2DFunctionalNanomaterials
Synthesis,Characterization,andApplications
EditedbyGaneshS.Kamble
Editor
Dr.GaneshS.Kamble
DepartmentofEngineeringChemistry
KolhapurInstituteofTechnology
CollegeofEngineering Kolhapur416234 India
Cover
CoverImage:©theasis/GettyImages
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformationcontained inthesebooks,includingthisbook,tobefreeof errors.Readersareadvisedtokeepinmindthat statements,data,illustrations,proceduraldetailsor otheritemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailablefrom theBritishLibrary.
BibliographicinformationpublishedbytheDeutsche Nationalbibliothek
TheDeutscheNationalbibliothekliststhis publicationintheDeutscheNationalbibliografie; detailedbibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>.
©2022WILEY-VCHGmbH,Boschstr.12,69469 Weinheim,Germany
Allrightsreserved(includingthoseoftranslation intootherlanguages).Nopartofthisbookmaybe reproducedinanyform–byphotoprinting, microfilm,oranyothermeans–nortransmittedor translatedintoamachinelanguagewithoutwritten permissionfromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhennot specificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34677-6
ePDFISBN: 978-3-527-82395-6
ePubISBN: 978-3-527-82394-9
oBookISBN: 978-3-527-82396-3
Typesetting Straive,Chennai,India PrintingandBinding
Printedonacid-freepaper
10987654321
Contents
Foreword xvii
Preface xxi
1GrapheneChemicalDerivativesSynthesisandApplications: State-of-the-ArtandPerspectives 1
MaximK.Rabchinskii,MaksimV.Gudkov,andDinaYu.Stolyarova
1.1Introduction 1
1.2GrapheneOxide:SynthesisMethodsandChemistryAlteration 3
1.3GrapheneOxideReductionandFunctionalization 6
1.4ApplicationsofCMGs 13
1.5ConcludingRemarks 15 Acknowledgments 15 References 16
22D/2DGrapheneOxide-LayeredDoubleHydroxide NanocompositefortheImmobilizationofDifferent Radionuclides 21
PaulmanickamKoilrajandKeikoSasaki
2.1Introduction 21
2.2SynthesisofGO/LDHComposite 22
2.2.1Co-precipitation 22
2.2.2HydrothermalPreparation 23
2.2.3Self-AssemblyofLDHNanosheetswithGONanosheets 24
2.3RemovalofRadionuclides 24
2.3.1U(VI)Removal 24
2.3.2SorptionofEu(III)withthePresenceofGOonLDH 25
2.3.3Co-remediationAnionicSeO4 2 andCationicSr2+ 26
2.4Conclusion 29 References 29
32DNanomaterialsforBiomedicalApplications 31 PolirajuKalluruandRavirajVankayala
3.1Introduction 31
3.1.1PhotothermalandPhotodynamicTherapy 31
3.1.2BioimagingandDrug/GeneDelivery 34
3.1.3Biosensors 37
3.1.4AntibacterialActivity 39
3.1.5TissueEngineeringandRegenerativeMedicine 41
3.2Conclusions 43 References 43
4NovelTwo-DimensionalNanomaterialsforNext-Generation Photodetectors 47 KhurelbaatarZagarzusemandZumuukhorolMunkhsaikhan
4.1Introduction 47
4.22DMaterialsforPDs 49
4.2.1Graphene 49
4.2.2TMDs(TransitionMetalDichalcogenides) 49
4.2.3MXenes(2DTransitionMetalCarbides/Nitrides) 50
4.2.4Xenes(Monoelemental2DMaterials) 50
4.3ThePhysicalMechanismEnablingPhotodetection 50
4.4CharacterizationParametersforPhotodetectors 51
4.4.1Responsivity 51
4.4.2Detectivity 52
4.4.3ExternalQuantumEfficiency 52
4.4.4Gain 52
4.4.5ResponseTime 52
4.4.6NoiseEquivalentPower 52
4.5SynthesisMethodsfor2DMaterials 53
4.5.1MechanicalExfoliation 53
4.5.2LiquidExfoliation 53
4.5.3ChemicalVaporDeposition(CVD) 53
4.6PhotodetectorsBasedon2DMaterials 55
4.6.1PhotodetectorsBasedonGraphene 55
4.6.2PhotodetectorsBasedonMoS2 55
4.6.3PhotodetectorsBasedonBP 55
4.7PhotodetectorsBasedon2DHeterostructures 56
4.8ConclusionsandOutlook 58 References 58
52DNanomaterialsforCancerTherapy 63 NareshKuthala
5.1Introduction 63
5.22DNanomaterialsforCancerTherapy 64
5.2.12DNanomaterialsforCombinationPTTwithPDT 64
5.2.22D-NanomaterialsforCombinationPTTTherapywith Radiotherapy(RT) 68
5.2.32DNanomaterialsforCombinationPTTTherapywithSonodynamic Therapy(SDT) 70
5.2.42DNanomaterialsforCombinationPTTTherapywithImmune Therapy(ImT) 73
5.3SummaryandFuturePerspectives 76 References 76
6GrapheneandItsDerivatives–Synthesisand Applications 81 AmerAl-Nafiey
6.1Introduction 81
6.2Graphite 81
6.2.1Define 81
6.2.2SyntheticGraphite 82
6.2.3CharacterizedandPropertiesofGraphite 82
6.2.3.1Structure 82
6.2.4Applications 84
6.3GrapheneOxide 84
6.3.1Define 84
6.3.2SyntheticofGrapheneOxide 84
6.3.3CharacterizedandPropertiesofGrapheneOxide 84
6.3.3.1Structure 84
6.3.3.2PropertiesofGrapheneOxide 87
6.3.3.3ApplicationsofGrapheneOxide 88
6.3.3.4FewExamples 88
6.4ReducedGrapheneOxide 89
6.4.1Define 89
6.4.2SyntheticofReducedGrapheneOxideorReductionof GrapheneOxide 89
6.4.2.1ThermalReductionofGO 90
6.4.2.2PhotocatalyticMethod 94
6.4.2.3ElectrochemicalMethod 95
6.4.2.4OtherMethods 95
6.4.3Characterized,Structure,andPropertiesofReduced GrapheneOxide 95
6.4.3.1Structure 96
6.4.3.2PropertiesandApplicationsofReducedGrapheneOxide 97
6.5Graphene 98
6.5.1Define 98
6.5.2SynthesisofGraphene 98
6.5.2.1ChemicalVaporDeposition(CVD) 101
6.5.2.2EpitaxialGrowth 102
6.5.2.3MechanicalExfoliation 104
6.5.2.4ChemicalReductionofGrapheneOxide(GO) 105
6.5.3Characterized,Structure,andPropertiesofGraphene 105
6.5.3.1SurfaceProperties 105
6.5.3.2ElectronicProperties 105
6.5.3.3OpticalProperties 106
6.5.3.4MechanicalProperties 107
6.5.3.5ThermalProperties 107
6.5.3.6PhotocatalyticProperties 108
6.5.3.7MagneticProperties 109
6.5.3.8CharacterizationsofGraphene 109
6.5.3.9Morphology(SEM,TEM,andAFM) 109
6.5.3.10RamanSpectroscopy 111
6.5.3.11X-rayPhotoelectronSpectroscopy(XPS) 111
6.5.3.12UV–VisibleSpectroscopy 112
6.5.3.13X-rayDiffraction(XRD) 114
6.5.3.14ThermogravimetricAnalysis(TGA) 114
6.5.3.15FTIRSpectroscopy 115
6.5.4ApplicationofGraphene 116 References 116
7RecentTrendsinGraphene–LatexNanocomposites 125 AnandKrishnamoorthy
7.1Introduction 125
7.2PolymerLattices–AnOverview 125
7.3Graphene–Background 127
7.4PreparationandFunctionalizationofGraphene 128
7.5Graphene–LatexNanocomposites:PreparationPropertiesand Applications 129
7.6Conclusions 137 References 138
8AdvancedCharacterizationandTechniques 141 RajaMurugesan
8.1Introduction 141
8.2CharacterizationTechniques 141
8.2.1OpticalTechniques–DynamicLightScattering(DLS) 141
