-images.Animalsandplantshavenosuchinorganicsemiconductors.Inbiologicalsystems,molecularphotoswitchesareextensivelyemployedin photoreceptors.Vision,forexample,usesthe cis-to-trans photoisomerizationofretinaltocontroltheconformationofrhodopsinandinitiatethetransductioncascade togenerateneuralsignals,whilephototaxisof Chlamydomonas isactivatedbythe trans-to-cis photoisomerizationofretinalinthechannelrhodopsin.Inplants,the photoisomerizationofphytochromesplaysakeyroleincontrollingtheirbiologicalactivity.Theseingenioususesoforganicmoleculesforthedetectionofphotons inbiologicalsystemsindicatethatmolecularphotoswitcheshavethepotentialtobe appliedintheconstructionofvarioustypesofphoton-workingreagentsanddevices.
Acharacteristicfeatureofmoleculesistheirsmallsize(∼ 1nm).Ifasingle moleculewouldworkasone-bitmemory,ultimatehigh-density(1Pbit/inch2 )opticalmemorycouldberealized.Conductanceswitchesarekeycomponentsofalmost allelectronicdevices.Fabricationofsingle-moleculephotoswitchesisthefirststep towardmolecularelectronics.Photoswitchingofsingle-moleculefluorescencehas revolutionizedfluorescencemicroscopyimaging.Thesuper-resolutiontechnique realizesaresolutionofafewtensofnanometers.Forsuchapplications,molecular photoswitchesarerequiredtopossesssuperiorproperties,suchasthermalstability ofbothisomers,fatigueresistance,highsensitivity,rapidresponse,andreactivity inthesolidstate.Amongthem,thermalstabilityandfatigueresistanceareindispensableproperties.Althoughtremendouseffortsweremadeinthe1970–1980s
x Preface
toprovidethethermalirreversibilitytomolecularphotoswitchesinordertoapply themtoopticalmemorymedia,allattemptstomodifyexistingphotoswitchable moleculesfailed,becausetherewasnoguidingprincipleonhowtopreparesuch thermallystablemolecularphotoswitches.
In1988,theserendipitousdiscoveryofdiarylmaleicanhydrides,whichundergo thermallyirreversiblephotoswitchingreactions,pavedthewaytosolvetheproblem.Inferringfromexperimentalaswellastheoreticalanalysis,themolecular designprincipleofthermallyirreversiblemolecularphotoswitcheswasestablished.Thisnewclassofmolecularphotoswitchesisnamed“Diarylethene.”The well-designeddiarylethenesprovideoutstandingphotoswitchingperformance: bothisomersarethermallystableformorethan470000yearsat30 ∘ C,photocyclization(coloration)/photocycloreversion(decoloration)canberepeatedformore than104 cycles,thequantumyieldofcyclizationreactioniscloseto1(100%),and theresponsetimesofbothphotocyclizationandphotocycloreversionreactions arelessthan20ps.Manyofthediarylethenederivativesundergophotoswitching reactionseveninthecrystallinephase.Inthisbook,thediscoveryanddevelopment ofdiarylethenesaredescribedcomprehensivelyfromthebasicconceptstotheir applications.
Iwishtoexpressmydeepappreciationtomycolleagues,Prof.M.Morimoto,Prof. S.Kobatake,Prof.K.Matsuda,Prof.T.Fukaminato,Prof.K.Uchida,Prof.T.Kawai, Prof.T.Tsujioka,andDr.K.Unofortheirkindhelpandsupporttopreparethe manuscript.Finally,Ialsoexpressmythankstomyfamily,Setsuko,Fumi,and Hisafumifortheircontinualencouragementtocompletethisbook.
December2020 MasahiroIrie
1.1GeneralIntroduction
Biologicalsystemshavedevelopedvariouskindsofphotoactiveorganstoadapt themselvestoenvironmentalelectromagneticradiation,sunlight.Inbiological systems,lightisusedintwowaysasshowninFigure1.1.Inplants,forexample, photosyntheticsystemshaveevolvedtoutilizelightasanenergysource.Carbohydratesareproducedfromwaterandcarbondioxideinplantsbyusinglight asanenergysource.Anothercategoryistheuseoflightforinformationaccess andtransmission.Lightisusedtoseekoutoptimumconditionsfortheirlife. Photoresponsivebiologicalsystemsforvision,phototaxis,andphototropismhave evolvedtorecognizesurroundingconditionsandtoaccessexternalinformation.In theformer,photoinducedelectrontransferisakeychemicalreaction.Ontheother hand,photoisomerizationplaysakeyroleinthelatter.
Photoisomerizationisoneofthefundamentalreactionsinphotochemistry[1–3]. Trans–cis isomerization,sigmatropicrearrangements,andelectrocyclicrearrangementsaretypicalexamples.Moleculescapableofthesereversiblephotoisomerizationreactionsarecalledphotochromicmoleculesormolecularphotoswitches [4–10].Thetwoisomersdifferfromeachothernotonlyintheirabsorptionand fluorescencespectrabutalsointheirgeometricalstructures,oxidation/reduction potentials,refractiveindices,anddielectricconstants.
Scheme1.1showstypicalexamplesofphotoswitchablemoleculesandwhenthey werediscovered.Thethreeuppermolecules,azobenzene[11],spirobenzopyran [12],andbridgedimidazoledimer[13],belongtoT-type(thermallyreversible) molecularphotswitches,inwhichphotogeneratedright-sidecoloredisomersare thermallyunstableandspontaneouslyrevertbacktoleft-sidecolorlessisomersin thedark.Azobenzeneundergoesalargechangeingeometricalstructureduringthe photoisomerizationreactionfromthe trans- tothe cis-form.Thedistancebetween4 and4′ carbonatoms(thelongaxisofthemolecule)decreasesfrom0.90to0.55nm andthedipolemomentincreasesfrom0.5to3.1D[14,15].Aspirobenzopyran derivative,6-nitro-1′ ,3′ ,3′ -trimethylspiro[2H -1-benzopyran-2,2′ -indoline],converts fromalesspolarspiro-formtoapolarmerocyanine-formuponirradiationwith UVlight.Itisreportedthatthedipolemomentofthespiro-formis6.2D,whileit
DiaryletheneMolecularPhotoswitches:ConceptsandFunctionalities, FirstEdition.MasahiroIrie. ©2021WILEY-VCHGmbH.Published2021byWILEY-VCHGmbH.
