SupramolecularChemistryonSurfaces
2DNetworksand2DStructures
EditedbyNeilR.Champness
Editor
Prof.NeilR.Champness UniversityofBirmingham SchoolofChemistry
Edgbaston B152TTBirmingham UnitedKingdom
CoverImage: Reproducedfrom “On-surfacechemicalreactions characterisedbyultrahighresolution scanningprobemicroscopy”
A.Sweetman,N.R.Champness,and A.Saywell. Chem.Soc.Rev.,2020, 49, 4189–4202,withpermissionfrom TheRoyalSocietyofChemistry.
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>
©2022WILEY-VCHVerlagGmbH&Co. KGaA,Boschstr.12,69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34491-8
ePDFISBN: 978-3-527-81668-2
ePubISBN: 978-3-527-81670-5
oBookISBN: 978-3-527-81669-9
Typesetting Straive,Chennai,India
Printedonacid-freepaper 10987654321
Contents
Preface ix
1Two-DimensionalSupramolecularChemistryonSurfaces 1 NeilR.Champness References 6
2CharacterisationandInterpretationofOn-SurfaceChemical ReactionsStudiedbyUltra-High-ResolutionScanningProbe Microscopy 9 AdamSweetman,NeilR.Champness,andAlexSaywell
2.1Introduction 9
2.2SPMUnderUHVConditions 10
2.2.1On-SurfaceReactions 11
2.2.2CharacterisationofMolecule-SubstrateSystemsviaSTM 12
2.2.3ncAFM 15
2.3PracticalStepsinAccomplishingSub-MolecularImaging 16
2.3.1SamplePreparation 16
2.3.1.1DepositionofOrganicMoleculesatLowTemperature 17
2.3.1.2CODeposition 17
2.3.1.3DecouplingLayers 18
2.3.2ConstructionoftheqPlusSensor 18
2.3.3TipPreparation 19
2.3.3.1TipFunctionalisation 19
2.3.4PracticalConsiderationsforImaging 21
2.3.4.1DriftandCreep 21
2.3.4.2AmplitudeCalibration 22
2.3.4.3ApparentDissipationandMechanicalCouplingoftheSensor 22
2.3.4.4Crosstalk 22
2.3.4.5ForceInversion 23
2.4InterpretationofSub-MolecularContrastattheSingleBondLevel 23
2.4.1ForcesintheTip-SampleJunction 24
2.4.1.1Non-siteSpecificInteractions–The‘Background’ 24
2.4.1.2LocalDispersionInteractions–The‘Halo’ 24
2.4.1.3PauliRepulsion–The‘CarbonBackbone’ 24
2.4.1.4ChemicalBonding 25
2.4.1.5LocalElectrostaticInteractions 25
2.4.2ResponseoftheProbeParticle–DistortionsinImaging 25
2.4.2.1FlexibilityofAdsorbedCO 26
2.4.2.2Electrostatics 28
2.4.2.3ChemicalSensitivity 29
2.5CharacterisingOn-SurfaceReactionswithncAFM 29
2.5.1PracticalConsiderationsforCharacterisingOn-SurfaceReactions 31
2.5.2SynthesisandCharacterisationofGrapheneBasedNanostructures 32
2.5.3StudyingtheEvolutionofOn-SurfaceReaction 34
2.6Conclusions 38 Acknowledgements 39 References 39
3ComplexityinTwo-DimensionalMulticomponent Assembly 43
KunalS.Mali,JoanTeyssandier,NereaBilbao,andStevenDeFeyter
3.1Introduction 43
3.2Two-ComponentSelf-AssembledSystems 45
3.2.1Two-ComponentSystems:Host–GuestArchitectures 46
3.2.1.1HostNetworksfromIntrinsicallyPorousBuildingBlocks 46
3.2.1.2HostNetworksfromSelf-AssemblyofBuildingBlocks 49
3.2.1.3Two-ComponentSystems:Host–GuestArchitecturesBasedon Surface-ConfinedTwo-DimensionalCovalentOrganicFrameworks (2D-sCOFs) 57
3.2.2Two-ComponentSystems:Non-Host–GuestArchitectures 59
3.3Three-ComponentSystems 62
3.3.1Three-ComponentSystems:Two-ComponentHostNetwork + Guest 62
3.3.2Three-ComponentSystems:One-ComponentHostNetwork + Two DifferentGuests 65
3.3.3Three-ComponentSystems:Non-host–GuestSystems 69
3.4Four-ComponentSystems 71
3.4.1Four-ComponentSystems:Host–GuestArchitectures 72
3.4.2Four-ComponentSystems:Non-host–GuestArchitectures 75
3.5SummaryandPerspectives 76 References 76
4ComplexityinTwo-DimensionalAssembly:UsingCoordination Bonds 81 NianLinandJingLiu
4.1Introduction 81
4.2AsymmetricLinkers 82
4.3MultipleTypesofLinkers 86
4.4Multiple-Level(Hierarchical)Interaction 88
4.5MultipleBindingModes 90
4.6SummaryandOutlook 97 References 97
5ComplexityinTwo-DimensionalAssembly:Quasicrystalline Structures 103 S.AlexKandel
5.1History 103
5.2RandomTilings 104
5.3QuasicrystallineTilings 108 References 114
6UsingSelf-AssemblytoControlOn-SurfaceReactions 117 ZhantaoPeng,LingboXing,andKaiWu
6.1Introduction 117
6.2MediatingOn-SurfaceReactionSelectivity 119
6.3MediatingOn-SurfaceReactionPathway 124
6.4MediatingOn-SurfaceReactionSite 125
6.5BriefSummaryandPerspective 130 Acknowledgement 131 References 131
7CovalentlyBondedOrganicStructuresviaOn-Surface Synthesis 135 CanWang,HaimingZhang,andLifengChi
7.1Introduction 135
7.2Dehalogenation 136
7.2.1UllmannCoupling 136
7.2.2SonogashiraCoupling 141
7.2.3HeckReaction 141
7.3Dehydrogenation 143
7.3.1(SP3 -C)AlkanePolymerisation 143
7.3.2(SP2 -C)ArylandAlkeneCyclodehydrogenation 145
7.3.2.1Aryl–ArylDehydrogenationCoupling 145
7.3.2.2Bottom-UpFabricationofGrapheneNanoribbons(GNRs) 148
7.3.2.3Homo-CouplingofTerminalAlkene 150
7.3.3(SP1 -C)Alkyne–GlaserCoupling 151
7.3.4HierarchicalDehydrogenationofX—HBonds(X = NandC) 152
7.4DehydrationReaction 153
7.4.1Schiff-BaseReaction 153
7.4.2ImidisationCondensationReaction 156
7.4.3BoronicAcidCondensation 156
7.4.4DecarboxylativePolymerisation 157
7.4.5DimerisationandCyclotrimerisationofAcetyls 159
7.5OtherReactions 159
7.5.1ClickReaction 159
7.5.1.1Azide–AlkyneCycloaddition 159
7.5.1.2Diels–AlderReaction 160
7.5.2Bergman-LikeReaction 161
7.5.3N-HeterocyclicCarbenesFormationandDimerisation 162
7.5.4 σ-BondMetathesis 163
7.5.5DiacetylenePolymerisation 164
