AtomicandNanoScaleMaterials forAdvancedEnergyConversion
Volume1
EditedbyZongyouYin
Editor
Prof.ZongyouYin
AustralianNationalUniversity ResearchSchoolofChemistry
SullivansCreekRoad
2601Canberra Australia
CoverImage: ©amiak/Shutterstock
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedbythe DeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>
©2022WILEY-VCHGmbH,Boschstr.12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34892-3
ePDFISBN: 978-3-527-83138-8
ePubISBN: 978-3-527-83139-5
oBookISBN: 978-3-527-83140-1
Typesetting Straive,Chennai,India
Printedonacid-freepaper
10987654321
Volume1
1Introduction 1 ZongyouYin
PartIEmergingNanomaterialsforElectrochemical(EC) EnergyConversion 3
22D-Materials-FreeHeterostructuresforECEnergy Conversion 5 KamranDastafkanandChuanZhao
2.1HeterostructuresforElectrochemicalWaterSplitting 5
2.1.1MetalOxide-BasedHeterostructures 5
2.1.2MetalHydroxide-BasedHeterostructures 9
2.1.3MetalPnictide-BasedHeterostructures 10
2.1.4MetalChalcogenide-BasedHeterostructures 11
2.1.5Metal/Carbon-BasedHeterostructures 15
2.1.6Mixed-PhaseMetallicHeterostructures 16
2.1.7MulticomponentMetal-BasedHeterostructures 17
2.1.7.1MetalOxide-BasedMulticomponentHeterostructures 17
2.1.7.2MetalNon-oxide-BasedMulticomponentHeterostructures 22
2.1.7.3MixedMetalOxide/MetallicNon-oxideMulticomponent Heterostructures 24
2.2HeterostructuresforElectrochemicalCO2 ReductionReaction 24
2.2.1Metal/MetalOxide-BasedHeterostructures 25
2.2.2MetalOxide-BasedHeterostructures 28
2.2.3Metal/Heteroatom-DopedPorousCarbon-BasedHeterostructures 29
2.2.4MetalOxide/PorousCarbon-BasedHeterostructures 31
2.2.5MetalNon-oxide-BasedHeterostructures 31
2.2.6MulticomponentMetal-BasedHeterostructures 32
2.2.6.1MetalOxide-BasedMulticomponentHeterostructures 34
2.3HeterostructuresforElectrochemicalN2 ReductionReaction 38
2.3.1MetalOxide-BasedHeterostructures 38
2.3.2Metal/Heteroatom-DopedPorousCarbon-BasedHeterostructures 39
2.3.3MetalNon-oxide-BasedHeterostructures 41
2.4ChallengesandFutureOpportunities 43 References 45
32D-Materials-BasedHeterostructuresforECEnergy Conversion 53 ZhengqingLiu
3.1Advancesof2DMaterials-BasedHeterostructures 53
3.2WaterSplitting 54
3.2.1HydrogenEvolutionReaction(HER) 68
3.2.1.1HERon2D–0DHeterostructures 68
3.2.1.2HERon2D–1DHeterostructures 71
3.2.1.3HERon2D–2D(vanderWaals)Heterostructures 73
3.2.1.4HERon2D–3DHeterostructures 76
3.2.2OxygenEvolutionReaction(OER) 80
3.2.2.1OERon2D–0DHeterostructures 80
3.2.2.2OERon2D–1DHeterostructures 82
3.2.2.3OERon2D–2D(vanderWaals)Heterostructures 85
3.2.2.4OERon2D–3DHeterostructures 87
3.2.3OverallWaterSplitting(OWS) 90
3.2.3.1OWSon2DBinary/TernaryCompounds 91
3.2.3.2OWSonHeteroatom-Doped2DMaterials 93
3.2.3.3OWSon2D–0DHeterostructures 95
3.2.3.4OWSon2D–1DHeterostructures 96
3.2.3.5OWSon2D–2D(vanderWaals)Heterostructures 98
3.2.3.6OWSon2D–3DHeterostructures 100
3.3CO2 ReductionReaction(CRR) 103
3.3.1CRRon2D–0DHeterostructures 105
3.3.2CRRon2D–1DHeterostructures 106
3.3.3CRRon2D–2D(vanderWaals)Heterostructures 107
3.4N2 ReductionReaction(NRR) 109
3.4.1NRRon2D–0DHeterostructures 110
3.4.2NRRon2D–1DHeterostructures 113
3.4.3NRRon2D–2DHeterostructures 115
3.5ChallengeandOpportunity 117 References 118
4SuperlatticesforECEnergyConversion 129 HangYinandZongyouYin
4.1ECWaterSplitting 129
4.1.1ECHydrogenEvolutionReaction(HER) 129
4.1.2ECOxygenEvolutionReaction(OER) 132
4.1.3ECOverallWaterSplitting(OWS) 137
4.2ECCO2 ReductionReaction(CRR) 143
4.3ChallengeandOpportunity 145 References 145
5PolymorphicPhaseEngineeredStructures(PPESs)forEC
EnergyConversion 147
NasirUddin,ZiyangLu,andZongyouYin
5.1Introduction 147
5.2PPESforECWaterSplitting 148
5.2.1ECHydrogenEvolutionReaction(HER) 150
5.2.1.1EC-HERonSinglePhases 151
5.2.1.2EC-HERonHetero-phases 152
5.2.1.3EC-HERonMulti-phases 155
5.2.2ECOverallWaterSplitting(OWS) 156
5.2.2.1ECOWSonSinglePhases 157
5.2.2.2ECOWSonHetero-Phases 158
5.3PPESforECN2 ReductionReaction(NRR) 160
5.3.1ECNRRonCrystallinePhases 160
5.3.2ECNRRonAmorphousPhases 164
5.4ChallengeandOpportunity 166 References 167
6Rare-earthNanomaterialsforECEnergyConversion 171
TongWu,MingziSun,BolongHuang,andYapingDu
6.1RareEarthNanomaterialsforECReactions 171
6.1.1SimpleOxides 171
6.1.1.1ECN2 ReductionReaction(NRR)onY2 O3 Nanosheet 171
6.1.2AlloysandIntermetallics 174
6.1.2.1ECHydrogenEvolutionReaction(HER) 174
6.1.3Ce-dopingandCeO2 -incorporatedTransitionMetal-based Catalysts 180
6.1.3.1ECHydrogenEvolutionReaction(HER) 180
6.1.3.2ECOxygenEvolutionReaction(OER) 183
6.1.3.3ECN2 ReductionReactiononBi4 V2 O11 /CeO2 185
6.2ChallengeandOpportunity 187 References 188
PartIIEmergingNanomaterialsforPhotochemical(PC) EnergyConversion 191
72D-MaterialsFreeHeterostructuresforphotochemicalEnergy Conversion 193 WeiChenandGuohuaJia
7.12D-MaterialsFreeHeterostructures 193
7.1.1HeterostructureforPCWaterSplitting 198
7.1.1.1MetalChalcogenide/PhosphideSemiconductor–Semiconductor Heterostructure 198
7.1.1.2Oxide(TiO2 )Semiconductor–SemiconductorHeterostructure 200
7.1.1.3Non-nobleMetalsandSemiconductorHeterostructures 204
7.1.1.4OtherHeterostructures 204
7.1.2PCCO2 ReductionReaction 207
7.1.2.1TiO2 BasedSemiconductorHeterostructures 212
7.1.2.2Chalcogenide/OxideSemiconductor-BasedHeterostructures 212
7.1.2.3OtherHeterostructures 218
7.1.3PCN2 ReductionReaction 218
7.1.4ChallengeandOpportunity 221 References 222
8VanderWaalsHeterostructuresinPhotocatalyticEnergy Conversion 225
BikeshGupta,HanLi,JulieTournet,HarkH.Tan,ChennupatiJagadish, ShaowenCao,andSivaK.Karuturi
8.1Introduction 225
8.2Fabricationof2D/2DHeterostructures 226
8.2.1VerticalHeterostructures 226
8.2.1.1MechanicalExfoliationandTransfer 226
8.2.1.2ChemicalVaporDeposition 228
8.2.1.3Liquid-BasedTechniques 229
8.2.1.4Liquid-PhaseAssembly 229
8.2.1.5Layer-by-LayerAssembly 231
8.2.2LateralHeterostructures 234
8.32D/2DHeterostructuresforPhotocatalyticRedoxReactions 236
8.3.1WaterSplitting 239
8.3.2CO2 Reduction 243
8.3.3N2 Reduction 246
8.3.4FuelCells 248
8.4Mixed-DimensionalHeterostructuresforPhotocatalyticRedox Reaction 249
