AmorphousNanomaterials
Preparation,CharacterizationandApplications
LinGuo
Author ProfLinGuo BeihangUniversity SchoolofChemistry No.37XueyuanRoad HaidianDistrict 100191Beijing China
Cover CoverDesign:Wiley CoverImage:©VAlex/Shutterstock
Allbookspublishedby Wiley-VCH arecarefullyproduced.Nevertheless, authors,editors,andpublisherdonot warranttheinformationcontainedin thesebooks,includingthisbook,to befreeoferrors.Readersareadvised tokeepinmindthatstatements,data, illustrations,proceduraldetailsorother itemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>.
©2021WILEY-VCHGmbH,Boschstr. 12,69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc. usedinthisbook,evenwhennot specificallymarkedassuch,arenotto beconsideredunprotectedbylaw.
PrintISBN: 978-3-527-34747-6
ePDFISBN: 978-3-527-82634-6
ePubISBN: 978-3-527-82635-3
oBookISBN: 978-3-527-82636-0
Typesetting SPiGlobal,Chennai,India PrintingandBinding
Printedonacid-freepaper
10987654321
Contents
Foreword xi
Preface xiii
1Introduction 1
1.1IntroductionofAmorphousMaterials 1
1.2StructuralDifferencesbetweenAmorphousMaterialsandCrystals 3
1.2.1CrystalsandQuasicrystals 3
1.2.2AmorphousMaterials 5
1.3HistoryofAmorphousMaterials 7
1.3.1EstablishmentofCrystallography 8
1.3.2EnlightenmentofAmorphousMaterials 9
1.3.3ModernAmorphousMaterials1-DisorderedElementarySubstance 10
1.3.4ModernAmorphousMaterials2-MetallicGlass 11
1.3.5ModernAmorphousMaterials3-NontraditionalAmorphous Nanomaterials 14
1.4GrowthMechanismsofAmorphousNanomaterials 15
1.4.1ClassicalNucleationTheory 15
1.4.2MultistepTransformationMechanismwithAmorphous Participation 17
1.4.3ComplexGrowthProcessinSolution 19
1.5SummaryandOutlook 19 References 20
2LocalStructureandElectronicStateofAmorphous Nanomaterials 23
2.1SphericalAberration-CorrectedTransmissionElectronMicroscopy 23
2.1.1Introduction 23
2.1.2SphericalAberration-CorrectedTransmissionElectronMicroscopy 24
2.1.3ElectronEnergyLossSpectroscopyinTEM 28
2.1.4ApplicationsinAmorphousNanomaterialCharacterization 34
2.1.5SummaryandOutlook 41
2.2X-rayAbsorptionFineStructureSpectrum 41
2.2.1Introduction 41
2.2.2ExtendedX-rayAbsorptionFineStructure 42
2.2.3X-rayAbsorptionNear-EdgeStructure 45
2.2.4ApplicationinAmorphousNanomaterialCharacterization 47
2.2.5SummaryandOutlook 51 References 52
3DefectCharacterizationofAmorphousNanomaterials 61
3.1Introduction 61
3.2PositronAnnihilationSpectrum 64
3.3ElectronParamagneticResonance 71
3.4PhotoluminescenceSpectroscopy 79
3.5SummaryandOutlook 82 References 84
4Synthesisof0DAmorphousNanomaterials 89
4.1Introduction 89
4.2Bottom-UpMethod 90
4.2.1Solution-BasedChemicalMethod 90
4.2.2ThermalTreatmentMethod 98
4.2.3OtherMethods 101
4.3Top-DownMethod 104
4.4SummaryandOutlook 106 References 106
5Synthesisof1DAmorphousNanomaterials 111
5.1Introduction 111
5.2Hydrothermal/SolvothermalMethod 113
5.3ChemicalPrecipitationMethod 116
5.4ElectrochemicalDepositionMethod 120
5.5TemplatingMethod 122
5.6OtherSyntheticMethods 124
5.7SummaryandOutlook 131 References 132
6Synthesisof2DAmorphousNanomaterials 137
6.1Introduction 137
6.2ThermalDecompositionMethod 138
6.3ExfoliationMethod 139
6.4DepositionMethod 143
6.4.1PhysicalVaporDepositionMethod 143
6.4.2ElectrodepositionMethod 143
6.5ChemicalPrecipitationMethod 147
6.6TemplatingMethod 148
6.7PhaseTransformationMethod 151
6.8Sol–GelMethod 151
6.9ElementDopingMethod 152
6.10SummaryandOutlook 155 References 155
7Synthesisof3DAmorphousNanomaterials 163
7.1Introduction 163
7.2Template-EngagedStrategies 163
7.2.1CoordinatingEtchingMethod 164
7.2.2Acid/AlkaliEtchingMethod 166
7.2.3RedoxEtchingMethod 169
7.2.4Self-TemplatedMethod 171
7.3ElectrochemicalMethod 173
7.4Hydrothermal/SolvothermalMethod 174
7.5CommonSolutionMethod 176
7.6Laser/Ultrasonic-AssistedSolutionMethod 177
7.7OtherSyntheticMethods 179
7.8SummaryandOutlook 182 References 183
8SynthesisofAmorphous-CoatedandAmorphous-Doped Nanomaterials 189
8.1Introduction 189
8.2AmorphousCoatedNanomaterialsbyALD 190
8.2.1AmorphousMetalOxideCoating 190
8.2.2AmorphousMetalFluorideCoating 192
8.3Amorphous-CoatedNanomaterialsWithDifferentDimensions 193
8.3.11DAmorphous-CoatedNanomaterials 193
8.3.1.1HomojunctionStructure 193
8.3.1.2HetrojuctionStructure 197
8.3.22DAmorphous-CoatedNanomaterials 198
8.3.2.1Carbon-BasedNanomaterials 198
8.3.2.2Ni-BasedNanomaterials 200
8.3.2.3OtherMetal-basedNanomaterials 201
8.3.33DAmorphous-CoatedNanomaterials 202
8.3.3.1SilicaCoating 202
8.3.3.2CarbonCoating 204
8.3.3.3MetalOxideCoating 205
8.3.3.4MetalSulfideCoating 207
8.4Amorphous-DopedorHybridNanomaterials 208
8.4.12DAmorphous-DopedNanomaterials 208
8.4.23DAmorphous-DopedNanomaterial 211
8.5SummaryandOutlook 215 References 215
9ApplicationsofAmorphousNanomaterialsin Electrocatalysis 223
9.1Introduction 223
9.2FundamentalsofElectrocatalysis 225
9.3AmorphousNanomaterialsasElectrocatalystsforWaterSplitting 226
9.3.1AmorphousNanomaterialsforHER 226
9.3.1.1AmorphousSingleMetallicNanomaterialsforHER 226
9.3.1.2AmorphousBinaryMetallicNanomaterialsforHER 232
9.3.1.3AmorphousCompositeNanomaterialsforHER 234
9.3.2AmorphousNanomaterialsforOER 237
9.3.2.1AmorphousSingleMetallicNanomaterialsforOER 238
9.3.2.2AmorphousBinaryMetallicNanomaterialsforOER 241
9.3.2.3AmorphousPolynaryMetalNanomaterialsforOER 244
9.3.2.4AmorphousCompositesforOER 246
9.3.3AmorphousNanomaterialsforORR 248
9.3.3.1AmorphousNobleMetal-basedNanomaterialsforORR 249
9.3.3.2Amorphous3dMetal-basedNanomaterialsforORR 249
9.3.4AmorphousNanomaterialsforCRR 251
9.3.5AmorphousNanomaterialsforNRR 252
9.3.6AmorphousNanomaterialsasBifunctionalElectrocatalysts 253
9.3.6.1AmorphousNanomaterialsasBifunctionalElectrocatalystsofHERand OER 254
9.3.6.2AmorphousNanomaterialsasBifunctionalElectrocatalystsofORRand OER 256
9.4SummaryandOutlook 256 References 258
10ApplicationsofAmorphousNanomaterialsinBatteries 269
10.1Introduction 269
10.2NegativeElectrodesinBatteries 269
10.2.1AmorphousPhosphorusCompounds 269
10.2.2AmorphousSiliconCompounds 273
10.2.3AmorphousTransitionMetalOxides 280
10.2.3.1AmorphousIronOxides 280
10.2.3.2AmorphousTitaniumOxides 281
10.2.3.3AmorphousVanadium-BasedOxides 282
10.2.3.4AmorphousTin-BasedOxides 288
10.2.4AmorphousCarbon 289
10.3PositiveElectrodesinBatteries 295
10.3.1AmorphousFerricPhosphate 295
10.3.2AmorphousVanadium-BasedOxides 300
10.3.3AmorphousMetalPolysulfides 302
10.4SummaryandOutlook 304
References 306
11ApplicationsofAmorphousNanomaterialsin Supercapacitors 317
11.1Introduction 317
11.2ApplicationsinElectricDouble-LayerCapacitors 318
11.3ApplicationsinPseudocapacitors 324
11.3.1AmorphousMetalOxides 325
11.3.2AmorphousMetalSulfides 334
11.3.3OtherAmorphousNanomaterials 337
11.4SummaryandOutlook 341
References 342
12ApplicationsofAmorphousNanomaterialsin Photocatalysis 347
12.1Introduction 347
12.2PhotocatalyticDegradation 349
12.3PhotocatalyticDecompositionofWater 355
12.4Photo-Electrocatalysis 359
12.5AmorphousNanomaterialasCocatalystinPhotocatalysis 363
12.6OtherApplicationsinPhotocatalysis 366
12.7SummaryandOutlook 370 References 370
13EngineeringApplicationsofAmorphousNanomaterials 375
13.1Introduction 375
13.2MechanicalPropertiesofAmorphousNanomaterials 376
13.2.1AmorphousAlloys/Metals 376
13.2.2AmorphousNonmetallicMaterials 382
13.3StrategyforEnhancingtheMechanicalPerformance 386
13.3.1IntroductionofMicro/NanosecondPhase 387
13.3.2IntroductionofMicro/Nano-Inhomogeneity 393
13.3.3SurfaceModification 395
13.3.4AmorphousBasedCompositeMaterials 396
13.4SummaryandOutlook 401
References 402
Index 407
Foreword
Thewordisunityofopposites,whichisdescribedasYinandYanginChinesephilosophy.Inthefieldofmaterials,thestructures,features,orpropertiesofmaterialsare alsounitiesofopposites,forexample,hydrophilicityandhydrophobicityintheinterfaceofmaterials,anisotropy,andisotropyinarrangementofmaterials,strength,and toughnessinmechanicsofmaterials.