Energy Information
Photosynthesis (Photoinducedelectrontransfer)
Photoresponsiveness (Photoisomerization)
Vision
Phototaxis
Phototropism
Figure1.1 Theuseoflightinbiologicalsystems.
increasesto13.9Dinthemerocyanineform[16].Thebluecolorofthemerocyanine formdisappearsinlessthananhoureveninhigh-Tg polymermatricesinthedarkat roomtemperature[17].Acolorlessbridgedimidazoledimer,1,8-TPID-naphthalene (TPID:dimeroftriphenylimidazolylradicals),turnsgreenintolueneuponirradiationwithUVlight,andthegreencolordisappearsinlessthanafewseconds afterswitchingofftheUVlight[18].Theimidazoledimerexhibitsextremelyquick response.
Thetwolowermolecules,furylfulgide[19]anddiarylethene[20–22],undergo P-type(thermallyirreversiblebutphotochemicallyreversible)photoswitchingreactions.IntheP-typemolecularphotoswitches,photogeneratedright-sidecoloredisomersarethermallystableandpracticallyneverreturntotheright-sidecolorless isomersinthedarkatroomtemperature.Althoughmanymolecularphotoswitches havebeensofarreported,P-typechromophoresareveryrare.Thefamilies,furylfulgidesanddiarylethenes,aretwosuchrareexamplesexhibitingP-typereactivity. Theprimarydifferencebetweenfurylfulgidesanddiarylethenesisfatigueresistance. Photoinducedcoloration/decolorationcyclesofwell-designeddiarylethenederivativescanberepeatedmorethan104 timesmaintainingadequatephotoswitching ability(seeSection3.3),whereasinmostcasesthecorrespondingcyclesoffurylfulgidesarelimitedtolessthan102 times.
Theinstantpropertychangesofphotoswitchablemoleculesbyphotoirradiation withoutanyadditionalprocessleadtotheiruseinvariousphotoresponsivematerials andphotonicdevices.Whenthebistablemoleculesareincorporatedintomaterials, theelectronicstructurechangescanbeappliedtoopticalmemorymediaandconductancephotoswitches,whilethegeometricalstructurechangescanbeappliedto light-drivenactuatorsandothers.Molecularphotoswitchesusedinsuchapplicationsarerequiredtofulfillfollowingproperties.
1.Thermalstabilityofbothisomers
2.Fatigue-resistance
3.Highsensitivity
4.Rapidresponse
5.Reactivityinthesolidstate.
Molecularphotoswitchesbelongingtothediarylethenefamilyfulfilltheabove requirementssimultaneously.Diarylethenesarederivativesofstilbene.Whenthe phenylringsofstilbenearereplacedwithfive-memberedheterocyclicrings,such
Azobenzene
Spirobenzopyran
Bridgedimidazoledimer
Furylfulgide
Diarylethene
Scheme1.1 Molecularphotoswitchesandyearswhentheywerediscovered.
asthiopheneorfuranrings,bothopen-andclosed-ringisomersbecomethermally stableandphotoinducedcoloration/decolorationcyclescanberepeatedmanytimes. Thebestphotoswitchingperformanceofwell-designeddiarylethenesissummarized asfollows.
1.Bothisomersarethermallystable:half-lifetimesatroomtemperatureareas longas470000yearsat30 ∘ C.
2.Photoinducedcoloration/decolorationcanberepeatedformorethan104 cycles.
3.Thequantumyieldofcyclization(coloration)reactioniscloseto1(100%).
4.Responsetimesofbothcolorationanddecolorationreactionsarelessthan20ps.
5.Manyofdiarylethenederivativesundergophotoswitchingeveninthesingle crystallinephase.
1.2DiscoveryofDiaryletheneMolecularPhotoswitches
Thediarylethenemolecularphotoswitcheswereserendipitouslydiscoveredduring thecourseofastudyonphotoresponsivepolymers[23].Variouspolymershaving molecularphotoswitches,suchasspirobenzopyran,azobenzene,orstilbene,inthe sidegroupsormainchainshavebeenpreparedinanattempttomodulatetheir conformationsbyphotoirradiation.Whenazobenzenechromophoresareincorporatedintoapolymerbackbone,thesolutionviscositywasfoundtoreversiblychange uponalternateirradiationwithUVandvisiblelight[24].BeforeUVlightirradiation, thepolymerhasarod-likeextendedconformation.UponUVlightirradiation,the azobenzeneunitsconvertfromthe trans- tothe cis-formandtheisomerizationkinks thepolymerchain,resultinginacompactconformationandadecreaseintheviscosity,asshowninFigure1.2.Notonlyviscositybutalsootherproperties,suchaspH, solubility,andsol–gelphasetransitiontemperature,weresuccessfullymodulated uponphotoirradiation[23–28].
Justlikeazobenzene,stilbenealsoundergoesthe trans–cis photoisomerization reaction.Thephotoresponsivepolymerresearchwasextendedtopolymershaving stilbeneunits.Apolymerhavingstilbeneunitsinthebackbonecanbepreparedby 1,4-additionradicalpolymerizationof2,3-diphenylbutadiene,whichisprepared fromacetophenone,asshowninFigure1.3[29].Uponirradiationwith313-nm light,thepoly(2,3-diphenylbutadiene)efficientlyunderwentphotocyclizationreactionstoproduceapolymerhavingyellowcoloreddihydrophenanthreneunitsina deaerateddichloromethanesolution,insteadofthe trans–cis photoisomerization. The trans–cis photoisomerizationofstilbeneunitsinthebackbonewasstrongly suppressedduetorigidityofthepolymerchain.Thedihydrophenanthreneunits readilyreturnedtotheinitial2,3-diphenyl-2-buteneunitsandtheyellowcolor disappearedinlessthan10minutesatroomtemperature.