7.6ConclusionandPerspectives 165 References 166
8HybridOrganic-2DTMDHeterointerfaces:TowardsDevices Using2DMaterials 171 YuL.HuangandAndrewT.S.Wee
8.1Introduction 171
8.2AtomicStructures 172
8.2.1Pristine2DTMDs 172
8.2.2Organic/2DTMDInterfaces 174
8.3SurfaceFunctionalisationof2DTMDsbyOrganics 177
8.3.1DefectEngineering 177
8.3.2PhaseEngineering 179
8.4FundamentalElectronicProperties 180
8.4.1EnergyLevelAlignment 181
8.4.2InterfacialChargeTransfer 184
8.4.3ScreeningEffect 190
8.5ApplicationsinDevices:Organic-2DTMDp–nHeterojunctions 192
8.6Conclusion 193
Acknowledgements 194 References 194
9SurfaceSelf-AssemblyofHydrogen-BondedFrameworks 199 NicholasPearceandNeilR.Champness
9.1Introduction 199
9.2CarboxylicAcidSupramolecularSynthons 200
9.3Imide-MelamineSupramolecularSynthons 205
9.4FromHydrogen-bondingSynthonstoCovalently-organic Frameworks 211
9.5HeteromolecularHydrogen-bondingSynthons 213
9.6Conclusions 215 References 215
Index 219
Preface
Thefieldofsupramolecularchemistryhasdevelopedfromitsinceptiontonow influencethinking,strategies,andapplicationacrossthechemicalandmaterials sciences.Whilstremarkableprogresshasbeenmadeinmanyfields,theneedto interfacesupramolecularsystemstotherealworldhasspurredinterestinperformingsupramolecularchemistryonsurfaces.Thegoalofstudyingsupramolecular self-assemblyprocesseshasinturnengenderednewideas,newconcepts,and ultimatelyanewfieldofstudy.
Interestingly,thisfreshresearchfocushasbroughttogetherexpertsfrommany differentbackgroundscreatingnewinterdisciplinaryconnections,notablybetween syntheticchemistsandphysicists.Surface-basedsupramolecularchemistryisa trulymultidisciplinaryfield.Indeed,thefieldhasrapidlydevelopedandtheoriginal focusonhydrogen-bondedsystemshasbeenjoinedbytheexploitationofother supramolecularinteractions.Similarly,someresearchershavemovedtowardsusing self-assemblyprocessesthatenabletheformationofcovalentbondsandhence robustchemicalsystems,suchasnanoscalegraphenes.
Thesestudiesrelyoncharacterisationtechniques,particularlyscanning-probe microscopies,thatenablemolecular,andevensubmolecular,resolution.Notonly dosuchapproachesresultinvisuallyinspiringimagestheyalsoallowappreciation ofsupramolecularstructureswithalevelofdetailthatisrarelyachievablein traditionalsupramolecularchemistry.Inturn,thishasledtothediscoveryofcomplexquasi-crystallinearraysandhighlycomplexarrangements.Thesefascinating structuressparktheimaginationandmovebeyondmuchthathasbeenachievedin supramolecularchemistry.
Alltheseremarkabledevelopmentsandnewavenuesofresearchhavespurred increasingattentiontohowthesesystemsmaybeexploitedindevicesoperatingat thesingle-moleculelevel.Theinteractionbetweenthesurfaceandthoseabsorbed moleculesallowsdirectinteractionbetweenmolecularsystemsandthemacroscopic worldandhasledtoincreasinginterestindevelopingdevices,particularlyemployingelectronicproperties.Thus,thefieldisdevelopingfromsimplecuriosityand structuralfascinationtowardsapplications.
Itistimelytoevaluateprogressinthefieldandtoappreciatewherethefocushas beenandwhereitisgoing.Hence,thiscollectionsurveysthefieldfromthepoint ofviewofexpertswhohavedevotedtheirendeavourstodevelopthisnewareaof
x Preface science.Iamgratefultoallthoseauthorsfortheirexcellentcontributionsandfor soclearlyexpoundingtheirvisionoftheresearcharea.Ihopethatthechapters containedhereinwillinspirethemanyresearchersinthefieldbutalsothosewho currentlysitaroundtheperipheryofthisactivitywhetherchemist,physicist,orthe nextgenerationofscientist.
NeilR.Champness Birmingham April2021
Two-DimensionalSupramolecularChemistryonSurfaces
NeilR.Champness
UniversityofBirmingham,SchoolofChemistry,Edgbaston,BirminghamB152TT,UK
Supramolecularchemistryrepresentsoneofthecentralthemesofmodernchemical sciences.Crossingtraditionalboundariesofchemistry,materialsscience,biology, andphysics,thefieldofsupramolecularchemistryaffordsopportunitiestocreate newmoleculesandmaterials,withfarreachingimplicationsformanyanddiverse applications.ThesignificanceofsupramolecularchemistryliesbehindtwoNobel Prizes,1987[1]and2016[2–4],andisnownotonlyafieldinitsownrightbutis alsoacentralunderpinningthemeinalmostanyareaofchemistry.Theprimary principleofsupramolecularchemistryistheuseofnon-covalentinteractionstocreateandcontrolself-assembledstructures.Alargerangeofinteractionsisavailable tothesupramolecularchemisttoinfluenceandcontrolself-assemblyprocesses. Fromhydrogenbonds[5–7]andhalogenbonds[8,9]to π-interactions[10,11], coordinationbonds[12,13]andthemechanicalbond[2,3,14–16],interactions ofdifferentstrengthsandvaryingdegreesofgeometricalpreferencesareavailable todesignandcreatestructures.Wheninitsinfancy,supramolecularchemistry focussedpredominantlyonsyntheticstrategiesincombinationwithunderstanding thefundamentalpropertiesofthenon-covalentinteractionsemployed.Overrecent years,thefieldhasdevelopedtosuchanextentthatitisnowcommonplacetofocus efforttowardsapplicationsandtheserangeacrossavastspectrum.Supramolecular chemistryissowide-rangingthatitsrelevancecanbeappliedtodiversefields,from biology[17,18]andmedicine[18,19]tonewmaterials[20,21]andenergy-related applications[22,23].