8.4.1WaterSplitting 249
8.4.2CO2 Reduction 253
8.4.3N2 Reduction 257
8.4.4FuelCells 260
8.5ChallengesandPerspectives 260
8.5.1PreciseControlovertheOptical,Electronic,andStructural Properties 261
8.5.2MechanisticUnderstandingofCatalysis 261
8.5.3CommercialApplications 261 Acknowledgments 262 References 262
9SuperlatticesforPCEnergyConversion 275 HangYinandZongyouYin
9.1PCWaterSplitting 275
9.1.1PCHydrogenEvolutionReaction(HER) 275
9.1.2PCOverallWaterSplitting(OWS) 279
9.2ChallengeandOpportunity 282 References 282
10PolymorphicPhaseEngineeredStructures(PPESs)forPC
EnergyConversion 285
NasirUddin,ZiyangLu,andZongyouYin
10.1PPESforPCWaterSplitting 285
10.1.1PCHydrogenEvolutionReaction(HER) 286
10.1.1.1PC-HERonSinglePhases 286
10.1.1.2PC-HERonHeterophases 288
10.1.1.3PC-HERonMultiphases 289
10.1.2PCOverallWaterSplitting(OWS) 290
10.1.2.1PCOWSonHeterophases 291
10.1.2.2PCOWSonMultiphases 292
10.2PPESforPCCO2 ReductionReaction(CRR) 294
10.2.1PCCRRonSinglePhases 295
10.2.2PCCRRonMultiphases 297
10.3PPESforPCN2 ReductionReaction(NRR) 300
10.3.1PC-NRRonSinglePhases 300
10.3.2PC-NRRonHeterophases 302
10.4ChallengeandOpportunity 303
References 304
11Rare-earthNanomaterialsforPCEnergyConversion 309 TongWu,MingziSun,BolongHuang,andYapingDu
11.1ComplexOxides 309
11.1.1Perovskites 309
11.1.1.1PC-HER 309
11.1.1.2PCCO2 ReductionReaction 311
11.1.2Tantalates 311
11.1.2.1PC-HERonK4 Ce2 Ta10 O ∼30 311
11.1.3Niobates 314
11.1.3.1PC-HERonH1 x Lax Ca2 x Nb3 O10 314
11.2Ce-BasedPhotocatalysts 317
11.2.1PC-HERonCdS/CeO2 317
11.2.2PC-NRRonCeO2 /FeS2 319
11.3ChallengeandOpportunity 321
References 321
12Non-noblePlasmonicEnhancement(NNPE)forPCEnergy Conversion 325 ChaoYangandShaowenCao
12.1Introduction 325
12.2NNPEWaterSplitting 326
12.2.1PCHydrogenEvolutionReaction(HER) 326
12.2.1.1PCHERonNon-nobleMetals 326
12.2.1.2PCHERonNon-nobleMetalOxides 327
12.2.1.3PCHERonOtherNon-nobleMetalCompounds 328
12.2.2PCOxygenEvolutionReaction(OER) 328
12.2.3PCOverallWaterSplitting(OWS) 330
x Contents
12.3NNPECO2 ReductionReaction(CRR) 331
12.3.1PCCRRonNon-nobleMetals 331
12.3.2PCCRRonNon-nobleMetalOxides 333
12.4NNPEN2 ReductionReaction(NRR) 335
12.4.1PCNRRonNon-nobleMetals 336
12.4.2PCNRRonNon-nobleMetalOxides 336
12.5ChallengeandOpportunity 337 References 338
PartIIIEmergingNanomaterialsforPhotoelectrochemical (PEC)EnergyConversion 341
132DMaterials-FreeHeterostructuresforPECEnergy Conversion 343
WeiChenandGuohuaJia
13.12DMaterials-FreeHeterostructures 343
13.1.1PECSystemforWaterSplitting 343
13.1.1.1HeterostructureasPECPhotocathodesforHER 345
13.1.1.2HeterostructureasaPECPhotoanodeforOER 348
13.1.2PECSystemforCO2 ReductionReaction 351
13.1.2.1HeterojunctionofSemiconductorsasaPECPhotocathodeforCO2 ReductionReaction 352
13.1.2.2Non-nobleCocatalystonSemiconductorasPECPhotocathodeforCO2 ReductionReaction 353
13.1.3PECSystemforN2 ReductionReaction(NRR) 355
13.1.4ChallengeandOpportunities 355 References 359
142D-Materials-basedHeterostructuresforPECEnergy Conversion 361
BikeshGupta,JulieTournet,HarkH.Tan,ChennupatiJagadish,and SivaK.Karuturi
14.1Introduction 361
14.1.1PhotoelectrochemicalDeviceConfigurations 362
14.1.2MaterialRequirementsforPhotoelectrocatalysis 363
14.2Rolesof2DMaterialsinPhotoelectrochemicalSystems 365
14.2.12DMaterialsasPhotoabsorbersandSensitizers 365
14.2.22DMaterialsasCocatalysts 366
14.2.32DMaterialsasElectronAcceptors/Transporters 366
14.2.42DMaterialsasProtectiveLayers 367
14.3HeterostructureBandEnergeticsattheInterface 367
14.42DMaterialsHeterostructuresforPhotoelectrocatalyticRedox Reactions 369
14.4.1WaterSplitting 369
14.4.1.10D/2DHeterostructures 369
14.4.1.21D/2DHeterostructures 373
14.4.1.32D/2DHeterostructures 374
14.4.1.43D/2DHeterostructures 376
14.4.2CO2 ReductionReaction 378
14.5ChallengesandOutlook 380 Acknowledgments 380 References 381
15PolymorphicPhaseEngineeredStructures(PPES)forPEC EnergyConversion 389 NasirUddinandZongyouYin
15.1Photoelectrochemical(PEC)EnergyConversion 389
15.1.1PolymorphicPhase-EngineeredStructures(PPES) 389
15.2PPESforPECOverallWaterSplitting(OWS) 389
15.2.1PEC-OWSonSinglePhases 390
15.2.2PEC-OWSonHetero-Phases 392
15.3PPESforPECNitrogenReductionReaction 394
15.4ChallengeandOpportunity 396 References 397
16Rare-earthNanomaterialsforPECEnergyConversion 399 TongWu,MingziSun,BolongHuang,andYapingDu
16.1ComplexOxides 399
16.1.1PECWaterSplitting 399
16.1.1.1Ln2 Ti2 O7 399
16.1.1.2Ln2 Zr2 O7 399
16.1.1.3Nd2 Sn2 O7 //Fe2 O3 400
16.2Ce-BasedPhotoelectrocatalysts 404
16.2.1PECWaterSplitting 404
16.2.1.1TiO2 /Ce 404
16.2.1.2CeO2 406
16.3ChallengeandOpportunity 409 References 409
17Non-NoblePlasmonEnhancement(NNPE)forPECEnergy Conversion 411 SandraSajiandZongyouYin
17.1NNPEforWaterSplitting 411
17.1.1WaterSplittingonNon-nobleMetals 411
17.1.1.1Ti 411
17.1.1.2Al 412
17.1.1.3Cu 416
17.1.1.4Bi 421
17.1.2WaterSplittingonNon-nobleCompounds 425
17.1.2.1WO3 x 425
17.2ChallengeandOpportunity 426 References 427
PartIVEmergingNanomaterialsforPhotovoltaic(PV) EnergyConversion 429
182D-MaterialsFreeHeterostructuresforPhotovoltaicEnergy Conversion 431 WeiChenandGuohuaJia
192D-Materials-basedHeterostructuresforPVEnergy Conversion 449 ChunH.Mak,Jung-HoYun,HoiY.Chung,YunH.Ng,andHsien-YiHsu
20Perovskite–SiTandemSolarCells 481 DishengYaoandHongxiaWang
21III–VCompoundSemiconductorNanowireSolarCells 531 ZiyuanLi,HarkH.Tan,ChennupatiJagadish,andLanFu
22Rare-EarthNanomaterialsforPVEnergyConversion 559 TongWu,MingziSun,BolongHuang,andYapingDu
23Non-noblePlasmonEnhancement(NNPE)forPVEnergy Conversion 581 Jung-HoYun,ChunHongMak,Hsien-YiHsu,andYunHauNg
PartVClustersforEnergyConversion 611
24ElectrochemicalEnergyConversionwithClusters 613 ZhengqingLiu,SandraE.Saji,andZongyouYin
25PhotochemicalEnergyConversionwithClusters 655 XiaoshanZhang,SandraE.Saji,andZongyouYin
26PhotoelectrochemicalEnergyConversionwithClusters 695 KailiLiuandZongyouYin
PartVISingleAtomsforEnergyConversion 719
27ElectrochemicalEnergyConversionwithSingleAtoms 721 PeilongLu,SandraE.Saji,HaitaoZhao,andZongyouYin