Ontheotherhand,inthepresenceofairthedihydrophenanthreneunits convertedtophenanthreneunitsbyhydrogeneliminationandthereversibilitywaslost.Topreventhydrogeneliminationandprovidereversibilityeven underaeratedconditions,2,3-dimesitylbutadienewasdesigned(Figure1.4A(a)). Thesynthesisof2,3-dimesitylbutadienewasattemptedbyphotoreductionof 2,4,6-trimethylacetophenone,asshowninFigure1.4B(a).But,thesynthesis ofpinacolfailedbecauseofthebulkysizeofthemesitylgroup.Toreduce sterichindrance,themesitylenewasreplacedwith2,5-dimethylthiophene (Figure1.4A(b)).AccordingtothesyntheticrouteshowninFigure1.4B(b), 2,3-bis(2,5-dimethyl-3-thienyl)butadienewassuccessfullysynthesizedfrom 2,5-dimethyl-3-acetylthiophene.Thebutadienewaspolymerizedtopoly(2,3-bis(2,5dimethyl-3-thienyl)butadiene)by1,4-additionradicalpolymerization.
Figure1.2 Schematic illustrationofthe photoinducedconformational changeofapolymerhaving azobenzeneunitsinthe backbone.
1.2DiscoveryofDiaryletheneMolecularPhotoswitches 5
Figure1.3 Asynthesisrouteofpoly(2,3-diphenylbutadiene)anditsphotochemicaland thermalreactions.
Thepolymerhaving2,3-dithienyl-2-buteneunitswasdissolvedinbenzene andthesolutionwasirradiatedwith313-nmlight.Thecolorlesssolutionturned yellow(��max ∼ 430nm)alongwiththeformationofcyclizedclosed-ringisomers. Theyellowcolordisappeareduponirradiationwithvisiblelight.Incontrastto poly(2,3-diphenylbutadiene),theyellowcoloroftheclosed-ringisomerunits wasfoundtoremainstableovernightinthedark.Theyellowclosed-ring unitswerestableevenat100 ∘ Candreturnedtotheinitialcolorlessopen-ring isomerunitswithvisiblelight.Thedithienyletheneunitinthepolymerwas unprecedentedlyfoundtoundergoathermallyirreversiblephotoswitchingreaction.Poly(2,3-bis(2,5-dimethyl-3-furyl)butadiene)alsounderwentthethermally irreversiblephotoswitchingreaction.Theamazingresultledustostudythe photochemistryofthemonomerunit,2,3-di(2,5-dimethyl-3-thienyl)-2-buteneand itsderivativesindetail.Thisisthecourseofserendipitousdiscoveryofdiarylethene molecularphotoswitches.
Sincethediscoveryofthermallyirreversiblediarylethenemolecularphotoswitchesinthemiddleof1980s,varioustypesofdiarylethenederivativeshavebeensynthesizedtoimprovetheirphotoswitchingperformance. Figure1.5showsalistofmaindiarylethenederivativesdevelopedinKyushu UniversityandRikkyoUniversityuntil2017.UponirradiationwithUV light,2,3-di(2,5-dimethyl-3-thienyl)-2-buteneunderwenta cis–trans isomerizationinadditiontothecyclizationreaction.Topreventtheunfavorable
Figure1.4 (A)Synthesisofpolymershaving(a)2,3-dimesitylbuteneunitsand (b)2,3-di(2,5-dimethyl-3-thienyl)buteneunitsinthebackbone.(B)(a)Asyntheticrouteto prepare2,3-dimesitylbutadiene.(b)Syntheticroutesandphotochemicalreactionsof poly(2,3-di(2,5-dimethyl-3-thienyl)butadiene)and poly(2,3-di(2,5-dimethyl-3-furyl)butadiene).
cis–trans photoisomerization,acyclicbridge,suchasmaleicanhydrideor maleimide,wasintroduced.Althoughdiarylethenederivativeswiththe maleicanhydrideormaleimidebridgeshowedphotocyclizationreactivityin lesspolarsolvents,thereactivitywasstronglysuppressedinpolarsolvents, suchasmethanoloracetonitrile.Toprovidephotoswitchingreactivityeven inpolarsolvents,theethenebridgeswerereplacedwithperfluorocycloalkenes withfour-,five-,andsix-memberedrings[30].The1,2-bis(2-methyl-1benzothiophen-3-yl)perfluorocycloalkenesunderwentreversiblephotoinduced cyclization/cycloreversionreactionsinpolarmethanolandacetonitrile.Among thethreederivativeshavingfour-,five-,andsix-memberedrings,five-membered 1,2-bis(2-methyl-1-benzothiophen-3-yl)perfluorocyclopentenewasfoundtooffer
1.2DiscoveryofDiaryletheneMolecularPhotoswitches 7
Figure1.5 Developmentofdiarylethenemolecularphotoswitches. thehighestresistancetophotofatigue.Sincethen,perfluorocyclopentenederivatives havebeenmainlystudied.
Althoughdiarylethenephotoswitchesexhibitbrilliantcolorchangesupon photoirradiation,mostofthemarenonfluorescentorveryweaklyfluorescent inbothisomerforms.Itwasalong-standingambitiontopreparephotoswitchablefluorescentdiaryletheneswithoutattachingfluorescentchromophorestothe diarylethenes.In2011,sulfonederivativesof1,2-bis(2-ethyl-6-aryl-1-benzothiophen3-yl)perfluorocyclopentenewerefoundtoexhibitverystrongfluorescence (fluorescencequantumyield ∼ 0.9)intheclosed-ringisomers[31].Theturn-on modefluorescentdiarylethenesarenowextensivelyappliedtosuper-resolution fluorescencemicroscopyinmaterialsscienceandbiologicalsystems.Diarylethenes areabletoswitchbothabsorption(color)andfluorescenceemissionupon photoirradiation.
Atfirstsight,themoststrikingphenomenonobservedinmolecularphotoswitches isaphotoinducedinstantaneouscolorchange.Figure1.6showsphotosofthecolor
Figure1.6 Colorchangesofdiarylethenederivatives 1–7 intolueneuponirradiationwith UVandvisiblelight.