Inadditiontothetraditionalcrystallography,amorphousmaterialsareastrong complementtocondensedmatterphysics.Eventhoughthepresentprogressof solidmaterialsismainlybasedoncrystalwithregularatomicarrangement,the ruleofthedisorderedwordisalsofascinating.Nanoscaleamorphousmaterialsare veryimportantmemberofthenon-crystallinesolidsfamilyandhaveemergedas anewcategoryofadvancedmaterials.Comparedtothecrystallinecounterpart, amorphousnanomaterialswithisotropicnaturealwaysexhibitfastiondiffusion, relievedstrain,andhigherreactivity,whichenableamorphousnanomaterialsto exhibithighperformanceincatalysis,energystorageandmechanics,aswellas otherinterestingproperties.
Basedontherichexperienceofnanomaterials,Prof.LinGuohasmadegreatcontributionsforthepropellingofnanoscaleamorphousmaterials.Heisoneofthe earliestscientistswhoputforwardtheuniversalsyntheticstrategiesofnanoscale amorphousmaterialswithregularmorphologies.Healsocreativelydiscovereda seriesofsystematiclawsbetweenamorphousstructureandthepropertyofcatalysis,energystorage,mechanics,optics,etc.AsthecolleagueandfriendofProf.Guo, Ihaveknownhimformorethantwentyyears.Weoftendiscussabouttheprobable andpotentialapplicationsofnanoscaleamorphousmaterials.Heisanintelligent scientistwithactivemindandinexhaustiblecreativity.
Thisbookisanexcellenttreatisefornanoscaleamorphousmaterials,inwhich someemerginginnovativemethodstofabricatewell-definedamorphousnanomaterialsaresystematicallyreviewed,including0D,1D,2D,3D,andcoatedordoped amorphousnanomaterials.Apartfromthat,somepowerfultechniquesforthecharacterizationofamorphousmaterialsarealsoaddressed.Finally,hefocusedonthe variousfascinatingapplicationsrelatedtoamorphousnanomaterials,including theapplicationsinelectrocatalysis,batteries,supercapacitors,photocatalysis,and mechanics.
xii Foreword
Thisbookisinformativeandvaluableinprofessionalandcommondisciplines, whichisalsoeasytounderstand.Ibelievethatthisbookisofgreatsignificanceto promotetherapiddevelopmentofamorphousnanomaterials.Ithasreferencevalue forthoseengagedinrelatedscientificresearch. MemberoftheChineseAcademyofSciences FellowofAmericanAcademyofEngineering AcademicianofthethirdWorldAcademyofSciences
18January2021,Beijing
Preface
Throughoutthehistoryofhumanscienceandtechnologydevelopment,thesurge ofnewtechnologyisalwaysaccompaniedbythediscoveryandimprovementof newmaterials.Fromtheancientbone,stone,porcelain,metalmaterialstothe modernhigh-performanceplastics,alloys,micromaterials,andnanomaterials, whicharestilldevelopingconstantly,allofthemhaveleftadeepimprintin thehistoryofhumandevelopment.Comparedwiththetraditionalcrystalline materialswithaperiodicarrangementofbasicunits,amorphousmaterialsare ofshort-range-orderedandlong-range-disorderedstructure.Unlikecrystalline counterparts,amorphousmaterialsdonothavegrainboundaries,crystaldefects, segregation,andanisotropy,soamorphousmaterialsshowmacroscopichomogeneityandisotropy.Forexample,amorphousalloyshaveauniquedisordered structuresimilartoglass.Theysharethecharacteristicsofbothmetalandglass andthushaveuniquephysical,chemical,andmechanicalproperties.Intheaspect ofmechanicalstrength,amorphousalloyshavehighstrength,highhardness,high wearandcorrosionresistance,highfatigueresistance,lowelasticmodulus,and largeelasticstrainlimit,whichisconsideredtohaveabroadpotentialapplicationin thefieldofengineeringmechanics,biomedicalscience,andaerospace.Inaddition tomechanicalproperties,amorphousmaterialshavealsoattractedwideattention becauseoftheirhighlyunsaturatedcentersonthesurfaceandhomogeneous catalyticcentersinchemicalandstructuralenvironments,whichmakethemnot onlyasmodelcatalystsbutalsoasthepracticalones.
Inaddition,theemergenceofnanomaterialshasbeenoneofthemostimportant developmentsinthefieldofmaterialssincethelatetwentiethcentury.Nanomaterialsrefertoanewclassoffunctionalmaterialsinwhichatleastonedimension ofmaterialsisreducedtothenanoscale.Becausetheircharacteristicsizeissimilar totheDeBrogliewavelengthofelectrons,theelectronmovementinthiskindof materialsislimited,andtheirenergyischangedfromcontinuousstatetodiscontinuousenergylevel,therebyderivingmanynewphysicalandchemicalproperties, whichhavebeenwidelyconcernedbythescientificcommunity.Inrecentyears, nanoscaledamorphousmaterialshavegraduallyenteredpeople’sfieldofvisionand showntheirexcellentperformancecomparabletoorevenbetterthanthatofcrystallinenanomaterialsinvariousfields,suchascatalysis,electrochemicalenergystorage,mechanicalengineering,andsoon.
Althoughmanystudieshaveverifiedthebroadapplicationprospectsofamorphousnanomaterialsasemergingmaterialsinmanyfields,theirdevelopmentis stillinitsinfancy,andmanyscientificandtechnicalproblemsneedtobesolved.For example,amorphousmaterialsarewidelybelievedtohaveshort-rangeorder,but theiratomicarrangementformsarestillunabletobeaccuratelydefinedevenwith thedevelopmentofelectronmicroscopytechniquestoday.Besides,itisdifficultto controlthegrowthmodeofamorphousmaterialsandobtainspecificstructures withregularmorphologybecauseoftheirlong-range-disorderedinternalstructure. Asinglepreparationmethodcanonlyobtainamorphousnanomaterialswith specificstructures,whichisfarfromsatisfyingtheresearchontheperformance regulationofamorphousmaterials.Inaddition,theatomicdisorderinamorphous materialsaffordsalargenumberofunsaturatedcenters.Intheory,theseabundantactivesitesshouldhavehighercatalyticperformancethanthoseofcrystal nanomaterialinanorderofmagnitude,buttheexistingstudieshavenotbeen realizedyet.
