Visit to download the full and correct content document: https://ebookmass.com/product/novel-electrochemical-energy-storage-devices-materi als-architectures-and-future-trends-1st-edition-feng-li/

More products digital (pdf, epub, mobi) instant download maybe you interests ...

Dielectric Materials for Energy Storage and Energy
Harvesting Devices Shailendra Rajput & Sabyasachi
Parida & Abhishek Sharma & Sonika
https://ebookmass.com/product/dielectric-materials-for-energystorage-and-energy-harvesting-devices-shailendra-rajputsabyasachi-parida-abhishek-sharma-sonika/

Transition Metal Oxides for Electrochemical Energy Storage Jagjit Nanda
https://ebookmass.com/product/transition-metal-oxides-forelectrochemical-energy-storage-jagjit-nanda/

Nanowire Energy Storage Devices: Synthesis, Characterization and Applications Liqiang Mai
https://ebookmass.com/product/nanowire-energy-storage-devicessynthesis-characterization-and-applications-liqiang-mai/

Energy Storage Devices for Renewable Energy-Based Systems: Rechargeable Batteries and Supercapacitors 2nd Edition Nihal Kularatna
https://ebookmass.com/product/energy-storage-devices-forrenewable-energy-based-systems-rechargeable-batteries-andsupercapacitors-2nd-edition-nihal-kularatna/




Advances
in Supercapacitor and Supercapattery: Innovations in Energy Storage Devices Mohammad Khalid
https://ebookmass.com/product/advances-in-supercapacitor-andsupercapattery-innovations-in-energy-storage-devices-mohammadkhalid/
Nanostructured, Functional, and Flexible Materials for Energy Conversion and Storage Systems 1st Edition Alagarsamy Pandikumar
https://ebookmass.com/product/nanostructured-functional-andflexible-materials-for-energy-conversion-and-storage-systems-1stedition-alagarsamy-pandikumar/
Emerging 2D Materials and Devices for the Internet of Things: Information, Sensing and Energy Applications (Micro and Nano Technologies) 1st Edition Li Tao (Editor)
https://ebookmass.com/product/emerging-2d-materials-and-devicesfor-the-internet-of-things-information-sensing-and-energyapplications-micro-and-nano-technologies-1st-edition-li-taoeditor/
Energy Storage Systems: System Design and Storage Technologies Armin U. Schmiegel
https://ebookmass.com/product/energy-storage-systems-systemdesign-and-storage-technologies-armin-u-schmiegel/

Thermoelectric Materials and Devices Lidong Chen
https://ebookmass.com/product/thermoelectric-materials-anddevices-lidong-chen/

NovelElectrochemicalEnergyStorage Devices
Materials,Architectures,andFutureTrends
FengLi
LeiWen
Hui-mingCheng
Authors
FengLi InstituteofMetalResearch
ChineseAcademyofSciences 72WenhuaRoad Shenyang110016 China
LeiWen InstituteofMetalResearch ChineseAcademyofSciences 72WenhuaRoad Shenyang110016 China
Hui-mingCheng InstituteofMetalResearch ChineseAcademyofSciences 72WenhuaRoad Shenyang110016 China
Cover CoverImage:©TarikVision/Getty Images
Allbookspublishedby Wiley-VCH arecarefullyproduced.Nevertheless, authors,editors,andpublisherdonot warranttheinformationcontainedin thesebooks,includingthisbook,to befreeoferrors.Readersareadvised tokeepinmindthatstatements,data, illustrations,proceduraldetailsorother itemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>.
©2021WILEY-VCHGmbH,Boschstr. 12,69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc. usedinthisbook,evenwhennot specificallymarkedassuch,arenotto beconsideredunprotectedbylaw.