28PhotochemicalEnergyConversionwithSingleAtoms 773
HaijiaoLuandZongyouYin
29Photoelectrochemical(PEC)EnergyConversionwith SingleAtoms 787
MahmoudM.AbdelnabyandZongyouYin
30FuturePerspectives 815
ZongyouYin
Index 817
Introduction
ZongyouYin
ResearchSchoolofChemistry,AustralianNationalUniversity,Canberra,ACT2601,Australia
Therapidincreaseinpopulationandeconomicgrowthbringsariseinenergy demand,whichstillmainlyreliesonnaturalfossilfuels,suchaspetroleum, naturalgases,coals,andoils,andaccountsforover70%ofthetotalprimaryenergy supply.Unfortunately,thisresultsingreenhousegasemissionstotheatmosphere. ThecurrentlyavailablecleanH2 energytechnologyforemissionreductionstill reliesonfossilfuelstoproduceH2 fuel,primarilythroughsteammethanereformingathightemperatures.However,itbringswiththeconcernsofrequiringcostly high-temperaturematerialsandemittinggreenhousegas.Aswidelyknown,the greenhousegasesinducedbyglobalwarmingmayseriouslythreatenlow-lying coastalislandsandcitiesbyrisingsealevelsandradicallydamaginghomesby extremeweatherevents,causingtragichabitatandeconomicloss.Additionally, afterseawateradsorption,greenhousegaseswillaccelerateoceanacidification, threatencalcifyingspeciesinoceans,andbreakmarinefoodchains,destroying manyrelatedjobsandeconomiesintheworld.Therefore,advancingmaterialsscienceandmanufacturinganddevelopingsustainablesolutionstoaddresstheenergy crisisandtheglobalwarmingissueareimportanttoglobalresearchatthecurrent stage,especiallyundertheimpetusoftheworldimplementingtheParisAgreement. Inthisbook,multipleviewsfromdifferentanglesweretakentopresentanddiscusstheup-to-datecriticalmaterialsscienceandcatalytictechnologiesforenergy conversion.
1.Fourtypesofheavily-researchedenergyconversionprocesses/technologies,i.e. electrochemistry,photochemistry,photoelectrochemistry,andphotovoltaics,are encompassed.Theyaresystematicallycategorizedinchaptersandintegrated withdifferentbutlogicalcontentrelatedtomaterialsscienceandengineeringto befurtheraddressed.
2.Foreachenergyconversiontechnology,differentdimensionalnanomaterials, i.e.zero-dimension(0D),one-dimension(1D),two-dimension(2D),threedimension(3D),clusters,andsingleatoms,areincludedandevaluatedbased ontheiruniquedimension-inducedinteriorproperties,andtheirmeritsvs. demeritsinapplications.
AtomicandNanoScaleMaterialsforAdvancedEnergyConversion,FirstEdition.EditedbyZongyouYin. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
3.Polymorphicphasesofsolidmaterials,suchascrystalstructuralphases,singlephase,heterophases,andmulti-phases,arediscussedespeciallyfortheemerging 2Dmaterialsfromtheviewofphaseformation,control/tuning,interfacing,and impactsonfunctionalities.
4.Therearevariousfunctionalstructures,2D-freeheterojunctions,and2D-based heterostructures,including2D–0D,2D–1D,2D–2D,and2D–3Dheterostructures, periodictandem,andsuperlatticestructurescombinedintodifferentenergyconversiontechnologiesbasedontheireffectsonenergybandprofiles,chargetransportbehavior,andtheperformance.
5.Acompletecomparisonfrommaterials,synthesis,reactionconditionsofenergy conversion,energyconversionefficiency,andperformancestabilityhasbeen madeandpresentedintablesofthechapters,providingreaderswithastraightforwardconvenientreferencefortheirresearchand/orstudy.
Thisbook’sexpectedaudiencesincluderesearchers(Master/PhDdegreecandidates,postdoctoralfellows,researchscientists,andacademicstaff)inacademiaand academicR&Dresearchersandindustrialtechniqueconsultantsandengineersof therelatedfields.Theaudienceswithanoccasionalneedforthisbookareuniversity lecturers,postgraduates,undergraduates,andpractitionersfromeducationinstitutionsandgovernmentfunding-policy-makingofficialsfrompublicservicesofthe relatedfields.
Withbroad,significanttopiccoverageinenergyconversion,ourproposedbookis expectedtobringthefollowingbenefitstothereaders:
1.Thebrand-newforefrontnano-to-atomicmaterialsscienceandknowledge enableresearcherstocarryoutfurtherscientificexploration.
2.EmergingenergyconversionstrategiesasthebasisenableR&Dscientists/ engineerstodevelopmodernpracticalenergytechnologies.
3.Up-to-dateenergyconversionscienceandtechnologyprovidelecturers/learners withstate-of-the-artclassroomknowledge.
4.Thefirst-handscienceandtechnologydatainformationhelpsgovernmentofficialstocreatesustainablefundingpoliciesfortheircountries.
EmergingNanomaterialsforElectrochemical(EC)Energy Conversion
2D-Materials-FreeHeterostructuresforECEnergy Conversion
KamranDastafkanandChuanZhao
TheUniversityofNewSouthWales,SchoolofChemistry,AnzacParade,Kensington,Sydney,NSW2052, Australia
2.1HeterostructuresforElectrochemicalWaterSplitting
Electrochemicalwatersplittingislargelyconsideredahighlyefficientprotocol forenergyconversion.Theinvolvedhalfreactionsofoxygenevolutionreaction (OER)andhydrogenevolutionreaction(HER),respectively,occurringatanode andcathodearemulti-electrontransferprocesseswithsluggishchargetransfer kinetics.Therefore,anelectrocatalystisusedattheelectrodetodecreaseOERand HERenergybarriersattheelectrode–electrolyteinterfaceandincreasetheFaradaic chargetransferrate[1].Avastvarietyofelectrocatalyst(nano)materialshavebeen developedtodate.However,thechallengessuchashighenergybarrierofcatalyticallyactivesites,lowsurfacechargetransfer,slowinterfacialmasstransport,and poorintrinsicactivityandelectronconductivitystillremain[2].Heterostructured nanomaterials,consistingoftwoormoreinterfacedcatalyticallyactivematerials, havebeenincreasinglydevelopedashighlyefficientelectrocatalystsforenergy conversionprocesses.Alternatively,thesematerialscanbeconsistedofoneelectrochemicallyactivephase,providingthereactionactivesites,andsupportivephase(s), whichaugmenttheredoxandstructuralpropertiesofthemainactivematerial [3].Inthissection,weintroducevariousclassesofmetal-basedheterostructured catalystswithregardtodifferentstructuresofelectrocatalyticallyactivephase, includingmulticomponentheterostructures,forelectrochemicalwatersplitting reactions.Therecentadvancementsontheheterointerfacesinheterostructured electrocatalystsarereviewed,asshowninTable2.1.