Thisbookwaswrittenbasedonguaranteeingtheintegrityofthecontent, combinedwithmyexperienceinthepreparationofamorphousnanomaterials, characterization,andapplicationresearchpracticeformanyyears,notonly expoundsthelatestinformationofamorphousnanomaterialpreparationtechnologyandcutting-edgeprogress,butalsotakeintoaccountsomeapplicationsof amorphousnanomaterials.Thisbookfocusesontheelaborationofresearchideas andmethods,aimingtoenablereaderstohaveafullunderstandingofamorphous nanomaterials,andeasytograspthepreparationprinciple,applicationrange,and existingtechnicalbottlenecks,soastobettercarryoutinnovativeresearchinthe future.Thisbookisdividedinto13chapters,themaincontentsofeachchapter arewrittenasfollows:Chapter1mainlyintroducestheintrinsiccharacteristics, developmenthistory,andgeneralpreparationmethodsofamorphousnanomaterials;Chapters2and3introducethelocalstructure,electronicstates,anddefects ofamorphousnanomaterials,mainlyfromtheperspectiveofcharacterization techniques.InChapters4–7,thelatestprogressofpreparationmethodsof0D–3D amorphousnanomaterialsismainlyintroduced.InChapter8,themainwaysand structuralcharacteristicsofhybridnanomaterials,suchasamorphouscoating ordopednanomaterial,areintroduced.Chapters9–13describetheapplications ofamorphousnanomaterialsincatalysis,electrochemicalenergystorage,and mechanicalengineering.
Iwouldlovetoexpressmyspecialthankstomygroupmemberswhohavecontributedtothescientificresearchandintheprocessofbookwritingandrevision:Wei Zhou,GuangshengWang,HuaWang,YujieZhu,XiaotianWang,LidongLi,Jianxin Kang,ZhaoYang,XiaoyiQiu,JieYang,ZhiCai,BaohongZhang,BinbinJia,Jian Yu,YanZhang,JuzheLiu,XiaogangNiu,LeqingDeng,XiangyuChen,FengshiLi, HeweiZhao,JianweiNai,etc.Besides,IwouldalsoliketothankProf.YongjunTian, Prof.XiaodongHan,Prof.XunWang,Prof.ZhiyongTang,Prof.JingZhang,Prof.Yi Luo,Prof.ShiheYang,Prof.XiangfengDuan,Prof.NicholasKotov,Prof.LiminLiu, Prof.XiaodongLi,Prof.LinGu,Prof.LiminLiu,andProf.RobertRitchiefortheir
Preface xv
greatsupportandhelpinscientificresearchcooperation.Iwouldalsoliketoextend mysincerethankstotheNationalNaturalScienceFoundationofChina(51532001) foritsfundingandsupport.
IwouldliketoexpressmysincerethankstoProf.LeiJiang,Prof.YadongLi, Prof.HongjieZhang,Prof.YuliangLi,Prof.JihongYu,andProf.JinghongLifor theirvaluablecommentsandsuggestionsforthisbook.Iwouldalsoliketothank theWileyPressandeditorsfortheirenthusiastichelpduringthepublicationof thisbook.
Thisbookisdedicatedtothoseresearchscholars,students,andbusinesspeople whoareengagedintheresearchofamorphousnanomaterials.Becauseoftherapid developmentinthefieldofamorphousnanomaterials,theemergenceofnewknowledgeandtheories,andtheinexperienceoftheauthor,somemistakesinevitablyexist inthebook.Wehopethatexpertsandreaderscanprovidevaluablesuggestionsfor timelysupplementandrevision.
17January2021
LinGuo BeihangUniversity,Beijing
1.1IntroductionofAmorphousMaterials
Amorphousmaterialisatypeofsubstanceinwhichbasicunitsdonotexhibit long-rangeorder(LRO)inspacebutmaintainsomeorderedcharacteristicsonlyin therangeofseveralatomicscope.Differentfromthetraditionalcrystallinematerials withregularlyarrangedbasicunits,amorphousmaterialsarecharacterizedby atomicshort-range(<1nm)orderandlong-range(>1nm)disorder[1].Compared withanisotropiccrystallinematerials,amorphousmaterialsexhibitmacroscopic homogeneityandisotropy.Basedonthisinternalstructure,amorphousmaterials donothavecrystaldefectssuchasdislocations,grainboundaries,etc.,which endowstheamorphousmaterialswithsomeexcellentmechanicalpropertiessuch ashighstrength,highhardness,highwearresistance,highfatigueresistance, etc.Atthesametime,amorphoussurfaceexhibitsahighdegreeofunsaturated atomicsitesordanglingbonds,whichmadeitdesirableasamodelcatalystora practicalcatalyst.Thus,thespecialdisorderedsurfacestructurecanprovidemore activesitesthantraditionalcrystalnanomaterialsandfurtherimprovethecatalytic performance.Apartfromthat,thespecialatomicarrangementofamorphous structurescaneffectivelyregulatetheelectronicstateofthematerial,leadingto optimizedtransmissionofelectronsorionsincatalysis.Therefore,theintensive studyofamorphousmaterialshasimportantimplicationsfortheoreticaland practicalexplorationofsolidmaterials.
Intheorderofaggregates,modernphysicsclassifiesconventionalmaterialsas solid,liquid,gaseous,andplasmastates(Figure1.1),whichareconsideredtobethe basicmaterialformsthatmakeupoursurroundings.Inadditiontothethreestates ofsolid,liquid,andgas,whicharecommoninourperception,theplasmastate consistsofgaseousmoleculesafterionization(thermalionization,photoionization, impactionization,etc.),anditsbehaviorismainlycontrolledbytheCoulomb interactionbetweenionsandelectrons.Thefourbasicstatesarestableundersome certainconditions,andthetransformationofphysicalstatesfromonetoanotheris duetothechangeofthermodynamicstatessuchastemperature,volume,enthalpy, andentropy.
Comparedtothestablebasicstates,theamorphousmaterialsareconsidered tobeametastableintermediatestateofliquid-to-solidtransition.Itsprecursoris
AmorphousNanomaterials:Preparation,CharacterizationandApplications, FirstEdition.LinGuo. ©2021WILEY-VCHGmbH.Published2021byWILEY-VCHGmbH.
Figure1.1 Theresearchscopeof amorphousmaterials. T , V , H ,and S arethe abbreviationoftemperature,volume, enthalpy,andentropy,respectively.
aviscousliquid(supercooledliquid)thathasbeguntoundergoagglomeration transformation.Inaddition,thenextstageofanamorphousstructureisacrystal withastableperiodicarrangementoftheconstituentelements.Amorphous materialshaveaphasesimilartothatofsolid,whichisembodiedasasolidshape. Meanwhile,ithasadisorderedatomicarrangementsimilartothatofliquid. Withoutthefixedatomicpatternascrystal,orthedynamicequilibriumasliquid andgas,amorphousmaterialsareintervenedformattedsolidwithunstablestate.
Fewmaterialsresearchesinthepasthundredyearsdiscussedtheamorphous system,mostofthestudieshaveusedcrystalmodelstoconstructtheirperceptions abouttheformationofmaterials.Asufficientlysoundtheoreticalsystemhas beenestablishedforcrystalsowingtoitsaccuratelyandconstantlyregularatomic arrangements.Comparedwiththesetraditionalcrystallinematerials,amorphous materialsonlyhaveshort-rangeorder(SRO)in1–2atomicscope,withouttranslationalsymmetryorrotationalsymmetry(Figure1.2).Thisdisorderedstatemakes theamorphousmaterialextremelydifficulttobestudiedbothexperimentallyand theoretically.Forexample,eventhoughthesphericalaberrationcorrectiontechniqueandcryo-electronmicroscopytechniquehaveachievedgreatdevelopment today,themostbasicstructuralortheatomicarrangementofamorphousmaterials hasnotbeeneffectivelysolved.
Inbothexperimentalobservationandtheoreticalreasoning,thesystematical arrangementandtheregularmorphologyarethebasisofscientificresearch,which
Side view (a)(b) Top view
1.2StructuralDifferencesbetweenAmorphousMaterialsandCrystals 3 cangreatlysimplifypeople’scognitiveprocess.Theestablishmentofcrystallography reliesheavilyonatheoreticalsystembasedonmathematics.However,amongall thesubstancesthatmakeuptheworld,theoneswiththeperfectandregular arrangementaretheonlyveryspecialcases.Thechaoticanddisorderedstateis therealcornerstoneofthewholecondensedmatter.Fromthewateronwhich lifedepends,tothelifeitself,tothevastuniverse,toclustersofatomstensof thousandsoftimessmallerthanbacteria,theyarenotarrangedinregularform. Meanwhile,eventhoughscientistshaveacompleteandsystematicunderstanding oncrystalmaterials,researchesarenecessarytobecarriedoutwiththeamorphous counterparts,suchasglass,plasticsandrubber,tofurtherexploretheirirreplaceable roleinmanykeyareas.