8.2.2OpticalSpectroscopy 144
8.2.3NMR-NuclearMagneticResonanceSpectroscopy 145
8.2.4InfraredSpectroscopy(IR)andRamanSpectroscopy 145
8.2.5X-RayPhotoelectronSpectroscopy(XPS) 146
8.2.6CharacterizationBasedonInteractionswithElectronsorElectron Microscopy(EM) 147
8.2.6.1ScanningElectronMicroscopy(SEM) 147
8.2.6.2TransmissionElectronMicroscopy(TEM) 149
8.2.6.3ScanningTransmissionElectronMicroscopy(STEM) 150
8.2.6.4ScanningTunnelingMicroscopy(STM) 151
8.2.7AtomicForceMicroscopy(AFM) 151
8.2.8KelvinProbeForceMicroscopy(KPFM) 152
8.2.9X-Ray-BasedTechniques 152 References 154
92DNanomaterials:SustainableMaterialsforCancerTherapy Applications 157 MamtaChaharandSaritaKhaturia
9.1Introduction 157
9.2Typesof2DNanomaterials 158
9.3MethodsfortheSynthesisof2DNanomaterials 160
9.4MechanismofCancerTheranostics 162
9.5Applicationsof2DNanomaterials 163
9.6Conclusion 163 References 169
10RecentAdvancesinFunctional2DMaterialsforFieldEffect TransistorsandNonvolatileResistiveMemories 175 AdnanYounis,JawadAlsaei,BasmaAl-Najar,HaceneManaa,PranayRajan, ElHadiS.Sadki,AichaLoucif,andShamaSehar
10.1Introductionto2DMaterials 175
10.2ElectronicBandStructurein2DMaterials 176
10.3ElectronicTransportPropertiesof2DMaterials 178
10.4Two-DimensionalMaterialsinFieldEffectTransistors 180
10.4.1FieldEffectTransistors 180
10.4.2TheRiseof2DMaterialsResearchinFETs 180
10.4.3Graphene-BasedFieldEffectTransistors 181
10.4.42DTransitionMetalDichalcogenides(TMDCs)inTransistors 183
10.5Two-DimensionalMaterialsasNonvolatileResistiveMemories 184
10.5.1NonvolatileResistiveMemoriesBasedonGrapheneandIts Derivatives 185
10.5.2ResistiveSwitchingMemoriesin2DMaterials“Beyond”Graphene 187
10.5.2.1Solution-ProcessedMoS2 -BasedResistiveMemories 187
10.5.2.2Solution-ProcessedBlackPhosphorousNonvolatileResistive Memories 188
10.5.2.3EmergingNVMBasedonHexagonalBoronNitride(h-BN) 188
10.6ConclusionsandOutlook 189 References 190
x Contents
112DAdvancedFunctionalNanomaterialsforCancer Therapy 199
RajKumar,NaveenBunekar,SunilDutt,PulikantiG.Reddy,AbhishekK. Gupta,KeshawR.Aadil,VivekK.Mishra,ShivendraSingh,andChandrani Sarkar
11.1Introduction 199
11.22DNanomaterialsClassification 202
11.2.1GrapheneFamilyNanomaterials 202
11.2.2TransitionMetalDichalcogenides(TMDs) 203
11.2.3LayeredDoubleHydroxides(LDHs) 205
11.2.4Carbonitrides(MXenes) 206
11.2.5BlackPhosphorus(BP) 206
11.3CancerTherapy 208
11.3.1MechanismofActioninCancerTherapy 212
11.3.1.1ModeofActionof2DNanomaterials 212
11.3.2PhotodynamicTherapyforCancerCellTreatment 215
11.3.2.1MechanismofPhotodynamicTherapy 215
11.3.2.22DNanomaterialsasPhotosensitizerforPDT 217
11.3.2.3Applicationof2DNanomaterialsinPhotodynamicTherapy 217
11.3.32DNanomaterials-CancerDetection/Diagnosis/Theragnostic 218
11.4TissueEngineering 219
11.5Conclusion 220
Acknowledgment 221 References 221
12SynthesisofNanostructuredMaterialsViaGreenandSol–Gel Methods:AReview 235
AnkitS.Bartwal,RahulThakur,SumitRingwal,andSatishC.Sati
12.1Introduction 235
12.2MethodsUsedinNanostructuredSynthesis 236
12.2.1GreenMethodofNanoparticlesSynthesis 236
12.2.2Sol–GelMethodofNanoparticlesSynthesis 236
12.2.3GreenMethodofNanocompositesSynthesis 241
12.2.4Sol–GelMethodofNanocomposites 241
12.3Discussion 241
12.4Conclusion 244 References 244
13StudyofAntimicrobialActivityofZnONanoparticlesUsing LeavesExtractof Ficusauriculata BasedonGreenChemistry Principles 249 GurpreetKour,AnkitS.Bartwal,andSatishC.Sati
13.1Introduction 249
13.2MaterialsandMethods 250
13.2.1Chemicals 250
13.2.2Methodology 250
13.2.3AntimicrobialActivity 251
13.3ResultsandDiscussion 251
13.3.1CharacterizationofSynthesizedZinc-OxideNanoparticles (ZnONPs) 251
13.3.1.1XRDAnalysis 251
13.3.1.2FT-IRAnalysis 252
13.3.1.3SEMAnalysis 254
13.3.1.4TEMAnalysis 254
13.3.2AntibacterialActivity 254
13.4Conclusion 255 Acknowledgments 255 References 255
14PiezoelectricPropertiesofNa1 x Kx NbO3 near x = 0.475, MorphotropicPhaseRegion 257
SurendraSinghandNarayanS.Panwar
14.1Introduction 257
14.2ExperimentalProcedure 259
14.3ResultsandDiscussion 260 References 262
15SynthesisandCharacterizationofSDCNano-Powderfor IT-SOFCApplications 265
BharatiB.Patil
15.1Introduction 265
15.1.1SolidOxideFuelCells(SOFCs) 265
15.1.2IntermediateTemperatureSolidOxideFuelCells(IT-SOFCs) 266
15.1.3WhySamarium-DopedCeria(SDC)Material? 266
15.1.4VariousSynthesisMethodsforSDC 267
15.1.5WhySDCSynthesisbyCombustionProcess? 268
15.1.6WhySDCSynthesisbyGlycineNitrateCombustion Process(GNP)? 268
15.1.7ApplicationsofSDCMaterialRelatedtoIntermediateTemperatureSolid OxideFuelCells 269
15.1.7.1ApplicationsofSDCasSOFCElectrolyte 269
15.1.7.2ApplicationsofSDCtoMakeCompositeAnode 269
15.1.7.3ApplicationsofSDCtoMakeCompositeCathode 270
15.1.7.4ApplicationsofSDCasanInterlayer 270
15.1.7.5ApplicationsofSDCasanAdditionalAnodeLayer 270
15.2Experimental 270
15.2.1PowderSynthesis 270
15.2.2PowderCharacterization 271
15.3ResultsandDiscussion 272
15.3.1TG-DTGStudy 272
15.3.2XRDAnalysis 272
15.3.3PowderMicrostructure 276
15.3.3.1SEMAnalysis 276
15.3.3.2TEMAnalysis 277
15.3.3.3EDAXAnalysis 277
15.3.3.4BETAnalysis 278
15.3.4ElectricalProperties 278
15.4Conclusions 281 Acknowledgments 281 References 282
16Introductionof2DNanomaterialsandTheirPhotocatalytic Applications 285 KallappaRamchandraSanadi
16.1Introduction 285
16.2DefinitionsofNanomaterials 286
16.3HistoryofNanotechnology 286
16.3.1Top-downApproach 286
16.3.2Bottom-upApproach 286
16.4ClassificationofNanomaterials 286
16.4.1Zero-Dimensional(0-D) 287
16.4.2One-Dimensional(1-D) 287
16.4.3Three-Dimensional(3-D) 287
16.4.4Two-Dimensional(2-D) 287
16.4.4.1SyntheticMethods 288
16.5CharacterizationTechniquesfor2DNanomaterials 290
16.6Applicationsof2DNanomaterials 291
16.7PhotocatalyticApplication 291
16.7.1WhyPhotocatalyst? 291
16.7.2BriefHistoryofPhotocatalysis 292
16.7.3PrinciplesofHeterogeneousPhotocatalysis 292
16.7.4PhotocatalyticStudyof2DNanomaterials 293
16.7.5ChallengesBehind2DNanomaterialsasaPhotocatalyst 294 References 294
17GrapheneandItsAnalogous2D-LayeredMaterialsforFlexible PersistentEnergyStorageDevicesinConsumer Electronics 297