2.1.1MetalOxide-BasedHeterostructures
Thesurfacechemistryandelectrocatalyticperformanceoftransitionmetaloxides (TMOs)arehinderedbytheexistenceofasignificantelectricalresistance,which limitsthechargetransferforthesurfaceFaradaicprocess.ConstructingheterojunctionswithotherTMOsisaneffectiveapproachforoptimizingthecatalytic AtomicandNanoScaleMaterialsforAdvancedEnergyConversion,FirstEdition.EditedbyZongyouYin. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
22D-Materials-FreeHeterostructuresforECEnergyConversion
Table2.1 Recentadvancesinnonpreciousmetal-basednano-heterostructuredelectrocatalystsfor electrochemicalwatersplittingreactions(OERandHER).
HeterointerfacesElectrocatalystApplication
Experimental conditionsResultsReferences
MetaloxidesCuCo@CuCoOx HERSupport:NFa)
Electrolyte: KOH1M Scanrate:2b)
OER
OWSe)
Co3 O4 @CeO2
OERElectrode:RDEh)
Electrolyte: O2 -saturated KOH1M
Scanrate:5
�� 10 :112mVc)
b:55mVdec 1 d) [4]
�� 10 :190mV
b:88mVdec 1
CV10 :1.53Vf)
S:100h(at 0.15V)g)
�� 10 :270mV
b:60mVdec 1
S:10h(at1.6V) [5]
Metal hydroxides
NiFeLDHs@NiOOERElectrode:NF
Electrolyte: KOH1M Scanrate:5
Ni-BDC@Ni(OH)2 OERElectrode:GCEi)
Electrolyte: KOH1M
Scanrate:1
MetalpnictidesFeP@Ni2 PHERElectrode:NF
Electrolyte: KOH1M Scanrate:1
OER
�� 10 :210mV
b:72mVdec 1
S:50h(at50and 100mAcm 2 ) [6]
�� 10 :320mV
b:41mVdec 1
S:20h [7]
�� 10 :14mV
b:22.7mVdec 1 [8]
�� 10 :154mV
b:24.2mVdec 1
OWSCV10 :1.42V
S:40h(at 100mAcm 2 )
MoP@Ni2 PHERElectrode:NF
Electrolyte: O2 -saturated KOH1M
OER
�� 10 :75mV
b:100.2mVdec 1
Scanrate:5 [9]
�� 20 :309mVj)
b:77.6mVdec 1
Scanrate:1
OWSCV10 :1.55V
S:15h
Table2.1 (Continued)
2.1HeterostructuresforElectrochemicalWaterSplitting
HeterointerfacesElectrocatalystApplication
Experimental conditionsResultsReferences
Co@Ni3 NHERElectrode:CCk)
Electrolyte: N2 -saturated KOH1M
OER
Scanrate:2
Metal chalcogenides
Ni3 S2 @MoS2
HERElectrode:CC
Electrolyte:
O2 -saturated KOH1M
Scanrate:5
CoSe2 @MoSe2 HERElectrode:GCE
Electrolyte: N2 -saturated KOH1M
Scanrate:5
MoSSe@NiSe2 HERElectrode:NF
Electrolyte:
H2 SO4 0.5M
Scanrate:0.5
�� 10 :194mV
b:156mVdec 1
S:24h [10]
�� 10 :307mV
b:57mVdec 1
S:24h
�� 10 :173mV
b:39mVdec 1
S:48h (100mAcm 2 ) [11]
�� 10 :218mV
b:76mVdec 1 [12]
�� 10 :69mV
b:42.1mVdec 1 [13]
Metal/CarbonNi2 B@g-C3 N4
HERElectrode:GCE
Electrolyte:KOH 1M
Scanrate:2
Co2 P@WC@NCHERElectrode:GCE
Electrolyte:
H2 SO4 0.5M
Scanrate:5
Mixed-phase metallic
c-CoP@a-CoOx HERElectrode:CC
Electrolyte:KOH 1M
Scanrate:5
OER
�� 10 :707mV
b:221mVdec 1 [14]
�� 10 :91mV
b:40mVdec 1
S:50h(at 120mV) [15]
�� 10 :132mV
b:89mVdec 1
S:24h [3]
�� 10 :232mV
b:67mVdec 1
S:24h
OWSCV10 :1.66V
S:30h (continued)
Table2.1 (Continued)
HeterointerfacesElectrocatalystApplication
Ni3 S2 @Ni-Fe-OHOERElectrode:NF
Electrolyte:KOH 1M
Scanrate:0.5 �� 10 :165mV b:93mVdec 1 S:50h (100mAcm 2 )
a)Nifoam.
b)InmVs 1
c)Overpotential.
d)Tafelslope.
e)Overallwatersplitting.
f)Cellvoltage.
g)Stability.
h)Rotatingdiskelectrode.
i)Glassycarbonelectrode.
j)Overpotentialat20mAcm 2
k)Carboncloth.
activityofsingleTMOstowardOERandHER.Intherecentyears,developing Mott–Schottky-typenanohybridsofnon-noblemetalsandsemiconductoroxides hasbeenawell-practicedstrategytomakehighlyefficientheterostructureswith optimalintrinsicelectrocatalyticactivity[17].
Houetal.preparedpromotedactivesitesincore–shellnanowirearraysof bimetallicCuCo-andCuCoOx -mixedoxidessupportedbynitrogen-dopedcarbon (CuCo@CuCoOx @NC)asaMott–Schottkyhybridcatalystforhighlyefficientand stableoverallwatersplitting[4].Thereportedmetal–semiconductornanohybrid consistedofCuConanoalloysanddefectiveCuCoOx phases,synthesizedasa conductivecore–shellarchitecturewithnanowiremorphology,andwasshown effectivetosupplyacontinuouselectrontransportbetweenmetallicandsemiconductorphases.CuCo@CuCoOx @NCheterostructuredepictedalowoverpotential of112mVandTafelslopeof55mVdec 1 forHERandalowoverpotentialof 190mVforOER,bothatacurrentdensityof10mAcm 2 .Furthermore,alowcell voltageof1.53Vwasneededtooperatefullwatersplittingtoreach10mAcm 2 withasubstantialstabilityof100hours.Theachievedhighsurfaceconductivity andtheenlargedactivesurfaceareawithabundantactivesitesandacontinuous electrontransportremarkingtheefficientactivitywereinducedbyasynergisticeffectemergingbetweennanoalloymetallic,defectivemixedoxide,and heteroatom-dopedcarbonaceousphases.