Therefore,increasingattentionhasbeenreceivedtothestudyofamorphous materials.In1995, Science publishedaspecialissue: ThroughtheGlassLightly.It inviteddozensoftopscientiststoputforwardtheideasforthefutureofsciencein thetwenty-firstcentury. PhilipWarrenAnderson ofPrincetonUniversity,whowon the1977NobelPrizeinPhysicsforhisfundamentaltheoreticalresearchonthe electronicstructureofmagneticanddisorderedsystems,believedthatthedeepest andmostinterestingunsolvedprobleminsolidstatestheoryisprobablythetheory ofthenatureofglassandglasstransition.Thiscanbethenextbreakthroughinthe comingdecade[2].
Tenyearslaterin2005,atthecommemorationofthe125thanniversaryof Science,anotherspecialissuenamed WhatDon’tWeKnow invitedmanyofthemost influentialscientistsinvariousfieldstoraise125scientificproblemsthatneedto besolvedurgentlyinthisnewcentury.Amorphousmaterialwasstilllistedamong them: Whatisthenatureoftheglassystate? [3]Moleculesinaglassarearranged muchlikethoseinliquidsbutaremoretightlypacked.Whereandwhydoesliquid endandglassbegin?
Atthe9thInternationalConferenceonbulkamorphousalloys,heldinXiamen Universityin2012, TakeshiEgami fromOakRidgeNationalLaboratoryandTennesseeStateUniversity,oneofthemostfamousscientistsinthefieldofamorphous materialsandphysics,concludedtheconferencebysaying,theamorphousfield isanareawithouttextbooks,andaspiringyoungpeopleshouldactivelyengagein researchareaswheretextbooksarenotyetavailable.
1.2StructuralDifferencesbetweenAmorphous MaterialsandCrystals
Accordingtothearrangementofatoms,modernscienceclassifiessolidmaterials intothreecategories:crystals,quasicrystalandamorphousmaterials(Figure1.3).
1.2.1CrystalsandQuasicrystals
Forcrystalmaterials,theatomicarrangementhasbothtranslationalsymmetryand rotationalsymmetry.Inrealspace,itsstructuralelements(atomsormolecules) arearrangedperiodicallyinthree-dimensionalspaceaccordingtocertainrules. Therefore,periodicityisconsideredasthemostessentialcharacteristicofacrystal
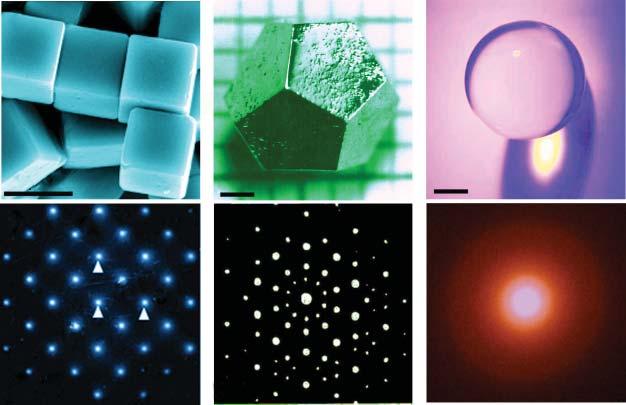
Figure1.3 Themorphologiesofthreedifferentkindsofsolidmaterials,aswellastheir correspondingelectrondiffractionpatterns.(a,b)Crystal,(c,d)quasicrystal,and(e,f) amorphousmaterials.Source:Panels(a,b)ReproducedwithpermissionfromZhangetal. [4].Copyright2009,RoyalSocietyofChemistry.Panel.(c)Reproducedwithpermissionfrom Fisheretal.[5].Copyright2000,ElsevierInc.Panel.(d)Reproducedwithpermissionfrom Zhangetal.[6].Copyright1985,TaylorandFrancisGroup.Panel.(f)Reproducedwith permissionfromYueetal.[7].Copyright2015,ScienceChinaPress.
structure.Itsmorphologyismostlymanifestedasahighlysymmetricalpolyhedron (Figure1.3a).Inreciprocalspace,theperiodicallyarrangedstructuralunitsofa singlecrystalmaterialwouldproducediffractionspotswithtranslationandrotation repeatability.Thediffuseddiffractionspotsformdiamondpatternscenteredon thetransmissionspot(Figure1.3b).Thediamondangleandedgelengtharethe directtransformationofcrystallatticeparameters.Forpolycrystallinematerials,the diffractionpatternsaresharpdiffractionringscenteredonthetransmissionspot.
Forquasicrystalmaterials(Figure1.3c),theatomicarrangementhasrotational symmetrybutdoesnothavetranslationalsymmetry.Itsbiggestfeatureisthesymmetrythatisincompatiblewiththetraditionalcrystalspacelattice(e.g.fifthsymmetric axis).Inreciprocalspace,italsoexhibitssimilardiffractionpatternsasthecrystals withregularanddiffuseddiffractionspots(Figure1.3d).Thedifferenceisthatthere isonlyrotationregularityandnotranslationregularity.
Becausecrystalsandquasicrystalshavegreatconsistencyinstructure,modern solid-statephysicsisalsoaccustomedtoclassifyingquasicrystalstogetherintocrystals,i.e.materialswithsharpdiffractionspots(i.e.periodicarrangementofatomsin realspace)ascrystals,whichhavethefollowingcharacteristics:
(1)Theatomicarrangementofcrystalunitshaslong-rangesymmetryand regularity.
1.2StructuralDifferencesbetweenAmorphousMaterialsandCrystals 5
(2)Crystalsshowself-limitation,whichmeansnatural-growncrystalswithout externalinterferencewilleventuallygrowintoregularmorphologieswithhigh symmetry.Itisthegeometricbasisforthedeterminationofcrystals.
(3)Crystalsobeythelawofconstancyofinterfacialangles,whichisthefirstlawof geometriccrystallography,andisalsothebasisforjudgingcrystalsinmorphology.Itstatesthattheanglesbetweentwocorrespondingfacesonthecrystalsof anysolidchemicalormineralspeciesareconstantandarecharacteristicofthe species.Thelawholdsforanycrystals,regardlessofsize,localityofoccurrence, orwhethertheyarenaturalorman-made.
(4)Singlecrystalsareanisotropic.
(5)Crystalmaterialhasafixedmeltingpoint,anditstemperatureremains unchangedduringthephasetransitionprocess.
(6)CrystalscanproduceX-raydiffractionwithspecificregularity:Itisthebasisfor moderncrystallographytojudgewhetherasubstanceisacrystalornot.
1.2.2AmorphousMaterials
Foramorphousmaterials,thearrangementofatomsdoesnothavelong-range symmetry,neitherrotationalsymmetrynortranslationalsymmetry.Inrealspace, itisgenerallybelievedthatamorphousmaterialshaveSROonlyinafewangstroms butdonothaveLRO.Itcannotspontaneouslyembodyregularmorphology(except spherical),soamorphousmaterialsaregenerallyknownasformless(Figure1.3e). Inreciprocalspace,thediffractionpatterndoesnothaveanydiffractionspotsor sharpringsbutacirculardiffractionhalo(Figure1.3f).
Comparedwithcrystallinematerials,thefeaturesofamorphousmaterialscanbe summarizedasfollows:
(1)Atomsinamorphousmaterialsonlyhavefixedatomicarrangementrulesinthe nearestandthenextneighbor(<1nm).Theorderofthelongerrangeisstill unclear.
(2)Becauseofthelong-rangedisorderoftheatomicarrangement,theregular morphologyofamorphousmaterialscannotbeobtainedbynaturalgrowth undernon-limitedconditions.Therefore,amorphousmaterialsalwaysembody asformlessorspherical,drivenbysurfaceenergy.
(3)Amorphousmaterialisphysicallyandchemicallyisotropic:thehomogeneityof theatomicenvironmentdeterminesthattheyarenotasanisotropicascrystals;
(4)Comparedwithcrystal,theamorphousmaterialismetastable.Theamorphous structurewillrelaxtocrystallinestateatahigh-temperature/high-pressureprocessing.