Theoriginsofsupramolecularchemistrylieinsolution-basedsystems,usingintermolecularinteractionstocreatesupermolecules.Fromtheseorigins,supramolecularchemistryisnowobservedinmostphases,notablyinthesolid-state,through crystalengineering[24,25],inliquidcrystals[26]andionicliquids[27],andeven inthegasphase[28].Itwasonlynaturalthatsupramolecularchemistrystrategies wouldcometobeappliedtothetwo-dimensional(2D)environmentofsurfaces (Figure1.1).Thisseeminglynaturalprogressionalsoraisedanumberofchallenges topractitionersofthesubject,notleastintermsofappreciatingthisquitedifferent
SupramolecularChemistryonSurfaces:2DNetworksand2DStructures,FirstEdition. EditedbyNeilR.Champness. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
2 1Two-DimensionalSupramolecularChemistryonSurfaces
environmentandperhapsmostimportantlythedifferenttechniquesthatareused tocharacteriseandinterpretsurface-basedmolecularsystems.
(b)
1Two-DimensionalSupramolecularChemistryonSurfaces 3
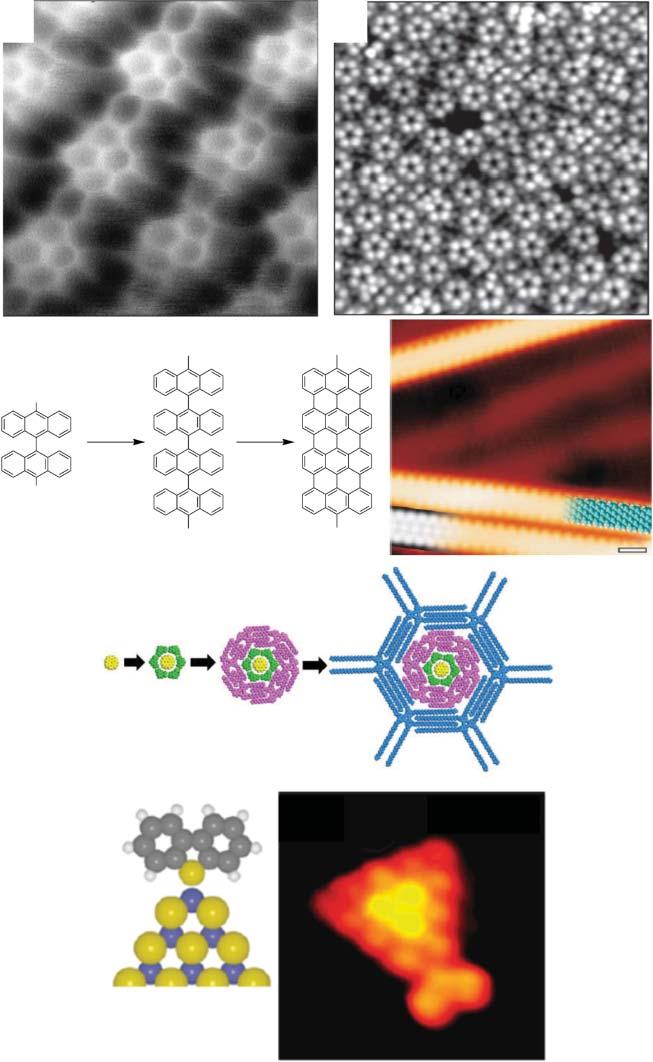
Figure1.1 Examplesoftwo-dimensionalsupramolecularchemistryonsurfacesdiscussed withinthisvolume.(a)ncAFMimageofahydrogen-bondednaphthalene-1,4:5,8tetracarboxylicdiimide(NTCDI)islandonaAg:Si(111)–(√3 × √3)R30∘ surfaceacquired at77K.Theimagerevealssub-moleculardetailsoftheself-assembledstructure;(b)STM imageofself-assembledarraysofferrocene-carboxylicacid(FcCOOH);eachbrightfeature representsaseparateFcCOOHmolecule,whichthenassembleintopentamershighly reminiscentofaPenrosetilingarrangement;(c)Surface-assistedC–Ccouplingreaction usedtopreparestraightgraphenenanoribbonsfrombianthrylmonomers,includingaSTM imageofnanoribbon,followingcyclodehydrogenationat400 ∘ C,withpartlyoverlaid molecularmodel(rightinblue)andadensity-functionaltheorymodel(bottomleftingrey); (d)Schematicrepresentationofastrategyusedtoprepareamulticomponentsystemusing a‘core–shell’approach.Eachcolourrepresentsadifferentmolecularbuildingblock; (e)SchematicrepresentationandSTMimageshowingdibenzothiopheneboundtothe cornervacancyofaS-edge-terminatedMoS2 nanocluster.Source:Imagesreproducedwith permissionasfollows:(a)Sweetmanetal.[29];(b)reproducedwithpermissionfrom SpringerNaturefromWasioetal.[30];(c)reproducedwithpermissionfromSpringerNature fromCaietal.[31];(d)Malietal.[32];(e)reprintedandadaptedwithpermissionfrom Tuxenetal.[33].Copyright(2010)AmericanChemicalSociety.
Whereasthetechniquesappliedtocharacterisingsolutionphase,orsolid-state, supramolecularsystemsarecommonacrosssyntheticchemistry,forexample, NMRspectroscopy,massspectrometry,andX-raydiffraction,characterisationof surface-boundmoleculesisaquitedistinctdomain.Themostcommonapproaches tocharacterisingmolecularspeciesonsurfacesarescanningprobemicroscopies (SPM).Specifically,techniquessuchasscanning-tunnellingmicroscopy(STM)[34] andatomicforcemicroscopy(AFM)[35]representthedominantcharacterisation methodsusedintheanalysisofsurface-basedsupramolecularsystems.Theseimagingmicroscopiescanbe,andoftenare,supplementedbyotherapproaches,suchas X-rayphotoelectronspectroscopy(XPS),butSPMapproachesprovideinvaluable insightintospecificmoleculararrangementsallowingdeterminationofthegeometricstructureoforganicmoleculeswithmolecularresolution.Morerecently,the developmentofnoncontactatomicforcemicroscopy(ncAFM)[36]allowsthecharacterisationofsupramolecularsystemswithsub-molecularresolution[37].Theuse ofSPMcharacterisationtechniquesinitselfpresentsopportunities,whicharerarely availabletothoseworkinginotherphases,notleastbecausesuchmicroscopies functionatthemolecular,orevensub-molecular,levelandasaresultinformation, bothstructuralandelectronic,canbegatheredforindividualmoleculesanddefined self-assembledarrays.Incomparison,techniquessuchasNMRspectroscopy orX-raydiffractionrelyuponthesignalfromcomparativelylargenumbersof molecules.Thus,thecharacterisationofsurface-basedsupramolecularsystemscan giveadetailedpictureofthestructuresandeventransformationsbetweendifferent arrangementswithahighdegreeofresolution.Thecomplexities,challenges,and advantagesofdifferentSPMtechniquesarediscussedinmoredetailbySweetman, Champness,andSaywellinthisvolume.
