Biofuel Cells
Materials and Challenges
Edited by Inamuddin, Mohd Imran Ahamed,
Rajender Boddula, and Mashallah Rezakazemi
This edition first published 2021 by John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA and Scrivener Publishing LLC, 100 Cummings Center, Suite 541J, Beverly, MA 01915, USA © 2021 Scrivener Publishing LLC
For more information about Scrivener publications please visit www.scrivenerpublishing.com.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at http://www.wiley.com/go/permissions.
Wiley Global Headquarters
111 River Street, Hoboken, NJ 07030, USA
For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com.
Limit of Liability/Disclaimer of Warranty
While the publisher and authors have used their best efforts in preparing this work, they make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives, written sales materials, or promotional statements for this work. The fact that an organization, website, or product is referred to in this work as a citation and/or potential source of further information does not mean that the publisher and authors endorse the information or services the organization, website, or product may provide or recommendations it may make. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for your situation. You should consult with a specialist where appropriate. Neither the publisher nor authors shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages. Further, readers should be aware that websites listed in this work may have changed or disappeared between when this work was written and when it is read.
Library of Congress Cataloging-in-Publication Data
ISBN 9781119724698
Cover image: Russell Richardson
Cover design by Russell Richardson
Set in size of 11pt and Minion Pro by Manila Typesetting Company, Makati, Philippines Printed in the USA
10 9 8 7 6 5 4 3 2 1
Arushi Chauhan and Pramod Avti
Rabisa Zia, Ayesha Taj, Sumaira Younis, Haq Nawaz Bhatti, Waheed S. Khan and Sadia Z. Bajwa
4.5
4.6
5.3
5.3.3
5.3.4
7.2.2
7.3
6.3.4.1
6.3.4.2
7.4
7.3.6.1
7.3.6.2
7.3.6.3
7.3.6.4
7.3.7
7.3.8
7.4.1
8.1
8.3 Glucose Oxidize (GOs) as Enzyme Catalyst in Glucose
8.4 General Experimental Technique for Fabrication of Enzyme GOs Immobilized Electrodes for Glucose Oxidation
8.5 General Method of Characterization of Fabricated Enzyme
Mohd Nur Ikhmal Salehmin, Rosmahani Mohd Shah, Mohamad Azuwa Mohamed, Ibdal Satar and Siti Mariam Daud
12
Munjal, Deepak Kumar Yadav,
Kishore Sharma and Gurmeet Singh
13.4
13.4.3
13.4.4
13.3.2.11
16
Naveen Patel, Dibyajyoti Mukherjee, Ishu Vansal, Rama Pati Mishra and Vinod Kumar Chaudhary
Joel Joseph, Muthamilselvi Ponnuchamy, Ashish Kapoor and Prabhakar Sivaraman
Preface
Rapid industrialization and urbanization associated with the environment changes call for reduced pollution and thereby least use of fossil fuels. Biofuel cells are bioenergy resources and biocompatible alternatives to conventional fuel cells. Biofuel cells are one of the new sustainable renewable energy sources that are based on the direct conversion of chemical matters to electricity with the aid of microorganisms or enzymes as biocatalysts. The gradual depletion of fossil fuels, increasing energy needs, and the pressing problem of environmental pollution have stimulated a wide range of research and development efforts for renewable and environmentally friendly energy. Energy generation from biomass resources by employing biofuel cells is crucial for sustainable development. Biofuel cells have attracted considerable attention as micro- or even nano-power sources for implantable biomedical devices, such as cardiac pacemakers, implantable self-powered sensors, and biosensors for monitoring physiological parameters.
This book covers the most recent developments and offers a detailed overview of fundamentals, principles, mechanisms, properties, optimizing parameters, analytical characterization tools, various types of biofuel cells, all-category of materials, catalysts, engineering architectures, implantable biofuel cells, applications and novel innovations and challenges in this sector. This book is a reference guide for the peoples working in the areas of energy and environment. This book is an essential reference guide for readers, students, faculty, engineers, industrialists, energy chemists, material scientists, electrochemists biotechnologists, microbiologists, and environmentalists who would like to understand the science behind the advanced renewable energy, advanced materials and flexible implantable devices, etc. This book includes the seventeen chapters and the summaries are given below.