HimadriTanayaDas,K.Hariprasad,andT.E.Balaji
17.1Introduction 297
17.2BriefSketchoftheTypesofSCandItsWorkingMechanism 298
17.3EvolutionofElectrodeMaterialsforFlexibleSupercapacitors 300
17.4DevelopingGrapheneElectrodeswithDifferentNanocomposites 304
17.4.1OtherCarbon-BasedNanomaterialswithGraphene 304
17.4.2UsingOrganicCompositeswithGraphene 306
17.4.3ConductivePolymerwithGraphene 306
17.4.4CombiningGraphenewithOtherMetalOxides/Hydroxides 308
17.4.5CombiningGraphenewithOther2D-LayeredMaterials 308
17.5NovelTechnologiestoDevelopFlexibleGraphene-Based Supercapacitors 310
17.6Conclusion 311
17.7FutureAspects 313 References 313
182DDichalcogenides 317
RamS.Singh,VarunRai,andArunK.Singh
18.1Introduction 317
18.1.1WhatAre2DDichalcogenides? 317
18.1.2Properties 318
18.2MethodsofSynthesis 321
18.2.1Top-DownMethod 321
18.2.1.1MicromechanicalExfoliation 321
18.2.1.2LiquidExfoliation 322
18.2.1.3ChemicalIntercalationandExfoliation 322
18.2.1.4ElectrochemicalExfoliation 322
18.2.1.5ThinningbyThermalAnnealing,Laser,andChemicalEtching 323
18.2.2Bottom-UpMethod 323
18.2.2.1ChemicalVaporDeposition 323
18.2.2.2Solvo-Thermal 324
18.2.2.3MolecularBeamEpitaxy 325
18.3ModificationofProperties 325
18.4Applications 327
18.4.1Optoelectronics 327
18.4.2Sensors 329
18.4.3Spintronics 329
18.4.4Photocatalysis 329
18.4.5BiomedicalApplications 330
18.5Conclusion 330 Acknowledgment 330 References 331
19RecentTrendsonGraphene-BasedMetalOxide NanocompositesTowardPhotoelectrochemicalWater SplittingApplication 335 KashinathLellalaandMouniRoy
19.1Introduction 335
19.1.1BasicofPhoto-Anode/Cathode 335
19.1.2PropertiesofPEC 336
19.1.3ImportanceofCatalyst/Electrode 336
19.1.4FundamentalConceptofPhoto-ElectrochemicalWaterSplitting 337
19.1.4.1Light–CatalystInteraction 337
19.1.4.2Electron–HolePair 337
19.1.4.3CarrierTransportation-Separation 338
19.1.4.4WaterSplittingReaction 339
19.1.4.5NatureofElectrolyte 339
19.1.4.6Catalysis 339
19.1.4.7CrystallinityandSize 340
19.1.4.8TemperatureandPressure 340
19.1.4.9HeterogeneousElectronTransfer 340
19.1.4.10pHDependency 340
19.2GrapheneandGraphene-BasedNanocomposites 340
19.2.1Graphene 340
19.2.2Graphene-BasedNanocomposites 341
19.3SynthesisofGraphene-BasedMetalOxideNanocomposites 342
19.4ApplicationofGraphene–MetalOxideCompositesToward PhotoelectrochemicalWaterSplitting 345
19.5SummaryandFuturePerspective 349 References 349
202DMOFsNanosheets 357 ArezouMohammadinezhad
20.1Introduction 357
20.2SyntheticStrategies 357
20.2.1Top-DownMethod 358
20.2.1.1SonicationExfoliation 358
20.2.1.2MechanicalExfoliationMethod 359
20.2.1.3ChemicalExfoliation 359
20.2.1.4Langmuir–BlodgettMethod 359
20.2.1.5Solvent-InducedExfoliation 359
20.2.2Bottom-UpMethod 359
20.2.2.1InterfacialSynthesisMethod 360
20.2.2.2Surfactant-AssistedMethod 360
20.2.2.3TemplateMethod 360
20.2.2.4SonicationSynthesisMethod 360
20.2.3OtherSynthesisMethods 361
20.3Applicationsof2DMOFsNanosheets 361
20.3.1GasSeparation 361
20.3.2EnergyConversionandStorage 361
20.3.3Catalysis 362
20.3.4SensingPlatforms 362
20.3.5Biomedicine 362
20.4Compositesof2DMOFNanosheets 362
20.5Conclusion 363 References 363
21IntroductionandApplicationsof2DNanomaterials 369 AttaU.Rehman,FatimaAfzal,MuhammadT.Ansar,AmnaSajjad,and MuhammadA.Munir
21.1Introduction 369
21.2Applicationsof2DNanomaterials 371
21.2.1Photodetectors 371
21.2.2Phototransistors 371
21.2.3p–nJunctionPhotodetectors 372
21.2.4Field-EffectTransistors 373
21.2.5GasSensors 373
21.2.6Lithium-IonBatteries 374
21.2.7Lithium-IonBatteryAnodes 374
21.2.8Lithium-IonBatteryCathodes 375
21.2.9GrapheneasCurrentCollector 376
21.2.10GrapheneinSupercapacitors 376
21.2.11GrapheneNanocompositeswithDistinctMaterials 377
21.2.12DopingandSurfaceModifications 378
21.2.13GrapheneforGasSensors 379
21.3Conclusion 379 References 380
222DNanomaterialsforPhotocatalysisand Photoelectrocatalysis 383 GubbalaV.Ramesh,N.MahendarReddy,MuvvaD.Prasad,D.Saritha,and KolaRamesh
22.1Introduction 383
22.2PhotocatalyticCO2 Reduction 385
22.3PhotoelectrocatalyticCO2 Reduction 388
22.4PhotocatalyticHydrogenProduction 391
22.5PhotoelectrocatalyticHydrogenProduction 395
22.6PhotocatalyticDyeDegradation 397
22.7Conclusion 401 References 402
Index 413
Foreword
Nanoscalematerialsornanomaterialshaveattractedmuchattentioninthelast fewdecades.Zero-dimensional(0D)fullerenes,semiconductingquantumdots, andmetalnanoparticles,aswellasone-dimensional(1D)nanowiresandcarbon nanotubesstartedthenanomaterialsrevolutionattheendofthelastcentury.In thepastdecade,weobservedashiftfromtraditionalbulkmaterialsproduction anddevicemanufacturingbysubtractiveprocessestoadditivemanufacturing andself-assemblyfromnanoparticles.Iexpectthetwenty-firstcenturytobecome acenturyofnanomaterials,whennanoparticleswillbeassembledinmultiple controlledways(guidedbyartificialintelligence,AI)tocreatematerialswiththe requiredcombinationsofproperties.Andalargeroleinshapingthefuturematerial technologybelongstotwo-dimensional(2D)materials.Separationofgraphene layersanddiscoveryofattractivephysicalpropertiesinsingle-andfew-layer graphenein2004attractedinteresttoother2Dmaterials.Boronnitride,transition metaldichalcogenides,andoxides/hydroxideswereproducedin2Dstatefrom widelyavailableprecursorswithweakvanderWaalsbondingbetweenthelayers. Moreover,newmaterialsthatdon’thaveweaklybondedlayeredprecursors,suchas 2Dsilicon,germanium,tin,phosphorus,boron,andothers,weresynthesized.Even metal–organicframeworks(MOFs)havebeenproducedin2Dstate.Transition metaldichalcogenides,andmorerecentlydiscoveredcarbidesandnitrides,known asMXenes,formverylargefamiliesof2Dmaterialswithdozensofwell-defined stoichiometricstructures,butalsoavirtuallyinfinitenumberofsolidsolutions. Finetuningofpropertiesispossiblebyformingthose“2Dalloys.”Ultrathin2D materialsoffer:
● Electronicpropertiesrangingfrommetallictosemiconductingtoinsulating;
● Subnanometerthicknessleadingtomechanicalflexibilityandopticaltransparency;
● Highsurfaceareaavailableforadsorption,catalysis,andreversiblesurfaceredox reactionsusedinenergystorage.