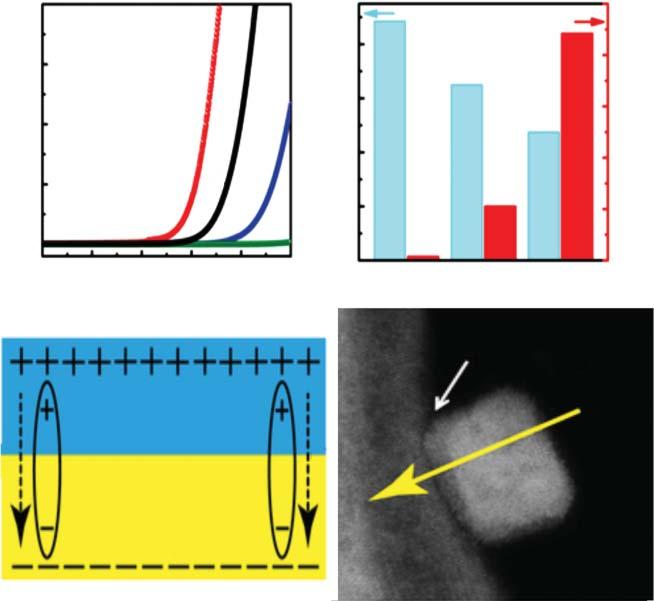
Liuetal.[5]reportedCo3 O4 @CeO2 nanohybridsmadebyanelectrongasand oxygenvacancyapproachforadvancedOER.Bothmetaloxideswereselectedbased onintrinsicpropertiestoinduceahighconcentrationofoxygenvacanciesandan electron-gasbehavioronCe3+ centersattheCo3 O4 –CeO2 interface.Theobserved electron-gasfeatureactsatransportchannelallowingahighchargecarrierdensity andagoodelectronconductivityforthenanohybridcatalyst.Achievingnanocube andnanosheetmorphologiesforCeO2 andCo3 O4 phases,respectively,ledtoa
(a) Co3O4/CeO2
(b) IrO2
(c) Co3O4 (d) CeO2
Figure2.1 (A)PolarizationcurvesofCo3 O4 ,CeO2 ,andCo3 O4 @CeO2 nanohybridand commercialIrO2 catalystsforOER.(B)Comparisonsoftheobtainedoverpotentialsofata currentdensityof10mAcm 2 andtherecordedcurrentdensitiesatapotentialof1.6V. (C)SchematicrepresentationoftheinterfacialelectronicstructureinCo3 O4 @CeO2 nanohybrid.(D)High-angleannulardark-field(HAADF).ImageofCo3 O4 @CeO2 nanohybrid. Source:ReprintedwithpermissionfromLiuetal.[5].Copyright2019,Wiley.
remarkableOERperformancewithalowoverpotentialof270mVatacurrent densityof10mAcm 2 andahighturnoverfrequency(TOF)of0.25s 1 compared withpureCeO2 andCo3 O4 phasesandalsooutperformedthebenchmarkIrO2 catalyst(Figure2.1).
2.1.2MetalHydroxide-BasedHeterostructures
Transitionmetal-basedhydroxides(TMOHs)arecommonlyknownashighlyactive materialsforanodicOERprocessofwaterelectrolysis.Currently,NiFe-based binarysystemsincludingNiFe-mixedhydroxides(NiFe(OH)x )andNiFe-layered doublehydroxides(NiFeLDHs)arerecognizedasnoble-metal-freebenchmark OER-catalyzingheterogeneouscatalysts[18].Intherecentyears,tremendous effortshavebeendedicatedtoincreasetheefficiencyofTMOHcatalystbydifferent strategies,i.e.precise/systematicmetaldoping,ternaryhydroxideformationwith
anothertransitionmetal,surfaceactivesitemodification,andheterostructuring [19].
Sirisomboonchaietal.fabricatedcore–shellheterostructurecatalystsconsisting ofbinaryNiFeLDHsdecoratedonhierarchicalNiOmicroflakes(NiFeLDHs@NiO) throughatwo-stephydrothermal/post-thermalannealingprocedureforefficient OER[6].Thedevelopedarchitectureachievedalowoverpotentialof265mV affording10mAcm 2 currentdensityduetothewell-establishedcomposite microstructurewithstronginterfaceconnectionandinterfacialinteractionsthat facilitatedelectrontransferbetweencatalystsurfaceandNifoamascurrentcollector.Atthesametime,porouscore–shellarraymorphologysubstantiallyimproved thecontactsurfacebetweencompositecatalystandhydroxylreactantsinthe alkalineKOHelectrolyte,wheretheinterlayerNiOphasebetweenNiFeLDHsand NifoamsubstrateactsasamediatorforeffectiveelectrontransporttoNifoam.The outerultrathinNifeLDHnanosheetsalsowerewell-wrappedoverNiOmicroflakes, maximizingtheactivesiteexposuretohydroxylspecies.Moreover,theobserved apparentactivityofNiFeLDHs@NiOwasevenfurtheredthroughinsituintercalatingNiFeLDHnanoarraysinformamideorganicsolventat30 ∘ Cforjustfive minutesthroughanultrasoundtreatment.Thisresultedinpromotedionictransport andgasdiffusionchannelswithintheLDHslabs.ThetreatedNiFeLDHs@NiO heterostructurethenexhibitedstablehighcurrentdensitiesof50and100mAcm 2 for50hoursandasignificantlyreducedoverpotentialfor10mAcm 2 to210mV. ItwasdiscussedthatNiOintheintercalatedcompositecatalysteffectivelyfacilitatedthecleavageofHO—Hbondandthesubsequentconversionoftheadsorbed OHspeciestomolecularoxygen.NiFeLDHswithextendedinterlayerspacesalso suppliedabundantexposedactivesitesandhighlyincreasedmasstransferroutes foriondiffusion/electrontransferatthecatalyst–electrolyteinterface.
Zhuetal.[7]describedtheutilizationofaheterostructuredNi-basedmetal–organicframework(Ni–MOF)andNihydroxideforefficientOER,owingtothe inherentfeaturesofextendedsurfacewithcoordinativelyunsaturatedmetalspecies andfastmasstransportofMOFsandinnateelectrocatalyticactivityofNi(OH)2 . OnecommonproblemofusingMOFsascatalystmaterialsistheirrelatively fastautonomousaggregationratethatcanbeobviatedthroughtheformationof nano-heterointerfaceswithTMOsandTMOHs.ThedevelopedNi-BDC@Ni(OH)2 (BDC:1,4-benzenedicarboxylate,C8 H4 O4 )hybridnanosheetsweresynthesized byasonication-assistedsolutionmethod.WhilethelargesurfaceareaofNi-BDC MOFwasmaintained,couplingwithNi(OH)2 significantlyimprovedtheelectronic structureofNiatomsinNi(OH)2 phase,whicheffectivelyresultedinahigh densityofNisiteswithhighoxidationstatesasOERmainactivesites.Asaresult, Ni-BDC@Ni(OH)2 revealed5.5and20.6timeshigheractivity(82.5mAcm 2 )at 1.6Vvs.reversiblehydrogenelectrode(RHE)thanthoseofuncoupledNi-BDC (15.1mAcm 2 )andNi(OH)2 (4.0mAcm 2 ),respectively.
2.1.3MetalPnictide-BasedHeterostructures
Coordinatingtransitionmetalphosphides(TMPs)ornitrideswitheachotheror othertransitionmetalsinonestructurehasbeennotedasarationaldesignstrategy
2.1HeterostructuresforElectrochemicalWaterSplitting 11 thatsuppliesaugmenteddensityofthesurfaceactivesitesandinterfacialelectron conductivity[20].Inparticular,bimetallicTMPshavebeenstudiedinmuchdetails, owingtotheirsuperiorperformancewithrespecttomonometallicphosphide catalysts[21].Phosphorousatomiswidelyrecognizedasanefficiententityforthe non-noblemetalcatalystsforalkalineHER,mostlyregardingitsinnatecharacteristicasagoodprotonacceptorthatcanattenuatethemetal–hydrogenbonding strengthandacceleratethehydrogendesorptionduringtheHER[22].