Afurtheraspectofthedetailedimagingwithmolecularresolutionisthatthis allowscharacterisationofstructuresthatwouldproveextremelychallengingbyany othertechnique.UsingSPMtechniquesallowsreadyidentificationofdefectswithin supramoleculararraysbutintriguinglyallowsthestudyofextendedstructures,
1Two-DimensionalSupramolecularChemistryonSurfaces
whichdonotpossesslong-rangeorder,withmolecularresolution.Thisapproach hasbeenappliedtothestudyofrandom,entropicallystabilised,rhombustilings [29,38,39],amolecularPenrosetile[30],quasicrystallinestructures[40],and fascinatingassembliesthatexhibitthestructureofSerpi ´ nskitriangles[41].The complexissueswithstudyingandcharacterisingquasicrystalline2Darraysare discussedindetailbyKandelinthisbook.
Thestudyofsupramolecularchemistryonsurfacesprobablybeganwithearly studiesofhydrogen-bondedassemblies[42–44]buthasspreadtoemployother non-covalentinteractionsincludingcoordinationbonds[45,46]andweakervan derWaalsinteractions[47–49].Theuseofdifferentintermolecularinteractions isdiscussedthroughoutchaptersinthisvolume.Inparticular,Mali,Teyssandier, Bilbao,andDeFeyterdiscusstheuseofhydrogenbondsandvanderWaals interactionstocreatecomplexstructureswhereastheapplicationofcoordination bondsispresentedbyLinandLiu.Itwillbecomecleartothereaderthatthechoice ofintermolecularinteractioninfluencesthechoiceofexperimentalconditions used,includingdepositionconditions,useofultra-highvacuum(UHV)orstudies atthesolid–solutioninterface,andeventhenatureofthesurfaceemployedfor surfaceself-assembly.Theinteractionsbetweensurface,substrate,solution,and self-assembledarrayareallimportantindeterminingthesubtleenergeticbalance betweendifferentproducts[50].
Thesestudieshavenowdevelopedfurthertocreatecovalentlylinkedstructures includingnanographenes[51,52]andcovalent-organicframeworks(COFs)[53]. Allofthesestrategiespresenttheirowndistinctadvantages,anddisadvantages,but importantlyrepresentabroadpaletteforresearcherstoemployandexplore.Weaker interactionssuchashydrogenbonds,vanderWaalsinteractions,andevencoordinationbonds,formreversiblyandthereforefacilitatetheformationofwellorganised, andrelativelydefect-free,supermoleculestructuresovercomparativelylargeareas. Creatinglargerdefectfreestructurescanbemorechallengingusingcovalentbonds althoughtheuseofreversibly-formedbondssuchasimines[54]hasbeendeveloped toaidinthisrespect.Nanographenes,wherecarbon–carbonbondsareanabsolute requirement,presentquitedifferentchallengesbutremarkableadvanceshavebeen madeinthisarea.InthisvolumePeng,Xing,andWudiscusstheuseofintermolecularinteractionstocontrolon-surfacereactionsandWang,Zhang,andChipresent developmentsinthefieldofon-surfacereactionstocreatecovalentlybondedsystems.
Anothermajorchallengethatrequiresthoughtwhenoneconsiderssurface-based supramolecularchemistryarethereactionenvironmentandconditions.Firstly,itis typicaltouseasurfacethatisatomicallyflatoratleastclosetoatomicallyflat.This ratherstringentrequirementfacilitatestheuseofSPMcharacterisationandsimultaneouslycontrolstheintroductionofsurface-basedreactivesitestotheself-assembly process.Eventhoughatomicallyflatsurfacesarecommonlyused,itwouldbeamistaketoconsiderthesurfaceasaninnocentbystanderintheself-assemblyprocess. Indeed,adsorptionbetweenthesurfaceandthemoleculesinvolvedinself-assembly isessentialtoallowtheformationofasurface-boundorsurface-supported, supramolecularstructure[50].Arangeofsurfacesareavailabletoresearchers
1Two-DimensionalSupramolecularChemistryonSurfaces 5 investigatingsuchsystemsbutsomearemorecommonthanothers,notably highly-orientedpyrolyticgraphite(HOPG)andAu(111).However,insomeareas ofstudy,thesurfaceplaysanintegralroleinthereactionprocessprovidingactive sites,suchasmetalatoms,whichcatalysetheformationofaspecificproduct[55].
Theothermajoraspectthatinfluencestheself-assemblyprocessistheexperimentalconditionsoftheexperiment.SPMtechniquescanbeusedinbothUHV conditionsorattheinterfacebetweensurfaceandsolution.Thesequitedifferent conditionspresentbothadvantagesanddisadvantagesdependingonthespecificmoleculesandreactionprocessesbeinginvestigated.Forexample,studying moleculesandself-assembledaggregatesinUHVconditionscanleadtohigher resolutionimaging,inpartbecauselowertemperatures(belowthefreezingpointof solvents)canbeaccessed.Additionally,ncAFMimagingspecificallyrequiresUHV conditions.However,theintroductionofmoleculestothesurfacetypicallyinvolves sublimation,andhenceheatingofthesample.Sublimationisnotalwayspossible andthermaldegradationisasignificantimpedimentforcomplexmolecules.Milder electrospraydepositiontechniqueshavebeendeveloped[56]buttheuseofthis approachisnotyetwidespread.Incontrast,studiesatthesolution-solidinterface directlyimageself-assembledstructuresinthepresenceofsolvent.Intermsof preparativeconditions,thisapproachisquitestraightforward,simplyimaging attheinterfacebetweenadropofsolventcontainingthemoleculesofinterest andthesubstrate.Althoughthisapproachoffersmanyadvantagesthechoiceof solvent,whichislimitedbytherequirementsforimaging,canclearlyinfluence theself-assemblyprocess,potentiallywithsolventmoleculesinteractingoreven co-adsorbingwiththetargetspecies.Althoughimagestendtohavelowerresolution thanUHVstudies,thisisnotalwaysthecaseandremarkableexamplesofmolecular resolutionwithAFMhavebeenreported[49].
Ultimately,thepossibilitiesthatarisefromthevariousapproachestocreate supramolecularstructuressuggestthepossibilityofcreatingmolecularleveldevices andtheapplicationof2Dmaterials.Theadvancesinthisareaareillustratedin thechapterbyHuangandWeewheretheydiscusstherapidlyadvancingfieldthat studies2Dtransitionmetaldichalcogenidesandtheirpotentialintegrationwith organicmoleculesformultifunctionalflexibledevices.