PrintISBN: 978-3-527-34579-3
ePDFISBN: 978-3-527-82104-4
ePubISBN: 978-3-527-82106-8
oBookISBN: 978-3-527-82105-1
Typesetting SPiGlobal,Chennai,India PrintingandBinding
Printedonacid-freepaper 10987654321
Contents
Preface xiii
Abbreviations xv
1Introduction 1
1.1EnergyConversionandStorage:AGlobalChallenge 1
1.2DevelopmentHistoryofElectrochemicalEnergyStorage 3
1.3ClassificationofElectrochemicalEnergyStorage 4
1.4LIBsandECs:AnAppropriateElectrochemicalEnergy Storage 6
1.5SummaryandOutlook 10 References 10
2MaterialsandFabrication 15
2.1MechanismsandAdvantagesofLIBs 15
2.1.1Principles 15
2.1.2AdvantagesandDisadvantages 16
2.2MechanismsandAdvantagesofECs 18
2.2.1Categories 18
2.2.2EDLCs 18
2.2.3Pseudocapacitor 20
2.2.4HybridCapacitors 21
2.3RoadmapofConventionalMaterialsforLIBs 22
2.4TypicalPositiveMaterialsforLIBs 23
2.4.1LiCoO2 Materials 23
2.4.2LiNiO2 andItsDerivatives 25
2.4.3LiMn2 O4 Material 26
2.4.4LiFePO4 Material 27
2.4.5Lithium–Manganese-richMaterials 28
2.4.6CommercialStatusofMainPositiveMaterials 28
2.5TypicalNegativeMaterialsforLIBs 29
2.5.1Graphite 29
2.5.2SoftandHardCarbon 31
2.6NewMaterialsforLIBs 33
2.6.1NanocarbonMaterials 33
2.6.2Alloy-BasedMaterials 35
2.6.3MetalLithiumNegative 39
2.7MaterialsforConventionalECs 39
2.7.1PorousCarbonMaterials 40
2.7.2TransitionMetalOxides 41
2.7.3ConductingPolymers 42
2.8ElectrolytesandSeparators 42
2.8.1Electrolytes 42
2.8.2Separators 45
2.9EvaluationMethods 46
2.9.1EvaluationCriteriaforLIBs 46
2.9.2TheoreticalGravimetricandVolumetricEnergy Density 46
2.9.3PracticalEnergyandPowerDensityofLIBs 47
2.9.4CycleLife 48
2.9.5Safety 48
2.9.6EvaluationMethodsforECs 49
2.10ProductionProcessesfortheFabrication 50
2.10.1Design 50
2.10.2Mixing,Coating,Calendering,andWinding 51
2.10.3ElectrolyteInjectingandFormation 51
2.11Perspectives 51 References 53
3FlexibleCells:TheoryandCharacterizations 67
3.1LimitationsoftheConventionalCells 67
3.1.1MechanicalPropertiesofConventionalMaterials 67
3.1.2LimitationsofConventionalArchitectures 68
3.1.3LimitationsofElectrolytes 69
3.2MechanicalProcessforBendableCells 69
3.2.1EffectofThickness 70
3.2.2EffectofFlexibleSubstratesandNeutralPlane 71
3.3MechanicsofStretchableCells 72
3.3.1WavyArchitecturesbySmallDeformationBuckling Process 72
3.3.2WavyArchitecturesbyLargeDeformationBuckling Process 74
3.3.3IslandBridgeArchitectures 75
3.4StaticElectrochemicalPerformanceofFlexibleCells 76
3.5DynamicPerformanceofFlexibleCells 77
3.5.1BendingCharacterization 78
3.5.2StretchingCharacterization 78
3.5.3ConformabilityTest 79
3.5.4StressSimulationbyFiniteElementAnalysis 79
3.5.5DynamicElectrochemicalPerformanceDuring Bending 83
3.5.6DynamicElectrochemicalPerformanceDuring Stretching 85
3.6SummaryandPerspectives 90 References 90
4FlexibleCells:MaterialsandFabrication Technologies 95
4.1ConstructionPrinciplesofFlexibleCells 95
4.2SubstrateMaterialsforFlexibleCells 95
4.2.1PolymerSubstrates 96
4.2.2PaperSubstrate 97
4.2.3TextileSubstrate 98
4.3ActiveMaterialsforFlexibleCells 98
4.3.1CNTs 98
4.3.2Graphene 99
4.3.3Low-DimensionalMaterials 99
4.4ElectrolytesforFlexibleLIBs 101
4.4.1InorganicSolid-stateElectrolytesforFlexibleLIBs 102
4.4.2Solid-statePolymerElectrolytesforFlexibleLIBs 104