(5)Thereisnofixedmeltingpointforamorphousmaterial.Itonlyshowedaglass transitiontemperature.Thereisnounchangedtemperatureplatformduringthe phasetransitionprocess.
(6)AmorphousmaterialdoesnotproduceregularX-raydiffraction.Thetypical X-raydiffractionpatternofamorphousmaterialisahumpataspecificlocation, ratherthanaseriesofpeaksincrystal.Itstypicalelectronicdiffractionpattern isdiffractionhalos.
Itcanbefoundthattheessentialdifferencebetweenamorphousstructureand crystalsliesintheLRO,andthesimilarityliesinthehighSRO.
SROmeansthatamorphousatomsonlyhaveahighdegreeoflocalcorrelation, whichistheresultofthestrongchemicalbondsbetweenthenearestneighbor (includingthenextneighbor)atomstomaintainasafixedcomponentsolid.This makestheshort-rangestructureofamorphousmaterialssimilartothatofcrystals, sotheSROisconsideredasthestructuralfeatureofamorphousmaterials.A largenumberofsimulationsandexperimentsshowthattheshort-orderscaleof amorphouscrystalsshouldbelessthan1nm.
Commondiffractionmethods,suchasX-raydiffractionandselectiveelectron diffraction,arebasedonLRO.Therefore,itisdifficulttodirectlyobtainshort-range informationsandimagesofamorphousmaterial.Toinvestigatetheaveragestructureinformationsuchastheradialdistributionfunction(RDF),theanalysisof atomicorderingofamorphousstructuresisgenerallybasedonthefittingresultsof diffraction(electron,neutron,etc.)orspectroscopy(X-rayfinestructureabsorption spectroscopy,nuclearmagneticresonancespectroscopy,etc.).
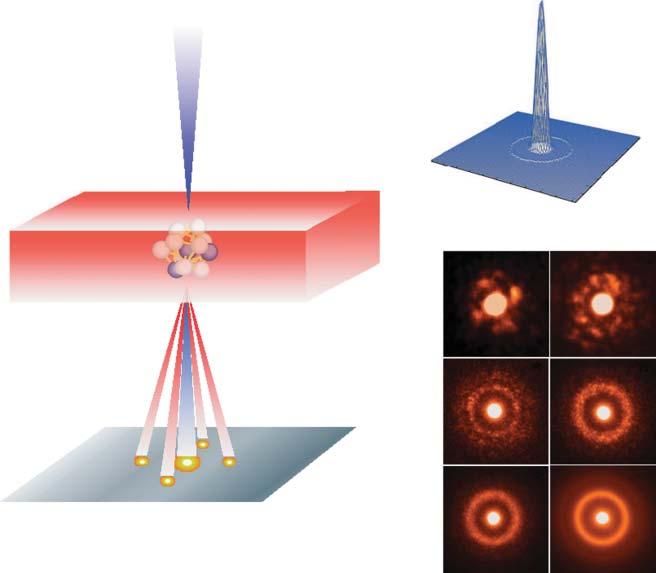
In2011, ChenMingwei and AkihisaInoue [8]ofNortheasternUniversityusedthe mostadvancedsphericalaberrationcorrectingtransmissionelectronmicroscopy (AC-TEM)technologytoreducethediameterofthecoherentelectronbeamto 3Åfornanobeamelectrondiffraction(NBED)analysistoreplacetraditional selectiveareaelectrondiffraction(SAED).Itwasfoundintheexperimentthat underthe3.6Åelectronbeam,thediffractionpatternofamorphousmaterial showedobviouspatterning(Figure1.4).Forthefirsttime,theorderofthe atomicneighborandthenext-nearestneighborstructureoftheamorphousalloy wasobserved.
Forcrystalmaterials,themostessentialfeatureistheorderlyarrangementof structuralelements.Itenablescrystalresearchtobebasedonmathematicsand establishedstandardmodels.Thestructuralorcompositionalchangescouldbe fullystudiedrelyontheestablishedmodel.Itcanalsointroducevariousdefects onthebasisofthemodelandestablishamaterial–structure–propertyrelationship toadjusttheperformancebytuningmaterials.Thiskindofsystematicresearch cannotonlyensurethecontinuousfollow-upofthetheoreticalresearchtothe explanationofexperimentalphenomenabutalsomakemedium-andlong-term predictionsofexperimentalresults.Theexperimentaldatawillbefedbacktothe theoreticalsystematthesametime,makingitcompleteandmoreaccurate,forming aperfectclosedloopbetweentheoryandexperiment.
Mostofthemodernstructuredetectionwasbuiltbasedoncrystalmodels.Thus, foramorphousmaterialswithouttheLRO,theexistinganalysismethodscanonly giveaverageatomicinformationinthestatisticalcategory,whichisdifficulttoget theaccuratestructuralinformation.Therefore,wehavenotbeenabletoaccurately establishthestructuralmodelofamorphousmaterialstosortoutthecomplex long-rangeinteractionsbeyondtheatomicscale.Theexistedamorphousresearch studieshaveestablishedtherelationshipbetweenmaterials,kineticunits,and propertiesinmetallicglasssystems.Apartfromit,manyotherresearchstudies arestillindividualresultsthatareonlyintheexperimentalobservationstageto
1.3HistoryofAmorphousMaterials 7
Figure1.4 Theordereddiffractionpatternsofamorphousmaterialundercoherent electronnanobeamwithdifferentdiameters.Source:Reproducedwithpermissionfrom Hirataetal.[8].Copyright2011,NaturePublishingGroup.
summarizethephenomenologicalrules.Thissituationmaybeimprovedwiththe developmentofthebasicphysicsandexperimentalcharacterizationtechnique.
1.3HistoryofAmorphousMaterials
Throughoutthehistoryofhumanscienceandtechnology,thebreakthroughofnew technologiesisalwaysaccompaniedbythediscoveryofnewmaterials.Fromancient bone,stone,porcelain,metalmaterialstomodernhigh-performanceplastics,alloys, micro–nanomaterials,whicharestilldeveloping,allofthemhaveleftadeepimprint inthehistoryofsciencedevelopment.Alongwiththeprogressingofscientifictheory,thewaysofexploringnewmaterialshavechangedsignificantly(Figure1.5).For example,ittookthousandsofyearsforhumanbeingstodistinguishandadjustthe compositionofsubstances.Fromoriginalboneandstonetoolstosyntheticbronze andirontools,wefinallyobtainedengineeringmaterialsbasedonpreciselycontrolledalloysandcomposites.Allofthemprovidethestrongsupportfortheindustrializationprocessofhumanbeings.Ontheotherhand,ittookdecadestoachieve materialdesignandcontrolfrommacroscaletonanoscale,thentoatomicscale,layingastructuralfoundationfordiscoveryofmodernhighperformanceenergystorage
High
MesosopicNano–microClusterSingle-atom
Figure1.5 Thedevelopmentofmaterialsresearchindifferentresearchorientations. andconversionmaterials.Thisacceleratedproceedingwasstronglysupportedby variousphysicalandchemicaltheoriesandmethodsandalsosupportedbytherapid developmentofcharacterizationmethods.Onthebasisofthesedevelopment,scientistscontinuetofocusonatomicorderingofthematerials,whichwasexpectedto furtherimprovethemechanicalorcatalyticpropertiesoftraditionalcrystalmaterials.Withthehelpofthemostadvancedcharacterizationmethods,suchasspherical AC-TEMandsynchrotronradiationX-rayfineabsorptionspectroscopy,scientists trytoadjusttheatomicarrangementtoobtainnewstructuralmaterials,suchas metastablematerials,high-entropyalloys,metallicglass,andamorphousmaterials.
1.3.1EstablishmentofCrystallography
Theconceptofamorphousisdefinedinthecomparisonofcrystallographyinsolid physics.Therefore,thehistoryofamorphousmaterialsshouldbecomparedwith thatofcrystallography.
On25June2012, theGeneralAssemblyoftheUnitedNations adoptedaresolution,whichproclaimingtheyear2014asthe InternationalYearofCrystallography Itwasnamedtocommemorate MaxvonLaue,whowontheNobelPrizeinPhysics of1917,about100yearsagoforcharacterizingthecrystalstructurebyX-ray.Atthe sametime,itwasalsocommemorated JohannesKepler proposedthefamousarticle ANewYear’sGiftofHexagonalSnow 400yearsago(1611).Hewasregardedasthe firstonetoconceptualizethesymmetryofcrystalswhichwereformedbyaregular accumulationofsphericalelements.Itwasconsideredtobethebeginningofthe establishmentoftraditionalcrystallography.