1.2DiscoveryofDiaryletheneMolecularPhotoswitches 9 changesofdiarylethenederivativesinsolution.Whenthetoluenesolutionsofthe derivativesareirradiatedwithUVlight,thecolorlesssolutionsturnyellow,orange, red,violet,blue,cyan,andgreen.Thechemicalstructuresofthederivativesare shownbelowinthephotos.Thesecolorsdisappearuponirradiationwithvisible light.Thephotoinducedcoloration/decolorationcyclesuponalternateirradiation withUVandvisiblelightcanberepeatedmanytimes.
Thecolorchangesareascribedtotheelectronicstructurechangesofthe derivativesfromtheopen-totheclosed-ringisomers.Twotypicalexamplesofthe electronicstructurechangesareshowninFigure1.7.1,2-Bis(2,5-dimethyl-3-thienyl) perfluorocyclopentene(3)and1,2-bis(3,5-dimethyl-2-thienyl)perfluorocyclopentene (1)undergoreversibleelectrocyclicrearrangements.Theelectrocyclicreactions involverearrangementsofpositionsofsingleanddoublebondsinamolecule. Duringthereactions,anewsinglebondismadebetweenthecentralreactivecarbon atomsbythecyclizationreactionandthebondisbrokenastheringisopened.
Figures1.7showsthechemicalstructuresoftheopen-andtheclosed-ring isomersandtheirabsorptionspectra.Inbothderivatives 3 and 1,uponirradiation withappropriatewavelengthoflight(��1 or ��3 )asinglebondisformedbetween thecentralreactivecarbonatomsandthedoublebondschangetheposition.Upon irradiationwithanotherwavelengthoflight(��2 or ��4 )thesinglebondisbroken andthemoleculereturnstotheinitialstructure.Thecoloriscontrolledbythe lengthof π-conjugation.Intheopen-ringisomers,twothiopheneringshaveno particularinteractionandthespectraarecomparabletosubstitutedthiophenes.In theclosed-ringisomers,the π-conjugationlengthdependsontheattachedposition ofthiopheneringstotheethenebridge.Whenthethiopheneringsareattachedto theethenebridgeat3-position,suchasderivative 3, π-conjugationisdelocalized throughoutthemoleculeintheclosed-ringisomerandthedelocalizationresultsin redcolor.The π-conjugationisfurtherextendedwhenphenylgroupsaresubstituted at5-and5′ -positionsofthethiophenerings,suchas 5.Thelong π-conjugation shiftstheabsorptionbandtolongerwavelengths,resultinginbluecolor.On theotherhand,whenthethiopheneringsareattachedtotheethenebridgeat 2-position,suchasderivative 1, π-conjugationislocalizedinthecentralpart.The short π-conjugationresultsinyellowcolorintheclosed-ringisomer.
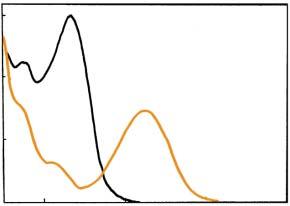
Thephotoswitchingbetweentwodiscretestatesandthermalirreversibilityof thetwostatesareindispensableforapplicationstomemorymediaandswitching devices.Thebistabilityisabasiccharacteristicofdiarylethenes.Althoughthechemicalstructuresofthetwoisomerssuggestphotoswitchingbetweentwodiscrete states,ingeneral,theabsorptionandfluorescencespectragraduallychangeupon photoirradiationinensemblesystems.Figure1.8bshowsthephotoswitchingperformanceof1,2-bis(2-ethyl-6-phenyl-1-benzothiophene-1,1-dioxide-3-yl)perfluorocyclopentene(8,Figure1.8a)intheensemblesystemin1,4-dioxane.Thegradual analogincreaseinthefluorescenceintensityuponirradiationwithUVlight indicatesachangeintheconcentrationsofthetwoisomers.UponUVirradiation
Figure1.7 Chemicalstructuresandabsorptionspectraofopen-andclosed-ringisomers of(a) 3 and(b) 1 in n-hexane.
theopen-ringisomersconverttothefluorescentclosed-ringisomersandtheconcentrationratiooftheclosed-ringisomersincreases,causingthegradualincrease inthefluorescenceintensity.Subsequently,uponirradiationwithvisiblelight theratiooftheclosed-ringisomersdecreases,resultingindisappearanceofthe fluorescence.Theswitchingbetweenthetwodiscretestatescannotbediscerned fromthephotoswitchingperformanceintheensemblesystem.
1.2DiscoveryofDiaryletheneMolecularPhotoswitches
Figure1.8 (a)Chemicalstructuresofopen-andclosed-ringisomersof 8.(b)Fluorescence photoswitchingof 8 uponirradiationwithUVandvisiblelightintheensemblesystemin 1,4-dioxane.(c)Fluorescencephotoswitchingof 8 atthesingle-moleculelevelinaZeonex polyolefinfilm.
Digital on/off photoswitchingbetweentwodiscretestateswasconfirmed bymeasuringtheswitchingresponseatasingle-moleculelevel.Figure1.8c showsthefluorescencephotoswitchingofasinglemoleculeofderivative 8 upon alternateirradiationwithUVandvisiblelight.UponirradiationwithUVlight, thefluorescenceabruptlyswitchesfromthe off -statetothe on-state,whileupon irradiationwithvisiblelightthe on-stateabruptlyreturnstothe off -state.The digitalphotoswitchingresponsedefinitelyindicatesthatdiarylethenephotoswitch 8 hasbistablestates.Thephotoisomerizationbetweentwodiscreteisomerstates expressedbythetwochemicalstructuresisexperimentallyevidenced.
References
1 Barltrop,J.A.andCoyle,J.D.(1975). ExcitedStatesinOrganicChemistry.London:Wiley.
2 Turro,N.J.(1978). ModernMolecularPhotochemistry.MenloPark,CA:Benjamin/CummingsPub.
3 Balzani,V.,Ceroni,P.,andJuris,A.(2014). PhotochemistryandPhotophysics: Concept,Research,Applications.Weinheim:Wiley-VCH.