However,themostvaluablefeatureof2Dmaterialscomparedtoallothernanoparticlesistheirabilitytobeassembledintodenseandstrongheterostructuresbyusing flatlayersasbuildingblocks–thinkofbuildingahouseusingavarietyofbricks orassemblingtoysusingLegoblocks.Whilegrapheneattractedmuchattentionin
thepast15years,theworldismovingfromexploitingasingle“wondermaterial” toutilizationofdozensandhundredsof2Dbuildingblockswithrichchemistry toassemblematerialsanddevicesforfutureadvancedtechnologies.Thisapproach allows:
● Assemblyofhybridmaterialswithcombinationsofpropertiesthatnoconventional(single)materialcanprovide;
● Buildinghybridandcompositematerialsbyself-assemblyoradditivemanufacturingtechniquesanddeviceassemblywithoutwaste;
● Creatingextremelyanisotropicmaterials;
● Complexshapesandintegrationwith0Dand1Dmaterialsintothree-dimensional (3D)materialsandstructures;
● Buildingentiredevices,e.g.bycombiningmaterialswithsemiconducting,metallic,andinsulatingproperties.
Nanomaterialsmadeofprecise2Dstructurescanhaveremarkableproperties, buttocreatefunctionaldevicesonealsoneedsdiverseandtunableproperties.For example,matchingworkfunctionsofmaterialsallowscontrolofjunctionsinelectronicsandsolarcells.Manyapplicationsrequirematerialsthatoffercombinations ofproperties,suchas:
● Conductivity + redoxability = energystorage;
● Conductivity + catalyticability = electrocatalysts;
● Conductivity + transparency + color = opticaldevices;
● Plasmonresonanceinnear-IRrange + biocompatibility = photothermaltherapy.
Effortsofmaterialchemistsarecurrentlydirectedtowardsynthesisofnew2D materialswiththerequiredstructureandproperties.Also,chemicalfunctionalizationofsurfacesallowsfurthertuningof2Dnanosheets.Materialscientistsandengineersassemblethemintofilms,fibers,complexshapes,anddevicesthatcanperform certainfunctions.The2Dmaterialsalreadyfindapplicationsinenergyconversion andstorage,electronics,medicine(includingdrugdeliveryandtheranostics),environmentclean-up,catalysis(includingelectrocatalysisandphotocatalysis),sensing, andmanyotherfields.
Thisbookincludesmorethan20chapterscoveringthesynthesis,modification, characterization,andapplicationsof2Dandrelatednanomaterials.Graphene anditsderivatives,suchasgrapheneoxide,metaloxides,doublehydroxides, dichalcogenides,MOFs,aswellastheirhybridsandnanocomposites,havededicatedchapters.Someothermaterialsaredescribedinchaptersdealingwith biomedical,photonic,photocatalytic,energystorage,andelectronicapplications of2Dmaterials.Since2Dmaterialsandstructuresexpandtoagreatlengthinto chemistry,physics,medicine,andmanyengineeringdisciplines,nosinglebookcan provideacompletecoverage.Still,thisbookcoversmanyrepresentativematerials andimportanttopics,withafocusonchemistryandchemicalapplicationsof2D materials.Itwillbeofinteresttostudentsandresearchersnotonlyinchemistry butalsoinotheradjacentfields,astheimportanceof2Dmaterialsinphysics,
Foreword xix medicine,environmentalscience,andengineeringissteadilyincreasingandtheir applicationsarequicklyexpanding.
Philadelphia,April3,2021
CharlesT.andRuthM.Bach
DistinguishedUniversityProfessor Director,A.J.DrexelNanomaterialsInstitute
DrexelUniversity Philadelphia,PA,USA
Preface
Inthetwenty-firstcentury,two-dimensional(2D)nanomaterialsarewidelyconsideredtoconstitutethebasisofthenexttechnologicalrevolutionwhichholdsagreat potentialinadvancingscienceandtechnology.The2Dnanomaterialhasmadean insightfulimpactonthesociety;itiscloselyassociatedtothewell-beingofhuman kind.Therateofadvancementsin2Dnanomaterialissohighthatprospectusdeveloperscontinuouslylookforstrategiestohackitwiththeseadvancements.More recently,the2Dcrystallineallotropeofcarbon,i.e.graphene,hasbroughtanew cheeringintocarbonnanomaterialswhichgreatlyprogresspracticalapplications. Therefore,theworldwideresearcherscanbemotivatedtobethefutureleaderswho wouldmakefundamentalcontributions.Thepresentbookisasincereattemptin thisdirection.
Theemergingdevelopmentinthefieldof2Dnanomaterialsanditsdevicesare playingavitalroleinmotivatingthegrowthofanation’seconomybecauseofits impendingcontributiontomanufacturingprocessesandinventiveproducts.Thus, theaimsofdeveloping2Dfunctionalizednanomaterialistoextendbroadapplicationstowardenergystoragedevices,cancercellapplications,photocatalysts,and photoelectrocatalyst,etc.,ingrowingharmonywiththeprinciplesofGreenChemistry,andmorebroadlyGreenEngineeringandTechnology.
TheBook“2DFunctionalNanomaterials”summarizesthescientificcontributionsoftheadvancementinfabricationsandapplicationsof2Dnanomaterials, reportedfromdifferentveinsofchemistryandtheotherrelatedtechnologiesover thelastdecade.Thecontributionshavebeenmadebydifferentresearchersand distinguishedscientistsfromallovertheworld.Thebookincludedthevarious2D nanomaterialsandtheiruniquepropertieswiththeirwiderangeofapplications. Thechaptersincludevariousmostup-to-dateandnoteworthytopicssuchas synthesisandpropertiesofgraphene;grapheneoxide-basedLDHnanocomposite; graphene-latexnanocomposites;novel2Dnanomaterials;2Ddichalcogenides;2D metal–organicframeworksanditsvariousapplicationssuchascancertherapy; energystoragedevices;photocatalysis–photoelectrocatalysisandelectronicdevices andalotsmore.Inresponsetotheincreaseddemandsforthevariousapplications, thisbookisdesignedtoupdatethelatestadvancementsintheresearchand developmentofnanomaterialswithfocusonthetechnologiesforthesynthesisand characterizationof2Dmaterials.Thisbookiscontributedbyagroupofleading
scientists,researchers,andprofessorswhoaredirectlyworkingonthesubjected areas.Allchaptersincludefundamentalsofthe2Dnanomaterialsinvestigated thatwillofferstudents,researchers,andacademicianstheopportunitytoevaluateandselectthetechnologiesthatleadtobenefitrelatedtotheapplicationof nanotechnologiesforcleanenergyandenvironmentconservation.
Webelievethatthisbookisextremelyusefulfortheresearcherswhoworkinthe 2Dnanomaterialapplicationsandalsoservesanexcellenttextbookandreference fortheresearchscholars,college/universityundergraduates,andgraduateswhoare interestedintheareasofmaterials,energy,andcancertherapyapplications.
IamindebtedtomyPh.D.ResearchGuideDr.MansingA.Anuse,Ex.Professor, andHeadofDepartmentofChemistry,ShivajiUniversity,Kolhapur,forhisconstant encouragementandsupport.IamthankfultomySummerResearchFellowship mentorProfessor,SrinivasanKannan,Director,CouncilofScientificandIndustrial Research-CentralSaltandMarineChemicalsResearchInstitute(CSIR-CSMCRI), Bhavnagar,Gujarat,India,forencouragement.IamgratefultomyPostDoctoral ResearchAdvisorProfessor,Yong-ChienLing,DepartmentofChemistry,National TsingHuaUniversity,Taiwan,forhisongoingsupportandencouragement.
IamalsohighlygratefulandIdedicatethisbooktoProf.C.N.R.Rao,Director, JawaharlalNehruCentreforAdvancedScientificResearch(JNCASR),Bangalore, India,bywhomIreallyinspiredofhisdistinguishedcontributioninChemistry.
IamalsogratefultoProf.YuryGogotsi,Director,A.J.DrexelNanomaterials Institute,DrexelUniversity,Philadelphia,Pennsylvania,USA,forgivingavaluable “Foreword”forthisbook.