Chapter 1 provides details about the factors that influence electron transfer in two categories of bioelectro catalysis, the enzymatic and the microbial catalysis. Anodic and cathodic relevant reactions for these two types of
biocatalysts are discussed in addition to their applications. Challenges for preparation of electrodes as well as various techniques and strategies for immobilization of enzymes and bacteria are discussed in details.
Chapter 2 highlights recent progress in implantable and wearable biofuel cell technologies and their breakthrough applications particularly in living bodies. Important parameters such as sufficient and stable power output, long duration, biocompatibility, biofouling, inflammation that need to be resolved before being converted into a commercial product are discussed.
Chapter 3 discusses some of the challenges and factors that affect the overall performance and efficiency of biofuel cells. Biofuel cell development is an emerging versatile technological platform for harvesting the desired energy requirements of miniature implantable medical devices to meet the challenges of various biomedical applications under physiological conditions.
Chapter 4 discusses the basic structure of an enzymatic fuel cell. Various enzymes used in enzymatic biofuel cells and their modes of electron transport are mentioned. Enzyme immobilization strategies and different materials for enzyme immobilization are also detailed. Finally, the advantages and prospects with pertaining challenges are discussed.
Chapter 5 provides general knowledge about microbial fuel cell technology. The basic working principle of the microbial fuel cell is discussed. Furthermore, the components of microbial fuel cell technology, i.e. reactor configurations, anode and cathode materials and type of substrates are elaborated. The application, challenges, and prospects of microbial fuel cell technology are also presented.
Chapter 6 summarizes the basic principles of the biofuel cells and their uses in various fields. The discussion is mainly focused on the flexibility to the biofuel cells, recent advances and the challenges that are faced by flexible biofuel cells.
Chapter 7 discusses various types of carbon-based nanomaterials in the biofuel domain. Detailed discussion on carbon-based nanomaterials like cellulose starch, glucose, carbon nanoparticles, nanographene, carbon nanotubes and carbon nanofibers are presented. Finally, a separate section on carbon-based nanomaterials is presented.
Chapter 8 discusses different types of biofuel cells with special emphasis on glucose-based biofuel cell. Advantages of using glucose oxidase as a natural enzyme-catalyst in these cells are described. Prevention of loss of efficiency at high temperatures due to denaturation of enzymes using polyols is discussed.
Chapter 9 summarizes the basic working principles of various configurations of photoelectrochemical fuel cells that suit different applications and their performance. Several promising applications are also discussed including wastewater treatment, power generation, fuel production, and a wide range of contaminant degradation.
Chapter 10 focuses on various engineering architectures for biofuel cells. It explores the attractiveness of biofuel cells as energy sources. Various routes for design and fabrication of these cells, material options available, relevant characterization techniques, perspectives and future challenges are discussed.
Chapter 11 discusses the history and classification of biofuel cells and biochemical reactions. The classification of biofuel cells comprises bio-electro chemicals producing whole organisms, producing hydrogen gas, etc. Additionally, various commercial applications of biofuel cells are discussed in detail.
Chapter 12 addresses the development and experimental progress of oxygen reduction reactions cathode catalyst for biofuel cells applications. Classification, mechanism, activity and performance of oxygen reduction reaction cathode catalyst are discussed in details. Additionally, various aspects concerning their electrochemical activity and their limitations in terms of technological applications are highlighted in this chapter.
Chapter 13 starts with an introduction for working mechanisms of fuel cells, biofuel cells, and the microbial desalination cell. A major focus is given to explore various configurations of desalination cells designed so far. The chapter concludes with a discussion on the factors affecting the performance and efficiency of desalination cells.
Chapter 14 discusses the types, designs, working principles, applications of biofuel cells and conventional fuel cells. It explains in detail about the types of various fuel cell and biofuel cells such as molten carbonate, proton exchange membrane, direct methanol, solid oxide, alkaline, phosphoric acid fuel cells, microbial, enzymatic, glucose, photochemical and flexible biofuel cells as well as their advantages, limitations, and applications.
Chapter 15 deliberates on different classes of biofuel cells with a focus on wearable biofuel cells, fuel used and bioelectricity generation outlining possible bioelectronic applications. The issues, challenges and scalability of biofuel cells are discussed and addressed through a proposed sustainable solution roadmap.
Chapter 16 discusses different types of anodes that are currently being utilized in biofuel cells. The main idea of this chapter is to deliver
xx Preface
information related to recent advancements in the field of anode materials, along with their capability to improve the overall performance of biofuel cells.