4 Brown,G.H.(1971). Photochromism.NewYork:Wiley-Interscience.
5 Crano,J.C.andGuglielmetti,R.J.(1998). OrganicPhotochromismandThermochromicCompounds.NewYork:PlenumPress.
6 Bouas-Laurent,H.andDürr,H.(2001).Organicphotochromism. PureAppl. Chem. 73:639–665.
7 Dürr,H.andBouas-Laurent,H.(eds.)(2003). Photochromism:MoleculesandSystems.Amsterdam:Elsevier.
8 Irie,M.,Yokoyama,Y.,andSeki,T.(eds.)(2013). NewFrontiersinPhotochromism.Tokyo:Springer.
9 Tian,H.andZhang,J.(eds.)(2016). PhotochromicMaterials:Preparation,PropertiesandApplications.Weinheim:Wiley-VCH.
10 Yokoyama,Y.andNakatani,K.(eds.)(2017). Photon-WorkingSwitches.Tokyo: Springer.
11 Hartley,G.S.(1937).Thecis-formofazobenzene. Nature 140:281.
12 Fischer,E.andHirshberg,Y.(1952).Formationofcolouredformsofspiropyrans bylow-temperatureirradiation. J.Chem.Soc.:4522–4524.
13 Iwahori,F.,Hatano,S.,andAbe,J.(2007).Rationaldesignofanewclass ofdiffusion-inhibitedHABIwithfastback-reaction. J.Phys.Org.Chem. 20: 857–863.
14 Hampson,G.C.andRobertson,J.M.(1941).Bondlengthsandresonanceinthe cis-azobenzenemolecule. J.Chem.Soc.:409–413.
15 Bullock,D.J.W.,Cumper,C.W.N.,andVogel,A.I.(1965).Physicalpropertiesand chemicalconstitution.PartXLLIII.Theelectricdipolemomentsofazobenzene, azopyridines,andazoquinolines. J.Chem.Soc.:5316–5323.
16 Malic,E.,Weber,C.,Richter,M.etal.(2011).Microscopicmodeloftheopticalabsorptionofcarbonnanotubesfunctionalizedwithmolecularspiropyran photoswitches. Phys.Rev.Lett. 106:097401.
17 Kryszewski,M.,Nadolski,B.,North,A.M.,andPethrick,R.A.(1980).Kinetic matrixeffects(responseanddensitydistributionfunction):ringclosurereaction ofindolinobenzospiropyransinglassypoly(alkylmethacrylates). J.Chem.Soc., FaradayTransII 76:351–368.
18 Fujita,K.,Hatano,S.,Kato,D.,andAbe,J.(2008).Photochromismofaradical diffusion-inhibitedhexaarylbiimidazolederivativewithintensecolorationand fastdecolourationperformance. Org.Lett. 10(14):3105–3108.
19 Heller,H.G.andOliver,S.(1981).Photochromicheterocyclicfulgides.Part1. Rearrangementreactionsof(E)-α-3-furylethylidene(isopropylidene)succinic anhydride. J.Chem.Soc.PerkinTrans.1:197–201.
20 Irie,M.andMohri,M.(1988).Thermallyirreversiblephotochromicsystems. Reversiblephotocyclizationofdiarylethenederivatives. J.Org.Chem. 53(4): 803–808.
21 Irie,M.(2000).Diarylethenesformemoriesandswitches. Chem.Rev. 100(5): 1685–1716.
22 Irie,M.,Fukaminato,T.,Matsuda,K.,andKobatake,S.(2014).Photochromism ofdiarylethenemoleculesandcrystals:memories,switchesandactuators. Chem. Rev. 114:12174–12277.
23 Irie,M.(1990).Photoresponsivepolymers. Adv.Polym.Sci. 94:27–67.
24 Irie,M.,Hirano,Y.,Hashimoto,S.,andHayashi,K.(1981).Photoresponsive polymers.2.Reversiblesolutionviscositychangeofpolyamideshavingazobenzeneresiduesinthemainchain. Macromolecules 14(2):262–267.
25 Irie,M.andSchnabel,W.(1981).Photoresponsivepolymers.Onthedynamics ofconformationalchangesofpolyamideswithbackboneazobenzenegroups. Macromolecules 14(5):1246–1249.
26 Irie,M.andTanaka,H.(1983).Photoresponsivepolymers.5.Reversiblesolubilitychangeofpolystyrenehavingazobenzenegroups. Macromolecules 16(2): 210–214.
27 Irie,M.andSchnabel,W.(1985).Onthedynamicsofphotostimulatedconformationalchangeofpolystyrenewithpendantazobenzenegroupsinsolution. Macromolecules 18(3):394–398.
28 Irie,M.andIga,R.(1986).Photoresponsivepolymers.9.Photostimulated reversiblesol-geltransitionofpolystyrenewithpendantazobenzenegroups incarbondisulfide. Macromolecules 19(10):2480–2484.
29 Irie,M.andSchnabel,W.(1982).Photochemicalconversionof poly-2,3-diphenylbutadienetopoly-9,10-dimethylenephenanthrene. Eur.Polym.J. 18:15–18.
30 Hanazawa,M.,Sumiya,R.,Horikawa,Y.,andIrie,M.(1992).Thermallyirreversiblephotochromicsystems.Reversiblephotocyclizationof 1,2-bis(2-methylbenzo[b]thiophen-3-yl)perfluorocycloalkenederivatives. J.Chem. Soc.,Chem.Commun. 3:206–207.
31 Uno,K.,Niikura,H.,Morimoto,M.etal.(2011).Insitupreparationofhighly fluorescentdyesuponphotoirradiation. J.Am.Chem.Soc. 133:13558–13564.
2.1BasicConcepts
Thermalirreversibilityisanessentialandindispensablepropertyfortheapplicationsofmolecularphotoswitchestomemorymediaandswitches.Although tremendouseffortsweremadeinthe1970–1980stoprovidethethermalirreversibilitytomolecularphotoswitches,allattemptstomodifyexistingphotoswitchable moleculesfailed.Wehadtowaituntilthethermallystablemolecularphotoswitches wereserendipitouslydiscovered.Inthebeginningandthemiddleofthe1980s, itwasfoundthatfurylfulgidesanddiarylethenesundergothermallyirreversible photoswitching.Thephotogeneratedcoloredisomerspracticallyneverrevertback tothecolorlessisomersatroomtemperature.Althoughtheyundergothermally irreversiblephotoswitching,thereasonwhythemoleculesshowthethermal stabilitywasnotunderstood.Itwasacrucialtasktorevealthereason.Thebasic principlebehindthethermallyirreversiblephotoswitchingreactivitywaselucidated usingboththeoreticalandexperimentalapproaches,asfollows.