Iwouldliketoexpressmygratitudetoallchapterauthorsfortheirenthusiasticand collaborativecontributions.Itcouldnotbepossibletoundertakethischallenging workandenablingmetoaccomplishitwithouttheirsupportandtimelyresponse. Allthecontributorshavejustifiedtheirinvolvementinthisbookbypresentingthe workonlatestareasof2Dnanomaterialsandtheirapplications.
IamverymuchthankfultotheWiley-VCHforacceptingourbookproposaland IalsowishtosincerelythankDr.LifenYang,ProgramManager,andMs.Katherine Wong,SeniorManagingEditoroftheWiley-VCH,fortheirkindsupport,valuable suggestions,motivation,andco-operationduringthepreparationofthisbook,withoutwhichitwouldhavebeenextremelydifficulttocompletethistaskontime.I admiretheirdistinguishedhelpingnature.
ItgivesmegreatpleasuretoacknowledgeShivajiUniversity,Kolhapur,andKolhapurInstituteofTechnology’s,CollegeofEngineering(Autonomous),Kolhapur, fortheirencouragement.
Mostofall,Icouldnotpossiblyfinishwithoutthankingmyfamilyfortheirpersistentlove,continuoussupport,andforbearanceduringthewritingofthisbook.They havebeenaconstantsourceofinspiration.Ialsothankmywonderfulson“Takshil” foralwaysmakingmehappyandforcooperatingmewiththeunderstandingwhen Iwasworkingforthisbookinsteadofplayinggameswithhim.Ihopethatinfuture hewillreadthisbookandrealizewhyIusedtosparesomuchtimeinfrontofmy computer.
Preface xxiii
Asaneditor,Iwouldliketoreceivesuggestionsandopinionforthebookat ganeshchemistry2010@gmail.com
14April,2021
Dr.GaneshS.Kamble DepartmentofEngineeringChemistry KolhapurInstituteofTechnology CollegeofEngineering Kolhapur416234,India
GrapheneChemicalDerivativesSynthesisandApplications: State-of-the-ArtandPerspectives
MaximK.Rabchinskii 1 ,MaksimV.Gudkov 2 ,andDinaYu.Stolyarova 3
1 IoffeInstitute,CentreofNanoheterostructurePhysics,26Politekhnicheskaya,194021SaintPetersburg, Russia
2 RussianAcademyofSciences,N.N.SemenovFederalResearchCenterforChemicalPhysics,Departmentof PolymerandCompositeMaterials,4KosyginaStreet,Building1,119991,Moscow,Russia
3 NRC“KurchatovInstitute”,ComplexofNBICSTechnologies,1AkademikaKurchatovapl.,123182,Moscow, Russia
1.1Introduction
Afteritsrediscoveryin2004byGeimandNovoselov[1],graphenehasprovided astunningscientificandtechnologicalexcitementforresearchersinvarious disciplines,becomingthefirsttwo-dimensional(2D)crystalbroadlystudied[2,3]. However,afterthefirstdecadeofextensiveresearch,anexplosionofinteresthas beenfollowedbythefadeofenthusiasm.Suchashiftisduetoseveralreasons. Firstly,alargesetofnew2Dmaterials,suchashexagonalboronnitride(h-BN), molybdenumdisulfide(MoS2 ),silicene,etc.,hasbeenisolatedandwidelyinvestigated,demonstratingtohavenewunprecedentedproperties,differingfromthose ingraphene[4,5].Secondly,despitealargenumberoftechniquesdevelopedfor thegraphenesynthesis,ithasappearedthattransitionfromobtainingasingleideal laboratorysampletolarge-scaleproductionofhigh-qualitygraphenestillexhibiting itsuniquepropertiesisamajorchallenge,stillnotresolved[6].Thislimitstherealizationoftheproposedrevolutionaryapplicationsofgraphene.Finally,thanksto greatattentionattractedatthestart,duringalmosttwodecadesofgraphenehistory thekeyfeaturesofitsphysicshasbeenthoroughlystudied,leaving,asseemed,only detailsandquestionsofitsfurtherintegrationintopracticalapplications.
Giventhesefacts,themainfocuswithinthefieldofgrapheneresearchhasprogressivelyshiftedtoanotherkeyfeatureofthismaterial–versatilechemistryand itsinfluenceonthepropertiesofgraphene.Apartfrombeinga2Dcrystalderived fromthegraphitestructure,grapheneatthesametimerepresentsaquasi-infinite π-conjugatedpolyaromatichydrocarbonmacromolecule[7].Thismeansgraphene canundergoalargesetofchemicalreactionsfromorganicsynthesisandchemistry ofaromaticcompounds,eventhoughseverallimitationsduetothedifferencein theoverallreactivityhavetobeconsidered.Usingthesereactions,variousorganic
2DFunctionalNanomaterials:Synthesis,Characterization,andApplications,FirstEdition. EditedbyGaneshS.Kamble. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
1GrapheneChemicalDerivativesSynthesisandApplications:State-of-the-ArtandPerspectives groups,containinghalogens(Br,Cl,F,I),chalcogens(O,S),pnictogens(N,P, Sb,Bi),orsiliconaswellasmorecomplexfunctionalities,suchasalkylandaryl hydrocarbons,canbecovalentlyattachedtoedgesorbasalplaneofgraphene[8,9]. Furthermore,substitutionalmodificationofgraphene,duringwhichcarbon atomsarereplacedbyatomslikenitrogen,phosphorous,sulfur,orboron,canbe performedaswell(dopingprocess)[10].
Inturn,theintroductionoffunctionalgroupsordopingprovidesthereconfigurationofthe π-conjugatedelectroncloudduetothepartialconversionof sp2 -hybridizedcarbonatomsintosp3 -hybridizedones,aswellastotheelectronwithdrawingorelectron-donatingeffectofthemodifyingmoieties[11].Accordingly,anintriguingopportunitytotailortheelectronicstructureofthegraphene layerand,thus,itsphysicalandchemicalproperties,arises.Graphenetransport characteristicsandbandstructure,opticalabsorptionandluminescence,field emission,magnetic,andthermalpropertiescanbetunedinadesirablemannervia modificationofgraphenechemistry[8,9,12].Inotherwords,chemicalderivatizationallowstorenderthephysicsofgraphenesignificantlymorevariable,altering theintrinsicpropertiesofgrapheneandgivingrisetonewinspiringfeatures,not observableinpristinegraphene.Theintroductionoforganicgroupstographene edgesorbasalplanealsoprovidesanopportunitytocontrollablymodifythe reactivityofmaterial,itschemiresistive,andwettingproperties[13].Thisextends theperformanceofgrapheneinvariousapplicationsduetotheadjustmentofits characteristicsforcertainconditions.
Hence,itisnotasurprisethatalargefamilyofgraphenechemicalderivatives, chemicallymodifiedgraphenes(CMGs)orfunctionalizedgraphenes(FGs),has appearedduringthelastdecades,displacingpristinegraphenefromthepositionof acentralresearchsubjectwithinthefieldofnanocarbonscience.Stoichiometric CMGs(C1 X1 ,whereX–modifyingfunctionality),suchasfluorographeneand graphane,havebeensynthesizedaswellasalargenumberofnon-stoichiometric (C1 X<1 )graphenederivativeshasbeensynthesized.Speakingaboutgraphenefunctionalization,onecannotcircumventgrapheneoxide(GO)–graphenelayer,which basalplaneandedgesarecovalentlyfunctionalizedwithasetofoxygen-containing groups[14].Startingasjustaprecursorforthegraphenesynthesis,GOpromptly appearedtobeafacilestartingplatformfortheCMGsynthesis.Thisfeature arisesfromtheeaseoflarge-scaleproductionofGOvialiquid-phaseexfoliationof graphiteandthepresenceofoxygenicgroups[15],whichcanbesubstitutedwith otherorganicgroupsviasimplemethodsfromorganicchemistry.Thisisincontrast tothefunctionalizationofpristinegraphene,whichrequiresspecialreactivespecies thatcanformcovalentadductswiththesp2 carbonstructuresingraphene[16].
Asaresult,themodificationofGOhasgivenrisetonumerousCMGsandupto dateisakeyapproachforthesynthesisofthefunctionalizedgraphenes[8].