Chapter 17 discusses the emerging alternative sources of renewable energy in the form of biofuel cells. The fundamental concepts, and types of biofuel cells and their applications are explained. The prospects of biofuel cells as substitutes of conventional technologies and their potentialities in novel applications are presented.
Bioelectrocatalysis for Biofuel Cells
Casanova-Moreno Jannu1, Arjona Noé2 and Cercado Bibiana2*
1CONACYT-Centro de Investigación y Desarrollo Tecnológico en Electroquímica S.C., Pedro Escobedo, Mexico
2Centro de Investigación y Desarrollo Tecnológico en Electroquímica S.C., Pedro Escobedo, Mexico
Abstract
Bioelectrocatalysis is the acceleration of reactions that occur on an electrode via a biological component, be it an enzyme, a cellular organelle or a whole cell. Enzymatic reactions on the anode are mainly the oxidation of saccharides and alcohols, while the oxidative metabolism of bacteria is exploited for removal of short-chain organic acids. In the cathode, the main enzyme-controlled reaction is the reduction of dioxygen, while microbial catalysis tends to obtain hydrogen and methane-like energy vectors. One of the challenges in bioelectrocatalysis is the preparation of electrodes. The techniques for immobilization of enzymes and organelles include the use of polymers and composites and the naturallyoccurring adhesion of bacteria to the solid material forming a biofilm on the electrode. Given the importance of the support material, numerous efforts have been directed to modifying materials that improve the adhesion of enzymes and bacteria, as well as electron transfer. The control of electron transfer is performed by the modification of the pH in the medium, the use of mediators, and the application of a potential difference in an electrolytic cell. The applications of electrochemical cells in bioelectrocatalytic operation include energy conversion, enzymatic sensors and gaseous fuel production in microbial bioelectrochemical systems.
Keywords: Bioelectrocatalysis, biofuel cell, enzymatic electrocatalysis, microbial electrocatalysis
*Corresponding author: bcercado@cideteq.mx
Inamuddin, Mohd Imran Ahamed, Rajender Boddula, and Mashallah Rezakazemi (eds.) Biofuel Cells: Materials and Challenges, (1–52) © 2021 Scrivener Publishing LLC
1.1 Introduction: Generalities of the Bioelectrocatalysis
Electrochemical catalysis or electrocatalysis is used to describe charge transfer-based reactions occurring on an electrode. This term was employed for first time in 1936 by Santos and Schimickler [1]. The electrocatalysis is focused on increasing the reaction rate of an electrochemical process (oxidation/reduction), involving a dissociative chemisorption or a reaction step on an electrode surface and thus, the electrocatalysis depends on the ad/ desorption of reactants and products, and on the formation of an electrochemical double layer. An electrocatalytic cycle is composed of three stages: 1) mass transport of electroactive species from bulk to the interface, 2) the electrocatalytic reaction, and 3) transport of products to bulk. Additionally, stage 2 involves the adsorption of reactants, the electron transfer, and the desorption of products. Consequently, the art of electrocatalysis consists of identifying the barriers of an electrochemical reaction to adjust the properties of the electrochemical interface (electrode and/or solution) with the aim of remove or at least, decrease the energy barriers (activation energies).
The practical role of electrocatalysis implies the science of designing the electrochemical interface properties. Hence, the morphological and electronic properties of the electrocatalyst, together with the electrolyte characteristics, become important to analyze. On the other hand, the activation energy of electrocatalytic reactions also depends on the electrode potential, thus enabling a fine control of the reactions. Consequently, electrocatalysis focuses on minimizing electrode overpotential, and increasing the reaction rate via the decrease of activation energies for a specific reaction.
The relation between electrocatalysis and microorganisms was presented in 1910 when a yeast was used as catalyst in a fuel cell. In the 60s microbial fuel cells (MFC) were used to exploit human waste from spacecraft, and only about 40 years later, the MFCs gained worldwide attention due to the use of industrial wastewater as fuel [2]. Entire microbial cells, organelles and biological molecules have been utilized as catalyst in biofuel cells. The molecules for energy conversion in living eukaryotic cells are utilized as biocatalyst and as model reactions. The reactions are complex and involve the action of nucleotides nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD). The nucleotides in the cell are reduced to NADH and FADH2 by protons coming from a chain of oxidation reactions belonging to the microbial catabolic metabolism. The cyclic oxidation and reduction of nucleotides enables the transport of charge in the mitochondria and thus in the microbial cell. The energy pathways in prokaryote cells involve a chain of transmembrane enzymatic proteins. The c-type cytochromes in the outer cell
membrane enable direct contact cell-electrode and research in molecular biology shows that cytochromes are responsible for extracellular electron transfer (EET).