Thisbookbringstogetherperspectivesfromresearchleadersinthefield.Itcan beseenthatacrossthebreadthofthesubject,therearemanyfascinatingexamples ofapplyingsupramolecularchemistrytothedevelopmentofsurface-basedarrays. Whetherthroughthedirectimplementationofhydrogenbonds,coordination bonds,orwell-designedvanderWaalsinteractions,orthroughthecontrolled formationofcovalently-bondedarrays,itisclearthatstrategiesforcreating2D arraysonsurfacesarewelldeveloped.Athemethatcommonlyarisesthroughout thecontributionsisthatofcomplexity.Itisnotasurprisethatthissubjecthas becomeprominentinthefieldofsurface-basedsupramoleculararrayswhenone considersthespecificityoftheSPMcharacterisationtechniquesemployedfor characterisation.Whenoneappliesatechniquethataffordsmolecularresolution, allowingdetailedappreciationofextendedframeworks,theircomplexitybecomes allthemoreapparent,drawingtheattentionofresearchersandhencebecominga
1Two-DimensionalSupramolecularChemistryonSurfaces
focusforinvestigation.Remarkablediscoverieshavebeenmadeacrossthefieldand inturn,spurnewendeavours.Anemergingaspectofthefieldistheimplementation ofsyntheticstrategiestowardsnewapplicationswithelectronicpropertiesofnew structuresreceivingnotableattention.However,otherdirectionsofresearchare alsoemergingatthesolid–solutioninterface,forexample,applyingthechiralityof surfacearrays.Exploitingtheinterplaybetweensurface-basedarraysandsolution chemistrypromisestobeofsignificanceinapplicationsrangingfromsensingtothe interfacewithbiologicalprocesses.
Insummary,asiscommonfornewareasofscience,thefieldnowstandsata crossroads.Theoriginsofthefieldhavebeenbasedondevelopinganunderpinningmethodologyforbothsynthesisandcharacterisationandanappreciationof themanyfactorsthataffectsurface-basedsupramolecularassembly.Increasingly, thereisafocusondevelopingthesefascinating2Dmaterialsforspecificapplicationsandfortheirincorporationintodevices.Iamconfidentthatalltheauthorsof theotherchapterswillagreethatthereisapromisingandbrightfutureforthearea of2Dchemistryonsurfaces.
References
1 Lehn,J.-M.(1988). Angew.Chem.Int.Ed.Engl. 27:89–112.
2 Stoddart,J.F.(2017). Angew.Chem.Int.Ed. 56:11094–11125.
3 Sauvage,J.-P.(2017). Angew.Chem.Int.Ed. 56:11080–11093.
4 Feringa,B.L.(2017). Angew.Chem.Int.Ed. 56:11060–11078.
5 Li,Z.T.andWu,L.Z.(eds.)(2015). HydrogenBondedSupramolecularMaterials Heidelberg:Springer.
6 Prins,L.J.,Reinhoudt,D.N.,andTimmerman,P.(2001). Angew.Chem.Int.Ed. 40:2382–2426.
7 Gonzalez-Rodriguez,D.andSchenning,A.P.H.J.(2011). Chem.Mater. 23: 310–325.
8 Metrangolo,P.,Meyer,F.,Pilati,T.etal.(2008). Angew.Chem.Int.Ed. 47: 6114–6127.
9 Gilday,L.C.,Robinson,S.W.,Barendt,T.A.etal.(2015). Chem.Rev. 115: 7118–7195.
10 Hunter,C.A.andSanders,J.K.M.(1990). J.Am.Chem.Soc. 112:5525–5534.
11 Bauzá,A.,Deyà,P.M.,andFrontera,A.(2015).Anion-π interactionsin supramolecularchemistryandcatalysis.In: NoncovalentForces,Challenges andAdvancesinComputationalChemistryandPhysics,vol.19(ed.S.Scheiner), 471–500.NewYork:Springer.