Yuetal.synthesizedporoushybridiron–di-nickelphosphides(FeP@Ni2 P)supportedoncommercialNifoamasahigh-performancebifunctionalmaterialforoverallwatersplittinginalkalinemedia[8].Thishybridwashighlyefficienttowardboth OERandHERwithaPt-likeperformance( 14mVoverpotentialat 10mAcm 2 ) andasuperioractivitythanIrO2 (154mVoverpotentialat10mAcm 2 ).Asabifunctionalcatalyst,FeP@Ni2 Prequiredonly1.42Vcellvoltagetodeliver10mAcm 2 and1.72Vachievingapracticalcurrentdensityof500mAcm 2 forindustrial considerations.Excellentdurabilityof40hoursat500mAcm 2 corroboratedthis bifunctionalhybridcatalyst,outperformingthecurrentindustrialbenchmark materialsthatrequire2.40Vtoproduce400mAcm 2 .
Duetal.[9]preparedhierarchicalMoP@Ni2 PheterostructuresonNifoamsubstrateasabifunctionalcatalystforoperatingHERandOER.Throughaninsitucontrollablehydrothermal-phosphidationprocess,MoP@Ni2 Pwasmadewithoptimal electrocatalyticpropertiesemergingfromtheiruniquehierarchicalheterostructures andcollaborativeinteractionsofthebimetallicphosphides.TheproposedbifunctionalMoP@Ni2 P@NFelectroderequiredalowcellvoltageof1.55Vtodelivera currentdensityof10mAcm 2 inalkalinemedia(Figure2.2).
Zhuetal.[10]reportedinsitugrownepitaxialheterojunctionaseffectivestrategy tomakehigh-performancenitride-basedmaterialsforelectrochemicalwatersplitting.ArraysofCo@Ni3 Nnanowireswerepreparedbyanatomicepitaxialin-growth methodwithananoconfinementeffecttoaugmenttheinterfaceproperties.Surface nano-heterojunctionengineeringandfine-tuningtheinterfaceelectronicstructure wereallcarriedoutbyathermalannealingprocessonNiCo2 O4 spinelprecursor underoptimizedconditionswithaprecisecontrolovermorphology.Thisinsitu in-growthstrategyatnanometricscalepromotedtheelectrontransferbetweentwo differentdomainsoftheepitaxialinterfaceandresultedinasignificantincreasein theintrinsicactivityforbothHERandOER.Almost15and19timeslargerelectrocatalyticTOFswereachievedforthehybridCo@Ni3 Nnanowireswithrespect tothepureNi3 NnanorodsforHERandOER,respectively.Thenanoconfinement effectbetweenmetallicCoandNi3 Nnanowirescausedelectronicbindingenergy shiftandhighlyboostedtheFaradaicchargetransferacrosstheCo–Ni3 Ninterface.
2.1.4MetalChalcogenide-BasedHeterostructures
Stronginterfacialinteractionsandelectroniccouplingeffecthavealsobeen observedwhenhybridizingtwoormoretransitionmetalchalcogenides,i.e.sulfides,selenides,andtellurides[23].Suchchalcogenide-basedheterocatalystscan furtherimprovetheelectrocatalyticperformanceofindividualmetalchalcogenides orthecorrespondingmetalliccatalyststhroughoptimizingelectronicstructureat
Figure2.2 (a)SchematicillustrationofthesynthesisofMoP@Ni2 Pheterostructures. (b)Scanningelectronmicroscopy(SEM)and(c)HRTEMimagesofMoP@Ni2 P heterostructures.Comparisonoflinearsweepvoltammetry(LSV)polarizationcurvesof MoP@Ni2 Pheterostructuresfor(d)OERand(e)HER.Source:Reprintedwithpermission fromDuetal.[9].Copyright2017,RoyalSocietyofChemistry.
theirinterfacewhileatthesametimebenefitingfromvariousmorphologiesofthe coupledphases.
Wangetal.[11]describedanasymmetricelectrodedesignforalkalinewatersplittingconsistingofbimetallicdisulfideheterostructuresascathode(Ni3 S2 @MoS2 ) andthesameheterostructurehybridizedwithasuperficialamorphousbimetallichydroxidephaseasanode(Ni3 S2 @MoS2 @NiFe(OH)x )tooperateefficient HERandOERreactions,respectively.Anoptimizedhydro/solvothermalprocess
2.1HeterostructuresforElectrochemicalWaterSplitting 13 assistedbyanelectrodepositionstepwasadaptedtoallowtheinsitugrowthofthe (bi)-transitionmetaldisulfides(TMDSs)andmixedhydroxides.Theobtainedcathodeandanodeexhibitedafirst-rateactivityandpromotedelectrolysisdurability. Thecathode,Ni3 S2 @MoS2 ,showedanoverpotentialof173mVat 100mAcm 2 forHER,andtheanode,Ni3 S2 @MoS2 @NiFe(OH)x ,demonstratedanoverpotential of309mVatthesamecurrentdensity.TightinterstratificationamongtheTMDS nanosheetarraysandtheamorphousbimetallichydroxidesresultedinaugmented intermediate(OH /H+ )adsorption,electronconductivity,chargetransfer,and enhancedsurfaceactivesites.Atwo-electrodealkalinewaterelectrolyzerwas developedbytheproposedcathodeandanode,whichexhibitedlowoverallcell voltagesof1.55and1.71Vtodelivercurrentdensitiesof10and100mAcm 2 , respectively.
Zhaoetal.[12]synthesizedCo,Mo-basedtransitionmetaldiselenide(TMDSe) heterostructureswithdensewateradsorption/dissociationactivesitesforefficient alkalineHER.Usually,TMDSematerialsaresuitableelectrocatalystsforHERin acidicmediabutprovideweakperformanceinalkalineelectrolytes,becauseof thehighlysluggishwaterdissociationkinetics.Theproposedheterostructurewith CoSe2 quantumdotsanchoredonMoSe2 nanosheets(CoSe2 @MoSe2 )increased thewateradsorptionsitesduetotheCoSe2 incorporationonthebasalplanesof MoSe2 topromotewaterdissociation.Asaresult,theCoSe2 @MoSe2 heterostructureexhibitedadvancedHERin1MKOHwithanoverpotentialof218mVto produceacurrentdensityof10mAcm 2 ,whichismorethan100mVandnearly 200mVsmallerthantherequiredoverpotentialvaluesforsingleCoSe2 andMoSe2 phases,respectively.Itwassuggestedthatthewateradsorptionandthesubsequent dissociationprocesswereexpeditedsignificantlyduetotheenrichededgesite CoSe2 phaseandthepopulatedadsorptioncombinationsitesforH*intermediate onMoSe2 phase.
Zhouetal.reportedacceleratedHERactivityandkineticsonaternarychalcogenidestructureconsistingofMo-mixedsulfoselenideparticlescombinedwitha self-supportingporousNidiselenide[13].Throughafaciletwo-stepselenization processatmoderatetemperature(600 ∘ C)withsubsequentinsitugrowthof MoSSeverticallayers,theternaryMoSSe@NiSe2 catalystwassynthesizedon three-dimensional(3D)Nifoamscaffold.TheadvantagesoftheTMDSedeveloped heterostructurearehighconductivity,double-gyroidstructure(3D,porous,and largedensityofexposededgesites),andtheemergenceofcatalyticsynergybetween theTMDSephases.Asaresult,alowoverpotentialof69mVwasobtainedtoachieve acurrentdensityof 10mAcm 2 in0.5MH2 SO4 electrolytecomparedwiththe highoverpotentialsof118and153mVneededforthebinaryMoS2 phaseonNiSe2 self-supportedfoamandthesingleNiSe2 foam,respectively.AlowTafelslopeof 42.1mVdec 1 exhibitedadvancedTafelmechanismforhydrogendesorptionon MoSSe@NiSe2 heterostructureduringHERthatwasmuchlowerthanTafelslopes ofbinaryMoS2 (58.5mVdec 1 )andpristineNiSe2 (46.4mVdec 1 )phases.Also,no obviousdegradationofcathodiccurrentdensitieswasobservedforthedeveloped TMDSeheterostructureafter1000continuousCVcycles(Figure2.3).