The reactions occurring in the living cells involve different catalytic proteins or enzymes; thus charge transfer through biological molecules has required many years of investigation. Enzymes can act in the electrolyte, or be immobilized at the electrode, and electron transfer achieved via either mediated or direct form. The contact of the enzyme with the substrate is achieved via physical or covalent adsorption. The type of contact is a function of the location of the active site in the enzyme, which can be in the periphery or in the core of the catalytic protein. The electrode material for immobilization of the bioelectrocatalyst is one of the main issues. Thus, the intrinsic properties of the electrode such as porosity and conductivity must be improved via doping, template construction or addition of nanomaterials. Another concern in bioelectrocatalysis is the lifetime of the enzymatic electrodes, which are very sensitive to environmental conditions. Plenty of strategies using polymers have been proposed, including encapsulation, cross-linking, anchoring, and self-assembly with the aim of improving the electron transfer between the enzyme and the electrode. This process can be explained by different mechanisms like percolation though immobile redox centers, collision of mobile centers, and conduction through a conjugated backbone. The direct transfer occurs via electron tunneling from the active site in the enzyme and the electrode.
In the following sections, reactions of general interest in cells catalyzed by enzymes and microorganisms are described in the first instance. The next section focuses on advances in electrode material development, as well as enzyme immobilization and bacterial biofilm preparation strategies. Finally, in the last sections the phenomena that occur in the transfer of electrons at the enzymatic and bacterial level are described, and two cases of application of bioelectrocatalysis are presented.
1.2 Reactions of Interest in Bioelectrocatalysis
1.2.1
Enzyme Catalyzed Reactions
Oxidoreductases (EC group 1†) are enzymes that are capable of catalyzing reactions in which electron transfer is involved and have been used as
†Enzyme Commission (EC) numbers classify enzymes according to the reaction they catalyze. Therefore, two different enzymes (from two different organisms, for example) catalyzing the same reaction will share the same EC code.
fuel cell components since the early 1960s. In 1962, Davis and Yarborough reported an increase in the potential of a cell in which glucose oxidase was present in one of the electrode compartments (the other being a Pt/ O2 one) [3]. Two years later, Yahiro et al. reported the first polarization curves using glucose oxidase (GOx), D-amino acid oxidase and yeast alcohol dehydrogenase in bioanodes that they coupled with Pt cathodes for O2 reduction [4]. Since then, most of the enzymatic biofuel research has been centered in the oxidation of glucose and the reduction of oxygen. This has been driven by the abundance of both substances in our biosphere; while oxygen is abundant in the atmosphere, glucose is used as a source of energy by almost all living beings. Because of their predominance in the literature, this section will focus on the mechanistic description of enzymatic oxidation of glucose and reduction of oxygen by glucose oxidase and laccase, respectively. Other enzymes, like glucose dehydrogenase [5, 6] and bilirubin oxidase [5] can perform similar reactions through different mechanisms and can be useful in some situations. Furthermore, a variety of other fuels (e.g. carbohydrates [7, 8], alcohols [9, 10], lipids [11] and organic acids [12] and oxidants (mainly H2O2 [13]) have been employed in enzymatic biofuel cells. However, it is out of the scope of this work to review them all in detail. Rather, it is expected that the information presented here will allow the readers to perform similar literature search for their particular enzyme of interest.