The2,3-diphenyl-2-buteneunit,showninFigure1.3,underwentathermallyreversiblephotoswitchingreactioninadeaeratedsolution,whilethe 2,3-di(2,5-dimethyl-3-thienyl)-2-buteneunit,showninFigure1.4B(b)exhibitedathermallyirreversiblereactivity.Thephotogeneratedclosed-ringformof 2,3-di(2,5-dimethyl-3-thienyl)-2-butenewasfoundtoremainstableandpracticallyneverreturnedtotheopen-ringformatroomtemperature.Inaddition,the open-ringisomerwasstableevenatelevatedtemperaturesandnothermochromic reactionwasobserved.Torevealthereasonwhythediarylethenehavingphenyl ringsandthathavingthiopheneringsexhibitsuchadifferentreactivity,semiempiricalmodifiedneglectofdiatomicoverlap(MNDO)calculationwascarriedout fordiarylethenederivatives 9–12 (Scheme2.1)[1].
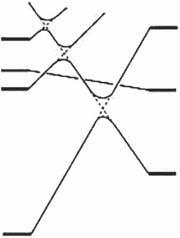
Forelectrocyclicreactions,twomodesofgeometricalstructurechanges,conrotatoryanddisrotatory,aredistinguished,asshowninScheme2.2.Accordingtothe Woodward-Hoffmannrule[2]basedon π-orbitalsymmetriesfor1,3,5-hexatriene (HT),whichisthesimplestmolecularframeworkoftheabovemolecules,theconrotatorycyclizationreactiontocyclohexadiene(CHD)isbroughtaboutbylightand thedisrotatorycyclizationbyheat.
DiaryletheneMolecularPhotoswitches:ConceptsandFunctionalities, FirstEdition.MasahiroIrie. ©2021WILEY-VCHGmbH.Published2021byWILEY-VCHGmbH.
10o:X=NH
11o:X=O
12o:X=S
10c:X=NH
11c:X=O
12c:X=S
Scheme2.1 Electrocyclicreactionsofdiarylethenes 9 12
Scheme2.2 Disrotatoryandconrotatorycyclizationreactionsofhexatriene.
Thecycloreversionreactionisallowedphotochemicallyintheconrotatorymode andthermallyinthedisrotatorymode.Fromthissimplesymmetryconsideration oftheHTmolecularframework,thethermalstabilityoftheopen-ringisomerof 2,3-di(2,5-dimethyl-3-thienyl)-2-buteneandthermalirreversibilityinthecycloreversionreactioncannotbeexplained.Astateenergycalculationisnecessarytodiscuss thethermalstability.
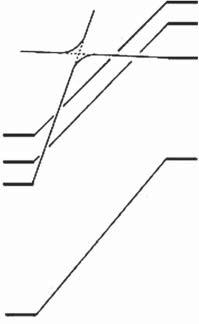
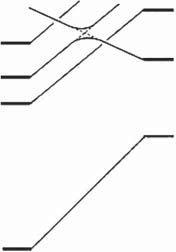
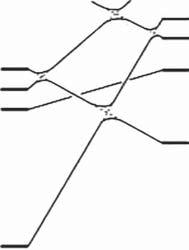
Figures2.1and2.2showthestatecorrelationdiagramsfortheelectrocyclicreactionsof 9 and 11 indisrotatoryandconrotatorymodes,respectively.Fulllinesin thefiguresshowthatinterconnectingstatesbelongtothesamesymmetrygroups. Therelativegroundstateenergydifferencesbetweentheopen-andclosed-ringisomersareshowninTable2.1.Thetwoheterocyclicringswereassumedtobein theparallelorientationforthedisrotatoryreactionandintheantiparallelorientationfortheconrotatoryreaction.AscanbeseenfromFigure2.1,orbitalsymmetry allowsthedisrotatorycyclizationsinthegroundstatesfrom 9o to 9c andfrom 11o to 11c.Therelativegroundstateenergiesoftheclosed-ringisomersof 9 and 11 are, however,175and113kJ/molhigherthantherespectiveenergiesoftheopen-ring isomers.Thisindicatesthattheopen-ringisomersarestableandthethermalcyclizationreactiondoesnottakeplacepracticallyinbothcases.
Onthecontrary,orbitalsymmetryforbidstheconrotatorycyclizationsinthe groundstatesfrom 9o to 9c andfrom 11o to 11c,becauseeach S0 open-ringisomer statecorrelateswithahighlyexcitedstateoftheclosed-ringisomer,asshown inFigure2.2.Ontheotherhand,nosuchlargebarrierexistsinthe S1 statefor 9o andthe S2 statefor 11o.Thisindicatesthatelectrocyclicreactionsofboth
″ (51,45)
′ (50,46)
Figure2.1 Statecorrelationdiagramsfortheelectrocyclicreactionsindisrotatorymode. 9c and 11c aretheclosed-ringisomers,inwhichtwohydrogensattachedtothereactive centralcarbonsareinacisconfiguration.
A(50,46)
A(48,48)
B(49,47)
A(48,48)
A(48,48)
A(50,45)
B(49,47)
A(50,46)
A(42,42)
B(43,41)
A(42,42)
A(42,42)
A(42,42)
B(43,41)
A(44,40)
Figure2.2 Statecorrelationdiagramsfortheelectrocyclicreactionsinconrotatorymode. 9c and 11c areclosed-ringisomers,inwhichtwohydrogensattachedtothereactivecentral carbonsareinatransconfiguration.
1,2-diphenyletheneand1,2-bis(3-furul)etheneareallowedinthephotochemically excitedstates.
Whatshouldbediscussedhereisthestabilityoftheclosed-ringisomers. Figure2.2showsthatinboth 9c and 11c, thecycloreversionreactionsintheground statehavetoovercomeenergybarriers,andthebarrierscorrelatewithground stateenergydifferencesbetweentheopen-andclosed-ringisomers.Thecalculated
2ReactionMechanism
Table2.1 Relativegroundstateenergydifferencesbetweentheopen-andclosed-ring isomers.