12 Chakrabarty,R.,Mukherjee,P.S.,andStang,P.J.(2011). Chem.Rev. 111: 6810–6918.
13 Lanigan,N.andWang,X.(2013). Chem.Commun. 49:8133–8144.
14 Stoddart,J.F.(2009). Chem.Soc.Rev. 38:1802–1820.
15 Ba˛k,K.M.,Porfyrakis,K.,Davis,J.J.,andBeer,P.D.(2020). Mater.Chem.Front. 4:1052–1073.
16 Mena-Hernando,S.andPérez,E.M.(2019). Chem.Soc.Rev. 48:5016–5032.
17 Peng,H.-Q.,Niu,L.-Y.,Chen,Y.-Z.etal.(2015). Chem.Rev. 115:7502–7542.
18 Williams,G.T.,Haynes,C.J.E.,Fares,M.etal.(2021). Chem.Soc.Rev. 50: 2737–2763.
19 Smith,D.K.(2018). Chem.Commun. 54:4743–4760.
20 Stupp,S.I.andPalmer,L.C.(2014). Chem.Mater. 26(1):507–518.
21 Amabilino,D.B.,Smith,D.K.,andSteed,J.W.(2017). Chem.Soc.Rev. 46: 2404–2420.
22 Griffin,S.L.andChampness,N.R.(2020). Coord.Chem.Rev. 414:213295.
23 Cordova,K.E.andYaghi,O.M.(2017). Mater.Chem.Front. 1:1304–1309.
24 Desiraju,G.R.(2013). J.Am.Chem.Soc. 135:9952–9967.
25 Aakeröy,C.,Champness,N.R.,andJaniak,C.(2010). CrystEngComm 12:22–43.
26 Saez,I.M.andGoodby,J.W.(2005). J.Mater.Chem. 15:26–40.
27 Hayes,R.,Warr,G.G.,andAtkin,R.(2015). Chem.Rev. 115:6357–6426.
28 Schalley,C.A.(2000). Int.J.MassSpectrom. 194:11–39.
29 Blunt,M.O.,Russell,J.,Giménez-López,M.C.etal.(2008). Science 322: 1077–1081.
30 Wasio,N.A.,Quardokus,R.C.,Forrest,R.P.etal.(2014). Nature 507:86–89.
31 Cai,J.,Ruffieux,P.,Jaafar,R.etal.(2010). Nature 466:470–473.
32 Mali,K.S.,Pearce,N.,DeFeyter,S.,andChampness,N.R.(2017). Chem.Soc. Rev. 46:2520–2542.
33 Tuxen,A.,Kibsgaard,J.,Gøbel,H.etal.(2010). ACSNano 4:4677–4682.
34 Binnig,G.,Rohrer,H.,Gerber,C.,andWeibel,E.(1982). Appl.Phys.Lett. 40: 178–180.
35 Binnig,G.,Quate,C.F.,andGerber,C.(1986). Phys.Rev.Lett. 56:930–933.
36 Gross,L.,Mohn,F.,Moll,N.etal.(2009). Science 325:1110–1114.
37 Sweetman,A.M.,Jarvis,S.P.,Sang,H.etal.(2014). Nat.Commun. 5:3931.
38 Stannard,A.,Russell,J.C.,Blunt,M.O.etal.(2012). Nat.Chem. 4:112–117.
39 Steeno,R.,Minoia,A.,Gimenez-Lopez,M.C.etal.(2021). Chem.Commun. 57: 1454–1457.
40 Urgel,J.I.,Écija,D.,Lyu,G.etal.(2016). Nat.Chem. 8:657–662.
41 Shang,J.,Wang,Y.,Chen,M.etal.(2015). Nat.Chem. 7:389–393.
42 Barth,J.V.,Weckesser,J.,Cai,C.etal.(2000). Angew.Chem.Int.Ed. 39: 1230–1234.
43 Griessl,S.,Lackinger,M.,Edelwirth,M.etal.(2002). SingleMol. 3:25–31.
44 Theobald,J.A.,Oxtoby,N.S.,Phillips,M.A.etal.(2003). Nature 424:1029–1031.
45 Stepanow,S.,Lingenfelder,M.,Dmitriev,A.etal.(2004). Nat.Mater. 3:229–233.
46 Dong,L.,Gao,Z.,andLin,N.(2016). Prog.Surf.Sci. 91:101–135.
47 Elemans,J.A.A.W.,Lei,S.,andFeyter,S.D.(2009). Angew.Chem.Int.Ed. 48: 7298–7332.
48 Elemans,J.A.A.W.,DeCat,I.,Xu,H.,andDeFeyter,S.(2009). Chem.Soc.Rev. 38:722–736.
49 Tobe,Y.,Tahara,K.,andDeFeyter,S.(2021). Chem.Commun. 57:962–977.
50 Song,W.,Martsinovich,N.,Heckl,W.M.,andLackinger,M.(2013). J.Am.Chem. Soc. 135:14854–14862.
8 1Two-DimensionalSupramolecularChemistryonSurfaces
51 Narita,A.,Wang,X.-Y.,Feng,X.,andMüllen,K.(2015). Chem.Soc.Rev. 44: 6616–6643.
52 Song,S.,Su,J.,Telychko,M.etal.(2021). Chem.Soc.Rev. 50:3238–3262.
53 Jin,Y.,Hu,Y.,Ortiz,M.etal.(2020). Chem.Soc.Rev. 49:4637–4666.
54 Xu,L.,Zhou,X.,Yu,Y.etal.(2013). ACSNano 7:8066–8073.
55 Judd,C.,Haddow,S.L.,Champness,N.R.,andSaywell,A.(2017). Sci.Rep. 7: 14541.
56 Saywell,A.,Magnano,G.,Satterley,C.J.etal.(2010). Nat.Commun. 1:75.
CharacterisationandInterpretationofOn-SurfaceChemical ReactionsStudiedbyUltra-High-ResolutionScanningProbe Microscopy
AdamSweetman 1 ,NeilR.Champness 2 ,andAlexSaywell 3
1 UniversityofLeeds,SchoolofPhysicsandAstronomy,LeedsLS29JT,UK
2 UniversityofBirmingham,SchoolofChemistry,Edgbaston,BirminghamB152TT,UK
3 UniversityofNottingham,SchoolofPhysicsandAstronomy,NottinghamNG72RD,UK
2.1Introduction
Thedevelopmentofsupramolecularchemistryonsurfacesisreliantupondetailed characterisationatthemolecularlevel.Avarietyofapproacheshavebeenemployed tounderstandthedetailedarrangementofmoleculesinself-assembledarraysbut thedominantandtypicallymostinformativetechniquesarebaseduponscanning probemicroscopy(SPM).Inthemainscanning-tunnellingmicroscopy(STM)[1] hasbeenhighlysuccessfulinestablishingadetailedappreciationofthestructure ofsupramolecularsystems,oftenatthemolecularlevel,butitcanbehelpfulto supplementthisapproachwithothertechniquesthatallowananalysisofthechemicalspeciationorotherstructuralfeaturesthatSTMcannotprobe.Forexample, X-rayphotoelectronspectroscopy(XPS)[2–5]allowsinvestigationofthechemical compositionofmoleculeswithinsupramoleculararrays,andtechniquessuchas X-raystandingwave(XSW)analysis[6]canprobethemolecularconformationsof adsorbedmolecules.However,STMandatomicforcemicroscopy(AFM)[7]arethe mostcommontechniquesusedtostudysurface-basedsupramolecularstructures. Indeed,SPMsfacilitatethecharacterisationofsinglemoleculesandassembliesof molecules,confinedtoasupportingsubstrate,onthemolecularorsub-molecular level.ThedefiningcharacteristicofallvariantsofSPMistheuseofaprobetomeasureaspecificprobe–sampleinteractionoveragridofpoints,whichisusedtogeneratean‘image’ofawell-definedspatialregionofthesurface;oftenresultingin resolutiononthesub-Ångströmlevel.
Conceptually,theprobeisterminatedwithasingleatomanditistheinteraction betweenthisatomandthemolecule-substratesystemwhichismeasured.Theoriginsofthisprobe–sampleinteractiondeterminetheinterpretationoftheresulting imagebutcommonlytheinformationacquiredprovidesarelativelysimplepathway tounderstandingstructuralarrangements.Thus,theterminatingatomattheapex oftheprobeistypicallybroughttowithinafewÅngströmofthesurfaceand,due tothestrongdistancedependenceoftheprobe–surfaceinteractions,themeasured
SupramolecularChemistryonSurfaces:2DNetworksand2DStructures,FirstEdition. EditedbyNeilR.Champness.
©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
2CharacterisationandInterpretationofOn-SurfaceChemicalReactions
interactionisdominatedbythepositionandpropertiesofthesingleterminating atom.InbothSTM[1]andAFM[7],thecurrentflowbetweentheprobeandsample ortheprobe–sampleinteractionforce,respectively,aremeasured.Inthemajority ofapplications,theprobeiseitherformedfromametalwire,sharpenedmechanicallyoretchedchemically,oranetchedsilicontip.Theresolutionobtainablecanbe furtherimprovedwhentheapexofthetipisfunctionalisedwithawell-definedterminatingspecies,suchasCO[8],providingaprobewithadefinedsizeandknown intermolecularchemistry.Itisthelocalisednatureoftheprobe–sampleinteraction measuredbytheprobe,whichgivesrisetothehighspatialresolutionwhichallows thestudyofmolecule-substratesystemsontheatomicandmolecularlevel.
AnoteworthyfeatureofallSPMtechniquesisthattheacquireddatadirectly correspondstoreal-spacemeasurements,whichallowanimageofthesurface tobeproduced.ThisisdistinctfromtechniquessuchasX-raycrystallography andlow-energyelectrondiffraction(LEED)whereensemblereciprocalspace measurementsareconvertedtoproduceareal-spacestructure.Suchimagesofthe surface,particularlyofmolecule-substratesystems,mayoftenofferwhatappears tobeaneasilyaccessibleviewofmolecularstructureand/orreactionprocesses. However,greatcareshouldalwaysbetakenwheninterpretingthedataacquired; theacquireddataprovidesawealthofinformationontheelectronicandchemical structureofthesystemunderstudywhichisdistinctfrom,althoughoftenrelated to,thetopographyoftheadsorbedmolecules.