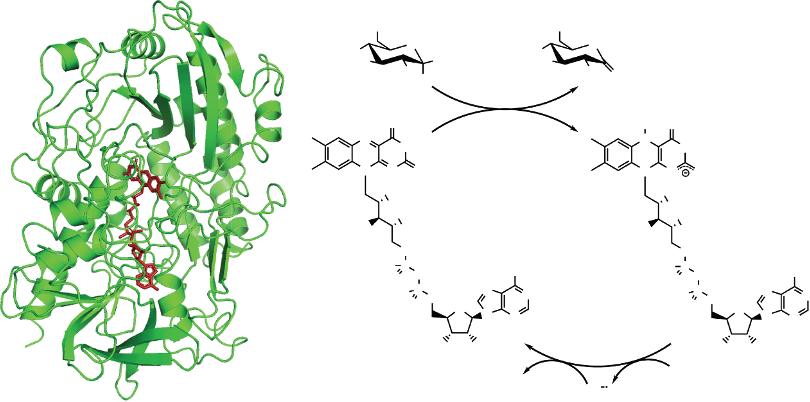
Glucose can be oxidized by a variety of enzymes including glucose oxidase and glucose dehydrogenase. Of these, glucose oxidase has been the one massively preferred, due to its high specificity and good turnover and stability [14, 15]. Glucose oxidase (EC 1.1.3.4) is a dimeric flavoprotein that oxidizes β-D-glucose into D-glucono-δ-lactone while reducing molecular dioxygen (from here on simply referred to as oxygen) to hydrogen peroxide. In each subunit, an active site is deeply buried in a funnel-shaped pocket that contains a non-covalently bound flavin adenine dinucleotide (FAD) cofactor (Figure 1.1a). The N5 atom of this molecule is situated 13–18 Å from the surface and acts as the first electron acceptor in a so called “ping-pong” mechanism [16]. This first half reaction (enzyme reduction) takes place through simultaneous donation of a proton and a hydride from the glucose to the His516 residue and FAD, respectively. Although literature frequently states that the product of this half-reaction is FADH2, there is evidence of the resulting negative charge in the flavin moiety. Therefore, the reduced state of the cofactor is better described as the anionic form FADH [17]. The second half-reaction is the reoxidation of FADH to FAD, reducing an oxygen molecule to peroxide. This last process takes place in two one-electron steps that produces two intermediates,
a semiquinone radical for the FAD flavine moiety and a superoxide anion radical for the O2 molecule (Figure 1.1b). Although not directly participating in the electron transfer, it is believed that His559 and Glu412 help with the pH control in the active site.
Glucose oxidase is produced by a variety of animals, plants, bacteria, algae and fungi. However, only GOx extracted from this last kingdom (mainly from Aspergillus and Penicillium genera) have gained industrial application, partly because they fall under the “generally recognized as safe” category of the U.S. Food and Drug Administration [14]. In academic fuel cell research, GOx produced by Aspergillus niger is highly preferred mainly due to its commercial availability. A few studies have been reported using GOx from Penicillium funiculosum 46.1 but the enzyme extraction and purification from the cell culture needs to be performed [13].
Although efficient, it is thought that wild-type glucose oxidase is not at its catalytic maximum, and therefore directed evolution experiments have been performed to find better versions of the enzyme. In a recent study, Petrović et al. found several GOx mutants that presented a higher rate for glucose oxidation reaction, as well as a smaller Michaelis-Menten constant (KM). Molecular dynamics simulations and X-ray crystallographic information revealed that a key mutation was the exchange of Met556 for a valine residue modifying the shape of the active site. In wild-type GOx, the His516 residue can have two conformations, a catalytic and an inactive
Figure 1.1 (a) Structure of a subunit of glucose oxidase from Aspergillus niger elucidated by Hecht et al. [18] (PDB code 1GAL), showing the FAD cofactor buried in the protein. (b) Catalytic reaction of glucose oxidase. The semiquinone radical FAD intermediate is not shown for clarity.
one, in which its imidazole side chain flips into a cavity near the active site (Figure 1.2a). When Met556 was substituted for a valine, the cavity became smaller, effectively locking the His516 into the catalytic position [15]. This is reflected in the relative values of the calculated free energies (ΔG) for the catalytic and non-catalytic states. While in wild-type GOx both states have similar ΔG, the mutated enzyme clearly shows thermodynamic and kinetic preference for the catalytic state (Figure 1.2c). This example shows that deeper understanding of the mechanistic subtleties can have convenient implications in the industrial applications that employ GOx, including fuel cells.