4.5ElectrolytesforFlexibleECs 104
4.6NonconductiveSubstrates-BasedFlexibleCells 107
4.6.1Paper-BasedFlexibleCells 108
4.6.2Textiles-BasedFlexibleCells 112
4.6.3PolymerSubstrates-BasedFlexibleCells 117
4.7CNTandGraphene-BasedFlexibleCells 121
4.7.1Free-standingGrapheneandCNTsFilmsforSCs 121
4.7.2Free-standingGrapheneandCNTFilmsforLIBs 122
4.7.3FlexibleCNTs/GrapheneCompositeFilmsforthe Cells 125
4.8ConstructionofStretchableCellsbyNovel Architectures 127
4.8.1StretchableCellsBasedonWavyArchitecture 127
4.8.2StretchableCellsBasedonIsland-Bridge Architecture 129
4.9ConclusionandPerspectives 130
4.9.1MechanicalPerformanceImprovement 131
4.9.2InnovativeArchitectureforStretchableCells 132
4.9.3ElectrolytesDevelopment 132
4.9.4PackagingandTabs 132
4.9.5IntegratedFlexibleDevices 133 References 133
5ArchitecturesDesignforCellswithHighEnergy Density 147
5.1StrategiesforHighEnergyDensityCells 147
5.2GravimetricandVolumetricEnergyDensityof Electrodes 149
5.3ClassificationofThickElectrodes:BulkandFoam Electrodes 151
5.4DesignandFabricationofBulkElectrodes 153
5.4.1AdvantagesofBulkElectrodes 153
5.4.2LowTortuosity:TheKeyforBulkElectrodes 155
5.5CharacterizationandNumericalSimulationof Tortuosity 157
5.5.1CharacterizationofTortuositybyX-rayTomography 157
5.5.2NumericalSimulationofTortuosityonRatesby CommercialSoftware 158
5.6FabricationMethodsforBulkElectrodes 159
5.7ThickElectrodeswithRandomPoreStructure 160
5.7.1Pressure-lessHigh-temperatureSinteringProcess 160
5.7.2ColdSinteringProcess 161
5.7.3SparkPlasmaSinteringTechnology 162
5.7.4BriefSummaryforSinteringTechnologies 165
5.8ThickElectrodeswithDirectionalPoreDistribution 165
5.8.1IterativeExtrusionMethod 165
5.8.2Magnetic-InducedAlignmentMethod 168
5.8.3CarbonizedWoodTemplateMethod 168
5.8.4IceTemplatesMethod 172
5.8.53D-PrintingforThickElectrodes 173
5.8.6BriefSummaryforBulkElectrodes 175
5.9Carbon-BasedFoamElectrodeswithHighGravimetric EnergyDensity 178
5.9.1GrapheneFoam 179
5.9.2CNTsFoam 181
5.9.3CNT/GrapheneFoam 181
5.10Carbon-BasedThickElectrodes 182
5.10.1LowElectronicConductiveMaterial/CarbonFoam 182
5.10.2LargeVolumeVariationMaterials/CarbonFoam 186
5.10.3CompactGrapheneElectrodes 188
5.10.4SummaryforCarbonFoamElectrodes 189
5.11ThickElectrodesBasedontheConductivePolymer Gels 191
5.12SummaryandPerspectives 193 References 195
6MiniaturizedCells 205
6.1Introduction 205
6.1.1DefinitionoftheMiniaturizedCellsandTheir Applications 205
6.1.2ClassificationofMiniaturizedCells 206
6.1.3DevelopmentTrendsoftheMiniaturizedCells 207
6.2EvaluationMethodsfortheMiniaturizedCells 209
x Contents
6.2.1EvaluationMethodsforElectricDouble-layerm-ECs 210
6.2.2Evaluationmethodsform-LIBsandm-ECs 211
6.3ArchitecturesofVariousMiniaturizedCells 212
6.4MaterialsfortheMiniaturizedCells 213
6.4.1ElectrodeMaterials 213
6.4.2ElectrolytesfortheMiniaturizedCells 214
6.5FabricationTechnologiesforMiniaturizedCells 215
6.5.1FabricationofMiniaturizedCellswith2DParallelPlate Configuration 216
6.6FabricationTechnologiesfor2DInterdigitatedCells 220
6.7PrintingTechnologiesfor2DInterdigitatedCells 222
6.7.1AdvantagesofPrintingTechnologies 222
6.7.2ClassificationofPrintingTechniques 222
6.7.3ScreenPrintingforMiniaturizedCells 224