Despitetheprogressmadewithinthefieldofgraphenederivatization,theresearch onCMGssynthesisandcharacterizationisstillfarfromover.Thedetailedunderstandingontheeffectsofmodifyingfunctionalitiesonthephysicalpropertiesof CMGsstillnotachieved[17].Particularly,theoriginofopticalabsorbanceorfluorescenceinGOorfluorographeneisstillunderdiscussionwithmanycontroversial
1.2GrapheneOxide:SynthesisMethodsandChemistryAlteration 3
modelssuggestedtoexplaintheparticularroleoffunctionalizationparametersin thisquestion[18,19].NewmethodsforthesynthesisofnewstoichiometricCMGs apartfromfluorographeneandgraphaneaswellasforthepreciseintroductionof asinglecertaintypeoffunctionalgroupinthedesiredconcentrationarestillunder development.Thissectionsummarizesandcomparestherecentresultswithinthe fieldofgraphenechemicalderivatives,startingfromfeaturesofGOsynthesisand concludingbysomeremarksontheperspectivesofCMGsapplications.
1.2GrapheneOxide:SynthesisMethodsandChemistry Alteration
ThehistoryofGObeganlongbeforetheterm“graphene”wasproposed.Itstarted withgraphiteoxide,thesynthesisofwhichwasproposedbackin1859byBrodie[20]. Theproductwasnamedgraphiticacidorgraphiteoxideandwasmoderatelystudied forover150years[21].Theboomaroundgrapheneamongotherthingshasrevived theinterestofthescientificcommunitytographiteoxide.Itwasdiscoveredthatupon anincreaseinpHfromacidictoneutralgraphiteoxideexfoliatestomonolayerGO sheetsduetotheCoulombrepulsionofsimilarlychargeddissociatingfunctional groups.
HereitmustbeemphasizedthatGOandgraphiteoxidearedistinctmaterials, althoughthesenamesareoftenusedassynonyms.Theformeroneisasingle graphenelayercovalentlymodifiedwithoxygenmoieties,whereasthelatterone isastackofnon-exfoliatedGOsheets.Generallyspeaking,thenomenclaturefor two-dimensionalcarbonsandCMGsisstillnotalignedwell,withimproperand arbitrarynamingofgraphenederivatives.Thistopicisfinelydiscussedbythe Carbon editorialboardintherecentarticlebyBiancoetal.[22].
GOisanon-stoichiometricgraphenederivativewithageneralformulaofthetype Cx Hy Oz withtheamountofhydrogenonaverageestimatedas y = 0.8,whereasthe carbon-to-oxygenratio(so-calledoxidationdegree,C/O)canvaryfrom1.5to2.5 [23].GOcarriesalargesetofoxygen-containingfunctionalgroups,suchashydroxyls,epoxides,carboxyls,carbonyls,esterandlactolstructures,peroxides,etc.Numerousmodels,rangingfromtheHofmannandRuessmodelstoLerf–Klinowskiand Dekanyones[24,25],havebeenbeingprogressivelydevelopedtodescribethechemistryofGO,reflectingtheevolutionofthesynthesisprotocolsandmethodsapplied forthestudyingofGO.Thisprocesshaspointedoutthattheoxidationdegree,defectivenessofgraphenelayer,andthespecificcompositionofoxygenicgroupscanbe controlledbythechoiceofacertainmethodofsynthesisandparameterswithinit.
Particularly,theBrodiemethodbasedontheheatingofgraphiteat60 ∘ Cforfew days(threetofourdays)inthemixtureofKClO3 andHNO3 resultsinGOwithahigh C/Oratioofapproximately2.5–2.7withanintroductionofN-containingfunctionalities,particularlynitrates.ApplicationofthemodificationsoftheBrodiemethod byStaudenmaier(additionofconcentratedH2 SO4 andportionedintroductionof KClO3 )andHofmann(theuseofnon-fumingnitricacid)resultsinanalmostequal C/Oratioandfunctionalizationpredominantlybybasal-planeepoxideswithlow
1GrapheneChemicalDerivativesSynthesisandApplications:State-of-the-ArtandPerspectives contentofedge-locatedcarboxylsandcarbonyls(lessthan1at.%)[26,27].Atthe sametime,theelementalanalysisoftheGOsynthesizedbythesemethodshasindicatedtheabsenceofsulfurandnitrogenspeciesinthematerial.
Higheroxidationdegreeandintroductionofasubstantialamountofcarboxyland carbonylsisachievedbytheGOsynthesisviathemethodproposedbyHummers andOffermanwiththereplacementofKClO3 andfumingHNO3 byKMnO4 and KNO3 inconcentratedH2 SO4 [28].ThishasallowedtoreducetheC/Oratioof thesynthesizedGOtotwoandlesswithsimultaneousincreaseinthecontentof carbonyls/carboxylsbyalmostthreetimestoapproximately3–4at.%[29].However, theHummersmethodalsoresultsintheappearanceofthetracesofsulfurand nitrogeninGO(upto6at.%)[30],likelytobeduetothecovalentlybondedsulfates andnitratesoradsorbedsulfuricandnitricacids[31].Thiseffect,despiteintuitive beingadisadvantagefortheGOsynthesis,canbeappliedforthedopingand chemicalmodificationofGO,whichwillbediscussedfurther.
Noteworthy,inthecaseoflargegraphiteflakes >50 μm,theproductofthe Hummersmethodinevitablycontainsunder-oxidizedparticleswithagraphitecore andanoxidizedshell,carryingoxygenicgroups.Moreover,thematerialsproduced byBrodie,Staudenmaier,orHummersmethodshaveshownadefectivestructureof thegraphenelayerwiththepresenceofholes,rips,andwrinklesduetotheoxidation reaction[32].AsolutionfortheseproblemswasforthefirsttimeproposedbyKovtyukhovabasedonthepre-expansionofgraphiteinaconcentratedH2 SO4 ,K2 S2 O8 , andP2 O5 forseveralhoursat80 ∘ S [33].Thisincreasedtheyieldofthefullyoxidized graphiteflakes.AnotherapproachwasproposedbyMarcanoetal.[15],which hasbecomethemostpopularamongothervariationsoftheHummersmethod andisknownasaTourmethod.TheauthorsproposedusingamixtureofH2 SO4 andH3 PO4 (9:1)insteadofconcentratedsulfuricacidaswellassixequivalentsof KMnO4 insteadofthreeaccordingtothestandardHummersprocedureandexcludingNaNO3 fromthereaction.ThishasresultedinthefurtherreductionoftheC/O ratioto1.7–1.9,anincreaseintheconcentrationoftheedge-locatedoxygenmoieties (carbonyls/carboxyls)[29],andamoreregularstructurewithfewerdefectsinthe basalplanecomparedtotheothermethods.However,thevolumeofacidsrequired fortheprocedureaccordingtotheproposedmethodisincrediblyhigh(400mlper 3gofgraphitevs.69mlinthestandardHummersmethod),whichisasignificant drawbackthathinderstoapplythisprocessforthemassproductionofGO.