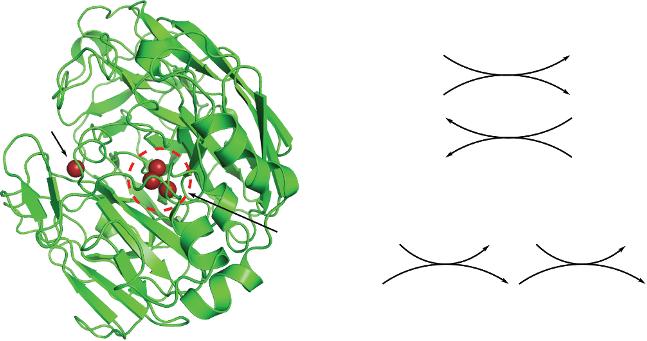
Laccase (EC 1.10.3.2) is a multicopper “blue” oxidase that has a variety of organic and inorganic compounds as its natural substrates, including phenols, phosphates, acids, ketones and amines. In all cases, O2 acts as the final electron acceptor. It is formed by a single polypeptide chain that folds into three different beta barrel domains. Its active site contains four copper atoms, classified as T1, T2, T3α and T3β, according to their spectroscopic and paramagnetic properties. T1 Cu, for example, presents an intense blue color because of its coordination with nitrogen and sulfur atoms from the
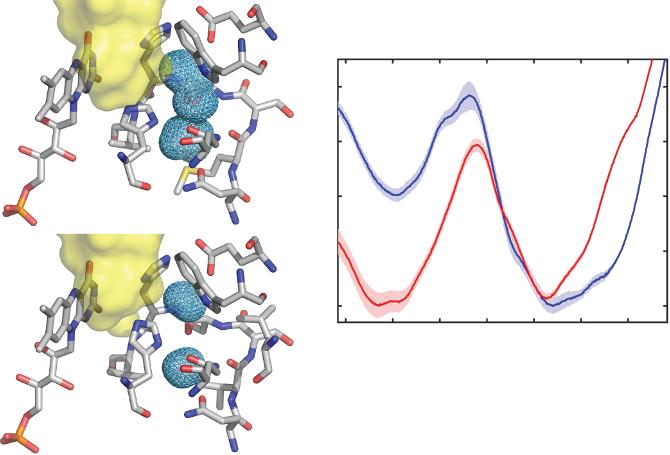
Figure 1.2 Molecular dynamics studies of the structure of the active site of wild-type (a) and mutant (b) glucose oxidase. The meshed area in the right-hand side is a pocket in which His516 can flip into. In the mutant form, the pocket is reduced in size and divided in two parts, effectively locking His516 out. (c) Comparison of the calculated free energy for the catalytic and non-catalytic states in wild-type (curve 1) and mutant (curve 2) GOx. Republished with permission, from ACS Catal., Dušan Petrović et al., 7, 2017, 6188–6197 available online at https://pubs.acs.org/doi/10.1021/acscatal.7b01575; further permissions related to the material excerpted should be directed to the ACS.
neighboring histidine and cysteine residues. On the other hand, T2 Cu presents similar absorption to aquo or hydroxo Cu complexes [19]. In the enzyme oxidized state, all the Cu atoms have an oxidation state of +2.
The T1 Cu is located in a substrate binding pocket close to the enzyme surface and is the initial electron acceptor in a one-electron oxidation of the substrates (Figure 1.3a). It has been proposed that the His458 and Asp206 residues can form hydrogen bonds with some of the substrates, helping to maintain them in the adequate conformation for electron transfer. Furthermore, computer simulations have shown that the H atom at ε-N in His458 is less than 5 Å away from the OH in phenolic substrates making it likely to participate in the electron transfer [20]. Once the T1 Cu2+ has been reduced to Cu1+, it transfers electrons one by one to the cluster formed by the other three Cu atoms (Figure 1.3b). This tri-nuclear cluster (TNC) is buried deeper inside the enzyme at the interface between two domains. In this cluster, oxygen is reduced to two molecules of water in a four-electron process that takes place as two sequential steps. First, the water is reduced in a two-electron process to a peroxide-level intermediate by the two T3 Cu1+ ions. Then, electrons from the T1 and T2 Cu1+ ions further reduce this intermediate to water (Figure 1.3c) [21, 22].
Different sources for laccase include insects, bacteria, fungi and plants like the Japanese lacquer tree (Toxicodendron vernicifluum), where it was first extracted from [24] and which gives the enzyme its name. Laccase varieties extracted from fungi present the highest redox potentials [21, 22], and are thus preferred for biocathode development. Laccase isolated from different species presents some variations in their amino acid sequence [20]. Laccase from Trametes versicolor is particularly popular in
Figure 1.3 (a) Structure of laccase from Trametes versicolor elucidated by Bertrand et al. [23] (PDB code 1KYA), showing the surface T1 Cu and the buried trinuclear cluster (TNC). Mechanism of substrate oxidation (b) and oxygen reduction (c) by laccase.
electrochemistry research and thus, the numbering of the residues in the previous paragraph is referred to the numbering in this species.