6.7.4InkjetPrinting 228
6.8ElectrochemicalDepositionMethodfor2DInterdigitated Cells 228
6.9LaserScribingfor2DInterdigitatedCells 231
6.10InSituElectrodeConversionfor2DInterdigitated Cells 234
6.11FabricationTechnologiesfor3DIn-planeMiniaturized Cells 236
6.11.13DPrintingfor3DInterdigitatedConfigurationCells 236
6.11.23DInterdigitatedConfigurationbyElectrodeposition 239
6.12FabricationofMiniaturizedCellswith3DStacked Configuration 240
6.12.13DStackedConfigurationbyTemplateDeposition 241
6.12.23DStackedConfigurationbyMicrochannel-Plated DepositionMethods 245
6.13IntegratedSystems 247
6.14SummaryandPerspectives 249 References 250
7SmartCells 263
7.1DefinitionofSmartMaterialsandCells 263
7.1.1DefinitionofSmartCells 263
7.1.2DefinitionofSmartMaterials 263
7.2TypeofSmartMaterials 264
7.2.1Self-healingMaterials 264
7.2.2Shape-memoryAlloys 265
7.2.3Thermal-respondingPTCThermistors 266
7.2.4ElectrochromicMaterials 267
7.3ConstructionofSmartCells 268
7.3.1Self-healingSiliconAnodes 268
7.3.2AqueousSelf-healingElectrodes 271
7.3.3Liquid-alloySelf-healingElectrodeMaterials 273
7.3.4Thermal-respondingLayer 274
7.3.5Thermal-respondingElectrodesBasedonthePTC Effect 276
7.3.6IonicBlockingEffect-BasedThermal-responding Electrodes 278
7.4ApplicationofShape-memoryMaterialsinLIBsand ECs 280
7.4.1Self-adaptingCells 280
7.4.2Shape-memoryAlloy-BasedThermalRegulator 281
7.5Self-heatingandSelf-monitoringDesigns 282
7.5.1Self-heating 283
7.5.2Self-monitoring 285
7.6IntegratedElectrochromicArchitecturesforEnergy Storage 286
7.6.1IntegrationPossibilities 286
7.6.2IntegratedElectrochromicECs 287
7.6.3IntegratedElectrochromicLIBs 289
7.7SummaryandPerspectives 291 References 292
Index 301
Preface
Electrochemicalenergystorageinthecells(herethesearelithiumionbatteries (LIBs)andelectrochemicalcapacitors(ECs))hasbeenrecognizedasthemost promisingtechnologyforportableelectronicsaswellasstationaryandvehicle applications.Existingtechnologiesstillfaceperformanceandcostchallenges, includingbarriersinspecificenergy,energydensity,servicelife,andenergyefficiencyathighrates.Overthepastdecades,portableelectronicshavebeenusedin everyaspectofourdailylife.Oneofthekeycomponentsoffutureportabledevices isthecompatiblecellwithanultrahighenergydensityandspecificfeatures(e.g. miniaturization,integration,flexibility,andsmartfunctions).
Developingadvancedcellsalwaysrequiresthediscoveryofnewmaterials,new electrochemistry,andanincreasedunderstandingoftheprocessesonwhichthe devicesdepend.Theoverallperformanceofthecellsislimitedbythefundamentalbehavioroftheusedmaterials,includingelectrodeactivematerials,electrolytes, separators,andothercomponents.Unfortunately,theconventionalfabricationtechnologyandarchitecturesofelectrodesbasedonthesematerialshavealmostreached theirlimits,whichcannotsatisfyfuturerequirements.Therefore,forthecomingera ofportableelectronics,weurgentlyneedtoreconsiderhowwerationallydesignand intelligentlyfabricateadvancedandintelligentcells.Weneedtonotonlyconstruct novelconfigurationsofmaterials,electrolytes,separators,and,asresults,thecells tomeetthedesiredcriteriabutalsodevelopsmarttechnologiestofabricatethese electrochemicalenergystoragedevicesinaneconomicallyviableandtime-efficient manner.