In1699, NicolasSteno observedalargenumberoforesandproposedthattheangle betweentwoidenticalcrystalplanesofacrystalisalwaysconstant,regardlessofthe sizeandshapeofthecrystalplane[9].Itwascalled thelawofconstantanglesofthe crystalplane,whichwasgenerallyrecognizedas thefirstlawofcrystals.Priortothe discoveryofX-ray,thelawlaidthefoundationforcrystalidentification,andtheword Crystal begantobeusedtodescribesubstanceswithregularmorphologiesandfixed angles.
In1784, ReneJustHayuy proposedthateachcrystalplanewassimplycomposed ofblocksofthesamesizeandshape.Thecrystalstructurewasdescribedasaregularthree-dimensionalarrangement,whichwasaninfiniterepetitionofcellsinthe three-dimensionaldirections.Onthisbasis, WilliamHallowesMiller proposedin 1839thateachcrystalplanecanbedescribedbythreesimpleintegers(h, l, k).That wasthe MillerIndex ,aswasstillusedtoday.
Attheendofthenineteenthcentury,fromamathematicalpointofview,scientists putforward32-pointgroupstodescribethesymmetryofcrystalshapeand230space groupstonominatethesymmetryofmicroelements.Untilnow,allthegeometric structurecharacteristicsofcrystallographyhavebeenbasicallyperfected.
In1912,onthebasisof Laue’swork, WilliamLawrenceBragg proposedthefamous BraggFormula,whichlaidthefoundationfortheestablishmentofmoderncrystallographyandthecharacterizationofcrystalstructure.Later,togetherwithhisfather WilliamHenryBragg,hequicklycharacterizedthecrystalstructureofvarioussubstances,andestablishedmoderncrystallographysoon[10].
Fromthedevelopmenthistoryofcrystallography,wecanfindthattheconfirmationofcrystalstructurecharacteristicsdependsonthediscoveryofX-ray,butthe establishmentofgeometrictheoryincrystallographywasfarbeforethecharacterizationofitscrystalstructure.Alltheelementsinthedefinitionofcrystals(crystalsaresolidswithregularperiodicrepetitivearrangementofinternalparticlesin three-dimensionalspace)aredeterminedbeforeX-raydiscovery.Theestablishment ofcrystallographydependsmoreonthemathematics-inducedtheoreticalsystem andphysics-drovetechnique.
1.3.2EnlightenmentofAmorphousMaterials
Theuseofnaturalamorphousmaterialscanbetracedbacktoprehistorictimeswhen Obsidian wasusedasacuttingtool.Itwasakindofnaturalglass,formedbythe suddencoolingofmagmafromvolcaniclava.Withasharpfracturesurface,people useditforcuttingasknives.
Becauseofthesimplicityofpreparation,glasshasbecomethefirstamorphous materialtobepreparedonalargescale.Thehistoryofglasspreparationcanbe tracedbacktotheancient Babylonians and Greeks 5000yearsago,butitwasnot untilthetwelfthcenturythatglassbegantobemanufacturedinbatchesasanindustrialmaterial.Subsequently,thelarge-scalepreparationoftransparentglassrapidly promotedthedevelopmentofscienceandtechnologyatthattime.Forexample, GalileoGalilei’shome-madetelescopediscoveredtheunevensurfaceofthemoon in1609,refuted Aristotle’stheoryofperfectcelestialbodies,andopenedthecurtain
ofmodernastronomy; IsaacNewton observedthedispersionoflightwithaprismin 1666.Ayearlaterin1676, AntonievanLeeuwenhoek,thefatherofthemicroscope, reportedobservationsofredbloodcellsandmicrobes,whichlaidthefoundation ofmodernbacteriologyandprotozoologyinbiology.Thistransparent,stable,and processablematerialprovidedfullytechnicalsupportforthedevelopmentinmany disciplines.
1.3.3ModernAmorphousMaterials1-DisorderedElementary Substance
Theresearchonamorphousmaterialsisstillcenteredonthecomparisonwith crystals.Therearethreemostusednamesofdisordermaterials. Amorphous is theearliestandmostwidelyusedexpressionapproach. Glass isanothernameof disordermaterial,especiallyinthemetallicglassfield. Non-crystal isusedinthe fieldofbiomineralization.
Amorphous,themostcommonEnglishexpressionofdisorderedmaterials,originatedfromGreek,whereaisaprefix,indicatingno;morphouscomesfrommorph, referringtomorphology.Theoriginalmeaningofthiswordismaterialwithoutmorphology.Itcanbeseenthatbeforetheestablishmentofmoderncrystallography, peoplehaveknownthemorphologicaldifferencesbetweenamorphousmaterials andcrystallinematerialstodistinguishthem.
In1840, JustusvonLiebig,theGermanorganicchemistandfatherofmineral nutrition,describedinhisclassicbook OrganicChemistryinitsApplicationtoAgricultureandPhysiology thatwhensulfurisheatedto160 ∘ C,andthenquicklypouring intocoldwater,sulfurdoesnotcrystallizebutturnssoftandtransparent.Inthis chapter,hewrote“suchsolidbodiesarecalledamorphous”.Thismaybetheearliest reportthatcomparedmorphologiesbetweenamorphousandcrystallinematerials. Liebig thenpublishedinthefamousmedicaljournal ProvincialMedicalandSurgical Journal in1840,pointedoutthatintheextractionofquinine(themaincomponent ofantimalarialdrugs),quinineobtainedfromquinolinetincturemainlyexistsin theformofamorphous[11].Itdoesnotaffectitsmedicalvaluebutcangreatly enhancethenaturalquinoline.In1862, G.Gore pointedoutin OntheProperties ofElectro-DepositedAntimony thatwhenantimonywaselectrodeposited,different depositionconditionswouldleadtotwokindsofantimonymonomerswithdifferent structures,i.e.crystallineantimonyandamorphousantimony[12].Healsoreported thatamorphousantimonyshoweddifferentphysicalandchemicalproperties.
Beforethetwentiethcentury,theresearchonamorphousmaterialswasstillinthe enlightenmentstage.Theamorphousmaterialsweremainlyfoundinthepreparationoftraditionalcrystalmaterials.Themorphologyandpropertiesofthis“novel” materialwerefullycomparedwithtraditionalcrystalmaterials.BecauseX-rayhas notbeendiscoveredandmoderncrystallographyhasnotbeendeveloped,theessentialcharacteristicsofthedisorderedstructurehavenotbeendiscovered.However, thesestudiesareveryimportantforthedevelopmentofamorphousandcrystal. Forexample,inthestudyofamorphoussulfur, Liebig arguedthatthesoftnessof amorphoussulfurprovedaremarkablefactthatthesmallestparticlesthatmake
1.3HistoryofAmorphousMaterials 11 upasolidaremovabletosomeextentandnotperfectlyconnected.ModernX-ray crystallographyverifiedthatthestructureofamorphoussulfurshouldbeaspiral chainstructurechangedfromtheS8 ringofcrystalsulfur,endoweditwiththesame elasticityasrubber.Thereisnodoubtthat,therecognitionatthattimehasagreat significanceintheresearchofsolidscience.
Theamorphousstateofphosphorusisthesameasthatofsulfur.Similartothe octahedralringstructuralunitofsulfurcrystal,thestructuralunitofphosphorus crystalisaregulartetrahedralstructurecomposedofphosphorusatoms(P4 ).When thecrystalofwhitephosphorusismeltedatahightemperature,P4 tetrahedron transformsintoachain-connectedstructure.Then,theamorphousredphosphorus couldbeobtainedbyquenching,maintainingitschain-likestructure.Recently, amorphousphosphorushasshowedgreatapplicationsinlithium-ionbatteriesand sodium-ionbatteriesbecauseoftheirsuperhightheoreticalcapacity.Similarly,in somepolymermaterials,whentheasymmetryoftheatomsconnectedchanges irregularly,thepolymerwillformarandomstereomer,whichwillbehaveasan amorphousstate.Becauseofthecomplexityofthemolecularstructureofultralong chains,theatomicarrangementmodesofamorphousnonmetallicelementssuch asamorphoussulfur,redphosphorus,andamorphouspolymersarenotclearyet. However,theyallhaveglasstransitiontemperaturessimilartothoseofglass,so theybelongtothecategoryofamorphous.