1.2.2 Reactions Catalyzed by Microorganisms
Conventional MFCs utilize abiotic cathodes; however, limitations for the oxygen reduction reaction are present similarly to what is observed in electrochemical cells. The use of biocathodes as an alternative to metallic cathodes was proposed in 2005 [25].
The biocathodes are classified as a function of the terminal electron acceptor available. Aerobic biocathodes utilize oxygen as final acceptor; of electrons electron transfer occurs via the reduction of a mediator such as iron and manganese and then, the mediator is oxidized by the bacteria. Anaerobic biocathodes directly reduce acceptors such as nitrate and sulfate [26].
Suitable bacteria for indirect electron transfer are Leptothix discophora, which is able to reduce MnO2 to manganese ion [27], and Acidithiobacillus ferrooxidans an iron oxidizing bacterium [28], while consortia of electroactive microorganisms for direct transfer can be found in sediment and anaerobic sludge [29, 30]. An exhaustive evaluation of the electroactivity capabilities of diverse bacterial species was reported by Cournet et al. [31].
Hybrid microbial bioelectrochemical systems include MFC fueled by light energy; this is achieved by oxygenic photosynthetic organisms, such as microalgal and cyanobacteria species. Photosynthetic organisms transfer electrons to the anode, or to heterotrophic microorganisms which in turn transfer the charge to the anode.
Energy pathways in cyanobacteria occur in the thylakoid membranes containing respiratory electron transfer chain components and in the cytoplasmic membrane with a respiratory electron transfer short chain. Since the electron transfer is not adapted for extracellular electron transport, mutant strains for electron export ability are being obtained [32]. Synechocystis have three respiratory terminal oxidase complexes for the reduction of oxygen and mutants lacking respiratory terminal oxidases showed increased ferricyanide reduction rate [32]. However, the mechanism for electron excretion to the periplasmatic space and beyond remains unresolved; hypothesis on the presence of nanowires, an assimilatory metal reduction pathway, and endogenous mediators have been stated.
One additional advantage of MFCs over general bioreactors is the possibility to adapt the microbial metabolism of the inoculum as function of the set electrode potential. This procedure enables one to increase the selectivity of the reactions and the galvanic mode of operation can become electrolytic [33].
Table 1.1 Cell potential for typical reactions with microbial bioanode, and a microbial biocathode.
Microbial bioanode
CH3COOH + 2H2O → CO2 + 8H+ + 8e
E = −0.280/NHE
Anode
Cathode
2H+ + 2e → H2
E = −0.414 V/NHE
Microbial biocathode
Cell potential
E = 0.134 V
2H2O → O2 + 4H+ + 4e- CO2 + 8 H+ + 8 e → CH4 + 2H2O E = 1.064 V
E = 0.820 V/NHE
E = −0.244V/NHE
Microbial electrolysis cells (MECs) operate under a constant electrical supply, therefore this energy represents an operation cost and a decrease in the energy efficiency. To overcome these issues, the use of alternative energy technologies has been proposed [34]. Another alternative to reduce the cost of energy supply is an intermittent operation for conversion of CO2 into organic products using a biocathode [35].
The thermodynamic cell voltage for hydrogen production from acetate oxidation in a bioanode, and methane production in a biocathode from water oxidation is resumed in Table 1.1. At least theoretically, these reactions present advantages compared to totally abiotic processes for gaseous fuel production. For instance, water electrolyzers require 1.23 V for hydrogen formation while the bioelectrolysis of water requires 0.134 V.
1.3 Immobilization of Biocatalyst
1.3.1
Immobilization of Enzymes on Electrodes
Although the first proof-of-concept biofuel cells employed the enzymes freely in solution [3, 4] this approach is poorly applicable in practice. Enzymes are costly and losing them with the fuel and oxidant flow turns operation expensive. Therefore, most of the reported enzymatic biofuels include enzymes immobilized on the electrode surface.
A number of strategies have been developed to immobilize enzymes on solid supports and a significant number of reviews have been published