Inthisbook,wewillpresentacomprehensiveintroductionofthedevelopmentsof innovativematerials,architecturesanddesignconsiderationsintheelectrode,and cellconfigurations,togetherwiththerecenttechnologiesusedtoachievethesenovel designs.Aswewantedtowriteabookforresearchers,engineers,andstudents,we tryourbesttounderstandthecurrentapplicationofthecellsinportableelectronic products.
ThewritingofthisbookwascompletedbyProf.FengLiandDr.LeiWen,and Prof.Hui-mingChengrevisedit.TheoriginofthisbookisfromthemeetingofProf. LiandDr.ZaiYuinChinaNano2017atBeijing.Dr.Yuwishedthatwecanwritea bookaboutourresearch.Itisahardworkforusandnewchancetothinkabout ourresearchinsight.In2018,wewroteanoutlineofthebookandpassedittothe
reviewersforapproval.Afterthat,webeganwritingthebook.AlthoughDr.ZaiYu hasleftWiley,Ms.ArunaPragasamisveryhappytocontinuetocontactandhelp us.Ononeoccasion,Prof.LiwenttoTsinghuaUniversityandtalkedwithProf. QiangZhangandProf.JiaqiHuang.WeknowthatDr.ShaoyuQianisresponsible forauthorofChinaregion.Sheansweredourquestionswithpatienceduringthe writing.
Finally,wewouldliketothankallscientistswhohavebeenhelpfulinthepreparationofthisbookandallcolleagueswhokindlydevotedtheirtimeandeffortsto contributechaptersanddiscussions.WethankDr.HongzeLuo,fromCouncilfor ScientificandIndustrialResearch(CSIR),SouthAfrica,forpreparingthedraftof Chapter6;Dr.ZhigangZhao,fromSuzhouInstituteofNano-techandNano-Bionics (SINANO),China,forhishelpinthepreparationofElectrochromicCellssection inChapter7;Drs.JiLiangandHouFeng,MrsHaoLi,NanLi,andMissPengyi LufromTianjinUniversity,China,fortheirhelpfuldiscussionandinitialdrafting ofChapters3and4;andDr.LiqunWang,TianjinNormalUniversity,forthehelpfuldiscussionindraftingChapter6.WewouldliketothankMr.HaoruiShenand HuicongYang,PhDcandidatesinourlab.Mr.Shenhelpedintheplottingoffigures. Mr.HuicongYangisthefirstreaderofthisbookandgavevaluableadvicestoward theentirebook.
WealsothankthefinancialsupportfromNationalNaturalScienceFoundationof China(Nos.51525206,51927803,52020105010,51972313,52072378and51902316), MOST(2016YFA0200102and2016YFB0100100),theStrategicPriorityResearch ProgramoftheChineseAcademyofSciences(XDA22010602),LiaoningRevitalizationTalentsProgram(No.XLYC1908015),YouthInnovationPromotionAssociation oftheChineseAcademyofSciences(No.Y201942)andChinaPetrochemical Cooperation(No.218025).TheBureauofIndustryandInformationTechnologyof Shenzhenforthe“2017GrapheneManufacturingInnovationCenterProject”(No. 201901171523).