1.3.4ModernAmorphousMaterials2-MetallicGlass
Metallicglassisthemostabundantandwidelyusedmaterialinmodernamorphous scientificresearch.Becauseitsmainsyntheticprocessandpropertyaresimilarto traditionalglass,itisusuallyjustnamedasglass.
Learnfromthequenchingtechnologyinglassandsmelt,theearliestattempts weremadetocondensehigh-temperaturemetalvapors(Bi,Ga,Sn,etc.)on ultra-low-temperature(2–4K)substratesanduselargeinstantaneoustemperature differencestostabilizedisorderstructure.In1934,Germanscientist Krammer used vapordepositiontoobtainthefirstsystematicpreparationofamorphousalloys. Subsequently,avarietyofmetals,includingsemiconductorssuchasAsandTe, wereproducedbygas-phasequenching[13].However,thesereportshavenotattract muchattentiontoamorphousmaterialsbecausethepreparationprocessismore limitedandcannotbeextendedtoothermaterials.
Formetalsandalloys,thetraditionalmanufacturingprocesshaslongbeenmeltingandcolling.Becausethedifferenceoflocalstructureanddensitybetweenmetal liquidsandtheirsolidsisverysmall,ithasbeenthoughtthatmetalliquidscannotbe overcooled.However,in1952, TurnbullDavid discoveredthatmetalscanbesupercooledto20%oftheirmeltingtemperature,breakingthisconclusionandproviding atheoreticalbasisforthedevelopmentofamorphousalloys[14].In1960, PolDuwez etal.[15]reportedin Nature thataband-shapedamorphousAu–Sialloywassynthesizedbyquenchingforthefirsttime.Heimprovedtherapidquenchingprocesswith aquenchingrateof106 Ks 1 ,madethedisorderedatomsinthemetalmeltunable torearrangetocrystal,andobtainamorphousmaterial.Sincethen,metallicglass
hasquicklyattractedwidespreadattention,andquenchinghasalsobecomeabasic methodforsynthesizingamorphousmaterials.
Especiallysince1988, Inoue etal.[16]summarizedthreeexperimentalrulesfor obtainingbulkamorphousalloys:
(1)Thealloyshouldbecomposedofmorethanthreealloyelements;
(2)Thereshouldbemorethan12%atomicsizedifferencebetweenthemain elements;
(3)Themixingheatbetweentheelementsshouldbenegative.
Thepreparationofmetallicglasswerethenpromotedfromlow-dimensional materialstobulkamorphousmaterials.Manyexcellentcharacteristicsarefully utilized,soithasbecomearesearchfieldwithimportantapplicationprospects.
Metallicglassshowesauniquedisorderedstructure,withoutdefectssuchas dislocationsandgrainboundariesinthecrystal,endowingthemwithmanyunique superiorproperties.Forexample,intermsofmechanicalproperties,metallicglasses exhibithighstrength,highhardness,highwearresistanceandcorrosionresistance, highfatigueresistance,lowelasticmodulus,largeelasticstrainlimit,etc.Thus, metallicglasspossessesbroadpotentialapplicationsinthefieldsofengineering mechanics,biologicalsciences,andaerospace.Forexample,theamorphousalloys inalmosteveryalloysystemhaveachievedseveraltimeshigherstrengththanthe crystallinematerial.In2011, ZhangTao etal.[17]developedaCoTaBternaryalloy withacompressivestrengthof6.0GPaandaspecificstrengthof650Nmg 1 ,which reachedthehighestrecordforthestrengthofmetalmaterials.
Atthesametime,theintroductionofmicro/nanoscaleheterogeneousstructures orthesecondphaseinbulkamorphousmaterialscouldsignificantlyimprovethe toughnessofamorphousmaterials.In2007,accordingtoPoisson’sratiocriterion, WangWeihua etal.[18]adjustedthecompositionoftheZr–Cu–Ni–Almetallicalloy andpreparedanamorphousalloysystemwithamultilevelmicroscaleheterogeneousstructure,whichshowedhighstrength(1.7GPa)andverylargecompressive plasticity(strain > 150%).Theseamorphousalloyscanevenbebentto90∘ atroom temperature(Figure1.6a–d).In2008, WLJohnson etal.[19]improvedthecompositionoftheamorphousalloyandcontrolledthecontentofeachcomponentto synthesizetheZr–Ti–Nb–Cu–Bemetallicalloywithamicron-scaleprecipitatedsecondphase.Forthefirsttime,thefracturedeformationhasbeenincreasedtomore than10%,andupto14%.Atthesametime,thefracturetoughnessoftheamorphous alloyreached170MPam0.5 ,indicatinganexcellenttoughness(Figure1.6e–g).
Inadditiontothemechanicalproperties,anotherkeypointforamorphous materialsattractingwidespreadattentionistheapplicationincatalysis.Amorphous materialsexhibithighactivitysurfacewithunsaturatedcoordinationsitesand uniquelocalenvironmentwithuniformchemicalstatesandatomicstructures. Whetherintheoreticalresearchasamodelcatalystorperformanceexplorationas apracticalcatalyst,thestudyofamorphousmaterialsissignificant.Forexample, in1925, Constable systematicallydiscussedthedifferencebetweenamorphous materialsandcrystallinematerialsduringtheprocessofcatalyticdecomposition bycalculatingtheactivecenterandpointedoutthatamorphousmaterialsmay

Figure1.6 Amorphousmetalwithmicro/nanomicrostructure.(a–d)Amorphousalloywith micron-scaleheterogeneousstructureanditsmechanicalpropertiesand(e–g)amorphous alloywithmicron-scalesecondphaseanditsmechanicalproperties.Source:Panels (a–d)reproducedwithpermissionfromLiuetal.[18].Copyright2007,AAAS.Panels (e–g)reproducedwithpermissionfromHofmannetal.[19].Copyright2008,Nature PublishingGroup.
havehigherperformance[20].In1980,attheSeventhInternationalConference onCatalysis, GerardV.Smith oftheUniversityofSouthernIllinoisatCarbondale [21]firstlydemonstratedamorphousalloysasanewsystemforcatalyticreactions.In1983, Brower etal.reportedin Nature thatPd-basedmetallicglasshas ahigherselectivitythancrystallinePdinhydrogenationreactions[22],which graduallyopenedthepreludetotheresearchofamorphousalloysinthefield ofcatalysis.
However,becauseofthelowspecificsurfacearea,amorphousalloysalwaysfailed tofullyrealizethetrueactivity.Forexample,inthedegradationofdirectblue, thedegradationabilityofiron-basedamorphousalloy(46h)isnotsignificantly improved,comparedtopureiron(>50h).However,aftergrindingtheamorphous alloytothemicronlevel,itcancompletelydegradethesameconcentrationof
dyeinashortperiodof1h[23],showingtheimportantapplicationprospectsof micro–nanoamorphousmaterialsinthefieldofcatalysis.
1.3.5ModernAmorphousMaterials3-NontraditionalAmorphous Nanomaterials
Thepolymerizedamorphousstructurerepresentedbyamorphoussulfurandpolymers,andthemultielementmetallicamorphousstructurerepresentedbymetallic glasstogetherconstitutethetraditionalamorphousmaterials.Theydemonstrate thestandardstructurecharacteristicsofamorphousrepresentedbyglass,whichis theexistenceofglasstransitiontemperature.Theformeramorphousstructureis formedduetothecomplexityofthebasicchainstructure,theflexibilityandthe homogeneoussitewouldconfusetheconnectionofmonomer.Theconstructionof latteramorphousstructureisduetothehindranceofmultiplecomponentsduring quenching,whichpreventtheatomstomovetotheregularpositionincrystal. Inadditiontothesetwostrategies,bymanipulatingthesynthesisstepandintroducingadditionalfactorstodisrupttheregulararrangement,itispossibletoobtain amorphousnanomaterialswhosecompositionsarebasicallythesameastheircrystals,butwhosestructureischaotic.Thesematerialsalsohavearandomatomic arrangement.Herewesimplysummarizesomemethodsandwilldescribethemin chapter4tochapter8.
1.Formostmetal,theamorphousstructureofasingleelementalmetalcannotbe easilyobtainedbyquenching.Bymeansofchemicalsynthesisinsolution,the strongreducingagentNaBH4 /KBH4 couldbeusedtorapidlyreducethetransitionmetalfromthesolvent.Thisprocessissimilartothefastquenchingof metallicglassinwhichmetalatomsarefrozenatthedisorderedarrangement insolution.Apartfromit,theresidualsmallatomsBhindersthenucleationand regulararrangementofmetalatoms,producingamorphousM–Bnanomaterials.