Nise2
MoS2/Nise2 foam MoS2(1–x)Se2x/Nise2
Figure2.3 (a)SchematicgrowingprocessofternaryMoS2(1 x ) Se2x particlesonporous NiSe2 foam.(b,c)TypicalSEMandtransmissionelectronmicroscopy(TEM)imagesofthe grownternaryMoS2(1 x ) Se2x particlesonporousNiSe2 foam.(Scalebar:SEM,1 μm;TEM, 5nm.)(d)ComparisonofthepolarizationcurvesofMoS2(1 x ) Se2x @NiSe2 andMoS2 /NiSe2 hybridcatalystswithpureNiSe2 andPtwireelectrodesforHERand(e)thecorresponding Tafelplots.(f)Polarizationcurvesoftheas-preparedternaryMoS2(1 x ) Se2x @NiSe2 hybrid electrodesafter1000CVcyclesandafterthestabilitytest.(g)Extracted C dl valuesforpure NiSe2 ,MoS2(1 x ) Se2x @NiSe2 ,andMoS2 /NiSe2 hybridcatalysts.Source:Reprintedwith permissionfromZhouetal.[13].Copyright2016,Naturepublications.
2.1.5Metal/Carbon-BasedHeterostructures
Asamainchallengefortheelectrocatalyticwatersplitting,multi-electronand multi-chargetransferprocesseswithslowkinetics,hindertheefficiencyofHER andOERreactions.Totacklethisissue,aninterestingapproachistodesignnew (multi)phasearchitectureswithexposedactivesitesandacceleratedchargetransfer kinetics[18e,24].Thediffusionpathforionicreactants,i.e.OH /H+ foralkaline/acidicwatersplitting,respectively,canbeshortenedusingone-dimensional buildingblocksforconstructing3Dhetero-hierarchicalstructures[25].Thecontact betweentheexposedsurfaceandelectrolytecanbelargelyincreasedtoafford superiorFaradaicelectrontransfer.Forexample,asabinaryTMO,spinelcopper cobaltitehasintrinsicallypromisingelectrocatalyticactivityandratecapabilitythat arisefrominter-metallicsynergybetweenCuandCo.WhenhybridizingCuCoO4 withcarbonquantumdots(CuCoO4 @CQDs),therestrictionsoflowelectrical conductivityandperformancerobustnessweresignificantlyresolved[26].The surfaceofcarbonquantumdots(CQDs)withrichnegativechargesfacilitatesthe electrostaticinteractionswithTMOswithpositivelychargedsurface.
Caoetal.showedtheenhancementinHERactivitybyestablishingheterojunctionbetweenNiborideandgraphiticcarbonnitride(Ni2 B@g-C3 N4 )[14]. Particularly,owingtoitsfunctionasagoodsemiconductor,g-C3 N4 isusedinthe energyconversionresearch.ThroughafacileprocessofdirectlyheatingtheN-rich ureaasprecursorinasemi-closedenvironmentandpost-thermalcalcination, Ni2 Bnanoparticleswereencapsulatedintog-C3 N4 bulklayers.Asaresult,highly enlargedspecificsurfacearea,exfoliationoftheselayers,andpromotedelectronic conductivitywereachievedbyheterostructuringthesemetal/carbonphases. Thepreparedcompositecatalystdemonstratedanimprovedonsetoverpotential of300mV,aTafelslopeof221mVdec 1 ,andanoverpotentialof707mVat 10mAcm 2 withrespecttotheoriginalg-C3 N4 material.
Gaoetal.constructedanano-heterojunctionbetweencobaltphosphideand tungstencarbidestructuresthatwerecoveredwithnitrogen-dopedcarbonthrough aone-stepannealingtreatmentofthecorrespondingpolyoxometalatestructuresNa9 (NH4 )5 [{(B-α-PW9 O34 )Co3 (OH)(H2 O)2 (Ale)}2 Co] 35H2 O(Co7 P6 W18 )and dicyandiamide(DCA).Also,theydemonstratedahighlyefficientHERperformancefortheresultedheterostructure(Co2 P@WC@NC)[15].Conductingcobalt phosphorizationandtungstencarbonizationstepsatthesametimeinaconfined spacemadeanisolatedCo2 P@WCnano-heterojunctionphase.Thedeveloped heterostructureexhibitedadvancedhydrogenelectrocatalysiswithasmalloverpotentialof91mVtoaffordacurrentdensityof 10mAcm 2 inacidicmedia, asmallTafelslopeof40mVdec 1 (remarkingaTafelmechanisticpathwayfor themolecularhydrogendesorptionfromthecatalystsurface),andanexcellent long-termstabilityfor50hoursatanoverpotentialof120mV.Itwasrevealedthat thefacilitatedHERkineticsensuedfromtheemergingsynergisticinteractions betweenCo2 P,WC,andpyridinicNCphasesintheheterostructure.Theproposed hybridcatalystalsoshowedfavorableHERperformancesinothermediawith neutralandalkalinepHvalues.
2.1.6Mixed-PhaseMetallicHeterostructures
Heterostructuringwithtenaciousinterfacialinteractionsbetweendifferentstructuresoftransitionmetal-basedcatalysts,i.e.oxides,hydroxides,chalcogenides,pnictides,andalsoconductive(nano)carbons,isalsoaviableapproachtoenablecatalytic synergyandtoimproveelectrontransferkineticsamongtheseOER-andHER-active materials[27].
Yuetal.synthesizedcrystallineCoPnanoparticlesembeddedinamorphousCoOx nanoplatesthroughacombinedsolvothermallow-temperaturephosphidationprocedure[3].Thedesignedc-CoP@a-CoOx hybridcatalystpresentedaremarkable bifunctionalperformanceforalkalinewatersplitting,andtheauthorsattributedthis bifunctionalitytotheestablishedcatalyticsynergybetweenCophosphideandoxide phases.ThecouplingofcrystallineCoPandamorphousCoOx phasesconstructed ahybridmatrixwithwell-formedphaseboundaryandanelectronicallyimproved nanointerfacewithsynergisticeffectbetweentheCophases.Asaresult,theproposedCo-basedheterostructureshowedadvancedOERandHERactivitiescomparedwiththecorrespondingbenchmark,IrO2 andPt/Ccatalysts.Inaddition,an efficientandstableoverallwatersplittingperformancewasachievedbyapplyingthe c-CoP@a-CoOx heterostructureasbothanodeandcathodeinalkalinemediawhere alowcellvoltageof1.66Vwasobtainedtodeliveracurrentdensityof10mAcm 2 withgoodstabilityfor30hours.
Zhangetal.[28]constructedMophosphide-multiwalledcarbonnanotubes (MoP@MWCNT)hybridcatalystwithefficientHERactivityovertheentirepH rangeinelectrolytes.WhileMoPisgenerallyrecognizedforitsintrinsicHER activity,makingthewell-formedMoPfinenanostructureswithfullyexposed edgesites,incontrasttothedominantbasalsites,alongwithenlargedsurface activesitesisachallengingtask.However,hybridizingthecrystallineMoPwith carbonaceousphasessuchascarbonnanotubes(CNTs)isafeasibleapproach toincreasetheintrinsicactivityofMoP.Atwo-stepsynthesisprocedureinvolvingwetchemicalroutecoupledwithgas–solidphosphorizationwasutilizedto transformtheMoOx (OH)y @MWCNTintermediatestructuretoMoP@MWCNT catalyst.TheproposedhybridstructurecontainsfineMoPnanoparticles,uniformly distributedonthesidewallsofCNTsandexhibitinghighlyactiveandstableHER performanceinallpH-valuemedia.Lowoverpotentialsof83,102,and86mV wereobtainedforMoP@MWCNTheterostructuretodeliveracurrentdensityof 10mAcm 2 inacidic(0.5MH2 SO4 ),neutral(1Mphosphatebuffer),andalkaline (1MKOH)electrolytes.Furthermore,theachievedoverpotentialswerestablefor continuousHERprocessof40hours.Thewell-crystallizedtextureoftheanchored MoPnanoparticlesoverMWCNTswasdescribedtosignificantlyinfluencethe intrinsicactivity.Besides,theincorporationofMWCNTssignificantlydecreased therateofaggregationandsinteringofMoPnanocrystalsduringthesynthesis andalsoenhancedtheelectricalconductivityandelectronicinteractionswiththe small-sizedcrystallineMoPphase.