CompoundDisrotatory(kJ/mol)Conrotatory(kJ/mol)
1,2-Diphenylethene(9)175114
1,2-Di(3-pyrrolyl)ethene(10)13565
1,2-Di(3-furyl)ethene(11)11338
1,2-Di(3-thienyl)ethene(12)51 14
isomer
Figure2.3 Correlationbetweenthegroundstateenergydifferencebetweenopen-and closed-ringisomersandtheenergybarrier.
energydifferencesareshowninTable2.1.Whentheenergydifferenceislarge,as inthecaseof 9,theenergybarrierbecomessmallandthecycloreversionreaction takesplacereadily.Ontheotherhand,theenergybarrierbecomeslargewhenthe energydifferenceissmall.Inthiscase,thecycloreversionreactionhardlytakes place.Thecorrelationbetweenthegroundstateenergydifferenceandtheenergy barrieriswellexplainedbytheHoriuti–PolanyiruleasshowninFigure2.3.The energydifferenceinthegroundstatesbetweentheopen-andclosed-ringisomers controlsthestabilityoftheclosed-ringisomers.
Thenextquestioniswhatcausesthedifferenceinthegroundstateenergylevelsof thetwoisomers.First,strainenergiesofthesix-memberedringsoftheclosed-ring isomerswerecompared.Theoptimizedgeometriesoftheclosed-ringisomers, 9c and 11c,however,showedalmostidenticalsix-memberedringstructuresandthe ring-straincouldnotexplaintheenergydifference.Next,thearomaticitychange fromtheopen-totheclosed-ringisomerswasexamined.Duringthecyclizationreaction,phenylandheterocyclicringschangethestructuresasshowninScheme2.3. Thearomaticityoftheringsislostduringthecyclizationreactions.Theenergydifferencesbetweentheright-andleft-sidegroupswerecalculatedandareshownin Table2.2.Thearomaticstabilizationenergyofthearylgroupscorrelateswellwith
Scheme2.3 Thestructurechangesofphenylandfive-memberedheterocyclicringsalong withthecyclizationreactions.
Table2.2 Aromaticstabilization energydifferences.
thegroundstateenergydifference.Thehighestenergydifferencewascalculatedfor thephenylgroupandthelowestoneforthethienylgroup.Destabilizationdueto destructionofthearomaticringduringthecyclizationreactionincreasestheenergy oftheclosed-ringform.Thearomaticityisthekeymolecularpropertythatcontrols thethermalstabilityoftheclosed-ringisomers.
ThetheoreticalpredictionwasconfirmedbythesynthesisofdiarylethenederivativeswithvarioustypesofarylgroupsasshowninFigure2.4.Whenthearylgroups arethiophene,benzothiophene,thiazole,oroxazolerings,whichhavelowaromatic stabilizationenergy,theclosed-ringisomersarestable(morethan12hoursat 80 ∘ C).Ontheotherhand,photogeneratedclosed-ringisomersofdiarylethenes withindolerings,whichhaveintermediatearomaticstabilizationenergy,undergo thermallyreversiblephotoswitching(half-lifetimeat80 ∘ Cof 16c:2.5hours).The closed-ringisomersofdiarylethenederivativeswithphenylringsreadilyreturned backtoopen-ringisomers(half-lifetimeat20 ∘ Cof 18c:1.5minutes).
Fromtheabovetheoreticalandexperimentalresults,theguidingprincipleforthe synthesisofthermallyirreversiblediarylethenesisdefinedasfollows.
Thethermallyirreversiblephotoswitchingdiarylethenescanbepreparedby employingarylgroupswithlowaromaticstabilizationenergy.
2ReactionMechanism
ThermallyStable
ThermallyUnstable
Figure2.4 Thermalstabilityofdiarylethenederivatives.Anyappreciablechangeofthe absorptionintensityoftheclosed-ringisomerwasnotobservedinthethermallystable derivativesformorethan12hoursat80 ∘ C.
2.2TheoreticalStudy
Thewell-studiedphotoinducedcyclizationandcycloreversionreactionsbetween 1,3,5-hexatriene(HT)andcyclohexadiene(CHD)provideausefulframeworkfor understandingthebasicreactionmechanismofdiarylethenes.Asthefirststep, potentialenergysurfacesofamodeldiarylethene,1,2-bis(cyclopenta-1,3-dien-2-yl)ethene,werecalculatedusingacompleteactivespaceself-consistentfield(CASSCF) method[3].Figure2.5shows S0 and S1 potentialenergysurfacesalongthereaction coordinate.Onboth S0 and S1 surfacesthereexisttwominima,aclosed-ring isomer(CHDandCHD*)andanopen-ringisomer(HTandHT*).Transition structures(TS0 andTS1 )werealsocharacterizedoneachpotentialenergysurface. Severalconicalintersectionminima(indicatedbycrosses)existonbothclosed-and open-ringsidesofthepotentialsurfaceinadditiontoCI3 .CI3 isthemostimportant conicalintersection,becauseitistheonlyonethatprovidesapathwaytowardboth openandclosedminimaonthegroundstate.Attheconicalintersectiongeometry, onehasatriangulararrangementbetweenthreeunpairedelectronsbelonging tocarbonatomsinthetwo5-memberedrings.Thesethreeelectronsareweakly
Ring-openingreactioncoordinate
Figure2.5 Potentialenergysurfacesofamodeldiarylethene. Source:Reprintedwith permissionfromRef.[3].Copyright2003AmericanChemicalSociety.
coupled π-electrons,andafourth(belongingtoathree-electronallylfragment) isanuncoupledspectator.ItisworthnotingthattheTS1 barrierdoesnotexist intheCHD/HTsystem.Thebarrierisprobablyduetostericconstraintfromthe sigma-bondframework.