2.ForSiandGe,whicharethemostimportantmaterialsinthesemiconductor field,theirindustrialamorphousmaterialscannotbeobtainedbythequenching method.Thatwasbecauseofthedistinctdifferencebetweenthesix-coordinated structureintheliquidphaseandthefour-coordinatedstructureinthesolid phase.Therefore,thepreparationofamorphoussiliconreliesongas-phasesynthesismethods.However,thedirectlyobtainedamorphoussiliconfromgaseous Siexhibitahighconcentrationofdanglingbonds.Theproductwithabundant defectsshowsnopracticalindustrialvalue.Thus,amorphousSialwaysreplace toamorphoussilicon–hydrogenalloyobtainedfromthedecompositionofthe precursorsilane(SiH4 )bythevapordepositionmethodortheglowdischarge method.Hydrogenatomscansaturatethedanglingbonds,therebyreducingthe concentrationoftheparamagneticcenterbyfourtofiveordersofmagnitude.In additiontotheadvantageofsimpleandconvenient,theamorphoussiliconcan beeasilyadjustedtoap-typeorn-typesemiconductormixingasmallamountof borane(BH3 )orphosphane(PH5 ).
3.Amorphousnanomateriaslcouldalsobeachievedinsolutionbyadjustingthe decompositionprocessoftheprecursortoobtainapartiallydecomposedproduct
1.4GrowthMechanismsofAmorphousNanomaterials 15 inwhichtheobtainedamorphousstructureisstabilizedbytheremainingatoms/ coordinationgroups.Forexample,asthemostpolarsolvent,watermoleculescan easilycoordinatewithcationstoformionichydrates,whichiswidelyusedinthe formationofamorphousoxides,hydroxides,etal.Forexample,inthepreparationoftitaniumdioxideinsolution,theamorphoushydratedoxidationstateis usuallyobtainedfirst.Inthefieldofbiomineralization,amorphouscalciumcarbonateisthemostimportantintermediatestateinthemineralizationprocess.Its formationalsodependsonthewatercarriedoutfromthesolution.Itscompositionismostlyunderstoodashydratedcalciumcarbonate.TheadditionofMg ions,polyacids,aminoacids,etc.,whichmimicthebiologicalenvironment,can effectivelyimprovethestabilityofitsamorphousstructure.
4.Inadditiontotheintroductionofadditionalcomponents,takingawaythestructuralatomstoconstructanunsaturatedenvironmentcanalsodestroytheregular atomicarrangementoftheoriginalstructuretoobtainanamorphousstructure. Forexample,theclassicsemiconductormaterialtitaniumdioxidecanchangeto black/bluetitaniumdioxidewithalargenumberofoxygenatomsmissingunder astrongreducingatmosphere.Duetotheabundantoxygendefects,itssurface usuallyexhibitsanamorphousstructure.Thisisauniversalmethodformany transitionmetaloxideslikeZnO,CeO2 ,SnO2 .
5.Forultrasmall/ultrathinnanomaterials,thepositionoftheatomsonthesurface couldbeaffectedbysurfaceligand.Thedesignofsurfaceligandscouldreducethe orderofatomstoconstructamorphousstructures.Alargenumberofamorphous noblemetalparticlesandamorphousultrathintwo-dimensionalmaterialscould beachievedthroughthismethod.
1.4GrowthMechanismsofAmorphousNanomaterials
1.4.1ClassicalNucleationTheory
Inclassicalnucleationtheory,theformationofsolidparticlesinsolutionunderwent twophases:nucleationandgrowth.In1950, Lamer proposedanucleationmechanismintheenergycategorybasedonthesulfurcolloidsynthesisprocessinsolution, whichisstilloneofthemostsignificantself-consistentnucleationmechanisms[24]. Atthenucleationstage,thetransitionfromtheliquidphasetothesolidphase occurs.Therearetwokindsofenergychangesduringthistransition.Oneisthe volumefreeenergy(ΔGV ),whichisdefinedasthedecreaseofthefreeenergyof atomsduringthetransitionfromthefreestateinthesolutiontothecrystalnuclei. Theotheristhesurfacefreeenergy(ΔG�� ),whichdemonstratestheincreasingof thesystem’sfreeenergyfromthegeneratednewinterfaces.Ifthenewlygenerated nucleusisregardedasasphere,theformerisapositivecorrelationfunctionof volume(thecubeoftheradius r ), ΔGV = 4/3πr 3 ⋅��RT ln(c/c0 ),where c0 isthe concentrationofthesupersaturatedsolution.Thelatterisapositivecorrelation functionofthesurfacearea(thesquareof r ), ΔG�� = 4πr 2 ⋅�� s l .Thismakesacritical radius r *inthenucleationprocess,whichexhibitanegativecorrelationwiththe
concentrationofthesupersaturatedsolution.Therefore,ahigherconcentration supersaturationleadtosmallernucleationradius.
Takingtheformationofmetalmaterialsasanexample.Duringthenucleation stage,theoriginalprecursorsarereducedtometalatomswithexternalstimulation(reductiveagentadding,heating,irradiating,etc.).Theseatomswillbethe modularunitsforthesubsequentconstructionofthecrystalstructure.Withthe decompositionoftheprecursor,theconcentrationofmetalatomsincreases.Once theatomicconcentrationexceedstheminimumsupersaturationpoint,theatoms exhibitatendencytoaggregatespontaneouslytoreducesurfaceenergy(surface energyishigherthanvolumeenergybeforethecriticalradius).Insomewhere ofthesolution,affectedbythermodynamicfluctuations,initialnucleiwould instantaneouslyaggregatebyatomsandseparatefromsolution.Oncetheinitial clustersareformed,theseseedsthenacceleratetheirgrowthbyadsorbingfree atomsinthesolution,leadingtothedecreaseoftheconcentrationofatomsinthe solution.Atthistime,thedecompositionoftheprecursorisstillcontinuingand thefreeatomiscontinuouslyadded.Iftheatomicconcentrationdropsrapidly belowtheminimumsupersaturation,noadditionalnucleationwilloccurandthe resultingproductwillexhibitamonodispersesize.Withthecontinuoussupply ofatomsthroughtheprecursordecomposition,theinitialnucleuswillgrowinto nanocrystalswithanincreasingsize,untilachievinganequilibriumstatebetween thesurfaceatomsonthenanocrystalsandthefreeatomsinthesolution.
Oncetheseedisformed,itssizewouldincreasebycontinuousadditionoffree atoms.Fromtheperspectiveofchemicaldeposition,whenatomsareaddedtoa solidsurface,theatomsdiffuseontheirsurfaceuntiltheyencounterastepposition wheretheycanbeincorporated.Atthesametime,asparticlesizeincreases,the volumefreeenergyreduces(favorableforgrowth)andsurfaceenergyincreases (favorablefordissolution).Thedynamicinteractionofgrowthanddissolution leadstotheformationofanisotropicnanocrystalwithspecificmorphology.With thedevelopmentofin-situelectronmicroscopes,thestructureandshapeofseeds andnanocrystalsproducedatdifferentstagescouldbeobserved.Therefore,alarge numberofstudieshavereportedtoexploretherelationshipbetweentheinitial seedandthefinalnanocrystal.Afterthenucleationandgrowthoftheseed,the generatednewnanocrystalwillstillgothroughcomplexevolution,suchasOstwald ripening,Kirkendalldiffusion,orientedattachment,etc.Finally,nanoparticleswith specificstructureandmorphologyareobtained.
Atypicalsynthesismethoddevelopedundertheguidanceofthistheoryisthe hotinjectionmethodproposedby A.P.Alivisatos and PengXiaogang oftheUniversityofCalifornia[25].Coldstocksolutionisquicklyinjectedintotherapidly stirred,hotsolvent,andalargenumberofcrystalnucleiareinstantaneouslygenerated.Themonomerconcentrationisrapidlyreducedbelowthesupersaturation threshold,andfurthernucleationissuppressed.Thismethodseparatesthenucleationfromgrowth,yieldingparticlesofonesize.Itisaclassicmethodforpreparing monodispersenanoparticles,especiallyquantumdots.
However,thetheoryisstillanidealmodelunderextremeconditions,whilethe actualreactionsandprocessesthatoccurinsolutionarefarmorecomplicated.