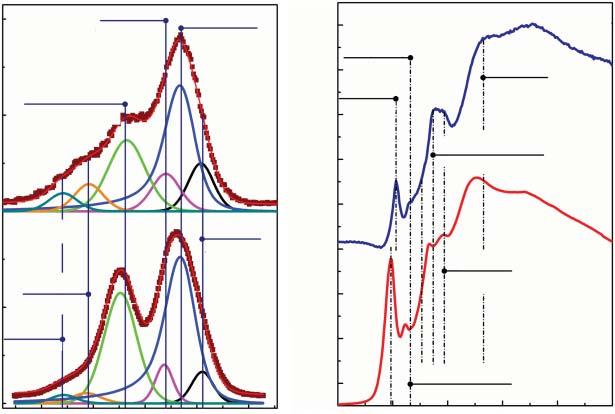
Substantialalterationintherelativecontentofbasal-planeandedge-locatedoxygenicgroupscanbeachievedbythegraphiteoxidationusingK2 Cr2 O7 intheH2 SO4 solutioninthepresenceofNaNO3 firstproposedin2010[34].Itturnedoutthatthe GOobtainedbythismethodhasalowoxidationdegree[35,36],C/O = 2.8–3.1, duetotheminornumberoftheintroducedbasal-planehydroxylsandepoxides. Atthesametime,theoverallconcentrationsofcarboxylsandcarbonylsappeartobe higherthanthoseintheGOproducedbytheconventionalHummersorTourmethods,reachingupto7at.%aswasdemonstratedbyX-rayphotoelectronandX-ray absorptionspectra(Figure1.1).Nevertheless,thematerialisfairlystableinaqueoussolutions,andGOplateletshavealateralsizeof10–100 μm.Asaresult,this methodprovidesanintriguingopportunitytoobtainpartiallyoxidizedGOwitha
290288286284282
280285290295300305
Figure1.1 (a)C1sX-rayphotoelectronand(b)CKedgeX-rayabsorptionspectraofGO samples,synthesizedviaHummersandK2 Cr2 O7 oxidationmethods.
highnumberofchemicallyreactivecarboxylgroupswithouttheneedforadditional stepsofGOtreatment.Furthermore,itcanbesuggestedthatoxidationofgraphite withthemixofK2 Cr2 O7 andKMnO4 introducedindifferentrelativeconcentrations canbeperformed,allowingonetomanagetheoxidationdegreeofGOand,more importantly,therelativecontentofcarboxyls,carbonyls,andhydroxyls/epoxides. Besidesthechoiceofthespecificmethodofgraphiteoxidation,thechemistryof GOcanbetunedalsobytheadjustmentofthewateramount,temperatureanddurationofthereaction,typeofthegraphiteused,andexclusionofacertainreagent. Chenetal.haveshownthatacarefulcontrolofthewateraddedduringtheoxidationallowsonetomodulatethecontentofhydroxylandepoxidegroupsonGOsheets withoutsacrificingtheirstructuralintegrity[37].Atthesametime,theincreaseof thetemperatureupto95 ∘ Cinthelackofwaterresultsintheselectiveformation ofcarboxylgroups.Recently,wehaveshownthattheincreaseintheintercalation timeinthemodifiedHummersmethodfromseveralhourstoaboutaweekwith theuseofNaNO3 leadstofirstnitrationandthendopingofGOwithintroductionofupto5at.%ofnitrogen,predominantlyinthegraphiticform[38].Atthe sametime,covalentattachmentofsulfuricacidestersduringtheHummersoxidationwiththeirretentionuponthewashinginorganicsolventswasdemonstratedby Dimievetal.[39]andfurtherdiscussedbyGrovemanetal.[40].Thisdemonstrates thatapartfromoxygenicgroups,otherfunctionalgroupscanbeintroducedtoGO duringitssynthesisforvariouspracticalpurposeseventhoughatfirstsighttheycan beregardedascontaminatingimpurities.
Nevertheless,despitetheseachievements,GOsynthesisviavariousmethodsof liquid-phaseexfoliationgivesonlylimitedopportunitiesintheadjustmentofthe
1GrapheneChemicalDerivativesSynthesisandApplications:State-of-the-ArtandPerspectives chemistryofGOanditsderivatives.Theexactprinciplesandmechanismslying behindthegraphiteoxidationarestillnotwellunderstoodandareunderdiscussion, restrictingthedevelopmentofnewstrategiesfortheGOsynthesiswitharequired setofoxygenicorotherorganicgroups[41].Giventhis,electrochemicalsynthesis ofGOhasbeguntoberegardedasafacilestrategyforthispurpose.Electrochemicallysynthesizedgrapheneoxide(EGO)havingaC/Oratioof1.7,lowerthanthat inGOobtainedvialiquid-phaseexfoliation,canbeobtainedinhighyieldwithfew defects[42,43].However,thepoweroftheelectrochemicalsynthesismethodsisnot onlytheproductionofhighlyoxidizedGObutthesynthesisofvariousfunctionalizedgraphenelayers.Owingtothepossibilityofusingvariouselectrolytes,such asammonium,chloride,hydroxide,phosphate,nitrate,bromicandchloricacid, etc.,onecanobtaingraphenelayersfunctionalizedbynitrogen,sulfur,orhalogen (Cl,Br,I)containingorganicgroups.Moreover,onceobtainedEGOcanbeagainsubjectedtotheelectrochemicaloxidationorreductionusinganotherelectrolyte,allowingcomplexfunctionalizationofthegraphenelayerwithvarioustypesoforganic moieties.Thankstothisandsimplicityofthemethod,thepossibilityofre-usingof theelectrolytes,andabsenceofnecessityofexcessivewashing,theelectrochemical methodsareregardedasanextstepinthelarge-scalesynthesisofGOandCMGs withacontrollablechemistry[44].
1.3GrapheneOxideReductionandFunctionalization
TheveryfirststrategyproposedforthefurtherGOprocessingwasitsconversion intopristinegraphenebytheso-calledreductionprocess–eliminationofalloxygenicgroupstorestorethe π-conjugatedgraphenenetwork.Expectationswerethat suchanapproachwouldbeafacileroutetolarge-scaleandlow-costproductionof graphenewithdozensofvariousreductionmethodsproposed[23,45].However, theobtainedmaterial,reducedgrapheneoxide(rGO),appearedtobefarfromboth thetheoreticallyinvestigatedidealgrapheneandpristinegraphenesynthesizedvia mechanicalcleavageofgraphiteorchemicalvapordeposition(CVD)method[46]. Thisisduetotheincompleteremovaloftheoxygenicgroupsandtheintroduction ofstructuraldefectsduringthereductionprocesswhichevermethodisapplied.The contaminationofGOwiththeresidualsofthereducingagentappearedtobeanother significantproblem.ThetypicalvaluesoftheC/OratioreachedupontheGOreductionareabout12–40[23],typicallyoneorderofmagnitudelowercomparedtoCVD graphene.TheonlymethodforGOreductiondemonstrateduptodatetoprovidethe C/O > 75andaminornumberofstructuraldefectsishigh-temperatureannealing at1000–1100 ∘ Cintheinertatmosphere–aprocesscomparablebyitscomplexity andyieldwithCVDgrowthofgraphenewiththestillworsestructuralqualityofthe material.Apparently,theGOreductionhasentrenchedinthegraphenecommunity asasecondratemethodtomanufacturegrapheneforseveralapplicationswhere thequalityofgrapheneisnotthedeterminingfactor.Despitealargenumberofnew eco-friendly,facile,andhigh-yieldmethodsforGOreductionarestillbeingdevelopeduptodate,thisstrategyforthesynthesisofpristinegraphene,demonstrating suchdesiredphysicalproperties,becomesregardedasadeadend.
However,allchangesifweproceedfromconsideringGOreductionasjustaroute toobtainpristinegraphenetoregardingitasaversatilemethodforthesynthesis ofgraphenelayerfunctionalizedwithvariousorganicgroups.Withinthisconcept, theinitialpresenceofoxygenicgroupsinGO,theirtendencytoretainorbeingsubstitutedbyothermoieties,andincorporationofotherelementsintothegraphene networkuponreductiondrasticallytransformfromaproblemintoanadvantageous feature.ThegoaloftheGOprocessingshiftstothealterationofthetype,number,andspatialdistributionofmodifyingmoieties,takingthecontrolonthe π-conjugationrateandlocalelectronicstructureofgraphenelayersalongwiththeir chemicalreactivity(Figure1.2)[8,9,11].Theintroductionofdefectsalsocanbe translatedintoabeneficialfeaturewithinthisconcept,owingtotheirinfluenceon theelectronicstructureandpropertiesofgraphene.
Note,GOinheritsthisversatilityinfurthermodificationfromitsinitialchemistry, whereaspristinegrapheneischemicallyinert,withchemicalmodificationrequiring eithercomplicatedradiativemethodsorradicalchemistry[16,47].