Abbreviations
AAOanodizedaluminumoxide
ACactivatedcarbon,alternativecurrent
AGVautomatedguidedvehicles
ALDatomiclayerdeposition
CASChineseAcademyofSciences
CCCDconstantcurrentchargeanddischarge
CMCsodiumcarboxymethylcellulose
CNFcellulosenanofiber
CNTcarbonnanotubes
CVcyclicvoltammetry
CVDchemicalvapordeposition
DECdiethylcarbonate
DMCdimethylcarbonate
DMEdimethoxyethane
DOEDepartmentofEnergy
DOL1,3-dioxolane
EBIDelectronbeam-induceddeposition
ECelectrochemicalcapacitors,ethylenecarbonate
EDLCelectricdouble-layercapacitors
EGexfoliatedgraphene
EISelectrochemicalimpedancespectroscopy
ELDelectrolyticdeposition
EMCethylmethylcarbonate
EPDelectrophoreticdeposition
ETPTAethoxylatedtrimethylolpropanetriacrylate
EVelectricvehicle
EVAethylenevinylacetate
FTOfluorine-dopedtinoxide
GCEgelcompositeelectrolyte
GCPgraphene-coatedpaper
GICgraphiteintercalationcompound
GNSgraphenenanosheet
GOgrapheneoxide
xvi Abbreviations
HEVhybridelectricvehicle
HFPhexafluoropropylene
IDCInternationalDataCorporation
IHPinnerHelmholtzplane
IoTInternetofThings
IREAInternationalRenewableEnergyAgency
ITOindiumtinoxide
LBLlayer-by-layer
LCOLiCoO2
LDHlayerdoublehydroxides
LFPLiFePO4
LIBlithiumionbattery
LIBOBlithiumbis(oxalato)borate
LIPONlithiumphosphorusoxynitrides
LLZOLi7 La3 Zr2 O12
LMOLiMn2 O4
LTOLi4 Ti5 O12
m-LIBsmicrolithiumionbatteries
m-ECsmicroelectrochemicalcapacitors
MCMBmesocarbonmicrobeads
mtoemilliontonoilequivalent
NASICONsodiumsuperionicconductor
NCALiNix Coy Al1 x y O2
NEDONewEnergyandIndustrialTechnologyDevelopmentOrganization
NiCdnickelcadmium
NiMHnickelmetalhydride
NMCLiNix Mny Co1 x y O2
NMP N -methyl-2-pyrrolidone
NWnanowire
OHPouterHelmholtzplane
P3DTpoly(3-decylthiophene)
P3OTpoly(3-octylthiophene-2,5-diyl)
P3OPyPSSpoly(3-octylpyrrole)poly(styrenesulfonate)
PAApolyacrylicacid
PAAmpolyacrylamide
PANIpolyaniline
PCpropylenecarbonate
PDApolydopamine
PDMSpolydimethylsiloxane
PEpolyethylene
PEDOTpoly(3,4-ethylenedioxythiophene)
PEOpolyoxyethylene
PEGpolyethyleneglycol
PENpolyethylenenaphthalate
PESpolyethersulfone
Abbreviations xvii
PETpolyethyleneterephthalate
PIpolyimide
PMMApolymethylmethacrylate
PPpolypropylene
PPOpropyleneoxide
PPYpolypyrrole
PSSpoly(styrenesulfonate)
PTCpositivetemperaturecoefficient
PTFEpolytetrafluoroethylene
PUpolyurethane
PVApolyvinylalcohol
PVDFpolyvinylidenedifluoride
PVPpolyvinylpyrrolidone
rGOreducedgrapheneoxide
RFradiofrequency
RTILroom-temperatureionicliquid
SEsolidelectrolyte
SEBSstyrene–ethylene–butylene–styrene
SEIsolidelectrolyteinterphase
SEMscanningelectronmicroscope
SHEstandardhydrogenelectrode
SHPself-healingpolymer
SPEsolidpolymerelectrolyte
SPRPStrategicPriorityResearchProgram
TEABF4 tetraethylammoniumtetrafluoroborate
TEMtransmissionelectronmicroscope
TMOtransitionmetaloxides
MWCNTsmulti-walledcarbonnanotubes
UPyureidopyrimidinone
UVultraviolet
Introduction
Theworldiswitnessingincreasingrequirementsforenergytomeettheneedsof modernsociety.Duetothedrasticclimatewarmingaroundtheworld,developing innovativesustainablecleanenergy(wind,tidal,solarenergy,etc.)withhighenergy efficiencyisextremelyimportant.However,variousrenewableenergytoelectricity arequitefluctuatingovertime,anddevelopingreliableenergystoragesystemsis animportantwaytosolvethesechallenges.Therefore,tosatisfytheincreasing socialandindustrialdemands,betterelectrochemicalenergystoragedevicesshould beused.
Onthispoint,searchingfornovelelectrochemicalenergystoragesystemwith exceptionalelectrochemicalpropertiesforenergystorageisessential.Inthischapter, wewillfirstgiveabriefintroductiontowardvariouselectrochemicalenergystorage devices,includingelectrochemicalcapacitors(ECs)andlithiumionbatteries(LIBs).