Zouetal.[16]reportedanultrafastmethodtoformnickelsulfidenanosheet arrays,coatedwithamorphousNiFebinaryhydroxides(Ni3 S2 @Ni–Fe–OH)and
2.1HeterostructuresforElectrochemicalWaterSplitting 17 supportedonNifoamscaffoldasacompositecatalystforefficientOERathigh currentdensities.ThehybridNi3 S2 @Ni–Fe–OHcatalystwasgrowninsituatopof Nifoamthroughafastandfacileinterfaceinteractionderivedbytheimmersionof hydrothermallysynthesizedNifoam-supportedNi3 S2 intoanironcationicsolution at100 ∘ Cforfiveseconds.TheintegrationofbinaryNiFehydroxidelayerwith highlyintrinsicallyactivesitesandtheconductiveandstablenanoarraysofNi sulfidephaseallowedforhighlyboostedcatalyticactivityinboth1MKOHand concentrated(30%)KOHelectrolyteandresultedinhighstructuralrobustness, electronicconductivity,andstrongcoupledinterfacebetweenthehydroxideand sulfidephases.Ni3 S2 @Ni–Fe–OHheterostructuredemonstratedalmost3and2.5 timeshigheractivitywithrespecttotheuncoupledNi3 S2 phaseandalsoIrO2 benchmarkOERcatalystathighcurrentdensities.Particularly,thisdelivered currentdensitiesof10and100mAcm 2 withoverpotentialsof165and300mV in1MKOHandhighcurrentdensityof1000mAcm 2 withanoverpotentialof 479mVin30%KOH.StableperformanceforcontinuousOERof50hourswas observedatvariouscurrentdensitiesof100,500,and1000mAcm 2 (Figure2.4).
2.1.7MulticomponentMetal-BasedHeterostructures
Inpractice,variousinterphasicincorporationsbetweencatalyticallyactiveand supportingphasesexistinmulticomponentheterostructurewheretheoverall surfaceandinterfacepropertiesareboostedtooutperformthesingle-component catalysts.Themostimportantaspectsofsuchimprovedfunctionalityincludethe enhancementin(i)electrochemicallysurfaceareaandnumberofactivesites; (ii)extentofexposureofsurfaceactivesitestoelectrolyte;(iii)intrinsicactivityof thecatalyticallyactivephase(s)throughaugmentedinteractionwiththeinterfacial intermediates;(iv)surfaceandinterfacefeaturessuchaselectronconductivity, chargetransfer,andmasstransport;(v)morphologyandnanoarray-basedsurfaces suchascore–shell,sandwich,andmultidimensionalstructures;(vi)theelectrocatalyticsynergybetweeninterfacedstructures;and(vii)insituphenomena duringtheelectrocatalysissuchascatalystsurfacereconstructionandelectron redistributionthatcouldpositivelyaffectthedynamicstabilityandactivationof activephase(s)andfurthermodulationofthecatalystelectronicstructure[29]. Here,weintroducerecentadvancesonmulticomponentheterostructuredcatalyst materialsforelectrochemicalwatersplitting(Table2.2).
2.1.7.1MetalOxide-BasedMulticomponentHeterostructures
2.1.7.1.1TransitionMetal/MetalOxideHeterointerfaces
TMOsandhydroxidesareamongthemoststudiedelectrocatalystsforwatersplittingreactions.Themainconsiderationforthedevelopmentofthecorresponding multicomponentheterostructuresisthemodulationoftheirstructure,composition, andmorphologywithrespecttotheinterfacedcomponents[37].Thetypicaland mainTMOsinvolvemono-andbimetallicoxides,mixed(hydro)oxides,spinel oxides,andperovskitesalongwithlayeredstructures,i.e.LDHs[38].Incorporation ofwateradsorption/dissociationcentersbasedonTMOshasbeenaneffective
22D-Materials-FreeHeterostructuresforECEnergyConversion
Figure2.4 (a)SchematicpreparationofNi–Fe–OH@Ni3 S2 @NFbyquicklyimmersing Ni3 S2 @NFinapreheatedaqueoussolutionwithFe3+ ionsat100 ∘ Cforfiveseconds. (b,c)TypicalSEMofNi3 S2 @NFandNi–Fe–OH@Ni3 S2 @NF.(d)LSVcurvesof Ni–Fe–OH@Ni3 S2 @NF(black),Ni3 S2 @NF(gray),IrO2 (lightgray),andNifoam(dashline) forOERin1MKOH.(e)ChronopotentiometriccurvesofNi–Fe–OH@Ni3 S2 @NF(black)and IrO2 (lightgray)in1MKOH.(f)ChronopotentiometriccurveofNi–Fe–OH@Ni3 S2 @NFin 30wt%KOHwithacurrentdensityof1Acm 2 .(g)SchematicillustrationoftheOER activityenhancementusingNi–Fe–OH@Ni3 S2 @NFheterostructure.Source:Reprinted withpermissionfromZouetal.[16].Copyright2017,Wiley.
2.1HeterostructuresforElectrochemicalWaterSplitting 19
Table2.2 Recentadvancesinnonpreciousmetal-basedmulticomponentnano-heterostructured electrocatalystsforelectrochemicalwatersplittingreactions(OERandHER).
HeterointerfacesElectrocatalyst
Application Experimental conditionsResults
Metal/metal oxide Co3 O4 @CoO@CoOERSupport:RDE
Electrolyte: KOH1M
Scanrate:5
FeOOH@Co@FeOOHOERElectrode:NF
Electrolyte: NaOH1M
Scanrate:5
Eonset :1.58V
b:73.3mVdec 1
S:15h (at1.58V)
�� 20 :250mV
b:32.0mVdec 1
S:50h a)
Metal/metal oxide/nonmetal
Metal non-oxide/ nonmetal
Co@CoOx @N,O,S-CHERElectrode:NF
Electrolyte: KOH1M
Scanrate:2
Co3 O4 x @C@Fe2 y Co y O3 OERElectrode:RDE
Electrolyte: KOH1M
Scanrate:5
Co9 S8 @Zn0.8 Co0.2 S@COERElectrode:GCE
Electrolyte: KOH0.1M
Scanrate:10
NiP@NiFeP@NP-CHERElectrode:RDE
Electrolyte: KOH1M
OER
Scanrate:1
�� 10 :61mV
b:78mVdec 1
S:20h (at10mAcm 2 )
Eonset :1.52V
�� 10 :350mV
b:37.6mVdec 1
�� 10 :292mV
b:52mVdec 1
S:6h(at1.58V) [23h]
�� 10 :238mV
b:68mVdec 1 [34]
�� 10 :250mV
b:58mVdec 1
OWSCV10 :1.53V
S:20h(at 100mAcm 2 )
Metal oxide/metallic non-oxide
CoP@MoS2 @CNTsHERElectrode:GCE
Electrolyte: H2 SO4 0.5M
Scanrate:5
CoS@β-Co(OH)2 @MoS2+x OWSElectrode:NF
Electrolyte: KOH0.1M
Scanrate:2
�� 10 :12mV
b:42.0mVdec 1
S:60000s (at50mV) [35]
�� 10 :350mV
S:100000s (at10mAcm 2 ) [36]
a)Long-termstabilityrecordedatcurrentdensitiesof20,50,100,and200mAcm 2