TwogenerallinescanbedistinguishedintheconversionofGOintoCMGwith thecovalentlyattachedfunctionalgroups:(i)selectivereductionoroxidationof GOwiththeretentionorincreaseinconcentrationofacertainoxygenicgroup; (ii)substitutionalmodificationofGOwiththeremovalandpartialreplacement oftheoxygen-containingfunctionalitiesbyotherorganicgroups.Synthesisof carboxyl-richCMGsiswidelydisseminatedwithintheselectiveGOreduction. Suchaninterestarisesfromthewell-establishedchemistryofcarboxylicacid derivativesinorganicsynthesisandefficiencyofcarboxylsasanchoringpoints forthefurthercovalentmodificationwithbiomolecules,polymers,etc.Typically, selectivereductionofGOwiththepreservationofcarboxylscanbeperformedby low-temperatureannealingat150–200 ∘ C,whichislowerthanthetemperature
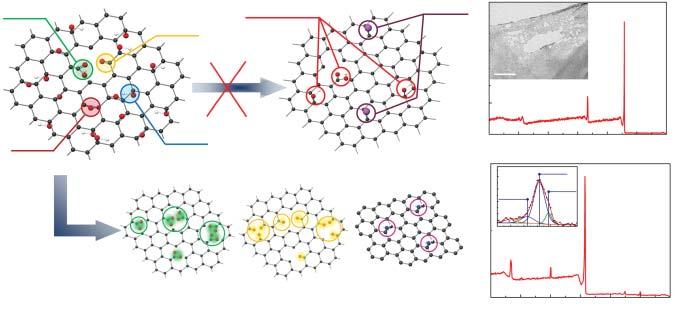
Figure1.2 TwostrategiesfortheGOprocessing:reductionandfunctionalization.Inthe formercase,thedesiredpristinegraphenecannotbeobtainedduetotheretainedoxygenic moieties,contaminationofgraphenelayer,andformationsofdefects.Oppositely,these featuresbecomeadvantageousinthecaseofgraphenefunctionalizationtosynthesize graphenechemicalderivatives.Source:AdaptedfromRefs.[8,9,11].
8
1GrapheneChemicalDerivativesSynthesisandApplications:State-of-the-ArtandPerspectives oftheirdissociation[48]or,chemically,byusingNaBH4 [49],thioureadioxide, orhydrazine[50,51].However,theconcentrationofcarboxylsintheinitialGOis low,varyingfrom2to4at.%withtheonlyexceptionofGOsynthesizedviaaforementionedoxidationwithpotassiumdichromate,whichcontainsupto6at.%of carboxyls[29,35].Thus,reductionmethodsthatadditionallyincreasethenumber ofcarboxylsareactivelydevelopednowadays,althoughonlyfewwerepresented. Pumeraetal.havesynthesizedcarboxyl-enrichedgraphenefromGOusingthe Kolbe–Schmittreaction[52],resultinginupto11at.%ofcarboxylgroupswitha negligibleamountofotheroxygenicgroups.Anotherapproachwassuggestedby Jankovskyetal.byrepetitiveoxidationofGOwithpotassiumpermanganatein anacidicenvironment[53].Theappliedmethodallowstoreachthecontentof carboxylsofupto11–15at.%,althoughalargenumberofhydroxylsarealsopresent inthematerial.Acarefultreatmentwithacombinationofhydrogenperoxideand ammoniaoranotherbasecanbeappliedtoobtaincarboxylatedgraphene,although thismethodalsoprovidescuttingofgraphenelayertonanoflakes[54].Apartfrom theliquidchemistrymethods,thephotochemicalsynthesisofthecarboxylated graphenecanbeperformed.Particularly,wehaveshownthatconcentrationof carboxylscanbeengineeredwithintherangeof3–11at.%withsimultaneous reductionofbasal-planegroupsviatheUVirradiationofGOfilmsintheargon atmosphere(Figure1.3)[55,57].Theproposedmethodiscompatiblewiththe lithographytechniques,butitsyieldissufficientlylowercomparedtoliquid-phase carboxylationprotocols.
Lessattentionisgainedtothecarbonylatedgraphenederivatives,although carbonylgroupscanactascentersforthenon-covalentcoordinationbondingandcontributetothereversiblepseudocapacitanceorbondingofmetal cations[58–60].Carbonylsaremorestablethancarboxyls,andbasal-plane
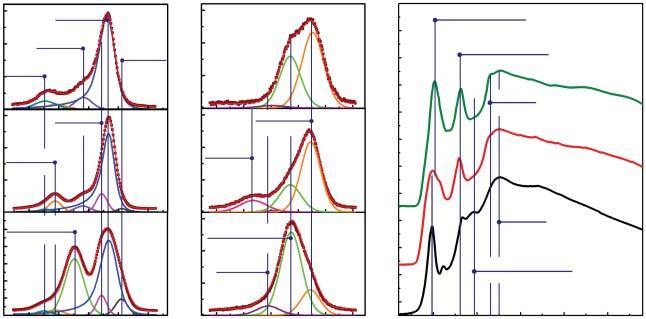
Figure1.3 (a)C1s,(b)O1sX-rayphotoelectron,and(c)CKedgeX-rayabsorptionspectra oftheinitialGO,carbonylatedgraphene(C-ny),carboxylatedgraphene(C-xy)synthesized accordingtothemethodsdescribedinRefs.[55,56].Source:AdaptedfromRabchinskii etal.[55,56].
functionalities[48]easilyretainintheGOuponitsreductionviasoftreducing agentsorlow-temperatureannealing.Theproblemis,analogouslytothecaseof carboxyls,thelowinitialcontentofcarbonyls–ofabout2–3at.%.Toovercome this,GOreductionmethods,inducingtheeliminationofhydroxylsaccordingto theE1cBmechanismwiththeprogressiveformationofcarbonyls,canbeapplied. Recently,wehaveshownthatsuchaprocessproceedsinthecaseofGOreduction usingsodiumsilicate[61],resultinginthecarbonylatedgraphenewithupto9at.% ofcarbonyls(Figure1.3).Almostatthesametime,Yuetal.havepresentedan alternativewaytosynthesizecarbonylatedgraphenecoveredbyupto6at.%via theGOtreatmentwiththediluteH3 PO4 andNaH2 PO4 solutions[62].Inboththe synthesizedCMGs,carbonylisthepredominantoxygenicgroup,withnegligible contentofotherfunctionalities.
CarboxylatedandcarbonylatedCMGsarerelativelystablegraphenederivatives regardinglow-temperatureheating,andotherexternaleffectsmaybeappliedduring theirapplication.However,thedrawbackiseitherperforationofthegraphenelayer ordisruptionofgrapheneflakestosmalleroneduringthesynthesissincecarboxyls andcarbonylslocateattheedgesofthegraphenenetwork(Figure1.4),requiring theincreaseofitsextenttoappearinlargenumbers[54,55,57].Carboxylscanbe attachedtotheGObasalplaneaswell[63],butinalowernumberandbeingless stable.Thus,structuralmodificationofthegraphenelayeruponcarboxylationand carbonylationandcorrespondingalterationofthegraphenechargetransportmust beconsidered.Ontheotherhand,performedinacontrollableway,thisfeature expandstheapplicationofCMGswithinthefieldofdesalination,considering theperforatedstructureofcarboxylated/carbonylatedgraphenewithchemically activeedgesofholes.
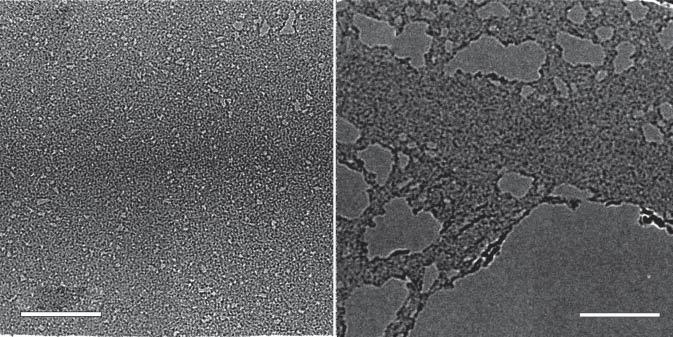
ApartfromthesetwooxygenicCMGs,preferentialfunctionalizationbyhydroxylsorepoxidesisdemonstratedtobeperformed.Onesuchtypeofreactionis hydroborationwhichcangiveGOwithanalmoststoichiometriccomposition (a) 100 nm50 nm (b)
Figure1.4 Transmissionelectronmicroscopyimagesofthe(a)carbonylatedgrapheneand (b)carboxylatedgrapheneplateletsobtainedaccordingtothemethoddiscussedinRefs. [55,56].Source:Rabchinskiietal.[55,56].