ThischapterseekstoprovideabackgroundtoSPMstudiesofmolecule-substrate systemsandhowtheycanbeemployedtounderstandself-assembledstructures andinparticularsurface-basedreactionprocesses.Thechapterwillfocusonthe underlyingtheoryandexperimentalconsiderationsthatarerequiredtoconductand interprettheinvestigationofon-surfacesynthesisreactionsusinghigh-resolution SPMmethodologies.However,thespecificexamplesdiscussedinthischapter alsoprovidetheunderpinningconceptsthatcanbeappliedtorelatedareasof surface-basedmolecularassemblysuchasthosediscussedintheotherchapters withinthisvolume.ThechapterprovidesdetailsofthebasicpremiseofSPM studiesformolecule-substratesystems,includinganoverviewoftheexperimental conditions(Section2.2),andprovidesanin-depthdiscussionofthetechnical aspectsofperformingnoncontactatomicforcemicroscopy(ncAFM)experiments (Section2.3).Thephysicalprocessesunderlyingtheprobe–moleculeinteraction willbeusedasabasisfordiscussionofimageinterpretation(Section2.4),and inthefinalsection(Section2.5)examplesofon-surfacereactionsinvestigatedby SPMwillbegiven;focusingspecificallyontheformationofgraphenestructures (includinggraphenenanoribbons–GNRs)andcyclisationreactions(e.g.Bergman cyclisation).
2.2SPMUnderUHVConditions
AlthoughtherearemanyexamplesoftheimplementationofSPMinambient,liquid, andevenelectrochemicalenvironments,herewespecificallyfocusontheultra-high
2.2SPMUnderUHVConditions 11 vacuum(UHV)studiesconductedatcryogenictemperatures(e.g. <5K–achievable usingliquidhelium).AUHVenvironmentisusuallyavitalprerequisiteforthe formationofatomicallyflatandcleansubstrates.AllSPMtechniquesworkoptimally,withregardstothecharacterisationofmolecularspecies,whenlargeareas (>100nm2 )offlatsurfaceareaccessible.Theselargeatomicallyflatregionsfacilitatesub-molecularandatomicresolution,whichisinitselfaprerequisiteforthe characterisationofon-surfacechemicalreactions.
SamplepreparationunderUHVconditionsallowscontaminant-freesurfacesto beproduced(simplybylimitingexposuretocontaminantspecies),offersaccurate temperaturecontrolforsamplepreparation(withspecifictemperaturesrequiredto formcertainsurfacereconstructions),andfacilitatestheuseofthecleaningproceduresdescribedinSection2.3.TypicallyUHVchambersallowpressuresdownto ∼10 10 mbar,andlower,tobeobtained.CryogenicSPMsystemsalsoallowsamples tobecooledto <5K(inhibitingbothmoleculardiffusionandtheprogressofchemicalreactions–requiredtostudyintermediatestatesofon-surfacereactions).
ThereishoweveradisconnectbetweentheuseofUHVandtheenvironment inwhichindustrialscale,orevenlab-based,chemicalreactionsoftentakeplace: specificallywithregardstotheenvironmentinwhichsolution-phasereactionsare performed.Ingeneral,itisnotpossibletointroducesolventsintoUHV(asthehigh vapourpressureofmanysolventsrendersthemincompatiblewithaUHVenvironment),meaningthatreactionsinvestigatedbySPMunderUHVarestudiedinthe absenceofsolvents.Inaddition,studyingsuchmolecule-substratesystemsunder UHV,asopposedtoambientconditions,givesrisetoseveralchallenges(including theinherenttechnicaldifficultiesofsimplymovingsamplesaroundinaUHVenvironment).Mostnotableistheissueoftransferringthemoleculestoasurfaceheldin UHV.Inthesimplestcase,acrucibleloadedwiththemoleculesunderstudycanbe introducedtotheUHVsystemwithsubsequentthermalevaporationusedtoproduce asub-monolayertomulti-layerfilmuponthesubstrate.However,inmanycases,the moleculesmaybenon-volatileorthermallylabileandinsuchcases,oneofavariety ofalternativetechniqueshastobeemployed[9].
ThereareseveralbenefitsinutilisingUHV-SPMcomparedtoothercharacterisationtechniques.Themoleculestobestudieddonothavetobecrystalline(asis thecaseforsomediffraction-basedtechniques)andonlyverysmallquantitiesof materialarerequiredforstudybySPM(comparedto,forexample,nuclearmagnetic resonance[NMR]).Combinedwiththeexceptionallyhighspatialresolutionoffered bySPM,thetechniquehasrecentlygainedimportanceasacharacterisationtechniquethatcanprovide‘realspace’characterisationofmolecule-substratesystems, whichbothcomplementsandenhancesthechemicalandstructuralcharacterisation offeredbyensembleaveragingtechniques.
2.2.1On-SurfaceReactions
AnobviousconsiderationwithregardstocharacterisationutilisingSPMtechniques isthatthemoleculesinvestigatedhavetobestudiedonasupportingsubstrate;prohibitingthestudyofsolventconfinedsystems.TheoperationalmechanicsofSPM
2CharacterisationandInterpretationofOn-SurfaceChemicalReactions
lendthemselvestothestudyofsystemsconfinedtoa2Dsubstrateandprovidean invaluabletechniqueforinvestigatingchemicalreactionsupon,apotentiallyreactiveand/orcatalytic[10],surface(seereviews[10–15]andreferencestherein).Asthe systemstobestudiedareonasubstrate,thisprecludestheuseoftransmissionelectronmicroscopy(TEM)whichcanalsobeusedinprincipletoprovideatomic-level resolution,butisgenerallyunsuitableforthestudyofmolecule-substratesystems duetothethicknessofthesubstratesrequired.
ThemajorbenefitofcharacterisationviaSPMisthelevelofspatialresolutionachievable(verticalresolutionoflessthan5pmandsub-angstromlateral resolutionisroutine).Thisisbaseduponsensitivemeasurementsoftheprobe–substrate/moleculeinteraction(videinfra).Astheprobeplaysavitalpartinthe measurements,oneneedstoconsideritsshape,anditselectronicandchemical properties,asthesecanpotentiallygiverisetoavarietyof‘artefacts’(Section2.3 discussesthisindetail).Anadditionalbenefitofconfiningachemicalreactiontoa 2Dplaneisthepotentialtocontrolreactionsviadifferentmethodologiestothose availableinsolution[16].Thetechniquehasalsobeenshowntoallowdifferent stagesduringtheprogressionofachemicalreactiontobestudied(i.e.initial,final, andevenintermediatestates)[13].