1.1EnergyConversionandStorage:AGlobalChallenge
NobelchemistryprizewinnerRichardSmalleyhadsaid:“Energyisthesinglemost importantproblemfacinghumanitytodayandenergyisthelargestenterpriseon Earth”[1].Nowadays,theenergygenerationstillmainlyreliesonfossilfuels(oil, coal,andgas),whichoccupy80%oftotalenergyneedsintheworld.Ontheother hand,thefossilfuelsarestilltobethedominantprimaryenergyresourcesformany yearsinthefuture.Therefore,limitedsuppliesofthefossilfuelsmakeitimperativethatcombustion-basedenergysourcesshouldbereplacedbycleanandrenewableenergy[2].Thesesustainableenergiesmainlyincludehydropower,solar,wind, geothermal,andtidalenergy.
Figure1.1showstheglobalenergyconsumption.In2018,thegrowthrateofglobal energyconsumptionis2.9%,whichwasthehighestratesince2010.Althoughfossil energystilloccupiesthemostenergyconsumptionaroundtheworld,therenewableenergyalsomadeasignificantincreaseinrecentyears[4].Gasandrenewables havethemostobviousincreaseamongvariousenergysourcessince2010[3].
Moreimportantly,therenewableenergysectoralsohassignificantsocialandeconomicimpact.In2018,atleast11millionpeoplewereemployedintherenewable energysectoraroundtheworld.Comparedwiththedatain2016,thegrowthrate NovelElectrochemicalEnergyStorageDevices:Materials,Architectures,andFutureTrends, FirstEdition.FengLi,LeiWen,andHui-mingCheng. ©2021WILEY-VCHGmbH.Published2021byWILEY-VCHGmbH.
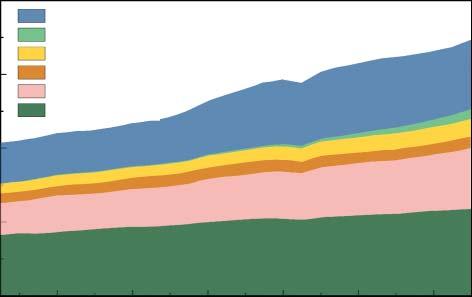
Figure1.1 Amountsofglobalenergyconsumption.

Figure1.2 Globalrenewableenergyemploymentindexedbytechnology.Source: InternationalRenewableEnergyAgency[4].©2018,IRENA-InternationalRenewable EnergyAgency.
ofemploymentwas5.3%[4].AsshowninFigure1.2,thetopfiveemploymentin therenewableenergysectoronthelistincludessolarphotovoltaic,liquidbiofuels, hydropower,windenergy,andsolarheating.
Electricityhasbeenconsideredasthemosteffectivewaytoexploreandutilize variousrenewableenergieseffectively.Comparedwithotherformsofenergy, electricityhasmanyobviousadvantagesasfollows[5]:(i)Convenience:electricity canbeeasilytransformedintothedesiredformsofenergy,suchasheat,light,and mechanicalenergy.(ii)Easycontrol:electricitycansimplybeoperatedandtuned.

Figure1.3 Applicationofelectrochemicalenergystorage,AGV(automatedguided vehicles).
(iii)Flexibility:electricitycanbeeasilytransferredbytransmissionline.(iv)Cheap: comparedwithotherformsofenergy,electricityisaneconomicalform,whichhas beenwidelyusedfordomesticandindustrialapplications.(v)Lowtransmission loss:electricitycanbeeasilytransmittedwithhighefficiencyfromthepowerplant totheuser.
Althoughelectricityhasmanyadvantages,therenewableenergy-basedelectricity isquitefluctuatingovertime.Forexample,thecloudsconstantlyaltertheoutputof solarenergysystemsandwindcannotblowatafixedspeed.Unfortunately,gridhas afixedfrequencyof50Hz,whichwasdeterminedbyturbinesinpowerplants.These mustbematchedtoavoidthefluctuationofgrid.Therefore,thecleanenergy-based electricityrequirestobestoredanddeliveredforcommercialusage.
Asaresult,renewableenergycallsforthedevelopmentofelectricitystorage devices.Amongthesevariouselectrochemicalenergystoragesystems,ECsand variousbatterieshaveshowedgreatpotentialnotonlyinthepoweringportable electronicsbutalsointhetransportationsector.