Figure2.5showsthepotentialenergysurfacesalongthereactioncoordinate ofthehydrocarbonmodelsystem.Amorerealisticmodelisdithienylethene. Thepotentialenergysurfacesof1,2-di(3-thienyl)ethene(12)werecalculatedas afunctionofthedistancebetweentworeactivecarbonatoms,R(C—C),using theCASSCFmethod,asshowninFigure2.6[4].Tounderstandthereaction mechanism,amolecularmechanics-valencebond(MMVB)computationofthe dynamicswasalsocarriedout.Three-dimensionalrepresentationofthestructures of S0 and S1 potentialenergysurfacesandtwo-dimensionalsimplifiedoneofthe structuresareshowninFigures2.7and2.8,respectively[5].Thesefiguresalso outlineminimumenergypathscorrespondingtoring-closingandring-opening reactionsofthedithienylethene.
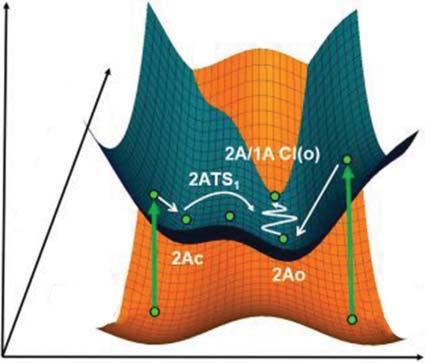
UponirradiationwithUVlight,theopen-ringisomerisexcitedtotheallowed Frank–Condonstate(1BFC(o)),whichiscloseinenergytoa2Asurface,andfast internalconversiontothe2Astatetakesplace.Theexcitedwavepacketmovesdown alongthe2Asurfaceandtheconicalintersection(2A/1ACI(o))canthenbeaccessed viavibrationsorthogonaltothereactionpass,asshowninFigure2.7.Attheconical intersection,efficientdecaytothegroundstatepotentialenergysurfacetakesplace, leadingtotheformationoftheclosed-ringisomer.Thismechanismaccountsforthe highlyefficientandultrafastring-closingreaction.
Thering-openingreactiontakesplaceasfollows.Uponirradiationwithvisible light,theclosed-ringisomerisexcitedtotheallowed1BFrank–Condonstate (1BFC(c)).TheexcitedwavepacketmovesawayfromtheFrank–Condonregion
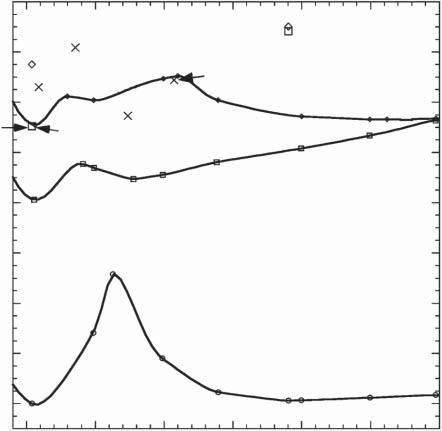
Figure2.6 Potentialenergysurfacesofground(1A)andtwoexcited(2Aand1B)statesof 12 asafunctionofthedistancebetweentworeactivecarbonatoms. Source:Reprintedwith permissionfromRef.[4].Copyright2004AmericanChemicalSociety. alongthe1Bsurfaceandrapidlyfallsfromthe1Bto2Asurfaceviaaconical intersection(1B/2ACI(c)).Themoleculeexcitedwithlightofshorterwavelengths havingvibrationalexcessenergyonthe2Asurfacegoesovertheenergybarrier 2ATS1 morereadilyandreaches2A/1Aconicalintersection(2A/1ACI(o)).The conicalintersectionisreachedviavibrationsorthogonaltotheinitialmotioninthe valley.Attheconicalintersection,fastdeactivationtothegroundstatetakesplace andthustheopen-ringisomerisformed.Theroutesuggeststhatthermalactivation orexcesskineticenergyonthe2Asurfaceisrequiredforthering-openingreaction totakeplace.Thisagreeswithexperimentalobservationthatthering-opening reactionhasanactivationenergyandthatthequantumyieldincreasesupon irradiationwithlightofshorterwavelengths(seeSection3.1.3)[7,8].
2.3ReactionDynamics
2.3.1CyclizationReaction
AsdiscussedinthetheoreticalstudyinSection2.2,diarylethenesareanticipatedto undergoveryfastring-closing(cyclization)reactions.Therapidcyclizationdynamicswasconfirmedusingfemtosecondabsorptionspectroscopiesinsolutionaswell asinthesinglecrystallinephase[9].
EnergyBranchingspace
Closed-ring isomer
Open-ring isomer
Reactioncoordinate
Figure2.7 Schematicrepresentationofthestructuresof S 0 (orange)and S 1 (green) potentialenergysurfacescorrespondingtothephotoisomerizationbetweenopen-and closed-ringisomersofadiarylethene.Reactioncoordinateisthedistancebetweenthetwo reactivecarbonatoms. Source:AdaptedfromRef.[5]withpermissionfromthePCCPOwner Societies.
1B/2ACl(c) 1BFC(o)
1BFC(c)
Closed-ringisomerOpen-ringisomer
Figure2.8 Outlineoftworeactionpathscorrespondingtoring-openingandring-closing reactionsofadiarylethene.Thelettersinparentheses,cando,representclosed-and open-ringisomerstructures,respectively. Source:ReprintedwithpermissionfromRef.[6]. Copyright2014AmericanChemicalSociety.
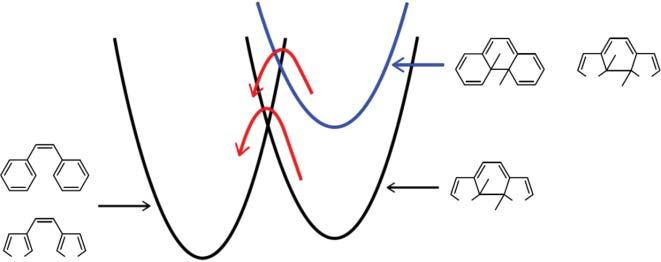
Thecyclizationreactionof1,2-bis(2,4-dimethyl-5-phenyl-3-thienyl)perfluorocyclopentene(5)wasinvestigatedinthesinglecrystallinephase.Insolution,the open-ringisomerhastwoconformations,photoactiveantiparallelandphotoinactiveparallelones,asshowninScheme2.4.Coexistenceofthetwoconformations insolutionpreventsclearresolutionofspectralfeaturesassociatedwiththe