TwomainvariantsofSPMhavecommonlybeenemployedtostudyon-surface reactions;STMandAFM.Inparticular,aspecificvariantofAFM,ncAFM,providesasub-molecularresolutionthatallowscharacterisationofthespatialposition ofchemicalgroupswithinamolecule,aswellasfacilitatingnotonlytheobservation ofsinglechemicalbonds[8]butprovidingamethodologytodistinguishthebond order(i.e.single,double,ortriplecarbon–carbonbondspecies)[17].ItisimportanttonotethatthespecificaspectsofncAFM(discussedindetailthroughoutthis chapter)providesub-molecularresolution,andtherefore,sub-molecularresolution ncAFMispartofthefamilyofSPMtechniques,itisnotsimplyamodeofoperationthatcanbeappliedtootherSPMsystemsandrequires,atleastinthecurrent implementation,aspecificexperimentalset-up.
Thelevelofsub-molecularresolutionprovidedbyncAFMcanbeusedtocomplementtraditionalcharacterisationtechniques(e.g.NMR,GLC,LEED)and,for example,allowsalevelofsingle-moleculecharacterisation,whichcanaidinthe structuraldeterminationofcompletelynewspecies(typifiedbytheroleofncAFM inthecharacterisationofaplanar,proton-poorcompoundincombinationwith computationalstudies[18])aswellasdistinguishingbetweenthestructureof asphaltenes(polycyclicaromatichydrocarbonswithincrudeoil;whosestructural analysisisatremendouschallengefornon-spatiallyresolvedtechniques)[19].
2.2.2CharacterisationofMolecule-SubstrateSystemsviaSTM
Therearemany‘flavours’ofSPMalldesignatedbyaconfusingmenagerieof acronyms,includingbutnotlimitedtoSTM,ncAFM,KPFM(Kelvinprobeforce microscopy),andSNOM(scanningnear-fieldopticalmicroscopy).Thearchetypal exampleofthissetofmethodsisSTM.IncommonwithallSPMmethodologies, STMworksbyscanningaprobeacrossasurface,inthiscasewithanappliedbias
2.2SPMUnderUHVConditions 13 (relativetotheprobe–whichisusuallydefinedasgrounded).Theconductingtip (usuallymetallic)ismovedinastraightlineacrossaconducting/semi-conducting surfaceandtheinteractionbetweentheprobeandthetipmeasured(inthecase ofSTM,themeasuredquantityisthemagnitudeofthecurrentflowduetoelectronstunnellingbetweentheprobeandthesurface,orviceversa).Detailsofthe conceptsunderpinningSTMaregiveninseveralexcellenttextbooks[20,21],but insummary,thesalientpointsare:(i)thesubstrateisbiasedrelativetotheprobe (typicallyintherange ±2V),(ii)theresultantflowofelectronsbetweentheprobe andthemolecule/substrateisrecorded,(iii)themagnitudeofthistunnel-current (I )hasanexponentialdependenceonthedistancebetweentheprobeandthe substrate/molecule,and(iv)theverticalprobeposition(z)canbevariedinorder togiveaconstantcurrentastheprobeismovedlaterallyacrossthesurface(this feed-backmodeisknownasaconstant-currentoperation)or(v)theverticalprobe positioniskeptconstantandthecurrentisrecordedatvariouslateralpositions abovethesubstrate/molecule,knownasconstant-heightmode(seeFigure2.1).
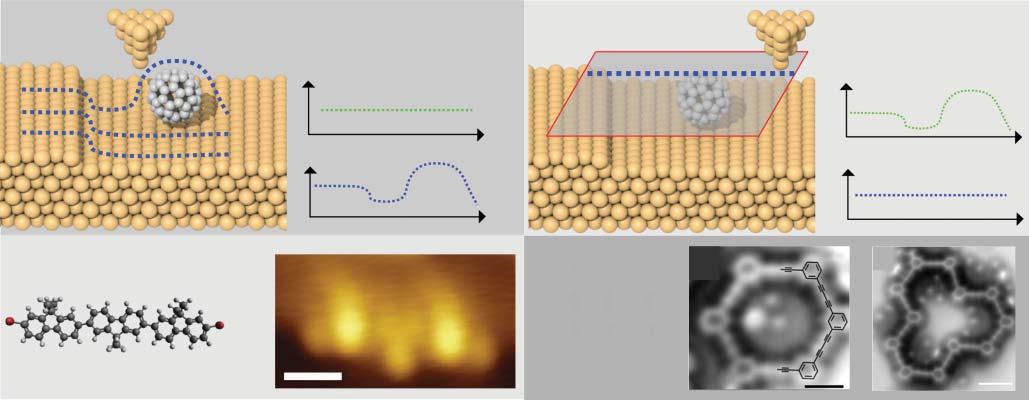
AnSTMimageisproducedbyobtainingaseriesoflinescans(shownin Figure2.1a),whicharethencombinedtoforma2Dimage.Inconstantcurrent mode, I ismaintainedatafixedset-point,typicallyafewpicoamperes,andthe resultantimage,therefore,showsthevariationin z astheprobeisscannedover thesurface.Inconstantheightmode,imageswillshowthevariationin I withtip position.Itisimportanttonotethatthemeasuredcurrent,forafinitebiasvoltage, isproportionaltothesumofthecontributionsforthelocaldensityofstates(LDOS) fromwhichtunnellingispossible[20,21];i.e.themeasuredcurrentisrelatedtothe electronicstructureofthemolecule/substrate,andisnotnecessarilywellcorrelated tothespatialpositionoftheatomicnuclei.Inthisrespect,thepathoftheprobein constant-currentmodedoesnotsimplyprovideatopographicheightbutisbetter interpretedasamapoftheLDOS.Thisissuemanifestsinthecharacterisation ofmoleculeswheremolecularorbitalsareoftendelocalisedoverthemolecular speciesunderstudy.Therefore,preventingthepositionofindividualatoms,within similarchemicalenvironments(e.g.conjugatedaromaticcarbons),frombeing resolvedastheywilloftenformpartofthesamefeatureobservedwithinanSTM image.However,incaseswhereelectroniccharacterislocalisedoverspecific chemicalmoieties,STMimagesmaybecompared(atleastasanapproximation) tothechemicalstructureofthemoleculeunderstudy.Anexampleofthisis showninFigure2.1cwherethestructureofabrominatedterfluorenemolecule (α,ω-dibromoterfluorene[DBTF])canbecomparedwithaconstant-currentSTM image[22];featuresrelatedtotheperipheralBratomsandcentralfluorenegroups arevisible.Suchelectronicstructuresareoftencomparedwithdensityfunctional theory(DFT)basedsimulationsofSTMimages,whichcanhelpidentifymolecular structureandconformations[24].
WhileSTMcanprovidesub-molecularresolution,itsuffers,incommonwith allSPMtechniques,withregardstothenon-trivialinterpretationoftheacquired data.AlthoughDFTstudiesusedinconjunctionwithSTMdataoftenoffergood agreementandprovideaplausibleinterpretationoftheresults(intermsofamore completeappreciationoftheexpectedLDOS),anoverrelianceonDFTcanlead