AsshowninFigure1.3,variouselectrochemicalenergystoragehasbeenwidely usedineveryaspectinourdailylife,suchasaerospace(satellites,rockets,andaircrafts),transportation(cars,trains,andships),portableelectronicgadgets(mobile phones,laptops,anddigitalcameras),andindustryfields[6].Theever-growing advancementofelectrochemicalenergystoragetechnologyhasgreatlypromoted thedevelopmentofhumansociety.Itcanbeanticipatedthatelectrochemical energystoragematerialsandtechnologyplaymoreimportantroleinhumanlife.
1.2DevelopmentHistoryofElectrochemicalEnergy Storage
AsshowninFigure1.4,thefirstelectrochemicalenergystoragechemistryinhistory isBaghdadbattery,whichconsistedofaceramicpot,atubeofcopper,arodofiron, andvinegarelectrolyte.Thisancientbatteryhas ∼2.0voltsofelectricity[8].

Figure1.4 ReplicaofBaghdadbatteryfoundinIraq.Source:Aquiziam[7].
ThefirstmodernbatterywasinventedbytheItalianscientistVoltain1799,which wascalledas“Voltapile.”ThisbatterywasastackofAgandZndisks,andthemetal diskswereseparatedbysaltwater-soakedcloth[9].Voltafoundthatthesinglepile couldonlyproduce1.0–2.0voltsofelectricity.Toincreasethevoltageoutput,several “Voltapiles”couldbeconstructedsidebyside.Theprocessesthatoccurinthedevice werelaterdemonstratedbyHumphryDavyandMichaelFaraday,whichdescribed thatitistheoccurrenceofchemicalreactionsthatisresponsiblefortheproduction ofelectricity[9].
Thisfindingmarkedtheemergenceoftheelectrochemistry.Consequently, theresearchanddevelopmentofvariouselectrochemicalenergystoragesystems becameactiveinthenineteenthandtwentiethcenturies.[10]Thesimplehistoryof electrochemicalenergystorageisshowninFigure1.5.
1.3ClassificationofElectrochemicalEnergyStorage
Theelectrochemicalenergystoragehasalmostpenetratedeveryaspectinourdaily life,whichcanefficientlystoreandconvertenergyreversiblybetweenchemical energyandelectricalenergyinanenvironmentallyfriendlyway.
Therearemanyrequirementsthatelectrochemicalenergystorageneedstofulfill forvariousapplicationfields,suchasportableelectronics,electricvehicles(EVs), andpowertools.Themainrequirementsfordifferentelectrochemicalenergy storageincludehighgravimetric/volumetricenergydensity,longcyclelife,low cost,safety,andeasyfabrication[11].Theseattributesaremainlydeterminedbythe intrinsicpropertiesofthematerialsandchemistryconstitutingtheelectrochemical
1.3ClassificationofElectrochemicalEnergyStorage

Figure1.5 Developmentofvariouselectrochemicalenergystorage.
Figure1.6 Classificationof electrochemicalenergystorage.EDLC, electricdouble-layercapacitors.
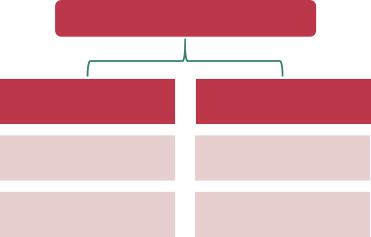
energystorage[12].Basedonthechargestoragemechanism,theelectrochemical energystoragetechnologyhastwomaincategories:ECsandbatteries,asshownin Figure1.6.
AsshowninFigure1.6,ECshavetwomechanismstostoreelectricity:double-layer capacitanceandpseudocapacitance.Double-layercapacitanceisbasedonionic adsorption,whereaspseudocapacitanceisanelectrochemicalprocess.Electrochemicalbatterieshavetwobroadcategories,primaryandsecondarybatteries.A primarybatteryisonethatcannoteasilyberechargedafteroneuse.Anexampleof aprimarybatteryisthedrycell,whichwascommonlyusedtopowerremotesand clocks.Insuchcells,aZncontaineractsasthenegativeandacarbonrodactsas thepositive.Asecondarybatterycanberechargedtotheiroriginalpre-discharge status,suchasLIBs,NiCd,andNiMHbatteries.